Abstract
Background
Racial disparities among clinical trial participants present a challenge to assess whether trial results can be generalized into patients representing diverse races and ethnicities. The objective of this study was to evaluate the impact of race and ethnicity on treatment response in patients with advanced non‐small cell lung cancer (aNSCLC) treated with programmed cell death‐1 (PD‐1) or programmed cell death‐ligand 1 (PD‐L1) inhibitors through analysis of real‐world data (RWD).
Materials and Methods
A retrospective cohort study of 11,138 patients with lung cancer treated at hospitals within the Mount Sinai Health System was performed. Patients with confirmed aNSCLC who received anti‐PD‐1/PD‐L1 treatment were analyzed for clinical outcomes. Our cohort included 249 patients with aNSCLC who began nivolumab, pembrolizumab, or atezolizumab treatment between November 2014 and December 2018. Time‐to‐treatment discontinuation (TTD) and overall survival (OS) were the analyzed clinical endpoints.
Results
After a median follow‐up of 14.8 months, median TTD was 7.8 months (95% confidence interval, 5.4–not estimable [NE]) in 75 African American patients versus 4.6 (2.4–7.2) in 110 White patients (hazard ratio [HR], 0.63). Median OS was not reached (18.4–NE) in African American patients versus 11.6 months (9.7–NE) in White patients (HR, 0.58). Multivariable Cox regression conducted with potential confounders confirmed longer TTD (adjusted HR, 0.65) and OS (adjusted HR, 0.60) in African American versus White patients. Similar real‐world response rate (42.6% vs. 43.5%) and disease control rate (59.6% vs. 56.5%) were observed in the African American and White patient populations. Further investigation revealed the African American patient group had lower incidence (14.7%) of putative hyperprogressive diseases (HPD) upon anti‐PD‐1/PD‐L1 treatment than the White patient group (24.5%).
Conclusion
Analysis of RWD showed longer TTD and OS in African American patients with aNSCLC treated with anti‐PD‐1/PD‐L1 inhibitors. Lower incidence of putative HPD is a possible reason for the favorable outcomes in this patient population.
Implications for Practice
There is a significant underrepresentation of minority patients in randomized clinical trials, and this study demonstrates that real‐world data can be used to investigate the impact of race and ethnicity on treatment response. In retrospective analysis of patients with advanced non‐small cell lung cancer treated with programmed cell death‐1 or programmed cell death‐ligand 1 inhibitors, African American patients had significantly longer time‐to‐treatment discontinuation and longer overall survival. Analysis of real‐world data can yield clinical insights and establish a more complete picture of medical interventions in routine clinical practice.
Keywords: Non‐small cell lung cancer, Real‐world evidence, Electronic health records, Overall survival, Immunotherapy
Short abstract
Racial disparities in clinical trials affect whether trial results can be generalized for diverse patient populations. This article evaluates real‐world data to assess the impact of race and ethnicity on treatment response in patients with advanced non‐small cell lung cancer treated with PD‐1 or PD‐L1 inhibitors.
Introduction
Evidence‐based medicine (EBM) has been the guiding principle in today's clinical practice. The main source of evidence for EBM comes from randomized clinical trials (RCTs). Although RCTs are rigorously designed and adequately powered in a well‐controlled target population and have high internal validity because of unbiased methodology with prespecified clinical endpoints, there are notable weaknesses associated with RCTs. RCTs are generally carried out in a rather homogeneous patient subpopulation and under ideal circumstances; therefore, the trial results may not be necessarily generalizable to a much more heterogeneous patient population in real‐world clinical settings [1, 2]. As defined by the U.S. Food and Drug Administration (FDA) (https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence), real‐world data (RWD) are data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources including electronic health records, registries, and claims databases. RWD augment results from RCTs on the effectiveness of the approved medications in a broader, more diverse patient population and help to establish a more complete picture of a medication in clinical practice [3, 4].
Anti‐programmed cell death‐1 (PD‐1) and anti‐programmed cell death‐ligand 1 (PD‐L1) inhibitors represent a new class of immunotherapy drugs for systemic treatment of cancers [5]. Currently, three anti‐PD‐1/PD‐L1 inhibitors, nivolumab, pembrolizumab, and atezolizumab, have been approved by the FDA for the treatment of advanced non‐small cell lung cancer (aNSCLC) in the metastatic disease setting. There are significant racial disparities in registration trials of these drugs leading to FDA approvals. Notably, only 1%–4% of the trial participants are Black or African American and a subgroup analysis based on race and ethnicity was not even attempted in these trials [6, 7, 8, 9, 10]. This disparity raises questions if trial results can be generalized to patients with diverse races and ethnicities and if race/ethnicity impacts treatment response to anti‐PD‐1/PD‐L1 therapies. A recent study of RWD in patients with aNSCLC treated with nivolumab or pembrolizumab examined clinical outcome data based on overall survival (OS) and compared the results with those from registration trials [11]. However, only 5.7% of the patients in the study cohort were African American, and a race‐based analysis, again, was not performed.
The Mount Sinai Health System (MSHS) is a hospital network in New York City. Because of their geographic locations in highly diverse communities, MSHS hospitals are distinguished among most hospital networks by a high percentage of minority patient populations, with approximately 40% of patients cared for by the MSHS being African American or Hispanic (https://www.mountsinai.org/). In this study, we first queried MSHS electronic medical record (EMR) databases to identify patients with aNSCLC treated with anti‐PD‐1/PD‐L1 inhibitors. We then assessed patient outcomes using time‐to‐treatment discontinuation (TTD) and OS as clinical endpoints. Instead of progression‐free survival (PFS), TTD was selected as an intermediate clinical endpoint because it has been shown TTD exhibited consistently higher correlation with OS in RWD [12]. The primary objectives of this retrospective, observational study were to investigate (a) if there are differences in treatment response among patients with different races and ethnicities, and (b) if there are other demographic and clinical characteristics associated with clinical outcomes in patients with aNSCLC treated with anti‐PD‐1/PD‐L1 therapy. To avoid any potential bias and misinterpretation caused by imbalances of the analyzed confounders, we also performed multivariable analyses.
Materials and Methods
Data Sources
MSHS EMR databases were used for this study. Demographic and clinical variables were obtained by either directly querying structured tables or abstracting the relevant information from unstructured clinical reports. Race and ethnicity in the MSHS EMR databases are self‐reported. The final race category was derived from the most common recorded race and ethnicity values. Those identifying as Other and White/Caucasian, Other and Black or African American Other and Hispanic/Latino were placed into the White, African American, and Hispanic groups, respectively. Those identifying as Asian along with Pacific Islander or Other were categorized as Asian.
Clinical Endpoints
TTD was computed as: “the last administration date” minus “the first administration date” plus one. The treatment discontinuation event was defined as permanent discontinuation of treatment. Permanent discontinuation was determined when one of the following conditions was met (https://www.focr.org/rwe): (a) having a subsequent line of systemic therapy after the anti‐PD‐1/PD‐L1‐containing regimen; (b) having a date of death while on the anti‐PD‐1/PD‐L1‐containing regimen; (c) having a gap of more than 120 days between the last administration of anti‐PD‐1/PD‐L1 therapy and the patient's last visit, if no other systemic therapy could be identified after the anti‐PD‐1/PD‐L1 treatment. Patients without permanent discontinuation were censored at their last administration date of the anti‐PD‐1/PD‐L1 therapy. OS was computed as the time from the first administration date of the anti‐PD‐1/PD‐L1 therapy to the death date recorded in the MSHS death registry. The OS event was defined as death. Patients without an event were censored at their last visit date.
Treatment follow‐up time was reported as the known function time: the time from the first administration date of the anti‐PD‐1/PD‐L1 therapy to the last visit date (or end of study date in the case of the event, death). This information is useful to determine the relevant time frame of the Kaplan‐Meier curves where enough time has passed for events to occur. It is standard to report the follow‐up rate for cohort studies using methods such as the percentage method or Clark's completeness index. Here we used a version of the simplified person‐time method (SPT) [13], in which individuals who died or had the event were treated as the same as those followed to the end of the study versus those who dropped out:
where N is the number of individuals in the study, τ is the time defining the end of the study, T i and C i are the time to the event and censoring for the ith individual, and N τ is the potential total follow‐up time.
Treatment response to anti‐PD‐1/PD‐L1 therapy was curated from patients’ progress reports written by medical oncologists based on their interpretation of imaging reports. As the determination of radiographic response oftentimes did not follow the RECIST criteria [14] in real‐world clinical practice, we only captured the exact language used by the oncologists that describe response in three categories: response, stable disease, and progressive disease.
Results
Cohort Selection
The study cohort was identified from MSHS EMR databases at a cutoff date of December 15, 2018, for the first treatment date, and follow‐up time went through May 31, 2019. A total of 11,138 patients had International Classification of Diseases (ICD) codes (ICD‐9: 162.*; ICD‐10: C34.*) for lung cancer diagnosis, and 1,780 of those had systemic treatment including at least one drug listed in the National Comprehensive Cancer Network guideline for non‐small cell lung cancer (NSCLC). Of these 1,780 patients, 314 received nivolumab, pembrolizumab, or atezolizumab between November 2014 and December 2018 for any line of treatment. After more extensive chart reviews, 249 of these 314 patients were confirmed as having been treated with nivolumab, pembrolizumab, or atezolizumab for aNSCLC. Concurrent chemotherapy treatment was also noted. Demographic, clinical, and treatment characteristics of the 249 patients are shown in Table 1. In comparison with published clinical studies of patients with aNSCLC treated with anti‐PD‐1/PD‐L1 therapy (Table 2), 30.1% of the 249‐patient cohort in this study are African Americans, significantly higher than those in registration trials (1%–4%) [6, 7, 8, 9, 10] or in a previous study of RWD (6%) [11].
Table 1.
Characteristics of a cohort of 249 patients with aNSCLC who received nivolumab, pembrolizumab, or atezolizumab in the metastatic setting in MSHS hospitals
| Characteristics | n (%) | TTD | OS | ||
|---|---|---|---|---|---|
| Median (95% CI), mo | p value | Median (95% CI), mo | p value | ||
| All | 249 (100) | 5.6 (3.7–7.8) | 17.4 (11.9–NE) | ||
| Age at PD1/PD‐L1 inhibitor initiation (IQR) | 67.3 (61.3–74.3) | ||||
| Age categories at PD1/PD‐L1 inhibitor initiation, yr | .582 | .961 | |||
| <50 | 7 (2.8) | 3.3 (2.8–NR) | 17.4 (17.4–NE) | ||
| 50–64 | 92 (36.9) | 5.3 (2.6–8.5) | NR (11.2–NE) | ||
| 65–74 | 96 (38.6) | 5.6 (3.7–7.8) | 14.6 (11.2–NE) | ||
| 75+ | 54 (21.7) | 9.7 (3.5–NE) | 15.9 (9.9–NE) | ||
| Gender | .639 | .031 | |||
| Female | 122 (49.0) | 5.6 (3.5–8.5) | NR (16.4–NE) | ||
| Male | 127 (51.0) | 5.6 (3.3–8.8) | 12.5 (9.7–NE) | ||
| Race/ethnicity | .342 | .161 | |||
| White | 110 (44.2) | 4.6 (2.4–7.2) | 11.6 (9.7–NE) | ||
| African American | 75 (30.1) | 7.8 (5.4–NE) | NR (18.4–NE) | ||
| Asian | 24 (9.6) | 4.9 (2.8–NE) | NR (12.6–NE) | ||
| Hispanic | 35 (14.1) | 4.7 (2.6–12.9) | 17.4 (9.9–NE) | ||
| Other race | 4 (1.6) | ‐ | ‐ | ||
| Unknown/missing | 1 (0.4) | ‐ | ‐ | ||
| Histology | .187 | .91 | |||
| Nonsquamous cell carcinoma | 189 (75.9) | 5.6 (3.3–7.3) | 16.4 (11.3–NE) | ||
| Squamous cell carcinoma | 48 (19.3) | 7.9 (4.6–NE) | NR (11.5–NE) | ||
| NSCLS, NOS | 12 (4.8) | 3.4 (1.4–NE) | NR (7.5–NE) | ||
| Smoking status | .014 | .145 | |||
| Current or former smoker | 207 (83.1) | 6.6 (4.9–8.5) | 15.9 (11.5–NE) | ||
| Current smoker | 90 (36.1) | 8.3 (6–12.5) | 19.5 (12.6–NE) | ‐‐ | |
| Former smoker | 117 (47.0) | 5.3 (3.0–7.9) | 11.9 (10.9–NE) | ‐‐ | |
| Never Smoker | 38 (15.3) | 3.5 (2.4–5.1) | NR (13.2–NE) | ||
| Not Reported | 4 (1.6) | ‐ | ‐ | ||
| Line of treatment | .369 | .106 | |||
| 1 | 113 (45.4) | 7.2 (4.4–12.2) | NR (13.2–NE) | ||
| 2 or higher | 136 (54.6) | 5.3 (3.0–7.8) | 14.6 (10.9–NE) | ||
| 2 | 102 (41) | 4.9 (2.8–8.8) | 14.6 (10.5–NE) | ‐‐ | |
| 3 or higher | 34 (13.7) | 5.4 (3.0–8.5) | 11.9 (9.9–NE) | ‐‐ | |
| PD‐L1 status | .569 | .118 | |||
| Negative | 52 (20.9) | 4.9 (3.0–NE) | 18.4 (10.5–NE) | ||
| Positive (>1%) | 78 (31.3) | 6.6 (4.9–12.5) | NR (17.4–NE) | ||
| Positive, 1–49% | 35 (14.1) | 5.3 (2.1–13.4) | NR (17.4–NE) | ‐‐ | |
| Positive, ≥50% | 43 (17.3) | 7.9 (4.9–NE) | NR (12.5–NE) | ‐‐ | |
| Unknown or not tested | 119 (47.8) | 4.7 (2.8–7.8) | 11.6 (9.9–NE) | ||
| EGFR mutation/ALK fusion | .222 | .255 | |||
| Mutation present | 25 (10.0) | 3.5 (2.8–NE) | 8.6 (5.6–NE) | ||
| Mutation absent | 157 (63.1) | 5.6 (3.5–7.3) | 18.4 (11.9–NE) | ||
| Not tested | 67 (26.9) | 7.9 (4.4–NE) | NR (11.5–NE) | ||
| Immunotherapy drug | .186 | .412 | |||
| Atezolizumab | 59 (23.7) | 2.8 (2.1–6.6) | NR (10.9–NE) | ||
| Nivolumab | 84 (33.7) | 6.0 (3.3–12.9) | 11.6 (8.6–NE) | ||
| Pembrolizumab | 102 (41.0) | 7.3 (4.9–12.7) | NR (13.2–NE) | ||
| More than one | 4 (1.6) | ‐ | ‐ | ||
| Concurrent with chemotherapy | .053 | .138 | |||
| No | 201 (80.7) | 4.4 (3.0–6.5) | 15.9 (11.5–NE) | ||
| Yes | 48 (19.3) | 8.3 (7.2–NR) | NR (11.6–NE) | ||
p values were based on log‐rank test.
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; IQR, interquartile range; MSHS, Mount Sinai Health System; NE, not estimable; NOS, not otherwise specified NR, not reached; NSCLC, non‐small cell lung cancer; PD‐1, programmed cell death one; PD‐L1, programmed cell death ligand one; ‐ not computed due to small sample size within group; ‐‐ subgroup, p values were computed from the higher level grouping.
Table 2.
Comparison of the cohort in this study to clinical trial cohorts and a published RWD cohort
| Characteristics | Nivolumab (Checkmate 017), n = 135 [7] | Nivolumab (Checkmate 057), n = 292 [6] | Pembrolizumab (Keynote 001), n = 495 [8] | Pembrolizumab (Keynote 189), n = 410 [25] | Atezolizumab (OAK), n = 425 [9] | Atezolizumab (IMpower150), n = 400 [10] | RWD, n = 1,344 [11] | This study, n = 249 |
|---|---|---|---|---|---|---|---|---|
| Age median (range), yr | 62 (39–85) | 61 (37–84) | 64 (28–93) | 65 (34–84) | 63 (33–82) | 63 (31–89) | 69 (32–85) | 67 (39–89+) |
| Gender, male (%) | 111 (82.2) | 151 (51.7) | 261 (52.7) | 254 (62.0) | 261 (61.4) | 240 (60.0) | 747 (55.6) | 127 (51.0) |
| Race/ethnicity (%) | ||||||||
| White | 122 (90.4) | 267 (91.4) | 406 (82.0) | Not reported | 302 (71.1) | 322 (80.5) | 935 (69.6) | 110 (44.2) |
| Asian | 4 (3.0) | 9 (3.1) | 64 (12.9) | Not reported | 85 (20.0) | 56 (14.0) | 41 (3.1) | 24 (9.6) |
| Black/African American | 6 (4.4) | 7 (2.4) | 20 (4.0) | Not reported | 5 (1.2) | 3 (0.8) | 76 (5.7) | 75 (30.1) |
| Other a | 1 (0.7) | 9 (3.1) | 5 (1.0) | Not reported | 13 (3.1) | 6 (1.5) | 114 (8.5) | 39 (15.6) |
| Unknown | 2 (1.5) | – | – | Not reported | 20 (4.7) | 13 (3.3) | 178 (13.2) | 1 (0.4) |
| Smoking (%) | ||||||||
| Current/former smoker | 121 (89.6) | 231 (79.1) | 369 (74.5) | 362 (88.3) | 341 (80.2) | 318 (79.5) | 1,183 (88.0) | 207 (83.1) |
| Never smoker | 10 (7.4) | 58 (19.9) | 126 (25.5) | 48 (11.7) | 84 (19.8) | 82 (20.5) | 150 (11.2) | 38 (15.3) |
| Unknown | 4 (3.0) | 3 (1.0) | – | – | – | – | 11 (0.8) | 4 (1.6) |
| Histology (%) | ||||||||
| Squamous | 135 (100) | – | 85 (17.1) | – | 112 (26.3) | – | 427 (31.8) | 48 (19.3) |
| Nonsquamous | – | 292 (100) | 401 (81.0) | 394 (96.1) | 313 (73.6) | 400 (100) | 872 (64.9) | 189 (75.9) |
| NSCLC, NOS | – | – | 7 (1.4) | 16 (3.9) | – | – | 45 (3.3) | 12 (4.8) |
| Unknown | – | – | 2 (0.4) | – | – | – | – | – |
| OS (95% CI), mo | 9.2 (7.3–13.3) | 12.2 (9.7–15.0) | 12.2 (9.3–14.7) | 22.0 (19.5–25.2) | 13.8 (11.8–15.7) | 19.2 (17.0–23.8) | 8.0 (7.4–9.0) | 17.4 (11.9–NE) |
Other may include Hispanic/Latino.
Abbreviations: CI, confidence interval; NE, not estimable; NOS, not otherwise specified; NSCLC, non‐small cell lung cancer; OS, overall survival; RWD, real‐world data.
Assessment of Clinical Outcomes
The median time to follow‐up after the initiation of anti‐PD‐1/PD‐L1 treatment was 14.8 months (mean 16.9 months). In all patients 65.4% had complete follow‐up, either their last visit date was <120 before the end of the study or they died during the study and had a last visit date within 120 days of their death. The person‐time follow‐up rates, a measure of the completeness of the data, were estimated to be 80.0% and 77.3% for TTD and OS, respectively (in Materials and Methods). Spearman's rank correlation coefficient between the TTD and OS for all individuals, including censored individuals, is 0.78 (similar results were found by methods accounting for censoring: supplemental online Appendix 2). Initial TTD and OS analyses were performed using all 249 individuals without any consideration of potential confounders. Median TTD was 5.6 months (95% confidence interval [CI], 3.7–7.8) with 142 events (Fig. 1A), and median OS was 17.4 months (95% CI, 11.9–NE) with 91 events (Fig. 2A). Additional summary information and statistics for cohort follow‐up may be found in the supplemental online Appendices and in supplemental online Table 1.
Figure 1.
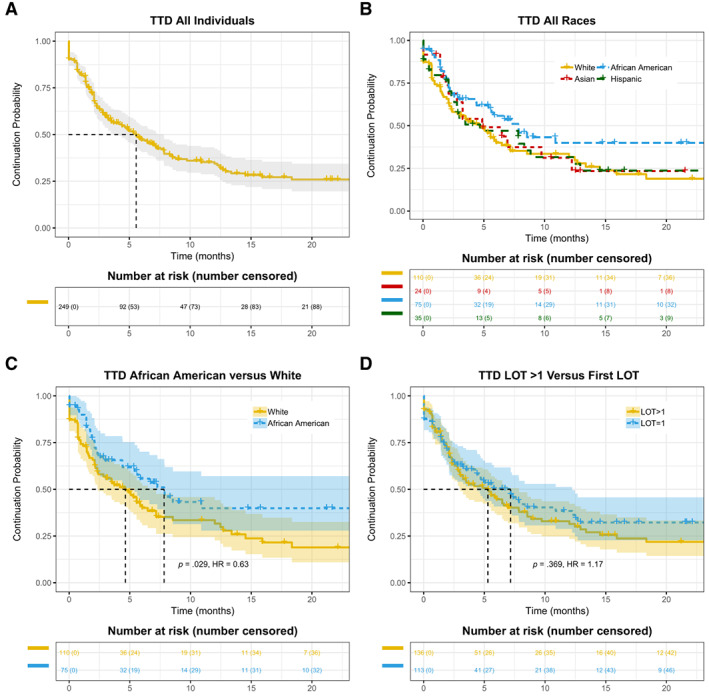
Kaplan‐Meier curves of TTD in patients with advanced non‐small cell lung cancer treated with anti‐programmed death 1/programmed death‐ligand 1 antibodies. (A): The entire cohort. (B): Patients stratified by race and ethnicity. (C): Comparison between African American versus White patients. (D): Patients stratified by lines of therapy. Abbreviations: HR, hazard ratio; LOT, line of treatment; TTD, time‐to‐treatment discontinuation.
Figure 2.
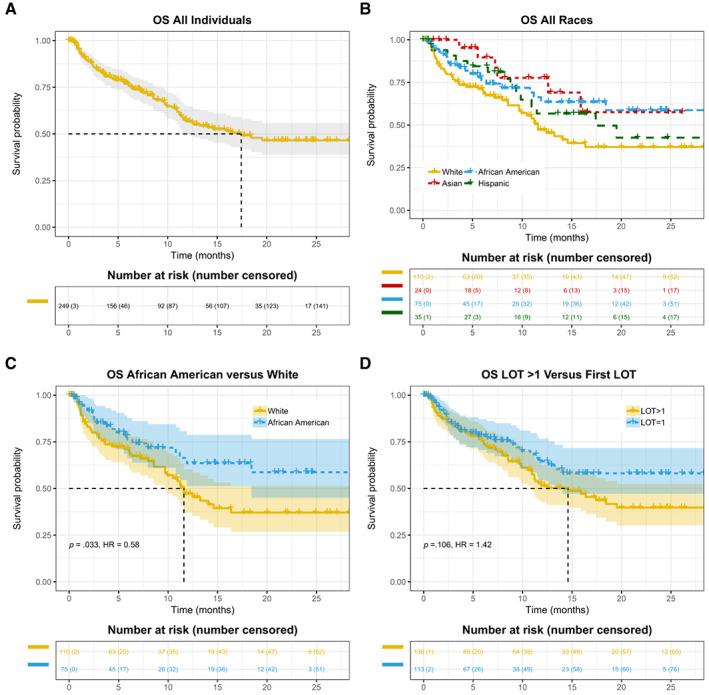
Kaplan‐Meier curves of OS in patients with advanced non‐small cell lung cancer treated with anti‐programmed death 1/programmed death‐ligand 1 antibodies. (A): The entire cohort. (B): Patients stratified by race and ethnicity. (C): Comparison between African American versus White patients. (D): Patients stratified by lines of therapy. Abbreviations: HR, hazard ratio; LOT, line of treatment; OS, overall survival.
Clinical Outcomes in Patients with Different Races and Ethnicities
As a primary objective of the study, we examined TTD and OS among different race/ethnicity groups. Differences in TTD and OS across various groups were assessed using the Kaplan‐Meier method and log‐rank score test. Hazard ratios (HRs) were computed using Cox proportional hazards models (supplemental online Table 2 and supplemental online Appendices 1 and 2 for additional methods and results for single variable HRs). TTD appears to be longer in African American patients versus other race/ethnicity groups (Table 1 and Fig. 1B). We subsequently assessed the statistical significance of different TTD in African American versus White patients. As shown in Fig. 1C, the median TTD was 7.8 months (95% CI, 5.4–NE) in the African American group (n = 75), and 4.6 months (95% CI, 2.4–7.2) in the White group (n = 110; HR for treatment discontinuation or death, 0.63; p = .029). We then asked if longer TTD as a surrogate clinical endpoint in African American patients is associated with improved OS and performed Kaplan‐Meier analysis of survival indexed to anti‐PD‐1/PD‐L1 initiation date (Fig. 2B, C). As shown in Fig. 2C, the median OS was not reached (95% CI, 18.4–NE) in the African American group and was 11.6 months (95% CI, 9.7–NE) in the White group (HR for death, 0.58; p = .033).
Unlike randomized clinical trials in which covariates are balanced in different treatment arms, results from observational studies based on RWD could be confounded by possible covariates due to unbalanced patient characteristics [15]. To examine whether inherent unbalanced covariates may be drivers of the difference in outcome between groups, we further tested the difference of TTD and OS in African American versus White patients with aNSCLC treated with anti‐PD‐1/PD‐L1 therapy via multivariable Cox proportional hazards models with adjusted hazard ratios (aHRs) using the Wald test for significance (Fig. 3). Small numbers within a category lead to unstable estimates of HRs, thus patients with unknown smoking status or other/unknown race were excluded from this analysis. Consistent with Kaplan‐Meier analysis (Fig. 1B, C), results of Cox proportional hazards models also showed longer TTD in African American patients reflected by aHR of less than 1 for treatment discontinuation or death, when White patients were used as the reference (Fig. 3A; aHR, 0.60; p = .065). Better survival in African American patients in the above‐described Kaplan‐Meier analysis was confirmed by Cox proportional hazards models (Fig. 3B; aHR, 0.60; p = .062). Although there were only 24 Asian patients in our cohort, they also exhibited a statistically significant longer OS in comparison with the White patient group (Fig. 3B; aHR, 0.32; p = .023). We further harmonized the data using propensity scores (a form of cohort matching) to balance treatment variables among the different patient groups and found similar results between the African American and White groups, even after adjustment for clinical characteristics within the matched treatment group (supplemental online Appendices 1 and 2 for additional methods and results; supplemental online Figure 1–2; supplemental online Tables 4–6). Additionally, we performed subgroup analysis within smokers and by line of treatment (LOT) to control for potential confounding and bias due to complex interactions between variables for OS (see supplemental online appendix 2—Additional Results; supplemental online Tables 6, 7). Within the smoking cohort (which also excludes individuals with anaplastic lymphoma kinase [ALK]/ epidermal growth factor receptor [EGFR] mutations), the first LOT cohort, and the second LOT cohort, the OS HRs for the African American group versus the White group remain consistent with the overall cohort (range between 0.55 and 0.61; supplemental online Tables 6, 7).
Figure 3.
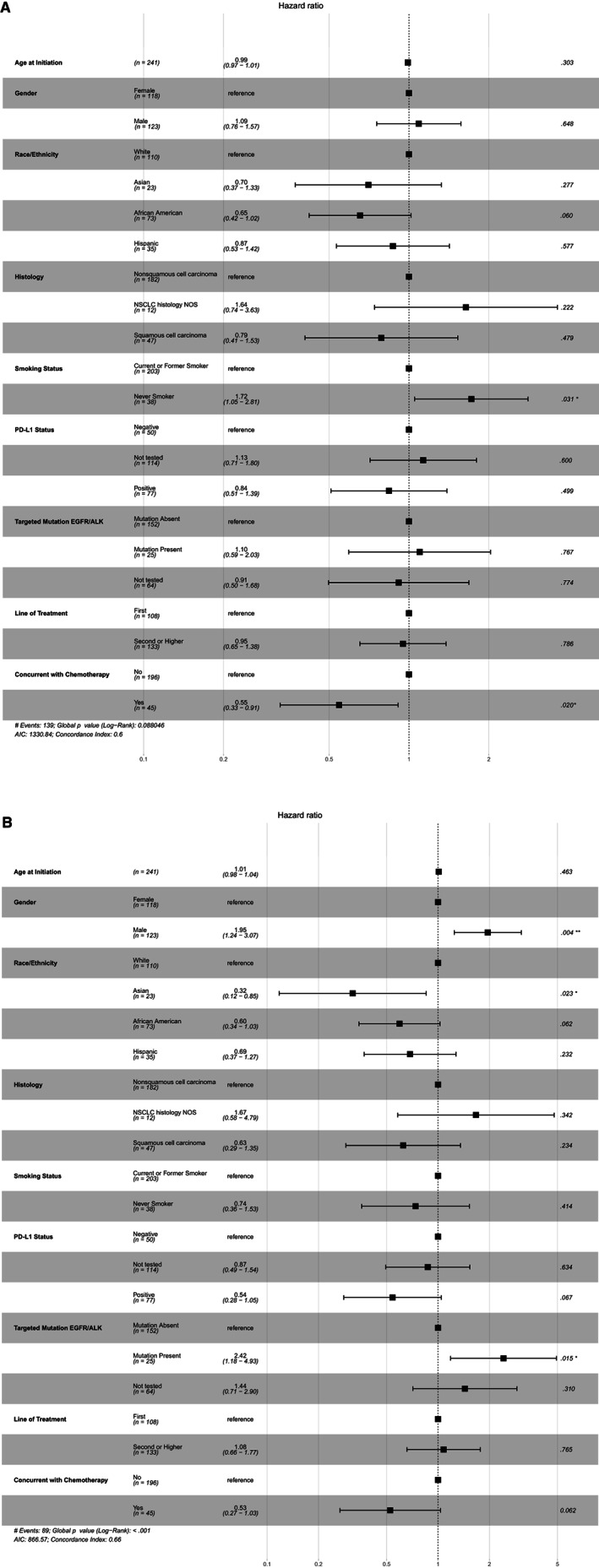
Analysis of (A) time‐to‐treatment discontinuation and (B) overall survival by multivariable Cox proportional hazards models. For each demographic or clinical variable, other variables were adjusted in the Cox proportional hazards models. For each analyzed variable, one subgroup was set as the reference group, and the other subgroups were compared with the reference group. The adjusted hazards ratio with 95% confidence internal and the p values are shown. * <0.05, **<0.005. Abbreviations: AIC, akaike information criterion; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; NOS, not otherwise specified; NSCLC, non‐small cell lung cancer; PD‐L1, programmed cell death‐ligand 1.
For reasons that still remain unclear, others have noted that some of the patients with aNSCLC treated with anti‐PD‐1/PD‐L1‐containing regimen experience unexpected acceleration of disease progression, referred to as hyperprogressive disease (HPD) [16]. We determined HPD upon anti‐PD‐1/PD‐L1 treatment based on time‐to‐treatment failure (TTF) <2 months, an approximate definition used in previous clinical studies [17, 18], specifically when one of the following conditions are met: (a) patients died within 60 days after the initiation of treatment and the death was not due to immune‐related adverse events (irAEs), and (b) patients had documented disease progression within 60 days after the initiation of treatment. A total of 41 (16.5% of the cohort) patients with putative HPD were identified, in line with two prospective clinical studies of HPD in NSCLC in which 13.8% [19] and 18.9% [18] of the anti‐PD‐1/PD‐L1 treated patients in these respective studies developed HPD (in these studies HPD was more precisely determined by comparing tumor growth rate before and after the treatment). When the 249 patients in this study were stratified by race/ethnicity, fewer African American (11/75, 14.7%) patients had HPD than White patients (27/110, 24.5%). We then removed patients with putative HPD and performed Kaplan‐Meier analysis of OS (Fig. 4A). Although there was still a separation of Kaplan‐Meier curves between the two groups 10 months after treatment initiation, the overall difference was less significant (HR, 0.73; p = .331) in comparison to Fig. 2C. To test the statistical significance of the impact of HPD on survival difference, we randomly removed 11 and 27 patients from the African American and White groups respectively, computed the distribution of p values from 1,000 simulations, and the result indicated the difference of HPD had significant impact on OS (p < .001). Additionally, within the African American and White cohorts, we performed a 60‐day landmark analysis in which individuals who died within 60 days of anti‐PD‐1/PD‐L1 initiation (or those with less than 60 days of follow‐up) were excluded (n = 50). These individuals had high overlap with the putative HPD cohort (24 of the 38 HPD individuals were among these 50), and the HR between the African American and White groups remained similar (HR, 0.62; p = .13; Fig. 4B).
Figure 4.
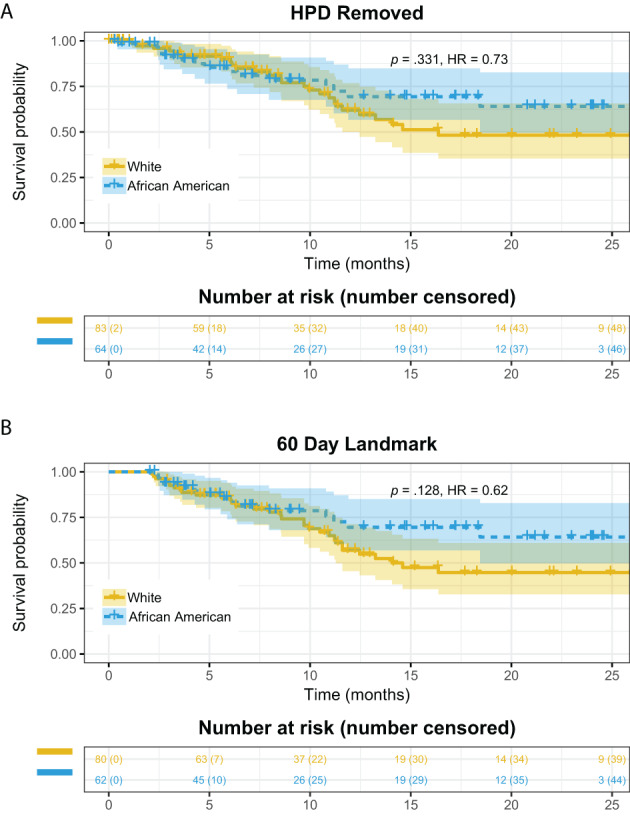
(A): Kaplan‐Meier (KM) curve of overall survival (OS) in patient groups (African American vs. White) after removing patients with putative HPD. (B): Sixty‐day landmark analysis for African American versus White individuals. Individuals with 60 days or less survival time (either because of censoring or death) were excluded and KM curves are compared for OS. Abbreviations: HPD, hyperprogressive disease; HR, hazard ratio.
Finally, we investigated the response rate and disease control rate in the African American and White patient populations through manual curation of progress notes written by medical oncologists based on their assessment of imaging reports (see Materials and Methods). Surprisingly, even though the two patient groups had significantly different TTD and OS, the response rate and disease control rate are almost identical (Table 3), further supporting the notion that favorable clinical outcomes in African American patients are mainly due to lower incidence of HPD rather than higher treatment response rate.
Table 3.
Response rate and disease control rate in African American versus White patient populations
| Response category | African American (n = 75) | White (n = 110) |
|---|---|---|
| Response | 20 | 30 |
| Stable disease | 8 | 9 |
| Progressive disease | 19 | 30 |
| Unknown | 28 | 41 |
| R + SD + PD | 47 | 69 |
| Response rate, % | 42.6 | 43.5 |
| Disease control rate (R + SD), % | 59.6 | 56.5 |
Abbreviations: PD, progressive disease; R, response; SD, stable disease.
Assessing the Impact Other Variables Have on Clinical Outcomes
We analyzed various demographic and clinical variables to identify patient characteristics that impacted TTD or OS (Table 1; supplemental online Table 2; OS in supplemental online Tables 6–8). Multivariable Cox proportional hazards models were used to assess the effect of these variables on TTD or OS with the aHRs (Fig. 3; supplemental online Table 5). For each tested variable, all other variables were included as covariates in the Cox proportional hazards models. Age at anti‐PD‐1/PD‐L1 initiation, gender, tumor histology, PD‐L1 expression, and status of EGFR mutation or ALK fusion did not impact TTD, whereas current and former smokers seemed to have longer TTD than nonsmokers (Table 1; supplemental online Table 2; Fig. 3A). For OS, men appear to have worse survival in comparison with women in all patients (aHR, 1.95; Fig. 3B; p = .004; supplemental online Table 2) and in all subgroups tested, including (a) smokers in supplemental online Table 6, (b) first and second or higher line of therapy in supplemental online Table 7, and (c) African American and White race in supplemental online Table 8. Patients with positive PD‐L1 expression had longer OS than those with negative PD‐L1 (aHR, 0.54; p = .067; Fig. 3B). The presence of EGFR mutation or ALK fusion is associated with significantly worse survival (aHR, 2.42; p = .015; Fig. 3B). See Supplemental online Appendix 2 for additional information on other clinical variables such as body mass index and chronic obstructive pulmonary disease.
Notably, there was a difference in OS when patients received anti‐PD‐1/PD‐L1 in different settings of LOT (Table 1; supplemental online Table 2). The median OS was not reached, 14.6, or 11.9 months when patients received anti‐PD‐1/PD‐L1 regimen as the first, second, or third LOT, respectively. We then combined patients who were treated with anti‐PD‐1/PD‐L1 in the second or later LOT setting into one group. Although LOT had no effect on TTD (Fig. 1D), we observed numerically longer OS in the first‐line setting (Fig. 2D), although the difference was not statistically significant (p = .106). Multivariable analysis further suggested that LOT is not strongly correlated with OS after adjusting for other variables (Fig. 3B).
Discussion
In this retrospective study, we examined clinical outcomes of patients with aNSCLC who received anti‐PD‐1/PD‐L1 therapy in hospitals of MSHS, a large urban health care system. One of the primary objectives of the study is to investigate if race/ethnicity impacted response to anti‐PD‐1/PD‐L1 therapy in patients with aNSCLC. The unique characteristics of MSHS with high percentage of minority patient populations allowed us to address this important question with clinical implications. We indeed observed a significant longer OS in African American patients in comparison with White patients. As multiple studies indicated that African American patients have similar survival to White patients with NSCLC [20, 21, 22, 23], our results suggest race/ethnicity is likely a predictive factor of response to anti‐PD‐1/PD‐L1 inhibitors in aNSCLC rather than a prognostic factor. In addition to OS, our data revealed that TTD is also longer in African American patients, suggesting the underlying reason for the improved OS is potentially due to longer treatment duration. We recognized the caveat of RWD where demographic and clinical variables are not balanced in different patient groups. To rule out possible covariates such as gender, smoking status, PD‐L1 expression, oncogenic mutations, LOT, and others that may confound our analysis, we applied Cox proportional hazards models for survival analysis, adjusting for all available variables as covariates, and reached the same conclusion.
Notably, the African American and White patient groups had similar response rates to anti‐PD‐1/PD‐L1 based on real‐world assessment of radiographic response (Table 3). African American patients had longer TTD on anti‐PD‐1/PD‐L1 therapy, and we postulate that longer treatment duration in this patient population could be at least partially due to less toxicity to treatment. An abstract presented in the 2019 American Society of Clinical Oncology (ASCO) annual meeting [24] showed that African American patients with NSCLC treated with immune checkpoint inhibitors had significantly fewer irAEs than White patients, supporting our hypothesis. Our further investigation of anti‐PD‐1/PD‐L1‐associated HPD suggests fewer African American patients with HPD is also a possible reason of overall better outcome in this patient population, and future studies in additional cohorts are warranted.
The estimated median OS indexed to the initiation date of anti‐PD‐1/PD‐L1 treatment was 17.4 months in our cohort. When we further break down the data according to the lines of therapy, the median OS was not reached in the first‐line setting and was 14.6 months when anti‐PD‐1/PD‐L1 inhibitors were administered as the second LOT (Table 1). This is similar to the OS in registration trials of nivolumab [6, 7], pembrolizumab [8, 25], or atezolizumab [9, 10, 26] (Table 2). However, the estimated median OS in this study is longer than what was described in the report also based on RWD analysis by Khozin et al. in which the median OS of aNSCLC treated with nivolumab or pembrolizumab was 8.0 months [11]. Whereas the cutoff date in the RWD data used by Khozin et al. is March 2016 and only 17% of the anti‐PD‐1/PD‐L1 treatment was in the first‐line setting, the cutoff date in our study is May 2019 and 45% of the cohort received anti‐PD‐1/PD‐L1 as the first LOT, perhaps explaining the difference given anti‐PD‐1/PD‐L1 in the first‐line setting resulted in longer OS in clinical trials. Unexpectedly, the Khozin et al. study did not find differences of OS when LOT was taken into consideration (median OS indexed to anti‐PD‐1 initiation date was 8.3, 8.0, 7.1, or 9.3 months for LOT 1, 2, 3, and 4+, respectively) [11]. Our data showed a trend of longer OS when anti‐PD‐1/PD‐L1 was administered in the first‐line setting whereas there are no differences in TTD (Figs. 1D, 2D), but it did not reach statistical significance. The benefit of OS in the first‐line setting is even less significant in multivariable analysis (Fig. 3B). Longer OS in patients treated with anti‐PD‐1/PD‐L1 in the first versus second LOT setting has been consistently observed in clinical trials of pembrolizumab (Keynote 024 and Keynote 189 vs. Keynote 001 and Keynote 010) [8, 25, 27, 28, 29] and atezolizumab (IMpower 150 and IMpower 130 vs. OAK) [9, 10, 26]. Therefore, the observations by Khozin et al. and this study are counterintuitive because the results suggest delaying anti‐PD‐1/PD‐L1 treatment of aNSCLC until patients have progressed on less effective regimens may prolong overall survival after disease diagnosis.
In addition to race and lines of therapy, gender, PD‐L1 expression, and tyrosine kinase inhibitor (TKI) sensitizing mutation status also impacted OS in our cohort. Female gender, PD‐L1 positivity, and absence of TKI sensitizing EGFR mutations and ALK fusion are associated with longer OS. These results are consistent with subgroup analysis in several clinical trials [6, 8, 9, 26, 27] as well as analysis of RWD [11], demonstrating the validity of our data and analysis methods. In contrast to race/ethnicity stratified analysis in which longer OS is correlated with longer TTD in African American patients, gender, PD‐L1 expression, and TKI sensitizing alterations did not impact TTD (Fig. 3A vs. Fig. 3B), suggesting favorable outcome reflected by longer OS in female patients, patients with PD‐L1 expression, or patients with wild‐type EGFR, and ALK is not due to longer treatment duration. The clinical implication is that anti‐PD‐1/PD‐L1 therapy may provide long term benefit in these patients even if the treatment is discontinued because of irAE. Indeed, it has been noted that patients who discontinued PD‐1 checkpoint antibodies continue to benefit from the treatment in advanced melanomas [30], as well as in a small cohort study of several other solid tumor types presented at the 2019 ASCO annual meeting [31].
We also examined anti‐PD‐1/ PD‐L1 treatment as a single agent versus in combination with chemotherapy. In multivariable Cox regression analysis, patients that received combination had longer TTD (aHR, 0.55; p = .020) and overall survival (aHR, 0.53; p = .062). This is somewhat expected as most of these individuals received the combination therapy in the first‐line setting. The regimen of anti‐PD‐1/PD‐L1 as a single agent or in combination with chemotherapy was included as a covariate and the results suggest it did not affect our overall conclusion regarding the impact of race on TTD (Fig. 3A) and OS (Fig. 3B).
We recognize there are several limitations in our study. First, although African American patients account for 30.1% of the patients in this study, our cohort size is relatively small. Further studies are required to confirm our findings when a larger cohort with balanced race representations becomes available. Furthermore, in this study as well as the majority of the published studies, race and ethnicity are self‐reported, and more rigorous assessment of racial disparities in clinical research should incorporate genetic data. Second, although we performed Cox regression adjusting demographic and clinical variables in different race groups to avoid any potential confounding issues, there are other variables that would impact OS, but the data are sparse in EMR. For example, we could not assess the impact of the Eastern Cooperative Oncology Group performance status score because of the lack of data. Although tumor mutation burden has been postulated as a response marker to anti‐PD‐1/PD‐L1 independent of PD‐L1 expression [32, 33], the data are lacking in our cohort. Third, our determination of HPD was based on TTF <2 months, which is only an approximation of HPD defined by accelerated tumor growth rate upon anti‐PD‐1/PD‐L1 therapy. Fourth, radiographic treatment response in this study was not based on the RECIST criteria. Additionally, 33.3% of the cohort did not have information about response, of which two thirds is likely attributed to short follow‐up times of <120 days, because of either death or censoring. Consequently, response rate should be interpreted cautiously. Finally, the median follow‐up time in this study was 14.8 months. Although it is sufficient for us to derive results that are statistically significant or display a strong trend, longer follow‐up time would improve statistical power of the analysis.
Conclusion
Overall, this RWD‐based study confirmed many observations reported in prospective clinical trials of anti‐PD‐1/PD‐L1 inhibitors. To the best of our knowledge, this is the first report to describe the impact of race/ethnicity on clinical outcomes to anti‐PD‐1/PD‐L1 therapy in patients with aNSCLC. As it is impractical to conduct clinical trials to study racial disparities in treatment response or the impact of LOT on survival, further analyses of RWD with large cohorts are warranted to confirm our findings.
Author Contributions
Conception/design: Kristin L. Ayers, Shuyu D. Li
Provision of study material or patients: Rajwanth Veluswamy, Juan Wisnivesky, Fred R. Hirsch, William K. Oh
Collection and/or assembly of data: Tommy Mullaney, Xiang Zhou, Kyeryoung Lee, Meng Ma, Scott Jones, Li Li, Arielle Redfern, Whitney Jappe, Zongzhi Liu, Howard Goldsweig, Kamlesh K. Yadav, Nicholas Hahner, Matthew Dietz, Michelle Zimmerman, Tony Prentice, Shuyu D. Li, Eric E. Schadt, Rong Chen
Data analysis and interpretation: Kristin L. Ayers, Jane J. Liu, Scott Newman, Rajwanth Veluswamy, Juan Wisnivesky, Fred R. Hirsch, William K. Oh, Shuyu D. Li, Eric E. Schadt
Manuscript writing: Kristin L. Ayers, Shuyu D. Li, Eric E. Schadt
Final approval of manuscript: Kristin L. Ayers, Tommy Mullaney, Xiang Zhou, Jane J. Liu, Kyeryoung Lee, Meng Ma, Scott Jones, Li Li, Arielle Redfern, Whitney Jappe, Zongzhi Liu, Howard Goldsweig, Kamlesh K. Yadav, Nicholas Hahner, Matthew Dietz, Michelle Zimmerman, Tony Prentice, Scott Newman, Rajwanth Veluswamy, Juan Wisnivesky, Fred R. Hirsch, William K. Oh, Shuyu D. Li, Eric E. Schadt, Rong Chen
Disclosures
Kristin L. Ayers: Sema4 (E); Tommy Mullaney: Sema4 (E); Xiang Zhou: Sema4 (E); Jane J. Liu: Sema4 (E); Kyeryoung Lee: Sema4 (E); Meng Ma: Sema4 (E); Scott Jones: Sema4 (E); Li Li: Sema4 (E); Arielle Redfern: Sema4 (E); Whitney Jappe: Sema4 (E); Zongzhi Liu: Sema4 (E); Howard Goldsweig: Sema4 (E); Kamlesh K. Yadav: Sema4 (E); Nicholas Hahner: Sema4 (E); Matthew Dietz: Sema4 (E); Michelle Zimmerman: Sema4 (E); Tony Prentice: Sema4 (E); Scott Newman: Sema4 (E); Shuyu D. Li: Sema4 (E); Eric E. Schadt: Sema4 (E); William K. Oh: Sema4 (E); Rong Chen: Sema4 (E); Juan Wisnivesky: Sanofi, Banook, GlaxoSmithKline (H), Sanofi (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting information
Appendix S2: Supporting information
Figure S1 Supporting information
Figure S2 Supporting information
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Shuyu D. Li, Email: shuyudanli@gmail.com.
Eric E. Schadt, Email: eric.schadt@sema4.com.
Rong Chen, Email: rong.chen@sema4.com.
References
- 1. Booth CM, Tannock IF. Randomised controlled trials and population‐based observational research: Partners in the evolution of medical evidence. Br J Cancer, 2014;110:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katkade VB, Sanders KN, Zou KH. Real world data: An opportunity to supplement existing evidence for the use of long‐established medicines in health care decision making. J Multidiscip Healthc 2018;11:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basch E, Schrag D. The evolving uses of "real‐world" data. JAMA, 2019;321:1359–1360. [DOI] [PubMed] [Google Scholar]
- 4. Khozin S, Blumenthal GM, Pazdur R. Real‐world data for clinical evidence generation in oncology. J Natl Cancer Inst 2017;109. [DOI] [PubMed] [Google Scholar]
- 5. Tang J, Shalabi A, Hubbard‐Lucey VM. Comprehensive analysis of the clinical immuno‐oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 9. Rittmeyer A, Barslei F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 11. Khozin S, Carson KR, Zhi J et al. Real‐World outcomes of patients with metastatic non‐small cell lung cancer treated with programmed cell death protein 1 inhibitors in the year following U.S. regulatory approval. The Oncologist 2019;24:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stewart M, Norden AD, Dreyer N et al. An exploratory analysis of real‐world end points for assessing outcomes among immunotherapy‐treated patients with advanced non‐small‐cell lung cancer. JCO Clin Cancer Inform 2019;3:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xue X, Agalliu I, Kim MY et al. New methods for estimating follow‐up rates in cohort studies. BMC Med Res Methodol 2017;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) . Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 15. Presley CJ, Tang D, Soulos PR et al. Association of broad‐based genomic sequencing with survival among patients with advanced non‐small cell lung cancer in the community oncology setting. JAMA 2018;320:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Champiat S, Besse B, Marabelle A. Hyperprogression during immunotherapy: Do we really want to know? Ann Oncol 2019;30:1028–1031. [DOI] [PubMed] [Google Scholar]
- 17. Kato S, Goodman A, Walavalkar V et al. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim CG, Kim KH, Pyo KH et al. Hyperprogressive disease during PD‐1/PD‐L1 blockade in patients with non‐small‐cell lung cancer. Ann Oncol 2019;30:1104–1113. [DOI] [PubMed] [Google Scholar]
- 19. Ferrara R, Mezquita L, Texier M et al. Hyperprogressive disease in patients with advanced non‐small cell lung cancer treated with PD‐1/PD‐L1 inhibitors or with single‐agent chemotherapy. JAMA Oncol 2018;4:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bryant AS, Cerfolio RJ. Impact of race on outcomes of patients with non‐small cell lung cancer. J Thorac Oncol 2008;3:711–715. [DOI] [PubMed] [Google Scholar]
- 21. Brzezniak C, Satram‐Hoang S, Goertz HP et al. Survival and racial differences of non‐small cell lung cancer in the United States military. J Gen Intern Med 2015;30:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osuoha CA, Callahan KE, Ponce CP et al. Disparities in lung cancer survival and receipt of surgical treatment. Lung Cancer 2018;122:54–59. [DOI] [PubMed] [Google Scholar]
- 23. Zheng L, Enewold L, Zahm SH et al. Lung cancer survival among Black and White patients in an equal access health system. Cancer Epidemiol Biomarkers Prev 2012;21:1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah NJ, Blackburn M, Cook MR et al. Real‐world outcomes of underrepresented patient populations treated with immune checkpoint inhibitors (ICIs): African American descent, poor ECOG performance status, and chronic viral infections. 2019;37(suppl_15):2587–2587. [Google Scholar]
- 25. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 26. West H, McCleod M, Hussein M et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2019;20:924–937. [DOI] [PubMed] [Google Scholar]
- 27. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 28. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Updated analysis of KEYNOTE‐024: Pembrolizumab versus platinum‐based chemotherapy for advanced non‐small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol 2019;37:537–546. [DOI] [PubMed] [Google Scholar]
- 29. Gadgeel, SM , Garassino MC, Esteban E et al. KEYNOTE‐189: Updated OS and progression after the next line of therapy (PFS2) with pembrolizumab (pembro) plus chemo with pemetrexed and platinum vs placebo plus chemo for metastatic nonsquamous NSCLC. J Clin Oncol 2019;37(suppl 15):9013–9013. [Google Scholar]
- 30. Schadendorf D, Wolchok JD, Hodi FS et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bassanelli M, Giannarelli D, Migliorino MR et al. Immunotherapy discontinuation and outcome: A multicenter real‐life experience. J Clin Oncol 2019;37(suppl 15):e14075–e14075. [Google Scholar]
- 32. Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singal G, Miller PG, Agarwala V et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non‐small cell lung cancer using a clinicogenomic database. JAMA 2019;321:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting information
Appendix S2: Supporting information
Figure S1 Supporting information
Figure S2 Supporting information


