Abstract
Dementia accompanied by memory loss is considered one of the most common neurodegenerative diseases worldwide, and its prevalence is gradually increasing. Known risk factors for dementia include genetic background, certain lifestyle and dietary patterns, smoking, iron overload, insulin resistance, and impaired glucose metabolism in the brain. Here, we review recent evidence on the regulatory role of lipocalin 2 (LCN2) in dementia from various perspectives. LCN2 is a neutrophil gelatinase‐associated protein that influences diverse cellular processes, including the immune system, iron homeostasis, lipid metabolism, and inflammatory responses. Although its functions within the peripheral system are most widely recognized, recent findings have revealed links between LCN2 and central nervous system diseases, as well as novel roles for LCN2 in neurons and glia. Furthermore, LCN2 may modulate diverse pathological mechanisms involved in dementia. Taken together, LCN2 is a promising therapeutic target with which to address the neuropathology of dementia.
Keywords: dementia, insulin resistance, iron homeostasis, lipocalin 2 (LCN2), neuroinflammation
The role of LCN2 in neuronal cell damage in the brain of dementia patients.
![]()
1. INTRODUCTION
Dementia is a common neurodegenerative disorder with a markedly increasing worldwide prevalence. 1 , 2 , 3 The term dementia is used as a general, umbrella term for vascular dementia (VD), Lewy body dementias (LBD), frontotemporal dementia (FTD), and Alzheimer's disease (AD). The major neurological features of dementia are cognitive impairment and neuroinflammation. 4 , 5 Chronic neuroinflammation, neuronal loss, oxidative stress, amyloid beta accumulation, and impaired synaptic plasticity lead to memory loss. 6 , 7 Furthermore, a chronic inflammatory state gives rise to the activation of the tau kinase, increasing the formation of intracellular neurofibrillary tangles, leading to synapse dysfunction. 6
Multiple risk factors for dementia have been reported, including genomic mutations, impaired lipid metabolism, and impaired glucose metabolism. 8 Furthermore, several studies have reported that changes in the metabolic state, including blunt insulin sensitivity and hyperglycemia, are directly linked to the onset and development of dementia, especially memory deficits. 6 , 9 Thus, a complete understanding of the risk factors for dementia will allow the underlying mechanisms to be characterized and targeted for therapeutic approaches.
Lipocalin 2 (LCN2), also known as neutrophil gelatinase‐associated lipocalin (NGAL), functions in the regulation of the immune system and in inflammatory processes. 10 , 11 LCN2 is an antibacterial protein that acts by sequestering iron during bacterial infection and has recently been reported to be involved in various pathophysiological conditions in various organs and tissues, including the heart, lung, liver, kidney, and brain. 12 , 13
Recent studies have suggested that LCN2 modulates cellular activity in the central nervous system (CNS) and peripheral nervous system through the activation of glia, the control of iron accumulation, and the regulation of neuroinflammation, which is defined as an inflammatory response and neurotransmitter secretion within the CNS. 14 , 15 , 16 , 17 Additionally, LCN2 can cross the blood‐brain barrier (BBB) by binding with the melanocortin 4 receptor (MC4R) in hypothalamic neurons. 18 Another study demonstrated that cancer cells are growing in the LCN2/SLC22A17 system in the leptomeningeal metastasis mouse model. 19
Lipocalin 2 likely regulates cell differentiation by modulating iron transport and cell death signaling, such as controlling the activation of the nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB). LCN2 ultimately controls neuroinflammation by modulating the production of cytokines, including interleukin‐1 beta (IL‐1β), tumor necrosis factor‐α (TNF‐α), and interleukin‐6 (IL‐6) secreted from glia. 15 In contrast, LCN2 also has neuroprotective functions in the brain by suppressing the secretion of pro‐inflammatory cytokines. 18
In addition, modulating LCN2 may be useful for preventing BBB disruptions and white matter atrophy that lead to neurological diseases, including stroke and dementia. 14 , 20 Furthermore, LCN2 is involved in the accumulation of iron that occurs within brain neurons of patients with dementia and is related to cognitive dysfunction. 21 LCN2 can also decrease synaptic plasticity through its involvement in controlling active mobility through iron‐dependent signaling. 22 Moreover, LCN2 is also strongly related to an impaired metabolic state, such as impaired glucose metabolism, thus leading to cognitive decline. 23 , 24 Finally, LCN2‐deficient mice display increased insulin resistance under hyperglycemia and heightened blood glucose levels compared to control mice. 23
Here, we review the evidence for the involvement of LCN2 in dementia, focusing on neuroinflammation, iron accumulation, and metabolic alterations. These findings highlight the importance of the role of LCN2 in the CNS and suggest that LCN2 is key to modulating the neuropathology of dementia.
2. DEMENTIA
Dementia is one of the most common disorders affecting elderly people, and the number of patients with dementia is gradually increasing globally. 1 Dementia is generally classified into four subtypes and is used as a general term to describe a syndrome of progressive cognitive decline, including AD, LBD, FTD, and VD. 25 , 26 The main clinical pathologies of dementia are objective cognitive deficits, language impairments, and difficulties with executive function or judgment. 5
Alzheimer's disease, the most common type of dementia, is characterized by the excessive accumulation of amyloid beta, neuronal damage, BBB disruption, tau hyperphosphorylation, and neurofibrillary tangles in several brain regions, including the hippocampus and the entorhinal cortex. 27 , 28 , 29 Patients with AD show atrophy and degeneration of the cortex, as well as limbic and hippocampal regions, compared to healthy controls, which leads to memory loss. 30 , 31
Vascular dementia, the second most common type of dementia globally, represents approximately 20% of all dementia cases and results from neuronal damage caused by oxygen and glucose deprivation in various brain regions. 4 VD, considered a multi‐infarct type of dementia, is influenced by lifestyle, dietary patterns, and vascular dysfunctions such as small vessel disease and lacunar infarctions. 4 , 32 , 33
Lewy body dementias, the third most common type of dementia, results from the excessive accumulation of alpha‐synuclein protein, called Lewy bodies, in neurons. 34 LBD leads to cognitive impairments with recurrent visual hallucinations, lethargy, and a decrease in attention, as well as parkinsonism. 34 , 35
Frontotemporal dementia is a general type of dementia that affects the frontal and temporal lobes and mainly occurs in younger individuals, as opposed to the generally older age of patients with AD. A hallmark of FTD is the high accumulation of neurofibrillary tangles in the frontal and parietal lobes. 36 This type of dementia leads to a lack of judgment, inappropriate social behavior, and memory dysfunction. 37 Importantly, the cognitive decline in dementia results from neuronal damage in the cerebral cortex, synaptic dysfunction, neuroinflammation, and impaired cerebral metabolism. 5 , 38 , 39
Vascular dementia is caused by impaired vascular homeostasis, which aggravates cortical and hippocampal neuronal loss in the ischemic state, ultimately contributing to cognitive decline. 39 In addition, changes in metabolic biomarkers contribute to impaired cerebral metabolism and ultimately damages neurons and glia by decreasing the supply of nutrients and oxygen. 38
Numerous risk factors for dementia have been identified, including genetic predispositions, vascular damage, inflammation, comorbidities, as well as various lifestyle and physiological factors. 8 , 40 Furthermore, a number of studies have demonstrated a correlation between dementia and metabolic changes, such as insulin resistance (impaired intracellular insulin functioning) and glucose imbalance. 9 , 41 In fact, Bergantin et al 9 found that dysregulation of cellular Ca2+ signaling represents an important risk factor, suggesting that it aggravates brain insulin resistance, resulting in memory loss.
Neuroinflammation aggravates neuronal loss and activates glia, ultimately leading to memory loss in dementia through excessive secretion of pro‐inflammatory cytokines and inflammatory mediators. 42 , 43 In the dementia brain, astrocytes and microglia are activated and secrete several inflammatory cytokines and neurotransmitters such as glutamate, 44 , 45 whereas neurons are damaged and show impaired synaptic plasticity against oxidative stress condition. 46 Increased BBB permeability in dementia induces the infiltration of circulating immune cells such as T‐lymphocytes. 47 A previous study found that brain insulin resistance, including impaired insulin receptor signaling and insulin‐like growth factor‐1 (IGF‐1) signaling, leads to cognitive dysfunction in dementia by aggravating neuronal cell death and synaptic dysfunction. 48 Additionally, excessive iron accumulation observed in the caudate nucleus and putamen of VD patients contributes to neuronal synapse connectivity dysfunction and memory loss. 49
Thus, the onset and progression of dementia are linked to many factors, which may contribute to the neuropathology observed in dementia. These previous studied have highlighted that there are few pathological differences between the different types of dementia. 8 Further understanding of the potential risk factors for dementia is critical, as the onset of dementia results from diverse routes. Therefore, identifying specific risk factors for the onset and development of the disease is necessary for its prevention and treatment.
3. ROLES OF LCN2 IN DEMENTIA
Lipocalin 2 is a glycoprotein of the lipocalin superfamily comprised of 160–180 amino acids, which contributes to innate immunity and inflammatory responses. 10 , 11 , 50 Two specific membrane receptors of LCN2 have been reported: megalin (known as LRP2, expressed by kidney epithelial cells) and 24p3R (known as solute carrier SLC22A17, expressed in various tissues, including the brain). 51 , 52 LCN2 can bind and transport both lipids and small hydrophobic molecules into the cell, and also control matrix metalloproteinases in blood vessels to mediate neurovascular remodeling. 53 , 54
Several studies have shown that LCN2 exerts multiple physiological cellular processes, such as inflammatory responses, and iron metabolism in the brain. 12 , 15 , 55 , 56 , 57 LCN2 is an important modulator of innate responses, and exerts antibacterial and bacteriostatic effects through an interplay with the bacterial iron‐laden siderophore. 57 LCN2 acts as a key controller of inflammation mediated through the toll‐like receptor 2 (TLR2) and TLR4 dependent pathways. 55 Moreover, LCN2 blood levels are positively correlated with insulin resistance in females, as demonstrated in some clinical studies. 56 Although the detailed mechanisms behind the role of LCN2 in these responses remain to be elucidated, the relevance between LCN2 and various cellular mechanisms should be highlighted in order to understand the roles of LCN2 in the brain.
In the CNS, increased LCN2 is observed in patients with neurodegenerative disorders such as AD and Parkinson disease, and ultimately aggravates the neuropathophysiology of such diseases. 50 , 58 , 59 LCN2 is mainly produced in glia under oxidative stress in the brain 50 and can disrupt the BBB by boosting astrocyte and brain endothelial cell damage. 14 One study demonstrated that LCN2‐deficient condition leads reduced cerebral cortex damage in a VD mouse model by decreasing neuroinflammation. 8
Lipocalin 2 causes neuronal death, induces synaptic dysfunction, activates microglia, reduces white matter mass, and leads to microvasculature damage in the hippocampus by promoting the expression of vascular endothelial growth factor (VEGF). 8
Several studies have shown that the level of LCN2 in the blood increases in patients with neurological diseases, including AD, mild cognitive impairment, and Parkinson's disease. 59 , 60 Mechanically, LCN2 regulates cellular responses by activating NF‐κB and hypoxia‐inducible factor‐1‐α (HIF‐1‐α) induction. 61 Other in vivo and in vitro studies have reported that LCN2 is involved in the aggravation of pro‐inflammatory responses and the inhibition of neuroprotective cellular pathways in the brain by boosting the release of pro‐inflammatory cytokines such as TNF‐α. 58 , 62 Together, these findings suggest that LCN2 mediates the inflammatory state in the brain. Recently, LCN2 has emerged as a potential diagnostic marker for dementia, 63 based on the observation of elevated LCN2 in the plasma of patients with dementia 64 and in the cerebrospinal fluid (CSF) of patients with VD. 65 In fact, a previous study demonstrated that LCN2 deficiency attenuates white matter damage and improves cognitive dysfunction in animal models of VD. 34 Furthermore, in an in vitro model of AD, astrocytes secreted high amounts of LCN2, and elevated LCN2 ultimately boosted amyloid beta aggregation and amyloid beta‐induced cell death in astrocytes and endothelial cells. 58 , 66 Recent studies have also suggested that LCN2 is a strong and specific biomarker for VD. 38 , 67 , 68 , 69 , 70
The relationship between the level of LCN2 and the pathological progression of dementia has been reported in previous studies. One study suggested that the CSF level of LCN2 is elevated in the brains of patients with VD. 50 In addition, one cross‐sectional study showed that the plasma level of LCN2 was higher in patients with AD than in normal subjects. 71
Based on these findings, we suspect that the levels of LCN2 may differ across the various models of dementia due to the difficulties in measuring the levels of LCN2 during the progress of dementia. Thus, although a strong relationship between neurodegenerative disease progression and LCN2 levels in the CNS and blood have been reported, clinicians should use caution when using LCN2 levels as a clinical indicator of neurodegenerative disease progression.
To summarize, LCN2 contributes to inflammatory responses and regulates various cell signaling pathways, affecting neurons and glia in the brains of patients with dementia. The fact that dementia is also linked to multiple mechanisms related to LCN2, such as inflammatory responses, iron metabolism, and cellular apoptosis, highlights the importance of the relationship between LCN2 and the neuropathology of dementia. Below, we review the roles of LCN2 in dementia from various perspectives.
In the CNS, iron is an essential biomolecule for cellular homeostasis, including DNA repair, DNA synthesis, oxidation, immune cell activation, synapse development, and cell division; it also acts as a neurotransmitter, such as in the development of neurodegenerative diseases. 72 , 73 , 74 Specifically, iron is known to play a role in the development of neurodegenerative diseases by controlling monoamine neurotransmitters such as dopamine and serotonin and ultimately contributes to the regulation of cognitive functions, including emotional‐ and arousal‐related behaviors. 75
Iron is also a co‐factor for multiple cellular physiological mechanisms, such as axonal myelination, oxidative mitochondrial metabolism (by promoting adenosine triphosphate [ATP] production), and the synthesis of neurotransmitters that depend on tyrosine hydroxylase. 76 , 77 , 78 Iron is also a critical regulator of the oxidative stress response and is thus considered a redox‐active transition metal. 79 Iron acts as an antioxidant regulator by catalyzing the formation of reactive oxygen species (ROS). 80
Moreover, iron is an essential metal for the maintenance of energy consumption in the brain ‐ the most energy‐consuming metabolic organ in the body. 81 , 82 Furthermore, iron acts as a co‐factor in axonal myelination, mitochondrial function, and neurotransmission in the CNS. 83
The main route of iron uptake begins with intestinal absorption in the gut, where dietary Fe3+ is reduced to Fe2+ by duodenal cytochrome B (DcytB), and divalent metal transporter 1 (DMT1) brings Fe2+ into the gut intestinal cells. 84 , 85 Thus, dietary intake of iron contributes to the balance of iron in the body and can offset iron deficiency. 86 Intracellular iron transport requires iron transporters, such as DMT1 and transferrin receptor 1 (TfR1), 87 as well as iron regulatory protein 1 (IRP1). 88 Iron is encapsulated in hemoglobin cells in the blood, and a small portion of iron in the body binds to iron storage proteins such as ferritin and transferrin. 89 , 90 Iron enters the brain mainly through the BBB. 91
In vitro inflammatory conditions induce microglial polarization and activation. 92 During neuroinflammation, microglia are activated and polarized into the pro‐inflammatory M1 phenotype and the anti‐inflammatory M2 phenotype in vitro. 92 A previous study showed that M1 microglia secrete pro‐inflammatory cytokines and nitric oxide, whereas M2 phenotype microglia produce anti‐inflammatory cytokines such as IL‐4, and IL‐13. 92 Owing to the functional diversity of microglia, microglia are classified by M1, M2a, M2b, and M2c classifications, 93 , 94 , 95 and M1/M2 polarization states could be identified in in vitro conditions. 96 Previous in vivo studies have shown that the M2 microglia population contributes to the neuroprotective response after stroke, 97 , 98 whereas M1 microglial populations aggravate the neuronal damage after stroke. 97
Since it is difficult to specifically detect M1 and M2 microglia phenotypes in the brain, some studies have suggested that polarization can be estimated using several surface markers, including classical M1 markers CD11b, 99 CD16, 100 CD32 98 and CD86, 101 and the classical M2 marker CD206. 102
Microglial activation is very important for controlling neuroinflammatory responses and is directly related to iron uptake. 103 Under inflammatory conditions, the increased production of pro‐inflammatory mediators increases the uptake of non‐transferrin‐bound serum iron (NTBI) as well as ferritin storage by upregulating both DMT1 and ferritin. 82 In the inflammatory state, various proteins related to iron metabolism, such as DMT1 and ferritin, contribute to microglial polarization and microglia function. 82 Within the NTBI uptake pathway in cells, Fe3+ is reduced at the cell surface to Fe2+ through an endogenous ferrireductase and conveyed into the cytosol through DMT1. During the transferrin‐bound iron (TBI) uptake pathway, iron is mainly combined with transferrin as Fe3+ and subsequently enters endosomes through endocytosis. 82 Microglia can bind to NTBI and TBI as iron forms in the CSF. 104 In addition, previous studies in rats have shown a positive correlation between microglia polarization and the amount of microglial iron uptake. 82 , 103 Furthermore, a recent study reported that knockdown of the ferritin 3 heavy chain homolog (Fer3HCH) leads to mitochondrial dysfunction by decreasing mitochondrial respiration. 105
Iron deficiency has been reported to cause abnormal brain development and dysfunctions in cognition, motor function, and social behavior patterns. 81 Excessive accumulation of iron in the brain damages AD‐related brain regions, leading to neuronal loss in the frontal, parietal and hippocampal areas. 106 , 107 , 108 , 109 The high accumulation of iron in microglia in the cortex and hippocampus of AD patients means that these microglia could be used as a monitoring marker for AD. 108 Iron chelators could also be used as treatment options for AD based on clinical trial data. Indeed, the iron‐chelating drug deferoxamine has been shown to reduce amyloid plaque formation, and prevent memory loss. 109 , 110
Other studies have demonstrated that cerebral iron overload is directly linked to the development of neurodegenerative diseases, such as dementia, 111 by boosting mitochondrial dysfunction and microglial activation. 112 In addition, several studies have suggested that iron directly regulates AD neuropathology by boosting amyloid beta peptide aggregation and amyloid beta plaque accumulation, thereby leading to cognitive decline. 113 , 114 Iron deficiency aggravates mitochondrial function and oxidative stress, whereas iron overload results in oxidative stress. 115 , 116 It has also been suggested that lipid peroxidation, a typical feature of ferroptosis, is an early step in the development of AD. 117
A recent study using the APP/PS1 AD mouse model demonstrated that excessive iron accumulation in microglia triggers brain dysfunction and changes in brain metabolism. 118 Subsequently, another study suggested that iron dysregulation is the principal factor behind AD neuropathology and needs to be addressed in order to find a cure for the disease. 119
These findings show that imbalances in iron levels in the brain contribute to diverse cellular signaling pathways and can aggravate AD neuropathology. Combined, they suggest that the specific mechanisms underlying the link between iron accumulation and brain functions need to be identified in order to find therapeutic solutions for dementia.
4. LCN2 AND IRON HOMEOSTASIS
Lipocalin 2, an acute‐inflammatory phase‐related mediator, is quickly secreted in response to inflammatory stimulation. 15 LCN2 plays a crucial role in various cellular responses, such as in the defense against bacterial infections through the regulation of iron accumulation in the cell, inflammatory signaling, and apoptotic signaling. 120 LCN2 stimulates glia such as astrocytes and microglia and regulates their production of anti‐ and pro‐inflammatory cytokines under inflammatory conditions via its involvement in iron accumulation. 16 , 121 LCN2 is emerging as a promising player in the search for novel dementia treatments because it can regulate inflammatory cytokines and iron accumulation in the CNS. 19 , 122
Previous studies have found that LCN2 knockout mice show iron dysregulation in both the peripheral system and the CNS, subsequently leading to synaptic dysfunction and impaired neurogenesis. 22 , 123 , 124 , 125 A recent study reported that LCN2 could regulate neurogenesis and spine density in hippocampal neurons, as well as neuronal connectivity by modulating iron loading. 124 Ferreira et al 124 showed that LCN2 knockout mice display an increase in neuronal differentiation, which is involved in cognitive function. Another study reported that LCN2‐deficient mice displayed more anxiety and depressive behavior as well as cognitive decline, 125 and LCN2 has also been shown to mediate iron import into the brain. 51 Furthermore, Xia et al. reported that LCN2 knockout mice show a high level of iron and a severe oxidative stress compared to normal mice. 126 Conversely, increases in LCN2 levels in the CSF are positively correlated with iron accumulation in basal ganglia regions and elevated levels of transferrin in the CSF. 127
Previous studies have also reported that the expression of LCN2 in the brain is positively correlated with excessive iron overload in various brain regions such as the cerebral cortex. 59 , 128 LCN2 stimulates ferritin expression and iron storage in astrocytes under amyloid beta toxicity, 66 and has been reported to mediate the import and export of iron into cells under inflammatory conditions. 129 In fact, recent studies have found that LCN2 contributes to iron homeostasis to regulate the flow of iron from cells into the circulating system involving hepcidin and ferroportin in the brain, 11 and that it plays a critical role as an iron transport protein by binding to the LCN2 receptor. 15 LCN2 deficiency has also been reported to impair iron export in cells. 130
Taken together, the existing evidence suggests that LCN2 is key to the regulation of excessive iron accumulation and inflammation in the brain of dementia patients. Further investigations into the mechanism(s) underlying the link between iron homeostasis and LCN2 in the brain are needed, as iron imbalance is a critical problem leading to memory loss in patients with dementia.
5. LCN2 IN DEMENTIA: FOCUS ON NEUROINFLAMMATION
Neuroinflammation is considered an early diagnostic marker of dementia as it is observed from early to late stages of dementia. 131 , 132 Neuroinflammation is involved in the activation of glia such as microglia and astrocytes in dementia‐related brain regions, 133 as well as in the associated cognitive decline. 134 One study demonstrated that the LCN2 receptor is highly expressed in microglia, astrocytes, and neurons under inflammatory conditions. 135 Another study suggested that the LCN2 promoter provides binding sites for the inflammatory NF‐κB pathway and CCAAT/enhancer‐binding protein (C/EBP) during inflammation. 136
In addition, LCN2 induces the polarization of microglia through the activation of NF‐κB signaling as well as the activation of the signal transducer and transcription 3 (STAT3) pathway. 137 LCN2 knockout mice display a neuroprotective phenotype, which manifests as reduced neuroinflammation under ischemic stroke conditions. 138 , 139 Additionally, LCN2 has been reported to promote the activation of astrocytes, and reactive astrocytes are known to stimulate microglial activation under neuroinflammation. 140 Importantly, activated astrocytes control the expression of inflammatory mediators, transporters, and neurotransmitters and influence neuronal metabolism. 141 , 142 , 143 , 144
Lipocalin 2 can induce reactive astrocytes through its neurotoxic properties, and subsequently promote cell death. 144 LCN2 activates both astrocytes and microglia, and exerts neurotoxic effects in neurons, involving memory functions. 144 Previous in vitro studies have reported that LCN2 is secreted in cultured astrocytes upon lipopolysaccharide stimulation and that it plays a role as an autocrine server. 145 , 146 For example, Lee et al 145 found that LCN2 secretion in glia aggravates neuronal apoptosis mediated by iron and the bcl2 interacting mediator of cell death (BIM) protein, while also boosting neuronal motility. LCN2 also contributes to the inflammatory response by regulating the phagocytic capacity of bacterial clearance in astrocytes and microglia. 147 , 148
To summarize, these findings show that LCN2 induces the activation of microglia and astrocytes, regulates their functions, causes neuronal cell death, and influences neuronal function when in a state of neuroinflammation. Therefore, the modulation of LCN2 levels in the brain may be key to reducing pro‐inflammatory responses that occur in the brain of individuals with dementia.
6. LCN2 IN DEMENTIA: FOCUS ON METABOLIC ALTERATIONS
Recent studies have emphasized and investigated the positive correlations between metabolic syndromes such as diabetes, obesity, and dementia. 149 , 150 Numerous studies have reported that metabolic changes commonly observed in patients with metabolic syndromes, such as hyperglycemia, dyslipidemia, hypertension, and insulin resistance, are strongly related to the neuropathology of dementia. 151 , 152 , 153 Clinically, diabetic neuropathy, such as memory loss, is a complication of diabetes. 154 , 155
Several studies have suggested that LCN2 is linked to inflammatory responses in metabolic disorders, including obesity, 156 in which the pathway involves NF‐κB, 157 C/EBP, 158 and estrogen response elements. 157 , 159 Other studies have reported that elevated LCN2 levels are observed in animal models of diabetes and obesity 160 , 161 and that LCN2 aggravates insulin resistance as well as lipid metabolism. 162 Furthermore, blood levels of LCN2 are reported to be positively correlated with total body fat mass and glycated hemoglobin (HbA1c), 163 as well as with hyperglycemia and insulin resistance in patients with metabolic syndrome. 164 , 165 , 166 Recent studies have also reported that the levels of LCN2 in blood serum, adipose tissue, and liver are increased in models of obesity. 160 , 167 In fact, Yan et al 160 found that LCN2 expression in adipocytes leads to insulin resistance in adipocytes, and that LCN2 could control insulin sensitivity in patients with obesity. Based on this data, the authors suggested that LCN2 likely regulates the secretion of adipokines in adipose tissues, and ultimately controls both the inflammatory state and metabolic balance. 160
The expression and secretion of LCN2 are higher in mature adipocytes than in preadipocytes 23 and are induced by various inflammatory cytokines and factors. 168 Finally, the change of fat mass leads to increased cytokines and adipokines, and subsequently, aggravates the state of inflammation, and increases vascular damage. LCN2 influences vascular remodeling, regulates atherosclerotic plaque formation caused by metabolic changes, and is ultimately involved in the onset of VD. 168 , 169 , 170
To summarize, the current evidence shows that LCN2 is involved in insulin sensitivity, glucose metabolism, vascular homeostasis, and hyperglycemia related to metabolic syndromes. These strong correlations between LCN2 and metabolic factors should be highlighted and considered in the search for therapeutic options for the treatment and prevention of dementia, considering that the onset and development of dementia are strongly related to metabolic disorders, including hyperglycemia and insulin resistance.
7. CONCLUSIONS
Here, we emphasize that LCN2 has crucial pathogenic roles in dementia through the regulation of iron homeostasis, neuroinflammation, and insulin resistance. The onset and progression of dementia are influenced by a variety of LCN2‐mediated mechanisms including inflammation, insulin resistance, iron accumulation, immune response, neuronal cell damage, and glia dysfunction.
Given the significant evidence supporting the involvement of LCN2 in the demented brain, we believe that the level of LCN2 in the brain is a critical factor in the regulation of risk factors for dementia. Thus, we reviewed the roles of LCN2 in dementia and came to the following three conclusions: first, LCN2 contributes to excessive iron accumulation and is ultimately involved in the neuropathology of dementia (Figure 1A,B), suggesting that LCN2 influences neuronal cell apoptosis by regulating iron accumulation in the brain. Second, LCN2 accelerates neuroinflammation by regulating the activation and function of glia and subsequently aggravates the induced neuroinflammation by regulating NF‐kB signaling and the STAT3 pathway. Appropriate modulation of LCN2 may enhance the neuropathology of dementia (Figure 1C). Finally, LCN2 can control metabolic homeostasis, including insulin sensitivity, hyperglycemia, and dyslipidemia through the modulation of NF‐κB, C/EBP signaling, HbA1c level, and body fat mass (Figure 2).
FIGURE 1.
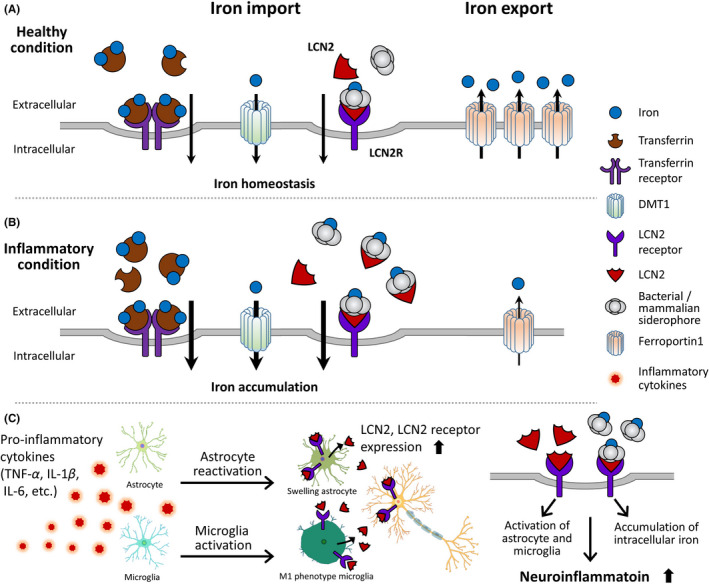
The role of LCN2 in iron accumulation and neuroinflammation. (A,B) LCN2 is related to the import and export of iron into neuronal cells. (B) Under neuroinflammation conditions, LCN2 binds with many bacterial/mammalian siderophores and subsequently increases iron accumulation from the extracellular space into the intracellular space. (C) LCN2 triggers astrocyte reactivation and swelling as well as the induction of M1 microglial phenotype. Finally, LCN2 activates the production of pro‐inflammatory cytokines in astrocytes and microglia, and promotes the accumulation of intracellular iron, leading to the aggravation of neuroinflammation. DMT1, divalent metal transporter 1; IL‐1β, interleukin‐1 beta; IL‐6, interleukin −6; LCN2, lipocalin 2; LCN2R, lipocalin 2 receptor; TNF‐α, tumor necrosis factor‐α
FIGURE 2.
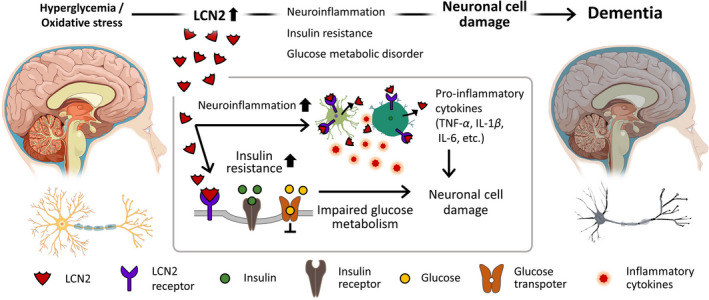
The role of LCN2 in neuronal cell damage under hyperglycemia and oxidative stress. Hyperglycemia and oxidative stress cause cognitive decline in the brains of patients with dementia. Elevated levels of LCN2 contribute to the increase in insulin resistance and the aggravation of neuroinflammation in the brain. Ultimately, these responses result in neuronal cell death. IL‐1β, interleukin‐1 beta; IL‐6, interleukin −6; LCN2, lipocalin 2; TNF‐α, tumor necrosis factor‐α
The modulation of LCN2 levels may contribute to the attenuation of the neuropathology of dementia, given that recent research focused on the chemotherapy in CNS disease including dementia as well as brain tumor. 171 The immunotherapy using LCN2 neutralization may be a promising clinical approach to treat and prevent neuropathology in dementia, suggesting that LCN2 deficiency markedly suppresses neuroinflammation based on preclinical studies for various CNS disorders, 172 such as traumatic brain damage, 173 experimental autoimmune encephalomyelitis, 174 and stroke. 175 Thus, we emphasize that LCN2 may be a critical target for the treatment and prevention of dementia.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Daejin Lim and Jae‐ho Jeong and Juhyun Song contributed to the writing of text and provided the table and figures. Juhyun Song wrote, revised, and finalized the whole manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: NRF‐2019R1F1A1054111 awarded to Juhyun Song, and grant number NRF‐2017R1A6A3A04006167 awarded to Jae‐ho Jeong).
Contributor Information
Jae‐ho Jeong, Email: jeongjaeho@chonnam.ac.kr.
Juhyun Song, Email: juhyunsong@chonnam.ac.kr.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Lee CM, Woodward M, Batty GD, et al. Association of anthropometry and weight change with risk of dementia and its major subtypes: a meta‐analysis consisting 2.8 million adults with 57 294 cases of dementia. Obes Rev. 2020;21(4):e12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao M, Hu PP, Zhang YL, et al. Enriched physical environment reverses spatial cognitive impairment of socially isolated APPswe/PS1dE9 transgenic mice before amyloidosis onset. CNS Neurosci Ther. 2018;24(3):202‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hildreth KL, Church S. Evaluation and management of the elderly patient presenting with cognitive complaints. Med Clin North Am. 2015;99(2):311‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3(11):862‐872. [DOI] [PubMed] [Google Scholar]
- 8. Kim JH, Ko PW, Lee HW, et al. Astrocyte‐derived lipocalin‐2 mediates hippocampal damage and cognitive deficits in experimental models of vascular dementia. Glia. 2017;65(9):1471‐1490. [DOI] [PubMed] [Google Scholar]
- 9. Bergantin LB. A link between brain insulin resistance and cognitive dysfunctions: targeting Ca2+/cAMP signalling. Cent Nerv Syst Agents Med Chem. 2020;20(2):103‐109. [DOI] [PubMed] [Google Scholar]
- 10. Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482(1–2):9‐24. [DOI] [PubMed] [Google Scholar]
- 11. Xiao X, Yeoh BS, Vijay‐Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu Rev Nutr. 2017;37:103‐130. [DOI] [PubMed] [Google Scholar]
- 12. Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917‐921. [DOI] [PubMed] [Google Scholar]
- 13. Asimakopoulou A, Weiskirchen S, Weiskirchen R. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front Physiol. 2016;7:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egashira Y, Hua Y, Keep RF, Iwama T, Xi G. Lipocalin 2 and blood‐brain barrier disruption in white matter after experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2016;121:131‐134. [DOI] [PubMed] [Google Scholar]
- 15. Jha MK, Lee S, Park DH, et al. Diverse functional roles of lipocalin‐2 in the central nervous system. Neurosci Biobehav Rev. 2015;49:135‐156. [DOI] [PubMed] [Google Scholar]
- 16. Holland R, McIntosh AL, Finucane OM, et al. Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav Immun. 2018;68:183‐196. [DOI] [PubMed] [Google Scholar]
- 17. Feng J, Wang JX, Du YH, et al. Dihydromyricetin inhibits microglial activation and neuroinflammation by suppressing NLRP3 inflammasome activation in APP/PS1 transgenic mice. CNS Neurosci Ther. 2018;24(12):1207‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mosialou I, Shikhel S, Liu JM, et al. MC4R‐dependent suppression of appetite by bone‐derived lipocalin 2. Nature. 2017;543(7645):385‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chi Y, Remsik J, Kiseliovas V, et al. Cancer cells deploy lipocalin‐2 to collect limiting iron in leptomeningeal metastasis. Science. 2020;369(6501):276‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li W, Lo EH. Leaky memories: impact of APOE4 on blood‐brain barrier and dementia. J Cereb Blood Flow Metab. 2020;40(9):1912‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dekens DW, Naude PJW, Keijser JN, Boerema AS, De Deyn PP, Eisel ULM. Lipocalin 2 contributes to brain iron dysregulation but does not affect cognition, plaque load, and glial activation in the J20 Alzheimer mouse model. J Neuroinflammation. 2018;15(1):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin‐2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc Natl Acad Sci USA. 2011;108(45):18436‐18441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Law IK, Xu A, Lam KS, et al. Lipocalin‐2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59(4):872‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Lam KS, Kraegen EW, et al. Lipocalin‐2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53(1):34‐41. [DOI] [PubMed] [Google Scholar]
- 25. Karantzoulis S, Galvin JE. Distinguishing Alzheimer's disease from other major forms of dementia. Expert Rev Neurother. 2011;11(11):1579‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chertkow H, Feldman HH, Jacova C, Massoud F. Definitions of dementia and predementia states in Alzheimer's disease and vascular cognitive impairment: consensus from the Canadian conference on diagnosis of dementia. Alzheimers Res Ther. 2013;5(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matthews FE, Stephan BC, McKeith IG, et al. Two‐year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? J Am Geriatr Soc. 2008;56(8):1424‐1433. [DOI] [PubMed] [Google Scholar]
- 28. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moon WJ, Lim C, Ha IH, et al. Hippocampal blood‐brain barrier permeability is related to the APOE4 mutation status of elderly individuals without dementia. J Cereb Blood Flow Metab. 2020:e0271678X20952012. 10.1177/0271678X20952012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arbizu J, Larumbe R, Gamez C, Marti J, Martinez‐Lage J, Richter J. Correlations between brain SPECT and neuropsychology assessments in mild and moderate stages of Alzheimer's disease. Rev Esp Med Nucl. 1999;18(4):252‐260. [PubMed] [Google Scholar]
- 31. Aisen PS. Can rofecoxib delay a diagnosis of Alzheimer's disease in patients with mild cognitive impairment? Nat Clin Pract Neurol. 2005;1(1):20‐21. [DOI] [PubMed] [Google Scholar]
- 32. Ferrer I. Cognitive impairment of vascular origin: neuropathology of cognitive impairment of vascular origin. J Neurol Sci. 2010;299(1–2):139‐149. [DOI] [PubMed] [Google Scholar]
- 33. Szu JI, Obenaus A. Cerebrovascular phenotypes in mouse models of Alzheimer's disease. J Cereb Blood Flow Metab. 2021:e0271678X21992462. 10.1177/0271678X21992462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zupancic M, Mahajan A, Handa K. Dementia with lewy bodies: diagnosis and management for primary care providers. Prim Care Companion CNS Disord. 2011;13(5):PCC.11r01190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine DN, Lee JM, Fisher CM. The visual variant of Alzheimer's disease: a clinicopathologic case study. Neurology. 1993;43(2):305‐313. [DOI] [PubMed] [Google Scholar]
- 37. Tsai PH, Teng E, Liu C, Mendez MF. Posterior cortical atrophy: evidence for discrete syndromes of early‐onset Alzheimer's disease. Am J Alzheimers Dis Other Dement. 2011;26(5):413‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2018;14(3):280‐292. [DOI] [PubMed] [Google Scholar]
- 40. van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 5):v2‐v7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Folch J, Olloquequi J, Ettcheto M, et al. The involvement of peripheral and brain insulin resistance in late onset Alzheimer's dementia. Front Aging Neurosci. 2019;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mondal A, Bose D, Saha P, et al. Lipocalin 2 induces neuroinflammation and blood‐brain barrier dysfunction through liver‐brain axis in murine model of nonalcoholic steatohepatitis. J Neuroinflammation. 2020;17(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang T, Guo R, Zhang F. Brain perivascular macrophages: recent advances and implications in health and diseases. CNS Neurosci Ther. 2019;25(12):1318‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: the role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res. 2006;6(5):237‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perez‐Nievas BG, Serrano‐Pozo A. Deciphering the astrocyte reaction in Alzheimer's disease. Front Aging Neurosci. 2018;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silva MC, Ferguson FM, Cai Q, et al. Targeted degradation of aberrant tau in frontotemporal dementia patient‐derived neuronal cell models. Elife. 2019;8:e45457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mietelska‐Porowska A, Wojda U. T Lymphocytes and inflammatory mediators in the interplay between brain and blood in Alzheimer's disease: potential pools of new biomarkers. J Immunol Res. 2017;2017:e4626540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF‐1 resistance, IRS‐1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moon Y, Han SH, Moon WJ. Patterns of brain iron accumulation in vascular dementia and Alzheimer's dementia using quantitative susceptibility mapping imaging. J Alzheimers Dis. 2016;51(3):737‐745. [DOI] [PubMed] [Google Scholar]
- 50. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood‐brain barrier. Neurobiol Dis. 2010;37(1):13‐25. [DOI] [PubMed] [Google Scholar]
- 51. Devireddy LR, Gazin C, Zhu X, Green MR. A cell‐surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123(7):1293‐1305. [DOI] [PubMed] [Google Scholar]
- 52. Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil‐gelatinase‐associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579(3):773‐777. [DOI] [PubMed] [Google Scholar]
- 53. Bolignano D, Coppolino G, Lacquaniti A, Buemi M. From kidney to cardiovascular diseases: NGAL as a biomarker beyond the confines of nephrology. Eur J Clin Invest. 2010;40(3):273‐276. [DOI] [PubMed] [Google Scholar]
- 54. Elneihoum AM, Falke P, Axelsson L, Lundberg E, Lindgarde F, Ohlsson K. Leukocyte activation detected by increased plasma levels of inflammatory mediators in patients with ischemic cerebrovascular diseases. Stroke. 1996;27(10):1734‐1738. [DOI] [PubMed] [Google Scholar]
- 55. Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826(1):129‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cakal E, Ozkaya M, Engin‐Ustun Y, Ustun Y. Serum lipocalin‐2 as an insulin resistance marker in patients with polycystic ovary syndrome. J Endocrinol Invest. 2011;34(2):97‐100. [DOI] [PubMed] [Google Scholar]
- 57. Nocon AL, Ip JP, Terry R, et al. The bacteriostatic protein lipocalin 2 is induced in the central nervous system of mice with west Nile virus encephalitis. J Virol. 2014;88(1):679‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Naude PJ, Nyakas C, Eiden LE, et al. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer's disease. FASEB J. 2012;26(7):2811‐2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim BW, Jeong KH, Kim JH, et al. Pathogenic upregulation of glial lipocalin‐2 in the Parkinsonian dopaminergic system. J Neurosci. 2016;36(20):5608‐5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dekens DW, Naude PJ, Engelborghs S, et al. Neutrophil gelatinase‐associated lipocalin and its receptors in Alzheimer's disease (AD) brain regions: differential findings in AD with and without depression. J Alzheimers Dis. 2017;55(2):763‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leung L, Radulovich N, Zhu CQ, et al. Lipocalin2 promotes invasion, tumorigenicity and gemcitabine resistance in pancreatic ductal adenocarcinoma. PLoS One. 2012;7(10):e46677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang G, Weng YC, Han X, Whaley JD, McCrae KR, Chou WH. Lipocalin‐2 released in response to cerebral ischaemia mediates reperfusion injury in mice. J Cell Mol Med. 2015;19(7):1637‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song J, Kim OY. Perspectives in lipocalin‐2: emerging biomarker for medical diagnosis and prognosis for Alzheimer's disease. Clin Nutr Res. 2018;7(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Choi J, Lee HW, Suk K. Increased plasma levels of lipocalin 2 in mild cognitive impairment. J Neurol Sci. 2011;305(1–2):28‐33. [DOI] [PubMed] [Google Scholar]
- 65. Llorens F, Hermann P, Villar‐Pique A, et al. Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia. Nat Commun. 2020;11(1):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mesquita SD, Ferreira AC, Falcao AM, et al. Lipocalin 2 modulates the cellular response to amyloid beta. Cell Death Differ. 2014;21(10):1588‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Llorens F, Schmitz M, Knipper T, et al. Cerebrospinal fluid biomarkers of Alzheimer's Disease show different but partially overlapping profile compared to vascular dementia. Front Aging Neurosci. 2017;9:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hermann P, Romero C, Schmidt C, Reis C, Zerr I. CSF biomarkers and neuropsychological profiles in patients with cerebral small‐vessel disease. PLoS One. 2014;9(8):e105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Barry Erhardt E, Pesko JC, Prestopnik J, Thompson J, Caprihan A, Rosenberg GA. Biomarkers identify the Binswanger type of vascular cognitive impairment. J Cereb Blood Flow Metab. 2019;39(8):1602‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosenberg GA. Binswanger's disease: biomarkers in the inflammatory form of vascular cognitive impairment and dementia. J Neurochem. 2018;144(5):634‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eruysal E, Ravdin L, Kamel H, Iadecola C, Ishii M. Plasma lipocalin‐2 levels in the preclinical stage of Alzheimer's disease. Alzheimers Dement (Amst). 2019;11:646‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med. 2019;133:221‐233. [DOI] [PubMed] [Google Scholar]
- 73. Eid R, Arab NT, Greenwood MT. Iron mediated toxicity and programmed cell death: a review and a re‐examination of existing paradigms. Biochim Biophys Acta Mol Cell Res. 2017;1864(2):399‐430. [DOI] [PubMed] [Google Scholar]
- 74. Carpenter KLH, Li W, Wei H, et al. Magnetic susceptibility of brain iron is associated with childhood spatial IQ. NeuroImage. 2016;132:167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim J, Wessling‐Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem. 2014;25(11):1101‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317‐337. [DOI] [PubMed] [Google Scholar]
- 77. Lill R, Hoffmann B, Molik S, et al. The role of mitochondria in cellular iron‐sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta. 2012;1823(9):1491‐1508. [DOI] [PubMed] [Google Scholar]
- 78. Gassen M, Youdim MB. The potential role of iron chelators in the treatment of Parkinson's disease and related neurological disorders. Pharmacol Toxicol. 1997;80(4):159‐166. [DOI] [PubMed] [Google Scholar]
- 79. Lane DJR, Ayton S, Bush AI. Iron and Alzheimer's disease: an update on emerging mechanisms. J Alzheimers Dis. 2018;64(s1):S379‐S395. [DOI] [PubMed] [Google Scholar]
- 80. Lawen A, Lane DJ. Mammalian iron homeostasis in health and disease: uptake, storage, transport, and molecular mechanisms of action. Antioxid Redox Signal. 2013;18(18):2473‐2507. [DOI] [PubMed] [Google Scholar]
- 81. Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41‐58. [DOI] [PubMed] [Google Scholar]
- 82. McCarthy RC, Sosa JC, Gardeck AM, Baez AS, Lee CH, Wessling‐Resnick M. Inflammation‐induced iron transport and metabolism by brain microglia. J Biol Chem. 2018;293(20):7853‐7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Salvador GA. Iron in neuronal function and dysfunction. BioFactors. 2010;36(2):103‐110. [DOI] [PubMed] [Google Scholar]
- 84. Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(Suppl 6):1559S‐1566S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wessling‐Resnick M. Iron Imports. III. Transfer of iron from the mucosa into circulation. Am J Physiol Gastrointest Liver Physiol. 2006;290(1):G1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285‐297. [DOI] [PubMed] [Google Scholar]
- 87. Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776‐781. [DOI] [PubMed] [Google Scholar]
- 88. Song N, Wang J, Jiang H, Xie J. Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson's disease. Free Radic Biol Med. 2010;48(2):332‐341. [DOI] [PubMed] [Google Scholar]
- 89. Correnti C, Strong RK. Mammalian siderophores, siderophore‐binding lipocalins, and the labile iron pool. J Biol Chem. 2012;287(17):13524‐13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202(2):199‐211. [DOI] [PubMed] [Google Scholar]
- 91. McCarthy RC, Kosman DJ. Iron transport across the blood‐brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci. 2015;72(4):709‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677‐686. [DOI] [PubMed] [Google Scholar]
- 94. Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurogibol. 2016;142:23‐44. [DOI] [PubMed] [Google Scholar]
- 95. Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sarlus H, Heneka MT. Microglia in Alzheimer's disease. J Clin Invest. 2017;127(9):3240‐3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063‐3070. [DOI] [PubMed] [Google Scholar]
- 98. Jin Q, Cheng J, Liu Y, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;40:131‐142. [DOI] [PubMed] [Google Scholar]
- 99. Liu LQ, Liu XR, Zhao JY, et al. Brain‐selective mild hypothermia promotes long‐term white matter integrity after ischemic stroke in mice. CNS Neurosci Ther. 2018;24(12):1275‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jiang M, Liu X, Zhang D, et al. Celastrol treatment protects against acute ischemic stroke‐induced brain injury by promoting an IL‐33/ST2 axis‐mediated microglia/macrophage M2 polarization. J Neuroinflammation. 2018;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li R, Liu W, Yin J, et al. TSG‐6 attenuates inflammation‐induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J Neuroinflammation. 2018;15(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shu ZM, Shu XD, Li HQ, et al. Ginkgolide B protects against ischemic stroke via modulating microglia polarization in mice. CNS Neurosci Ther. 2016;22(9):729‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Urrutia P, Aguirre P, Esparza A, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126(4):541‐549. [DOI] [PubMed] [Google Scholar]
- 104. Gaasch JA, Lockman PR, Geldenhuys WJ, Allen DD, Van der Schyf CJ. Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochem Res. 2007;32(7):1196‐1208. [DOI] [PubMed] [Google Scholar]
- 105. Wan Z, Xu J, Huang Y, et al. Elevating bioavailable iron levels in mitochondria suppresses the defective phenotypes caused by PINK1 loss‐of‐function in Drosophila melanogaster. Biochem Biophys Res Commun. 2020;532(2):285‐291. [DOI] [PubMed] [Google Scholar]
- 106. Tao Y, Wang Y, Rogers JT, Wang F. Perturbed iron distribution in Alzheimer's disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta‐analysis. J Alzheimers Dis. 2014;42(2):679‐690. [DOI] [PubMed] [Google Scholar]
- 107. Bulk M, Abdelmoula WM, Nabuurs RJA, et al. Postmortem MRI and histology demonstrate differential iron accumulation and cortical myelin organization in early‐ and late‐onset Alzheimer's disease. Neurobiol Aging. 2018;62:231‐242. [DOI] [PubMed] [Google Scholar]
- 108. van Duijn S, Bulk M, van Duinen SG, et al. Cortical iron reflects severity of Alzheimer's disease. J Alzheimers Dis. 2017;60(4):1533‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Telling ND, Everett J, Collingwood JF, et al. Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer's disease. Cell Chem Biol. 2017;24(10):1205‐1215.e1203. [DOI] [PubMed] [Google Scholar]
- 110. Guo C, Wang T, Zheng W, Shan ZY, Teng WP, Wang ZY. Intranasal deferoxamine reverses iron‐induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34(2):562‐575. [DOI] [PubMed] [Google Scholar]
- 111. Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(8):551‐564. [DOI] [PubMed] [Google Scholar]
- 112. Mena NP, Urrutia PJ, Lourido F, Carrasco CM, Nunez MT. Mitochondrial iron homeostasis and its dysfunctions in neurodegenerative disorders. Mitochondrion. 2015;21:92‐105. [DOI] [PubMed] [Google Scholar]
- 113. Ayton S, Wang Y, Diouf I, et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry. 2019;25(11):2932‐2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res. 2018;341:154‐175. [DOI] [PubMed] [Google Scholar]
- 115. Xu W, Barrientos T, Andrews NC. Iron and copper in mitochondrial diseases. Cell Metab. 2013;17(3):319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82–83:969‐974. [DOI] [PubMed] [Google Scholar]
- 117. Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE‐bound proteins in early Alzheimer disease: insights into the role of lipid peroxidation in the progression of AD. Brain Res. 2009;1274:66‐76. [DOI] [PubMed] [Google Scholar]
- 118. McIntosh A, Mela V, Harty C, et al. Iron accumulation in microglia triggers a cascade of events that leads to altered metabolism and compromised function in APP/PS1 mice. Brain Pathol. 2019;29(5):606‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Belaidi AA, Bush AI. Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem. 2016;139(Suppl 1):179‐197. [DOI] [PubMed] [Google Scholar]
- 120. Ferreira AC, Da Mesquita S, Sousa JC, et al. From the periphery to the brain: Lipocalin‐2, a friend or foe? Prog Neurogibol. 2015;131:120‐136. [DOI] [PubMed] [Google Scholar]
- 121. Thomsen MS, Andersen MV, Christoffersen PR, Jensen MD, Lichota J, Moos T. Neurodegeneration with inflammation is accompanied by accumulation of iron and ferritin in microglia and neurons. Neurobiol Dis. 2015;81:108‐118. [DOI] [PubMed] [Google Scholar]
- 122. Chia WJ, Dawe GS, Ong WY. Expression and localization of the iron‐siderophore binding protein lipocalin 2 in the normal rat brain and after kainate‐induced excitotoxicity. Neurochem Int. 2011;59(5):591‐599. [DOI] [PubMed] [Google Scholar]
- 123. Nairz M, Theurl I, Schroll A, et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin‐2. Blood. 2009;114(17):3642‐3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ferreira AC, Santos T, Sampaio‐Marques B, et al. Lipocalin‐2 regulates adult neurogenesis and contextual discriminative behaviours. Mol Psychiatry. 2018;23(4):1031‐1039. [DOI] [PubMed] [Google Scholar]
- 125. Ferreira AC, Pinto V, Da Mesquita S, et al. Lipocalin‐2 is involved in emotional behaviors and cognitive function. Front Cell Neurosci. 2013;7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xiao X, Yeoh BS, Saha P, Olvera RA, Singh V, Vijay‐Kumar M. Lipocalin 2 alleviates iron toxicity by facilitating hypoferremia of inflammation and limiting catalytic iron generation. Biometals. 2016;29(3):451‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Khalil M, Renner A, Langkammer C, et al. Cerebrospinal fluid lipocalin 2 in patients with clinically isolated syndromes and early multiple sclerosis. Mult Scler. 2016;22(12):1560‐1568. [DOI] [PubMed] [Google Scholar]
- 128. Dong M, Xi G, Keep RF, Hua Y. Role of iron in brain lipocalin 2 upregulation after intracerebral hemorrhage in rats. Brain Res. 2013;1505:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xu G, Ahn J, Chang S, et al. Lipocalin‐2 induces cardiomyocyte apoptosis by increasing intracellular iron accumulation. J Biol Chem. 2012;287(7):4808‐4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Barasch J, Hollmen M, Deng R, et al. Disposal of iron by a mutant form of lipocalin 2. Nat Commun. 2016;7:e12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385‐393. [DOI] [PubMed] [Google Scholar]
- 132. Brys M, Pirraglia E, Rich K, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30(5):682‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42(3):233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE. Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement. 2013;9(1):76‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Selkoe DJ, American College of P , American Physiological S . Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140(8):627‐638. [DOI] [PubMed] [Google Scholar]
- 137. Giaccone G, Arzberger T, Alafuzoff I, et al. New lexicon and criteria for the diagnosis of Alzheimer's disease. Lancet Neurol. 2011;10(4):298‐299.author reply 300–291. [DOI] [PubMed] [Google Scholar]
- 138. Jin M, Kim JH, Jang E, et al. Lipocalin‐2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2014;34(8):1306‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Berard JL, Zarruk JG, Arbour N, et al. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60(7):1145‐1159. [DOI] [PubMed] [Google Scholar]
- 140. Ovanesov MV, Ayhan Y, Wolbert C, Moldovan K, Sauder C, Pletnikov MV. Astrocytes play a key role in activation of microglia by persistent Borna disease virus infection. J Neuroinflammation. 2008;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Escartin C, Bonvento G. Targeted activation of astrocytes: a potential neuroprotective strategy. Mol Neurobiol. 2008;38(3):231‐241. [DOI] [PubMed] [Google Scholar]
- 142. Okada S, Nakamura M, Katoh H, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12(7):829‐834. [DOI] [PubMed] [Google Scholar]
- 143. Custer SK, Garden GA, Gill N, et al. Bergmann glia expression of polyglutamine‐expanded ataxin‐7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9(10):1302‐1311. [DOI] [PubMed] [Google Scholar]
- 144. Bi F, Huang C, Tong J, et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci USA. 2013;110(10):4069‐4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lee S, Lee WH, Lee MS, Mori K, Suk K. Regulation by lipocalin‐2 of neuronal cell death, migration, and morphology. J Neurosci Res. 2012;90(3):540‐550. [DOI] [PubMed] [Google Scholar]
- 146. Lee S, Park JY, Lee WH, et al. Lipocalin‐2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009;29(1):234‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Xing C, Wang X, Cheng C, et al. Neuronal production of lipocalin‐2 as a help‐me signal for glial activation. Stroke. 2014;45(7):2085‐2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lee S, Lee J, Kim S, et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007;179(5):3231‐3241. [DOI] [PubMed] [Google Scholar]
- 149. Arnold SE, Arvanitakis Z, Macauley‐Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang J, Chen C, Hua S, et al. An updated meta‐analysis of cohort studies: diabetes and risk of Alzheimer's disease. Diabetes Res Clin Pract. 2017;124:41‐47. [DOI] [PubMed] [Google Scholar]
- 151. Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369(19):1863‐1864. [DOI] [PubMed] [Google Scholar]
- 152. Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(1):75‐89. [DOI] [PubMed] [Google Scholar]
- 153. Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9(1):36‐45. [DOI] [PubMed] [Google Scholar]
- 154. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64‐74. [DOI] [PubMed] [Google Scholar]
- 155. Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta‐analysis of longitudinal studies. Intern Med J. 2012;42(5):484‐491. [DOI] [PubMed] [Google Scholar]
- 156. Banjara M, Ghosh C. Sterile neuroinflammation and strategies for therapeutic intervention. Int J Inflam. 2017;2017:e8385961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhao P, Stephens JM. STAT1, NF‐kappaB and ERKs play a role in the induction of lipocalin‐2 expression in adipocytes. Mol Metab. 2013;2(3):161‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin‐17 target genes. J Biol Chem. 2006;281(34):24138‐24148. [DOI] [PubMed] [Google Scholar]
- 159. Seth P, Porter D, Lahti‐Domenici J, Geng Y, Richardson A, Polyak K. Cellular and molecular targets of estrogen in normal human breast tissue. Cancer Res. 2002;62(16):4540‐4544. [PubMed] [Google Scholar]
- 160. Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56(10):2533‐2540. [DOI] [PubMed] [Google Scholar]
- 161. Kanaka‐Gantenbein C, Margeli A, Pervanidou P, et al. Retinol‐binding protein 4 and lipocalin‐2 in childhood and adolescent obesity: when children are not just "small adults". Clin Chem. 2008;54(7):1176‐1182. [DOI] [PubMed] [Google Scholar]
- 162. Semba T, Nishimura M, Nishimura S, et al. The FLS (fatty liver Shionogi) mouse reveals local expressions of lipocalin‐2, CXCL1 and CXCL9 in the liver with non‐alcoholic steatohepatitis. BMC Gastroenterol. 2013;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Luo Y, Ma X, Pan X, et al. Serum lipocalin‐2 levels are positively associated with not only total body fat but also visceral fat area in Chinese men. Medicine (Baltimore). 2016;95(30):e4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Russell DW, Halford RW, Ramirez DM, Shah R, Kotti T. Cholesterol 24‐hydroxylase: an enzyme of cholesterol turnover in the brain. Annu Rev Biochem. 2009;78:1017‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tan BK, Adya R, Shan X, et al. Ex vivo and in vivo regulation of lipocalin‐2, a novel adipokine, by insulin. Diabetes Care. 2009;32(1):129‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bhusal A, Rahman MH, Lee IK, Suk K. Role of hippocampal lipocalin‐2 in experimental diabetic encephalopathy. Front Endocrinol (Lausanne). 2019;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008;22(6):1416‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Oberoi R, Bogalle EP, Matthes LA, et al. Lipocalin (LCN) 2 mediates pro‐atherosclerotic processes and is elevated in patients with coronary artery disease. PLoS One. 2015;10(9):e0137924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wu G, Li H, Zhou M, et al. Mechanism and clinical evidence of lipocalin‐2 and adipocyte fatty acid‐binding protein linking obesity and atherosclerosis. Diabetes Metab Res Rev. 2014;30(6):447‐456. [DOI] [PubMed] [Google Scholar]
- 170. Xiao Y, Xu A, Hui X, et al. Circulating lipocalin‐2 and retinol‐binding protein 4 are associated with intima‐media thickness and subclinical atherosclerosis in patients with type 2 diabetes. PLoS One. 2013;8(6):e66607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Akushevich I, Yashkin AP, Kravchenko J, Kertai MD. Chemotherapy and the RISK of Alzheimer's disease in colorectal cancer survivors: evidence from the medicare system. JCO Oncol Pract. 2021:OP2000729. 10.1200/OP.20.00729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wang G, Weng YC, Chiang IC, et al. Neutralization of lipocalin‐2 diminishes stroke‐reperfusion injury. Int J Mol Sci. 2020;21(17):6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Almeida‐Suhett CP, Li Z, Marini AM, Braga MF, Eiden LE. Temporal course of changes in gene expression suggests a cytokine‐related mechanism for long‐term hippocampal alteration after controlled cortical impact. J Neurotrauma. 2014;31(7):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nam Y, Kim JH, Seo M, et al. Lipocalin‐2 protein deficiency ameliorates experimental autoimmune encephalomyelitis: the pathogenic role of lipocalin‐2 in the central nervous system and peripheral lymphoid tissues. J Biol Chem. 2014;289(24):16773‐16789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ni W, Zheng M, Xi G, Keep RF, Hua Y. Role of lipocalin‐2 in brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2015;35(9):1454‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


