Abstract
Introduction: Some cannabinoids have been identified as anti-inflammatory agents; however, their potential therapeutic or prophylactic applications remain controversial. The aim of this systematic review was to provide a timely and comprehensive insight into cannabinoid-mediated pro- and anti-inflammatory cytokine responses in preclinical in vivo studies.
Methods and Materials: A systematic search was conducted using PubMed, Web of Science, EMBASE, and Scopus. Eligible studies where cannabinoids had been evaluated for their effect on inflammation in animal models were included in the analysis. Data were extracted from 26 of 4247 eligible full text articles, and risk of bias was assessed using the SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) tool. Studies examined cannabidiol (CBD; n=20); cannabigerol (CBG; n=1); delta 9-tetrahydrocannabinol (THC; n=2); THC and CBD separately (n=1); and THC and CBD in combination (n=2).
Results: Tumor necrosis factor alpha, interleukin (IL)-1β, IL-6, and interferon gamma were the most commonly studied pro-inflammatory cytokines and their levels were consistently reduced after treatment with CBD, CBG, or CBD+THC, but not with THC alone. The association between cannabinoid-induced anti-inflammatory response and disease severity was examined. In 22 studies where CBD, CBG, or CBD in combination with THC were administered, a reduction in the levels of at least one inflammatory cytokine was observed, and in 24 studies, some improvements in disease or disability were apparent. THC alone did not reduce pro-inflammatory cytokine levels (n=3), but resulted in improvements in neuropathic pain in one study.
Conclusions: This review shows that CBD, CBG, and CBD+THC combination exert a predominantly anti-inflammatory effect in vivo, whereas THC alone does not reduce pro-inflammatory or increase anti-inflammatory cytokines. It is anticipated that this information could be used to inform human clinical trials of cannabinoids, focusing on CBD and CBG to reduce inflammation across a range of pathophysiological processes.
Keywords: anti-inflammatory, cannabinoids, cannabis, CBD, cytokines, inflammation, systematic review, THC
Introduction
Cannabis sativa is a complex botanical containing ∼500 phytochemicals of which 60 or more belong to the phytocannabinoid class. These phytochemical compounds have been used for >6000 years to treat diseases.1 Cannabinoid compounds act on the endocannabinoid system (ECS) that consists of cannabinoid type 1 (CB1) and type 2 (CB2) receptors, their endogenous ligands (endocannabinoids), and the enzymes responsible for their synthesis and degradation.2 Primarily, CB2 receptors, and to a lesser extent CB1 receptors, are involved in regulating the immune response, diminishing inflammatory responses by inhibiting the release of pro-inflammatory mediators and through immune cell modulation.2 Cannabinoids also modulate several noncannabinoid receptors and ion channels and act through various receptor-independent pathways—for example, by delaying the reuptake of endocannabinoids and neurotransmitters (such as anandamide and adenosine) and by enhancing or inhibiting the binding action of certain G-protein-coupled receptors.2,3 Reported therapeutic benefits of exogenous cannabinoids (plant derived and synthetic) on the ECS include analgesic, anti-inflammatory, anti-emetic, and anticonvulsive effects as well as improved muscle tone, mood state, cognition, and appetite.3–8 The ECS is therefore an emerging target to treat a wide range of pathological conditions, particularly those related to inflammation.9
Inflammation is a complex sequence of events that occurs in response to harmful stimuli, and is a vital part of the immune response to damage caused by trauma, infection, or environmental factors. Whereas the inflammatory response is essential and beneficial to a host in the short term, excessive or persistent inflammation can cause pain, tissue damage, and is associated with many chronic conditions such as diabetes, dementia, multiple sclerosis (MS), and rheumatoid arthritis.10 Cytokines are potent soluble immune mediators that are modulated in various disease states.11 Pro-inflammatory responses are characterized by increased levels of cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-1β, and interferon gamma (IFN-γ), and these drive the progression of chronic disease.12 Conversely, anti-inflammatory cytokines such as IL-10 mediate the opposing effect.12 Chronic inflammatory conditions can be prevented and better managed by regulating or inhibiting excessive or persistent inflammation through anti-inflammatory compounds that act on the ECS, such as exogenous cannabinoids.13
In recent times, regulations that previously restricted scientific study of cannabis and its constituents have become more relaxed internationally. As a result, interest in the therapeutic potential of cannabis has increased.14 Despite this recent research intensity, systematic reviews undertaken to critically appraise human trials of medicinal cannabis have, in general, found only modest benefit to patients15–18; yet, many cannabis users report great benefit for conditions for which compelling evidence is lacking.19 It is timely to critically evaluate the preclinical in vivo evidence so that interventions can be appropriately targeted in subsequent human trials. Therefore, in this review, we focus on the animal models of inflammation that researchers have used to investigate the therapeutic effects of cannabinoids. We aimed to systematically summarize and review studies that evaluate the in vivo effect of cannabinoids on pro- and anti-inflammatory cytokines.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for transparent reporting of methodology and results.20 After several scoping searches, a comprehensive search of articles was conducted on the in vivo anti-inflammatory activity of cannabis-derived compounds in four different online bibliographical databases: PubMed, Web of Science, EMBASE, and Scopus. As given in Table 1, the structure of the search strategy combined anti-inflammatory agents and cannabis and laboratory animals with the appropriate MeSH terms or synonyms. Articles that solely investigated synthetic cannabinoids and endocannabinoids were excluded during the selection process.
Table 1.
Population, Intervention, Comparison, Outcomes Illustrating the Systematic Search Keyword Strategy
| Population |
| “Animals, laboratory” MeSH terms or synonyms were (laboratory animals) OR (animal, laboratory) OR (laboratory animal) OR (in vivo) OR (rodent) OR (rat) OR (mouse) OR (murine) OR (rabbit) OR (gerbil) OR (hamster) OR (pig) OR (cat) OR (dog) OR (primate) OR (guinea pig) |
| Intervention |
| “Cannabis” MeSH terms or synonyms: (hashish) OR (marijuana) OR (cannabinoids) OR (dronabinol) OR (nabiximol) OR (levonantradol) OR (nabilone) OR (Savitex) (tetrahydrocannabinol) OR (THC) OR (cannabidiol) OR (CBD) OR (cannabiniol) OR (CBN) OR (Medicinal Plants) OR (Medicinal Herbs) |
| Outcome |
| “antiinflammatory agents” with the following MeSH terms or synonyms: (anti inflammatory agents) OR (agents, antiinflammatory) OR (antiinflammatories) OR (anti-inflammatory agents) OR (agents, anti-inflammatory) OR (agents, anti inflammatory) OR (anti-inflammatories) OR (anti inflammatories) |
Note: Population, intervention, and outcome searches were combined with the Boolean operator “AND.” Comparison terms were not specified due to the broad nature of the review.
Study selection
Non-English articles, as well as reviews, meta-analyses, conference articles or proceedings, theses, editorials/letters, patents, case reports, clinical studies, ex vivo, in vitro and in silico and any reports other than in vivo studies, were excluded from this systematic review. Articles where cytokine analysis was undertaken by a quantitative antibody-based assay such as enzyme-linked immunosorbent assay (ELISA) were included. Quantification methods of specific markers are considered advantageous compared with more qualitative or semiquantitative methods such as immunohistochemistry or polymerase chain reaction.
The systematic search was run in each of the four databases and the records from database inception through to January 1, 2019 were imported and saved to reference management software EndNote™ X7 (Thompson Reuter, CA). At the time the search was run (by F.R.H. and G.Z.S.), database alerts were set up and new articles collated until July 1, 2020 for article finalization.
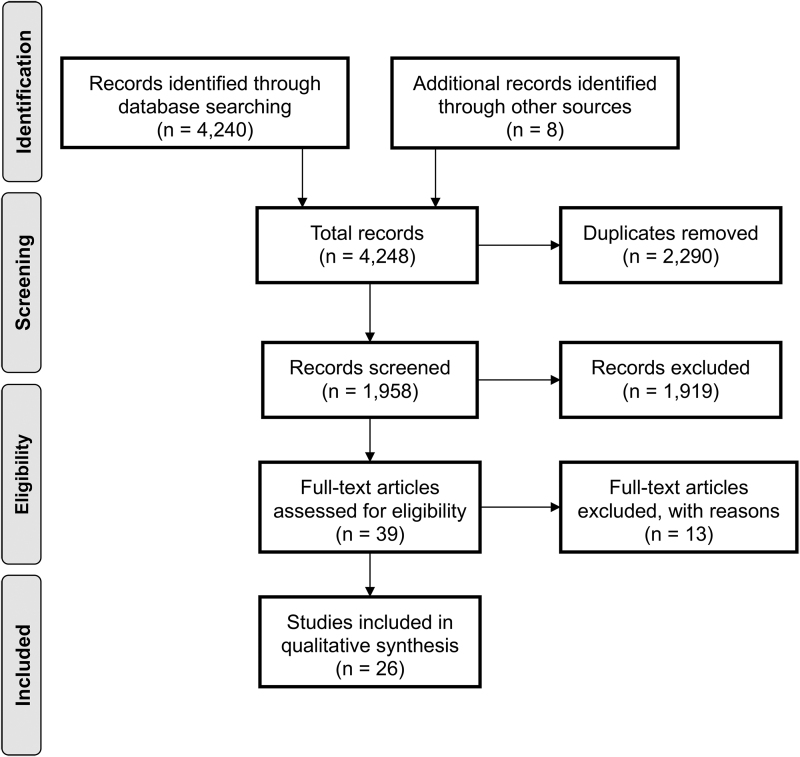
Figure 1 illustrates the study selection process (PRISMA). Using EndNote, duplicate references were removed, and remaining titles and abstracts were screened against study inclusion/exclusion criteria (by F.R.H.). The titles and abstracts of remaining articles were then screened and if there was any doubt regarding the eligibility of an article, the full text was retrieved for clarification. Articles deemed eligible by one reviewer were further assessed by a second independent reviewer (L.S.D.) to ensure inclusion criteria were met. All authors completed data extraction and resolved any disagreements by reviewing and discussing the full articles.
FIG. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing data extraction process.
Risk of bias assessment
The SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) risk of bias (RoB) tool was used to establish consistency and avoid discrepancies in assessing RoB in systematic reviews of animal interventions.21 Details of the tool and the rationale for the included categories are described in Higgins et al.22 Two authors (F.R.H. and L.S.D.) independently answered the questions from the SYRCLE RoB tool. Disagreements in the answers were resolved by discussion with a co-author (G.Z.S.) until consensus was achieved.
Results
Study selection
The search strategy resulted in 26 studies that met the inclusion criteria, 21 animal models were used across 3 species, with the rodent being the most common (mouse: n=15;23–37 rat: n = 9).38–46 One study used porcine,47 and one used both canines and rodents48 (inflammatory cytokines were assessed in rodents only). Because of high numbers of unclear or unreported items, or the vast majority of one type of RoB in the other domains, it was decided that a meta-analysis was not appropriate. In lieu of this, a qualitative synthesis approach was taken to summarize the evidence across the following domains: study characteristics, inflammatory cytokine responses to cannabinoids, efficacy of cannabinoids, and dose response.
Study characteristics
Study characteristics are detailed in Table 2. The studies were undertaken in several countries: Brazil (n=8),29–32,38,41–43 United States (n=4),23,26,39,48 China (n=3),28,37,44 Israel (n=3),27,35,36 Italy (n=3),24,25,40 Australia (n=2),34,45 Spain (n=1),47 and international collaborations with three or more countries (n=2).33,46 Studies were carried out between 2006 and 2020, with the majority of studies (n=20) supported independently by competitive funding or nonprofit organizations,23,26,28–45 three supported by joint industry/independent funding,46–48 and three studies did not disclose funding sources.24,25,27 The 2019 impact factor value of the journals in which these studies were published (InCites) ranged from 2.067 to 6.633 (median=3.412), two studies were published in a peer-reviewed journal without an impact factor (InCites).27,42 No conflict of interest (COI) was declared by 11 studies23,25,28,29,31,33,34,37,42,44,46; in 10 cases COI was not disclosed.24,26,30,35,36,38–41,45 In two studies, authors declared patents on cannabidiol-(CBD) related drugs,32,43 and in two studies there was disclosure of affiliation or research agreements with industry partners, but the authors stated no COI,27,47 and in one study a scientific advisory role was disclosed with the industry partner cofunding the research.48
Table 2.
In Vivo Study Characteristics Including Species, Model, Specimen Examined, Cannabis Intervention Details, Study Methodology, Measures, Inflammatory Cytokines, and Results
| Study | Animal species/sex/age | Model | Specimen | Cannabis administration/dosage | Methods | Measures | Inflammatory cytokine(s) measured/effect directionality | Results |
|---|---|---|---|---|---|---|---|---|
| Al-Ghezi et al.23 | Female C57BL/6 mice (6–8 weeks) | EAE | Spleen; serum; cecal content | CBD+THC (10 mg/kg each; 1:1 ratio); vehicle; daily i.p. injection, beginning 10 days after disease induction | EAE was induced using MOG/CFA emulsion (subcutaneous injection) and Bordetella Pertussis toxin (i.p. injection). The role of the microbiota was studied in relation to the mechanism of cannabinoids on clinical symptoms of MS | Histopathology; inflammatory cytokines; microbiome profiling; LPS and SCFA quantification | ↓ IL-17A ↑ IL-10 No change: IFN-γ |
CBD+THC treatment reduced EAE severity by enhancing anti-inflammatory responses reflected in IL-10 and TGF-β levels. Treatment also limited gut microbiota such as Akkermansia muciniphila linked to EAE reflected by reduced microbial metabolites and LPS while increasing SCFA production |
| Arruza et al.47 | Male long white Landrace piglets (1–2 days) | HI brain damage | Brain; lung; BALF | CBD (1 mg/kg); CBD+WAY100635 (5-HT1A receptor antagonist, 0.1 mg/kg); vehicle; single IV administration | Carotid arteries were exposed and wrapped with elastic bands. HI was induced by pulling out the elastic bands until complete interruption of carotid blood flow occurred for 30 min. Total lung compliance, oxygenation index, and extravascular lung water content were monitored for 6 h after HI brain damage | Respiratory and cardiac functional outcome measures; histopathology; IL-1β; oxidative stress markers | CBD vs. vehicle: ↓ IL-1β (lung tissue) CBD+WAY100635 vs. vehicle (lung tissue): No change |
CBD mitigated negative HI-induced effects on total lung compliance, oxygen index and reduced histological lung damage. For CBD alone, decreases in IL-1β were observed, combined with reduced protein content in BALF and extravascular lung water. Positive effects are hypothesized to be owing to anti-inflammatory changes in the brain induced by CBD only treatment |
| Barichello et al.38 | Male Wistar rats (estimated 8–10 weeks based on weight) | Neuroinflammation-pneumococcal meningitis | Brain, CSF | CBD (2.5, 5, or 10 mg/kg) once, or daily for 9 days after meningitis induction; placebo; i.p. injection | Meningitis was induced by inoculating rats with Streptococcus pneumoniae. Rats (single dose group) were killed 6 h after meningitis induction. On day 10, rats receiving chronic CBD treatment completed the inhibitory avoidance task before killing and collection of hippocampal and frontal cortical tissue | Inflammatory cytokines; BDNF; behavioral assessment (inhibitory avoidance task) | Acute treatment: no change: TNF-α (hippocampus); IL-6; IL-1β Chronic treatment: ↓ TNF-α (frontal cortex); unchanged in hippocampus |
Acute CBD treatment did not revert increases in TNF-α, IL-6, or IL-1β resulting from pneumococcal meningitis induction. Chronic treatment with CBD (10 mg/kg) prevented cognitive impairment in rats induced with pneumococcal meningitis. CBD administration at 2.5, 5 and 10 mg/kg reduced TNF-α concentrations in the frontal cortex |
| Borrelli et al.24 | Male ICR mice (estimated 7–9 weeks based on weight) | Colitis (IBD) | Colon | CBD (1–10 mg/kg daily for gross macroscopic evaluations and 5 mg/kg for all other measures) for six consecutive days, commencing 3 days before DNBS administration; control NR | Colitis was induced with intracolonic administration of DNBS. Mice were killed 3 days after DNBS administration. Macroscopic evaluation and histology performed | Histopathology; inflammatory cytokines; nitrites; oxidative stress markers; endocannabinoid levels | ↓ IL-1β; ↑ IL-10 | 5 mg/kg CBD reduced pro-inflammatory IL-1β and increased anti-inflammatory IL-10 concentrations in full-thickness colonic tissues. Attenuation of DNBS-induced damage in colon tissue was greatest at 5 mg/kg CBD treatment. CBD reduced nitrite production, oxidative stress marker and endocannabinoid levels, but no effect on COX-2 and FAAH mRNA expression |
| Borrelli et al.25 | Male ICR mice (estimated 7–9 weeks based on weight) | Colitis (IBD) | Colon | Preventative protocol: CBG (1–30 mg/kg) daily for six consecutive days commencing 3 days before DNBS administration; curative protocol: CBG (1–30 mg/kg) injected for two consecutive days starting 24 h after DNBS administration | As per Borrelli et al.24 | Inflammatory cytokines; oxidative stress markers; nitrites; intestinal permeability; SOD activity; histopathology; IHC | ↓ IL-1β; IFN-γ; ↑ IL-10 | Preventative and curative protocols (30 mg/kg): CBG reduced colon injury, colon weight:length ratio (from 1 mg/kg preventative protocol and from 5 mg/kg curative protocol). Curative protocol only (30 mg/kg): CBG reduced pro-inflammatory IL-1β and IFN-γ and increased anti-inflammatory IL-10, improved IHC, intestinal permeability, decreased MPO, SOD, iNOS, but had no effect on COX-2. Preventative protocol only (30 mg/kg): CBG partially improved IHC |
| Britch et al.39 Experiment 1 |
Male and female Sprague–Dawley rats (aged 60–90 days) | Localized inflammatory pain | Hind-paw | THC (0.0–4.0 mg/kg); CBD (0.0–10.0 mg/kg) i.p. administration twice daily for 3 days (repeated dose group). On day 4, naive rats received single dose of THC, CBD, or vehicle (acute dose group) and assessments performed | Inflammation was induced by intraplantar injection of CFA. Assessments were carried out 0.5–4.0 h postinjection on day 4 | Allodynia; nociceptive behavior; weight-bearing; locomotor activity; body weight | Not measured | THC and CBD increased mechanical threshold, and weight bearing on inflamed paw. THC had anti-hyperalgesic effects and reduced body weight; CBD did not. THC reduced locomotor activity while CBD showed the opposite effect |
| Britch et al.39 Experiment 2 |
Male and female Sprague–Dawley rats (60–90 days) | Systemic inflammation | Serum | THC (2.0 mg/kg); CBD (10 mg/kg); vehicle; i.p. administration twice daily for 3 days. On day 4 (4 h after treatment) serum samples obtained for cytokine analysis | Inflammation was induced by intraplantar injection of CFA or mineral oil (no pain control was present) | Inflammatory cytokines | THC: No change: IFN-γ; TNF-α; IL-1β; IL-6; IL-10 CBD: ↓ IFN-γ; IL-10; IL-1β; TNF-α (only male in CFA model); ↑ IL-6 |
Sex, dosing frequency, and treatment varied cytokine responses independently. Serum cytokines were largely unaffected by THC. In contrast, CBD generally exerted reduction in serums in IFN-γ, IL-10, and IL-1β production, and to some extent with TNF-α, with increases in IL-6 |
| Costa et al.40 | Male Wistar rats (200–220 g) (estimated ∼6 weeks based on weight) | Chronic neuropathic pain; inflammatory pain | Hind-paw; spinal cord; plasma | Chronic neuropathic pain: CBD (2.5, 5, 10, and 20 mg/kg); Inflammatory pain: 20 mg/kg); vehicle; oral administration daily for 7 days, beginning on day 7 after disease induction | Unilateral neuropathic pain was induced by constriction injury of the sciatic nerve in the right hind paw. Inflammatory pain was induced using CFA (intraplantar injection). 14 days after disease induction (24 h after last CBD dose), behavioral evaluations carried out and rats killed. Biochemical assays and hind paw tissue assessed | TNF-α; oxidative stress markers; nociceptive behavior | No change: TNF-α (measured in neuropathic model only) | Hyperalgesia to mechanical and thermal stimuli was reduced in both pain models after daily oral CBD treatment. CBD activity was associated with reduced pro-oxidant markers and normalized overall antioxidant capacity. No reductions in TNF-α or NF-κB were observed |
| Elliott et al.26 | Female C57BL/6 mice (6–8 weeks) | EAE | Serum | CBD (20 mg/kg); vehicle; i.p. administration daily, beginning 9 days after disease induction, until day 25 | Disease induction as per Al-Ghezi et al.23 Clinical scores of EAE assessed. Serum cytokines measured | Inflammatory cytokines | Serum: ↓ IFN-γ; IL-17 | CBD treatment delayed onset of disease, and led to reduction in EAE severity, T cell infiltration and pro-inflammatory markers |
| Gallily et al.27 | Female Sabra mice (6–8 weeks old) | Inflammatory pain | Hind-paw; plasma | Parenteral dose (single i.p. injection): Purified CBD (1, 5, 25, and 50 mg/kg); Cannabis clone 202 (5, 25 and 50 mg/kg); vehicle. Oral dose (single administration): Purified CBD (10, 25, 50 and 100 mg/kg); Cannabis clone 202 (10, 25, 50, 100, and 150 mg/kg); vehicle |
Inflammation was induced using 60 μg zymosan injection into the subplanter surface of the right paw. Therapeutic agents were given immediately after disease induction and compared with aspirin and tramadol. Paw swelling and pain perception were assessed 2-, 6-, and 24-h postinjection | TNF-α; paw edema; nociceptive behavior | ↓ TNF-α | CBD at 5 mg/kg achieved maximal effect on inhibition of inflammation (evident by reduced TNF-α) and antinociceptive effect. These beneficial effects were greater compared with tramadol and aspirin |
| Li et al.28 | C57BL mice; sex and age NR (weight 22–26 g) | Acute pancreatitis | Pancreas; lungs; plasma | CBD (0.5 mg/kg) or O-1602 (synthetic cannabinoid; 10 mg/kg); 2 doses 30 min before the first cerulein injection and immediately before the fifth (of 6) cerulein injection; saline; all i.p. administration | Pancreatitis was induced by hourly cerulein (i.p. injection) for a total of six doses. At 3 h after final cerulein injection, mice were killed. Plasma, lungs and pancreas were collected for assessment | Histopathology; inflammatory cytokines; pancreatic enzyme activity; MPO activity; GPR55 expression | CBD: ↓ IL-6; TNF-α O-1602: ↓ TNF-α |
Either CBD or O-1602 attenuated acute pancreatitis pathophysiology, pancreatic enzyme activity, reduced MPO activity, protein expression, and rescued IHC outcomes. Plasma: CBD reduced IL-6 and TNF-α and O-1602 reduced TNF-α only. Pancreatic tissue: CBD reduced IL-6, O-1602 did not |
| Napimoga et al.41 | Male Wistar rats (age NR; estimated ∼7–10 weeks based on weight) | Experimental periodontitis | Gingival tissue/bone | CBD (5 mg/kg); vehicle, i.p. administration daily for 30 days after disease induction | Periodontal disease was induced using a ligature around the first molars of each treated rat. Rats were killed after 30 days where mandibles and gingival tissue were taken for further assessment | Histopathology; inflammatory cytokines; MPO activity | ↓ IL-1β; TNF-α | CBD-treated animals had reduced bone loss in periodontal disease along with decreased expression of RANKL/RANK; an NF-κB activator. The MPO demonstrated a lessening of neutrophil migration, associated with reductions in levels of IL-1β and TNF-α |
| Ribeiro et al.30 | Male C57BL/6 mice (∼60 days) | Acute lung injury | Lung; BALF | CBD (0.3, 1.0, 10, 20, 30, 40, and 80 mg/kg); vehicle, single i.p. administration 60 min after disease induction | Acute lung injury was induced by intranasal instillation of Escherichia coli LPS. Total inflammatory cells combined with BALF was used to assess leukocyte migration to the lungs | Inflammatory cytokines; MPO activity; protein concentration (for normalizing read-out in BALF samples) | ↓ TNF-α; IL-6 | CBD administration reduced lung leukocyte migration, the production of pro-inflammatory cytokines, and vascular permeability, mediated by adenosine A2A receptor signaling to promote anti-inflammatory effects |
| Ribeiro et al.29 | Male C57BL/6 mice (8–10 weeks) | Acute lung injury | Lung; BALF | CBD (20 and 80 mg/kg); vehicle, single i.p. administration 6 h after disease induction | As per Ribeiro et al.30 Mice were killed 24 h after disease induction. Pulmonary mechanics were assessed and various biochemical assays performed | Inflammatory cytokines; pulmonary mechanics; MPO activity; histopathology; protein concentration (for normalizing read-out in BALF samples) | ↓ TNF-α (CBD 20 and 80 mg/kg); IL-6 (80 mg/kg CBD) | CBD (both doses or 80 mg/kg CBD only) successfully reduced inflammatory cytokines and chemokines, leukocyte migration, and MPO activity in BALF. Both doses of CBD improved pulmonary mechanics and lung function |
| Soares et al.42 | Male adult Wistar rats (estimated 10–12 weeks based on weight) | Ischemia/reperfusion kidney injury | Kidney | CBD (5 mg/kg); saline, single infusion via the aorta immediately before the release of renal pedicle (clamped bilaterally for 45 min) | Kidney injury was induced by incising the abdominal wall and dissecting the renal pedicle bilaterally. A clamp was applied to pedicle bilaterally for 45 min followed by reperfusion. Rats were killed 24 h after disease induction. Kidneys were retrieved for further analysis | Inflammatory cytokines; MPO activity; oxidative stress markers; cannabinoid receptor signaling | ↓ TNF-α; IL-1β | CBD exhibited a protective effect in kidney injury and reduced pro-inflammatory cytokines induced by ischemia/reperfusion. CBD reduced damage caused by oxidative stress, but had no effect on nitrate levels. No changes were observed in the expression of cannabinoid receptors after treatment |
| Sonego et al.31 | Male Swiss mice (10 weeks) | Experimental tardive dyskinesia | Brain | Experiment 1: CBD (60 mg/kg) and haloperidol (2–3 mg/kg) i.p. administration daily for 21 days; vehicle. Experiment 2: CBD (60 mg/kg) or GW9662 (PPAR-γ antagonist; 2 mg/kg) and haloperidol (3 mg/kg) i.p. administration daily for 21 days; vehicle |
Orofacial dyskinesia was induced by haloperidol. Behavioral assessments carried out 24 h after final injection, then striatum extracted for analyses | Inflammatory cytokines; oxidative stress markers; locomotor activity; vacuos chewing movements | ↓ TNF-α; IL-1β No change: IL-6 ↑ IL-10 (only in conjunction with haloperidol 3 mg/kg) |
Chronic CBD administration alleviated orofacial dyskinesia but did not improve locomotor activity. In striatum, CBD attenuated microglial activation, oxidative stress, and pro-inflammatory cytokines TNF-α; IL-1β, and increased anti-inflammatory IL-10. CBD reduced TNF-α and IL-6 mRNA expression; no treatment affected IL-1β mRNA expression. When mice were pretreated with PPAR-γ antagonist, this blocked positive behavioral effects of CBD, suggesting PPAR-γ receptor involvement |
| Verrico et al.48 | Female, C57BL/6J mice (6–10 weeks). Note: canine data not reported as inflammatory cytokines not assessed | Experimental inflammation (local and systemic) | Ear; serum | Croton oil-induced inflammation: CBD oil (100 μL of 10 mg/mL); vehicle; single topical administration 2 h after disease induction. LPS-induced inflammation: CBD (1, 10, and 100 μg) i.p. administration or CBD (100 μg) topical administration at LPS injection site 2 h after disease induction; control |
Croton oil (localized) and LPS (systemic) models of experimental inflammation (localized applied topically to ear and systemic via i.p. injection, respectively). Collection of ear tissue 1–4 h after croton oil and serum samples 2 h after LPS administration | Inflammatory cytokines; oxidative stress markers; MPO activity; in vivo assessment of cannabidiol bioavailability | ↓ TNF-α; IL-6 ↑ IL-10 |
Local CBD administration reduced MPO activity, circulating TNF-α, and the development of edema. Systemic TNF-α and IL-6 were reduced in a dose-dependent manner; 100 μg topical CBD generated similar anti-inflammatory effects to i.p. administration. CBD alone increased IL-10. Liposomal CBD was more bioavailable than naked CBD and was able to reduce inflammatory cytokines at a faster rate |
| Vuolo et al.43 | Male adult Wistar rats (8 weeks) | Experimental asthma | Serum | CBD (5 mg/kg); vehicle, i.p. administration once daily on final 2 days of OVA challenge | Asthma was induced by inoculation with OVA (i.p. injection). 14 days after initial injection, rats were boosted with OVA or alum. After another 7 days, rats were challenged with aerosol with OVA or saline (30 min/day for 3 days). 24 h after the final OVA challenge, animals were killed and blood samples taken for inflammatory marker assessment | Inflammatory cytokines | ↓ Th1 cytokines: TNF-α; IL-6; and Th2 cytokines: IL-4; IL-5; IL-10; IL-13 Note: IL-10 results taken from Figure 2C; abstract reports opposing effect |
CBD treatment reduced all measured cytokines (all of which are specifically implicated in asthma pathogenesis). BALF and lung function were not quantified |
| Vuolo et al.32 | BALB/c mice; sex and age NR (weight 20–25 g) | Experimental asthma | Lung; BALF | CBD (5–10 mg/kg); OVA; vehicle, single i.p. administration | Mice were inoculated with sterile OVA (i.p. injection). Mice were anesthetized and airway responsivity after methacholine challenge was assessed 24 h after the final treatment | Inflammatory cytokines; lung function; cannabinoid receptors; metalloproteinase protein content | ↓ IL-4; IL-5; IL-13 | CBD generally reduced inflammatory cytokines; this effect was not reversed when CB1 and CB2 receptors were individually blocked. Double blockade of CB1 and CB2 receptors did reverse effects of CBD and increased levels of IL-4, IL-5, and IL-13 in BALF. CBD reversed increases in collagen fiber content in alveolar septa. CBD at the highest dose (10 mg/kg) reversed changes in static lung elastance and inflammatory cell infiltration in lung tissue |
| Wang et al.33 | Female C57BL/6J mice (10–12 weeks) | Hepatic steatosis | Liver; serum | CBD (5 or 10 mg/kg); vehicle, daily i.p. administration for 11 days during ethanol exposure | Chronic plus binge ethanol feeding was used to induce liver injury and steatosis | TNF-α; biochemical assays; MPO activity; oxidative stress markers; histopathology | ↓ TNF-α Note: only fold change; absolute concentration not presented |
CBD treatment attenuated both oxidative and nitrative stress together with ethanol-induced liver steatosis. Leukocyte infiltration and neutrophil-mediated inflammation was also reduced |
| Watt et al.34 | Male C57BL/6J×C3H/Hej mice (12 months) | AβPPswe/PS1ΔE9 AD model | Brain (hippocampus and cortex) | CBD (50 mg/kg) daily; vehicle, daily i.p. injection for a total of 7 weeks | Double transgenic mice were used for AD model. Treatment commenced 3 weeks before behavioral testing and continued throughout, for a total of 7 weeks | Inflammatory cytokines; behavioral assessments; disease markers (Aβ40,42); protein expression of IBA1; BDNF; PPAR-γ | No change: TNF-α; IL-1β | CBD treatment restored deficits in spatial learning and social recognition memory, and marginally reduced hippocampal insoluble Aβ40, but no effect on other measures |
| Wei et al.44 | Sprague-Dawley rats (sex and age NR; estimated males ∼9–10 weeks based on weight). Note: C57/BL mouse data NR as inflammatory cytokines not assessed | Colitis | Colon; serum | CBD (1 mg/kg); O-1602 (synthetic atypical cannabinoid, 10 mg/kg control (ethanol solution), i.p. administration daily for 5 days | Colitis was induced by TNBS via intrarectal instillation daily. CBD/O-1602 treatment was administered 30 min before drug injection | Inflammatory cytokines; histopathology; MPO activity; GPR55 gene and protein expression; ATP-mediated colon muscular activity | ↓ IL-6 No change: TNF-α |
CBD and O-1602 treatment reduced disease severity mediated by decreased MPO activity and IL-6 but not TNF-α levels. CBD also increased GPR55 expression and reversed abnormal colonic mobility induced by TNBS |
| Weiss et al.35 | Female NOD/LtJ (known as NOD/ShiLtJ since 2007) mice (6–12 weeks) | Autoimmune type 1 diabetes | Pancreatic tissue; plasma | CBD (5 mg/kg); vehicle, daily i.p. administration (5 times/week for 2–4 weeks) | Urinary glucose was tested 1–2 times/week. Plasma was obtained from treated and untreated mice and assayed for inflammatory cytokines | Inflammatory cytokines; urinary glucose, pancreatic histopathology | ↓ TNF-α; IFN-γ | CBD treated mice had lower incidence of diabetes, delayed disease onset and reduced severity of insulitis and reduction of pro-inflammatory cytokines TNF-α, and IFN-γ in plasma |
| Weiss et al.36 | Female NOD/LtJ and BALB/c mice (all 11–14 weeks) | Autoimmune type 1 diabetes | Pancreas; plasma | CBD (5 mg/kg); vehicle, daily i.p. administration (5 times/week for 4 weeks) | Mice were observed until 24 weeks of age. Urinary glucose was tested 1–2 times/week. Plasma was obtained from treated and untreated mice and assayed for cytokines | IL-6; urinary glucose; histopathology | ↓ IL-6 | CBD-treated mice were less prone to diabetes development, had normal intact islet with limited immune cell infiltration along with reductions in IL-6 levels in plasma compared with untreated mice |
| Wong et al.45 | Australian Albino Wistar rats (sex and age NR; estimated males ∼56 days based on weight) | Obesity | Adipose, plasma | THC (10 mg/kg); vehicle, daily i.p. administration for 10 days | Method of inducing obesity not reported. After a 48-h washout period (after final injection), rats were killed. Epididymal fat pads were removed for analyses | Histopathology; TNF-α; obesity markers; apoptosis marker | No change: TNF-α | THC improved obesity markers reflected by reduced overall body weight and food intake, free fatty acid levels, and increased gluconeogenesis. THC increased macrophage infiltration, TNF-α expression (gene but not plasma conc.) and hypertrophy in adipose tissue |
| Zhang et al.37 | BALB/c mice (6 weeks); sex NR | Retinal damage | Retina | THC (1 or 2 mg/kg); vehicle; i.p. administration daily for 2 months | Pathological and morphological changes in the retina induced by THC administration were evaluated | Inflammatory cytokines; oxidative and ER stress markers; apoptosis markers; histopathology; retinal function | ↑ TNF-α; IL-1β; IL-6 Note: only fold change; absolute concentration not presented |
THC caused retinal damage evident by presence of apoptotic photoreceptor cells. THC also enhanced oxidative and ER stress along with increased pro-inflammatory cytokines in retinas |
| Zhou et al.46 | Female Lewis rats (9–14 weeks) | EAE | Spinal cord | THC:CBD (10:10 and 1:20; 215 mg/kg); oral gavage daily on days 6–18 after disease induction | Disease induction by myelin basic protein gp 69–88 (subcutaneous injection) in CFA. Measures of neuropathic pain induced behavioral changes and neurological disability scoring was used to assess therapeutic effect of CBD | TNF-α; neurological disability scoring; thermal sensory testing; behavior for neuropathic pain; BDNF activity | ↓ TNF-α; THC:CBD 10:10 more effective at the initial time point than THC:CBD 1:20 | Both combination cannabinoid formulas decreased TNF-α and increased BDNF and improved behavioral assessments and neurological disability scoring. Both formulations were able to delay peak onset of disease, however the THC:CBD 10:10 formulation delayed time to peak neurological disability by an additional day compared with THC:CBD 1:20 |
Aβ, beta-amyloid; AD, Alzheimer's disease; BALB, Bagg Albino; BALF, bronchoalveolar lavage fluid; BDNF, brain-derived neurotrophic factor; CB1, cannabinoid type 1; CB2, cannabinoid type 2; CBD, cannabidiol; CBG, cannabigerol; CFA, complete Freund's adjuvant; COX-2, cyclooxygenase-2; CSF, cerebral spinal fluid; DNBS, 2,4,6-dinitrobenzene sulfonic acid; EAE, experimental autoimmune encephalitis; ER, endoplasmic reticulum; FAAH, fatty acid amide hydrolase; HI, hypoxic-ischemic; IBA1, ionized calcium binding adaptor molecule 1; IBD, inflammatory bowel disease; ICR, Institute of Cancer Research; IFN-γ, interferon gamma; IHC, immunohistochemistry; IL, interleukin; iNOS, inducible nitric oxide synthase; i.p., intraperitoneal; IV, intravenous; LPS, lipopolysaccharide; MOG, myelin oligodendrocyte glycoprotein; MPO, myeloperoxidase; mRNA, messenger RNA; MS, multiple sclerosis; NF-κB, nuclear factor-κB; NO, nitric oxide; NOD, nonobese diabetic; NR, not reported; OVA, ovalbumin; PPAR-γ, peroxisome proliferator-activated receptor gamma; RANKL/RANK, receptor activator of nuclear factor kappa-B ligand; SCFA, short chain fatty acid; SOD, superoxide dismutase; TGF-β, transforming growth factor beta; THC, delta 9-tetrahydrocannabinol; TNBS, 2,4,6-trinitrobenzenesulfonic acid; TNF-α, tumor necrosis factor alpha.
The characteristics of the included studies are summarized in Table 2. In brief, two studies investigated the effect of cannabinoids on systemic inflammation,31,38 one study on Alzheimer's disease,34 one study the effect of ischemia-induced brain hypoxia on the lung,47 one study on osteoarthritis,48 and three studies on MS23,26,46 with one focusing on the gut microbiome and the association with inflammation.23 Two studies were conducted in nonobese diabetes,35,36 one study on obesity,45 and another three studies on pain27,39,40 (neuropathic and/or inflammatory). A further four studies examined the effect of cannabinoids on the respiratory system; of these, two studies used an acute lung injury model29,30 and two experimental allergic asthma.32,43 One study examined renal ischemia–reperfusion injury42 and another alcohol-induced steatosis.33 Cannabinoid effects on the colon were investigated in three studies,24,25,44 acute pancreatitis in one study,28 periodontitis in one study,41 and the retina in another.37 Most commonly, cannabinoids were administered intraperitoneally.23–39,41,43–45 Drug administration was intravenous or intra-arterial in two studies42,47 and oral in two studies.40,46 One study compared liposomally packaged to “naked” or unpackaged CBD oil and also examined topically applied CBD.48
Five studies included dose–response testing.27,29,30,32,38 Looking across all studies there was wide variability in the dose of CBD administered. CBD dose ranged from 0.5 to 80 mg/kg.29,30 There was also variability in duration of treatment from a single dose29,32 to 2 months of daily administration.37
The effects of cannabinoids on inflammatory cytokines were explored through enzyme immunoassay, most commonly ELISA. Pro-inflammatory cytokines investigated included TNF-α,27–31,33–35,37–44,46,48 IL-1β,24,25,31,34,37–39,41,42,47 IL-6,28–31,36–39,43,44,48 IFN-γ,23,25,26,35,39 IL-4,32,43 IL-5,32,43 IL-17,23,26 and IL-13.32,43 The anti-inflammatory cytokine IL-10 was also investigated in seven studies.23–25,31,39,43,48
RoB within and across studies
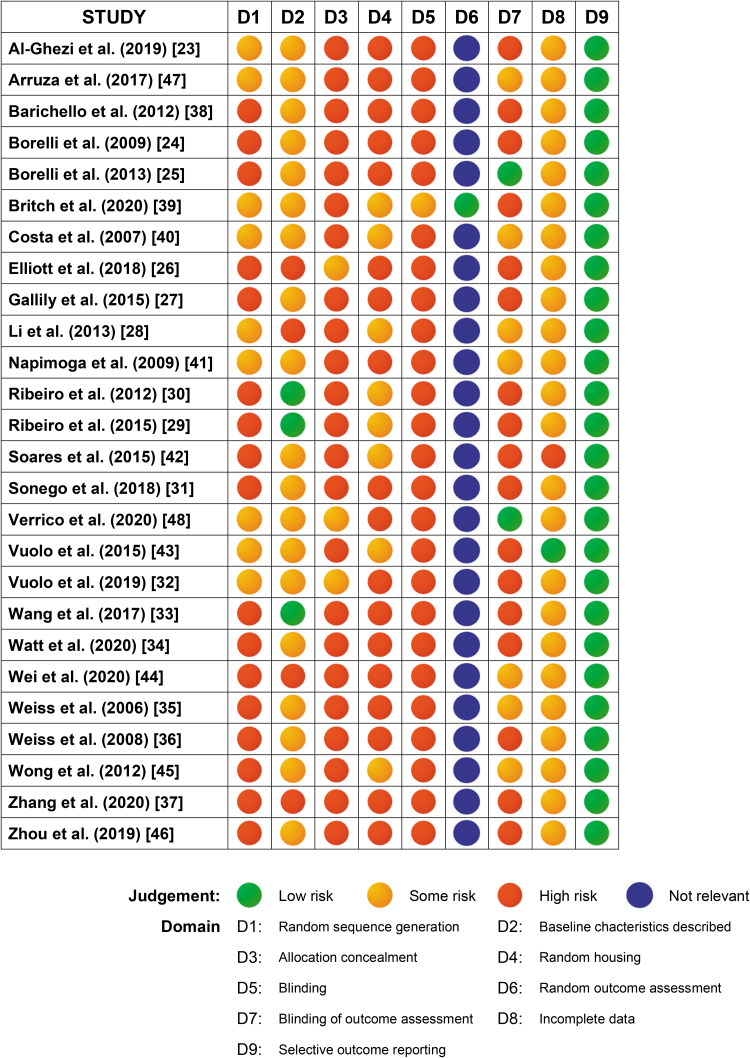
Figure 2 provides the RoB assigned to each study in each of the SYRCLE items. Random sequence generation was partially described in 9 of the 26 studies.23,28,32,39–41,43,47,48 Baseline characteristics of the animals including species, age, sex, and weight were comprehensively reported in three of the studies29,30,33; these characteristics were described with less detail in a further 19 studies.23–25,27,31,32,34–36,38–43,45–48 Allocation to treatment group was not widely described, with three studies giving some detail,26,32,48 whereas the majority did not include this information. Eight studies provided limited information pertaining to random housing,28–30,39,40,42,43,45 whereas the remaining studies did not describe this element of the experimental protocol. Information about caregiver and investigator blinding to the intervention that animals received was provided in one of the studies.39 Random outcome assessment was reported in one study.39 One study stipulated that the outcome assessor was blinded throughout analyses,48 another eight studies partially reported this information,25,28,35,40,41,44,45,47 or it pertained to histological analyses only. All the studies, with two exceptions27,43 had incomplete outcome data; most commonly, the numbers of animals included in the analyses varied and did not reflect the number of animals in each experimental group at the commencement of the study. In one study numbers of animals in the experimental groups were not reported.42 All studies were free of other sources of bias.
FIG. 2.
Risk of bias assessment for each study (rows) and each item (columns) of the SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) risk of bias tool.
Inflammatory cytokine responses to cannabinoids
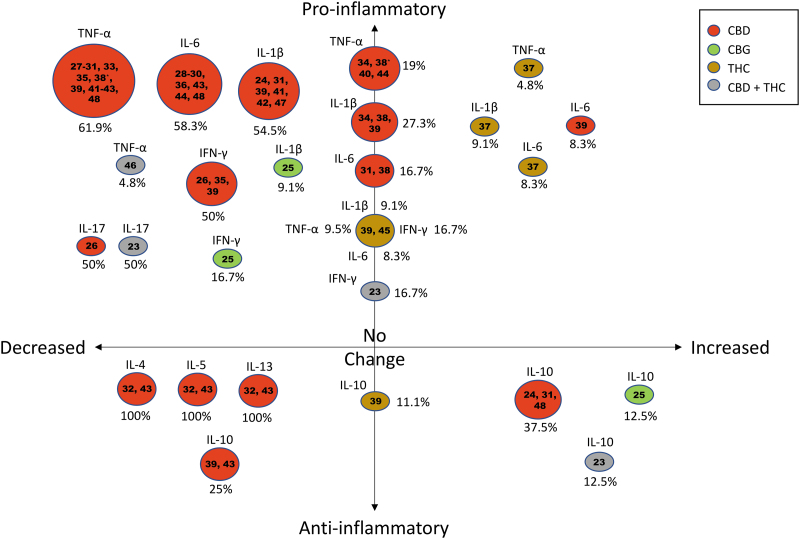
As given in Figure 3, the most commonly studied pro-inflammatory cytokines were TNF-α (n=19),27–31,33–35,37–46,48 IL-1β (n=10),24,25,31,34,37–39,41,42,47 followed by IL-6 (n=11),28–31,36–39,43,48 and IFN-γ (n=5).23,25,26,35,39 IL-10, an anti-inflammatory cytokine, was also investigated in seven studies.23–25,31,39,43,48 These results are summarized hereunder in the context of each cytokine to facilitate interpretation of immune responses.
FIG. 3.
Change in inflammatory cytokine levels from included studies are summarized in the bubble plot together with the cannabinoids assessed. The bubble plot is depicted in four quadrants: decreasing levels of pro-inflammatory cytokines (top left), increasing levels of pro-inflammatory cytokines (top right), decreasing levels of anti-inflammatory cytokines (bottom left), and increasing levels of anti-inflammatory cytokines (bottom right). Percentages were calculated based on the number of studies showing the observed change in the specific cytokine levels for a particular cannabinoid, divided by the total number of studies for that specific cytokine (some studies may be calculated twice to reflect treatment with different cannabinoids used in a single study). *Differing results for the effects of CBD on TNF-α within a single study. CBD, cannabidiol; CBG, cannabigerol; IL, interleukin; THC, delta 9-tetrahydrocannabinol; TNF-α, tumor necrosis factor alpha.
After CBD administration, TNF-α levels were broadly decreased, except in three studies34,40,44 and the female animals in one study,39 where TNF-α levels did not change. In one study, TNF-α levels were reduced in the frontal cortex after chronic administration, but did not change in the hippocampus after acute or chronic administration of CBD.38 Three studies examined the effect of delta 9-tetrahydrocannabinol (THC); two of these studies showed that THC had no effect on TNF-α,39,45 whereas one study showed THC increased TNF-α.37
CBD treatment resulted in a reduction in levels of IL-1β in five of eight instances.24,31,41,42,47 Cannabigerol (CBG) reduced IL-1β in the study that evaluated this cannabinoid,25 whereas in the two THC studies where IL-1β was measured, the effects were mixed as given in Table 2 and Figure 3.37,39
Eleven studies examined the effects of cannabinoids on IL-6, finding in most cases that CBD reduced IL-6 except for three studies including only female animals in one of these studies.31,38,39 Two studies looked at the effects of THC on IL-6 and one study found no effect,39 the other showed an increase in IL-6.37
Reductions in IFN-γ were observed in all five cases where it was measured (three CBD, one CBG, and one CBD+THC in combination),23,25,26,35,39 but only in spleen tissue and not serum in one study after treatment with combined CBD and THC.23 Similarly, the anti-inflammatory cytokine IL-10 was upregulated in four of seven studies: two CBD,24,48 one CBG,25 and spleen tissue but not serum in combined CBD+THC treatment.23 In one CBD study, IL-10 was unchanged.39 Less commonly measured cytokines IL-5, IL-13, and IL-17 were all reduced in the instances that they were measured.23,26,32,43 IL-4 decreased in both studies where it was measured.32,43
Efficacy of cannabinoids
The effects of cannabinoids on inflammatory cytokines are as given in Figure 3. In each of the studies, administration of the cannabinoids CBD, CBG, or a combination of CBD and THC led to some improvement in outcome measures in the treated groups with the exception of one study where there was no observable reduction in pro-inflammatory cytokine-mediated inflammation with CBD.34 In this study pro-inflammatory cytokines were unchanged after CBD treatment; however, social cognition memory and spatial learning deficits were attenuated dose dependently.
The three studies where THC alone was administered resulted in no decrease in pro-inflammatory cytokines.37,39,45 TNF-α, measured by ELISA, was unchanged in an obese mouse model,45 whereas IL-1β, IL-6, IFN-γ, TNF-α, and the anti-inflammatory IL-10 did not change significantly in a rat pain model.39 In a retinal damage mouse model THC increased pro-inflammatory cytokines and markers of oxidative stress.37 In this study, the authors concluded that their results suggested potential mechanisms for retinal damage caused by cannabis abuse. CBG treatment resulted in a reduction of each of the pro-inflammatory cytokines (IL-1β, IFN-γ) measured and an increase in anti-inflammatory cytokine IL-10.25 Where CBD was used as an intervention, there was a decrease in pro-inflammatory cytokines in most instances. However, CBD treatment did not attenuate pro-inflammatory IL-6 in models of experimental tardive dyskinesia and pneumococcal meningitis.31,38
In 23 of the 24 studies where the cannabinoids CBD, CBG, or CBD in combination with THC were administered, a reduction in the levels of at least 1 inflammatory cytokine was observed, and in all those 24 studies, some improvements in disease or disability were apparent. CBD was reported to attenuate disease in acute lung damage28,30 and hypoxic–ischemic brain-mediated lung injury.47 CBD attenuated cognitive impairment,34,38 autoimmune encephalomyelitis,26 tardive dyskinesia,31 pain,27,39,40 colitis,24,44 acute pancreatitis,28 periodontitis,41 renal injury,42 and alcohol-induced liver disease.33 In addition, CBD reduced incidence of disease in diabetes,35,36 and cytokine activity associated with experimental asthma32,43 and local and systemic inflammation.48 A combination of THC and CBD suppressed neuroinflammation in experimental MS46 and attenuated MS-induced neuroinflammation by preventing gut microbial dysbiosis.23 CBG attenuated colitis.25 THC did not reduce pro-inflammatory cytokine levels in any of the three studies where it was used alone.37,39,45 However, THC did attenuate neuropathic pain in one study39 (Experiment 1), reduced body weight, food intake, fatty acid levels, and promoted adipocyte hypertrophy in one study,45 and caused retinal damage in another study.37
Dosing
Five studies reported on the effects of different doses of CBD.27,29,30,32,38 CBD had a dose-dependent effect on TNF-α.27,29,30 A bell-shaped dose response of purified CBD (oral administration) was observed in one study, where serum TNF-α was reduced at 25 and 50 mg/kg, but not at 10 or 100 mg/kg, similarly lowered at 5 mg (intraperitoneal [i.p.] administration), but not at 1, 25, or 50 mg/kg.27 When compared with vehicle in induced lung injury, a single dose of 80 mg/kg CBD resulted in a greater reduction in TNF-α than 20 mg/kg (p<0.0001 vs. p<0.05, respectively).29,30 IL-6 levels were unchanged after CBD at 20 mg/kg, but did reduce at 80 mg/kg (p<0.05). Cytokine levels in lung tissue in a parallel series of experiments showed that CBD reduced IL-4, IL-5, and IL-13 levels at doses of 5 and 10 mg/kg (both p<0.05).32,43 There was no difference in effects at different CBD doses at 2.5, 5.0, and 10.0 mg/kg in a rodent model of pneumococcal meningitis.38
A series of studies showed that i.p. administration of CBD reduced circulating TNF-α and IL-6 levels in a dose-responsive manner and that in mice treated i.p. with increasing doses of CBD (1, 10, or 100 μg). A single topical CBD dose of 100 μg generated an anti-inflammatory effect similar to that of 100 μg administered i.p.48 In addition, orally administered liposomal CBD began to significantly reduce rising TNF-α levels within an hour of administration, whereas an additional hour was required before orally administered naked CBD significantly reduced TNF-α. (p<0.05, p<0.01 at 4-h postadministration).48 Circulating TNF-α was decreased by 50% among mice to which croton oil+CBD was administered, compared with croton oil alone in a local inflammation model of the ear.48
Duration of cannabinoid administration was variable between the studies ranging from a single dose29,32 to 2 months of daily administration.37 Of interest, the study with the longest duration of CBD treatment (50 mg/kg i.p. for 7 weeks) was the only study where CBD did not reduce either of the pro-inflammatory cytokines that were studied (TNF-α and IL-1β).34 A single administration at 20 mg/kg was beneficial in reducing TNF-α and IL-6 in one study29 but had no effect on TNF-α in another.40
Two THC:CBD formulations were trialed in one study46 and both resulted in reductions of TNF-α: 10:10 THC:CBD delayed time to peak onset of disease and initially reduced TNF-α more at day 12 (p<0.01), but both groups were similar by day 18 (p<0.05). However, the 10:10 formulation delayed time to peak neurological disability by an additional day compared with 1:20. Other studies stated that different doses of cannabinoids were used, in some cases animals were administered a standard dose for the cytokine part of the study (CBD 5 mg/kg; CBG 30 mg/kg; and CBD 20 mg/kg).24,25,40 CBD was administered at 5 or 10 mg/kg; however, it is not clear from the results which dose the animals had received in this mouse model of liver steatosis.33
Discussion
The aim of this study was to systematically review preclinical studies that evaluate the in vivo effect of cannabinoids on the production of inflammatory cytokines with the purpose of informing human clinical trials. Twenty-six studies were reviewed, using 3 cannabinoids, 21 animal models with 9 cytokines measured. In general, CBD and CBG had a positive effect in reducing inflammation in most disease states. This effect was not observed in experiments where THC alone was used as an intervention.
Intervention efficacy and dosage
The 26 studies investigated CBD (21), CBG (1), CBD+THC (2), and THC (2), and 1 study investigated CBD and THC separately in 2 sets of experiments conducted at different time points reported in the same article. CBD treatment was able to reduce pro-inflammatory cytokines in >90% of the CBD studies, and in all cases, disease or disability was alleviated by CBD administration. Conversely, THC alone did not affect pro- or anti-inflammatory cytokines and only appeared to be efficacious in modulating experimentally induced pain in one study.39
In all experimental conditions where the non-neurological peripheral tissues were targeted, CBD/CBG reduced the pro-inflammatory profile of each cytokine tested, with the exception of one study.44 Here, no effect on TNF-α levels after CBD treatment was observed in a rat colitis model, which may be attributable to a relatively small dose (1 mg/kg). In several studies that looked at the brain31,34,38 and pain,27,39,40 CBD treatment resulted in less consistent reduction of pro-inflammatory cytokines. The variability in results between the effects of CBD on neurological versus peripheral tissues could be because of the predilection of CBD to act as an inverse agonist on CB2 receptors that are predominantly distributed in microglia and immune cells throughout the body.49,50 CBD's antagonistic action on CB2 receptors has been shown to reduce the release of pro-inflammatory cytokines.51 In the single study that investigated THC compared with CBD effects on the nervous system, THC did not induce a reduction in pro- or anti-inflammatory cytokines. In the CBD-treated groups, reduction of inflammatory cytokines was observed (IL-6, IFN-γ, and TNF-α). THC did not reduce experimentally induced hind-paw edema39 (Experiment 1), whereas CBD did.39,40 However, THC dose dependently reduced pain-related behaviors, compared with CBD, suggesting that THC may be more beneficial than CBD for reducing inflammatory pain despite serum levels of pro-inflammatory cytokines being unaffected after THC treatment. THC has a greater affinity for CB1 receptors that are primarily located on nerve cells in the brain and spinal cord.52 Furthermore, THC may modulate pain through a different pathway such as presynaptic inhibition of GABAergic transmission, which has antinociceptive actions.53
Inflammatory cytokine levels were measured in a variety of tissues including brain, colon, spinal cord, spleen, and serum. Only one study compared the levels of two cytokines in two different tissues.23 This study found that IL-17a was reduced in both serum and spleen after combined CBD+THC treatment.23 IFN-γ was reduced in the spleen, yet not in the serum samples. These data suggest that local tissues where inflammation is present may be preferentially targeted by cannabinoids, whereas systemic markers are less affected. This previously reported phenomenon54 should be considered when planning future studies. Similar observations have been made when comparing tissue to serum cytokines in people with osteoarthritis and fractures.55 Therefore, it may be more accurate to measure inflammatory cytokines in their target tissues peripherally as systemic markers may not accurately represent cannabinoid effects.
The most common dose of CBD used across the studies was 5–20 mg/kg (0.4–1.6 mg/kg human equivalent dose). Where studies compared doses of CBD in some cases, increased doses led to a better response29,30 and in others the opposite occurred.43 Similarly, where a single concentration was used, high levels of CBD did not necessarily cause a reduction in inflammation34 and duration of therapy did not necessarily predict efficacy. A nonlinear dose–response was also observed in some studies, where increasing dose eventually resulted in reduced efficacy.27,32,38,43 It is widely accepted that physiological responses to CBD are variable,27,56,57 which could explain this finding, especially given that cytokines were frequently measured in only a small number of animals.
Where CBG was used, it was efficacious in reducing inflammation (colitis),25 suggesting that CBG could be potently anti-inflammatory most likely because of its partial agonistic effects on CB2 receptors.58 This is supported by in vitro and gene expression studies.59–61 Only one study examined CBD+THC in two combinations. Both formulations decreased TNF-α; however, at 10:10 THC:CBD delayed onset of disease appears to have a potential advantage over the 1:20 formulation in terms of behavioral, neurological, and disability outcomes.
Although in the majority of studies, the cannabinoid intervention was delivered i.p., it was also efficacious when administered by oral gavage.40,46 One study compared topically applied versus i.p. administered CBD in a systemic inflammation model and found that they were equally efficacious at a dose of 100 μg,48 suggesting that topical treatments could be a simple and acceptable method of administration, which could have particular utility in skin disorders. More studies are needed to confirm this and the optimal carrier across the skin barrier (e.g., lipid-based, nanodelivery systems). The same study also compared orally gavaged “naked” CBD with a liposomally packaged CBD (both administered at 20 mg/kg) and found liposomal CBD was more bioavailable than naked CBD and reduced inflammatory cytokines at a faster rate, indicating that innovation in delivery systems could lead to more potent and swiftly acting formulations.
Studies methodological quality
Although bias was generally similar for all studies, it is apparent that protocols used for in vivo studies with animal models are heterogeneous and often lacking in detail. Evidence shows that awareness and use rates of RoB tools such as SYRCLE, the Animal Research: Reporting of In Vivo Experiments guidelines, and the Gold Standard Publication Checklist are low.62 None of the 26 studies in this review fully reported details of allocation, randomization, and blinding according to the SYRCLE RoB criteria. Hirst et al.63 found that failure to randomize significantly increased effect sizes, and that randomization, allocation concealment, and blinding reduced effect sizes, especially where outcomes were subjective.
Although baseline characteristics were in general, adequately reported, characteristics between groups were not compared. Adequate description of random sequence generation, random housing, and allocation concealment was lacking throughout the studies; only one study reported on caregiver blinding,39 and only one study43 stated that investigators were blinded throughout the process. Incomplete data reporting was also a consistent finding. The adoption of a more rigorous reporting structure, for example, a flow diagram would make it easier to interpret the findings and to understand the attrition process.
Recommendations for future research
These current studies show that cannabinoids are in general efficacious in reducing inflammation and treating a wide variety of disease models. However, the number of in vivo studies in this field is relatively small and many cannabinoids have not been explored in terms of their anti-inflammatory properties. In vitro studies are also of great importance within the field,64,65 and examining these will help to contextualize mechanisms more clearly and evaluate the likelihood of making the translational leap to human clinical studies. Further studies to examine other lesser known cannabinoids and terpenes from Cannabis sativa (e.g., THCA, CBN, alpha-pinene, and linalool) might unearth uncharted therapeutic benefits.66 Studies to examine optimal dose67 and duration of treatment as well as the development of standardized or synthetic cannabinoids might increase efficacy and more predictable outcomes.
The incorporation of cannabinoids into more sophisticated delivery systems such as lysosomes would enable more effective treatments to be developed and trialed for inflammation-driven human diseases. Given the differences that were observed between serum and tissue cytokine levels, in the same animal model in one study,23 site of administration may also be worth considering. For topical preparations to treat skin conditions, CBD and CBG demonstrated the most consistency in reducing inflammation. For potentially efficacious doses for human studies, the human equivalent dose‡ for a 70 kg person68 of CBG is 56.8–170.3 mg/day (0.8–2.4 mg/kg), mean=113.5±80.3; and for CBD is 2.8–851.4 mg/day (0.04–12.16 mg/kg), mean=185.4±249.1 mg.
Limitations of this study
Some cannabinoids included in this study were underrepresented as they have not been widely studied in terms of their anti-inflammatory effect in vivo, namely CBG and THC, which makes it difficult to extrapolate the conclusions drawn in the studies to form a complete picture of efficacy. However, anti-inflammatory effects of THC are not anticipated to be strong given its high affinity for CB1 receptors and its function as a partial agonist of CB2 receptors.
Bias assessment tools do not assess the quality and adequacy of the study methodologies; they can only evaluate the reporting. The reporting of measures against bias are largely optional and therefore, reporting rates of these measures may not be reliable indicators of scientific rigor.69 Other parameters may not have been captured by the SYRCLE RoB tool such as sample size calculation, and perhaps the inclusion of such indices should be considered in future.
It is well documented that results from animal studies do not always translate to parallel findings in humans,70,71 and that some anti-inflammatory effects of cannabinoids may be disease or person specific, and not generalizable.18 However, preclinical trials are important to enhance our understanding of the safety and efficacy of novel compounds and drugs, and have utility in predicting whether or not to proceed to human trials.
Conclusions
The in vivo studies examined in this review show that cannabinoids have the potential to treat inflammatory disease. Worldwide, three of five people die because of chronic inflammatory diseases such as stroke, respiratory diseases, cancer, and diabetes.72 Therefore, the results of these in vivo studies would suggest that CBD and CBG may be harnessed as an effective treatment for inflammatory disease, and that whereas THC may be helpful in reducing pain, there is less evidence to support its use as an anti-inflammatory therapy. Given the paucity of preclinical studies, more work is necessary to better determine the efficacy of cannabinoids as anti-inflammatory therapies. Nonetheless, this present review is important as it has for the first time, systematically evaluated in vivo studies using these novel compounds and summarized their potential as anti-inflammatory agents.
Abbreviations Used
- Aβ
beta-amyloid
- AD
Alzheimer's disease
- BALB
Bagg Albino
- BALF
bronchoalveolar lavage fluid
- BDNF
brain-derived neurotrophic factor
- CB1
cannabinoid type 1
- CB2
cannabinoid type 2
- CBD
cannabidiol
- CBG
cannabigerol
- CFA
complete Freund's adjuvant
- COI
conflict of interest
- COX-2
cyclooxygenase-2
- CSF
cerebral spinal fluid
- DNBS
2,4,6-Dinitrobenzene sulfonic acid
- EAE
experimental autoimmune encephalitis
- ECS
endocannabinoid system
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- FAAH
fatty acid amide hydrolase
- HI
hypoxic-ischemic
- IBA1
ionized calcium-binding adaptor molecule 1
- IBD
inflammatory bowel disease
- ICR
Institute of Cancer Research
- IFN-γ
interferon gamma
- IHC
immunohistochemistry
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- i.p.
intraperitoneal
- IV
intravenous
- LPS
lipopolysaccharide
- MOG
myelin oligodendrocyte glycoprotein
- MPO
myeloperoxidase
- mRNA
messenger RNA
- MS
multiple sclerosis
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- NOD
nonobese diabetic
- NR
not reported
- OVA
ovalbumin
- PPARγ
peroxisome proliferator-activated receptor gamma
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RANKL/RANK
receptor activator of nuclear factor kappa-B ligand
- RoB
risk of bias
- SCFA
short chain fatty acid
- SOD
superoxide dismutase
- SYRCLE
SYstematic Review Center for Laboratory animal Experimentation
- TGF-β
transforming growth factor-beta
- THC
delta 9-tetrahydrocannabinol
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- TNF-α
tumor necrosis factor alpha
Authors' Contributions
F.R.H. and G.Z.S. conceptualized the study, designed and conducted the search strategy. F.R.H. sorted the EndNote library and drafted the article with assistance from G.Z.S. F.R.H. and L.S.D. extracted the data, assessed study eligibility, and conducted the RoB assessment; discrepancies on RoB were resolved with G.Z.S. C.K.L. provided guidance on inflammation and animal model aspects of the article, and data visualization. All authors checked data extraction accuracy, confirmed eligibility of included studies, and read and approved the final version of the article.
Author Disclosure Statement
G.Z.S. and L.S.D. are part of the NICM Health Research Institute at Western Sydney University. As a medical research institute, NICM receives research grants and donations from foundations, universities, government agencies, individuals and industry. Sponsors and donors provide untied funding for work to advance the vision and mission of the Institute. The project that is the subject of this article was not undertaken as part of a contractual relationship with any organization other than the funding declared hereunder. It should also be noted that NICM conducts clinical trials relevant to this topic area, for which further details can be provided on request. G.Z.S. has received funding from Australian Natural Therapeutics Group to conduct investigator-initiated cannabis research; this company did not have any role in the conduct of this research.
Funding Information
G.Z.S.'s contribution was supported by funding from a National Health and Medical Research Council (NHMRC)-Australian Research Council Dementia Research Development Fellowship (APP1102532) and a NHMRC Investigator Grant (APP1195709).
Cite this article as: Henshaw FR, Dewsbury LS, Lim CK, Steiner GZ (2021) The effects of cannabinoids on pro- and anti-inflammatory cytokines: a systematic review of in vivo studies, Cannabis and Cannabinoid Research 6:3, 177–195, DOI: 10.1089/can.2020.0105.
Based on the body surface area normalisation method; where HED (mg/kg)=animal dose (mg/kg)×animal Km/Human Km.
References
- 1. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1973;28:437–448 [Google Scholar]
- 2. Argenziano M, Tortora C, Bellini G, et al. . The endocannabinoid system in pediatric inflammatory and immune diseases. Int J Mol Sci. 2019;20:5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mechoulam R. The pharmacohistory of Cannabis sativa. Cannabinoids as Therapeutic Agents. Chapman and Hall/CRC: Boca Raton, FL, 2019 [Google Scholar]
- 4. Iversen LL. The science of marijuana. Oxford University Press: Oxford, UK, 2001 [Google Scholar]
- 5. Vaccani A, Massi P, Colombo A, et al. . Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144:1032–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, et al. . Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Srug Safety. 2011;6:237–249 [DOI] [PubMed] [Google Scholar]
- 7. Borgwardt SJ, Allen P, Bhattacharyya S, et al. . Neural basis of Δ-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–973 [DOI] [PubMed] [Google Scholar]
- 8. Devinsky O, Cilio MR, Cross H, et al. . Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiurchiù V, Battistini L, Maccarrone M. Endocannabinoid signalling in innate and adaptive immunity. Immunology. 2015;144:352–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol. 2014;29:23–28 [DOI] [PubMed] [Google Scholar]
- 11. Franceschi C, Capri M, Monti D, et al. . Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105 [DOI] [PubMed] [Google Scholar]
- 12. Zhang J-M, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haney M, Hill MN. Cannabis and cannabinoids: from synapse to society. Neuropsychopharmacol. 2018;43:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lakhan SE, Rowland M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72:735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martín-Sánchez E, Furukawa TA, Taylor J, et al. . Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10:1353–1368 [DOI] [PubMed] [Google Scholar]
- 18. Whiting PF, Wolff RF, Deshpande S, et al. . Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473 [DOI] [PubMed] [Google Scholar]
- 19. Reinarman C, Nunberg H, Lanthier F, et al. . Who are medical marijuana patients? Population characteristics from Nine California Assessment Clinics. J Psychoact Drugs. 2011;43:128–135 [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hooijmans CR, Rovers MM, De Vries RB, et al. . SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodo. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PC, et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Ghezi ZZ, Busbee PB, Alghetaa H, et al. . Combination of cannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigates experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. Brain Behav Immun. 2019;82:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borrelli F, Aviello G, Romano B, et al. . Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009;87:1111–1121 [DOI] [PubMed] [Google Scholar]
- 25. Borrelli F, Fasolino I, Romano B, et al. . Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013;85:1306–1316 [DOI] [PubMed] [Google Scholar]
- 26. Elliott DM, Singh N, Nagarkatti M, et al. . Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front Immunol. 2018;9:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallily R, Yekhtin Z, Hanuš LO. Overcoming the bell-shaped dose-response of cannabidiol by using cannabis extract enriched in cannabidiol. Pharmacol Pharm. 2015;6:75 [Google Scholar]
- 28. Li K, Feng JY, Li YY, et al. . Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas. 2013;42:123–129 [DOI] [PubMed] [Google Scholar]
- 29. Ribeiro A, Almeida VI, Costola-de-Souza C, et al. . Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol Immunotoxicol. 2015;37:35–41 [DOI] [PubMed] [Google Scholar]
- 30. Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, et al. . Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. Eur J Pharmacol. 2012;678:78–85 [DOI] [PubMed] [Google Scholar]
- 31. Sonego AB, Prado DS, Vale GT, et al. . Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARgamma receptors. Brain Behav Immun. 2018;74:241–251 [DOI] [PubMed] [Google Scholar]
- 32. Vuolo F, Abreu SC, Michels M, et al. . Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur J Pharmacol. 2019;843:251–259 [DOI] [PubMed] [Google Scholar]
- 33. Wang YP, Mukhopadhyay P, Cao ZX, et al. . Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci Rep. 2017;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watt G, Shang K, Zieba J, et al. . Chronic treatment with 50 mg/kg cannabidiol improves cognition and moderately reduces Aβ40 levels in 12-month-old male AβPPswe/PS1ΔE9 transgenic mice. J Alzheimers Dis. 2020;74:937–950 [DOI] [PubMed] [Google Scholar]
- 35. Weiss L, Zeira M, Reich S, et al. . Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–151 [DOI] [PubMed] [Google Scholar]
- 36. Weiss L, Zeira M, Reich S, et al. . Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54:244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Z, Li R, Lu H, et al. . Systemic administration with tetrahydrocannabinol causes retinal damage in BALB/c mice. Hum Exp Toxicol. 2020;39:290–300 [DOI] [PubMed] [Google Scholar]
- 38. Barichello T, Ceretta RA, Generoso JS, et al. . Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur J Pharmacol. 2012;697:158–164 [DOI] [PubMed] [Google Scholar]
- 39. Britch SC, Goodman AG, Wiley JL, et al. . Antinociceptive and immune effects of delta-9-tetrahydrocannabinol or cannabidiol in male versus female rats with persistent inflammatory pain. J Pharmacol Exp Ther. 2020;373:416–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costa B, Trovato AE, Comelli F, et al. . The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83 [DOI] [PubMed] [Google Scholar]
- 41. Napimoga MH, Benatti BB, Lima FO, et al. . Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int Immunopharmacol. 2009;9:216–222 [DOI] [PubMed] [Google Scholar]
- 42. Soares RZ, Vuolo F, Dall'Igna DM, et al. . Evaluation of the role of the cannabidiol system in an animal model of ischemia/reperfusion kidney injury. Rev Bras Ter Intensiva. 2015;27:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vuolo F, Petronilho F, Sonai B, et al. . Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediators Inflamm. 2015;2015:538670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei D, Wang H, Yang J, et al. . Effects of O-1602 and CBD on TNBS-induced colonic disturbances. Neurogastroenterol Motil. 2020;32:e13756. [DOI] [PubMed] [Google Scholar]
- 45. Wong A, Gunasekaran N, Hancock DP, et al. . The major plant-derived cannabinoid delta(9)-tetrahydrocannabinol promotes hypertrophy and macrophage infiltration in adipose tissue. Horm Metab Res. 2012;44:105–113 [DOI] [PubMed] [Google Scholar]
- 46. Zhou T, Ahmad TK, Alrushaid S, et al. . Therapeutic impact of orally administered cannabinoid oil extracts in an experimental autoimmune encephalomyelitis animal model of multiple sclerosis. Biochem Biophys Res Commun. 2019;516:373–380 [DOI] [PubMed] [Google Scholar]
- 47. Arruza L, Pazos MR, Mohammed N, et al. . Cannabidiol reduces lung injury induced by hypoxic-ischemic brain damage in newborn piglets. Pediatr Res. 2017;82:79–86 [DOI] [PubMed] [Google Scholar]
- 48. Verrico CD, Wesson S, Konduri V, et al. . A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain. 2020;161:2191–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. . Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petitet F, Jeantaud B, Reibaud M, et al. . Complex pharmacology of natural cannabivoids: evidence for partial agonist activity of Δ9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–PL6 [DOI] [PubMed] [Google Scholar]
- 51. Lunn CA, Fine JS, Rojas-Triana A, et al. . A novel cannabinoid peripheral cannabinoid receptor-selective inverse agonist blocks leukocyte recruitment in vivo. J Pharmacol Exp Ther. 2006;316:780–788 [DOI] [PubMed] [Google Scholar]
- 52. Thapa D, Cairns EA, Szczesniak A-M, et al. . The cannabinoids Δ8THC, CBD, and HU-308 act via distinct receptors to reduce corneal pain and inflammation. Cannabis Cannabinoid Res. 2018;3:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127:935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Šumanović-Glamuzina D, Čulo F, Čulo MI, et al. . A comparison of blood and cerebrospinal fluid cytokines (IL-1β, IL-6, IL-18, TNF-α) in neonates with perinatal hypoxia. Bosn J Basic Med Sci. 2017;17:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krämer HH, Eberle T, Üçeyler N, et al. . TNF-alpha in CRPS and “normal” trauma—Significant differences between tissue and serum. Pain. 2011;152:285–290 [DOI] [PubMed] [Google Scholar]
- 56. McGilveray IJ, Pharmacokinetics of cannabinoids. Pain Res Manag. 2005;10(Suppl. A):15A–22A [DOI] [PubMed] [Google Scholar]
- 57. Lim SY, Sharan S, Woo S. Model-based analysis of cannabidiol dose-exposure relationship and bioavailability. Pharmacotherapy. 2020;40:291–300 [DOI] [PubMed] [Google Scholar]
- 58. Navarro G, Varani K, Reyes-Resina I, et al. . Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1–CB2 heteroreceptor complexes. Front Pharmacol. 2018;9:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Granja AG, Carrillo-Salinas F, Pagani A, et al. . A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J Neuroimmune Pharmacol. 2012;7:1002–1016 [DOI] [PubMed] [Google Scholar]
- 60. Gugliandolo A, Pollastro F, Grassi G, et al. . In vitro model of neuroinflammation: efficacy of cannabigerol, a non-psychoactive cannabinoid. Int J Mol Sci. 2018;19:1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valdeolivas S, Navarrete C, Cantarero I, et al. . Neuroprotective properties of cannabigerol in Huntington's disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics. 2015;12:185–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ma B, Xu J-K, Wu W-J, et al. . Survey of basic medical researchers on the awareness of animal experimental designs and reporting standards in China. PLoS One. 2017;12:e0174530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hirst JA, Howick J, Aronson JK, et al. . The need for randomization in animal trials: an overview of systematic reviews. PLoS One. 2014;9:e98856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nallathambi R, Mazuz M, Ion A, et al. . Anti-inflammatory activity in colon models is derived from δ9-tetrahydrocannabinolic acid that interacts with additional compounds in cannabis extracts. Cannabis Cannabinoid Res. 2017;2:167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Romano B, Borrelli F, Fasolino I, et al. . The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013;169:213–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gallily R, Yekhtin Z, Hanuš LO. The anti-inflammatory properties of terpenoids from cannabis. Cannabis Cannabinoid Res. 2018;3:282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Malfait AM, Gallily R, Sumariwalla PF, et al. . The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–9566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661 [DOI] [PubMed] [Google Scholar]
- 69. Reichlin TS, Vogt L, Würbel HJ. The researchers' view of scientific rigor—survey on the conduct and reporting of in vivo research. PLoS One. 2016;11:e0165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McGonigle P, Ruggeri BJ. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87:162–171 [DOI] [PubMed] [Google Scholar]
- 71. Van der Worp HB, Howells DW, Sena ES, et al. . Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tunstall-Pedoe H. Preventing Chronic Diseases: A Vital Investment. WHO Global Report. Geneva: World Health Organization, 2005, pp. 200. Available at www.who.int/chp/chronic_disease_report/en (accessed January27, 2021). In: Oxford University Press; 2006