Abstract
Simple Summary
MicroRNAs are small non-coding RNAs, acting as post-transcriptional regulators of gene expression. In the last two decades, their role in cancer as oncogenes (oncomir), as well as tumor suppressors, has been extensively demonstrated. Recently, epitranscriptomics, namely the study of RNA modifications, has emerged as a new field of great interest, being an additional layer in the regulation of gene expression. Almost all classes of eukaryotic RNAs, including miRNAs, undergo epitranscriptomic modifications. Alterations of RNA modification pathways have been described for many diseases—in particular, in the context of malignancies. Here, we reviewed the current knowledge on the potential link between epitranscriptomic modifications of miRNAs and cancer.
Abstract
MicroRNAs are pervasive regulators of gene expression at the post-transcriptional level in metazoan, playing key roles in several physiological and pathological processes. Accordingly, these small non-coding RNAs are also involved in cancer development and progression. Furthermore, miRNAs represent valuable diagnostic and prognostic biomarkers in malignancies. In the last twenty years, the role of RNA modifications in fine-tuning gene expressions at several levels has been unraveled. All RNA species may undergo post-transcriptional modifications, collectively referred to as epitranscriptomic modifications, which, in many instances, affect RNA molecule properties. miRNAs are not an exception, in this respect, and they have been shown to undergo several post-transcriptional modifications. In this review, we will summarize the recent findings concerning miRNA epitranscriptomic modifications, focusing on their potential role in cancer development and progression.
Keywords: microRNA, cancer, epitranscriptomics, m6A, m5C, A-to-I editing, m7G
1. Introduction
MicroRNAs (miRNAs) are a class of short, non-coding RNAs that control gene expression at the post-transcriptional level via either translational repression or mRNA degradation.
Since miRNAs act as pervasive regulators of gene expression, it is not surprising that they were involved in normal animal development and in a variety of biological processes [1,2]. The aberrant expression of miRNAs is also associated with many human diseases [3,4].
A large amount of literature documents the wide involvement of miRNAs in cancer as key players in the development and progression of different malignancies (reviewed in reference [5]), as diagnostic/prognostic biomarkers (reviewed in reference [6]) and as potential therapeutic targets [7].
One hundred and seventy-two post-transcriptional modifications of RNAs have been reported thus far [8], collectively known as the “epitranscriptome” [9]. Some of these epitranscriptomic modifications have been thoroughly investigated, unraveling their contribution to RNA stability and/or activity [10,11,12]. The most common and best-characterized epitranscriptomic modifications include N6-methyl-Adenosine (m6A) [13], pseudoUridine (Ψ) [14], Adenosine-to-Inosine (A-to-I) editing [15] and 5-methyl-Cytidine (m5C) [16].
In epigenetics, a widely exploited paradigm postulates that DNA methylation and histone modifications are installed by “writer” enzymes, recruit “reader” proteins and are removed by “eraser” enzymes [17]. Although it has been proposed that the same general view may hold true for epitranscriptomic modifications, the intrinsic features of RNA imply that “readers” and “erasers” may be dispensable for some modifications [18]. “Writer” enzymes have been identified for all major RNA modifications [19,20,21,22,23,24]. Otherwise, “reader” proteins have been described only for m6A [25] and m5C [26]. Several RNA modifications directly affect the RNA structure and/or base pairing, thus requiring no “reader” proteins to exert their functions. This is obvious for A-to-I editing, which changes the identity of a base, and it has also been demonstrated for Ψ [27,28]. Furthermore, while it has been suggested that m6A can be removed from modified RNA molecules [29,30], most epitranscriptomic modifications are apparently not dynamic. On the one hand, because of the very short half-life of most eukaryotic RNAs, specific “eraser” enzymes might be dispensable at least for some epitranscriptomic modifications that may actually be removed through the rapid turnover of modified RNA molecules. On the other hand, epitranscriptomic modifications on more stable RNA molecules (e.g., rRNAs) may lack any “eraser” enzymes simply because reverting such modifications is not beneficial to the cell. Accordingly, no “eraser” enzyme has been identified yet for m5C, Ψ, A-to-I editing and many other epitranscriptomic modifications [31,32].
The first evidence of an epitranscriptomic modification in miRNAs was reported in 2004 [33]. From that moment on, the role of the epitranscriptomics of miRNAs in cancer promotion and progression started to be elucidated. Notably, epitranscriptomic modifications have also been described in RNAs targeted by miRNAs, positively or negatively affecting miRNA:target interactions.
In this review, we will focus on the current evidence supporting the role in cancer of epitranscriptomic modifications of miRNAs and of miRNA-targeted RNAs.
2. miRNAs: Biogenesis and Functions
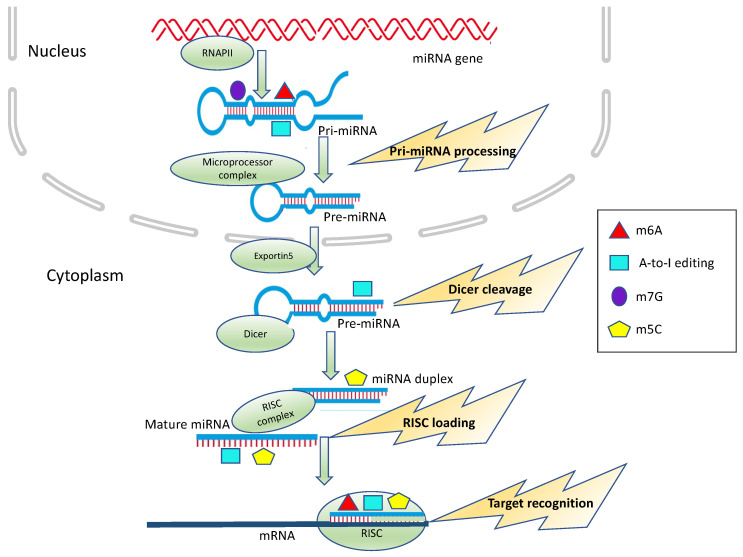
miRNAs are a class of small (18–24 nt) non-coding RNAs that are processed from long primary miRNAs (pri-miRNAs) generally transcribed by RNA Polymerase II [34,35,36] and harbor one or more hairpin structure [37]. Pri-miRNA processing starts in the nucleus, where the Microprocessor complex, formed by the RNase III enzyme DROSHA, the RNA-binding protein Di George Syndrome Critical Region Gene 8 (DGCR8) and other proteins [38,39] catalyzes the endonucleolytic cleavage of the pri-miRNA to yield a ~70-nt-long hairpin pre-miRNA [40].
Pre-miRNAs are then exported to the cytoplasm by Exportin-5 [41,42,43]. In the cytoplasm, pre-miRNAs undergo further cleavage by DICER, which removes the terminal loop of the hairpin to yield a duplex consisting of the mature miRNA (guide strand) base-paired to the passenger strand [44,45].
Mature miRNAs interact with the RNA-binding proteins belonging to the Argonaute family (AGO), thus becoming integral components of the RNA-Induced Silencing Complex (RISC) (reviewed in references [46,47]). The mature miRNA within RISC recruits the complex onto target RNA molecules by base-pairing between a “seed” region (nt 2–7) at the 5′ end of the miRNA and the 3′ UTR of the target RNA [48,49,50], leading to gene silencing through translation repression and mRNA decay.
MiRNA expression is tightly controlled in cells by mechanisms acting at both the transcriptional and post-transcriptional levels (reviewed in references [51,52,53]). The titration of miRNAs by competing endogenous RNAs (ceRNAs) adds a further layer of regulation of miRNA activity [54,55,56,57]. These long RNA molecules (which can be mRNAs, lncRNAs, pseudogene-encoded RNAs or circRNAs) sequester miRNAs, thereby preventing their interaction with other targets, whose repression is therefore relieved.
MiRNAs participate in gene regulatory networks that control diverse biological processes in multicellular organisms, such as animal development (reviewed in reference [1]), cell fate specification and differentiation [58], the immune response [59] and inflammation [60]. Changes in the miRNA expression levels have been associated with a wide range of human diseases, including diabetes, cardiovascular and kidney disease and cancer [3,4]. A huge number of miRNAs are downregulated or upregulated in human cancers, where they exert oncogenic or tumor suppressor functions, depending on the cellular context. Alterations of miRNAs in different malignancies have been linked to genetic deletion or amplification, as well as to DNA methylation of the miRNA genomic loci, to the modulation of the pri-mRNA transcription level by transcription factors or to the dysregulation of one or more steps in miRNA biogenesis (reviewed in reference [5]). Recently, epitranscriptomics is emerging as an additional layer of the regulation of the miRNA function in cancer.
3. Epitranscriptomic Modifications of miRNA in Cancer
3.1. N6-Methyl-Adenosine (m6A)
m6A was first reported in the 1970s in mammalian RNAs [61,62,63]. A full comprehension of the role of this modification took several decades. In 1997, the protein Methyltransferase-like (METTL) 3 was identified as the first “writer” of m6A in mammalian cells [23]. Further investigations have shown that m6A is installed by a nuclear complex comprised of METTL3, METTL14 and WT1-Associated Protein (WTAP) [22]. Further components of this complex include KIAA1429, RNA Binding Motif Protein 15 (RBM15) and Zinc Finger CCCH-Type Containing 13 (ZC3H13) [64,65,66].
Several members belonging to the YTH (YT521-B homology) family, such as human YT521-B (also known as YTHDC1), YTHDC2, YTHDF1, YTHDF2 and YTHDF3, have been identified as m6A-binding or “reader” proteins [18,25]. The members belonging to the DF family likely confine m6A-modified RNAs in specific cytoplasmic liquid–liquid phase separation compartments [67].
Several other “reader” proteins have been shown to bind m6A-modified RNAs thanks to a so-called “m6A switch” [68]. Indeed, m6A installation may trigger a conformational switch that allows the binding of these “reader” proteins, which, in fact, do not directly bind to the m6A residue itself [13]. This mechanism is exploited by several members of the hnRNP (heterogeneous nuclear ribonucleoprotein) family. Finally, insulin-like growth factor 2 mRNA-binding proteins (IGF2BP) were also reported to bind m6A-modified RNAs, promoting their stability [69].
Although two enzymes able to “erase” m6A from mammalian RNAs have been reported, i.e., FTO Alpha-Ketoglutarate-Dependent Dioxygenase (FTO) and AlkB Homolog 5, RNA Demethylase (ALKBH5) [29,30], the specificity and the relevance of these enzymes in physiological conditions are still a matter of debate [70].
About 0.1–0.4% of all adenosines in global cellular RNAs are modified as m6A, and this modification accounts for ~50% of all methylated ribonucleotides [61]. m6A was found in all classes of cellular RNAs: mRNAs (in particular, in long internal exons, locations upstream of stop codons and the 3′-UTR regions) [25,71,72]; ribosomal RNAs; transfer RNAs and various non-coding RNAs [73,74,75].
The first report of m6A modification in miRNAs dates back to 2014, when Yuan and colleagues [76] reported this epitranscriptomic modification in miR-125b. Surprisingly, the authors identified NOP2/Sun RNA Methyltransferase 2 (Nsun2), a well-characterized m5C “writer” [21,77], as the “writer” enzyme of this modification. Furthermore, their data suggested that a m6A modification may prevent pri-miR-125b-2 processing into mature miR-125b [76].
In 2015, two pivotal contributions by Alarcón and colleagues [78,79] provided evidence that a m6A modification by METTL3 globally enhances miRNA processing in mammalian cells. Mechanistically, a novel nuclear m6A “reader”, namely hnRNPA2B1, binds to m6A-modified pri-miRNAs and interacts with the Microprocessor complex, promoting miRNA processing in a METTL3-dependent manner. Interestingly in Arabidopsis thaliana, an ortholog of METTL3 has been recently shown to mediate m6A installation on pri-miRNAs, thus promoting an interaction with the Microprocessor complex, suggesting that the regulation of miRNA processing by m6A is a widely conserved mechanism [80].
Neither FTO nor ALKBH5 were proven yet to be able to catalyze the demethylation of miRNAs. However, it is worth mentioning that DEAD-box RNA helicase 3 (DDX3) controls the methylation status of microRNAs and interacts with both the AGO2 and the ALKBH5 enzymes [81], suggesting a possible role of ALKBH5 in miRNA demethylation.
In cancer, the relevancy of m6A in miRNA maturation was first unveiled for miR-126 in hepatocellular carcinoma (HCC) [82]. The authors showed that a METTL14-dependent m6A modification on pri-miR-126 is required for its processing by the Microprocessor complex. Indeed, in hepatocellular carcinoma, METTL14 is downregulated, thus decreasing m6A on pri-miR-126 and reducing the miR-126 abundance. Furthermore, their findings support the hypothesis that the miR-126 modification by METTL-14 is instrumental to prevent cell invasion, as assessed by in vitro assays.
From that moment on, increasing evidence has disclosed the relevance of the m6A modification of miRNA in cancer progression. Most of the literature confirms that m6A mainly promotes pri-miRNA processing and that the deregulation of the enzymes involved in writing or reading m6A is correlated with tumor onset. Notably, alteration of the m6A deposition on miRNAs is not only a common feature of different tumors but also participates in tumorigenesis processes (Figure 1 and Table 1).
Figure 1.
Epitranscriptomic modification impacts on miRNA processing and activity. m6A, A-to-I editing, m5C and m7G can affect different steps of miRNA biogenesis, including Microprocessor cleavage, Dicer cleavage and RISC loading or alter target recognition and binding.
Table 1.
Effects of m6A modification of miRNAs in cancer.
| Cancer Type | m6A-Modified miRNA(s) |
Increase/ Decrease 1 |
Effects on miRNA Processing/Function | Effects on Tumor Progression | Reference |
|---|---|---|---|---|---|
| Colorectal Cancer | miR-1246 | ↑ | processing | Up-regulation of mature of miR-1246 results in the reduction of SPRED2, thus activating the RAF/MEK/ERK pathway | [85] |
| miR-375 | ↓ | processing | Down-regulation of mature miR-375 increases the expression of its targets YAP1 and SP1 thus increasing proliferation, and migration and invasion | [83] | |
| miR-483, miR-676 miR-877 |
n.d. | processing | miR-483, miR-676 and miR-877 modulate mitochondrial metabolism by targeting electron transport chain genes | [90] | |
| miR-17-5p let-7a-5p |
↑ | Binding to targets | n.d. | [91] | |
| Pancreatic cancer | miR-25-3p | ↑ | processing | Up-regulation of mature miR-25-3p results in the reduction of PHLPP2, leading to AKT activation. | [89] |
| miR-17-5p let-7a-5p |
↑ | Binding to targets | n.d. | [91] | |
| Hepatocellular Carcinoma | miR-126 | ↓ | processing | Down-regulation of mature miR-126 which acts as a tumor suppressor | [82] |
| Bladder cancer | miR-221/222 | ↑ | processing | Up-regulation of mature miR-221/222 results in the reduction of PTEN, leading to proliferation | [84] |
| Gallbladder cancer | miRNA-92 | ↑ | processing | Up-regulation of mature miRNA-92 results in the reduction of PTEN, thus activating PI3K/AKT signaling | [88] |
| Ovarian cancer | miR-126 | ↑ | processing | Up-regulation of mature miR-126-5p results in the reduction of PTEN, thus activating the PI3K/Akt/mTOR pathway | [87] |
| Gastric cancer | miR-17-5p let-7a-5p |
↑ | Binding to targets | n.d. | [91] |
| Lung cancer (brain metastasis) | miR-143-3p | ↑ | processing | Up-regulation of mature miR-143-3p promotes the metastatic potential of lung cancer via regulation of angiogenesis and microtubules through VASH1 | [86] |
1 increase (↑) or decrease (↓) of the epitranscriptomic modification (n.d., not detected; SPRED2, Sprouty Related EVH1 Domain Containing 2; YAP1, yes-associated protein 1; SP1, Sp1 Transcription Factor; PHLPP2, PH Domain And Leucine Rich Repeat Protein; PTEN, Phosphatase 2 Phosphatase And Tensin Homolog; VASH1, Vasohibin 1.
Colorectal cancer (CRC) displays a low expression of METTL14, and this is associated with an impaired m6A modification and processing of pri-miR-375. Low levels of miR-375 result in the overexpression of its targets YAP1 (Yes-associated protein 1) and SP1, thus increasing cell proliferation, migration and invasion [83].
The overexpression of METTL3 in bladder, colorectal, lung, ovarian and gallbladder cancers promotes the processing of several oncomirs, such as miR-221/222 [84], miR-1246 [85], miR-143 [86], miR-126 [87] and miRNA-92 [88]. These miRNAs have tumor suppressors as targets; therefore, the m6A-dependent accumulation of miRNAs results in promoting tumor progression. The same mechanism was described by Zhang and colleagues [89], who recently showed that, in response to cigarette smoke condensate in pancreatic ductal adenocarcinoma, there was an accumulation of mature miR-25-3p, caused by the overexpression of METTL3, which enhanced the m6A modification of pri-miR-25-3p. Increased miR-25-3p resulted in the repression of its target PH Domain and Leucine-Rich Repeat Protein Phosphatase 2 (PHLPP2), thus triggering AKT activation. Interestingly, a novel m6A reader, namely NF-κB-Associated Protein (NKAP), facilitated the interaction of pri-miR-25 with DGCR8, thus promoting the maturation of miR-25-3p [89].
A recent report identified a further putative m6A “reader” protein, RALY (also known as hnRNPCL2), which interacts with miR-483, miR-676 and miR-877 in CRC and is required for their m6A-dependent processing. Mechanistically, RALY interacts with DROSHA and DGCR8 to enhance pri-miRNA processing [90].
Recently, it has been proposed that a m6A modification reduces the ability of miRNAs to suppress target mRNA translation. Indeed, Konno and colleagues [91] showed that the m6A modification of let-7a-5p and miR-17-5p caused a large structural change in the RISC complex, which affected the target RNA recognition. In pancreatic and CRC tissues, the m6A levels on let-7a-5p and miR-17-5p increased without affecting the miRNA expression level.
3.2. A-to-I Editing
A-to-I editing is catalyzed by enzymes highly conserved in vertebrates, called Adenosine Deaminases Acting on RNA (ADAR) [92]. Mammalian genomes encode for three members of the ADAR family: ADAR1, ADAR2 and ADAR3 [93].
ADAR enzymes bind double-stranded (ds) regions of coding and noncoding RNAs [94]; in RNAs forming imperfect dsRNA structures, A-to-I editing involves only one or two adenosines (site selective editing), while, in the case of long perfect dsRNA regions, the random modification of several A residues is observed (hyper-editing) [95,96,97].
Inosine is recognized by the cellular machinery as guanosine, causing a change in the RNA sequence. As a consequence, depending on the modification site, this type of RNA editing can influence the RNA stability [98,99,100], splicing [101,102,103], localization and translation, as well as redefine its interactions with specific factors [104,105]. In mRNAs, the modification of A-to-I can lead to a codon change, thus affecting the primary structure of the encoded protein [106,107].
A-to-I editing mainly targets noncoding regions of RNA, such as introns and UTRs, containing repetitive Alu elements and Long Interspersed Elements (LINEs) that fold into dsRNA structures recognized by ADARs [108].
In most types of cancer, the activity of ADAR enzymes is significantly decreased, as witnessed by the extensive hypoediting of Alu RNAs, as well as by the reduced expression of ADAR enzymes [109].
The first evidence of the editing of a miRNA was shown in 2004 by Luciano and colleagues [33], who reported A-to-I conversion within the miR-22 precursor in Homo sapiens and Mus musculus. Soon after, it was shown that the A-to-I editing of pri-miR-142 prevents processing by DROSHA [110]. ADAR enzymes have a degree of specificity for different miRNA precursors, depending on their secondary structure [111]. The ADAR1 interaction with DICER was associated with enhanced miRNA processing in oral squamous cells carcinoma [112] and in melanoma [113], although, in both cases, the authors did not assess the editing of the miRNA precursors. Furthermore, ADAR editing has been shown to affect the DICER-dependent processing of viral miRNAs [114]. Of note, ADARs can also alter miRNA metabolism independently from their editing activity [115,116,117].
Several examples showed that the A-to-I editing of miRNA precursors inhibits the biogenesis of mature miRNAs (Figure 1 and Table 2). The deregulation of ADAR1 and/or ADAR2 in glioblastoma and in chordoma affects the expression levels of miR-21, miR-221 and miR-222 [118] and of miR-10a and miR-125a [119], respectively. Furthermore, the impairment of let-7 biogenesis by means of ADAR1-mediated A-to-I editing drives leukemia stem cells renewal [120].
Table 2.
Effects of A-to-I editing of miRNAs in cancer.
| Cancer | A-to-I-Modified miRNA(s) |
Increase/ Decrease 1 |
Effects on miRNA Processing/Function | Effects on Tumor Progression | Reference |
|---|---|---|---|---|---|
| Glioma | mir-376a-5p | ↓ | Binding to targets | Unedited miR-376a-5p promotes aggressive glioma growth, by its ability to target RAP2A and concomitant inability to target AMFR | [125] |
| miR-221/222 miR-21 |
↓ | processing | Up-regulation of mature miR-221/222 and miR-21 results in the repression of its targets p27Kip1 and PDCD4, thus increasing proliferation and migration of glioblastoma | [118] | |
| miR-589-3p | ↓ | Binding to targets | Editing within miR-589–3p retargets the miRNA from the protocadherin PCDH9 to the metalloprotease ADAM12, which is involved in glioblastoma cell invasion. | [129] | |
| Melanoma | miR-455-5p | ↓ | Binding to targets | Unedited miR-455-5p but not the edited form targets the tumor suppressor gene CPEB1, thus promoting tumor growth and metastasis | [127] |
| miR-378a-3p | ↓ | Binding to targets | Edited miR-378a-3p but not the unedited form specifically targets the PARVA oncogene, thus preventing the progression of melanoma towards the malignant phenotype | [128] | |
| Chordoma | miR-10a miR-125a |
↑ | processing | Down-regulation of miR-10a and miR-125a expression and upregulates expression of their target genes | [119] |
| Chronic myeloid leukemia | let-7 | ↑ | processing | Down-regulation of mature let-7 results in increased LIN28B expression and enhanced self-renewal | [120] |
| Thyroid cancer | miR-200b | ↑ | Binding to targets | Edited miR-200b has weakened activity against its target gene ZEB1, an epithelial–mesenchymal transition (EMT) marker | [131] |
| Lung cancer | miR-381 | ↑ | n.d. | Edited miR-381 enhances the growth of non-small-cell lung cancer cells as compared to the unedited form | [134] |
1 increase (↑) or decrease (↓) of the epitranscriptomic modification (n.d., not detected; RAP2A, Ras-Related Protein Rap-2a; AMFR, Autocrine Motility Factor Receptor; PDCD4, Programmed Cell Death 4; PCDH9, Protocadherin 9; ADAM12, ADAM Metallopeptidase Domain 12; CPEB1, Cytoplasmic Polyadenylation Element-Binding Protein 1; PARVA, Parvin Alpha).
A further mechanism by which A-to-I editing alters miRNA functions is the remodulation of potential targets of mature miRNAs [121]. Indeed, A-to-I editing in the “seed” sequence causes a loss-of-function when no more targets are recognized [122] or a gain-of-function when a new target is recognized by the edited miRNA [123,124]. Therefore, changes in the relative abundance of the edited and unedited forms of the miRNA lead, in turn, to altered gene expression profiles.
In this context, in 2012, it has been shown that the loss of mir-376a-5p editing results in the increased invasiveness of glioblastoma multiforme (GBM). Mechanistically, unedited miR-376a-5p promotes aggressive glioma growth by its ability to target Ras-Related Protein Rap-2a (RAP2A), a member of the RAS oncogene family, and the concomitant inability to target Autocrine Motility Factor Receptor (AMFR) [125]. These findings were further corroborated by the discovery that miRNA hypoediting is widespread in GBM [126].
Similarly, the impairment of ADAR-mediated editing of miR-455-5p enhances the progression in melanoma. Indeed, unedited miR-455-5p targets the tumor suppressor Cytoplasmic Polyadenylation Element-Binding Protein 1 (CPEB1), thus promoting metastasis, while edited miR-455-5p exerts the opposite effect [127]. In a follow-up of their work, the authors showed that, in melanoma, the editing of miR-378a-3p allows the targeting of the oncogene Parvin Alpha (PARVA). Hence, the loss of miR-378a-3p editing promotes melanoma progression [128].
In the brain, ADAR2 edits the seed sequence of miR-589-3p. In glioblastoma, the editing of miR-589-3p decreases. Higher levels of the unedited version of miR-589-3p promote proliferation and invasion by targeting the tumor suppressor Protocadherin 9 (PCDH9). On the contrary, editing within miR-589-3p retargets the miRNA to the ADAM Metallopeptidase Domain 12 (ADAM12) to contrast the progression of the tumor [129].
In different contexts, miRNA A-to-I editing stimulates the progression of the tumor by altering the selection of miRNA targets. In thyroid cancer, the slight overexpression of ADAR1 corresponds to a higher expression of ZEB1, a master regulator of Epithelial–Mesenchymal Transition (EMT). It has been demonstrated that editing of the seed sequence of miR-200b by ADAR1 impairs its ability to inhibit ZEB1 expression, favoring the progression of the cancer [130,131].
Another target of A-to-I editing is miR-381, a microRNA involved in stemness and chemoresistance [132,133] that is overedited in non-small cell lung carcinoma (NSCLC) cell lines harboring the genomic amplification of ADAR1. Edited miR-381 promotes cell viability [134].
Besides these examples on specific miRNAs, a global analysis of miRNA sequencing data from healthy and cancerous tissues unveiled that miRNA editing is frequently dysregulated in cancer [130,135,136,137,138,139].
3.3. 5-Methylcytosine (m5C)
m5C is one of the most representative post-transcriptional RNA modifications [140], and it has long been known to be present in all three kingdoms of life [141,142].
m5C was originally reported in tRNAs, rRNAs [62] and coding RNAs [143]; later, it was identified in other noncoding RNAs, thanks to technologies such as bisulfite treatment and Next-Generation Sequencing (NGS) [144,145,146].
The synthesis of m5C is catalyzed by the seven members of the NOL1/NOP2/SUN domain (NSUN) family of methyltransferases [147] or by DNA methyltransferase-2 (DNMT2) [148]. These enzymes are responsibles for the methylation of rRNAs, tRNAs [149,150,151,152,153], mRNAs [154,155,156], lncRNAs [157], vault-RNAs [158], enhancer-RNAs [145], mitochondrial tRNAMet [159] and mitochondrial 12S rRNA [160].
In vitro and in vivo studies have demonstrated that aly/REF nuclear factor (ALYREF) is a putative “reader” of m5C sites on mRNAs and that, following the knockdown of NSUN2, ALYREF loses its RNA-binding ability and is retained in the nucleus, suggesting a role for m5C in mRNA exports from the nucleus [26]. A further m5C “reader” is Y-Box-Binding Protein 1 (YBX1) that recognizes and binds m5C-modified mRNAs and stabilizes their target mRNAs by recruiting ELAV-like Protein 1 (ELAVL1) [161,162].
m5C “writers” and “readers” are primarily implicated in fundamental cancer-related processes such as cell differentiation, motility [163,164], proliferation [165,166], cell cycle progression [167] and senescence [155].
In particular, NSUN2 is aberrantly expressed and plays important roles in the development and pathogenesis of different types of tumors, such as breast, colorectal, lung, skin, ovarian and bladder cancers [168].
The distribution of m5C in small RNAs is poorly understood so far; nevertheless, this modification has been recently highlighted in vault RNAs (vtRNAs) [158], piwi-associated RNAs (piRNAs) [169] and miRNAs [91,170,171]. m5C deposition regulates the processing of vault ncRNAs into small vault RNAs (svRNAs) [158,172].
m5C has been only recently characterized in miRNAs. Interestingly, methylation, but not an abundance of miR-200c-3p and miR-21-3p, was increased in pancreatic and colorectal cancer tissues, as well as in serum samples from pancreatic and colorectal cancer patients [91] (Figure 1 and Table 3).
Table 3.
Effects of m5C and m7G modifications of miRNAs in cancer.
| Cancer | Modified miRNA(s) |
Increase/ Decrease 1 |
Effects on miRNA Processing/Function | Effects on Tumor Progression | Reference |
|---|---|---|---|---|---|
| Glioma | miRNA-181a-5p (m5C) |
↑ | Binding to targets | Cytosine-methylated miRNA-181a-5p loses its ability to target the mRNA of the pro-apoptotic protein BIM | [169] |
| Colorectal cancer; gastric cancer; pancreatic cancer | miR-200c-3p miR-21-3p (m5C) |
↑ | Binding to targets | n.d. | [88] |
| Lung cancer | let-7 family (m7G) |
n.d. | processing | m7G methylation within miRNAs regulates cell migration | [173] |
| Colon cancer | let-7e (m7G) |
↓ | processing | Down-regulation of mature let-7e results in the activation of its targets HMGA2 thus stimulating colon cancer cell viability and mobility | [174] |
1 Increase (↑) or decrease (↓) of epitranscriptomic modifications (n.d., not detected; HMGA2 High Mobility Group AT-hook 2).
Cheray and colleagues [170] proposed that the DNMT3A/AGO4 complex promotes the methylation of cytosine residues of miRNAs at CG dinucleotides. In glioblastoma-derived cell lines and glioblastoma tumor samples, the methylation of mature miR-181a-5p by the DNMT3A/AGO4 complex inhibits the recognition of its target mRNA BIM, a proapoptotic gene, also known as B-cell chronic lymphocytic leukemia/lymphoma (Bcl-2)-like 11 (BCL2L11) [170]. This preliminary evidence highlights that the profiling in tumor samples of m5C in miRNAs deserves further investigation.
Recently, we described that m5C is widely spread in human miRNAs in various sequence contexts by taking advantage of a novel NGS analysis of bisulfite-treated small RNAs (BS-miRNA-seq) [171].
Finally, 5mC is oxidized by the Ten-eleven translocation (TET) enzymes both in DNA and RNA. TET enzymes are Fe(II)- and 2-oxoglutarate-dependent dioxygenases that mediate the conversion of 5mC to 5hmC, then to 5-formylcytosine (5-fC) [175] and, finally, to 5-carboxylcytosine (5-caC) [18,176,177]. In DNA, these subsequent conversions have the purpose of demethylating 5mC [178], but it is still not clear if this mechanism is conserved in RNA.
hm5C has been detected in RNA isolated from different mouse and human tissues, including the brain, heart, pancreas and spleen [179]. Transcriptome-wide analyses of hm5C in mouse and in Drosophila RNAs have revealed the presence of 5hmC on hundreds of messenger RNAs, mainly in UC-rich motifs [180,181]. The deposition of hm5C in mRNAs has been associated with the differentiation of murine embryonic stem cells and brain development in Drosophila via controlling the mRNA stability or translation, respectively.
To date, decreased levels of hm5C in RNA have been shown in tumor tissues, such as CRC and hepatocellular carcinoma [176].
Interestingly, we recently unraveled not only the presence of m5C but, also, of hm5C on several miRNAs in human cancer cell lines [171]. However, no evidence of the role of hm5C modification in miRNAs in tumors has yet been reported.
3.4. N7-Methylguanosine (m7G)
m7G is a positively charged modification installed cotranscriptionally at the 5’ Caps of eukaryotic mRNAs [182]. This modification protects and stabilizes transcripts from exonucleolytic degradation [183] and influences all the events responsible for the processing of the mRNA molecules, from transcript elongation to translation [184,185].
Notably, the presence of internal m7G sites was found not only in tRNA and rRNA molecules [186,187,188] but also in mammalian mRNAs [188]. Internal m7G could affect mRNA translation, and this modification typically occurs near the start and stop codons in a GA-enriched motif [189].
The enzyme responsible for this internal m7G modification is METTL1, which cooperates with the cofactor WD Repeat Domain 4 (WDR4) [189,190]. Interestingly, METTL1 has been linked to tumor vascular invasion and poor prognosis in hepatocellular carcinoma [173,191].
Recently, by high-throughput screening, several miRNAs were identified as harboring internal m7G sites [192]. In particular, METTL1-dependent m7G was discovered in a subset of tumor-suppressor miRNAs involved in the inhibition of cell migration, including the let-7 family. METTL1-mediated m7G occurs on pri-miRNA within G-rich regions that display the propensity to form G-quadruplexes, i.e., structures known to be inhibitory to miRNA processing [174,193,194] (Figure 1 and Table 3).
Indeed, m7G in the let-7 family affects G-quadruplex formations, thus facilitating the formation of a canonical stem-loop structure and miRNA processing [192]. In line with this study, Liu and colleagues showed that, in colon cancer, the downregulation of METTL1 leads to a decrease in the let-7e levels. The alteration of let-7e expression affects its downstream target High Mobility Group AT-hook 2 (HMGA2), thus promoting cell proliferation, invasion and EMT [195].
4. Epitranscriptomic Modifications of miRNA Targets
4.1. m6A in miRNA Targets
The installation of m6A in miRNA targets may affect the pairing with miRNAs, thus affecting miRNA functions. Such a mechanism was first suggested in 2015, when Ke and colleagues [72] reported that the majority of m6A sites on mRNAs were in the last exon. The authors also reported a significant overlap between the m6A residues and AGO-binding sites. This finding was further corroborated by a later study [196].
Importantly, m6A modification may positively or negatively affect miRNA–mRNA pairing by several distinct mechanisms. On the one hand, m6A modifications within miRNA-binding sites may directly affect the miRNA:mRNA duplex stability. On the other hand, m6A modifications nearby AGO-binding sites may alter the mRNA secondary structure and/or recruit other RNA-binding proteins, thus modifying the mRNA accessibility to miRNA. Thus far, a few examples have been reported.
In the liver, YAP is a target of miR-582-3p, which binds at residues 313-321 of YAP 3′ UTR. Such binding is enhanced by m6A modifications of YAP 3′ UTR at residue 355. In HCC, the m6A modification at residue 355 of YAP 3′ UTR is impaired, resulting in the loss of YAP repression by miR-582-3p [197].
In cancer cell lines, m6A modification promotes the recruitment of IGF2BP1 onto Serum Response Factor (SRF) mRNA 3′ UTR. Interestingly, IGF2BP1 acts as a m6A “reader”, and its recruitment is instrumental to reduce miRNA-mediated AGO binding to SRF. Therefore, m6A deposition on the 3′ UTR of SRF mRNA relieves the negative post-transcriptional regulation by miRNAs [198]. Furthermore, a genome-wide analysis of m6A in glioma stem cells highlighted that m6A deposition on the 3′ UTR of several mRNAs may affect their targeting by different miRNAs [199]. On the other hand, Cheng and colleagues [200] showed an effect at odds with this. Indeed, in neuroblastoma, m6A modification in N-Myc 3′ UTR near a miR-98-binding site is necessary to promote the miR-98-mediated post-transcriptional repression of N-Myc. Overall, these examples highlight that the effect of m6A within mRNA 3′ UTRs on miRNA targeting is ambiguous.
m6A not only affects miRNA:mRNA interactions but, also, miRNA:ceRNA interactions. Yang and colleagues [201] showed that the m6A modification of linc-RNA-1281 is critical for its interaction with miRNAs belonging to the let-7 family. In nasopharyngeal carcinoma, lncRNA FAM225A acts as a ceRNA, sequestering miR-590-3p and miR-1275, thus activating FAK/PI3K/Akt signaling; in this scenario, m6A modification contributes to this mechanism by increasing the lncRNA FAM225A stability [202]. Finally, LINC00958, a ceRNA acting to sponge miR-3619-5p in HCC, is also stabilized through m6A modification by METTL3 [203].
4.2. A-to-I Editing in miRNA Targets
A-to-I editing can influence microRNA targeting. Indeed, when A-to-I editing occurs within the miRNA-binding site in the 3′ UTR of a mRNA, this can remodulate the interactions between miRNA and mRNA in different ways.
The editing of the 3′ UTR of Rho GTPase Activating Protein 26 (ARHGAP26) by ADAR1 blocks the interaction with miR-30b-3p and miR-573 to favor the expression of the protein [204]. On the contrary, in HCC cells, for instance, the RNA editing catalyzed by ADAR1 of 3′ UTR of Aryl hydrocarbon Receptor (AhR) creates a miR-378-binding site to negatively regulate the expression of this protein [205]. Through a similar mechanism, editing of the 3′ UTR of the mRNA encoding for the tumor suppressor Phosphatase and Actin Regulator 4 (PHACTR4) mediated by ADAR1 prevents the binding of miR-196a-3p. Accordingly, the decreased activity of ADAR1 in gastric cancer results in the repression of PHACTR4 by miR-196-3p [206].
Interestingly, a pan-cancer RNA editing study highlighted that the 3′ UTR of the Mouse double minute 2 homolog (MDM2) oncogene underwent A-to I editing in 11 out of the 14 cancer types investigated within a region of the 3′ UTR complementary to the miR-200 “seed” region. This editing impaired MDM2 repression by miR-200 [207].
The above-mentioned examples suggest that A-to-I editing within regions of 3′ UTR pairing with miRNA “seed” affects miRNA binding. However, a genome-wide report pinpointed that the editing of A residues lying outside of the miRNA:mRNA pairing region affects the mRNA structure, thus modulating the accessibility to AGO2-miRNA complexes [208].
4.3. m5C in miRNA Targets
To date, little is known about the mechanisms through which m5C can regulate the function of miRNAs, but, interestingly, a possible role for m5C in miRNA targeting was suggested by the overlap between the AGO2-binding sites and m5C positions reported in the 3′ UTR of human mRNAs [209].
5. Methodological Challenges and the Potential Limits of Current Knowledge
Our understanding of the mechanisms underlying the epitranscriptomic regulation of miRNAs is still potentially biased by the relatively small number of modifications which have been widely investigated in miRNAs, with most reports focused on m6A and A-to-I editing. Indeed, further mechanisms through which epitranscriptomic modifications may affect miRNA function are conceivable. For example, the recent report by Konno and colleagues suggested that m5C modification in position 9 of miR-200-3p might affect the interaction with AGO proteins [91]. However, further investigation will be required to assess whether this may represent a general paradigm. Epitranscriptomic modifications might also modulate miRNA half-life, or control miRNA subcellular localization (either in membrane enclosed organelles, or by liquid-liquid phase separation) or secretion of modified miRNAs. Secreted miRNAs have been widely reported as potential biomarkers for a variety of diseases and they also serve as signaling molecules to mediate cell-cell communications [210,211].
Our current knowledge of the modifications installed on miRNAs is likely incomplete because of technical limits which still prevent application of several high-throughput NGS methods for the quantification and characterization of epitranscriptomic modification to miRNAs. Indeed, most epitranscriptomic modifications are investigated through NGS methods which exploit the block of reverse transcription at the modified nucleobase of interest by means of different biochemical treatments (i.e., antibody cross-linking or chemical modifications). These protocols result in reads truncated at the modified positions, thus allowing the genome-wide mapping of the epitranscriptomic modification at single-nucleotide resolution [212,213]. Those methods cannot be applied to mature miRNAs, as premature reverse trascriptase termination would yield reads too short to be effectively mapped on the genome. Currently, single-nucleotide resolution analysis in miRNAs is only possible for those modifications assessed through methods based on mismatched nucleotides introduced during the reverse transcription step. Furthermore, techniques relying on unmodified nucleobases conversion (e.g., bisulfite treatment) require dedicated pipelines for data analysis as alignment of short reads is severely affected by complexity reduction associated with those techniques [171].
Finally, emerging methods to assess epitranscriptomic modifications through third generation sequencing are best-suited for long RNA molecules and cannot be easily adapted to the study of miRNAs [214].
6. Conclusions
In this review, we summarized the current knowledge on the epitranscriptomic modifications of miRNAs that play a role in cancer development and/or progression. In most cases, epitranscriptomic modifications exert their effect on miRNAs by affecting either their biogenesis or their binding to target mRNAs.
The current lack of mature, high-throughput technologies to profile epitranscriptomic modifications of miRNAs, with the notable exception of A-to-I editing, is likely one of the main reasons why the prognostic/diagnostic use of the “miRNA epitranscriptome” has been poorly explored thus far. Nevertheless, a plethora of studies focused on the mechanistic role of specific modifications in cancer development and/or progression point out how promising the miRNA epitranscriptome is.
Adaptation of the existing NGS methods to yield techniques allowing cheap, high-throughput and quantitative assessments of epitranscriptomic modifications in miRNAs could potentially propel research in this field by allowing mechanistic investigations of further modifications. Furthermore, such methodologies would be fundamental to investigate the potential prognostic role of miRNAs, including miRNA secreted into extracellular fluids.
Author Contributions
V.F. is responsible for the ideation; V.D.P., E.L., E.O., C.C., I.L., V.F. performed the literature search V.D.P., E.L., E.O., C.C., I.L., V.F. drafted the work and I.L., C.C., V.F. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by a Sapienza Università di Roma grant (“Ateneo Medi 2019 #RM11916B7A048AA0”) to C.C.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alberti C., Cochella L. A Framework for Understanding the Roles of MiRNAs in Animal Development. Development. 2017;144:2548–2559. doi: 10.1242/dev.146613. [DOI] [PubMed] [Google Scholar]
- 2.Tüfekci K.U., Meuwissen R.L.J., Genç Ş. The Role of MicroRNAs in Biological Processes. In: Yousef M., Allmer J., editors. miRNomics: MicroRNA Biology and Computational Analysis. Humana Press; Totowa, NJ, USA: 2014. pp. 15–31. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 3.Paul P., Chakraborty A., Sarkar D., Langthasa M., Rahman M., Bari M., Singha R.S., Malakar A.K., Chakraborty S. Interplay between MiRNAs and Human Diseases. J. Cell. Physiol. 2018;233:2007–2018. doi: 10.1002/jcp.25854. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z., Shi J., Gao Y., Cui C., Zhang S., Li J., Zhou Y., Cui Q. HMDD v3.0: A Database for Experimentally Supported Human MicroRNA-Disease Associations. Nucleic Acids Res. 2019;47:D1013–D1017. doi: 10.1093/nar/gky1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali Syeda Z., Langden S.S.S., Munkhzul C., Lee M., Song S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020;21:1723. doi: 10.3390/ijms21051723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y., Li Q., Zhang R., Dai X., Chen W., Xing D. Circulating MicroRNAs: Biomarkers of Disease. Clin. Chim. Acta. 2021;516:46–54. doi: 10.1016/j.cca.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Forterre A., Komuro H., Aminova S., Harada M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers. 2020;12:1852. doi: 10.3390/cancers12071852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccaletto P., Machnicka M.A., Purta E., Piątkowski P., Bagiński B., Wirecki T.K., de Crécy-Lagard V., Ross R., Limbach P.A., Kotter A., et al. MODOMICS: A Database of RNA Modification Pathways. 2017 Update. Nucleic Acids Res. 2018;46:D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saletore Y., Meyer K., Korlach J., Vilfan I.D., Jaffrey S., Mason C.E. The Birth of the Epitranscriptome: Deciphering the Function of RNA Modifications. Genome Biol. 2012;13:175. doi: 10.1186/gb-2012-13-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peer E., Rechavi G., Dominissini D. Epitranscriptomics: Regulation of MRNA Metabolism through Modifications. Curr. Opin. Chem. Biol. 2017;41:93–98. doi: 10.1016/j.cbpa.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Wiener D., Schwartz S. The Epitranscriptome beyond M6A. Nat. Rev. Genet. 2020 doi: 10.1038/s41576-020-00295-8. [DOI] [PubMed] [Google Scholar]
- 12.Nachtergaele S., He C. The Emerging Biology of RNA Post-Transcriptional Modifications. RNA Biol. 2017;14:156–163. doi: 10.1080/15476286.2016.1267096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaccara S., Ries R.J., Jaffrey S.R. Reading, Writing and Erasing MRNA Methylation. Nat. Rev. Mol. Cell Biol. 2019;20:608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Ma S., Yi C. Pseudouridine: The Fifth RNA Nucleotide with Renewed Interests. Curr. Opin. Chem. Biol. 2016;33:108–116. doi: 10.1016/j.cbpa.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg E., Levanon E.Y. A-to-I RNA Editing—Immune Protector and Transcriptome Diversifier. Nat. Rev. Genet. 2018;19:473–490. doi: 10.1038/s41576-018-0006-1. [DOI] [PubMed] [Google Scholar]
- 16.Trixl L., Lusser A. The Dynamic RNA Modification 5-Methylcytosine and Its Emerging Role as an Epitranscriptomic Mark. Wiley Interdiscip. Rev. RNA. 2019;10:e1510. doi: 10.1002/wrna.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janzen W.P., Wigle T.J., Jin J., Frye S.V. Epigenetics: Tools and Technologies. Drug Discov. Today Technol. 2010;7:e59–e65. doi: 10.1016/j.ddtec.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y., Dominissini D., Rechavi G., He C. Gene Expression Regulation Mediated through Reversible M6A RNA Methylation. Nat. Rev. Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 19.Cortese R., Kammen H.O., Spengler S.J., Ames B.N. Biosynthesis of Pseudouridine in Transfer Ribonucleic Acid. J. Biol. Chem. 1974;249:1103–1108. doi: 10.1016/S0021-9258(19)42947-5. [DOI] [PubMed] [Google Scholar]
- 20.Koonin E.V. Pseudouridine Synthases: Four Families of Enzymes Containing a Putative Uridine-Binding Motif Also Conserved in DUTPases and DCTP Deaminases. Nucleic Acids Res. 1996;24:2411–2415. doi: 10.1093/nar/24.12.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohnsack K.E., Höbartner C., Bohnsack M.T. Eukaryotic 5-Methylcytosine (M5C) RNA Methyltransferases: Mechanisms, Cellular Functions, and Links to Disease. Genes. 2019;10:102. doi: 10.3390/genes10020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., Jia G., Yu M., Lu Z., Deng X., et al. A METTL3–METTL14 Complex Mediates Mammalian Nuclear RNA N 6 -Adenosine Methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokar J.A., Shambaugh M.E., Polayes D., Matera A.G., Rottman F.M. Purification and CDNA Cloning of the AdoMet-Binding Subunit of the Human MRNA (N6-Adenosine)-Methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 24.Bass B.L., Weintraub H. An Unwinding Activity That Covalently Modifies Its Double-Stranded RNA Substrate. Cell. 1988;55:1089–1098. doi: 10.1016/0092-8674(88)90253-X. [DOI] [PubMed] [Google Scholar]
- 25.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M., et al. Topology of the Human and Mouse m 6 A RNA Methylomes Revealed by m 6 A-Seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 26.Yang X., Yang Y., Sun B.-F., Chen Y.-S., Xu J.-W., Lai W.-Y., Li A., Wang X., Bhattarai D.P., Xiao W., et al. 5-Methylcytosine Promotes MRNA Export—NSUN2 as the Methyltransferase and ALYREF as an M5C Reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newby M.I., Greenbaum N.L. Sculpting of the Spliceosomal Branch Site Recognition Motif by a Conserved Pseudouridine. Nat. Struct. Biol. 2002;9:958–965. doi: 10.1038/nsb873. [DOI] [PubMed] [Google Scholar]
- 28.Kierzek E., Malgowska M., Lisowiec J., Turner D.H., Gdaniec Z., Kierzek R. The Contribution of Pseudouridine to Stabilities and Structure of RNAs. Nucleic Acids Res. 2014;42:3492–3501. doi: 10.1093/nar/gkt1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.-G., et al. N 6-Methyladenosine in Nuclear RNA Is a Major Substrate of the Obesity-Associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.-M., Li C.J., Vågbø C.B., Shi Y., Wang W.-L., Song S.-H., et al. ALKBH5 Is a Mammalian RNA Demethylase That Impacts RNA Metabolism and Mouse Fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbieri I., Kouzarides T. Role of RNA Modifications in Cancer. Nat. Rev. Cancer. 2020;20:303–322. doi: 10.1038/s41568-020-0253-2. [DOI] [PubMed] [Google Scholar]
- 32.Haruehanroengra P., Zheng Y.Y., Zhou Y., Huang Y., Sheng J. RNA Modifications and Cancer. RNA Biol. 2020;17:1560–1575. doi: 10.1080/15476286.2020.1722449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luciano D.J., Mirsky H., Vendetti N.J., Maas S. RNA Editing of a MiRNA Precursor. RNA. 2004;10:1174–1177. doi: 10.1261/rna.7350304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y., Kim M., Han J., Yeom K.-H., Lee S., Baek S.H., Kim V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez A., Griffiths-Jones S., Ashurst J.L., Bradley A. Identification of Mammalian MicroRNA Host Genes and Transcription Units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y.-K., Kim V.N. Processing of Intronic MicroRNAs. EMBO J. 2007;26:775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of Novel Genes Coding for Small Expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 38.Denli A.M., Tops B.B.J., Plasterk R.H.A., Ketting R.F., Hannon G.J. Processing of Primary MicroRNAs by the Microprocessor Complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 39.Gregory R.I., Yan K., Amuthan G., Chendrimada T., Doratotaj B., Cooch N., Shiekhattar R. The Microprocessor Complex Mediates the Genesis of MicroRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., et al. The Nuclear RNase III Drosha Initiates MicroRNA Processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 41.Yi R., Qin Y., Macara I.G., Cullen B.R. Exportin-5 Mediates the Nuclear Export of Pre-MicroRNAs and Short Hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohnsack M.T., Czaplinski K., Görlich D. Exportin 5 Is a RanGTP-Dependent DsRNA-Binding Protein That Mediates Nuclear Export of Pre-MiRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lund E., Güttinger S., Calado A., Dahlberg J.E., Kutay U. Nuclear Export of MicroRNA Precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 44.Ketting R.F., Fischer S.E., Bernstein E., Sijen T., Hannon G.J., Plasterk R.H. Dicer Functions in RNA Interference and in Synthesis of Small RNA Involved in Developmental Timing in C. Elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutvágner G., McLachlan J., Pasquinelli A.E., Bálint É., Tuschl T., Zamore P.D. A Cellular Function for the RNA-Interference Enzyme Dicer in the Maturation of the Let-7 Small Temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 46.Gebert L.F.R., MacRae I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoda M., Kawamata T., Paroo Z., Ye X., Iwasaki S., Liu Q., Tomari Y. ATP-Dependent Human RISC Assembly Pathways. Nat. Struct. Mol. Biol. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krek A., Grün D., Poy M.N., Wolf R., Rosenberg L., Epstein E.J., MacMenamin P., da Piedade I., Gunsalus K.C., Stoffel M., et al. Combinatorial MicroRNA Target Predictions. Nat. Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 49.Lewis B.P., Burge C.B., Bartel D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are MicroRNA Targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 50.Chipman L.B., Pasquinelli A.E. MiRNA Targeting: Growing beyond the Seed. Trends Genet. TIG. 2019;35:215–222. doi: 10.1016/j.tig.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schanen B.C., Li X. Transcriptional Regulation of Mammalian MiRNA Genes. Genomics. 2011;97:1–6. doi: 10.1016/j.ygeno.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saj A., Lai E.C. Control of MicroRNA Biogenesis and Transcription by Cell Signaling Pathways. Curr. Opin. Genet. Dev. 2011;21:504–510. doi: 10.1016/j.gde.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michlewski G., Cáceres J.F. Post-Transcriptional Control of MiRNA Biogenesis. RNA. 2019;25:1–16. doi: 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karreth F.A., Tay Y., Perna D., Ala U., Tan S.M., Rust A.G., DeNicola G., Webster K.A., Weiss D., Perez-Mancera P.A., et al. In Vivo Identification of Tumor- Suppressive PTEN CeRNAs in an Oncogenic BRAF-Induced Mouse Model of Melanoma. Cell. 2011;147:382–395. doi: 10.1016/j.cell.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tay Y., Kats L., Salmena L., Weiss D., Tan S.M., Ala U., Karreth F., Poliseno L., Provero P., Di Cunto F., et al. Coding-Independent Regulation of the Tumor Suppressor PTEN by Competing Endogenous MRNAs. Cell. 2011;147:344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumazin P., Yang X., Chiu H.-S., Chung W.-J., Iyer A., Llobet-Navas D., Rajbhandari P., Bansal M., Guarnieri P., Silva J., et al. An Extensive MicroRNA-Mediated Network of RNA-RNA Interactions Regulates Established Oncogenic Pathways in Glioblastoma. Cell. 2011;147:370–381. doi: 10.1016/j.cell.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A Long Noncoding RNA Controls Muscle Differentiation by Functioning as a Competing Endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galagali H., Kim J.K. The Multifaceted Roles of MicroRNAs in Differentiation. Curr. Opin. Cell Biol. 2020;67:118–140. doi: 10.1016/j.ceb.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta A., Baltimore D. MicroRNAs as Regulatory Elements in Immune System Logic. Nat. Rev. Immunol. 2016;16:279–294. doi: 10.1038/nri.2016.40. [DOI] [PubMed] [Google Scholar]
- 60.Tahamtan A., Teymoori-Rad M., Nakstad B., Salimi V. Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei C.M., Gershowitz A., Moss B. Methylated Nucleotides Block 5′ Terminus of HeLa Cell Messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- 62.Desrosiers R., Friderici K., Rottman F. Identification of Methylated Nucleosides in Messenger RNA from Novikoff Hepatoma Cells. Proc. Natl. Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perry R.P., Kelley D.E. Existence of Methylated Messenger RNA in Mouse L Cells. Cell. 1974;1:37–42. doi: 10.1016/0092-8674(74)90153-6. [DOI] [Google Scholar]
- 64.Schwartz S., Mumbach M.R., Jovanovic M., Wang T., Maciag K., Bushkin G.G., Mertins P., Ter-Ovanesyan D., Habib N., Cacchiarelli D., et al. Perturbation of M6A Writers Reveals Two Distinct Classes of MRNA Methylation at Internal and 5′ Sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patil D.P., Chen C.-K., Pickering B.F., Chow A., Jackson C., Guttman M., Jaffrey S.R. M(6)A RNA Methylation Promotes XIST-Mediated Transcriptional Repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wen J., Lv R., Ma H., Shen H., He C., Wang J., Jiao F., Liu H., Yang P., Tan L., et al. Zc3h13 Regulates Nuclear RNA M6A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol. Cell. 2018;69:1028–1038.e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ries R.J., Zaccara S., Klein P., Olarerin-George A., Namkoong S., Pickering B.F., Patil D.P., Kwak H., Lee J.H., Jaffrey S.R. M6A Enhances the Phase Separation Potential of MRNA. Nature. 2019;571:424–428. doi: 10.1038/s41586-019-1374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu B., Su S., Patil D.P., Liu H., Gan J., Jaffrey S.R., Ma J. Molecular Basis for the Specific and Multivariant Recognitions of RNA Substrates by Human HnRNP A2/B1. Nat. Commun. 2018;9:420. doi: 10.1038/s41467-017-02770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang H., Weng H., Sun W., Qin X., Shi H., Wu H., Zhao B.S., Mesquita A., Liu C., Yuan C.L., et al. Recognition of RNA N6-Methyladenosine by IGF2BP Proteins Enhances MRNA Stability and Translation. Nat. Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mauer J., Jaffrey S.R. FTO, M6 Am, and the Hypothesis of Reversible Epitranscriptomic MRNA Modifications. FEBS Lett. 2018;592:2012–2022. doi: 10.1002/1873-3468.13092. [DOI] [PubMed] [Google Scholar]
- 71.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive Analysis of MRNA Methylation Reveals Enrichment in 3′ UTRs and near Stop Codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ke S., Alemu E.A., Mertens C., Gantman E.C., Fak J.J., Mele A., Haripal B., Zucker-Scharff I., Moore M.J., Park C.Y., et al. A Majority of M6A Residues Are in the Last Exons, Allowing the Potential for 3′ UTR Regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou C., Molinie B., Daneshvar K., Pondick J.V., Wang J., Van Wittenberghe N., Xing Y., Giallourakis C.C., Mullen A.C. Genome-Wide Maps of M6A CircRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns That Are Distinct from MRNAs. Cell Rep. 2017;20:2262–2276. doi: 10.1016/j.celrep.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y., Fan X., Mao M., Song X., Wu P., Zhang Y., Jin Y., Yang Y., Chen L.-L., Wang Y., et al. Extensive Translation of Circular RNAs Driven by N 6 -Methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linder B., Grozhik A.V., Olarerin-George A.O., Meydan C., Mason C.E., Jaffrey S.R. Single-Nucleotide-Resolution Mapping of M6A and M6Am throughout the Transcriptome. Nat. Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan S., Tang H., Xing J., Fan X., Cai X., Li Q., Han P., Luo Y., Zhang Z., Jiang B., et al. Methylation by NSun2 Represses the Levels and Function of MicroRNA 125b. Mol. Cell. Biol. 2014;34:3630–3641. doi: 10.1128/MCB.00243-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brzezicha B., Schmidt M., Makalowska I., Jarmolowski A., Pienkowska J., Szweykowska-Kulinska Z. Identification of Human TRNA:M5C Methyltransferase Catalysing Intron-Dependent M5C Formation in the First Position of the Anticodon of the Pre-TRNA Leu (CAA) Nucleic Acids Res. 2006;34:6034–6043. doi: 10.1093/nar/gkl765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alarcón C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alarcón C.R., Lee H., Goodarzi H., Halberg N., Tavazoie S.F. N6-Methyladenosine Marks Primary MicroRNAs for Processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhat S.S., Bielewicz D., Gulanicz T., Bodi Z., Yu X., Anderson S.J., Szewc L., Bajczyk M., Dolata J., Grzelak N., et al. MRNA Adenosine Methylase (MTA) Deposits M6A on Pri-MiRNAs to Modulate MiRNA Biogenesis in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA. 2020;117:21785–21795. doi: 10.1073/pnas.2003733117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shah A., Rashid F., Awan H.M., Hu S., Wang X., Chen L., Shan G. The DEAD-Box RNA Helicase DDX3 Interacts with M6A RNA Demethylase ALKBH5. Stem Cells Int. 2017;2017:8596135. doi: 10.1155/2017/8596135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma J., Yang F., Zhou C., Liu F., Yuan J., Wang F., Wang T., Xu Q., Zhou W., Sun S. METTL14 Suppresses the Metastatic Potential of Hepatocellular Carcinoma by Modulating N 6 -methyladenosine-dependent Primary MicroRNA Processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 83.Chen X., Xu M., Xu X., Zeng K., Liu X., Sun L., Pan B., He B., Pan Y., Sun H., et al. METTL14 Suppresses CRC Progression via Regulating N6-Methyladenosine-Dependent Primary MiR-375 Processing. Mol. Ther. 2020;28:599–612. doi: 10.1016/j.ymthe.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Han J., Wang J., Yang X., Yu H., Zhou R., Lu H.-C., Yuan W.-B., Lu J., Zhou Z., Lu Q., et al. METTL3 Promote Tumor Proliferation of Bladder Cancer by Accelerating Pri-MiR221/222 Maturation in M6A-Dependent Manner. Mol. Cancer. 2019;18 doi: 10.1186/s12943-019-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng W., Li J., Chen R., Gu Q., Yang P., Qian W., Ji D., Wang Q., Zhang Z., Tang J., et al. Upregulated METTL3 Promotes Metastasis of Colorectal Cancer via MiR-1246/SPRED2/MAPK Signaling Pathway. J. Exp. Clin. Cancer Res. CR. 2019;38:393. doi: 10.1186/s13046-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H., Deng Q., Lv Z., Ling Y., Hou X., Chen Z., Dinglin X., Ma S., Li D., Wu Y., et al. N6-Methyladenosine Induced MiR-143-3p Promotes the Brain Metastasis of Lung Cancer via Regulation of VASH1. Mol. Cancer. 2019;18:181. doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Bi X., Lv X., Liu D., Guo H., Yao G., Wang L., Liang X., Yang Y. METTL3-Mediated Maturation of MiR-126-5p Promotes Ovarian Cancer Progression via PTEN-Mediated PI3K/Akt/MTOR Pathway. Cancer Gene Ther. 2020 doi: 10.1038/s41417-020-00222-3. [DOI] [PubMed] [Google Scholar]
- 88.Lin R., Zhan M., Yang L., Wang H., Shen H., Huang S., Huang X., Xu S., Zhang Z., Li W., et al. Deoxycholic Acid Modulates the Progression of Gallbladder Cancer through N 6 -Methyladenosine-Dependent MicroRNA Maturation. Oncogene. 2020;39:4983–5000. doi: 10.1038/s41388-020-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J., Bai R., Li M., Ye H., Wu C., Wang C., Li S., Tan L., Mai D., Li G., et al. Excessive MiR-25-3p Maturation via N 6 -Methyladenosine Stimulated by Cigarette Smoke Promotes Pancreatic Cancer Progression. Nat. Commun. 2019;10:1858. doi: 10.1038/s41467-019-09712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun L., Wan A., Zhou Z., Chen D., Liang H., Liu C., Yan S., Niu Y., Lin Z., Zhan S., et al. RNA-Binding Protein RALY Reprogrammes Mitochondrial Metabolism via Mediating MiRNA Processing in Colorectal Cancer. Gut. 2020 doi: 10.1136/gutjnl-2020-320652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konno M., Koseki J., Asai A., Yamagata A., Shimamura T., Motooka D., Okuzaki D., Kawamoto K., Mizushima T., Eguchi H., et al. Distinct Methylation Levels of Mature MicroRNAs in Gastrointestinal Cancers. Nat. Commun. 2019;10:3888. doi: 10.1038/s41467-019-11826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bass B.L., Nishikura K., Keller W., Seeburg P.H., Emeson R.B., O’Connell M.A., Samuel C.E., Herbert A. A Standardized Nomenclature for Adenosine Deaminases That Act on RNA. RNA. 1997;3:947–949. [PMC free article] [PubMed] [Google Scholar]
- 93.Barraud P., Allain F.H.-T. ADAR Proteins: Double-Stranded RNA and Z-DNA Binding Domains. Curr. Top. Microbiol. Immunol. 2012;353:35–60. doi: 10.1007/82_2011_145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagner R.W., Yoo C., Wrabetz L., Kamholz J., Buchhalter J., Hassan N.F., Khalili K., Kim S.U., Perussia B., McMorris F.A. Double-Stranded RNA Unwinding and Modifying Activity Is Detected Ubiquitously in Primary Tissues and Cell Lines. Mol. Cell. Biol. 1990;10:5586–5590. doi: 10.1128/MCB.10.10.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gott J.M., Emeson R.B. Functions and Mechanisms of RNA Editing. Annu. Rev. Genet. 2000;34:499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- 96.Bass B.L. RNA Editing by Adenosine Deaminases That Act on RNA. Annu. Rev. Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wahlstedt H., Ohman M. Site-Selective versus Promiscuous A-to-I Editing. Wiley Interdiscip. Rev. RNA. 2011;2:761–771. doi: 10.1002/wrna.89. [DOI] [PubMed] [Google Scholar]
- 98.Stellos K., Gatsiou A., Stamatelopoulos K., Perisic Matic L., John D., Lunella F.F., Jaé N., Rossbach O., Amrhein C., Sigala F., et al. Adenosine-to-Inosine RNA Editing Controls Cathepsin S Expression in Atherosclerosis by Enabling HuR-Mediated Post-Transcriptional Regulation. Nat. Med. 2016;22:1140–1150. doi: 10.1038/nm.4172. [DOI] [PubMed] [Google Scholar]
- 99.Levanon E.Y., Eisenberg E., Yelin R., Nemzer S., Hallegger M., Shemesh R., Fligelman Z.Y., Shoshan A., Pollock S.R., Sztybel D., et al. Systematic Identification of Abundant A-to-I Editing Sites in the Human Transcriptome. Nat. Biotechnol. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- 100.Amin E.M., Liu Y., Deng S., Tan K.S., Chudgar N., Mayo M.W., Sanchez-Vega F., Adusumilli P.S., Schultz N., Jones D.R. The RNA-Editing Enzyme ADAR Promotes Lung Adenocarcinoma Migration and Invasion by Stabilizing FAK. Sci. Signal. 2017;10:eaah3941. doi: 10.1126/scisignal.aah3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kapoor U., Licht K., Amman F., Jakobi T., Martin D., Dieterich C., Jantsch M.F. ADAR-Deficiency Perturbs the Global Splicing Landscape in Mouse Tissues. Genome Res. 2020;30:1107–1118. doi: 10.1101/gr.256933.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang S.J., Shen H., An O., Hong H., Li J., Song Y., Han J., Tay D.J.T., Ng V.H.E., Bellido Molias F., et al. Cis- and Trans-Regulations of Pre-MRNA Splicing by RNA Editing Enzymes Influence Cancer Development. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-14621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y.-T., Chang I.Y.-F., Liu H., Ma C.-P., Kuo Y.-P., Shih C.-T., Shih Y.-H., Kang L., Tan B.C.-M. Tumor-Associated Intronic Editing of HNRPLL Generates a Novel Splicing Variant Linked to Cell Proliferation. J. Biol. Chem. 2018;293:10158–10171. doi: 10.1074/jbc.RA117.001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keegan L.P., Gallo A., O’Connell M.A. The Many Roles of an RNA Editor. Nat. Rev. Genet. 2001;2:869–878. doi: 10.1038/35098584. [DOI] [PubMed] [Google Scholar]
- 105.Valente L., Nishikura K. ADAR Gene Family and A-to-I RNA Editing: Diverse Roles in Posttranscriptional Gene Regulation. Prog. Nucleic Acid Res. Mol. Biol. 2005;79:299–338. doi: 10.1016/S0079-6603(04)79006-6. [DOI] [PubMed] [Google Scholar]
- 106.Sommer B., Köhler M., Sprengel R., Seeburg P.H. RNA Editing in Brain Controls a Determinant of Ion Flow in Glutamate-Gated Channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-J. [DOI] [PubMed] [Google Scholar]
- 107.Burns C.M., Chu H., Rueter S.M., Hutchinson L.K., Canton H., Sanders-Bush E., Emeson R.B. Regulation of Serotonin-2C Receptor G-Protein Coupling by RNA Editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 108.Nishikura K. Functions and Regulation of RNA Editing by ADAR Deaminases. Annu. Rev. Biochem. 2010;79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paz N., Levanon E.Y., Amariglio N., Heimberger A.B., Ram Z., Constantini S., Barbash Z.S., Adamsky K., Safran M., Hirschberg A., et al. Altered Adenosine-to-Inosine RNA Editing in Human Cancer. Genome Res. 2007;17:1586–1595. doi: 10.1101/gr.6493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang W., Chendrimada T.P., Wang Q., Higuchi M., Seeburg P.H., Shiekhattar R., Nishikura K. Modulation of MicroRNA Processing and Expression through RNA Editing by ADAR Deaminases. Nat. Struct. Mol. Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishiguro S., Galipon J., Ishii R., Suzuki Y., Kondo S., Okada-Hatakeyama M., Tomita M., Ui-Tei K. Base-Pairing Probability in the MicroRNA Stem Region Affects the Binding and Editing Specificity of Human A-to-I Editing Enzymes ADAR1-P110 and ADAR2. RNA Biol. 2018;15:976–989. doi: 10.1080/15476286.2018.1486658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu X., Fu Y., Huang J., Wu M., Zhang Z., Xu R., Zhang P., Zhao S., Liu L., Jiang H. ADAR1 Promotes the Epithelial-to-Mesenchymal Transition and Stem-like Cell Phenotype of Oral Cancer by Facilitating Oncogenic MicroRNA Maturation. J. Exp. Clin. Cancer Res. 2019;38:315. doi: 10.1186/s13046-019-1300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yujie Ding M.M., Shi X., Ji J., Su Y. ADAR1p150 Regulates the Biosynthesis and Function of MiRNA-149* in Human Melanoma. Biochem. Biophys. Res. Commun. 2020;523:900–907. doi: 10.1016/j.bbrc.2019.12.110. [DOI] [PubMed] [Google Scholar]
- 114.Iizasa H., Wulff B.-E., Alla N.R., Maragkakis M., Megraw M., Hatzigeorgiou A., Iwakiri D., Takada K., Wiedmer A., Showe L., et al. Editing of Epstein-Barr Virus-Encoded BART6 MicroRNAs Controls Their Dicer Targeting and Consequently Affects Viral Latency. J. Biol. Chem. 2010;285:33358–33370. doi: 10.1074/jbc.M110.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heale B.S.E., Keegan L.P., McGurk L., Michlewski G., Brindle J., Stanton C.M., Caceres J.F., O’Connell M.A. Editing Independent Effects of ADARs on the MiRNA/SiRNA Pathways. EMBO J. 2009;28:3145–3156. doi: 10.1038/emboj.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ota H., Sakurai M., Gupta R., Valente L., Wulff B.-E., Ariyoshi K., Iizasa H., Davuluri R.V., Nishikura K. ADAR1 Forms a Complex with Dicer to Promote MicroRNA Processing and RNA-Induced Gene Silencing. Cell. 2013;153:575–589. doi: 10.1016/j.cell.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vesely C., Tauber S., Sedlazeck F.J., Tajaddod M., von Haeseler A., Jantsch M.F. ADAR2 Induces Reproducible Changes in Sequence and Abundance of Mature MicroRNAs in the Mouse Brain. Nucleic Acids Res. 2014;42:12155–12168. doi: 10.1093/nar/gku844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomaselli S., Galeano F., Alon S., Raho S., Galardi S., Polito V.A., Presutti C., Vincenti S., Eisenberg E., Locatelli F., et al. Modulation of MicroRNA Editing, Expression and Processing by ADAR2 Deaminase in Glioblastoma. Genome Biol. 2015;16:5. doi: 10.1186/s13059-014-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuang L., Lv G., Wang B., Li L., Dai Y., Li Y. Overexpression of Adenosine Deaminase Acting on RNA 1 in Chordoma Tissues Is Associated with Chordoma Pathogenesis by Reducing MiR-125a and MiR-10a Expression. Mol. Med. Rep. 2015;12:93–98. doi: 10.3892/mmr.2015.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zipeto M.A., Court A.C., Sadarangani A., Delos Santos N.P., Balaian L., Chun H.-J., Pineda G., Morris S.R., Mason C.N., Geron I., et al. ADAR1 Activation Drives Leukemia Stem Cell Self-Renewal by Impairing Let-7 Biogenesis. Cell Stem Cell. 2016;19:177–191. doi: 10.1016/j.stem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kume H., Hino K., Galipon J., Ui-Tei K. A-to-I Editing in the MiRNA Seed Region Regulates Target MRNA Selection and Silencing Efficiency. Nucleic Acids Res. 2014;42:10050–10060. doi: 10.1093/nar/gku662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y., Liang H. When MicroRNAs Meet RNA Editing in Cancer: A Nucleotide Change Can Make a Difference. BioEssays News Rev. Mol. Cell. Dev. Biol. 2018;40 doi: 10.1002/bies.201700188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kawahara Y., Zinshteyn B., Sethupathy P., Iizasa H., Hatzigeorgiou A.G., Nishikura K. Redirection of Silencing Targets by Adenosine-to-Inosine Editing of MiRNAs. Science. 2007;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kawahara Y., Megraw M., Kreider E., Iizasa H., Valente L., Hatzigeorgiou A.G., Nishikura K. Frequency and Fate of MicroRNA Editing in Human Brain. Nucleic Acids Res. 2008;36:5270–5280. doi: 10.1093/nar/gkn479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choudhury Y., Tay F.C., Lam D.H., Sandanaraj E., Tang C., Ang B.-T., Wang S. Attenuated Adenosine-to-Inosine Editing of MicroRNA-376a* Promotes Invasiveness of Glioblastoma Cells. J. Clin. Investig. 2012;122:4059–4076. doi: 10.1172/JCI62925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paul D., Sinha A.N., Ray A., Lal M., Nayak S., Sharma A., Mehani B., Mukherjee D., Laddha S.V., Suri A., et al. A-to-I Editing in Human MiRNAs Is Enriched in Seed Sequence, Influenced by Sequence Contexts and Significantly Hypoedited in Glioblastoma Multiforme. Sci. Rep. 2017;7:2466. doi: 10.1038/s41598-017-02397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shoshan E., Mobley A.K., Braeuer R.R., Kamiya T., Huang L., Vasquez M.E., Salameh A., Lee H.J., Kim S.J., Ivan C., et al. Reduced Adenosine-to-Inosine MiR-455-5p Editing Promotes Melanoma Growth and Metastasis. Nat. Cell Biol. 2015;17:311–321. doi: 10.1038/ncb3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Velazquez-Torres G., Shoshan E., Ivan C., Huang L., Fuentes-Mattei E., Paret H., Kim S.J., Rodriguez-Aguayo C., Xie V., Brooks D., et al. A-to-I MiR-378a-3p Editing Can Prevent Melanoma Progression via Regulation of PARVA Expression. Nat. Commun. 2018;9:461. doi: 10.1038/s41467-018-02851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cesarini V., Silvestris D.A., Tassinari V., Tomaselli S., Alon S., Eisenberg E., Locatelli F., Gallo A. ADAR2/MiR-589-3p Axis Controls Glioblastoma Cell Migration/Invasion. Nucleic Acids Res. 2018;46:2045–2059. doi: 10.1093/nar/gkx1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Xu X., Yu S., Jeong K.J., Zhou Z., Han L., Tsang Y.H., Li J., Chen H., Mangala L.S., et al. Systematic Characterization of A-to-I RNA Editing Hotspots in MicroRNAs across Human Cancers. Genome Res. 2017;27:1112–1125. doi: 10.1101/gr.219741.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ramírez-Moya J., Baker A.R., Slack F.J., Santisteban P. ADAR1-Mediated RNA Editing Is a Novel Oncogenic Process in Thyroid Cancer and Regulates MiR-200 Activity. Oncogene. 2020;39:3738–3753. doi: 10.1038/s41388-020-1248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Formosa A., Markert E.K., Lena A.M., Italiano D., Finazzi-Agro’ E., Levine A.J., Bernardini S., Garabadgiu A.V., Melino G., Candi E. MicroRNAs, MiR-154, MiR-299-5p, MiR-376a, MiR-376c, MiR-377, MiR-381, MiR-487b, MiR-485-3p, MiR-495 and MiR-654-3p, Mapped to the 14q32.31 Locus, Regulate Proliferation, Apoptosis, Migration and Invasion in Metastatic Prostate Cancer Cells. Oncogene. 2014;33:5173–5182. doi: 10.1038/onc.2013.451. [DOI] [PubMed] [Google Scholar]
- 133.Wang Z., Yang J., Xu G., Wang W., Liu C., Yang H., Yu Z., Lei Q., Xiao L., Xiong J., et al. Targeting MiR-381-NEFL Axis Sensitizes Glioblastoma Cells to Temozolomide by Regulating Stemness Factors and Multidrug Resistance Factors. Oncotarget. 2015;6:3147–3164. doi: 10.18632/oncotarget.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anadón C., Guil S., Simó-Riudalbas L., Moutinho C., Setien F., Martínez-Cardús A., Moran S., Villanueva A., Calaf M., Vidal A., et al. Gene Amplification-Associated Overexpression of the RNA Editing Enzyme ADAR1 Enhances Human Lung Tumorigenesis. Oncogene. 2016;35:4407–4413. doi: 10.1038/onc.2015.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gong J., Wu Y., Zhang X., Liao Y., Sibanda V.L., Liu W., Guo A.-Y. Comprehensive Analysis of Human Small RNA Sequencing Data Provides Insights into Expression Profiles and MiRNA Editing. RNA Biol. 2014;11:1375–1385. doi: 10.1080/15476286.2014.996465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng Y., Li T., Ren R., Shi D., Wang S. Revealing Editing and SNPs of MicroRNAs in Colon Tissues by Analyzing High-Throughput Sequencing Profiles of Small RNAs. BMC Genom. 2014;15:S11. doi: 10.1186/1471-2164-15-S9-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pinto Y., Buchumenski I., Levanon E.Y., Eisenberg E. Human Cancer Tissues Exhibit Reduced A-to-I Editing of MiRNAs Coupled with Elevated Editing of Their Targets. Nucleic Acids Res. 2018;46:71–82. doi: 10.1093/nar/gkx1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Behroozi J., Shahbazi S., Bakhtiarizadeh M.R., Mahmoodzadeh H. Genome-Wide Characterization of RNA Editing Sites in Primary Gastric Adenocarcinoma through RNA-Seq Data Analysis. Int. J. Genom. 2020;2020:#6493963. doi: 10.1155/2020/6493963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen J., Wang L., Wang F., Liu J., Bai Z. Genomic Identification of RNA Editing Through Integrating Omics Datasets and the Clinical Relevance in Hepatocellular Carcinoma. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Amos H., Korn M. 5-Methyl Cytosine in the RNA of Escherichia Coli. Biochim. Biophys. Acta. 1958;29:444–445. doi: 10.1016/0006-3002(58)90214-2. [DOI] [PubMed] [Google Scholar]
- 141.Dunn D.B. The Isolation of 5-Methylcytidine from RNA. Biochim. Biophys. Acta. 1960;38:176–178. doi: 10.1016/0006-3002(60)91219-1. [DOI] [PubMed] [Google Scholar]
- 142.Motorin Y., Lyko F., Helm M. 5-Methylcytosine in RNA: Detection, Enzymatic Formation and Biological Functions. Nucleic Acids Res. 2010;38:1415–1430. doi: 10.1093/nar/gkp1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dubin D.T., Taylor R.H. The Methylation State of Poly A-Containing-Messenger RNA from Cultured Hamster Cells. Nucleic Acids Res. 1975;2:1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amort T., Soulière M.F., Wille A., Jia X.-Y., Fiegl H., Wörle H., Micura R., Lusser A. Long Non-Coding RNAs as Targets for Cytosine Methylation. RNA Biol. 2013;10:1002–1008. doi: 10.4161/rna.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aguilo F., Li S., Balasubramaniyan N., Sancho A., Benko S., Zhang F., Vashisht A., Rengasamy M., Andino B., Chen C., et al. Deposition of 5-Methylcytosine on Enhancer RNAs Enables the Coactivator Function of PGC-1α. Cell Rep. 2016;14:479–492. doi: 10.1016/j.celrep.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.David R., Burgess A., Parker B., Li J., Pulsford K., Sibbritt T., Preiss T., Searle I.R. Transcriptome-Wide Mapping of RNA 5-Methylcytosine in Arabidopsis MRNAs and Noncoding RNAs. Plant. Cell. 2017;29:445–460. doi: 10.1105/tpc.16.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reid R., Greene P.J., Santi D.V. Exposition of a Family of RNA m(5)C Methyltransferases from Searching Genomic and Proteomic Sequences. Nucleic Acids Res. 1999;27:3138–3145. doi: 10.1093/nar/27.15.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goll M.G., Kirpekar F., Maggert K.A., Yoder J.A., Hsieh C.-L., Zhang X., Golic K.G., Jacobsen S.E., Bestor T.H. Methylation of TRNAAsp by the DNA Methyltransferase Homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 149.Bourgeois G., Ney M., Gaspar I., Aigueperse C., Schaefer M., Kellner S., Helm M., Motorin Y. Eukaryotic RRNA Modification by Yeast 5-Methylcytosine-Methyltransferases and Human Proliferation-Associated Antigen P120. PLoS ONE. 2015;10:e0133321. doi: 10.1371/journal.pone.0133321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tuorto F., Liebers R., Musch T., Schaefer M., Hofmann S., Kellner S., Frye M., Helm M., Stoecklin G., Lyko F. RNA Cytosine Methylation by Dnmt2 and NSun2 Promotes TRNA Stability and Protein Synthesis. Nat. Struct. Mol. Biol. 2012;19:900–905. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 151.Schosserer M., Minois N., Angerer T.B., Amring M., Dellago H., Harreither E., Calle-Perez A., Pircher A., Gerstl M.P., Pfeifenberger S., et al. Methylation of Ribosomal RNA by NSUN5 Is a Conserved Mechanism Modulating Organismal Lifespan. Nat. Commun. 2015;6:6158. doi: 10.1038/ncomms7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu R.-J., Long T., Li J., Li H., Wang E.-D. Structural Basis for Substrate Binding and Catalytic Mechanism of a Human RNA:M5C Methyltransferase NSun6. Nucleic Acids Res. 2017;45:6684–6697. doi: 10.1093/nar/gkx473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M., Lyko F. RNA Methylation by Dnmt2 Protects Transfer RNAs against Stress-Induced Cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xing J., Yi J., Cai X., Tang H., Liu Z., Zhang X., Martindale J.L., Yang X., Jiang B., Gorospe M., et al. NSun2 Promotes Cell Growth via Elevating Cyclin-Dependent Kinase 1 Translation. Mol. Cell. Biol. 2015;35:4043–4052. doi: 10.1128/MCB.00742-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tang H., Fan X., Xing J., Liu Z., Jiang B., Dou Y., Gorospe M., Wang W. NSun2 Delays Replicative Senescence by Repressing P27 (KIP1) Translation and Elevating CDK1 Translation. Aging. 2015;7:1143–1155. doi: 10.18632/aging.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Q., Li X., Tang H., Jiang B., Dou Y., Gorospe M., Wang W. NSUN2-Mediated M5C Methylation and METTL3/METTL14-Mediated M6A Methylation Cooperatively Enhance P21 Translation. J. Cell. Biochem. 2017;118:2587–2598. doi: 10.1002/jcb.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun Z., Xue S., Zhang M., Xu H., Hu X., Chen S., Liu Y., Guo M., Cui H. Aberrant NSUN2-Mediated m 5 C Modification of H19 LncRNA Is Associated with Poor Differentiation of Hepatocellular Carcinoma. Oncogene. 2020;39:6906–6919. doi: 10.1038/s41388-020-01475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hussain S., Sajini A.A., Blanco S., Dietmann S., Lombard P., Sugimoto Y., Paramor M., Gleeson J.G., Odom D.T., Ule J., et al. NSun2-Mediated Cytosine-5 Methylation of Vault Noncoding RNA Determines Its Processing into Regulatory Small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Van Haute L., Dietmann S., Kremer L., Hussain S., Pearce S.F., Powell C.A., Rorbach J., Lantaff R., Blanco S., Sauer S., et al. Deficient Methylation and Formylation of Mt-TRNA Met Wobble Cytosine in a Patient Carrying Mutations in NSUN3. Nat. Commun. 2016;7:12039. doi: 10.1038/ncomms12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Metodiev M.D., Spåhr H., Polosa P.L., Meharg C., Becker C., Altmueller J., Habermann B., Larsson N.-G., Ruzzenente B. NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S RRNA and Coordination of Mitoribosomal Assembly. PLoS Genet. 2014;10:e1004110. doi: 10.1371/journal.pgen.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang Y., Wang L., Han X., Yang W.-L., Zhang M., Ma H.-L., Sun B.-F., Li A., Xia J., Chen J., et al. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal MRNA Decay. Mol. Cell. 2019;75:1188–1202.e11. doi: 10.1016/j.molcel.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 162.Chen X., Li A., Sun B.-F., Yang Y., Han Y.-N., Yuan X., Chen R.-X., Wei W.-S., Liu Y., Gao C.-C., et al. 5-Methylcytosine Promotes Pathogenesis of Bladder Cancer through Stabilizing MRNAs. Nat. Cell Biol. 2019;21:978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 163.Flores J.V., Cordero-Espinoza L., Oeztuerk-Winder F., Andersson-Rolf A., Selmi T., Blanco S., Tailor J., Dietmann S., Frye M. Cytosine-5 RNA Methylation Regulates Neural Stem Cell Differentiation and Motility. Stem Cell Rep. 2016;8:112–124. doi: 10.1016/j.stemcr.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trixl L., Amort T., Wille A., Zinni M., Ebner S., Hechenberger C., Eichin F., Gabriel H., Schoberleitner I., Huang A., et al. RNA Cytosine Methyltransferase Nsun3 Regulates Embryonic Stem Cell Differentiation by Promoting Mitochondrial Activity. Cell. Mol. Life Sci. CMLS. 2018;75:1483–1497. doi: 10.1007/s00018-017-2700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang W. MRNA Methylation by NSUN2 in Cell Proliferation. Wiley Interdiscip. Rev. RNA. 2016;7:838–842. doi: 10.1002/wrna.1380. [DOI] [PubMed] [Google Scholar]
- 166.Bi J., Huang Y., Liu Y. Effect of NOP2 Knockdown on Colon Cancer Cell Proliferation, Migration, and Invasion. Transl. Cancer Res. 2019;8 doi: 10.21037/tcr.2019.09.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hong J., Lee J.H., Chung I.K. Telomerase Activates Transcription of Cyclin D1 Gene through an Interaction with NOL1. J. Cell Sci. 2016;129:1566–1579. doi: 10.1242/jcs.181040. [DOI] [PubMed] [Google Scholar]
- 168.Chellamuthu A., Gray S.G. The RNA Methyltransferase NSUN2 and Its Potential Roles in Cancer. Cells. 2020;9:1758. doi: 10.3390/cells9081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Y., Zhang X., Shi J., Tuorto F., Li X., Liu Y., Liebers R., Zhang L., Qu Y., Qian J., et al. Dnmt2 Mediates Intergenerational Transmission of Paternally Acquired Metabolic Disorders through Sperm Small Non-Coding RNAs. Nat. Cell Biol. 2018;20:535–540. doi: 10.1038/s41556-018-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cheray M., Etcheverry A., Jacques C., Pacaud R., Bougras-Cartron G., Aubry M., Denoual F., Peterlongo P., Nadaradjane A., Briand J., et al. Cytosine Methylation of Mature MicroRNAs Inhibits Their Functions and Is Associated with Poor Prognosis in Glioblastoma Multiforme. Mol. Cancer. 2020;19:36. doi: 10.1186/s12943-020-01155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carissimi C., Laudadio I., Lorefice E., Azzalin G., De Paolis V., Fulci V. Bisulfite MiRNA-Seq Reveals Widespread CpG and Non-CpG 5-(Hydroxy)Methyl-Cytosine in Human MicroRNAs. RNA Biol. 2021 doi: 10.1080/15476286.2021.1927423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sajini A.A., Choudhury N.R., Wagner R.E., Bornelöv S., Selmi T., Spanos C., Dietmann S., Rappsilber J., Michlewski G., Frye M. Loss of 5-Methylcytosine Alters the Biogenesis of Vault-Derived Small RNAs to Coordinate Epidermal Differentiation. Nat. Commun. 2019;10:2550. doi: 10.1038/s41467-019-10020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Barbieri I., Tzelepis K., Pandolfini L., Shi J., Millán-Zambrano G., Robson S.C., Aspris D., Migliori V., Bannister A.J., Han N., et al. Promoter-Bound METTL3 Maintains Myeloid Leukaemia by M6A-Dependent Translation Control. Nature. 2017;552:126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rouleau S.G., Garant J.-M., Bolduc F., Bisaillon M., Perreault J.-P. G-Quadruplexes Influence Pri-MicroRNA Processing. RNA Biol. 2018;15:198–206. doi: 10.1080/15476286.2017.1405211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang H.-Y., Xiong J., Qi B.-L., Feng Y.-Q., Yuan B.-F. The Existence of 5-Hydroxymethylcytosine and 5-Formylcytosine in Both DNA and RNA in Mammals. Chem. Commun. Camb. Engl. 2016;52:737–740. doi: 10.1039/C5CC07354E. [DOI] [PubMed] [Google Scholar]
- 176.Huang W., Lan M.-D., Qi C.-B., Zheng S.-J., Wei S.-Z., Yuan B.-F., Feng Y.-Q. Formation and Determination of the Oxidation Products of 5-Methylcytosine in RNA. Chem. Sci. 2016;7:5495–5502. doi: 10.1039/C6SC01589A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huber S.M., van Delft P., Mendil L., Bachman M., Smollett K., Werner F., Miska E.A., Balasubramanian S. Formation and Abundance of 5-Hydroxymethylcytosine in RNA. Chembiochem Eur. J. Chem. Biol. 2015;16:752–755. doi: 10.1002/cbic.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu X., Zhang Y. TET-Mediated Active DNA Demethylation: Mechanism, Function and Beyond. Nat. Rev. Genet. 2017;18:517–534. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- 179.Fu L., Guerrero C.R., Zhong N., Amato N.J., Liu Y., Liu S., Cai Q., Ji D., Jin S.-G., Niedernhofer L.J., et al. Tet-Mediated Formation of 5-Hydroxymethylcytosine in RNA. J. Am. Chem. Soc. 2014;136:11582–11585. doi: 10.1021/ja505305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lan J., Rajan N., Bizet M., Penning A., Singh N.K., Guallar D., Calonne E., Li Greci A., Bonvin E., Deplus R., et al. Functional Role of Tet-Mediated RNA Hydroxymethylcytosine in Mouse ES Cells and during Differentiation. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Delatte B., Wang F., Ngoc L.V., Collignon E., Bonvin E., Deplus R., Calonne E., Hassabi B., Putmans P., Awe S., et al. Transcriptome-Wide Distribution and Function of RNA Hydroxymethylcytosine. Science. 2016;351:282–285. doi: 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- 182.Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A.J. Reovirus Messenger RNA Contains a Methylated, Blocked 5′-Terminal Structure: M-7G(5′)Ppp(5′)G-MpCp- Proc. Natl. Acad. Sci. USA. 1975;72:362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Furuichi Y., LaFiandra A., Shatkin A.J. 5′-Terminal Structure and MRNA Stability. Nature. 1977;266:235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- 184.Pei Y., Shuman S. Interactions between Fission Yeast MRNA Capping Enzymes and Elongation Factor Spt5. J. Biol. Chem. 2002;277:19639–19648. doi: 10.1074/jbc.M200015200. [DOI] [PubMed] [Google Scholar]
- 185.Muthukrishnan S., Both G.W., Furuichi Y., Shatkin A.J. 5′-Terminal 7-Methylguanosine in Eukaryotic MRNA Is Required for Translation. Nature. 1975;255:33–37. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- 186.Wallace R.B., Aujame L., Freeman K.B. Chemical and Physical Properties of Mammalian Mitochondrial Aminoacyl-Transfer RNAs II. Analysis of 7-Methylguanosine in Mitochondrial and Cytosolic Aminoacyl-Transfer RNAs. Biochim. Biophys. Acta BBA Nucleic Acids Protein Synth. 1978;518:321–325. doi: 10.1016/0005-2787(78)90188-0. [DOI] [PubMed] [Google Scholar]
- 187.Choi Y.C., Busch H. Modified Nucleotides in T1 RNase Oligonucleotides of 18S Ribosomal RNA of the Novikoff Hepatoma. Biochemistry. 1978;17:2551–2560. doi: 10.1021/bi00606a015. [DOI] [PubMed] [Google Scholar]
- 188.Guy M.P., Phizicky E.M. Two-Subunit Enzymes Involved in Eukaryotic Post-Transcriptional TRNA Modification. RNA Biol. 2015;11:1608–1618. doi: 10.1080/15476286.2015.1008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang L.-S., Liu C., Ma H., Dai Q., Sun H.-L., Luo G., Zhang Z.S., Zhang L., Hu L., Dong X., et al. Transcriptome-Wide Mapping of Internal N7-Methylguanosine Methylome in Mammalian Messenger RNA. Mol. Cell. 2019;74:1304–1316.e8. doi: 10.1016/j.molcel.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Alexandrov A., Martzen M.R., Phizicky E.M. Two Proteins That Form a Complex Are Required for 7-Methylguanosine Modification of Yeast TRNA. RNA. 2002;8:1253–1266. doi: 10.1017/S1355838202024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tian Q.-H., Zhang M.-F., Zeng J.-S., Luo R.-G., Wen Y., Chen J., Gan L.-G., Xiong J.-P. METTL1 Overexpression Is Correlated with Poor Prognosis and Promotes Hepatocellular Carcinoma via PTEN. J. Mol. Med. Berl. Ger. 2019;97:1535–1545. doi: 10.1007/s00109-019-01830-9. [DOI] [PubMed] [Google Scholar]
- 192.Pandolfini L., Barbieri I., Bannister A.J., Hendrick A., Andrews B., Webster N., Murat P., Mach P., Brandi R., Robson S.C., et al. METTL1 Promotes Let-7 MicroRNA Processing via M7G Methylation. Mol. Cell. 2019;74:1278–1290.e9. doi: 10.1016/j.molcel.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mirihana Arachchilage G., Dassanayake A.C., Basu S. A Potassium Ion-Dependent RNA Structural Switch Regulates Human Pre-MiRNA 92b Maturation. Chem. Biol. 2015;22:262–272. doi: 10.1016/j.chembiol.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 194.Pandey S., Agarwala P., Jayaraj G.G., Gargallo R., Maiti S. The RNA Stem-Loop to G-Quadruplex Equilibrium Controls Mature MicroRNA Production inside the Cell. Biochemistry. 2015;54:7067–7078. doi: 10.1021/acs.biochem.5b00574. [DOI] [PubMed] [Google Scholar]
- 195.Liu Y., Zhang Y., Chi Q., Wang Z., Sun B. Methyltransferase-like 1 (METTL1) Served as a Tumor Suppressor in Colon Cancer by Activating 7-Methyguanosine (M7G) Regulated Let-7e MiRNA/HMGA2 Axis. Life Sci. 2020;249:117480. doi: 10.1016/j.lfs.2020.117480. [DOI] [PubMed] [Google Scholar]
- 196.Das Mandal S., Ray P.S. Transcriptome-Wide Analysis Reveals Spatial Correlation between N6-Methyladenosine and Binding Sites of MicroRNAs and RNA-Binding Proteins. Genomics. 2021;113:205–216. doi: 10.1016/j.ygeno.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 197.Zhang X., Xu Y., Qian Z., Zheng W., Wu Q., Chen Y., Zhu G., Liu Y., Bian Z., Xu W., et al. CircRNA_104075 Stimulates YAP-Dependent Tumorigenesis through the Regulation of HNF4a and May Serve as a Diagnostic Marker in Hepatocellular Carcinoma. Cell Death Dis. 2018;9:1091. doi: 10.1038/s41419-018-1132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Müller S., Glaß M., Singh A.K., Haase J., Bley N., Fuchs T., Lederer M., Dahl A., Huang H., Chen J., et al. IGF2BP1 Promotes SRF-Dependent Transcription in Cancer in a M6A- and MiRNA-Dependent Manner. Nucleic Acids Res. 2019;47:375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Visvanathan A., Patil V., Abdulla S., Hoheisel J.D., Somasundaram K. N6-Methyladenosine Landscape of Glioma Stem-Like Cells: METTL3 Is Essential for the Expression of Actively Transcribed Genes and Sustenance of the Oncogenic Signaling. Genes. 2019;10:141. doi: 10.3390/genes10020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cheng J., Xu L., Deng L., Xue L., Meng Q., Wei F., Wang J. RNA N6-Methyladenosine Modification Is Required for MiR-98/MYCN Axis-Mediated Inhibition of Neuroblastoma Progression. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-64682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang D., Qiao J., Wang G., Lan Y., Li G., Guo X., Xi J., Ye D., Zhu S., Chen W., et al. N6-Methyladenosine Modification of LincRNA 1281 Is Critically Required for MESC Differentiation Potential. Nucleic Acids Res. 2018;46:3906–3920. doi: 10.1093/nar/gky130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zheng Z.-Q., Li Z.-X., Zhou G.-Q., Lin L., Zhang L.-L., Lv J.-W., Huang X.-D., Liu R.-Q., Chen F., He X.-J., et al. Long Noncoding RNA FAM225A Promotes Nasopharyngeal Carcinoma Tumorigenesis and Metastasis by Acting as CeRNA to Sponge MiR-590-3p/MiR-1275 and Upregulate ITGB3. Cancer Res. 2019;79:4612–4626. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- 203.Zuo X., Chen Z., Gao W., Zhang Y., Wang J., Wang J., Cao M., Cai J., Wu J., Wang X. M6A-Mediated Upregulation of LINC00958 Increases Lipogenesis and Acts as a Nanotherapeutic Target in Hepatocellular Carcinoma. J. Hematol. Oncol. J. Hematol. Oncol. 2020;13:5. doi: 10.1186/s13045-019-0839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang Q., Hui H., Guo Z., Zhang W., Hu Y., He T., Tai Y., Peng P., Wang L. ADAR1 Regulates ARHGAP26 Gene Expression through RNA Editing by Disrupting MiR-30b-3p and MiR-573 Binding. RNA. 2013;19:1525–1536. doi: 10.1261/rna.041533.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nakano M., Fukami T., Gotoh S., Takamiya M., Aoki Y., Nakajima M. RNA Editing Modulates Human Hepatic Aryl Hydrocarbon Receptor Expression by Creating MicroRNA Recognition Sequence. J. Biol. Chem. 2016;291:894–903. doi: 10.1074/jbc.M115.699363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cho C.J., Jung J., Jiang L., Lee E.J., Kim D.-S., Kim B.S., Kim H.S., Jung H.-Y., Song H.-J., Hwang S.W., et al. Combinatory RNA-Sequencing Analyses Reveal a Dual Mode of Gene Regulation by ADAR1 in Gastric Cancer. Dig. Dis. Sci. 2018;63:1835–1850. doi: 10.1007/s10620-018-5081-9. [DOI] [PubMed] [Google Scholar]
- 207.Zhang L., Yang C.-S., Varelas X., Monti S. Altered RNA Editing in 3′ UTR Perturbs MicroRNA-Mediated Regulation of Oncogenes and Tumor-Suppressors. Sci. Rep. 2016;6:23226. doi: 10.1038/srep23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Brümmer A., Yang Y., Chan T.W., Xiao X. Structure-Mediated Modulation of MRNA Abundance by A-to-I Editing. Nat. Commun. 2017;8:1255. doi: 10.1038/s41467-017-01459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Squires J.E., Patel H.R., Nousch M., Sibbritt T., Humphreys D.T., Parker B.J., Suter C.M., Preiss T. Widespread Occurrence of 5-Methylcytosine in Human Coding and Non-Coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fong M.Y., Zhou W., Liu L., Alontaga A.Y., Chandra M., Ashby J., Chow A., O’Connor S.T.F., Li S., Chin A.R., et al. Breast-Cancer-Secreted MiR-122 Reprograms Glucose Metabolism in Premetastatic Niche to Promote Metastasis. Nat. Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu R., Rai A., Chen M., Suwakulsiri W., Greening D.W., Simpson R.J. Extracellular Vesicles in Cancer—Implications for Future Improvements in Cancer Care. Nat. Rev. Clin. Oncol. 2018;15:617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 212.Suzuki T., Ueda H., Okada S., Sakurai M. Transcriptome-Wide Identification of Adenosine-to-Inosine Editing Using the ICE-Seq Method. Nat. Protoc. 2015;10:715–732. doi: 10.1038/nprot.2015.037. [DOI] [PubMed] [Google Scholar]
- 213.Hauenschild R., Tserovski L., Schmid K., Thüring K., Winz M.-L., Sharma S., Entian K.-D., Wacheul L., Lafontaine D.L.J., Anderson J., et al. The Reverse Transcription Signature of N-1-Methyladenosine in RNA-Seq Is Sequence Dependent. Nucleic Acids Res. 2015;43:9950–9964. doi: 10.1093/nar/gkv895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao L., Zhang H., Kohnen M.V., Prasad K.V.S.K., Gu L., Reddy A.S.N. Analysis of Transcriptome and Epitranscriptome in Plants Using PacBio Iso-Seq and Nanopore-Based Direct RNA Sequencing. Front. Genet. 2019;10:253. doi: 10.3389/fgene.2019.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]