Objective:
Efficacy and safety of long-acting cabotegravir (CAB) and rilpivirine (RPV) dosed intramuscularly every 4 or 8 weeks has been demonstrated in three Phase 3 trials. Here, factors associated with virologic failure at Week 48 were evaluated post hoc.
Design and methods:
Data from 1039 adults naive to long-acting CAB+RPV were pooled in a multivariable analysis to examine the influence of baseline viral and participant factors, dosing regimen and drug concentrations on confirmed virologic failure (CVF) occurrence using a logistic regression model. In a separate model, baseline factors statistically associated with CVF were further evaluated to understand CVF risk when present alone or in combination.
Results:
Overall, 1.25% (n = 13/1039) of participants experienced CVF. Proviral RPV resistance-associated mutations (RAMs), HIV-1 subtype A6/A1, higher BMI (associated with Week 8 CAB trough concentration) and lower Week 8 RPV trough concentrations were significantly associated (P < 0.05) with increased odds of CVF (all except RPV trough are knowable at baseline). Few participants (0.4%) with zero or one baseline factor had CVF. Only a combination of at least two baseline factors (observed in 3.4%; n = 35/1039) was associated with increased CVF risk (25.7%, n = 9/35).
Conclusion:
CVF is an infrequent multifactorial event, with a rate of approximately 1% in the long-acting CAB+RPV arms across Phase 3 studies (FLAIR, ATLAS and ATLAS-2M) through Week 48. Presence of at least two of proviral RPV RAMs, HIV-1 subtype A6/A1 and/or BMI at least 30 kg/m2 was associated with increased CVF risk. These findings support the use of long-acting CAB+RPV in routine clinical practice.
Keywords: antiretroviral therapy, cabotegravir, HIV, long-acting, multivariable analysis, rilpivirine, virologic response
Introduction
Current antiretroviral therapy (ART) consists of a combination of two or more oral agents from at least two drug classes, such as an integrase strand transfer inhibitor (INSTI) as well as one or two nucleoside reverse transcriptase inhibitors (NRTIs) [1,2]. Cabotegravir (CAB), an INSTI, and rilpivirine (RPV), a non-NRTI (NNRTI), have been developed as the first long-acting, injectable, two-drug ART regimen administered intramuscularly monthly or every 2 months for the maintenance of virologic suppression in people living with HIV-1 [3,4].
Long-acting CAB+RPV is indicated for the treatment of HIV-1 infection in virologically suppressed adults (HIV-1 RNA <50 copies/ml), per positive results from three Phase 3/3b studies. The Phase 3 FLAIR (NCT02938520) and ATLAS (NCT02951052) studies [3,4] demonstrated that long-acting CAB+RPV dosed every 4 weeks (Q4W) was noninferior to daily oral ART in maintaining virologic suppression in stably suppressed participants through 48 weeks; noninferiority was established within each study and in a pooled analysis [5]. In addition, long-acting CAB+RPV dosed every 8 weeks (Q8W) demonstrated noninferior efficacy to Q4W dosing with a similar safety profile in the Phase 3b ATLAS-2M (NCT03299049) study [6]. In the FLAIR, ATLAS and ATLAS-2M studies [3,4,6], confirmed virologic failure (CVF; two consecutive plasma HIV-1 RNA measurements ≥200 copies/ml) was rare, occurring in 1% (n = 17/1636) of participants in the long-acting CAB+RPV arms combined. Similar CVF rates were observed among participants who continued daily oral therapy in FLAIR and ATLAS, with three out of 283 (1.1%) and four out of 308 (1.3%) participants meeting the CVF criterion, respectively.
Identifying the factors associated with virologic outcome is important to holistically understand any ART regimen and to assist healthcare professionals/prescribers regarding patient selection. Although few participants experienced CVF across the long-acting CAB+RPV arms of the Phase 3 studies, it is important to identify factors that may have predisposed this minority of participants to CVF. This information will help inform clinicians and patients, allowing them to assess the potential benefits and risks of this novel long-acting therapy. Early analyses in the individual studies sought to identify any participant, viral or pharmacokinetic factors that may warrant further investigation in relation to virologic outcome. One factor that appeared initially to be associated with CVF was the L74I integrase polymorphism [3–5]; however, its role in virologic outcome was unclear.
The small number of participants who experienced CVF in the three studies prevented the drawing of meaningful conclusions when the trials were analysed individually. Therefore, a post-hoc multivariable analysis was performed using pooled data from long-acting CAB+RPV participants in FLAIR, ATLAS and ATLAS-2M to explore potential drug (pharmacokinetic and dosing regimen), viral and participant factors predictive of Week 48 virologic failure.
Materials and methods
Study population
Data from participants randomized to long-acting CAB+RPV Q4W or Q8W dosing within the Phase 3/3b FLAIR, ATLAS or ATLAS-2M (subset naive to CAB+RPV at entry) studies through Week 48 were pooled in a post-hoc analysis. FLAIR, ATLAS and ATLAS-2M are randomized, multicentre, parallel-group, open-label, noninferiority Phase 3/3b studies evaluating long-acting CAB+RPV dosed Q4W vs. continuing standard of care (SOC) oral therapy (FLAIR and ATLAS) or long-acting CAB+RPV dosed Q8W vs. Q4W (ATLAS-2M). These studies were designed similarly and conducted in similar timeframes, facilitating the meaningful pooling of the data. The full study designs and eligibility criteria have been published previously (Figure, Supplemental Digital Content 1, study designs) [3,4,6]. Participants were at least 18 years of age and virologically suppressed (plasma HIV-1 RNA <50 copies/ml) without evidence of any major INSTI or NNRTI resistance-associated mutations (RAMs) (except K103N). Baseline characteristics have been presented previously and were broadly similar across the studies [3,4,6]. FLAIR participants were ART naive at study entry and underwent induction with a three-drug regimen for 20 weeks to achieve virologic suppression before initiating randomized therapy, while ATLAS and ATLAS-2M participants were ART experienced before study entry. ATLAS-2M included a proportion of participants (approximately half) who rolled over from the SOC or long-acting CAB+RPV Q4W arm of the ATLAS study. To obtain a consistent pooled study population with aligned pharmacokinetic parameters and time on therapy, participants in ATLAS-2M who rolled over from ATLAS with prior CAB+RPV exposure had only ATLAS data included in the current analysis. All studies were conducted in accordance with the Declaration of Helsinki [7]. All participants provided written informed consent, and the study protocols were approved by the investigational review board.
Factors explored for association with virologic failure
For each participant, data on the following 10 covariates were collected and evaluated as potential contributors to virologic failure based on demographics, virus, drug exposure or dosing regimen: L74I (excluding mixtures with L74M) integrase polymorphism at baseline, prespecified INSTI mutation [excluding L74I (excluding mixtures with L74M)] at baseline, RPV RAM(s) at baseline, NNRTI RAM(s) (excluding RPV RAMs) at baseline, HIV-1 subtype A6/A1, female sex at birth, BMI at baseline, dosing regimen and Week 8 CAB and RPV trough concentrations (population pharmacokinetic post-hoc estimates of concentrations 4 weeks following the first long-acting injections).
Full details of the methodology are presented in Supplemental Digital Content 2, resistance methodology. Prespecified RAMs used in this analysis are presented in Table, Supplemental Digital Content 3, resistance mutations.
Initial classification of viral subtypes was performed by Monogram Biosciences (PhenoSense GT or GenoSure Archive assays), except participants without CVF in FLAIR, which were classified by Q2 Solutions. Given the strong correlation between L74I and subtype A6 [8], further analysis was performed to extend the original A/A1 subtyping to include the A6 subtype based on the 20 June 2020 version of the Los Alamos National Laboratory library (research grade, HIV Sequence Database; http://www.hiv.lanl.gov/).
CAB and RPV trough concentrations at Week 8 (i.e. 4 weeks after the first injections) and CVF through Week 48 were evaluated by regimen (Q8W vs. Q4W) and BMI. BMI was included due to previous population pharmacokinetic analyses that indicated BMI values above at least 30 kg/m2 are associated with initially slower long-acting CAB absorption [9,10]. Pharmacokinetic plasma samples were analysed for CAB and RPV concentrations using liquid chromatography with tandem mass spectrometry methods, as presented previously [3,4].
Statistical analysis
To explore potential factors associated with CVF through Week 48, univariate descriptive and multivariable logistic regression analyses were conducted. In addition, a multivariable Cox regression analysis was performed to assess time to CVF with time-updated observed pharmacokinetic concentrations. Models were estimated using Firth's penalized likelihood approach. For the univariate analysis, each of the 10 prespecified factors were examined individually in relation to CVF outcome (yes/no). For the multivariable analysis, two models were utilized: full models containing all the prespecified covariates, and reduced models that were identified using a conventional backwards elimination variable selection algorithm to identify predictors of CVF. In the backwards elimination, the covariate with the largest P value was removed and the model was refitted and repeated with the remaining covariates until no covariate yielded a P value more than 0.20. Finally, a separate model exploring those factors present at baseline was fitted, applying a stepwise selection algorithm to further determine if CVF risk could be differentiated according to the presence of one, two, or three baseline factors. A significance level of 0.3 was required to allow a variable into the model, and a significance level of 0.05 was required for a variable to stay in the model. The maximum value of Youden's J statistic was used to identify an optimal cut-off value providing the best trade-off between sensitivity and specificity; for reporting purposes, this is presented by CVF rates according to zero/one/at least two factors (Figure, Supplemental Digital Content 4, analyses conducted).
The primary outcome of interest was the occurrence of CVF as a more clinically relevant indicator of treatment failure; however, virologic success (HIV-1 RNA <50 copies/ml at Week 48; per the FDA Snapshot algorithm) was also examined in the baseline factor model. In the Cox regression model, the same initial 10 factors were examined, except the original Monogram Biosciences subtype assignment was retained -- A, A1, AG combined vs. B, C vs. B and other vs. B -- and time-updated observed CAB and RPV trough concentrations were employed.
Results
Participants
Across the three studies, 17 out of 1636 (1.0%) participants met the criterion for CVF; three had prior CAB+RPV exposure before ATLAS-2M study entry and one had met the CVF criterion during oral lead-in. For the current analysis, in total, 1039 participants naive to CAB+RPV were included after excluding those with prior exposure (n = 391), those who never received a long-acting injection (n = 22) and those with a missing value for one or more of the covariates (n = 184). This resulted in a total CVF population of 13 of 1039 (1.3%) for this analysis (Figure, Supplemental Digital Content 5, participant disposition). The distribution of the 10 prespecified covariates among participants with CVF in the pooled analysis is summarized in Table 1.
Table 1.
Summary of covariates per participant.
| Study | IDa | CAB PKb ≤Q1 | RPV PKb ≤Q1 | HIV-1 subtype A6/A1 | Baseline IN L74Ic | Baseline INSTI mutationd | Baseline Proviral RPV RAMe | Baseline NNRTI RAMf | Female sex at birthg | BMI ≥30 kg/m2 | Q8W |
| ATLAS-2M | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| ATLAS-2M | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| ATLAS | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| ATLAS | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| FLAIR | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| FLAIR | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| FLAIR | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| ATLAS-2M | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| ATLAS-2M | 9 | ✓ | ✓ | ||||||||
| ATLAS | 10 | ✓ | ✓ | ✓ | |||||||
| ATLAS-2M | 11 | ✓ | ✓ | ✓ | ✓ | ||||||
| ATLAS-2M | 12 | ✓ | ✓ | ||||||||
| ATLAS-2M | 13 | ✓ |
CAB, cabotegravir; CVF, confirmed virologic failure; IN, integrase; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PK, pharmacokinetics; Q1, first quartile; Q8W, every 8 weeks; RAM, resistance-associated mutation; RPV, rilpivirine.
Participants were from study sites in Russia (n = 6), France (n = 2), US (n = 2), Spain (n = 1), South Africa (n = 1), Canada (n = 1). Follow-up at Russian study sites did not find clustering of failures at any one particular site, reducing the likelihood of drug administration errors being the cause.
CAB and RPV PK refer to Week 8 trough concentrations (4 weeks following first injections); Q1 refers to the lowest quartile of the Week 8 trough concentration.
Excluding mixtures with L74M.
Excluding L74I (excluding mixtures with L74M).
Reduced RPV susceptibility was observed with 11/13 participants at CVF. Baseline genotype and phenotype were analysed using viral RNA from plasma samples in FLAIR and viral DNA from peripheral blood mononuclear cell samples in ATLAS and ATLAS-2M.
Excluding RPV RAMs.
There were six out of 1039 (<1%) participants included in the analysis whose self-reported gender differed from sex at birth – none of these six participants reported CVF.
Predictors of virologic failure
In the univariate analysis, the presence of each of the 10 factors was associated with a higher proportion of participants meeting the CVF criterion, except for preexisting INSTI mutation(s) [excluding L74I (excluding mixtures with L74M)] (Fig. 1). In the multivariable logistic regression analysis, four factors were statistically associated (P < 0.05) with an increased odds of CVF [odds ratio (95% confidence interval), P value] in the final model: RPV RAMs at baseline [40.36 (8.81–>99), <0.001], log2 of Week 8 RPV trough concentration [5.00 (1.79–16.67), 0.002] per halving of value, baseline HIV-1 subtype A6/A1 [5.92 (1.62–22.89), 0.008] and baseline BMI [1.13 (1.02–1.24), 0.020] per unit increase (Table 2). The elimination of Week 8 CAB trough concentration from the model likely reflects the known association between CAB pharmacokinetics and BMI [9,10]. Other variables, namely female sex at birth, baseline NNRTI RAM(s) excluding RPV RAMs and baseline L74I integrase polymorphism, had no statistically significant association with increased odds of CVF. The multivariable Cox regression analysis of time to CVF results were comparable with the logistic regression results, with the addition of time-updated observed log2 CAB trough (P = 0.049) and L74I (excluding mixtures with L74M) (P = 0.005) meeting statistical significance (Table, Supplemental Digital Content 6, multivariable Cox regression analysis). The use of Q8W vs. Q4W regimen was not associated with increased odds of CVF in either model.
Fig. 1.
Univariate analysis of CVF outcome by 10 prespecified factors.
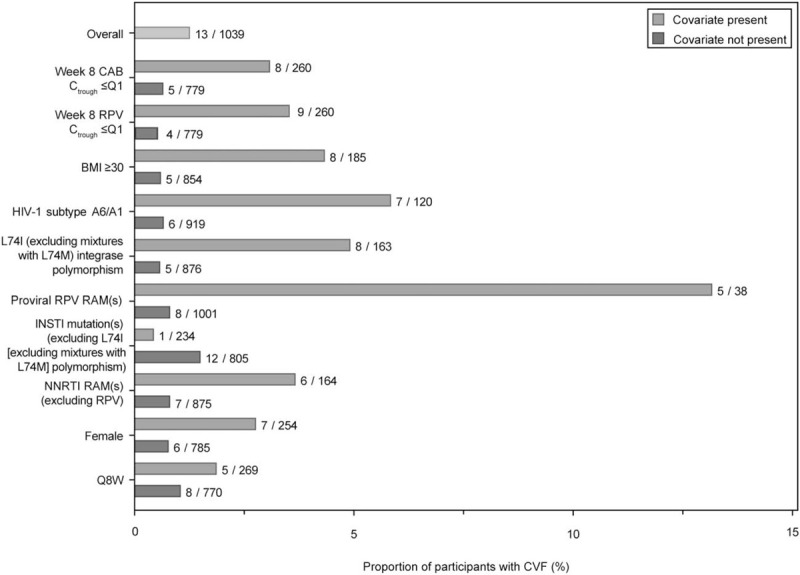
BMI (kg/m2); CAB, cabotegravir; Ctrough, trough concentration; CVF, confirmed virologic failure; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; Q1, first quartile; Q8W, every 8 weeks; RAM, resistance-associated mutation; RPV, rilpivirine.
Table 2.
Multivariable logistic regression analysis of confirmed virologic failure through Week 48.
| N | Parameter | Full model OR (95% CI), Pa | Backwards elimination model OR (95% CI), Pa |
| 1039 | RPV RAM(s) at baseline | 30.23 (6.25–>99), <0.001 | 40.36 (8.81–>99), <0.001 |
| Log2 of post hoc Week 8 RPV trough concentration | 3.85 (1.15–14.29)b, 0.029 | 5.00 (1.79–16.67)b, 0.002 | |
| Baseline HIV-1 subtype A6/A1 | 2.37 (0.34–22.14), 0.394 | 5.92 (1.62–22.89), 0.008 | |
| BMI (kg/m2) at baseline | 1.08 (0.96–1.22), 0.192 | 1.13 (1.02–1.24), 0.020 | |
| Prespecified INSTI polymorphism (excluding L74I [excluding mixtures with L74M]) at baseline | 0.16 (0.01–1.05), 0.057 | 0.14 (0.01–0.91), 0.038 | |
| NNRTI RAM(s) (excluding RPV RAMs) at baseline | 2.64 (0.72–9.21), 0.137 | 2.78 (0.78–9.63), 0.111 | |
| Q8W regimen | 2.76 (0.65–11.68), 0.164 | 2.77 (0.67–11.38), 0.156 | |
| L74I (excluding mixtures with L74M) INSTI polymorphism at baseline | 2.51 (0.33–13.85), 0.347 | Eliminated from model | |
| Female (sex at birth) | 1.09 (0.26–4.36), 0.899 | Eliminated from model | |
| Log2 of post hoc Week 8 CAB trough concentration | 0.66 (0.25–1.74), 0.395 | Eliminated from model |
CAB, cabotegravir; CI, confidence interval; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; OR, odds ratio; Q8W, every 8 weeks; RAM, resistance-associated mutation; RPV, rilpivirine.
95% penalized profile confidence intervals and penalized likelihood ratio P values are provided. Backwards elimination used a significance threshold of alpha = 0.2. CAB and RPV pharmacokinetic parameters were log2-transformed; therefore, the corresponding odds ratios are per halving of each variable.
Results are reciprocal of these so that all ORs are in same direction.
Baseline factors associated with confirmed virologic failure
The baseline factor analysis evaluated covariates that are potentially knowable to prescribers before starting long-acting CAB+RPV (i.e. at baseline; excluding CAB/RPV plasma concentrations); BMI of at least 30 kg/m2 was chosen as a dichotomous variable compared with the continuous variables used above. Proviral RPV RAMs, BMI at least 30 kg/m2 and HIV-1 subtype A6/A1 were significantly associated with an increased risk of CVF. These three baseline factors were further evaluated to determine if CVF risk increased when one or more were present. In the pooled dataset, most participants had either zero (70.5%, n = 732/1039) or one (26.2%, n = 272/1039) contributing baseline factor; however, very few of these had CVF [zero factors, 0.41% (n = 3/732); one factor, 0.37% (n = 1/272)]. Correspondingly, virologic success rates were high at 94.8% (n = 694/732) for those with zero factors and 96.0% (n = 261/272) for those with one factor at Week 48 (Table 3). The combination of at least two contributing baseline factors was uncommon in the study population (3.37%, n = 35/1039), though was present in nine out of 13 participants with CVF (Figure, Supplemental Digital Content 7, CVF outcome by presence of key baseline factors). Of all participants with at least two factors, 25.7% (n = 9/35) had CVF, whilst 71.4% (n = 25/35) maintained suppression. Only one participant had all three factors present at baseline; this participant had CVF (n = 1/1039, <1%). The model sensitivity and specificity of having any two contributing baseline factors was considered optimal given the 69% sensitivity and 97.5% specificity, with a positive predictive value (PPV) of 26% and a negative predictive value (NPV) of 99.6%. Any one contributing baseline factor had a PPV less than 1%, NPV 98%, sensitivity 8% and specificity 74%.
Table 3.
Week 48 outcomes by presence of key baseline factors of rilpivirine resistance-associated mutation(s), HIV-1 subtype A6/A1 and BMI at least 30 kg/m2.
| Baseline factors | Virologic successan (%) | CVFbn (%) |
| None of the three factors | 694/732 (94.8) | 3/732 (0.41) |
| Any one of the three baseline factors | 261/272 (96.0) | 1/272 (0.37) |
| HIV-1 subtype A6/A1 alone | 90/95 (94.7) | 1/95 (1.1) |
| BMI ≥30 kg/m2 alone | 147/153 (96.1) | 0/153 (0) |
| RPV RAM(s) alone | 24/24 (100) | 0/24 (0) |
| At least two of the three baseline factors | 25/35 (71.4) | 9/35 (25.7) |
| RPV RAM(s) + HIV-1 subtype A6/A1 | 2/3 (66.7) | 1/3 (33.3) |
| RPV RAM(s) + BMI ≥30 kg/m2 | 7/10 (70.0) | 3/10 (30.0) |
| HIV-1 subtype A6/A1 + BMI ≥30 kg/m2 | 16/21 (76.2) | 4/21 (19.0) |
| All three baseline factors | 0/1 (0) | 1/1 (100) |
| TOTAL | 980/1039 (94.3) | 13/1039 (1.25) |
| [95% CI (exact method)] | (92.74–95.65) | (0.67–2.13) |
CI, confidence interval; CVF, confirmed virologic failure; RAM, resistance-associated mutation; RPV, rilpivirine.
Based on the FDA Snapshot algorithm of HIV-1 RNA <50 copies/ml.
Defined as two consecutive measurements of HIV-1 RNA ≥200 copies/ml.
Association between HIV-1 subtype and integrase L74I relative to confirmed virologic failure
Given the L74I integrase polymorphism was common at the time of failure among participants with CVF and the near complete overlap with HIV-1 subtype A6/A1 (Table 1), baseline L74I incidence was retrospectively analysed in all participants. Most (88.3%, n = 106/120) participants with HIV-1 subtype A6/A1 also had L74I; of those, 6.6% (n = 7/106) had CVF, representing 53.8% (n = 7/13) of participants with CVF in this analysis. HIV-1 subtype C was uncommon (7.4%, n = 77/1039) in the pooled population, 9.1% (n = 7/77) of whom also had L74I. One participant with CVF had both HIV-1 subtype C+L74I (14.3%, n = 1/7). Subtype B was the most common subtype (72.7%, n = 755/1039) and was not associated with L74I carriage (Figure, Supplemental Digital Content 8, subtype distribution by the presence of L74I). No participant with CVF had both subtype B+L74I (n = 0/41), while four participants with CVF had subtype B alone (0.6%, n = 4/714) without L74I (Tables, Supplemental Digital Content 9/10, association between HIV-1 subtype, L74I and CVF).
Pharmacokinetics in relation to virologic outcome
Week 8 plasma CAB and RPV trough concentrations in participants with CVF overlapped with those for participants who did not meet the CVF criterion. Most participants with CVF (n = 9/13, 69%) had plasma concentrations for both drugs below the median plasma concentrations, with seven out of 13 (54%) participants with CVF having both Week 8 CAB and RPV plasma trough concentrations in the lowest quartiles of exposure, irrespective of dosing regimen (Q8W vs. Q4W) (Fig. 2a). There was a wide range of Week 8 CAB and RPV trough concentrations in participants with high BMI (≥30 kg/m2), with a trend towards lower Week 8 CAB troughs (n = 6/8 in the first concentration quartile) in participants with CVF (Fig. 2b).
Fig. 2.
Cabotegravir and rilpivirine trough concentrations at Week 8 (i.e. 4 weeks after first injections) and CVF through Week 48 (pooled Phase 3/3b) by regimen (a) and baseline BMI category (b).
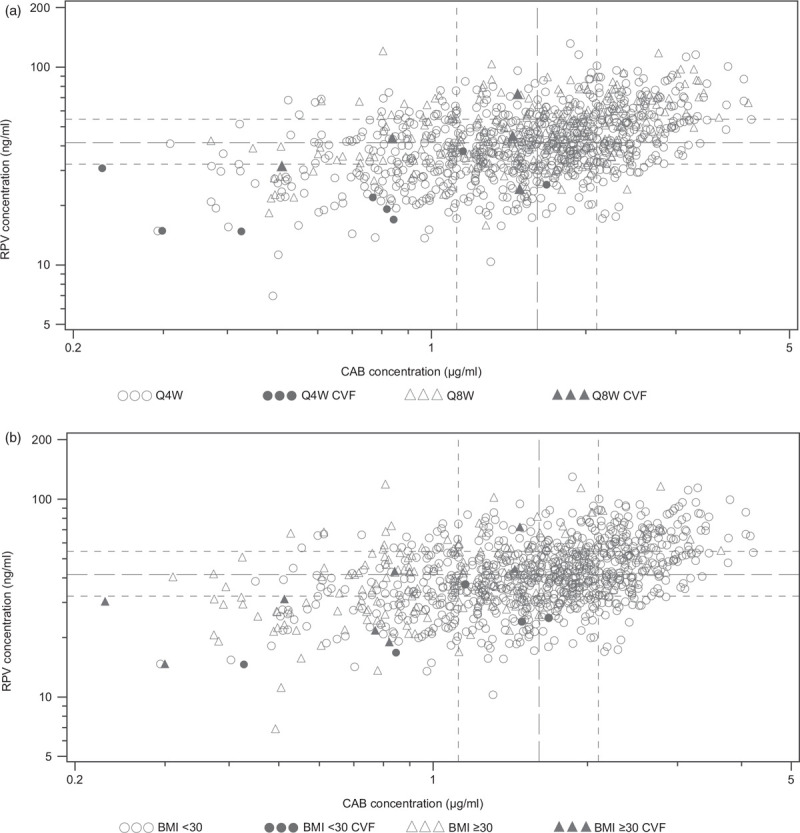
The median (Q1, Q3) for Week 8 RPV trough concentration is 41.50 (32.10, 54.30) and for Week 8 CAB trough concentration is 1.61 (1.12, 2.10). CAB, cabotegravir; CVF, confirmed virologic failure; Q1, first quartile; Q3, third quartile; Q4W, every 4 weeks; Q8W, every 8 weeks; RPV, rilpivirine. The median (Q1, Q3) for Week 8 RPV trough concentration is 41.50 (32.10, 54.30) and for Week 8 CAB trough concentration is 1.61 (1.12, 2.10). BMI (kg/m2); CAB, cabotegravir; CVF, confirmed virologic failure; Q1, first quartile; Q3, third quartile; RPV, rilpivirine.
Discussion
Patient, regimen, pharmacokinetic and viral factors are associated with virologic outcomes from ART [11,12]. Approximately 1% of participants on long-acting CAB+RPV met the CVF criterion through Week 48, and the results of this post-hoc pooled analysis support the initial study observations of a multifactorial relationship between participant and virologic factors and the occurrence of CVF. Four factors, proviral RPV RAMs, HIV-1 subtype A6/A1, baseline BMI and Week 8 RPV plasma trough concentration, were statistically associated with CVF occurrence in the logistic regression analysis. Further evaluation of the baseline covariates (proviral RPV RAMs, HIV-1 subtype A6/A1 and BMI ≥30 kg/m2) showed that at least two baseline factors were required to increase risk of CVF. The presence of any factor alone was not associated with CVF. The presence of proviral RPV RAMs was not known before enrolment in ATLAS and ATLAS-2M and may reflect prior NNRTI exposure with underlying drug resistance, or transmitted drug resistance, which would cause reduced susceptibility to RPV. Although proviral RPV RAMs were associated with the greatest increase in odds of CVF, no participant (n = 24; Table 3) with proviral RPV RAMs alone experienced CVF, supporting a multifactorial relationship. Prespecified INSTI mutations did not increase risk of CVF in the multivariable analysis, although notably this category included mutations that were not all RAMs (Table, Supplemental Digital Content 3, resistance mutations).
HIV-1 subtype A6/A1 was the most common viral subtype in participants with CVF and was significantly associated with CVF in the multivariable logistic regression model. However, in vitro, CAB (our unpublished observations) and RPV susceptibility is broadly similar across HIV-1 subtypes [13,14]. All but one of the participants with CVF and baseline integrase L74I polymorphism also had subtype A6/A1, consistent with previous findings showing L74I is associated with HIV-1 subtype A6 virus, a clade uncommon outside the Russian Federation [8,15,16]. Of the participants with HIV-1 subtype A6/A1 who had CVF, all but one (from Canada) were from Russia; however, these participants were distributed across several study sites, suggesting that drug administration errors did not contribute to higher CVF rates. Despite the correlation between HIV-1 subtype A6/A1 and L74I, L74I was not significantly associated with CVF in the multivariable logistic regression model. Furthermore, a post-hoc analysis in FLAIR demonstrated that this polymorphism was relatively common in the trial participants, 94.4% (n = 51/54) of whom did not meet the CVF criterion [3]. This is consistent with L74I not being considered an INSTI RAM, although it can contribute to reduced susceptibility if present with additional INSTI mutations [17,18]. Recent research has shown a lower genetic barrier for L74M and L74I mutations in the A6 subtype, suggesting a lower barrier for INSTI resistance [16]. Subsequent to the initial finding that the L74I integrase polymorphism was present in all three FLAIR participants with CVF who received long-acting CAB+RPV, in-vitro investigation revealed no differential sensitivity to CAB between viral subtypes A1 or B with or without L74I [19]. Further, the results of this analysis suggest both HIV-1 subtype A6/A1 and L74I are required to increase the odds of CVF. In contrast, the CVF rate in participants with L74I within other subtypes (Table, Supplemental Digital Content 10, association between HIV-1 subtype, L74I and CVF) is similar to the overall population (1.8 vs. 1.3%). This is evident with subtype B, for which the absence of participants with HIV-1 subtype B+L74I who had CVF suggests no influence of the L74I polymorphism on CVF occurrence within that subtype. Further research is warranted to understand the interplay of L74I with other subtypes.
Baseline BMI was another covariate found to be statistically associated with CVF. Previous research has shown that long-acting CAB absorption is slower [9], and mean trough concentration following the first intramuscular dose is lower, in high BMI (≥30 kg/m2) compared with low BMI (<30 kg/m2) individuals [10]. However, by Week 20–24, drug concentrations are comparable. There is no apparent association between BMI and long-acting RPV concentration [10]. The logistic model identified BMI as a more sensitive covariate than Week 8 CAB plasma trough concentration, which may indicate a greater importance of ensuring delivery of the long-acting injection into the muscle of individuals with high BMI. Hence, longer needle lengths should be considered for participants with high BMI [20]. None of the participants with CVF and high BMI were recorded as having been injected with more than 1.5-inch needle lengths; therefore, it remains unclear whether the use of longer needles would have influenced clinical outcomes.
Week 8 RPV plasma trough concentration was also significantly associated with CVF in this multivariable analysis. However, a low RPV trough concentration alone was rarely observed without the presence of other significant baseline factors among participants with CVF, indicating that low initial RPV concentration alone is not predictive of CVF. Dosing regimen (Q8W vs. Q4W) was not significantly associated with CVF in the model, a result supported by individual and pooled study results that demonstrate comparable CVF rates across long-acting CAB+RPV arms [3–5,21]. In summation, the Q8W regimen was not identified as an independent predictor of CVF occurrence (Table 2 and S2), and noninferior efficacy has been demonstrated between Q8W and Q4W dosing as well as indirectly between Q8W and SOC [21,22]. The initial part of both dosing regimens (Q8W and Q4W) is the same, that is a 3 ml intramuscular injection of each drug (CAB, 600 mg; RPV, 900 mg). CAB and RPV plasma concentrations are therefore comparable between both dosing regimens until 4 weeks after the first injection, after which there is some divergence in CAB and RPV trough plasma concentrations between regimens. However, there does remain overlap in CAB and RPV concentrations between both dosing regimens and between participants with and without CVF, precluding clear establishment of a pharmacokinetic/pharmaco-dynamic relationship [6].
The fact that three of the four significant covariates associated with a potential increased risk of CVF can be considered at baseline may be useful information to clinicians considering long-acting CAB+RPV therapy. In participants with zero or one baseline factor, the CVF rate was less than 0.5%. However, any combination of at least two factors appears to increase CVF risk. Virologically suppressed patients without known or suspected resistance to CAB or RPV are suitable candidates for long-acting CAB+RPV. If the patient's treatment history is unclear, additional consideration may be warranted, particularly if the patient also has an HIV-1 subtype A6/A1 and/or high BMI.
Limitations
The evaluation of many covariates relative to the low number of CVFs may have stressed the limits of modelling. Also, some covariates in the model are known to be associated with each other and may have impacted the model building process and selection of factors. For these correlated pairs, the ‘other factor’ would have been selected if both were not included in the full model. Sensitivity analyses were conducted by dropping one of the pair of correlated factors; the overall conclusions were unchanged except for the substitution of the correlated factor in the absence of the other. In addition, the use of cell-associated HIV-1 DNA testing via peripheral blood mononuclear cells has been shown to be discordant with plasma resistance testing [23,24]. Finally, as this study was post hoc, it carries the inherent limitations associated with this type of analysis.
Conclusion
Approximately 1% of participants receiving long-acting CAB+RPV Q4W or Q8W through Week 48 developed CVF in the pooled Phase 3/3b study population. A multivariable analysis showed that a combination of at least two factors, proviral RPV RAMs, HIV-1 subtype A6/A1 and BMI at least 30 kg/m2, increased the risk of CVF; however, such a combination was rare in this pooled population. Consideration of these factors before use of this novel regimen may help to further minimize CVF risk. The results of this analysis should be contextualized with the high suppression rates observed in the Phase 3/3b studies. Taken together, the findings support the use of long-acting CAB+RPV in routine clinical practice in a broad patient population.
Acknowledgements
We thank everyone who has contributed to the success of the study: all study participants and their families, and the clinical investigators and their staff. FLAIR, ATLAS and ATLAS-2M were funded by ViiV Healthcare and Janssen Research & Development. Professional medical writing and editorial assistance was provided by Daniel Williams, MSc, at SciMentum (Nucleus Global) and funded by ViiV Healthcare.
A.C., P.P., D.D., Y.W., S.W., V.V.E., H.C., S.L.F., M.B., C.L.T., M.S.C., J.J., S.V., K.V., D.M., W.S., J.V.L. participated in the research design.
V.V.E., H.C., S.L.F., C.L.T., M.S.C., J.J., C.T.W. conducted experiments for the study analyses.
A.C., D.D., Y.W., S.W., S.V. performed statistical analyses.
A.C., J.S., C.F.P., D.R.K., R.Q., P.P., J.W.P., D.D., M.B., C.L.T., M.S.C., J.J., S.V., K.V., D.M., M.A., W.S., J.V.L. interpreted the findings.
All authors were involved in the drafting and review of the manuscript and approved the final version.
Conflicts of interest
A.C. is an employee of ViiV Healthcare and stockholder of GlaxoSmithKline.
J.S. reports personal fees and nonfinancial support from Gilead Sciences, Merck, ViiV Healthcare/GlaxoSmithKline, Teva, AbbVie, Virology Education and nonfinancial support from WHO outside the submitted work.
C.F.P. received grants and personal fees from Gilead Sciences, ViiV Healthcare and Merck Sharp and Dohme, and personal fees from Janssen and Theratechnologies outside the submitted work.
D.R.K. has received research support and consulting honoraria from ViiV Healthcare, Gilead Sciences and Merck, and consulting honoraria from GlaxoSmithKline outside the submitted work.
R.Q., P.P., J.W.P., D.D., Y.W., M.B., C.L.T., M.S.C, J.J., C.T.W., M.A., W.S. and J.V.L. are employees of ViiV Healthcare and stockholders of GlaxoSmithKline. D.M. was an employee of ViiV Healthcare during the conduct of this work and is a stockholder of GlaxoSmithKline. S.W. and S.L.F. are employees and stockholders of GlaxoSmithKline. H.C., S.V. and K.V. are employees and stockholders of Janssen, Pharmaceutical Companies of Johnson & Johnson. V.V.E. is an employee of Janssen, Pharmaceutical Companies of Johnson & Johnson.
Supplementary Material
Current affiliation: Brii Biosciences, Durham, North Carolina, USA.
Supplemental digital content is available for this article.
References
- 1. U.S. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2020. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/15/virologic-failure. [Accessed 25 February 2020]. [Google Scholar]
- 2. World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens. 2019. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1. [Accessed 31 October 2019]. [Google Scholar]
- 3.Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard PM, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–1135. [DOI] [PubMed] [Google Scholar]
- 4.Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–1123. [DOI] [PubMed] [Google Scholar]
- 5.Rizzardini G, Overton ET, Orkin C, Swindells S, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: week 48 pooled analysis of phase 3 ATLAS and FLAIR trials. J Acquir Immune Defic Syndr 2020; 85:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overton ET, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 48-week results: a randomised, multicentre, open-label, phase 3b, noninferiority study. Lancet 2021; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 7.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 8.Lapovok I, Laga V, Kazennova E, Bobkova M. HIV type 1 integrase natural polymorphisms in viral variants circulating in FSU countries. Curr HIV Res 2017; 15:318–326. [DOI] [PubMed] [Google Scholar]
- 9.Han K, Patel P, Baker M, Margolis D, Spreen W, Ford S. Population pharmacokinetics of cabotegravir in healthy adult subjects and HIV-1 infected patients following administration of oral tablet and long-acting intramuscular injection. Poster presented at 22nd International AIDS Conference; 23–27 July 2018. Amsterdam, the Netherlands: Poster WEPDB0205; 2018. [Google Scholar]
- 10.Patel P, Ford S, Crauwels H, Han K, Rossenu S, Neyens M, et al. Pharmacokinetics of cabotegravir and rilpivirine long-acting injectables in HIV-infected individuals through 48 weeks in the FLAIR and ATLAS phase 3 studies. Poster presented at IDWeek; 2–6 October 2019. Washington, DC, USA: Poster 2495; 2019. [Google Scholar]
- 11.Deeks SG. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clin Infect Dis 2000; 30: Suppl 2: S177–S184. [DOI] [PubMed] [Google Scholar]
- 12.Kiweewa F, Esber A, Musingye E, Reed D, Crowell TA, Cham F, et al. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLoS One 2019; 14:e0211344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmon SL, Mohri H, Spreen W, Markowitz M. GSK1265744 demonstrates robust in vitro activity against various clades of HIV-1. J Acquir Immune Defic Syndr 2015; 68:e39–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvez V, Marcelin AG, Vingerhoets J, Hill A, Hadacek B, Moecklinghoff C. Systematic review to determine the prevalence of transmitted drug resistance mutations to rilpivirine in HIV-infected treatment-naive persons. Antivir Ther 2016; 21:405–412. [DOI] [PubMed] [Google Scholar]
- 15.Schlösser M, Kartashev VV, Mikkola VH, Shemshura A, Saukhat S, Kolpakov D, et al. HIV-1 sub-subtype A6: settings for normalised identification and molecular epidemiology in the Southern Federal District, Russia. Viruses 2020; 12:475. [Google Scholar]
- 16.Kirichenko A, Lapovok I, Baryshev P, van de Vijver DAMC, van Kampen JJA, Boucher CAB, et al. Genetic features of HIV-1 integrase sub-subtype A6 predominant in Russia and predicted susceptibility to INSTIs. Viruses 2020; 12:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanford University. HIV drug resistance database. https://hivdb.stanford.edu/. [Accessed 2 April 2020]. [Google Scholar]
- 18.Wensing AM, Calvez V, Ceccherini-Silberstein F, Charpentier C, Günthard HF, Paredes R, et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med 2019; 27:111–121. [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffrey J, St Clair M, Wang P, Wang C, Li Z, Fridell R, et al. HIV A1 or B do not differentially impact cabotegravir in vitro potency or durability. Abstract presented at Conference on Retroviruses and Opportunistic Infections (CROI); 8–11 March 2020. Boston, MA, USA; 2020. [Google Scholar]
- 20. CABENUVA Product Monograph including patient medication information. 2020. [Google Scholar]
- 21.Overton E, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, et al. Cabotegravir R rilpivirine every 2 months is noninferior to monthly dosing: Week 48 results from the ATLAS-2M study. Poster presented at Conference on Retroviruses and Opportunistic Infections (CROI); 8–11 March 2020. Boston, MA, USA: Presentation 3334; 2020. [Google Scholar]
- 22.Chounta V, Snedecor S, WuS, Van de Velde N. Comparability of 48-week efficacy and safety of cabotegravir and rilpivirine longacting every 8 weeks to standard of care in suppressed people living with HIV-1. Poster presented at Glasgow; 5–8 October 2020. Virtual. Poster P011; 2020. [Google Scholar]
- 23.Sharkey M, Triques K, Kuritzkes DR, Stevenson M. In vivo evidence for instability of episomal human immunodeficiency virus type 1 cDNA. J Virol 2005; 79:5203–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaugerre C, Braun J, Charreau I, Delarue S, Nere ML, de Castro N, et al. Comparison of resistance mutation patterns in historical plasma HIV RNA genotypes with those in current proviral HIV DNA genotypes among extensively treated patients with suppressed replication. HIV Med 2012; 13:517–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


