Highlights
-
•
Spontaneous remission (SR) of cancer is a rare but well-documented phenomenon.
-
•
Mechanisms of SR are described in detail.
-
•
Knowing the intricacies of SR would help in devising novel treatment strategies.
Keywords: Spontaneous regression, Tumour immunology, Oncolytic virus, BCG, T-VEC
Abstract
Many diseases heal spontaneously. The common cold, for example, remedies itself within a few days in people with an uncompromised immune system. If a disease with a poor prognosis heals in the absence of a targeted therapeutic, many even call it a miracle cure. Such is the case with the spontaneous regression (SR) of malignant neoplasms, a rare but well-documented phenomenon that finds its first mention in the Ebers Papyrus of 1550 BCE. Given the challenges associated with current cancer treatment modalities such as rapidly evolving drug resistance mechanisms, dose-limiting side effects, and a failure to completely eliminate cancer cells, knowledge of how a tumour heals itself would be immensely helpful in developing more effective therapeutic modalities. Although the intricate mechanisms of SR have yet to be fully elucidated, it has been shown that infection-mediated immune system activation, biopsy procedures, and disruptions of the tumour microenvironment play pivotal roles in the self-healing of many tumours. Bacterial and viral infections are especially well-documented in instances of SR. Insights from these findings are paving the way for novel therapeutic strategies. Inspired by bacteria-mediated SR, Bacillus Calmette-Guérin (BCG) has been used as an approved treatment option for non-muscle-invasive bladder cancer (NMIBC). Similarly, Talimogene laherparepvec (T-VEC), the first engineered oncolytic herpes simplex virus (HSV), has been approved by the United States Food and Drug Administration for the treatment of some forms of advanced melanoma. Here we describe the current understanding of SR, explore its therapeutic significance, and offer perspectives on its future.
Graphical abstract
Introduction
In 2020, 19.3 million new cancer cases and approximately 10 million cancer deaths occurred worldwide, with breast cancer being the most prevalent one and lung cancer, one with the highest mortality rate [1]. To effectively counter this surge, intense efforts have been focused on developing novel drug molecules with enhanced efficacy and tolerable side effects. From drugs conjugated to tumour-targeted antibodies [2] and nanoparticles-based theranostics [3], the quests for developing better therapeutics are underway with varying degrees and definitions of success. Despite the lethal intensity of malignant tumours, at times, a ‘miraculous’ phenomenon occurs: a spontaneous remission of cancer. The term spontaneous remission or spontaneous regression (SR) of cancer translates into the recovery of a patient from cancer in the absence of a disease-specific treatment or in the presence of inadequate therapy [4]. The recovery, therefore, does not involve conventional cancer treatment modalities such as chemotherapy or radiotherapy. Given the current prevalence of cancer cases, we believe that efforts must focus also on unraveling the mechanisms of SR. A lucid comprehension of the incidence patterns and the underlying mechanisms of SR could decipher contiguous mechanisms of cancer progression and lead to the development of efficacious treatment modalities. This review focuses on the current understanding of spontaneous remission, its possible causes, and clinical significance.
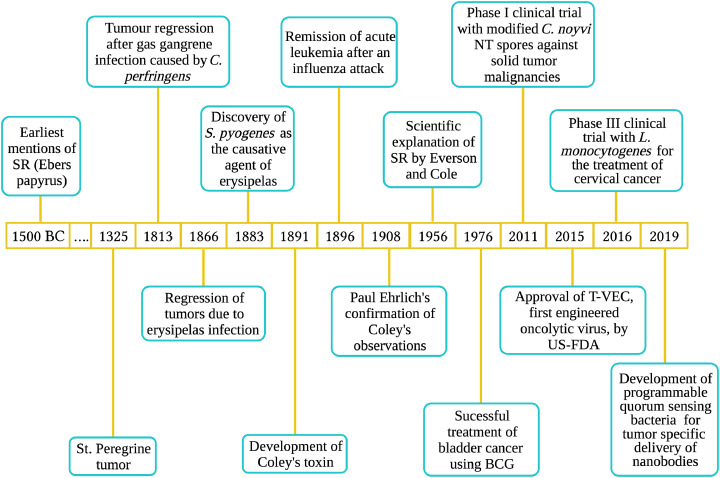
SR and its therapeutic potential have been known for millennia. Mention of indirect induction of SR as a treatment strategy is available in Ebers papyrus, one of the oldest Egyptian medical documents written during 1550 BCE that describes cures for various diseases. An Egyptian physician, Imhotep, recommended the application of poultice on tumours followed by an incision to induce an infection at the affected area [5]. The infection, he reasoned, could promote tumour regression. Perhaps, the most famous case of SR is the one reported from Italy in the 12th century. Peregrine Laziosi, a Catholic priest, had experienced recovery from a tumour that afflicted one of his tibiae. Suffering from the tumour, he also developed a severe infection on the skin, necessitating an imminent amputation of his leg. Interestingly, before the surgery, he recovered from the tumour [6,7]. Due to this incident, tumours that show spontaneous regression are also called “St. Peregrine tumour” [8].
So, how SR occurs? Despite notable exceptions, acute infections has been a common denominator for a large number of SR cases. Although the idea of infections triggering the immune system to eliminate tumours was proven early on, two German physicians, Wilhelm Busch and Friedrich Fehleisen, built the scientific basis for this concept (Fig. 1). They independently discovered that erysipelas (Greek for “red skin”; an infection caused by bacteria such as Streptococcus pyogenes) could lead to tumour regression [9]. Further, their studies paved the way for indirect interventions to regress many forms of aggressive tumours. Treatment modalities thus evolved include the use of bacteria-based formulations such as Coley's Toxin, Bacillus Calmette-Guerin (BCG), and oncolytic virus-mediated strategies.
Fig. 1.
Spontaneous remission of cancer as reported over time. From its earliest mention in Ebers Papyrus to the development of nanobodies-delivering designer bacteria are shown.
Coley's Toxin
The idea of inducing an infection to promote collateral regression of tumours gained momentum with the advent of Coley's Toxin. In 1891, Dr. William Bradley Coley, an American bone surgeon and cancer researcher, developed a formulation, the Coley's Toxin. It consisted of heat-inactivated Streptococcus pyogenes and Serratia marcescens. At New York's Memorial Hospital, Coley used the vaccine to treat a sarcoma patient successfully [10]. After that, many cancer patients underwent treatment with the Toxin with promising results. Even when administered at the advanced stages of cancer, the Toxin brought forth remarkable recoveries [10,11]. Nevertheless, for the formulation to be effective, Coley maintained, a few conditions were to be met, including induction of an acute infection that makes the patient feverish with considerably elevated body temperature [12]. The inoculation should be given daily or on every alternate day for a month or two with gradually increasing dosage. The Toxin should be injected directly into the tumour. Further, a six-month course of weekly injections is needed to avoid relapse [12]. Coley's Toxin was effective against many cases of sarcoma, lymphoma, melanomas, and myelomas. Although it was a breakthrough treatment strategy, with time, advanced treatment modalities, such as radiation therapy and chemotherapy, gained credence and momentum. A lack of understanding of the exact mechanism of action of the Toxin, the risks associated with infecting patients with potentially pathogenic bacteria, and varied and often unpredictable responses to the treatment led eventually to the relinquishment of this treatment strategy in its original form. The infection-induced remission of tumours nevertheless shed light on the vital roles of the immune system in fighting cancer, as substantiated by prominent scientists of that period, including Paul Ehrlich [9]. The last successful use of Coley's Toxin was reported in China in 1980 when a patient with terminal liver cancer was given 68 injections of the Toxin for 34 weeks [11,13]. Coley's efforts have laid the foundation for developing anticancer immunotherapy regimens. He is deservedly considered as father of cancer immunotherapy.
BCG (Bacillus Calmette-Guerin) and SR
During the 20th century, substantial progress in the field of immunology enabled researchers to revisit Coley's treatment strategy with a new perspective and deepened insight. Inspired by the findings of Coley, for instance, Lloyd Old and colleagues repurposed the well-known antituberculosis bacterial formulation, Bacillus Calmette-Guerin (BCG). It comprises an attenuated strain of Mycobacterium bovis [14] (BCG was developed by two French Scientists, Albert Calmette and Camille Guerin, in 1921). During the 1950s, Old studied the effect of BCG on solid tumours and Ehrlich ascites cells implanted in mice [15]. The formulation regressed the tumours considerably.
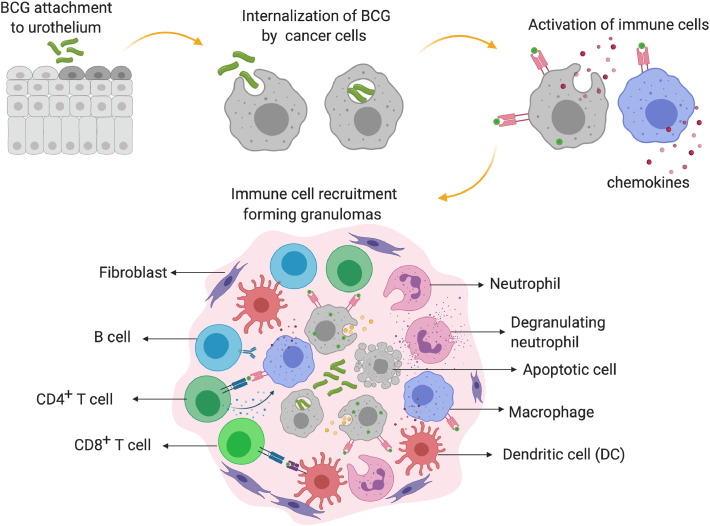
Further evidence of BCG's efficacy came in 1976 when many recurrent superficial bladder tumours were treated successfully with it [16]. A timeline of these and subsequent developments is available in Fig. 1. Later studies attested the utility of BCG to induce tumour remission via activating the immune system. Although the precise anti-tumour mechanism of BCG is not clear, the internalization of BCG by the tumour cells, the subsequent display of antigens on their surface and the consequent release of cytokines, such as IL-2, TNF-α, INF-γ, IL-4, and IL-6 from infiltrating T-lymphocytes, are thought to induce the remission (Fig. 2). Supporting this postulate, mice depleted of CD4+ and CD8+ T-cells exhibited only a negligible response to BCG [17]. In addition to the T-cells, granulocytes, macrophages, and dendritic cells also play pivotal roles in the remission; the number of these cells increases considerably during the treatment [18], [19], [20]. BCG therapy, nevertheless, has its caveats. After the remission, the tumour returned in nearly 30% of the patients. Nevertheless, by far, BCG is the only bacterial therapeutic used for the treatment of non-muscle invasive bladder cancer (NMIBC) for the past 40 years [18].
Fig. 2.
Postulated anti-tumour mechanism of BCG in non-muscle invasive bladder cancer. BCG gets internalized in the cancer cells of the urothelium and and in the antigen-presenting cells (APCs). The urothelial cells and the activated APCs present the BCG antigens on their surface, leading to cytokine/chemokine release and granuloma formation. These granulomas, comprising macrophages, dendritic cells (DCs), neutrophils, T cells, B cells, and fibroblasts, trigger innate and adaptive immune responses and destroy the cancer cells.
Oncolytic viruses (OV)
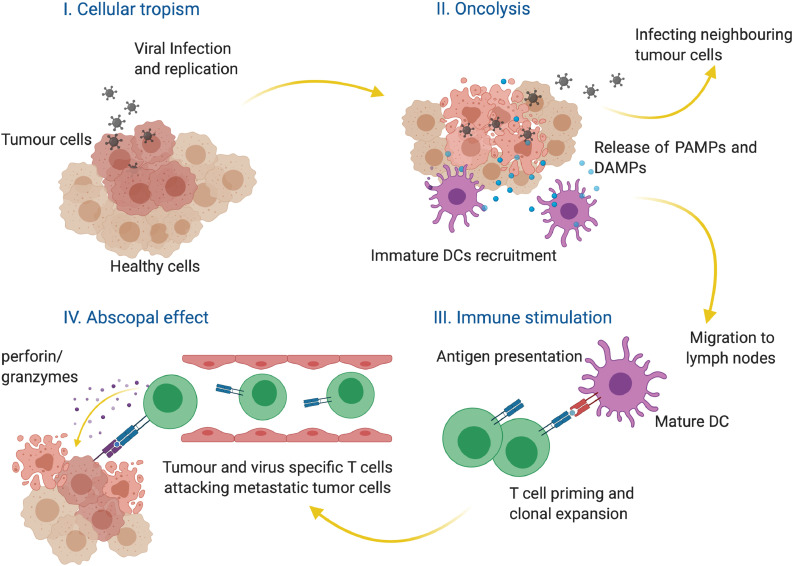
One of the earliest examples of virus-orchestrated SR was reported in 1896 when influenza cured a patient of leukaemia (Fig. 1) [21]. Measles-induced remission of Burkitt's and Hodgkin lymphoma [22,23] and several similar cases were reported subsequently [24]. SR after viral infections thus paved the way for the development of a new mode of cancer treatment- cancer virotherapy. Initially (between 1950–60), wild-type viruses were used to induce the remission, which worked successfully in some cases but proven to be dangerous to many patients due to uncontrolled infection [25]. The risks associated with the use of natural viruses led eventually to the dissolution of this treatment modality. However, by the end of the 20th century, the field of cancer virotherapy regained propulsion with the advent of genetically modified viruses [9]. The inherent ability of viruses to enter specific host cells (cellular tropism), to replicate therein and to subsequently lyse the host, and to exert bystander toxicity to the neighboring cells make them potential therapeutic agents (Fig. 3). Furthermore, upon exposure of the viral PAMPs (Pathogen-associated molecular patterns), the immune system primes the T-cells to effect contemporaneous elimination of metastasized tumours (the so-called “abscopal effect”) [26] (Fig. 3). Viral species such as adeno, herpes, vaccinia, polio, and measles are design-optimized and evaluated in clinical settings as monotherapy or as combination therapies [27]. Talimogene laherparevec (T-VEC) is the first engineered oncolytic herpes simplex virus (HSV) that received United States Food and Drug Administration's approval for the treatment of advanced melanoma [28,29]. T-VEC is a double-edged weapon that not only destroys the target tumour cells at the site of injection but could also initiate localized as well as systemic immune response [30]. Locally, the lysed tumour cells release tumour-associated antigens. These antigens attract members of the mononuclear phagocyte system-especially the macrophages-promoting clearance of the dying and dead tumour cells. The dendritic cells then present these viral antigens to T-helper cells and cytotoxic T-cells and thereby mount a formidable immune response at the systemic level [30]. Insights into the mechanism of action of T-VEC have also led to the development of novel combinatorial therapeutics. For example, T-VEC, in combination with ipilimumab (an inhibitor of the immune-suppressor protein, CTLA-4), has entered clinical trials for melanoma (https://clinicaltrials.gov/ct2/show/NCT01740297). Similarly, the efficacy of T-VEC in combination with pembrolizumab (a monoclonal antibody that blocks another immune checkpoint protein PD-1) to improve the outcome of melanoma is currently under investigation (https://clinicaltrials.gov/ct2/show/NCT02263508).
Fig. 3.
Oncolytic viruses. Many OVs selectively replicate in cancer cells (cellular tropism) and lyse the cells. The resultant release of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) recruits and activates dendritic cells (DCs). DCs then migrate to lymph nodes and present the antigens to the T cells. These activated cytotoxic T cells undergo clonal expansion. They induce apoptosis in target cells via the release of perforin and granzymes.
SR of specific cancers
Interestingly, some forms of cancer seem to possess a peculiar propensity to regress spontaneously. There are several examples of such remissions (Tables 1, 2). Among them, metastatic melanoma (MM), leukaemia, lung cancer, and Merkel cell carcinoma merit special mention. MM is especially interesting as it can regress from end-stage disease. In one such case, a patient was suffering from MM for nearly ten years. When diagnosed, she was already at stage IV of the melanoma with a poor prognosis. However, after episodes of fever, most of her tumours disappeared, and the remaining ones stabilized (Table 1). The frequency of SR of MM is 1 in 400 patients. Although it is often associated with immune response elicited by cytotoxic T cells [31], the SR in MM can also occur in the absence of infection; Table 2. MM has also shown regression in response to the DTP (for diphtheria-tetanus-pertussis) vaccine [33].
Table 1.
Clinical characteristics of SR cases linked to infection reported during 2018–2020.
| Patient age and gender | Cancer type showing SR | Diagnostic methods | Features of the remission | Associated history or details | Follow up to confirm the SR | Reference |
|---|---|---|---|---|---|---|
| 41 Y F | MM | CT, chest X-ray, MRI | SR was observed after episodes of fever No sign of reoccurrence when tested subsequently |
The fever episodes were not tretaed with any medication. It is a remarkable recovery from stage IV melanoma with dissappearance of the most of the tumours and stabilization of the remaining ones. | > 2.5 Y | [31] |
| 58 Y M | AML | Bone marrow biopsy, blood counts, CT | Myeloblast counts significantly reduced (from a 25% increase) to normal levels after two weeks of gastrointestinal infection (GI). | The patient suffered GI due to which intravenous antibiotics were given. Blood transfusions were performed. |
> 2 Y | [34] |
| 40 Y M | AML | Bone marrow biopsy, blood counts, flow-cytometric analysis, CT | Blast cell percentage came down to normal levels after three weeks since the diagnosis. | S. aureus growth was obsreved in blood cultures, and antibiotics were given. The patient had episodes of fever. Frequent blood transfusions were required. | 6 M | [43] |
| 42 Y F | AML | CT, blood counts, bone marrow biopsy, cytogenetics, flow cytometry | Significant decrease in blast cell count and simultaneous recovery from pneumonia after four weeks of detection in 2017. Blast cell counts were normalized in the subsequent four weeks. | The patient was previously diagnosed with AML in 2000 and underwent chemotherapy. Also, allogenic blood stem cell transplantation was performed. 17 years later, the patient suffered severe pneumonia and again developed AML (relapse). Antibiotics were given to treat pneumonia and the AML regressed |
14 M | [35] |
| 31 Y F | ALL | Blood counts, flow-cytometric analysis, bone marrow biopsy | Blood samples came negative for ALL. CR was observed in 42 days after the 1st diagnosis. The patient experienced a relapse of ALL twice. After each relapse, the patient exhibited septic shock followed by SR. |
The patient was pregnant when diagnosed with ALL for the first time. She experienced fever, pancytopenia, and inflammation and delivered a healthy preterm child. Blood transfusions were on during the treatment. |
NA | [44] |
| 47 Y M | Non-Hodgkin FL | Blood counts, Bone marrow and lymph node biopsy, CT, PET | Three months after diagnosis, blood counts were normal and the CT scan showed regression in lymphadenopathy and splenomegaly. | The patient had H. pylori infection during this course and was given antibiotics. | 2 Y | [38] |
| 76 Y M | Myelodysplasia | Blood count , CT | The patient was diagnosed with transfusion-dependent myelodysplasia in2011, followed by urinary bladder cancer in 2014. During the treatment of bladder cancer with BCG (2015-2016), SR was observed for myelodysplasia. | The patient suffered anemia since 2008 and developed myelodysplasia in 2011. Blood transfusions were on. The patient developed a bladder tumour, for which tumour resection was performed and an intravesical BCG course was on . Bladder cancer showed no reoccurrence. |
NA | [45] |
(Y- years, M-months, F-female, M-male, CR- complete recovery, CT- computed tomography, MRI- magnetic resonance imaging, MM- metastatic melanoma, AML- acute myeloid leukemia ALL- acute lymphoblastic leukemia, FL- follicular lymphoma, BCG- Bacillus Calmette-Guerin, NA- not available, PET- positron emission tomography, NA- Not available)
Table 2.
Clinical characteristics of SR cases reported during 2018-2020 that are not associated with preceding infection.
| Patient age and gender | Cancer type showing SR | Diagnostic methods | Features of the remission | Associated history or details | Follow up to confirm the SR | Reference |
|---|---|---|---|---|---|---|
| 86 Y M | Pulmonary metastatic melanoma (MM) | Biopsy, X-ray, PET,CT | 12-mm round lesion in the right lung detected in 2014. It was not seen in 2015 while mediastinal metastasis was progressive. Significant shrinkage of metastatic mediastinal mass was observed in 2017 with no relapse of pulmonary metastasis. | MM was detected in 2012 on the left forearm. In 2014, it metastasized to the right lung and mediastinal lymph nodes. | NA | [46] |
| 75 Y M | MM | CT, MRI, Biopsy, PET,CT | Tumour metastasized in the left mandibular ramus, shrunk completely. | NA | 2 Y | [47] |
| 72 Y M | AML | Blood counts, bone-marrow biopsy | Seven weeks afterthe diagnosis, the skin lesion showed infiltration of myeloblasts, but blood counts showed no sign of AML. | The patient was suffereing from comorbidities, such as ischemic and valvular heart disease and chronic kidney disease. Blood transfusions were performed. AML relapsed after one year; the patient died in few days. |
- | [48] |
| 73 Y M | NSCLC | CT/, PET, biopsy | After 14 months of the biopsy procedure, tumour mass in the lungs decreased substantially. | Before the biopsy, the patient suffered non-invasive bladder cancer, for which he had completed a BCG instillation course of six-weeks (one month before the lung biopsy). | NA | [49] |
| 74 Y F | NSCLC | X-ray, CT, biopsy, PET, CT | After one year of discontinuation of chemotherapy, the lung tumour showed SR | The patient received two cycles of chemotherapy for advanced lung cancer. She then developed drug-induced hepatitis and took a short break from chemotherapy. The therapy was then resumed and continued for several months, and later abandoned. Few months before SR, the patient had been taking herbal medicine of Orostachys japonicus extract. |
NA | [40] |
| 77 Y M | NSCLC | X-ray, CT, biopsy | Near CR was observed within 24 months of initial diagnosis. | It was andvanced-stage lung cancer that regressed | > 1 Y | [50] |
| 56 Y F | NSCLC | CT, PET, biopsy | Six weeks after the diagnosis, tumour mass on the right lower lobe decreased while the size of swollen mediastinal lymph nodes increased. | Advanced-stage lung cancer metastasized to mediastinal lymph nodes while regressing at its site of origin | NA | [51] |
| 57 Y M | Lung cancer | Biopsy, CT, PET | Tumour mass on the lower lobe of the left lung significantly decreased in three months and further reduced when examined after a month. | The patient was a cigarette smoker. | NA | [52] |
| 35 Y M | DLBCL | PET,CT, biopsy | Three months after the biopsy, regression of abnormal accumulation of cells in the small intestine and lymph nodes was observed. | The patient did not take any medication or had any infection. | 3 Y | [39] |
| 88 Y F | DLBCL | Biopsy, PET,CT | Lymphoma remission was observed three months after the biopsy. | The patient died ~two yrs after remission due to progressive respiratory insufficiency. | NA | [53] |
| 66 Y M | DLBCL | Biopsy, CT | Two months post-diagnosis, DLBCL-leg type purple tumour in the left lower leg started to regress. | No systremic therapy was used to initiate the regression. | NA | [54] |
| 32 Y F | Classical HL | Biopsy, PET,CT | Complete regression after two years of diagnosis. | The patient also exhibited thyroid papillary cancer at the time of diagnosis. However, SR was not observed for the same. | 3.5 Y | [55] |
| 74 Y M | Hepatosplenic T-cell lymphoma | PET,CT, surgical biopsy | Complete disappearance of lesions in the liver after one and a half months of diagnosis. | Surgical biopsy was suspected as the trigger of the SR. | NA | [56] |
| 96 Y F | MCC | Biopsy | Regression of tumour mass after two weeks of the biopsy. CR was observed subsequently. | The patient used topical tea tree oil | NA | [57] |
| 69 Y M | MCC | Biopsy | Five weeks after diagnosis, the nodular lesion on the head regressed. | NA | 4 Y | [58] |
| 78 Y M | MCC | Biopsy, CT, PET | After the biopsy, tumour nodules present on the head gradually decreased. |
The patient died in 10 months after developing tumour lesions in the rib regions. |
- | [59] |
| 83 Y F | MCC and SCC | Biopsy | The nodule on the nose showed regression in one month, after which surgical resection of the tumour was performed. | No systemic therapy was used. | > 10 Y | [60] |
| 79 Y F | HCC with pulmonary metastasis | CT | Two months after the diagnosis, a significant decrease in liver tumour mass was observed while nodules in the lung completely disappeared. Tumour leison of the hepatic lobe also showed signs of regression | ~Five years after initial follow-ups, the patient developed a solid mass on the liver and several nodules in the lungs, and the patient died within few weeks. | - | [61] |
| 78 Y M | CRC | Colonoscopy, biopsy, CT | Two months after the diagnosis, colectomy was performed, which showed the absence of cancer cells. | NA | 1 Y | [62] |
| 66 Y F | RCC | MRI, CT, biopsy | SR observed after renal mass biopsy. | It was a low-grade carcinoma with oncolytic features. | NA | [63] |
(Y- years, M-months, F-female, M-male, CR- complete recovery, MM- metastatic melanoma, AML- acute myeloid leukemia, NSCLC- non-small cell lung cancer, DLBCL- diffuse large B-cell lymphoma, HL-Hodgkin lymphoma, MCC-Merkel cell carcinoma, SCC-Squamous cell carcinoma, CRC-colorectal cancer, RCC-renal cell carcinoma, PET, positron emission tomography, CT- computed tomography, MRI- magnetic resonance imaging, NA - not available)
Leukaemia, which accounts for 3.1% of global cancer deaths [1], is another cancer that is prone to SR. Bradley and colleagues reviewed several cases of SR of leukaemia that were reported from 1949 to 2019 [34]. Fifty cases were of acute myeloid leukaemia (AML), and four were acute lymphocytic leukaemia (ALL). For leukaemia, out of the five cases reported in 2018, infection-driven remissions were seen in four cases (Table 1). Unfortunately, many of the remissions of AML were not long-lasting: a high relapse rate (< 1Y) was reported among the patients [34,35]. Nevertheless, design-optimized, infection-based therapies for AML might yield a better prognosis. During the last leg of eradication of smallpox, it has been observed that the smallpox vaccine could induce remission of chronic lymphocytic leukaemia [36]. In another instance, an acute flare-up of hepatitis B virus healed a patient of splenic marginal-zone lymphoma [37]. The virus build-up and consequent immune response cleared atypical lymphocytes from the blood and bone marrow of the patient. Concerning SR of lymphoma, three cases of diffuse large B-cell lymphoma (DLBCL), one of follicular lymphoma (FL), and one of Hodgkin lymphoma (HL) (Tables 1, 2) were reported in the past three years. Some of these SRs-especially those associated with FL-could be attributed to Helicobacter pylori infection, a known activating factor in the patient's immune response against the lymphoma cell [38]. DLBCL, on the other hand, is an aggressive cancer with a less prognostic probability of SR. Nevertheless, such remissions do occur. For example, Tanaka and colleagues presented a case of SR of DLBCL [39]. In the patient's biopsy sample, rampant apoptosis of the cancer cells were observed, suggesting that the regression could have occurred via T-cell-mediated induction of apoptosis [39].
Lung cancer accounts for 18% of cancer deaths, making it the leading cause of cancer death [1]. Zhang et al. reported 14 cases of remission of lung cancer, barring patients who have received any chemotherapy and radiotherapy [32]. Among cancers of the lung, SR of non-small cell lung cancer (NSCLC) is very rare. Interestingly, among the reported SR cases of lung cancer, none showed an association with concurrent or preceding infections (Table 2). Instead, such regressions were associated with postoperative trauma. Further, one of the patients attested using the herbal extract of Rock pine (Orostachys japonicus) [40] as a potential causative agent for the remission. Rock pine, widely grown on the rocks in Korea, Japan, and China, has several anticancer compounds [41]. The possibility that some of these compounds in the extract contributing to the remission cannot be ruled out.
Merkel cell carcinoma (MCC) is an aggressive skin cancer with a frequency of 0.7 out of one lakh population and shows a high rate of metastasis [42]. Several cases of SR have been documented for MCC in the past three years. Other less common cases of SR include the regression of cancers of the liver, kidney, and colorectal areas (Table 2).
Decoding SR with a therapeutic perspective
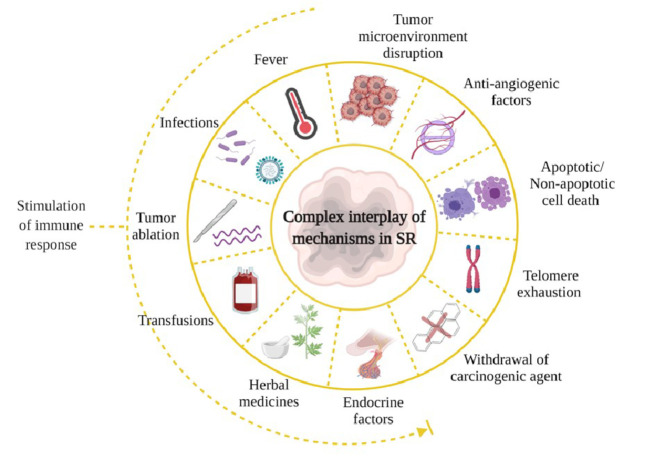
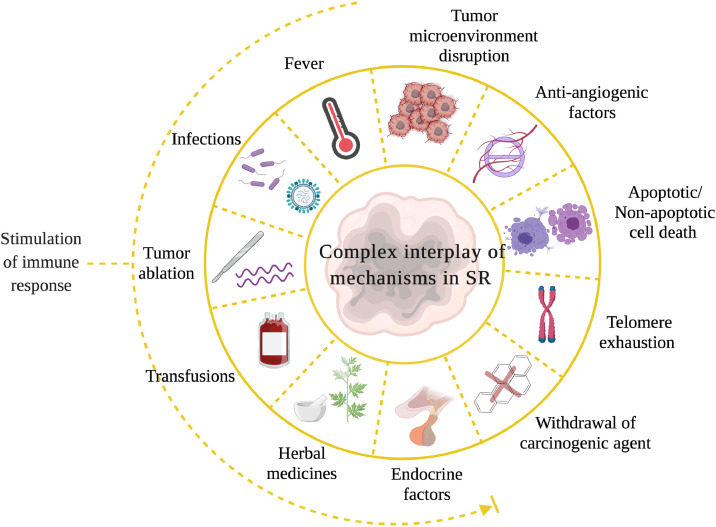
Different forms of cancer originate via a complex interplay among signaling cascades induced by genomic, metabolomic, and proteomic aberrations. Further, their often-counterintuitive modes of sustenance and progression mean that the spontaneous remission of these cancers could as well involve diverse inputs and mechanisms (Fig. 4). Febrile infections are responsible for immune cell infiltrations and cytokine cascades, leading to SR in many cases. Nevertheless, as we have mentioned before, not all cases of SR can be traced to bacterial or viral infection (Table 2). In such scenarios, there must exist hitherto poorly understood mechanisms that heal the tumour. Based on medical and scientific observations, some possible whys and wherefores are as follows: Partial excision of a tumour impairs blood supply to the remaining malignant tissue. Since tumour cells require an ample supply of blood (so much so that the cells themselves promote angiogenesis to meet this vampiric need), cutting it off could starve the cells to death [4]. Not surprisingly, therefore, many SR cases have been attributed to biopsy procedures [4]. Another interesting possibility lies in tumour ablation procedures that could set forth an avalanche of tumour-derived antigens in circulation. Studies have shown that the antigens thus released could act as a therapeutic vaccine [64] (Fig. 4).
Fig. 4.
Possible mechanisms involved in SR of cancers. Factors that stimulate an immune response, including fever and infection, may promote SR. Similarly, disruption in the tumour microenvironment, biopsy and ablation procedures and the induction of cell death by various means, may also induce SR.
Techniques that use radiofrequency waves, high-intensity focused ultrasound, or cryoablation are potential activators of immune cells [64]. In addition to its hormetic effect, exposure to small doses of radiation (such as those received during radiographic examinations) has been shown to induce tumour remission [65]. Low-level radiation (≤ 0.1 Gy for acute irradiation, for example) is capable of pro-SR immunomodulation in experimental settings. Whole- or half-body exposures to low dose radiations [66] as enablers of anticancer immune defense is gaining considerable momentum. Blood transfusion procedures performed in leukemic patients, and use of herbal extracts are also implicated as potential triggers of SR [67,40]. Hormonal influence in SR of breast and prostate cancers and withdrawal of carcinogenic agents could promote the remission [4]. Such non-targeted bystander inputs could lead to processes such as telomerase inhibition, promoting cytostatic or cytocidal outcomes in cancer cells [24] (Fig. 4). Concerning the latter, not only apoptosis but non-apoptotic cell death pathways [68] could also facilitate SR. Such pathways can be considered for future interventional or surrogate therapeutic strategies. Changes in the tumour microenvironment (TME) due to presence of metalloproteinase inhibitors and anti-angiogenesis factors are also believed to play a role in the regression [69] (Fig. 4).
Kwok et al. analyzed SR cases of chronic lymphocytic leukaemia (CLL). They excluded patients with infections, second malignancies, or are undergoing immunosuppressive therapies from their study [70] and compared regressing and non-regressing CLL using flow-cytometry, single nucleotide polymorphism (SNP) analysis, and exome sequencing. It was found that CLL clone in residual SR tumours gradually acquires a low-proliferation state with reduced expression of migration-specific markers. Therefore, it is postulated that in specific genomic backgrounds, changes in microenvironmental factors, such as B-cell-receptor stimulation, can spontaneously regress CLL [70]. This is the only major study published to date that had analyzed the transcriptomic and genomic data to understand the intricacies of SR.
SR and immunity
Since immune response plays crucial roles in tumour regression, many SR cases could also be considered as immunity-mediated tumour remissions. In fact, SR linked to infections influenced the discovery of different anti-cancer therapies that work by boosting the immune system to recognize and attack the cancer cells [71]. The field of cancer immunotherapy (CI) has evolved from clinically-induced infections (Coley's toxins) in the 19th century and cytokine therapy and cancer vaccines in the 20th century to current approaches, such as immune checkpoint inhibitors, oncolytic viruses and Chimeric antigen receptor (CAR)-T cell therapy [72,9]. Different CIs-although they differ in their mechanism of action-work to bolster a weakened immune system. Immuno-onco therapies are broadly classified into active and passive therapies [72]. Active immunotherapy involves triggering the host immune response using cytokines or cancer-specific antigens leading to immunological memory. Passive therapies include direct targeting of tumours with monoclonal antibodies (mABs) or modified immune cells, as in the case of adoptive cell transfer therapy (ACT), without inducing immunological memory [73]. Establishing CI as the fourth pillar of cancer treatment modality (along with chemotherapy, radiation and surgery), the number of CI drugs has been on the rise and many molecules are undergoing clinical trials [74]. CI nevertheless has its drawbacks, such as immunological toxicity [75]. Studies showing that gut microbiomes could influence an individual's anti-tumour response to immunotherapeutic agents are quite intriguing [75], [76], [77]. It has been reported that bacteria from the Bifidobacteriaceae and Bacteroidaceae family enhance the response to anti-PD-L1 (anti-programmed cell death protein ligand- 1) and anti-CTL4 (anti-cytotoxic T-lymphocyte antigen 4S) therapies, respectively [78,79].
Future perspective
Occurrences of spontaneous regression have been reported for many types of cancer. Despairingly, the frequency of SR is very low; one in 60,000–1,00,000 cancer cases [4,9,80]. Melanomas, lymphoma, leukaemia, neuroblastoma, renal cell carcinoma, and germ cell cancers have shown higher frequency of SR [81,8]. The reasons behind higher percentages of SR in certain types of cancer are poorly understood. Understanding the mechanistic aspects of SR of cancer could pave the way for innovative strategies to improve cancer outcomes.
Learning from the drawbacks of Coley's toxin, the search for efficacious recombinant microbes as cancer therapeutic agents began in the 21st century [82]. The genetically engineered bacteria thus created express cytotoxic proteins or toxins to elicit an anti-tumour immune response. For example, programmable bacteria could grow into the core of tumours and deliver anti-CD47 nanobodies into B-cell-lymphoma [83]. A welcome addition to this targeted repertoire is a class of engineered non-pathogenic bacteria that can prime cytotoxic T-cells to induce tumour regression [84]. Anaerobic bacteria could be an apt choice to enhance the targeted delivery into the deep interiors of solid tumours due to their penchant for hypoxic environments [85,82].
Given that antibiotics are available for most bacterial strains, it might be easier to regulate the possibility or severity of infection in such treatment regimens. Interestingly, in addition to full-grown bacteria, bacterial spores have been explored for their tumoricidal effect. When the spores of certain bacterial species germinate, get metabolically active, and grow under conducive conditions, they could promote tumour regression. In this context, Clostridium novyi NT (non-toxic) spores have been extensively studied. These spores have entered phase 1 clinical trials-alone or in combination with chemotherapeutic drugs-for the treatment of solid tumours that are not responding to conventional treatments [82]. When it comes to the fate of the tumour cells under self-regression, apoptosis could be the prevalent mechanism. However, exceptions do occur. During SR of some neuroblastoma, for example, the tumour cells undergo a RAS-associated, caspase-independent, non-apoptotic form of cell death [86]. Therefore, in addition to apoptosis, regulated cell death (RCD) mechanisms, such as ferroptosis, necroptosis, pyroptosis, parthanatos, etc. [68] may play a crucial role in SR. Given that many tumour cells resist natural or drug-induced apoptosis, exploring non-apoptotic RCD mechanisms involved during the self-regression would provide valuable insights of therapeutic relevance. As we have discussed earlier, understanding of infection-based immune system activation against tumours has led to the development of more efficacious treatment strategies of bacteriotherapy and virotherapy. However, research on the non-infection-based mechanisms of SR is still in its infancy.
It is quite challenging to deduce all the factors responsible for spontaneous or indirectly induced tumour regression. Bio-banking tumour biopsy samples of those patients who experienced SR without undergoing any cancer-specific treatments will allow retrospective studies [44]. Investigating SR case reports in the light of diet and microbiome composition of the recovered patient may aid in developing novel therapies with reduced side effects. Further, insights from these studies may help predispose and orient one's lifestyle towards conditions that are barren for cancer cells to flourish.
CRediT authorship contribution statement
Gudapureddy Radha: Writing – review & editing. Manu Lopus: Writing – review & editing.
Declaration of Competing Interest
None to be declared
Acknowledgment
The authors thank UM-DAE Centre for Excellence in Basic Sciences for financial support. All figures used in this article were created using BioRender Software
References
- 1.Sung H., Ferlay J., Siegel R.L. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021 doi: 10.3322/caac.21660. [Published online ahead of print February 4, 2021] [DOI] [PubMed] [Google Scholar]
- 2.Thomas A., Teicher B.A., Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17(6):e254–e262. doi: 10.1016/S1470-2045(16)30030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madamsetty V.S., Mukherjee A., Mukherjee S. Recent trends of the bio-inspired nanoparticles in cancer theranostics. Front Pharmacol. 2019;25(10):1264. doi: 10.3389/fphar.2019.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COLE W.H., EVERSON T.C. Spontaneous regression of cancer: preliminary report. Ann. Surg. 1956;144(3):366–383. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson W.R., Ebbell B. The papyrus ebers; the greatest egyptian medical document. J. Egypt Archaeol. 1938;24(1):250–251. [Google Scholar]
- 6.St. Peregrine PG.T. O.S.M.-The patron saint of cancer patients. CA Cancer J. Clin. 1967;17(4):181–182. [PubMed] [Google Scholar]
- 7.Saint Peregrine JR. O.S.M.-The patron saint of cancer patients. Can. Med. Assoc. J. 1974;111(8):827. 824. [PMC free article] [PubMed] [Google Scholar]
- 8.Pakhmode V. Understanding the possible mechanisms of spontaneous regression of oral cancer. J. Oral Maxillofac. Pathol. 2007;11(1):2–4. [Google Scholar]
- 9.Dobosz P., Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol. 2019;10:2965. doi: 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoption Cann S.A., Van Netten J.P., Van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad. Med. J. 2003;79(938):672–680. [PMC free article] [PubMed] [Google Scholar]
- 11.Kucerova P., Cervinkova M. Spontaneous regression of tumour and the role of microbial infection-possibilities for cancer treatment. Anticancer Drugs. 2016;27(4):269–277. doi: 10.1097/CAD.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coley W.B. Contribution to the knowledge of sarcoma. Ann. Surg. 1891;14(3):199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas J.A., Badini M. The role of innate immunity in spontaneous regression of cancer. Indian J. Cancer. 2011;48(2):246–251. doi: 10.4103/0019-509X.82887. [DOI] [PubMed] [Google Scholar]
- 14.Luca S., Mihaescu T. History of BCG vaccine. Maedica. 2013;8(1):53–58. (Buchar) [PMC free article] [PubMed] [Google Scholar]
- 15.Old L.J., Clarke D.A., Benacerraf B. Effect of Bacillus calmette-guérin infection on transplanted tumours in the mouse. Nature. 1959;184(Suppl 5):291–292. doi: 10.1038/184291a0. [DOI] [PubMed] [Google Scholar]
- 16.Morales A., Eidinger D., Bruce A.W. Intracavitary Bacillus Calmette Guerin in the treatment of superficial bladder tumors. J. Urol. 1976;116(2):180–182. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 17.Ratliff T.L., Ritchey J.K., Yuan J.J.J., Andriole G.L., Catalona W.J. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J. Urol. 1993;150(3):1018–1023. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 18.Fuge O., Vasdev N., Allchorne P., Green J.S. Immunotherapy for bladder cancer. Res. Rep. Urol. 2015;7:65–79. doi: 10.2147/RRU.S63447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redelman-Sidi G., Glickman M.S., Bochner B.H. The mechanism of action of BCG therapy for bladder cancer-a current perspective. Nat. Rev. Urol. 2014;11(3):153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 20.Pettenati C., Ingersoll M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018;15(10):615–625. doi: 10.1038/s41585-018-0055-4. [DOI] [PubMed] [Google Scholar]
- 21.Dock G. The influence of complicating diseases upon leukæmia.*. Am. J. Med. Sci. 1904;127(4):563–592. Published online. [Google Scholar]
- 22.A.M. Taqi, M.B. Abdurrahman, A.M. Yakubu, A.F. Fleming Regression of hodgkin's disease after measles. Lancet. 198;1(8229):1112. [DOI] [PubMed]
- 23.Bluming A.Z., Ziegler J.L. Regression of burkitt's lymphoma in association with measles infection. Lancet. 1971;2(7715):105–106. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 24.Kelly E., Russell S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. Published online. [DOI] [PubMed] [Google Scholar]
- 25.Larson C., Oronsky B., Scicinski J. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget. 2015;6(24):19976–19989. doi: 10.18632/oncotarget.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamarin D., Holmgaard R.B., Subudhi S.K. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6(226):226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ylösmäki E., Cerullo V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020;65:25–36. doi: 10.1016/j.copbio.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Andtbacka R.H.I., Kaufman H.L., Collichio F. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 29.Zheng M., Huang J., Tong A., Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol. Ther. Oncol. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conry R.M., Westbrook B., McKee S., Norwood T.G. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum. Vaccin. Immunother. 2018;14(4):839–846. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wrotek S., Brycht Ł., Wrotek W., Kozak W. Fever as a factor contributing to long-term survival in a patient with metastatic melanoma: a case report. Complement Ther. Med. 2018;38:7–10. doi: 10.1016/j.ctim.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 32.J. Zhang, H. Wang, C. Li, H. Qian Chance to rein in a cancer-spontaneous regression of lung carcinoma (1988–2018): a 30-year perspective. 2020; 13(5):1190-1196. [PMC free article] [PubMed]
- 33.Tran T., Burt D., Eapen L., Keller O.R. Spontaneous regression of metastatic melanoma after inoculation with tetanus-diphtheria-pertussis vaccine. Curr. Oncol. 2013;20(3):e270–e273. doi: 10.3747/co.20.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley T., Zuquello R.A., Aguirre L.E. Spontaneous remission of acute myeloid leukemia with NF1 alteration. Leuk Res. Rep. 2020;13 doi: 10.1016/j.lrr.2020.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rautenberg C., Kaivers J., Germing U., Haas R., Schroeder T., Kobbe G. Spontaneous remission in a patient with very late relapse of acute myeloid leukemia 17 years after allogeneic blood stem cell transplantation. Eur. J. Haematol. 2019;103(2):131–133. doi: 10.1111/ejh.13245. [DOI] [PubMed] [Google Scholar]
- 36.Hansen R.M., Libnoch J.A. Remission of chronic lymphocytic leukemia after smallpox vaccination. Arch Intern. Med. 1978;138(7):1137–1138. [PubMed] [Google Scholar]
- 37.Fujimoto K., Endo T., Nishio M. Complete remission of splenic marginal zone lymphoma after an acute flare-up of hepatitis B in a hepatitis B virus carrier. Int. J. Hematol. 2009;90(5):601–604. doi: 10.1007/s12185-009-0426-y. [DOI] [PubMed] [Google Scholar]
- 38.Morigi A., Casadei B., Argnani L., Cavo M., Zinzani P.L. Spontaneous remission of follicular lymphoma. Hematol. Oncol. 2019;37(5):626–627. doi: 10.1002/hon.2653. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y., Ishihara M., Miyoshi H., Hashimoto A., Shinzato I., Ohshima K. Spontaneous regression of diffuse large B-cell lymphoma in the small intestine with multiple lymphadenopathy. J. Clin. Exp. Hematop. 2019;59(1):17–21. doi: 10.3960/jslrt.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon H.Y., Park H.S., Cho M.S., Shim S.S., Kim Y., Lee J.H. Spontaneous remission of advanced progressive poorly differentiated non-small cell lung cancer: A case report and review of literature. BMC Pulm. Med. 2019;19(1):210. doi: 10.1186/s12890-019-0978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee G.S., Lee H.S., Kim S.H., Suk D.H., Ryu D.S., Lee D.S. Anti-cancer activity of the ethylacetate fraction from orostachys japonicus for modulation of the signaling pathway in HepG2 human hepatoma cells. Food Sci. Biotechnol. 2014;23(1):269–275. [Google Scholar]
- 42.Paulson K.G., Park S.Y., Vandeven N.A. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018;78(3):457–463. doi: 10.1016/j.jaad.2017.10.028. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helbig D., Quesada A.E., Xiao W., Roshal M., Tallman M.S., Knorr D.A. Spontaneous remission in a patient with acute myeloid leukemia leading to undetectable minimal residual disease. J. Hematol. 2020;9(1-2):18–22. doi: 10.14740/jh606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Höres T., Wendelin K., Schaefer-Eckart K. Spontaneous remission of acute lymphoblastic leukemia: a case report. Oncol. Lett. 2018;15(1):115–120. doi: 10.3892/ol.2017.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray N.P., Fuentealba C., Salazar I., Salazar A., Lopez M.A., Minzer S. Transitory spontaneous remission of myelodysplasia in an elderly man while receiving intravesical bacillus calmette-guérin for bladder cancer: a case report and review of the literature. Case Rep. Hematol. 2018;2018 doi: 10.1155/2018/9750532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kappauf H., Esser G. Metachronous spontaneous remission of melanoma lung metastasis and mediastinal lymph node metastases. Oncol. Res. Treat. 2018;41(3):135–138. doi: 10.1159/000485626. [DOI] [PubMed] [Google Scholar]
- 47.Schlabe J., Shah K.A., Sheerin F., Payne M.J., Fasanmade A.A. Complete spontaneous regression of a metastatic melanoma of the mandible: a case report and follow-up recommendations. Int. J. Oral. Maxillofac. Surg. 2018;47(12):1519–1522. doi: 10.1016/j.ijom.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Grunwald V.V., Hentrich M., Schiel X. Patients with spontaneous remission of high-risk MDS and AML show persistent preleukemic clonal hematopoiesis. Blood Adv. 2019;3(18):2696–2699. doi: 10.1182/bloodadvances.2019000265. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shatola A., Nguyen K.N., Kamangar E., Daly M.E. Spontaneous regression of non-small cell lung cancer: a case report and literature review. Cureus. 2020;12(1):e6639. doi: 10.7759/cureus.6639. Published online January 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooi K.H., Cheo T., Soon G.S.T., Leong C.N. Spontaneous regression of locally advanced nonsmall cell lung cancer: a case report. Med. 2018;97(31) doi: 10.1097/MD.0000000000011291. (United States) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui T., Mizuno T., Kuroda H. Spontaneous regression of lung squamous cell carcinoma with synchronous mediastinal progression: a case report. Thorac. Cancer. 2018;9(12):1778–1781. doi: 10.1111/1759-7714.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esplin N., Fergiani K., Legare T.B., Stelzer J.W., Bhatti H., Ali S.K. Spontaneous regression of a primary squamous cell lung cancer following biopsy: a case report. J. Med. Case Rep. 2018;12(1) doi: 10.1186/s13256-018-1589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snijder J., Mihyawi N., Frolov A., Ewton A., Rivero G. Spontaneous remission in diffuse large cell lymphoma: a case report. J. Med. Case Rep. 2019;13(1) doi: 10.1186/s13256-018-1937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toberer F., Mechtersheimer G., Jaschinski H., Enk A., Hakim-Meibodi L., Haenssle H.A. Spontaneous regression of primary cutaneous diffuse large B-cell lymphoma, leg type. Acta Derm. Venereol. 2018;98(6):608–609. doi: 10.2340/00015555-2921. [DOI] [PubMed] [Google Scholar]
- 55.Pasvolsky O., Berger T., Bernstine H., Hayman L., Raanani P., Vidal L. Spontaneous regression of hodgkin lymphoma: case report and review of the literature. Acta Haematol. 2019;141(1):14–18. doi: 10.1159/000494422. [DOI] [PubMed] [Google Scholar]
- 56.Nakamoto R., Okuyama C., Oka S. Complete Spontaneous regression of hepatosplenic T-cell lymphoma after surgical biopsy. Clin. Nucl. Med. 2020;45(2):e88–e91. doi: 10.1097/RLU.0000000000002782. [DOI] [PubMed] [Google Scholar]
- 57.S. Branch, K. Maloney, S.M. Purcell Case report spontaneous regression of merkel cell carcinoma. 2018; 101(4):301-305. [PubMed]
- 58.Marcoval J., Valentí-Medina F., Penín R.M., Bermejo J. Complete spontaneous regression of the primary tumor in merkel cell carcinoma. Actas Dermosifiliogr. 2018;109(8):752–754. doi: 10.1016/j.ad.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi Y., Nakamura M., Kato H., Morita A. Distant recurrence of merkel cell carcinoma after spontaneous regression. J. Dermatol. 2019;46(4):e133–e134. doi: 10.1111/1346-8138.14652. [DOI] [PubMed] [Google Scholar]
- 60.Nagase K., Inoue T., Koba S., Narisawa Y. Case of probable spontaneous regression of merkel cell carcinoma combined with squamous cell carcinoma without surgical intervention. J. Dermatol. 2018;45(7):858–861. doi: 10.1111/1346-8138.14335. [DOI] [PubMed] [Google Scholar]
- 61.Chohan M.B.Y., Taylor N., Coffin C., Burak K.W., Bathe O.F. Spontaneous regression of hepatocellular carcinoma and review of reports in the published english literature. Case Rep. Med. 2019;2019 doi: 10.1155/2019/9756758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karakuchi N., Shimomura M., Toyota K. Spontaneous regression of transverse colon cancer with high-frequency microsatellite instability: a case report and literature review. World J. Surg. Oncol. 2019;17(1):19. doi: 10.1186/s12957-018-1552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srivastava A., Meyer A.R., Pierorazio P.M., Rowe S.P., Allaf M.E., Gorin M.A. Spontaneous Regression of a low-grade renal cell carcinoma with oncocytic features after renal mass biopsy. Clin. Genitourin. Cancer. 2018;16(6):e1083–e1085. doi: 10.1016/j.clgc.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Butterfield L.H. Cancer vaccines. BMJ. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sasaki J., Kurihara H., Nakano Y., Kotani K., Tame E., Sasaki A. Apparent spontaneous regression of malignant neoplasms after radiography: report of four cases. Int. J. Surg. Case Rep. 2016;25:40–43. doi: 10.1016/j.ijscr.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janiak M.K., Wincenciak M., Cheda A., Nowosielska E.M., Calabrese E.J. Cancer immunotherapy: how low-level ionizing radiation can play a key role. Cancer Immunol. Immunother. 2017;66(7):819–832. doi: 10.1007/s00262-017-1993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitterbauer M., Fritzer-Szekeres M., Mitterbauer G. Spontaneous remission of acute myeloid leukemia after infection and blood transfusion associated with hypergammaglobulinaemia. Ann. Hematol. 1996;73(4):189–193. doi: 10.1007/s002770050226. [DOI] [PubMed] [Google Scholar]
- 68.Nirmala J.G., Lopus M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020;36(2):145–164. doi: 10.1007/s10565-019-09496-2. [DOI] [PubMed] [Google Scholar]
- 69.Ricci S.B., Cerchiari U. Spontaneous regression of malignant tumors: Importance of the immune system and other factors (review) Oncol. Lett. 2010;1(6):941–945. doi: 10.3892/ol.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwok M., Oldreive C., Rawstron A.C. Integrative analysis of spontaneous CLL regression highlights genetic and microenvironmental interdependency in CLL. Blood. 2020;135(6):411–428. doi: 10.1182/blood.2019001262. [DOI] [PubMed] [Google Scholar]
- 71.Lizée G., Overwijk W.W., Radvanyi L., Gao J., Sharma P., Hwu P. Harnessing the power of the immune system to target cancer. Annu. Rev. Med. 2013;64:71–90. doi: 10.1146/annurev-med-112311-083918. [DOI] [PubMed] [Google Scholar]
- 72.Abbott M., Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Sem. Oncol. Nurs. 2019;35(5) doi: 10.1016/j.soncn.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Galluzzi L., Vacchelli E., Bravo-San Pedro J.M., Buqué A. Classification of current anticancer immunotherapies. Oncotarget. 2014;30(24):12472–12508. doi: 10.18632/oncotarget.2998. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang J., Shalabi A., Hubbard-Lucey V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 2018;29(1):84–91. doi: 10.1093/annonc/mdx755. [DOI] [PubMed] [Google Scholar]
- 75.Dai Z., Zhang J., Wu Q. Intestinal microbiota: a new force in cancer immunotherapy. Cell Commun. Signal. 2020;18(1):90. doi: 10.1186/s12964-020-00599-6. Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gopalakrishnan V., Spencer C.N., Nezi L. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Routy B., Le Chatelier E., Derosa L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. Jan 5. [DOI] [PubMed] [Google Scholar]
- 78.Sivan A., Corrales L., Hubert N., Williams J.B. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vétizou M., Pitt J.M., Daillère R., Lepage P. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jessy T. Immunity over inability: the spontaneous regression of cancer. J. Nat. Sci. Biol. Med. 2011;2(1):43–49. doi: 10.4103/0976-9668.82318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Papac R.J. Spontaneous regression of cancer: possible mechanisms. In Vivo. 1998;12(6):571–578. (Brooklyn) [PubMed] [Google Scholar]
- 82.Sedighi M., Zahedi Bialvaei A., Hamblin M.R. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med. 2019;8(6):3167–3181. doi: 10.1002/cam4.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dougan M., Dougan S.K. Programmable bacteria as cancer therapy. Nat. Med. 2019;25(7):1030–1031. doi: 10.1038/s41591-019-0513-4. [DOI] [PubMed] [Google Scholar]
- 84.Sockolosky J.T., Dougan M., Ingram J.R. Durable antitumor responses to sedsedCD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. U S A. 2016;113(19):E2646–E2654. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forbes N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer. 2010;10(11):785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitanaka C., Kato K., Ijiri R. Increased ras expression and caspase-independent neuroblastoma cell death: possible mechanism of spontaneous neuroblastoma regression. J. Natl. Cancer Inst. 2002;94(5):358–368. doi: 10.1093/jnci/94.5.358. [DOI] [PubMed] [Google Scholar]