Significance
Plants can detect the proximity and density of neighboring vegetation using phytochrome photoreceptors. In shade-sensitive species, canopy cover drives stem elongation, facilitating light foraging. In deep shade, where resources are severely limited, excessive elongation growth can be detrimental to plant survival. In these conditions, phytochrome-A signaling suppresses shade avoidance. Here, we provide a molecular mechanism controlling this response. We show that phytochrome A promotes early-evening increases in the expression of the circadian-clock components TIMING OF CAB EXPRESSION 1 (TOC1), PSEUDO RESPONSE REGULATOR 7 (PRR7), EARLY FLOWERING 3 (ELF3), and ELF4, encoding proteins that collectively limit hypocotyl elongation. This indicates that light signals are integrated with the circadian clock to control plant development in stressful environments.
Keywords: phytochrome A, shade avoidance, circadian clock
Abstract
Shade-avoiding plants can detect the presence of neighboring vegetation and evoke escape responses before canopy cover limits photosynthesis. Rapid stem elongation facilitates light foraging and enables plants to overtop competitors. A major regulator of this response is the phytochrome B photoreceptor, which becomes inactivated in light environments with a low ratio of red to far-red light (low R:FR), characteristic of vegetational shade. Although shade avoidance can provide plants with a competitive advantage in fast-growing stands, excessive stem elongation can be detrimental to plant survival. As such, plants have evolved multiple feedback mechanisms to attenuate shade-avoidance signaling. The very low R:FR and reduced levels of photosynthetically active radiation (PAR) present in deep canopy shade can, together, trigger phytochrome A (phyA) signaling, inhibiting shade avoidance and promoting plant survival when resources are severely limited. The molecular mechanisms underlying this response have not been fully elucidated. Here, we show that Arabidopsis thaliana phyA elevates early-evening expression of the central circadian-clock components TIMING OF CAB EXPRESSION 1 (TOC1), PSEUDO RESPONSE REGULATOR 7 (PRR7), EARLY FLOWERING 3 (ELF3), and ELF4 in photocycles of low R:FR and low PAR. These collectively suppress stem elongation, antagonizing shade avoidance in deep canopy shade.
Plants have evolved molecular mechanisms to detect competitors and avoid shading. Above-ground neighbor-detection signals include alterations in light quality, mechanical stimulation, and accumulation of volatiles such as the plant hormone ethylene (1). Shade-avoidance responses include the elongation of hypocotyls and petioles to elevate leaves. Prior to canopy closure, plants detect neighbors through the touching of leaf tips and perception of far-red light (FR, 700 to 800 nm) signals reflected from green tissue (2). The relative enrichment of the local light environment with FR reduces the red to far-red ratio (R:FR), which inactivates phytochrome B (phyB), phyD, and phyE photoreceptors and initiates shade avoidance. Within a canopy, red (R, 600 to 700 nm), blue (B, 400 to 500 nm), and ultraviolet-B (UV-B) (280 to 315 nm) wavelengths are also filtered, leading to an environment with significantly reduced R:FR, a depletion of photosynthetically active radiation (PAR) and reduced UV-B (1).
Low R:FR–induced stem elongation is driven largely by inactivation of phyB (1). This results in the stabilization and activation of a subgroup of phytochrome-interacting basic helix–loop–helix (bHLH) transcription factors termed PHYTOCHROME INTERACTING FACTORS (PIFs). PIFs 3, 4, and 5 show enhanced protein stabilization in low R:FR and promote stem elongation in these conditions (3, 4). PIF7 undergoes dephosphorylation and nuclear localization in low R:FR, performing a major role in shade avoidance (5, 6). PIFs 4, 5, and 7 have been shown to bind to auxin-biosynthesis gene promoters and increase their expression in low R:FR, elevating auxin and promoting stem elongation (5, 7). Excessive elongation is prevented by multiple negative feedback loops. Low R:FR increases the abundance of proteins that interact with and inhibit PIF function through the formation of heterodimers. These include the bHLH transcription factor LONG HYPOCOTYL IN FAR-RED (HFR1) (8) and atypical bHLH proteins PHYTOCHROME RAPIDLY REGULATED 1 (PAR1) and PAR2 (9). Other PIF-binding negative regulators include the gibberellin-regulated DELLA proteins (10), the cryptochrome photoreceptor (11, 12), and the circadian clock components ELF3, TOC1, and PRR proteins (13–16).
Stem elongation responses are exaggerated by the combination of very low R:FR and low PAR present in canopy shade. These conditions promote the activity of phyA (17). In contrast to other phytochromes, phyA is relatively light labile in the active Pfr form. Enhanced light lability and unique signaling properties enable phyA to signal in FR through a high irradiance response (HIR) mode (18). Arabidopsis mutants deficient in phyA display exaggerated shade-avoidance responses to low R:FR (19, 20) and reduced survival in deep vegetational shade (17). It is therefore assumed that phyA antagonizes shade avoidance to promote survival in dense canopies (17). Laboratory studies support this hypothesis and have shown that the effectiveness of phyA in suppressing shade-induced hypocotyl elongation is inversely correlated with both R:FR and PAR. Maximum phyA-mediated hypocotyl inhibition occurs in very low R:FR and low PAR conditions, consistent with dense canopy cover (21). However, understanding of the molecular mechanisms controlling this response remains incomplete. In photosynthetically limited conditions, shade avoidance is predominantly driven by increased sensitivity to auxin, in a response involving PIF4 and PIF5 (7, 22, 23). phyA has been shown to weaken auxin signaling by directly binding to auxin/indole acetic acid (AUX/IAA) proteins (24) and repressing brassinosteroid signaling (25). Here, we show that, in deep canopy shade, phyA also promotes TOC1, PRR7, ELF3, and ELF4 expression to suppress stem elongation and prevent unnecessary shade-avoidance responses in severely light-deficient environments.
Results
Low R:FR Causes Low-Amplitude Circadian Rhythms.
Rapid hypocotyl-elongation responses to shade are regulated by the circadian clock and display sensitivity to the time of day (20). The clock regulates PIF transcript levels, while photoreceptors control PIF protein abundance. This coincidence between clock and photoreceptor signaling underlies the rhythmic control of plant growth in day/night cycles (23). Furthermore, photoreceptors act to entrain the circadian clock, highlighting the complex interplay between light and circadian signaling (26, 27). We therefore wished to understand how prolonged growth in vegetational shade affects circadian-clock function. The impact of reduced R:FR on the relative transcript abundance of the circadian-clock components CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and TOC1 was first assessed in free running conditions. CCA1 and TOC1 transcripts reach peak abundance in the morning and evening, respectively, and form a transcription–translation feedback loop within the circadian oscillator (28). Plants were entrained to 12-h light/12-h dark photocycles of either high R:FR (5) or low R:FR (0.05) (SI Appendix, Fig. S1 A and B) for 4 d before transfer to continuous light (LL) on day 5. Seedlings were sampled for analysis of transcript abundance every 4 h, for 2 d, starting on day 6 (Fig. 1 A and B). In high R:FR, CCA1 (Fig. 1A) and TOC1 (Fig. 1B) both had circadian rhythms of transcript abundance; CCA1 transcript levels peaked at the start of the subjective day, and TOC1 transcript levels peaked at the end. For plants grown in low R:FR, transcript oscillations appeared to be arrhythmic (Fig. 1 A and B). Under low R:FR, CCA1 assumed a low transcript abundance, whereas TOC1 transcripts assumed a relatively high abundance (Fig. 1 A and B).
Fig. 1.
Low R:FR damps circadian rhythms of CCA1 and TOC1. Ler seedlings were entrained in white light (12-h light/12-h dark) in either R:FR = 5 (open circles) or R:FR = 0.05 (filled circles) for 4 d. Plants were transferred to 24-h LL on day 5. The 6-, 7-, and 8-d-old-seedlings were sampled for RNA analysis. The mean relative transcript abundance of two independent experiments is shown (n = 2) for (A) CCA1 and (B) TOC1 normalized to ACTIN2 ± SE. (C and D) Luciferase activity of (C) CCA1pro::LUC and (D) TOC1pro::LUC (Col-0) at R:FR ratios of 0.05 (red line), 0.5 (brown line) and 1.2 (blue line) in light/dark cycles (0 to 48 h), followed by free-run (48 to 168 h) conditions. Dark-gray shading indicates night, while light-gray shading indicates subjective night under continuous light conditions. Plants were grown for 7 d at a PAR of 70 µmol ⋅ m−2 ⋅ s−1 at the R:FR ratio indicated and then transferred to 47 µmol ⋅ m−2 ⋅ s−1 for 3 d to entrain, maintaining the same R:FR ratios. The 10-d-old plants in clusters of 12 were dosed with luciferin, and imaging commenced 24 h later (time 0 on the graph). Data represent means ± SE (n = 6). (E) Comparison of period length and RAE using data derived from FFT-NLLS analysis of CCA1pro::LUC and TOC1pro::LUC free-run data. Data represent means ± SE (n = 6). (F and G) Period-length box plots of CCA1pro::LUC (F) and TOC1pro::LUC (G). Different letters indicate statistically significant differences at P < 0.05 by one-way ANOVA and Tukey’s post hoc test.
We reasoned that the altered abundance of CCA1 and TOC1 transcripts under low R:FR (Fig. 1 A and B) might result from low R:FR–mediated effects on promoter activity. Diel and circadian rhythms of CCA1 and TOC1 promoter activity were monitored at three different R:FR ratios of continuous light; high (1.2), intermediate (0.5), and low (0.05) R:FR, which correspond with direct sunlight, moderate crowding by neighboring vegetation, and densely crowded conditions, respectively (SI Appendix, Fig. S2 A–C). Transgenic plants expressing CCA1pro::LUC (Fig. 1C) and TOC1pro::LUC (Fig. 1D) had robust rhythms of promoter activity across the range of R:FR ratios tested in light/dark cycles (first 48 h of data acquisition), suggesting that low R:FR does not disrupt light input to the clock. After 24 h of continuous low R:FR, CCA1pro::LUC and TOC1pro::LUC oscillations had reduced amplitude compared to oscillations in high and intermediate R:FR conditions (Fig. 1 C and D). CCA1pro::LUC and TOC1pro::LUC assumed relatively low and high signals, respectively, which is consistent with transcript-abundance data (Fig. 1 A and B) and their reciprocal transcriptional repression (29, 30). Relative amplitude error (RAE) from fast Fourier transform nonlinear least squares (FFT-NLLS) analysis indicates the closeness of fit of the experimental data to a sine wave (where 0 is a perfect fit, 1 is no fit, and values >0.5 are often taken to suggest arrhythmia). FFT-NLLS analysis of CCA1pro::LUC and TOC1pro::LUC data after 24 h of constant conditions indicated that plants in low R:FR had greater RAE than plants grown in high and intermediate R:FR (Fig. 1E). This suggests that the rhythmic robustness of the oscillation was weakened by low R:FR. Decreases in R:FR ratio also significantly reduced period lengths of rhythms of both CCA1pro::LUC and TOC1pro::LUC (Fig. 1 F and G), suggesting that a very low R:FR ratio can accelerate the pace of the circadian clock.
TOC1 Transcripts Are Elevated at Dusk in Photocycles of Deep Shade.
Adding supplementary FR to a background of white light reduces R:FR without simulating the light-limiting conditions within a canopy. Such treatments mimic neighbor-proximity cues prior to canopy closure and are commonly used in shade-avoidance studies (20, 31). The failure of low R:FR to reduce the amplitude of circadian rhythms in light/dark cycles (Fig. 1 C and D, first 48 h) led us to hypothesize that this rhythmicity was maintained by robust photoreceptor signaling under light/dark cycles. We reasoned that, in deep shade (where PAR and R:FR are simultaneously reduced), this might not occur. Measurements of deep-shade spectra outdoors in Bristol, United Kingdom, found that a R:FR of <0.1 occurred in environments where PAR reached <15 µmol ⋅ m−2 ⋅ s−1 (SI Appendix, Fig. S1 C and D). We therefore analyzed the effect of R:FR on CCA1 and TOC1 rhythmicity under light/dark cycles at a PAR of 5 µmol ⋅ m−2 ⋅ s−1 to mimic deep shade. CCA1 and TOC1 promoter activity was recorded under three cycles of high R:FR (0.9) followed by three cycles of low R:FR (0.05) before return to four cycles of high R:FR (Fig. 2A and SI Appendix, Fig. S2 D and E). To additionally examine the effect of photoperiod on this response, experiments were performed in both 12-h light/12-h dark (Fig. 2 B and C) and 8-h light/16-h dark cycles (Fig. 2 D and E). Although the peak luciferase signal was reduced compared with experiments using a greater PAR, CCA1pro::LUC and TOC1pro::LUC activity remained rhythmic, with traces having kurtoses typical of plants in light/dark cycles (Fig. 2 B and C; compare to the first three cycles of Fig. 1 C and D). CCA1pro::LUC rhythms appeared unaffected by low-R:FR treatment in a background of low PAR in 12-h light/12-h dark cycles (Fig. 2 B and F). In 8-h light/16-h dark cycles, the amplitude of CCA1pro rhythms was significantly reduced following the first full day of low-R:FR treatment (Fig. 2 D and G). Surprisingly, transfer to low R:FR at low PAR increased TOC1pro::LUC activity at dusk and during the night, potentially explaining the reduced amplitude of CCA1 in Fig. 2D. This effect was reversed following transfer back to high R:FR (Fig. 2 C, E, H, and I). Low R:FR–mediated increases in TOC1pro::LUC amplitude did not occur in an identical experiment performed at a higher PAR, confirming the restriction of this response to low PAR (SI Appendix, Fig. S3). Transfer to low R:FR at low PAR also increased TOC1 transcript accumulation at dusk (Fig. 3A and SI Appendix, Fig. S1 E and F).
Fig. 2.
Low R:FR elevates TOC1 abundance at dusk in photocycles of deep shade. (A). Experimental design. Plants were grown in 12-h light/12-h dark or 8-h light/16-h dark cycles (PAR = 70 μmol ⋅ m−2 ⋅ s−1, R:FR = 5) for 7 d before transfer to PAR = 5 μmol ⋅ m−2 ⋅ s−1, R:FR = 0.9 for 4 d. Imaging was carried out during 10 light/dark cycles (PAR = 5 μmol ⋅ m−2 ⋅ s−1, R:FR = 0.9 (3 d), 0.05 (3 d), and 0.9 (4 d). (B–E) Luciferase activity of CCA1pro::LUC (B and D) and TOC1pro::LUC (C and E) from plants grown in 12-h light/12-h dark (B and C) or 8-h light/16-h dark (D and E) conditions. Gray shading indicates period of darkness, and red shading indicates photoperiods with R:FR = 0.05. Data represent means ± SE (n = 6). (F–I) Amplitude of CCA1pro::LUC (F and G) and TOC1pro::LUC (H and I) rhythmic expression for each light treatment for 12-h light/12-h dark- (F and H) and 8-h light/16-h dark- (G and I) grown plants. Data represent means ± SD (n = 6). Statistically significant differences at P < 0.05 by one-way ANOVA and Tukey’s post hoc test are indicated with *.
Fig. 3.
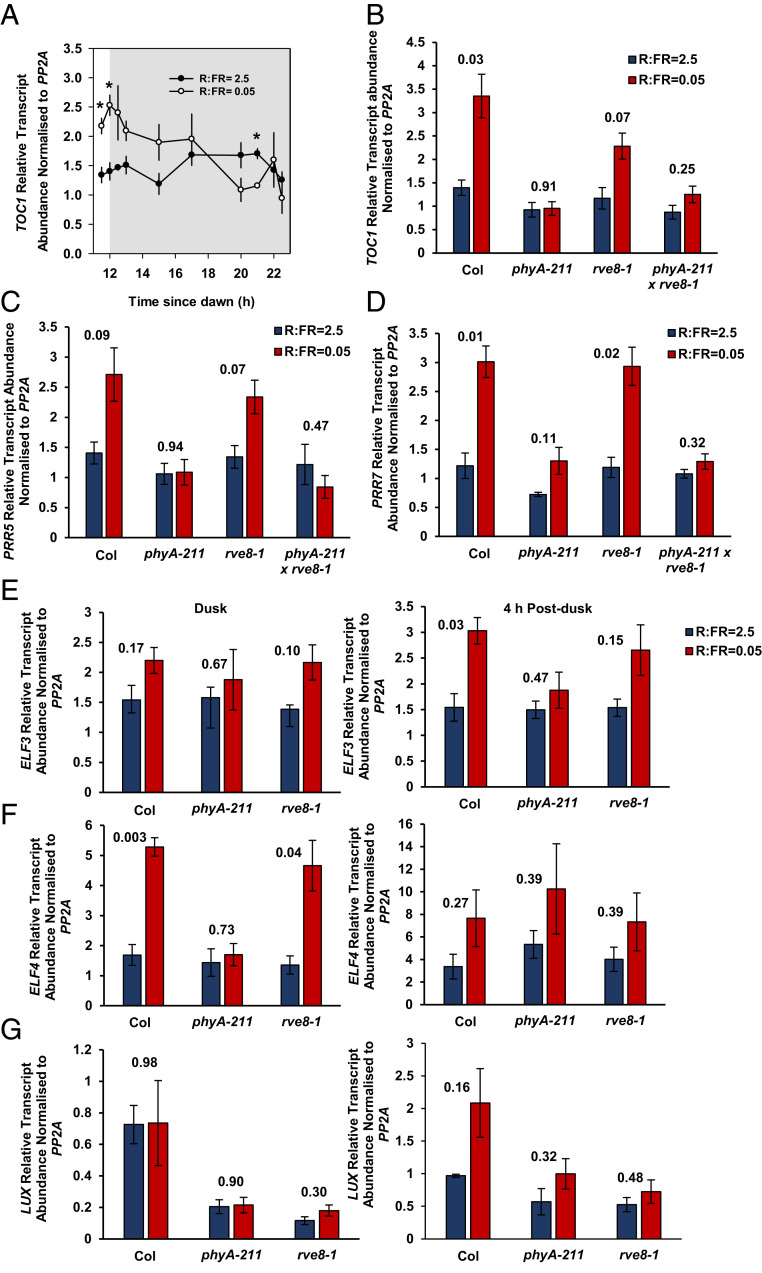
phyA elevates TOC1, PRR7, ELF3, and ELF4 transcripts in photocycles of deep shade. (A) TOC1 relative transcript abundance normalized to PP2A in Col-0 grown in 12-h photoperiods. Seedlings were germinated in 12-h light/12-h dark cycles of white light (70 µmol ⋅ m−2 ⋅ s−1, R:FR = 5) for 48 h before transfer to 12-h light/12-h dark cycles at 5 µmol ⋅ m−2 ⋅ s−1 and a R:FR of 2.5 or 0.05 for a further 5 d. Sampling was performed at the indicated times, and gray shading indicates the dark period. Data represent means ± SE (n = 3). Statistically significant differences at P < 0.05 between high and low R:FR–treated plants at each time point are indicated with *. (B) TOC1 relative transcript abundance at dusk normalized to PP2A in phyA-211, rve8-1, and phyA-211/rve8-1 mutants grown in 8-h photoperiods. Seedlings were germinated in 8-h light/16-h dark cycles of white light (70 µmol ⋅ m−2 ⋅ s−1, R:FR = 5) for 48 h before transfer to 8-h light/16-h dark cycles at 5 µmol ⋅ m−2 ⋅ s−1 and a R:FR of 2.5 or 0.05 for a further 3 d. P values from t tests comparing the log-transformed ΔΔCT values within genotypes are shown. (C and D) Relative transcript abundance of PRR5 (C) and PRR7 (D) normalized to PP2A in phyA-211, rve8-1, and phyA-211/rve8-1 mutants. Plants were grown as in B (n = 3). (E–G) Relative transcript abundance of ELF3 (E), ELF4 (F), and LUX (G) normalized to PP2A at dusk and 4 h postdusk in phyA-211 and rve8-1 mutants. Plants were grown as in B (n = 3). P values from t tests comparing the log-transformed ΔΔCT values within genotypes are shown.
phyA and REVEILLE8 Increase the Abundance of TOC1 Transcripts in Deep Shade.
Low-R:FR treatment in a background of low PAR consistently increased dusk levels of the TOC1 transcript in both Ler and Col-0 backgrounds (Fig. 3 A and B and SI Appendix, Fig. S4A). phyA has been shown to increase TOC1 transcript abundance during FR-mediated seedling de-etiolation (32). Consistent with these data, phyA-1 mutants did not elevate TOC1 transcript in simulated deep shade (Fig. 3B and SI Appendix, Fig. S4A), confirming the involvement of phyA in this response. TOC1 is absent from chromatin immunoprecipitation-sequencing and RNA-sequencing datasets identifying gene promoters bound directly by phyA in FR light, suggesting an indirect regulatory mechanism (33). phyA does, however, bind the promoter of the circadian-clock component REVEILLE8 (RVE8) in these conditions (33). RVE8 is an activator of gene expression that associates with the evening element within promoters such as the TOC1 promoter to promote histone acetylation (34). We found that increased TOC1 transcript accumulation under deep-shade conditions partially requires RVE8 (Fig. 3B). Double mutants deficient in phyA and RVE8 resembled phyA mutants, suggesting that phyA-mediated elevation of TOC1 transcript abundance in deep shade involves RVE8-dependent and -independent mechanisms (Fig. 3B). RVE8 transcript abundance was not significantly altered by deep shade or dependent on phyA, suggesting that phyA may regulate RVE8 activity at the posttranscriptional or posttranslational level (SI Appendix, Fig. S4B).
phyA Increases the Abundance of PRR7, ELF3, and ELF4 Transcripts in Deep Shade.
phyA signaling inhibits hypocotyl elongation in conditions that simulate plant canopy shade but not neighbor proximity (21). TOC1 binds to and inhibits PIF3 (14) and PIF4 (35) activity through cobinding to PIF target promoters, leading to the suppression of PIF target-gene expression. PRR proteins act with TOC1 in transcriptional waves to regulate the timing of PIF activity and hypocotyl elongation in diel cycles (15). In addition, PRR5 and PRR7 bind to and inhibit PIF proteins to suppress hypocotyl elongation in low R:FR (16). Because phyA directly binds the PRR5 promoter (33), we hypothesized that phyA might also regulate the abundance of PRR5 and PRR7 transcripts in deep shade. RVE8 has been shown to promote expression of PRR5 (34), so rve8 and phyA/rve8 mutants were included in the analysis. PRR5 and PRR7 transcripts were quantified at dusk in short (8-h) photocycles of low PAR (5 µmol ⋅ m−2 ⋅ s−1) with R:FR values of 2.5 and 0.05. Low R:FR repeatedly induced PRR5 transcript accumulation in wild-type (WT) and rve8 mutants, but these differences were not statistically significant (P < 0.05) by t test analysis comparing within genotypes across three independent repeats. Mutants deficient in phyA consistently displayed no low R:FR–mediated induction of PRR5 (Fig. 3C). Significant increases in the PRR7 transcript occurred following low-R:FR treatment in WT and rve8 mutants but not phyA or phyA/rve8 mutants, confirming a role for phyA, but not RVE8, in mediating this response (Fig. 3D).
In addition to TOC1, PRR5, and PRR7, the evening-complex component ELF3 physically interacts with PIF4 to suppress its activity during the early night (13, 36). Together with the evening-complex components ELF4 and LUX ARRYTHMO (LUX), ELF3 also suppresses PIF4 and PIF5 transcription during this time (37). Therefore, the role of phyA in regulating the transcript abundance of evening-complex components was investigated under simulated deep-shade conditions. RVE8 promotes the transcription of ELF4 and LUX (34), so the rve8 mutant was included in these analyses. Transcript abundance was measured at dusk and 4 h after dusk to capture the peaks in all transcripts. No significant increase in ELF3 transcript abundance occurred in low R:FR–treated WT seedlings at dusk (Fig. 3E). A clear phyA-mediated increase was, however, observed at 4 h after dusk. rve8 mutant responses to low R:FR resembled WT plants but were not statistically significant over three biological repeats, suggesting a possible role for RVE8 in this response (Fig. 3E). Significant increases in ELF4 transcript abundance were observed in low R:FR–treated plants at dusk but not 4 h after dusk. These increases required phyA but not RVE8 (Fig. 3F). No significant low R:FR–mediated increases in LUX transcript abundance occurred at either time point (Fig. 3G). Collectively, these data suggest that phyA elevates TOC1, PRR7, ELF3, and ELF4 transcript abundance in deep shade with an additional, partial requirement for RVE8 in the TOC1 response.
TOC1, PRR7, ELF3, and ELF4 Inhibit Shade Avoidance in Photocycles of Deep Shade.
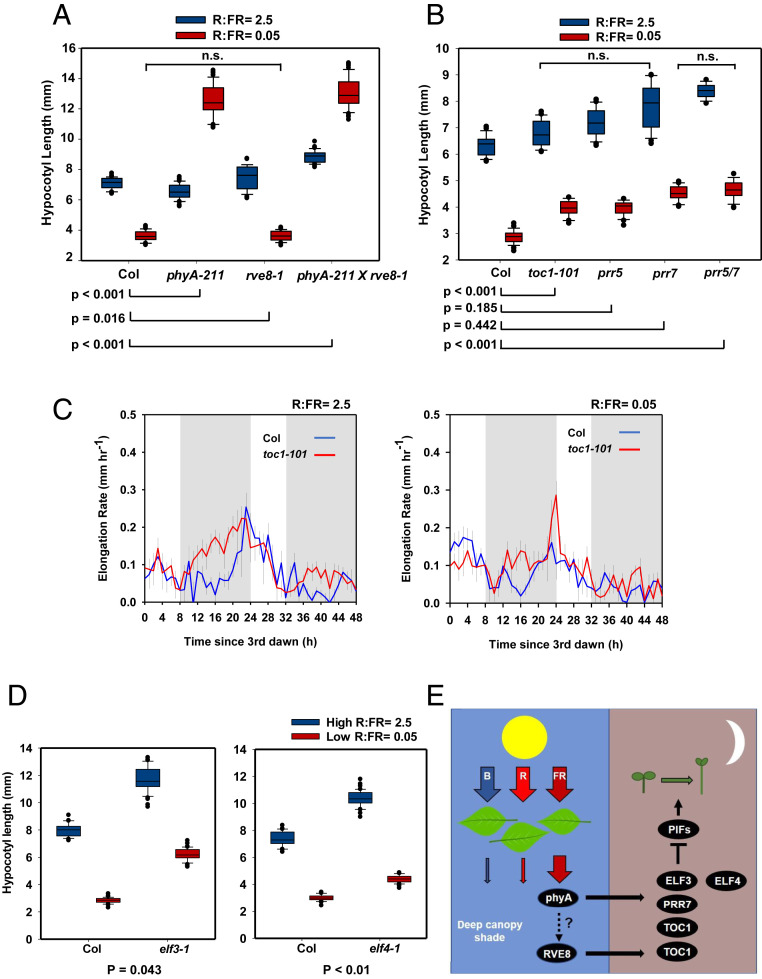
We hypothesized that the elevation of TOC1, PRR7, ELF3, and ELF4 in photocycles of deep shade might function to inhibit shade avoidance through suppression of PIF4 and PIF5 activity. No inhibition of PIF4 or PIF5 transcript abundance was observed in low R:FR, suggesting that any regulation is posttranscriptional (SI Appendix, Fig. S5). Such data are consistent with observations that only two out of three evening-complex components are up-regulated in these conditions (Fig. 3 E and G). ELF3 operates independently of evening-complex function to repress PIF4 activity (13), while ELF4 has been shown to facilitate ELF3 import into the nucleus (38). We first quantified hypocotyl-elongation responses to R:FR in low PAR. Low R:FR significantly inhibited hypocotyl elongation in WT plants. phyA mutants displayed significantly elongated hypocotyls in these conditions, whereas rve8 mutants resembled WT plants (Fig. 4A; all differences between pairs samples are statistically significant unless marked with n.s). Collectively, these data suggest that inhibition of hypocotyl elongation in deep shade requires phyA with no significant role for RVE8. A two-way ANOVA with genotype and R:FR ratios as factors found highly significant interactions between genotype and R:FR ratios for phyA mutant combinations, supporting this conclusion.
Fig. 4.
TOC1, PRR7, ELF3, and ELF4 inhibit shade avoidance in deep shade. (A and B) Hypocotyl lengths of (A) phyA-211, rve8-1, and phyA-211/rve8-1 and (B) toc1-101, prr5, prr7, and prr5/7 mutants. Seedlings were germinated in 8-h light/16-h dark cycles of white light (70 µmol ⋅ m−2 ⋅ s−1, R:FR = 5) for 48 h before transfer to 8-h light/16-h dark cycles at a PAR of 5 µmol ⋅ m−2 ⋅ s−1 and R:FR or 2.5 or 0.05 for a further 5 d. Data are shown as box plots representing the first, second, and third quartiles with whiskers representing the 10th and 90th percentiles (n ≥ 22). All differences between pairs of samples are statistically different unless stated (n.s. = P > 0.05 using a Tukey post hoc test). P values for two-way ANOVA comparing mutant to WT using genotype and R:FR ratios as factors are shown below each plot. (C) Growth rate of Col-0 and toc1-101 seedlings in deep shade. Seedlings were germinated in 8-h light/16-h dark cycles white light (70 µmol ⋅ m−2 ⋅ s−1, R:FR = 5) for 48 h before transfer to 8-h light/16-h dark cycles (5 µmol ⋅ m−2 ⋅ s−1 and a R:FR of 2.5 or 0.05). Growth rate was analyzed using time-lapse IR photography. Measurements were recorded for 48 h, starting at dawn on the third day after germination. Data were smoothed using a 3-h rolling average to reduce noise. Unshaded areas indicate the light period, and shaded areas indicate the dark period (n = 12 ± SE). (D) Hypocotyl lengths of elf3-1 and elf4-1 mutants. Seedlings were grown and analyzed as in A. All differences between pairs of samples are statistically significant (n ≥ 37). (E) Hypothetical model showing phyA-mediated inhibition of hypocotyl elongation in deep shade. As sunlight passes through a dense canopy, red (R), green, and blue (B) light are depleted to very low levels, while far-red (FR) light is enriched. phyA activity is enhanced in low R:FR and low PAR. TOC1, PRR7, ELF3, and ELF4 transcripts are increased at dusk/early evening in a mechanism requiring phyA. The elevation of TOC1 transcripts partially requires RVE8 through an unknown mechanism. Increased TOC1, PRR7, ELF3, and ELF4 collectively inhibit hypocotyl elongation in deep shade. This is likely to occur through increased inhibition of PIF activity through cobinding of TOC1, PRR7, and ELF3 at PIF target promoters (13, 14, 16).
In low PAR, toc1, prr5, prr7, and prr5/7 mutants displayed longer hypocotyls than WT seedlings in high and low R:FR (Fig. 4B; all differences between pairs of samples are statistically significant unless marked with n.s). The inability of these plants to fully suppress hypocotyl elongation in low R:FR suggests these components inhibit this response in deep shade. A two-way ANOVA with genotype and R:FR ratios as factors found statistically significant interactions between genotype and R:FR ratios for toc1 and prr5/7 mutant combinations, supporting this conclusion. To temporally compare elevations of TOC1 with patterns of seedling growth, the hypocotyl-elongation rate of the toc1-101 mutant was analyzed in low PAR at high and low R:FR in 8-h light/16-h dark cycles using time-lapse IR photography (Fig. 4C). The data presented are from the 48-h period starting at the third dawn postgermination, when the majority of hypocotyl elongation occurred. In both high and low R:FR, the rate of hypocotyl elongation was higher in toc1-101 compared to WT throughout the night of day 3, consistent with previous reports (14). Low-R:FR treatment resulted in a suppression of growth rate at the end of the night. This peak in growth rate was restored in the toc1-101 mutant, confirming a role for TOC1 in shade-avoidance suppression in deep shade.
As ELF3 and ELF4 transcripts were also up-regulated by phyA during the early evening (Fig. 3 E and F), hypocotyl-length responses to low R:FR in low PAR were quantified in elf3 and elf4 mutants. Both mutants had longer hypocotyls than WT plants in high and low R:FR (Fig. 4D; all differences between pairs of samples are statistically significant). The elf3 mutant hypocotyls were longer than the elf4 mutant, particularly in low-R:FR conditions. This suggests a role for ELF3 in suppressing hypocotyl elongation in deep shade, with a smaller role for ELF4. A two-way ANOVA with genotype and R:FR ratios as factors found highly significant interactions between the genotype and R:FR ratios for both elf3 and elf4 mutants, supporting this conclusion.
Discussion
Here, we show that a very low R:FR ratio reduces the amplitude of oscillations of the core clock components CCA1 and TOC1 in free running conditions. This supports previous findings from experiments using monochromatic FR (27) and analyses of the clock output gene COLD, CIRCADIAN RHYTHM, AND RNA BINDING 2 (CCR2) (39). Both transcript abundance and luciferase data show that, under very low R:FR, CCA1 expression becomes low, whereas TOC1 expression becomes high (Fig. 1). Given that CCA1 and TOC1 act as reciprocal transcriptional repressors (29, 30), it is possible that transfer to low R:FR elevates TOC1 expression, which subsequently suppresses CCA1 expression.
Low R:FR reduced the amplitude (CCA1) and damped (TOC1) circadian oscillations in continuous light but not in light/dark cycles (Fig. 1 C and D). The damping of circadian oscillations of TOC1::LUC in continuous low R:FR might result from a loss of synchronicity between cells or tissues, maintenance of synchronicity but a reduction in amplitude, or a combination of both. It was hypothesized that weakening the entraining stimulus (by lowering PAR to levels to simulate deep shade) may cause low R:FR–induced damping of oscillations in light/dark cycles. Despite having lower amplitudes than in high PAR conditions, oscillations of CCA1 and TOC1 continued under light/dark cycles of deep shade (Fig. 2). An unexpected observation was that, in TOC1pro::LUC but not CCA1pro::LUC, activity was increased in these conditions. Elevated TOC1 promoter activity in deep shade was accompanied by an increase in TOC1 transcript abundance (Figs. 2 C and E and 3 A and B). The increase of TOC1 but not CCA1 expression under low-R:FR photocycles suggests that the TOC1 elevation does not represent a general response of circadian entrainment to a higher total photon irradiance of light caused by FR supplementation (SI Appendix, Figs. S1 and S2). Intriguingly, the daily elevations of TOC1 observed at dusk in deep shade in 12-h photoperiods are not accompanied by reduced CCA1 promoter activity the following dawn. In contrast, a reduction in CCA1 promoter activity occurred at dawn following TOC1 elevation in 8-h photoperiods. This suggests that there is an interaction between the photoperiod and the response of the circadian oscillator to simulated deep shade (Fig. 2 H and I).
Consistent with previous studies (27, 32, 33), we show that phyA and, to a lesser extent, RVE8 mediate low R:FR–induced increases in TOC1 transcript abundance at low PAR (Fig. 3 A and B). Low R:FR did not increase RVE8 transcript abundance (SI Appendix, Fig. S4B), suggesting that the elevation of the TOC1 transcript does not occur via linear transcriptional activation. One possible explanation relates to alternative splicing. Multiple splice variants of RVE8 are present in Arabidopsis following temperature transfer (40). phyA and phyB can mediate alternative splicing of multiple transcripts in R (41), though it is unknown whether FR can trigger a similar response. Therefore, phyA could potentially induce alternative splicing of RVE8 transcripts in low R:FR to control RVE8 activity in these conditions.
TOC1 mediates the circadian gating of hypocotyl elongation through direct interaction with PIFs, suppressing their transcriptional activity (14, 33). We therefore hypothesized that phyA-mediated elevation of TOC1 may contribute toward the antagonism of shade avoidance in resource-limited conditions. End-point data showed that TOC1, but not RVE8, significantly inhibits hypocotyl elongation in deep shade (Fig. 4A). Although the elevation of TOC1 expression in deep shade antagonizes shade avoidance, the small contribution of RVE8 to this response might fall below the level of detection (Figs. 3 A and B and 4A). Analyses of hypocotyl-elongation rates (Fig. 4C) suggest that low R:FR in a background of low PAR suppresses the peak of elongation growth at the end of the night in a TOC1-dependent manner.
In addition to TOC1, we observed phyA-mediated elevations of PRR7, ELF3, and ELF4 transcripts during early evening in photocycles of deep shade. PRR7 and ELF3 bind directly to PIFs, repressing their transcriptional activity (13, 14), while ELF4 facilitates ELF3 import into the nucleus (38). Mutants deficient in these signaling components all had long hypocotyls in low-PAR conditions, consistent with their well-characterized phenotypes in higher PAR (14, 16, 42). Importantly, phyA was unable to completely suppress hypocotyl elongation in toc1, prr7, elf3, and elf4 mutant backgrounds in low R:FR, suggesting that these repressors contribute to this response in WT plants. These observations align with de-etiolation studies in monochromatic FR, during which toc1, prr7, and elf4 mutants displayed longer hypocotyls than WT controls (43–45). We propose that elevations in TOC1, PRR7, and ELF3 transcripts increase TOC1, PRR7, and ELF3 proteins, which, in combination, inhibit hypocotyl elongation in deep shade through directly suppressing PIF protein activity. Direct phyA-mediated weakening of auxin signaling through increased AUX/IAA stability would provide additional suppression of hypocotyl elongation in these conditions (24). In short days, the timing of peak PIF abundance is shifted into the night, when it is protected from photoreceptor-mediated degradation (22, 46). The combination of short photoperiods and low PAR would therefore limit photoreceptor-mediated regulation of PIF abundance and increase the importance of inhibitor interactions in the regulation of PIF activity (13, 15). Antagonism of shade avoidance by TOC1/PRR7/ELF3 on short days might additionally enhance survival when hypocotyls are longest and resources are most limited (Fig. 4E).
Mutant analyses identified a role for ELF4 in mediating FR signaling by phyA to the circadian oscillator (27). The data presented here suggest that phyA links deep-shade perception to TOC1, PRR7, ELF3, and ELF4. In addition to suppressing shade avoidance, the elevation of these proteins might contribute to robust circadian entrainment under conditions in which light and sugar availability are severely limited (26, 47). Intriguingly, increased abundance of TOC1, PRR5, and ELF4 transcript homologs has also been recorded in densely planted sorghum (48), suggesting that deep-shade elevation of evening clock components may be a widespread adaptation of plants growing under dense vegetational canopy.
Materials and Methods
The majority of Arabidopsis mutants and transgenic lines used in this study have been reported elsewhere: toc1-101 in Col-0 (14), rve8-1 in Col-0 (32), phyA-211 in Col-0 (49), phyA-1 in Ler (19), prr5-3 in Col-0 (50), prr7-3 in Col-0 (50), prr5-11/prr7-11 in Col-0 (51), elf3-1 in Col-0 (52), elf4-1 in Col-0 (53), CCA1pro::LUC in Col-0 (54), and TOC1pro::LUC in Col-0 (54). phyA-211/rve8-1 double mutants were produced by crossing single mutants using standard procedures. Homozygosity for the rve8-1 mutation was confirmed by PCR testing using RVE8 RP, RVE8 LP, and LBb1 primers (SI Appendix, Table S1). Homozygosity of the phyA-211 mutation was confirmed by the presence of an etiolated phenotype in FR.
Growth Conditions.
Seeds were surface sterilized in 70% (vol/vol) ethanol, followed by a 20% (vol/vol) sodium hypochlorite treatment before washing in sterile H2O. These were suspended in 0.1% wt/vol agar and individually placed on a 3:1 mixture of compost (Levington F2) and silver sand for hypocotyl-length analysis and RT-qPCR. The 0.5× Murashige and Skoog medium, pH 5.8 was used for luciferase imaging. Seeds were stratified in darkness at 4 °C for 72 h and germinated in controlled-climate chambers (Microclima 1600E, Snijder Scientific). Temperature was maintained at 20 °C with 70% humidity. PAR (400 to 700 nm) was adjusted from 70 to 5 µmol ⋅ m−2 ⋅ s−1 using neutral density filters (Lee Filters). Supplementary FR light-emitting diodes (LEDs) (peak 730 nm) were used to adjust the R:FR ratio within a range of 0.05 to 5 as specified in experiments. Light measurements were recorded at the soil surface using an Ocean Optics Flame spectrophotometer and analyzed using Oceanview software (Ocean Optics) and SigmaPlot version 13 (Systat Software, Inc.).
Analysis of Transcript Abundance.
RNA was extracted using the Spectrum Plant Total RNA Kit (Sigma-Aldrich) according to manufacturer’s instructions. DNA was removed from the eluted RNA using the Amplification Grade DNase I kit (Sigma-Aldrich). A total of 1 mg RNA was used for complementary DNA (cDNA) synthesis using the Applied Biosystems High Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). RT-qPCR was carried out using the Brilliant III Ultra-Fast SYBR Green qPCR Master Mix kit according to the manufacturer’s instructions (Agilent Technologies). Relative transcript abundance was calculated using the ∆∆Ct algorithm (55), normalized to the abundance of ACTIN2 or PP2A (AT1G13320) transcript, as specified in the figures. Three technical and two to five biological repeats were performed for each data point as indicated in figure legends. qPCR primers are provided in SI Appendix, Table S1.
Luciferase Imaging.
Groups of seedlings were cultivated in plastic rings to produce pools of bioluminescence signal for analysis (54). Luciferase bioluminescence was recorded using a Lumintek electron multiplying charge coupled device (EM-CCD) imaging system (Photek) controlled by Image32 software (Photek) and custom control scripts (45-s integrations, EM gain setting 2,700). Monochromatic B, R, and FR LEDs were modulated to deliver PAR at 47 or 5 µmol ⋅ m−2 ⋅ s−1 and R:FR ratios as indicated in the experiments. The 7-d-old plants were moved to specified experimental conditions 72 h before image acquisition to entrain for three cycles of 12-h light/12-h dark or 8-h light/16-h dark. A total of 100 mL sterile 5 mM luciferin (potassium salt of D-luciferin, Melford Laboratories) was added 24 h before data acquisition. Images were captured at 60-min intervals preceded by a dark delay of 2 min to eliminate chlorophyll autofluorescence from the bioluminescence signal. Time-course data were further analyzed using the FFT-NLLS algorithm within the Biological Rhythm Analysis Software Suite (BRASS; Fig. 1) or BioDare (Fig. 2) software (www.biodare2.ed.ac.uk).
Hypocotyl-Length Measurement.
End-point hypocotyl-length data were extracted from images using Fiji (56). Hypocotyls were measured from the shoot apical meristem to the shoot–root junction. For time-lapse IR photography, a custom-built 8 × 8 array of 880-nm IR LEDs was controlled with a 24-h timer. Time-lapse images were captured with a modified Nikon D80 digital single lens reflex (DSLR) camera with its IR-blocking filter removed, a SIGMA 105-mm macro lens, and an IR pass filter (>850 nm) (Zomei) operated with digiCamControl version 2.0.0 remote camera tethering freeware (digicamcontrol.com). Hypocotyl lengths from individual images and time-lapse image stacks were manually measured from images using Fiji.
Statistical Analyses.
SigmaPlot version 13 was used to plot and analyze quantitative data (Systat Software, Inc.).
Supplementary Material
Acknowledgments
We thank Elena Monte (Centre for Research in Agricultural Genomics, Barcelona, Spain) for the donation of toc1-101 seed, Stacey Harmer (University of California, Davis, CA) for the donation of rve8-1 seed, Rob McClung (Dartmouth College, Hanover, NH) for prr5-3 and prr7-3 mutants, Takeshi Mizuno for prr5-11/7-11 mutants, and Anthony Hall (Earlham Institute, Norwich, United Kingdom) for the donation of transgenic lines containing CCA1::LUC and TOC1::LUC. This work was funded by Biotechnology and Biological Sciences Research Council (BBSRC) Grants BB/L01369X, BB/M008711/1, BB/R002045/1, and GEN BB/P013511/1, the Bristol Centre for Agricultural Innovation, and The Leverhulme Trust Grant RPG-2018-216.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108176118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Fiorucci A. S., Fankhauser C., Plant strategies for enhancing access to sunlight. Curr. Biol. 27, R931–R940 (2017). [DOI] [PubMed] [Google Scholar]
- 2.de Wit M., et al., Plant neighbor detection through touching leaf tips precedes phytochrome signals. Proc. Natl. Acad. Sci. U.S.A. 109, 14705–14710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorrain S., Allen T., Duek P. D., Whitelam G. C., Fankhauser C., Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53, 312–323 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Leivar P., et al., Dynamic antagonism between phytochromes and PIF family basic helix-loop-helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in Arabidopsis. Plant Cell 24, 1398–1419 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L., et al., Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 26, 785–790 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X., et al., Shade-induced nuclear localization of PIF7 is regulated by phosphorylation and 14-3-3 proteins in Arabidopsis. eLife 7, e31636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornitschek P., et al., Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 71, 699–711 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Hornitschek P., Lorrain S., Zoete V., Michielin O., Fankhauser C., Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 28, 3893–3902 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galstyan A., Cifuentes-Esquivel N., Bou-Torrent J., Martinez-Garcia J. F., The shade avoidance syndrome in Arabidopsis: A fundamental role for atypical basic helix-loop-helix proteins as transcriptional cofactors. Plant J. 66, 258–267 (2011). [DOI] [PubMed] [Google Scholar]
- 10.de Lucas M., et al., A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Pedmale U. V., et al., Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 164, 233–245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma D., et al., Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl. Acad. Sci. U.S.A. 113, 224–229 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto C., López-Salmerón V., Davière J. M., Prat S., ELF3-PIF4 interaction regulates plant growth independently of the evening complex. Curr. Biol. 25, 187–193 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Soy J., et al., Molecular convergence of clock and photosensory pathways through PIF3-TOC1 interaction and co-occupancy of target promoters. Proc. Natl. Acad. Sci. U.S.A. 113, 4870–4875 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martín G., et al., Circadian waves of transcriptional repression shape PIF-regulated photoperiod-responsive growth in Arabidopsis. Curr. Biol. 28, 311–318.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., et al., Central clock components modulate plant shade avoidance by directly repressing transcriptional activation activity of PIF proteins. Proc. Natl. Acad. Sci. U.S.A. 117, 3261–3269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanovsky M. J., Casal J. J., Whitelam G. C., Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: Weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 18, 788–794 (1995). [Google Scholar]
- 18.Li J., Li G., Wang H., Wang Deng X., Phytochrome signaling mechanisms. Arabidopsis Book 9, e0148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson E., Bradley M., Harberd N. P., Whitelam G. C., Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of daylength extensions). Plant Physiol. 105, 141–149 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salter M. G., Franklin K. A., Whitelam G. C., Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426, 680–683 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Martínez-García J. F., et al., The shade avoidance syndrome in Arabidopsis: The antagonistic role of phytochrome A and B differentiates vegetation proximity and canopy shade. PLoS One 9, e109275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hersch M., et al., Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 111, 6515–6520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozue K., et al., Rhythmic growth explained by coincidence between internal and external cues. Nature 448, 358–361 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Yang C., et al., Phytochrome A negatively regulates the shade avoidance response by increasing auxin/indole acidic acid protein stability. Dev. Cell 44, 29–41.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Song B., et al., Phytochrome A inhibits shade avoidance responses under strong shade through repressing the brassinosteroid pathway in Arabidopsis. Plant J. 104, 1520–1534 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Somers D. E., Devlin P. F., Kay S. A., Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282, 1488–1490 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Wenden B., et al., Light inputs shape the Arabidopsis circadian system. Plant J. 66, 480–491 (2011). [DOI] [PubMed] [Google Scholar]
- 28.McClung C. R., Plant circadian rhythms. Plant Cell 18, 792–803 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alabadí D., et al., Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293, 880–883 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Gendron J. M., et al., Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc. Natl. Acad. Sci. U.S.A. 109, 3167–3172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roig-Villanova I., Martínez-García J. F., Plant responses to vegetation proximity: A whole life avoiding shade. Front. Plant Sci. 7, 236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tepperman J. M., Zhu T., Chang H.-S., Wang X., Quail P. H., Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc. Natl. Acad. Sci. U.S.A. 98, 9437–9442 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F., et al., Arabidopsis phytochrome A directly targets numerous promoters for individualized modulation of genes in a wide range of pathways. Plant Cell 26, 1949–1966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu P. Y., Devisetty U. K., Harmer S. L., Accurate timekeeping is controlled by a cycling activator in Arabidopsis. eLife 2, e00473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J.-Y., Oh E., Wang T., Wang Z.-Y., TOC1-PIF4 interaction mediates the circadian gating of thermoresponsive growth in Arabidopsis. Nat. Commun. 7, 13692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H., Nusinow D. A., Into the evening: Complex interactions in the Arabidopsis circadian clock. Trends Genet. 32, 674–686 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Nusinow D. A., et al., The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475, 398–402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrero E., et al., EARLY FLOWERING4 recruitment of EARLY FLOWERING3 in the nucleus sustains the Arabidopsis circadian clock. Plant Cell 24, 428–443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiménez-Gómez J. M., Wallace A. D., Maloof J. N., Network analysis identifies ELF3 as a QTL for the shade avoidance response in Arabidopsis. PLoS Genet. 6, e1001100 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James A. B., Syed N. H., Brown J. W., Nimmo H. G., Thermoplasticity in the plant circadian clock: How plants tell the time-perature. Plant Signal. Behav. 7, 1219–1223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shikata H., et al., Phytochrome controls alternative splicing to mediate light responses in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 111, 18781–18786 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McWatters H. G., et al., ELF4 is required for oscillatory properties of the circadian clock. Plant Physiol. 144, 391–401 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Más P., Alabadí D., Yanovsky M. J., Oyama T., Kay S. A., Dual role of TOC1 in the control of circadian and photomorphogenic responses in Arabidopsis. Plant Cell 15, 223–236 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaczorowski K. A., Quail P. H., Arabidopsis PSEUDO-RESPONSE REGULATOR7 is a signaling intermediate in phytochrome-regulated seedling deetiolation and phasing of the circadian clock. Plant Cell 15, 2654–2665 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikis E. A., Khanna R., Quail P. H., ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J. 44, 300–313 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Niwa Y., Yamashino T., Mizuno T., The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol. 50, 838–854 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Haydon M. J., Mielczarek O., Robertson F. C., Hubbard K. E., Webb A. A. R., Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502, 689–692 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kebrom T. H., McKinley B. A., Mullet J. E., Shade signals alter the expression of circadian clock genes in newly-formed bioenergy sorghum internodes. Plant Direct 4, e00235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed J. W., Nagatani A., Elich T. D., Fagan M., Chory J., Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104, 1139–1149 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michael T. P., et al., Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302, 1049–1053 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Nakamichi N., Kita M., Ito S., Yamashino T., Mizuno T., PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol. 46, 686–698 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Covington M. F., et al., ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13, 1305–1315 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doyle M. R., et al., The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature 419, 74–77 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Noordally Z. B., et al., Circadian control of chloroplast transcription by a nuclear-encoded timing signal. Science 339, 1316–1319 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Pfaffl M. W., A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindelin J., et al., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.