Abstract
Multiple nanotherapeutics have been approved for patients with cancer, but their effects on survival have been modest and, in some examples, less than those of other approved therapies. At the same time, the clinical successes achieved with immunotherapy have revolutionized the treatment of multiple advanced-stage malignancies. However, the majority of patients do not benefit from the currently available immunotherapies and many develop immune-related adverse events. By contrast, nanomedicines can reduce — but do not eliminate — the risk of certain life-threatening toxicities. Thus, the combination of these therapeutic classes is of intense research interest. The tumour microenvironment (TME) is a major cause of the failure of both nanomedicines and immunotherapies that not only limits delivery, but also can compromise efficacy, even when agents accumulate in the TME. Coincidentally, the same TME features that impair nanomedicine delivery can also cause immunosuppression. In this Perspective, we describe TME normalization strategies that have the potential to simultaneously promote the delivery of nanomedicines and reduce immunosuppression in the TME. Then, we discuss the potential of a combined nanomedicine-based TME normalization and immunotherapeutic strategy designed to overcome each step of the cancer-immunity cycle and propose a broadly applicable ‘minimal combination’ of therapies designed to increase the number of patients with cancer who are able to benefit from immunotherapy.
The widely accepted paradigm of nanomedicine — enhanced permeability and retention (EPR) — assumes that cytotoxic drugs can be delivered selectively to tumours using nanomedicines (defined as drug-loaded nanoparticles of 1–1,000 nm in diameter) to increase efficacy and minimize the risk of systemic adverse effects. However, this approach has thus far conferred only modest improvements in the survival outcomes of patients with cancer1 (Supplementary Table 1). By contrast, immune-checkpoint inhibition (ICI) has provided unprecedented improvements in the survival outcomes of a subset of patients. However, ICI is currently estimated to benefit <13% of patients with cancer2 and a substantial fraction of patients receiving these therapies will develop immune-related adverse events3. As a result, research interest in nanomedicine is shifting rapidly towards the adaptation of delivery platforms for improving the percentage of patients who derive clinical benefit from ICI and other immunotherapies4,5. Two paradigms for the application of nanomedicines to the potentiation of immunotherapy are currently emerging: systemic administration of nanomedicines that have a tumour-priming effect; and local or extratumoural administration of nanomedicines to induce local and/or systemic antitumour immunity. The first paradigm is supported by data from a successful phase III trial, in which women with metastatic triple-negative breast cancer (TNBC) received the combination of nab-paclitaxel plus the anti-programmed cell death 1 ligand 1 (PD-L1) antibody atezolizumab6. Various manifestations of the second paradigm, such as the delivery of vaccines using lipid-based nanomedicines to promote antitumour immunity, are the focus of preclinical and clinical investigations (for example, NCT02410733).
We hypothesize that the pathophysiology of the tumour microenvironment (TME; Supplementary Figure 1) limits the uniform delivery of both systemically administered and locally applied nanomedicines, thus compromising their efficacy even when they accumulate in tumours1,7,8. Therefore, we propose that nanomedicines should incorporate not only anticancer drugs but also agents that ‘normalize’ the various components and physiology of the TME, resulting in improved tumour perfusion and reduced levels of hypoxia. This normalization effect has the potential to facilitate not only drug delivery1 but also that of oxygen to slow tumour progression and convert an immunosuppressed TME into an immunostimulatory TME9,10. We propose that nanotechnology will improve the implementation of immunotherapies by facilitating the delivery of specific combinations and schedules of TME-normalizing agents, cytotoxic agents and immunotherapies. In this Perspective, we first summarize the evidence indicating how the TME limits the efficacy of both nanomedicines and immunotherapies, followed by discussions of how normalizing the TME can improve drug delivery and the outcomes of patients receiving immunotherapy. We then summarize how nanomedicine-based approaches might overcome the mechanisms of resistance to immunotherapies. Finally, we propose strategies that involve re-engineering and/or developing new nanomedicines with the aim of optimizing the effectiveness of immunotherapies.
Role of the TME in treatment resistance
We hypothesize that the pathophysiology of the TME of primary tumours and their distant metastases often limits the efficacy of nanomedicines and immunotherapies by limiting the accumulation, distribution and function of drugs and immune cells9–11. Angiogenic and fibrotic signalling mediates this pathophysiology and directly — and indirectly through induction of hypoxia — induces immunosuppression.
Distribution of nanomedicines
Data from clinical studies published in 2017 (REFS12,13) confirm the existence of the EPR effect in patients with cancer and that this effect is correlated with the response to nanomedicines. However, the benefits of EPR are compromised by a substantial level of spatial intratumour and intertumour heterogeneity in drug distribution, both in patients with tumours of the same type and between multiple tumours in the same patient12,13. This heterogeneity might explain the disparate results obtained with nab-paclitaxel in the metastatic and adjuvant settings in patients with pancreatic ductal adenocarcinoma (PDAC; Supplementary Table 1). A dysfunctional tumour vasculature, resulting from abnormal angiogenesis and desmoplasia (leading to tumour fibrosis), limits the uniform distribution of nanomedicines independently of their physicochemical properties by reducing tumour blood flow and hindering the transport of nanomedicines from blood vessels to cancer cells (reviewed in detail elsewhere1,8,14,15). Reduced tumour blood flow also limits the availability of oxygen and induces acidity, resulting in immunosuppression9–11. Similar to the distribution of nanomedicines12,13, the level of immune cell infiltration can even differ between the primary tumour and metastases in the same patient16,17.
Cancer-immunity phenotypes
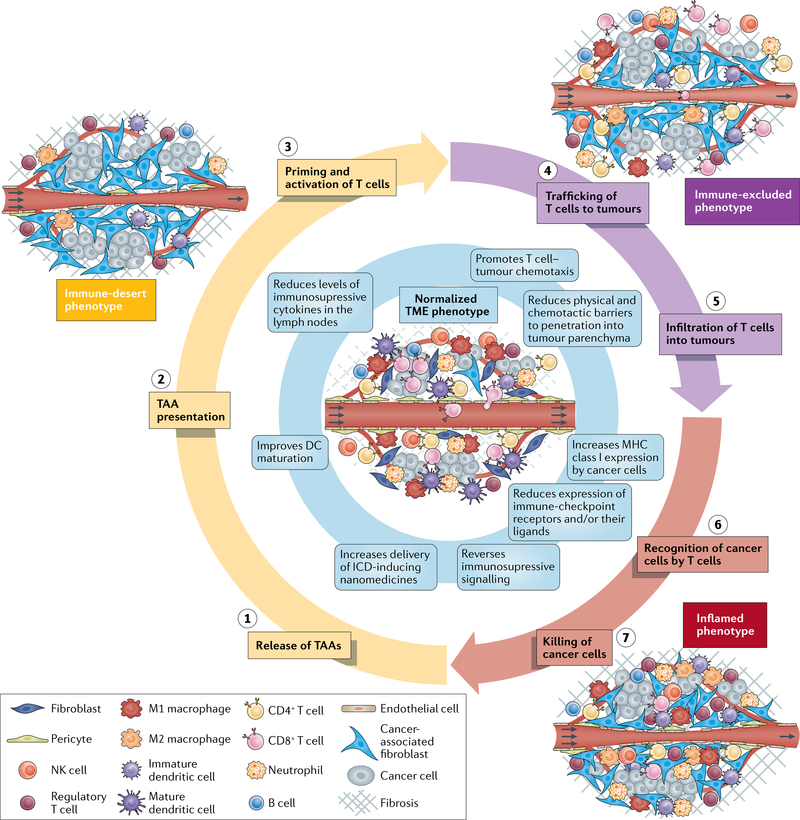
The immune phenotype of the TME influences sensitivity to immunotherapies, including ICI and can be broadly classified into three main phenotypes18 (FIG. 1). First, the immune-desert phenotype, which lacks antitumour immune cells, is characterized by immunological ignorance (a lack of antigens and/or their presentation), tolerance (a lack of response to antigen presentation) and a lack of T cell priming18 (FIG. 1). Tumours of this phenotype are the least responsive to ICI. Indeed, patients with tumours that lack pre-existing cytotoxic T cells and have a less clonal repertoire of T cell receptors (TCRs) have worse outcomes than those with tumours of other immune phenotypes19. The pathophysiology of the TME contributes to this phenotype. VEGF, an angiogenic growth factor induced by hypoxia, promotes immunological ignorance by inhibiting dendritic cell (DC) maturation, thereby reducing the extent of antigen presentation20. Hypoxia also promotes immune tolerance by inducing the expression of chemokines that recruit pro-tumour CD4+CD25+FOXP3+ regulatory T (Treg) cells21,22. Thus, hypoxia and hypoxia-induced signalling contribute to the lack of T cell priming characteristic of the immune-desert phenotype.
Fig. 1 |. Cancer-immunity TME phenotypes affecting responsiveness to immunotherapy18.
Three distinct cancer-immunity phenotypes exist and can affect responsiveness to immunotherapies in different ways. These phenotypes reflect tumours at different phases of the seven-step cancer-immunity cycle that must be completed repeatedly for immunotherapies to be effective. Tumour microenvironment (TME) normalization (centre, blue shading) promotes perpetuation of the cycle. In tumours of the immune-desert phenotype (yellow shading), the TME and the often limited number of immune cells within the tumour are immunosuppressed. The host immune system permits cancer cell growth owing to a lack of antigen recognition, immune tolerance and/or a failure to prime cytotoxic T cells. Some of these processes are affected by hypoxia. In tumours of the immune-excluded phenotype (purple shading), immune cell infiltration is restricted to the periphery and/or the stroma. The stromal factors that promote this phenotype similarly inhibit the delivery of nanomedicines and/or oxygen. In tumours of the inflamed phenotype (red shading), immune cells are stimulated by pro-inflammatory cytokines and are able to move throughout the tumour parenchyma. However, various inhibitory factors, which are often induced by hypoxia, lead to a reduction in antitumour immunity. Normalization of the vasculature by targeting angiogenic factors (such as VEGF and/or angiopoietin-2) and/or immune checkpoints and normalization of the tumour extravascular compartment by reprogramming cancer-associated fibroblasts (CAFs) to produce a less dense extracellular matrix (ECM) are two strategies that can be applied alone or in combination to normalize the entire TME and improve perfusion, oxygen delivery and drug distribution. Improper use of these strategies, however, can lead to excessive vessel pruning or CAF and/or ECM depletion, which might accelerate tumour progression and metastasis. Anti-programmed cell death 1 (PD-1) or programmed cell death 1 ligand 1 (PD-L1) antibodies can also normalize blood vessels in some tumour types and could promote the growth of mature blood vessels that are protected from pruning by antiangiogenic agents, which primarily target immature vessels. Signalling pathways such as those activated by VEGF, angiopoietin-2, CXCL12/CXCR4 and transforming growth factor-β, which can all be targeted to normalize the TME, are themselves immunosuppressive. As a result of these effects, the combination of therapies targeting these pathways with immunotherapies is a promising approach. Thus, specific TME normalization strategies based on the TME immune phenotype of the target tumour could increase both the response rates and the magnitude of responses to immunotherapies. DC, dendritic cell; ICD, immunogenic cell death; NK, natural killer; TAA, tumour-associated antigen.
Second, the immune-excluded phenotype is characterized by immune cells localized to the tumour periphery or stroma18, impeded by immature vessels and extravascular stroma and characterized by increased expression of transforming growth factor-β (TGFβ), an excessive extracellular matrix (ECM) and a high density of cancer-associated fibroblasts (CAFs)23–25 (FIG. 1). Tumours of this phenotype have more potential for sensitivity to ICI than those of the immune-desert phenotype because T cells present in the stroma might become active and proliferate, despite being unable to easily infiltrate the parenchyma19,23. Desmoplasia is a major cause of this phenotype, as CAFs secrete chemokines such as TGFβ and stromal cell-derived factor 1α, which prevent cytotoxic T lymphocytes (CTLs) from migrating to cancer cells23–25. TGFβ also induces the development of a Treg phenotype in naive CD4+ T cell precursors22. CAFs have immunosuppressive subsets and are also able to produce and maintain the dense desmoplasia that blocks CTL migration26. Angiogenesis is another major cause of this phenotype, as angiogenic signalling dysregulates the expression of adhesion molecules on the vessel wall, thereby reducing the extent of leukocyte binding and limiting their flux into tumours11. The demonstration that T cell motility is slower in hypoxic regions of tumours is consistent with evidence that angiogenesis and desmoplasia cause hypoxia and can directly exclude immune cells through both signalling and physical means27,28.
Third, tumours of the inflamed phenotype have T cells located in the parenchyma and express pro-inflammatory cytokines, indicating the presence of a failed antitumour immune response18. Owing to the presence of large numbers of T cells with TCRs against tumour-associated antigens, this phenotype has the most potential for sensitivity to ICI, although it often also contains many hypoxia-suppressed immune cells23 (FIG. 1). Indeed, activation of VEGF signalling recruits immunosuppressive cells, including Treg cells, myeloid-derived suppressor cells and M2-like tumour-associated macrophages (TAMs) to the tumour29. Hypoxia also promotes the recruitment of Treg cells21 and even certain CAF phenotypes can recruit immunosuppressive cells26. Additionally, the expression of immune checkpoint molecules, which reduces the activity of immune cells, is a key feature of the inflamed phenotype. VEGF upregulates the expression of several immune-checkpoint proteins on T cells30–32. Similarly, hypoxia — via hypoxia inducible factor 1α signalling — upregulates the expression of immune-checkpoint receptors and/or their respective ligands on myeloid-derived suppressor cells, TAMs, DCs and cancer cells33,34. Even though T cells are present in the parenchyma of tumours of the inflamed phenotype, acidity35, CAFs26 and collagen density36 might all reduce their level of cytotoxic activity.
Abnormal angiogenesis can induce immunosuppression in tumours of all three cancer-immunity TME phenotypes and, in turn, certain immune cells can induce angiogenesis37 thus creating a vicious cycle. We hypothesize that by normalizing the constituents and physiology of the TME, nanomedicine delivery will be homogenized1,8,14,15, immune-cell infiltration and function will be increased9,10,38 and this vicious cycle will be broken10,38,39, even in patients with primary tumours and/or metastases featuring heterogeneous and varied TME phenotypes12,13,16,17.
Normalizing the TME
We have developed two broad strategies to ‘normalize’ the TME10: normalizing the tumour vasculature with judicious use of direct or indirect antiangiogenic therapies (AATs) so that it becomes more morphologically and functionally similar to the vasculature of nonmalignant tissues9,40–45; and reprogramming CAFs to reduce ECM levels, decompress the blood vessels and improve the extent of intratumoural drug penetration8,14,46,47 (FIG. 1). In contrast to normalization, depletion of the TME could contribute to disease progression10,47.
Normalization of tumour blood vessels
Judicious use of AATs can reduce the size of abnormally large pores in the tumour vessel wall, making the wall less permeable to fluid and repairing the chaotic structure of the vascular network without excessive vessel depletion; more than a dozen FDA-approved agents are able to mediate this effect10 (Supplementary Table 2). Decreasing the size of the pores increases tumour perfusion and reduces the interstitial fluid pressure (IFP)14. Perfusion correlates with the accumulation of nanomedicines48,49, and the reduction in IFP also contributes to the increased transport of nanomedicines with a diameter of at least ~30 nm (or potentially as large as liposomes, depending on the cancer type) across the vessel wall50,51. Data from more than a dozen hypothesis-generating clinical trials demonstrate that patients whose tumour blood vessels are normalized and/or those who have improved levels of tumour perfusion and/or oxygenation after AAT have better outcomes10. Nonetheless, mathematical modelling simulations suggest that AATs do not improve the vascular function of tumours with an abundance of compressed vessels, such as PDACs52, consistent with the clinical failure of AAT in patients with PDAC53. In fact, extended use of AATs often results in excessive pruning of the tumour vasculature, which exacerbates hypoperfusion10. Unaffected by IFP and angiogenesis54,55, tumour vessel compression is an effect of the accumulation of mechanical compressive forces within the tumour — termed solid stress — generated by rapid cancer cell growth and stored in intratumour and surrounding host tissue structural components56.
Normalization of extravascular factors
Solid stress generated by proliferating cancer cells and CAFs is transmitted by the dense ECM to the tumour vasculature, resulting in compressed vessels that limit the delivery of both oxygen and therapeutics of all sizes14,46,56,57. Similar to impairment of T cell migration to the tumour parenchyma23–25, components of the tumour extravascular space also hinder the diffusion of therapeutics from blood vessels to cancer cells1,8,14. Collagen is one such barrier58, and co-injection with bacterial collagenase increases the distribution of intratumourally administered oncolytic viral vectors in a patient-derived xenograft model of melanoma59. Similarly, the hormone relaxin modifies the structure and content of collagen to promote diffusion of macromolecular agents in mouse models of several common solid tumours60. Sulfated glycosaminoglycans are another barrier, as demonstrated by the observation that matrix metalloproteinase 1 (MMP1) and MMP8 overexpression reduces the density of this ECM component in a mouse model of soft-tissue sarcoma, thereby increasing the diffusion of oncolytic viral vectors61. Nonetheless, care must be exercised with such ablative strategies owing to the risk of inducing disease progression10,47. Rather, TME-normalizing CAF-reprogramming agents, such as paricalcitol (which is an agonist of the vitamin D receptor on CAFs), can promote the development of a quiescent stromal phenotype62, can reduce the ECM density and IFP to near-normal values and can also alleviate solid stresses, thus decompressing tumour blood vessels. These agents have been referred to as ‘mechanotherapeutics’46,63.
One promising strategy for the development of mechanotherapeutics involves repurposing commonly used drugs that inhibit TGFβ signalling in CAFs for use in patients with cancer (such as tranilast57,64, metformin65, pirfenidone66 and others; Supplementary Table 3). Of these, angiotensin system inhibitors (ASIs), which are commonly used antihypertensives, are promising TGFβ-inhibiting mechanotherapeutics owing to the large numbers of retrospective studies indicating they provide benefit to patients. Indeed, data from a series of retrospective studies involving patients with non-small-cell lung cancer (NSCLC), PDAC, ovarian cancer, hepatocellular carcinoma, glioblastoma, oesophageal cancer or renal cell carcinoma (RCC) and a prospective phase II trial involving patients with locally advanced PDAC, show that repurposing angiotensin II receptor blockers (ARBs) to reprogramme the stroma has the potential to extend the survival of patients with cancer67–73 (Supplementary Table 4). ASIs are able to reprogramme CAFs and promote transvascular transport74, thus improving both the intratumoural distribution and the effectiveness of nanomedicines75. In particular, ARBs such as losartan reduce the activation of TGFβ signalling and ECM production by CAFs more effectively than other ASIs8,74,75. In fact, a phase II trial has shown that adding losartan to standard-of-care neoadjuvant leucovorin, 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) followed by chemoradiotherapy led to a resection rate of 61% and a median overall survival duration of 33 months in patients with locally advanced PDAC76. These encouraging findings have led to a multi-institutional randomized phase II trial (NCT03563248).
Normalizing the TME for immunotherapy
Improvements in tumour perfusion and oxygenation increase responsiveness to ICI77, and multiple preclinical studies have shown that vascular normalization with AATs can increase the antitumour potency of these agents78–80, whole cancer cell vaccines81 and adoptive cell therapy (ACT)82. Phase III trials showing the effectiveness of combining the anti-VEGFA antibody bevacizumab with atezolizumab and either paclitaxel or nab-paclitaxel in the first-line treatment of patients with metastatic nonsquamous NSCLC83 and the small-molecule VEGFR1–3 inhibitor axitinib with avelumab84 or pembrolizumab85 in patients with advanced-stage RCC also highlight the importance of angiogenesis to the efficacy of ICI. The role of vascular normalization in increasing responsiveness to immunotherapy, in part by ameliorating hypoxia, is also supported by clinical evidence that increased tumour perfusion correlates with responsiveness to bevacizumab in patients with advanced-stage NSCLC10,86. Emerging data also indicate that stimulation of immune cells via ICI can induce vascular normalization in some, but not all, preclinical models77,80,87. Thus, the combination of vascular normalization and ICI could promote a positive feedback loop in which improved vascular function alleviates hypoxia and further improves the efficacy of both therapeutic modalities38,39,80. This feedback loop provides an additional rationale for the combinations of AATs and ICI that are either FDA approved or currently undergoing clinical evaluation11,38,39 (Supplementary Table 2).
Beyond vascular normalization, mechanotherapeutics are also currently under clinical investigation in combination with nanomedicines and ICI (Supplementary Table 3), and those that interfere with TGFβ signalling in CAFs (such as losartan74, tranilast57,64 and pirfenidone66) might, in particular, improve the efficacy of immunotherapies88. TGFβ signalling has multiple roles in tumour progression, although TGFβ signalling through CAFs is immunosuppressive23,88,89. Specifically, in mouse models of metastatic cancer, TGFβ promotes immune evasion, while inhibition of TGFβ signalling produces a potent T cell response89 and potentiates the efficacy of ICI25. In patients with metastatic urothelial carcinomas, TGFβ expression and desmoplasia exclude T cells from the tumour parenchyma, thereby contributing to the immune-excluded phenotype, which is associated with a poor response to ICI23 (FIG. 1). Similarly, biopsy samples of lung metastases obtained from women with breast cancer also typically have a pattern of fibrosis that excludes T cells24. In other experiments, inhibition of TGFβ signalling in mouse models has been shown to promote the systemic antitumour immune response induced by radiation in mouse models — the so-called abscopal effect90. Finally, inhibition of TGFβ signalling upregulates the expression of adhesion molecules (such as intercellular adhesion molecule 1 or vascular cell adhesion molecule 1) on tumour blood vessels that are used by lymphocytes to extravasate38.
In support of these mechanistic studies, a prospective analysis of samples obtained from patients with PDAC demonstrated an increase in the expression of genes associated with T cell activation, and an accompanying retrospective analysis revealed better outcomes among patients receiving long-term ARBs versus those not receiving long-term ARBs after adjustment for other covariates68. By contrast, a retrospective analysis of samples from patients with NSCLC who received anti-programmed cell death 1 (PD-1) or anti-PD-L1 antibodies with or without angiotensin-converting enzyme (ACE) inhibitors found fewer antitumour M1 macrophages and memory-activated T cells in samples from patients who were also receiving ACE inhibitors, leading to worse patient outcomes, even after adjustment for other risk factors91. This result contradicts the findings of a preclinical study indicating that losartan reduces the incidence of lung metastases in mouse models by blocking monocyte recruitment92. Although ACE inhibitors, similar to ARBs, also inhibit components of the renin–angiotensin–aldosterone system, they might act in opposition to ARBs that induce stromal normalization because ACE inhibitors indirectly inhibit both angiotensin II receptor 1 and 2 signalling, unlike losartan, which inhibits receptor 1 only74. Thus, mechanotherapeutic strategies, particularly those involving inhibition of TGFβ signalling, could be used to promote the effects of both nanomedicines and immunotherapies through numerous mechanisms. This rationale supports an ongoing clinical trial in which patients with locally advanced PDAC are receiving losartan in combination with nivolumab, FOLFIRINOX and stereotactic body radiotherapy (NCT03563248).
Nanomedicines in immunotherapy
ICI has revolutionized the treatment of patients with cancer, and antibodies targeting PD-1, PD-L1 or cytotoxic T lymphocyte-associated protein 4 (CTLA-4) have been approved by various regulators as monotherapies or in combination with other treatments for more than a dozen metastatic and/or advanced-stage malignancies, including NSCLC, TNBC, melanoma, Merkel cell carcinoma, hepatocellular carcinoma, RCC, urothelial carcinoma, lymphoma, cervical cancer, gastric or gastro-oesophageal junction adenocarcinoma, colorectal cancer and head and neck squamous cell carcinoma. These agents provide durable responses in a subset of patients and some of these patients are ‘cured’; however, an estimated 87% of patients currently do not derive long-term benefit from ICI2. Thus, new strategies are needed to improve the response rates to such therapies in patients with ICI-resistant disease.
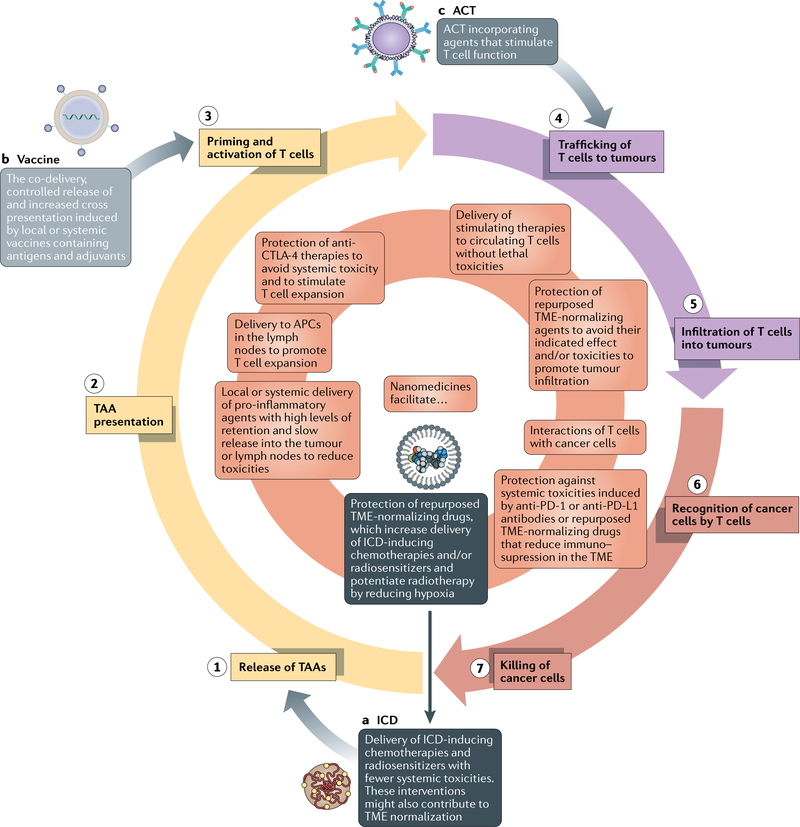
Antitumour immunity must be perpetuated in order to enable durable responses to immunotherapy (FIG. 2). The cancer-immunity cycle consists of: (1) release of tumour-associated antigens; (2) antigen presentation; (3) priming and activation of T cells; (4) trafficking of T cells to tumours; (5) infiltration of T cells into tumours; (6) recognition of cancer cells by T cells; and (7) killing of cancer cells18. Tumours of the immune-desert TME phenotype cannot progress beyond steps 1–3 owing to a lack of immune cells within and surrounding the tumour, while those of the immune-excluded phenotype cannot progress beyond steps 4 and 5 owing to a lack of T cells in the tumour parenchyma. Tumours of the inflamed TME phenotype cannot overcome steps 6 and 7 owing to T cell exhaustion. Various nanomedicine-based approaches have been developed in an attempt to address steps 1–7, while TME normalization might also be beneficial in this regard.
Fig. 2 |. How nanomedicines can be used to perpetuate the cancer-immunity cycle.
The goal of nanomedicine-based immunotherapy is to ensure that the cancer-immunity cycle (the seven numbered steps) perpetuates. Initially, in order to ensure that T cells are capable of attacking cancer cells, we highlight three nanomedicine-based starting points: (a) immunogenic cell death (ICD)-inducing therapy, (b) vaccines and (c) nanoparticle-loaded T cells. Therapies specifically relevant to each of these starting points are in dark grey, light grey and grey boxes, respectively. After one of these initiations, various types of nanomedicines described in this Perspective can turn the cycle forward (the inner orange circle). ACT, adoptive cellular therapy; APC, antigen-presenting cell; CTL A-4, cytotoxic T lymphocyte-associated protein 4; DC, dendritic cell; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; TAA, tumour-associated antigen; TME, tumour microenvironment.
Tumour-associated antigen release
A lack of, or limited, antigen release is a major challenge for successful immunotherapy, especially in tumours of the immune-desert phenotype93,94. Underlining the importance of antigens, ICI is most effective in patients with solid tumours with a high tumour mutational burden, including melanoma, NSCLC and bladder cancer. Only isolated responses to ACT and ICI combinations have been observed among patients with tumour types with a lower tumour mutational burden95. Thus, varying numbers of neoantigens are produced and released across all types of solid tumours96. Additionally, the number and type of neoantigens are unique to each lesion, which might necessitate a personalized immunotherapeutic approach97.
Inducing immunogenic cell death (ICD) using chemotherapies with immunogenic effects or selected doses of ionizing radiation has the potential to prime tumours for a response to immunotherapy by promoting the release of signals that can be recognized by the immune system and thus enhance systemic antitumour immunity98. Signals are released from cancer cells undergoing ICD into the microenvironment and possibly the systemic circulation, where they induce DCs to present neoantigens to T cells, a process that is required for activation of tumour-specific T cells99,100. By reducing the delivery of small-molecule chemotherapies to nonmalignant tissues, and limiting the necessary dose of radiotherapy, nanomedicine-based chemotherapeutics and radiosensitizers, respectively, could improve the efficacy of ICI and minimize toxicities.
Chemotherapy-induced ICD.
The rationale for using nanomedicines rather than small molecules as ICD-inducing chemotherapy is not only the expected reduction in toxicities owing to the EPR effect, but also the potential of the former to normalize the TME. However, metronomic chemotherapy (delivered using frequent low-dose administration) can also normalize the TME, increase tumour perfusion and alleviate hypoxia, thus reprogramming an immunosuppressive TME to an antitumour immunity phenotype44,101 similar to the effects of certain cytotoxic nanomedicines64. Data from mathematical modelling suggest that these two approaches induce TME normalization through similar mechanisms101. The key feature of both strategies is the delivery of sustained drug concentrations. Nanomedicines achieve this effect by prolonging the length of time spent in the circulation and gradually releasing the cytotoxic payload, whereas metronomic strategies achieve the same effect by maintaining consistent drug concentrations through frequent administration. The schedule, dose and type of chemotherapy administered using nanomedicine-based ICD is also important, yet still undefined. Some chemotherapeutic agents seem to promote more ICD and/or other steps of the cancer-immunity cycle than others102. Nonetheless, while the mechanisms and optimal scheduling remain unclear, the use of nab-paclitaxel as a nanomedicine-based chemotherapy to avoid the requirement of immunosuppressive glucocorticoids and potentiate the efficacy of ICI in patients with advanced-stage TNBC fulfils the initial potential of this approach6.
We hypothesize that normalizing the TME using AAT and/or mechanotherapeutics in order to increase penetration of cytotoxic nanomedicines capable of both ICD induction and TME normalization could increase the number of cancer cells undergoing ICD and, in turn, promote further TME normalization, thereby increasing responses to ICI64 (FIG. 2). Data from a clinical trial combining metronomic chemotherapy, vascular normalization and ICI provide support for further investigations of this approach103. The preliminary results of this phase II in a small cohort of women with recurrent ovarian, fallopian tube or primary peritoneal cancers revealed that metronomic cyclophosphamide combined with bevacizumab and pembrolizumab resulted in a 37.5% overall response rate103, whereas the highest response rate reported with pembrolizumab alone among women with gynaecological cancers is 8% in the KEYNOTE-100 trial104.
Radiation-induced abscopal effects.
The abscopal effect involves inducing ICD in one or more superficial lesions, which can then induce a systemic immune response against distant metastases105–109. Besides chemotherapy, locally administered ionizing radiation can also induce ICD and stimulate the immune system to attack distant metastases. However, radiation oncologists must optimize the radiation dose and level of fractionation when scheduling radiotherapy specifically for the induction of abscopal effects, as preclinical data indicate that fractionated, rather than single-dose, radiotherapy can induce ICD110,111 while high-dose radiotherapy limits immunogenicity owing to activation of enzymatic digestion of DNA112. When effective, radiation increases the diversity of the TCR repertoire found in tumours113. Indeed, the European Medicines Agency approval of intratumoural injections of NBTXR3 hafnium oxide nanoparticles designed to enhance the radiation-induced abscopal effect for patients with locally advanced soft-tissue sarcomas demonstrates the validity of this approach114 (Supplementary Table 1).
Similar to intratumoural injections of hafnium oxide nanoparticles, some elements of cancer treatment regimens could include locally administered nanomedicines. Nonetheless, these agents still must overcome the physical barriers posed by the TME of the target tumour, while the local TME and that of distant metastases will likely continue to be immunosuppressive. In this setting, nanomedicine encapsulation increases the retention of chemotherapies within the tumour, enables delayed release of the active payload and reduces the amount of drugs released into the systemic circulation, thus avoiding or reducing the risk of toxicities. Mechanotherapeutics that normalize the extravascular component of the TME increase the distribution of locally administered nanomedicines75,115, although the optimal approach to normalizing the TME of distant metastases within an appropriate period of time after local delivery of ICD-inducing therapy is unclear. TME normalization before radiotherapy improves outcomes by alleviating hypoxia9,116, although the effects of TME normalization on ICD require further investigation64. Separately, administration of nanomedicines after radiotherapy might be an effective method of perpetuating the cancer-immunity cycle. Indeed, radiotherapy improves the distribution of nanomedicines in mouse models, in part by normalizing the TME117,118. An alternative approach involves the delivery of nanomedicines targeting P-selectin, which consist of fucoidan-based nanoparticles with a high level of affinity for this protein. Radiotherapy can be used to increase the cell surface expression of P-selectin, thus improving the delivery and efficacy of this formulation in preclinical models119.
Cancer antigen presentation
Immature DCs must capture released tumour-associated antigens, followed by maturation, lymph node migration and education of naive T cells, despite the presence of an immunosuppressive TME. Immunological adjuvants, which stimulate DCs during antigen detection and processing, can increase the strength of antigen presentation. Otherwise, immune tolerance might occur, during which an immature DC captures an antigen but is not stimulated and therefore fails to process the antigen and activate T cells120.
Dendritic cell maturation.
Cancer antigen presentation can be increased by agents that induce DC maturation, although agents designed to induce this effect have, thus far, shown poor pharmacokinetics when administered systemically121. One clinically investigated, non-nanomedicine-based strategy involves intratumourally injecting an agonist of Toll-like receptor 9 (TLR9) (such as SD-101) to induce DC maturation and subsequent antigen presentation. This strategy has been shown to be well-tolerated and leads to promising response rates in the injected lesion as well as in distant metastases when administered in combination with ICI in patients with anti-PD-1-antibody-naive advanced-stage melanomas in a phase Ib/II trial122. Similarly, intratumourally injected activators of stimulator of interferon-genes (STING) agonists (such as SD-100) are currently in early-phase clinical trials with preliminary data indicating limited response rates123. Indeed, a potential problem involves the overstimulation of T cells, resulting in their death and a reduction in antitumour immunity124. Nonetheless, such therapies could be useful in combination with ICI in patients with tumours with an immune-desert TME phenotype.
Compared with small molecules, locally administered nanomedicines are likely to be retained in the TME for longer periods of time, thereby increasing the ratio of intratumour to systemic drug distribution. For example, a nanomedicine-conjugated TLR7 and TLR8 agonist promotes DC maturation and potentiates the effects of ICI in mouse models125. STING agonists are also candidates for encapsulation in locally and systemically administered nanomedicine formulations126,127. Such cytokines can also be targeted to collagen, thus enabling them to be retained in tumours more effectively128,129.
Nanomedicine-based vaccines induce antigen presentation and make release dispensable.
Vaccines are able to initiate antigen presentation, although their effects upon cancer treatment have, thus far, been modest. Nanomedicine-based approaches confer various advantages over conventional methods of delivery of subunit vaccines, which contain only the antigenic parts. First, nanomedicine-based vaccines improve the delivery of antigens to lymphoid tissues130. Without nanotechnology, the delivery of subunit vaccines can require repeat local injections, such as injections of mRNAs into inguinal lymph nodes131 and peptides into subcutaneous tissues132, which can be invasive and lead to antigen tolerability133. Subcutaneously administered nanomedicines, particularly of a diameter of 10–100 nm, enable more efficient delivery and retention of vaccines in draining lymph nodes and facilitate the controlled release of antigens and adjuvants, thus increasing the level of antigen presentation120. Second, nanomedicine-based vaccines might be targeted for uptake by DCs and other antigen-presenting cells (APCs) using targeting moieties and by tuning their physicochemical properties134. Third, nanomedicine-based vaccines can promote antigen cross-presentation and increase the level of CTL activation that otherwise skews T cells towards a CD4+ phenotype when exposed to conventional vaccines. Specifically, nanomedicine-based vaccines promote endosomal release via pH-sensitive delivery systems that release antigens in the endosome, which enables antigen engulfment by APCs for presentation to CTLs by MHC class I molecules, while conventional vaccines rely on extracellular antigens (soluble proteins or peptides) that are presented to CD4+ T cells via MHC class II molecules135. Fourth, nanomedicine-based vaccines enable the co-delivery of antigens and adjuvants, which increases the extent of antigen presentation and cross-presentation and promotes the development of CD4+ T helper antitumour phenotypes136.
Various sources of antigens for antitumour vaccines are available. Whole-cell vaccines increase the scope of antigens delivered, are personalized and benefit from nanomedicine-based approaches137. For example, in a preclinical study, ICD was induced in cancer cells that were removed from mice that had been previously loaded with a nanomedicine-based adjuvant. Upon subsequent reintroduction, this vaccine elicited systemic antitumour responses that increased the efficacy of ICI138. Furthermore, intravenously administered DC-based vaccines generated by ex vivo exposure to tumour antigens are also capable of priming T cells in patients139. Besides cells, DNA and mRNA can also be used as subunit vaccines to encode protein-based or peptide-based antigens. mRNA can serve as both antigen and adjuvant because, as a single strand, it is a ligand for TLR7 and TLR8 (REF.140). DNA and mRNA can be administered with or without the use of viral vectors. A general consensus exists that nanomedicine-based approaches protect nucleic acids from degradation and are safer than viral vectors. Nanomedicine-based strategies designed to improve the intravenous administration of RNA-based vaccines have entered clinical development. Specifically, intravenously administered RNA lipoplexes can be selectively targeted to DCs without ligands by adjusting the net charge to slightly negative, which targeted the particles to the spleen and generated de novo T cell responses in three patients with melanoma141. Nanomedicines are generally the same, or a similar, size as pathogens so they can be efficiently taken up by DCs142,143. Furthermore, controlling the diameter of the nanoparticles promotes distribution to tissues enriched with APCs. In mouse models, 200 nm diameter RNA lipoplexes are retained in the spleen, although smaller nanoparticles with a diameter of 70 nm preferentially accumulate in the lymph nodes after systemic injection144.
Nanomedicine-based delivery of antigens to DCs can be well controlled using subcutaneous or intranodal administration145. Indeed, subcutaneous injections can preferentially target the uptake of nanomedicines to lymph nodes via the lymphatic drainage by adjusting the diameter of the nanoparticles to <100 nm (REF.146). Once in lymph nodes, surface characteristics of nanovaccines also affect distribution within lymph nodes. Specifically, an array of glycans on the nanomedicine surface can enable antigens to reach the follicles in which B cells reside147. Besides size and surface decoration, another consideration for the optimal delivery of vaccines is that soluble tumour antigens must be released into the cytosol of DCs in order to be processed for presentation to CTLs, although such antigens are often trapped and retained in endosomes. As mentioned previously, nanomedicines sensitive to the acidic pH of endosomes can be controlled to enable antigen release into the cytosol of APCs. This construct works as a monotherapy and also synergizes with ICI in tumour-bearing mice148. Additionally, the nanocarrier itself can act as an adjuvant to the vaccine. Specifically, researchers have screened polymers148 and lipids149 that can assemble into nanocarriers for antigens and themselves act as adjuvants stimulating the STING signalling pathway while also identifying characteristics of the chemical structure that lead to this effect. Vascular normalization can also improve the efficacy of vaccines81, although the benefits likely arise from improvements in T cell infiltration and activity, suggesting that the administration of AATs after vaccines is worth exploring.
Priming and expanding T cells
Nanomedicines that promote antigen presentation often also result in increased priming and expansion of T cells, as the process of antigen presentation is very closely related to priming, and DCs are able to directly stimulate naive T cells. Antigen presentation is completed when naive T cells in lymphoid organs are primed to become effector or memory T cells and expand into differentiated cytotoxic T cells; therefore, drugs that stimulate this process are desirable. Ipilimumab, an anti-CTLA-4 antibody, promotes the priming and expansion of T cells, although it also introduces substantial toxicities especially when simultaneously administered with anti-PD-1 antibodies150. The ability of DCs to prime T cells is dependent on the TCRs, co-stimulatory molecules and cytokine stimulation. Delivering such stimuli to lymphoid organs could stimulate the priming and expansion of T cells, although such an approach could also induce autoimmune toxicities if lacking selectivity. Thus, encapsulating the stimuli in a nanomedicine could prove beneficial.
Systemically administered IL-2 globally activates the immune system and can lead to autoimmune toxicities. Unfortunately, IL-2 and other simulators of T cell expansion and activity, such as antibodies that activate CD137, also result in a ‘cytokine storm’ effect when binding to circulating lymphocytes. Fortunately, anchoring IL-2 and CD137 stimulatory antibodies to the surface of liposomes enables the passive delivery of these agents to tumours and tumour-draining lymph nodes to induce a therapeutic effect without notable toxicities in a mouse model151. Similar results were observed with IL-2, anti-PD-1 antibodies and anti-CTLA-4 antibodies bound to a collagen-binding domain that accumulates within tumours129. Besides inducing toxicities, systemic IL-2 mediates the expansion of immunosuppressive cells that could limit the effectiveness of ICI. Thus, bempegaldesleukin, a PEGylated CD122-preferential IL-2 pathway agonist, was developed to target antitumour T cells as opposed to pro-tumour Treg cells and is currently being tested in combination with nivolumab in a phase III clinical trial involving patients with metastatic RCC (NCT03729245). Only 11% of patients had grade 3–4 adverse events in early-phase trials of this agent as a monotherapy152. The promising results achieved with such agents in clinical trials support the notion that stimulating the priming and expansion of T cells can enhance the effectiveness of ICI.
Agents that prime and expand T cells could be delivered to lymphoid organs as nanomedicines and thus potentially avoid encountering the TME, although many patients with advanced-stage cancers have undergone surgery and might not have an intact lymphatic system at one or more tumour locations. Indeed, such agents carried in nanomedicines engineered to bind with albumin and traffic to lymph nodes enable more effective targeting of APCs towards expanding T cells and boost the effectiveness of ACT153. Additionally, circulating T cells can be programmed with a chimeric antigen receptor (CAR) in vivo using polymeric nanomedicines carrying DNA154. In combination with, or separate from, these strategies, normalization of the TME could reduce the secretion of immunosuppressive cytokines from the tumour to the draining lymph nodes, thereby also promoting the expansion of T cells in lymph nodes. However, once these expanded T cells reach an immunosuppressive TME, they could lose their activity. This concern remains relevant, even for ACT, which reduces the need for priming and expansion of T cells in patients by doing so ex vivo. Thus, strategies that aim to prime and expand T cells in lymph nodes could still benefit from TME normalization and/or intratumour T cell stimulation.
T cell trafficking and infiltration
Trafficking.
Once primed, T cells must traffic out of lymph nodes and through the bloodstream to tumours, followed by infiltration from the vasculature or tumour periphery into the tumour parenchyma to encounter cancer cells. DCs located in tumour-draining lymph nodes present antigens to and stimulate naive T cells, which then enter the bloodstream. Unfortunately, solid tumours often produce chemokines that reduce the extent of T cell trafficking into tumours155. Additionally, the chemokines that solid tumours do produce often do not match the chemokine receptors expressed by T cells, thereby resulting in limited chemotaxis156. Vascular normalization, which either polarizes TAMs to an antitumour M1-like phenotype that is associated with secretion of the chemokine CXCL9 that binds to CXCR3 on T cells157 or modifies the TME and peripheral T cells to express CX3CL1 and its receptor32 to stimulate trafficking to the tumour, is one strategy that might overcome this problem (FIG. 1).
Infiltration.
After trafficking to the site of the tumour, T cells must infiltrate from blood vessels into the parenchyma in order to reach cancer cells. Because tumours often have an abnormal vasculature with limited blood flow, increasing the density and distribution of perfused vessels enables T cells to flow closer to cancer cells before extravasating and migrating. Thus, perfusion-enhancing vascular and stromal normalization improves the distribution of T cells within the TME24,25,81. Even after flowing deep into the tumour vasculature, T cells158 and natural killer cells159 both face barriers to extravasation, owing to reduced expression of adhesion molecules in the immature tumour vasculature. Thus, vascular normalization with AATs that increase the maturity of perivascular and endothelial cells can enhance T cell adhesion, extravasation and infiltration32,82,160.
Once extravasated, T cell migration from blood vessels to the parenchyma is often limited by the desmoplasia. Thus, reprogramming CAFs to reduce the levels of ECM, beyond increasing perfusion and intravascular T cell transport, increases extravascular T cell migration towards cancer cells23–25. Potential stroma-normalizing drugs have been approved by the FDA for other diseases or indications. These drugs include losartan, as described previously74,75, plerixafor24 for stem cell mobilization prior to haematopoietic stem cell transplantation and pirfenidone66 for the treatment of idiopathic pulmonary fibrosis. These drugs have a proven record of clinical use in other indications; therefore, data from retrospective studies could support their application to the treatment of patients with cancer71 (Supplementary Table 4). Moreover, these stroma-normalizing drugs could also reduce the toxicities of cancer therapies161. Incorporating these drugs into nanomedicines could reduce the systemic effects of these agents (for example, the antihypertensive effects of losartan), while enhancing their normalizing effects within tumours. As an example, we developed a nanoformulation consisting of an ARB (valsartan) bound to pH-sensitive polymers, thus creating a nano-ARB, which abrogates the blood-pressure-reducing effect of losartan while increasing the extent of TME normalization25. Accordingly, this nano-ARB improved the antitumour effects of ICI in multiple mouse models of metastatic breast cancer25. Other examples of ARB-containing nanoformulations include telmisartan glycolipid micelles162 and brush-arm star polymers163. Apart from the repurposing of approved drugs, nanomedicines can deliver experimental agents that reprogramme the tumour-associated stroma to a quiescent phenotype. For example, PEGylated polyethylenimine-coated gold nanoparticles that deliver all-trans retinoic acid and small interfering RNAs (siRNAs) against heat-shock protein 47 are able to reprogramme CAFs in mouse xenograft models of PDAC164. Furthermore, the widely used AAT sunitinib (Supplementary Table 2) has been encapsulated into a polymeric micelle delivery system that enhanced the efficacy of an antitumour vaccine in a mouse model of advanced-stage melanoma165. However, a major challenge for the clinical implementation of these nanoformulations will be to demonstrate meaningful clinical benefit over relatively inexpensive, small-molecule generic drugs such as ARBs, which have already led to unprecedented levels of benefit in a phase II clinical trial in patients with locally advanced PDAC76.
Recognition of cancer cells by T cells
In addition to the delivery of antigens and immune-activating compounds to APCs for the promotion of memory T cell responses against tumours, nanomedicines could potentially be engineered to promote the recognition of cancer cells by T cells following infiltration of the TME (FIG. 2). The potential of this strategy is supported by the clinical success of bispecific antibody technology in the clinic for the treatment of lymphoma and some types of leukaemia166. These bispecific T cell engagers (BiTEs) bind to T cells using a conserved component of the TCR (mainly CD3) and to antigens expressed by cancer cells. The first BiTEs to reach the clinic were catumaxomab, which binds to EpCAM on the cell surface of carcinomas167, and blinatumomab, which targets CD19 and has been approved by the FDA for patients with acute lymphoblastic leukaemias166.
The ongoing experience with anti-EGFRvIII CAR T cell therapy in patients with glioblastoma illustrates the importance of recognition of cancer cells by T cells and the potential of BiTEs. Histological evaluations of tumour material from a cohort of ten patients with glioblastoma who received intravenously administered anti-EGFRvIII CAR T cells demonstrated that, although the treatment was not effective in inducing disease regression and resulted in the recruitment of Treg cells, CAR T cells were successfully delivered to the tumour, and a loss of cancer cells harbouring the target antigen was observed168. In order to improve this approach, researchers engineered these CAR T cells to secrete BiTEs that target CD3+ T cells to the wild-type antigen and administered them intraventricularly to mouse models of glioblastoma. This approach increased the range of cancer cells targeted without increasing the incidence of toxic effects on nonmalignant cells bearing the wild-type antigen169. The local administration of BiTE-secreting CAR T cells provides an example of how overactivation of T cells can be avoided, as demonstrated by the lack of nonspecific effects on nonmalignant cells and the ability of the BiTE to direct Treg cell cytotoxicity against EGFR-mutant cancer cells. A nanoparticle has been conjugated with an anti-human epidermal growth factor receptor 2 (HER2) antibody and calreticulin, which provides an ICD signal that promotes phagocytosis. This multivalent bispecific nanobioconjugate engager stimulated the phagocytosis of cancer cells. Thus, although HER2 targeting was used for recognition, HER2-negative cancer cells were also killed because the phagocytosis of cancer cells released additional cancer cell antigens170.
TME normalization could aid in the recognition of cancer cells by T cells. Both T cells and natural killer cells recognize cancer cells via MHC class I molecules, yet hypoxia causes cancer cells to shed these molecules171,172. Vascular normalization, achieved by inhibiting either nitric oxide171,172 or VEGF signalling, increases the expression of MHC class I molecules32. Indeed, vascular normalization also promotes the antigen-specific migration of T cells32. Thus, combining TME normalization with one or more of the strategies described in this section might alleviate hypoxia and immunosuppression in the TME and enable increasing levels of cancer cell recognition by T cells.
Killing of cancer cells
If the cancer-immunity cycle proceeds until T cells recognize yet fail to kill cancer cells, inhibition of the PD-1 immune checkpoint, which suppresses T cell activity, using an anti-PD-1 or anti-PD-L1 antibody could stimulate antitumour immunity. However, these inhibited T cells then produce enzymes such as indoleamine 2,3-dioxygenase (IDO) that reduce their activity, thus making IDO a rational target. Nonetheless, attempts to inhibit this enzyme in combination with pembrolizumab in a phase III trial involving patients with unresectable or metastatic melanoma failed to provide any improvements in efficacy, which raises questions about the dosing and scheduling of these agents173 as well as the extent of co-expression of PD-L1 and IDO174. These questions must be addressed before nanomedicines that combine inhibition of PD-L1 and IDO can be considered175.
Besides stimulating a desmoplastic reaction that causes solid stress in CAFs, vessel compression and hypoxia (FIG. 1), TGFβ also suppresses T cell killing by promoting the development of T cells of a tumour-promoting phenotype. Thus, nanomedicines have been developed that inhibit TGFβ signalling in T cells by actively targeting CD8+ T cells176, ex vivo in preparing cells for ACT177, or passively through the EPR effect178. Indeed, cells loaded with delivery platforms that become activated after cellular recognition are promising approaches for supporting T cell-mediated cancer cell killing. Specifically, loading nanomedicines into T cells enables the delivery of T cell-stimulating agents without accumulation in nonmalignant tissues, with activation upon cancer cell binding observed in mouse models179–181.
Along with TGFβ, hypoxia might inhibit cancer cell lysis, in part by inducing epithelial-to-mesenchymal transition in NSCLC cells, and thus interfere with the receptors and signalling molecules at the T cell–cancer cell junction182. In this scenario, hypoxia delays the development of CTLs, although in general it is considered to enhance cancer cell lysis34. Mechanotherapeutics that inhibit TGFβ signalling could alleviate hypoxia while also interfering with signalling pathways that limit T cell signalling. Furthermore, because impaired cancer cell lysis occurs in the tumour parenchyma, we hypothesize that agents that aim to modulate exhausted T cells must penetrate deep into tumours. Thus, using TME normalization strategies to increase the penetration of nanomedicines that reverse T cell exhaustion is worth exploring.
Re-engineering nanomedicines
In most patients with solid tumours, the TME of both the primary tumour and the metastases compromises responsiveness owing to limited drug delivery and increased levels of immunosuppression. Thus, in this Perspective we have described how various nanomedicines and TME normalization strategies can facilitate progression through the cancer-immunity cycle. Nonetheless, treatments that overcome only one step of this cycle are insufficient for most patients with cancer. Indeed, even in patients with primary tumours with increased levels of nanomedicine uptake and a favourable immune TME phenotype, the metastases could have different distributions of both nanomedicines12,13 and immune cells16,17. Even in patients with a single primary lesion, treatments designed to overcome one step of the cancer-immunity cycle might simply reveal the next mechanism of treatment resistance. Thus, we propose using minimal combinations of agents that could facilitate progression through the entire cancer-immunity cycle while limiting the incidence of adverse events. In order to initiate the cycle, nanomedicine-based vaccines or ACT incorporating nanomedicines could be administered either locally or systemically. Alternatively, nanomedicine-based TME-normalization strategies that precede ICD induction, either involving nanomedicine-based chemotherapy or radiotherapy-sensitizing agents, can initiate the cycle. If an adjuvant is not administered before, or with, the vaccine, additional nanomedicines should be administered that stimulate DC maturation and thus enable antigen presentation and subsequent priming and expansion of antitumour T cells (FIG. 2). TME normalization following T cell activation can increase T cell trafficking to, and infiltration into, tumours. Finally, TME normalization and nanomedicines targeting T cells can also promote the recognition and killing of cancer cells by T cells. Thus, combinations of drugs will likely be necessary to potentiate the effectiveness of immunotherapies in patients with solid tumours (FIGS 2,3). Nonetheless, the extent of resistance mediated by the various steps of the cancer-immunity cycle varies between patients, within patients and likely also within a single tumour over time183. Thus, in order to avoid the risk of toxicities in patients with tumours with a high level of nanomedicine uptake and a favourable immune TME phenotype, the number of agents should be reduced.
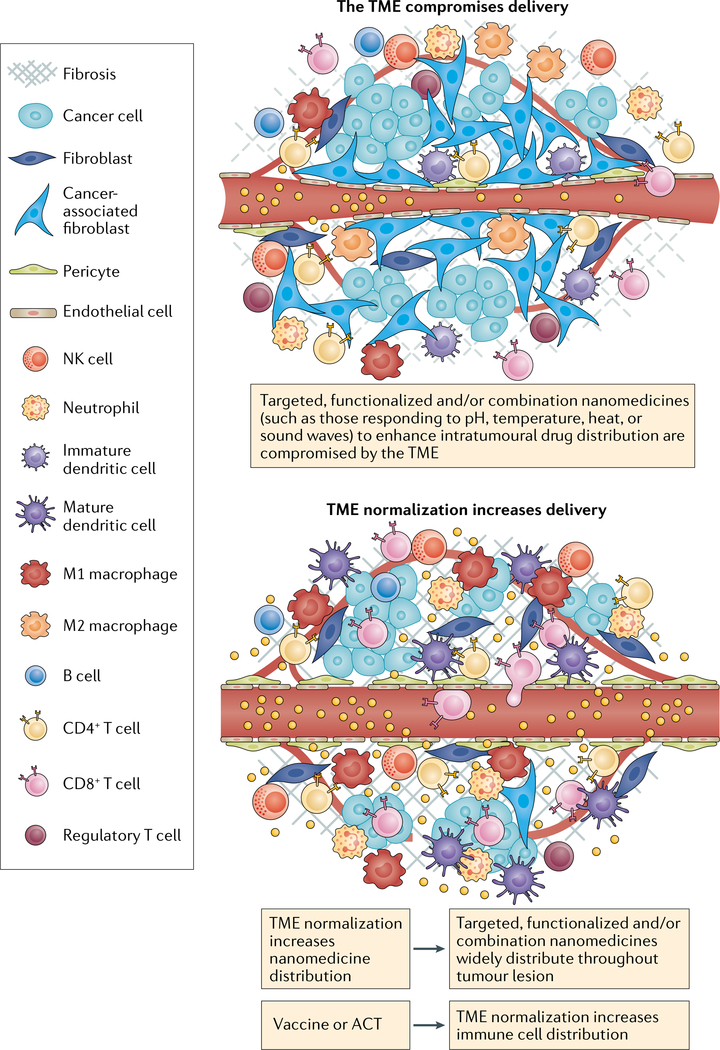
Fig. 3 |. Normalizing the TME to increase the penetration of combination therapies.
Targeted or stimuli-responsive nanomedicines often have a limited level of distribution within tumours because the tumour microenvironment (TME) limits blood flow and therefore the extent of tumour penetration. With insufficient penetration and a limited density of antitumour immune cells, the advantage of these nanomedicines compared with passively accumulating and releasing nanomedicines is reduced. TME-normalizing therapies increase and homogenize the intratumour distribution of immune cells and nanomedicines. Nanomedicine-based vaccines and autologous transferred T cells carrying nanomedicines increase the number of antitumour T cells in the host. If followed by TME normalization, a larger percentage of these T cells can migrate to the tumour parenchyma. Similarly, functionalized nanomedicines following TME normalization can penetrate the tumour parenchyma in higher fractions thereby reaching their target and/or stimuli and demonstrating a larger improvement over passive nanomedicine. Thus, normalizing the TME could increase the effectiveness of targeted nanomedicines that combine cytotoxic agents and immunotherapies. ACT, adoptive cellular therapy; NK, natural killer.
Combination nanomedicines
Given the various drugs necessary to facilitate progression of the cancer-immunity cycle in patients with solid tumours, extensive research has led to the development of nanomedicines that combine multiple immunotherapies, including: chemotherapy and immune-modulators; radiosensitizers, including photothermal and photodynamic therapies and immune-modulators; RNA interference-based immune-modulators; and RNA-based or other vaccines loaded with adjuvants184. In fact, several features of nanomedicines, such as coordinating drug interactions within the core of nanomedicines, controlling the release chemistry of a drug–polymer linker, and/or conjugating a drug or antibody on the surface of the nanomedicine, might enable the synchronous administration of multiple agents, including nucleic acids, into single nanomedicines. These features enable the control of pharmacokinetics, drug concentrations, drug ratios and release rates of bioactive payloads, thus enabling the optimization of synergistic effects185,186. For example, self-assembled micelles carrying oxaliplatin in the core and dihydroartemisinin in the shell are able to synergistically induce ICD and increase the efficacy of ICI in subcutaneously grown tumours187. A similar approach, relying on the delivery of external radiation, involves the delivery of two agents that promote ICD (chemotherapy and a photodynamic therapy sensitizer), which are located in the nanoparticle core and shell, respectively109. A nanomedicine consisting of a hollow MnO2 nanoparticle containing doxorubicin plus a photodynamic agent that decomposes to eventually supply oxygen to the TME has been engineered to deliver a photodynamic therapy sensitizer to provide an additional level of functionality188. A nanomedicine featuring four components (a tumour antigen-targeting antibody, a recombinant form of IL-29 with an extended half-life, an anti-PD-1 antibody and a T cell stimulatory vaccine) demonstrated robust antitumour effects in mouse models of melanoma with a high tumour burden189. Fucoidan-based nanoparticles are antiangiogenic and have been combined with an anti-PD-L1 antibody and anti-CD3 and anti-CD28 moieties, thereby forming another quadruple combination190. Another formulation involving a three-in-one liposome-based nanomedicine has been developed with the aim of normalizing the TME prior to the administration of CAR T cells to patients with solid tumours. This liposome binds to angiogenic tumour vessels via an iRGD ligand that enhances the transit of tumour-specific nanomedicines across the vessel wall followed by the delivery of a PI3K inhibitor to inhibit immunosuppressive cancer cells and an α-galactosylceramide agonist that stimulates T cells191. Indeed, direct modifications such as equipping nanomedicines with targeting agents192,193 and providing nanomedicines with triggers that are either TME-responsive (such as tumour pH, redox status or specific enzyme activity)25,194 or that can be activated by external stimuli (such as heat, light, magnetic fields or sound waves) to enable spatiotemporal control of drug activity195,196 have been pursued by several investigators. However, an extensive discussion of this research is mostly outside the scope of this Perspective because these strategies are unlikely to reach their full potential without prior systemic TME normalization (FIG. 3). Furthermore, it is unclear to what extent specifically targeted nanomedicines increase the intratumour distribution of these agents, especially for nanomedicines administered after TME normalization.
The specific clinical implementation of these various nanomedicine-based combination therapy approaches will depend on whether these therapies should be scheduled simultaneously, sequentially or are contraindicated. All the drugs described in this Perspective, even repurposed drugs like losartan that already have regulatory approval for other indications, can unfortunately have serious adverse effects in certain patients; therefore, combination nanomedicines and treatment schedules incorporating more than one agent must be carefully designed to avoid administering an unnecessary number of drugs to patients. For example, because TME normalization is beneficial throughout the cancer-immunity cycle whereas ICI can be toxic and is more important at specific stages of the cycle, strategies that combine repurposed TME-normalizing agents with an ICI in a single nanomedicine could be counterproductive. Similarly, agents designed to normalize the TME need not precede vaccines, so there is no need to integrate them into a single nanomedicine. The experience with combined administration of ipilimumab plus nivolumab to patients with metastatic melanoma, in whom the combination was more efficacious but also more toxic than sequential administration, demonstrates the risks associated with combining different immunotherapies150. Nonetheless, the FDA approval of the liposomal combination of daunorubicin and cytarabine for patients with newly diagnosed therapy-related acute myeloid leukaemia or acute myeloid leukaemia with myelodysplasia-related changes confirms the validity of using nanomedicines to concurrently deliver more than one drug to patients with cancer.
Imaging nanomedicines
Clinical imaging of nanomedicines that emit PET or MRI signals has confirmed the heterogeneous distribution of nanomedicines in patients with solid tumours, as originally discovered in preclinical studies12,13. Further development of these targeted imaging approaches could also provide information on TME status197 and the activity of immunotherapies in patients198. These probes will be useful in evaluating the benefit of TME-normalizing strategies in reducing the heterogeneity of nanomedicine distribution and testing predictive markers of delivery and responsiveness to nanomedicines. Current predictive approaches, including tumour histology, flow cytometry and whole-genome sequencing, provide a partial view of the TME conditions that affect the antitumour immune response. Direct imaging of nanomedicines has the potential to facilitate the direct visualization of dynamic events that could complement, or possibly even replace, these other methods. Thus, advances in TME normalization could help advance the development of nanomedicines and vice versa.
Gene therapies as nanomedicines
The encapsulation of oligonucleotide vaccines in nanomedicines might help avoid nucleic acid degradation, immune detection, renal clearance and the difficulties associated with cell entry. In the example of mRNA-loaded nanomedicines, encapsulation might also enable the production of therapeutic proteins in tumours, as demonstrated in mouse models of PDAC199. The silencing effects of siRNAs or the protein translation from mRNAs are transient, although more permanent alterations of the TME and long-lasting therapeutic responses can be achieved by modifying the DNA of tumour cells. This strategy can be achieved by using nanomedicines to deliver DNA to the nuclei of tumour cells, as well as by nanomedicines that incorporate CRISPR–Cas9 gene editing technology. CRISPR–Cas systems are an important tool that enables precision genome editing. For in vivo use, however, delivery systems are required that transport the necessary components to the nucleus of the target cells. PEGylated nanoparticles have been developed that can deliver CRISPR–Cas9 and have antitumour efficacy in cell lines and in mouse models200. However, owing to the bacterial origins of the enzymes, humans often have pre-existing adaptive immunity to Cas9 proteins201. Hence, novel strategies must be developed to overcome this and various other challenges202.
Innovative approaches involving the encapsulation of oligonucleotides have been developed and used to deliver several anticancer drugs to experimental animals and a drug to patients with Alzheimer disease186. One strategy involves the combination of ICD-inducing chemotherapy with a nanomedicine enabling the delivery of plasmid DNA encoding a PD-L1 trap with expression localized to the TME, which enables reduced levels of toxicity compared with systemic ICI203. Another strategy involves the potentiation of a DC vaccine by interfering with the adenosine signalling pathway using a nanomedicine containing an siRNA against CD73 (also known as 5′-nucleotidase, an enzyme that converts AMP into adenosine). This approach enhances T cell activity and reduces that of immunosuppressive cells in the TME204. Other strategies include the layer-by-layer self-assembly of polymers encapsulating siRNAs and chemotherapy205, a polymer construct of metformin harbouring siRNA in a core-membrane structured lipid–polycation–hyaluronic acid nanomedicine206 and poly-ion complex (PIC) micelles. In this latter approach, the charge of nucleic acids can be neutralized by oppositely charged block ionomers, thereby facilitating the formation of stable micelles186,207. Various researchers have conjugated cyclic arginine–glycine–aspartate (cRGD) ligands to PIC micelles in an attempt to knockdown VEGF and other targets in various cancer models208–210. In order to improve the uptake and minimize accumulation in nontarget organs, smaller diameter PIC micelles have been developed involving a Y-shaped block catiomer with a specific chain length designed to match the negative charges of the oligonucleotide strand211. Similar to the strategy of using cRGD ligands on the surface of PIC micelles to target endothelial cells, plasmid DNA encoding tumour necrosis factor-related apoptosis-inducing ligand encapsulated in branched polyethylenimine can be targeted to pancreatic stellate cells via uptake through the fibroblast growth factor receptor212. The clinical success, including safety, of the lipid-based RNA interference nanomedicine patisiran in patients with amyloidosis demonstrates the feasibility of using such approaches to deliver oligonucleotides213; however, the experience with phase I trials of nanomedicines designed to deliver siRNAs to solid tumours indicates the need to improve both the safety and efficiency of delivery214,215.
Conclusions
The use of cytotoxic nanomedicines is based on the assumption of selective accumulation in tumours relative to most nonmalignant tissues. This targeting enables chemotherapies to accumulate more selectively in tumours relative to nonmalignant tissues, which reduces the risk of toxicities. We previously suggested, approximately 30 years ago, that the efficacy of this strategy is limited by the physical barriers posed by tumours7. Indeed, various clinical studies have since confirmed our preclinical observations of the heterogeneity of nanomedicine accumulation and distribution in single tumours, between multiple tumours within the same patient and between patients with the same cancer type. Nonetheless, strategies to promote the delivery of nanomedicines have not received clinical approval, and much of the research in this area is now focused on the delivery of immunotherapies. Pathological angiogenesis and desmoplasia compromise drug and oxygen delivery alike, thus resulting in hypoxia and acidity and causing immunosuppression. Here, we propose that normalizing the TME in order to improve the delivery of nanomedicines will also reverse immunosuppression, thereby potentiating the effectiveness of immunotherapies delivered using nanomedicines. Furthermore, nanomedicines designed to deliver repurposed FDA-approved drugs should be used to normalize the TME.
Despite intense clinical investigation, much more research is still needed in order to effectively combine immunotherapies, including identifying predictive biomarkers and biomarkers of response and gaining a better understanding of the temporal effects of the various therapies, thus enabling the development of more effective nanomedicines that have fewer toxicities. A better understanding of these aspects will guide the combination and personalization of therapies that is necessary to increase the percentage of patients with cancer who derive benefit from ICI from the current level of 13%2. The limited percentage of patients who currently benefit from ICI alone indicates an urgent need for novel combination strategies.
Personalization — requiring validation and measurement of biomarkers beyond expression of the immune-checkpoint receptors or their ligands — could take years to develop and be expensive to implement. Furthermore, many patients cannot afford and/or tolerate biopsy sampling and/or other invasive procedures for the procurement of samples for biomarker analysis. Finally, the TME immune phenotype can change with treatment, over time and can also vary by location; even the TME of the metastases can differ from that of other metastases and of the primary tumour in the same patient. In other words, the biomarkers used to select a treatment for a patient’s primary tumour might be ineffective against their metastases. In this Perspective, we propose a minimum combination of therapies that potentially could address all seven steps of the cancer-immunity cycle in patients with systemic, heterogeneous disease. Treatment can be initiated using either ACT, a vaccine carrying an antigen and an adjuvant, or using a repurposed TME-normalizing agent followed by ICD-inducing chemotherapy and/or radiotherapy. Once T cells are primed and expanded, TME normalization combined with an anti-PD-1 or anti-PD-L1 antibody could perpetuate the cycle. Nanomedicines have the potential to enhance the efficacy and safety of each of these treatment modalities.
Supplementary Material
Acknowledgements
The authors apologize to authors whose work could not be cited owing to space constraints. In general, the authors focused on systemically and locally administered particle-based therapies, so the scope of this article does not include certain nanotechnologies developed for the delivery of immunotherapies, such as injectable scaffolds, which are currently under clinical investigation (NCT01753089). The authors thank M. Kalli (University of Cyprus) for assistance with the preparation of the figures, K. Kakimi (University of Tokyo) for helpful discussions and A. Osada (NanoCarrier Co., Ltd), Y. Huang (Cyrus Tang Haematology Center), M. R. Martin (University of Tokyo), V. Melo (University of Tokyo) and Z. Amoozgar and D. Fukumura (Massachusetts General Hospital) for critical input into the manuscript. The research leading to these results has received funding from the National Foundation for Cancer Research, the Ludwig Center at Harvard, the Jane’s Trust Foundation, the Advanced Medical Research Foundation, the US National Cancer Institute grants P01-CA080124, R01-CA098706, R01-CA208205 and U01-CA224348 and the US Department of Defense Breast Cancer Research Program Innovator Award W81XWH-10-1-0016 (to R.K.J.), the European Research Council grant 838414 and the INFRASTRUCTURE/1216/0052 grant co-financed by the European Regional Development Fund and the Republic of Cyprus through the Research Promotion Foundation (to T.S.), and the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research B (JP16H03179) and Young Scientists B (JP25750172) to H.C. R.K.J. is a recipient of an Outstanding Investigator Award R35-CA197743 from the U.S. National Cancer Institute. J.D.M was supported by a JSPS Postdoctoral Fellowship, P16731.
Footnotes
Competing interests
J.D.M. became a full-time employee of NanoCarrier during the preparation of this manuscript. R.K.J. has received honoraria from Amgen, has acted as a consultant for Chugai, Merck, Ophthotech, Pfizer, SPARC, SynDevRx and XTuit, owns equity in Enlight, Ophthotech and SynDevRx and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund. H.C. and T.S. declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41571-019-0308-z.
References
- 1.Jain RK & Stylianopoulos T Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam A & Prasad V Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open. 2, e192535–e192535 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R & Hellmann MD Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Riley RS, June CH, Langer R & Mitchell MJ Delivery technologies for cancer immunotherapy. Nat. Rev. Drug. Discovery 18, 175–196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg MS et al. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 19, 587–602 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Schmid P et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Jain RK Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 50, 814s–819s (1990). [PubMed] [Google Scholar]
- 8.Chauhan VP & Jain RK Strategies for advancing cancer nanomedicine. Nat. Mater. 12, 958–962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin JD, Seano G & Jain RK Normalizing function of tumor vessels: progress, opportunities and challenges. Annu. Rev. Physiol. 81, 505–534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukumura D, Kloepper J, Amoozgar Z, Duda DG & Jain RK Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 23, 4190–4202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramanathan RK et al. Correlation between ferumoxytol uptake in tumor lesions by MRI and response to nanoliposomal irinotecan in patients with advanced solid tumors: a pilot study. Clin. Cancer Res. 23, 3638–3648 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Stylianopoulos T, Munn LL & Jain RK Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer 4, 292–319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauhan VP, Stylianopoulos T, Boucher Y & Jain RK Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2, 281–298 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Baine MK et al. Characterization of tumor infiltrating lymphocytes in paired primary and metastatic renal cell carcinoma specimens. Oncotarget 6, 24990–25002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller P et al. Metastatic spread in patients with non-small cell lung cancer is associated with a reduced density of tumor-infiltrating T cells. Cancer Immunol. Immunother. 65, 1–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen DS & Mellman I Elements of cancer immunity and the cancer–immune set point. Nature 541, 321–330 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Tumeh PC et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veglia F & Gabrilovich DI Dendritic cells in cancer: the role revisited. Curr. Opin. immunol. 45, 43–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Facciabene A et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475, 226–230 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Togashi Y, Shitara K & Nishikawa H Regulatory T cells in cancer immunosuppression — implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16, 356–371 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Mariathasan S et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen IX et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl Acad. Sci. USA 116, 4558–4566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan VP et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl Acad. Sci. USA 116, 10674–10680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa A et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479. e410 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Rytelewski M et al. Merger of dynamic two-photon and phosphorescence lifetime microscopy reveals dependence of lymphocyte motility on oxygen in solid and hematological tumors. J. Immunother. Cancer 7, 78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatfield SM et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 7, 277ra230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maenhout SK, Thielemans K & Aerts JL Location, location, location: functional and phenotypic heterogeneity between tumor-infiltrating and non-infiltrating myeloid-derived suppressor cells. Oncoimmunology 3, e956579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voron T et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palazon A et al. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32, 669–683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallin JJ et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 7, 12624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noman MZ et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noman MZ et al. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C569–C579 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calcinotto A et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 72, 2746–2756 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Kuczek DE et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 7, 68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzone M & Bergers G Regulation of blood and lymphatic vessels by immune cells in tumors and metastasis. Annu. Rev. Physiol. 81, 535–560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munn LL & Jain RK Vascular regulation of anti-tumor immunity. Science 365, 544–555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y et al. Improving immune–vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 18, 195–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain RK Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Jain RK Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Goel S et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmeliet P & Jain RK Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug. Discov. 10, 417–427 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Jain RK Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viallard C & Larrivée B Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20, 409–426 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Jain RK, Martin JD & Stylianopoulos T The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whatcott CJ, Han H & Von Hoff DD Orchestrating the tumor microenvironment to improve survival for patients with pancreatic cancer: normalization, not destruction. Cancer J. 21, 299–306 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stapleton S, Allen C, Pintilie M & Jaffray DA Tumor perfusion imaging predicts the intra-tumoral accumulation of liposomes. J. Control. Rel. 172, 351–357 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Toy R et al. Multimodal in vivo imaging exposes the voyage of nanoparticles in tumor microcirculation. ACS Nano 7, 3118–3129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chauhan VP et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin JD et al. Dexamethasone increases cisplatin-loaded nanocarrier delivery and efficacy in metastatic breast cancer by normalizing the tumor microenvironment. ACS Nano 13, 6396–6408 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Stylianopoulos T & Jain RK Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl Acad. Sci. USA 110, 18632–18637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayson GC, Kerbel R, Ellis LM & Harris AL Antiangiogenic therapy in oncology: current status and future directions. Lancet 388, 518–529 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Chauhan VP et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26, 14–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stylianopoulos T et al. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 73, 3833–3841 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stylianopoulos T et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papageorgis P et al. Tranilast-induced stress alleviation in solid tumors improves the efficacy of chemo-and nanotherapeutics in a size-independent manner. Sci. Rep. 7, 46140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ & Jain RK Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60, 2497–2503 (2000). [PubMed] [Google Scholar]
- 59.McKee TD et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 66, 2509–2513 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Brown E et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 9, 796–800 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Mok W, Boucher Y & Jain RK Matrix metalloproteinases-1 and −8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 67, 10664–10668 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Sherman MH et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheridan C Pancreatic cancer provides test bed for first mechanotherapeutics. Nat. Biotechnol. 37, 829 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Panagi M et al. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 10, 1910–1922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Incio J et al. Metformin reduces desmoplasia in pancreatic cancer by reprogramming stellate cells and tumor-associated macrophages. PLoS One 10, e0141392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polydorou C, Mpekris F, Papageorgis P, Voutouri C & Stylianopoulos T Pirfenidone normalizes the tumor microenvironment to improve chemotherapy. Oncotarget 8, 24506–24517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Y et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 116, 2210–2219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H et al. Use of angiotensin system inhibitors is associated with immune activation and longer survival in nonmetastatic pancreatic ductal adenocarcinoma. Clin. Cancer Res. 23, 5959–5969 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geller A et al. Angiotensin system inhibitors during induction chemotherapy for esophageal adenocarcinoma: analysis of survival. J. Clin. Oncol. 36, e16066 (2018). [Google Scholar]
- 70.Pinter M et al. Use of inhibitors of the renin–angiotensin system is associated with longer survival in patients with hepatocellular carcinoma. United Eur. Gastroenterol. J. 5, 987–996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinter M & Jain RK Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci. Transl. Med. 9, eaan5616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levin VA, Chan J, Datta M, Yee JL & Jain RK Effect of angiotensin system inhibitors on survival in newly diagnosed glioma patients and recurrent glioblastoma patients receiving chemotherapy and/or bevacizumab. J. Neurooncol. 134, 325–330 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Cleary JM et al. FOLFOX plus ziv-aflibercept or placebo in first-line metastatic esophagogastric adenocarcinoma: a double-blind, randomized, multicenter phase 2 trial. Cancer 125, 2213–2221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chauhan VP et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y & Jain RK Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA 108, 2909–2914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy JE et al. A phase II study of neoadjuvant FOLFIRINOX in combination with losartan followed by chemoradiotherapy in locally advanced pancreatic cancer: R0 resection rate and clinical outcomes. JAMA Oncol. 5, 1020–1027 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng X et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J. Clin. Invest. 128, 2104–2115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allen E et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak9679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmittnaegel M et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 9, eaak9670 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Shigeta K et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 10.1002/hep.30889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang Y et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shrimali RK et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Socinski MA et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378, 2288–2301 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Motzer RJ et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rini BI et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Heist RS et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc. Natl Acad. Sci. USA 112, 1547–1552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian L et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chakravarthy A, Khan L, Bensler NP, Bose P & De Carvalho DD TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9, 4692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tauriello DV et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Vanpouille-Box C et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 75, 2232–2242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Medjebar S et al. Angiotensin-converting enzyme inhibitor prescription is associated with decreased progression-free survival (PFS) and overall survival (OS) in patients with lung cancers treated with PD-1/PD-L1 immune checkpoint blockers. J. Clin. Oncol. 37, e20512 (2019). [Google Scholar]
- 92.Regan DP et al. The angiotensin receptor blocker losartan suppresses growth of pulmonary metastases via AT1R-independent inhibition of CCR2 signaling and monocyte recruitment. J. Immunol. 202, 3087–3102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Newick K, O’Brien S, Moon E & Albelda SM CAR T cell therapy for solid tumors. Annu. Rev. Med. 68, 139–152 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Segal NH et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 68, 889–892 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Stevanović S et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 356, 200–205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu Z, Ott PA & Wu CJ Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 18, 168–182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soliman HH nab-Paclitaxel as a potential partner with checkpoint inhibitors in solid tumors. Onco Targets Ther. 10, 101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schuler G & Steinman R Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 186, 1183–1187 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albert ML, Sauter B & Bhardwaj N Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86 (1998). [DOI] [PubMed] [Google Scholar]
- 101.Mpekris F, Baish JW, Stylianopoulos T & Jain RK Role of vascular normalization in benefit from metronomic chemotherapy. Proc. Natl Acad. Sci. USA 114, 1994–1999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Voorwerk L et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 25, 920–928 (2019). [DOI] [PubMed] [Google Scholar]
- 103.Zsiros E et al. A phase II trial of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide for recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer. Gynecol. Oncol. 154, 23 (2019). [Google Scholar]
- 104.Matulonis U et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann. Oncol. 30, 1080–1087 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Min Y et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 12, 877–882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng J et al. Photosensitizer micelles together with IDO inhibitor enhance cancer photothermal therapy and immunotherapy. Adv. Sci. 5, 1700891 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Q et al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 31, 1802228 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Chen Z et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano 12, 8633–8645 (2018). [DOI] [PubMed] [Google Scholar]
- 109.He C et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 7, 12499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dewan MZ et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demaria S et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005). [PubMed] [Google Scholar]
- 112.Vanpouille-Box C et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Twyman-Saint Victor C et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bonvalot S et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 20, 1148–1159 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Liu J et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 109, 16618–16623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Batchelor TT et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl Acad. Sci. USA 110, 19059–19064 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stapleton S et al. Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intra-tumoral fluid dynamics. ACS Nano 12, 7583–7600 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Miller MA et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med. 9, eaal0225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shamay Y et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci. Transl. Med. 8, 345ra387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Irvine DJ, Swartz MA & Szeto GL Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 12, 978–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramanjulu JM et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 564, 439–443 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Milhem MM et al. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced melanoma who are naïve to anti-PD-1 therapy. J. Clin. Oncol. 37, 9534 (2019). [Google Scholar]
- 123.Meric-Bernstam F et al. Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J. Clin. Oncol. 37, 2507 (2019). [Google Scholar]
- 124.Larkin B et al. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. J. Immunol. 199, 397–402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nuhn L et al. Nanoparticle-conjugate TLR7/8 agonist localized immunotherapy provokes safe antitumoral responses. Adv. Mater. 30, 1803397 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Wilson DR et al. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine 14, 237–246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koshy ST, Cheung AS, Gu L, Graveline AR & Mooney DJ Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosyst. 1, 1600013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Momin N et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl. Med. 11, eaaw2614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishihara J et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci. Transl. Med. 11, eaau3259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Irvine DJ, Hanson MC, Rakhra K & Tokatlian T Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 115, 11109–11146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sahin U et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Ott PA et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hailemichael Y et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 19, 465–472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rosalia RA et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 40, 88–97 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Yuba E et al. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 35, 3091–3101 (2014). [DOI] [PubMed] [Google Scholar]
- 136.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A & Moon JJ Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ali OA, Huebsch N, Cao L, Dranoff G & Mooney DJ Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 8, 151–158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan Y et al. Immunogenic cell death amplified by co-localized adjuvant delivery for cancer immunotherapy. Nano Lett. 17, 7387–7393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carreno BM et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 348, 803–808 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heil F et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Kranz LM et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–400 (2016). [DOI] [PubMed] [Google Scholar]
- 142.Kanchan V & Panda AK Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials 28, 5344–5357 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Li X, Sloat BR, Yanasarn N & Cui Z Relationship between the size of nanoparticles and their adjuvant activity: data from a study with an improved experimental design. Eur. J. Pharm. Biopharm. 78, 107–116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tseng Y-C, Xu Z, Guley K, Yuan H & Huang L Lipid–calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials 35, 4688–4698 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jewell CM, López SCB & Irvine DJ In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl Acad. Sci. USA 108, 15745–15750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oussoren C & Storm G Liposomes to target the lymphatics by subcutaneous administration. Adv. Drug. Deliv. Rev. 50, 143–156 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Tokatlian T et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 363, 649–654 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luo M et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 12, 648–654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miao et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Wolchok JD et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Y, Li N, Suh H & Irvine DJ Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 9, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bentebibel S-E et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 9, 711–721 (2019). [DOI] [PubMed] [Google Scholar]
- 153.Ma L et al. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith TT et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 12, 813–820 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feig C et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harlin H et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 69, 3077–3085 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rolny C et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19, 31–44 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Taggart D et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc. Natl Acad. Sci. USA 115, E1540–E1549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Melder RJ et al. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med. 2, 992–997 (1996). [DOI] [PubMed] [Google Scholar]
- 160.Hamzah J et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453, 410–414 (2008). [DOI] [PubMed] [Google Scholar]
- 161.Pinter M, Kwanten WJ & Jain RK Reninangiotensin system inhibitors to mitigate cancer treatment-related adverse events. Clin. Cancer Res. 24, 3803–3812 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu Y et al. Inhibition of tumor-promoting stroma to enforce subsequently targeting AT1R on tumor cells by pathological inspired micelles. Biomaterials 161, 33–46 (2018). [DOI] [PubMed] [Google Scholar]
- 163.Golder MR et al. Reduction of liver fibrosis by rationally designed macromolecular telmisartan prodrugs. Nat. Biomed. Eng. 2, 822–830 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Han X et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 9, 3390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huo M et al. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J. Control. Rel. 245, 81–94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Topp MS et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, singlearm, phase 2 study. Lancet Oncol. 16, 57–66 (2015). [DOI] [PubMed] [Google Scholar]
- 167.Heiss MM et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int. J. Cancer 127, 2209–2221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Rourke DM et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Choi BD et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Yuan H et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat. Nanotechnol. 12, 763–769 (2017). [DOI] [PubMed] [Google Scholar]
- 171.Siemens DR et al. Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: role of nitric oxide. Cancer Res. 68, 4746–4753 (2008). [DOI] [PubMed] [Google Scholar]
- 172.Sethumadhavan S et al. Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. PLoS One 12, e0187314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Long GV et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 20, 1083–1097 (2019). [DOI] [PubMed] [Google Scholar]
- 174.Krähenbühl L et al. A longitudinal analysis of IDO and PDL1 expression during immune- or targeted therapy in advanced melanoma. Neoplasia 20, 218–225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cheng K et al. Sequentially responsive therapeutic peptide assembling nanoparticles for dual-targeted cancer immunotherapy. Nano Lett. 18, 3250–3258 (2018). [DOI] [PubMed] [Google Scholar]
- 176.Schmid D et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 8, 1747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zheng Y, Tang L, Mabardi L, Kumari S & Irvine DJ Enhancing adoptive cell therapy of cancer through targeted delivery of small-molecule immunomodulators to internalizing or noninternalizing receptors. ACS Nano 11, 3089–3100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu Z, Wang Y, Zhang L & Huang L Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano 8, 3636–3645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stephan MT, Moon JJ, Um SH, Bershteyn A & Irvine DJ Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16, 1035–1041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tang L et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Siriwon N et al. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol. Res. 6, 812–824 (2018). [DOI] [PubMed] [Google Scholar]
- 182.Terry S et al. Acquisition of tumor cell phenotypic diversity along the EMT spectrum under hypoxic pressure: consequences on susceptibility to cell-mediated cytotoxicity. Oncoimmunology 6, e1271858 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Karasaki T et al. An immunogram for the cancer-immunity cycle: towards personalized immunotherapy of lung cancer. J. Thorac. Oncol. 12, 791–803 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Nam J et al. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 4, 398–414 (2019). [Google Scholar]
- 185.Kinoh H et al. Nanomedicines eradicating cancer stem-like cells in vivo by pH-triggered intracellular cooperative action of loaded drugs. ACS Nano 10, 5643–5655 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Cabral H, Miyata K, Osada K & Kataoka K Block copolymer micelles in nanomedicine applications. Chem. Rev. 118, 6844–6892 (2018). [DOI] [PubMed] [Google Scholar]
- 187.Duan X et al. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat. Commun. 10, 1899 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang G et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 8, 902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Moynihan KD et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 22, 1402–1410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chiang C-S et al. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 13, 746–754 (2018). [DOI] [PubMed] [Google Scholar]
- 191.Zhang F et al. Nanoparticles that reshape the tumor milieu create a therapeutic window for effective T cell therapy in solid malignancies. Cancer Res. 78, 3718–3730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Miura Y et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano 7, 8583–8592 (2013). [DOI] [PubMed] [Google Scholar]
- 193.Quader S et al. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. J. Control. Release 258, 56–66 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Wong C et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl Acad. Sci. USA 108, 2426–2431 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mura S, Nicolas J & Couvreur P Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013). [DOI] [PubMed] [Google Scholar]
- 196.Arvanitis CD et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc. Natl Acad. Sci. USA 115, E8717–E8726 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mi P et al. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat. Nanotechnol. 11, 724–730 (2016). [DOI] [PubMed] [Google Scholar]
- 198.Kulkarni A et al. Reporter nanoparticle that monitors its anticancer efficacy in real time. Proc. Natl Acad. Sci. USA 113, E2104–E2113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Uchida S et al. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials 82, 221–228 (2016). [DOI] [PubMed] [Google Scholar]
- 200.Wang H-X et al. Nonviral gene editing via CRISPR/Cas9 delivery by membrane-disruptive and endosomolytic helical polypeptide. Proc. Natl Acad. Sci. USA 115, 4903–4908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Charlesworth CT et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25, 249–254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Glass Z, Lee M, Li Y & Xu Q Engineering the delivery system for CRISPR-based genome editing. Trends Biotechnol. 36, 173–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Song W et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 9, 2237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jadidi-Niaragh F et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J. Control. Release 246, 46–59 (2017). [DOI] [PubMed] [Google Scholar]
- 205.Deng ZJ et al. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 7, 9571–9584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhao Y et al. Polymetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 7, 11822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kataoka K et al. Spontaneous formation of polyion complex micelles with narrow distribution from antisense oligonucleotide and cationic block copolymer in physiological saline. Macromolecules 29, 8556–8557 (1996). [Google Scholar]
- 208.Christie RJ et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano 6, 5174–5189 (2012). [DOI] [PubMed] [Google Scholar]
- 209.Kim HJ et al. Multifunctional polyion complex micelle featuring enhanced stability, targetability, and endosome escapability for systemic siRNA delivery to subcutaneous model of lung cancer. Drug. Deliv. Transl. Res. 4, 50–60 (2014). [DOI] [PubMed] [Google Scholar]
- 210.Nishida H et al. Systemic delivery of siRNA by actively targeted polyion complex micelles for silencing the E6 and E7 human papillomavirus oncogenes. J. Control. Release 231, 29–37 (2016). [DOI] [PubMed] [Google Scholar]
- 211.Watanabe S et al. In vivo rendezvous of small nucleic acid drugs with charge-matched block catiomers to target cancers. Nat. Commun. 10, 1894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jiang H et al. New path to treating pancreatic cancer: TRAIL gene delivery targeting the fibroblast-enriched tumor microenvironment. J. Control. Release 286, 254–263 (2018). [DOI] [PubMed] [Google Scholar]
- 213.Adams D et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018). [DOI] [PubMed] [Google Scholar]
- 214.Setten RL, Rossi JJ & Han SP The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 18, 421–446 (2019). [DOI] [PubMed] [Google Scholar]
- 215.Singh A, Trivedi P & Jain NK Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 46, 274–283 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.