Abstract
Fibromyalgia (FM) is a generalized chronic pain condition whose pathophysiology is poorly understood, and both basic and translational research are needed to advance the field. Here we used the Sluka model to test whether FM-like pain in mice would produce detectable brain modifications using resting-state (rs) functional Magnetic Resonance Imaging (fMRI). Mice received intramuscular acid saline treatment, images were acquired at 7T 5 days post-treatment, and pain thresholds tested 3 weeks post-scanning. Data-driven Independent Component Analysis revealed significant reduction of functional connectivity (FC) across several component pairs, with major changes for the Retrosplenial cortex (RSP) central to the default mode network, and to a lesser extent the Periaqueductal gray (PAG), a key pain processing area. Seed-to-seed analysis focused on 14 pain-related areas showed strongest FC reduction for RSP with several cortical areas (somatosensory, prefrontal and insular), and for PAG with both cortical (somatosensory) and subcortical (habenula, thalamus, parabrachial nucleus) areas. RSP-PAG FC was also reduced, and this decreased FC tended to be positively correlated with pain levels at individual subject level. Finally, seed-voxelwise analysis focused on PAG confirmed seed-to-seed findings and, also detected reduced PAG FC with the anterior cingulate cortex, increasingly studied in aversive pain effects. In conclusion, FM-like pain triggers FC alterations in the mouse, which are detected by rs-fMRI and are reminiscent of some human findings. The study reveals the causal fingerprint of FM-like pain in rodents, and indicates that both RSP and PAG connectional patterns could be suitable biomarkers, with mechanistic and translational value, for further investigations.
Keywords: Fibromyalgia, Sluka model, mice, resting-state fMRI, retrosplenial cortex, periaqueductal gray, biomarker
Introduction
Fibromyalgia (FM) is a pain condition characterized by chronic widespread musculoskeletal pain, co-morbid with fatigue, memory deficits, sleep and mood disruption (McBeth & Mulvey 2012, Sarzi-Puttini et al 2018). The disease affects 2–8% of the population with higher prevalence in women, and current treatments involve psychological and pharmacological therapies (Clauw 2014). Increasing evidence supports a role for both peripheral small fiber neuropathy and central mechanisms (Clauw 2014), but aetiology of the disease remains essentially unknown.
Neuroimaging studies using functional and structural Magnetic Resonance Imaging (MRI) have shown evidence for brain alterations in FM patients. These include morphological changes at multiple brain sites, as well as increased activity notably in frontal and anterior cingulate cortex, somatosensory areas, insula, thalamus and amygdala, with evidence that pharmacological interventions reduce these brain activities (rev in ref (Sawaddiruk et al 2017)). Studies have also shown significant decrease of functional connectivity (FC) in descending pain regulatory pathways and disrupted FC of the pain matrix (Cifre et al 2012), possibly indicative of central sensitization (rev in Cagnie et al 2014). Whether these modifications of brain activity are causally linked to FM pathophysiology, however, has not been established.
Animal models are extremely powerful tools to understand neural mechanisms underlying chronic pain conditions, as well as to implement new treatments. Several rodent models have been developed to induce FM-like symptoms, and involve repeated muscle insults (Sluka et al 2001), stress or biogenic amine depletion (rev in ref (DeSantana et al 2013)). In all cases, widespread pain is produced without manifest peripheral tissue damage, therefore reflecting some aspects of the clinical situation. Animal models of FM also incorporate signs of comorbidities, such as emotional disturbances, whose pathophysiology could be explored (Nagakura 2015). Animal studies have identified some molecular mechanisms contributing to FM-like symptoms that involve neurotrophins and neuromodulators, ion channels, and several intracellular signalling cascades (DeSantana et al 2013). Our own work has demonstrated a role for lysophosphatidic acid receptors, using an intermittent psychological stress model that induces chronic abdominal stress in female mice (Ueda & Neyama 2017).
Importantly, the criteria for the diagnosis of FM has evolved since the tender point determination in 1990 via the inclusion of symptom severity criteria in 2010. In 2016, the criteria further refined the generalized pain syndromes related to polysymptomatic distress in FM by excluding the regional pain syndromes (Benlidayi 2019). At present however, there is no gold standard for the diagnosis of FM, and polysymptomatic distress comes from multiple causes, such as genetic, environmental, hormonal, neural factors (Häuser et al 2019, Ramírez-Tejero et al 2018) and immunological alterations (Ramírez-Tejero et al 2018, Sluka & Clauw 2016, Littlejohn & Guymer 2018, Metyas et al 2017). Accordingly, the several animal conditions that have been proposed to model FM-like generalized pain also involve distinct brain mechanisms. The most typical example is observed in the differential opioid sensitivity, where morphine analgesia was observed in the Sluka model using repeated muscle insults (Nishiyori et al 2010), but not in the intermittent cold- and psychological stress models (Sluka et al 2002; Ueda & Neyama 2017). Therefore, in the future, the comparison of functional MRI features in FM patients and animal models could be an interesting approach to stratify patients based on these distinct pathological mechanisms, and should help refine both diagnostic and treatment. Further, mouse studies have the strong advantage that both genes and brain networks can be manipulated, and the combined information of functionally connected brain regions and key molecules involved in generalized pain disease will uncover causal processes, and provide basic understanding of molecular/network mechanisms leading to different aspects of FM-like pain. Before moving to complex experimental manipulations, however, it is critical to establish whether generalized pain is detectable in mice using functional MRI.
Here we used the simple and well-accepted Sluka model (Sluka et al 2001) of FM, in order to test whether and how FM-like pain alters brain communication in mice. We thus investigated resting-state FC patterns in mice receiving intramuscular pH 7.2 (control) or pH 4.0 (FM) treatment using functional MRI (fMRI). We found a reduction of FC across pain-related anatomical regions, some of which are reminiscent of the human findings. Moreover, this decreased FC was positively correlated with pain levels at individual subject level. This study reveals the causal fingerprint of FM-like pain in rodents, and provides translational validity to the experimental mouse model for further studies.
Methods
General timeline
The overall experimental strategy is summarized in Fig. 1A and image acquisition and analysis detailed in Suppl Fig. S1.
Fig. 1. Experimental design & Independent component analysis of FM-like pain effects on whole brain functional connectivity (WBFC).
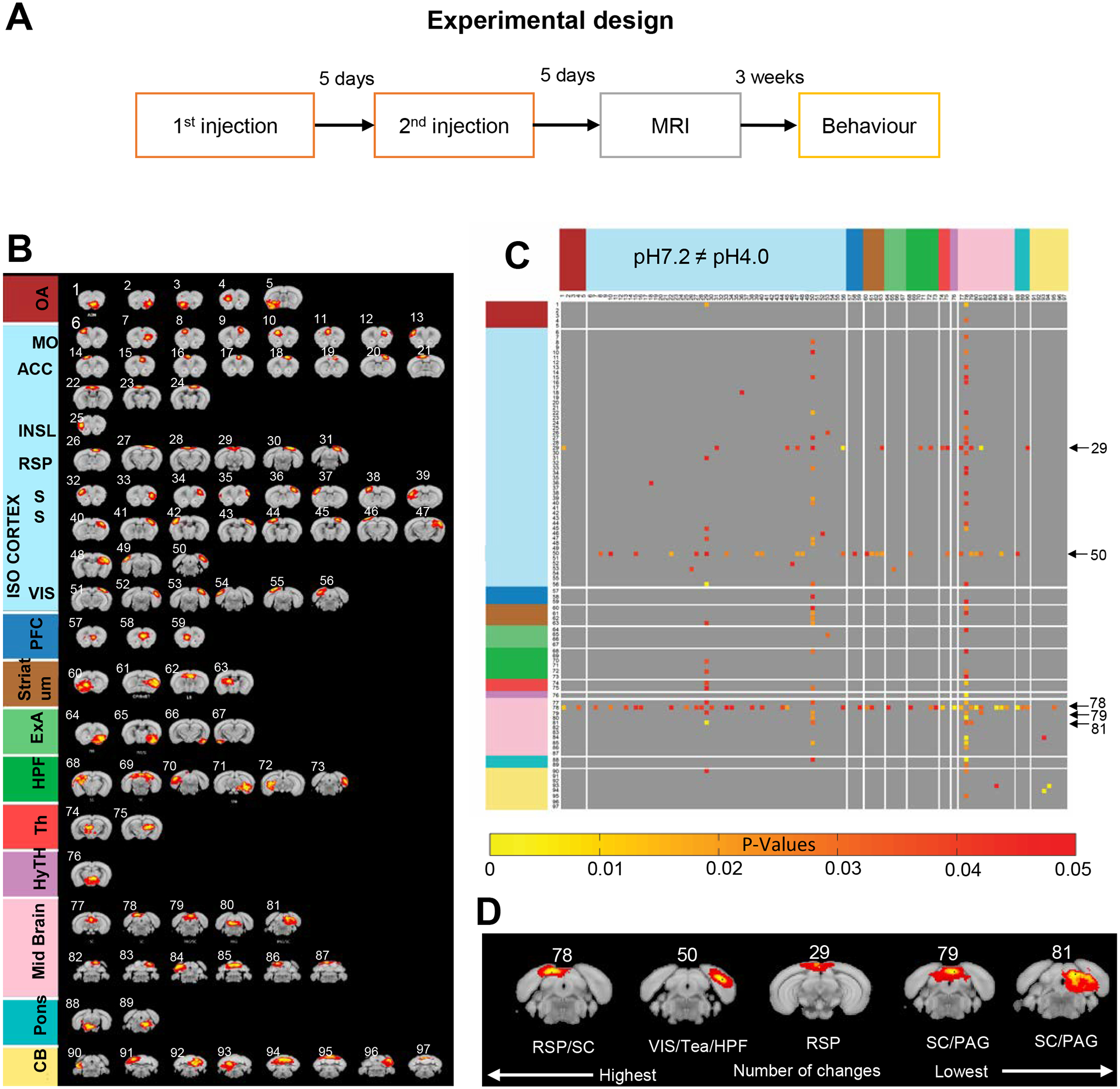
(A) Overview of the experimental design. Acid saline (pH4.0) or neutral saline (pH7.2) solution was injected in the left gastrocnemius muscle of each mouse twice, with a 5-day interval, and scanning was performed 5 days after the second injection. Three weeks after MRI scanning, mice were tested for pain responses. (B) Spatial ICA performed on the pooled datasets (pH7.2 and pH4.0) identifies 97 anatomically-defined components displayed here in the rostrocaudal order, based on their correspondence with the Allen Mouse Brain Connectivity Atlas 2011. (C) Generalized pain effects on WBFC. The symmetric correlation matrix represents significant FC differences induced by FM-like pain across 97 components (two-tailed t-tests). Red-yellow scaling color bar indicates pH4.0-induced FC modification; black arrows on the right side of the matrix identify the top-five ranked components (highest number of modifications, see Suppl Table S1). (D) Magnification of the top-five components. Periaqueductal gray (PAG), Retrosplenial cortex (RSP), visual area (VIS), hippocampal formation (HPF), Temporal association areas (Tea) and Superior colliculus (SC).
Animals.
C57BL/6J commercial male mice (8–12 weeks old) were purchased from Charles River. Mice were maintained in the animal facility of Douglas Mental Health University Institute, housed in a temperature (21±1°C) and humidity (55±10%) controlled room with a 12 h light: 12 h dark cycle (light on between 08:00 and 20:00 h). Animals were five per cage. Food and water were available ad libitum. Animals were habituated for a week in the animal facility before any manipulation. All experiments were performed following the guidelines and ethics on animal experimentation established by the Canadian Council of Animal Care and by the Animal Care Committees of McGill University/Douglas Mental Health University Institute, Montreal, Canada (protocol number 7466).
Animal preparation and testing.
Neutral saline (pH7.2) or acid saline (pH4.0, adjusted by 0.1 N HCL) was injected (20 uL of solution) in the left gastrocnemius muscle of each mouse twice, with a 5-day interval, to induce generalized pain as previously described (Sluka et al 2001) (n=10/10 control mice (pH7.2) /acid saline treated mice (pH4.0). Five days after the second injection, MRI was performed, after which the animals went back to their home cage. Mice were then tested for pain responses three weeks after image acquisition (Fig. 1A).
MRI experiment: anesthesia and physiological parameters.
Animals were anesthetized in a chamber using isoflurane at 5% volume mixed with oxygen for five minutes. Animals were then moved inside the scanner and isoflurane anesthesia maintained to 1.5–2%. Body temperature during scanning was maintained using a warm airflow over the animal set at 37 C. Respiration rate was monitored and maintained between 60 and 90 breaths per minute using 1025-IBP-50 Small Animal Monitoring Gating System (SA instruments).
Rs fMRI – image acquisition.
Data acquisition was achieved using a 7T small animal scanner (BioSpec 70/30USR, Bruker) equipped with a Cryoprobe. All fMRI images were obtained following Spin Echo EPI pulse sequence with parameters: FOV: 3.5 × 1; Matrix: 128 × 80; slices: 15; Resolution: 139 × 125 × 700 μm3; TE/TR: 1/1.50 s; flip angle 90; Repetitions: 400; Total acquisition time: 10 minutes for each mouse (representative raw image in Suppl Fig. S2A). To check the fMRI image quality, we determined the temporal signal to noise ratio (tSNR) (mean/SD) for 400 volumes (Suppl Fig. 2C).
Rs fMRI - data analysis.
The FC preprocessing pipeline involved six steps as follows: (i) denoising using Advanced Normalized tools (ANTs) (https://github.com/ANTsX/ANTs/blob/master/Examples/DenoiseImage.cxx) (Suppl Fig. 2B); (ii) motion correction using ANTs and FMRIB Software Library (FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki)) toolboxes; (iii) registration in two steps, first normalizing all the subject’s data using a template created by the existing subject’s mean volumes using ANTs, and second, registering these normalized data with the high-resolution Allen Mouse Brain template (http://help.brain-map.org/display/mouseconnectivity/API, annotation/mouse_2011), which we down-sampled as in our previous studies (Mechling et al 2016, Boulos et al 2019, Charbogne et al 2017, Nasseef et al 2019) using the MAGeT tool (Nasseef et al 2018) (Suppl Figure S2D) to achieve an isotropic voxel resolution 150 × 150 × 150 μm3 suitable for further annotation and analyses based on Allen Mouse Brain Atlas 2011 reference regions; (iv) smoothing with a Gaussian Kernel of full width half maximum (FWHM) at 0.3 by Analysis of Functional NeuroImages (AFNI (https://afni.nimh.nih.gov/)); (v) cropping using a brain mask generated according to grey and white matter segmentation using the Allen mouse brain template space and applied to all subjects; (vi) bandpass filtering at a frequency of window between 0.01Hz to 0.1 Hz by AFNI tool (Nasseef 2015, Nasseef et al 2019).
For post-processing analysis, we applied three different methods: (i) Independent component analysis (ICA) (Charbogne et al 2017, Nasseef et al 2018, Nasseef et al 2019), (ii) seed-based voxelwise analysis and (iii) seed-to seed analysis (Ben Hamida et al 2018, Boulos et al 2019, Charbogne et al 2017, Nasseef et al 2019).
High component ICA was performed to identify functional brain nodes using combined subjects from pH7.2 and pH4.0 groups using GIFT tools (Group ICA of fMRI Toolbox, v1.3i, www.nitrc.org/projects/gift/). ICASSO was applied to examine the stability and reliability of the components, by randomization and bootstrapping, and a quality index was assigned to each component (value range 0 to 1) (Mechling et al 2016, Nasseef et al 2019). Then, FC analysis was performed by an in-house built script written in MATLAB following our previous studies (Boulos et al 2019, Nasseef et al 2019). We computed correlation coefficients across all the components for each subject using MATLAB. Finally, group comparison was performed using two sample t-test (P<0.05, one/two tail). Low ICA was also performed to detect large brain networks using data from the pH7.2 and pH4.0 groups separately, using the same GIFT tools, and quality index was measured as was done for high component ICA.
Seed-based voxelwise analysis was performed to study whole-brain FC patterns of Periaqueductal gray (PAG), as described in our previous work (Nasseef et al 2019). Correlation analysis was done as follows: (i) the mean of each seed time series was used to produce a whole brain correlation map (Nasseef et al 2019) transformed into Fishers Z scores and (iii) tested for significant differences between pH7.2 and pH4.0 groups using t-test (threshold T > 5; significance/alpha level 0.001; one/two tail). All results were family-wise error rate (EWER) corrected following clustering with significance/alpha level 0.05 (Nasseef et al 2019). For 3D representation of the mouse brain for all the data, we used Mango (http://ric.uthscsa.edu/mango/) and BrainNet Viewer (https://www.nitrc.org/projects/bnv/) tools.
Seed-to-seed analysis was performed using 14 pain-related brain regions. Prefrontal cortex (PFC), Anterior cingulate area (ACA), Caudate putamen (CP), Insular cortex (INS), Amygdala (AMY), Habenula (HAB), Hypothalamus (HY), Somatosensory cortex (SS), Thalamus (TH), Retrosplenial cortex (RSP), Hippocampus (HIP), PAG, Superior colliculus (SC) and Parabrachial nucleus (PBN) seeds were generated manually based on the 2011 Allen Mouse Brain Atlas, and mean time series were extracted for seed-to-seed FC analysis including statistical significance test. First, correlation coefficients were computed using a single t-test (P<0.05, FDR correction). For group comparison, a two-sample t-test (P<0.05; one/two tail; FDR correction) was applied to investigate lower/higher functional connections across the selected seeds.
RSP-PAG receiver operator characteristic (ROC) curve analysis was performed using a MATLAB based extension function (https://www.mathworks.com/matlabcentral/fileexchange/52442-roc-curve) to investigate the characteristic of brain functional connectivity between two groups (Fayyaz et al 2012).
Pain Sensitivity test.
Mechanical hypersensitivity was measured using von Frey testing after 3 weeks of fMRI scanning performed, as described previously (Ma et al 2017). Mice were habituated for 2 hours in individual plexiglass enclosures with a mesh floor before testing. The paw withdrawal threshold (PWT) was measured using an electronic von Frey anesthesiometer (IITC Life Science, Woodland Hills, CA, USA) equipped with a 10μl rigid pipette tip. While it was applied perpendicularly to the plantar surface of the mouse hind paw, the pressure was manually increased until a positive response was observed, such as a brisk withdrawal or flinching of the hind paw. The values (gram) of applied forces triggering a positive response were recorded. The two hind paws were measured alternatively. Three measurements of PWT were obtained for each paw, with intervals of 10 to 15 min. The PWT of each paw was an average of all measurements. The mean ± SEM of PWT of 4 weeks after injection of pH7.2 and pH4.0 was compared using Student t-test (SigmaPlot, SPSS Inc., Chicago, IL, USA). The significance level was set at p< 0.05.
Correlation between imaging and behavioural data.
Fischer transformed Z scores of seed pair correlation coefficients (18 seeds pairs, see Fig. 2D) for each subject, and their paw withdrawal thresholds (left/right mean, g) were correlated by Pearson analysis using MATLAB (corrcoef’ function).
Fig. 2. Seed-to-seed FC analysis across pain-related areas.
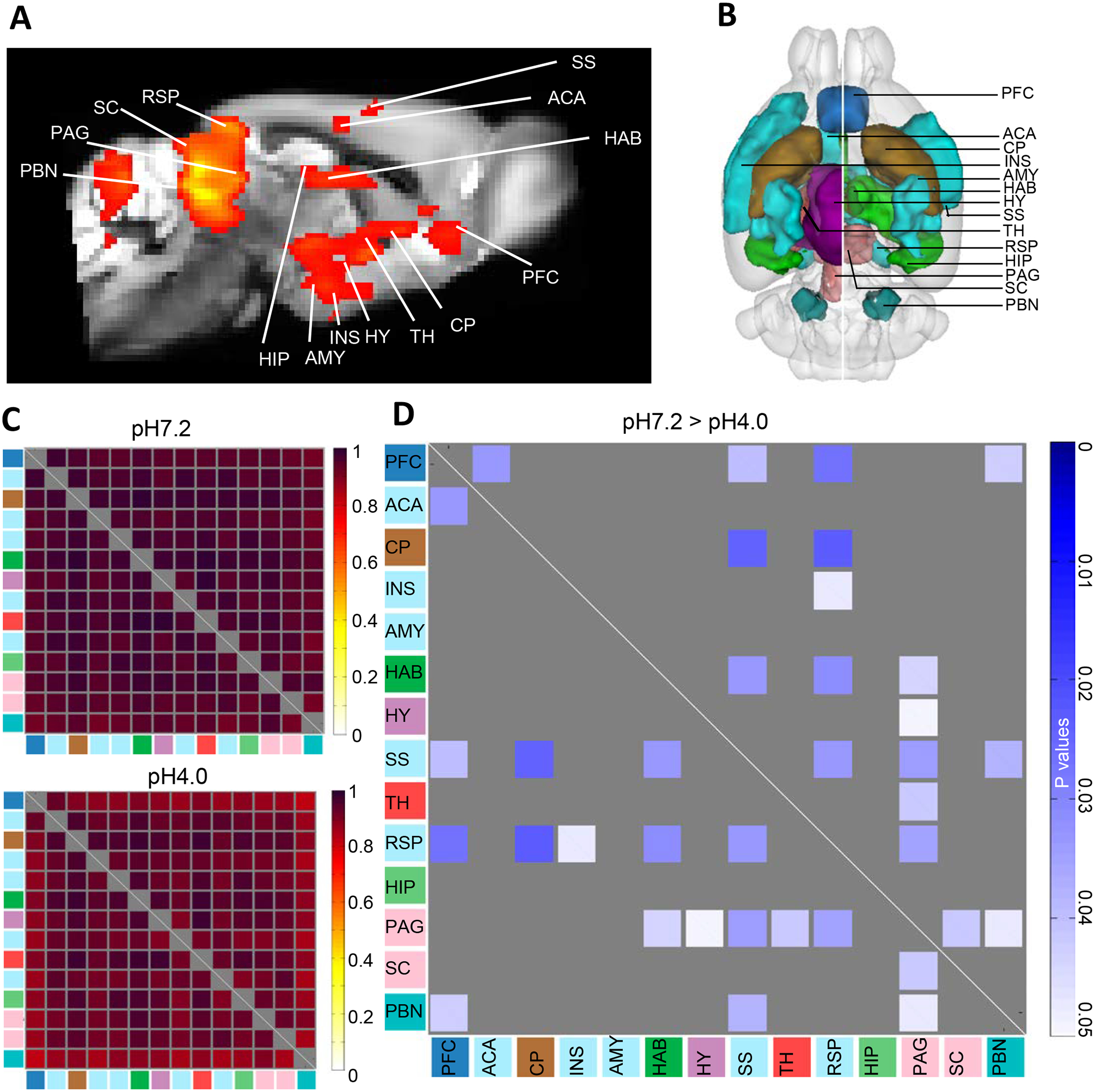
(A) Low component ICA identifies a pain network in the pH7.2 group, which was used to construct a seed-based pain cluster; (B) 3D illustration of the 14 generated pain-related seeds based upon the mouse the Allen Brain Connectivity Atlas 2011; (C) Mean correlation coefficients for pH7.2 (top) and pH4.0 (bottom) groups; (D) The group comparison matrix shows p values using t-test (P<0.05, n=10, one tail, FDR correction), and identifies significant lower seed-to-seed FC in the FM group (pH7.2 > pH4.0). Color code used for seed annotation is identical to Fig. 1A. The color bar (right) indicates significant p-value scaling. Prefrontal cortex (PFC); Anterior cingulate area (ACA); Caudate putamen (CP); Insular cortex (INS); Amygdala (AMY); Habenula (HAB); Hypothalamus (HY); Somatosensory cortex (SS); Thalamus (TH); Retrosplenial cortex (RSP); Hippocampus (HIP); Periaqueductal gray (PAG); Superior colliculus (SC); Parabrachial nucleus (PB).
Results
Independent component analysis reveals a whole brain FC signature for FM-like pain.
Mice received acid saline (pH4.0, n=10) or saline (pH7.2, n=10) treatment twice with a 5-days interval, and were scanned under resting state conditions 5 days after the second injection (see methods and Fig. 1A). Details of the MRI procedures and analyses are summarized in Suppl Fig. S1).
We first conducted a data-driven analysis on the whole brain. We applied spatial independent component analysis (ICA) combining the two groups (pH7.2, n=10; pH4.0, n=10; 10 min acquisition) and generated 100 components (100-ICASSO). We validated the quality, stability and reliability of those components using the ICASSO toolkit (Mechling et al 2016, Nasseef et al 2019). Fifty percent components showed a quality index of 0.9, indicating high stability for these components. After visual inspection and considering low quality indexes (IQ<0.3), we removed 3 components from the analysis, and the remaining 97 components were annotated using the 2011 Allen Mouse Brain Connectivity Atlas (Fig. 1B).
FC between each component pair was analyzed for each group separately, then group comparison was performed (see methods) to create a group comparison matrix (Fig. 1C) showing the FM-like pain effect. Data are shown as a matrix, with color-coded p values for all significant changes induced by FM-like pain (two-tailed t-test). Further one-tailed analysis (Suppl Table S2) showed significant reduced FC across several components, whereas no increase of FC was detected, suggesting that generalized pain overall reduces brain communication. A total of 170 significant FC reductions (Suppl Table S1) therefore constitute the fingerprint of FM-like pain on the whole brain FC.
To quantify the generalized pain effect on FC and highlight most significant modifications, we further ranked the components by the number of significant FC alterations (Suppl Table S1). The top-5 included components 78, 50, 29, 79, 81 (Fig. 1D), overlapped with Retrosplenial cortex (RSP)/Superior colliculus (SC), Visual area VIS)/Temporal association areas (Tea) / hippocampal formation (HPF), RSP, and Superior Colliculus (SC)/ Periaqueductal gray matter (PAG). Altogether therefore, the data-driven ICA analysis demonstrates that FM-like pain induced by the Sluka model induces strongest reduction of brain communication across components of the cortex, as well as centers belonging to the Default Mode Network (DMN) and, to a lower extent in this analysis, key pain centers.
Seed-to-seed analysis confirms major FC reduction for RSP and PAG.
We then ran a low component ICA on the control pH7.2 group (see methods), and found a network overlapping main pain-related areas (Fig. 2A), indicating that functional communication across these brain areas is detectable in the mouse brain (quality index > 0.9). This component was not found in the pH4.0 group, suggesting that FC disruption has occurred in this network.
We therefore focused on pain-related areas to conduct a hypothesis-driven analysis and generated 14 seeds using the 2011 Allen Mouse Brain Connectivity Atlas (Fig. 2B) corresponding to key regions involved in pain processing at both subcortical and cortical levels. We extracted time series of all 14 seed regions individually for both pH7.2 and pH4.0 subjects. We computed correlation coefficients for each subject individually for both groups, and mean correlation coefficients of both groups are presented on Fig. 2C, highlighting generally lower correlation coefficients across seeds in the pH4.0 group compared to the pH 7.2 group. Group comparison using two sample t-test (n=10, p<0.05, one tail, FDR correction) further identified significant FC reduction in pH4.0 group (pH7.2>pH4.0) and group difference p values are presented in Fig. 2D. Remarkably, both RSP and PAG showed the highest number of changes with other seeds of the pain cluster. RSP, the core DMN center, showed reduced FC with several other cortical areas involved in the processing of pain perception and aversion (PFC, INS and SS), as well as CP involved in activity, HAB considered another aversion center and PAG known as one of the best-established pain centers. Conversely, PAG FC was also reduced with several seeds, including the RSP, as well as other cortical and subcortical areas involved in aversive aspects of pain perception (SS, SC, HAB) and pain processing (TH, PBN).
In summary, RSP and PAG showed the highest number of reduced correlated connectivity with other seeds (RSP, 6; PAG, 7), and also showed lower FC between them. This second analysis therefore confirms findings from the data-driven ICA analysis, to support predominant alteration of RSP and PAG FC upon the induction of FM-like pain.
FC between RSP and PAG correlates with pain sensitivity in the Sluka model.
A pilot experiment on a separate cohort indicated that, under our experimental conditions, generalized pain develops and lasts for at least 3 weeks (Suppl Figure S3). Thus, for the MRI cohort, we performed behavioural pain testing after, rather than before MRI scanning, to avoid potential confounds that may be introduced by pain testing on the whole brain FC. Mechanical hypersensitivity was measured using electronic Von Frey testing, three weeks after scanning the animals that had received intramuscular injection of saline (pH7.2) or acidic saline (pH4.0) in the left paw. The mean of left and right paw withdrawal thresholds was significantly lower for pH4.0 mice compared to control pH7.2 mice (Fig. 3A, **p<0.01). This observation indicates that FM-like pain in the Sluka model had indeed taken place in this cohort, and remained significant beyond the scanning sessions.
Fig. 3. Positive correlation between FC and pain sensitivity.
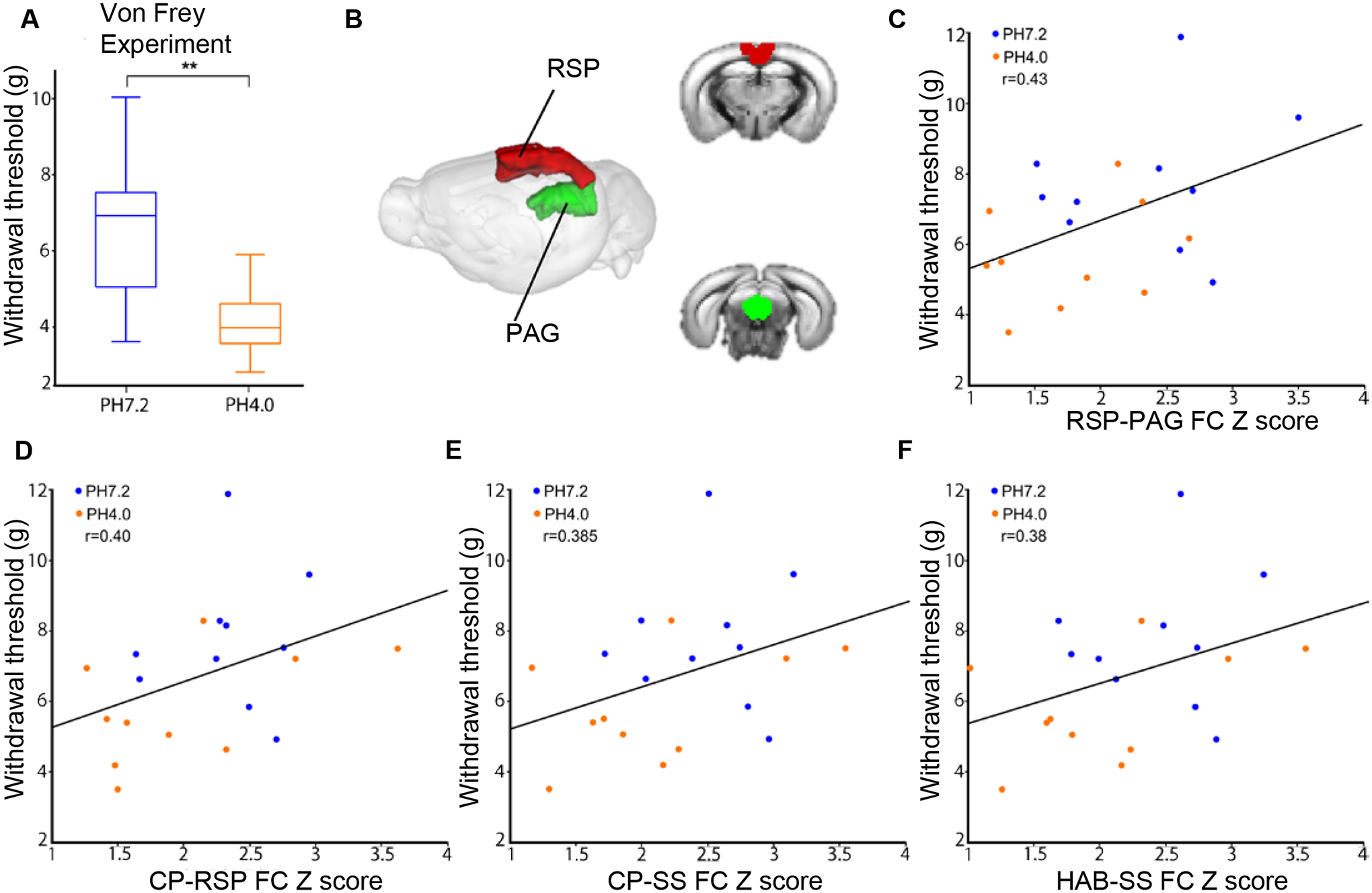
(A) Pain sensitivity was measured using the von Frey Test assay. Data are expressed as paw withdrawal threshold in grams and are represented using a box plot with t-test (n=10/10, **P<0.01); (B) 3D representation of PAG and RSP seeds in the mouse brain. (C) Although not significant (> p 0.05, see Suppl Table S2), highest positive correlation was found between tactile allodynia and PAG-RSP FC (z scores) (pH4.0, orange circle; pH7.2, blue circle; n=10–10), shown for each subject as a scatter plot with the regression line. Positive correlation was also found between hyperalgesia and (D) CP-RSP FC (z scores), (E) CP-SS FC (z scores) and (F) HAB-SS FC (z scores) respectively. Habenula (HAB); Periaqueductal gray (PAG); Retrosplenial cortex (RSP); Somatosensory cortex (SS).
Next, we tested whether behavioural pain responses to stimulation with von Frey fibers and FC deficits are correlated. We considered the 18 seed pairs, which had shown a significant FC reduction in the seed-to-seed analysis (Fig. 2D), and transformed correlation coefficients into z-scores (see methods) for each subject. Pearson correlation analysis between these scores and individual behavioural data (mean paw withdrawal threshold (g)) was performed (Suppl Table S2). Highest positive correlation was found for the RSP-PAG seed pair (r=0.43) with a trend to significance (p=0.057) (Fig. 3B–C). The next seed pairs showing positive correlation were CP-RSP (r=0.40, p=0.078), CP-SS (r=0.385, p=0.093) and HAB-SS (r=0.38, p=0.94) (Fig. 3D–F). These data indicate that modifications of RSP-PAG connectivity best correlate with the higher pain sensitivity of mice subjected to the Sluka pain model.
Seed-based voxelwise analysis maps brain wide FC reduction for PAG.
Results from ICA and seed-to-seed analyses prompted us to refine investigation of FM-like pain effects on FC. We chose to extend the characterization of pain-induced FC alterations of the PAG, which is largely studied in animal pain research. We investigated FC of the PAG seed (Fig. 4A) with the entire brain. Seed-to-voxel FC analysis was performed for each group, and group comparison mapping was conducted using t-test (t>5, n=10, P<0.001, FWER correction). We found a significant decrease of PAG FC in the pH4.0 group relative to pH7.2 with many brain areas (Fig. 4B). In sum, voxels showing significant alterations overlapped with the DMN (RSP, SC and entorhinal area) and with PAG itself, as well as areas processing sensory and aversive aspects of pain (TH, Anterior cingulate area or ACA, INS), and more general reward/aversion processing areas (NAC, CP, HAB).
Fig. 4. Seed-voxelwise analysis of FM-like pain effects on PAG FC.
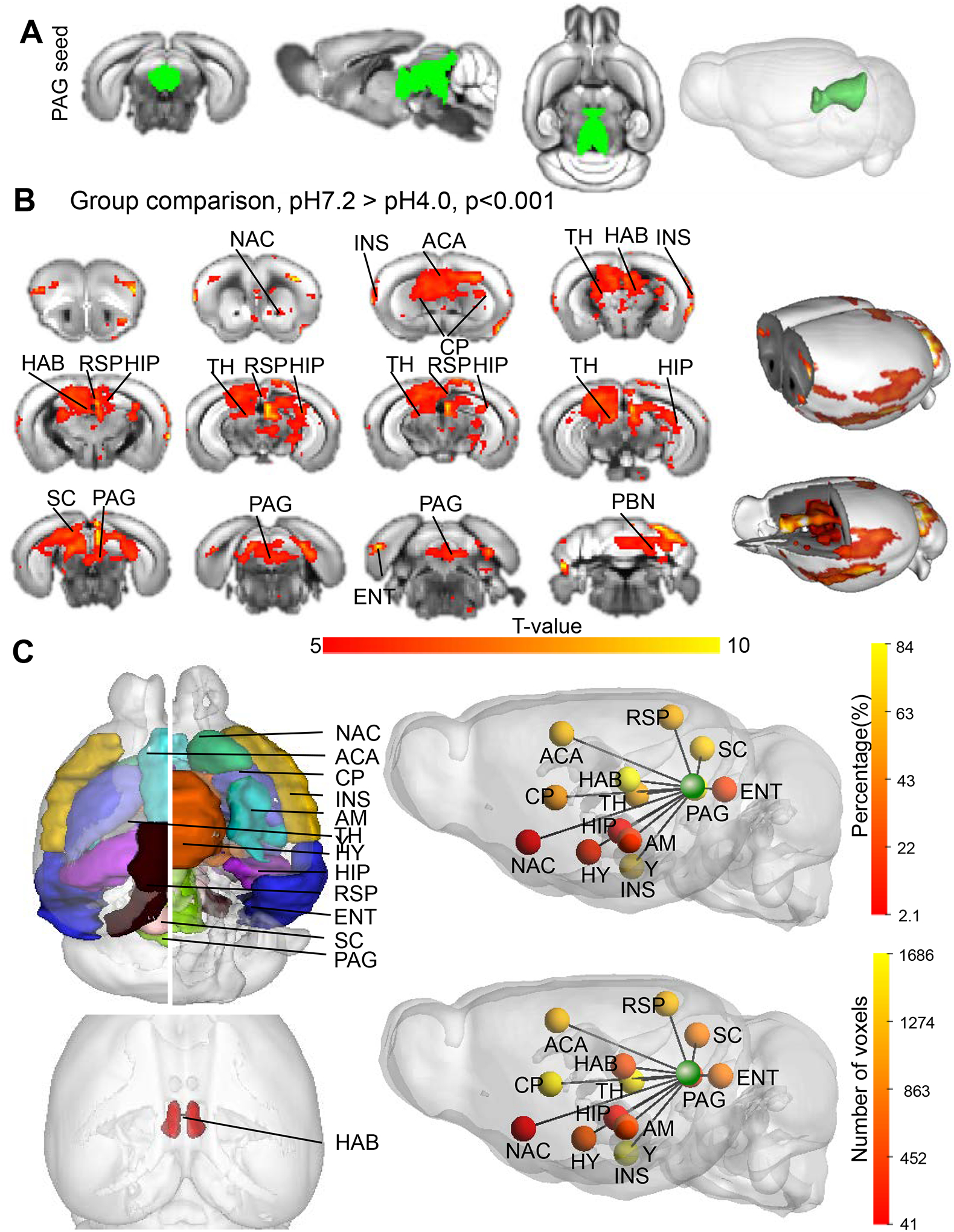
(A) 3D representation of the PAG seed created from the Allen Mouse Brain Connectivity Atlas (2011); (B) PAG connectivity is significantly reduced with 13 annotated brain regions between the groups. Red to yellows voxels represent significant decrease of FC (pH7.2 versus pH4.0) by t-test (t values > 5, cc=0.001, pH7.2 n=10, pH4.0 n=10) FWER corrected); (C) Quantification of pain effects on FC of the PAG seed. 3D representation of both PAG and target seeds is shown in the left panel. Scaled color bars show significance of the pain effect expressed as % voxels modified in the target region (top-right, percentage) or number of voxels modified in the target region (bottom-right, number of voxels) modified by FM-like pain in the target seed (see also Table 1). Each target is represented by a sphere color-coded to show the level of FC reduction. Nucleus accumbens (NAC); Anterior cingulate area (ACA); Caudate putamen (CP); insular cortex (INS), Amygdala (AMY), Habenula (HAB), Hypothalamus (HY), Thalamus (TH), Retrosplenial cortex (RSP), Hippocampus (HIP) Periaqueductal gray (PAG), Superior colliculus (SC), Entorhinal area (ENT) and Parabrachial nucleus (PBN).
Next, we quantified FC reduction of PAG with these regions. To do so we created seeds for all the identified target areas, and counted the number of voxels of these seeds showing significant FC difference with PAG as we previously described (Nasseef et al 2019, Boulos et al 2019). We ranked the target areas based on the percent of voxels significantly modified by FM-like pain (Table 1) and results are schematized in Fig. 4C, which displays a 3D-brain representation of the data. FC reduction was strongest with: HAB (84%), PAG (48%), HIP (36.9%), SC (36.7%), ACA (31.1%), RSP (26.6%), TH (24.3%) and CP (16.9%). It is interesting to note that this analysis, which covers all voxels of the brain and uses different statistical methods, identifies reduced communication of the PAG with most regions that we earlier identified using seed-to-seed analyses. Data from this analysis therefore confirms altered communication across the main pain/aversion centers of the brain.
Table 1. Quantification of the seed-voxelwise analysis for the PAG seed.
T-maps from PAG seed-voxelwise analysis reveal brain areas (target seeds) showing significant FC reduction upon pH4.0 treatment. Target seeds were created for quantification (Fig. 4C) Columns from left to right show: the ranking (Rank), the seed name (Seed), the total number of voxels composing the seed (Number of voxels), the number of voxels showing significant FC change between the groups (overlapping voxels), and the percent voxels with significant changes in FC in this seed (percent). Seeds are ranked from highest to lowest percent FC modifications with the PAG seeds.
| Rank | Seed | Number of voxels | Overlapping voxels | Percent |
|---|---|---|---|---|
| 1 | HAB | 144 | 121 | 84 |
| 2 | PAG | 1392 | 669 | 48 |
| 3 | HIP | 4570 | 1686 | 36.9 |
| 4 | SC | 664 | 244 | 36.7 |
| 5 | ACA | 1875 | 584 | 31.1 |
| 6 | RSP | 2210 | 587 | 26.6 |
| 7 | TH | 5000 | 1217 | 24.3 |
| 8 | CP | 7064 | 1197 | 16.9 |
| 9 | ENT | 4494 | 223 | 4.9 |
| 10 | INS | 2803 | 126 | 4.5 |
| 11 | HY | 3828 | 146 | 3.8 |
| 12 | AMY | 2028 | 45 | 2.2 |
| 13 | NAC | 1960 | 41 | 2.1 |
Discussion
Fibromyalgia is characterized by widespread chronic pain and co-occurring psychological dysfunctions (McBeth & Mulvey 2012, Sarzi-Puttini et al 2018), however mechanisms underlying the origin and progress of the disease are poorly understood. In humans, MRI-based imaging studies have described altered brain activity and FC in brain areas known to process sensory and emotional information, including pain pathways (Sawaddiruk et al 2017), and a number of animal models have been developed to investigate neural bases of fibromyalgia pain (DeSantana et al., 2013). In the present study we used the rodent Sluka model to investigate whether FM-like pain effects could be detected using mouse resting-state (rs) fMRI, and if so, whether altered FC patterns would have mechanistic and/or translational value for further investigations of FM pain in animals.
We used both unbiased (ICA) and seed-based (seed-to-seed and seed-voxelwise) analytic approaches, and all three approaches indicated that FM-like pain in the Sluka model triggers significant FC modifications across the brain. The different analyses altogether indicated that acid-saline treatment in mice produces a significant reduction of communication between several brain areas, including those relevant to pain processing at both cortical and subcortical levels. Pain states are therefore detectable in the mouse using rsfMRI, and manifest under the form of FC deficits. More sophisticated studies in the future may reveal both increased and decreased communication across brain networks.
A most important finding was that both unbiased (Fig. 1) and seed-based (Fig. 2) analyses concurred to reveal strongest alterations of FC patterns for two brains areas: (i) the RSP, considered the core component of the default mode network (DMN) in mice (Sforazzini et al 2014, Stafford et al 2014), first identified in humans and primates (Greicius et al 2003, Raichle et al 2001, Vincent et al 2007), known as a highly interconnected brain network reflecting overall intrinsic properties of brain organization, and showing abnormal FC in disease conditions (Broyd et al 2009) and (ii) the PAG, a crucial hub for the modulation of nociceptive information (Basbaum et al 2009, Reichling et al 1988). The seed-to-seed analysis also indicated that, in addition to reduced FC with many brain areas, RSP and PAG show diminished FC between them. Of note, it is likely that the functional communication between RSP and PAG is indirect, as no obvious direct physical connection is detected in whole brain connectivity mapping ((https://connectivity.brain-map.org/projection). Finally, our correlation analysis between FC deficits and pain thresholds of individual animals (Fig. 3) revealed that the strongest positive correlation is found between FC of the RSP-PAG pair (among 18 pairs) and pain levels. The data together therefore suggest a possible contribution of correlated RSP-PAG activity to the manifestation of FM-like pain in the Sluka mouse model. Remarkably, alterations of DMN-PAG FC is also observed in human pain patients, and was proposed to be a key feature of spontaneous attentional fluctuations towards and away from pain (Kucyi & Davis 2015). A human study also showed engagement of the posterior cingulate cortex, a component of the human DMN, in FM pain catastrophizing (Lee et al 2018). Whether similar processes exist in mice is unknown, however, the notion that reduced RSP-PAG connectivity could be a biomarker (Suppl Figure S4) with both mechanistic and translational value deserves further investigations.
Beyond the RSP-PAG pair, data-driven ICA and seed-to-seed analyses showed a number of other brain communication deficits for RSP, which may provide circuitry-level insights into generalized pain, and perhaps parallel some of the human findings. RSP showed disrupted communication with PFC, INS and SS, all involved in the integration of pain information and aversion processing, and further, the PFC also showed lowest FC with other cortical areas (ACA and SS). At mechanistic level, modification of aversive information processing at high-order level is consistent with numerous recent pain studies focusing, in particular, on dysfunction of the anterior cingulate cortex (Bliss et al 2016, Juarez-Salinas et al 2019). In FM patients, decreased FC was found across a number of cortical areas (medial prefrontal cortex, anterior and posterior cingulate areas, somatosensory cortex SII), as well as between the caudate and posterior cingulate areas and the insula (Cifre et al 2012), most of which are DMN-related areas, suggesting potential common pain signatures in rodent and human cortices, which may deserve further characterization. Within this line also, altered SS FC is a hallmark of sustained pain in FM patients (Kim et al 2015), and our data show broad disruption of SS FC with both cortical (PFC, RSP) and subcortical (CP, HAB, PAG and PBN) areas in the Sluka mice.
At subcortical level, RSP FC was diminished with CP and HAB. Modification of HAB FC (with RSP, as well as SS and PAG) is also of interest, as this small brain structure is considered an aversion center (Boulos et al 2017, Fakhoury 2017). Considerable preclinical evidence has shown involvement of HAB in pain and analgesia (Shelton et al 2012a), and more recent studies in humans revealed significant correlated activity between HAB, PAG and the caudate under noxious heat stimulation (Shelton et al 2012b). Further focus on habenula function and connectivity along pain chronification (Borsook et al 2016) could be an interesting research path in the context of fibromyalgia research.
The analysis of PAG connectivity was also of particular interest. Our seed-to-seed analysis using 14 pain-related brain regions (Fig. 2) revealed that, beyond RSP, PAG FC was reduced with HAB, HY, SS, TH, RSP, SC and PBN, all with a demonstrated role in animal models of pain (Aimone & Gebhart 1987, Barriere et al 2019, de Oliveira et al 2017, Roeder et al 2016, Shelton et al 2012a). Further seed-based voxelwise analysis, refining the investigation of FM-like pain effects on FC (Fig. 4) added several brain regions, including ENT (a component of the DMN), ACA (including ACC) and INS (cortical areas processing sensory and aversive aspects of pain), and to a lesser extent NAC, HIP and AMY (reward/aversion and cognition-related regions). The present study showing negative PAG FC with brain regions is consistent with human studies, where FM patients showed the reduction of PAG FC with regions related to motor/executive functions, the salience network and the DMN, which correlated with measures of negative affect (Coulombe et al 2017), with ACC, TH and CP, and significant negative correlation between depression and PAG FC with TH and ACC (Cifre et al 2012, Cagnie et al 2014). However, as there is also a report that FM patients showed increased PAG FC with Insula, ACC and PFC (Truini et al 2016), the similarity between the animal model and human studies awaits further studies using different FM-like animal models, as well as more studies of FM patients with different characteristics. Thus, although a direct parallel between mouse and human findings is difficult to establish at this stage, it is possible that PAG connectivity may be a good indicator to further investigate pain states by fMRI in the two species.
In conclusion, findings from this study demonstrate that rs-fMRI is a suitable approach to detect modifications of FC patterns under FM-like pain conditions. It will be important in the future to determine commonalities or differences of these modifications in other mouse models of chronic pain, and how FC evolves along pharmacological or genetic intervention to reduce FM-like pain. Both RSP and PAG connectivity may be appropriate targets towards these goals.
Supplementary Material
Acknowledgments.
We thank the staff at the animal facility of the Neurophenotyping Center Douglas Research Center (Montréal, Canada) and DaWoon Park for animal care and Axel Mathieu for his assistance with MRI acquisition.
Funding.
This work was supported by US National Institute of Health (National Institute of Drug Addiction, grant # 05010 and National Institute on Alcohol Abuse and Alcoholism, grant #16658), the Canada Fund for Innovation and the Canada Research Chairs to BK and McGill University HBHL grant to HU.
Footnotes
Conflict of interest. None of the authors has a conflict of interest to disclose.
Ethical approval. All experiments were performed following guidelines and ethics of animal experimentation established by the Canadian Council of Animal Care (protocol number 7466) and by the Animal Care Committees of McGill University/Douglas Mental Health University Institute, Montreal, Canada.
References
- Aimone LD, Gebhart GF. 1987. Spinal monoamine mediation of stimulation-produced antinociception from the lateral hypothalamus. Brain Res 403: 290–300 [DOI] [PubMed] [Google Scholar]
- Barriere DA, Hamieh AM, Magalhaes R, Traore A, Barbier J, et al. 2019. Structural and functional alterations in the retrosplenial cortex following neuropathic pain. Pain 160: 2241–54 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. 2009. Cellular and molecular mechanisms of pain. Cell 139: 267–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamida S, Mendonca-Netto S, Arefin TM, Nasseef MT, Boulos LJ, et al. 2018. Increased Alcohol Seeking in Mice Lacking Gpr88 Involves Dysfunctional Mesocorticolimbic Networks. Biological psychiatry 84: 202–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlidayi IC. 2019. Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatology international 39: 781–91 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Kaang BK, Zhuo M. 2016. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 17: 485–96 [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. 2016. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 68: 282–97 [DOI] [PubMed] [Google Scholar]
- Boulos LJ, Darcq E, Kieffer BL. 2017. Translating the Habenula-From Rodents to Humans. Biological psychiatry 81: 296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos LJ, Nasseef MT, McNicholas M, Mechling A, Harsan LA, et al. 2019. TouchScreen-based phenotyping: altered stimulus/reward association and lower perseveration to gain a reward in mu opioid receptor knockout mice. Scientific reports 9: 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. 2009. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 33: 279–96 [DOI] [PubMed] [Google Scholar]
- Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. 2014. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Seminars in arthritis and rheumatism 44: 68–75 [DOI] [PubMed] [Google Scholar]
- Charbogne P, Gardon O, Martin-Garcia E, Keyworth HL, Matsui A, et al. 2017. Mu Opioid Receptors in Gamma-Aminobutyric Acidergic Forebrain Neurons Moderate Motivation for Heroin and Palatable Food. Biological psychiatry 81: 778–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, et al. 2012. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosomatic medicine 74: 55–62 [DOI] [PubMed] [Google Scholar]
- Clauw DJ. 2014. Fibromyalgia: a clinical review. JAMA 311: 1547–55 [DOI] [PubMed] [Google Scholar]
- Coulombe MA, Lawrence KS, Moulin DE, Morley-Forster P, Shokouhi M, et al. 2017. Lower Functional Connectivity of the Periaqueductal Gray Is Related to Negative Affect and Clinical Manifestations of Fibromyalgia. Frontiers in neuroanatomy 11: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira R, de Oliveira RC, Falconi-Sobrinho LL, da Silva Soares R Jr., Coimbra NC. 2017. 5-Hydroxytryptamine2A/2C receptors of nucleus raphe magnus and gigantocellularis/paragigantocellularis pars alpha reticular nuclei modulate the unconditioned fear-induced antinociception evoked by electrical stimulation of deep layers of the superior colliculus and dorsal periaqueductal grey matter. Behav Brain Res 316: 294–304 [DOI] [PubMed] [Google Scholar]
- DeSantana JM, da Cruz KM, Sluka KA. 2013. Animal models of fibromyalgia. Arthritis research & therapy 15: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhoury M 2017. The habenula in psychiatric disorders: More than three decades of translational investigation. Neurosci Biobehav Rev 83: 721–35 [DOI] [PubMed] [Google Scholar]
- Fayyaz A, Ghanim U, Sung-Ho K. 2012. A neighborhood method for statistical analysis of fMRI data. Open Journal of Biophysics 2012 [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America 100: 253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuser W, Clauw D, Fitzcharles M-A. 2019. Fibromyalgia as a chronic primary pain syndrome: issues to discuss. Pain 160: 2651–52 [DOI] [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. 2004. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nature medicine 10: 712–18 [DOI] [PubMed] [Google Scholar]
- Juarez-Salinas DL, Braz JM, Etlin A, Gee S, Sohal V, Basbaum AI. 2019. GABAergic cell transplants in the anterior cingulate cortex reduce neuropathic pain aversiveness. Brain 142: 2655–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Loggia ML, Cahalan CM, Harris RE, Beissner FDPN, et al. 2015. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis & rheumatology (Hoboken, N.J.) 67: 1395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. 2015. The dynamic pain connectome. Trends Neurosci 38: 86–95 [DOI] [PubMed] [Google Scholar]
- Lee J, Protsenko E, Lazaridou A, Franceschelli O, Ellingsen DM, et al. 2018. Encoding of Self-Referential Pain Catastrophizing in the Posterior Cingulate Cortex in Fibromyalgia. Arthritis & rheumatology (Hoboken, N.J.) 70: 1308–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn G, Guymer E. Seminars in immunopathology 2018, 40: 291–300. Springer; [DOI] [PubMed] [Google Scholar]
- Ma W, St-Jacques B, Rudakou U, Kim YN. 2017. Stimulating TRPV1 externalization and synthesis in dorsal root ganglion neurons contributes to PGE2 potentiation of TRPV1 activity and nociceptor sensitization. European journal of pain (London, England) 21: 575–93 [DOI] [PubMed] [Google Scholar]
- McBeth J, Mulvey MR. 2012. Fibromyalgia: mechanisms and potential impact of the ACR 2010 classification criteria. Nat Rev Rheumatol 8: 108–16 [DOI] [PubMed] [Google Scholar]
- Mechling AE, Arefin T, Lee HL, Bienert T, Reisert M, et al. 2016. Deletion of the mu opioid receptor gene in mice reshapes the reward-aversion connectome. Proceedings of the National Academy of Sciences of the United States of America 113: 11603–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metyas S, Rezk T, Arkfeld D, Leptich T. 2017. Autoinflammation and immunomodulation in inflammatory fibromyalgia syndrome-a review. Current rheumatology reviews 13: 98–102 [DOI] [PubMed] [Google Scholar]
- Nagakura Y 2015. Recent Advancements in Animal Models of Fibromyalgia. MYOPAIN 23: 104–11 [Google Scholar]
- Nasseef MT. 2015. Measuring directed functional connectivity in mouse fMRI networks using Granger Causality. University of Trento [Google Scholar]
- Nasseef MT, Devenyi GA, Mechling AE, Harsan LA, Chakravarty MM, et al. 2018. Deformation-based Morphometry MRI Reveals Brain Structural Modifications in Living Mu Opioid Receptor Knockout Mice. Frontiers in psychiatry 9: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseef MT, Singh JP, Ehrlich AT, McNicholas M, Park DW, et al. 2019. Oxycodone-Mediated Activation of the Mu Opioid Receptor Reduces Whole Brain Functional Connectivity in Mice. ACS Pharmacology & Translational Science 2: 264–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyori M, Nagai J, Nakazawa T, Ueda H. 2010. Absence of morphine analgesia and its underlying descending serotonergic activation in an experimental mouse model of fibromyalgia. Neuroscience letters 472: 184–87 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America 98: 676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Tejero JA, Martínez-Lara E, Rus A, Camacho MV, Del Moral ML, Siles E. 2018. Insight into the biological pathways underlying fibromyalgia by a proteomic approach. Journal of proteomics 186: 47–55 [DOI] [PubMed] [Google Scholar]
- Reichling DB, Kwiat GC, Basbaum AI. 1988. Anatomy, physiology and pharmacology of the periaqueductal gray contribution to antinociceptive controls. Prog Brain Res 77: 31–46 [DOI] [PubMed] [Google Scholar]
- Roeder Z, Chen Q, Davis S, Carlson JD, Tupone D, Heinricher MM. 2016. Parabrachial complex links pain transmission to descending pain modulation. Pain 157: 2697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzi-Puttini P, Atzeni F, Masala IF, Salaffi F, Chapman J, Choy E. 2018. Are the ACR 2010 diagnostic criteria for fibromyalgia better than the 1990 criteria? Autoimmun Rev 17: 33–35 [DOI] [PubMed] [Google Scholar]
- Sawaddiruk P, Paiboonworachat S, Chattipakorn N, Chattipakorn SC. 2017. Alterations of brain activity in fibromyalgia patients. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 38: 13–22 [DOI] [PubMed] [Google Scholar]
- Sforazzini F, Schwarz AJ, Galbusera A, Bifone A, Gozzi A. 2014. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. NeuroImage 87: 403–15 [DOI] [PubMed] [Google Scholar]
- Shelton L, Becerra L, Borsook D. 2012a. Unmasking the mysteries of the habenula in pain and analgesia. Prog Neurobiol 96: 208–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, et al. 2012b. Mapping pain activation and connectivity of the human habenula. J Neurophysiol 107: 2633–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Clauw DJ. 2016. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 338: 114–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. 2001. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 24: 37–46 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rohlwing J, Bussey R, Eikenberry S, Wilken J. 2002. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered μ-and δ-, but not κ-, opioid receptor agonists. Journal of Pharmacology and Experimental Therapeutics 302: 1146–50 [DOI] [PubMed] [Google Scholar]
- Stafford JM, Jarrett BR, Miranda-Dominguez O, Mills BD, Cain N, et al. 2014. Large-scale topology and the default mode network in the mouse connectome. Proceedings of the National Academy of Sciences of the United States of America 111: 18745–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Tinelli E, Gerardi MC, Calistri V, Iannuccelli C, et al. 2016. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clinical and experimental rheumatology 34: S129–33 [PubMed] [Google Scholar]
- Uchida H, Nagai J, Ueda H. 2014. Lysophosphatidic acid and its receptors LPA1 and LPA3 mediate paclitaxel-induced neuropathic pain in mice. Molecular pain 10: 1744-8069-10–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Neyama H. 2017. LPA1 receptor involvement in fibromyalgia-like pain induced by intermittent psychological stress, empathy. Neurobiology of pain (Cambridge, Mass.) 1: 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Neyama H, Sasaki K, Miyama C, Iwamoto R. 2019. Lysophosphatidic acid LPA1 and LPA3 receptors play roles in the maintenance of late tissue plasminogen activator-induced central poststroke pain in mice. Neurobiology of Pain 5: 100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, et al. 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


