Abstract
BACKGROUND:
Older patients with acute decompensated heart failure (ADHF) have severely impaired physical function (PF) and quality-of-life (QOL). However, relationships between impairments in PF and QOL are unknown but are relevant to clinical practice and trial design.
METHODS:
We assessed 202 consecutive patients hospitalized with ADHF in the multicenter Rehabilitation Therapy in Older Acute HF Patients (REHAB-HF) Trial. PF measures included Short Physical Performance Battery (SPPB) and 6-minute Walk Distance (6MWD). Disease-specific QOL was assessed by Kansas City Cardiomyopathy Questionnaire (KCCQ). General QOL was assessed by the Short Form-12 (SF-12) and EuroQol-5D-5L. PF was evaluated as a predictor of QOL using stepwise regression adjusted for age, sex, race, and NYHA class.
RESULTS:
Participants were 72±8 years, 54% women, 55% minority race, 52% with reduced ejection fraction, BMI 33±9kg/m2. Participants had severe impairments in PF (6MWD 185±99 meters, SPPB 6.0±2.5 units) and disease-specific QOL (KCCQ Overall Score 41±21, Physical Score 47±24) and general QOL (SF-12 Physical Score 28±9, EuroQol Visual Analogue Scale 57±23). There were modest, statistically significant correlations between 6MWD and KCCQ Overall, KCCQ Physical Limitation, and SF-12 Physical Scores (r=0.23, p<0.001, r=0.30, p<0.001, and r=0.24, p=0.001, respectively); and between SPPB and KCCQ Physical and SF-12 Physical Scores (r=0.20, p=0.004, and r=0.19, p=0.007, respectively). Both 6MWD and SPPB were correlated with multiple components of the EuroQol-5D-5L. 6MWD was a significant, weak predictor of KCCQ Overall Score and SF-12 Physical Score (estimate=0.05±0.01, p<0.001 and estimate=0.05±0.02, p=0.012, respectively). SPPB was a significant, weak predictor of KCCQ Physical Score and SF-12 Physical Score (estimate=1.37±0.66, p=0.040 and estimate=0.54±0.25, p=0.030, respectively).
CONCLUSION:
In older, hospitalized ADHF patients, PF and QOL are both severely impaired, but are only modestly related, suggesting that PF and QOL provide complementary information and assessment of both should be considered to fully assess clinically meaningful patient-oriented outcomes.
Keywords: physical function, quality of life, older adults, heart failure
INTRODUCTION
Acute decompensated heart failure (ADHF) is the leading cause of hospitalization in older adults and is associated with high rates of morbidity, mortality and health care expenditures. 1 Older patients with acute decompensated heart failure (ADHF) are commonly frail with severe impairments in many domains of physical function (PF), including balance, strength, mobility, and endurance.2–4 These impairments are further worsened during hospitalization 5 and some patients never recover baseline function.4–6 This occurs during the most vulnerable, high-risk period for re-hospitalizations, up to six months post discharge,7 and likely contributes to the high rates of re-hospitalizations with accompanying high costs.
ADHF patients also suffer from severe impairments in quality of life (QOL) in comparison to patients with chronic ambulatory HF.8–10 Several studies suggest that QOL, like physical function, is a predictive variable for disease progression, independent of other routine prognostic factors, such as left ventricular ejection fraction.11,12 Severe impairments in PF due to HF decompensation 3,13 are exacerbated by low mobility during hospitalization which leads to muscle weakness and physical deconditioning3,14,15 and worse QOL.16
In chronic HF patients, disease-specific and general QOL are assessed as indicators of chronic HF severity.17 Additionally, QOL is a predictor for development of an acute cardiovascular disorder and physical deconditioning.18 In chronic, stable HF patients, changes in patient-reported health status, as measured by the KCCQ and its subscales, are linked but not highly correlated with changes in functional capacity, including peak oxygen consumption and six-minute walk distance (6MWD).19 Previous studies have shown that exercise training and improvements in PF are inconsistently related with improvements in QOL.20,21 However, the relationship between PF and QOL has not been examined systematically in older, hospitalized ADHF patients. Therefore, the purpose of this study was to examine the relationship between PF and QOL in older patients hospitalized with ADHF.
METHODS
Study Design and Population
A total of 202 hospitalized ADHF first consecutively enrolled patients in the NIH-funded REHAB-HF trial (NCT02196038), a multicenter, randomized, attention-controlled, single-blind trial of a novel, progressive, multi-domain rehabilitation intervention versus attention control were included in this analysis. Details regarding the trial design and methods have been previously published.22,23 Briefly, patients across 7 US centers were ≥60 years old, hospitalized for ≥24 hours for ADHF, and included both HFrEF (defined as EF <45%) and HFpEF (defined as EF ≥45%) phenotypes. ADHF was based on at least one symptom of HF (exertional dyspnea or fatigue, swelling of legs or abdomen, orthopnea, or paroxysmal nocturnal dyspnea) and at least two signs of HF (pulmonary congestion by examination or x-ray film, elevated jugular or central venous pressure, peripheral edema, elevated B-type natriuretic peptide [>100 pg/ml] or N-terminal prohormone of B-type natriuretic peptide [>220 pg/ml]); and change in medical treatment consistent with HF (e.g., augmentation of diuretic agents). The diagnosis of ADHF was confirmed by a study cardiologist with expertise in HF. Additional inclusion criteria were age ≥60 years, independent with basic activities of daily living prior to admission, able to walk at least 4 meters at the time of enrollment (assistive device allowed) and expected to be discharged to home. All participants provided informed consent and the study was approved by the Institutional Review Board of each site.
Physical and Cognitive Function
Study variables were assessed during hospitalization for ADHF, after achieving clinical stability per clinician assessment but before hospital discharge. Trained assessors used standardized protocols to assess physical function measures. 6MWD was assessed by the method of Guyatt et al in cleared hallways; patients were allowed to use an assist device, slow down, or stop if necessary.24,25 The SPPB, a standardized measure in older adults’ patients of physical function across 3 domains: balance, mobility, and functional strength. The 3 components of the SPPB are static balance, 4-meter walk time, and time to complete 5 repeated chair stands. Each component is scored from 0–4 for a total score of 0–12 with higher scores representing better physical function, and a score <10 indicates risk for physical disability.26 Each component of the SPPB is independently associated with important outcomes including HF and mortality.27 Time to walk 4 meters was used to calculate gait speed, where <0.8 m/s is considered a limitation to community ambulation.28 Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA), which screens multiple domains of cognition, and is scored on a scale of 0–30 with a score <26 indicating at least mild cognitive impairment.29
Quality of Life Measures
The primary measure of QOL was the KCCQ, which is a HF disease-specific measure and an independent predictor of clinical outcomes such as hospitalization, recent hospitalization and mortality in outpatients with HF.30,31 We used multiple scores of the KCCQ: the Overall Summary Score, and the subscores: Physical Limitation Score, Total Symptom Score, Quality of Life Score, and Social Limitation Score. All scores are graded on a 0–100-point scale with higher scores indicating better QOL. A 5-point change in KCCQ is associated with cardiologists’ ratings of changes in clinical status in chronic HF outpatients and with all-cause mortality, cardiovascular death, hospitalization, and cardiologists’ rating of health status in patients with HF complicating acute myocardial infarction.32,33
General QOL was assessed using the 12-Item Short-Form Health Survey (SF-12). The SF-12 is composed of the Physical Composite Score (PCS) and the Mental Composite Score (MCS), which are independent. Both scores are graded on a 0–100-point scale with a higher score equating better QOL. The SF-12 is valid and reliable in older adults and in HF.34,35
The Euro-QOL was utilized to assess patient specific health utilities.36 It consist of five items that measure difficulties with 1) mobility; 2) self-care; 3) usual activities; 4) pain/discomfort; 5) anxiety/depression and the Visual Analogue Scale self-rating (0–100 scale) “thermometer” of the respondent’s overall health, with higher scores indicating better health status.37
Statistical Analyses
Pearson’s correlation examined association between PF as measured by SPPB and 6MWD and QOL as measured by KCCQ Overall, Clinical, and Physical Limitation Scores, SF-12 PCS and MCS, and EuroQol components. L regression models with stepwise selection were performed to identify independent predictors of the KCCQ Overall Score, Physical Limitation Score, SF-12 PCS, and Euro-QOL Visual Analogue Scale separately for6MWD and SPPB total score, including independent variables of age, sex, non-white race, and New York Heart Association class. A 2-tailed p< 0.05 was considered significant. The sample size of our study (n=202) provided 80% power to detect correlations as low as 0.20 between PF and QOL measures. All analyses were performed using SAS Enterprise Guide 7.1 (Cary, NC).
RESULTS
Participant characteristics are listed in Table 1. Mean age of the cohort was 72±8 years. Of the 202 participants, 54% were female, 52% were non-white, 52% had HFrEF, and 44% had a previous hospitalization within the prior 6 months of enrollment, with 61% of these recent hospitalizations being for HF. Participants had high rates of comorbidities, including hypertension, diabetes, dyslipidemia, chronic kidney disease, and obesity with a mean BMI of 33.2±8.8kg/m2.
Table 1.
Participant Characteristics
| Characteristics | N=202 |
|---|---|
| Age | 72.0 ± 7.5 |
| Women | 109 (54%) |
| Non-white | 105 (52%) |
| BMI (kg/m2) | 33.2 ± 8.8 |
| Ejection fraction < 45% | 106 (52%) |
| Days Hospitalized at Index Hospitalization, median (IQR) | 5 (3,7) |
| Patients with previous hospitalizations within 6 months | 89 (44%) |
| Patients with previous HF hospitalizations within 6 months | 55 (27%) |
| BNP (n=117) | 923 ± 958 |
| Pro-BNP (n=77) | 5968 ± 10066 |
| Comorbidities | |
| Diabetes mellitus | 111 (55%) |
| Hypertension | 187 (93%) |
| Hyperlipidemia | 140 (69%) |
| Chronic obstructive pulmonary disease | 65 (32%) |
| Chronic kidney disease | 67 (33%) |
| History of Stroke | 32 (16%) |
| Arthritis | 94 (47%) |
| History of Cancer | 42 (21%) |
| History of Depression | 33 (16%) |
| Physical Function | |
| SPPB total score | 6.0 ± 2.5 |
| 6-minute walk distance (m) | 185 ± 99 |
| Gait speed (m/s) | 0.61 ± 0.21 |
| Cognitive Function (MoCA Score) | 21.7±4.5 |
| Quality of Life Measures | |
| KCCQ Overall Score | 41 ± 21 |
| KCCQ Physical Limitation Score | 47 ± 24 |
| KCCQ Total Symptom Score | 33 ± 22 |
| KCCQ Quality of Life Score | 39 ± 25 |
| KCCQ Social Limitation Score | 42 ± 31 |
| SF-12 PCS | 28 ± 9 |
| SF-12 MCS | 44 ± 14 |
| EuroQol 5D-5L Components | |
| Walking | 2.5 ± 1.0 |
| Self-Care | 1.7 ± 0.9 |
| Usual Activities | 2.7 ± 1.2 |
| Pain/Discomfort | 2.4 ± 1.1 |
| Depression/Anxiety | 1.8 ± 1.0 |
| Visual Analog Scale (0–100) | 57 ± 23 |
Values presented as mean ± standard deviation or N (%) unless otherwise noted. Abbreviations: BMI – body mass index; BNP – B-type natriuretic peptide; Pro-BNP – n-terminal brain natriuretic peptide; SPPB – Short Physical Performance Battery; MoCA – Montreal Cognitive Assessment; KCCQ – Kansas City Cardiomyopathy Questionnaire; SF-12 – Short Form 12; PCS – Physical Composite Score; MCS – Mental Composite Score.
Participants had severe impairments in PF and QOL (Table 1). Mean total SPPB score was 6.0±2.5 (range: 1–12) units. Average 6MWD was 185±99 (range: 12–547) meters. Additionally, gait speed was impaired at 0.61±0.21 m/s. On average, participants had at least mild cognitive impairment as indicated by MoCA score <26. Impairments in QOL were broad, both in disease-specific and general QOL measures. The disease-specific KCCQ Overall Score was 41±21 (range: 4–99) and Physical Limitation Score 47±24 (range: 0–100). General QOL was very low in the SF-12 PCS 28±9 and SF-12 MCS 44±14. By the EuroQol, participants frequently reported problems with mobility, self-care, and usual activities as well as pain/discomfort and anxiety/depression.
Univariate relationships between PF and QOL measures are presented in Table 2. Notably, SPPB and the KCCQ Overall Score were unrelated, as well as the Total Symptom, Quality of Life, and Social Limitation scores, and the EuroQol Visual Analog Scale. The SPPB and KCCQ Physical Limitation Score had a modest but highly statistically significant correlation (r= 0.20, p=0.004, Figure 1). The SPPB was also correlated with measures of general QOL: the SF-12 PCS (r=0.19, p=0.007) and multiple EuroQol components: mobility, usual activities, and pain/discomfort. 6MWD showed modest, significant correlations with the KCCQ Overall (Figure 1), Physical Limitation, Total Symptom, and Social Limitation scores (r=0.23, p=0.0007, r= 0.30, p <0.0001, r=0.25, p<0.001, and r=0.15, p=0.039, respectively). For general QOL, 6MWD was correlated with the SF-12 PCS, and had highly statistically significant correlations with all components of the EuroQol, except the Visual Analog Scale. Partial correlation analysis adjusting for age and race did not meaningfully alter results.
Table 2.
Univariate Relationships of Physical Function and Quality of Life
| Measure | 6-minute walk distance | SPPB Score | ||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Participant Characteristics | ||||
| Age | −0.11 | 0.13 | −0.26 | <0.001 |
| Weight | −0.29 | <0.001 | −0.07 | 0.36 |
| Height | 0.13 | 0.07 | 0.05 | 0.47 |
| BMI | −0.37 | <0.001 | −0.10 | 0.17 |
| QOL Measures | ||||
| KCCQ Overall Score | 0.23 | <0.001 | 0.07 | 0.31 |
| KCCQ Physical Limitation Score | 0.30 | <0.001 | 0.20 | 0.004 |
| KCCQ Total Symptom Score | 0.25 | <0.001 | 0.12 | 0.10 |
| KCCQ Quality of Life Score | 0.09 | 0.21 | −0.03 | 0.66 |
| KCCQ Social Limitation Score | 0.15 | 0.039 | 0.01 | 0.91 |
| SF-12 PCS | 0.24 | 0.001 | 0.19 | 0.007 |
| SF-12 MCS | 0.06 | 0.39 | −0.03 | 0.64 |
| EQ-5D-5L Components | ||||
| Mobility | −0.40 | <0.001 | −0.36 | <0.001 |
| Self-Care | −0.25 | <0.001 | −0.20 | 0.004 |
| Usual Activities | −0.26 | <0.001 | −0.16 | 0.020 |
| Pain/Discomfort | −0.30 | <0.001 | −0.20 | 0.004 |
| Depression/Anxiety | −0.15 | 0.043 | −0.13 | 0.07 |
| Visual Analog Scale (0–100) | 0.12 | 0.10 | 0.06 | 0.41 |
Abbreviations: BMI – body mass index; SPPB – Short Physical Performance Battery; KCCQ – Kansas City Cardiomyopathy Questionnaire; SF-12 – Short Form 12; PCS – Physical Composite Score; MCS – Mental Composite Score; EQ-5D-5L – EuroQol 5D-5L
Figure 1.
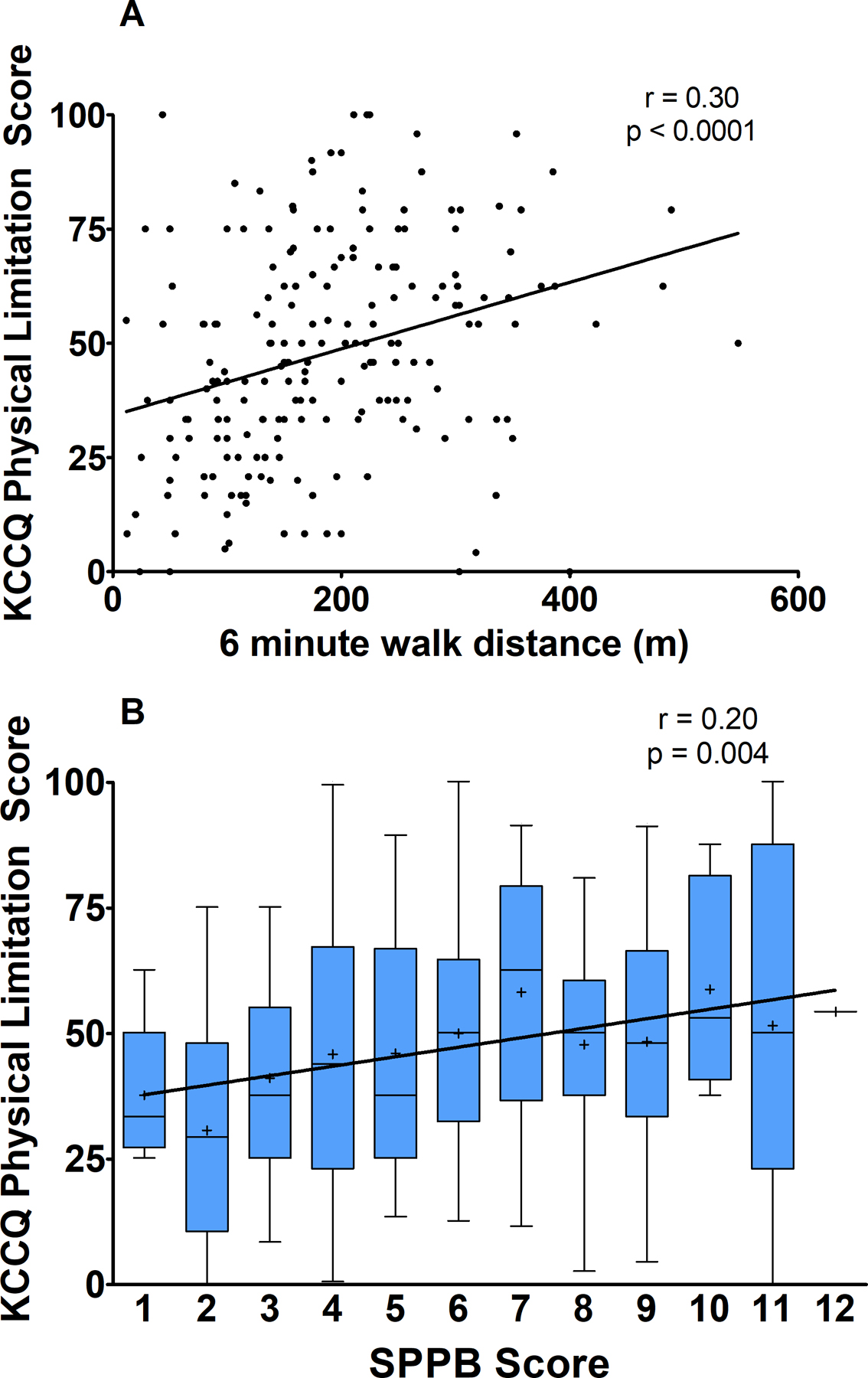
Relationship of physical function as measured by A) 6-minute walk distance and B) SPPB Total Score with QOL as measured by KCCQ Physical Limitation Score. In panel B, boxes represent 25th, 50th, and 75th percentiles, whiskers represent 95th percentiles, and (+) represents mean.
In stepwise regression, 6MWD, but not SPPB, was a modest but statistically significant predictor of the KCCQ Overall Score (Table 3). However, both the SPPB and 6MWD were statistically significant predictors for the KCCQ Physical Limitation Score, along with body mass index. For general QOL, only the SPPB was a significant predictor for SF-12 PCS, and neither 6MWD nor SPPB were predictors for the Visual Analog Scale.
Table 3.
Predictors of Quality of Life by Physical Function Measures
| Model R2 | Estimate | Partial R2* | p-value | |
|---|---|---|---|---|
| 6MWD models | ||||
| KCCQ Overall Score | 0.17 | 0.05±0.01 | 0.04 | <0.001 |
| KCCQ Physical Limitation Score | 0.10 | 0.05±0.02 | 0.04 | 0.012 |
| SF-12 PCS | 0.07 | -- | -- | -- |
| EuroQol Visual Analogue Scale | 0.03 | -- | -- | -- |
| SPPB models | ||||
| KCCQ Overall Score | 0.14 | -- | -- | -- |
| KCCQ Physical Limitation Score | 0.09 | 1.37±0.66 | 0.02 | 0.040 |
| SF-12 PCS | 0.06 | 0.54±0.25 | 0.03 | 0.030 |
| EuroQol Visual Analogue Scale | 0.03 | -- | -- | -- |
Stepwise regression parameter estimate presented with SE if entered model. All models adjusted for age, sex, BMI, non-white race, and NYHA class. Abbreviations: 6MWD – 6-minute walk distance; KCCQ – Kansas City Cardiomyopathy Questionnaire; SF-12 – Short Form 12; PCS – Physical Composite score; SPPB – Short Physical Performance Battery.
partial R2 for 6MWD or SPPB
DISCUSSION
In this study, we utilized multiple measures of PF, and three different QOL instruments to assess impairments in a well-characterized population of older patients with ADHF. Older patients with ADHF displayed severe impairments in many domains of PF and QOL across multiple measures, and that had considerable variation and range between individual patients. Our findings suggest that PF and QOL are only modestly related despite both being severely impaired in older, hospitalized ADHF patients. This finding suggests that PF and QOL are distinct, relatively independent domains and that they cannot be used as surrogates for each other (Figure 2). Thus, PF and QOL, while modestly related, should both be assessed to fully characterize clinically meaningful patient-oriented outcomes in older patients with ADHF.
Figure 2.
Impaired physical function and impaired quality of life are frequent in older patients hospitalized with ADHF. The only modest relation between these domains suggests the complementary information these assessments provide in characterizing impairments in patients with ADHF.
Impairments in PF were severe and broad in older ADHF patients. Marked PF impairments were present in multiple domains including balance, mobility, and strength as measured by the SPPB total score 6.0±2.5 units and endurance as measured by 6MWD of 185±99 meters, well below a threshold of ≤300 meters that indicates severe functional impairment.38 We have previously shown that these impairments are much more profound than in stable HF patients and are comparable to advanced HF outpatients awaiting left ventricular assist device implantation (204 m).39 These severe and broad impairments are likely due to combined effects of preexisting impairments from aging, comorbidities (including orthopedic limitations), chronic HF, chronic skeletal muscle dysfunction, and the recent insult of ADHF itself and exacerbated by immobility and deconditioning during hospitalization.40–42
Similarly, QOL was impaired across multiple measures, including disease-specific and general QOL measures. These levels of QOL impairment were substantially greater than previously seen in older, stable HFrEF and HFpEF patients, but less than QOL measurements seen in patients referred to a palliative care trial.9,10,43 That these impairments were not only disease-specific, but also present in the general SF-12 and EuroQol instruments suggests a broad impairment, possibly due to factors beyond HF symptomology, such as frailty, depression, and multiple comorbidities.
Despite the severity and broad range of impairments in both PF and QOL, our findings suggest that these domains are only modestly related. By univariate analysis, SPPB, a multiple-domain measure of PF, was not correlated to the Overall KCCQ score, although it was significantly but only modestly correlated to the Physical Limitation subscore only. That other subscores of the KCCQ (Total Symptom, Quality of Life, and Social Limitation) were not related to SPPB further emphasizes the distinct information of assessed by PF testing and QOL instruments. Although the 6MWD was correlated to the KCCQ and multiple subscores, these associations were again only modest (all r≤0.30), further supporting that 6MWD and QOL have only a small amount of overlap of information regarding the patient experience of disease manifestations. The SPPB and 6MWD had modest association with general QOL measures (SF-12 PCS), but correlation coefficients were small. In multivariate analyses, the SPPB and 6MWD were significant but weak predictors of QOL measures, and were less potent than simple demographic predictors such as age and body mass index. Altogether, these findings indicate that PF and QOL represent distinct domains of the ADHF profile, and should not be used interchangeably.
The clinical implications of our study support the concept that patient-reported outcome measures such as the KCCQ in addition to PF provide crucial information to the profile of patient health. The KCCQ offers unique information in that it quantifies, from a subjective patient perspective, the severity of HF symptoms and their impact on PF and social function and QOL in ADHF. Conversely, PF measures provide objective information regarding functional ability to complete ADLs, remain independent and community dwelling, as well as provide important prognostic indicators. Additionally, these measures also provide clear patient-centered targets for intervention.
This analysis extends previously published data from this study and other existing studies as it uniquely focused on association of PF and QOL in hospitalized, older patients with ADHF. Although the lack of a strong association in ADHF patients may be due to the recall period used in QOL measurements during which patients may had better PF, the finding of only a modest relationship between PF and QOL have also been identified in previous studies in stable outpatient HF populations.19,44 This was apparent in our recently reported study of stable patients with HFpEF that showed that improvements in health-related QOL and physical function in response to exercise training were largely independent.21 Additionally, QOL and PF measures have been shown to associate differently with clinical changes in patients with HFrEF.33
Our study has multiple strengths including: several validated, multi-domain PF measures multiple QOL instruments including both disease-specific and general QOL, and a large, diverse population of older, frail, hospitalized patients with ADHF. This study also has limitations. Because the study was designed to include only older, frail who were hospitalized with ADHF, the results may not be generalizable to other patient populations.22 Second, because we excluded non-independent and non-ambulatory patients, we potentially underestimated the severity of PF and QOL impairments in hospitalized ADHF patients. Finally, the cross-sectional study design does not allow for assessment of intervention of PF or QOL as an outcome.
Conclusion
In older patients hospitalized with acute ADHF, PF and QOL are severely impaired but only modestly related. This suggests that PF and QOL provide complementary information and assessment of both is required to fully characterize clinically meaningful patient-oriented outcomes in ADHF.
Key Points.
Physical function and quality of life are both severely impaired in older patients hospitalized with acute decompensated heart failure.
Physical function and quality of life impairments are only modestly related.
Physical function and quality of life are distinct, partly independent measures, and provide complementary information to fully characterize clinically meaningful patient-oriented outcomes in ADHF.
Why does this paper matter?
Older, hospitalized patients with acute decompensated heart failure have severely impaired physical function and quality of life. These impairments are only modestly related, meaning they are partly independent, and that assessment of both is needed to fully characterize their patient-centered outcomes.
ACKNOWLEDGEMENTS
Funding:
This study was supported in part by the following research grant awards from the National Institutes of Health (NIH): R01AG045551 and R01AG18915. It was also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine (Dr Kitzman), the Claude D. Pepper Older Americans Independence Center NIH Grants P30AG021332 (Dr Kitzman) and P30AG028716 (Dr Pastva), and the Wake Forest Clinical and Translational Science Award, NIH Grant UL1TR001420.
Footnotes
Conflict of Interest: Dr Kitzman has been a consultant for Corvia Medical, Bayer, Astra-Zeneca, Keyto, Boehringer-Ingleheim, and St Luke’s Medical Center in Kansas City, Kansas; received grant support from Astra-Zeneca, Novartis, Bayer, NovoNordisk, and St Luke’s Medical Center in Kansas City, Kansas; and owns stock in Gilead Sciences. Dr Mentz receives research support from the National Institutes of Health (U01HL125511–01A1 and R01AG045551–01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Regent, Medtronic, Merck, Novartis and Sanofi; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and Sanofi; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim. The other authors report no conflicts.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez E, Vidan MT, Serra JA, Fernandez-Aviles F, Bueno H. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97(19):1602–1606. [DOI] [PubMed] [Google Scholar]
- 3.Reeves GR, Whellan DJ, Patel MJ, et al. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients >/=60 Years Hospitalized With Acute Decompensated Heart Failure Versus Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2016;117(12):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66(1):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168(5):721–730. [DOI] [PubMed] [Google Scholar]
- 8.Reeves GR, Whellan DJ, O’Connor CM, et al. A Novel Rehabilitation Intervention for Older Patients With Acute Decompensated Heart Failure: The REHAB-HF Pilot Study. JACC Heart Fail. 2017;5(5):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers JG, Patel CB, Mentz RJ, et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J Am Coll Cardiol. 2017;70(3):331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konstam V, Salem D, Pouleur H, et al. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. SOLVD Investigations. Studies of Left Ventricular Dysfunction Investigators. Am J Cardiol. 1996;78(8):890–895. [DOI] [PubMed] [Google Scholar]
- 13.Chati Z, Zannad F, Jeandel C, et al. Physical deconditioning may be a mechanism for the skeletal muscle energy phosphate metabolism abnormalities in chronic heart failure. Am Heart J. 1996;131(3):560–566. [DOI] [PubMed] [Google Scholar]
- 14.Kortebein P, Symons TB, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(10):1076–1081. [DOI] [PubMed] [Google Scholar]
- 15.Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–273. [DOI] [PubMed] [Google Scholar]
- 16.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113(7):1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majani G, Pierobon A, Giardini A, et al. Relationship between psychological profile and cardiological variables in chronic heart failure. The role of patient subjectivity. Eur Heart J. 1999;20(21):1579–1586. [DOI] [PubMed] [Google Scholar]
- 18.Rumsfeld JS, MaWhinney S, McCarthy M Jr., et al. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA. 1999;281(14):1298–1303. [DOI] [PubMed] [Google Scholar]
- 19.Flynn KE, Lin L, Moe GW, et al. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012;163(1):88–94 e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rees K, Taylor RS, Singh S, Coats AJ, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2004(3):CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brubaker PH, Avis T, Rejeski WJ, Mihalko SE, Tucker WJ, Kitzman DW. Exercise Training Effects on the Relationship of Physical Function and Health-Related Quality of Life Among Older Heart Failure Patients With Preserved Ejection Fraction. J Cardiopulm Rehabil Prev. 2020;40(6):427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J. 2017;185:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastva AM, Duncan PW, Reeves GR, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials. 2018;64:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demers C, McKelvie RS, Negassa A, Yusuf S, Investigators RPS. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142(4):698–703. [DOI] [PubMed] [Google Scholar]
- 25.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. Journal of aging and physical activity. 2015;23(2):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich PA, Spertus JA, Jones PG, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47(4):752–756. [DOI] [PubMed] [Google Scholar]
- 31.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110(5):546–551. [DOI] [PubMed] [Google Scholar]
- 32.Kosiborod M, Soto GE, Jones PG, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115(15):1975–1981. [DOI] [PubMed] [Google Scholar]
- 33.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. [DOI] [PubMed] [Google Scholar]
- 34.Resnick B, Nahm ES. Reliability and validity testing of the revised 12-item Short-Form Health Survey in older adults. J Nurs Meas. 2001;9(2):151–161. [PubMed] [Google Scholar]
- 35.Cernin PA, Cresci K, Jankowski TB, Lichtenberg PA. Reliability and validity testing of the short-form health survey in a sample of community-dwelling African American older adults. J Nurs Meas. 2010;18(1):49–59. [DOI] [PubMed] [Google Scholar]
- 36.Reed SD, Li Y, Kamble S, et al. Introduction of the Tools for Economic Analysis of Patient Management Interventions in Heart Failure Costing Tool: a user-friendly spreadsheet program to estimate costs of providing patient-centered interventions. Circ Cardiovasc Qual Outcomes. 2012;5(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grady KL, Warner Stevenson L, Pagani FD, et al. Beyond survival: recommendations from INTERMACS for assessing function and quality of life with mechanical circulatory support. J Heart Lung Transplant. 2012;31(11):1158–1164. [DOI] [PubMed] [Google Scholar]
- 38.Yancy C, Jessup M, Bozkurt B, et al. 2013 ACC/AHA Guideline for the Management of Heart Failure. Circulation. 2013;128(16):e240–e319. [DOI] [PubMed] [Google Scholar]
- 39.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55(17):1826–1834. [DOI] [PubMed] [Google Scholar]
- 40.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306(9):H1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitzman DW, Haykowsky MJ, Tomczak CR. Making the Case for Skeletal Muscle Myopathy and Its Contribution to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3(4):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masoudi FA, Rumsfeld JS, Havranek EP, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. J Card Fail. 2004;10(5):368–373. [DOI] [PubMed] [Google Scholar]


