Highlights
-
•
Among all factors considered, maternal education contributes the most to racial/ethnic disparities in birth outcomes.
-
•
Mother’s health insurance coverage significantly helps explain racial/ethnic disparities in birth outcomes.
-
•
More research is needed to better account for Black-White disparities in birth outcomes.
Keywords: Racial and ethnic disparities, Birth outcomes, Preterm births, Mother’s education, Mother’s health insurance coverage, Low-birth-weight, Fairlie decomposition
Abstract
This study seeks to quantify and rank the contribution of selected factors to the observed racial/ethnic disparities in low-birth-weight births (LBWBs) and preterm births (PTBs). Based on vital statistics data on births to primiparous women and characteristics of mothers in the State of Nebraska during the period of 2005 to 2014 (n = 93,375), unadjusted and adjusted odds ratios were estimated to examine the association between selected variables and the odds of having LBWBs or PTBs. Fairlie decomposition analysis was performed to quantify the contribution of each selected factor to racial/ethnic differences in LBWB and PTB rates. The prevalence of PTBs was 9.1% among non-Hispanic White (NHW) women, as compared to 12.8% among non-Hispanic Black (NHB) women and 10.6% among Hispanic women. The corresponding prevalence of LBWBs in the three groups were 5.9%, 11.9%, and 7.2%, respectively. The higher educational attainment among NHW women, relative to NHB women accounted for 10% of the observed difference in LBWB rate between the two groups. Health insurance coverage was the second most important factor accounting for the observed disparities in birth outcomes. Addressing socioeconomic disadvantages in NHB and Hispanic women would be important for them to narrow their gaps with NHW women in LBWB and PTB prevalence. More research is needed to identify key factors leading to the disparities in birth outcomes between NHW and NHB women.
1. Introduction
Adverse birth outcomes such as preterm births (PTBs, being born before the 37th week of gestation) and low-birth-weight births (LBWBs, birth weight of less than 2,500 g) are common causes of infant mortality in the United States (Hoyert and Xu, 2011). In 2018, about 1 out of 10 newborns were preterm and about 1 out of 12 newborns were of low birth weight in the U.S. (Martin et al., 2019). Infants born before term or with low birth weights (LBW) are at increased risk of neurodevelopmental impairments and respiratory and gastrointestinal complications (Goldenberg et al., 2008). It was estimated that employer-sponsored health plans in the U.S. spent between $6 billion and $14 billion to care for preterm babies in 2013 (Grosse et al., 2017).
Relative to non-Hispanic White (NHW) mothers, racial and ethnic minority mothers are generally at higher risk for PTBs or LBWBs (McGrady et al., 1992, Orr et al., 2002, Ventura et al., 2001). In 2015, the national prevalence of PTBs in the U.S. was 8.91% among NHW mothers as compared to 13.23% among non-Hispanic Black (NHB) mothers and 9.03% among Hispanic mothers. The corresponding rates in LBWBs in these three groups were 6.93%, 13.35%, and 7.21%, respectively (Martin et al., 2017). Addressing racial and ethnic disparities in these adverse birth outcomes, especially the substantial gaps between NHW and NHB mothers, would be important for reducing PTBs, LBWBs, and infant mortality in the U.S. as specified in Healthy People (2020a).
Previous studies suggested that a host of factors could contribute to racial and ethnic disparities in birth outcomes including but not limited to socioeconomic disadvantages (Krieger, 1991, Blumenshine et al., 2010), maternal stress (Copper et al., 1996, Dole et al., 2003, Dunkel Schetter, 2011, Lu and Chen, 2004), perceived racism (Dominguez, 2008, Dominguez et al., 2008, Earnshaw et al., 2013, Dunkel and Tanner, 2012, Collins et al., 2004, Paradies, 2006), risky behaviors during pregnancy such as smoking, alcohol consumption, and substance abuse (Salihu and Wilson, 2007, Bernstein et al., 2000, Pollack et al., 2000, Putnam-Hornstein and Prindle, 2016, Roberts et al., 2018), maternal morbidity such as hypertension or diabetes (Guendelman et al., 2006, Lawrence et al., 2008), maternal age (Rauh et al., 2001, Geronimus, 1996), genetic and epigenetic factors (Simhan et al., 2003, Velez et al., 2009, Kramer and Hogue, 2009), access to prenatal care (Ickovics et al., 2003, Kotelchuck, 1994), and rural versus urban settings (Kent et al., 2013). While identifying these risk factors is helpful for developing targeted interventions to address birth outcome disparities, assessing the relative importance of each factor amongst identified factors in explaining racial and ethnic disparities is also needed for prioritizing strategies and interventions that will effectively reduce these disparities.
Decomposition analysis breaks group differences in the mean of an outcome into two parts: group differences in the magnitude of the determinants of the outcome in question and group differences in the effects of these determinants (Oaxaca, 1973, O'Donnell, et al., 2008). Despite growing efforts in adopting this technique in health disparities research (Sen, 2014), only a few studies have conducted decomposition analysis to quantify the contribution of different factors to the observed racial disparities in birth outcomes (Lhila and Long, 2012, Benmarhnia et al., 2017, Pitts et al., 2011). A converging finding from these studies is that among the selected factors included in the decomposition analysis, differences in maternal socioeconomic status contributed the most to the observed racial disparities in LBWBs or PTBs. While two of the studies (Lhila and Long, 2012, Pitts et al., 2011) documented higher rates of tobacco use during pregnancy in White women than in Black women, these studies did not single out tobacco use during pregnancy as a separate factor in the decomposition analysis of racial disparities in birth outcomes. Consequently, it remains unclear how racial differences in tobacco use during pregnancy has been linked to corresponding disparities in birth outcomes between White and Black women.
More research is also needed to better understand ethnic differences in birth outcomes between Hispanic and non-Hispanic White women. Findings from a report released by the Centers for Disease Control and Prevention in 2018 suggested an expanding gap in LBWB rate between NHW and Hispanic women since 2006 when the rates in the two groups were respectively 5.4% and 5.8% (Womack et al., 2018). By 2016, the respective rates in the two groups became 5.2% and 6.0%, which revealed that the gap between the two groups increased from 0.4 percentage points in 2006 to 0.8 percentage points in 2016. Elimination of this disparity in 2016 would translate into an aversion of over 7,000 LBWBs among Hispanic women based on 918,447 births given by Hispanic mothers during the same year (Martin et al., 2018).
This study seeks to use population-based data to assess the relative importance of mothers’ demographics, SES, body mass index (BMI), health status prior or during pregnancy, tobacco use during pregnancy, adequacy of prenatal care, and urban versus rural residence in explaining racial and ethnic differences in the incidence of PTBs and LBWBs. Implications of study findings can inform the development of relevant interventions to reduce racial and ethnic disparities in birth outcomes.
2. Materials and methods
2.1. Sample
For the period from 2005 to 2014, data on birth outcomes and characteristics of mothers in the State of Nebraska were collected from birth records reported to the vital records electronic registration system at the Nebraska Department of Health and Human Services. In consideration of the clustering of adverse birth outcomes among women with a history of giving preterm or underweight births (Su et al., 2018), only births to primiparous women with a gestational age of at least 22 weeks (0.1% of the sample had a missing gestational age or a gestational age less than 22 weeks) and a plausible birth weight for gestational age were included in the analysis. Implausible weights were determined using methods previously published (Talge et al., 2014), but briefly, z-scores were calculated separately by sex and gestational age (in weeks) using the median weight for each group and previously published standard deviations (Kramer et al., 2001), and babies with weights outside of −5 and 5 z-scores for term births (37 weeks or later), and −4 and 3 z-scores for preterm births were excluded from the study sample. Cases for this study were limited to those where race was categorized as NHW, NHB, or Hispanic (n = 93,375).
3. Measures
Frequencies of outcomes and explanatory variables of interest were provided separately by race and ethnicity categories. Dichotomous outcome variables included preterm birth status (preterm if less than 37 weeks gestation; else not preterm) and birth weight status, which was only analyzed for term birth babies (low birth weight if less than 2500 g; else not low birth weight). Gestational age was measured by obstetric estimate at delivery.
Explanatory variables of interest included mother’s age (younger than 21 years, 21 to 27 years, or 28 years or older); mother’s marital status (yes/no); mother’s education (less than 12 years, high school degree, some college, at least a college degree); insurance status at the time of birth (Medicaid, private, self-pay, other); tobacco use during pregnancy (yes/no); urban/rural designation of mother’s county of residence (urban/rural); body mass index (BMI) of mother before pregnancy (underweight (less than 18.5 kg/m2), normal weight (18.5 to 24.99 kg/m2), overweight (25.0 to 29.99 kg/m2), obese (30 kg/m2 or greater); pre-pregnancy diabetes (yes/no); gestational diabetes (yes/no); chronic hypertension (yes/no); pregnancy associated hypertension (yes/no); Cesarean-section (C-section) status (yes/no); and prenatal care utilization (Inadequate, Intermediate, Adequate, Intensive), calculated using Kotelchuck’s Adequacy of Prenatal Care Utilization (APNCU) index (Alexander and Kotelchuck, 1996), which compares the actual number of prenatal care visits to what would be expected for a baby of a given gestational age.
4. Statistical analyses
Unadjusted and adjusted odds ratios for race categories were calculated separately for the two outcomes of interest: preterm birth and low birth weight status. Both unadjusted and adjusted odds ratios for race were calculated using logistic regressions, but only the adjusted odds logistic regression controlled for all variables of interest.
This study utilized Fairllie decomposition specifically tailored for data with a dichotomous outcome (Fairlie, 1999), in order to quantify the contribution of individual variables to the difference in probabilities of outcomes (i.e. low weight births or preterm births) between minority groups (i.e. NHBs or Hispanics) and NHWs. The decomposition analysis identifies the contribution of independent variables to explaining the differences across groups by calculating the change in the average predicted probability resulting from replacing one independent variable at a time for one group while keeping all the other variables constant for the other group (Fairlie and Robb, 2007, Pagán et al., 2009). First, all three racial and ethnic groups of interest for this study were combined in order to obtain the pooled coefficient estimates used in the decomposition analyses. Then, separately for each minority group, a random sample of NHW cases of equal size to the minority sample size was chosen, and the distribution of the minority replaced the white distribution sequentially for each independent variable in order to calculate each independent variable’s contribution to the racial difference or ‘gap’ in probability of the outcome. The process of randomly sampling NHW cases and estimation of each variable’s contribution to the gap was repeated 1000 times. The order of variables was randomized on each run to address the issue of path dependence (Fairlie, 2017). Contributions to the gap by each factor are given both as a point estimate with associated standard error, as well as a percentage of the gap in the outcome between a minority group and NHWs. All statistical analyses were conducted using SAS version 9.4, and p-values less than 0.05 are considered statistically significant.
5. Results
There were substantial differences in birth outcomes and selected covariates across NHW, NHB, and Hispanic women in the study sample (Table 1). The rate of PTBs was 9% among NHW women, as compared to 12.8% among NHB women and 10.5% among Hispanic women. The corresponding rates of LBWBs among term births in the three groups were respectively 2.2%, 5.6%, and 3.0%.
Table 1.
Frequency and percent distribution for variables used in the analysis by race/ethnicity.
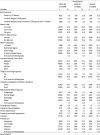 |
The three groups also differed in demographics, SES, tobacco use during pregnancy, health insurance coverage, obesity, adequacy of prenatal care, and other health indicators considered. Over 38% of NHW women had a college or higher degree, as compared to 9.7% for NHB women and 8.3% for Hispanic women. In terms of health insurance coverage, 25.1% of NHW women were covered by Medicaid, as compared to 67.2% and 59.8% among NHB and Hispanic women, respectively.
Based on unadjusted odds ratios (Table 2), among women with term births NHB women had higher odds of giving LBWBs than NHW women (OR = 2.68, 95% CI [2.34, 3.05]). This difference largely persisted in the adjusted model (OR = 2.38, 95% CI [2.04, 2.77]. Similar findings, though to a lesser extent, were also observed in the elevated odds of Hispanic women giving LBWBs when compared to NHW women.
Table 2.
Unadjusted and Adjusted Odds Ratios for Low Weight and Preterm Births.
 |
The higher unadjusted odds of giving PTBs by NHB women, relative to NHW women, were mitigated in the adjusted model though the difference was still statistically significant. This, however, was not the case for Hispanic women whereby their elevated odds of giving PTBs, relative to NHW women, in the unadjusted model became statistically insignificant when the effects from other included variables were considered.
Among women with term births, the rate of LBWBs among NHW women was 2.1% as compared to 5.5% among NHB women, resulting in a difference of 3.4% (Table 3). About 10% of this difference can be explained by education and 4.5% by health insurance coverage. Relative to NHW women, the lower education as well as the higher percentage of Medicaid coverage among NHB women contributed to their higher odds of having LBWBs. The higher concentration of NHB women in urban Nebraska as well as the lower use of tobacco during pregnancy by NHB women, however, helped mitigate their gap with NHW women in LBWB rate.
Table 3.
Nonlinear decomposition analysis of factors contributing to racial/ethnic gaps in Low Weight and Preterm birth outcomes.
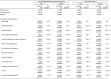 |
In terms of the difference in LBWB rate between NHW and Hispanic women, 54.2% of the gap in LBWB rate was explained by difference in educational attainment between the two groups. Relative to NHW women, Hispanic women were far less likely to use tobacco during pregnancy, which helped mitigate the gap in LBWB rate between the two groups by 23.2%.
As for the difference in PTB rate between NHW and NHB women, the gap in education between the two groups was the most important contributing factor, explaining 17.3% of the difference in PTB rate, followed by marital status, which accounted for 10.1% of the difference in PTB rate. That is, the lower marriage rate among NHB women, relative to NHW women, contributed to the higher PTB rate among NHB women.
Similarly, the dominant factor explaining the difference in PTB rate between NHW and Hispanic women was education, accounting for 64.3% of the difference. Differences in health insurance coverage between the two groups, especially the higher percentage of health insurance covered by self-pay among Hispanic women, also contributed to their higher rate of PTBs, relative to NHW women. The relatively lower prevalence of pregnancy-induced hypertension among Hispanic women, however, mitigated the difference in PTB rates between the two groups.
A more straightforward illustration of the relative importance of different factors in accounting for racial and ethnic differences in birth outcomes can be found in Fig. 1. Out of the four comparisons between NHW women and minority women in birth outcomes, mother’s education and health insurance coverage were the top two most important factors in three comparisons.
Fig. 1.
Leading Factors Contributing to the Observed Differences in Birth Outcomes Between Non-Hispanic White Women and Minority Women.
6. Discussion
Racial and ethnic disparities in birth outcomes in the United States and their persistence over time remain a public health challenge (Healthy People, 2020b). This study represents a rare effort in using the Fairlie decomposition methodology to rank the importance of various selected factors in explaining the observed racial and ethnic differences in birth outcomes. Overall, mother’s education and health insurance coverage turn out to be the most important factors, which highlights the necessity and fundamentality of addressing socioeconomic disadvantages among minority women in narrowing their gaps with White women in birth outcomes.
One of the leading factors contributing to racial disparities in these adverse birth outcomes, based on results from the decomposition analysis in this study, was mother’s education, which explained about 10% of the observed Black-White difference in LBWBs. This is consistent with findings from a previous study where it was suggested that between 13% and 28% of racial differences in LBWBs were explained by racial differences in endowments across included measures and the most important factor driving racial differences in LBWBs turned out to be SES (Lhila and Long, 2012). Women with higher education usually not only have better knowledge of prenatal care, they also tend to have more resources and support including higher income and better access to prenatal care, both of which can help explain their better birth outcomes. Results from this study revealed that 26.1% of NHB women had an education below high school as compared to 7.1% among NHW women, and the odds of having LBWBs or PTBs increased with each reduced level of education. By the time of pregnancy, educational attainment as measured by degree or diploma might no longer be a modifiable risk factor for many women. Improving educational attainment of NHB women and narrowing their gap with NHW women in this area thus requires a life course perspective whereby the socioeconomic disadvantages of NHB women can be addressed early in life (Lu et al., 2010).
Differences in health insurance coverage between NHW and NHB women turned out to be the second most important factor in explaining differences in LBWBs between the two groups, only after mother’s education. Health insurance coverage is important for access to health services; it can also serve as a proxy of economic status since eligibility for Medicaid is primarily based on household income. Over two thirds of NHB women in this study were covered by Medicaid as compared to one quarter among NHW women, which points to the importance of reducing the poverty rate among NHB women in improving their birth outcomes and reducing the racial gap in LBWB rate. The economic divide between White and Black women, as well as its persistence over time, underscores the need of addressing social and structural factors such as structural racism as important drivers of disparities in birth outcomes between the two groups (O’Brien et al., 2020).
Despite all the factors considered in the analysis, the majority of the differences in birth outcomes between NHB and NHW women remains unaccounted for. Similar findings were also reported from previous studies using the same methodology (Lhila and Long, 2012, Pitts et al., 2011). For example, in a study of Black-White disparities in birth outcomes using Vital Statistics birth records from the State of Georgia for the period 1994 to 2002, only 16% of the racial gap in LBWBs was explained by covariates included in the decomposition analysis (Pitts et al., 2011). Racial differences in LBWBs were also observed in extreme low-risk U.S. populations (Alexander et al., 1999). These findings point to the relevance of developing race-specific definitions of LBWBs based on physiological variations in fetal size and growth across racial groups (Gardosi, 2014), which might further our understanding of racial differences in LBWBs. More attention should also be paid to chronic stress associated with institutional and perceived racism, which was shown to be linked with adverse birth outcomes among women of color (Dominguez, 2008, Dominguez et al., 2008, Earnshaw et al., 2013).
The covariates included in the decomposition analysis accounted for more of the gaps between NHW and Hispanic women than they did in the case of the gaps between NHW and NHB women. Some of the most important factors contributing to the gap in LBWB rate between NHW and Hispanic women included education, tobacco use during pregnancy, and health insurance coverage. The relative socioeconomic disadvantages among Hispanic women, as indicated by lower education and higher poverty rate as proxied by Medicaid coverage, need to be addressed in order to reduce their LBWB rate. Relative to NHW women, one of the protective factors for Hispanic women was their lower rate of tobacco use during pregnancy, which mitigated the gap in LBWB rate between the two groups. This also implies that the relative advantage in LBWB rate among NHW women could have been more substantial had they managed to reduce their tobacco use during pregnancy to the level of Hispanic women.
In terms of the difference in PTB rate between NHW and Hispanic women, the dominant factor was education, accounting for 62.7% of the difference, followed by health insurance coverage and marital status. This again underscores the importance of improving socioeconomic status of Hispanic women in reducing their gap with NHW women in PTB rate. This issue will become more important with the increasing Hispanic population in Nebraska and their high rates of poverty and uninsurance. It was estimated that among the 190,000 Hispanics living in Nebraska (10% of the state population) in 2014, one quarter of them did not have health insurance and this rate of uninsurance became 49% among foreign-born Hispanics (Demographic profile of Hispanics in Nebraska, 2014). This was partially reflected in this study whereby 9.2% of Hispanic women in the study had to pay for their health insurance themselves, as compared to 2.5% among NHW women and 3.7% among NHB women. Besides lower income and lack of health insurance coverage, many Hispanic women, especially foreign-born Hispanic women had to overcome language barriers and potential acculturative stress before they could effectively navigate the health care system in the U.S. for prenatal care (Fuentes-Afflick et al., 2014).
7. Limitations of the study
As a state, Nebraska is not as racial diverse as many other states. Cautions should be taken before generalizing findings from this study to other states or the national level. The rate of preterm births in our sample (9.4%) comes a bit lower than corresponding estimates of 10% at the national level (Martin et al., 2019).
Numerous factors at the genetic, biological, demographic, social, economic, psychological, environmental, and medical level can impact birth outcomes and their disparities across racial/ethnic groups. Due to data restrictions, this study was only able to include a limited number of factors primarily focusing on demographics, SES, health insurance coverage, smoking during pregnancy, and health status prior or during pregnancy. Research is needed to incorporate more factors into the decomposition analysis, including factors on exposure to racism and psychosocial distress, to better account for racial and ethnic disparities in PTBs and LBWBs. This is especially important for identifying key factors contributing to Black-White disparities in LBWBs, most of which remained unexplained.
Despite their common use in health disparity research, race and ethnicity are imprecise, socially defined constructs with important limitations (Kaplan and Bennett, 2003). In our study, the variable of race and ethnicity was used as a risk marker, not a risk factor. We did not consider sub-group differences within each of the three major racial and ethnic groups considered, including potential differences based on nativity, which can be a topic of future research along this line.
Measurement errors cannot be ignored in analysis of birth outcomes using vital records. This is especially important when it comes to ensuring the accuracy of key measurements such as gestational age, birthweight, and smoking during pregnancy. In this study while we removed extreme outliers associated with gestational age and birthweight from our analysis, we could not find any documentation in the birth records that would allow us to verify or assess the accuracy of the data. Smoking during pregnancy could have been underreported due to potential social desirability bias. Future studies can evaluate the robustness of study findings by factoring in measurement errors or by using measures less prone to measurement errors (e.g., use cotinine assays for smoking status instead of reliance on self-report data).
8. Conclusions
Among the various factors included in this study, mother’s education contributed the most to the observed racial/ethnic differences in PTB and LBWB rates, followed by health insurance coverage. Addressing these socioeconomic disadvantages in NHB and Hispanic women would be important for them to narrow their gap with NHW women in LBWB and PTB rates. More research is needed to identify key factors leading to the disparities in birth outcomes between NHW and NHB women.
Acknowledgements
This study was supported by the Grant or Cooperative Agreement Number B01 OT009067, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Centers for Disease Control and Prevention or the Department of Health and Human Services. We want to thank Dong Liu, Ashvita Garg, Ming Qu, Peibei Sun, Norm Nelson, and Mark Miller for their assistance with data accessing and coding.
References
- Hoyert DL & Xu J. Deaths: preliminary data for 2011. National Vital Statistics Reports. 2012. http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. Accessed 23 July 2019. [PubMed]
- Martin J.A., Hamilton B.E., Osterman M.J.K., Births D.AK. vol 68, no 13. National Center for Health Statistics; Hyattsville, MD: 2019. (Final data for 2018. National Vital Statistics Reports). [PubMed] [Google Scholar]
- Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse S.D., Waitzman N.J., Yang N., Abe K., Barfield W.D. Employer-sponsored plan expenditures for infants born preterm. Pediatrics. 2017;140(4):e20171078. doi: 10.1542/peds.2017-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrady G.A., Sung J.F., Rowley D.L., Hogue C.J. Preterm delivery and low birth weight among first-born infants of black and white college graduates. Am J Epidemiol. 1992;136(3):266–276. doi: 10.1093/oxfordjournals.aje.a116492. [DOI] [PubMed] [Google Scholar]
- Orr S.T., Jame S.A., Blackmore P.C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore. Maryland. American Journal of Epidemiology. 2002;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- Ventura S.J., Martin J.A., Curtin S.C., Menacker F., Hamilton B.E. Births: final data for 1999. Natl Vital Stat Rep. 2001;49(1):1–100. [PubMed] [Google Scholar]
- Martin J.A., Hamilton B.E., Osterman M.J.K. Births: final data for 2015. National Vital Statistics. Report. 2017;66 [PubMed] [Google Scholar]
- Krieger N. Women and social class: A methodological study comparing individual, household, and census measures as predictors of black/white differences in reproductive history. Journal of Epidemiology and Community Health. 1991;45(1):35–42. doi: 10.1136/jech.45.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenshine P., Egerter S., Barclay C.J., Cubbin C., Braveman P.A. Socioeconomic disparities in adverse birth outcomes: a systematic review. American Journal of Preventive Medicine. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Copper R.L., Goldenberg R.L., Das A., Elder N., Swain M., Norman G., Ramsey R., Cotroneo P., Collin B.A., Johnson F. The preterm prediction study: Maternal stress is associated with spontaneous pretrem birth at less than 35 weeks' gestation. Am J Obstet Gynecol. 1996;175:1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- Dole N., Savitz D.A., Hertz-Picciotto I., Siega-Riz A.M., McMahon M.J., Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62(1):531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Lu M.C., Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. 2004;191(3):691–699. doi: 10.1016/j.ajog.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Dominguez T.P. Race, racism, and racial disparities in adverse birth outcomes. Clinical obstetrics and gynecology. 2008;51(2):360–370. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]
- Dominguez T.P., Dunkel-Schetter C., Glynn L.M., Hobel C., Sandman C.A. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association. 2008;27(2):194–203. doi: 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw V.A., Rosenthal L., Lewis J.B., Stasko E.C., Tobin J.N., Lewis T.T., Reid A.E., Ickovics J.R. Maternal Experiences with Everyday Discrimination and Infant Birth Weight: A Test of Mediators and Moderators Among Young, Urban Women of Color. Annals of Behavioral Medicine. 2013;45(1):13–23. doi: 10.1007/s12160-012-9404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel C., Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Current opinion in psychiatry. 2012;25(2):141. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.W., David R.J., Handler A., Wall S., Andes S. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. Am J Public Health. 2004;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y. A systematic review of empirical research on self-reported racism and health. Int J Epidemiol. 2006;35(4):888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Salihu H.M., Wilson R.E. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83(11):713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Bernstein I.M., Plociennik K., Stahle S., Badger G.J., Secker-Walker R. Impact of maternal cigarette smoking on fetal growth and body composition. Am J Obstet Gynecol. 2000;183(4):883–886. doi: 10.1067/mob.2000.109103. [DOI] [PubMed] [Google Scholar]
- Pitts M., Walker M., Armour B. A decomposition of the black-white differential in birth outcomes. Federal Reserve Bank of Atlanta Working Paper Series. 2011;1 [Google Scholar]
- Pollack H., Lantz P.M., Frohna J.G. Maternal smoking and adverse birth outcomes among singletons and twins. Am J Public Health. 2000;90(3):395–400. doi: 10.2105/ajph.90.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E. Putnam-Hornstein J.J. Prindle J.M. Leventhal Prenatal Substance Exposure and Reporting of Child Maltreatment by Race and Ethnicity Pediatrics. 138 3 2016 e20161273 e20161273. [DOI] [PubMed]
- Sarah C.M. Roberts, Amy A. Mericle, Meenakshi S. Subbaraman, Sue Thomas, Ryan D. Treffers, Kevin L. Delucchi, and William C. Kerr.Health Equity.Dec 2018.356-365. [DOI] [PMC free article] [PubMed]
- Guendelman S., Thornton D., Gould J., Hosang N. Obstetric complications during labor and delivery: assessing ethnic differences in California. Womens Health Issues. 2006;16(4):189–197. doi: 10.1016/j.whi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Lawrence J.M., Contreras R., Chen W., Sacks D.A. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5):899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- Rauh V.A., Andrews H.F., Garfinkel R.S. The contribution of maternal age to racial disparities in birthweight: a multilevel perspective. American Journal of Public Health. 2001;91(11):1815–1824. doi: 10.2105/ajph.91.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A.T. African American/White differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. 1996;42:589–597. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Simhan H.N., Krohn M.A., Roberts J.M., Zeevi A., Caritis S.N. Interleukin-6 promoter -174 polymorphism and spontaneous preterm birth. Am J Obstet Gynecol. 2003;189(4):915–918. doi: 10.1067/s0002-9378(03)00843-3. [DOI] [PubMed] [Google Scholar]
- Velez D.R., Fortunato S., Thorsen P., Lombardi S.J., Williams S.M., Menon R. Spontaneous preterm birth in African Americans is associated with infection and inflammatory response gene variants. Am J Obstet Gynecol. 2009;200(2):209.e1–209.e27. doi: 10.1016/j.ajog.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M.R., Hogue C.R. What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiologic Reviews. 2009;31(1):84–98. doi: 10.1093/ajerev/mxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthy People, 2020a. https://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives. (Accessed 25 July 2020).
- Healthy People Maternal, Infant, and Child Health. 2020 https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Maternal-Infant-and-Child-Health/data Accessed 24 June 2019. [Google Scholar]
- Ickovics J.R., Kershaw T.S., Westdahl C., Rising S.S., Klima C., Reynolds H., Magriples U. Group prenatal care and preterm birth weight: results from a matched cohort study at public clinics. Obstet Gynecol. 2003;102(5, Part 1):1051–1057. doi: 10.1016/s0029-7844(03)00765-8. [DOI] [PubMed] [Google Scholar]
- Kotelchuck M. The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. American Journal of Public Health. 1994;84(9):1486–1489. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S.T., McClure L.A., Zaitchik B.F., Gohlke J.M. Area-level risk factors for adverse birth outcomes: trends in urban and rural settings. BMC Pregnancy Childbirth. 2013;13:129. doi: 10.1186/1471-2393-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaxaca R. Male-female wage differentials in urban labor markets. International Economic Review. 1973;14(3):693. doi: 10.2307/2525981. [DOI] [Google Scholar]
- O'Donnell O. et al. Explaining Differences between Groups: Oaxaca Decomposition. Chapter 12 in Analyzing Health Equity Using Household Survey Data: A Guide to Techniques and Their Implementation. World Bank Publications. 2008. pp. 147–158.
- Sen B. Using the Oaxaca–Blinder decomposition as an empirical tool to analyze racial disparities in obesity. Obesity. 2014. 22(7):1750–1755, PMID: 24733610,https://doi.org/10.1002/oby.20755. [DOI] [PubMed]
- Lhila A., Long S. What is driving the black-white difference in low birthweight in the U.S.? Health Economics. 2012;21(3):301–315. doi: 10.1002/hec.1715. [DOI] [PubMed] [Google Scholar]
- Benmarhnia T., Huang J., Basu R., Wu J., Bruckner T.A. Decomposition Analysis of Black-White Disparities in Birth Outcomes: The Relative Contribution of Air Pollution and Social Factors in California. Environ Health Perspect. 2017;125(10):107003. doi: 10.1289/EHP490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack L.S., Rossen L.M., Martin J.A. National Center for Health Statistics; Hyattsville, MD: 2018. Singleton low birthweight rates, by race and Hispanic origin: United States, 2006–2016 NCHS Data Brief, no 306. [PubMed] [Google Scholar]
- Martin J.A., Hamilton B.E., Osterman M.J.K., Driscoll A.K., Births D.P. vol 67 no 1. National Center for Health Statistics; Hyattsville, MD: 2018. (Final data for 2016. National Vital Statistics Reports). [PubMed] [Google Scholar]
- Su D., Samson K., Garg A., Hanson C., Anderson Berry A.L., Lin G.e., Qu M. Birth history as a predictor of adverse birth outcomes: Evidence from state vital statistics data. Prev Med Rep. 2018;11:63–68. doi: 10.1016/j.pmedr.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge N.M., Mudd L.M., Sikorskii A., Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133(5):844–853. doi: 10.1542/peds.2013-3285. [DOI] [PubMed] [Google Scholar]
- M.S. Kramer R.W. Platt S.W. Wen K.S. Joseph A. Allen M. Abrahamowicz B. Blondel G. Breart A new and improved population-based Canadian reference for birth weight for gestational age Pediatrics 108 2 2001 e35 e35. [DOI] [PubMed]
- Alexander G.R., Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public health reports. 1996;111(5):408. [PMC free article] [PubMed] [Google Scholar]
- Fairlie R.W. The absence of the African-American owned business: An analysis of the dynamics of self-employment. Journal of Labor Economics. 1999;17(1):80–108. [Google Scholar]
- Fairlie R., Robb A. Why are black-owned businesses less successful than white-owned businesses: The role of families, inheritances, and business human capital,“. Journal of Labor Economics. 2007;25(2):289–323. [Google Scholar]
- Pagán J.A., Su D., Li L., Armstrong K., Asch D.A. Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med. 2009;37(6):524–530. doi: 10.1016/j.amepre.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Fairlie, RW. Addressing Path Dependence and Incorporating Sample Weights in the Nonlinear Blinder-Oaxaca Decomposition Technique for Logit, Probit and Other Nonlinear Models. SIEPR Discussion Paper No. 17-013. 2017. https://siepr.stanford.edu/sites/default/files/publications/17-013.pdf Accessed July 7, 2020.
- Lu M.C., Kotelchuck M., Hogan V., Jones L., Wright K., Halfon N. Closing the Black-White gap in birth outcomes: a life-course approach. Ethn Dis. 2010;20(1 Suppl 2):S2. [PMC free article] [PubMed] [Google Scholar]
- O’Brien R, Neman T, Seltzer N, Evans L, Venkataramani A. Structural racism, economic opportunity and racial health disparities: evidence from U.S. counties. SSM Popul Health 2020;11:100564. [DOI] [PMC free article] [PubMed]
- Alexander G.R., Kogan M.D., Himes J.H., Mor J.M., Goldenberg R. Racial differences in birthweight for gestational age and infant mortality in extremely low risk US populations. Paediatr Perinat Epidemiol. 1999;132:205–217. doi: 10.1046/j.1365-3016.1999.00174.x. [DOI] [PubMed] [Google Scholar]
- Gardosi J. Fetal growth and ethnic variation. Lancet Diabetes Endocrinol. 2014;2(10):773–774. doi: 10.1016/S2213-8587(14)70188-3. [DOI] [PubMed] [Google Scholar]
- Demographic profile of Hispanics in Nebraska, 2014. Pew Research Center. 2014. https://www.pewhispanic.org/states/state/ne/. Accessed 27 June 2019.
- Fuentes-Afflick E., Odouli R., Escobar G.J., Stewart A.L., Hessol N.A. Maternal acculturation and the prenatal care experience. Journal of Women's Health. 2014;23(8):688–706. doi: 10.1089/jwh.2013.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.B., Bennett T. Use of Race and Ethnicity in Biomedical Publication. JAMA. 2003;289(20):2709–2716. doi: 10.1001/jama.289.20.2709. [DOI] [PubMed] [Google Scholar]



