Abstract
Resistance among pathogenic bacteria to the existing antibiotics is one of the most alarming problems of the modern world. Alongwith reducing the use of antibiotics, and antibiotic stewardship, an alternative to antibiotics is much needed in the current scenario to combact infectious diseases. One alternative is to produce nanomaterials, especially, silver nanoparticles (AgNPs) against antibiotic-resistant bacteria. AgNPs are the most vital and fascinating nanoparticles because of their unique structural and functional properties and application against pathogenic bacteria. However, the synthesis of AgNPs remains a problem because of the chemicals and energy requirements and the byproducts of the reactions. Concerns have been raised about using chemically and physically synthesized nanoparticles because of their potential risks to the human body, animals, and environment. Green synthesis of these nanoparticles is a better alternative to physical and chemical approaches. Plant-based synthesis in turn is a method which can provide AgNPs that are cost-effective and eco-friendly as well as biocompatible. The specific features of size, morphology and shape of plant-based AgNPs give them the potency to fight multi-drug resistant bacteria. A detailed look into mechanistic aspects of the action of AgNPs against resistant bacteria with a focus on characteristic properties of AgNPs is required. This review discusses in detail these aspects and the potential of plant-based AgNPs as a solution to antibiotic resistance.
Keywords: Biogenic silver nanoparticles, Multi-drug resistant bacteria, Natural extracts
Biogenic silver nanoparticles; Multi-drug resistant bacteria; Natural extracts.
1. Introduction
The world health organization has declared antimicrobial resistance (AMR) among the top 10 global public health threats to human life (WHO, 2016). According to the WHO, a growing list of infections – including throat infections, foodborne diseases, tuberculosis, gonorrhoea, and pneumonia are offering harder fronts to antibiotics during the fight of these antibiotics against the causative agents of these infections. According to the United States Pharmacopeia, approximately 700,000 deaths per year occurs due to antimicrobial resistance which is likely to grow to 10 million by the year 2050 (Betts et al., 2018). Apart from deaths, antibiotic resistance is related to the burden on hospitals and thus the ultimate economic burden. Antibiotic resistant infections have been reported to cause 2.5 million hospital days in European union and 3.2 million hospital days in Thailand (ECDC, 2009).
Therefore, there comes a much-needed call for alternatives to antibiotics. Scientists have aimed at developing alternate techniques to address this challenge (Singh et al., 2020). Among the many advances made during this century, nanomaterial have found to be a better option in fighting against the multi-drug resistant bacteria (Kim et al., 2007) because of their noticeable properties like their large surface area to volume ratio (Khalil et al., 2013). The property of having large surface area to volume ratio enhances their affinity to membrane. Furthermore, the affinity towards the negatively charged membrane increases because of the presence of positive charge on the nanoparticles. Bacteria cannot easily become resistant to nanoparticles as nanoparticles at the same time target numerous routes for killing bacteria (Singh et al., 2020). Metallic nanoparticles including silver nanoparticles (AgNPs) are the best studied nanoparticles having the potential to address the problem of antibiotic resistance (Gemmell et al., 2006). Silver nanoparticles (AgNPs) are known to have key applications in the research area of multi-decipilines such as gene delivery in molecular biology, drug delivery in pharmaceutical industry, use in biosensors mainly in biochemistry, environmental sciences for handling the problems of pollution and agricultural sciences. AgNPs are now used in novel ways in the form of nanomedicine due to the fact that AgNPs posses unique physical and chemical properties (Gurunathan et al., 2015). Also AgNPs have good anti-fungal, anti-viral and anti-inflammatory properties (Ahmad et al., 2003) and above all they are very good antibacterial agents (Khalil et al., 2013) (Baruwati et al., 2009; Popescu et al., 2010). Silver nanoparticles (AgNPs) have characteristic features of low cytotoxicity and large surface area that proves AgNPs the best agents against both gram positive and gram negative bacteria (Biel et al., 2011). However, several studies have reported that cytotoxicity of these nanoparticles depends upon the exposure time and dose concentration. It has been identified that size and shape of nanoparticles also play part in cellular toxicity. Size of nanoparticles have been reported to be inversely related to cytotoxicity. Spherical shaped nanoparticles have found to have an average lethal dose ranging from 1 to 20 μg/ml while hexagonal, rod, and triangular shaped nanoparticles were found to be less potent (Hanan et al., 2018). To address the problem of multi-drug resistant bacteria, use of AgNPs formulations against the bacteria that have already developed multi-drug resistance is the best alternative to the use of antibiotics against such bacteria based on the distinctive features that AgNPs hold. However, there has been a debate about the safety an environmental concerns of AgNPs. In this review, we have mainly focused on the antibacterial aspects of using plant-based AgNPs against resistant bacteria.
2. Antibiotic resistant bacteria; a simple introduction
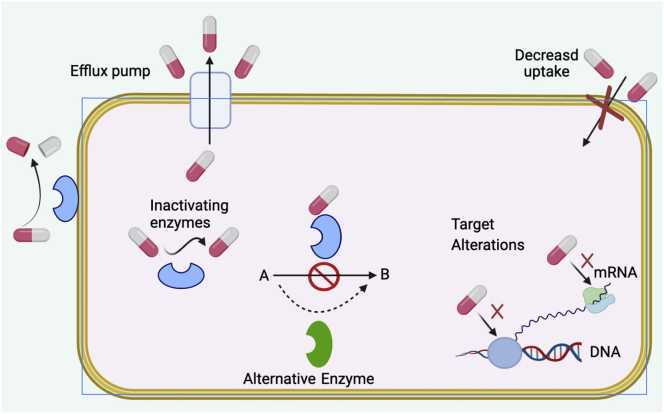
Different classes of antibiotics are used to fight against infectious microbes. Antibiotics eliminate such microbes through various antimicrobial mechanisms such as interference in transcription and translation, inhibition of enzymes, and alteration of membrane structure (Kohanski et al., 2010). However, some microbes have developed antibiotic resistance (Padiyara et al., 2018). Antibiotic resistance is one of the major cause of lack of efficiency of antimicrobial agents. This leads to consumption of higher drug doses, longer stays in hospitals, higher toxicity, and increased mortality (Pelgrift and Friedman, 2013). Various factors including over-prescription of antibiotics, misuse of antibiotics, overuse of antibiotics, their widespread agricultural use, and availability of fewer new antibiotics contribute towards antibiotic resistance (Ventola and therapeutics, 2015). are Among the recently known microorganisms that have developed resistance are Vancomycin resistant Staphylococcus aureus, Enterococcus faecum, and Enterococcus faecalis (Cetinkaya et al., 2000), carbapenem-resistant Enterobacteriaceae, penicillin-resistant Streptococcus pneumonia, and multidrug-resistant Acinetobacter baumannii, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Salmonella enterica and Vibrio cholera (Betts et al., 2018). Some of the possible known mechanisms used by bacteria to develop antibiotic resistance include decreased uptake and increased efflux pumps, acquiring and expression of drug resistant genes, modification of antimicrobial target, alteration of antimicrobial drugs through the development of drug degrading enzymes, production of competitive inhibitors, emergence of persister cells, biofilm formation, and swarming (Figure 1) (Blecher et al., 2011). These mechanisms ultimately cause lesser accumulation of antibiotics in bacterial cells which in turn lower the therapeutic level of the drug (Huh and Kwon, 2011). Thus, a higher and repeated dose of drugs will be required which leads to harmful effects both on humans and animals.
Figure 1.
Diagrammatic representation of the mechanism of antibiotic resistance in bacteria.
3. Solutions to antibiotic resistance
In order to tackle these problems, new drugs need to be developed. Scientists are now interested in rapid diagnosis and targeted therapy. This could be achieved by either modifying or completely avoiding the utilization of conventional antibiotics. Metal nanoparticles such as copper, silver, titanium, and zinc could be the possible alternative. These nanoparticles are antimicrobial in nature. Metals use different mechanisms i.e. damage to DNA and protein, membrane degradation, and generation of reactive oxygen species to kill microorganisms (Ahn et al., 2018). Metal nanoparticles possess unique tunable physical properties such as size and shape. Bulk metals can be transformed to metal nanoparticles (Singh et al., 2017b). This transformation not only reduces the size but also results in the formation of different shape nanoparticles such as spherical, octahedral, rod, and triangular shaped (Singh et al., 2015). These geometrical variations are very useful for antimicrobial applications as the antimicrobial activity of nanoparticles is dependent on the surface area available for biological components interactions. Therefore, metallic nanoparticles, especially, AgNPs have become one of the highly promising alternative to combat antibiotic resistance and fight resistant microbes (Huh and Kwon, 2011). Different studies have reported the antimicrobial applications of both biologically and chemically synthesized metallic nanoparticles. The results have shown that biologically synthesized nanoparticles possess higher antimicrobial effect as compared to chemically synthesized nanoparticles (Singh et al., 2016).
4. Why green synthesis of nanoparticles?
Metallic nanoparticles are more promising antimicrobial agents due to their large surface area to volume ratio (Gong et al., 2007). Among the different types of metal nanoparticles, magnesium, gold (Gu et al., 2003), copper, titanium, zinc (Schabes-Retchkiman et al., 2006), alginate (Ahmad et al., 2006), and silver nanoparticles (Rai et al., 2009) have been shown to possess strong antibacterial activities. Chemically synthesized nanoparticles use chemicals as a reducing agent, hence converting Ag ion to AgNPs. But because of the harmful effects and the low biocompatibility of the chemically synthesized nanoparticles, green synthesis of the nanoparticles is favored. Green synthesized nanoparticles are eco-friendly reducing agents (Park, 2014a). There is an ongoing debate about the toxic effects of AgNPs. The debate focus on whether chemically or physically synthesizing these nanoparticles especially AgNPs is safe or not. To address this issue, biologically synthesis of AgNPs is considered.
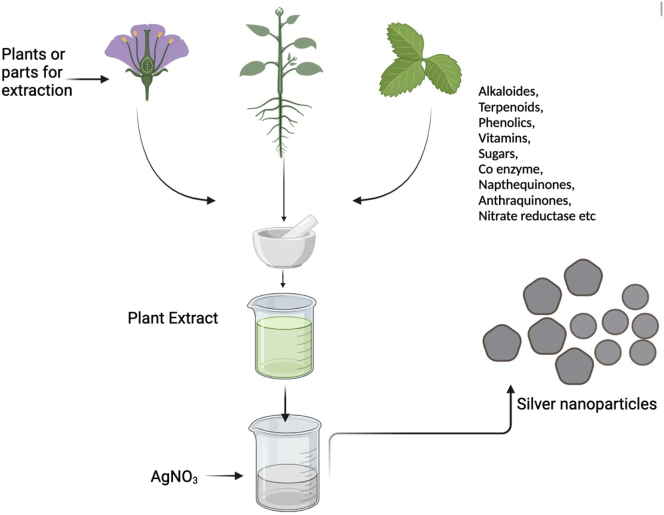
In the biogenic synthesis of AgNPs, plant parts are used to prepare an extract and the process is performed in a hot or cold solvent. Compounds found in plants such as ascorbic acid, reducing sugars, citric acid amino acids, alkaloids, terpenoids, and flavonoids present in different parts of plants acts as reducing agent, quickly converting Ag + to AgNPs (Barros et al., 2018). Carson et al. experimented with the biogenic synthesis of AgNPs from a medicinal plant. They prepared an aqueous extract from Dulcis trev (Verbenaceae) to synthesize AgNPs using microwave irradiation and its formation was confirmed by ultraviolet-visible spectroscopy. Other characterization techniques included X-ray photoelectron spectroscopy, Transmission electron microscopy and X-ray diffraction (Carson et al., 2020).
AgNPs based on living extracts or biosynthesized AgNPs have an advantage of being eco-friendly, clean and no use of toxic chemicals in the production process. Other advantages are this method being simple enough and the one-pot reaction, amenability to scale up, elimination of the toxic chemicals, increased biocompatibility, enhanced colloidal stability and the cost-effectivity. Thus, biosynthesized AgNPs are preferred over the chemical and physical methods of AgNPs production. In addition, they do not involve the use of use of abiotic factors such as maintaining high temperature, pressure maintenance, and use of energy. Bacteria, fungi, plants, plant-based compounds, algae, carbohydrates, and microorganisms are among the main source for the synthesis of green nanoparticles (Park, 2014b). The extracts of these organisms contain protein, amino acid, enzymes, carbohydrates, vitamins, and secondary metabolites which take part in AgNPs synthesis as both reducing and capping agents.
Plants are, in turn, better synthesizers among the other biological approaches owing to the amount of plant resources available. Plants also provide a superior platform for nanoparticle manufacturing. They contain harmless compounds and act as natural capping agents. Furthermore, the use of plant extracts lowers the cost of microbe isolation and culture medium, improving the cost-competitive viability of microorganism-based nanoparticle production (Sharma et al., 2009). The benefit of employing plants for nanoparticle production is that they are readily available, safe to handle, and contain a wide range of secondary metabolites. Several plants are now being researched for their function in nanoparticle production.
5. Silver nanoparticles (AgNPs) as a solution to antimicrobial resistance
AgNPs is very a viable alternative solution to antimicrobial resistance. A plethora of studies have confirmed that AgNPs possess enormous potential against pathogenic bacteria. For instance, in a study by Jia et al. (2007), AgNPs were mixed with Poly (Vinyl Alcohol) (PVA) nanofibers and the antibacterial properties were assessed against Staphylococus aureus and Escherchia coli. A significant reduction in bacterial activity confirmed that the nanoparticles coated fibers are efficient antibacterial agents. Similarly, in another study, AgNPs were chemically synthesized using silver nitrate and sodium borohydride and its antimicrobial activity was evaluated against S. aureus and E. coli wherein they observed marked activity of these nanoparticles especially against pathogenic E. coli (Kim et al., 2007). AgNPs have also been shown to be potent against biofilm formation in bacteria. Biofilms are structure developed when bacterial cells adhere to surfaces and make a conjugate. Palanisamy et al. (2014) investigated the antibacterial properties of chemically synthesized AgNPs in the formation of biofilms in P. aeruginosa. Results from the resistant strain indicated that 20 μg/mL of AgNPs inhibited growth with an inhibition rate of 56% in biofilm forming P. aeruginosa. This suggests that AgNPs can be used as an alternative to combat P. aeruginosa infections. Despite the properties discussed above, chemically synthesized AgNPs, however, possess problems of the use of potentially toxic environmentally unsafe chemicals. We will now highlight the importance of plants in conferring antibacterial properties to AgNPs.
5.1. Contribution of plants to the properties of antibacterial AgNPs
Medicinally important biomolecules such as amino acids, proteins, vitamins, alkaloids, phenolics, saponins, tannins and terpenoids present in the plant extracts are found to be involved in the reduction as well as stabilization of silver ions (Kulkarni and Muddapur, 2014). Variety of plants and plant extracts have been reported to facilitate the synthesis of AgNPs (Table 1). We will discuss different plants used and the influence they put on the different properties of these AgNPs (Figure 2).
Table 1.
AgNPs synthesis in different plants, their parts used and the morphological characteristics of biosynthesized AgNPs.
| Plants | Part | Shape | Size | Reference |
|---|---|---|---|---|
| Mentha pulegium | Leaves | Anisotropic | 5–50 nm | (Abd Kelkawi et al., 2016) |
| Styrax benzoin | Plant extract | Spherical | 12–38 nm | (Du et al., 2016) |
| Acorus Calamus | Rhizome | Spherical | 59 nm | (Nayak et al., 2015) |
| Ficus religiosa | Leaves | Spherical | 21 nm | (Nakkala et al., 2017) |
| Iresine herbstii | Leaves | Cubic | 44–64 nm | (Dipankar and Murugan, 2012) |
| Prunus persica | Plant extract | Spherical | 40–98 nm | (Kumar et al., 2017) |
| Aloe vera | Leaves | Spherical | 15.2 nm | (Chandran et al., 2006) |
| Carica papaya | Fruit | Cubic | 15 nm | (Jain et al., 2009) |
| Memecylon edula | Leaves | Triangular | 50–90 nm | (Elavazhagan and Arunachalam, 2011) |
| Handelia trichophylla | Plant extract | Spherical | 20–50 nm | (Yazdi et al., 2019) |
| Anacardium occidentale | Leaves | Spherical | 40–60 nm | (Balavigneswaran et al., 2014) |
| Synsepalum Dulcificum | Seed/leaves | Spherical | 4–26 nm | (Lateef et al., 2016) |
| Alphonsea sclerocarpa | Plant extract | Irregular | 33 nm | (Doddapaneni et al., 2018) |
| Solanum lycopersicum | Plant extract | Cubic | 21.11 nm | (Baran and Hilal, 2019) |
| Salvia officinalis | Leaves | Spherical | 41 nm | (Okaiyeto et al., 2020) |
Figure 2.
Schematic representation of the synthesis of AgNPs in plants.
5.1.1. Characterization of antibiotic silver nanoparticles
For characterization of AgNPs different types of analytical techniques are used including UV-vis spectroscopy, X-ray diffraction, Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). UV-vis spectroscopy is usually used to monitor the synthesis of AgNPs through the combined oscillation of electrons on their surface in resonance with incident light. This in turn gives surface plasmon resonance (SPR) absorption bands in the range of wavelength (400–500 nm) which are indictors of the presence of AgNPs (Raj et al., 2018). AgNPs synthesized using mustard seed extract have been characterized through UV-vis spectroscopy by Khatami et al. (2015). They studied the reaction mixture of seed extract and silver nitrate under UV-vis spectrophotometer to monitor the AgNPs synthesis. The maximum SPR peak was indicated in the range of 411 nm. Most recently the synthesis of AgNPs using leaf extract of plants including Tridax procumbens, Euphorbia hirta, and Azadirachta indica is confirmed by Surega et al. (2020) through analysis of absorption band obtained in the range of 400–500 nm.
For analysis and determination of the size and morphology of AgNPs, TEM and SEM are employed. Studies on the synthesis of AgNPs employ these techniques for the accurate determination of size and shape of these nanoparticles. For instance, Raj et al. (2018) employed TEM to identify the nanocrystalline structure of silver particles synthesized by using leaf extract of Enicostemma axillare. TEM image revealed that AgNPs were spherical with an average size of 18.12 nm having a thin organic layer coating acting as a capping agent. Field emission scanning electron microscopy is also used to characterize the surface morphology of AgNPs. Hemmati et al. (2019) observed the size and structure of AgNPs in colloidal solutions by studying both TEM and SEM micrograph pictures. These AgNPs were synthesized using the flower extract of Fritillaria. SEM image showed that larger particles are formed owing to agglomeration of AgNPs and TEM revealed the spherical morphology of these AgNPs with size ranging from 5 to 10 nm. In order to determine what functional groups play role in the fabrication of AgNPs, FTIR is applied for the biosynthesized AgNPs. In case of plant-based AgNPs, it is based on identification of functional groups of phytochemicals that have played role in reduction of silver salts to AgNPs. In the previously cited study by Hemmati et al. (2019), the biomolecules exploited as reducing and capping agents were analyzed by FTIR. Various peaks observed indicated the stability of nanoparticles. It has also been used to determine the interaction between AgNPs and protein molecules. FTIR analysis has demonstrated the occurrence of likely functional groups of phytochemicals contained in plant extracts exploited for the synthesis of AgNPs (Surega et al., 2020). For the confirmation of crystal structure, AgNPs are subject to XRD analysis. Ashraf et al. (2016) studied the crystal structure of biosynthesized AgNPs through XRD and revealed it to be face-centered cubic.
5.1.2. The role of AgNPs in antibiotic resistance is a function of their important properties
The antimicrobial activity of silver nanoparticles depends upon several factors including the type of microorganism, pH, temperature (Marambio-Jones and Hoek, 2010), different size, shapes (Zhou et al., 2012), and the concentration of AgNPs and zeta potential (Li et al., 2013; Pal et al., 2007). A general look at the above studies initiate the debate that size and shape among other properties play an important role in the antibacterial activities of the plant-based AgNPs (Table 2). Size and shape tends to be among the most important features of AgNPs which confer upon them their activity against pathogenic bacteria (Figure 3).
Table 2.
Table summarizing different studies reporting antimicrobial activity of plant-based silver nanoparticles (AgNPs).
| Plants name | Plants parts | Size (nm) | Shapes | Bacterial strain | Antibacterial potency | References |
|---|---|---|---|---|---|---|
| Justicia Adhatoda L. | Leaves | 5–50 nm | Spherical | P. aeruginosa | Inhibition the growth of bacteria | (Bose and Chatterjee, 2015) |
| Carica papaya L. | Fruit and leaves | 25–50 nm | Cubic | E. coli and P. aeruginosa | Inhibition the growth of bacteria and disrupting the membrane | (Jain et al., 2009) |
| Artemisia nilagirica | Leaves | 70–90 nm | Square/spherical/hexagonal | S. aureus, B. subtills, E. coli | Inhibition of the growth of bacteria and degradation of the membrane | (Vijayakumar et al., 2013) |
| Trianthema decandra | Roots | 15 nm | Cubic | P. aeruginosa and E. coli | Clear inhibition zone and membrane disrupting | (Geethalakshmi and Sarada, 2010) |
| Emblica officinalis | Fruit | 15 nm | Spherical | Staphylococcus, B. subtilis, E. coli, K. pneumonia | Disrupting cell membrane, permeability, and respiration function of the cell. Also, penetrate the bacteria and cause cell death. | (Ramesh et al., 2015) |
| Crataegus douglasii | Fruit | 29.28 nm | Spherical | S. aureus and E. coli | Produce a clear zone of inhibition in bacteria | (Ghaffari-Moghaddam and Hadi-Dabanlou, 2014) |
| Cleome viscosa . | Fruit | 20–50 nm | Spherical and irregular | S. aureus, B. subtilis, E. coli, K. pneumonia | Produce a clear zone of inhibition in bacteria | (Lakshmanan et al., 2018) |
| Prosopis farcta | Leaves | 10.8 nm | Spherical | S. aureus, B. subtilis, E. coli, P. aeruginosa. | Produce a clear zone of inhibition in bacteria | (Miri et al., 2015) |
| Petroselinum crispum | Leaves | 25–30 nm | Spherical | S. aureus, E. coli, K. pneumonia. | Prevent the growth of bacteria | (Roy et al., 2015) |
| Psidium guajava | Leaves | 25–35 nm | Spherical | A. faecalis and E. coli | Inhibit the growth of bacteria | (Wang et al., 2018) |
| Moringa olefira | Leaves | 9–11 nm | Spherical | E. faecalis, E. coli. S. aureus, P. aeruginosa, and K. pneumoniae. | Reduce the growth of bacteria | (Moodley et al., 2018) |
| Ceropegia thwaitesii | Leaves | 100 nm | Spherical | S. typhia and B. subtilis | Inhibit the growth of bacteria | (Muthukrishnan et al., 2015) |
| Holoptelea integrifolia | Leaves | 32–38 nm | Cubic | E. coli and S. typhimurium | Inhibit the growth of bacteria | (Kumar et al., 2019) |
| Curcuma longa L | Leaves | 15–40 nm | Spherical | P. aeruginosa, E. coli, and S. aureus | Reduce the growth and also kill the bacteria | (Maghimaa and Alharbi, 2020) |
| Aegle marmelos | Fruit | 159–181 nm | Cubic | P. aeruginosa, B. cereus, and S. dysenteries | Inhibit the growth of bacteria | (Devi et al., 2020) |
| Terminalia bellerica | Fruit | 10 nm | Spherical | K. pneumoniae and P. aeruginosa | Inhibit the growth of bacteria | (Andra et al., 2019) |
| Artemisia marschalliana | Stem, fruit, leaves | 5–50 nm | Spherical | B. cereus, A. baumannii, P. aeruginosa and S. aureus | Inhibit the growth of bacteria | (Salehi et al., 2016) |
| Borago officinalis | Leaves | 30–80 nm | Spherical | S. aureus, v. parahaemolyticus, P. aeruginosa, and E. coli | A clear zone of inhibition and led bacteria to death | (Singh et al., 2017a) |
| Solanum trilobatum | Fruit | 12.50–41.90 nm | Spherical | E. faecalis, K. pneumoniae, S. mutans, and E. coli | Inhibit the growth of bacteria | (Ramar et al., 2015) |
| Ocimum basilicum | Leaves | 3–25 nm | Spherical | S. aureus, P. aeruginosa, and E. coli. | Produce a clear zone of inhibition against bacteria | (Malapermal et al., 2017) |
| Pimpinella anisum | Seed | 3–16 nm | Spherical | S. pyogenes, A baumannii, K. pneumoniae, S typhi, and p. aeruginosa. | Inhibit the growth of bacteria and disrupting their membrane | (AlSalhi et al., 2016) |
| Handelia trichophylla | Aqueous extract | 20–50 nm | Spherical | E. coli, P. aeruginosa, S. aureus and B. subtilis. | Prevent the growth of bacteria | (Yazdi et al., 2019) |
| Terminalia arjuna | Bark extract | 30–50 nm | Spherical | E. coli | Produce a clear zone of inhibition against bacteria | (Ahmed et al., 2017) |
| Morinda citrifolia | Leaves | 10–60 nm | Cubic | E. aerogenes, E. coli, B. subtilis, K. pneumoniae, B. cereus and P. aeruginosa. | Inhibitory action against bacteria | (Sathishkumar et al., 2012) |
| Salvia splendens | Aqueous extract | 15–20 nm | Cubic | P. vulgaris, B. subtills and S. aureus. | Inhibit the growth of bacteria | (Rajendran and Prabha, 2015) |
| Allium cepa | Whole plant | 10–23 nm | Spherical | P. aeruginosa, B. subtilis, | produce a clear zone of inhibition against bacteria | (Gomaa, 2017a) |
| Grewia flaviscences | Leaves | 50–70 nm | Spherical | P. aeruginosa and Bacillus | Attach to the cell membrane of bacteria and disrupt membrane | (Sana et al., 2015) |
| Cannabis sativa | Leaves | 26.52 nm | Spherical | S. aureus, M. luteus, B. subtilis, K. pneumoniae, E. coli | Integrate the cell membrane of bacteria | (Chouhan and Guleria, 2020) |
| Nigella sativa | Seed | 34 nm | Cubic | S. aureus, E. coli, L. monocytogenes, P. aeruginosa | produce a clear zone of inhibition against bacteria | (Vijayakumar et al., 2020) |
| Lysiloma acapulcensis | Aqueous extract | 5 nm | Spherical | S. aureus, E. coli, P. aeruginosa. | produce a clear zone of inhibition against bacteria | (Diana et al., 2020) |
|
Tribulus Terrestris |
Fruit | 16–28 nm | Spherical | P. aeruginosa, S. aureus, E. coli and B. subtilis, | Inhibition the growth of bacteria and disrupting the membrane | (Gopinath et al., 2012a) |
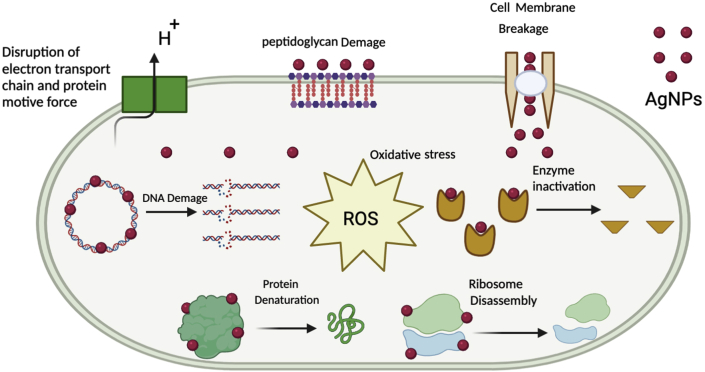
Figure 3.
A schematic representation of the probable mechanism of antimicrobial activity of AgNPs.
5.1.3. Importance of the size of plant-based AgNPs
Smaller nanoparticle having large interactive surface area is proved to be good bactericidal agents than large ones (Kvitek et al., 2008; Morones et al., 2005). The smaller nanoparticles have a greater surface area and is the most important property that affects antimicrobial activity, due to this it can provide high interaction area and give bactericidal effect more than larger particles (Gurunathan et al., 2014; Raza et al., 2016). For example, Li et al. (2013) reported that the antibacterial activity decreases with an increase in the size of the of the nanoparticles. Similarly, Yacamán et al. (2001) observed that the nanoparticles size less than 50 nm have an effective antimicrobial activity. It has been observed that nanoparticles with diameter between 5 to 15 nm have superior antimicrobial activity. According to Sondi and Salopek-Sondi (2004), greater antibacterial activity has been shown for smallest nanoparticles (5 nm). They easily get attached to the cell membrane, damage the cell, and increase the permeability of the membrane, and finally resulting in cell death. Similarly, Morones et al. (2005) reported that nanoparticles having diameter around 10 nm have potent activity against bacteria.
In a study, aqueous extract of Alternathera dentate was used to synthesize spherical shaped AgNPs with diameter of 50–100 nm. These AgNPs showed significant antibacterial activity against E. coli, E. faecalis, Klebsiella pneumonia and P. aeruginosa (Palanisamy et al., 2014). The extracts of Cocous nucifera was used to synthesize AgNPs of diameter 22 nm. These nanoparticles exhibited significant antibacterial activity against Bacillus subtilis, P. aeruginosa, K. pneumoniae, and Salmonella paratyphi (Mariselvam et al., 2014). Euphorbia hirta leaf extract was used to prepare 40–50 nm spherical shaped silver nanoparticles. These NPs were supposed to have strong antibacterial property against S. aureus and B. cereus. The Silver nanoparticles with size of 20–30 nm can be synthesized from the extract of Acalypha indica leaves. The nanoparticles produced had strong activity against V. cholera and E. coli (Krishnaraj et al., 2010). Leaf extract of Moringa oleifera had been used to prepare 57 nm sized silver nanoparticles with high antimicrobial potential against many types of pathogens i.e. C. krusei, S. aureus, K. pneumonia and C. tropicalis (Prasad and Elumalai, 2011). Leaf extract of Garcinia mangostana having reducing property as well as antibacterial property could be helpful in the biosynthesis of 35 nm sized Silver nanoparticles (Veerasamy et al., 2011). Extract of Cacumen platycladi has great antibacterial activity and can be used in biosynthesis of silver NPs. To synthesize hexagonal and cubic shaped silver nanocrystals with the size of 31–40 nm, bark of Cinnamon zeylanicum could be used (Huang et al., 2011).
5.1.4. Importance of the shape of plant-based AgNPs
Furthermore, different shapes of AgNPs exhibit several types of interaction with the cell membrane and show their different type of antimicrobial activity by damaging the membrane. It has been reported that when the shape of AgNPs is truncated triangular, hexagonal, and octahedral the antimicrobial activity will be highest against gram-negative bacteria E. coli (Alshareef et al., 2017; Chen and Carroll, 2002). Similarly, El-Zahry et al. (2015) showed that AgNPs in the shape of hexagonal exhibit the highest antibacterial activity while triangular AgNPs display no activity against E. coli. Similarly the antibacterial activity conducted against B. subtilis, K. planticola, K. pneumoniae, S. nematodiphila, and E. coli by spherical shape AgNPs synthesized from Planomicrobium sp. That show decrease in growth rate with the increased in concentration of AgNPs, which suggested that biologically AgNPs can be an effective bactericidal food covering material (Rajeshkumar and Malarkodi, 2014). Similarly biological synthesis of AgNPs from Bacillus brevis and check their activity against pathogenis such as Staphylococcus Aures and Salmonella Typhi which show significant result in the from of zone of inhibition in the diameter of 7 nm–19 nm (Saravanan et al., 2018). Similarly, plant extract of Boerhaavia diffusa was used to synthesize face centered cubic AgNPs with an average size of 25 nm. The antibacterial potential of these nanoparticles was evaluated against Aeromonas hydrophila, Pseudomonas fluorescens, and Flavobacterium branchiophilum. These AgNPs showed highest sensitivity to F. branchiophilum (Kumar et al., 2014). Spherical shaped AgNPs, were synthesized using the fruit body extract of Tribulus terrestris. The antibacterial activity of these AgNPs was observed against Bacillus subtilis, E. coli, P. aeruginosa, Streptococcus pyogens, and S. aureus (Gopinath et al., 2012b). Similarly (Das et al., 2013), synthesized spherical shaped AgNPs using the leaf extract of Sesbania grandiflora. These AgNPs showed significant antibacterial activity against human pathogens; S. aureus and S. enterica. A critical analysis of these examples shows that shape triggered by plant-based synthesis of AgNPs confers them potent antibacterial activity.
5.1.5. Concentration of AgNPs is important for combating antibiotic resistance
The concentration of nanoparticles is another important factor to be considered during the activity against antibiotic resistant bacteria. For instance, Kim et al. (2007) report that is at low concentration of AgNPs, the growth of E. coli was inhibited while the inhibitory effect on similar concentration was poor against S. aureus. In a report by Pazos-Ortiz et al. (2017), AgNPs fixed in poly-epsilon-caprolactone were tested against gram-positive and gram-negative bacteria which showed major antibacterial effects against these bacteria when applied in 12 mM concentrations. However, higher concentrations of AgNPs that are chemically synthesized is not safe for the environment or other body functions. Biologically synthesized AgNPs even if applied in higher concentrations could be more safer to use. Ahmed et al. (2017) investigated the antibacterial efficiency of Terminalia arjuna based AgNPs (TA-AgNPs) against E. coli. Different concentrations (50, 100, 150, and 200 μg/mL) were used to assess the antibacterial effectiveness of TA-AgNPs. At higher concentrations of the AgNPs solution (200 μg/mL), the development of the pathogens was highly repressed, indicating the role of concentration of AgNPs in effective antibacterial action of the TA-AgNPs. Besides, it was found that the inhibition zone increased with the increase in the concentration of TA-AgNPs. Similarly, Dinesh et al. (2015) synthesized AgNPs from Aloe vera extracts and used against the bacteria B. subtilis, K. pneumoniae, and S. typhi using the disk diffusion method. AgNPs showed inhibitory action at a maximum concentration of 150 mg/L against all three bacteria. The zone of inhibition observed was 80 mm for B. subtilis and 90 mm for both K. pneumoniae and S. typhi, suggesting that concentration of AgNPs is an important factor in considering the role of AgNPs against bacteria. Another study reported the antibacterial activity of plant based AgNPs and chemically synthesized AgNPs. The results revealed that both type of AgNPs showed antimicrobial potential against S. areus and E. coli. However, the maximum zone of inhibition in case of plant based AgNPs was higher as compared to chemically synthesized AgNPs i.e. 4mm and 1mm respectively. The maximum inhibitory concentration of plant based AgNPs in S. areus and E. coli was observed to be 10.24 mg/ml and 5.12 mg/ml respectively. For chemically synthesized AgNPs the MIC was found to be 10.24 mg/ml in both S. areus and E. coli (Mousavi-Khattat et al., 2018). It has also been reported that plant based AgNPs produced maximum inhibition zones when compared with the standard antibiotic i.e. streptomycin. In case of streptomycin the inhibition zones were 9.41 mm and 10.12 mm for S. areus and E. coli respectively. While for plant based AgNPs the inhibition zones were 12.47 mm and 16.27 mm in S. areus and E. coli respectively (Senthil et al., 2017). Another study reported the antibacterial activity of sodium borohydride based AgNPs revealing the MIC values to be 100ppm for both S. areus and E. coli. But in case of biosynthesized AgNPs the MIC values has been reported to be 31.25 and 62.5 ppm respectively for S. areus and E. coli (Ocsoy et al., 2017).
5.1.6. Zeta potential of AgNPs as an important features of plant-based AgNPs
Zeta potential is also an important property that can affect antimicrobial activities. The interface between cell membranes and nanoparticles is based on electrostatic adhesion. The activity of AgNPs against bacteria is based on the electrostatic attraction in which bacterial component has a strong negative charge and AgNPs are less charged and their activity decrease the zeta potential on the surface of both bacteria (Abbaszadegan et al., 2015). For example, according to El Badawy et al. (2011) positively charge AgNPs are more toxic toward Bacillus species than negatively charged AgNPs. This is because the cell surface of Bacillus species is negatively charged, and it causes repulsion of negatively charged AgNPs. In another study by Ahmad et al. (2017), E. coli and B. subtilis were treated with chitosan AgNPs which indicate significant alteration of the membranes of bacteria because of the positive charge during interaction with the cell surface (zeta potential -11.6 and -7.5 mV). A biologically synthesized AgNPs from Mikia scabrella leaf extract was tested against multidrug resistance gram negative bacteria such as the nosocomial pathogens of Pseudomonas aeruginosa, Acinetobacter sp., and Klebsiella pneumoniae. In the size range from 18-12 nm with spherical shape and -21.7mV zeta potential for stable AgNPs, that exhibited significant antimicrobial activity for therapeutic application in nanomedicine (Prabakar et al., 2013).
6. Mechanism of antibacterial activity of AgNPs
There are three general pathways on which they perform their antimicrobial activity: (1) Degradation of the cell membrane and cell wall, (2) entry into the cell and disruption, and (3) Oxidative stress (Dakal et al., 2016; Slavin et al., 2017).
6.1. Degradation of the cell membrane and cell wall
Bacterial cell wall and membranes perform mainly the function of protecting them against harmful microbes and different types of environmental stress. These membranes also facilitate the transport of different types of useful nutrients (Madigan et al., 2014; Silhavy et al., 2010). It is suggested that AgNPs bind to the bacterial cell wall by ionic bond and generate a high proton motive force strength to disrupt the action of the enzymes containing thiol groups (Sereemaspun et al., 2008). For example, the bactericidal effects of the Avicennia marina based AgNPs against the E. coli were observed to be due to the possible dissipation of the proton motive force (Gnanadesigan et al., 2012). The action of AgNPs depends on the composition of the cell membrane and cell. In a study by Singh et al. (2010), Argemone mexicana leaf extract was used to synthesize green nanoparticles and the antibacterial assays were done against E. coli and P. aeruginosa. The study suggested two-pronged antibacterial activity i.e., the bactericidal effects of the AgNPs and the membrane disrupting capability of the polymer subunit. The gram-positive bacteria have thicker cell wall because of the presence of lipopolysaccharides in lesser quantity in their wall and they provide a tough barrier to AgNPs as compared to gram-negative. The higher amount of lipopolysaccharides and less peptidoglycan in cell walls of gram-negative bacteria makes them thinner in composition along with the composition of the cell membrane. Their sustenance, composition and negative charge on them makes them adhesive to AgNPs (Aziz et al., 2015; Gopinath et al., 2017; Pal et al., 2007; Rai et al., 2012; Sowmya et al., 2018). As mentioned earlier, the activity of AgNPs against bacteria is based on the electrostatic attraction between bacterial surfaces and AgNPs (Abbaszadegan et al., 2015). Due to this attraction and activity, the inside environment of the cell and their membrane polarization changes leading to cell death (Abalkhil et al., 2017; Gomaa, 2017b). The bacteria which are not treated with AgNPs show no physiological and morphological changes while other bacteria that are treated with AgNPs show changes in their morphology, and their integrity is disturbed. Due to the disruption of the cell wall and permeability of the membrane, the components of the bacterial cell such as nucleic acid, proteins, enzyme, metabolites, and source of energy are released outside (Gomaa, 2017b; Li et al., 2013; Ravichandran et al., 2018; Yuan et al., 2017). Attachment of AgNPs to the cell wall of bacteria and its degradation is the basis of such mechanism of antimicrobial activity (Ansari and Alzohairy, 2018; McQuillan et al., 2012).
6.2. Intracellular penetration and damage caused by AgNPs
When the AgNPs enter a bacterial cell, it binds to the proteins and DNA and causes conformational changes thus converting it into other less stable states affecting their function (Bondarenko et al., 2013; Gogoi et al., 2006; Hsueh et al., 2015). Studies reported that the presence of sulfur and other amino groups on the membrane surface facilitates the entry of AgNPs to the cell which leads to the destruction of DNA. AgNPs interact with biomolecules such as DNA, protein, and lipids due to the interaction of AgNPs with these biomolecules that have adverse effects on bacteria. For example, Gomathi et al. (2017) reported that Datura stramonium based AgNPs have antibacterial activity against E. coli. In this study it has been concluded that Ag ions from AgNPs penetrate inside into bacteria cell and cause severe damage to bacteria and led cell to death. Similarly, Jyoti et al. (2016) reported that AgNPs with large surface area, synthesized from Urtica dioica interact with bacteria cell and penetrate bacterial cell and release Ag + ions which interact with phosphorous and sulfur compound such as DNA and inhibit their replication and led the cell to death.
6.3. Oxidative stress in treated bacterial cells
Oxidative stress is usually produced by the introduction of antimicrobials to the bacterial cell. One way that inhibit the growth of resistant microbes is the induction of reactive oxidative species (ROS) in these microbes. Normally antimicrobials are used to elevate the levels of ROS. But new research shows that the introduction of AgNPs to resistant microbes raises the levels of ROS which leads to these resistant species. Research conducted by Khan and Ali (2020) shows that the introduction of AgNPs results in exceeding the level of ROS in the resistant microbial species such as Xanthomonas citri, S. aureus, and Erwinia carotovora. The results showed that AgNPs applied to bacterial suspension increased the quantity of ROS which subsequently resulted in the inhibition of bacteria. According to Das et al. (2017) AgNPs mediated the increase in ROS when subjected to resistant strains E. coli and S. aureus. The study showed that when these resistant strains are treated with AgNPs they raise the levels of ROS in these microbes which cause cellular inhibition of these microbes. Similarly, according to Kim et al. (2011) AgNPs formed ROS which damage cell membrane, protein, and DNA of both bacteria E. coli and S. aureus.
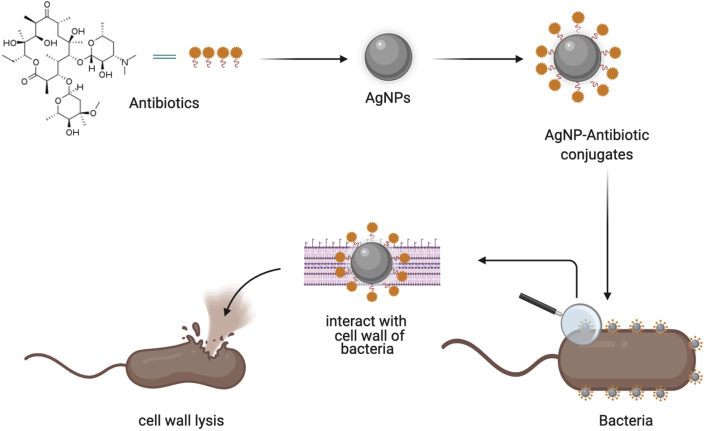
7. Combined synthesis of AgNPs with antibiotics to combat antibiotic resistance
AgNPs possess another importance function against resistance in the form conjugates with existing antibiotics (Figure 4). When antibiotics are attached with AgNPs, their stability, selectivity and functionality become enhanced and they target the drug very specifically (Kingsley et al., 2006). AgNPs conjugated with antibiotics (such as erythromycin, vancomycin, amoxicillin, clindamycin) showed enhanced antimicrobial activity against S. aureus and E. coli (Shahverdi et al., 2007). These antibiotic showed clear inhibition zones against both strains but erythromycin conjugated with AgNPs showed the highest activity against S. aureus with a clear zone of inhibition of 14 mm. In another set of experiments AgNPs conjugated with antibiotics (including gentamycin, tetracycline, ciprofloxacin, chloramphenicol) effectively targeted multidrug resistant bacteria (such as Micrococcus luteus, Staphylococcus epidermidis, and K. pneumoniae). The results showed that AgNPs-Antibiotics showed clear zones of inhibition (6–10 mm) when applied against these bacteria as compared to antibiotics alone (Tyagi, 2016).
Figure 4.
Illustration of the antibacterial potential of AgNPs conjugated with antibiotics.
Plant-based AgNPs have also found an important role in creating conjugates with antibiotics. According to Bonde et al. (2012), AgNPs synthesized via extracts of Murray koenigii were applied as combination with antibiotics such as ampicillin, streptomycin, gentamycin, and tetracycline and produced a synergistic effect against resistant pathogens such as E. coli and S. aureus. Gentamycin when combined with AgNPs showed the highest antibacterial activity against E. coli (4.06 fold increase) while tetracycline showed the highest activity against S. aureus (2.16 fold increase). In a study by Anjum et al. (2018) gentamycin was conjugated with AgNPs synthesized from Azadirachta indica which showed significant antibacterial activity against S. pneumoniae and S. aureus by interacting with the cell wall and inhibit the growth, and cause cell wall lysis, and inhibit bacterial replication. According to Halawani et al. (2020) biosynthesis of AgNPs from Rosa damascenes conjugated with cefotaxime showed maximum antibacterial activity against E. coli (zone of inhibition = 23–37 mm). Bio-fabricated AgNPs conjugated with antibiotics to which resistance has been developed can prove a way forward towards fighting multidrug resistance. In one of our studies, we observed that combination of AgNPs with a broad-spectrum Ciproflaxacin showed better antibacterial activity as compared to AgNPs alone and ciprofloxacin separately. The zones of inhibition zone of S. sonnei, S. typhi C. amalonaticus and E. coli in response to Cipro-AgNPs and ciprofloxacin were 33 mm, 35.5 mm, 35.5 mm and 38.5 mm, respectively. Therefore, it can be suggested that the AgNPs along with Ciprofloxacin might have worked in interaction and resulted in better antibacterial activity against pathogens (Adil et al., 2019). Several studies have reported that AgNPs functionalized with biomolecules possess more antibacterial potential as compared to AgNPs used alone (Some et al., 2020).
8. Conclusion
AgNPs have act against pathogenic bacteria because of their unique properties like small size, specific shape which provide a larger surface area for interaction with pathogenic bacteria to destroy them easily. AgNPs synthesized through green methods having many advantages like the cost-effectiveness, safety, and less toxicity to non-targeted living organisms. Plant-based AgNPs have distinctive antimicrobial properties that deem them fit for use as an alternative to antibiotics. They have been shown to have antimicrobial activity against both gram-positive and gram-negative bacteria which depends on size, shape, concentration, and zeta potential. Antimicrobial activity of AgNPs alone or in combination with some antibiotic drugs is also promising. Green AgNPs can be conjugated with many antibiotics that increase their stability and functionality and show enhanced antimicrobial activity against many bacteria. However, future studies must focus on the toxicological and toxicokinetic actions of nanoparticles as antibacterial agents.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge the University of Malakand for providing opportunity to work in a conducive environment and collaborate with other colleagues.
References
- Abalkhil T.A., Alharbi S.A., Salmen S.H., Wainwright M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017;31:411–417. [Google Scholar]
- Abbaszadegan A., Ghahramani Y., Gholami A., Hemmateenejad B., Dorostkar S., Nabavizadeh M., Sharghi H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J. Nanomater. 2015;2015:1–8. [Google Scholar]
- Abd Kelkawi A.H., Kajani A.A., Bordbar A.-K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2016;11:370–376. doi: 10.1049/iet-nbt.2016.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adil M., Khan T., Aasim M., Khan A.A., Ashraf M. Evaluation of the antibacterial potential of silver nanoparticles synthesized through the interaction of antibiotic and aqueous callus extract of Fagonia indica. AMB Exp. 2019;9:1–12. doi: 10.1186/s13568-019-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerf. 2003;28:313–318. [Google Scholar]
- Ahmad A., Wei Y., Syed F., Tahir K., Rehman A.U., Khan A., Ullah S., Yuan Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017;102:133–142. doi: 10.1016/j.micpath.2016.11.030. [DOI] [PubMed] [Google Scholar]
- Ahmad Z., Pandey R., Sharma S., Khuller G.K. Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Indian J. Chest Dis. Allied Sci. 2006;48:171–176. [PubMed] [Google Scholar]
- Ahmed Q., Gupta N., Kumar A., Nimesh S. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artificial Cell Nanomed. Biotechnol. 2017;45:1192–1200. doi: 10.1080/21691401.2016.1215328. [DOI] [PubMed] [Google Scholar]
- Ahn S., Singh P., Jang M., Kim Y.-J., Castro-Aceituno V., Simu S.Y., Kim Y.J., Yang D.-C. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264. 7 macrophages via NF-κB and AP-1 pathways. Colloid. Surf. B: Biointerf. 2018;162:398–404. doi: 10.1016/j.colsurfb.2017.11.037. [DOI] [PubMed] [Google Scholar]
- AlSalhi M.S., Devanesan S., Alfuraydi A.A., Vishnubalaji R., Munusamy M.A., Murugan K., Nicoletti M., Benelli G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 2016;11:4439. doi: 10.2147/IJN.S113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshareef A., Laird K., Cross R. Shape dependent antibacterial activity of silver nanoparticles on Escherichia coli and Enterococcus faecium bacterium. Appl. Surf. Sci. 2017;424:310–315. [Google Scholar]
- Andra S., Balu S., Ramoorthy R., Muthalagu M., Manisha V.S. Terminalia bellerica fruit extract mediated synthesis of silver nanoparticles and their antimicrobial activity. Mater. Today: Proc. 2019;9:639–644. [Google Scholar]
- Anjum F.R., Anam S., Rahman S., Ali A., Naveed A. Evaluation of antibacterial activity of plant based silver nanoparticles in Synergism with Antibiotics. Int. J. Biosci. 2018;13:250–261. [Google Scholar]
- Ansari M.A., Alzohairy M.A. One pot facile green synthesis of silver nanoparticles using seed extract of Phoenix dactylifera and their bactericidal potential against MRSA. Evid. base Compl. Alternative Med. 2018;2018:1–9. doi: 10.1155/2018/1860280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf J.M., Ansari M.A., Khan H.M., Alzohairy M.A., Choi I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 2016;6:20414. doi: 10.1038/srep20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Faraz M., Pandey R., Shakir M., Fatma T., Varma A., Barman I., Prasad R. Facile algae derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties. Langmuir. 2015;31:11605–11612. doi: 10.1021/acs.langmuir.5b03081. [DOI] [PubMed] [Google Scholar]
- Balavigneswaran C., Kumar T.S.J., Packiaraj R.M., Prakash S. Rapid detection of Cr (VI) by AgNPs probe produced by Anacardium occidentale fresh leaf extracts. Appl. Nanosci. 2014;4:367–378. [Google Scholar]
- Baran M.F., Hilal A. Antimicrobial activity of silver nanoparticles synthesized with extract of tomato plant against bacterial and fungal pathogens. Middle Black Sea J. Health Sci. 2019;5:67–73. [Google Scholar]
- Barros C.H., Fulaz S., Stanisic D., Tasic L. Biogenic nanosilver against multidrug-resistant bacteria (MDRB) Antibiotics. 2018;7:69. doi: 10.3390/antibiotics7030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruwati B., Polshettiwar V., Varma R.S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009;11:926–930. [Google Scholar]
- Betts J.W., Hornsey M., La Ragione R.M. Novel antibacterials: alternatives to traditional antibiotics. Adv. Microb. Physiol. 2018;73:123–169. doi: 10.1016/bs.ampbs.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Biel M.A., Sievert C., Usacheva M., Teichert M., Balcom J. Paper Presented at: International Forum of Allergy & Rhinology (Wiley Online Library) 2011. Antimicrobial photodynamic therapy treatment of chronic recurrent sinusitis biofilms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher K., Nasir A., Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence. 2011;2:395–401. doi: 10.4161/viru.2.5.17035. [DOI] [PubMed] [Google Scholar]
- Bondarenko O., Ivask A., Käkinen A., Kurvet I., Kahru A. Particle cell contact enhances antibacterial activity of silver nanoparticles. PloS One. 2013;8 doi: 10.1371/journal.pone.0064060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde S., Rathod D., Ingle A., Ade R., Gade A., Rai M. Murraya koenigii mediated synthesis of silver nanoparticles and its activity against three human pathogenic bacteria. Nanosci. Meth. 2012;1:25–36. [Google Scholar]
- Bose D., Chatterjee S. Antibacterial activity of green synthesized silver nanoparticles using Vasaka (Justicia adhatoda L.) leaf extract. Indian J. Microbiol. 2015;55:163–167. doi: 10.1007/s12088-015-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L., Bandara S., Joseph M., Green T., Grady T., Osuji G., Weerasooriya A., Ampim P., Woldesenbet S. Green synthesis of silver nanoparticles with antimicrobial properties using Phyla dulcis plant extract. Foodborne Patho. Dis. 2020;17:504–511. doi: 10.1089/fpd.2019.2714. [DOI] [PubMed] [Google Scholar]
- Cetinkaya Y., Falk P., Mayhall C.G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 2000;13:686–707. doi: 10.1128/cmr.13.4.686-707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- Chen S., Carroll D.L. Synthesis and characterization of truncated triangular silver nanoplates. Nano Lett. 2002;2:1003–1007. [Google Scholar]
- Chouhan S., Guleria S. Green synthesis of AgNPs using Cannabis sativa leaf extract: characterization, antibacterial, anti yeast and α amylase inhibitory activity. Mater. Sci. Energy Technol. 2020;3:536–544. [Google Scholar]
- Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B., Dash S.K., Mandal D., Ghosh T., Chattopadhyay S., Tripathy S., Das S., Dey S.K., Das D., Roy S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017;10:862–876. [Google Scholar]
- Das J., Das M.P., Velusamy P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim. Acta Mol. Biomol. Spectrosc. 2013;104:265–270. doi: 10.1016/j.saa.2012.11.075. [DOI] [PubMed] [Google Scholar]
- Devi M., Devi S., Sharma V., Rana N., Bhatia R.K., Bhatt A.K. Green synthesis of silver nanoparticles using methanolic fruit extract of Aegle marmelos and their antimicrobial potential against human bacterial pathogens. J. Tradit. Complement. Med. 2020;10:158–165. doi: 10.1016/j.jtcme.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana G., Borbón-Nuñez H.A., de León Jorge N.D., Ernesto G.M., Estrada I., Yanis T.-M., Hugo T., Marcela O.-M., Soto-Ramos A.G., Blanco A. Vol. 10. Nature Publisher Group; 2020. Green Synthesis of Silver Nanoparticles Using Lysiloma Acapulcensis Exhibit High Antimicrobial Activity. Scientific Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh D., Murugan K., Madhiyazhagan P., Panneerselvam C., Kumar P.M., Nicoletti M., Jiang W., Benelli G., Chandramohan B., Suresh U. Mosquitocidal and antibacterial activity of green synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi. Parasitol. Res. 2015;114:1519–1529. doi: 10.1007/s00436-015-4336-z. [DOI] [PubMed] [Google Scholar]
- Dipankar C., Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerf. 2012;98:112–119. doi: 10.1016/j.colsurfb.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Doddapaneni S.J.D., Amgoth C., Kalle A.M., Suryadevara S.N., Alapati K.S. Antimicrobial and anticancer activity of AgNPs coated with Alphonsea sclerocarpa extract. 3 Biotech. 2018;8:156. doi: 10.1007/s13205-018-1155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Singh H., Yi T.-H. Antibacterial, anti biofilm and anticancer potentials of green synthesized silver nanoparticles using benzoin gum (Styrax benzoin) extract. Bioproc. Biosyst. Eng. 2016;39:1923–1931. doi: 10.1007/s00449-016-1666-x. [DOI] [PubMed] [Google Scholar]
- ECDC . 2009. ECDC/EMEA Joint Technical Report: the Bacterial challenge: Time to React. [Google Scholar]
- El Badawy A.M., Silva R.G., Morris B., Scheckel K.G., Suidan M.T., Tolaymat T.M. Surface charge dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011;45:283–287. doi: 10.1021/es1034188. [DOI] [PubMed] [Google Scholar]
- El-Zahry M.R., Mahmoud A., Refaat I.H., Mohamed H.A., Bohlmann H., Lendl B. Antibacterial effect of various shapes of silver nanoparticles monitored by SERS. Talanta. 2015;138:183–189. doi: 10.1016/j.talanta.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Elavazhagan T., Arunachalam K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011;6:1265. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geethalakshmi R., Sarada D. Synthesis of plant mediated silver nanoparticles using Trianthema decandra extract and evaluation of their anti microbial activities. Int. J. Eng. Sci. Technol. 2010;2:970–975. [Google Scholar]
- Gemmell C.G., Edwards D.I., Fraise A.P., Gould F.K., Ridgway G.L., Warren R.E. Guidelines for the prophylaxis and treatment of methicillin resistant Staphylococcus aureus (MRSA) infections in the UK. J. Antimicrob. Chemother. 2006;57:589–608. doi: 10.1093/jac/dkl017. [DOI] [PubMed] [Google Scholar]
- Ghaffari-Moghaddam M., Hadi-Dabanlou R. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. J. Ind. Eng. Chem. 2014;20:739–744. [Google Scholar]
- Gnanadesigan M., Anand M., Ravikumar S., Maruthupandy M., Ali M.S., Vijayakumar V., Kumaraguru A. Antibacterial potential of biosynthesised silver nanoparticles using Avicennia marina mangrove plant. Appl. Nanosci. 2012;2:143–147. [Google Scholar]
- Gogoi S.K., Gopinath P., Paul A., Ramesh A., Ghosh S.S., Chattopadhyay A. Green fluorescent protein expressing escherichia coli as a model system for investigating the antimicrobial activities of silver nanoparticles. Langmuir. 2006;22:9322–9328. doi: 10.1021/la060661v. [DOI] [PubMed] [Google Scholar]
- Gomaa E.Z. Antimicrobial, antioxidant and antitumor activities of silver nanoparticles synthesized by Allium cepa extract: a green approach. J. Genet. Eng. Biotechnol. 2017;15:49–57. doi: 10.1016/j.jgeb.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa E.Z. Silver nanoparticles as an antimicrobial agent: a case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram negative bacteria. J. Gen. Appl. Microbiol. 2017;63:36–43. doi: 10.2323/jgam.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Gomathi M., Rajkumar P., Prakasam A., Ravichandran K. Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Res. Effic. Technol. 2017;3:280–284. [Google Scholar]
- Gong P., Li H., He X., Wang K., Hu J., Tan W., Zhang S., Yang X. Preparation and antibacterial activity of Fe3O4 Ag nanoparticles. Nanotechnology. 2007;18:285604. [Google Scholar]
- Gopinath V., MubarakAli D., Priyadarshini S., Priyadharsshini N.M., Thajuddin N., Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf. B Biointerf. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Gopinath V., MubarakAli D., Priyadarshini S., Priyadharsshini N.M., Thajuddin N., Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloid. Surf. B: Biointerf. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Gopinath V., Priyadarshini S., Loke M.F., Arunkumar J., Marsili E., MubarakAli D., Velusamy P., Vadivelu J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017;10:1107–1117. [Google Scholar]
- Gu H., Ho P., Tong E., Wang L., Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003;3:1261–1263. [Google Scholar]
- Gurunathan S., Han J.W., Kwon D.-N., Kim J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram negative and Gram positive bacteria. Nanoscale Res. Lett. 2014;9:1–17. doi: 10.1186/1556-276X-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S., Park J.H., Han J.W., Kim J.-H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA MB 231 human breast cancer cells: targeting p53 for anticancer therapy. Int. J. Nanomed. 2015;10:4203. doi: 10.2147/IJN.S83953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halawani E.M., Hassan A.M., El-Rab S.M.G. Nanoformulation of biogenic cefotaxime conjugated-silver nanoparticles for enhanced antibacterial efficacy against multidrug-resistant bacteria and anticancer studies. Int. J. Nanomed. 2020;15:1889. doi: 10.2147/IJN.S236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanan N.A., Chiu H.I., Ramachandran M.R., Tung W.H., Mohamad Zain N.N., Yahaya N., Lim V.J. Cytotoxicity of plant-mediated synthesis of metallic nanoparticles: a systematic review. Int. J. Mol. Sci. 2018;19:1725. doi: 10.3390/ijms19061725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati S., Rashtiani A., Zangeneh M.M., Mohammadi P., Zangeneh A., Veisi H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019;158:8–14. [Google Scholar]
- Hsueh Y.-H., Lin K.-S., Ke W.-J., Hsieh C.-T., Chiang C.-L., Tzou D.-Y., Liu S.-T. The antimicrobial properties of silver nanoparticles in Bacillus subtilis are mediated by released Ag+ ions. PloS One. 2015;10 doi: 10.1371/journal.pone.0144306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhan G., Zheng B., Sun D., Lu F., Lin Y., Chen H., Zheng Z., Zheng Y., Li Q. Biogenic silver nanoparticles by Cacumen platycladi extract: synthesis, formation mechanism, and antibacterial activity. Ind. Eng. Chem. Res. 2011;50:9095–9106. [Google Scholar]
- Huh A.J., Kwon Y.J. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Contr. Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Jain D., Daima H.K., Kachhwaha S., Kothari S. Synthesis of plant mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Digest J. Nanomater. Biostruct. 2009;4:557–563. [Google Scholar]
- Jia J., Duan Y.-Y., Wang S.-H., Zhang S.-F., Wang Z.-Y. Preparation and characterization of antibacterial silver containing nanofibers for wound dressing applications. J. US China Med. Sci. 2007;4:52–54. [Google Scholar]
- Jyoti K., Baunthiyal M., Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016;9:217–227. [Google Scholar]
- Khalil K.A., Fouad H., Elsarnagawy T., Almajhdi F.N. Preparation and characterization of electrospun PLGA/silver composite nanofibers for biomedical applications. Int. J. Electrochem. 2013;8:3483–3493. [Google Scholar]
- Khan T., Ali G.S. Variation in surface properties, metabolic capping, and antibacterial activity of biosynthesized silver nanoparticles: comparison of bio fabrication potential in phytohormone-regulated cell cultures and naturally grown plants. Roy. Soc. Chem. 2020;10:38831–38840. doi: 10.1039/d0ra08419k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatami M., Soltani Nejad M., Pourseyedi S. Biogenic synthesis of silver nanoparticles using mustard and its characterization. Int. J. Nanosci. Nanotechnol. 2015;11:281–288. [Google Scholar]
- Kim J.S., Kuk E., Yu K.N., Kim J.-H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kim S.-H., Lee H.-S., Ryu D.-S., Choi S.-J., Lee D.-S. Antibacterial activity of silver nanoparticles against Staphylococcus aureus and Escherichia coli. Kor. J. Microbiol. Biotechnol. 2011;39:77–85. [Google Scholar]
- Kingsley J.D., Dou H., Morehead J., Rabinow B., Gendelman H.E., Destache C.J. Nanotechnology: a focus on nanoparticles as a drug delivery system. J. Neuroimmune Pharmacol. 2006;1:340–350. doi: 10.1007/s11481-006-9032-4. [DOI] [PubMed] [Google Scholar]
- Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaraj C., Jagan E., Rajasekar S., Selvakumar P., Kalaichelvan P., Mohan N.J.C., Biointerfaces S.B. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloid. Surf. B: Biointerf. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kulkarni N., Muddapur U.J. Biosynthesis of Metal Nanoparticles: a Review. J. Nanotechnol. 2014;2014 [Google Scholar]
- Kumar D.A., Palanichamy V., Roopan S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014;127:168–171. doi: 10.1016/j.saa.2014.02.058. [DOI] [PubMed] [Google Scholar]
- Kumar R., Ghoshal G., Jain A., Goyal M. Rapid green synthesis of silver nanoparticles (AgNPs) using (Prunus persica) plants extract: exploring its antimicrobial and catalytic activities. J. Nanomed. Nanotechnol. 2017;8:1–8. [Google Scholar]
- Kumar V., Singh S., Srivastava B., Bhadouria R., Singh R. Green synthesis of silver nanoparticles using leaf extract of Holoptelea integrifolia and preliminary investigation of its antioxidant, anti inflammatory, antidiabetic and antibacterial activities. J. Environ. Chem. Eng. 2019;7:103094. [Google Scholar]
- Kvitek L., Panáček A., Soukupova J., Kolář M., Večeřová R., Prucek R., Holecova M., Zbořil R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs) J. Phys. Chem. 2008;112:5825–5834. [Google Scholar]
- Lakshmanan G., Sathiyaseelan A., Kalaichelvan P., Murugesan K. Plant mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L Assessment of their antibacterial and anticancer activity. Karbala Int. J. Modern Sci. 2018;4:61–68. [Google Scholar]
- Lateef A., Akande M.A., Azeez M.A., Ojo S.A., Folarin B.I., Gueguim-Kana E.B., Beukes L.S. Phytosynthesis of silver nanoparticles (AgNPs) using miracle fruit plant (Synsepalum dulcificum) for antimicrobial, catalytic, anticoagulant, and thrombolytic applications. Nanotechnol. Rev. 2016;5:507–520. [Google Scholar]
- Li J., Rong K., Zhao H., Li F., Lu Z., Chen R. Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J. Nanosci. Nanotechnol. 2013;13:6806–6813. doi: 10.1166/jnn.2013.7781. [DOI] [PubMed] [Google Scholar]
- Madigan M., Martinko J., Bender K., Buckley D., Stahl D. Benjamin-Cummings Pub Co; 2014. Brock Biology of Microorganisms Plus Masteringmicrobiology with Etext; pp. 2673–2702. [Google Scholar]
- Maghimaa M., Alharbi S.A. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B Biol. 2020;204:111806. doi: 10.1016/j.jphotobiol.2020.111806. [DOI] [PubMed] [Google Scholar]
- Malapermal V., Botha I., Krishna S.B.N., Mbatha J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum L using silver nanoparticles. Saudi J. Biol. Sci. 2017;24:1294–1305. doi: 10.1016/j.sjbs.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambio-Jones C., Hoek E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010;12:1531–1551. [Google Scholar]
- Mariselvam R., Ranjitsingh A., Nanthini A.U.R., Kalirajan K., Padmalatha C., Selvakumar P.M. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: arecaceae) for enhanced antibacterial activity. J. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014;129:537–541. doi: 10.1016/j.saa.2014.03.066. [DOI] [PubMed] [Google Scholar]
- McQuillan J.S., Groenaga Infante H., Stokes E., Shaw A.M. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology. 2012;6:857–866. doi: 10.3109/17435390.2011.626532. [DOI] [PubMed] [Google Scholar]
- Miri A., Sarani M., Bazaz M.R., Darroudi M. Plant-mediated biosynthesis of silver nanoparticles using Prosopis farcta extract and its antibacterial properties. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;141:287–291. doi: 10.1016/j.saa.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Moodley J.S., Krishna S.B.N., Pillay K., Govender P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018;9 [Google Scholar]
- Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- Mousavi-Khattat M., Keyhanfar M., Razmjou A.J.A.c. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. J. Nanomed. Biotechnol. 2018;46:S1022–S1031. doi: 10.1080/21691401.2018.1527346. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Bhakya S., Kumar T.S., Rao M. Biosynthesis, characterization and antibacterial effect of plant mediated silver nanoparticles using Ceropegia thwaitesii an endemic species. Ind. Crop. Prod. 2015;63:119–124. [Google Scholar]
- Nakkala J.R., Mata R., Sadras S.R. Green synthesized nano silver: synthesis, physicochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J. Colloid Interface Sci. 2017;499:33–45. doi: 10.1016/j.jcis.2017.03.090. [DOI] [PubMed] [Google Scholar]
- Nayak D., Pradhan S., Ashe S., Rauta P.R., Nayak B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015;457:329–338. doi: 10.1016/j.jcis.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Ocsoy I., Demirbas A., McLamore E.S., Altinsoy B., Ildiz N., Baldemir A.J. Green synthesis with incorporated hydrothermal approaches for silver nanoparticles formation and enhanced antimicrobial activity against bacterial and fungal pathogens. J. Mol. Liq. 2017;238:263–269. [Google Scholar]
- Okaiyeto K., Hoppe H., Okoh A.I. Plant based synthesis of silver nanoparticles using aqueous leaf extract of salvia officinalis: characterization and its antiplasmodial activity. J. Cluster Sci. 2020:1–9. [Google Scholar]
- Padiyara P., Inoue H., Sprenger M., Treatment Global governance mechanisms to address antimicrobial resistance. J. Res. Treat. 2018;11 doi: 10.1177/1178633718767887. 1178633718767887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Tak Y.K., Song J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle A study of the gram negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy N.K., Ferina N., Amirulhusni A.N., Mohd-Zain Z., Hussaini J., Ping L.J., Durairaj R. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J. Nanobiotechnol. 2014;12:2. doi: 10.1186/1477-3155-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. A new paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014;30:169–178. doi: 10.5487/TR.2014.30.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. New paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014;30:169–178. doi: 10.5487/TR.2014.30.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos-Ortiz E., Roque-Ruiz J.H., Hinojos-Márquez E.A., López-Esparza J., Donohué-Cornejo A., Cuevas-González J.C., Espinosa-Cristóbal L.F., Reyes-López S.Y. Dose dependent antimicrobial activity of silver nanoparticles on polycaprolactone fibers against gram positive and gram negative bacteria. J. Nanomater. 2017;2017:1–9. [Google Scholar]
- Pelgrift R.Y., Friedman A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Popescu M., Velea A., Lőrinczi A. Biogenic production of nanoparticles. Digest J. Nanomater. Biostruct. 2010;5:1035–1040. [Google Scholar]
- Prabakar K., Sivalingam P., Rabeek S.I.M., Muthuselvam M., Devarajan N., Arjunan A., Karthick R., Suresh M.M., Wembonyama J.P. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial gram negative bacterial pathogens. Colloids Surf. B Biointerfaces. 2013;104:282–288. doi: 10.1016/j.colsurfb.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Prasad T., Elumalai E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011;1:439–442. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Rai M.K., Deshmukh S., Ingle A., Gade A. Silver nanoparticles: the powerful nanoweapon against multidrug resistant bacteria. J. Appl. Microbiol. 2012;112:841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- Raj S., Mali S.C., Trivedi R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun. 2018;503:2814–2819. doi: 10.1016/j.bbrc.2018.08.045. [DOI] [PubMed] [Google Scholar]
- Rajendran R., Prabha A.L. AgNPs synthesis, characterization and antibacterial activity from Salvia splendens Sellow ex Roem. & Schult. plant extract. Int. J. Sci. Res. 2015;4:1086–1090. [Google Scholar]
- Rajeshkumar S., Malarkodi C. In vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioinorgan. Chem. Appl. 2014;2014 doi: 10.1155/2014/581890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramar M., Manikandan B., Marimuthu P.N., Raman T., Mahalingam A., Subramanian P., Karthick S., Munusamy A. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;140:223–228. doi: 10.1016/j.saa.2014.12.060. [DOI] [PubMed] [Google Scholar]
- Ramesh P., Kokila T., Geetha D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;142:339–343. doi: 10.1016/j.saa.2015.01.062. [DOI] [PubMed] [Google Scholar]
- Ravichandran A., Subramanian P., Manoharan V., Muthu T., Periyannan R., Thangapandi M., Ponnuchamy K., Pandi B., Marimuthu P.N. Phyto mediated synthesis of silver nanoparticles using fucoidan isolated from Spatoglossum asperum and assessment of antibacterial activities. J. Photochem. Photobiol. B Biol. 2018;185:117–125. doi: 10.1016/j.jphotobiol.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Raza M.A., Kanwal Z., Rauf A., Sabri A.N., Riaz S., Naseem S. Size and shape dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials. 2016;6:74. doi: 10.3390/nano6040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K., Sarkar C., Ghosh C. Plant mediated synthesis of silver nanoparticles using parsley (Petroselinum crispum) leaf extract: spectral analysis of the particles and antibacterial study. Appl. Nanosci. 2015;5:945–951. [Google Scholar]
- Salehi S., Shandiz S.A.S., Ghanbar F., Darvish M.R., Ardestani M.S., Mirzaie A., Jafari M. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomed. 2016;11:1835. doi: 10.2147/IJN.S99882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana S.S., Badineni V.R., Arla S.K., Boya V.K.N. Eco friendly synthesis of silver nanoparticles using leaf extract of Grewia flaviscences and study of their antimicrobial activity. Mater. Lett. 2015;145:347–350. [Google Scholar]
- Saravanan M., Barik S.K., MubarakAli D., Prakash P., Pugazhendhi A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018;116:221–226. doi: 10.1016/j.micpath.2018.01.038. [DOI] [PubMed] [Google Scholar]
- Sathishkumar G., Gobinath C., Karpagam K., Hemamalini V., Premkumar K., Sivaramakrishnan S. Phyto synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf. B Biointerf. 2012;95:235–240. doi: 10.1016/j.colsurfb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Schabes-Retchkiman P., Canizal G., Herrera-Becerra R., Zorrilla C., Liu H., Ascencio J. Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt. Mater. 2006;29:95–99. [Google Scholar]
- Senthil B., Devasena T., Prakash B., Rajasekar A.J. Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B: Biol. 2017;177:1–7. doi: 10.1016/j.jphotobiol.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Sereemaspun A., Hongpiticharoen P., Rojanathanes R., Maneewattanapinyo P., Ekgasit S., Warisnoicharoen W. Inhibition of human cytochrome P450 enzymes by metallic nanoparticles: a preliminary to nanogenomics. Int. J. Pharmacol. 2008;4:492–495. [Google Scholar]
- Shahverdi A.R., Fakhimi A., Shahverdi H.R., Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harbor Perspect. Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Gautam P.K., Verma A., Singh V., Shivapriya P.M., Shivalkar S., Sahoo A.K., Samanta S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections. Review. 2020;25 doi: 10.1016/j.btre.2020.e00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Jain D., Upadhyay M., Khandelwal N., Verma H. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Bios. 2010;5:483–489. [Google Scholar]
- Singh H., Du J., Yi T.-H. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artificial Cell Nanomed. Biotechnol. 2017;45:1310–1316. doi: 10.1080/21691401.2016.1228663. [DOI] [PubMed] [Google Scholar]
- Singh P., Kim Y.-J., Zhang D., Yang D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Singh P., Kim Y.J., Singh H., Mathiyalagan R., Wang C., Yang D.C.J. Biosynthesis of anisotropic silver nanoparticles by Bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J. Nanomater. 2015;2015 [Google Scholar]
- Singh P., Singh H., Ahn S., Castro-Aceituno V., Jiménez Z., Simu S.Y., Kim Y.J., Yang D.C. Pharmacological importance, characterization and applications of gold and silver nanoparticles synthesized by Panax ginseng fresh leaves. Artificial Cell Nanomed. Biotechnol. 2017;45:1415–1424. doi: 10.1080/21691401.2016.1243547. [DOI] [PubMed] [Google Scholar]
- Slavin Y.N., Asnis J., Häfeli U.O., Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017;15:1–20. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Some S., Sarkar B., Biswas K., Jana T.K., Bhattacharjya D., Dam P., Mondal R., Kumar A., Deb A.K., Sadat A. Bio-molecule functionalized rapid one-pot green synthesis of silver nanoparticles and their efficacy toward the multidrug resistant (MDR) gut bacteria of silkworms (Bombyx mori) RSC Adv. 2020;10:22742–22757. doi: 10.1039/d0ra03451g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram negative bacteria. J. Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Sowmya C., Lavakumar V., Venkateshan N., Ravichandiran V., Saigopal D. Exploration of Phyllanthus acidus mediated silver nanoparticles and its activity against infectious bacterial pathogen. Chem. Cent. J. 2018;12:1–9. doi: 10.1186/s13065-018-0412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surega R., Anita B., Ramakrishnan S., Gunasekaran K., Nakkeran S. Synthesis and characterization of AgNps using plant extracts. Int. J. Curr. Microbiol. Appl. Sci. 2020;9:1939–1947. [Google Scholar]
- Tyagi P.K. Use of biofabricated silver nanoparticles conjugated with antibiotic against multidrug resistant pathogenic bacteria. Biol. Insights. 2016;1:1–2. [Google Scholar]
- Veerasamy R., Xin T.Z., Gunasagaran S., Xiang T.F.W., Yang E.F.C., Jeyakumar N., Dhanaraj S.A.J. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011;15:113–120. [Google Scholar]
- Ventola C.L., therapeutics The antibiotic resistance crisis: part 1: causes and threats. J. Pharm. Therapeut. 2015;40:277. [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar M., Priya K., Nancy F., Noorlidah A., Ahmed A. Biosynthesis, characterisation and anti-bacterial effect of plant mediated silver nanoparticles using Artemisia nilagirica. Ind. Crop. Prod. 2013;41:235–240. [Google Scholar]
- Vijayakumar S., Divya M., Vaseeharan B., Chen J., Biruntha M., Silva L.P., Durán-Lara E.F., Shreema K., Ranjan S., Dasgupta N. Biological compound capping of silver nanoparticle with the seed extracts of blackcumin (Nigella sativa): a potential antibacterial, antidiabetic, anti-inflammatory, and antioxidant. J. Inorg. Organomet. Polym. Mater. 2020:1–12. [Google Scholar]
- Wang L., Wu Y., Xie J., Wu S., Wu Z. Characterization, antioxidant and antimicrobial activities of green synthesized silver nanoparticles from Psidium guajava L. leaf aqueous extracts. Mater. Sci. Eng. C. 2018;86:1–8. doi: 10.1016/j.msec.2018.01.003. [DOI] [PubMed] [Google Scholar]
- WHO . 2016. Antibiotic Resistance. [Google Scholar]
- Yacamán M.J., Ascencio J., Liu H., Gardea-Torresdey J. Structure shape and stability of nanometric sized particles. J. Vac. Sci. Technol. B: Microelect. Nanometer Struct. Proc. Measure. Pheno. 2001;19:1091–1103. [Google Scholar]
- Yazdi M.E.T., Amiri M.S., Hosseini H.A., Oskuee R.K., Mosawee H., Pakravanan K., Darroudi M. Plant-based synthesis of silver nanoparticles in Handelia trichophylla and their biological activities. Bull. Mater. Sci. 2019;42:155. [Google Scholar]
- Yuan Y.-G., Peng Q.-L., Gurunathan S. Effects of silver nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa from mastitis-infected goats: an alternative approach for antimicrobial therapy. Int. J. Mol. Sci. 2017;18:569. doi: 10.3390/ijms18030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Kong Y., Kundu S., Cirillo J.D., Liang H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J. Nanobiotechnol. 2012;10:19. doi: 10.1186/1477-3155-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.