Abstract
The use of plant materials in traditional medicine as a significant ingredient in synthesizing drugs in the form of decoctions had played a vital role due to their potential therapeutic action. The curry leaves, Murraya koenigii, and Micromelum minutum are two most common aromatic herbs widespread in Sri Lanka and many other Asian countries. They are rich in nutrition and exert several medicinal properties such as antidiabetic, antioxidant, antimicrobial, anti-inflammatory, and anti-carcinogenic due to various essential phytochemicals, minerals, and trace minerals. In this study, we determined the proximate composition, in vitro antioxidant activity, total phenol (TPC), flavonoid content (TFC), and antibacterial activity in both species using standard methods. Results showed that both species varied greatly in nutritional composition, antioxidant, and antibacterial activities. The nutrient composition in terms of ash, crude protein, and fat was significantly high in M. koenigii based on dry weight basis. In vitro antioxidant potential of M. koenigii and M. minutum was evaluated by means of the DPPH radical scavenging assay, and the IC50 values of M. koenigii and M. minutum were 107 ± 2 μg/mL and 208 ± 4 μg/mL, respectively. The TPC and TFC of M. koenigii were 101 ± 1 mg GAE/g and 9.75 ± 0.05 mg QE/g, and M. minutum were 80 ± 2 mg GAE/g and 9.16 ± 0.02 mg QE/g respectively. The antibacterial study was assessed against three bacterial strains. The study showed the highest inhibitory activity for M. koenigii and M. minutum against Staphylococcus aureus and Escherichia coli, respectively at 1.0 mg/mL concentration. The study indicated that M. koenigii possessed a significantly high TPC, and TFC correlated with higher antibacterial and antioxidant activity than M. minutum.
Keywords: Murraya koenigii, Micromelum minutum, IC50, Proximate composition, In vitro antioxidant activity, Total phenol content, Flavonoid content, Antibacterial activity
Murraya koenigii; Micromelum minutum; IC50; Proximate composition; In vitro antioxidant activity; Total phenol content; Flavonoid content; Antibacterial activity.
1. Introduction
The use of plant-based natural products in the treatment and prevention of diseases and health enhancement has led to the significant attention of the scientific community and the public nowadays. The availability of these medicinal plants provides a cost-effective source with lesser side effects to develop new drugs has drawn much attention among the researchers. Plant-based traditional medicine has a long history since ancient civilization and uses plant materials as a major ingredient in synthesizing drugs [1]. It is a widely accepted fact that the rapid development of deriving pharmacologically active drugs from medicinal herbs has a tremendous impact on current medicinal practices.
Curry leaves are fascinating house plants grown in Asia and are native to Sri Lanka, Bangladesh, and India. There are different varieties of curry leaves belonging to the family Rutaceae [2]. Out of the 2070 global species belonging to the family Rutaceae, only a few herbaceous varieties are available in Sri Lanka, including M. koenigii and M. minutum. However, M. koenigii is the most popular species and is commonly used as a flavouring agent and Ayurvedic medicine in Sri Lanka due to its numerous health benefits. They are rich in nutrition due to various essential phytochemicals, minerals, and trace minerals present in their leaves and seeds [3]. The M. koenigii leaves are extensively used in Asian cooking, from vegetables to many other dishes, due to their natural flavours [4]. Moreover, different plant parts of M. koenigii have also been used in indigenous medicine due to their various therapeutic actions. Furthermore, kola kenda or porridge made from curry leaves is famous for its nutritional value and efficacy in lowering blood cholesterol levels [5].
M. minutum is also used as a flavouring agent and is reported to have medicinal value in the southern part of Thailand and many other Asian countries due to the presence of coumarins in different plant parts [6]. Further, M. minutum roots are used to cure ringworms, tumors, fever and to regulate menstruation. The leaves and the bark are used to treat toothache and teething issues in babies, skin irritations caused by scabies, and a remedy for stomachache and headache. Besides that, the roots are used in decoction or infusions for diarrhea in children and as a carminative [7, 8].
M. koenigii and M. minutum reduce the number of medications needed to treat many metabolic and non-communicable diseases, including diabetics, cancers, cardiovascular diseases, and obesity [9, 10, 11, 12]. The majority of metabolic and non-communicable diseases are linked with higher mortality and morbidity rates [13]. Therefore, Nowadays, the research interests are more focused on evaluating the bioactivities of Natural products from herbal sources. The present study aimed to investigate the bioactive components and the nutritional composition of M. koenigii and M. minutum leaves. Additionally, the antimicrobial and antioxidant activities of both species were also tested.
2. Materials and methods
2.1. Apparatus and equipment
The apparatus and equipment used are as follows: Electronic analytical balance (Ohaus Corporation, USA), Rotary flask shaker (Jain scientific glassworks, India), Rotary flash evaporator (IKA RV 05 basic- Staufen, Germany), UV-visible spectrophotometer (BK-UV 1800 spectrophotometer – BIOBASE - Jinan, China). Muffle furnace (Thermo Scientific, USA) and Kjeldahl apparatus (Velp Scientifics, USA).
2.2. Collection and authentication of plant leaf samples
The mature M. koenigii and M. minutum leaf samples were collected from the Pitawala Pathana forest area of knuckles conservation forest (7° 18' - 7° 34′ N latitude and 80° 40' - 80° 54′ longitude), Sri Lanka. Samples were authenticated by the National Herbarium of the Royal Botanical Garden-Peradeniya. Voucher specimens were deposited in the National Herbarium of Peradeniya, Sri Lanka.
2.3. Preparation of samples
The M. koenigii and M. minutum leaf samples were separated and cleaned to remove all foreign matter adhered. Some of the leaf samples were used to analyze moisture and ash, and the rest were washed with clean water and air-dried for 3–4 weeks before being ground finely by using a mechanical blender. All the ground materials were kept in sterile bags in a dry place for later use.
Oven-dried, ground samples of each M. koenigii and M. minutum leaf samples were sieved to obtain particle size <1.40 mm. Each sample was extracted using ethanol with the ratio of solvent to dried samples at 10:1. The mixtures were kept in a steam bath for 1 h. Filtrates were collected using vacuum filtration. The filtrate was collected and concentrated by a rotary evaporator, and concentrated extracts were used for the preliminary phytochemical tests [8]. Air-dried ground samples of each M. koenigii and M. minutum leaf samples were extracted by maceration with methanol and concentrated by the rotary evaporator and used to analyze total phenolic content flavonoid content and DPPH radical scavenging activity.
2.4. Preliminary phytochemical screening of curry leaves
Preliminary phytochemical screening for alkaloids, flavonoids, unsaturated sterols, and triterpenes, saponins, and tannins was carried out for ethanol leaf extracts following standard methods previously reported for their identification and confirmation [14].
2.5. Determination of nutritional composition
The total moisture, ash, fat, crude protein, and carbohydrate content of each M. koenigii and M. minutum leaf were determined using AOAC official methods [15]. Carbohydrate content was determined by the difference method [15].
2.6. Determination of total phenolic content
Total phenolic content (TPC) of each M. koenigii and M. minutum leaf extract was analyzed using the Folin-Ciocalteu reagent by the method with slight modification [16]. Appropriately diluted plant extract (0.5 mL) was used with Folin-Ciocalteu reagent for this evaluation. Different concentrations of plant extract of each part for all the experiments were prepared by suitable dilutions based on the total phenol contents. The absorbance of each solution was measured at 760 nm. A calibration curve was plotted using gallic acid (20 μg/mL to 100 μg/mL) as the standard, and the total phenolic content was expressed as mg gallic acid equivalents per gram of dried sample (mg GAE/g).
2.7. Determination of total flavonoid content
The total flavonoid content (TFC) of each M. koenigii and M. minutum leaf extract was analyzed by the aluminum chloride colorimetry method [16]. The absorbance of each solution was measured at 415 nm. A calibration curve was plotted using gallic acid (100 μg/mL to 400 μg/mL) as the standard, and the total flavonoid content was expressed as mg gallic acid equivalents per gram of dried sample (mg GAE/g).
2.8. Determination of antioxidant activity
The antioxidant activity was measured using the DPPH radical scavenging ability assay [16]. M. koenigii and M. minutum leaf extract (1600 μL) of various concentrations (10, 25, 50, 75, 100, 250, and 500 μg/mL) were added to a methanolic solution of DPPH (40 μL, 0.25 mM). A dilution series of M. koenigii and M. minutum leaf extract was (0.01-0.035 mg/mL) prepared. DPPH reagent prepared in methanol (5 mg/100 mL, 2.0 mL) was added to each test sample (1.5 mL) and mixed with 0.5 mL of methanol. The mixture was allowed to stand for 15 min in the dark and absorbance was measured at 517 nm. Trolox was used as the reference standard. The percentage inhibition was calculated using Eq. (1).
| (1) |
where, As is the absorbance in the presence of standard or extracts and A0 is the absorbance of the control. The results were plotted as the % of scavenging activity against the concentration of the sample. The IC50 values were calculated using Prism 7 Release 2017, Statistical Software.
2.9. Determination of antibacterial activity
Antibacterial activity of the methanolic extract was determined by using S. aureus113, E. coli ATCC 1858, and P. aeruginosa ATCC 27853 bacterial species obtained from the bacterial culture collection of food and technology laboratory, Department of Agricultural Engineering of The Open University of Sri Lanka.
Antibacterial activity of the M. koenigii and M. minutum leaf extracts was measured using the slightly modified disk diffusion method described previously [17]. A single colony of bacteria was grown in 2 ml Muller Hinton Broth at 37 °C for 18 h. The content was centrifuged at 10000 g for 10 min to obtain the bacterial pellet. The supernatant was removed, and the bacterial pellet was re-suspended in 1 ml sterile 0.85 % NaCl. Then the content solution was serially diluted by adding 9 ml of sterile 0.85 % NaCl solution to obtain 5 × 10 5 CFU mL−1, and 100 μl of diluted bacterial suspension was spread on Mueller Hinton Agar (MHA) plates. An aliquot (10 μl) of M. koenigii and M. minutum leaf extract dilutions (0.2 mg/μL -1.0 mg/μL) was pipetted onto a sterile paper disc of 5.5 mm diameter (Whatman No1) on the agar surface. Ciprofloxacin (100 μgml−1) served as positive control while DMSO-containing disc served as the negative control. The plates were then inverted and incubated at 37 °C for 18 h. Microbial inhibition was determined by measuring the clear zone of inhibition around each disc and recorded as the diameter zone of inhibition (DIZ) in millimeters. All assays were performed independently three times with triplicates.
2.10. Statistical analyses
Statistical analysis was performed using SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). All experiments were carried out as means ± standard deviations of three replicates. Analysis of variance (ANOVA) followed by Tukey's post hoc test was used to establish the differences between means of various groups at the significance level fixed at P < 0.05.
3. Results and discussion
Fresh leaves of M. koenigii and M. minutum were subjected to proximate analysis; moisture, ash, crude protein, fat, and carbohydrate, as depicted in Table 1. Moisture content directly affects the freshness and flavour of curry leaves. As can be seen in Table 1, both M. koenigii and M. minutum leaves showed a similar moisture content on a wet basis. The high moisture content indicates a low shelf –life, and therefore, the plant material could be preserved by drying it to retain other valuable components for prolonged use.
Table 1.
Nutritional composition of M. koenigii and M. minutum leaves.
| Moisture content (g/100 g) | Ash content (g/100 g) | Protein content (g/100 g) | Fat content (g/100 g) | Carbohydrate content (g/100 g) | |
|---|---|---|---|---|---|
| M. Koenigii | 69.4 ± 0.7a | 2.75 ± 0.05a | 15.6 ± 0.4a | 11 ± 3a | 2.3 ± 0.4a |
| M. minutum | 70.4 ± 0.8a | 2.0 ± 0.1b | 14 ± 1b | 2.3 ± 0.6b | 11 ± 1b |
All data are presented as mean ± SD of the three replicates. Mean followed by a different letter in the same column differs significantly (p ≤ 0.05).
Ash content reflects the composition of inorganic materials, specifically minerals. Minerals are essential in maintaining normal physiological functions. The presence of trace minerals and vitamins protects tissue damage due to oxidative stress exerting antioxidant activity [18]. M. koenigii showed the highest ash content (2.75 g/100 g), significantly different from the ash content of M. minutum (2.0 g/100 g).
The crude protein content ranged from 14 to 15.6 g/100 g in both species. M. koenigii had a significantly higher (P < 0.05) protein content compared to M. minutum. Plant-based foods that provide more than 12% of their calorific value from protein are known to be a decent source of protein. Therefore, M. koenigii can serve as a good source of protein for individuals. Interestingly, the crude protein content of M. koenigii is relatively high compared to similar work reported [19].
Fat is among the essential substances for the function of life. In general, low-fat foods are known to be healthy foods, and therefore, fat content in food has a significant role in anyone's diet. According to our investigation, M. koenigii had a higher fat content of about 11g/100 g than M. minutum (2.3 g/100 g).
Carbohydrate composition is lowest in M. koenigii, 2.3 g/100g compared to M. minutum, 11 g/100g. Carbohydrates are an essential part of a balanced diet, mainly in providing energy. Both cellulose and soluble sugars are included in carbohydrates. Soluble sugars are readily absorbed by the human body, and cellulose cannot be absorbed. However, cellulose supplies the crude fiber requirement of the body. As shown in Table 1, the nutritional composition in terms of ash, protein, and fat was mostly high in M. koenigii, supporting its common use as a food in many cultures over the globe.
Therefore, the use of M. minutum mainly as a spice is justifiable. It is high in its nutritional composition, and M. minutum can also be used as a substitute, which is more often used in traditional medicine. Consumption of food, mainly plant-based food in our diet, provides us with essential nutrients and minerals required by our body, mainly for its vital functions. Wise and proper use of plant-based products in our daily diet will contribute to healthy individuals and the betterment of society.
Medicinal plants are rich in essential phytochemicals and many secondary plant metabolites accountable for the antimicrobial, anti-inflammatory effects, and various other known biological activities. Freshly prepared ethanolic extracts of M. koenigii and M. minutum were subjected to phytochemical analysis for the presence of various primary and secondary metabolites) responsible for the antibacterial properties. Phytochemical screening of the plant extracts under this study revealed a similar phytochemical profile, as depicted in Table 2. Alkaloids, flavonoids, triterpenes, tannins, and unsaturated steroids are the most critical types of phytochemicals found in these species. However, both extracts were devoid of saponins. Previous studies have also reported the absence of saponins in the curry leaf species under the study [20]. Alkaloids, flavonoids, polyphenols, and tannins were identified in several other investigations of the phytoconstituents of different plant parts of M. minutum [3, 4, 8].
Table 2.
Phytochemical screening of M. koenigii and M. minutum.
| Phytochemical | M. koenigii | M. minutum |
|---|---|---|
| Alkaloids | + | + |
| Flavonoids | + | + |
| Unsaturated steroids | + | + |
| Triterpenes | + | + |
| Saponins | - | - |
| Tannins | + | + |
The observed phytochemical compounds in these extracts have been reported to possess psychological activities and medicinal importance. Alkaloids are essential in analgesic, neuroprotective, antimicrobial, and antimalarial actions [21, 22]. Plant-derived flavonoids possess antidiarrheal, antimicrobial, antioxidant, and anti-inflammatory properties. Polyphenolic flavonoids make complexes with bacterial cell walls and exert biological functions [22]. Many other compounds, such as steroids and tannins, are potent antimicrobial agents [21, 23].
Terpenes are antimicrobial agents, and their mode of action is the weakening of tissue and the cell wall of the microorganisms. They also serve as anticancer and antidiabetic agents [24].
Therefore, the individual phytochemicals possess various biological activities, including antimicrobial, antioxidant, anti-inflammatory, antiplasmodial, and anticancer activities [25]. The potent biological activities of each phytochemical may enhance the discovery of novel antibiotic and antifungal agents against pathogens from the species M. koenigii and M. minutum.
Another critical group of phytoconstituents is phenolic compounds. These are considered the most abundant aromatic secondary metabolites within plants and range from simple phenolic acids to more complex tannins [26]. Two types of tannins are identified as hydrolyzable and non-hydrolyzable tannins. Commonly tannins are water-soluble and act as antibacterial agents, and are toxic due to their ability to form metal complexes [27].
The total phenolic content (TPC) of the methanolic leaf extracts of M. koenigii and M. minutum was determined by the Folin Ciocalteu method, as depicted in Table 3. Both species under study were rich in polyphenol content, and the TPC of the tested plants was significantly different (p < 0.05). While in comparison to the literature, it clearly shows that M. koenigii methanolic extract has a similar or a slightly higher value of phenolic content [28, 29]. However, the TPC of M. koenigii varied when extracted with different solvents [29].
Table 3.
Determination of total polyphenol content (TPC) and total flavonoid content (TFC) of M. koenigii and M. minutum leaf extracts.
| Species | TPC mg GAE/g of extract | TFC mg QE/g of extract |
|---|---|---|
| M. koenigii | 101 ± 1a | 9.75 ± 0.05a |
| M. minutum | 80 ± 2b | 9.16 ± 0.02b |
All the data is presented as mean ± SD of the three replicates. Mean followed by a different letter in the same column differs significantly (p ≤ 0.05).
Flavonoids are another group of phenolic compounds present in medicinal plants, exhibit antioxidant activity [30]. The results in Table 3 showed that the TFC in methanol extract of M. koenigii and M. minutum was in the range of 9.16–9.75 mg QE/g of extract. M. koenigii extract showed the highest TFC value, significantly different from M. minutum (p < 0.05).
The reported TPC and TFC of M. minutum were slightly lower in methanol extract, respectively [28, 31, 32]. The successful isolation of phenolic and flavonoid compounds may depend on various factors, sample size, storage conditions, weather, extraction method, and the presence of any interfering substances and the solvent. Aqueous methanol and ethanol at various percentages have been widely used as solvents to extract phenolic compounds from M. koenigii and M. minutum. However, no single solvent or a mixture of solvents was reported to extract phenolic compounds from these two species effectively.
Although the total phenolic content of M. koenigii was higher than that of M. minutum (Table 3), there was only a slight difference in the total flavonoid content of the two species. Therefore, these results indicate that the phenolic substances' quality may also have contributed to the higher antioxidant activity displayed by the M. koenigii species. Furthermore, various other phytochemicals such as terpenes are also known to be the major constituent exerting antioxidant activity, as confirmed by different comparative studies of M. koenigii [29].
As previously discussed, the phenolic compounds are major secondary metabolites that consist of a large group of biologically active compounds. Due to their redox properties, phenolics act as antioxidants and reducing agents. The phenolic hydroxyl groups have a remarkable ability to scavenge free radicals [28]. On the other hand, flavonoids are also considered important biological compounds with several biological activities such as antioxidant, anticancer, anti-inflammatory, anti-allergic, anti-angiogenic, and anti-allergic [33, 34, 35].
Both M. koenigii and M. minutum species are used for traditional medicinal purposes. Therefore, these plant extracts should contain phytochemicals with a high antioxidant value. Antioxidants.
Reduce different forms of free radicals, hydroperoxides (ROO∙), H2O2 by preventing the formation of active oxygen species. Therefore, antioxidants play a tremendous role in biological systems.
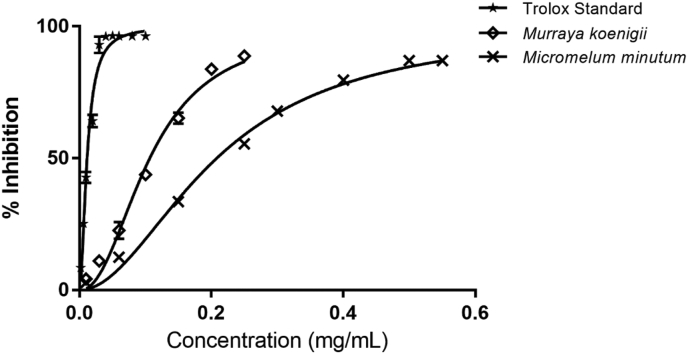
DPPH radical scavenging assay was used to evaluate the antioxidant potential. A stable free radical, DPPH, is used to study the radical scavenging activity of the plant extracts. The increased amount of an antioxidant in a given extract is responsible for the increased reduction of the absorbance of the DPPH solution. IC50 was used to compare the radical scavenging activity of the curry leaves samples, and the lower the IC50, the better it can scavenge radicals. Trolox was used as a positive standard with an IC50 of 11.5 ± 0.4. μg/mL.
In order to understand the antioxidant potential of the plant extracts, the IC50 curve with respect to the positive control was examined. In general, the IC50 of both M. koenigii and M. minutum methanol extracts increased with increasing concentrations of 0.1 mg/mL to 0.6 mg/mL, as depicted in Figure 1. However, the inhibition percentage of the DPPH assay indicated a significant difference (p < 0.05) of both species understudy with the standard antioxidant, Trolox. Furthermore, the reported IC50 values 107 ± 2 μg/mL and 208 ± 4 μg/mL of M. koenigii and M. minutum were significantly different (p < 0.05) as indicated in Table 4. The reported IC50 values for M. koenigii and M. minutum extracts are 440 μg/mL and 168.9 μg/mL respectively [32, 33]. These differences could be due to the differences in the maturation stage of leaves and environmental growth variations found in various geometrical locations.
Figure 1.
DPPH scavenging activity of curry leaves and Trolox standard. (O = Trolox standard,  = M. koenigii, X = M. minutum).
= M. koenigii, X = M. minutum).
Table 4.
Determination of the antioxidant activity of M. koenigii and M. minutum leaf extracts by DPPH free radical scavenging assay.
| Species | IC50 ± SD (μg/mL) |
|---|---|
| M. koenigii | 107 ± 2a |
| M. minutum | 208 ± 4b |
| Trolox standard | 11.5 ± 0.4c |
All the data is presented as mean ± SD of the three replicates. Mean followed by a different letter in the same column differs significantly (p ≤ 0.05).
The TPC and the antioxidant activities of M. koenigii and M. minutum methanol extracts vary in the order M. koenigii > M. minutum at a given concentration of an antioxidant, indicating that the total phenol concentration is responsible for the final antioxidant activity. Therefore, it is evident that the antioxidant activities of M. koenigii were correlated to various phenolic compounds. Previous studies have indicated carbazole alkaloids and terpenes [8] as the major components responsible for the antioxidant activities of M. koenigii. The presence of carbazole alkaloids and terpenes was further supported in our phytochemical analysis. Similarly. The presence of many versatile compounds such as coumarins and glycosides as antioxidants in M. minutum [36].
The antibacterial assay of the plant leaf extracts is done by the disk diffusion assay. The antibacterial susceptibility test of the methanolic leaf extracts from the M. koenigii and M. minutum showed varying degrees of antibacterial activities for the selected bacterial species (Table 5). The antibacterial standard used was ciprofloxacin, a broad-spectrum antibacterial drug [37]. It showed higher inhibitory properties towards all the studied gram-negative and positive bacterial species of the study.
Table 5.
Zone of inhibition affected by M. koenigii and M. minutum plant leaf extracts using the Disc Diffusion method.
| Plant species/Standard | Concentration mg/μL | Zone of inhibition (DIZ)/(mm) |
||
|---|---|---|---|---|
| SA | PA | EC | ||
| M. koenigii | 0.2 | 7.4 ± 0.2a | 5.7 ± 0.2b | 5.8 ± 0.2b |
| 0.6 | 9.7 ± 0.4a | 5.9 ± 0.2b | 6.0 ± 0.0b | |
| 1.0 | 11.9 ± 0.3a | 7.1 ± 0.3b | 6.2 ± 0.2c | |
| M. minutum | 0.2 | 6.1 ± 0.3a | 5.9 ± 0.2a | 5.8 ± 0.3a |
| 0.6 | 6.6 ± 0.3a | 5.9 ± 0.2b | 6.4 ± 0.2a’b | |
| 1.0 | 6.8 ± 0.3a | 6.0 ± 0.0b | 7.7 ± 0.3c | |
| Ciprofloxacin (μg/mL) | 100 | 18.0 ± 0.2a | 27.0 ± 0.0b | 29.1 ± 0.3c |
SA – S. aureus, PA – P. aeruginosa, EC – E. coli, DIZ – Diameter of Inhibitory Zone.
All the data is presented as mean ± SD of the three replicates. Mean followed by a different letter in the same row differs significantly (p ≤ 0.05).
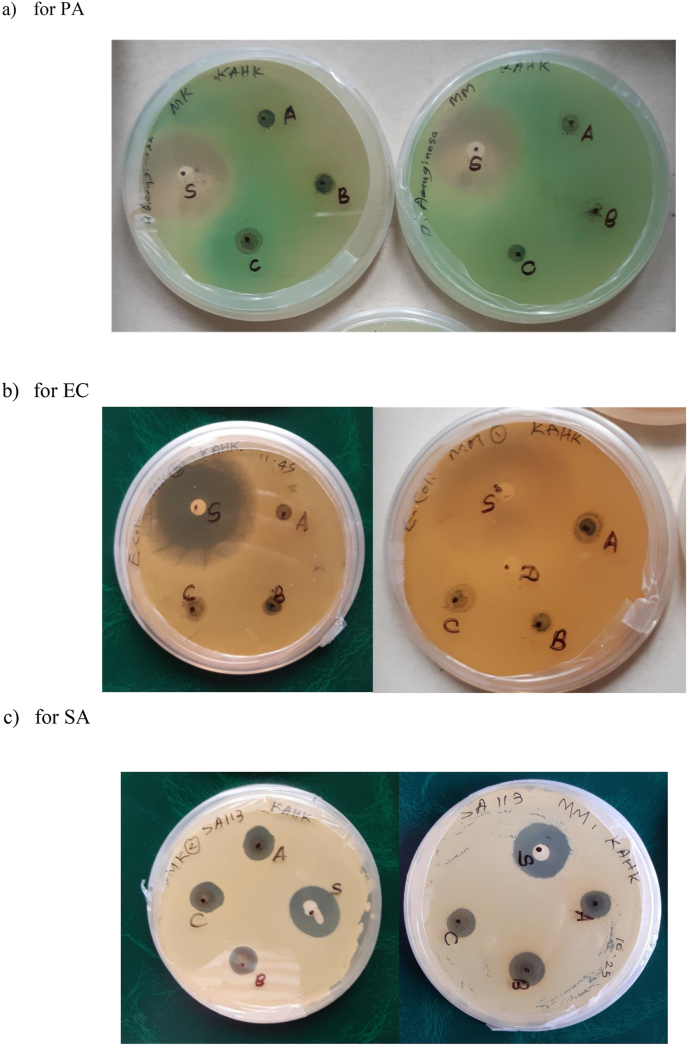
The inhibitory activity of all methanolic extracts (0.2. 0.6 and 1.0 mg/μL) varied against the tested isolates Staphylococcus aureus 113, Escherichia coli ATCC 1858, and Pseudomonas aeruginosa ATCC 27853 with DIZ values ranging from 5.7 to 11.9 mm as indicated in Figure 2. The least DIZ was produced by the M. koenigii leaf extract (0.2 mg/μL) on P. aeruginosa (5.7 mm). In comparison, the same extract exhibited the maximum value (11.9 mm) at a higher concentration (1.0 mg/μL) against S. aureus. As it could be seen from Table 5, the DIZ of M. koenigii leaf extracts towards the studied bacterial species were increased with the concentrations. However, the DIZ of the M. koenigii extracts at the concentrations of 0.2, and 0.6 mg/μL against E. coli and P. aeruginosa had no significant variation. The highest DIZ was observed towards gram-positive S. aureus (11.9 ± 0.3 mm) for 1 mg/mL extract of M. koenigii.
Figure 2.
Agar plates for Murraya koenigii (MK) and Micromelum minutum (MM), SA – S. aureus, PA – P. aeruginosa, EC – E. coli. (A-1.0 mg/μL, B- 0.6 mg/μL, C- 0.2 mg/μL, D-negative control (DMSO) S - Ciprofloxacin.
The M. minutum extract at each concentration (0.2. 0.6 and 1.0 mg/μL) had only a slight variation in DIZ against all the bacterial species tested and only had significantly (p < 0.05) different DIZ at the 0.6 and 1.0 mg/μL concentrations. Moreover, M. minutum showed its highest DIZ towards gram-negative E. coli (7.7 ± 0.3 mm for 1 mg/mL extract).
M. koenigii showed a pronounced inhibitor effect towards S. aureus and P. aeruginosa (p < 0.05) compared to M. minutum. Nevertheless, M. minutum showed higher antibacterial activity towards E. coli compared to M. koenigii (p < 0.05) as shown in Table 5. Previous reports support the current results regarding the antibacterial potential of M. koenigii [4]. However, no inhibition has not been detected for S. aureus at 0.2 mg/μL M. koenigii methanolic leaf extract [38]. According to our investigations, both 0.2 and 1.0 mg/μL methanolic leaf extracts of M. koenigii showed a significant DIZ, 7.4 ± 0.2 mm and 11.9 ± 0.3 mm respectively against S. aureus.
Although Chung et al. reported no inhibition was detected from M. minutum methanol leaf extract towards the bacterial species, S. aureus, E. faecalis, E. coli, and P. aeruginosa [39]. The current study indicated considerable DIZ towards S. aureus, P. aeruginosa, and E. coli. Furthermore, Nakahara et al. have reported significant DIZ values for S. aureus and E. coli for Mahanine, an isolated bioactive compound from M. minutum [40].
The antibacterial susceptibility pattern of bacterial isolates is shown in Table 6. The antibiotic ciprofloxacin exhibited the maximum zone of inhibition against the strain E.coli (29.1 mm) and the minimum against the strain S. aureus (18 mm) even at a lower concentration (100 μg/mL) compared to the leaf extracts. M. koenigii exhibited the maximum zone of inhibition against S. aureus (11.9 mm) and the minimum against E.coli (6.2 mm). On the contrary, E.coli was found to have the maximum zone of inhibition, and P. aeruginosa with the least DIZ reported in M.minutum. Since M. koenigii extracts exhibited maximum activity against S. aureus, mainly located on most healthy individuals' skin and mucous membranes causing pulmonary infections, meningitis, and many other urinary tract infections [41]. Therefore, M. koenigii extracts may find significant applications in treating skin rashes, pulmonary infections, and other urinary tract diseases. On the contrary, E.coli is associated with diarrhea, pneumonia, urinary tract inflammations, and M. minutum may be more effective in treating diarrhea, pneumonia, and urinary tract inflammations.
Table 6.
Antibiotic susceptibility pattern of bacteria species.
| Extract/Antibiotic | Concentration (μg/mL) | DIZ (mm) |
||
|---|---|---|---|---|
| SA | PA | EC | ||
| Ciprofloxacin | 100 | 18.0 ± 0.2a | 27.0 ± 0.0b | 29.1 ± 0.3c |
| M. koenigii | 1000 | 11.9 ± 0.3a | 7.1 ± 0.3b | 6.2 ± 0.2c |
| M .minutum | 1000 | 6.8 ± 0.3a | 6.0 ± 0.0b | 7.7 ± 0.3c |
SA – S. aureus, PA – P. aeruginosa, EC – E. coli, DIZ – Diameter of Inhibitory Zone.
All the data is presented as mean ± SD of the three replicates. Mean followed by a different letter in the same row differs significantly (p ≤ 0.05).
4. Conclusion
The present study revealed that the two medicinally important plants M. koenigii and M. minutum, exhibited good antioxidant potentials in the various assays conducted, TPC, TFC, and DPPH assay. A direct relationship was demonstrated in the study between the TPC, TFC, and antioxidant activity of M. koenigii and M. minutum leaf extracts, former species with greater antioxidant activity. The protective activity of these plants could be attributed to the presence of tannins, flavonoids, phenols, and alkaloids, as confirmed by the preliminary phytochemical screening of the methanol leaf extracts. Particularly, M. koenigii showed more dominant activities in the assays conducted compared to M. minutum. The nutrient composition in terms of ash, crude protein, and fat was mostly high in M. koenigii, confirming to be a better supplement for nutrients. The methanol extract showed significantly different towards S. aureus, E. coli, and P. aeruginosa. The highest inhibitory activity was observed in M. koenigii towards S. aureus, and M. minutum showed the highest inhibitory activity towards E. coli at 1.0 mg/mL concentration. Therefore, M. koenigii and M. minutum, leaves can be explored as a promising source of antioxidants and antibacterial compounds in pharmaceutical industry.
Declarations
Author contribution statement
Kumara K. A. H.: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kaushalya K. A. D.: Performed the experiments; Analyzed and interpreted the data.
Chandrika U. G., Alwis D. D. D. H.: Conceived and designed the experiments; Analyzed and interpreted the data.
Abeysinghe D. T: Performed the experiments; Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by National Research Council of Sri Lanka (18-063).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We wish to thank Mrs. T. Somaratne, Mr. K. C. K. Deraniyagala, and Mr. H. T. Arachchi for the laboratory facilities and technical support.
References
- 1.Chanda S., Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr. J. Microbiol. Res. 2009;3:981–996. [Google Scholar]
- 2.Gupta D., Kumar M., Gupta V. An in vitro investigation of antimicrobial efficacy of Euphorbia Hirta and Murraya koenigii against selected pathogenic microorganisms. Asian J. Pharmaceut. Clin. Res. 2018;11:359–363. [Google Scholar]
- 3.Taguiling M.A.L.G. Phytochemical screening and antibacterial activity of mircromelum minutum. Int. J. Green Herb. Chem. 2016;5:171–179. [Google Scholar]
- 4.Gahlawat D.K., Jakhar S., Dahiya P. Murraya koenigii (L.) Spreng: an ethnobotanical, phytochemical and pharmacological review. J. Pharmacogn. Phytochem. 2014;3:109–119. [Google Scholar]
- 5.Kesari A.N., Kesari S., Singh S.K., Gupta R.K., Watal G., Kesari G., N A., Kesari S., Singh S.K., Gupta R.K., Watal Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J. Ethnopharmacol. 2007;112:305–311. doi: 10.1016/j.jep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Itharat A E.-A.E., Houghton P.J. In vitro cytotoxic Activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharmacol. 2004;90:33–38. doi: 10.1016/j.jep.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Lekphrom R., Kanokmedhakul S., Kukongviriyapan V., Kanokmedhakul K. C-7 oxygenated coumarins from the fruits of micromelum minutum. Arch Pharm. Res. (Seoul) 2011;34:527–531. doi: 10.1007/s12272-011-0402-y. [DOI] [PubMed] [Google Scholar]
- 8.Gaurav N. Phytochemical analysis and antibacterial activity of different leaf extracts of Murraya koenigii. Int. J. Biochem. Biomol. 2016:1–5. [Google Scholar]
- 9.Samanta S.K., Kandimalla R., Gogoi B., Dutta K.N., Choudhury P., Deb P.K., Devi R., Pal B.C., Talukdar N.C. Phytochemical portfolio and anticancer activity of Murraya koenigii and its primary active component, mahanine. Pharmacol. Res. 2018;129:227–236. doi: 10.1016/j.phrs.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Koriem K., Aminuddin M., Kader A., Sheikh N. Antihyperglycemic, antihyperlipidemic and antiapoptotic activities of micromelum minutum seeds in diabetic rats. J. Mol. Genet. Med. 2013;(2013):1–8. [Google Scholar]
- 11.Lawal H.A., Atiku M.K., Khelpai D.G., Wannang N.N. Hypoglycaemic and hypolipidaemic effect of aqueous leaf extract of Murraya koenigii in normal and alloxan-diabetic rats. Niger. J. Physiol. Sci. 2008;23:37–40. doi: 10.4314/njps.v23i1-2.54919. [DOI] [PubMed] [Google Scholar]
- 12.Butt A., Waris N., Baqa K., Nazim U., Naz A., Abbasi S.R., Begum S. Comparative effect of Beta blocker-Atenolol and Murraya koenigii (L.) spreng (Curry leaves) on cardiac enzyme (CK-MB) level in male albino rats. Pak. J. Pharm. Sci. 2019;32:1643–1648. [PubMed] [Google Scholar]
- 13.Hu J., Wang J., Gan Q., Ran Q., Lou G., Xiong H., Peng C., Sun J., Yao R., Huang Q. Impact of red yeast rice on metabolic diseases: a review of possible mechanisms of action. J. Agric. Food Chem. 2020;68:10441–10455. doi: 10.1021/acs.jafc.0c01893. [DOI] [PubMed] [Google Scholar]
- 14.Farnsworth N.R. Ethnopharmacology and future drug development: the North American experience. J. Ethnopharmacol. 1993;38:145–152. doi: 10.1016/0378-8741(93)90009-t. [DOI] [PubMed] [Google Scholar]
- 15.AOAC . Maryland; USA: 2005. Official Methods of Analysis of the Association of Official Analytical Chemists International. [Google Scholar]
- 16.Ruth O., Hamid N., Ishmael C., Hamid N. Vernonia cinerea leaves as the source of phenolic compounds , antioxidants , and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018;122:533–544. [Google Scholar]
- 17.Somarathna T., Fernando W.M.A.D.B., Ranaweera K.K.D.S., Premakumara G.A.S., Abeysinghe T., Weerakkody N.S. Antimicrobial activity and phytochemical screening of Alpinia malaccensis (Ran-kiriya) against food-borne bacteria. J. Appl. Microbiol. 2018;125:1276–1285. doi: 10.1111/jam.14039. [DOI] [PubMed] [Google Scholar]
- 18.Mcdowell L., Wilkinson N., Madison R., Felix T. 2007. Vitamins and Minerals Functioning as Antioxidants with Supplementation Considerations. [Google Scholar]
- 19.Li A. Proximate and mineral composition of the marchubeh(Asparagus officinalis) World Dairy Food Sci. 2009;4:142–149. [Google Scholar]
- 20.Vats S., Singh M., Sardana H. Phytochemica screening and antimicrobial activity of roots of Murraya koenigii. Braz. J. Microbiol. 2011;42:1569–1573. doi: 10.1590/S1517-838220110004000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hetty Manurung R.A., Nugroho R.A., Puspita Sari Y., Chernovita R., Auliana Phytochemical analysis and antioxidant activity of leaves extracts of endemic plant jahe. Int. J. Sci. Technol. Res. 2019;8 [Google Scholar]
- 22.Hassan A., Akmal Z., Khan N. The phytochemical screening and antioxidants potential of schoenoplectus triqueter L. Palla. J. Chem. 2020:3865139. [Google Scholar]
- 23.Danish M., Hisamuddin, Robab M.I. Vitro studies on phytochemical screening of different leaf extracts and their antifungal activity against seed borne pathogen Aspergillus Niger. J. Plant Pathol. Microbiol. 2016;6:1–3. [Google Scholar]
- 24.B.C. Cox-Georgian D., Ramadoss N., Dona C. Therapeutic and medicinal uses of terpenes. In: Joshee N., Dhekney S., Parajuli P., editors. Medicinal Plants. Springer; 2019. Cham, Springer, Cham. [Google Scholar]
- 25.Abulude F.O. Phytochemical screnning and mineral contents of leaves of some Nigerian woody plants. Res. J. Phytochem. 2007;1:33–39. [Google Scholar]
- 26.Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyama H., Fujii K., Yamasaki O., Oono T., Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001;48:487–491. doi: 10.1093/jac/48.4.487. [DOI] [PubMed] [Google Scholar]
- 28.ChV S.I.M. Antioxidant and biological activities of three morphotypes of Murraya koenigii L. From uttarakhand. J. Food Process. Technol. 2013;4 [Google Scholar]
- 29.Zahin M., Aqil F., Husain F.M., Ahmad I. Antioxidant capacity and antimutagenic potential of Murraya koenigii. BioMed Res. Int. 2013:263509. doi: 10.1155/2013/263509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietta P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 31.Kassim N.K., Lim P.C., Ismail A., Awang K. Isolation of antioxidative compounds from Micromelum minutum guided by preparative thin layer chromatography-2,2-diphenyl-1-picrylhydrazyl (PTLC-DPPH) bioautography method. Food Chem. 2019;272:185–191. doi: 10.1016/j.foodchem.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 32.Sulaiman S.F., Sajak A.A.B., Ooi K.L., Supriatno, Seow E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011;24:506–515. [Google Scholar]
- 33.Ayoola G.A., Coker H.A.B., Adesegun S.A., Adepoju-bello A.A., Obaweya K., Ezennia E.C., Atangbayila T.O. Method spots test. Trop. J. Pharmaceut. Res. 2008;7:1019–1024. [Google Scholar]
- 34.Hossain M.A., AL-Raqmi K.A.S., AL-Mijizy Z.H., Weli A.M., Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac. J. Trop. Biomed. 2013;3:705–710. doi: 10.1016/S2221-1691(13)60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamtekar S., Keer V., Patil V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. Appl. Pharmaceut. Sci. 2014;4:61–65. [Google Scholar]
- 36.Kassim K., Cee L., A I., Awang K. Isolation of antioxidative compounds from Micromelum minutum guided by preparative thin layer chromatography-2,2-Diphenyl-1-picrylhydrazyl (PTLC-DPPH) bioautography method. Food Chem. 2018;272 doi: 10.1016/j.foodchem.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 37.Davis R., Markham A., Balfour J.A. Ciprofloxacin-an updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs. 1996;51:1019–1074. doi: 10.2165/00003495-199651060-00010. [DOI] [PubMed] [Google Scholar]
- 38.Jakhar S., Gahlawat D.K., Dahiya S., Swami U., Verma M., Dahiya P. Antibacterial and antioxidant potential of leaf and seed extracts of Murraya koenigii (linn.) spreng. Br. Microbiol. Res. J. 2015;10:1–7. [Google Scholar]
- 39.Chung P.Y., Chung L.Y., Ngeow Y.F., Goh S.H., Imiyabir Z. Antimicrobial activities of Malaysian plant species. Pharm. Biol. 2004;42:292–300. [Google Scholar]
- 40.Nakahara K., Trakoontivakorn G., Alzoreky N.S., Ono H., Onishi-Kameyama M., Yoshida M. Antimutagenicity of some edible Thai plants, and a bioactive carbazole alkaloid, mahanine, isolated from Micromelum minutum. J. Agric. Food Chem. 2002;50:4796–4802. doi: 10.1021/jf025564w. [DOI] [PubMed] [Google Scholar]
- 41.Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expet Rev. Dermatol. 2010;5:183–195. doi: 10.1586/edm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.