Abstract
Background:
Parkinson disease (PD) is the second most common neurodegenerative disease, affecting 2% of the population over 65 years of age. PD diagnosis is based on clinical examination and can only be confirmed during autopsy. In 2018, we reported that heme oxygenase-1 (HO-1), an inducible stress response protein important for heme catabolism and implicated in PD pathology, was higher in PD saliva relative to healthy controls, suggesting that salivary HO-1 may serve as a potential biomarker of PD.
Objectives:
To ascertain whether HO-1 protein levels are elevated in PD saliva relative to degenerative neurological, non-degenerative neurological and healthy controls.
Methodology:
The study included 307 participants comprising 75 participants with idiopathic PD and 3 control groups: 162 non-neurological, 37 non-PD degenerative neurological, and 33 non-degenerative neurological participants. Salivary HO-1 and total protein concentrations were measured using ELISA and BCA assay, respectively. Receiver operating characteristic (ROC) curves were used to estimate model discrimination. Analyses were adjusted by age, sex, total protein, and relevant comorbidities.
Results:
Elevated HO-1 concentrations were observed in the PD group and other neurodegenerative conditions compared to subjects with no neurological or non-degenerative neurological conditions. ROC curves using HO-1 levels and covariates yielded areas under the curve above 85% in models for PD or neurodegenerative conditions versus controls.
Conclusions:
Salivary HO-1 concentrations in combination with covariates may provide a biomarker signature that distinguishes patients with neurodegenerative conditions from persons without.
Classification of evidence:
This study provides Class III evidence that salivary HO-1 multivariable models can distinguish neurodegenerative conditions.
Keywords: Heme oxygenase-1, Parkinson disease, saliva, biomarker, neurodegenerative conditions
Introduction
Neurological disorders are the leading sources of disability for the global population. 1 Among these neurological disorders, Parkinson disease (PD) is the fastest growing in terms of prevalence. 1 PD currently affects 2% of the global population over 65 years of age. 2 In the U.S. alone, PD was estimated to be associated with $51.9 billion in total economic cost in 2017 for approximately 1 million individuals diagnosed with PD, with $25.4 billion in direct medical cost for diagnosis, treatments, medical appointments, and patient care. 3
Despite the increasing prevalence of PD worldwide and the rising total economic cost of this disease, the etiology of neurodegeneration in PD patients is still not well understood. The complexity of PD neuropathology is reflected in its clinical manifestations, which encompass motor symptoms like bradykinesia and resting tremors, as well as, non-motor symptoms such as constipation and REM sleep behavior disorder, which can occur years to decades before the onset of motor symptoms. 4 The diversity in PD symptom manifestation in addition to symptom overlap with other neurodegenerative and Parkinson-like conditions makes PD diagnosis difficult. The adoption of the United Kingdom Parkinson’s Disease Society Brain Bank (UKPDSBB) criteria and the Movement Disorder Society Diagnostic Criteria for PD (MDS-PD) as gold standards for diagnosis has facilitated the evaluation of patients with movement disorders, as it accounts for both non-motor and motor symptoms observed in PD and PD progression. 5 Nevertheless, PD is still prone to misdiagnosis with diagnostic accuracies at initial assessment ranging between 26% and 91%. 6 Though genetic screening, neuroimaging, and olfactory tests exist to assist in PD diagnosis, many are costly, labor-intensive, and not universally available.7–9
Several chemical analytes in saliva have been investigated as potential biomarkers for PD, including α-synuclein, protein deglycase DJ-1, and heme oxygenase-1 (HO-1), an inducible enzyme that degrades heme to biliverdin, ferrous iron, and carbon monoxide.10–16 The HMOX1 gene contains a panoply of regulatory elements that allows it to respond to numerous stressors such as heme, dopamine, nitric oxide, TH1 cytokines, and other oxidative and inflammatory stimuli.17–19 During acute oxidative stress-induced neurotoxicity, HO-1 has been shown to have cytoprotective effects; however, chronic expression of HO-1 has been proposed to be detrimental to cell health and survival. 20 Alongside hallmarks of PD pathogenesis, such as the formation of Lewy body inclusions and loss of dopaminergic neurons, post-mortem PD brain specimens also exhibit other core pathological features such as increased oxidative stress, excessive iron levels, mitochondria dysfunction, and macroautophagy in neurons of the substantia nigra.21–23 These features were mirrored during sustained upregulation of HO-1 in vitro experiments using rat astroglial cells and in a GFAP.HMOX1 transgenic mice model exhibiting a parkinsonian phenotype comprising of neurodegeneration, hypodopaminergia, altered gait, locomotor incoordination, and reduced olfaction.24–26 Post-mortem PD brain specimens were shown to have highly augmented HO-1 immunoreactivity in astrocytes of the substantia nigra and were recapitulated in peripheral biofluids of PD patients.10,27–29
Building upon considerable evidence implicating chronic HO-1 expression in PD pathogenesis from humans and animal models, we sought to evaluate HO-1 protein levels in human saliva as a potential biomarker for PD.24–26,28,29 In 2018, we reported the presence of full-length HO-1 protein in human saliva for the first time and determined that salivary HO-1 protein concentrations were significantly elevated in early-stage PD relative to healthy controls. 10 To further ascertain the specificity of salivary HO-1 for PD, we compared salivary HO-1 levels in PD participants against persons with other neurodegenerative disorders, non-degenerative neurological conditions, and in non-neurological controls in the current study. We anticipate that similar to our pilot study, salivary HO-1 of PD participants will be higher than control groups. The advent of a salivary biomarker would greatly facilitate the clinical evaluation of an increasingly prevalent neurological disease such as PD, in a non-invasive, simple, and relatively inexpensive manner.
Methods
Study design and population
This case-control study was approved by the Research Ethics Committee of the Jewish General Hospital (JGH; Montreal, Canada, No. 2019-1220). Eligible participants’ samples were identified based on the presence of a neurological condition (PD, non-PD degenerative neurological condition, or non-degenerative neurological condition) or lack thereof (non-neurological control). Samples of participant who matched our enrolment criteria from the JGH saliva biobank were included in this study and formed a random series. Participants in all groups with a history of cigarette smoking within the past year, history of oral cancer, active systemic inflammatory disease, current alcoholism, and drug abuse at the time of saliva collection in addition to evidence of atypical or familial parkinsonism were not included in our study, as per our exclusion criteria. All participants in the saliva biobank provided written informed consent.
Participants were recruited from the Departments of Medicine, Dentistry, Neurology, and Ophthalmology at the JGH. Participants clinically diagnosed with idiopathic PD or other neurological disorders were recruited from the Department of Neurology at the JGH with their diagnosis corroborated by serial evaluations. Idiopathic PD participants fulfilled the UKPDSBB Criteria. The sample size was determined based on the recommended minimum sample size criterion of greater than 10 event per degree of freedom proposed by Harrell. 30
Clinical and demographic data
Demographics and medical histories were taken via a medical history questionnaire and were available on all participants recruited to the JGH saliva biobank. PD participants’ Hoehn and Yahr (HY) scores were based on clinical status at the time of saliva collection noted in their medical charts.
Dosages and type of pro-dopamine medication varied between PD participants. Doses of pro-dopamine medications were provided in medical charts of 70 PD participants and were converted to levodopa equivalent daily dose (LEDD) using a conversion calculator (https://www.parkinsonsmeasurement.org/toolBox/levodopaEquivalentDose.htm).
Saliva collection and processing
Unstimulated whole saliva samples of participants were collected by passive drooling at least 30 minutes after food or liquid ingestion. Samples were kept at 4°C for a maximum of 3 hours prior to centrifugation at 7826 × g (10 000 rpm) for 20 minutes at 4°C to reduce viscosity and remove food debris. The supernatant was aliquoted and stored at −80°C until further analysis. COmplete™ Mini Protease Inhibitor Cocktail (Roche, Cat. No. 11836153001) was added upon the first thaw and samples were re-processed through centrifugation prior to use (same as above). Saliva samples used in this study were kept frozen and stored between October 2012 and January 2020. Long-term storage of saliva samples was previously determined to have no significant effect on composition. 31
Quantification of salivary HO-1 and total protein levels
Salivary HO-1 levels were measured using human HO-1 sandwich enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer instructions (Abbexa, Cat. No. abx252635). The detection range of the HO-1 ELISA kit is 0.156−10 ng/ml with a sensitivity of 0.1 ng/ml and intra-assay and inter-assay variabilities of <10%. Samples were diluted to 10% saliva and 90% dilution buffer for HO-1 measurements and undiluted for measurements of total protein via bicinchoninic acid assay (BCA; Bio-Rad DC TM Protein Assay, Cat. No. 500). HO-1 and total protein measurements were run in duplicate. The medical history of participants included in the study was available during quantification of salivary HO-1 and total protein levels of all samples.
Statistical analysis
Demographic and comorbidity data distributions were presented as means and standard deviations (or median and interquartile range if non-normal) for continuous variables, and frequencies and proportions of categorical variables. Student’s t-test (or Mann-Whitney test for non-normal) and Chi-Square test were used to compare continuous and categorical variables, respectively.
For the primary analyses, analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were used to assess the difference in levels of salivary HO-1 between the 3 control and PD groups, with and without adjustments for total protein measurements, age, sex, and relevant comorbidities. Relevant comorbidities were selected based on previous studies, 10 and included arthritis, thyroid conditions, cardiac conditions, and diabetes mellitus. We used univariate logistic models to distinguish between PD and non-neurological controls, PD and non-degenerative neurological controls, and PD and degenerative neurological controls. Multivariable logistic regression models were then constructed to adjust for the relevant comorbidities. Receiver operative characteristic (ROC) curves were developed to assess how well salivary HO-1 distinguishes cases and controls for each multivariable logistic model. All the models were internally validated using 10-fold cross-validation. The optimal cut-off for ROC curves were determined using the Youden index. The sample size of the control groups satisfies the minimum limiting sample requirement for multivariable regression analysis, as suggested by Harrell et al. 32
For secondary analyses, methods used in our primary analyses were used to assess the difference in the level of salivary HO-1 between participants with and without neurodegenerative conditions, the odds of a neurodegenerative condition, and how well salivary HO-1 can distinguish cases from non-neurodegenerative controls. Analyses also included linear regression models to evaluate the magnitude of change in HO-1 expected when total protein or LEDD increased, using the Pearson’s correlation to determine correlation. Additionally, ANCOVA was employed to assess the difference in mean values of HO-1 levels and LEDD between HY stages both against the non-PD participants and among PD participants.
Kolmogorov-Smirnov test and observation of Quantile-Quantile (Q-Q) plot was used to evaluate the normality of the sample population and Levene’s test served to ascertain the homogeneity of variance. Complete case analysis was used when the proportion of missing values was less than 5%. The alpha level was 0.05 for all analyses. Data were analyzed using R version 4.0.2.
Results
Demographic and comorbidity distribution among study groups
A total of 307 participants’ samples were included in the study (Figure 1). The groups comprised 75 PD, 37 degenerative neurological control (20 Alzheimer disease (AD) and 17 mild cognitive impaired participants (MCI)), 33 non-degenerative neurological control (16 multiple sclerosis, 12 epilepsy, 3 essential tremor, 3 stroke, and 3 participants with nerve pain), and 162 non-neurological control participants. Table 1 shows the demographics and comorbidity distribution among study groups and their relation to HO-1 between groups.
Figure 1.
Flowchart of study participants.
Table 1.
Demographic, comorbidity distribution, and salivary total protein between study groups and their relation to salivary HO-1.
| Groups | Non-neurological Control | Degenerative neurological Control | Non-degenerative neurological Control | PD | All non-neurodegenerative Control | All neurodegenerative condition | P value |
|---|---|---|---|---|---|---|---|
| N | 162 | 37 | 33 | 75 | 195 | 112 | |
| Protein (mean (SD)) (mg/ml) | 2.81 (1.39) | 3.39 (1.61) | 3.31 (1.34) | 3.99 (1.98) | 2.9 (1.39) | 3.79 (1.88) | <.01 |
| Age (mean(SD)) | 62.19 (12) | 80.14 (6) | 60.7 (14) | 72.65 (11) | 62 (12) | 75 (10) | <.01 |
| Female (%) | 99 (61%) | 21 (57%) | 24 (73%) | 18 (24%) | 99 (51%) | 39 (35%) | <.01 |
| Male (%) | 63 (39%) | 16 (43%) | 9 (27%) | 57 (76%) | 63 (32%) | 73 (65%) | |
| Arthritis (%) | 43 (27%) | 12 (32%) | 10 (27%) | 30 (40%) | 53 (27%) | 42 (38%) | .08 |
| No arthritis (%) | 119 (74%) | 25 (68%) | 23 (73%) | 45 (60%) | 142 (73%) | 70 (63%) | |
| Thyroid condition (%) | 29 (18%) | 4 (11%) | 10 (30%) | 16 (21%) | 39 (20%) | 20 (18%) | .76 |
| No-thyroid condition (%) | 133 (82%) | 33 (89%) | 23 (70%) | 59 (79%) | 156 (80%) | 92 (82%) | |
| Cardiac condition (%) | 9 (6%) | 9 (24%) | 3 (9%) | 12 (16%) | 12 (6%) | 21 (19%) | <.01 |
| No-cardiac condition (%) | 153 (94%) | 28 (76%) | 30 (91%) | 63 (84%) | 183 (94%) | 91 (81%) | |
| Diabetes (%) | 19 (12%) | 6 (16%) | 3 (9%) | 4 (5%) | 22 (11%) | 10 (9%) | .65 |
| No diabetes (%) | 143 (88%) | 31 (84%) | 30 (91%) | 71 (95%) | 173 (89%) | 102 (91%) |
Salivary HO-1 concentration
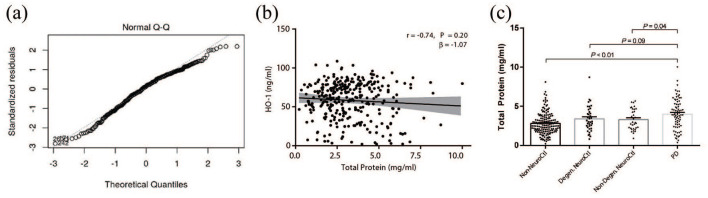
The residuals of HO-1 protein level measured by ELISA were determined to have a normal distribution based on the Q-Q plot and Kolmogorov-Smirnoff test (P > .05, Figure 2a). Additionally, Levene’s test for equal variance was assessed (P > .05).
Figure 2.
Salivary total protein and HO-1 levels between groups. A) Normal distribution visually assessed by Q-Q plot of salivary HO-1 protein level residuals and threshold 45°-line y=x B) Linear regression and correlation analyses between salivary HO-1 and total protein of study’s sample population. C) Unadjusted mean salivary total protein measured by BCA assay of non-neurological control group (n=162), degenrative neurological controls (n=37), and non-degenrative neurological controls(n=33) and PD group (n=75). Statistical analysis performed using ANOVA with a=0.05, error bars indicate the mean with SEM.
Table 2 shows ANOVA and ANCOVA analysis of salivary HO-1 between groups. ANOVA showed significantly higher salivary mean HO-1 protein concentration in the PD group (63.56 ng/ml) in comparison to non-neurological (55.79 ng/ml, P = .02) and non-degenerative neurological controls (50.55 ng/ml, P < .01). No significant difference was observed between PD and degenerative neurological controls (65.69 ng/ml, P = .65). Similar to results of the unadjusted analysis, salivary HO-1 levels were significantly elevated in the PD group (63.40 ng/ml) relative to non-neurological controls (55.13 ng/ml, P = .03) and non-degenerative neurological controls (51.61 ng/ml, P = .03) after adjusting for age, sex, total protein, and relevant comorbidities. The difference between the PD group and the degenerative neurological controls (60.93 ng/ml, P = .63) after adjustments remained non-significant. Additionally, a borderline association was observed between salivary HO-1 and age (P = .08) and thyroid conditions (P = .09), and a significant association was found with salivary total protein (P = .02).
Table 2.
Salivary HO-1 levels (adjusted by age, sex, total protein, and comorbidities).
| Primary analysis groups | n | Unadjusted analysis | Adjusted by age, sex, and comorbidities | ||||
|---|---|---|---|---|---|---|---|
| Mean (ng/ml) | 95% CI | P value | Mean (ng/ml) | 95% CI | P value | ||
| Non-neurological controls | 162 | 55.79 | 52.17−59.41 | .02 | 55.13 | 48.75−61.51 | .03 |
| Degenerative neurological controls | 37 | 65.69 | 58.12−73.26 | .65 | 60.93 | 51.25−70.62 | .63 |
| Non-degenerative neurological controls | 33 | 50.55 | 42.53−58.56 | <.01 | 51.61 | 42.19−61.03 | .03 |
| PD (reference) | 75 | 63.56 | 58.24−68.88 | 63.40 | 55.75−71.05 | ||
| Secondary analysis groups | n | Mean (ng/ml) | 95% CI | P value | Mean (ng/ml) | 95% CI | P value |
| Non-neurodegenerative controls (reference) | 195 | 54.90 | 51.61−58.20 | <.01 | 54.39 | 46.27−60.51 | .02 |
| Neurodegenerative conditions | 112 | 64.26 | 59.92−68.61 | 62.46 | 55.56−69.37 | ||
ANCOVA adjustments by total protein (Pprimary = .02; Psecondary = .02), age (Pprimary = .08; Psecondary = .08), sex (Pprimary = .51; Psecondary = .61), presence for arthritis (Pprimary = .68; Psecondary = .72), diabetes (Pprimary = .73; Psecondary = .69), thyroid (Pprimary = .09; Psecondary = .09), and heart conditions (Pprimary = .24; Psecondary = .27).
Subsequent secondary analysis of total protein data revealed no correlation between HO-1 and total protein concentrations (correlation coefficient (r) = −0.74, slope (β) = −1.07 per 1 mg, P = .20, Figure 2b). ANOVA showed higher mean total protein concentration in the PD subjects (3.99 mg/ml) relative to non-neurological (2.81 mg/ml, P < .01) and non-degenerative neurological controls (3.31 mg/ml, P = .04, Figure 2c). Higher salivary mean total protein concentration in PD subjects relative to degenerative neurological controls (3.39 mg/ml, P = .09, Figure 2c) was also observed, although this did not reach statistical significance. Although total protein was determined not to be associated with HO-1, it was kept as a factor in adjustments of HO-1 values in both primary and other secondary analyses.
Salivary HO-1 in non-neurodegenerative and neurodegenerative groups
Secondary analyses indicated no statistically significant difference in salivary HO-1 concentration between PD subjects and degenerative neurological controls (P = .63) and between non-neurological and non-degenerative neurological controls (P = .26, Supplementary Table 1). The sample population was re-classified in 2 groups comprising subjects with either neurodegenerative conditions (PD, AD, and MCI, n = 112) or subjects without neurodegenerative conditions (n = 195) to determine if HO-1 levels in participants with neurodegenerative conditions were significantly different from the other participants. Demographic and comorbidity data of the redefined groups are shown in Table 1.
With the latter classification, ANCOVA showed a significantly higher salivary mean HO-1 protein level in the neurodegenerative group (64.46 ng/ml) compared to the non-neurodegenerative controls (54.39 ng/ml, P = .02) after adjusting for total protein, age, sex, and covariates (Table 2). Furthermore, salivary HO-1 was found to be associated to total protein (P = .02) and borderline associations were found between HO-1 and age (P = .08) and thyroid conditions (P = .09, Table 2).
Logistic regression and ROC curve analyses
Table 3 shows that participants with PD were more likely to present higher salivary HO-1 levels than the non-neurological controls (OR = 1.02, P = .01) and non-degenerative neurological control (OR = 1.04, P < .01), regardless of their total protein, sex, age, and comorbidity data. Furthermore, logistic regression models of the re-classified groups showed that participants classified with a neurodegenerative condition showed greater odds of higher levels of salivary HO-1 than non-neurodegenerative controls, regardless of their total protein, sex, age, and comorbidity data (OR = 1.02, P < .01, Table 3).
Table 3.
Logistic regression models.
| PD v. | Neurodegenerative conditions v. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-neurological control | Degenerative neurological control | Non-degenerative neurological control | Non-neurodegenerative control | ||||||||||
| Univariate logistic regression model | |||||||||||||
| Predictor | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| HO-1 (ng/ml) | 1.01 | 1.00−1.03 | .02 | 1 | 0.98−1.01 | .63 | 1.03 | 1.01−1.04 | <.01 | 1.02 | 1.01−1.03 | <.01 | |
| Multivariate logistic regression model | |||||||||||||
| Predictor | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| HO-1 (ng/ml) | 1.02 | 1.00−1.03 | .01 | 1.00 | 0.97−1.02 | .75 | 1.04 | 1.01−1.07 | <.01 | 1.02 | 1.01−1.03 | <.01 | |
| Total protein (mg/ml) | 1.56 | 1.27−1.97 | <.01 | 1.25 | 0.94−1.70 | .13 | 1.34 | 0.97−1.93 | .10 | 1.39 | 1.15−1.68 | <.01 | |
| Age (per year) | 1.06 | 1.03−1.10 | <.01 | 0.89 | 0.82−0.94 | <.01 | 1.08 | 1.03−1.15 | <.01 | 1.11 | 1.08−1.15 | <.01 | |
| Sex | Female | 1 | (reference) | 1 | (reference) | 1 | (reference) | 1 | (reference) | ||||
| Male | 6.45 | 2.94−15.24 | <.01 | 10.45 | 3.36−38.14 | <.01 | 15.35 | 4.27−69.09 | <.01 | 3.27 | 1.75−6.25 | <.01 | |
| Arthritis | No | 1 | (reference) | 1 | (reference) | 1 | (reference) | 1 | (reference) | ||||
| Yes | 1.15 | 0.52−2.52 | .73 | 2.91 | 1.01−9.16 | .06 | 1.00 | 0.28−3.78 | 1.00 | 0.87 | 0.45−1.66 | .66 | |
| Thyroid condition | No | 1 | (reference) | 1 | (reference) | 1 | (reference) | 1 | (reference) | ||||
| Yes | 2.02 | 0.77−5.38 | .16 | 14.31 | 2.91−95.60 | <.01 | 1.75 | 0.40−8.75 | .47 | 0.87 | 0.39−1.92 | .73 | |
| Cardiac condition | No | 1 | (reference) | 1 | (reference) | 1 | (reference) | 1 | (reference) | ||||
| Yes | 1.89 | 0.65−5.56 | .24 | 0.99 | 0.28−3.68 | .99 | 1.19 | 0.24−7.29 | .84 | 2.57 | 1.10−6.24 | .03 | |
| Diabetes | No | 1 | (reference) | 1 | (reference) | 1 | (reference) | 1 | (reference) | ||||
| Yes | 0.21 | 0.05−0.73 | .02 | 0.08 | 0.01−0.39 | <.01 | 0.54 | 0.05−5.70 | .61 | 0.62 | 0.23−1.60 | .33 | |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
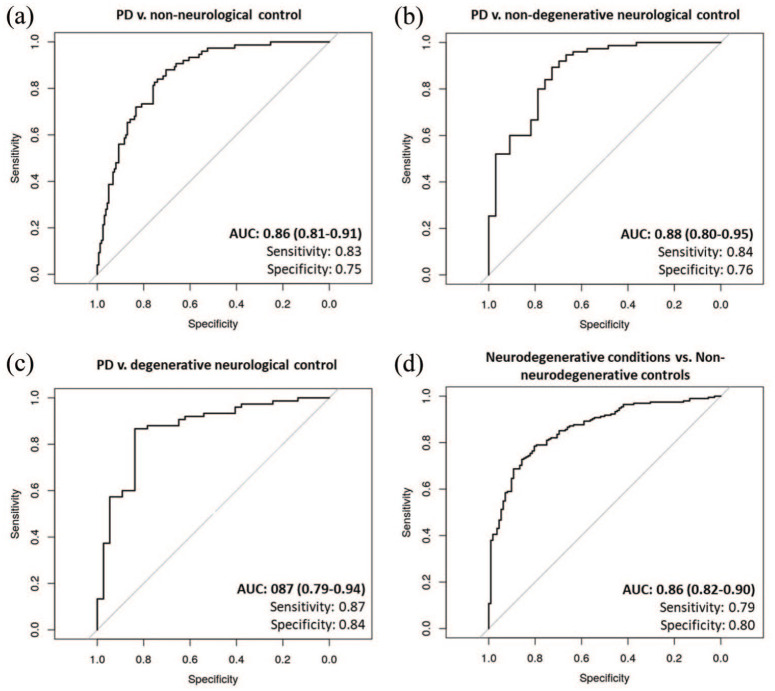
For the multivariable logistic regression models, the area under the ROC curve for the PD versus non-neurological controls model was 0.86 (95% Confidence Interval (CI) = 0.81%–0.91%; sensitivity = 0.83, specificity = 0.75), 0.88 (95% CI = 0.80–0.95; sensitivity = 0.84, specificity = 0.76) for PD versus non-degenerative neurological controls model and 0.87 (95% CI = 0.79–0.94; sensitivity = 0.87, specificity = 0.84) for PD versus degenerative neurological control model (Figure 3). The area under the ROC curve that separated the neurodegenerative conditions group from non-neurodegenerative controls was 0.86% (95% CI = 0.82–0.90; sensitivity = 0.79, specificity = 0.80, Figure 3).
Figure 3.
ROC curves of multivariate logistic regression analyses. Multivariable models assessing PD likelihood relative to A)non-neurological controls, B) non-degenrative neurological controls, and C)degentrative neurological control, and for D) neurodegenrative conditions relative to non-neurodegenrative controls.
HO-1, disease progression and LEDD
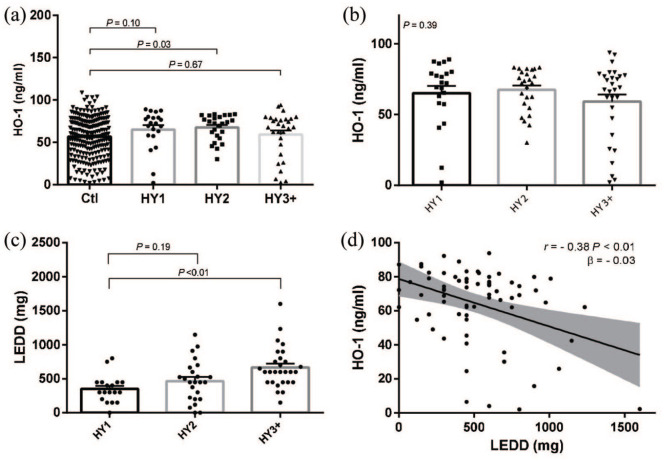
Additional secondary analyses revealed a significant difference in adjusted salivary HO-1 protein levels between non-PD controls (55.97 ng/ml, n = 232) and PD HY stage 2 subjects (67.04 ng/ml, P = .03, n = 24, Figure 4a). There was no significant difference in adjusted salivary HO-1 protein levels between non-PD controls and HY stage 1 (65.14 ng/ml, P = .10, n = 18) or HY stage 3 or greater (58.05 ng/ml, P = .68, n = 28, Figure 4a). Salivary HO-1 levels were not significantly different among PD participants at HY stage 1, 2, or greater than 3 by ANOVA (P = .39, Figure 4b).
Figure 4.
Relationship between HO-1, LEDD and HY stage. Disease progression measured by H&Y scale progression. A) HO-1 protein levels of all non-PD controls (n=232) relative to PD cases subdivided into their h&Y stage (HY1 n=18, HY2 n=24, HY3 n=28) and B) HO-1 protein levels and c) LEDD levels among PD patients (n=70) subdivided into their H&Y stage. Statistical analyses were conducted with a=0.05, error bars indicate the mean with SEm. D) Linear regression analysis of LEDD and salivary HO-1 and Pearson correlation.
Mean LEDD of PD patients in HY stage 1 was 441.31 mg (n = 18), 555.66 mg (n = 24) for HY stage 2, and 805.85 mg (n = 28) for HY stage 3 or greater. Mean LEDD were significantly higher in at HY stage 3 compared to PD participants at HY stage 1 (P < .01, Figure 4c). Furthermore, LEDD was correlated to salivary HO-1 (r = −0.38, β = −0.03 per 1 mg, P < .01, Figure 4d). For the less than 2.5% missing HY values, complete case analyses were used.
Discussion
In the present study we observed significantly higher levels of salivary HO-1 protein in PD subjects relative to participants without neurodegenerative conditions, and in participants with neurodegenerative conditions relative to the non-neurodegenerative condition group, regardless of comorbidities and total protein levels. The combination of these comorbidities, demographic information, salivary HO-1, and total protein concentrations may be used in models to assess the risk of PD and other neurodegenerative conditions.
HO-1 is an intracellular protein that has been implicated in PD pathology.24–26,28,29 Despite the lack of an N-terminus signal destining HO-1 to the secretory pathway, HO-1 has been detected in saliva and in other biofluids such as plasma, serum, and cerebrospinal fluid (CSF) in several diseased states.10,27,33–35 The method in which HO-1 is transported into these biofluids has yet to be fully elucidated. In a recent study, however, we reported the majority of HO-1 in various biofluids were localized in extracellular vesicles (EVs) with a substantial proportion being derived specifically from the central nervous system (CNS). 36 It is thus plausible that the levels of salivary HO-1 seen in this study in subjects with neurodegenerative conditions is reflective of the higher HO-1 levels previously reported in brain specimens of patients with these conditions.28,29,37–39
In 2018, we reported the presence of HO-1 protein in the saliva of PD participants and healthy controls without neurological conditions via ELISA and Western blot. 10 The findings for our previous study were reiterated here despite over a 30× fold difference in reported measurements. The difference in protein measurements may have been due to the matrix effects of saliva, which would have caused an obscuring of epitopes and reduced detection of analytes. To address and reduce matrix effects, pre-treatment, and dilution of the samples can be implemented.40,41 In the present study, dilution of the saliva samples was found to be sufficient to reduce the matrix effects.
Some of the limitations of our previous study 10 were addressed in the current study, such as adjusting for salivary total protein concentrations and the inclusion of neurological controls. Total protein concentrations were measured and added as a potential covariate for HO-1, after ascertaining that it was not a confounder. Measurements of total protein in saliva also showed higher total protein levels in PD subjects. This observation is in line with findings previously reported by others and is postulated to be caused by the autonomic dysfunction that is characteristic of PD subjects.11,42,43 This observation, combined with the frequent hypersiallorhea observed in PD patients would suggest that an altered total protein level may be due to excessive salivary flow rate. However, autonomic dysfunction in PD is heterogeneous and there are numerous reports of decreased salivary flow rate in PD patients.42–44 Both basal and stimulated salivary flow rate in PD patients during the off (absence of levodopa or prior to taking levodopa) state and on (with levodopa administration) state have been reported to be decreased, concomitant with increases in amylase secretion and total protein concentration.42,43 The hypersiallorhea in PD patients may be caused by autonomic dysfunction or dysphagia, and a reduction in saliva production may lead to increased salivary protein concentrations as reported here and elsewhere.11,42,43
Salivary HO-1 protein concentrations did not differ between the PD group and participants with other neurodegenerative conditions, suggesting that elevated salivary HO-1 protein is not unique to PD. This was confirmed in our secondary analysis. The lack of specificity of high salivary HO-1 levels in PD is not surprising as HO-1 has also been linked to a variety of neurodegenerative conditions including AD and MCI.33,45,46 Expression of HO-1 by induction of the HMOX1 gene has been reported to occur early in AD pathology with overexpression of HO-1 protein in participants afflicted with MCI, a frequent harbinger of AD. 37 Additionally, HO-1 protein and mRNA detected in other AD biofluids such as CSF, plasma, and serum, further attesting to the involvement in HO-1 induction in the pathophysiology of neurodegenerative conditions.33,45,46
Additional secondary analyses revealed a significant difference between non-PD controls and PD HY stage 2 participants. Participants at other stages of disease progression had higher HO-1 levels compared to non-PD controls, although this was statistically non-significant. These results may be due to the negative association found between salivary HO-1 and LEDD. During disease progression, motor symptoms of PD worsen and higher doses of pro-dopaminergic medications are typically prescribed. The increasing dosage of levodopa prescribed to participants at later stages of PD may have been sufficient to reduce HO-1 protein levels in saliva relative to subjects at earlier stages of PD, but insufficient to return them to basal levels. This may also explain the lack of statistically significant differences in HO-1 levels with disease progression. The molecular mechanism(s) responsible for the relationship between LEDD and HO-1 remains enigmatic in light of the fact that dopamine potently induces HMOX1 in cultured rodent astroglia. 26
Despite the lack of specificity of the salivary HO-1 assay for idiopathic PD, the test may prove valuable in clinically distinguishing degenerative from non-degenerative CNS disorders. For example, movement disorder specialists are often at a loss to differentiate idiopathic PD from neuroleptic-induced extrapyramidal disorders or vascular parkinsonism. Similarly, non-degenerative causes of MCI and dementia, including various toxic-metabolic encephalopathies or psychomotor retardation complicating severe depression, may confound the diagnosis of AD. The ability to accurately distinguish between degenerative and non-degenerative etiologies responsible for these clinical presentations by means of a salivary HO-1 protein or other simple biochemical assays would be a welcome development. In the ROC curves of multivariable logistic regression models, we demonstrated that these models can correctly classify a person having PD or a neurodegenerative condition apart from the control population with an accuracy over 85%.
Multivariable logistic regression models showed that the likelihood of having a neurodegenerative condition is higher in males, older age, and in subjects with increased salivary HO-1 and total protein concentration (Supplementary Figure 1). The association between neurodegenerative conditions such as PD and AD and age is not surprising as the worldwide prevalence of these conditions is known to increase with age.2,47 In addition, neural degeneration has been seen in normal aging at a rate of 4.7% per decade in the substantia nigra pars compacta with an exponential increase in neurodegeneration in human PD brain specimens with a 45% loss within the first decade. 48 This may tie into the oxidative damage and mitochondrial damage theory of aging, as these patients and models of these diseases do exhibit signs of oxidative stress and mitochondrial damage with age. The association of sex to neurodegenerative diseases is also not surprising as the prevalence of PD have been shown to be 1.40 times higher in males than females, while there is a 1.17 times female predominance in Alzheimer’s disease.2,47
The present findings suggest that models employing salivary HO-1, total protein concentration, age, sex, and relevant comorbidities may facilitate the clinical evaluation of patients suspected of harboring a neurodegenerative condition. The latter could be used in a manner analogous to the measurement of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in patients with systemic inflammation or infection. 49 Despite the availability of more specific analytes of inflammation, ESR and CRP are frequently employed as diagnostic markers, disease prognosticators, and indices of effective therapeutic intervention (eg, in the management of giant cell arteritis and other autoimmune conditions) on account of their sensitivities to disease presence and severity, low cost, and ease of implementation.49–51 The ESR enjoys widespread clinical utility notwithstanding the fact that it is relatively non-specific and can be influenced by factors including age, sex, heart disease, renal disease, and pregnancy.49,50 Similarly, CRP levels may be augmented by age, late pregnancy, cigarette smoking, obesity, and depression. 49 Along these lines, salivary HO-1 protein, albeit not specific to idiopathic PD (current study), may prove useful in differentiating neurodegenerative from non-degenerative neurological conditions, in the recruitment of appropriate subjects for clinical drug trials and as a surrogate marker of the therapeutic efficacy of disease-modifying medications when they become available.
Potential circulating biomarkers for diagnosis, prognosis, and monitoring have been identified in salivary secretions for various diseases, including PD, AD, Sjögren’s syndrome, and oral cancer.10–14,31,43,52–54 Among neurodegenerative diseases, like PD, the critical role of oxidative stress in disease pathogenesis have led to increasing research in the use of oxidatively modified nucleic acids, lipids, proteins, and antioxidants such as HO-1, advanced glycation end products, and reduced glutathione as salivary redox biomarkers for diagnostic or prognostic markers of disease.54–58 Saliva has several advantages in comparison to other biofluids for biomarker research such as its non-invasive method of fluid acquisition, the non-extensive requirement for personnel training for saliva specimen collection, and salivary composition being reflective of blood or CSF composition. 54 While there are advantageous attributes, the use of saliva as a diagnostic fluid is uncommon due to lower levels of analytes in saliva in comparison to other biological fluids. 59 Despite this, the search for salivary biomarkers of disease and research into their potential is key in providing an alternative diagnostic tool reflective of the physical well-being of an individual while also being a non-invasive and cheaper method of disease screening.
There were several limitations to the current study. First, the sample population included was recruited from a single site, did not include a dental examination to assess oral health and salivary gland dysfunction, and a replication cohort is lacking. Second, the sample sizes of the degenerative and non-degenerative neurological controls were smaller and contain a heterogeneous group of neurological conditions in comparison to the PD group. While the sample size of our groups satisfies the minimum limiting sample requirement for multivariable regression analysis suggested by Harrell and colleagues, a greater sample size for each control group would be desirable for future studies to further assess how HO-1 multivariable models can separate the groups. 32 Additionally, this study does not include PD-mimics such as participants with progressive supranuclear palsy and multiple system atrophy. Lastly, MDS-UPDRS scores were not available to corroborate HY staging for measurement of disease progression and severity and should be included in future studies for PD staging and additional confirmation of PD diagnosis.
The current study corroborates previous findings in which HO-1 was detected in human saliva and higher levels of HO-1 observed in participants with PD relative to healthy, non-neurological controls. Moreover, we now demonstrate significantly elevated salivary HO-1 levels in participants with other neurodegenerative conditions. Although high salivary HO-1 levels were not specific to the PD population, the multivariable models presented suggest the possible use of salivary HO-1 as a potential biomarker of neurodegenerative conditions such as PD, AD, and MCI when combined with total protein, demographic, and comorbidity data. The accuracy of these models to classify PD or a neurodegenerative condition was above 85%, indicating that such modeling may prove useful in the clinical setting. Conceivably, the inclusion of other potential biomarker(s) in models more specific to PD, such as salivary oligomeric α-synuclein, may confer added specificity to distinguish PD from other neurodegenerative disorders. Future studies would venture into the measurement of salivary HO-1 content of total EVs and CNS-derived EVs as the latter may further improve the accuracy of the HO-1 assay as a biomarker of neurodegeneration. 36
Supplemental Material
Supplemental material, sj-pdf-1-cns-10.1177_11795735211029114 for Salivary Heme Oxygenase-1: A Potential Biomarker for Central Neurodegeneration by Julia M Galindez, Lamin Juwara, Marisa Cressatti, Mervyn Gornitsky, Ana M Velly and Hyman M Schipper in Journal of Central Nervous System Disease
Acknowledgments
We thank Drs. R. Altman, J.A. Carlton, C.A. Melmed, D. Rabinovitch, and M. Sidel from the Department of Neurology, Jewish General Hospital, and volunteers Viet Hoang, Olivia Canie, Hafsa Barisé, and David Schipper for their assistance with participant recruitment and sample procurement. We would also like to thank Parkinson Canada and MITACs for funding our project.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Parkinson Canada Grant (MG and HMS) and MITACS Accelerate grant (JMG, LJ, AMV, MG, HMS)
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HMS and MG have served as officers of HemOx Biotechnologies. HMS has served as consultant to Osta Biotechnologies, Molecular Biometrics Inc., TEVA Neurosciences, and Caprion Pharmaceuticals. JMG now serves as an employee of Altasciences. MC, LJ, AMV, have no conflicts of interest to declare.
Author Contributions: All authors contributed to the study conception and design. Material preparation and data collection were performed by JMG. Statistical analysis was performed and critiqued by AMV, LJ and JMG. The first draft of the manuscript was written by JMG and edited by all authors. All authors read and approved the final manuscript.
Ethical approval: Approved by the Research Ethics Committee of the Jewish General Hospital (JGH; Montreal, Canada, No. 2019-1220)
Consent to participate: Informed consent was obtained from all individual participants whose samples were included in the study.
Consent for publication: All participants signed informed consent regarding publishing data obtained using their samples.
ORCID iD: Julia M Galindez  https://orcid.org/0000-0002-8075-4309
https://orcid.org/0000-0002-8075-4309
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Group GBDNDC. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017;16:877-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collaborators GBDPsD. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17:939-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang W, Hamilton JL, Kopil C, et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis. 2020;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Postuma RB. Prodromal Parkinson’s disease–using REM sleep behavior disorder as a window. Parkinsonism Relat Disord. 2014;20:S1-S4. [DOI] [PubMed] [Google Scholar]
- 5. Marsili L, Rizzo G, Colosimo C. Diagnostic criteria for Parkinson’s disease: from James Parkinson to the concept of prodromal disease. Front Neurol. 2018;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86:566-576. [DOI] [PubMed] [Google Scholar]
- 7. Miller DB, O’Callaghan JP. Biomarkers of Parkinson’s disease: present and future. Metabolism. 2015;64:S40-S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emamzadeh FN, Surguchov A. Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci. 2018;12:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morley JF, Cohen A, Silveira-Moriyama L, et al. Optimizing olfactory testing for the diagnosis of Parkinson’s disease: item analysis of the university of Pennsylvania smell identification test. NPJ Parkinsons Dis. 2018;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song W, Kothari V, Velly AM, et al. Evaluation of salivary heme oxygenase-1 as a potential biomarker of early Parkinson’s disease. Mov Disord. 2018;33:583-591. [DOI] [PubMed] [Google Scholar]
- 11. Masters JM, Noyce AJ, Warner TT, Giovannoni G, Proctor GB. Elevated salivary protein in Parkinson’s disease and salivary DJ-1 as a potential marker of disease severity. Parkinsonism Relat Disord. 2015;21:1251-1255. [DOI] [PubMed] [Google Scholar]
- 12. Shaheen HS, Mously S, Abuomira S, Mansour M. Salivary alpha-synuclein (total and oligomeric form): potential biomarkers in Parkinson’s disease. Egypt J Neurol Psychiatry Neurosurg. 2020;56:22. [Google Scholar]
- 13. Cao Z, Wu Y, Liu G, et al. alpha-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci Lett. 2019;696:114-120. [DOI] [PubMed] [Google Scholar]
- 14. Vivacqua G, Latorre A, Suppa A, et al. Abnormal salivary total and oligomeric alpha-synuclein in Parkinson’s disease. PLoS One. 2016;11:e0151156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388-6394. [PubMed] [Google Scholar]
- 17. Fukuda K, Richmon JD, Sato M, Sharp FR, Panter SS, Noble LJ. Induction of heme oxygenase-1 (HO-1) in glia after traumatic brain injury. Brain Res. 1996;736:68-75. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt J, Mertz K, Morgan JI. Regulation of heme oxygenase-1 expression by dopamine in cultured C6 glioma and primary astrocytes. Brain Res Mol Brain Res. 1999;73:50-59. [DOI] [PubMed] [Google Scholar]
- 19. Foresti R, Hoque M, Bains S, Green CJ, Motterlini R. Haem and nitric oxide: synergism in the modulation of the endothelial haem oxygenase-1 pathway. Biochem J. 2003;372:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schipper HM, Song W, Tavitian A, Cressatti M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog Neurobiol. 2019;172:40-70. [DOI] [PubMed] [Google Scholar]
- 21. Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12:25-31. [PubMed] [Google Scholar]
- 22. Mann VM, Cooper JM, Daniel SE, et al. Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann Neurol. 1994;36:876-881. [DOI] [PubMed] [Google Scholar]
- 23. Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann Neurol. 1998;44:S72-S84. [DOI] [PubMed] [Google Scholar]
- 24. Tavitian A, Cressatti M, Song W, et al. Strategic timing of glial HMOX1 expression results in either schizophrenia-like or Parkinsonian behavior in mice. Antioxid Redox Signal. 2020;32:1259-1272. [DOI] [PubMed] [Google Scholar]
- 25. Song W, Cressatti M, Zukor H, Liberman A, Galindez C, Schipper HM. Parkinsonian features in aging GFAP.HMOX1 transgenic mice overexpressing human HO-1 in the astroglial compartment. Neurobiol Aging. 2017;58:163-179. [DOI] [PubMed] [Google Scholar]
- 26. Schipper HM, Bernier L, Mehindate K, Frankel D. Mitochondrial iron sequestration in dopamine-challenged astroglia: role of heme oxygenase-1 and the permeability transition pore. J Neurochem. 1999;72:1802-1811. [DOI] [PubMed] [Google Scholar]
- 27. Mateo I, Infante J, Sanchez-Juan P, et al. Serum heme oxygenase-1 levels are increased in Parkinson’s disease but not in Alzheimer’s disease. Acta Neurol Scand. 2010;121:136-138. [DOI] [PubMed] [Google Scholar]
- 28. Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp Neurol. 1998;150:60-68. [DOI] [PubMed] [Google Scholar]
- 29. Castellani R, Smith MA, Richey PL, Perry G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996;737:195-200. [DOI] [PubMed] [Google Scholar]
- 30. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer Series in Statistics. Springer; 2001:xxii, 568 p. [Google Scholar]
- 31. Cressatti M, Juwara L, Galindez JM, et al. Salivary microR-153 and microR-223 levels as potential diagnostic biomarkers of idiopathic Parkinson’s disease. Mov Disord. 2020;35:468-477. [DOI] [PubMed] [Google Scholar]
- 32. Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143-152. [DOI] [PubMed] [Google Scholar]
- 33. Schipper HM, Chertkow H, Mehindate K, Frankel D, Melmed C, Bergman H. Evaluation of heme oxygenase-1 as a systemic biological marker of sporadic AD. Neurology. 2000;54:1297-1304. [DOI] [PubMed] [Google Scholar]
- 34. Maes OC, Kravitz S, Mawal Y, et al. Characterization of alpha1-antitrypsin as a heme oxygenase-1 suppressor in Alzheimer plasma. Neurobiol Dis. 2006;24:89-100. [DOI] [PubMed] [Google Scholar]
- 35. Pennisi G, Cornelius C, Cavallaro MM, et al. Redox regulation of cellular stress response in multiple sclerosis. Biochem Pharmacol. 2011;82:1490-1499. [DOI] [PubMed] [Google Scholar]
- 36. Cressatti M, Galindez JM, Juwara L, et al. Characterization and heme oxygenase-1 content of extracellular vesicles in human biofluids. J Neurochem. Published online September 3, 2020. doi: 10.1111/jnc.15167 [DOI] [PubMed] [Google Scholar]
- 37. Schipper HM, Bennett DA, Liberman A, et al. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging. 2006;27:252-261. [DOI] [PubMed] [Google Scholar]
- 38. Youssef P, Chami B, Lim J, Middleton T, Sutherland GT, Witting PK. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci Rep. 2018;8:11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernandez-Mendivil C, Arreola MA, Hohsfield LA, Green KN, Lopez MG. Aging and progression of beta-amyloid pathology in Alzheimer’s disease correlates with microglial heme-oxygenase-1 overexpression. Antioxidants (Basel). 2020;9:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Browne RW, Kantarci A, LaMonte MJ, et al. Performance of multiplex cytokine assays in serum and saliva among community-dwelling postmenopausal women. PLoS One. 2013;8:e59498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiu ML, Lawi W, Snyder ST, Wong PK, Liao JC, Gau V. Matrix effects—a challenge toward automation of molecular analysis. J Assoc Lab Autom. 2010;15:233-242. [Google Scholar]
- 42. Tumilasci OR, Cersosimo MG, Belforte JE, Micheli FE, Benarroch EE, Pazo JH. Quantitative study of salivary secretion in Parkinson’s disease. Mov Disord. 2006;21:660-667. [DOI] [PubMed] [Google Scholar]
- 43. Fedorova T, Knudsen CS, Mouridsen K, Nexo E, Borghammer P. Salivary acetylcholinesterase activity is increased in Parkinson’s disease: a potential marker of parasympathetic dysfunction. Parkinsons Dis. 2015;2015:156479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Proulx M, de Courval FP, Wiseman MA, Panisset M. Salivary production in Parkinson’s disease. Mov Disord. 2005;20:204-207. [DOI] [PubMed] [Google Scholar]
- 45. Schipper HM, Cisse S, Stopa EG. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol. 1995;37:758-768. [DOI] [PubMed] [Google Scholar]
- 46. Smith MA, Kutty RK, Richey PL, et al. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer’s disease. Am J Pathol. 1994;145:42-47. [PMC free article] [PubMed] [Google Scholar]
- 47. Collaborators GBDD. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:88-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283-2301. [DOI] [PubMed] [Google Scholar]
- 49. Bray C, Bell LN, Liang H, et al. Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. WMJ. 2016;115:317-321. [PubMed] [Google Scholar]
- 50. Alende-Castro V, Alonso-Sampedro M, Vazquez-Temprano N, et al. Factors influencing erythrocyte sedimentation rate in adults: new evidence for an old test. Medicine (Baltimore). 2019;98:e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lapic I, Padoan A, Bozzato D, Plebani M. Erythrocyte sedimentation rate and C-reactive protein in acute inflammation. Am J Clin Pathol. 2020;153:14-29. [DOI] [PubMed] [Google Scholar]
- 52. Burbelo PD, Bayat A, Lebovitz EE, Iadarola MJ. New technologies for studying the complexity of oral diseases. Oral Dis. 2012;18:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer’s disease in saliva: a systematic review. Dis Markers. 2019;2019:4761054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maciejczyk M, Zalewska A, Gerreth AK. Salivary redox biomarkers in selected neurodegenerative diseases. J Clin Med. 2020;9:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Choromanska M, Klimiuk A, Kostecka-Sochon P, et al. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int J Mol Sci. 2017;18:2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klimiuk A, Maciejczyk M, Choromanska M, Fejfer K, Waszkiewicz N, Zalewska A. Salivary redox biomarkers in different stages of dementia severity. J Clin Med. 2019;8:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Challacombe SJ, Percival RS, Marsh PD. Age-related changes in immunoglobulin isotypes in whole and parotid saliva and serum in healthy individuals. Oral Microbiol Immunol. 1995;10:202-207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cns-10.1177_11795735211029114 for Salivary Heme Oxygenase-1: A Potential Biomarker for Central Neurodegeneration by Julia M Galindez, Lamin Juwara, Marisa Cressatti, Mervyn Gornitsky, Ana M Velly and Hyman M Schipper in Journal of Central Nervous System Disease