Abstract
Individuals with serious mental disorders (SMD) may have a higher risk of vitamin D (VIT-D) deficiency. They also experience higher mortality because of coronavirus disease 2019 (COVID-19) infection. Therefore, we have conducted a comprehensive review to examine the significance of VIT-D for public health and public mental health during the ongoing COVID-19 pandemic. This review had three specific aims, from a global perspective to (a) create a profile of VIT-D and review the epidemiology of VIT-D deficiency, (b) explore VIT-D deficiency as risk factor for SMD and COVID-19 infections and (c) examine the effectiveness of VIT-D supplementation for both conditions. We found that, in terms of SMD, the evidence from laboratory and observational studies points towards some association between VIT-D deficiency and depression or schizophrenia. Mendelian randomisation studies, however, suggest no, or reverse, causality. The evidence from intervention studies is conflicting. Concerning COVID-19 infection, on proof of principle, VIT-D could provide a plausible defence against the infection itself and against an adverse clinical course. But data from observational studies and the first preliminary intervention studies remain conflicting, with stronger evidence that VIT-D may mitigate the clinical course of COVID-19 infection rather than the risk of infection in the first place. From a public health and public mental health point of view, based on the currently limited knowledge, for individuals with SMD, the benefits of VIT-D optimisation through supplementation seem to outweigh the risks. VIT-D supplementation, however, should not substitute for vaccination or medical care for COVID-19 infection.
Keywords: coronavirus, COVID-19, mental disorder, mental health, meta-analysis, public health, supplementation, vitamin D
Introduction
In the ongoing coronavirus disease 2019 (COVID-19) pandemic, vitamin D (VIT-D) has emerged as a substance of potential public health importance in individuals with serious mental disorder (SMD). Individuals with SMD are one of the groups at higher risk of an adverse outcome from a COVID-19 [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] infection. Three large cohort studies have indicated an approximately two-fold increased risk of mortality.1–3 It remains unclear whether this increased risk is due to the infection itself or due to a more adverse clinical course once infected. 1 Despite this higher mortality risk, individuals with SMD have been prioritised for vaccinations in only very few countries. 4 Therefore, it is even more important to identify other interventions that could help individuals with SMD, who are at risk of worse health outcomes during the ongoing COVID-19 pandemic, both in terms of physical and mental health. 1 VIT-D may be one substance that could improve both mental and physical health outcomes.
VIT-D deficiency has been associated with SMD, including psychosis and bipolar disorder and depression. At the same time, VIT-D may modify the risk of respiratory tract infections (RTIs).5–8 The UK Scientific Advisory on Nutrition (SCAN), in a revision of its previous conclusion, 9 has suggested that low dose VIT-D supplementation between 10 µg (400 IU) and 25 µg (1000 IU) may be of some benefit in reducing the risk of acute respiratory tract infection (ARTI). 10 Because of this, VIT-D has increasingly been explored in the context of COVID-19 infections. The United Kingdom (UK) National Health Service (NHS) and the UK National Institute for Health and Care Excellence (NICE) currently recommend taking 10 µg (400 IU) per day for the general population between October and early March to compensate for lack of ultraviolet B (UVB) exposure.11,12 At the same time, these recommendations point out that there currently is not enough evidence to support taking VIT-D to prevent or treat COVID-19 infections. The South London and Maudsley NHS Foundation Trust protocol for VIT-D prophylaxis during the COVID-19 pandemic, in acknowledgement of the special vulnerabilities of patients with SMD, has recommended that all patients of the hospital should be prescribed 100 µg (4000 IU) per day for 4 weeks. 13
With the COVID-19 pandemic passing through various stages and waves with substantial global differences, the evidence regarding VIT-D is still evolving. Therefore, we have conducted a comprehensive review to examine the significance of VIT-D for public health and public mental health during the ongoing COVID-19 pandemic. This review had three specific aims, from a global perspective to (a) create a profile of VIT-D and review the epidemiology of VIT-D deficiency, (b) explore VIT-D deficiency as risk factor for severe mental disorder (SMD) and COVID-19 infections and (c) examine the effectiveness of VIT-D supplementation for both conditions.
Method
We conducted a narrative review with a profile of VIT-D and clinical and public health aspects of VIT-D deficiency as a starting point. We then explored the potential benefits of VIT-D regarding our two chosen outcomes: SMD and COVID-19 infections. In terms of proof of principle and association, we examined laboratory and observational studies. For causality, we examined Mendelian randomisation and intervention studies. For this narrative review, we performed a literature search using the PubMed/Medline database. For SMD, as most evidence is available for depression and schizophrenia, we explored these two conditions in more detail. Prevention of depressive and psychotic relapses is also a major public mental health concern during the current COVID-19 pandemic.
For the search for observational studies, we used ‘vitamin D deficiency’ AND (‘depression’ OR ‘schizophrenia OR psychosis’ OR ‘COVID-19’ OR ‘coronavirus’).
We complemented the presentation of observational data with studies using a Mendelian randomisation approach, as a method less affected by confounding and reverse causation. Mendelian randomisation studies use genetic variation to investigate causal relations and between potentially modifiable risk factors and health outcomes in observational studies. 14 For the search of Mendelian randomization studies, we used vitamin D AND (mental disorder OR depression OR schizophrenia OR psychosis).
For the search for intervention studies, we used ‘vitamin D’ AND (‘depression’ OR ‘schizophrenia OR psychosis’ OR ‘COVID-19’ OR ‘coronavirus’) AND (‘trial OR meta-analysis’). Due to the large number of studies available, we focused on the available meta-analyses for the outcome depression. For outcomes related to COVID-19 infection, we reviewed the individual trials. We did not consider the only meta-analysis available at the time of writing. This meta-analysis only summarised three studies, all of which were heterogenous. Therefore, we judged the summary estimates as potentially unreliable. 15
We included all relevant papers published between 1 January 2010 and 30 September 2020, and (b) at the time of the revision including relevant papers published between 1 September 2020 and 24 March 2021. We considered English language papers only. For intervention studies we excluded studies in which VIT-D was co-supplemented with other substances. We further excluded studies concerning children, pregnancy, post-natal conditions and specific somatic conditions. We complemented the literature review with references to national guidelines regarding VIT-D use from the UK, Australia, and the United States (US). We also used COVID-19 statistics regarding infection, testing and death rates published by Worldometer. 16 For assessing the role of geographical latitude as a proxy of VIT-D exposure, we compared COVID-19 infection and mortality rates in the 13 countries that lie on the equator.
VIT-D: profile
Biochemistry
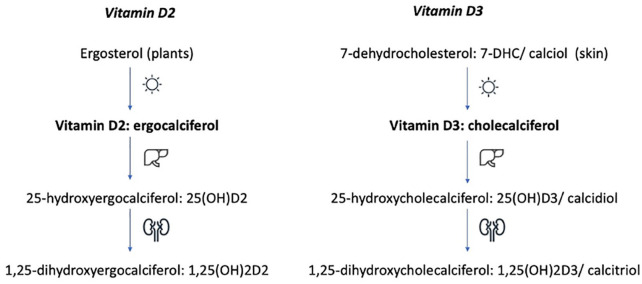
VIT-D is a fat-soluble vitamin. Chemically, it is a secosteroid that exists in two forms: VIT-D2 (ergocalciferol) and VIT-D3 (cholecalciferol). 17 VIT-D2 is synthesised by plants and fungi from ergosterol – a sterol found in the cell membranes. VIT-D3 is synthesised by humans under the influence of UVB light from 7-dehydrocholesterol (7-DHC) (calciol) – a sterol found in the skin. 18 VIT-D2 and D3 are not biologically active. Both forms need to undergo two steps of hydroxylation. The first step takes place in the liver to form 25(OH)D and the second in the kidneys to form 1,25(OH)2D (Figure 1).17,19–21 25(OH)D is the major circulating form of VIT-D through the body. 21 1,25(OH)2D2 and 1,25(OH)2D3 are the biologically active forms of VIT-D (1,25(OH)2D). 25(OH)D has a half-life of about 15 days. 1,25(OH)2D has a much shorter half-life, measured in hours. 19 The longer half-life makes 25(OH)D the preferred serum marker to assess VIT-D status. Laboratory reference ranges vary. The normal range tends to lie between 20 and 100 ng/ml (50–250 nmol/l).
Figure 1.
Vitamin D synthesis and hydroxylation.
Function
Classically, VIT-D is associated with bone health. Jointly with the parathyroid hormone (PTH), VIT-D plays a major role in calcium and phosphate metabolism. PTH stimulates the conversion of 25(OH)D into the active form of VIT-D, 1,25(OH)2D. VIT-D provides negative feedback for PTH. 22 VIT-D contributes to the regulation of calcium and phosphate in three target organs, kidney, intestine and bones. In the kidney, VIT-D enhances tubular calcium reabsorption. The effects of VIT-D on renal phosphate transport, however, are poorly understood. In the intestine, VIT-D enhances absorption of calcium and phosphate. In the bones, the actions of VIT-D are complex. VIT-D can activate both osteoblasts and osteoclasts. Depending on the net result, VIT-D could promote bone formation, bone resorption or have a neutral effect on bone metabolism. By regulation of bone turnover, VIT-D also contributes to the regulation of plasma calcium levels.22,23 But VIT-D has many other functions beyond bone metabolism. VIT-D receptors (VIT-DRs) are found in multiple tissues including brain, kidney, lung, cardiovascular system, breast, prostate, colon and immune cells, such as B and T lymphocytes.24–26 We will discuss the effects of VIT-D on brain, lung and immune function in more detail during this review.
Source and daily intake
The extent of photochemically produced VIT-D depends on geographical latitude, season, time of day, skin type, age, actual duration sun exposure and amount of skin exposed.18,27 At higher latitudes, more UVB radiation, of wavelength between 290 and 315 nm, is absorbed by the ozone layer of the atmosphere. Thus, the further away from the equator, the less it is possible to rely on sunlight to produce VIT-D. During the winter months, very little, if any VIT-D can be produced photochemically in regions above 33° North (i.e. north of Los Angeles) or below 33° South (i.e. south of Cape Town). 27 In latitudes above about 50° (Canada and northern Europe), VIT-D can be produced in significant amounts only during the six summer months of the year. The amount of UVB light to be absorbed depends on melanin content in the skin. 27 Increased skin pigmentation can lead to a reduction of photochemical VIT-D synthesis of up to 50-fold.28,29 Additionally, the amount of 7-DHC decreases with age, which reduces the capacity of the skin to synthesise VIT-D. 28 Life in institutionalised or homebound settings, clothes covering the whole body and use sunscreen may further reduce photochemical VIT-D synthesis.
VIT-D can also be taken up through foods. Recommendation of daily intake vary between countries, depending on age but not on gender (Table 1).11,19,30,31 The requirements for pregnant and lactating women remain the same as the corresponding age group.19,30
Table 1.
| Age | Australia | Europe | UK | US |
|---|---|---|---|---|
| VIT-D intake µg/day a | ||||
| >1 year | 5 b | 10 c | 8.5–10 d | 10 b |
| 1–18 years | 5 | 15 | 10 | 15 |
| 18–50 years | 5 | 15 | 10 | 15 |
| 51–70 years | 10 | 15 | 15 | |
| >70 years | 15 | 15 | 20 | |
Conversion factor from µg to IU = 40, 1 µg cholecalciferol = 0.2 µg 25(OH)D; 1 IU = 0.025 µg cholecalciferol or 0.005 µg 25(OH)D.
Infants 0–12 months.
Infants 7–11 months.
Babies up to 1 year of age.
IU, international units; UK, United Kingdom; US, United States; VIT-D, vitamin D.
Very few foods contain VIT-D. The best food sources of VIT-D include oily fish and fish oils. Liver, red meat, cheese and egg yolk also contain VIT-D. Mushrooms with enhanced VIT-D contents due to UVB exposure are a further option. Then, there are vitamin fortified foods such as some breakfast cereals, fat spreads, milk and dairy products and plant milk alternatives (Table 2).26,32 The availability of VIT-D fortified foods varies between countries. For instance, in the US, milk is VIT-D fortified, in the UK it is not.11,19
Table 2.
| Food item | VIT-D content µg/100 g servinga,b | Food item | VIT-D content µg/100 g servinga,b |
|---|---|---|---|
| High | Low | ||
| Herring, grilled | 16.1 | Prawns | Traces |
| Salmon farmed, grilled | 7.8 | Cod baked | Traces |
| Trout, grilled | 8.2 | Sea bass | Traces |
| Eggs, chicken, boiled c | 3.2 | Egg white raw | Traces |
| Beefburger grilled | 1.9 | Hamburger take-away | 0.2 |
| Fat spread, low fat, not polyunsaturated (26–39%) | 8.4 | Vegetable oil, average | Traces |
| Baking fat and margarine (75–90%) | 8.8 | Butter salted | 0.9 |
| Shiitake mushrooms sundried | 4.0 | Shiitake mushrooms fresh | 0.25 |
| Cornflakes, fortified | 4.7 | Cornflakes, unfortified | – |
| Dried skimmed milk fortified | 2.7 | Skimmed milk | 0 |
Conversion factor from µg to IU = 40, 1 µg cholecalciferol = 0.2 µg 25(OH)D; 1 IU = 0.025 µg cholecalciferol or 0.005 µg 25(OH)D.
Conversion factor from g to ounce (oz) = 0.035.
Medium egg weighs between 53 and 63 g.
IU, international units; VIT-D, vitamin D.
VIT-D deficiency: clinical and public health aspects
Definition
There is no consensus of what a constitutes an optimal VIT-D level. Previously suggested ranges are higher than current recommendations (Table 3).26,33 In older adults, the International Osteoporosis Foundation (IOF) suggests a VIT-D concentration of 30 ng/ml (75 mmol/l) for prevention of hip fractures. 34 For all the UK population, it is currently recommended that, in order to protect musculoskeletal health, the VIT-D concentration should not fall below 25 ng/ml (62.5 nmol/l) at any time of the year. 35 For the US, the National Institute of Health (NIH) suggests that VIT-D concentrations of less than 30 ng/ml constitute VIT-D deficiency leading to rickets in infants and osteomalacia in adults. Serum concentrations between 30 and 50 ng/ml may still bear some risk of VIT-D inadequacy. 19 An expert panel of the European Food Safety Agency considers a VIT-D concentration of 50 nmol/l (20 ng/ml) a suitable target value for all population groups. 31
Table 3.
Vitamin D status as measured by 25(OH)D serum concentration.
| VIT-D status | Global consensus recommendations on prevention and management on nutritional rickets 33 | Recommendations Holick 26 | ||
|---|---|---|---|---|
| 25(OH)D a | ||||
| ng/ml | nmol/l | ng/ml | nmol/l | |
| Sufficiency | >20 | >50 | 30–60 | 75–150 |
| Insufficiency | 12–19 | 30–50 | 20–29 | 50–74 |
| Deficiency | <12 | <30 | <20 | <50 |
| Intoxication | >100 b | >250 b | >150 | 375 |
Conversion factor ng/ml to nmol/l = 2.5.
With hypercalcaemia, hypercalciuria and suppressed PTH.
PTH, parathyroid hormone; VIT-D, vitamin D.
Clinical signs
Subclinical VIT-D deficiency can result in osteoporosis, increased falls and possibly fractures. Overt VIT-D deficiency results in hypocalcaemia and or hypophosphataemia. In children, overt VIT-D deficiency can lead to rickets and osteomalacia. In adults, overt VIT-D deficiency can lead to osteomalacia. VIT-D deficiency may lead to secondary hyperparathyroidism. 36 Many individuals with VIT-D insufficiency, however, may be asymptomatic.
Prevalence
The prevalence of VIT-D deficiency varies worldwide depending on UVB exposure and possibility to compensate with VIT-D containing foods (Table 4).37–39 Although countries in lower latitudes have more access to UVB radiation, VIT-D deficiency may still be more common. This can occur if available UV light is not exploited, and VIT-D food sources are limited (populations at risk). Samples are often heterogenous. This can make comparison of prevalence rates difficult.
Table 4.
Proportion of vitamin D insufficiency and deficiency in adults of varying age ranges between 18 and 99 years around the world collated from published reviews.
| Country of originating study | Palacios and Gonzalez 37 | Lips et al. 38 /van Schoor and Lips 39 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Insufficiency or deficiency | ||||||||||||
| VIT-D ng/ml a | ||||||||||||
| <12 | <20 | <12 | <20 | <12 | <20 | <10 | <20 | <10 | <20 | <10 | <20 | |
| All | Males | Females | All | Males | Females | |||||||
| Africa | ||||||||||||
| Egypt | 72 | |||||||||||
| Egypt b | 40–77 | |||||||||||
| Morroco c | 52 | |||||||||||
| Nigeria | 5 | 34 | 52 | |||||||||
| Tanzania | 1 | |||||||||||
| America North/Central | ||||||||||||
| Canada | 2 | 20 | 2–21 | |||||||||
| Canada d | 2 | 64 | ||||||||||
| Canada e | 7 | 89 | ||||||||||
| Mexico | 0.2 | 10 | 2 | 10 | 2 | 44 | ||||||
| Puerto Rico | 32 | |||||||||||
| US | 6 | 34–37 | 2–9 | 18–54 | ||||||||
| America South | ||||||||||||
| Brazil | 28 | |||||||||||
| Brazil b | 14 | 41–58 | ||||||||||
| Chile | 13 | 27 | 27–60 | |||||||||
| Ecuador | 22 | |||||||||||
| Guatemala b | 46 | |||||||||||
| Asia | ||||||||||||
| Bangladesh | 36 | 80 | ||||||||||
| Bahrain | 50 | 86 | ||||||||||
| India | 66 | |||||||||||
| Israel | 9–12 | 41–50 | 15–23 | 51–60 | 14–27 | 28–78 | ||||||
| Iran | 51 | 19–34 | 85 | |||||||||
| Jordan | 2 | 14 | cf. Palacios and Gonzalez 37 | |||||||||
| Korea | 8 | 62 | ||||||||||
| Lebanon | 40–58 | 60–63 | ||||||||||
| Lebanon b | 37 | 94 | 56 | 95 | ||||||||
| Malaysia | 70 | |||||||||||
| Pakistan | 58 | |||||||||||
| Saudi Arabia | 42–53 | 84–90 | ||||||||||
| Sri Lanka | 0 | 34 | 6 | 59 | ||||||||
| Syria | 61 | |||||||||||
| Thailand | 6% | |||||||||||
| Vietnam | 1 | 3 | ||||||||||
| Australia/Oceania | ||||||||||||
| Australia | 4 | 31 | ||||||||||
| Fiji | 3 | 11 | ||||||||||
| New Zealand | 0–2 | 42–54 | 2–6 | 51–52 | ||||||||
| Europe | ||||||||||||
| Belgium | 7 | 51 | ||||||||||
| Croatia c | 14 | 63 | ||||||||||
| Denmark | 14 | 52 | 0 | 24 | ||||||||
| Estonia d | 1 | 29 | cf. Palacios and Gonzalez 37 | |||||||||
| Estonia e | 8 | 73 | cf. Palacios and Gonzalez 37 | |||||||||
| Finland | 15 | 65 | 0.2 | 7 | ||||||||
| Finland b | 7 | 60 | ||||||||||
| France | 6 | 35 | ||||||||||
| Germany | 16 | 57 | 17 | 58 | 4 | 55 | ||||||
| Great Britain d | 3 | 15 | ||||||||||
| Great Britain e | 16 | 47 | ||||||||||
| Greece | 29 | |||||||||||
| Hungary | 57 | |||||||||||
| Iceland | 4 | 34 | ||||||||||
| Ireland | 6 | 45 | ||||||||||
| Italy b | 76 | |||||||||||
| Netherlands | 2 | 29 | ||||||||||
| Netherlands b | 4 | 33 | ||||||||||
| Norway | 40 | 0.1 | 19 | |||||||||
| Poland c | 25 | 92 | ||||||||||
| Scotland | 35 | 78 | ||||||||||
| Russia b | 47 | |||||||||||
| Russia, Northern Indigenous | 2–53 | 8–84 | ||||||||||
| Slovakia | 15 | |||||||||||
| Slovenia | 31 | 66 | ||||||||||
| Spain | 34 | |||||||||||
| Sweden b | 0.8 | 17 | 0 | 16 | ||||||||
| Switzerland | 38 | 6 | >50 | |||||||||
| Turkey | 66 | 79 | ||||||||||
| UK | 21 | |||||||||||
Studies including hospital patients, pregnant women and children excluded.
Conversion factor ng/ml to nmol/l = 2.5.
Older individuals with varying age ranges from 60+ years.
Postmenopausal women.
Summer.
Winter.
UK, United Kingdom; US, United States; VIT-D, vitamin D.
Factors associated with VIT-D deficiency
There are several factors associated with VIT-D deficiency. Association, however, does not necessarily imply causality, either in terms of the VIT-D deficiency itself, or in terms of risk of SMD or COVID-19 infection. We explore the limitation of such factors in more detail later, when examining the association between VIT-D deficiency, geographical latitude and death from COVID-19 infection.
Reduced sun exposure
VIT-D deficiency can occur when access to UVB radiation or nutritional sources are restricted (see section Source and daily intake). Migrants moving from lower to higher geographical latitudes and institutionalised populations have all been found to have an increased risk of VIT-D deficiency.38,39 Women from countries where it is customary to cover large parts of the skin may also be at risk, 37 as may be individuals who avoid sun exposure for cultural or health reasons. 40 The Oslo Health Study (HUBRO) may serve as an example. In this cross-sectional study, VIT-D concentration taken between May 2000 and January 2001 were compared between individuals born in Norway (which is above 50° latitude) and individuals born in Pakistan (which is largely below 33° but where it is traditional to cover the body). Individuals born in Norway had a mean standardised 25(OH)D concentration of 28.4 (95% CI 26.9–29.9) ng/ml; 15% had a 25(OH)D concentration of less than 20 ng/ml and 0.1% of less than 10 ng/ml. Individuals born in Pakistan had a mean standardised 25(OH)D concentration of 11.0 (95% CI 9.1–13.0) ng/ml; 92% had a 25(OH)D concentration of less than 20 ng/ml and 52% of less than 10 ng/ml. 41 In the context of the ongoing COVID-19 outbreak, prolonged periods of quarantine are likely to increase the risk of VIT-D deficiency. This risk may be particularly higher in individuals with darker skin and/or older age. In consequence, populations living in, or originating from, countries in lower latitudes closer to the equator may be more affected by prolonged quarantines if they do not compensate through nutritional intake of VIT-D. 42 Additionally, older individuals run a higher risk of VIT-D deficiency, reducing the age-related decline of renal function may also impair conversion of VIT-D into its biological active forms, 1,25(OH)2D. 43 Finally, air pollution decreases the permeability for UVB radiation. 24
Obesity
Obesity may also lead to VIT-D deficiency through a mechanism under genetic control. In a pooled analysis of 21 genetic studies with 42,024 participants, a higher body mass index (BMI) was associated with lower 25(OH)D serum concentrations. But, at the same time, lower 25(OH)D concentration did not seem to lead to higher BMI. In this study, a 10% higher BMI was associated with a 4.2% lower 25(OH)D concentration. 44 Two large American studies have shown that more than 40% of all patients admitted to hospital for a COVID-19 infection are obese.45,46
Age
There is an overlap between risk factors for VIT-D deficiency and need for hospital care for, or death from, COVID-19 infection. Older age has been identified as a major risk factor of death. 46
Ethnicity
According to a preliminary report of the OpenSAFELY Collaborative in the UK, Black and Asian individuals have a two-fold higher risk of dying from a COVID-19 infection compared with White individuals. 47 These findings seem also hold true for individuals with psychotic disorders. A further retrospective cohort study of individuals treated with antipsychotics also found COVID-19 infection to be significantly associated with Black ethnicity, older age and obesity. 48 In the most recent retrospective cohort study at the time of writing, Black individuals with VIT-D serum concentrations of less than 40 ng/ml ran a higher risk of COVID-19 infection than black individuals with VIT-D serum concentrations of 40 ng/ml or greater. The risk of COVID-19 infection seemed to be VIT-D serum concentration dependent. For White individuals, no significant association was identified. 49
Drug interactions
Orlistat is a slimming drug that reduces the absorption of fat and therefore VIT-D uptake. Enzyme induction and/or interference with the pregnane X receptor (PXR) can accelerate VIT-D metabolism. Enzyme induction and PXR activation can work in conjunction. PXR can bind to VIT-D responsive elements. Many drugs from various classes are PXR ligands.50,51 From a psychiatric point of view, the PXR ligands most likely encountered in clinical practice are steroidal anti-inflammatory drugs, the antiepileptic carbamazepine, the herbal antidepressant St John’s wort (Hypericum perforatum) and the herbal sedative Kava Kava (Piper methysticum).
Other risk factors
Smoking is a further risk factor for VIT-D deficiency.52,53 This is possibly related to accelerated of skin aging in smokers with increased wrinkling and hyperpigmentation.24,54 Adhering to a strict vegan diet may also increase the risk of VIT-D deficiency. 55 Some medical conditions can also increase the risk of VIT-D deficiency. These include (a) malabsorption syndromes such as irritable bowel syndrome or coeliac disease, (b) chronic kidney disease impairing the transformation of VIT-D from its biologically inactive form 25(OH)D to its biological active form 1,25(OH)2D and (c) taking medications that interfere with VIT-D uptake or accelerated VIT-D metabolism.
Vitamin D supplements
Dietary supplements exist in two forms, VIT-D2 and VIT-D3. VIT-D2 supplements are plant-based, originating from UV-irradiated ergosterol in yeast. VIT-D3 supplements are animal-based, originating from UV-irradiated 7-dehydrocholesterol in lanolin (sheep wool grease). 26 As an animal product, VIT-D3 supplements are not suitable for vegans. 56 In the UK, VIT-3 formulations are most common. Many over-the-counter available formulations also contain calcium-carbonate. Different VIT-D preparations may vary in pharmacokinetic properties depending on mode of administration (intramuscular versus oral) and type of VIT-D. 57 VIT-D2 has been suggested to be inferior to VIT-3 in terms of pharmacokinetic properties, including affinity to VIT-D binding protein, speed of deactivation and clearance.57–59 However, differences in clearance performance between VIT-D3 and VIT-D2 may diminish with higher baseline concentrations and increasing doses. 57 One meta-analysis of seven intervention studies has suggested that VIT-D3 was more effecting raising 25(OH)D concentrations than VIT-D2, when given intramuscularly but not when given orally. 60
The UK Food Standard Agency suggests that long-term exposure to doses of 25 µg (1000 IU) may be well tolerated. Higher exposures such as 45 µg (1800 IU) may be tolerated short-term under medical supervision. 23 US recommendations suggest a toxicity threshold of daily intakes between 250 µg (10,000 IU) and 1000 µg (40,000 IU)/day. 19 Both the US and Australia provide more detailed recommendations for safe upper levels for daily VIT-D intake (Table 5).19,30
Table 5.
| Age | Males | Females | Males | Females | |
|---|---|---|---|---|---|
| AUS | US | ||||
| Vitamin D intake µg/day a | |||||
| 0–12 months | 25 | 25 | 25 | 25 | 0–6 months |
| 38 | 38 | 7–12 months | |||
| 1–18 years | 80 | 80 | 73 | 73 | 1–3 years |
| 75 | 75 | 4–8 years | |||
| 100 | 100 | 9–13 years | |||
| 18+ years | 80 | 80 | 100 | 100 | |
| Pregnancy | 80 | 80 | |||
| Lactation | 80 | 80 | |||
Conversion factor from µg to IU = 40, 1 µg cholecalciferol = 0.2 µg 25(OH)D; 1 IU = 0.025 µg cholecalciferol or 0.005 µg 25(OH)D.
AUS, Australia; IU, international units; VIT-D, vitamin D.
Adverse effects
Excess VIT-D may result in hypercalcemia and hypercalciuria. Excessive use can lead to renal and cardiovascular toxicity due to tissue calcification in the heart, blood vessels and kidney. The evidence, however, remains conflicting. A Cochrane systematic review reported a 3.2-fold increased risk of hypercalcaemia with active forms of VIT-D, 1,25(OH)2D or alfacalcidiol – a VIT-D analogue. However, there was no statistically increased risk of hypercalcaemia with supplemental VIT-D2 or VIT-D3. VIT-D combined with calcium increased the risk of nephrolithiasis by 20%. 61 A more recent meta-analysis also found that long-term VIT-D use was associated with hypercalcaemia and hypercalciuria, but not with nephrolithiasis. These findings were irrespective of VIT-D dose and calcium co-administration. 62 An update of this meta-analysis concerning monthly high dose VIT-D supplementation did not either show any increased risk of nephrolithiasis, although there was a trend towards hypercalcaemia. 63 A randomised controlled trial (RCT) from the same research group with 5110 participants in New Zealand concluded that monthly supplementation of 100,000 international units (IU) VIT-D over a median of 3.3 years did not affect the incidence rate of hypercalcaemia or nephrolithiasis. 64 VIT-D remains, however, contraindicated in conditions associated with hypercalcaemia and nephrolithiasis. VIT-D should be used only with caution in patients with sarcoidosis because of increased conversion of 25(OH)D to 1,25(OH)2D. 65 Finally, there are several drugs that can increase calcium serum concentrations. Such include thiazide diuretics and theophylline. 65 Lithium has also been associated with hypercalcaemia and hyperparathyroidism. 66 Varying prevalence rates have been reported. One Swedish study of patients with bipolar disorder estimated that 26% patients treated with lithium had hypercalcaemia, compared with 1.5% not treated with lithium and 3% in a control population. 67 Routine monitoring of calcium concentrations is already standard for patients treated with lithium. Monitoring of serum calcium concentrations could be extended to other individuals with SMD, if excessive VIT-D use was suspected, or if there was deterioration in mental status without any obvious reason.
Vitamin D and serious mental disorders
VIT-D deficiency has been linked to several severe mental and neurodevelopmental disorders, including depression and schizophrenia, autism and ADHD.68–70 However, it remains unclear whether the link between VIT-D deficiency and such disorders is causal or just associative.
Proof of principle
Laboratory findings
A role for VIT-D in the brain was suggested when both 25(OH)D and 1,25(OH)2D were found in the cerebrospinal fluid of 46 patients with no endocrine disorders, who had undergone lumbar puncture for a suspected or confirmed disk prolapse. 71 A year later, it was demonstrated in a rodent model that 25(OH)D and 1,25(OH)2D could cross the blood brain area, albeit in small amounts. 72 An immunohistochemical study performed on human brain tissue showed that the VIT-D3 receptor (VIT-DR) was present in the brain; 1α- hydroxylase (1α-OHase), required to convert VIT-D into its active form, was also found. For both, immunoreactivity was present in varying amounts in the prefrontal cortex, cingulate gyrus, hippocampus, caudate, putamen, amygdala, thalamus, substantia nigra, lateral geniculate nuclei, hypothalamus and cerebellum. Additionally, 1α-OHase immunoreactivity was present in the basal forebrain. The wide distribution of the VIT-DR and 1α-OHase suggested a diverse role for VIT-D for cerebral functions. 73 VIT-D seems to play a crucial role for the developing, adult and aging brain. 74 Yet many functions are still not well understood. Studying inborn errors of VIT-D metabolism is one way to explore the role of VIT-D in the central nervous system (CNS). VIT-D 1α-OHase deficiency, formerly called pseudo VIT-D deficiency rickets, is caused by a mutation affecting CYP27B1 that converts 25(OH)D to 1,25(OH)2D leading to hypocalcaemic rickets. However, the impact of VIT-D 1α-OHase deficiency on the CNS has been described mainly in terms of symptoms associated with hypocalcaemia, seizures, depression and anxiety. 75
Neurodevelopment
Nutritional deficits including VIT-D deficiency can interfere with these neurodevelopmental processes. Timing, degree, and duration of VIT-D deficiency are all factors that are poorly understood. 76 Rodent experiments have shown that VIT-D deficiency can affect brain morphology. This can result in increase of volume of the lateral ventricles and decrease of cortical thickness – two morphological changes associated with psychosis.77,78 Further changes observed in rodent experiments include increase of mitosis in some brain areas, decrease of apoptosis and reduction of neurotrophic factors such as glia-derived neurotrophic factor (GDNF) and nerve growth factor (NGF). 77 VIT-D deficiency may also adversely affect the development of the dopaminergic system possibly through modulation of dopamine turnover and GDNF activity. 77 L-Type voltage gated Ca 2+ channels (L-VSCC), mediating a range of neuronal processes, including excitability, gene expression, long-term potentiation and depression, have been identified as a target for VIT-D.79,80 L-VSCC have been linked to brain aging, neuronal vulnerability and schizophrenia.80,81 Rodent experiments have further shown that pre-natal VIT-D deficiency can affect dopamine neurotransmission in adult life and lead to an enhanced response to pro-psychotic agents.77,82,83 Further rodent experiments have shown that 1,25(OH)2D2 can partially protect against neurotoxic damage from 6-hydroxydopamine (6OHDA) when given over a longer time,77,84 or against serotonin and dopamine depletion caused by toxic doses of methamphetamine. 85 Both effects may be GDNF mediated.84,85 Besides, VIT-D can also mediate the synthesis of various neurotransmitters such as acetylcholine, noradrenalin, serotonin and dopamine.79,86,87
Neurodegeneration
VIT-D has neuroprotective properties. In consequence, VIT-D deficiency may lead to neurodegeneration. The neuroprotective properties of VIT-D seem related to anti-inflammatory and antioxidant properties. 88 In one in vitro experiment, primary cortical glial cultures were exposes to a bacterial endotoxin. This led to an accommodation of nitrite, reactive oxygen species (ROS), interleukin-6 (IL-6) and macrophage inflammatory protein-2 (MIP-2). It was found that 1,25(OH)2D3 reduced the production of these pro-inflammatory substances. 89 Downregulation of L-VSCC protects against 6-hydroxydopamine-mediated neurotoxicity. Protection from glutamate-induced neurotoxicity is a further option.79,90,91 Finally, as outlined previously, VIT-D modulates GDNF and NGF. GDNF acts on the basal ganglia, whereas NGF acts on the cholinergic neurons in the basal forebrain. Via these pathways, VIT-D could potentially reduce the risk of Parkinson disease and cognitive impairment. 79
Observational studies
Depression
The suspicion of a link between depression and VIT-D deficiency stems from studies of seasonal affective disorder (SAD). SAD is characterised by seasonal mood swings with decreases in mood in the autumn and winter months. 17 It has also been shown that sunlight affects serotonin turnover in the brain, that serotonin turnover is lowest in winter and serotonin production is directly related to bright sunlight exposure. 92 Three meta-analyses have examined the association between VIT-D concentrations and depression.93–95 All three analyses identified an inverse association between VIT-D concentration and depression. However, it proved difficult to pool studies, and two meta-analyses reported heterogeneity.93,95 One meta-analysis reported publication bias, 93 and another meta-analysis could not rule out publication bias. 94 Cut-off points for VIT-D insufficiency and deficiency varied somewhat.
Three observational studies have been published since the most recent meta-analysis.96–98 In these studies, the relationship between depression and VIT-D deficiency was less clear. One study failed to find a significant difference in participants with non-psychotic depression and controls. 97 Another study, following older adults with a depressive disorder over 2 years, found that an increase in VIT-D concentrations was significantly associated with improved frailty scores when adjusted for depression, but not independently with improvement of depression. 98
Schizophrenia
The risk of schizophrenia has been linked to factors also associated with VIT-D deficiency. These concern individuals (a) born during winter or spring, (b) born in higher latitudes of either hemisphere, (c) living in urban areas and (d) with darker skin living in colder climates.77,79
Five meta-analyses have examined the association between VIT-D concentrations and schizophrenia or psychosis.99–103 All five meta-analyses identified an inverse association between VIT-D concentration and schizophrenia. However, four meta-analyses reported heterogeneity.99–102 One meta-analysis did not test for heterogeneity. 103 Three meta-analyses did not find any evidence for publication bias.99–101 The remaining two meta-analyses had not tested for publication bias.102,103 As for the meta-analyses concerning depression, the cut-off points for VIT-D insufficiency and deficiency varied somewhat. There have been six observational studies published since the most recent meta-analysis.96,97,104–107 Four studies found a low VIT-D concentration in individuals with psychosis or schizophrenia,96,105–107 two of which concerned first episode psychosis.105,106 Two studies failed to find such an association.97,104 One of these studies was substantially larger than all other observational studies. 97 There were no intervention studies testing the use of VIT-D in individuals with schizophrenia.
Mendelian randomisation studies
A recent genome-wide association study (GWAS) of 417,580 European UK biobank participants, using a Mendelian randomisation approach, explored the relationship between VIT-D concentrations and several phenotypes including several mental disorders. There were several candidate phenotypes, including major depression, bipolar, disorder or schizophrenia, with causal (direct or indirect) effects on VIT-D concentration. The findings suggested that SMDs were associated with behaviours leading to reduced production of VIT-D rather than VIT-D deficiency leading to SMD. 108 Another recent study exploring the causality between 25(OH)D and depression reported similar findings. Depression led to lower 25(OH)D concentrations, but lower 25(OH)D did not lead to depression. 109 Previous Mendelian randomisation studies had failed to find shared genetic effects between 25(OH)D and major depressive disorder.110–114 However, all studies have some limitations. Findings from populations with predominant European ancestry cannot be generalised to populations with non-European ancestry. Statistical power can become a problem, when investigating the impact of either extremely high or low 25(OH)D concentrations. 113 Potential antenatal adverse effects of VIT-D deficiency on the risk of SMD in offspring cannot be determined. 108 A life-time perspective for both risk factors and outcomes under study cannot inform on the impact of risk factors on outcomes during a specific sensitive period. 112 A potential impact of VIT-D deficiency in childhood on the risk of SMD later in life can therefore not be explored with this approach. Finally, all studies used data from a few major genetic databanks with overlap and partly duplication of results.
Intervention studies
Depression
Eight meta-analyses investigated the effect of VIT-D supplementation on depression (Supplemental Table S1).115–122 Three meta-analyses found VIT-D to be effective as judged by the reduction of depression scores. VIT-D doses and routes of administration differed. However, most studies used oral VIT-D administration. Five meta-analyses reported heterogeneity115,118,119,121,122 One study did not assess heterogeneity. 120 Publication bias was present in two studies.118,122 Two studies did not assess publication bias.119,120
Two more clinical trials have been published subsequent to the latest meta-analysis.123,124 The first study assessed the effect of oral VIT-D supplementation with 40 µg on depression and anxiety in 158 Chinese patients with low 25(OH)D concentrations over a period of 6 months. Whereas symptoms of anxiety improved significantly, symptoms of depression did not. 123 However, the findings of this study are difficult to interpret, since information on blinding and placebo use in the control group is not given. The second study was conducted as a randomised controlled trial in 46 Indian patients with major depression and concurrent VIT-D deficiency over 12 weeks. The intervention group received 75,000 µg single parenteral dose of VIT-D in addition to treatment as usual. At the end of follow-up, the intervention had significantly improved symptoms of depression, severity of illness and quality of life. 124
Summary of findings
The laboratory evidence suggests that VIT-D may have a role in the pathophysiology of SMD.
Most of the evidence from observational studies points towards some association between VIT-D deficiency and depression and VIT-D and schizophrenia. Mendelian randomisation studies suggest no or reverse causality; VIT-D deficiency is more likely consequence but not a cause of SMD, possibly related to social withdrawal and isolation indoors. 17 The evidence from intervention studies is conflicting; the effect of VIT-D may be most prominent when correcting an underlying VIT-D deficiency.
Vitamin D and COVID-19 infections
Proof of principle
The precise mechanism by which VIT-D exerts any protective effect against infection is unknown. Vitamin-D is nonetheless known to play a role in the immune system. As demonstrated for brain cells, immune cells also express 1α- hydroxylase (1α-OHase) and VIT-DR. 69
Several pathways have been suggested by which VIT-D could reduce the risk of viral infections: (a) actions on the innate and adaptive immune system, (b) antioxidant actions and (c) renin-angiotensin system (RAS) inhibition in the context of COVID 19 infection.69,125–127 The actions of VIT-D on these pathways are not completely understood.
Actions on the immune system
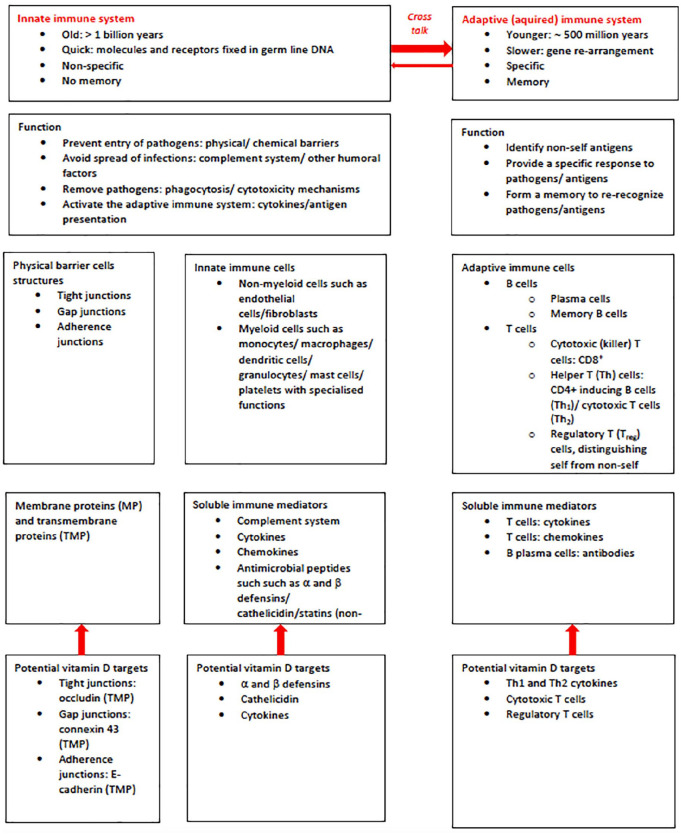
Vit-D can exert actions on both the innate and the adaptive part of the immune system (Figure 2).125,128–134
Figure 2.
Immune system: schematic overview and potential vitamin D targets.
Innate immune system
The innate immune system is the first line of defence with a rapid response to invading pathogens. It is non specific. The innate immune system has both cell and humoral components. The innate immune system aims at rapidly recognising and eliminating pathogens, thereby limiting the infection. Cellular physical barriers prevent pathogens from entry.125,126,133 VIT-D is involved in the upregulation of genes that encode proteins required to maintain cellular barriers and junction integrity. 133 Innate immune mediators include human cathelicidin (leucine-leucine-37/LL-37) and human α and β defensins. These ‘alarmins’ are peptides with antimicrobial activity against bacteria and fungi as well as viruses125,135 VIT-D can induce human cathelicidin and defensins as part of the rapid (innate) response system. This can reduce viral replication and pro-inflammatory cytokines. VIT-D may reduce the generation of pro-inflammatory cytokines, such as TNF-α, INF-γ, IL-6, IL-12p70; IL-17 and IL-21.134,136–142 At the same time, VIT-D may also increase the generation of anti-inflammatory cytokines, such as IL-10.137,140,143 VIT-D may also act synergistically with IL-2 to stimulate other proteins that regulate the immune response. 134 The net effect of shifting the balance towards anti-inflammatory cytokine activity may decrease the probability of a cytokine storm. This could then also decrease the likelihood of a potentially lethal cytokine storm, which can result from alarmins being released in excess during severe injuries or maximal stimulation. 144 Specifically, VIT-D may be able to reduce the pro-inflammatory IL-6. 145 This is particularly relevant since IL-6 may have a key role in the generation of cytokine storms and therefore in the pathophysiology of a COVID-19 infection. This assumption is supported by studies that have shown that higher IL-6 concentrations are associated with higher COVID-19 disease severity and mortality.146,147 One study investigated inflammatory markers in 154 patients with COVID-19 in relation to VIT-D deficiency. VIT-D deficient patients had significantly higher concentrations of IL-6 and a significantly higher mortality. 148
Adaptive immune system
The adaptive (acquired) immune system is the second line of defence with a protracted response to invading pathogens. It is highly specific to particular pathogens. The adaptive immune system has antibody response and a cell-mediated response. The antibody response involves B lymphocytes and T1-helper cells (Th1). The cell-mediated response involves cytotoxic T lymphocytes and T2 helper cells (TH2). The adaptive immune system aims at eliminating a specific pathogen and creating longer-lasting immunity. The actions of VIT-D seem directed mainly at T cells.125,130,134
Antioxidant properties
VIT-D may also have some antioxidant properties. 125 In rodent experiments, it has been shown that VIT-D can increase the expression of glutathione reductase (GR). GR facilitates the conversion of glutathione disulphide to glutathione. Glutathione reduces oxidative stress by scavenging hydroxyl radicals, ROS and other electrophiles. 149
Renin-angiotensin inhibition
RAS is an essential regulator of lung function. The angiotensin converting enzyme-2 (ACE-2) plays an essential role. ACE-2 converts angiotensin-2 into angiotensin. 150 Whereas angiotensin-2 is a vasoconstrictor, angiotensin is a vasodilator. Therefore, altered ACE-2 function plays a role in the development of hypertension. ACE-2 deficiency is associated with hypertension. ACE-2 overexpression is associated with attenuation of hypertension. 150 ACE-2 is also expressed highly in the lungs. Already in the early 2000s, ACE-2 was implicated as a functional receptor for SARS-CoV. 151 Coronavirus mediated acute respiratory distress syndrome (ARDS) may result from down-regulation of ACE-2. This results in upregulation of angiotensin-2. Actions of angiotensin-2 on the angiotensin-2 type 1a (AT1a) receptor leads to pulmonary oedema and impaired lung function. Actions of angiotensin-2 on the AT2 receptor, however, may be lung-protective. 152 SARS-CoV-2 is closely related to SARS-CoV. Like SARS-CoV, SARS CoV-2 also binds with high affinity to ACE-2. The virus uses ACE-2 to enter target cells in the lung and may affect lung function in the same way.153,154 Based on animal models, it has been suggested that VIT-D can suppress RAS and angiotensin-2 activity. In humans, this has been put to the test in 184 normotensive individuals. In this study, lower VIT-D levels were associated with (a) higher angiotensin-2 concentrations and (b) a blunted renal plasma flow response to exogenous angiotensin-2 infusion. 155
Evidence from observational studies regarding COVID-19 infections
Since the beginning of 2020, a multitude of observational studies have been published, examining the relationship between vitamin status and risk of COVID-19 infection or adverse clinical course, including death. These studies have been summarised in five meta-analyses (Supplemental Table S2).156–160 The results regarding an association between VIT-D deficiency and risk of COVID-19 infection were conflicting.156–158,160 Two meta-analyses found an increased risk of COVID-19 infection associated with lower 25(OH)D concentrations.156,158 For the third meta-analysis, the result was ambiguous; the lower confidence interval (CI) was 1. 157 The fourth meta-analysis did not find any significant association. 160 Three meta-analyses explored the severity of COVID-19 infection in relation to VIT-D status.156,159,160 All three studies found that lower 25(OH)D concentrations were associated with a more severe clinical course. Two meta-analyses reported on risk of death with COVID-19 infection.156,160 One meta-analysis reported a significant association with VIT-D deficiency. 160 The other meta-analysis had conflicting results, depending on regression model used. 156 All five meta-analyses reported heterogeneity. One meta-analyses reported publication bias. 157 Another meta-analysis reported publication bias for the subgroup analysis regarding risk of infection. 158 No Mendelian randomisation studies have been published assessing the relationship between VIT-D status and risk of COVID-19 infection or adverse clinical course.
Country/region comparisons
Several studies have related COVID-19 infections or deaths in a country or region to the assumed VIT-D status in that country or region.42,161–168 However, such studies are difficult to interpret. Reporting of COVID-19 infections and deaths varies between countries. 169 Reporting of VIT-D deficiency also varies, rendering population-based mean or median VIT-D concentrations unreliable. A Mendelian randomisation analysis from the US indicated a relationship between geographical latitude as a proxy for VIT-D status and COVID-19 associated mortality. The relationship was most prominent in Afro-Americans and less so in others. The mortality excess was related to geographical latitude with the steepest gradient at 44° north. 170
Still, latitude and VIT-D deficiency may correlate less well than often assumed. VIT-D status is dependent not only on exposure to sunlight but also on compensatory dietary intake, including access to VIT-D fortified foods and ethnicity.37–39,49,171 COVID-19 infections or deaths in the Southern hemisphere 165 may just reflect the much smaller landmass and population in the Southern hemisphere. Also, in the Southern hemisphere, the COVID-19 pandemic began during the summer months. Seasonal VIT-D fluctuations may not necessarily account for seasonal fluctuations of COVID-19 infection. Temperature and humidity are factors to influence the survival of many respiratory viruses including SARS-CoV, Middle Eastern respiratory syndrome (MERS)-CoV and influenza. This may also apply to COVID-19. 169 Finally, underreporting may play a role. Exploring the 13 countries through which the equator passes shows that latitude per se is a poor measure for the risk of or death from COVID-19 infection. There was a considerable variation in COVID-19 cases and deaths between countries. For reasons still unknown, the COVID-19 infection and mortality rates are much more unfavourable in the South American than for most African equatorial countries. Variations in country size and test availability cannot fully account for this observation (Table 6).
Table 6.
COVID-19 cases and death in the 13 countries through which the equator passes.
| Country a | Latitude | COVID-19 as of 24 March 202116 (Worldometer: Coronavirus https://www.worldometers.info/coronavirus/) last updated 13.07 GMT | |||||
|---|---|---|---|---|---|---|---|
| N tests/1 M pop | N cases/1 M pop | N deaths/1 M pop | Ratio n cases/n deaths/1 M pop | World ranking n cases | World ranking n deaths | ||
| Ecuador | 2°N–5°S | 61,951 | 17,580 | 925 | 19.0 | 98 | 42 |
| Colombia | 12°N–4°S | 240,726 | 45,777 | 1215 | 37.7 | 54 | 28 |
| Brazil | 6°N–34°S | 133,861 | 56,805 | 1399 | 40.6 | 39 | 23 |
| Sao Tome and Principe | 0° | 52,194 | 9721 | 153 | 63.5 | 123 | 108 |
| Gabon | 3°N–4°S | 267,214 | 7987 | 48 | 166.4 | 126 | 144 |
| Republic of Congo | 4°N–5°S | 16,472 | 1703 | 24 | 71.0 | 160 | 160 |
| Democratic Republic of Congo | 6°N–14°S | ||||||
| Uganda | 4°N–2°S | 19,717 | 871 | 7 | 124.4 | 181 | 184 |
| Kenya | 5°N–5°S | 26,094 | 2255 | 37 | 60.9 | 151 | 147 |
| Somalia | 12°N–2°S | 640 | 28 | 22.9 | 188 | 154 | |
| Maldives | 8°N–1°S | 1,128,428 | 41,632 | 121 | 344.1 | 61 | 116 |
| Indonesia | 6°N–11°S | 44,519 | 5357 | 154 | 145 | 113 | |
| Kiribati | 3°N–11°S | No data available | |||||
Countries listed geographically from West to East.
1 M pop, 1 million population; COVID-19, coronavirus disease 2019.
Evidence from intervention studies regarding COVID-19 infection
We identified seven studies examining VIT-D as an intervention.172–178 Three studies were intervention trials,172,173,175 two studies used mixed methods and two studies were observational (Table 7).174,176,177,178 The results were mixed. Doses and modes of administration varied. Five studies explored COVID-19 associated mortality.172,173,176–178 Of these, two studies found a decreased risk of death,176,177 whereas two did not.173,178 One study reported fewer deaths in the intensive care unit (ICU), but the result was not significant. 172 Three studies explored admission to ICU.172,173,178 Of these, one study found a significant association with decreased ICU admissions. 172 Another trial bordered on significance. 178 Two trials explored length of stay. Both did not find any association with VIT-D treatment.173,178 One study examined the time to recovery from COVID-19 infection in terms of negative RNA serology. 175 Significantly more patients receiving VIT-D became seronegative within 21 days. However, there was no difference in mean time to seronegativity. 175 One observational study explored the risk of COVID-19 infection with habitual VIT-D use in 8297 biobank patients. A significant association became only evident after adjustment for confounders. 174 The results from these studies can be considered as only preliminary. The studies are heterogenous and vary in mode of VIT-D administration. Only two studies used a placebo as a comparator.173,175 Lack of power and blinding are further concerns.
Table 7.
Vitamin D supplementation and COVID-19. Evidence from intervention studies.
| Study | Type of study | Sample | Age (years) | Sex | Intervention | Control | Follow up | Results |
|---|---|---|---|---|---|---|---|---|
| Ma et al., 174 UK | Prospective study of an intervention | 8297 adults with COVID-19 test results in the UK biobank I: 363 VIT-D users C: 7934 VIT-D non-users |
I: 59.1 SD 8.1 C: 57.4 SD 8.6 (p < 0.001) |
I: M 39% F 61% C: M 50% F 50% |
Habitual use of VIT-D | No VIT-D use | COVID-19 test results for ca. 4 months | Risk of COVID 19 infection I: 13.5%, C: 16.8% Unadjusted OR 0.78 CI 0.57, 1.05) (p = 0.105) Adjusted OR 0.67 CI 0.46, 0.98 (p = 0.038) |
| Murai et al. 173 | Multi-centre RCT, double blind | 237 of 240 randomised pts hospitalised with moderate to severe COVID-19 infection I: 119 C: 118 |
Mean I: 56.5 SD 13.8 C: 56.0 SD 15.0 (NS) |
I: M 59%, F 41% C: M 53% F 55% (NS) |
5000 µg VIT-D3 as a single dose | Placebo | Ca. 4 months | Median LOS I: 7 IQR 4, 10 days C: 7 IQR 5, 13 days HR 1.07 CI 0.82, 1.39 (p = 0.59) In-hospital mortality I: 7.6%, C: 5.1% Δ 2,5 CI −4.1, 9.2% (p = 0.43) Admission to ICU I: 16.0%, C: 21.2% Δ −5.2 CI −15.1, 4.7% (p = 0.30) |
| Annweiler et al., 176 France a | Post hoc open label intervention study | 66 pts, nursing home residents I: 57 C: 9 |
Mean I: 87.7 SD 9.3 C: 87.4 SD 7.2 (NS) |
I: M 23% F 77% C: M33% F 67% (NS) |
2000 µg VIT-D3 Bolus VIT-D in the week following a suspected or confirmed COVID-19 infection or in the preceding month |
None | Mean 36 SD 17 days |
Death I: 17.5%, C: 55.6%) Adjusted HR 0.11 CI 0.03, 0.48 (p = 0.003) Survival time I: Longer survival (p = 0.002) Severity of COVID-19 I: Inverse OS CI (p = 0.001) |
| Annweiler et al., 177 France | Post hoc open label intervention study | 77 pts hospitalised in a geriatric unit I1: 29 I2: 16 C: 32 |
I1: 88 IQR 87, 93 I2: 85 IQR 84–89 C: 88 IQR 84–92 (NS) b |
I1: M 31% F 69% I2: M 69% F 31% C: M 59% F 41% (p = 0.02) |
I1: Bolus VIT-D3 po taken regularly during the year before the COVID-19 pandemic, either 1250 µg VIT-D3 every month or 2000–2500 µg VIT-D3 every 2–3 months I2: Bolus VIT 3 po 2000 µg VIT-D3 within a few hours after COVD-19 diagnosis and no previous VIT-D supplements |
None | 14 days (from hospitalisation) |
Death
I1: 6.9%, I2: 18.8%, C 31.3% Adjusted HR I1 versus C 0.07 CI 0.01, 0.61 (p = 0.017) Adjusted HR I2 versus C 0.37 CI 0.06, 2.21 (0.28) Survival time I1 versus C: longer survival (p = 0.015) I2 versus C: no difference in survival time (p = 0.32) I1 versus I2: no difference in survival time (p = 0.32) Severity of COVID-19 I1 versus C: fewer pts with severe COVI-19 (p = 0.033) I2 versus C: no difference (p = 0.40) |
| Entrenas Castillo et al., 172 Spain | Open label trial, blinded at the point of analysis | 76 pts hospitalised with COVID-19 infection I: 50 C: 26 |
Mean I: 53.1 SD 10.8 C: 52.8 SD 9.4 (NS) |
I: M 54% F 46% C: M 69% F 31% (NS) |
532 µg 25(OH)D3 (=2660 µg VIT D3) on admission, 266 µg 25(OH)D3 (=1330 µg VIT D3) on day 3 and 7 and then weekly until ICU admission or discharge | None | Until ICU admission, death, or hospital discharge | Admission to ICU I: 2%, C: 50%, Adjusted OR 0.03 CI 0.003, 0.25 Death in ICU I: 0%, C: 15.4% c (p = 0.894) d |
| Rastogi et al., 175 India | RCT (SHADE study) | 40 pts with mild or asymptomatic COVID-19 infection with VIT-D deficiency, i.e., 25(OH)D conc <20 ng/ml I: 16 C: 24 |
Median I: 50 IQR 36, 51 C: 47.5 IQR 39.3, 49.2 (NS) |
I: M 38% F 62% C: M 58% F 42% (NS) |
1500 µg VIT D3 for 7 days with a serum conc of >50 ng/ml 25(OH)D as therapeutic target If after 7 days 25(OH)D <50 ng/ml 1500 µg VIT D3 for a further 7 days until day 14 |
Placebo | 21 days | COVID-19 RNA −ve after 21 days I: 62.5%, C: 20.8% (p < 0.018) No difference in mean duration to COVID-19 −ve ↓ fibrinogen I: more ↓ (p < 0.01) Other inflammatory parameters: No difference regarding D-dimer, CRP, ferritin, procalcitonin |
| Hernandez et al., 178 Spain | Retrospective study of an intervention (case control) | 216 pts hospitalised with COVID-19 infection I: 19 C: 197 |
Median I: 60 IQR 59, 75 C: 61 IQR 47.5, 70 (NS) |
I: M 37% F 63% C: M 62% F 38% (p = 0.03) |
Oral vitamin D supplements >3 months | No VIT-D use | 21 days | Median LOS I: 8 IQR 6, 14 days C: 12 IQR 8.0, 16.0 days (p = 1.07) ICU admission I: 5.3%, C: 25.4% (p = 0.05) Death I: 10.5%, C: 10.4% (p = 0.999) |
Conversion factor from µg to IU = 40, 1 µg cholecalciferol = 0.2 µg 25(OH)D.
Not specified by authors whether mean or median, in view of IQR use most likely median.
Of all ICU patients.
Own calculation.
1 IU = 0.025 µg cholecalciferol or 0.005 µg 25(OH)D.
C, control; CI, 95% confidence interval; conc, concentration; COVID-19, coronavirus disease 2019; F, female; HR, hazard ratio; I, intervention; ICU, intensive care unit; IU, international units; LOS, length of stay; M, male; NS, not significant; OR, odds ratio; po, per os; pts, participants; RCT, randomised controlled trial; re, regarding; SD, standard deviation; VIT-D: vitamin D; −ve, negative; Δ, difference.
Summary
The laboratory evidence suggests that VIT-D in principle could provide a defence against COVID-19 infection and against an adverse clinical course. The potential ability of VIT-D to reduce anti-inflammatory cytokines mitigate a cytokine storm and inhibit RAS may be particularly important. The evidence from observational studies does not point consistently towards some association between VIT-D deficiency and risk of COVID-19 infection. There is stronger, but not unanimous, evidence for an association between VIT-D deficiency and an adverse clinical course from COVID-19 infection. Inferences from geographical latitude seem unreliable. The available intervention studies provide conflicting results. Methodological flaws and lack of statistical power limits the validity of several studies. Larger and more methodical trials are required to assess the effectiveness in the prevention and treatment of COVID-19 infection as well as VIT-D dosing and mode of administration. Three larger trials are currently in preparation. The CARED trial will examine whether a single high dose of oral cholecalciferol improves the respiratory outcomes as compared with placebo among 1264 adult COVID-19 patients at moderate risk of clinical complications. 179 The VIVID trial will evaluate the efficacy of daily Vit-D3 supplementation for 4 weeks to reduce disease severity in persons with newly diagnosed COVID-19 infection and to prevent infection in their closest household members. This trial plans to recruit 2700 participants. 180 The COVIT-TRIAL will compare the effect of a single oral high dose of cholecalciferol versus a single oral standard dose of cholecalciferol on 14-day all-cause mortality rate in 260 older adults infected with SARS-CoV-2 at higher risk of deterioration. 181
Conclusion
VIT-D deficiency seem associated with mental disorders such as depression and schizophrenia. But VIT-D deficiency is more likely consequence than a cause of SMD. On proof of principle, VIT-D in could provide a plausible defence against COVID-19 infection and against an adverse clinical course. But data from observational studies and the first preliminary intervention studies remain conflicting, with stronger evidence that VIT-D may mitigate the clinical course of COVID-19 infection rather than the risk of infection in the first place.
Individuals with SMD may have a higher risk of VIT-D deficiency. They also experience higher mortality COVID-19 infection.1–3 Therefore, it is not unreasonable to assume that individuals with SMD may also experience more adverse effects from COVID-19 infection. VIT-D is relatively cheap, widely available, and easy to administer as an oral supplement. Within the recommended dose-range, adverse effects seem rare. From a public health and public mental health point of view, based on the currently limited knowledge, for individuals with SMD, the benefits of VIT-D optimisation through supplementation seem to outweigh the risks. At the same time, it is important to medically supervise VIT-D supplementation to prevent inappropriate VIT-D use in high doses that could lead to toxicity. VIT-D supplementation should not substitute for vaccination or medical care for COVID-19 infection.
Supplemental Material
Supplemental material, sj-docx-1-tpp-10.1177_20451253211027699 for Vitamin D in the time of the coronavirus (COVID-19) pandemic – a clinical review from a public health and public mental health perspective by Ursula Werneke, Fiona Gaughran and David M. Taylor in Therapeutic Advances in Psychopharmacology
Acknowledgments
We would like to thank Felix Filson for his assistance with literature management.
Footnotes
Conflict of interest statement: UW received funding for educational activities on behalf of Norrbotten Region (Masterclass Psychiatry Programme 2014-2018 and EAPM 2016, Luleå, Sweden): Astra Zeneca, Eli Lilly, Janssen, Novartis, Otsuka/Lundbeck, Servier, Shire, and Sunovion. UW has received a lecture honorarium from Lundbeck and is scheduled to deliver further educational activities.
DT has received research funding and lecture honoraria from Janssen, Mylan, Recordati, Lundbeck, and Sunovion.
FG has received support or honoraria from, Lundbeck, Otsuka and Sunovion, and has a family member with past professional links to Lilly and GSK. FG is in part supported by the National Institute for Health Research’s (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Maudsley Charity and the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.UW is Associate Editor and DT is Editor-in-Chief of Therapeutic Advances in Psychopharmacology. Therefore, the peer review process was managed by alternative members of the Editorial Board and the submitting editors were not involved in the decision-making process.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Psychiatry, Sunderby Hospital, Luleå, Region Norrbotten, Sweden.
ORCID iDs: Ursula Werneke  https://orcid.org/0000-0002-5023-3254
https://orcid.org/0000-0002-5023-3254
David M. Taylor  https://orcid.org/0000-0002-2557-1710
https://orcid.org/0000-0002-2557-1710
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ursula Werneke, Sunderby Research Unit – Psychiatry, Department of Clinical Sciences, Umeå University, Umeå, Sweden.
Fiona Gaughran, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College and National Psychosis Service, South London & the Maudsley NHS Foundation Trust, London, UK.
David M. Taylor, Maudsley Hospital, Pharmacy Department Denmark Hill, King’s College London and Institute of Pharmaceutical Science, London, UK
References
- 1. Maripuu M, Bendix M, Öhlund L, et al. Death associated with coronavirus (COVID-19) infection in individuals with SMD in Sweden during the early months of the outbreak – an exploratory cross-sectional analysis of a population-based register study. Front Psychiatry 2021; 11: 609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry 2021; 20: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkins JL, Masoli JAH, Delgado J, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci 2020; 75: 2224–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Picker LJ, Dias MC, Benros ME, et al. Severe mental illness and European COVID-19 vaccination strategies. Lancet Psychiatry 2021; 8: 356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine (Baltimore) 2019; 98: e17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pham H, Rahman A, Majidi A, et al. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis. Int J Environ Res Public Health 2019; 16: 3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner H, Holleczek B, Schöttker B. Vitamin D insufficiency and deficiency and mortality from respiratory diseases in a cohort of older adults: potential for limiting the death toll during and beyond the COVID-19 pandemic? Nutrients 2020; 12: 2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017; 356: i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Scientific Advisory Committee on Nutrition (SACN UK). Rapid review: vitamin D and acute respiratory tract infections, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/944108/SACN_June2020_VitaminD_AcuteRespiratoryTractInfections.pdf (2020, accessed 12 March 2021).
- 10. The Scientific Advisory Committee on Nutrition (SACN UK). Update of rapid review: vitamin D and acute respiratory tract infections, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/945179/SACN_December2020_VitaminD_AcuteRespiratoryTractInfections.pdf (2020, accessed 12 March 2021).
- 11. National Health Service (NHS UK). Vitamins and minerals – vitamin D, https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d/ (2020, accessed 21 March 2021).
- 12. National Institute for Health and Care Excellence (NICE). Covid-19 rapid guideline: vitamin D [NG 187], https://www.nice.org.uk/guidance/ng187 (2020, accessed 12 March 2021). [PubMed]
- 13. Whiskey E, Gaughran F. Protocol for vitamin D prophylaxis during COVID-19 pandemic. London: South London and Maudsley Foundation Trust, 2020. [Google Scholar]
- 14. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018; 362: k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM 2021; 114: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Worldometer. Coronavirus, https://www.worldometers.info/coronavirus/ (2021, accessed 24 March 2021).
- 17. Lerner PP, Sharony L, Miodownik C. Association between mental disorders, cognitive disturbances and vitamin D serum level: current state. Clin Nutr ESPEN 2018; 23: 89–102. [DOI] [PubMed] [Google Scholar]
- 18. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014; 21: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Institute of Health OoDS. Vitamin D, fact sheet for health professionals, https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (2018, accessed 20 May 2020).
- 20. Zarei A, Hulley PA, Sabokbar A, et al. 25-Hydroxy- and 1α,25-dihydroxycholecalciferol have greater potencies than 25-Hydroxy- and 1α,25-dihydroxyergocalciferol in modulating cultured human and mouse osteoblast activities. PLoS One 2016; 11: e0165462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holick MF, Chen TC, Lu Z, et al. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res 2007; 22(Suppl. 2): V28–V33. [DOI] [PubMed] [Google Scholar]
- 22. Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr Physiol 2016; 6: 561–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Expert Group on Vitamins and Minerals. Safe upper levels for vitamins and minerals. Food Standard Agency, London. https://cot.food.gov.uk/sites/default/files/vitmin2003.pdf (2003, accessed 19 May 2020).
- 24. Mousavi SE, Amini H, Heydarpour P, et al. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: evidence and potential mechanisms. Environ Int 2019; 122: 67–90. [DOI] [PubMed] [Google Scholar]
- 25. Gardner DG, Chen S, Glenn DJ. Vitamin D and the heart. Am J Physiol Regul Integr Comp Physiol 2013; 305: R969–R977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 27. Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol 2013; 5: 51–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holick MF. Vitamin D: a millenium perspective. J Cell Biochem 2003; 88: 296–307. [DOI] [PubMed] [Google Scholar]
- 29. Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982; 1: 74–76. [DOI] [PubMed] [Google Scholar]
- 30. Australian Health and National Medical Research Council (NHMRC), New Zealand Ministry of Health (MoH). Nutrient reference values. Vitamin D, https://www.nrv.gov.au/nutrients/vitamin-d (2014, accessed 20 May 2020).
- 31. European Food Safety Authority (EFSA). Dietary reference values for vitamin D, https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2016.4547 (2016, accessed 13 October 2020). [DOI] [PMC free article] [PubMed]
- 32. Finglas PM, Roe M, Pinchen HM, et al. McCance and Widdowson’s the composition of foods. 7th Summary Edition ed. Cambridge: The Royal Society of Chemistry, 2015. [Google Scholar]
- 33. Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 2016; 101: 394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int 2010; 21: 1151–1154. [DOI] [PubMed] [Google Scholar]
- 35. Scientific Advisory Committee on Nutrition (SACN). Vitamin D and health, https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (2016, accessed 13 October 2020).
- 36. Dawson-Hughes B. Vitamin D deficiency in adults: definition, clinical manifestations, and treatment. In: Mulder JE. (ed): UptoDate, https://www.uptodate.com/contents/vitamin-d-deficiency-in-adults-definition-clinical-manifestations-and-treatment?search=vitamin%20D%20deficiency&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (2020, accessed 12 March 2021).
- 37. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 2014; 144 Pt A: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lips P, Cashman KD, Lamberg-Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol 2019; 180: 23–54. [DOI] [PubMed] [Google Scholar]
- 39. van Schoor N, Lips P. Global overview of vitamin D status. Endocrinol Metab Clin North Am 2017; 46: 845–870. [DOI] [PubMed] [Google Scholar]
- 40. Benskin LL. A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front Public Health 2020; 8: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cashman KD, Dowling KG, Škrabáková Z, et al. Standardizing serum 25-hydroxyvitamin D data from four Nordic population samples using the Vitamin D Standardization Program protocols: shedding new light on vitamin D status in Nordic individuals. Scand J Clin Lab Invest 2015; 75: 549–561. [DOI] [PubMed] [Google Scholar]
- 42. Kara M, Ekiz T, Ricci V, et al. ‘Scientific Strabismus’ or two related pandemics: coronavirus disease and vitamin D deficiency. Br J Nutr 2020; 124: 736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clemens TL, Zhou XY, Myles M, et al. Serum vitamin D2 and vitamin D3 metabolite concentrations and absorption of vitamin D2 in elderly subjects. J Clin Endocrinol Metab 1986; 63: 656–660. [DOI] [PubMed] [Google Scholar]
- 44. Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 2013; 10: e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williamson E, Walker AJ, Bhaskaran KJ, et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020: 2020.2005.2006.20092999. [Google Scholar]
- 48. Govind R, Fonseca de, Freitas D, Pritchard M, et al. Clozapine treatment and risk of COVID-19 infection: retrospective cohort study. Br J Psychiatry. Epub ahead of print 27 July 2020. DOI: 10.1192/bjp.2020.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meltzer DO, Best TJ, Zhang H, et al. Association of vitamin D levels, race/ethnicity, and clinical characteristics with COVID-19 test results. JAMA Network Open 2021; 4: e214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gröber U, Kisters K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinol 2012; 4: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang B, Xie W, Krasowski MD. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics 2008; 9: 1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cutillas-Marco E, Fuertes-Prosper A, Grant WB, et al. Vitamin D deficiency in South Europe: effect of smoking and aging. Photodermatol Photoimmunol Photomed 2012; 28: 159–161. [DOI] [PubMed] [Google Scholar]
- 53. Lange NE, Sparrow D, Vokonas P, et al. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am J Respir Crit Care Med 2012; 186: 616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome. J Dermatol Sci 2017; 85: 152–161. [DOI] [PubMed] [Google Scholar]
- 55. Ho-Pham LT, Vu BQ, Lai TQ, et al. Vegetarianism, bone loss, fracture and vitamin D: a longitudinal study in Asian vegans and non-vegans. Eur J Clin Nutr 2012; 66: 75–82. [DOI] [PubMed] [Google Scholar]
- 56. Wilson LR, Tripkovic L, Hart KH, et al. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proc Nutr Soc 2017; 76: 392–399. [DOI] [PubMed] [Google Scholar]
- 57. Ocampo-Pelland AS, Gastonguay MR, Riggs MM. Model-based meta-analysis for comparing Vitamin D2 and D3 parent-metabolite pharmacokinetics. J Pharmacokinet Pharmacodyn 2017; 44: 375–388. [DOI] [PubMed] [Google Scholar]
- 58. Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr 2006; 84: 694–697. [DOI] [PubMed] [Google Scholar]
- 59. Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem 1984; 21: 81–86. [DOI] [PubMed] [Google Scholar]
- 60. Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 2012; 95: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014; 10: CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr 2016; 104: 1039–1051. [DOI] [PubMed] [Google Scholar]
- 63. Malihi Z, Wu Z, Lawes CMM, et al. Adverse events from large dose vitamin D supplementation taken for one year or longer. J Steroid Biochem Mol Biol 2019; 188: 29–37. [DOI] [PubMed] [Google Scholar]
- 64. Malihi Z, Lawes CMM, Wu Z, et al. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: results from a randomized controlled trial. Am J Clin Nutr 2019; 109: 1578–1587. [DOI] [PubMed] [Google Scholar]
- 65. Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev 2016; 37: 521–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet 2012; 379: 721–728. [DOI] [PubMed] [Google Scholar]
- 67. Meehan AD, Udumyan R, Kardell M, et al. Lithium-associated hypercalcemia: pathophysiology, prevalence, management. World J Surg 2018; 42: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Berridge MJ. Vitamin D deficiency: infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am J Physiol Cell Physiol 2018; 314: C135–C151. [DOI] [PubMed] [Google Scholar]
- 69. Kočovská E, Gaughran F, Krivoy A, et al. Vitamin-D deficiency as a potential environmental risk factor in multiple sclerosis, schizophrenia, and autism. Front Psychiatry 2017; 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Viani-Walsh D, Kennedy-Williams S, Taylor D, et al. Vitamin D deficiency in schizophrenia implications for COVID-19 infection. Ir J Psychol Med. Epub ahead of print 11 September 2020. DOI: 10.1017/ipm.2020.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Balabanova S, Richter HP, Antoniadis G, et al. 25-Hydroxyvitamin D, 24, 25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D in human cerebrospinal fluid. Klin Wochenschr 1984; 62: 1086–1090. [DOI] [PubMed] [Google Scholar]
- 72. Pardridge WM, Sakiyama R, Coty WA. Restricted transport of vitamin D and A derivatives through the rat blood-brain barrier. J Neurochem 1985; 44: 1138–1141. [DOI] [PubMed] [Google Scholar]
- 73. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 2005; 29: 21–30. [DOI] [PubMed] [Google Scholar]
- 74. Cui X, Gooch H, Petty A, et al. Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol 2017; 453: 131–143. [DOI] [PubMed] [Google Scholar]
- 75. Miller WL. Genetic disorders of Vitamin D biosynthesis and degradation. J Steroid Biochem Mol Biol 2017; 165: 101–108. [DOI] [PubMed] [Google Scholar]
- 76. Cui X, Gooch H, Groves NJ, et al. Vitamin D and the brain: key questions for future research. J Steroid Biochem Mol Biol 2015; 148: 305–309. [DOI] [PubMed] [Google Scholar]
- 77. Harms LR, Burne TH, Eyles DW, et al. Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab 2011; 25: 657–669. [DOI] [PubMed] [Google Scholar]
- 78. Takahashi T, Suzuki M. Brain morphologic changes in early stages of psychosis: implications for clinical application and early intervention. Psychiatry Clin Neurosci 2018; 72: 556–571. [DOI] [PubMed] [Google Scholar]
- 79. DeLuca GC, Kimball SM, Kolasinski J, et al. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol 2013; 39: 458–484. [DOI] [PubMed] [Google Scholar]
- 80. Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001; 21: 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gooch H, Cui X, Anggono V, et al. 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl Psychiatry 2019; 9: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kesby JP, Burne TH, McGrath JJ, et al. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: an animal model of schizophrenia. Biol Psychiatry 2006; 60: 591–596. [DOI] [PubMed] [Google Scholar]
- 83. Kesby JP, O’Loan JC, Alexander S, et al. Developmental vitamin D deficiency alters MK-801-induced behaviours in adult offspring. Psychopharmacology (Berl) 2012; 220: 455–463. [DOI] [PubMed] [Google Scholar]
- 84. Smith MP, Fletcher-Turner A, Yurek DM, et al. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res 2006; 31: 533–539. [DOI] [PubMed] [Google Scholar]
- 85. Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci 2006; 1074: 261–271. [DOI] [PubMed] [Google Scholar]
- 86. Kim SH, Seok H, Kim DS. Relationship between serum vitamin D levels and symptoms of depression in stroke patients. Ann Rehabil Med 2016; 40: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Puchacz E, Stumpf WE, Stachowiak EK, et al. Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Brain Res Mol Brain Res 1996; 36: 193–196. [DOI] [PubMed] [Google Scholar]
- 88. Cannell JJ. Vitamin D and autism, what’s new? Rev Endocr Metab Disord 2017; 18: 183–193. [DOI] [PubMed] [Google Scholar]
- 89. Huang YN, Ho YJ, Lai CC, et al. 1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures. J Neuroinflammation 2015; 12: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taniura H, Ito M, Sanada N, et al. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res 2006; 83: 1179–1189. [DOI] [PubMed] [Google Scholar]
- 91. Ibi M, Sawada H, Nakanishi M, et al. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 2001; 40: 761–771. [DOI] [PubMed] [Google Scholar]
- 92. Lambert GW, Reid C, Kaye DM, et al. Effect of sunlight and season on serotonin turnover in the brain. Lancet 2002; 360: 1840–1842. [DOI] [PubMed] [Google Scholar]
- 93. Li H, Sun D, Wang A, et al. Serum 25-hydroxyvitamin D levels and depression in older adults: a dose-response meta-analysis of prospective cohort studies. Am J Geriatr Psychiatry 2019; 27: 1192–1202. [DOI] [PubMed] [Google Scholar]
- 94. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry 2013; 202: 100–107. [DOI] [PubMed] [Google Scholar]
- 95. Ju SY, Lee YJ, Jeong SN. Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. J Nutr Health Aging 2013; 17: 447–455. [DOI] [PubMed] [Google Scholar]
- 96. Faivre S, Roche N, Lacerre F, et al. Vitamin D deficiency in a psychiatric population and correlation between vitamin D and CRP. Encephale 2019; 45: 376–383. [DOI] [PubMed] [Google Scholar]
- 97. Ikonen H, Palaniswamy S, Nordström T, et al. Vitamin D status and correlates of low vitamin D in schizophrenia, other psychoses and non-psychotic depression – the Northern Finland Birth Cohort 1966 study. Psychiatry Res 2019; 279: 186–194. [DOI] [PubMed] [Google Scholar]
- 98. van den Berg KS, Hegeman JM, van den Brink RHS, et al. A prospective study into change of vitamin D levels, depression and frailty among depressed older persons. Int J Geriatr Psychiatry 2021; 36: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhu JL, Luo WW, Cheng X, et al. Vitamin D deficiency and schizophrenia in adults: a systematic review and meta-analysis of observational studies. Psychiatry Res 2020; 288: 112959. [DOI] [PubMed] [Google Scholar]
- 100. Firth J, Carney R, Stubbs B, et al. Nutritional deficiencies and clinical correlates in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull 2018; 44: 1275–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Valipour G, Saneei P, Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J Clin Endocrinol Metab 2014; 99: 3863–3872. [DOI] [PubMed] [Google Scholar]
- 102. Belvederi Murri M, Respino M, Masotti M, et al. Vitamin D and psychosis: mini meta-analysis. Schizophr Res 2013; 150: 235–239. [DOI] [PubMed] [Google Scholar]
- 103. Adamson J, Lally J, Gaughran F, et al. Correlates of vitamin D in psychotic disorders: a comprehensive systematic review. Psychiatry Res 2017; 249: 78–85. [DOI] [PubMed] [Google Scholar]
- 104. Peitl V, Silić A, Orlović I, et al. Vitamin D and neurotrophin levels and their impact on the symptoms of schizophrenia. Neuropsychobiology 2020; 79: 179–185. [DOI] [PubMed] [Google Scholar]
- 105. Coentre R, Canelas da, Silva I. Symptomatic correlates of vitamin D deficiency in first-episode psychosis. Psychiatry J 2019; 2019: 7839287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lally J, Ajnakina O, Singh N, et al. Vitamin D and clinical symptoms in first episode psychosis (FEP): a prospective cohort study. Schizophr Res 2019; 204: 381–388. [DOI] [PubMed] [Google Scholar]
- 107. Fond G, Godin O, Schürhoff F, et al. Hypovitaminosis D is associated with depression and anxiety in schizophrenia: results from the national FACE-SZ cohort. Psychiatry Res 2018; 270: 104–110. [DOI] [PubMed] [Google Scholar]
- 108. Revez JA, Lin T, Qiao Z, et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun 2020; 11: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mulugeta A, Lumsden A, Hyppönen E. Relationship between serum 25(OH)D and depression: causal evidence from a bi-directional Mendelian randomization study. Nutrients 2020; 13: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Meng X, Li X, Timofeeva MN, et al. Phenome-wide Mendelian-randomization study of genetically determined vitamin D on multiple health outcomes using the UK Biobank study. Int J Epidemiol 2019; 48: 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Libuda L, Laabs BH, Ludwig C, et al. Vitamin D and the risk of depression: a causal relationship? Findings from a Mendelian randomization study. Nutrients 2019; 11: 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Milaneschi Y, Peyrot WJ, Nivard MG, et al. A role for vitamin D and omega-3 fatty acids in major depression? An exploration using genomics. Transl Psychiatry 2019; 9: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Michaëlsson K, Melhus H, Larsson SC. Serum 25-hydroxyvitamin D concentrations and major depression: a Mendelian randomization study. Nutrients 2018; 10: 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Taylor AE, Burgess S, Ware JJ, et al. Investigating causality in the association between 25(OH)D and schizophrenia. Sci Rep 2016; 6: 26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jamilian H, Amirani E, Milajerdi A, et al. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: a systematic review and meta-analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry 2019; 94: 109651. [DOI] [PubMed] [Google Scholar]
- 116. Vellekkatt F, Menon V. Efficacy of vitamin D supplementation in major depression: a meta-analysis of randomized controlled trials. J Postgrad Med 2019; 65: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Firth J, Teasdale SB, Allott K, et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019; 18: 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition 2015; 31: 421–429. [DOI] [PubMed] [Google Scholar]
- 119. Shaffer JA, Edmondson D, Wasson LT, et al. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosom Med 2014; 76: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014; 6: 1501–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab 2014; 99: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lázaro Tomé A, Reig Cebriá MJ, González-Teruel A, et al. Efficacy of vitamin D in the treatment of depression: a systematic review and meta-analysis. Actas Esp Psiquiatr 2021; 49: 12–23. [PubMed] [Google Scholar]
- 123. Zhu C, Zhang Y, Wang T, et al. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav 2020; 10: e01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vellekkatt F, Menon V, Rajappa M, et al. Effect of adjunctive single dose parenteral vitamin D supplementation in major depressive disorder with concurrent vitamin D deficiency: a double-blind randomized placebo-controlled trial. J Psychiatr Res 2020; 129: 250–256. [DOI] [PubMed] [Google Scholar]
- 125. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020; 12: 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rondanelli M, Miccono A, Lamburghini S, et al. Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds-practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Alternat Med 2018; 2018: 5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 2015; 7: 8251–8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Molnar C, Gair J. Concepts of biology – 1st Canadian edition. Chapter 23: the immune system. BC Campus. https://opentextbc.ca/biology/part/chapter-23-the-immune-system/ (2015, accessed 17 March 2021). [Google Scholar]
- 129. Carrillo Muñoz JL, Castro García FP, Gutiérrez Coronado, et al. Physiology and pathology of innate immune response against pathogens. In: Rezaei N. (ed.) Physiology and pathology of immunity. Intech Open. https://www.intechopen.com/books/physiology-and-pathology-of-immunology/physiology-and-pathology-of-innate-immune-response-against-pathogens (2017, accessed 17 March 2021). [Google Scholar]
- 130. Chun RF, Liu PT, Modlin RL, et al. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol 2014; 5: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Koenderman L, Buurman W, Daha MR. The innate immune response. Immunol Lett 2014; 162: 95–102. [DOI] [PubMed] [Google Scholar]
- 132. Daha MR. Grand challenges in molecular innate immunity. Front Immunol 2011; 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res 2011; 55: 96–108. [DOI] [PubMed] [Google Scholar]
- 134. Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol 2009; 183: 5458–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 2005; 17: 359–365. [DOI] [PubMed] [Google Scholar]
- 136. Sharifi A, Vahedi H, Nedjat S, et al. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS 2019; 127: 681–687. [DOI] [PubMed] [Google Scholar]
- 137. Jeffery LE, Henley P, Marium N, et al. Decreased sensitivity to 1,25-dihydroxyvitamin D3 in T cells from the rheumatoid joint. J Autoimmun 2018; 88: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jeffery LE, Qureshi OS, Gardner D, et al. Vitamin D antagonises the suppressive effect of inflammatory cytokines on CTLA-4 expression and regulatory function. PLoS One 2015; 10: e0131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li W, Liu Z, Tang R, et al. Vitamin D inhibits palmitate-induced macrophage pro-inflammatory cytokine production by targeting the MAPK pathway. Immunol Lett 2018; 202: 23–30. [DOI] [PubMed] [Google Scholar]
- 140. Khoo AL, Chai LY, Koenen HJ, et al. Vitamin D(3) down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine 2011; 55: 294–300. [DOI] [PubMed] [Google Scholar]
- 141. Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One 2020; 15: e0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. McNally P, Coughlan C, Bergsson G, et al. Vitamin D receptor agonists inhibit pro-inflammatory cytokine production from the respiratory epithelium in cystic fibrosis. J Cyst Fibros 2011; 10: 428–434. [DOI] [PubMed] [Google Scholar]
- 143. Giraldo DM, Cardona A, Urcuqui-Inchima S. High-dose of vitamin D supplement is associated with reduced susceptibility of monocyte-derived macrophages to dengue virus infection and pro-inflammatory cytokine production: an exploratory study. Clin Chim Acta 2018; 478: 140–151. [DOI] [PubMed] [Google Scholar]
- 144. Yang D, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev 2017; 280: 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Silberstein M. Correlation between premorbid IL-6 levels and COVID-19 mortality: potential role for vitamin D. Int Immunopharmacol 2020; 88: 106995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Liu Z, Li J, Chen D, et al. Dynamic interleukin-6 level changes as a prognostic indicator in patients with COVID-19. Front Pharmacol 2020; 11: 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jain A, Chaurasia R, Sengar NS, et al. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep 2020; 10: 20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lei GS, Zhang C, Cheng BH, et al. Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrob Agents Chemother 2017; 61: e01226–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 2012; 2012: 256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kuba K, Imai Y, Rao S, et al. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med (Berl) 2006; 84: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020; 181: 281–292.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Martín Giménez VM, Inserra F, Tajer CD, et al. Lungs as target of COVID-19 infection: protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci 2020; 254: 117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010; 55: 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kazemi A, Mohammadi V, Aghababaee SK, et al. Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis. Adv Nutr. Epub ahead of print 5 March 2021. DOI: 10.1093/advances/nmab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Liu N, Sun J, Wang X, et al. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis 2021; 104: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Teshome A, Adane A, Girma B, et al. The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis. Front Public Health 2021; 9: 624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Munshi R, Hussein MH, Toraih EA, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol 2021; 93: 733–740. [DOI] [PubMed] [Google Scholar]
- 160. Pereira M, Dantas Damascena A, Galvão Azevedo LM, et al. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. Epub ahead of print 4 November 2020. DOI: 10.1080/10408398.2020. [DOI] [PubMed] [Google Scholar]
- 161. Rhodes JM, Subramanian S, Laird E, et al. Perspective: vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med 2020; 289: 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 2020; 32: 1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Laird E, Rhodes J, Kenny RA. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J 2020; 113: 81. [PubMed] [Google Scholar]
- 164. Marik PE, Kory P, Varon J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med Drug Discov 2020; 6: 100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Panarese A, Shahini E. Letter: covid-19, and vitamin D. Aliment Pharmacol Ther 2020; 51: 993–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Singh S, Kaur R, Singh RK. Revisiting the role of vitamin D levels in the prevention of COVID-19 infection and mortality in European countries post infections peak. Aging Clin Exp Res 2020; 32: 1609–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Tang L, Liu M, Ren B, et al. Sunlight ultraviolet radiation dose is negatively correlated with the percent positive of SARS-CoV-2 and four other common human coronaviruses in the U.S. Sci Total Environ 2020; 751: 141816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Whittemore PB. COVID-19 fatalities, latitude, sunlight, and vitamin D. Am J Infect Control 2020; 48: 1042–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sajadi MM, Habibzadeh P, Vintzileos A, et al. Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020; 3: e2011834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kohlmeier M. Avoidance of vitamin D deficiency to slow the COVID-19 pandemic. BMJ Nutr Prev Health 2020; 3: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Meltzer DO, Best TJ, Zhang H, et al. Association of vitamin D deficiency and treatment with COVID-19 incidence. medRxiv 2020: 2020.05.08.20095893. [Google Scholar]
- 172. Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. “Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study”. J Steroid Biochem Mol Biol 2020; 203: 105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA 2021; 325: 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ma H, Zhou T, Heianza Y, et al. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank. Am J Clin Nutr 2021; 113: 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rastogi A, Bhansali A, Khare N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J. Epub ahead of print 12 November 2020. DOI: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 176. Annweiler C, Hanotte B, Grandin de l’Eprevier C, et al. Vitamin D and survival in COVID-19 patients: a quasi-experimental study. J Steroid Biochem Mol Biol 2020; 204: 105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Annweiler G, Corvaisier M, Gautier J, et al. Vitamin D supplementation sssociated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID Quasi-Experimental Study. Nutrients 2020; 12: 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hernández JL, Nan D, Fernandez-Ayala M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab 2021; 106: e1343–e1353. [DOI] [PubMed] [Google Scholar]
- 179. Mariani J, Tajer C, Antonietti L, et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: a structured summary of a study protocol for a randomised controlled trial (CARED-TRIAL). Trials 2021; 22: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wang R, DeGruttola V, Lei Q, et al. The vitamin D for COVID-19 (VIVID) trial: a pragmatic cluster-randomized design. Contemp Clin Trials 2021; 100: 106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Annweiler C, Beaudenon M, Gautier J, et al. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials 2020; 21: 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tpp-10.1177_20451253211027699 for Vitamin D in the time of the coronavirus (COVID-19) pandemic – a clinical review from a public health and public mental health perspective by Ursula Werneke, Fiona Gaughran and David M. Taylor in Therapeutic Advances in Psychopharmacology