Abstract
Developing effective therapies for the treatment of advanced head-and-neck squamous cell carcinoma (HNSCC) remains a major challenge, and there is a limited landscape of effective targeted therapies on the horizon. NAD(P)H:quinone oxidoreductase 1 (NQO1) is a 2-electron reductase that is overexpressed in HNSCC and presents as a promising target for the treatment of HNSCC. Current NQO1-targeted drugs are hindered by their poor oxidative tolerability in human patients, underscoring a need for better preclinical screening for oxidative toxicities for NQO1-bioactivated small molecules. Herein, we describe our work to include felines and feline oral squamous cell carcinoma (FOSCC) patients in the preclinical assessment process to prioritize lead compounds with increased tolerability and efficacy prior to full human translation. Specifically, our data demonstrate that IB-DNQ, an NQO1-targeted small molecule, is well-tolerated in FOSCC patients and shows promising initial efficacy against FOSCC tumors in proof-of-concept single agent and radiotherapy combination cohorts. Furthermore, FOSCC tumors are amenable to evaluating a variety of target-inducible couplet hypotheses, evidenced herein with modulation of NQO1 levels with palliative radiotherapy. The use of felines and their naturally-occurring tumors provide an intriguing, often underutilized tool for preclinical drug development for NQO1-targeted approaches and has broader applications for the evaluation of other anticancer strategies.
Keywords: Comparative oncology, NQO1, Targeted therapy, Feline, Preclinical
Introduction
While early detection of head-and-neck squamous cell carcinoma (HNSCC) is often associated with excellent clinical outcomes achieved through local or locoregional treatment strategies [1], patients with advanced (Stage III or IV) and/or human papillomavirus negative-HNSCC suffer from high morbidity rates, afflicting over 350,000 patients per year worldwide [2]. Furthermore, the prognosis for patients experiencing recurrent and/or metastatic (R/M) disease remains grim, with median survival times of less than 1 year [3]. Despite the rapid development of targeted therapies directed at a myriad of molecular targets for many cancer types, an effective targeted approach for patients with unresectable and/or R/M HNSCC remains acutely limited. While HNSCC highly express epidermal growth factor receptor (EGFR) and exploit this mitogenic signaling circuit for growth and survival [4], targeting the EGFR pathway for improving progression-free and overall survival times has been largely disappointing. To date, only cetuximab has demonstrated an overall survival benefit in HNSCC patients with locally-advanced or R/M disease receiving standard-of-care (SOC) radiation or cytotoxic chemotherapies [3,5]. Even with the provocative activity of immunotherapies for a minority (<15%) of patients in the relapse setting [6], there remains a major need for additional targeted, well-tolerated approaches for the treatment of advanced HNSCC that has efficacy either as a single agent and/or in combination with SOC therapies [7,8].
From a precision medicine perspective, an emerging leverageable target is the 2-electron reductase, NAD(P)H:quinone oxidoreductase 1 (NQO1) that is overexpressed in a variety of malignancies [9], with robust overexpression in HNSCC [10,11]. Elevated NQO1 expression is correlated with advanced disease and lower overall survival in HNSCC and other cancers [9]. With NQO1’s role as a hypothesized driver of oncogenesis, inhibiting the function of NQO1 as a targeted strategy has been explored with unfortunately minimal anticancer efficacy [9]. However, an alternative mechanism of utilizing NQO1’s 2-electron reduction of quinones adopts an orthogonal approach, rather than inhibiting its function, to leverage NQO1 enzymatic properties for the bioactivation of small molecules for cancer-targeted cell death [9].
NQO1 enzymatic conversion of quinones to unstable hydroquinones leads to a cascade of futile redox-cycling, rapidly forming reactive oxygen species (ROS) in a NQO1-dependent manner, and ultimately cancer cell-specific death; a mechanism that has been recently reviewed [12]. The compound β-lapachone, which undergoes this ROS-generating NQO1-bioactivation mechanism, has shown efficacy against preclinical models of HNSCC [10] and has been assessed in human HNSCC clinical trials [13,14]. However, a significant clinical limitation to β-lapachone (and its prodrug derivative, ARQ-761) is the development of methemoglobinemia in human patients resulting in clinically significant anemia [[13], [14], [15]]. Lessons learned from these pivotal early phase I/II human clinical trials underscore the importance of stringent preclinical screening for redox-mediated toxicities being paramount when developing NQO1-bioactivatiable drugs. However, current strategies for preclinical compound triaging may not be sufficient to predict the most promising leads.
Deoxynyboquinone (DNQ) and its derivatives are potent NQO1-substrates that undergo NQO1-mediated redox cycling and rapid cancer cell death [9,16,17]. DNQ has superior properties, anticancer activity, and NQO1 selectivity when compared to β-lapachone [9,18]. Given their shared mechanism of action, the potential for compound-mediated anemia and oxidative stress is a crucial consideration. There is a need for rigorous evaluation of DNQ (and its derivatives) to evaluate its safety in higher organisms prior to human translation.
Development of novel treatments for human cancer relies heavily on mouse models to assess the safety and efficacy of candidate therapeutics. However, there is growing frustration with the staggering failure rates of clinical trial anticancer compounds that have previously shown safety and efficacy in rodent models, especially in HNSCC [[19], [20], [21]]. These discrepancies are likely a result of the inadequacies of the contrived nature of rodent models; namely, their homogenous tumor composition, lack of robust metastatic phenotype, and various other factors [19,20,22,23]. While human patient-derived xenograft models are an emerging method for assessing preclinical leads, these models still rely on subcutaneous rodent engraftment in an immune incompetent setting and do not represent the features of spontaneously-derived oncogenesis, nor do they recapitulate tumor establishment and progression [20,22]. Further safety evaluations are often conducted with healthy, non-tumor bearing animals which may not recapitulate safety concerns that arise in tumor-bearing, often terminally ill, Phase 1 patient populations. Comparative oncology's major goal is to identify companion animal cancers that mimic human disease [24,25]. Incorporation of tumor-bearing companion animals into preclinical anticancer drug development is a powerful strategy, but often underutilized in drug development [[24], [25], [26], [27], [28]]. Interestingly, while the utility of canine cancers are often discussed, the inclusion of feline cancers is rarely employed, despite significant advances characterizing the genomic and molecular underpinnings for some feline neoplasms [29,30].
Feline oral squamous cell carcinoma (FOSCC) is a naturally-occurring head-and-neck cancer with heterogeneous tumor populations found in domestic cats that mimics advanced, human papillomavirus-negative HNSCC in humans [19,22,23,31]. Domestic cats develop highly aggressive, non-resectable FOSCC, and multimodal therapy provides only minimal benefit with a median survival time of 3 mo and a 1-year survival rate of <10% [[32], [33], [34], [35], [36]]. As the most common oral malignancy observed in cats, FOSCC has a robust patient population, representing >60% of all feline oral neoplasia [37]. FOSCC recapitulates a variety of clinical aspects seen in late stage (III/IV) human HNSCC, including similar metastatic rates [19,38], bone invasion, comparable immune cell environments [21], frequency of spontaneous recurrence, and develops in the same environment as comparable human cancers [19,21,[38], [39], [40]]. Comparative genomic analyses have shown that many of the same genes are responsible for both human and feline HNSCC [19], specifically mutation of the tumor suppressor protein p53 (TP53), dysregulation of retinoblastoma signaling, and overexpression of EGFR [19,22,27,31,39,[41], [42], [43]]. Importantly for studies herein, FOSCC expresses high levels of NQO1 in a similar manner to its human counterpart [44].
Felines are rarely utilized in traditional oncology drug development. Domestic cats are uniquely sensitive and susceptible to oxidative injury, more so than humans [[45], [46], [47], [48], [49]] and canines [[47], [48], [49]], due to their distinctive hemoglobin structure [[45], [46], [47], [48], [49], [50]]. This increased vulnerability to oxidative damage theoretically allows for more sensitive screening of redox-toxicities, such as those that have been shown for NQO1-targeted agents in human patients. Specifically, for FOSCC, tolerability of NQO1-targeted therapy can be elucidated in tumor bearing cats along with proof-of-concept efficacy data, exemplifying the full value of this companion animal model for HNSCC.
Herein we utilize felines in combination with standard murine systems to prioritize and select an NQO1-targeted compound, IB-DNQ, to evaluate against advanced FOSCC. As a single-agent, IB-DNQ treatment exerts measurable cytoreductive activity and stabilizes disease progression in a small cohort of FOSCC patients. To enhance NQO1-targeted approaches, we explore utilizing radiotherapy to induce leverageable NQO1 expression in FOSCC tumors. Our data supports IB-DNQ + RT as a well-tolerated approach and provides initial evidence as a potential synergistic couplet, targeted therapeutic strategy for the treatment of refractory human HNSCC.
Materials and methods
Materials
DNQ, IB-DNQ, and respective derivatives were synthesized as described [16,44]. Details of the optimized larger scale synthesis of IB-DNQ utilized is provided in a supplemental file. Antibodies used herein: anti-NQO1 (mouse monoclonal, CST 3187) was purchased from Cell Signaling Technology (Danvers, MA) and anti-mouse secondary (ab6814) was purchased from Abcam (Abcam, Cambridge, MA). Dicoumarol was purchased from Acros Organics (Fairlawn, NJ), cytochrome c, menadione, and NADH were purchased from Sigma-Aldrich (St. Louis, MO).
Cell lines
One feline OSCC cell line was used in this study (SCCF3, provided by Thomas J. Rosol, The Ohio State University) and 3 human HNSCC cell lines (UMSCC14A, UMSCC25 and HN31, provided by David A. Boothman, Indiana University). Cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (100 IU/mL each). Cells were maintained at 37 °C in 5% CO2.
Immunohistochemistry (IHC)
All IHC samples were prepared through the University of Illinois Veterinary Diagnostic Laboratory. Formalin-preserved samples were fixed for 24 to 48 h, then paraffin-embedded, before cutting and mounting onto charged slides. Slides were pre-treated with Diva Decloaker (DV2004), endogenous peroxidase activity was blocked with Biocare Peroxidazed 1 (PX968) at room temperature (RT) for 5 min, rinsed with TBS wash buffer, and then incubated for 10 min at RT with Biocare Background Punisher (BP974). Slides were incubated with NQO1 primary antibody for 60 min, washed, and then incubated with the secondary antibody for 40 min. Slides were washed with TBS, then the reaction was developed using DAB substrate (IPK5010) for 5 min. Slides were counterstained with Cat hematoxylin. Cross-reactivity of anti-NQO1 antibody against feline tissue was validated by western blot and immunohistochemistry (IHC) as previously described; positive control: A549, negative control: HEK293 [44].
NQO1 protein scoring by IHC
Tumor tissue from 27 pet feline patients were assessed by IHC for NQO1 protein expression. Two board-certified veterinary pathologists blinded to treatment outcomes scored the cytoplasmic protein staining intensity from 0-3 according to a standardized scoring system [51]. The standardized scoring system classifies an intensity of 0 = no staining, 1 = weak staining in <50% of cells, 2 = strong staining in <50% of cells, or weak staining in >50% of cells, and 3 = strong staining in >50% of cells.
Measurement of methemoglobinemia in cat blood
Whole blood was obtained from healthy cats. 500 μL of blood was placed into microcentrifuge tubes, and compounds of interested were added to make a final concentration of 10 μM. Blood was incubated at 37 °C for 10 min. Methemoglobin was measured following a previously published method [52]. Briefly, 80 μL of blood was added to 1.2 mL of distilled water, inverted to hemolyze the red blood cells (RBCs), and left at room temperature for 3 min. Next, 0.24 mL of 0.5 M phosphate buffer pH 6.5 was added and the hemolysate was cooled on ice. The lysate was centrifuged at 5000 rpm for 5 min at 4 °C and designated solution S (sample). A second dilution, R (reference), was created by adding 0.2 mL of solution S to 1.0 mL of 0.1 M phosphate buffer, pH 6.5. One milligram K3Fe(CN)6 was added by adding 5 μL of 20% w/v solution in 0.1 M phosphate buffer, mixed, and left at room temperature for 5 min. The absorbance of solution S was read at 630 nm (=S1). Following this, 0.5 mg of KCN was added by adding 5 μL of a 10% w/v solution, inverted, and left for 3 min. Absorbance of this solution was read at 630 nm (=S2) on a SpectraMax ID3 Microplate Reader (Molecular Devices, San Jose, CA). The absorbance of solution R was read at 630 nm (=R1). 5 μL of 10% w/v KCN was added, mixed, and left for 3 min and then read at 630 nm (=R2); with calculated percent methemoglobin = 100(S1-S2)/6(R1-R2).
Measurement of hemolysis in cat blood
Whole blood was obtained from healthy cats. Four mL of whole blood in EDTA was combined with 20 mL of 0.9% saline and centrifuged for 5 min at 1500 rpm 4 °C. The supernatant was aspirated, and cells were washed 3 times with 5 mL of 0.9% saline. The pellet was resuspended in RBC buffer (10 mM Na2HPO4[pH 7.4], 150 mM NaCl, 1 mM MgCl2). 200 μL blood was placed in a microcentrifuge tube and compounds of interest were added to make a final concentration of 17.3 μM. 38 μL of RBC buffer were added to wells of a PCR plate, and 22 μL of treated blood was added to wells making a final drug concentration of 10 μM. Triton X was used as a positive control, DMSO and HPβCD as vehicle controls, and an untreated sample for negative control. The plate was sealed and incubated at 37 °C for 2 h. Cells were pelleted by centrifuging at 1500 rpm for 5 min. Supernatant was transferred to a clear bottomed 96 well plate and absorbance read at 540 nm on a SpectraMax ID3 Microplate Reader (Molecular Devices, San Jose, CA). Percent hemolysis calculation: 100 x ([ODsample-ODnegative control)/(ODpositive control-ODnegative control]).
IACUC guidelines and protocol numbers
All patient enrollment and treatment in the study, and mouse model work, was conducted in accordance with UIUC IACUC guidelines and approved protocols. The following approved IACUC protocols were utilized for work described herein: 11178, 18009, and 18175.
Maximum tolerated dose studies in mice for DNQ derivatives
First, compounds were prepared in basic HPβCD. Briefly, 60 mM HPβCD is made basic by the addition of 1 to 2 drops of 10 M NaOH (final pH 11−12). Solid DNQ (or derivatives) was weighed into a vial and HPβCD is added to the appropriate concentration. The slurry was vortexed and sonicated until all solid dissolves. The solution was then brought back to pH 8.0 to 8.5 with 1 M HCl (note compounds will precipitate out if pH ≤ 7.5). Maximum tolerated dose was assessed in 6 to 8-wk-old female C57BL/6 mice (Charles River Laboratories). A dose of compound was administered to 3 mice via intraperitoneal injection (i.p.). The mice were monitored for toxicity for 1, 4, 8, 12, and 24 h. Criteria used to assess toxicity included weight loss (>20%), unhealthy appearance, loose stools and/or labored/irregular respiration. If toxicity was observed during the first 24 h, mice were removed from the study and humanely euthanized, defining that dose as the Maximum tolerated dose for that compound of interest. If no clinical signs were observed, the next dose was administered, and observations were made in a similar manner to described above.
Measurement of methemoglobinemia in mice
CD-1 mice (The Jackson Laboratory) were randomized into treatment and control groups (n = 6 for treatment groups, n = 3 for control). All DNQ compounds (DNQ, IB-DNQ, IP-DNQ, P-DNQ, NP-DNQ) were solubilized to 1.0 mg/mL in HPBCD and sterile water. Mice were treated at 10.0 mg/kg IV by tail vein injection for each compound, and then sacrificed at 30- and 60-min post-injection with whole blood being collected and immediately placed into heparinized tubes for assessment. Methemoglobin was measured following a previously published method [52] and using exact protocol above described in Measurement of Methemoglobinemia in Cat Blood.
Measurement of hemolysis in mice
This experiment used the same blood from mice that was obtained in the methemoglobinemia study (described above). Percent hemolysis following exposure to compounds was determined using the erythrocyte osmotic fragility test [53]. Briefly, for each sample measured, 16 test tubes were prepared with 10 mL each of increasing concentrations of NaCl in solution (ranging from 0.0%−0.85%). 20 μL of blood was added to each tube, inverted, and left to stand at room temperature for 30 min. Samples were centrifuged at 2,000 rpm for 10 min. Absorbance for each sample was read at 540 nm on a SpectraMax ID3 Microplate Reader (Molecular Devices, San Jose, CA). Percent hemolysis = (OD sample/OD 0% NaCl solution) x 100. Maximal hemolysis achievable being 0% NaCl = 100% hemolysis.
In-vitro upregulation of NQO1 protein
Each cell line was grown to 80% to 100% confluency and then exposed to 8 Gy radiation. Following radiation, cell lines were incubated at 37 °C for 4 h. Cells were then collected and centrifuged at 1500 rpm for 5 min. Cell pellets were resuspended in 1 mL of PBS, centrifuged at 1500 rpm for 5 min and then resuspended with 75 to 100 uL of Mammalian Protein Extraction Reagent mixed with protease inhibitor cocktail solution. Cell suspensions were sonicated on ice 3 times for 10 s pulses and then centrifuged at 10,000 rpm for 10 min at 4 °C. Protein concentration of the supernatant was assessed using the Pierce BCA Assay (Thermo Fisher Scientific, Waltham, MA).
In-vivo upregulation of NQO1 in pet cats
Seven cats with FOSCC were enrolled and treated with radiation therapy to assess increase in NQO1 protein expression by tumor cells. Each cat was placed under general anesthesia and a representative tissue biopsy was taken of the tumor prior to irradiation, then each cat received 8 Gy radiation to the tumor and immediately surrounding normal tissue. Following completion of radiation, patients were recovered from anesthesia, then each cat was re-sedated at 2- and/or 4-h post irradiation and serial post-irradiation biopsies were collected at these time points. Biopsies were then placed in 10% formalin for 24 to 48 h and assessed for changes in NQO1 expressions by IHC (described below).
Quantification of NQO1 protein up-regulation
Cell lines: For each cell line, pre- and 4-h post-RT samples were collected as described above for IHC and captured digitally. Each sample was converted to grayscale in Adobe Photoshop, then opened in Image J Software where a color-based quantification look up table was imported and the photos then pseudocolored. Number of pixels for each color in the pseudocolored photos was quantified in Photoshop, and fold change was calculated comparing post-RT pseudocolored measurement to baseline pre-RT pseudocolored measurement.
Pet cats: For each patient enrolled, pre-, 2-h and/or 4-h post-RT samples were collected as described above and captured digitally. For each tumor biopsy, 3 separate and representative areas of tumor were analyzed using the same size pixel area (area of highest NQO1 intensity, moderate NQO1 intensity, and lowest NQO1 intensity). Samples were analyzed using Image J Software and a fluorescence-based quantification look up table. The 3 areas analyzed were averaged, and fold change was calculated comparing post-RT fluorescence measurement to baseline pre-RT fluorescence measurement.
Quantification of NQO1 enzymatic activity
For each cell line, a pre- and 4-h post-RT sample was collected, protein extracted, and concentration determined as described above. NQO1 activity assay was then performed using samples containing 20 to 40 μg of protein. In a 96-well clear bottom plate, 10 μl of protein supernatant was loaded into each well along with medium containing 77 μM cytochrome c, 200 μM NADH as the electron donor, and 10 μM menadione as the intermediate electron acceptor in Tris-HCL buffer (50 mM, pH 7.5). The rate of change of absorbance (A550 nm) was read on a SpectraMax ID3 plate reader (Molecular Devices, San Jose, CA) and the coefficient for cytochrome c (21.1 mM−1cm−1) was used to determine changes in concentration. These were repeated with 25 μM dicoumarol, and NQO1 activity was calculate as the dicoumarol inhibited oxidoreductase activity. At least 3 replicates were performed.
Single agent IB-DNQ in pet cats
Five cats were accrued into this pilot study. Each cat received a CT scan of the head prior to treatment for tumor size determination. Enrolled pet cats were then treated with 1.0 mg/kg IV IB-DNQ over 5 min [44] with varying dose-intensity treatment schedules of either daily, weekly, or bi-weekly. Following completion of the treatment plan, each cat received a follow-up CT scan to assess tumor response to treatment. Pre and post-treatment CT images were analyzed and tumor measurements obtained according to the RECIST guidelines [54] using sum of longest diameters where complete remission (CR) is no visible tumor remaining, partial remission (PR) is ≥30% decrease in tumor size, progressive disease (PD) is ≥20% increase in tumor size, and stable disease (SD) < 30% decrease in tumor size and <20% increase in tumor size. Overall biologic response = CR + PR + SD.
NQO1*2 Taqman SNP genotyping assay
Genomic DNA was extracted from cell lines or blood samples using a DNeasy Blood & Tissue Kit (Qiagen) and was diluted to 3 ng/µL in ultrapure water. A stock solution of Taqman Genotyping Master Mix (Thermo Fisher Scientific, 2.5 µL per reaction) and assay working stock (0.25 µL per reaction) was created, and 2.75 µL was dispensed for reach reaction into a 384-well plate. Diluted genomic DNA (2.25 µL per reaction) was added to each well, for a total reaction volume of 5 µL and the plate was sealed using an optical film. Three technical replicates were performed per sample. Positive control for NQO1*2 was MDA-MB-231 cell line. Negative control for NQO1*2 null was A549 cell line. Genotyping was performed using a QuantStudio 7 Flex Real-Time PCR system (polymerase activation: 95 °C, 10 min; denaturation: 95 °C, 15 s; annealing: 60 °C, 1 min; 40 cycles of denaturation and annealing). Data was analyzed using the Thermo Fisher Cloud Genotyping application. Primers used: Cat (VIC/FAM reporters): Forward primer sequence GCATTTCTGTGGCTTCCAAGTC; Reverse primer sequence: TGTGCCCGACGCTGTATG; Reporter 1 sequence TCAGCTGAGGTTCCAG; Reporter 2 sequence CAGCTGAGATTCCAG; Human (VIC/FAM reporters): Forward primer sequence GCATTTCTGTGGCTTCCAAGTC; Reverse primer sequence CTGGAGTGTGCCCAATGCTAT; Reporter 1 sequence ATGTCAGTTGAGGTTCTAA; Reporter 2 sequence ATGTCAGTTGAGATTCTAA; Dog (VIC/FAM reporters): Forward primer sequence CATTTCTGGTGGCTTCCAAGTC; Reverse primer sequence CATAGGAGTGTGCCCAATGCT; Reporter 1 sequence TAGGTCAGTTGAGGTTCCAG; Reporter 2 sequence TAGGTCAGTTGAGATTCCAG.
Combined radiation therapy and IB-DNQ in pet cats
Nineteen cats were accrued to assess effectiveness and safety of combined radiation therapy with IB-DNQ. Each cat received a CT scan of the head prior to treatment for radiation planning and to determine the tumor size. Each cat was put under general anesthesia and then treated with 4 weekly doses of 8 Gy radiation. The radiation treatment plan was created by a veterinary board-certified radiation oncologist. Following irradiation, cats were recovered from anesthesia, and 1.0 mg/kg of IB-DNQ was administered intravenously over 5 min [44] 2 to 4 h post completion of radiation (determined based on single agent radiation results described previously). At each treatment, a complete blood count, chemistry profile and urinalysis were performed to monitor for hematologic and non-hematologic toxicity. Following completion of the treatment protocol, a repeat CT scan was performed of the head to determine radiologic response to treatment. Biopsies were performed prior to treatment and baseline NQO1 protein expression semi-quantified by IHC scoring (described previously). Pre- and post-treatment CT images were analyzed and tumor measurements obtained according to the RECIST guidelines [54] using sum of longest diameters where CR is no visible tumor remaining, PR is ≥30% decrease in tumor size, PD is ≥20% increase in tumor size, and SD < 30% decrease in tumor size and < 20% increase in tumor size. Overall biologic response = CR + PR + SD.
Urine 8-OHdG measurement
For each pet cat treated with radiation and IB-DNQ, urine was collected via cystocentesis at each visit, centrifuged at 5000 rpm for 10 min, and the supernatant was frozen at -80 °C until analysis. Urine 8-OHdG was measured in each sample using an ELISA kit (Abcam 201734). The assay was run according to manufacturer recommendations, and samples were performed in duplicate or triplicate. Absorbance was measured at 450 nm on a SpectraMax ID3 Microplate Reader (Molecular Devices, San Jose, CA). Quantification of urinary 8-OHdG were normalized with urinary creatinine concentrations.
Statistical analyses
For each hematologic and biochemical parameter assessed in patients treated with IB-DNQ, and for urine 8-OHdG measurements, change from baseline was evaluated with an analysis of variance with post-hoc comparison made with Dunnett's multiple comparisons test. For NQO1 enzymatic activity assay, change from baseline was evaluated with an unpaired t test. Statistical analysis was performed with commercially available software (Prism 9, GraphPad Software Inc). Significance was defined as P < 0.05.
RESULTS
Tolerability of IB-DNQ and other derivatives in healthy felines
Multiple derivatives of DNQ, namely IB-DNQ, IP-DNQ, P-DNQ, and NP-DNQ, have similar activity as NQO1 substrates in vitro and similar activity against cancer cells in culture [16] (Supplemental Table 1). Murine toxicity studies revealed improvements as compared to DNQ, but minimal differences between the derivatives (Supplemental Table 1). Healthy research felines were treated with each derivative and their respective pharmacokinetic and tolerability parameters compared (Supplemental Table 1). The summation of these parameters in combination with our previously reported tolerability of IB-DNQ in healthy felines and efficacy in FOSCC cell culture [44], lead us to continue our efforts with IB-DNQ as the lead compound.
Treatment of IB-DNQ is tolerated and leads to biologic response in FOSCC patients
IB-DNQ achieves biologically-relevant anticancer concentrations in healthy research felines with minimal toxicity observed at 0.5-2.0 mg/kg I.V. [44]. For further assessment within an intended target population, the single-agent tolerability and preliminary anticancer activities of IB-DNQ against FOSCC tumors was assessed in 5 FOSCC patients utilizing wide-ranging dose intensity protocols for rapid safety and therapeutic evaluations (Fig. 1A). Prior to treatment, biopsied tumors were stained for NQO1 protein utilizing IHC and assigned a score of 0-3 for NQO1 protein using a standardized scoring system [51] in order to assess the correlation of NQO1 protein and response to therapy. Feline patient information is shown in Fig. 1A. To assess tumor response, pre- and post-treatment computed tomography (CT) scans were performed, as well as blood work to monitor for hematologic and non-hematologic toxicities.
Fig. 1.
Activity of IB-DNQ single agent against FOSCC. (A) Summary of feline patients in the initial IB-DNQ single agent trial and a description of the IB-DNQ dosing protocol. q.o.wk, once every other week; q.d., every day; q.wk., once a week; DSH, domestic short hair; DLH, domestic long hair. (B) Waterfall plot of patient response (RECIST) and NQO1 staining. Dashed lines denote marker for progressive disease (+20%) vs. partial response (−30%). All responses are considered SD (stable disease). (C) Representative IHC and CT images of 2 clinical cases (Patient 1, top, and Patient 3, bottom). IHC images are at 40x magnification. (D−K) Panel of key hematologic and non-hematologic markers tracked for safety and toxicity monitoring. Dotted lines represent clinical ranges associated for each marker. Not all patients had samples for every time point displayed, so n < 5 at some time points.
During this initial study, 5/5 patients were considered to have a biologic response defined as achievement of either CR, PR, or SD (Fig. 1B). The best responder with a 21% reduction in tumor size had a high NQO1 expressing tumor (Fig. 1C top), while the worst responder (17% increase in tumor size), presented with a low basal expression of NQO1 (Fig. 1C bottom). Correlation of NQO1 score and tumor reduction could not be determined with such a small cohort. Importantly, no patients developed any significant hematologic or non-hematologic (Fig. 1D−K) toxicities outside ranges consistent with tumor-bearing FOSCC patients. Additionally, the natural history of FOSCC is uniformly and rapidly progressive, so the documented reduction in tumor size in Patient 1 (21% decrease) with only single-agent IB-DNQ therapy was deemed as a clinically significant and favorable response. Of note, patients 3 and 5, who were dosed on a daily schedule of IB-DNQ, did not exhibit any tolerability differences as compared to weekly dosing patients, albeit an underpowered comparison. This small pilot study with a variable dosing schedule demonstrated IB-DNQ single-agent safety and modest effect against advanced feline tumors with differing basal levels of NQO1.
Radiotherapy induces NQO1 expression in FOSCC tumors
As an Nrf2-target gene [55,56], NQO1 can be upregulated via several mechanisms including RT [[57], [58], [59]], heat [60], and various pharmacologic agents [61,62]. Leveraging this induced NQO1 expression can increase efficacy of NQO1-targeted agents [10,[57], [58], [59], [60],[63], [64], [65], [66], [67], [68]]. In this way, we hypothesized that RT treatment prior to IB-DNQ exposure will increase NQO1 expression in tumors, increasing sensitivity to IB-DNQ; a sensitization that would be especially important for tumors with low initial NQO1 expression (i.e., IHC scores 0-1). RT is considered a marginally effective SOC for advanced HNSCC and FOSCC to control local disease and is often coupled with chemotherapy [33,35,36,[69], [70], [71]]. This use of RT affords an opportunity to exploit the purposeful upregulation of NQO1 and subsequent targeting with IB-DNQ, a strategy of induced synergistic coupling akin to other couplet anticancer regimens [72].
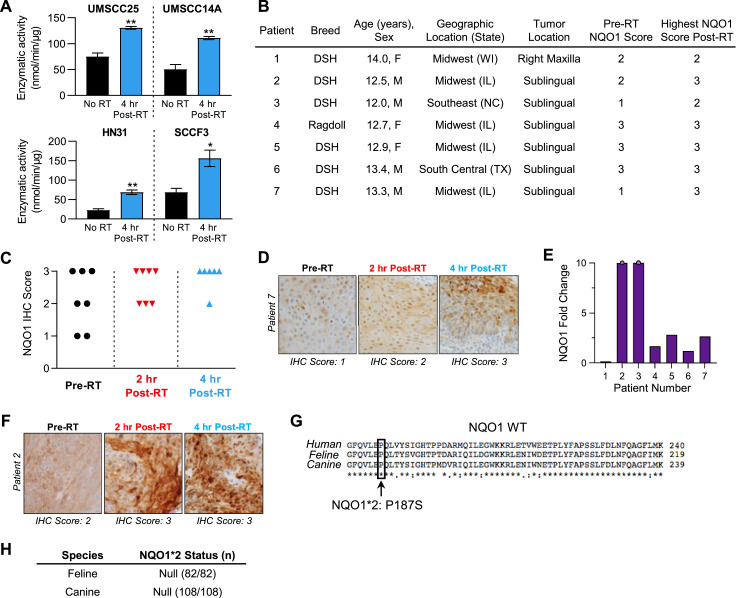
In agreement with previous reports demonstrating increased NQO1 protein levels in response to RT [[57], [58], [59]], we observed a similar increase in NQO1 enzymatic activity in low-NQO1 expressing HNSCC and FOSCC cell lines (Fig. 2A, SI Fig. 1). Boothman and coworkers [73] have demonstrated that low levels of NQO1 enzymatic activity (approximately 90-100 nmol/min/µg) are sufficient for the anticancer activity of NQO1-mediated redox cycling. Next, we sought to characterize this inducibility of NQO1 protein using RT in the natural disease setting (i.e., feline cancer patient) to define optimal timing and dosage. To determine if NQO1 expression can be induced in patients’ tumors, 7 domestic cats with FOSCC were enrolled into a small pilot study designed to allow for pre- and post-RT (2 and 4 h) biopsies to be collected and assessed for their NQO1 expression. Patient details are displayed in Fig. 2B. RT led to increases in NQO1 IHC scores (Fig. 2C), most dramatically in the low NQO1 scoring tumors (Fig. 2D). In 6/7 cases, NQO1 intensities increased upon RT treatment (Fig. 2E) with gains seen in already high NQO1 expressing tumors (Fig. 2F). Some degree of NQO1 expression heterogeneity was identified within individual tumor samples and partially accounts for the observed broad range of NQO1 upregulation across patient samples (Fig. 2F). These data provide evidence that RT can induce NQO1 expression in naturally occurring feline tumors regardless of basal pre-treatment levels, thereby justifying a sequential combinatorial approach with RT followed by IB-DNQ. Low NQO1 expressing tumors are predicted to derive the least potential benefit from single-agent NQO1 bioactivatable strategies (e.g., IB-DNQ), but these therapeutic limitations can be theoretically mitigated through the purposeful RT-induction of NQO1 that could sufficiently render these tumors susceptible to IB-DNQ.
Fig. 2.
Radiation induces leverageable NQO1 expression in FOSCC tumors. (A) In 3 human HNSCC cell lines and one FOSCC cell line, all which have low basal levels of NQO1 protein and enzymatic activity, NQO1 enzymatic activity is significantly increased at 4 h following 8 Gy radiation. Analysis: unpaired t test, P-values: *P < 0.05; **P < 0.01. (B) Summary of patients enrolled for serial biopsy post-radiation and their initial NQO1 IHC score. DSH, Domestic Short Hair. (C) Tracking NQO1 IHC scores pre-RT, 2 h, and 4 h post-RT. NQO1 scores converge to higher levels upon 8 Gy RT exposure. (D) Representative images of a low NQO1 tumor converting to a high NQO1 expressing tumor upon 8 Gy RT exposure. IHC images are at 40X magnification. (E) NQO1 relative intensities increase upon 8 Gy RT, showing that even high NQO1 expressing tumors have increased intensities of NQO1 staining post-RT treatment. Gray dots denote tumors with NQO1 fold change >10. (F) Representative image of an initially high NQO1 (score 2) staining tumor becoming a more intense NQO1 staining tumor (score 3). IHC images are at 40X magnification. (G) Sequence alignment of NQO1 primary sequence across human, feline, and canine. NQO1*2 polymorphism is defined as a P187S variant in human NQO1. (H) Summary of all feline and canine samples collected, which were all null for NQO1*2 polymorphism.
Of note in humans, NQO1 has genomic polymorphisms that lead to an unstable and catalytically inefficient NQO1 [74,75], with the NQO1*2 polymorphism being the most prevalent [[76], [77], [78]]. It is expected that NQO1 targeted therapy will have minimal anticancer effects in NQO1*2 patients [79]. While NQO1*2 is validated in human patients, this polymorphism has not been fully explored in companion animals. Utilizing qPCR, we did not observe an NQO1*2 polymorphism in feline (n = 82) nor canine (n = 108) samples that is similar to the polymorphism seen in human NQO1*2 (Fig. 2G,H, Supplemental Table 2).Though a relatively small sample set (n = 190), it does not appear that NQO1*2 is highly prevalent in companion animals and therefore upregulated expression of NQO1 would be predicted to yield an active enzyme during an induced synergistic couplet strategy.
Safety and efficacy of the combination IB-DNQ and palliative radiotherapy against FOSCC
To evaluate the safety, tolerability, and preliminary efficacy of weekly dosed palliative RT followed by intravenous IB-DNQ, nineteen FOSCC patients were prospectively accrued for evaluation of this treatment protocol. These patients originated from 7 US states and were accrued over 12 mo (Table 1). In this manner, these patients represent diverse local environments of FOSCC oncogenesis, as well as demonstrate the large patient population uniquely available for the conductance of high-value comparative cancer research to identify and validate investigational HNSCC therapies. Pre-treatment biopsies were collected and NQO1 expression analyzed by IHC and scored by 2 board-certified veterinary pathologists (Table 1).
Table 1.
Summary of all patients enrolled in IB-DNQ + RT FOSCC Trial.
| Patient Number | Breed | Age (y) | Sexa | Geographic Location (State) | Tumor Location | NQO1 Score | Tumor Response (%) |
|---|---|---|---|---|---|---|---|
| 1 | DSH/Siamese | 14.3 | M | Pacific Northwest (MT) | Lingual | 3 | -15 |
| 2 | DSH | 18.9 | F | Midwest (IL) | Maxilla | 3 | -40 |
| 3 | DMH | 13.8 | F | Midwest (MN) | Maxilla/Mandible | 2 | 0 |
| 4 | DSH | 12.7 | F | Southeast (VA) | Maxilla | 2 | 25 |
| 5 | DLH | 14.3 | M | South Central (MO) | Lingual | 3 | 5 |
| 6 | DSH | 16.6 | F | Midwest (IL) | Mandible | 1 | 1 |
| 7 | DSH | 16.8 | M | Midwest (IL) | Mandible | 2 | -56 |
| 8 | DSH | 12.2 | M | Midwest (IL) | Mandible | 2 | -27 |
| 9 | DSH/Siamese | 12.6 | F | Northeast (NY) | Mandible | 2 | -13 |
| 10 | DSH | 13.0 | M | Midwest (MI) | Mandible | 2 | -37 |
| 11 | DSH | 9.3 | M | Southwest (AZ) | Maxilla | 2 | -15 |
| 12 | DSH | 16.3 | F | Midwest (IL) | Mandible | 2 | -9 |
| 13 | DSH | 15.6 | F | Midwest (IL) | Maxilla | 1 | 12 |
| 14 | Maine Coon | 12.8 | M | South Central (TX) | Mandible | n.d. | -16 |
| 15 | DSH | 12.2 | M | Midwest (IL) | Maxilla | 2 | -8 |
| 16 | DLH | 15.1 | M | Midwest (IL) | Mandible | 2 | 8 |
| 17 | Siamese | 12.1 | F | Western (CO) | Lingual | 2 | 2 |
| 18 | DLH | 13.5 | F | Midwest (IL) | Maxilla | 2 | -26 |
| 19 | DSH | 14.3 | M | South Central (KS) | Maxilla | 2 | n/a |
DSH = domestic short hair; DMH = domestic medium hair; DLH = domestic long hair. n/a = not available, patient 19 was treated with 2 doses of IB-DNQ + RT prior to being withdrawn and the owner declined a follow-up CT scan.
All patients were castrated/spayed felines.
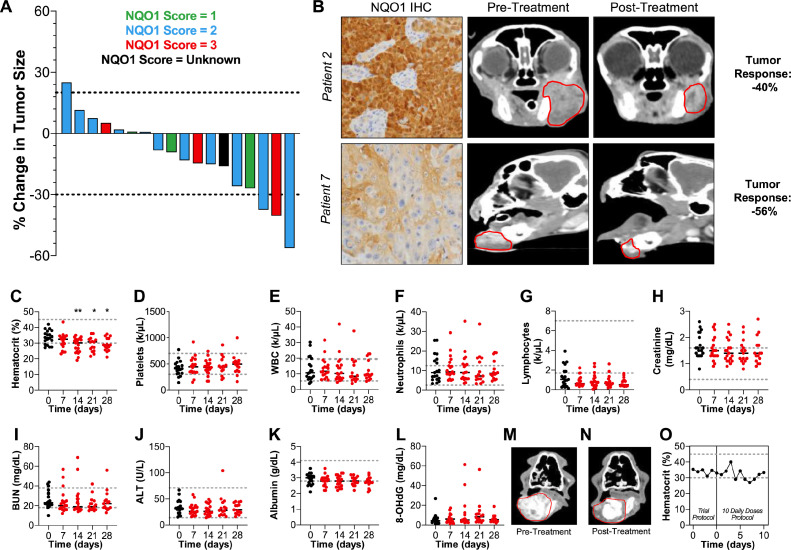
Based on our RT-induced NQO1 expression work (Fig. 2), regardless of initial NQO1 score, patients were treated with 4 weekly treatments of 8 Gy RT followed by IB-DNQ (1.0 mg/kg IV) 2 to 4 h post-RT. Cytoreductive therapeutic effect was determined by comparing CT scans prior to treatment and scans 1 wk following completion of treatment and tumor response was measured using RECIST guidelines [54]. Blood work and urinalyses were performed throughout to monitor toxicity markers. Eighteen patients had corresponding pre- and post-treatment CT scans to assess response, with 3/18 achieving PR, 14/18 with SD and 1/18 with PR, totaling 17/18 patients experiencing some overall biologic response (Fig. 3A, B). Treated pet cats did not demonstrate any clinically relevant hematologic or non-hematologic toxicities (Fig. 3C−K), including the absence of anemia-associated symptoms (tachycardia, exercise intolerance) and markers of oxidative damage as measured by 8-OHdG levels (Fig. 3L). Additionally, none of these patients experienced clinical signs associated with administration of IB-DNQ that were unexpected, or more severe, based on our previous work in healthy cats [44].
Fig. 3.
Efficacy and tolerability of IB-DNQ + RT in FOSCC patients. (A) Tumor responses of patients (n = 18) who received full protocol of RT (8 Gy) then 2 to 4 h later IB-DNQ (1.0 mg/kg I.V.) once a week for 4 cycles. Each patient corresponds to a separate bar and is colored based on their pre-treatment tumor biopsy NQO1 intensity using IHC. Tumor response was measured by percent tumor change compared to pre-treatment CT and post-treatment CT (taken 1 wk after protocol completion), tumor size was determined according to RECIST guidelines. (B) Representative images of pre-treatment NQO1 IHC, CT and post-treatment CT tumor response images. (C−K) Hematological and non-hematological markers tracked during the course of treatment (n = 18). Normal healthy clinical thresholds for each marker is denoted with a gray dotted line, feline cancer patients often have larger deviations outside normal physiological ranges. (L) Determination of oxidative damage associated with treatment by tracking levels of 8-hydroxy-2′–deoxyguanosine (8-OHdG) in the urine of feline patients. High levels are a clinical indication of oxidative damage, but absolute clinical “normal” thresholds are not agreed upon. (M−O) Pre-treatment and post-extended treatment of patient 14. The total treatment protocol for this patient was the standard RT (8 Gy), then IB-DNQ (1.0 mg/kg I.V.) 2-4 h later for 4 cycles, followed by an additional 10 daily treatments of IB-DNQ (1.0 mg/kg, I.V.) Disease was stable during this treatment course (M, N) and no clinically significant changes in hematocrit was observed during these additional IB-DNQ treatments (O). Gray dotted lines denote the standard threshold for hematocrit percentage for a non-tumor bearing, healthy feline. Statistical analysis for all panels: change from baseline was evaluated with an analysis of variance with post-hoc comparison made with Dunnett's multiple comparisons test, P-values: *P < 0.05; **P < 0.01.
During this study, patient 14 presented with severe soft tissue and bone involvement of FOSCC and experienced a visually dramatic decrease in tumor size with the initial 4 wk of treatment. Due to the observed robust clinical response to therapy and overall good systemic health, this patient was treated with IB-DNQ as a single agent for an additional 10 daily treatments. This patient's disease remained stable (Fig. 3M, N), and importantly no development of any hematologic toxicities was observed (Fig. 3O); findings which underscore the exceptional biologic tolerability of repeated IB-DNQ therapy, even in this late-stage feline cancer patient.
Discussion
Developing NQO1-bioactivated therapeutic approaches and the value of felines
We describe assessments of IB-DNQ (single agent and in combination with palliative RT) as a well-tolerated targeted therapy for advanced HNSCC. NQO1 overexpression in HNSCC and other cancer types is well-established [9], however current modalities (namely β-lapachone) have not yielded robust excitement due to anemic phenotypes observed in human clinical trials [[13], [14], [15]]. β-lapachone and DNQ (and their respective derivatives) both contain a quinone functional group that are potent substrates for NQO1’s enzymatic reduction, leading to unstable hydroquinone formation, ROS generation, and ultimately cancer cell death. Perhaps unsurprisingly with such a molecular mechanism, redox liabilities are a major consideration for in vivo tolerability and specific preclinical assessments for these liabilities are warranted.
Felines present as a valuable organism for preclinical drug development with their unique sensitivities to oxidative damage. While a higher therapeutic index for felines may appear to be a poor comparative metric for human translation, in the case of NQO1-mediated therapies where oxidative damage is an already established concern in humans, the use of such a matched toxicity-drug model sets a higher tolerability bar and thus prioritizes more tolerable therapies prior to human translation, a potentially cost saving strategy. The use of felines is even more advantageous for HNSCC drug development with the opportunity to use FOSCC patients, which not only better recapitulate human HNSCC tumors (discussed more below), but also allows for the evaluation of NQO1-targeted therapy tolerability in tumor bearing, late-stage patients; a physiological condition that better mimics patients receiving experimental therapy in a Phase 1 setting. Employing this type of matched toxicity-drug preclinical system may prove vital in predicting a given therapy's safety profile prior to assessment in human clinical trials, especially if a major toxicity liability is already well-reported.
The need to employ comparative oncology approaches for HNSCC drug development
The need for novel therapies against advanced HNSCC is not due to a lack of campaigns to fill this clinical void. The effectiveness of an anticancer compound is typically assessed with unnatural, rapidly growing, homogenous xenografts in immune deficient murine models. However, human tumors, the major target for all emerging anticancer therapies, are highly heterogeneous, often developed over an extended period of time, and have a variety of features that are difficult to fully model outside of a natural setting. This reliance on imperfect murine models leading to unoptimized therapeutic development is underscored by the relative dearth of current FDA-approved targeted therapies for the effective management of advanced stage, unresectable, and/or recurrent/metastatic (R/M) HNSCC disease settings [[19], [20], [21]].
Maximal utilization of companion animal cancer patients as unique and sophisticated experimental models for human therapeutic development relies on the identification of conserved tumor histologies between human beings and companion animals. While for some cancer types identifying this ideal mimic is obvious (e.g., osteosarcoma [80]), this is not the case in other cancer types that are of major interest. Much of comparative oncology research focuses on canines and their use in drug development, relegating felines to a subsidiary role. However, there are approximately 6 million feline cancer patients in the US alone, and many are afflicted with a variety of neoplasia that differ from dogs [81], and even the same tumor histologies can have vastly different biologic behaviors between the 2 species (e.g., compare canine [82] and feline [83] lymphoma). Therefore, feline patients can fill some unique voids of comparative oncology that canines simply cannot; FOSCC as a model for HNSCC is one such fruitful opportunity we sought to leverage.
FOSCC has been well-reported to recapitulate many of the aspects of HNSCC, namely its metastatic and heterogeneous nature [19,21,[38], [39], [40]]. Given FOSCC's high prevalence and therapeutically recalcitrant nature, there is clinical justification and ethical acceptance for rapid safety and activity assessments of drug candidates in this population and serves as a crucial consideration for proposing to utilize feline data to inform preclinical candidate selection. In our studies we were able to enroll multiple felines from diverse regions of the United States expeditiously, hallmarking the relative ease of establishing a robust patient population, as well as demonstrating the substantial need for new therapeutics for this feline cancer with human comparative value.
One of the most important commonalities between human HNSCC and FOSCC management is that many years of studies and clinical trials have demonstrated that radiotherapy will likely always be a backbone of treatment for these tumor types. With that in mind, a major goal of this study was to definitively establish that RT could induce NQO1 expression in naturally occurring tumors and be rationally combined with bioactivatable molecular strategies. Demonstrating this induction in vivo, provides evidence for a feasible couplet strategy in which a tumor is first irradiated then treated with IB-DNQ to increase anticancer efficacy. Of note, previous work has described β-lapachone (an NQO1 bioactivatable drug) as a radiosensitizer utilizing a co-dosing method [10]. This is a complementary approach to our work which utilizes the reverse; employing RT to sensitize tumors to IB-DNQ. Testing the feasibility of inducing NQO1 overexpression with RT was greatly empowered by the ability to ethically collect serial biopsies from pet cats presenting with OSCC tumors, a medical practice not permissible in human cancer patients based upon FDA's Good Clinical Practice and Human Protection Act. FOSCC tumors are amenable to testing the translational viability of an induced synergistic couplet regimen, and our results suggest the ability to preclinically test a wide-variety of target-inducible-based strategies for the treatment of cancer.
Challenges with feline-focused research
While applying felines to the drug development pipeline has many positives (discussed above) there are certain limitations that warrant distinction. Materials and scientific reagents for feline-focused research are relatively limited and require the validation of resources which cross-react with homologous feline targets (i.e., proteins, signaling pathways, etc.) An example of one such limitation is the lack of diverse feline genomic information; at the time of publication there are only 3 felines with variable amount of genome coverage reported on NCBI. These limitations can greatly constrain a variety of research avenues that rely on tools that are specific for feline targets (e.g., anti-PD-1/PD-L1 antibodies). Without a focused campaign on developing such tools, the use of felines for many therapeutic questions requires overcoming higher initial barriers to achieve research expediency, especially when compared to their canine counterparts.
Limitations and conclusions of current work
Our current study seeks to establish the potential of RT + IB-DNQ as an emerging well-tolerated targeted therapy in feline HNSCC models. While the value of using FOSCC patients is that data can be collected as a combination of safety and efficacy, our study design was not designed nor powered to demonstrate proven activity (similar to a Phase 1 clinical trial) with the utilization of a radiation-only control arm. However, when compared to a small scale historical study that employed a similar radiation treatment protocol to the one we utilized, pet cats did not benefit from radiation alone [84], which is in contrast to our study results whereby we observed an overall biologic response of 94.4% (17/18), albeit best responses were categorized as PRs achieved in only a minority of patients (~ 17%). A majority of these patients achieved SD, and it should be noted that 57% of those in the SD category did experience an overall decrease in their tumor size, despite not achieving PR using RECIST guidelines. While this is not an unexpected result with this proof-of-concept cohort given the late-stage and invasive nature of many cases, as well as using a coarse-fractionated RT protocol (often referred to as a palliative protocol), a more dose intense radiation protocol is likely necessary to exert more profound tumor responses as has been seen with this tumor type in cats [35]. The tolerability of daily IB-DNQ treatment documented in the current study paves the way towards evaluating curative-intent combinatorial strategies whereby IB-DNQ can be combined with definitive or stereotactic radiotherapy (SRT) fraction schemes. This work provides initial evidence for the translational potential of IB-DNQ as a targeted therapy for HNSCC and for IB-DNQ + RT for the treatment of advanced human HNSCC and demonstrates the powerful potential of using feline cancer patients for human anticancer drug development.
Acknowledgments
Author contributions
T.M.F. and P.J.H. conceived this study. A.P.L. executed the clinical aspect of this project, the research animal models, and contributed significantly to the in vitro studies in this project. M.W.B. synthesized IB-DNQ on large scale for the study and contributed to the in vitro studies. A.P.L. and M.W.B. processed data, generated figures, and wrote the manuscript with input from P.J.H and T.M.F. E.I.P. conducted MTD experiments in mice. The radiation therapy was planned and administered by K.A.S., and all tumor scoring was validated by boarded-veterinary pathologists J.P.S. and A.M.B. L.E.C performed large-scale analysis for NQO1*2 mutation in companion animals. M.W.B. and J.M.F. synthesized all derivatives of DNQ described herein.
Acknowledgments
The authors acknowledge Renee Walker in the Veterinary Diagnostic Laboratory for the work done in immunohistochemistry, and Rebecca Kamerer for her dedication to recruiting and providing care to the patients in the study.
Footnotes
✩Funding: We extend our gratitude to the University of Illinois, the Morris Animal Foundation (D17FE-007), and the NIH (R01-DE026836-02) for funding the work described herein.
✩✩Conflicts of interest: The University of Illinois has filed patents surrounding this work on which P.J.H. and E.I.P. are inventors. IB-DNQ and related technologies are licensed to Systems Oncology and Toray Industries; P.J.H. is on the S.A.B of Systems Oncology. M.W.B. and L.E.C. are members of the NIH Chemistry-Biology Interface Training Program (T32-GM136629). M.W.B. is an ACS Medicinal Chemistry Predoctoral Fellow, and an NCI F99/K00 predoctoral fellow (F99-CA253731).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2021.06.008.
Appendix. Supplementary materials
References
- 1.Le Tourneau C, Velten M, Jung GM, Bronner G, Flesch H, Borel C. Prognostic indicators for survival in head and neck squamous cell carcinomas: analysis of a series of 621 cases. Head Neck. 2005;27(9):801–808. doi: 10.1002/hed.20254. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 4.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53(15):3579–3584. [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, Soria A, Machiels J-P, Mach N, Mehra R. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. The Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 8.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Basté N, Neupane P, Bratland Å. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. The Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson EI, Hergenrother PJ. Deoxynyboquinones as NQO1-Activated Cancer Therapeutics. Acc Chem Res. 2015;48(10):2715–2723. doi: 10.1021/acs.accounts.5b00365. [DOI] [PubMed] [Google Scholar]
- 10.Li LS, Reddy S, Lin ZH, Liu S, Park H, Chun SG, Bornmann WG, Thibodeaux J, Yan J, Chakrabarti G. NQO1-Mediated Tumor-Selective Lethality and Radiosensitization for Head and Neck Cancer. Mol Cancer Ther. 2016;15(7):1757–1767. doi: 10.1158/1535-7163.MCT-15-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352) doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 12.Starcher CL, Pay SL, Singh N, Yeh IJ, Bhandare SB, Su X, Huang X, Bey EA, Motea EA, Boothman DA. Targeting Base Excision Repair in Cancer: NQO1-Bioactivatable Drugs Improve Tumor Selectivity and Reduce Treatment Toxicity Through Radiosensitization of Human Cancer. Front Oncol. 2020;10:1575. doi: 10.3389/fonc.2020.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khong HT, Dreisbach L, Kindler HL, Trent DF, Jeziorski KG, Bonderenko I, Popiela T, Yagovane DM, Dombal G. A phase 2 study of ARQ 501 in combination with gemcitabine in adult patients with treatment naïve, unresectable pancreatic adenocarcinoma. Journal of Clinical Oncology. 2007;25(18):15017. suppl. [Google Scholar]
- 14.Kawecki A, Adkins DR, Cunningham CC, Vokes E, Yagovane DM, Dombal G, Koralewski P, Hotko Y, Vladimirov V. A phase II study of ARQ 501 in patients with advanced squamous cell carcinoma of the head and neck. Journal of Clinical Oncology. 2007;25(18):16509. _suppl. [Google Scholar]
- 15.Gerber DE, Beg MS, Fattah F, Frankel AE, Fatunde O, Arriaga Y, Dowell JE, Bisen A, Leff RD, Meek CC. Phase 1 study of ARQ 761, a beta-lapachone analogue that promotes NQO1-mediated programmed cancer cell necrosis. Br J Cancer. 2018;119(8):928–936. doi: 10.1038/s41416-018-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkinson EI, Bair JS, Cismesia M, Hergenrother PJ. Efficient NQO1 substrates are potent and selective anticancer agents. ACS Chem Biol. 2013;8(10):2173–2183. doi: 10.1021/cb4005832. [DOI] [PubMed] [Google Scholar]
- 17.Bair JS, Palchaudhuri R, Hergenrother PJ. Chemistry and biology of deoxynyboquinone, a potent inducer of cancer cell death. J Am Chem Soc. 2010;132(15):5469–5478. doi: 10.1021/ja100610m. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Dong Y, Bey EA, Kilgore JA, Bair JS, Li LS, Patel M, Parkinson EI, Wang Y, Williams NS. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res. 2012;72(12):3038–3047. doi: 10.1158/0008-5472.CAN-11-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supsavhad W, Dirksen WP, Martin CK, Rosol TJ. Animal models of head and neck squamous cell carcinoma. Vet J. 2016;210:7–16. doi: 10.1016/j.tvjl.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nat Med. 2015;21(5):431–439. doi: 10.1038/nm.3853. [DOI] [PubMed] [Google Scholar]
- 21.Sparger EE, Murphy BG, Kamal FM, Arzi B, Naydan D, Skouritakis CT, Cox DP, Skorupski K. Investigation of immune cell markers in feline oral squamous cell carcinoma. Vet Immunol Immunopathol. 2018;202:52–62. doi: 10.1016/j.vetimm.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wypij JM. A naturally occurring feline model of head and neck squamous cell carcinoma. Patholog Res Int. 2013;2013 doi: 10.1155/2013/502197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piegols HJ, Takada M, Parys M, Dexheimer T, Yuzbasiyan-Gurkan V. Investigation of novel chemotherapeutics for feline oral squamous cell carcinoma. Oncotarget. 2018;9(69):33098–33109. doi: 10.18632/oncotarget.26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBlanc AK, Breen M, Choyke P, Dewhirst M, Fan TM, Gustafson DL, Helman LJ, Kastan MB, Knapp DW, Levin WJ. Perspectives from man's best friend: National Academy of Medicine's Workshop on Comparative Oncology. Sci Transl Med. 2016;8(324) doi: 10.1126/scitranslmed.aaf0746. 324ps325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBlanc AK, Mazcko CN. Improving human cancer therapy through the evaluation of pet dogs. Nat Rev Cancer. 2020;20(12):727–742. doi: 10.1038/s41568-020-0297-3. [DOI] [PubMed] [Google Scholar]
- 26.Riccardo F, Aurisicchio L, Impellizeri JA, Cavallo F. The importance of comparative oncology in translational medicine. Cancer Immunol Immunother. 2015;64(2):137–148. doi: 10.1007/s00262-014-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon CM: Cats, Cancer and Comparative Oncology. Vet Sci. 2015;2(3):111–126. doi: 10.3390/vetsci2030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kol A, Arzi B, Athanasiou KA, Farmer DL, Nolta JA, Rebhun RB, Chen X, Griffiths LG, Verstraete FJ, Murphy CJ. Companion animals: Translational scientist's new best friends. Sci Transl Med. 2015;7(308) doi: 10.1126/scitranslmed.aaa9116. 308ps321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley RM, Davis BW, Brashear WA, Farias FHG, Kuroki K, Graves T, Hillier LW, Kremitzki M, Li G, Middleton RP. A new domestic cat genome assembly based on long sequence reads empowers feline genomic medicine and identifies a novel gene for dwarfism. PLoS Genet. 2020;16(10) doi: 10.1371/journal.pgen.1008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granados-Soler JL, Bornemann-Kolatzki K, Beck J, Brenig B, Schutz E, Betz D, Junginger J, Hewicker-Trautwein M, Murua Escobar H, Nolte I. Analysis of Copy-Number Variations and Feline Mammary Carcinoma Survival. Sci Rep. 2020;10(1):1003. doi: 10.1038/s41598-020-57942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilgic O, Duda L, Sanchez MD, Lewis JR. Feline Oral Squamous Cell Carcinoma: Clinical Manifestations and Literature Review. J Vet Dent. 2015;32(1):30–40. doi: 10.1177/089875641503200104. [DOI] [PubMed] [Google Scholar]
- 32.Northrup NC, Selting KA, Rassnick KM, Kristal O, O'Brien MG, Dank G, Dhaliwal RS, Jagannatha S, Cornell KK, Gieger TL. Outcomes of cats with oral tumors treated with mandibulectomy: 42 cases. J Am Anim Hosp Assoc. 2006;42(5):350–360. doi: 10.5326/0420350. [DOI] [PubMed] [Google Scholar]
- 33.Hayes AM, Adams VJ, Scase TJ, Murphy S. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice. J Small Anim Pract. 2007;48(7):394–399. doi: 10.1111/j.1748-5827.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 34.Moore A. Treatment choices for oral cancer in cats. What is possible? What is reasonable? J Feline Med Surg. 2009;11(1):23–31. doi: 10.1016/j.jfms.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidel J, Lyons J, Tripp C, Houston R, Wheeler B, Ruiz A. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. J Vet Intern Med. 2011;25(3):504–510. doi: 10.1111/j.1939-1676.2011.0721.x. [DOI] [PubMed] [Google Scholar]
- 36.Sabhlok A, Ayl R. Palliative radiation therapy outcomes for cats with oral squamous cell carcinoma (1999-2005) Vet Radiol Ultrasound. 2014;55(5):565–570. doi: 10.1111/vru.12157. [DOI] [PubMed] [Google Scholar]
- 37.Stebbins KE, Morse CC, Goldschmidt MH. Feline oral neoplasia: a ten-year survey. Vet Pathol. 1989;26(2):121–128. doi: 10.1177/030098588902600204. [DOI] [PubMed] [Google Scholar]
- 38.Genden EM, Ferlito A, Bradley PJ, Rinaldo A, Scully C. Neck disease and distant metastases. Oral Oncology. 2003;39(3):207–212. doi: 10.1016/s1368-8375(02)00049-0. [DOI] [PubMed] [Google Scholar]
- 39.Snyder LA, Bertone ER, Jakowski RM, Dooner MS, Jennings-Ritchie J, Moore AS. expression and environmental tobacco smoke exposure in feline oral squamous cell carcinoma. Vet Pathol. 2004;41(3):209–214. doi: 10.1354/vp.41-3-209. [DOI] [PubMed] [Google Scholar]
- 40.Soltero-Rivera MM, Krick EL, Reiter AM, Brown DC, Lewis JR. Prevalence of regional and distant metastasis in cats with advanced oral squamous cell carcinoma: 49 cases (2005-2011) J Feline Med Surg. 2014;16(2):164–169. doi: 10.1177/1098612X13502975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathore K, Alexander M, Cekanova M. Piroxicam inhibits Masitinib-induced cyclooxygenase 2 expression in oral squamous cell carcinoma cells in vitro. Transl Res. 2014;164(2):158–168. doi: 10.1016/j.trsl.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Supsavhad W, Dirksen WP, Hildreth BE. Rosol TJ: p16, pRb, and p53 in Feline Oral Squamous Cell Carcinoma. Vet Sci. 2016;3(3):1–15. doi: 10.3390/vetsci3030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renzi A, De Bonis P, Morandi L, Lenzi J, Tinto D, Rigillo A, Bettini G, Bellei E, Sabattini S. Prevalence of p53 dysregulations in feline oral squamous cell carcinoma and non-neoplastic oral mucosa. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0215621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundberg AP, Francis JM, Pajak M, Parkinson EI, Wycislo KL, Rosol TJ, Brown ME, London CA, Dirikolu L, Hergenrother PJ. Pharmacokinetics and derivation of an anticancer dosing regimen for the novel anti-cancer agent isobutyl-deoxynyboquinone (IB-DNQ), a NQO1 bioactivatable molecule, in the domestic felid species. Invest New Drugs. 2017;35(2):134–144. doi: 10.1007/s10637-016-0414-z. [DOI] [PubMed] [Google Scholar]
- 45.Taketa F. Structure of the Felidae hemoglobins and response to 2,3-diphosphoglycerate. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1973;45(4):813–823. doi: 10.1016/0305-0491(73)90144-2. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton MN, Edelstein SJ. Cat hemoglobin. pH dependence of cooperativity and ligand binding. J Biol Chem. 1974;249(5):1323–1329. [PubMed] [Google Scholar]
- 47.Bunn HF. Regulation of Hemoglobin Function in Mammals. Amer Zool. 1980;20:199–211. [Google Scholar]
- 48.Harvey JW, Kaneko JJ. Mammalian erythrocyte metabolism and oxidant drugs. Toxicology and Applied Pharmacology. 1977;42(2):253–261. doi: 10.1016/0041-008x(77)90002-3. [DOI] [PubMed] [Google Scholar]
- 49.Harvey JW, Kaneko JJ. Erythrocyte enzyme activities and glutathione levels of the horse, cat, dog and man. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1975;52(4):507–510. doi: 10.1016/0305-0491(75)90226-6. [DOI] [PubMed] [Google Scholar]
- 50.Christopher MM, White JG, Eaton JW. Erythrocyte pathology and mechanisms of Heinz body-mediated hemolysis in cats. Vet Pathol. 1990;27(5):299–310. doi: 10.1177/030098589002700501. [DOI] [PubMed] [Google Scholar]
- 51.Ramos-Vara JA, Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry–the red, brown, and blue technique. Vet Pathol. 2014;51(1):42–87. doi: 10.1177/0300985813505879. [DOI] [PubMed] [Google Scholar]
- 52.Kuo YM, Nussbaum RL. Prolongation of Chemically-Induced Methemoglobinemia in Mice Lacking alpha-synuclein: A Novel Pharmacologic and Toxicologic Phenotype. Toxicol Rep. 2015;2:504–511. doi: 10.1016/j.toxrep.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coles E. Erythrocyte Fragility. In: Coles E, editor. Veterinary Clinical Pathology. 4th edn. Saunders Company; Canada: W.B: 1986. p. 29. Edited by. Edited by. [Google Scholar]
- 54.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13(3):176–183. doi: 10.1111/vco.12032. [DOI] [PubMed] [Google Scholar]
- 55.Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17(24):3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 56.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280(17):16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 57.Park HJ, Ahn KJ, Ahn SD, Choi E, Lee SW, Williams B, Kim EJ, Griffin R, Bey EA, Bornmann WG. Susceptibility of cancer cells to beta-lapachone is enhanced by ionizing radiation. Int J Radiat Oncol Biol Phys. 2005;61(1):212–219. doi: 10.1016/j.ijrobp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki M, Amano M, Choi J, Park HJ, Williams BW, Ono K, Song CW. Synergistic effects of radiation and beta-lapachone in DU-145 human prostate cancer cells in vitro. Radiat Res. 2006;165(5):525–531. doi: 10.1667/RR3554.1. [DOI] [PubMed] [Google Scholar]
- 59.Choi EK, Terai K, Ji IM, Kook YH, Park KH, Oh ET, Griffin RJ, Lim BU, Kim JS, Lee DS. Upregulation of NAD(P)H:quinone oxidoreductase by radiation potentiates the effect of bioreductive beta-lapachone on cancer cells. Neoplasia. 2007;9(8):634–642. doi: 10.1593/neo.07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park HJ, Choi EK, Choi J, Ahn KJ, Kim EJ, Ji IM, Kook YH, Ahn SD, Williams B, Griffin R. Heat-induced up-regulation of NAD(P)H:quinone oxidoreductase potentiates anticancer effects of beta-lapachone. Clin Cancer Res. 2005;11(24):8866–8871. doi: 10.1158/1078-0432.CCR-05-0818. Pt 1. [DOI] [PubMed] [Google Scholar]
- 61.Boothman DA, Meyers M, Fukunaga N, Lee SW. Isolation of x-ray-inducible transcripts from radioresistant human melanoma cells. Proc Natl Acad Sci U S A. 1993;90(15):7200–7204. doi: 10.1073/pnas.90.15.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia Z, Hallur S, Zhu H, Li Y, Misra HP. Potent upregulation of glutathione and NAD(P)H:quinone oxidoreductase 1 by alpha-lipoic acid in human neuroblastoma SH-SY5Y cells: protection against neurotoxicant-elicited cytotoxicity. Neurochem Res. 2008;33(5):790–800. doi: 10.1007/s11064-007-9496-5. [DOI] [PubMed] [Google Scholar]
- 63.Pink JJ. NAD(P)H:Quinone Oxidoreductase Activity Is the Principal Determinant of beta -Lapachone Cytotoxicity. Journal of Biological Chemistry. 2000;275(8):5416–5424. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 64.Song CW, Chae JJ, Choi EK, Hwang TS, Kim C, Lim BU, Park HJ. Anti-cancer effect of bio-reductive drug beta-lapachon is enhanced by activating NQO1 with heat shock. Int J Hyperthermia. 2008;24(2):161–169. doi: 10.1080/02656730701781895. [DOI] [PubMed] [Google Scholar]
- 65.Dong Y, Bey EA, Li LS, Kabbani W, Yan J, Xie XJ, Hsieh JT, Gao J, Boothman DA. Prostate cancer radiosensitization through poly(ADP-Ribose) polymerase-1 hyperactivation. Cancer Res. 2010;70(20):8088–8096. doi: 10.1158/0008-5472.CAN-10-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahn KJ, Lee HS, Bai SK, Song CW. Enhancement of radiation effect using beta-lapachone and underlying mechanism. Radiat Oncol J. 2013;31(2):57–65. doi: 10.3857/roj.2013.31.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamberti MJ, Vittar NB, da Silva Fde C, Ferreira VF, Rivarola VA. Synergistic enhancement of antitumor effect of beta-Lapachone by photodynamic induction of quinone oxidoreductase (NQO1) Phytomedicine. 2013;20(11):1007–1012. doi: 10.1016/j.phymed.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 68.Motea EA, Huang X, Singh N, Kilgore JA, Williams NS, Xie XJ, Gerber DE, Beg MS, Bey EA, Boothman DA. NQO1-dependent, Tumor-selective Radiosensitization of Non-small Cell Lung Cancers. Clin Cancer Res. 2019;25(8):2601–2609. doi: 10.1158/1078-0432.CCR-18-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 70.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 71.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 72.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 73.Li LS, Bey EA, Dong Y, Meng J, Patra B, Yan J, Xie XJ, Brekken RA, Barnett CC, Bornmann WG. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of beta-lapachone for pancreatic cancer therapy. Clin Cancer Res. 2011;17(2):275–285. doi: 10.1158/1078-0432.CCR-10-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegel D, Anwar A, Winski SL, Kepa JK, Zolman KL, Ross D. Rapid polyubiquitination and proteasomal degradation of a mutant form of NAD(P)H:quinone oxidoreductase 1. Mol Pharmacol. 2001;59(2):263–268. doi: 10.1124/mol.59.2.263. [DOI] [PubMed] [Google Scholar]
- 75.Asher G, Lotem J, Sachs L, Kahana C, Shaul Y. Mdm-2 and ubiquitin-independent p53 proteasomal degradation regulated by NQO1. Proc Natl Acad Sci U S A. 2002;99(20):13125–13130. doi: 10.1073/pnas.202480499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelsey KT, Ross D, Traver RD, Christiani DC, Zuo ZF, Spitz MR, Wang M, Xu X, Lee BK, Schwartz BS. Ethnic variation in the prevalence of a common NAD(P)H quinone oxidoreductase polymorphism and its implications for anti-cancer chemotherapy. Br J Cancer. 1997;76(7):852–854. doi: 10.1038/bjc.1997.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siegel D, McGuinness SM, Winski SL, Ross D. Genotype-phenotype relationships in studies of a polymorphism in NAD(P)H:quinone oxidoreductase 1. Pharmacogenetics. 1999;9(1):113–121. doi: 10.1097/00008571-199902000-00015. [DOI] [PubMed] [Google Scholar]
- 78.Ross D, Siegel D. NAD(P)H:Quinone oxidoreductase 1 (NQO1, DT-Diaphorase), Functions and Pharmacogenetics. Methods in Enzymology. 2004;382:115–144. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 79.Zhang K, Chen D, Ma K, Wu X, Hao H, Jiang S. NAD(P)H:Quinone Oxidoreductase 1 (NQO1) as a Therapeutic and Diagnostic Target in Cancer. J Med Chem. 2018;61(16):6983–7003. doi: 10.1021/acs.jmedchem.8b00124. [DOI] [PubMed] [Google Scholar]
- 80.Simpson S, Dunning MD, de Brot S, Grau-Roma L, Mongan NP, Rutland CS. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. 2017;59(1):71. doi: 10.1186/s13028-017-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.MacVean DW, Monlux AW, Anderson PS, Jr., Silberg SL, Roszel JF. Frequency of canine and feline tumors in a defined population. Vet Pathol. 1978;15(6):700–715. doi: 10.1177/030098587801500602. [DOI] [PubMed] [Google Scholar]
- 82.Zandvliet M. Canine lymphoma: a review. Vet Q. 2016;36(2):76–104. doi: 10.1080/01652176.2016.1152633. [DOI] [PubMed] [Google Scholar]
- 83.Paulin MV, Couronne L, Beguin J, Le Poder S, Delverdier M, Semin MO, Bruneau J, Cerf-Bensussan N, Malamut G, Cellier C. Feline low-grade alimentary lymphoma: an emerging entity and a potential animal model for human disease. BMC Vet Res. 2018;14(1):306. doi: 10.1186/s12917-018-1635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bregazzi VS, LaRue SM, Powers BE, Fettman MJ, Ogilvie GK, Withrow SJ. Response of feline oral squamous cell carcinoma to palliative radiation therapy. Vet Radiol Ultrasound. 2001;42(1):77–79. doi: 10.1111/j.1740-8261.2001.tb00907.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.