Trimethylamine N-oxide (TMAO) is a gut-derived circulating metabolite produced from the conversion of the dietary precursors L-carnitine and choline into trimethylamine (TMA) by intestinal microbiota. TMA is released into the portal circulation and then converted to TMAO in the liver by flavin-containing monooxygenase (FMO) enzymes. Elevated plasma TMAO concentrations are linked to the development of atherosclerosis in preclinical models and cardiovascular disease (CVD) events in humans, and portends higher mortality in other age-related disorders such as chronic kidney disease, diabetes and heart failure1, 2, and is associated with age-related cognitive decline in preclinical and human studies.3 Furthermore, aortic stiffness, measured by carotid-femoral pulse wave velocity (PWV), increases with advancing age and is a robust predictor of development of CVD and target organ damage to high blood flow, low resistance organs such as the brain and kidney in middle-aged/older adults. Although several studies demonstrate that plasma TMAO concentrations are elevated with aging4, 5, to date there have been no studies linking elevated TMAO with higher aortic stiffness.
In this issue of Hypertension, Brunt and colleagues6 performed a clever set of translational experiments to investigate the hypothesis that circulating TMAO contributes to the age-related increase in aortic stiffness and blood pressure (BP) with aging. First, they confirmed that circulating TMAO was elevated in older compared with younger adults, and that higher TMAO was correlated with higher carotid-femoral PWV. Importantly, the correlation remained significant after adjusting for standard CVD risk factors including BP, but the association was lost when age was included in the model. Similarly, elevated TMAO was associated with higher systolic BP and the association was also abolished with the adjustment for age. Together, these findings in humans represent the first link between the age-related increase in circulating TMAO with higher aortic stiffness and BP. However, given both aortic stiffness and BP are strong correlates of age, there remained the possibility that both outcomes were not, in fact, causally associated with TMAO. To address this limitation in the human study, complementary preclinical in vivo and ex vivo experiments were performed to determine whether there is indeed a mechanistic link between elevated TMAO and increased aortic stiffness. First, dietary supplementation of TMAO in young mice for 6 months and old mice for 3 months raised TMAO plasma concentrations in both groups of mice albeit higher in the old than the young. After early resistance to change at 3 months, TMAO supplementation in young mice resulted in ~70% higher aortic PWV compared with control low-choline fed mice after 6 months. In old mice, TMAO supplementation exacerbated the baseline higher aortic PWV only after 3 months indicating that increased stiffness occurred in one-half the TMAO exposure time with aged mice but with higher TMAO plasma concentrations than the young. If such findings translate to humans, this suggests there might be an opportunity for the prevention of premature aortic stiffening in younger adults with elevated TMAO, and that lowering TMAO in older adults might be a novel therapeutic strategy to attenuate aortic stiffness in middle-aged/older adults but this has not been tested in humans.
In addition to the increase in in vivo aortic PWW, the authors also tested ex vivo intrinsic aortic wall stiffness, assessed by the stress/strain relation, which quantifies the elastic modulus of the collagen-dominant region of stress-strain curve, i.e., where the stiffer collagen fibers bear the higher force. This is important because the increase in in vivo aortic PWV after 6 months in young mice and 3 months in older mice was accompanied by a parallel elevation in systolic BP, thus making it difficult to interpret whether the PWV changes were driven by increases in arterial distending pressure, or true alterations in intrinsic aortic wall stiffness. Indeed, the authors found an increased in elastic modulus of the collagen region in aortas from TMAO-supplemented young mice, and these changes occurred in the absence of changes in elastin (low-force) region of the stress/strain curve, or alterations in wall thickness and diameter. Finally, aortas from TMAO-supplemented young mice demonstrated an increase in advanced glycation end products (AGEs) accumulation, which form from non-enzymatic glycation of proteins and lipids and can mediate cross-linking of structural extracellular matrix proteins such as collagen-I, in the medial and adventitial layers. Interestingly, elevated AGEs occurred in the absence of alterations in total collagen-I or elastin, suggesting that AGE-related cross-linking of collagen, rather than an increase abundance of either extracellular matrix protein, may be one mechanism driving the greater TMAO-induced aortic stiffening.
Lastly, to test direct effects of TMAO on aortic wall stiffening, aortic rings from young mice that were incubated ex vivo with TMAO concentrations similar to in vivo supplemented concentrations, demonstrated an increase in intrinsic wall stiffness. Consistent with the idea that AGEs are a key mechanism involved, the TMAO-associated increase in aortic intrinsic stiffness was abolished with co-incubation of the pharmacological cross-link breaker alagebrium. Furthermore, given that previous studies found that TMAO is associated with vascular oxidative stress5, in separate experiments aortic rings were co-treated with the reactive oxygen species (e.g., superoxide anion) scavenger TEMPOL. TEMPOL abrogated the TMAO- associated increase in aortic stiffness, however, TEMPOL had no effect on aortic AGEs accumulation. As such, these data suggest that superoxide was mediating, at least in part, the TMAO-associated increase in aortic stiffness, but independently of AGE-associated cross-linking. Because it is known that AGEs binds to its specific receptor for AGEs (RAGE) resulting in an increase in NADPH-oxidase induced vascular oxidative stress7, it is plausible that AGEs could modulate aortic wall stiffness through RAGE-dependent oxidative stress without altering cross-links. Although this might be induced from circulating AGEs binding to transmembrane bound RAGEs, rather than AGEs in the arterial media and adventitia but circulating AGEs were not measured in the current study. Nevertheless, this study has identified the gut-derived metabolite TMAO as a possible novel mechanistic link between the gut microbiota and age-related aortic stiffness- henceforth called the “gut-arterial stiffness axis” (Figure).
Figure.
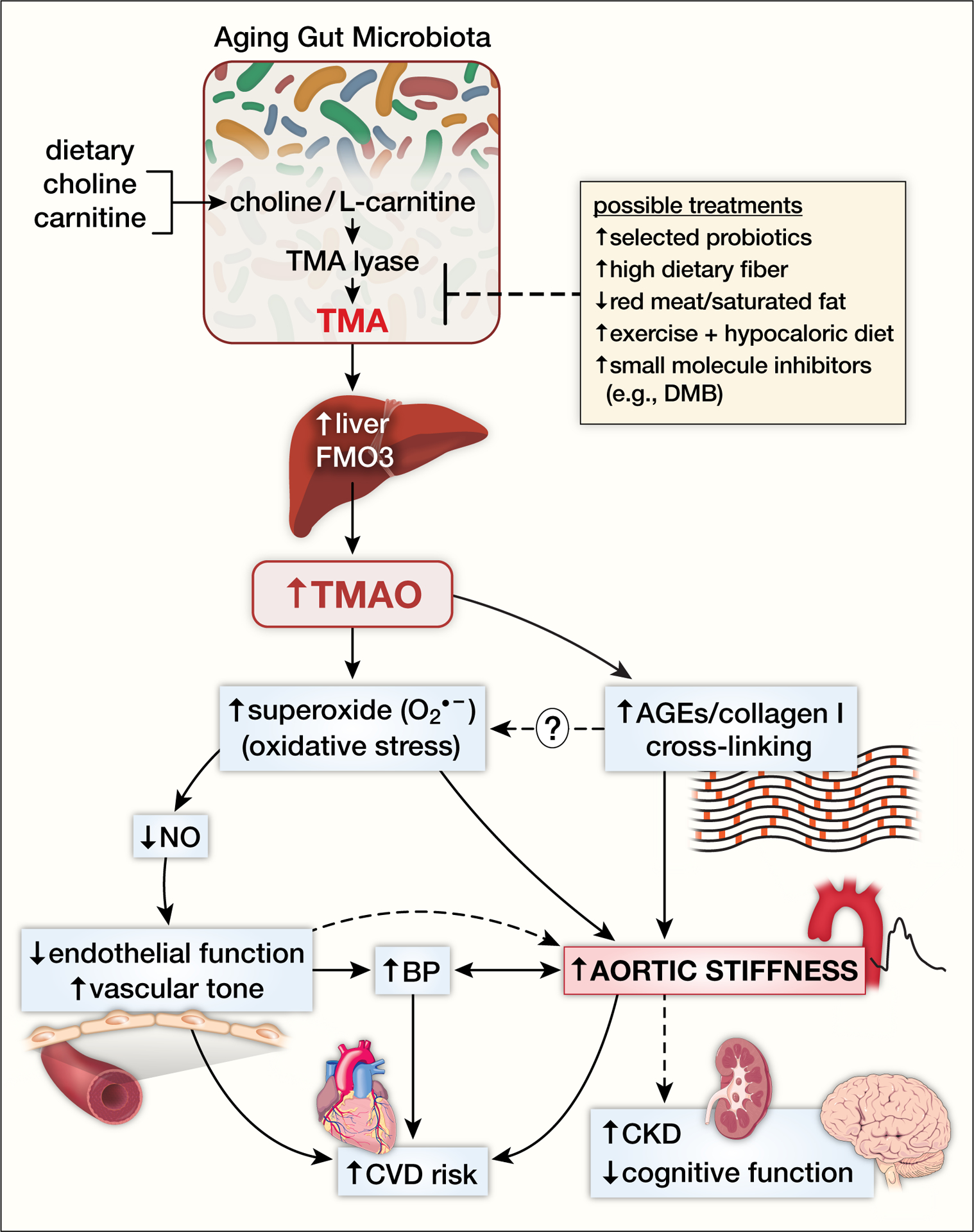
The gut-arterial axis stiffness with aging. Consumption of foods that contain choline and L-carnitine (e.g., red meat, high saturated fat dairy products) are converted to trimethylamine (TMA) by the enzyme TMA lyase by gut microbiota. TMA is released into the portal circulation where it is taken up by the liver and converted to trimethylamine N-oxide (TMAO) by the flavin containing monooxygenase (FMO) enzymes. In particular, FMO3 isoform expression is elevated in preclinical models with aging suggesting augmented conversion of TMA to TMAO. TMAO is then released from the liver into the systemic circulation where it is elevated in aged humans and increases superoxide anion-associated oxidative stress in the arterial wall leading to reductions in nitric oxide (NO)-mediated endothelial function and increases in vascular tone. TMAO-associated increased arterial oxidative stress and advanced glycation end products (AGEs) are associated with formation of cross-links between collagen and other structural proteins in the aortic wall. TMAO-related aortic stiffness is also associated with increases in blood pressure, but the causal direction of the relation is likely bidirectional. Aortic stiffness and endothelial dysfunction are associated with clinical cardiovascular disease (CVD) events in humans, and with the development of chronic kidney disease (CKD) and cognitive dysfunction with aging. Possible treatments to prevent or treat downstream effects of elevated TMAO include strategies that alter gut microbiota production of TMA including select probiotics, dietary fiber, decreased red meat/saturated fat consumption, exercise plus hypocaloric diet and novel small molecule inhibitors of gut-derived metabolites such as 3,3,-dimethyl-1-butanol (DMB).
This results of this interesting study provide insight into a potential new therapeutic target (i.e., TMAO) for attenuating age-related aortic stiffness, a strong risk factor for the development of CVD events and age-related target organ damage to high flow organs, such as the brain and kidney. Previous studies by this group3, 5 and others4 have reported that circulating TMAO is elevated in older adults, but how TMAO is elevated with advancing age is not entirely clear. However, in plasma of older mice, microbiota-derived TMA is not elevated, but liver expression of FMO3, the isoform of the FMO enzyme that converts TMA to TMAO is higher suggesting a greater hepatic capacity to convert TMA to TMAO occurs with aging.8 In addition, Brunt et al. (2020)5 reported that higher TMAO is associated with age-related vascular endothelial dysfunction, an important risk factor in the development and progression of CVD, through an oxidative stress-mediated reduction in NO bioavailability. Because NO-mediated endothelial function contributes in part to functional changes in arterial stiffness, vascular smooth muscle tone may be another mechanism modulating TMAO-associated aortic stiffness (Figure). As mentioned, the authors found higher abundance of AGEs in aortic rings incubated ex vivo with TMAO for 72 hours that resulted in increased intrinsic aortic stiffness. The cross-link breaker alagebrium prevented the increase in the TMAO-induced intrinsic aortic stiffness, but the short time-course of ex vivo incubation makes the finding surprising if AGEs- associated cross-linking is the underlying mechanism. However, there is little evidence that alagebrium disrupts cross-linked proteins and the authors did not provide evidence this occurred. Alternatively, there is some evidence that alagebrium itself has antioxidant properties9 so perhaps the beneficial effect of alagebrium on aortic stiffness in these short-term ex vivo experiments was from its antioxidant properties rather than disruption of cross-links. Taken together, oxidative stress appears to be an important mechanism contributing to this short-term increase in TMAO-induced aortic stiffness with a possible independent role of AGEs and cross-linking of structural proteins, while cross-linking structural proteins and/or other mechanisms may be involved in aortic stiffening from longer exposure to high TMAO with aging.
Perhaps the most obvious question generated from these new findings is whether TMAO is a viable therapeutic target to prevent the development of, or reverse, aortic stiffness in older adults. Brunt et al. (2019) previously demonstrated that suppression of circulating TMAO with broad spectrum antibiotics in old mice was associated with reversal of aortic stiffness (both aortic PWV and intrinsic wall stiffness) and endothelial dysfunction back to levels of young mice.8 However, broad spectrum, chronic antibiotic treatment in humans for CVD prevention is not a feasible strategy because of the risk of the development of antibiotic resistance and clinical trials of antibiotic treatment for CVD in humans have been negative.1 Alternatively, the authors mention several promising dietary strategies that could have more selective effects gut microbiota or TMA lyase on lowering circulating TMAO such as high soluble fiber, vegetarian/vegan diets (removing red meat/saturated fat from diet), and supplementation with some probiotics (Figure).1, 6 In addition, Erickson et al.10 found that 12 weeks of moderate/vigorous aerobic exercise (5 days/week) plus hypocaloric diet (−500 kcal/day) in middle-aged/older adults with obesity, resulted in greater percent reduction in TMAO compared with exercise plus eucaloric diet (Figure). However, whether the TMAO changes in the exercise + hypocaloric group were related to reductions in TMAO-dietary precursors or the negative energy balance could not be determined in this small study. Larger randomized, controlled trials featuring lifestyle interventions are needed to confirm and extend these findings.
Finally, small molecule inhibitors of gut-derived metabolites are being explored as a novel therapeutic strategy to prevent or treat cardiometabolic diseases.2 For example, the compound 3,3-dimethyl-1-butanol (DMB), a structural analog of choline that inhibits microbial TMA lyase enzyme activity resulting in reductions in TMA and TMAO, has shown initial promise in preclinical studies (Figure). In apolipoprotein knock-out mice on a choline- or carnitine- supplemented diet, treatment with DMB resulted in reduced circulating TMAO and attenuated macrophage foam cell formation and atherosclerosis development.11 In another study, DMB treatment of older mice for 8–10 weeks restored NO-mediated endothelium-dependent dilation by reversing vascular oxidative stress.5 Furthermore, exciting work in this area includes the recent development of next generation choline TMA lyase inhibitors, such as fluoromethylcholine, that are more potent than DMB and have limited systemic exposure in the host, but these have not been tested in human trials to date.1 In conclusion, elevated TMAO appears to be novel mechanism contributing to aortic stiffness that deserves further study. In addition to dietary and lifestyle strategies that should be explored further, more studies are needed to determine whether small molecule inhibitors of select gut-derived metabolites such as TMAO hold promise as a potential pharmacological strategy in the prevention of the development CVD with aging.
Sources of funding:
G.L. Pierce is supported by grants from the National Institutes of Health (R01 AG063790) and the American Heart Association (19TPA34910016). S.J. Roy is supported by a National Institutes of Health T32 Fellowship T32 HL007121.
References
- 1.Witkowski M, Weeks TL and Hazen SL. Gut Microbiota and Cardiovascular Disease. Circ Res. 2020;127:553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JM and Hazen SL. Targeting of microbe-derived metabolites to improve human health: The next frontier for drug discovery. J Biol Chem. 2017;292:8560–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt VE, LaRocca TJ, Bazzoni AE, Sapinsley ZJ, Miyamoto-Ditmon J, Gioscia-Ryan RA, Neilson AP, Link CD and Seals DR. The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. Geroscience. 2021;43:377–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM and Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP and Seals DR. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension. 2020;76:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunt VE, Casso AG, Gioscia-Ryan RA, Sapinsley ZJ, Ziemba BP, Clayton ZS, Bazzoni AE, VanDongen NS, Richey JJ, Hutton BF, Zigler MC, Neilson AP, Davy KP and Seals DR. The gut microbiome-derived metabolite trimethylamine N-oxide induces aortic stiffening and increases systolic blood pressure with aging in mice and humans. Hypertension. 2021;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM and Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94. [DOI] [PubMed] [Google Scholar]
- 8.Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, Gonzalez A, Vazquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R and Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol. 2019;597:2361–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, Kwon MK, Huh JY, Choi WJ, Jeong LS, Nagai R, Kim WY, Kim J, Lee GT, Lee HB and Ha H. Renoprotective antioxidant effect of alagebrium in experimental diabetes. Nephrol Dial Transplant. 2011;26:3474–84. [DOI] [PubMed] [Google Scholar]
- 10.Erickson ML, Malin SK, Wang Z, Brown JM, Hazen SL and Kirwan JP. Effects of Lifestyle Intervention on Plasma Trimethylamine N-Oxide in Obese Adults. Nutrients. 2019;11;179; 10.3390/nu11010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ and Hazen SL. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


