Abstract
BACKGROUND:
Historically, cancer has been considered a disease of the cell, caused by mutations in genes that control proliferation, differentiation, and death. In recent decades, however, the microenvironment surrounding the cancer cell has gained notoriety as a coconspirator in tumor initiation, progression, immune evasion, and treatment response. As tumors grow, they disrupt the structure and function of the surrounding tissue via physical and biochemical mechanisms. The resulting physical abnormalities affect both cancer cells and their microenvironment and fuel tumorigenesis and treatment resistance. The links between cancer biology and physics have provided opportunities for the discovery of new drugs and treatment strategies.
ADVANCES:
Here, we propose four distinct physical cancer traits that capture the biomechanical abnormalities in tumors: (i) elevated solid stress, (ii) elevated interstitial fluid pressure, (iii) increased stiffness and altered material properties, and (iv) altered tissue microarchitecture. Solid stresses are created as proliferating and migrating cells push and stretch solid components of the surrounding tissue. Being distinct from fluid pressure and close to zero in most normal tissues, solid stresses are large enough to compress blood and lymphatic vessels in and around tumors, impairing blood flow and the delivery of oxygen, drugs, and immune cells. Acting at organ, tissue, and cellular levels, solid stresses activate signaling pathways that promote tumorigenesis and invasiveness and induce treatment resistance. Elevated interstitial fluid pressure is caused by leakage of plasma from abnormally permeable tumor blood vessels and insufficient lymphatic drainage. As a result, the interstitial fluid leaks out of the tumor into the peritumor tissue, causing edema and elution of drugs and growth factors and facilitating invasion and metastasis through flow-induced shear stresses. Increased stiffness is caused by matrix deposition and remodeling. Traditionally used as a diagnostic marker, and more recently as a prognostic factor, increased stiffness activates signaling pathways that promote proliferation, invasiveness, and metastasis of cancer cells. Finally, when normal tissue architecture is disrupted by cancer growth and invasion, microarchitecture is altered. Stromal and cancer cells and extracellular matrix adopt new organization. This changes the interactions between an individual cell and its surrounding matrix and cells, which affects signaling pathways associated with invasion and metastasis.
OUTLOOK:
The tumor microenvironment is characterized by both biological and physical abnormalities. The growing appreciation of the role of tumor-stromal interactions in cancer has led to seminal discoveries that have resulted in previously unexplored targets and strategies for treatment. Understanding the key principles underlying the origins and consequences of the physical traits of cancer will be critical for improving treatment. Many of the concepts involved are nonintuitive and require deep and broad understanding of both the physical and biological aspects of cancer. Therefore, a rigorous but accessible description of physical cancer traits will assist research into the physical sciences of cancer—a highly multidisciplinary area—and help it remain an active and progressive subfield of cancer research.■
Graphical Abstract
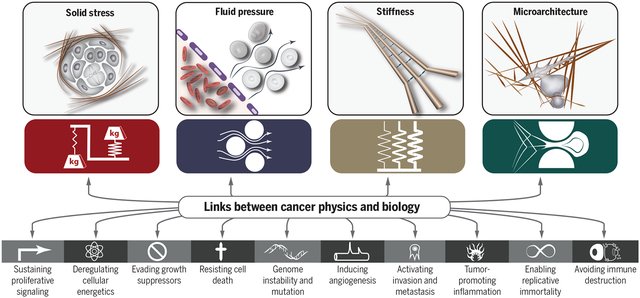
Physical traits of cancer. To provide a comprehensive framework for understanding the links between the physics of cancer and signaling pathways in cancer biology in terms of a small number of underlying principles, we propose four physical traits of cancer that characterize the major physical abnormalities shared by most if not all tumors.
The role of the physical microenvironment in tumor development, progression, metastasis, and treatment is gaining appreciation. The emerging multidisciplinary field of the physical sciences of cancer is now embraced by engineers, physicists, cell biologists, developmental biologists, tumor biologists, and oncologists attempting to understand how physical parameters and processes affect cancer progression and treatment. Discoveries in this field are starting to be translated into new therapeutic strategies for cancer. In this Review, we propose four physical traits of tumors that contribute to tumor progression and treatment resistance: (i) elevated solid stresses (compression and tension), (ii) elevated interstitial fluid pressure, (iii) altered material properties (for example, increased tissue stiffness, which historically has been used to detect cancer by palpation), and (iv) altered physical microarchitecture. After defining these physical traits, we discuss their causes, consequences, and how they complement the biological hallmarks of cancer.
Cancer is generally considered a disease of the cell, caused by mutations in genes that control cell proliferation, death, metabolism, and DNA repair. To create a unified conceptual framework for understanding the various manifestations of cancer, Hanahan and Weinberg proposed eight biological hallmarks that delineate the key features and properties of cancer cells (1). These biological hallmarks are useful for conceptualizing cancer at the cellular level, but we now know that the microenvironment surrounding the cancer cell acts as a coconspirator in tumor initiation and progression. As tumors grow, they disrupt the surrounding tissue biochemically and physically. They also recruit normal cells from the surrounding tissue, which further alter the matrix and cellular compositions of the tumor. These perturbations result in physical abnormalities associated with both cancer cells and the microenvironment in which they grow that influence tumor biology and response to treatment (2).
To provide a more comprehensive conceptual framework for cancer, we propose four additional traits stemming from the physical abnormalities of tumors. These are (i) elevated solid stress, (ii) elevated interstitial fluid pressure (IFP) and the resulting fluid flow in the interstitium, (iii) increased stiffness and altered material properties, and (iv) altered microarchitecture (Fig. 1). As discussed in this Review, these four physical traits are conceptually distinct but can interact synergistically. They also enable and exacerbate many of the biological hallmarks of cancer, thus facilitating cancer cell proliferation and invasion, immune system evasion, and resistance to therapies.
Fig. 1. Physical traits of cancer.
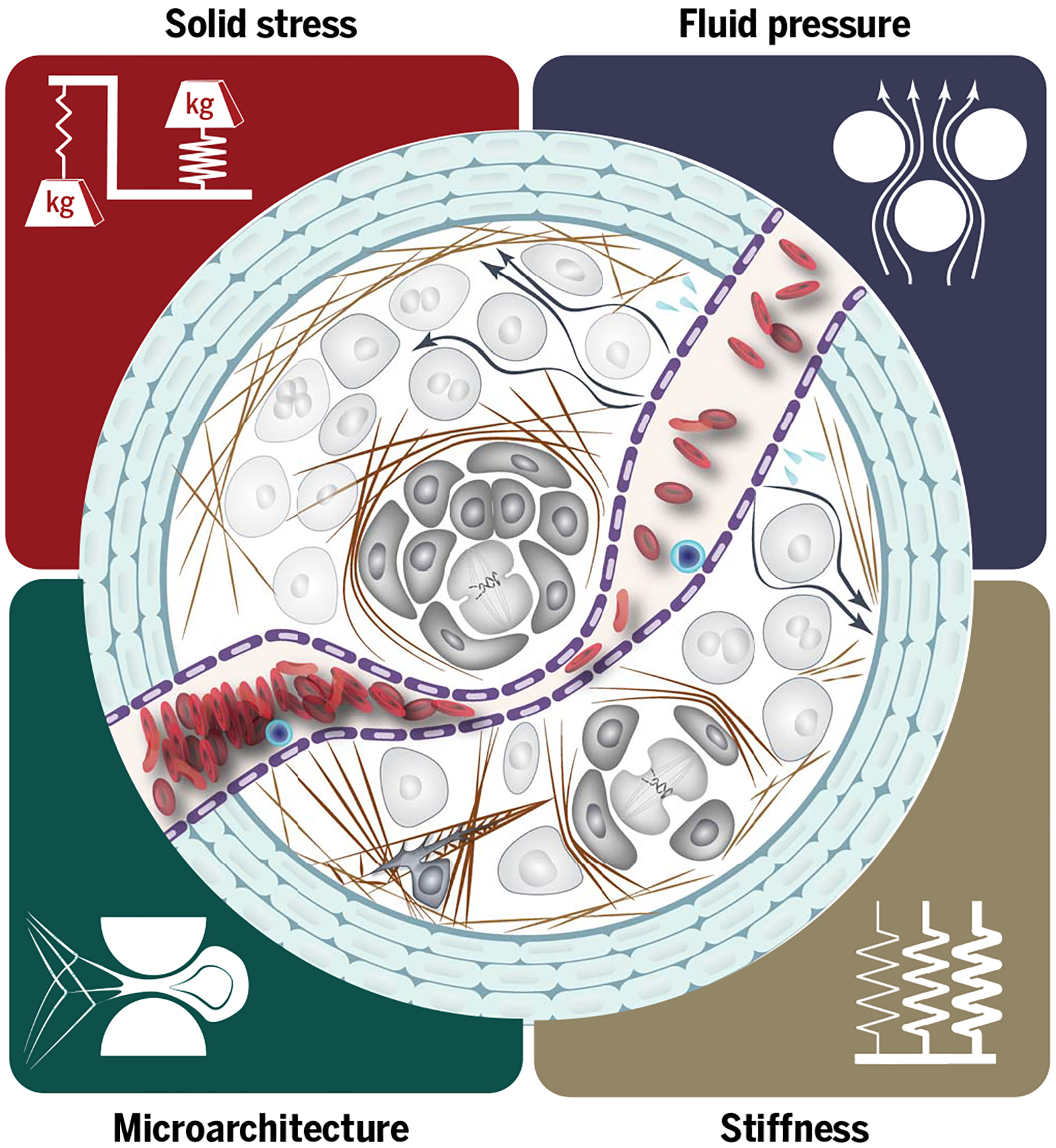
On the basis of the advancements of the past few decades, we suggest that the physical traits of cancer can be categorized into four major groups: (i) elevated solid stress, (ii) elevated interstitial pressure, (iii) increased stiffness, and (iv) altered architecture and geometry. Solid stresses and fluid pressure are the mechanical stresses (force per unit area) contained in, and transmitted by, solid and fluid phases of the tumor, respectively. Solid stresses and fluid pressure are reported in pascals or millimeters of mercury (1 mmHg ≅ 133.3 Pa). Stiffness (elasticity) is defined as the resistance of a material to deformation in response to an applied force, and elastic modulus is reported in pascals. Viscoelasticity defines the resistance of the material to deformation in response to a force applied at a given rate. Most soft tissues, including tumors, exhibit higher resistance to force (e.g., higher stiffness) when the force is applied at high rates. Solid stress, the latent or stored stress in a tissue, should not be confused with elasticity (stiffness) or viscoelasticity (time-dependent stiffness), which define how much or how fast, respectively, a tissue will deform if a force is applied. A tissue can be stiff (rigid) or soft (compliant), and, independently, it can be under compressive and/or tensile solid stresses (4) or, like most normal tissues, it can be unstressed. The proposed physical traits characterize most cancers, and their distinct origins and consequences make them indispensable to a comprehensive picture of cancer.
Solid stresses and elastic energy
Solid stresses, also known as residual stresses, are the mechanical forces (compressive, tensile, and shear) contained in—and transmitted by—solid and elastic elements of the extracellular matrix (ECM) and cells (3). Reported in pascals or millimeters of mercury (1 mmHg ≅ 133.3 Pa), solid stress values range from <100 Pa (0.7 mmHg) in glioblastomas to 10,000 Pa (75 mmHg) in pancreatic ductal adenocarcinomas (PDACs) (4). Multiple mechanisms, summarized in Fig. 2 and discussed below, are responsible for generating solid stress in tumors.
Fig. 2. Origins of the physical traits of cancer.
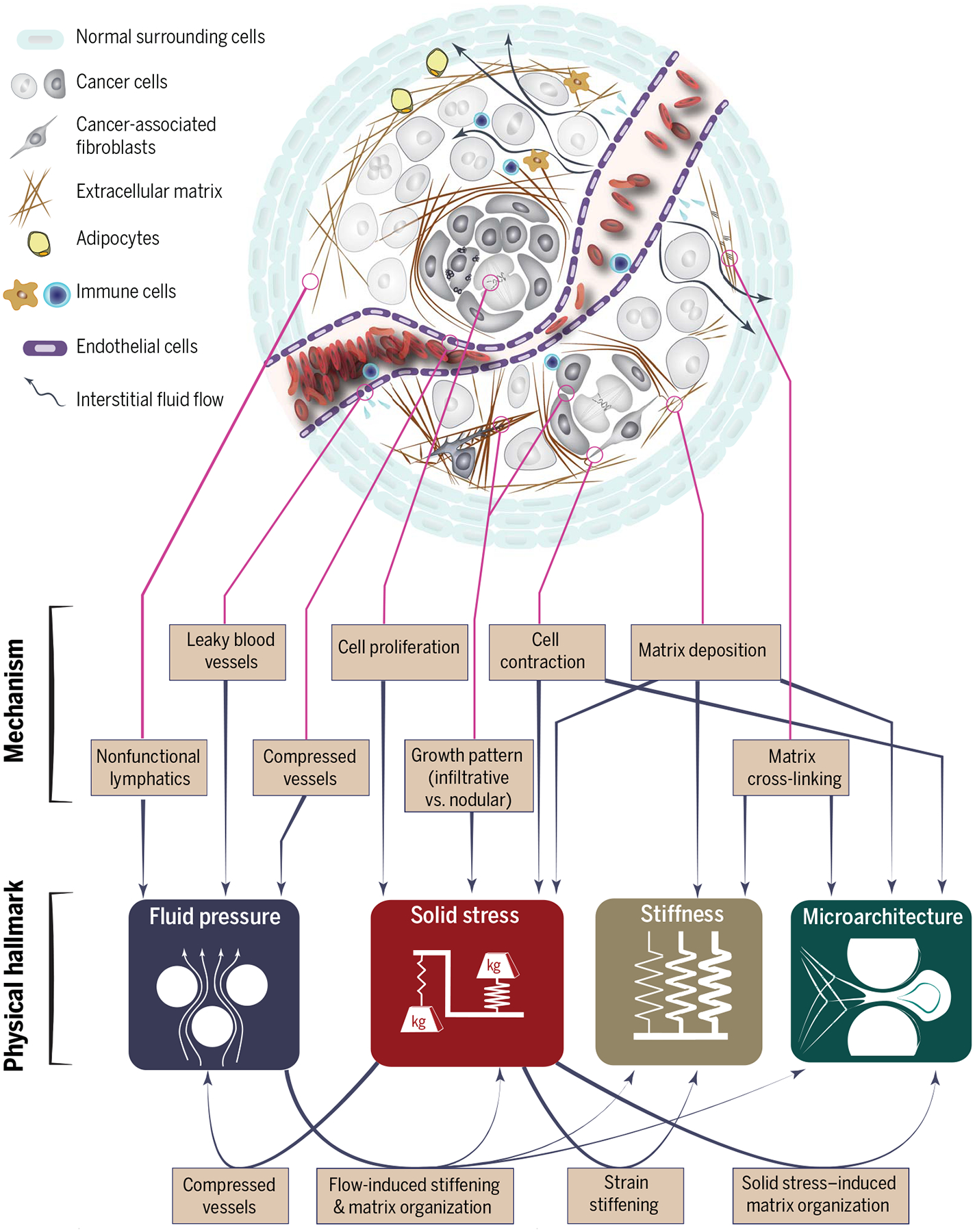
Physical interactions of cancer cells with stroma give rise to physical traits of tumors through distinct and interconnected mechanisms. Leaky and compressed blood vessels and nonfunctional lymphatics lead to increased interstitial fluid pressure within the tumor and interstitial fluid flow in the tumor margin. Cellular proliferation, matrix deposition, cell contraction, and abnormal growth patterns lead to compressive and tensile solid stresses. Matrix deposition and cross-linking cause increased stiffness in tumors. Cell contraction, matrix deposition, and cross-linking also alter the architecture of the tissue. The physical traits also interact with each other; solid stresses compress blood and lymphatic vessels and contribute to increased fluid pressure in tumors. Tensile solid stresses result in stretched and aligned matrix, and through strain-stiffening, solid stresses also increase tumor stiffness. Fluid flow activates fibroblasts, which then contribute to increased solid stresses and stiffness values and alter ECM architecture.
1) Increased tissue volume caused by cell infiltration, cell proliferation, and matrix deposition. The added volume pushes and displaces existing viscoelastic structures inside and out-side the tumor and gives rise to solid stresses in the tumor and the surrounding tissue (4, 5). As a result, when tumor cells are depleted through anticancer therapeutics, solid stress is decreased, and blood vessels are decompressed (6, 7).
2) Concerted displacement of normal tissue (8, 9). Some tumors grow as well-circumscribed, nodular masses, in which the tumor remains cohesive and pushes the surrounding tissue, generating considerable mechanical stresses. Other tumors are less cohesive and more infiltrative, interdigitating through the normal tissue by finding the path of least resistance or by creating space by virtue of cytotoxic and protease activities. In the latter case, there is less production of solid stress (5, 10).
3) Swelling of existing glycosaminoglycan matrix components such as hyaluronic acid (HA) owing to (electro)osmotic water absorption (11, 12). These components take up available water and swell, generating solid stress that is distinct from fluid pressure (12).
4) Actomyosin-mediated cell contractions. Fibroblasts, immune cells, and cancer cells can all contract matrix elements as they move around in a tumor or try to repair structural damage. Cell contraction generates tensile forces that contract ECM components (13), creating tension in some parts of the tumor, which are generally balanced by compression in other elements (4). Cancer-associated fibroblasts (CAFs) that are activated with transforming growth factor–β (TGF-β) become myofibroblasts and can generate large contractile forces (14).
The impact of solid stress on cancer cell biology was first recognized in 1997, when Helmlinger et al. found that accumulated solid stress inhibits the growth of tumor spheroids (3). These stresses are sufficiently large to compress and even collapse blood and lymphatic vessels (6, 7, 15). Vessel compression contributes to hypoxia (15, 16) and interferes with the delivery and/or efficacy of chemo-, radio-, and immunotherapies (17, 18). Solid stress may also have additional, direct effects on tumor biology, such as promoting the invasiveness of cancer cells (19) and stimulating tumorigenic pathways in colon epithelia (20) (Fig. 3).
Fig. 3. Pathways associated with the physical traits of cancer.
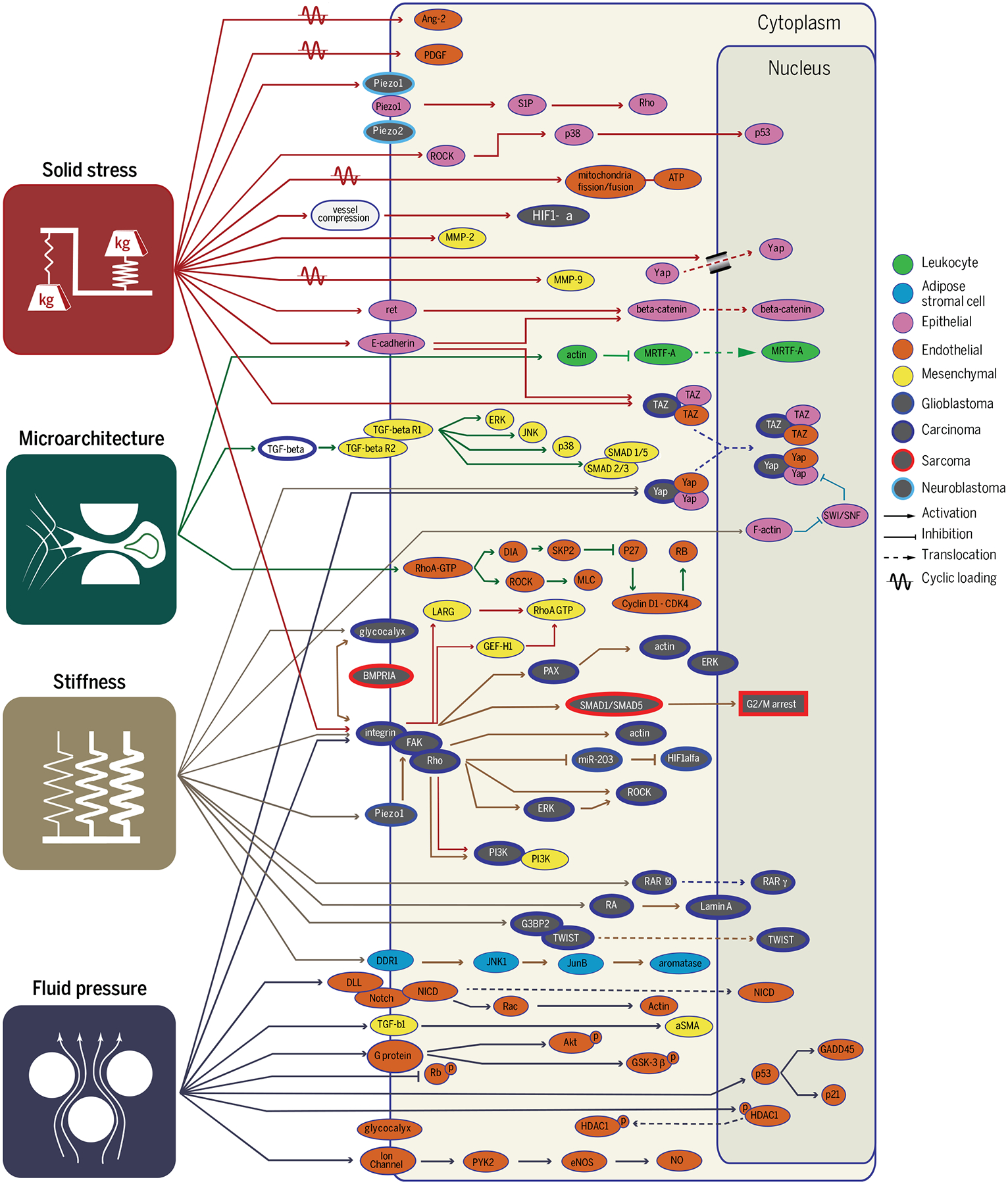
Physical traits of cancer activate a large cascade of mechanoresponsive pathways in cancer cells and stromal cells, including endothelial, epithelial, mesenchymal, and immune cells. Pathways such as integrin and YAP/TAZ are responsive to all four physical traits, whereas many other pathways appear to be more specific.
Cancer and normal cells have mechanosensitive machinery, such as cell-ECM (21) and cell-cell (22) adhesions and stretch-sensitive ion channels (23), that allows them to respond to applied forces. Solid stresses can also act on cells indirectly by deforming ECM components. For example, matrix-bound latent TGF-β, in-active upon synthesis and unable to bind to its cognate cell surface receptors, can be activated by myofibroblast-induced tensile forces on ECM (24). Other examples of ECM sensitivity to tensile forces include the unfolding of fibronectin in response to tensile forces (25), the enzymatic resistance of collagen fibers (26), and the tension-regulated interactions of fibronectin with collagen fibers (27).
The nucleus is also a mechanosensitive organelle capable of responding to solid stresses through the activity of nuclear pore complexes and associated proteins, which modulate the nuclear import of transcription factors when the nucleus is deformed (28–30). Nuclear perturbations, such as those generated by cells migrating through small pores, cause changes in gene expression and induction of DNA repair programs (31, 32).
YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif) have been identified as potent mechanoresponsive factors (33) that respond to physical cues, such as stretching (22, 34) and cell crowding (34), by translocating from the cytoplasm to the nucleus. YAP/TAZ mechanobiology is regulated by filamentous actin (F-actin) dynamics through Rho guanosine triphosphatases (GTPases), which probe the physical microenvironment via cell-matrix and cell-cell adhesion complexes at the cell surface (35). Thus, mechanosignaling by YAP and TAZ can be modulated by modifiers of actin and Rho GTPases such as cofilin, gelsolin, and F-actin–capping protein (CAP-Z). In a two-dimensional (2D) epithelial monolayer model of stretch, cells under tensile stresses showed activation of YAP/TAZ that led to cell proliferation (34) and induced cell cycle entry (22). Activation of the YAP/TAZ pathway contributes to tumor malignancy in many ways, including cell proliferation, cell cycle regulation, overcoming anoikis and mitochondria-induced apoptosis, inducing cancer stem cell functions, and accelerating fibrosis and desmoplasia by activating CAFs (36).
As cancer growth causes crowding of cells in the tissue, there is inevitably competition between cell populations for nutrients and free space. Cell competition has recently received attention because of its relevance in development, where it controls organ size and eliminates suboptimal cells (37), and in tumorigenesis, where cancer cells expand into new space by damaging and killing the normal surrounding cells (38). Compressive and tensile stresses, generated from the differential growth of cell layers, has been suggested to be a mechanical driver of cell competition (39). How cancer cells are able to outcompete the surrounding normal cells, which experience similar solid stresses at the tumor-host interface, remains an open question.
Because solid stresses are harbored in matrix components, many of the resulting problems can be reversed by drugs that degrade matrix components and reduce fibrosis. For example, losartan, an angiotensin receptor 1 blocker, reduces both collagen I and HA by inhibiting TGF-β (16). In preclinical models of PDAC, losartan alleviates solid stress and decompresses blood vessels, enhancing chemotherapy and increasing overall survival (16). This strategy is currently being tested in a randomized clinical trial (NCT01821729) based on promising results of a phase 2 trial (40). In another successful PDAC preclinical study, PEGPH20 (a pegylated recombinant human hyaluronidase) that reduced fibrosis in these tumors increased overall survival when combined with chemotherapy (41). Other approaches that have shown similar potential in preclinical models include inhibiting the vitamin D receptor (42), sonic hedgehog signaling (43), and C-X-C motif chemokine receptor 4 signaling (44). Targeting the vitamin D receptor is currently being tested in patients (NCT03472833). However, both PEGPH20 and sonic hedgehog targeting have been unsuccessful in clinical trials (45), highlighting the need for a deeper understanding of these pathways. Alleviating stress using these approaches may improve response to various treatments, such as immunotherapy (44, 46).
Interstitial fluid pressure
In most organs, the blood arrives via arteries and leaves via veins, and any excess tissue fluid is drained by lymphatic vessels. This maintains fluid homeostasis and results in near-zero IFP in most normal organs. This balance is disturbed by abnormalities in tumors, including hyperpermeable blood vessels and compression of blood and lymphatic vessels by solid stress. Leaky vessels, combined with a compromised drainage system, result in high IFP (Fig. 2), ranging from <1 kPa (7.5 mmHg) in brain tumors to 5 kPa (37 mmHg) in renal cell carcinomas. IFP is fairly uniform within a tumor and drops precipitously in the tumor margin, which generates a fluid flow toward lymphatic vessels in the surrounding normal tissue, where IFP is close to 0 mmHg. Note that IFP and solid stress are independent mechanical stresses with distinct origins and consequences (47).
High IFP in tumors was first reported in 1950 (48) and then later studied in detail through experiments and computational models (49, 50). Elevated fluid pressure drives interstitial flow in the tumor margin, exposing extravascular cells to shear stress. Because flow velocity and shear stresses depend strongly on the pore size between cells and matrix components, shear stresses likely vary widely, even along individual cell membranes. The shear stresses affect the biology of cancer and stromal cells in several ways (51) (Fig. 3), including activation of fibroblasts (51); modulation of endothelial sprouting (52), which affects angiogenesis and lymphangiogenesis (51); induction of matrix metalloproteinase (MMP) activity and cell motility (53); and activation of cancer cell migration (54) and invasion (55). Fluid flow has also been shown to induce cell cycle arrest through integrin signaling (56). Because immune cells are also responsive to interstitial flow, these fluid forces are likely also involved in regulation of immunity (51). Mechanisms for mechanotransduction of flow signals include sensors within the focal adhesions (54, 57), the cell glycocalyx (55), cell-cell junctions (58, 59), ion channels (60), Notch receptor (61), and cilia (62). The resulting signals can up-regulate TGF-β expression and activate YAP/TAZ down-stream pathways (51, 63, 64).
In addition to direct mechanotransduction mechanisms, fluid flow created by IFP gradients can affect tumor progression and treatment response in multiple ways. High IFP hinders the convection of drugs from the vasculature into the bulk of the tumor (2, 49). Moreover, the steep IFP gradient at the tumor boundary drives the flow of interstitial fluid from the tumor toward the surrounding tissue. This flow can promote tumor invasion and growth by facilitating the transport of growth factors and cancer cells into the surrounding normal tissue and peritumor lymphatics (65). The outward fluid flow may also facilitate angiogenesis in the tumor margin (52) and remove therapeutic agents from the tumor, reducing drug retention times (65). IFP has also been proposed as a diagnostic marker differentiating malignant from benign breast, head, and neck tumors (66, 67) and as a prognostic marker in some clinical studies (68).
Therapeutic strategies for correcting the fluid abnormalities in tumors have also been developed. One approach is to normalize the leaky and tortuous vasculature so that the intraluminal pressure operating within microvessels is not transmitted directly to the surrounding interstitium. Vascular normalization restores abnormal tumor vasculature to a more functional state closer to that of normal vessels. Using judicious doses of antiangiogenic therapy to normalize tumor vasculature (17, 65, 69), it is possible to increase pericyte coverage, decrease vessel leakiness, increase tumor vascular perfusion, and decrease IFP. In the clinic, there are many agents with the ability to normalize vessels, including bevacizumab, an antibody that blocks vascular endothelial growth factor A (VEGF-A) and inhibitors of VEGF receptor tyrosine kinases, which have been approved for more than a dozen tumor types (70). As mentioned in the previous section, accumulation of solid stress also disrupts the vascular flow in tumors by compressing the more fragile outflow vessels (veins and lymphatics), which contributes to the elevated IFP. Therefore, alleviating solid stress can also decompress blood and lymphatic vessels, resulting in better perfusion and more normal levels of IFP (7).
Stiffness (elasticity)
Stiffness, also known as rigidity or elastic modulus, is defined as the resistance of a material to deformation in response to a force applied at a very slow rate (quasi-statically). Stiffness is an intrinsic material property of the tissue—unlike solid or fluid mechanical stresses, which describe forces exerted on a material—and ranges from 1 kPa in brain tumors to 70 kPa in cholangiocarcinomas (2).
Increased tissue stiffness is the most tangible and best-recognized mechanical abnormality in tumors. Stiffness has traditionally been used as a diagnostic marker (71) and more recently as a prognostic factor (72, 73). In multiple cancer types, including breast (74), pancreatic (75), liver (76), and prostate (77), malignant tumors have been shown to be considerably stiffer than benign tumors. In 2006, stiffness sensing was implicated in determining cell lineage (78). Today, there are numerous studies showing how the material properties—in particular, the stiffness of the microenvironment—are central to many traits of cancer (79), including proliferation (80, 81), angiogenesis (82), metabolism (83), invasion (84–86), and migration and metastasis (87–89) (Fig. 3).
Stiffening promotes tumor progression in many tumor types, including breast (80, 90), pancreatic (85, 91), colorectal (92), and brain (93). Increased stiffness can also promote an invasive phenotype in cancer cells (84–86), induce invasion and metastasis (87, 88, 94, 95), enhance immune cell infiltration (90), facilitate the epithelial-mesenchymal transition through TGF-β (96), promote stem cell differentiation (97), alter growth factor secretion and signaling, and increase angiogenesis and vessel permeability (82).
One of the primary causes of matrix stiffening is increased deposition and cross-linking of ECM (Fig. 2). In fibrotic tumors, CAFs are primarily responsible for collagen production, and these cells have more actin stress fibers, alpha smooth muscle actin, and focal adhesions than nonactivated fibroblasts (14, 98). Collagen fibers can be cross-linked to different degrees by lysyl oxidase, and more cross-linking increases ECM stiffness. Transglutaminase 2, a calcium ion–dependent enzyme abundantly expressed by pancreatic cancer cells, also contributes to covalent collagen cross-linking and, consequently, activation of fibroblasts in pancreatic cancer (99). Increased ECM stiffness and TGF-β signaling activate fibroblasts to become CAFs, initiating a positive feedback loop that further enhances ECM stiffening. The profibrotic activation of cells in response to substrate stiffness can be perpetuated by mechanical memory, with microRNA 21 serving as one of the memory keepers (100).
Mechanical stresses can also alter the stiffness of the matrix through a phenomenon called strain-stiffening (101). Some collagen fibers are under tension, either because of cell contraction (102, 103) or because tumor growth causes local expansion, stretching the ECM (4). These tensile stresses increase the stiffness of the collagen network, which in turn further activates the focal adhesion contractility of the CAFs in their vicinity (104), leading to a vicious cycle of matrix deposition and stiffening. Matrix contraction by myofibroblasts is related to wound contraction, which resolves in wound healing but not in tumors (105, 106). In addition to stretching, the compressive stresses produced by tumor growth (5) can increase the stiffness of both normal and tumor tissue, as demonstrated with normal brain and glioma tissue (107). Strain-stiffening also happens at the subcellular level; mechanical stretch applied to nuclei can increase the stiffness of the nucleus through phosphorylation of emerin, one of the nuclear envelope proteins that provides structural stability to the nucleus (108).
There is considerable evidence that increased stiffness in breast tissue is associated with higher risk of breast cancer (72), and mammo-graphic density (which is related to tissue stiffness and density) has been proposed as a predictor of poor survival (73, 109). In PDAC, increased stiffness negatively correlates with the response to chemotherapy (91). Consistently, in lung adenocarcinoma tumors, the stiffness of regions with dense ECM increases with tumor stage. Interestingly, however, the stiffness of the cells showed an inverse relationship with tumor stage (110), suggesting that cytoskeleton stiffness, in addition to ECM stiffness, can be used to stage lung tumors.
Two major pathways that are sensitive to changes in stiffness are focal adhesion kinase (FAK), which is induced through integrin ligation (111), and the Hippo pathway transcription factors YAP and TAZ (112, 113). YAP activated by increased ECM stiffness through Rho-associated protein kinase (ROCK), myosin, and Src activation further promotes CAF activity, which fuels a feed-forward self-enhancing loop to maintain the CAF phenotype (113).
Matrix stiffness and tumor cell metabolism are interdependent (83, 114). The cell metabolic rate increases when cells migrate on stiff substrates or through confined spaces (115). On the other hand, targeting abnormal tumor metabolism with metformin, an agonist of adenosine monophosphate–activated protein kinase, reduces fibrosis and stiffness (83, 116). Although increased tumor stiffness increases the malignancy of tumors, there may also be opportunities to take advantage of the increased stiffness by developing mechanosensitive treatments (117). Other targets for reversing fibrosis include the angiotensin system, which modulates CAF activity through the TGF-β and CTGF signaling pathways, contributing to fibrosis (16). ECM components can also be targeted directly, for example, by enzymatic depletion of HA and/or collagenase (2).
Matrix architecture and cell geometry
Organs are constructed of collections of cells and matrix components arranged with specific microarchitecture, which has evolved to optimize the stability, efficiency, and function of the tissue. For example, gut epithelium exists in a 2D sheet, with the basement membrane on one side and the lumenal space on the other. This asymmetric arrangement, or polarization, allows the cell to respond to fluid forces on the luminal side, monitor the basement membrane and the underlying tissue through focal adhesions, and sense any changes in the neighboring cells through cadherin adhesions and gap junctions. Other cells require different microanatomy; for example, myocytes form aligned bundles in muscle tissue, and neurons exist as an interconnected linear network embedded in other tissues. These structures form during development, and in adult tissue, the immediate microenvironment (composition and geometry) of each cell serves as a cue for homeostasis or transformation and morphogenesis. For example, endothelial morphogenesis is triggered when blood flow stops and thrombosis fills the blood vessel lumen, resulting in vasculogenesis or angiogenesis. This process is, in part, induced by the lack of blood shear stresses and by the presence of the intravascular fibrin contacting the luminal side of the endothelial cells.
As tumors grow, both tumor and associated normal tissues are structurally disrupted in an ongoing, dynamic process that disturbs homeostasis. Cell overcrowding, protease activity, and changes in matrix production can all alter cell-matrix and cell-cell associations, signaling morphogenesis. Indeed, the much-studied epithelial-to-mesenchymal transition is an example of a morphogenic switch of cells from a 2D epithelial, surface-dependent geometry to a mesenchymal, infiltrative phenotype, where the cell is now comfortably surrounded by ECM.
The local tissue architecture plays a central role in cancer progression and treatment response, independent of the solid stress, fluid forces, and stiffness of the microenvironment (Fig. 3). A simple and familiar demonstration of the importance of architecture is surface-dependent growth. Normal and cancerous breast epithelial cells have drastically different morphology and growth rates when cultured in 3D matrices but are difficult to distinguish when cultured on 2D surfaces (118). This is an example of the dynamic reciprocity between tissue architecture, function, and neoplastic transformation, as cells not only create their environment but are also affected by it. Studies of 3D matrix architecture, mainly focused on collagen, show that collagen organization can be a prognostic biomarker (119) and that certain arrangements facilitate cancer cell migration, proliferation, and actomyosin contractility (120, 121). Studies designed to recapitulate various matrix architectures show that Rho/ROCK-mediated matrix alignment is a key step that promotes cancer cell migration in the early stages of invasion (122). Collagen alignment also modulates MMP-dependent mechanisms (123) and integrin β1 expression (124), which affect cells’ ability to migrate.
Many important discoveries regarding the influence of cell microenvironment on phenotype come from carefully designed in vitro studies. One of the earliest studies linking cell geometry to biological responses was reported by Folkman and Moscona in 1978 (125); they showed that DNA synthesis decreases as cell-substrate contact area is reduced. In another seminal study, Chen et al. were able to control growth and viability by confining cells to micropatterned islands, which controlled the extent of cell spreading while maintaining the total cell-substrate contact area (126). This model system has later been extended to micro- and nanopillar substrate systems (127) that allow specification of cell contact area in addition to substrate stiffness by varying pillar length, width, and spacing. Mechanistic studies using these model systems showed that cell proliferation is regulated by cell shape through two major mechanisms: (i) by regulating the cellular contractility through myosin light chain phosphorylation via ROCK (127, 128), and (ii) by phosphorylation of retinoblastoma protein (RB) (129). In addition to cell shape, there is recent evidence that cell volume can also affect cell response, including cell stiffness and stem cell fate, through water efflux (130). Surface features also affect the migration of cells, through a process called topotaxis (131).
Cell geometry also influences nuclear geometry, which can control gene expression (28, 29). Cells spread on a substrate have a more flattened nucleus than the same cells in 3D culture. Such shape dynamics and nuclear deformations affect perinuclear actin and micro-tubule networks (132), resulting in an altered arrangement of chromosomes, changes in gene expression, and YAP/TAZ nucleus translocation (34, 112). Cell and nuclear geometries are also altered during cell migration through constrictions. Migration of cancer cells (133–135), leukocytes (135), and primary mesenchymal stem cells (134) through pores smaller than their nucleus diameter results in severe compression and deformation of the nucleus, leading to loss of integrity of the nuclear envelope, herniation of chromatin via rupture through the nuclear membrane, and eventually DNA double-strand breaks (133–135), chromosomal aberration, and genomic instabilities (134).
The architecture of the local environment can also drastically affect migration (136). When cells are confined such that the plasma membrane experiences deformation, intercellular calcium ions are allowed to enter the cytoplasm through the stretch-activated ion channel Piezo1. This causes suppression of protein kinase A activity, which regulates migration of carcinoma cells via RhoA and Rac1 (137).
In addition to affecting cell migration, pore size and microarchitecture of the ECM determine the diffusion and convection of cytokines and therapeutic reagents. The key relevant parameters include pore size of vasculature for intravascular transport (138); pore size, charge, and orientation of ECM constituents (139, 140) for interstitial transport; and size, shape (e.g., spherical versus rod shape), and surface chemistry (e.g., cationic versus anionic) of the therapeutic reagents (141).
Outlook
The tumor microenvironment is aberrant both biologically and physically. The growing appreciation of the role of the physical microenvironment in cancer has led to several discoveries about the origins and consequences of the physical traits, which have resulted in new targets and treatment strategies in patients. Close collaboration between cancer biologists, clinicians, physical scientists, engineers, and data scientists will be required to ensure that research into the physical sciences of cancer—a highly multidisciplinary area—remains an active and progressive subfield of cancer research. Many of the concepts at play are nonintuitive and require rigorous and broad understanding of both the physical and biological aspects of cancer.
Continued growth of this subfield will require overcoming a number of challenges. As these proposed physical traits have received less research attention than their biological hallmark counterparts, the available tools for studying them are limited. Thus, more and improved in vivo and in vitro model systems are needed to recapitulate and study tumor physical abnormalities. Better model systems will aid in the discovery of solid stress–responsive pathways and delineate the biological consequences of solid stress from other traits, for example, increased stiffness. Similarly, additional measurement tools are needed to distinguish different causes of solid stress. Delineating the contribution of different factors to the accumulation of solid stress, such as cell-cell and cell-matrix interactions, is an unmet need with potential for revealing additional therapeutic targets for reducing solid stress in tumors and normalizing the tumor physical microenvironment.
While malignant and benign tumors have been shown to differ in stiffness (74–77) and IFP (66), similar comparative evaluations of other physical traits, specifically solid stress and microarchitecture, are lacking. Further-more, we know little about the origins of the differences in any of the physical traits of cancer in benign versus malignant tumors. Uncovering the potential mechanisms, such as infiltration of stroma, mutational loads, contractility of cancer and stromal cells, and collagen shell separating tumor stroma, should help researchers better understand the causes and consequences of physical cancer traits and their bidirectional links with the biological hallmarks of cancer.
Another promising area of research is the role that physical traits of cancer play in cancer cell biology at different stages of tumorigenesis, from early tumor formation to transformation, local invasion of basement membrane, and then dissemination and colonization at a distant site. The contribution and responsiveness of cancer cells to physical traits of cancer may vary at different stages of tumorigenesis and with different genetic aberrations. These factors may explain why certain transformed cells lose responsiveness to substrate stiffness (142), while other studies have shown that substrate stiffness promotes proliferation of cancer cells (80, 81).
Although it may appear that the physical traits of cancer discussed in this Review are specific to solid cancers, there is increasing evidence that they may also contribute to the progression and treatment response of hematological cancers. Phenomena such as swelling of lymph nodes, spleen, and even the liver subject both cancerous cells and normal immune cells to abnormal mechanical forces, which have yet to be studied in depth but may have important consequences on cell biology and anticancer immunity. Overcrowding of cells in the confined spaces of the vasculature and bone marrow results in a microenvironment with limited oxygen and nutrients and potentially important physical considerations that are currently unexplored. For example, in multiple myeloma (143), patients experience bone pain and spinal cord compression due to proliferation of cancer cells in confined spaces near nerves. The bone marrow niche—the origin of most liquid and hematopoietic cancers—represents a distinctive mechanical environment consisting of viscoelastic tissue bathed in flowing fluid and surrounded by bone (144). The role of the physical properties of the bone marrow niche has recently gained attention: ECM stiffness was shown to alter the proliferation and treatment response of myeloid leukemia in an in vitro model (145). The physical properties of the bone marrow also determine drug delivery and the progression and invasion of liquid cancers (146). Finally, similar to carcinoma and sarcoma cells, hematologic cancer cells are also subjected to shear stress in systemic circulation.
Despite numerous studies on the role of physical cancer traits in the progression and initial treatment response of several tumor types, recurrence and secondary treatment resistance have not been studied in depth in association with the physical tumor microenvironment. However, there is early evidence linking the physical traits to the recurrence of cancer; in a mouse model of breast cancer, compliant tumors had higher rates of recurrence than stiffer counterparts (147). In a clinical study of 175 patients with hepatocellular carcinoma (HCC), the stiffness of the spleen was an independent predictor of tumor recurrence (148), and in another study, recurrence rates of HCC after thermal ablation correlated with tissue stiffness (149). These limited but promising studies highlight the need for more thorough investigations of the role of physical traits of cancer in recurrence and secondary treatment resistance. Finally, emerging data indicate that obesity increases the incidence of cancer, aids tumor progression, impairs treatment response, and facilitates tumor recurrence, but that physical exercise can ameliorate many of these adverse consequences of obesity. How obesity and physical exercise differentially affect the physical traits of cancer may reveal previously unexplored ways to slow tumor progression and improve treatment response (116, 150).
ACKNOWLEDGMENTS
Funding:
NIH grants R01-CA208205 and U01-CA 224348, Outstanding Investigator Award R35-CA197743, and grants from the National Foundation for Cancer Research, Jane’s Trust Foundation, American Medical Research Foundation, and Ludwig Cancer Center at Harvard (R.K.J.); NIH R01-CA2044949 (L.L.M.); F32-CA216944, Johnson and Johnson Cancer Initiative, Boston University Dean’s Catalyst Award, and American Cancer Society Pilot Grant (H.T.N.).
Competing interests:
R.K.J. received an honorarium from Amgen; consultant fees from Merck, Ophthotech, Pfizer, SPARC, and SynDevRx; owns equity in Enlight, Ophthotech, and SynDevRx; and serves on the board of trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. L.L.M. owns equity in Bayer AG. Neither any reagent nor any funding from these organizations was used in this study.
Footnotes
The list of author affiliations is available in the full article online.
REFERENCES AND NOTES
- 1.Hanahan D, Weinberg RA, Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). doi: 10.1016/j.cell.2011.02.013; [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, Martin JD, Stylianopoulos T, The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng 16, 321–346 (2014). doi: 10.1146/annurev-bioeng-071813-105259; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK, Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol 15, 778–783 (1997). doi: 10.1038/nbt0897-778; [DOI] [PubMed] [Google Scholar]
- 4.Nia HT et al. , Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng 1, 0004 (2016). doi: 10.1038/s41551-016-0004; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seano G et al. , Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng 3, 230–245 (2019). doi: 10.1038/s41551-018-0334-7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padera TP et al. , Cancer cells compress intratumour vessels. Nature 427, 695 (2004). doi: 10.1038/427695a; [DOI] [PubMed] [Google Scholar]
- 7.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK, Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: Clinical implications. Cancer Res. 59, 3776–3782 (1999). [PubMed] [Google Scholar]
- 8.Heisenberg CP, Bellaïche Y, Forces in tissue morphogenesis and patterning. Cell 153, 948–962 (2013). doi: 10.1016/j.cell.2013.05.008; [DOI] [PubMed] [Google Scholar]
- 9.Irvine KD, Shraiman BI, Mechanical control of growth: Ideas, facts and challenges. Development 144, 4238–4248 (2017). doi: 10.1242/dev.151902; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández Moro C, Bozóky B, Gerling M, Growth patterns of colorectal cancer liver metastases and their impact on prognosis: A systematic review. BMJ Open Gastroenterol. 5, e000217 (2018). doi: 10.1136/bmjgast-2018-000217; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voutouri C, Polydorou C, Papageorgis P, Gkretsi V, Stylianopoulos T, Hyaluronan-derived swelling of solid tumors, the contribution of collagen and cancer cells, and implications for cancer therapy. Neoplasia 18, 732–741 (2016). doi: 10.1016/j.neo.2016.10.001; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grodzinsky AJ, Fields, Forces, and Flows in Biological Systems (Garland Science, 2011), chap. 4, pp. 139–173. [Google Scholar]
- 13.Simon DD, Horgan CO, Humphrey JD, Mechanical restrictions on biological responses by adherent cells within collagen gels. J. Mech. Behav. Biomed. Mater 14, 216–226 (2012). doi: 10.1016/j.jmbbm.2012.05.009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahai E et al. , A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020). doi: 10.1038/s41568-019-0238-1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stylianopoulos T et al. , Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. U.S.A 109, 15101–15108 (2012). doi: 10.1073/pnas.1213353109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauhan VP et al. , Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun 4, 2516 (2013). doi: 10.1038/ncomms3516; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain RK, Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). doi: 10.1016/j.ccell.2014.10.006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn LL, Jain RK, Vascular regulation of antitumor immunity. Science 365, 544–545 (2019). doi: 10.1126/science.aaw7875; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tse JM et al. , Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. U.S.A 109, 911–916 (2012). doi: 10.1073/pnas.1118910109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Sánchez ME et al. , Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523, 92–95 (2015). doi: 10.1038/nature14329; [DOI] [PubMed] [Google Scholar]
- 21.Kechagia JZ, Ivaska J, Roca-Cusachs P, Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol 20, 457–473 (2019). doi: 10.1038/s41580-019-0134-2; [DOI] [PubMed] [Google Scholar]
- 22.Benham-Pyle BW, Pruitt BL, Nelson WJ, Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 348, 1024–1027 (2015). doi: 10.1126/science.aaa4559; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coste B et al. , Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010). doi: 10.1126/science.1193270; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wipff P-J, Rifkin DB, Meister J-J, Hinz B, Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol 179, 1311–1323 (2007). doi: 10.1083/jcb.200704042; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith ML et al. , Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLOS Biol. 5, e268 (2007). doi: 10.1371/journal.pbio.0050268; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saini K, Cho S, Dooling LJ, Discher DE, Tension in fibrils suppresses their enzymatic degradation - A molecular mechanism for ‘use it or lose it’. Matrix Biol. 85–86, 34–46 (2020). doi: 10.1016/j.matbio.2019.06.001; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubow KE et al. , Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat. Commun 6, 8026 (2015). doi: 10.1038/ncomms9026; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby TJ, Lammerding J, Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol 20, 373–381 (2018). doi: 10.1038/s41556-018-0038-y; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S, Irianto J, Discher DE, Mechanosensing by the nucleus: From pathways to scaling relationships. J. Cell Biol 216, 305–315 (2017). doi: 10.1083/jcb.201610042; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elosegui-Artola A et al. , Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017). doi: 10.1016/j.cell.2017.10.008; [DOI] [PubMed] [Google Scholar]
- 31.Tajik A et al. , Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater 15, 1287–1296 (2016). doi: 10.1038/nmat4729; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A et al. , ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 158, 633–646 (2014). doi: 10.1016/j.cell.2014.05.046; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panciera T, Azzolin L, Cordenonsi M, Piccolo S, Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol 18, 758–770 (2017). doi: 10.1038/nrm.2017.87; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aragona M et al. , A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013). doi: 10.1016/j.cell.2013.07.042; [DOI] [PubMed] [Google Scholar]
- 35.Gaspar P, Tapon N, Sensing the local environment: actin architecture and Hippo signalling. Curr. Opin. Cell Biol 31, 74–83 (2014). doi: 10.1016/j.ceb.2014.09.003; [DOI] [PubMed] [Google Scholar]
- 36.Zanconato F, Cordenonsi M, Piccolo S, YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 (2016). doi: 10.1016/j.ccell.2016.05.005; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clavería C, Giovinazzo G, Sierra R, Torres M, Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44 (2013). doi: 10.1038/nature12389; [DOI] [PubMed] [Google Scholar]
- 38.Merino MM, Levayer R, Moreno E, Survival of the fittest: Essential roles of cell competition in development, aging, and cancer. Trends Cell Biol. 26, 776–788 (2016). doi: 10.1016/j.tcb.2016.05.009; [DOI] [PubMed] [Google Scholar]
- 39.Shraiman BI, Mechanical feedback as a possible regulator of tissue growth. Proc. Natl. Acad. Sci. U.S.A 102, 3318–3323 (2005). doi: 10.1073/pnas.0404782102; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy JE et al. , Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: A phase 2 clinical trial. JAMA Oncol. 5, 1020–1027 (2019). doi: 10.1001/jamaoncol.2019.0892; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Provenzano PP et al. , Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012). doi: 10.1016/j.ccr.2012.01.007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman MH et al. , Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014). doi: 10.1016/j.cell.2014.08.007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olive KP et al. , Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009). doi: 10.1126/science.1171362; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen IX et al. , Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl. Acad. Sci. U.S.A 116, 4558–4566 (2019). doi: 10.1073/pnas.1815515116; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang-Gillam A, Targeting stroma: A tale of caution. J. Clin. Oncol 37, 1041–1043 (2019). doi: 10.1200/JCO.19.00056; [DOI] [PubMed] [Google Scholar]
- 46.Chauhan VP et al. , Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl. Acad. Sci. U.S.A 116, 10674–10680 (2019). doi: 10.1073/pnas.1819889116; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chauhan VP et al. , Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26, 14–15 (2014). doi: 10.1016/j.ccr.2014.06.003; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young JS, Lumsden CE, Stalker AL, The significance of the tissue pressure of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J. Pathol. Bacteriol 62, 313–333 (1950). doi: 10.1002/path.1700620303; [DOI] [PubMed] [Google Scholar]
- 49.Jain RK, Baxter LT, Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: Significance of elevated interstitial pressure. Cancer Res. 48, 7022–7032 (1988). [PubMed] [Google Scholar]
- 50.Boucher Y, Baxter LT, Jain RK, Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: Implications for therapy. Cancer Res. 50, 4478–4484 (1990). [PubMed] [Google Scholar]
- 51.Swartz MA, Lund AW, Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 12, 210–219 (2012). doi: 10.1038/nrc3186; [DOI] [PubMed] [Google Scholar]
- 52.Song JW, Munn LL, Fluid forces control endothelial sprouting. Proc. Natl. Acad. Sci. U.S.A 108, 15342–15347 (2011). doi: 10.1073/pnas.1105316108; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qazi H, Shi ZD, Tarbell JM, Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression. PLOS ONE 6, e20348 (2011). doi: 10.1371/journal.pone.0020348; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polacheck WJ, Charest JL, Kamm RD, Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl. Acad. Sci. U.S.A 108, 11115–11120 (2011). doi: 10.1073/pnas.1103581108; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qazi H, Palomino R, Shi ZD, Munn LL, Tarbell JM, Cancer cell glycocalyx mediates mechanotransduction and flow-regulated invasion. Integr. Biol 5, 1334–1343 (2013). doi: 10.1039/c3ib40057c; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang SF et al. , Tumor cell cycle arrest induced by shear stress: Roles of integrins and Smad. Proc. Natl. Acad. Sci. U.S.A 105, 3927–3932 (2008). doi: 10.1073/pnas.0712353105; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD, Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl. Acad. Sci. U.S.A 111, 2447–2452 (2014). doi: 10.1073/pnas.1316848111; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lecuit T, Yap AS, E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol 17, 533–539 (2015). doi: 10.1038/ncb3136; [DOI] [PubMed] [Google Scholar]
- 59.Leckband DE, de Rooij J, Cadherin adhesion and mechanotransduction. Annu. Rev. Cell Dev. Biol 30, 291–315 (2014). doi: 10.1146/annurev-cellbio-100913-013212; [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto K et al. , Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat. Med 12, 133–137 (2006). doi: 10.1038/nm1338; [DOI] [PubMed] [Google Scholar]
- 61.Polacheck WJ et al. , A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552, 258–262 (2017). doi: 10.1038/nature24998; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toftgård R, Two sides to cilia in cancer. Nat. Med 15, 994–996 (2009). doi: 10.1038/nm0909-994; [DOI] [PubMed] [Google Scholar]
- 63.Wang KC et al. , Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. U.S.A 113, 11525–11530 (2016). doi: 10.1073/pnas.1613121113; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L et al. , Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540, 579–582 (2016). doi: 10.1038/nature20602; [DOI] [PubMed] [Google Scholar]
- 65.Jain RK, Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol 31, 2205–2218 (2013). doi: 10.1200/JCO.2012.46.3653; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nathanson SD, Nelson L, Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann. Surg. Oncol 1, 333–338 (1994). doi: 10.1007/BF03187139; [DOI] [PubMed] [Google Scholar]
- 67.Gutmann R et al. , Interstitial hypertension in head and neck tumors in patients: Correlation with tumor size. Cancer Res. 52, 1993–1995 (1992). [PubMed] [Google Scholar]
- 68.Fyles A et al. , Long-term performance of interstial fluid pressure and hypoxia as prognostic factors in cervix cancer. Radiother. Oncol 80, 132–137 (2006). doi: 10.1016/j.radonc.2006.07.014; [DOI] [PubMed] [Google Scholar]
- 69.Jain RK, Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005). doi: 10.1126/science.1104819; [DOI] [PubMed] [Google Scholar]
- 70.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK, Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol 15, 325–340 (2018). doi: 10.1038/nrclinonc.2018.29; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cochlin DL, Ganatra RH, Griffiths DF, Elastography in the detection of prostatic cancer. Clin. Radiol 57, 1014–1020 (2002). doi: 10.1053/crad.2002.0989; [DOI] [PubMed] [Google Scholar]
- 72.Boyd NF et al. , Evidence that breast tissue stiffness is associated with risk of breast cancer. PLOS ONE 9, e100937 (2014). doi: 10.1371/journal.pone.0100937; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maskarinec G et al. , Mammographic density as a predictor of breast cancer survival: The Multiethnic Cohort. Breast Cancer Res. 15, R7 (2013). doi: 10.1186/bcr3378; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evans A et al. , Differentiating benign from malignant solid breast masses: Value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br. J. Cancer 107, 224–229 (2012). doi: 10.1038/bjc.2012.253; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carrara S et al. , EUS elastography (strain ratio) and fractal-based quantitative analysis for the diagnosis of solid pancreatic lesions. Gastrointest. Endosc 87, 1464–1473 (2018). doi: 10.1016/j.gie.2017.12.031; [DOI] [PubMed] [Google Scholar]
- 76.Shahryari M et al. , Tomoelastography distinguishes noninvasively between benign and malignant liver lesions. Cancer Res. 79, 5704–5710 (2019). doi: 10.1158/0008-5472.CAN-19-2150; [DOI] [PubMed] [Google Scholar]
- 77.Rouvière O et al. , Stiffness of benign and malignant prostate tissue measured by shear-wave elastography: A preliminary study. Eur. Radiol 27, 1858–1866 (2017). doi: 10.1007/s00330-016-4534-9; [DOI] [PubMed] [Google Scholar]
- 78.Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). doi: 10.1016/j.cell.2006.06.044; [DOI] [PubMed] [Google Scholar]
- 79.Pickup MW, Mouw JK, Weaver VM, The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253 (2014). doi: 10.15252/embr.201439246; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paszek MJ et al. , Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). doi: 10.1016/j.ccr.2005.08.010; [DOI] [PubMed] [Google Scholar]
- 81.Ulrich TA, de Juan Pardo EM, Kumar S, The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167–4174 (2009). doi: 10.1158/0008-5472.CAN-08-4859; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bordeleau F et al. , Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. U.S.A 114, 492–497 (2017). doi: 10.1073/pnas.1613855114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tung JC et al. , Tumor mechanics and metabolic dysfunction. Free Radic. Biol. Med 79, 269–280 (2015). doi: 10.1016/j.freeradbiomed.2014.11.020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levental KR et al. , Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009). doi: 10.1016/j.cell.2009.10.027; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller BW et al. , Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: Inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med 7, 1063–1076 (2015). doi: 10.15252/emmm.201404827; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lang NR et al. , Biphasic response of cell invasion to matrix stiffness in three-dimensional biopolymer networks. Acta Biomater. 13, 61–67 (2015). doi: 10.1016/j.actbio.2014.11.003; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wirtz D, Konstantopoulos K, Searson PC, The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011). doi: 10.1038/nrc3080; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pathak A, Kumar S, Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. U.S.A 109, 10334–10339 (2012). doi: 10.1073/pnas.1118073109; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Charras G, Sahai E, Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol 15, 813–824 (2014). doi: 10.1038/nrm3897; [DOI] [PubMed] [Google Scholar]
- 90.Acerbi I et al. , Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol 7, 1120–1134 (2015). doi: 10.1039/c5ib00040h; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alvarez R et al. , Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br. J. Cancer 109, 926–933 (2013). doi: 10.1038/bjc.2013.415; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahbari NN et al. , Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med 8, 360ra135 (2016). doi: 10.1126/scitranslmed.aaf5219; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miroshnikova YA et al. , Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol 18, 1336–1345 (2016). doi: 10.1038/ncb3429; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isenberg BC, Dimilla PA, Walker M, Kim S, Wong JY, Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys. J 97, 1313–1322 (2009). doi: 10.1016/j.bpj.2009.06.021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gilkes DM, Semenza GL, Wirtz D, Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 14, 430–439 (2014). doi: 10.1038/nrc3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS, Matrix rigidity regulates a switch between TGF-β1–induced apoptosis and epithelial–mesenchymal transition. Mol. Biol. Cell 23, 781–791 (2012). doi: 10.1091/mbc.e11-06-0537; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pang M-F et al. , Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res. 76, 5277–5287 (2016). doi: 10.1158/0008-5472.CAN-16-0579; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Öhlund D, Elyada E, Tuveson D, Fibroblast heterogeneity in the cancer wound. J. Exp. Med 211, 1503–1523 (2014). doi: 10.1084/jem.20140692; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J et al. , Tissue transglutaminase mediated tumor–stroma interaction promotes pancreatic cancer progression. Clin. Cancer Res 21, 4482–4493 (2015). doi: 10.1158/1078-0432.CCR-15-0226; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li CX et al. , MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater 16, 379–389 (2017). doi: 10.1038/nmat4780; [DOI] [PubMed] [Google Scholar]
- 101.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA, Nonlinear elasticity in biological gels. Nature 435, 191–194 (2005). doi: 10.1038/nature03521; [DOI] [PubMed] [Google Scholar]
- 102.Samuel MS et al. , Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19, 776–791 (2011). doi: 10.1016/j.ccr.2011.05.008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han YL et al. , Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proc. Natl. Acad. Sci. U.S.A 115, 4075–4080 (2018). doi: 10.1073/pnas.1722619115; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choquet D, Felsenfeld DP, Sheetz MP, Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88, 39–48 (1997). doi: 10.1016/S0092-8674(00)81856-5; [DOI] [PubMed] [Google Scholar]
- 105.Munn LL, Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med 9, e1370 (2017). doi: 10.1002/wsbm.1370; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Greten FR, Grivennikov SI, Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019). doi: 10.1016/j.immuni.2019.06.025; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pogoda K et al. , Compression stiffening of brain and its effect on mechanosensing by glioma cells. New J. Phys 16, 075002 (2014). doi: 10.1088/1367-2630/16/7/075002; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guilluy C et al. , Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol 16, 376–381 (2014). doi: 10.1038/ncb2927; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bakker MF et al. , Supplemental MRI screening for women with extremely dense breast tissue. N. Engl. J. Med 381, 2091–2102 (2019). doi: 10.1056/NEJMoa1903986; [DOI] [PubMed] [Google Scholar]
- 110.Panzetta V et al. , Mechanical phenotyping of cells and extracellular matrix as grade and stage markers of lung tumor tissues. Acta Biomater. 57, 334–341 (2017). doi: 10.1016/j.actbio.2017.05.002; [DOI] [PubMed] [Google Scholar]
- 111.Sulzmaier FJ, Jean C, Schlaepfer DD, FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 14, 598–610 (2014). doi: 10.1038/nrc3792; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dupont S et al. , Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). doi: 10.1038/nature10137; [DOI] [PubMed] [Google Scholar]
- 113.Calvo F et al. , Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol 15, 637–646 (2013). doi: 10.1038/ncb2756; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DelNero P, Hopkins BD, Cantley LC, Fischbach C, Cancer metabolism gets physical. Sci. Transl. Med 10, eaaq1011 (2018). doi: 10.1126/scitranslmed.aaq1011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zanotelli MR et al. , Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nat. Commun 10, 4185 (2019). doi: 10.1038/s41467-019-12155-z; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Incio J et al. , Metformin reduces desmoplasia in pancreatic cancer by reprogramming stellate cells and tumor-associated macrophages. PLOS ONE 10, e0141392 (2015). doi: 10.1371/journal.pone.0141392; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu L et al. , Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci. Transl. Med 9, eaan2966 (2017). doi: 10.1126/scitranslmed.aan2966; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baker BM, Chen CS, Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci 125, 3015–3024 (2012). doi: 10.1242/jcs.079509; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conklin MW et al. , Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol 178, 1221–1232 (2011). doi: 10.1016/j.ajpath.2010.11.076; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Provenzano PP et al. , Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38 (2006). doi: 10.1186/1741-7015-4-38; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chaudhuri PK, Pan CQ, Low BC, Lim CT, Topography induces differential sensitivity on cancer cell proliferation via Rho-ROCK-Myosin contractility. Sci. Rep 6, 19672 (2016). doi: 10.1038/srep19672; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ, Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys. J 95, 5374–5384 (2008). doi: 10.1529/biophysj.108.133116; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fraley SI et al. , Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci. Rep 5, 14580 (2015). doi: 10.1038/srep14580; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Velez DO et al. , 3D collagen architecture induces a conserved migratory and transcriptional response linked to vasculogenic mimicry. Nat. Commun 8, 1651 (2017). doi: 10.1038/s41467-017-01556-7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Folkman J, Moscona A, Role of cell shape in growth control. Nature 273, 345–349 (1978). doi: 10.1038/273345a0; [DOI] [PubMed] [Google Scholar]
- 126.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE, Geometric control of cell life and death. Science 276, 1425–1428 (1997). doi: 10.1126/science.276.5317.1425; [DOI] [PubMed] [Google Scholar]
- 127.Tan JL et al. , Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. U.S.A 100, 1484–1489 (2003). doi: 10.1073/pnas.0235407100; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pirone DM et al. , An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol 174, 277–288 (2006). doi: 10.1083/jcb.200510062; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang S, Chen CS, Ingber DE, Control of cyclin D1, p27Kip1, and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell 9, 3179–3193 (1998). doi: 10.1091/mbc.9.11.3179; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo M et al. , Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl. Acad. Sci. U.S.A 114, E8618–E8627 (2017). doi: 10.1073/pnas.1705179114; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park J et al. , Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat. Mater 15, 792–801 (2016). doi: 10.1038/nmat4586; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramdas NM, Shivashankar GV, Cytoskeletal control of nuclear morphology and chromatin organization. J. Mol. Biol 427, 695–706 (2015). doi: 10.1016/j.jmb.2014.09.008; [DOI] [PubMed] [Google Scholar]
- 133.Denais CM et al. , Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016). doi: 10.1126/science.aad7297; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Irianto J et al. , DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol 27, 210–223 (2017). doi: 10.1016/j.cub.2016.11.049; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Raab M et al. , ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 (2016). doi: 10.1126/science.aad7611; [DOI] [PubMed] [Google Scholar]
- 136.Paul CD, Mistriotis P, Konstantopoulos K, Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 (2017). doi: 10.1038/nrc.2016.123; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hung WC et al. , Confinement sensing and signal optimization via Piezo1/PKA and myosin II pathways. Cell Rep. 15, 1430–1441 (2016). doi: 10.1016/j.celrep.2016.04.035; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chauhan VP et al. , Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol 7, 383–388 (2012). doi: 10.1038/nnano.2012.45; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK, Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60, 2497–2503 (2000). [PubMed] [Google Scholar]
- 140.Stylianopoulos T, Diop-Frimpong B, Munn LL, Jain RK, Diffusion anisotropy in collagen gels and tumors: The effect of fiber network orientation. Biophys. J 99, 3119–3128 (2010). doi: 10.1016/j.bpj.2010.08.065; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chauhan VP, Jain RK, Strategies for advancing cancer nanomedicine. Nat. Mater 12, 958–962 (2013). doi: 10.1038/nmat3792; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang H-B, Dembo M, Wang Y-L, Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol 279, C1345–C1350 (2000). doi: 10.1152/ajpcell.2000.279.5.C1345; [DOI] [PubMed] [Google Scholar]
- 143.Baur A et al. , Diffusion-weighted MR imaging of bone marrow: Differentiation of benign versus pathologic compression fractures. Radiology 207, 349–356 (1998). doi: 10.1148/radiology.207.2.9577479; [DOI] [PubMed] [Google Scholar]
- 144.Jansen LE, Birch NP, Schiffman JD, Crosby AJ, Peyton SR, Mechanics of intact bone marrow. J. Mech. Behav. Biomed. Mater 50, 299–307 (2015). doi: 10.1016/j.jmbbm.2015.06.023; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shin J-W, Mooney DJ, Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proc. Natl. Acad. Sci. U.S.A 113, 12126–12131 (2016). doi: 10.1073/pnas.1611338113; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chramiec A, Vunjak-Novakovic G, Tissue engineered models of healthy and malignant human bone marrow. Adv. Drug Deliv. Rev 140, 78–92 (2019). doi: 10.1016/j.addr.2019.04.003; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fenner J et al. , Macroscopic stiffness of breast tumors predicts metastasis. Sci. Rep 4, 5512 (2014). doi: 10.1038/srep05512; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marasco G et al. , Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J. Hepatol 70, 440–448 (2019). doi: 10.1016/j.jhep.2018.10.022; [DOI] [PubMed] [Google Scholar]
- 149.Zhang R et al. , Increased matrix stiffness promotes tumor progression of residual hepatocellular carcinoma after insufficient heat treatment. Cancer Sci. 108, 1778–1786 (2017). doi: 10.1111/cas.13322; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Incio J et al. , Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016). doi: 10.1158/2159-8290.CD-15-1177; [DOI] [PMC free article] [PubMed] [Google Scholar]


