Abstract
Purpose
To explore improvement in motor ability, function, health-related quality of life (HRQOL), and symptom severity in patients with sclerotic chronic graft-versus-host disease (ScGVHD) in response to treatment as well as the relationship among changes on such measures.
Methods
This study was a secondary analysis of data from 13 individuals with severe ScGVHD enrolled in a clinical trial evaluating the efficacy of imatinib mesylate (clinicaltrials.gov identifier: NCT00702689). Self-reported, clinician-reported, and performance-based indicators of motor ability, function, HRQOL, and symptom severity were assessed at baseline and 6 months following the administration of imatinib mesylate.
Results
Participants did not show statistically significant improvement on any measures over time. Approximately one-third of patients displayed clinically significant improvement on measures of motor ability (palmar pinch strength, dominant hand, 30.8%), functioning (Manual Ability Measure—36, 41.7%), HRQOL (Short Form 36 [SF-36] Mental Component Summary, 33.3%), and symptom severity (Lee Symptom Scale, 38.5%). Improvement in cGVHD symptom burden was correlated with improvement in function (Assessment of Motor and Process Skills [AMPS] and Disabilities of the Arm, Shoulder, and Hand [DASH] scores) and HRQOL (SF-36 Physical Component Summary scores).
Conclusions
Findings suggest the potential utility of administering patient-reported and performance-based functional measures, such as the DASH and the AMPS, to patients with cGVHD. By understanding the functional consequences of ScGVHD, interdisciplinary teams of health care providers, including rehabilitation professionals, can work to improve long-term outcomes.
Keywords: Chronic graft-versus-host disease, Sclerosis, Functional impairment, Symptom burden, Health-related quality of life, Allogeneic hematopoietic stem cell transplantation
Introduction
Worldwide, approximately 30,000 allogeneic hematopoietic stem cell transplants are performed each year to combat various hematologic diseases, around 87% of which are malignant [1]. Chronic graft-versus-host disease (cGVHD) is a common complication, affecting around half of allogeneic hematopoietic stem cell transplant survivors [2–4]. The disease occurs when the donor’s immune cells iatrogenically respond to the recipient’s tissues after identifying them as foreign. Chronic GVHD can manifest in one or more organs, including the skin, mouth, eyes, gastrointestinal tract, liver, lungs, joints/ fascia, and genitals [5]. The disease is associated with an inability to maintain employment [6], functional impairment [3], poor health-related quality of life (HRQOL) [6, 7], high symptom distress [4], and heightened risk of non-relapse mortality [2].
The sclerotic form of chronic graft-versus-host disease (ScGVHD) is a manifestation of skin cGVHD characterized by deep and superficial sclerosis [5] that appears in 15–23% of patients with cGVHD [8–10]. Compared to individuals with non-sclerotic cGVHD, patients having ScGVHD are likely to experience physical limitations [11], poor HRQOL [12], and a prolonged course of immunosuppressive therapy [10].
In order to improve the diagnosis, assessment, and treatment of cGVHD, there have been efforts to standardize outcome measurement in observational and drug efficacy trials. The 2014 National Institutes of Health (NIH) Consensus Development Project established recommendations for the diagnosis and severity scoring of cGVHD [5], as well as for the provision of ancillary and supportive care to patients with the disease [13]. In addition, these recommendations advocate for the use of a standard set of patient- and clinician-reported measures of HRQOL and disease severity to assess therapeutic response in this patient population [14]. Self-reported measures of symptom burden, cGVHD symptom severity, and HRQOL are associated with clinician-reported cGVHD severity [12, 15] and performance-based measures of motor ability [16]. However, none of the aforementioned indicators of clinical response evaluates activities of daily living (ADL) performance and/or disability. Adding self-reported and/or performance-based measures of functional impairment may allow clinicians to more fully characterize the impact of cGVHD on daily life and more effectively target treatment and rehabilitative efforts.
The inclusion of functional measures may be especially valuable in the context of cGVHD drug efficacy trials. Patients often fail to respond to the first line of systemic immunosuppressive therapy commonly used to alleviate cGVHD manifestations [10]. Given the ineffectiveness of traditional salvage therapy for many patients with ScGVHD in particular [9], imatinib mesylate has been investigated for its potential anti-fibrotic benefit. In preclinical models, imatinib mesylate inhibited platelet-derived growth factor and transforming growth factor β, both of which are implicated in the pathogenesis of fibrosis and systemic sclerosis [17]. Therefore, researchers have explored clinical response to the drug among patients with ScGVHD, though such researchers have typically not studied how treatment may influence the performance of ADLs [18–20].
In 2015, Baird and colleagues published a study investigating the degree to which imatinib mesylate improved range of motion (ROM) among patients with severe ScGVHD [19]. The authors found that 84% of patients (sample n = 13) displayed some improvement in ROM over a 6-month period. This was categorized as a partial response for 38.5% of patients and as stable disease for the remaining 45.5% of patients. The present study extends upon the findings of Baird et al. in a secondary analysis of the motor, functional, and HRQOL outcomes.
The objectives of this study were twofold. First, we explored whether administering imatinib mesylate to patients with ScGVHD was associated with improvements in motor ability, function, HRQOL, and cGVHD symptom severity. Given that most patients displayed improved ROM in response to imatinib mesylate [19], we predicted that patients would also experience statistical and clinical improvement in these areas. Second, we investigated whether changes in cGVHD symptom severity were associated with changes in motor ability, function, and HRQOL. We hypothesized that improvement in clinician-rated symptom severity and patient-reported cGVHD symptom burden would be related to motor, functional, and HRQOL gains.
Methods
This study was a secondary analysis of data from a single-arm prospective clinical trial investigating the efficacy of imatinib mesylate for patients with treatment-refractory ScGVHD (clinicaltrials.gov identifier: NCT00702689) [19]. The protocol was approved by the National Cancer Institute Institutional Review Board. Participants were primarily recruited from the NIH Natural History Study of cGVHD (clinicaltrials.gov identifier: NCT00092235). Individuals who met the 2005 NIH Consensus Group Criteria for ScGVHD [21] who agreed to participate in the study were enrolled at the NIH between December 2008 and February 2011. Data were collected at baseline, 3 months, and 6 months following the initiation of treatment. Six months was the primary endpoint of interest.
Eligibility criteria included biopsy-confirmed ScGVHD resulting in ROM deficits ≥ 25% in one or more joints [22], lack of cGVHD response to systemic corticosteroids or reliance on such medication to maintain stable disease, Karnofsky Performance Status Scale score ≥ 60%, and age ≥ 4 years. Exclusion criteria included pregnancy; concurrent receipt of other investigational agents; and residual/recurrent or relapsed metastatic disease. Baird and colleagues have reported detailed information on inclusion and exclusion criteria as well as dose escalation for this study [19].
Measures
Indicators of motor ability included the Jebsen-Taylor Hand Function Test (JTHFT), Grooved Pegboard Test (GPT), pinch strength, grip strength, and 2 Minute Walk Test (2MWT). The Disabilities of the Arm, Shoulder, and Hand (DASH); Human Activity Profile (HAP); Assessment of Motor and Process Skills version 7 (AMPS); and Manual Ability Measure (MAM-36) evaluated functional impairment. The Short-Form 36 version 2 (SF-36) measured HRQOL. Chronic GVHD symptom severity was assessed using the Lee Symptom Scale and Provider Global Rating (PGR).
A physiatrist assessed grip strength and 2MWT performance. A trained occupational therapist administered the AMPS, JTHFT, GPT, and pinch strength. A transplant oncologist with expertise in cGVHD completed the PGR. The same providers administered assessments at all time points and for all patients.
Motor ability
The JTHFT is a performance-based assessment of manual ability [23]. Scores reflect the time patients require to perform seven functional tasks with each hand. The GPT is a performance-based measure of motor speed and manual dexterity [24]. Participants are timed as they unilaterally insert grooved pegs into a 5 × 5 peg board. The 2MWT is a performance-based measure of physical ability that records the distance patients walk over the course of 2 min [25]. Grip strength was measured three times in the dominant hand using a dynamometer. Performance was then averaged across trials [26]. Key, Palmar, and Tip-to-Tip pinch strength were assessed in both hands using a pinch gauge [26].
Functional impairment
The DASH is a 30-item self-reported measure of upper extremity function, symptoms, and disability [27]. Scores range from 0 to 100, with higher scores indicating greater disability. The HAP is a 94-item self-reported measure of energy expenditure and physical fitness that produces a Maximum Activity Score (MAS) and an Adjusted Activity Score (AAS), which can range from 0 to 94 [28]. This measure has been validated for use in patients with cGVHD [29]. The AMPS is a performance-based assessment of the quality of one’s ADL performance [30]. After observing patients complete two ADL tasks, a trained occupational therapist rates performance on a 36-item scale, producing ADL motor skill and ADL process skill scores in logit units. The MAM-36 is a self-reported measure assessing the ease with which individuals can perform common daily activities that require the use of one or both hands [31]. Scores on this 36-item scale range from 0 to 100, with higher scores indicating greater manual ability.
Health-related quality of life
The SF-36 is a validated 36-item self-reported measure of general HRQOL [32]. This instrument assesses eight domains of physical and mental health: physical functioning, physical role functioning, bodily pain, perceptions of general health, vitality, social functioning, emotional role functioning, and mental health. Subscales are used to create Physical Component Summary (PCS) and Mental Component Summary (MCS) scores expressed as T-scores based on population norms, with a population mean of 50 and standard deviation of 10.
cGVHD symptom severity
The Lee Symptom Scale is a 30-item self-reported assessment of cGVHD-specific symptom burden [33]. This measure captures the extent the respondent is bothered by skin, eye/mouth, muscle/joint, nutrition, lung, energy, and psychological cGVHD symptoms. Responses are summed and linearly transformed to create summary scores that range from 0 to 100. Higher scores indicate greater cGVHD symptom burden. The validity and reliability of this measure have been established in patients with cGVHD [33]. For PGR scores, physicians rate the severity of patients’ signs and symptoms of cGVHD on an 11-point Likert scale. Possible scores range from 0 to 10. Higher scores reflect more severe cGVHD symptoms [34].
Statistical analysis
Descriptive statistics were calculated for the analytic sample’s demographic, cGVHD, motor, functional, and HRQOL characteristics. Wilcoxon signed-rank tests assessed change between baseline and 6 months. Spearman’s rank correlations evaluated the relationship between change in symptom severity (Lee Symptom Scale and PGR) and change in motor, functional, and HRQOL outcomes over this 6-month period. To reduce the likelihood of type I error, p values less than 0.01 were considered statistically significant. All statistical analyses were performed using R version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
The guidelines set forth by the 2014 cGVHD Response Criteria Working Group were used to identify minimal clinically important differences (MCID) for all measures [14]. Established MCID values were used for the AMPS [30], DASH [35], and PGR [14]. Half the standard deviation of baseline scores was used as the MCID for all other measures. MCID values obtained using this metric were similar to those reported in larger samples of patients with cGVHD or related conditions (See Supplemental Table 1) [29, 31, 36–39]. Consistent with previous work and the recommendations of the 2014 Response Criteria Working Group, half of the population standard deviation (5 points) was used as the MCID for SF-36 PCS and MCS scores [6, 7, 14]. The standard deviation of baseline Lee Symptom Scores was small in this study (0.5 SD = 2.37) compared with that in previous literature that used this same criterion to define MCID values (MCID between 6 and 7) [33, 37, 38]. Therefore, a more conservative score of 6.5 was used as the MCID on this measure. At baseline, despite differing patterns of responses on individual items, four patients (30.8% of the sample) reported Lee Symptom Scale Scores of 25, which contributed to the measure’s unexpectedly small standard deviation in this study.
Results
Patient characteristics
Twenty patients enrolled in the study. Six individuals were not available for primary endpoint analysis due to toxicity (1), relapse (1), or withdrawn consent (4). One additional patient experienced cGVHD progression between baseline and 3 months and was therefore taken off the study prior to assessment at 6 months. Demographic and disease characteristics presented in Table 1 reflect the analytic sample (n = 13). At enrollment, the median age of participants was 52.5 years (range, 6.8–60.9). A majority of patients were male (76.9%) and all identified as non-Hispanic white. Most patients were married (53.8%), lived in Midwestern or Northeastern states (84.6%), and one-third were working (30.8%).
Table 1.
Patient demographic and clinical characteristics
| Characteristics (n = 13) | Value |
|---|---|
| Agea, median years (range) | 52.5 (6.8–60.9) |
| <18 | 1 (7.7) |
| 18–29 | 2 (15.4) |
| 30–39 | 1 (7.7) |
| 40–49 | 2 (15.4) |
| ≥ 50 | 7 (53.8) |
| Gender, n (%) | |
| Male | 10 (76.9) |
| Female | 3 (23.1) |
| Race and ethnicity, n (%) | |
| White non-Hispanic | 13 (100.0) |
| Region of residency, n (%) | |
| Midwestern United States | 6 (46.2) |
| Northeastern United States | 5 (38.5) |
| Southern United States | 1 (7.7) |
| Western United States | 1 (7.7) |
| Employment status, n (%)b | |
| Working | 4 (30.8) |
| Not working | 6 (46.2) |
| Student | 3 (23.1) |
| Marital status, n (%) | |
| Married | 7 (53.8) |
| Not marriedc | 4 (30.8) |
| ≤ 18 years old | 2 (15.4) |
| Months from transplant, median (range) | 52.6 (12.7–121.0) |
| Months from cGVHD diagnosis, median (range) | 39.1 (4.8–112.9) |
| Number of affected organs, median (range) | 5 (2–7) |
| Source of transplant, n (%) | |
| Peripheral blood stem cells | 11 (84.6) |
| Bone marrow | 2 (15.4) |
| HLA match, n (%) | |
| Matchd | 12 (92.3) |
| Mismatch | 1 (7.7) |
| Conditioning regimen, n (%) | |
| Myeloablative | 7 (53.8) |
| Reduced intensity | 1 (7.7) |
| Non-myeloablative | 5 (38.5) |
| Diagnosis, n (%) | |
| Leukemiae | 5 (30.8) |
| Lymphoma | 3 (23.1) |
| Multiple myeloma | 2 (15.4) |
| Myelodysplastic syndromes | 2 (15.4) |
| Polycythemia vera myelofibrosis | 1 (7.7) |
| History of acute GVHD, n (%) | |
| Yes | 9 (69.2) |
| No | 4 (30.8) |
Demographic and disease characteristics for the analytic sample at study enrollment
Age at the time of consent
“Working” includes individuals who work full or part time. “Not work-ing” includes individuals who are retired, unemployed, or serve as homemakers
Includes individuals who are divorced
Match defined as having a 6/6 or 10/10 HLA match with donor
Includes both chronic and acute leukemia
Patients entered the study a median of 52.6 months post-transplant (range, 12.7–121.0 months) and 39.1 months following cGVHD diagnosis (range, 4.8–112.9 months). A majority of patients received myeloablative conditioning regimens (53.8%), grafts from HLA-matched donors (6/6 or 10/ 10; 92.3%), and peripheral blood stem cell transplants (84.6%). At baseline, all patients had severe NIH cGVHD global scores, severe NIH cGVHD skin organ scores, and a median of five affected organs (range, 2–7).
Change over time
Table 2 reports median scores on all outcomes at baseline and 6 months and statistical change over time. Table 3 summarizes clinical changes in motor ability, function, HRQOL, and symptom severity between baseline and 6 months. Patients did not display statistically significant change over time (see Table 2) nor did the majority of patients experience clinically significant improvement on any outcome of interest (see Table 3). MCID values used to categorize clinically significant change for all measures are also reported in Table 3.
Table 2.
Median motor ability, function, HRQOL, and symptom severity at baseline and 6 months and statistical change over time
| Measures | Median |
Wilcoxon signed-rank test | ||
|---|---|---|---|---|
| Na | Baseline | 6 months | p value | |
| Motor | ||||
| JTHFT | 13 | 45.2 | 49.4 | 0.685 |
| GPT | 13 | 101.5 | 106.6 | 0.735 |
| Grip strength (lbs) Pinch strength (lbs) | 12 | 50.8 | 54.8 | 0.230 |
| Tip-to-tip | 13 | 10.0 | 9.0 | 0.679 |
| Palmar | 13 | 14.0 | 16.0 | 0.401 |
| Key | 13 | 15.0 | 17.0 | 0.331 |
| 2MWT (ft) | 11 | 610.2 | 607.0 | 0.413 |
| Functional | ||||
| AMPS | ||||
| ADL motor skill | 13 | 1.7 | 1.5 | 0.340 |
| ADL process skill | 13 | 1.9 | 1.7 | 0.505 |
| DASH | 12 | 34.2 | 22.9 | 0.733 |
| HAP | ||||
| AAS | 12 | 69.0 | 69.5 | 0.247 |
| MAS | 12 | 78.0 | 79.5 | 0.552 |
| MAM-36 | 12 | 66.0 | 69.0 | 0.223 |
| Quality of life | ||||
| SF-36 | ||||
| PCS | 12 | 35.3 | 38.6 | 0.910 |
| MCS | 12 | 51.3 | 52.2 | 0.677 |
| Symptom severity | ||||
| Provider Global Rating | 13 | 6.0 | 6.0 | 0.551 |
| Lee Symptom Scale | 13 | 25.0 | 22.5 | 0.102 |
JTHFT, GPT, and pinch strength scores refer to performance with the dominant hand. Higher scores on the DASH, JTHFT, and GPT indicate poorer performance. Higher scores on the PGR and Lee Symptom Scale indicate greater symptom severity. On all other measures, higher scores indicate improved performance
Number of patients with data on each measure at both baseline and 6 months. Data for these individuals were used to calculate median scores at each time point. DASH, HAP, MAM-36, and SF-36 scores were missing from one patient due to young age (< 18 years old). For the 2MWT and grip strength, missing data were not collected
Table 3.
Clinically important change on assessments of motor ability, function, HRQOL, and symptom severity between baseline and 6 months
| Measures | MCIDs |
|||
|---|---|---|---|---|
| Na | Value | Improve, n (%) | Decline, n (%) | |
| Motor | ||||
| JTHFT | 13 | 15.32b | 1 (7.1) | 1 (7.1) |
| GPT | 13 | 13.82b | 2(15.4) | 4 (30.8) |
| Grip strength (lbs) | 12 | 13.95b | 1 (8.3) | 1 (8.3) |
| Pinch strength (lbs) | ||||
| Tip-to-tip | 13 | 2.60b | 1 (7.7) | 0 (0.0) |
| Palmar | 13 | 2.84b | 4 (30.8) | 1 (7.7) |
| Key | 13 | 2.50b | 3 (23.1) | 1 (7.7) |
| 2MWT (ft) | 11 | 50.35b | 1 (9.1) | 1 (9.1) |
| Functional | ||||
| AMPS | ||||
| ADL Motor skill | 13 | 0.3 logitsc | 2(15.4) | 3 (23.1) |
| ADL Process skill | 13 | 0.3 logitsc | 3 (23.1) | 3 (23.1) |
| DASH | 12 | 15c | 3 (25.0) | 3 (25.0) |
| HAP | ||||
| AAS | 12 | 8.81b | 3 (25.0) | 1 (8.3) |
| MAS | 12 | 5.03b | 3 (25.0) | 1 (8.3) |
| MAM-36 | 12 | 7.60b | 5 (41.7) | 2 (16.7) |
| Quality of life | ||||
| SF-36 | ||||
| PCS | 12 | 5c | 3 (25.0) | 2 (16.7) |
| MCS | 12 | 5c | 4 (33.3) | 2 (16.7) |
| Symptom severity | ||||
| Provider Global Rating | 13 | 2c | 2(15.4) | 2 (15.4) |
| Lee Symptom Scale | 13 | 6.50c | 5 (38.5) | 2 (15.4) |
JTHFT, GPT, and pinch strength scores refer to performance with the dominant hand. Higher scores on the DASH, JTHFT, and GPT indicate poorer performance. Higher scores on the PGR and Lee Symptom Scale indicate greater symptom severity. On all other measures, higher scores indicate improved performance
Number ofpatients with data on each measure at both baseline and 6 months. DASH, HAP, MAM-36, and SF-36 scores were missing from one patient due to young age (< 18 years old). For the 2MWT and grip strength, missing data were not collected
Half the standard deviation of baseline scores for individuals who also had scores at 6 months
Established MCID value
Clinically significant improvement on motor outcomes was most common for palmar pinch strength with the dominant hand (30.8%). Just over 30% of patients displayed clinically significant decline on the GPT with the dominant hand (30.8%). On most measures of motor ability, less than 10.0% of patients experienced clinically significant deterioration over time (JTHFT, 2MWT, grip strength, and pinch strength).
On functional outcomes, just under half of patients reported clinically significant improvement on the MAM-36 (41.7%) and one-quarter improved on the HAP AAS (25%), HAP MAS (25%), and DASH (25%). A similar proportion of patients had clinically important worsening on the DASH (25%), AMPS ADL Motor Skill (23.1%), and AMPS ADL Process Skill (23.1%).
Approximately one-third of patients displayed clinically significant improvement in HRQOL as measured by the SF-36 MCS (33.3%) and a quarter of patients reported clinically significant improvement on the SF-36 PCS (25.0%).
Between baseline and 6 months, over one-third of patients had clinically significant improvement on the Lee Symptom Scale (38.5%) and 15.4% experienced clinically significant improvements in PGR scores. However, the same proportion of patients (15.4%) displayed clinically significant worsening of PGR scores over this period.
Patients that Baird and colleagues classified as partial responders to imatinib mesylate (n = 5) [19] did not show a consistent pattern of clinically significant improvement or decline on any motor, functional, or HRQOL outcomes (See Supplemental Table 2).
Correlations among change scores on relevant measures
The relationship between change in cGVHD symptom severity and changes in motor ability, function, and HRQOL are reported in Table 4. Change in PGR scores was not significantly correlated with the change on any performance-based or patient-reported outcomes. Reduced symptom burden (Lee Symptom Scores) was strongly correlated with improved physical HRQOL (SF-36 PCS, r = −0.72, p = 0.008) and improved functioning as indicated by both AMPS ADL motor skill scores (r = −0.84, p < 0.001) and DASH scores (r = 0.71, p = 0.010). Change in symptom burden was not correlated with change on an cators of motor ability.
Table 4.
Relationship between changes in symptom burden, symptom severity, and measures of motor ability, function, and HRQOL
| Measures | Provider Global Rating |
Lee Symptom Scale |
||
|---|---|---|---|---|
| Correlation coefficient (ρ) | p value | Correlation coefficient (ρ) | p value | |
| Motor | ||||
| JTHFT | 0.082 | 0.789 | 0.194 | 0.526 |
| GPT | − 0.085 | 0.782 | 0.432 | 0.140 |
| Grip strength (lbs) | − 0.422 | 0.171 | − 0.438 | 0.154 |
| Pinch strength (lbs) | ||||
| Tip to tip | − 0.282 | 0.351 | − 0.214 | 0.482 |
| Palmar | − 0.332 | 0.268 | 0.438 | 0.134 |
| Key | 0.033 | 0.915 | − 0.612 | 0.026 |
| 2MWT (ft) | − 0.100 | 0.757 | − 0.357 | 0.255 |
| Functional | ||||
| AMPS | ||||
| ADL Motor skill | 0.409 | 0.166 | − 0.845 | < 0.001 |
| ADL Process skill | − 0.196 | 0.520 | − 0.057 | 0.853 |
| DASH | − 0.116 | 0.719 | 0.707 | 0.010 |
| HAP | ||||
| AAS | − 0.020 | 0.951 | − 0.248 | 0.437 |
| MAS | − 0.068 | 0.834 | 0.020 | 0.952 |
| MAM-36 | − 0.226 | 0.480 | − 0.103 | 0.751 |
| Quality of life | ||||
| SF-36 | ||||
| PCS | 0.306 | 0.334 | − 0.724 | 0.008 |
| MCS | − 0.342 | 0.276 | − 0.223 | 0.487 |
| Symptom severity | ||||
| Lee Symptom Scale | − 0.226 | 0.458 | ||
JTHFT, GPT, and pinch strength scores refer to performance with the dominant hand. Higher scores on the DASH, JTHFT, and GPT indicate poorer performance. Higher scores on the PGR and Lee Symptom Scale indicate greater symptom severity. Higher scores on all other measures indicate better performance. Italicized values indicate significant Spearman’s rank correlations between change scores (p ≤ 0.01)
Discussion
Symptom severity and HRQOL are important potential indicators of therapeutic response in patients with cGVHD [14]. Although ScGVHD causes significant impairment [11] and may respond poorly to treatment [9], functional performance is not routinely used as an outcome in clinical trials. Therefore, this study examined motor ability, function, and HRQOL in patients with ScGVHD following treatment with imatinib mesylate. A second aim of the present study was to investigate whether change in motor and functional abilities correlated with the change on established measures of cGVHD symptom severity. There was no statistically significant improvement on any outcome over time, although around a third of patients experienced clinically important improvement on measures of function, HRQOL, and symptom severity. In addition, reduced symptom burden correlated with improvement on two indicators of functioning.
In contrast to ROM results reported previously [19], motor ability, functional impairment, HRQOL, and cGVHD symptom severity remained relatively stable over time. Arai et al. and Inamoto et al. similarly failed to observe changes in patient-reported functional capacity and HRQOL, respectively, despite significant response to cGVHD treatment [18, 37]. Inamoto and colleagues found that such null findings were specific to patients who had been diagnosed with cGVHD more than 3 months prior to study enrollment [37]. A similar lack of responsiveness may have occurred in our analysis, given that most patients had long-standing treatment-refractory skin fibrosis.
Curtis et al. found that lower clinician-rated cGVHD severity was related to higher patient-reported HRQOL [15]. We did not identify such a relationship in this sample, which could be due to insufficient sensitivity of PGR scores or a lack of statistical power. Notably, change in clinician-reported symptom severity was not associated with change in symptom burden, corroborating findings from a previous cross-sectional study [15]. PGR scores capture clinician impressions of cGVHD symptom severity, whereas Lee Symptom Scores may reflect patients’ perceptions of the impact of such symptoms on daily life. These results support the continued use of both scales in patients with cGVHD given that they appear to assess relevant but distinct constructs.
Contrary to expectations, improvement in cGVHD symptom burden was not related to improvement in motor ability. Most of our indicators of motor capacity reflect manual ability (JTHFT, GPT, grip strength, and pinch strength), though the wrist was a target joint for only a third of patients (30.8%, see Supplemental Table 3). If the wrist and hand were not affected, burdened, or limited by ScGVHD, reduced symptoms and symptom burden could occur without parallel improvement on our measures of motor ability. The lack of an association between change in 2MWT distance and symptom burden could be due to the 2MWT’s insufficient sensitivity to clinical response among patients with cGVHD [16, 38]. As of 2014, the cGVHD Response Criteria Working Group no longer recommends using the 2MWT as an indicator of therapeutic response [14].
In this study, improved physical HRQOL (SF-36 PCS), enhanced ability to perform ADLs (AMPS ADL motor skill), and reduced upper extremity impairment (DASH) were associated with decreased symptom burden. Lee et al. similarly demonstrated a longitudinal association between reduced symptom burden and improved HRQOL in patients with cGVHD [33]. Among patients undergoing allogeneic hematopoietic stem cell transplantation, symptom distress is a predictor of reduced physical and mental health [40], poor HRQOL [41], and diminished likelihood of survival [42].
The relationship between symptom burden and functional impairment in patients with cGVHD has had limited study. Currently, the 2014 NIH Consensus Development Project recommends administering the HAP as an ancillary measure of therapeutic response in patients with cGVHD. This measure was originally intended to assess energy expenditure and physical fitness [28], though scores are often interpreted as reflecting physical functioning more generally [29, 38]. The HAP provides valuable information regarding the level of activity participation, but does not provide insight into the cause of limitations therein (e.g., ROM restrictions in the upper versus lower extremity, pain, fatigue, dyspnea, and weakness) to inform specific supportive care interventions for cGVHD patients.
In contrast, as patient-reported and performance-based accounts of disability and ADL limitations, respectively, the DASH and AMPS provide more detailed information about both the cause and extent of limitations in activity engagement. Neither of these measures evidenced ceiling or floor effects at any time point (see Figs. 1 and 2), and both have displayed reliability and validity in diverse clinical populations [30, 43]. Given their longitudinal association with symptom burden, the DASH and the AMPS may be useful indicators of functioning in patients with cGVHD and should be studied as potential ancillary measures of clinical response in future trials. Due to the importance of and distinction between self-reported and performance-based assessments of functioning [44], administering multiple measures may be necessary to inform care decisions.
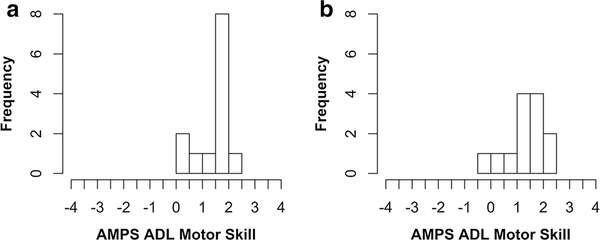
Fig. 1.
Histograms of AMPS ADL motor skill at baseline (a) and 6 months (b), n = 13. AMPS ADL motor skill scores are in logit units. At baseline, scores ranged from − 0.02 to 2.35. At 6 months, scores ranged from 0.18 to 2.44. There is no evidence of ceiling or floor effects at either time point (created using RStudio)
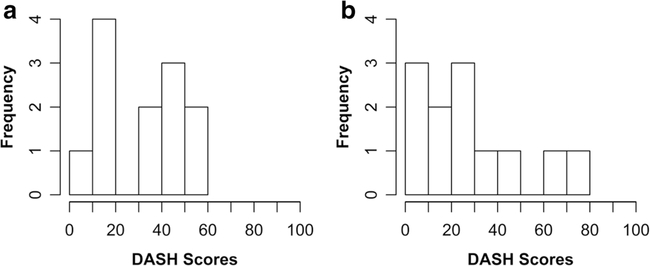
Fig. 2.
Histograms of DASH scores at baseline (a) and 6 months (b), n = 12. Possible scores range from 0 to 100, with higher scores indicating greater disability. At baseline, scores ranged from 6.7 to 55. At 6 months, scores ranged from 7.8 to 71.0. There is no evidence of ceiling or floor effects for DASH scores at either time point (created using RStudio)
More generally, such measures may allow clinicians to better understand the functional restrictions experienced by patients with cGVHD, thereby promoting individualized clinical care and rehabilitative efforts. Following effective assessments of patient limitations, occupational therapists, physical therapists, and physiatrists can work to address unmet rehabilitative needs among cancer patients [45]. Through the provision of orthotics, adaptive equipment, targeted exercises, ADL training, and patient education, rehabilitation professionals can work to aid patients with cGVHD. Future research should continue to explore the utility and feasibility of patient-reported and performance-based functional measures in patients with cGVHD and rehabilitation professionals’ ability to use patient scores to inform decisions regarding care.
The present study should be interpreted in the context of several limitations. Given the small size of our sample, it is unclear if null effects were due to a true lack of a relationship or due to insufficient statistical power. Although reducing our alpha level to 0.01 reduced the likelihood of type I error, it simultaneously lowered our power and increased the likelihood of type II error. Therefore, it is possible that significant relationships will emerge in future studies that examine larger samples of patients with ScGVHD. Data were collected as part of a clinical trial of patients with severe ScGVHD, so findings may not generalize to individuals with non-sclerotic or non-severe cGVHD. Finally, all results are correlational, so we cannot determine the directionality of relationships between constructs.
This study has several strengths. A wide range of assessments were administered to explore a comprehensive set of motor, functional, and HRQOL outcomes among patients with ScGVHD. The same clinicians collected data longitudinally to ensure consistent scoring. Further, to our knowledge, this is the first study to explore the use of the DASH, AMPS, MAM-36, and JTHFT in patients with cGVHD. Future studies should replicate and extend upon findings in larger and more diverse samples.
In conclusion, these results support the continued use of the Lee Symptom Scale and the SF-36 to measure therapeutic response in patients with cGVHD. The association between reduced symptom burden and improved ADL ability (AMPS ADL motor skill) and disability (DASH) indicates the potential importance of including these constructs when evaluating this patient population. The use of the DASH, AMPS, and other functional measures will enable rehabilitation professionals to provide targeted, evidence-based, and individualized care to patients with cGVHD in clinical practice.
Supplementary Material
Acknowledgments
We thank the patients who participated in this study for their contributions to our growing understanding of sclerotic chronic graft-versus-host disease. We also thank the NIH Chronic GVHD Multidisciplinary Team, the research nurses, Christiana Booher, and Bonnie Hodsdon for their respective contributions to this project. We thank the NIH Fellows Editorial Board for their editorial assistance.
Funding information
This research was supported by the National Institutes of Health Intramural Research Programs and the National Cancer Institute’s NCT00702689.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards The protocol was approved by the National Cancer Institute Institutional Review Board.
Disclaimer This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00520-019-05207-z) contains supplementary material, which is available to authorized users.
References
- 1.Niederwieser D, Baldomero H, Szer J, Gratwohl M, Aljurf M, Atsuta Y, Bouzas LF, Confer D, Greinix H, Horowitz M, Iida M, Lipton J, Mohty M, Novitzky N, Nunez J, Passweg J, Pasquini MC, Kodera Y, Apperley J, Seber A, (WBMT) AGftWNoBaMT (2016) Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant 51:778–785. 10.1038/bmt.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Francisco L, Carter A, Sun C-L, Baker KS, Gurney JG, McGlave PB, Nademanee A, O’Donnell M, Ramsay NKC, Robison LL, Snyder D, Stein A, Forman SJ, Weisdorf DJ (2007) Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood 110:3784–3792. 10.1182/blood-2007-03-082933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser CJ, Bhatia S, Ness K, Carter A, Francisco L, Arora M, Parker P, Forman S, Weisdorf D, Gurney JG, Baker KS (2006) Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood 108:2867–2873. 10.1182/blood-2006-02-003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun C-L, Kersey JH, Francisco L, Armenian SH, Baker Weisdorf DJ, Forman SJ, Bhatia S (2013) Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the Bone Marrow Transplantation Survivor Study. Biol Blood Marrow Transplant 19:1073–1080. 10.1016/j.bbmt.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME (2015) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. the 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21:389–401. 10.1016/j.bbmt.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SJ, Onstad L, Chow EJ, Shaw BE, Jim HSL, Syrjala KL, Baker KS, Buckley S, Flowers ME (2018) Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica 103:1535–1541. 10.3324/haematol.2018.192930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pidala J, Kurland BF, Chai X, Vogelsang G, Weisdorf DJ, Pavletic S, Cutler C, Majhail N, Lee SJ (2011) Sensitivity of changes in chronic graft-versus-host disease activity to changes in patient-reported quality of life: results from the Chronic Graft-versus-Host Disease Consortium. Haematologica 96:1528–1535. 10.3324/haematol.2011.046367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detrait MY, Morisset S, Peffault de Latour R, Yakoub-Agha I, Crocchiolo R, Tabrizi R, Bay J-O, Chevalier P, Barraco F, Raus N, Vigouroux S, Magro L, Mohty M, Milpied N, Blaise D, Socié G, Michallet M (2015) Pre-transplantation risk factors to develop sclerotic chronic GvHD after allogeneic HSCT: a multicenter retrospective study from the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Bone Marrow Transplant 50: 253–258. 10.1038/bmt.2014.244 [DOI] [PubMed] [Google Scholar]
- 9.Skert C, Patriarca F, Sperotto A, Cerno M, Filì C, Zaja F, Stocchi R, Geromin A, Damiani D, Fanin R (2006) Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica 91:258–261 [PubMed] [Google Scholar]
- 10.Uhm J, Hamad N, Shin EM, Michelis FV, Shanavas M, Gupta V, Kuruvilla J, Lipton JH, Messner HA, Seftel M, Kim D (2014) Incidence, risk factors, and long-term outcomes of sclerotic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol BloodMarrow Transplant 20:1751–1757. 10.1016/j.bbmt.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Martires KJ, Baird K, Steinberg SM, Grkovic L, Joe GO, Williams KM, Mitchell SA, Datiles M, Hakim FT, Pavletic SZ, Cowen EW (2011) Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood 118:4250–4257. 10.1182/blood-2011-04-350249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baird K, Steinberg SM, Grkovic L, Pulanic D, Cowen EW, Mitchell SA, Williams KM, Datiles MB, Bishop R, Bassim CW, Mays JW, Edwards D, Cole K, Avila DN, Taylor T, Urban A, Joe GO, Comis LE, Berger A, Stratton P, Zhang D, Shelhamer JH, Gea-Banacloche JC, Sportes C, Fowler DH, Gress RE, Pavletic SZ (2013) National Institutes of Health chronic graft-versus-host disease staging in severely affected patients: organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transplant 19:632–639. 10.1016/j.bbmt.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter PA, Kitko CL, Elad S, Flowers MED, Gea-Banacloche JC, Halter JP, Hoodin F, Johnston L, Lawitschka A,McDonald GB, Opipari AW, Savani BN, Schultz KR, Smith SR, Syrjala KL, Treister N, Vogelsang GB, Williams KM, Pavletic SZ, Martin PJ, Lee SJ, Couriel DR (2015) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. the 2014 Ancillary Therapy and Supportive Care Working Group report. Biol Blood Marrow Transplant 21: 1167–1187. 10.1016/j.bbmt.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ , Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, Pidala J, Olivieri A, Martin PJ, Przepiorka D, Pusic I, Dignan F, Mitchell SA, Lawitschka A, Jacobsohn D, Hall AM, Flowers MED, Schultz KR, Vogelsang G, Pavletic S (2015) Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response CriteriaWorking Group report. Biol Blood Marrow Transplant 21:984–999. 10.1016/j.bbmt.2015.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis LM, Grkovic L, Mitchell SA, Steinberg SM, Cowen EW, Datiles MB, Mays J, Bassim C, Joe G, Comis LE, Berger A, Avila D, Taylor T, Pulanic D, Cole K, Baruffaldi J, Fowler DH, Gress RE, Pavletic SZ (2014) NIH response criteria measures are associated with important parameters of disease severity in patients with chronic GVHD. Bone Marrow Transplant 49:1513–1520. 10.1038/bmt.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pidala J, Chai X,Martin P, Inamoto Y, Cutler C, Palmer J,Weisdorf D, Pavletic S, Arora M, Jagasia M, Jacobsohn D, Lee SJ (2013) Hand grip strength and 2-minute walk test in chronic graft-versus-host disease assessment: analysis from the Chronic GVHD Consortium. Biol Blood Marrow Transplant 19:967–972. 10.1016/j.bbmt.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon J, Spiera R (2011) Imatinib and the treatment of fibrosis: recent trials and tribulations. Curr Rheumatol Rep 13(1):51–58. 10.1007/s11926-010-0146-6 [DOI] [PubMed] [Google Scholar]
- 18.Arai S, Pidala J, Pusic I, Chai X, Jaglowski S, Khera N, Palmer J, Chen GL, Jagasia MH, Mayer SA, Wood WA, Green M, Hyun TS, Inamoto Y, Storer BE, Miklos DB, Shulman HM, Martin PJ, Sarantopoulos S, Lee SJ, Flowers MED (2016) A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clin Cancer Res 22:319–327. 10.1158/1078-0432.CCR-15-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baird K, Comis LE, Joe GO, Steinberg SM, Hakim FT, Rose JJ, Mitchell SA, Pavletic SZ, Figg WD, Yao L, Flanders KC, Takebe N, Sarantopoulos S, Booher S, Cowen EW (2015) Imatinib mesylate for the treatment of steroid-refractory sclerotic-type cutaneous chronic graft-versus-host disease. Biol Blood Marrow Transplant 21(6):1083–1090. 10.1016/j.bbmt.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro L, Mohty M, Catteau B, Coiteux V, Chevallier P, Terriou L, Jouet J-P, Yakoub-Agha I (2009) Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood 114:719–722. 10.1182/blood-2009-02-204750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers MED (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 11:945–956. 10.1016/j.bbmt.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Orthopaedic Surgeons (1965) Joint motion: method of measuring and recording. American Academy of Orthopaedic Surgeons, Chicago [Google Scholar]
- 23.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA (1969) An objective and standardized test of hand function. Arch Phys Med Rehabil 50:311–319 [PubMed] [Google Scholar]
- 24.Trites RL (1977) Grooved pegboard test – neuropsychological test manual. Royal Ottawa Hospital, Ottawa [Google Scholar]
- 25.Waters RL, Lunsford BR, Perry J, Byrd R (1988) Energy speed relationship of walking - standard tables. J Orthop Res 6:215–222. 10.1002/jor.1100060208 [DOI] [PubMed] [Google Scholar]
- 26.Mathiowetz V, Weber K, Volland G, Kashman N (1984) Reliability and validity of grip and pinch strength evaluations. J Hand Surg 9: 222–226 [DOI] [PubMed] [Google Scholar]
- 27.Hudak PL, Amadio PC, Bombardier C, Upper Extremity Collaborative Group (1996) Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder, and head). Am J Ind Med 29:602–608. [DOI] [PubMed] [Google Scholar]
- 28.Daughton DM, Fix AJ, Kass I, Bell CW, Patil KD (1982) Maximum oxygen consumption and the ADAPT quality-of-life scale. Arch Phys Med Rehabil 63:620–622 [PubMed] [Google Scholar]
- 29.Herzberg PY, Heussner P, Mumm FHA, Horak M, Hilgendorf I, von Harsdorf S, Hemmati P, Rieger K, Greinix H, Freund M, Lee SJ, Holler E, Wolff D (2010) Validation of the human activity profile questionnaire in patients after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 16:1707–1717. 10.1016/j.bbmt.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 30.Fisher AG, Jones KB (2011) Assessment of motor and process skills: volume 1 - development, standardization, and administration manual. 7th, Revised edn. Three Star Press, Inc, Fort Collins [Google Scholar]
- 31.Chen CC, Bode RK (2010) Psychometric validation of the manual ability Measure-36 (MAM-36) in patients with neurologic and musculoskeletal disorders. Arch Phys Med Rehabil 91:414–420. 10.1016/j.apmr.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Snow KK, Kosinski M, Gandek B, NEMCH (1993) SF-36 health survey: manual and interpretation guide. Health Institute, New England Medical Center, Boston [Google Scholar]
- 33.Lee SJ, Cook EF, Soiffer R, Antin JH (2002) Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant 8:444–452. 10.1053/bbmt.2002.v8.pm12234170 [DOI] [PubMed] [Google Scholar]
- 34.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC (2000) Assessing symptom distress in cancer patients: the MD Anderson Symptom Inventory. Cancer 89:1634–1646 [DOI] [PubMed] [Google Scholar]
- 35.Kennedy C, Beaton DE, Soloway S, McConnell S, Bombardier C (2011) The DASH and QuickDASH outcome measure User’s Manual, 3rd edn. Institute for Work & Health, Toronto [Google Scholar]
- 36.Abo S, Ritchie D, Denehy L, Panek-Hudson Y, Granger CL (2018) A hospital and home-based exercise program to address functional decline in people following allogeneic stem cell transplantation. Support Care Cancer 26:1727–1736. 10.1007/s00520-017-4016-x [DOI] [PubMed] [Google Scholar]
- 37.Inamoto Y, Martin PJ, Chai X, Jagasia M, Palmer J, Pidala J, Cutler C, Pavletic SZ, Arora M, Jacobsohn D, Carpenter PA, Flowers MED, Khera N, Vogelsang GB, Weisdorf D, Storer BE, Lee SJ (2012) Clinical benefit of response in chronic graft-versus-host disease. Biol Blood Marrow Transplant 18:1517–1524. 10.1016/j.bbmt.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inamoto Y, Pidala J, Chai X, Kurland BF, Weisdorf D, Flowers MED, Palmer J, Arai S, Jacobsohn D, Cutler C, Jagasia M, Goldberg JD, Martin PJ, Pavletic SZ, Vogelsang GB, Lee SJ, Carpenter PA (2014) Assessment of joint and fascia manifestations in chronic graft-versus-host disease. Arthritis Rheumatol 66:1044–1052. 10.1002/art.38293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell SA, Leidy NK, Mooney KH, Dudley WN, Beck SL, LaStayo PC, Cowen EW, Palit P, Comis LE, Krumlauf MC, Avila DN, Atlam N, Fowler DH, Pavletic SZ (2010) Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD). Bone Marrow Transplant 45:762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bevans MF, Mitchell SA, Barrett JA, Bishop MR, Childs R, Fowler D, Krumlauf M, Prince P, Shelburne N, Wehrlen L, Yang L (2014) Symptom distress predicts long-term health and well-being in allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant 20:387–395. 10.1016/j.bbmt.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bevans MF, Mitchell SA, Marden S (2008) The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Support Care Cancer 16:1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillay B, Lee SJ, Katona L, Burney S, Avery S (2014) Psychosocial factors predicting survival after allogeneic stem cell transplant. Support Care Cancer 22:2547–2555. 10.1007/s00520-014-2239-7 [DOI] [PubMed] [Google Scholar]
- 43.Raven EE, Haverkamp D, Sierevelt IN, Montfoort DO, Pöll RG, Blankevoort L, Tak PP (2008) Construct validity and reliability of the disability of arm, shoulder and hand questionnaire for upper extremity complaints in rheumatoid arthritis. J Rheumatol 35(12): 2334–2338. 10.3899/jrheum.080067 [DOI] [PubMed] [Google Scholar]
- 44.Simmonds MJ (2002) Physical function in patients with cancer: psychometric characteristics and clinical usefulness of a physical performance test battery. J Pain Symptom Manag 24(4):404–414 [DOI] [PubMed] [Google Scholar]
- 45.Holm LV, Hansen DG, Johansen C, Vedsted P, Larsen PV, Kragstrup J, Sondergaard J (2012) Participation in cancer rehabilitation and unmet needs: a population-based cohort study. Support Care Cancer 20:2913–2924. 10.1007/s00520-012-1420-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.