Abstract
Development COVID-19 vaccines in a record time has been an unprecedented global scientific achievement. However, the world has failed to ensure equitable access to what should have been a global public good. What options remain available to African countries to ensure immunization of their populations and ultimately overcome the pandemic?
Development COVID-19 vaccines in a record time has been an unprecedented global scientific achievement. However, the world has failed to ensure equitable access to what should have been a global public good. What options remain available to African countries to ensure immunization of their populations and ultimately overcome the pandemic?
Main text
Introduction: vaccine access globally, a tale of two worlds?
By the end of April, as the world just passed the one-year mark since the COVID-19 pandemic was declared, more than 150 million cases and 3 million deaths have been reported worldwide (https://covid19.who.int/), including 4,5 million cases (3%) and 120, 000 deaths (4%) in Africa (https://africacdc.org/covid-19/). Though the continent, home to 17% of the world population, shares a relatively smaller fraction of this global toll, the impact of COVID-19 has been devastating. Africa bears a disproportionate burden of infectious diseases in the face of chronic shortages in its healthcare workforce: any disruption to essential health services, any health care worker (HCW) infected, magnifies the impact of the pandemic. Health services for HIV, tuberculosis, and malaria on the continent have been severely hit by COVID-19 as access has been curtailed due to COVID-19 related movement restrictions. According to a survey by the Global Fund (The Global Fund, 2021), HIV testing has fallen by 41% on average, TB referral and screening by 28%–29%, and half of health facilities have recorded COVID-19 infections among HCWs. According to the United Nations Children’s Emergency Fund (UNICEF), interruptions in immunization campaigns will leave 80 million children under the age of 1 unvaccinated or under-vaccinated. Stringent measures promptly implemented to curb the pandemic have wreaked havoc on economies across the continent.
April also marked a contrasting historic milestone, with 1 billion doses of COVID-19 vaccines administered worldwide, following an exceptional scientific achievement that led to the development of multiple successful vaccines, many demonstrating an efficacy far exceeding the 50% threshold for authorization set by regulators, in less than a year. By end of 2020, two candidates (Pfizer BioNtech mRNA vaccine and AstraZeneca viral vectored vaccine) had already been approved for emergency use by World Health Organization (WHO) and/or the United States (US) Food and drug administration (FDA). The year was ending on an optimistic note with hope that containment of the SARS-CoV-2 virus to very low endemic levels was within reach, enabling lifting of restrictions and a progressive return to normalcy globally.
Based on the paradigm that “no one is secure until everyone is secure,” public health experts and global leaders urged that vaccines should be fairly and equitably available across the world, making appeals for a COVID-19 vaccine as a global common good (Yunus, Donaldson and Perron, 2020). The COVAX initiative, a multilateral coalition led by Gavi, the Vaccine Alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and the WHO, was set up to provide a framework for equitable access to COVID-19 vaccines worldwide. Aligned with pledges to “leave no one behind,” COVAX aims to allocate enough doses to support 92 funded low and middle income countries (LMICs), including 46 in Africa, to immunize 20% of their population in 2021, focusing on health workers and the most vulnerable groups.
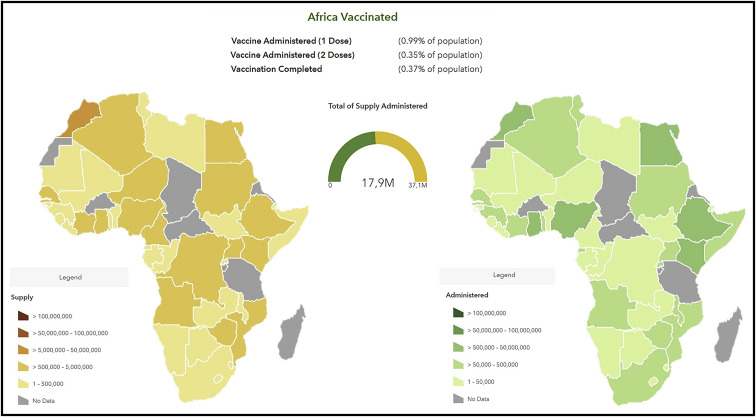
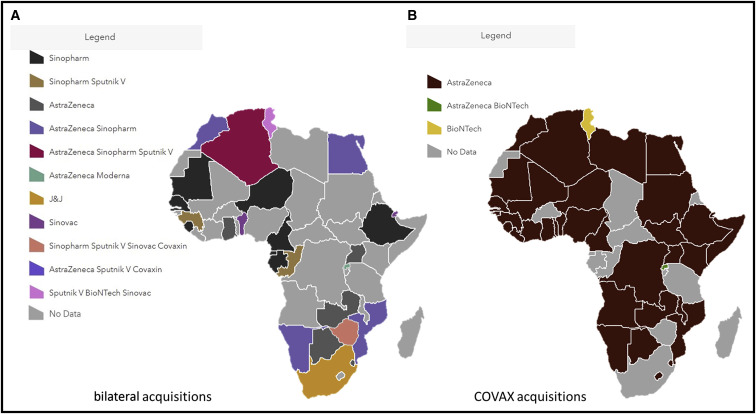
Despite these pledges for global equity, by the end of April 2021, 3 quarters of the 1 billion COVID-19 vaccine doses administered globally had been in given in 10 nations only (Kreier, 2021). African countries had barely administered 18 million COVID-19 vaccine doses out of the 37 million they had available (Figure 1 ). This represented less than 2% of all doses inoculated globally, corresponding to a coverage of only just 1.4% of the continent’s population. Vaccine access in Africa today is a case of history repeating itself, with the infamous episodes of inequitable access to HIV therapies or hoarding of the H1N1 vaccine doses by a few during the 2009 outbreak being re-enacted (Nkengasong et al., 2020). With a limited pool to choose from, African countries resorted to vaccines donated by the governments of China, Russia, and India to initiate their national COVID-19 immunization campaigns, a geopolitical phenomenon dubbed as “vaccine diplomacy,” often ahead of their inclusion on WHO Emergency Use Listing (EUL) with only limited efficacy and safety data publicly available. These vaccines included BBIBP-CorV vaccine by Sinopharm, China (used in 20 countries), Coronavac vaccine, by Sinovac, China (used in 4 countries) Sputnik V vaccine, by Gamaleya Institute, Russia (used in 6 countries), and Covaxin vaccine by Bharat Biotech, India (2 countries) (Figure 2 ). It is worth noting that COVID-19 vaccine rollout in Africa is marked by disparities at the regional level. At both extremes, 8 countries were yet to receive any supply and initiate immunization (Burkina Faso, Burundi, Chad, Central African Republic, Eritrea, Madagascar, Tanzania, and Saharawi Republic), whereas Seychelles, with close to 60% of its population fully vaccinated, is ranked at the second position globally, only behind Gibraltar.
Figure 1.
COVID-19 vaccine rollout in Africa: doses supplied and administered (by end of April 2021)
Figure 2.
COVID-19 vaccine rollout in Africa: doses obtained via multilateral (COVAX) or bilateral procurement mechanisms
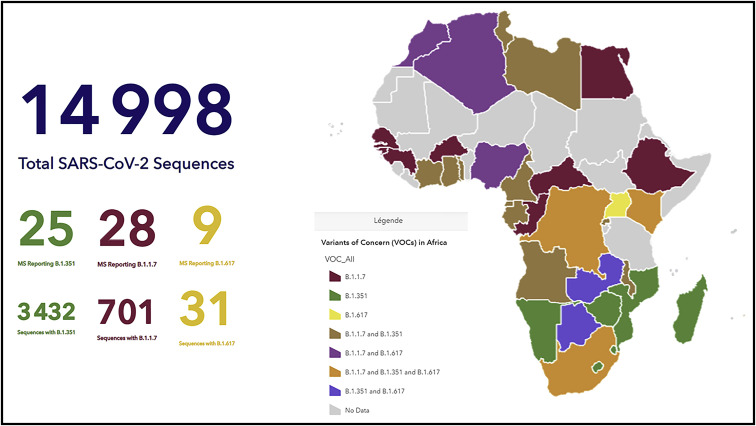
The rift in access between HICs and LIMCs is only widening. North America and Europe are already considering a return to normalcy, re-opening public spaces, lifting restrictions on public gatherings, and extending vaccination to adolescents using highly effective mRNA vaccines. In contrast, LMICs are facing devastating waves of the coronavirus, possibly driven by the rise of more transmissible variants of concern (VOC) such as the P.1 VOC first detected in Brazil or, more recently, the B.1.617 VOC in India, leading to overwhelmed healthcare services and unprecedented death tolls (Kuppalli et al., 2021). Africa is bracing itself to confront the same fate, as the B.1.351 SARS-CoV-2 VOC first detected in South Africa has been steadily spreading across the continent, and reports of the B1.1.7 and the B.1.617 VOC are on the rise, notably in incoming travelers (Figure 3 ).
Figure 3.
Map of SARS-CoV-2 variants of concern in Africa (by end of May 2021)
Here, we explore some of the factors explaining why Africa finds itself once again at the back of the queue for access to life saving therapeutics, the best practices at country level that should inform vaccine choice and scale up on the continent, and priorities to improve access to life saving vaccines during the response to this pandemic or any future outbreak.
Vaccine nationalism and the failure to ensure global equity
Despite financially committing to COVAX, HICs have prioritized national access over global equity. By concluding pre-purchase agreements directly with manufacturers and stockpiling enough doses to vaccinate their population several time over, a handful of wealthy nations have depleted vaccine supply available for LMICs (Callaway, 2020). Exports restrictions placed on vaccines, vaccine raw components, and on delivery supplies such as syringes and glass bottles, have further undermined the equity focused partnership (see Box 1 ). The self-centered approach is now culminating as wealthy nations move ahead to vaccinate younger age groups, mostly disregarding pleas to share excess doses to allow coverage of HCW and vulnerable populations elsewhere. As HICs bid to achieve an illusory return to normalcy within their own borders, the looming threat of new variants increases, as the virus continues to transmit and mutate unabated in other parts of the world. This situation has prompted South African President, Cyril Ramaphosa, to warn about an impending “vaccine apartheid.”
Box1.
The AstraZeneca vaccine and COVAX: exposing vulnerabilities in the current model for COVID-19 vaccine access in Africa?
The AstraZeneca vaccine, a successful venture between academia (the Jenner Institute at the University of Oxford) and the pharmaceutical sector, leading to the record speed development of an effective, easy to store and affordable vaccine, showcases the unprecedented worldwide mobilization for vaccine research and development. A landmark partnership between AstraZeneca and Serum Institute of India (SSI) for a licensed version of the vaccine (COVISHIELD) has been a further step to scale up production capacity and address demand for COVID-19 vaccines in the Global South. The AstraZeneca vaccine is currently the cornerstone of the COVAX initiative, representing the quasi totality of the 237 million vaccine doses forecasted for distribution during the first half of 2021 (www.gavi.org). With more than 17 million doses delivered in 35 AU member states by end of April, the vaccine is also the back bone of COVID-19 vaccine campaigns in Africa. However, 2 months after the first COVAX consignment landed in Ghana, providing optimism that the continent would finally join global efforts to roll out COVID-19 vaccination, technical challenges have cumulated, jeopardizing COVAX’s goal to ensure 20% coverage to immunize those most at risk and setting back timelines for access on the continent.
Limited global manufacturing capacity
Increased demand for vaccines in India, faced with a devastating up surge in COVID-19 cases, and government decision to redirect locally produced doses toward the domestic market has had repercussions around the globe (Kuppalli et al., 2021). As a consequence, SSI, the chief supplier for COVAX, has postponed delivery of the 90 million vaccine doses expected in March and April. Extra constraints following productions failures along the vaccine supply chain in Europe have led to further delays for delivery of AstraZeneca doses. The African countries which have been most efficient in administering doses received as part of their initial allocation, such as Rwanda and Ghana, need now to decide whether to reserve doses to vaccinate the most people fully, or to provide partial immunity to the most people while running the risk that many will miss their second dose.
Emerging variants of concern with a potential for immune evasion
The emergence of SARS-CoV-2 virus variants harboring key mutations in the spike protein of the virus, the main target for currently available vaccines, has been a cause for anxiety. In vitro studies have indicated the potential of the B.1.351 variant for escape from natural immunity or that conferred by vaccination (Table 2). These observations seemed substantiated by preliminary data from the phase I/II trial of the AstraZeneca vaccine in South Africa, with only minimal efficacy (10.4%) demonstrated against mild to moderate symptomatic infection with the B.1.351 variant, though the small sized study did not allow evaluation of protection against severe disease. Citing its commitment to ensure use of highly efficacious vaccines, the South African government decided to halt the rollout of the 1 million AstraZeneca vaccine doses it had just received, and instead opted to deploy the Johnson & Johnson COVID-19 vaccine Janssen, with reported higher efficacy (57%) against the variant, as part of a phase IIIb implementation study (Table 3). Meanwhile, both Africa CDC and WHO recommended that rollout of the vaccine should continue based on the favorable risk benefit balance.
Adverse events and vaccine hesitancy
Concurrently, concern in AU Member States has been mounting over reports of rare blood clotting disorders associated with the AstraZeneca vaccine in Europe. Reports of adverse events following immunization (AEFI) and investigation of their potential causal association with vaccine administration are a testimony to effective pharmacovigilance systems which enable constant update of information on vaccine risks and benefits. However, temporary pauses to rollout enacted by several European countries and ensuing restrictions limiting administration of the AstraZeneca vaccine to older age groups, have sparked suspicion in African countries. An earlier survey conducted by the Africa CDC and the London School of Hygiene & Tropical Medicine had indicated an initial willingness to undergo COVID-19 immunization with a safe and efficient vaccine, as demonstrated by an average acceptability rate of 79% in respondents across 15 countries (Samarasekera, 2021). The current situation has however had negative repercussions on vaccine uptake: the Democratic Republic of Congo (DRC) postponed deployment of the vaccine for close to a month, whereas Chad forfeited its COVAX allocation altogether. Furthermore, as wealthier nations are now turning away from AstraZeneca in favor of mRNA vaccines, while offering excess doses to COVAX for redistribution, there is a perception that second best options are being relegated to African countries, which could further contribute to build mistrust on the continent.
These setbacks have been exacerbated by a backdrop of insufficient readiness to achieve the last mile to deliver vaccine doses in the arms of an adult population not usually reached by routine immunization programs. It is becoming clear that the COVAX model, based on philanthropy and good will of wealthier nations, will not lead to equitable and fair vaccine access soon enough in the face of urgency. The market dominance of a few nations have sustainably undermined the multilateral initiative.
To ensure the continued relevance of the COVAX initiative, and ensure equitable access to vaccines for all, it is imperative that: 1) financial commitments by IHCs be increased, 2) excess doses stockpiled by wealthier nations be availed for redistribution to LMICs, targeting in priority those regions where the prevalence of COVID-19 cases is the highest, 3) the pool of vaccine manufacturers be diversified, and 4) the portfolio of vaccines to be distributed be expanded. Strategies should be applied to vaccinate the most people in the shortest time, such as delaying second doses or using a single dose in those with pre-existing immunity from prior infection. But most importantly for Africa, COVAX needs to be complemented by homegrown initiatives to attain a sufficient target of 60% herd immunity ahead of the emergence and spread of new virus variants.
Intrinsic factors limiting local access in Africa
The fact that Africa is lagging behind the rest of the world for access to vaccines reflects the paucity of sustainable national investments in domestic vaccine research and development (R&D) programs and industry. Though vaccines are clearly acknowledged as a cornerstone of the COVID-19 pandemic response, domestic production has not been a priority for national outbreak preparedness.
On the pull side of vaccine R&D, capacity for vaccine manufacturing is mostly lacking. Africa, home to a population of 1.3 billion and faced with a high prevalence of infectious diseases, only produces 1% of the doses it uses, despite a high need for vaccines to ensure health security (Abiodun et al., 2021). Around 10 local companies operate at some stage of the vaccine manufacturing value chain on the whole continent, from production of the vaccine ingredients to the “fill and finish” process. The Pasteur Institute in Dakar, Senegal, is the only institution that produces a prequalified WHO vaccine (for yellow fever). Moreover, vaccines in Africa are in large part supplied by external donors, such as GAVI or UNICEF. Having outsourced the procurement process, and faced with patents access issues, African nations have had few incentives to support a local vaccine industry.
According to the WHO landscape of COVID-19 vaccines, a total of 277 candidates were under development by end of April 2021. Africa had contributed a mere five, with four candidates being tested at Egypt National Research Centre, another one at a Nigeria-based private firm (Table 1 ), and with none having yet progressed past the pre-clinical evaluation phase. At the later phase of human clinical trials, there has been an initial reluctance to join global efforts to assess new COVID-19 candidate vaccines, even though the continent hosts a number of research sites with pre-established expertise for trials (Nkengasong et al., 2020). Public opinion relayed suspicions that Africa was being be used as testing ground by western researchers to assess unsafe vaccines (Samarasekera, 2021). One notable exception has been South Africa where several COVID-19 clinical trials have been undertaken (Tables 2 and 3 ). This underdeveloped R&D ecosystem and over-reliance on external financial influx for supplying vaccines may explain why most African countries, especially those with small populations, have remained minor players in the race to secure advanced market commitments.
Table 1.
Overview of the COVID-19 vaccine research landscape and evidence from Africa: preclinical studiesa
| Country | Developer | Vaccine Platform |
|---|---|---|
| Egypt | National Research Centre | DNA based |
| Egypt | National Research Centre | Inactivated whole virus |
| Egypt | National Research Centre | Non replicating viral vector (Influenza A H1N1) |
| Egypt | National Research Centre | Protein Subunit S, N, M&S1 protein |
| Nigeria | Helix Biogen Consult | Protein Subunit (S), E. coli based expression |
aReferenced from WHO COVID-19 candidate vaccine landscape and tracker (version of 30th April 2021)
Table 2.
Overview of the COVID-19 vaccine research landscape and evidence from Africa: COVID-19 vaccines authorized or under clinical evaluation in Africaa
| Vaccine/ Manufacturer | Platform | Dose (route) | Storage Temperature a | Efficacy (symptomatic COVID-19)a | Efficacy (hospitalization and death) a | Regulatory status | Evaluation in Africa (Country) | Trial No/Phase | Target population/sample sizeb | Efficacy against B.1.351 variant (Beta) | Efficacy against B. 1.1.7 variant (Alpha) | Efficacy against B. 1.617.2 variant (Delta) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BNT162 /Comirnaty | mRNA | 2 doses (Injectable) | 6 months at −70°C | 95% | 100% | WHO/EMA/FDA emergency use | South Africa | NCT04368728 | 16-85 years | Reduced neutralizing Ab titers in vitro (by 4.9) | Reduced neutralizing Ab titers in vitro (by 2.6) | Reduced neutralizing Ab titers in vitro (by 5.8) |
| (Pfizer BioNtech) | 2 weeks at −15 to −25°C | |||||||||||
| Phase 2/3 | n = 43,548 (across 152 sites and 6 countries) | 75% (in Qatar) | 89,5% (in Qatar) | 96% (in UK) | ||||||||
| 5 days up to 1 month at 2 −8°C (undiluted) | ||||||||||||
| 95;3% (in Israel, setting with 94·5% 501Y.V1) | ||||||||||||
| ChAdOx1 /Vaxzevria | Viral vector | 2 doses | 6 months at 2 −8°C | 63% | 100% | WHO/EMA/FDA emergency use | South Africa | PACTR202006922165132/NCT04444674 | 18-65 years n = 2130 | 10,4% (among 39 participants with Beta infection in South Africa) | 74.6% | 92% (in Qatar) |
| (Injectable) | Reduced neutralizing Ab titers in vitro (by 9) | |||||||||||
| (AstraZeneca & University of Oxford) | ||||||||||||
| phase 1b/2 | ||||||||||||
| 6 h at 30°C | Kenya | PACTR202005681895696 | 45-64 years | |||||||||
| phase 1b/2 | n = 400 | |||||||||||
| Ad26.COV2.S | Viral vector | 1 dose | 2 years at −20°C | 66% overall | 100% | WHO/EMA/FDA emergency use | South Africa | NCT04505722 | ≥ 18 years | 57% (setting with 95% Beta in South Africa) | / | |
| (Johnson & Johnson) | (Injectable) | 3 months at 2-8°C | 85% against severe disease | Phase 3 | n = 6,576 | |||||||
| mRNA-1273 | mRNA | 2 doses | −25 to −15°C | 94.1% | 100% | WHO/EMA/FDA emergency use | No | / | / | Reduced neutralizing Ab titers in vitro (by 6) Elevated neutralizing Ab titers upon boosting 6 to 8 months after the primary series |
No impact in vitro | |
| (Moderna) | (Injectable) | 30 days at 2–8°C | ||||||||||
| 24 h at 25°C | ||||||||||||
| Sputnik V | Viral vector | 2 doses (Injectable) | 91.6% | 100% | / | Guinea Conakry | (pre deployment pilot) | Older adults | Reduced neutralizing Ab titers in vitro (by 6.1) | No impact in vitro | ||
| (Gamaleya Institute) | n = 60 | |||||||||||
| BBIBP-CorV/Vero Cell | Inactivated | 2 doses (Injectable) | 2 years at 2-8°C | 78.1% | 78,1% (hospitalization) | WHO emergency use | Egypt | NCT04510207/ChiCTR2000034780 | ≥ 18 years | Reduced neutralizing Ab titers in vitro (by 1.5) | No impact in vitro | |
| (Sinopharm) | / (death) | Phase 3 | n = 45000 (across 4 countries) | |||||||||
| Morocco | ChiCTR2000039000 | ≥ 18 years | ||||||||||
| Phase 3 | n = 600 | |||||||||||
| CoronaVacc | Inactivated virus | 2 doses | 1 to 2 years at 2-8°C | 51% – 84% across trial sites | 85%-100% | WHO emergency use | / | / | / | Reduced neutralizing Ab titers in vitro (by 3.3) | Reduced neutralizing Ab titers in vitro (by 2) | |
| (Sinovac) | (Injectable) | |||||||||||
| Covaxinc | Inactivated virus | 2 doses | 2-8°C | 78% | 100% | / | No | / | / | / | No impact in vitro | |
| (Barhat Biotech) | (Injectable) | 28 days open vial policy | ||||||||||
| SARS-CoV-2 rS/Matrix-M1 Adjuvant | Protein subunit | 2 doses | 2-8°C | 90.4% | 100% | / | South Africa | NCT04533399 | 18-84 years | 60.1% in HIV- | 85.6% | |
| (Novavax) | (Injectable) | Phase 2 | n = 4400 | 50.1% overall (setting with 92.7% Beta) | ||||||||
| GRAd-COV2 | Viral vector | 1 or 2 doses | / | / | / | South Africa | EUCTR2020-005915-39 | ≥ 18 years | / | / | ||
| (ReiThera | (Injectable) | Phase 2/3 | ||||||||||
| hAd5-S+N | Viral vector | 1 dose | room temperature (oral formulation) | / | / | / | South Africa | NCT04710303 | 18-50 years | / | / | |
| (ImmunityBio) | (Injectable or oral) | phase 1 | n = 35 |
References are included in supplementary appendix. If data unavailable from peer reviewed literature, efficacy endpoint data were obtained from WHO strategic advisory group of experts (SAGE) public evidence assessment reports, or from manufacturer’s press releases.
Details obtained from listed clinical trial registers
Vaccines currently in use but which have not been evaluated in Africa
Table 3.
Overview of the COVID-19 vaccine research landscape and evidence from Africa: post marketing effectiveness studiesa
| Country | Title | Trial No | Vaccine | Sample size/age | Outcome |
|---|---|---|---|---|---|
| Egypt | Impact of COVID 19 Vaccine on Safety, Blood Elements, and Immunogenicity of the Egyptian Population | NCT04809948 | ChAdOx1 nCoV-19 /AstraZeneca | 800, 18-80 years | adverse side effects, hematological values; immunogenicity |
| Egypt | T Cell, Antibody and Cytokine Responses to Single and Double Doses of COVID-19 Vaccines in Egyptians | NCT04706143 | Lived attenuated (Sinopharm), mRNA (Pfizer/ BioNtech) and viral vector (Oxford/AastarZeneca) vaccines | 100, 25-65 years | T Cell, Antibody and Cytokine Responses |
| South Africa | Open-label, Single-arm Phase 3B Implementation Study to Monitor the Effectiveness of the Single-dose Ad26.COV2.S COVID-19 Vaccine Among Health Care Workers in South Africa (SISONKE study) | NCT04838795 | Ad26.COV2.S/Johnson & Johnson | 500,000, ≥ 18 years | effectiveness on severe COVID, hospitalizations and deaths in HCWs |
Referenced from WHO Landscape of observational study designs on the effectiveness of COVID-19 vaccination (version 19th April 2021)
Each nation is responsible for the regulation of medical products within its borders. However, with few regulatory entities up to international standards, African countries mostly rely on external stringent regulatory agencies. These ones act as surrogate decision makers for reviewing trial data and granting approval for new therapeutics and vaccines. Countries then use accelerated mechanisms to issue national marketing authorization. These various processes cause delays and prevent the use of i.e.: national rolling reviews of trial data to expedite approval.
Fragilities of African healthcare systems are a further impediment to deploying COVID-19 vaccines at scale and at speed. A World Bank assessment estimated that adequate readiness was in place in most LMICs to manage small initial consignments delivered through COVAX (The World Bank, 2021). This included having developed a national deployment and vaccination plan, defined priority target populations, established cold chain and logistics, procedures for safety surveillance and adverse effect monitoring, and trained vaccination teams. Notwithstanding, after the first tranche of the vaccines landed, countries soon realized that tackling the last mile to inoculate doses in people’s arms presented with additional operational stumbling blocks, notably ability to mobilize communities for vaccine uptake and to micromanage the logistics of delivery countrywide. By the end of April, only Morocco (9.5 million doses administered) and few other countries (Nigeria, Ethiopia, Egypt, Ghana, and Kenya) were nearing or surpassing the 1 million doses uptake mark (Figure 1).
The best practices and success stories at country level
Generating local evidence
With its pre-established expertise in large-scale clinical and vaccine trials, spanning decades long efforts and partnerships to develop effective treatments and vaccines against HIV and tuberculosis, South Africa has been a trailblazer for the evaluation of new COVID-19 vaccines. Experienced scientific teams led randomized clinical trials for a variety of vaccine candidates: from classical peptide based, to viral vector based, and onward to the innovative mRNA platforms. The early phase I to late stage III trials (Table 2) contributed significant evidence about safety and efficacy in more than 52,000 participants total, recruited across ethnic groups and including in HIV infected individuals, for three of the vaccines subsequently listed for emergency use by WHO (Pfizer mRNA, AstraZeneca ChAdOx1, and Johnson & Johnson Ad26.COV2.S vaccine candidates). South African trials provided invaluable data about the impact of the new B.1.351 SARS-CoV-2 variant on vaccine-induced immune responses, showing how efficacy was diminished compared to the earlier strain of the virus (Table 2). As a consequence, South African public health authorities decided to instead deploy the Johnson & Johnson candidate under a phase IIIb implementation trial. Named Sisonke (together) this national undertaking served two goals: to protect at risk frontline HCWs faced with a resurgence of cases and to provide a blueprint for the upcoming country vaccine mass roll-out while vaccine licensure was underway. The northern African countries of Morocco and Egypt adopted similar strategies in response to vaccine procurement barriers, negotiating participation to late stage trials of the BIPP vaccine candidate produced by Sinopharm, to generate local evidence on safety and efficacy and obtain preferential access for mass administration upon national approval of the vaccine candidate
National leadership and preparedness
Morocco, Ghana, and Rwanda managed to administer 90% or more of their vaccine supply 3 months after launching their national campaigns, whereas Seychelles had fully vaccinated close to 50% of its total population over the same period. Among the best practices that contributed to an efficient vaccine rollout, countries have highlighted setting up a proactive national immunization technical advisory group (NITAG), or equivalent, to provide evidence-based recommendations on immunization and accelerate national authorization of new vaccines (Seychelles and Morocco), demand generation via mass public sensitization campaigns (Morocco, Rwanda, and Seychelles), setting up electronic pre-registration portals (Morocco) and pre-listing eligible frontline workers and high risk groups (Rwanda), prepositioning essential commodities such as ultra-cold freezers and refrigerated vehicles (Rwanda), setting up ad hoc vaccination centers in prisons, nursing homes (Seychelles), or public markets (Rwanda), and outreach strategies with mobile vaccination teams (Seychelles).
Producing vaccines at the point of need
Some African countries are attempting to set up local manufacturing capacity as part of bilateral deals for vaccine access. In Northern Africa, Algeria negotiations with Russia for the purchase of the Sputnik V vaccine have included arrangements to allow technology transfer to a local production plan to kick start manufacturing of the vaccine by September 2021 (Algeria Press Services, 2021). According to its Minister of Health, Algeria endeavor is to address national demand for COVID-19 vaccines but also that from other African countries. Likewise, Egypt recently reached an agreement with China to ensure fill and finish production of 2 million doses of the Sinovac vaccine at the Vacsera facilities by June 2021, with plans for technology transfer to also allow local manufacturing of ingredients for the vaccine in the future (Bridge Consulting, 2021).
Which vaccine(s)?
The portfolio of safe and efficacious vaccines available to African countries has remained limited until now, even though considerations such as stability at room or regular fridge temperature or availability of a single dose regimen are paramount to efficiently vaccinating priority groups and remote populations in view of existing fragilities in healthcare services. The Pfizer mRNA vaccine, which demonstrates high effectiveness against COVID-19 disease, including against the B.1.351 SARS-CoV-2 VOC in Qatar (Table 2), has not been a prime choice for deployment as a consequence of logistical constraints for ultra-cold storage and the scarcity of doses available. Less than 320,000 doses of the vaccine have been delivered by COVAX among four countries (Cabo Verde, Tunisia, Rwanda, and South Africa). The AstraZeneca vaccine, available at cost via COVAX, and the one dose only Johnson & Johnson vaccine, both stable at regular fridge temperature, have been more attractive options. But concerns have cumulated over reports of extremely rare blood clotting events (Box 1) for both and suboptimal efficacy against the B.1.351 SARS-CoV-2 variant for the AstraZeneca vaccine specifically (Table 2). Inclusion of the Moderna, the Sinopharm, and the Sinovac vaccine candidates on WHO EUL on 29th April, 7th May and June 1st, respectively, has diversified the portfolio of available vaccines to six. A recent agreement between Moderna and Gavi will allow delivery of 34 million doses to funded LMICs until the end of 2021. The Novavax vaccine, which demonstrated good interim efficacy against SARS-CoV-2 VOC (Table 2), would equally represent an interesting addition to the portfolio of vaccines as 350 million doses have already been secured by COVAX, with first deliveries expected during the 3rd quarter of 2021.
Prioritize interventions to overcome the challenges
To address the formidable undertaking of rolling out COVID-19 vaccination at scale and at speed to 1.3 billion people, African Union (AU) member states have rallied behind an all of Africa approach. The COVID-19 Vaccine Development and Access Strategy (https://africacdc.org/covid-19/covid-19-resources/) was endorsed in August 2020, with the chief objective to complement COVAX efforts and immunize at least 60% of the African population to achieve herd immunity. The AU and Africa CDC have spearheaded a number of initiatives, closely aligned with priorities set forth in the strategy, aiming to strengthen capacity, provide regional stewardship, and coordination, and achieve a substantive impact through high level political engagement.
The africa CDC consortium for COVID-19 vaccine clinical trials (CONCVACT) and the pathogen genomics institute (PGI): Bolstering local R&D
Africa CDC CONCVACT has been established to promote investments in local innovation, as a push mechanism to support vaccines R&D in Africa. Current priorities include the development and evaluation of next generation COVID-19 vaccines, better adapted to the African context. Thermostable formulations allowing cold chain free storage will be key for mass vaccination campaigns in remote areas. Vaccines with alternative administration modalities (needle free, intranasal, or oral) will facilitate immunization of younger age groups or permit self-immunization. The hAd5-S+N vaccine candidate by ImmunityBio, which has entered a phase I trial in South Africa with plans for evaluation of a room-temperature oral capsule, is a representative example (Table 2). As hAd5-S+N targets both the spike (S) and nucleocapsid (N) viral proteins, it could provide T-cell-based immune protection against current VOC, or be used as a booster following (S) only based vaccines for a broader immune repertoire. The CureVac CVnCoV vaccine candidate, stable up to 3 months at standard fridge temperature, could be a game changer and bring mRNA based vaccines within close reach of African vaccine programs. Currently under late stage phase II/III evaluation in South America (Peru and Panama), CVnCoV has not been evaluated in Africa. The NDV-HXP-S vaccine, a candidate developed under the coordination of PATH Center for Vaccine Innovation and Access, is mass produced in eggs based using the same fast and affordable method used for influenza vaccines. It has recently entered clinical trials in South America (Brazil) and South East Asia (Vietnam and Thailand) with licensing agreements provided for local production (Zimmer, 2021). This type of partnership should be emulated on the continent to open the door to domestic manufacturing.
Substantial knowledge gaps equally remain for COVID-19 vaccines at the post-marketing stage which have been outlined in the WHO R&D Blueprint for COVID-19 vaccines. As countries move to expand COVID-19 vaccination campaigns beyond priority groups, administration to special populations (children, pregnant women and their infants, immunosuppressed individuals) will require additional safety data. Real world effectiveness data, notably in relation to existing and new SARS-CoV-2 VOC, will be crucial to inform national re-vaccination schedules or to adjust vaccine choice as necessary. The situation of Seychelles, which is facing a surge of COVID-19 cases despite a high immunization coverage, underscores the need and relevance of such studies, of which there are only few currently ongoing on the continent (Table 3).
These research efforts will need coupling with early warning systems based on genomic surveillance of emerging SARS Cov2 variants. To date, more than 1 million coronavirus genome sequences have been uploaded on the GISAID online sequencing data repository globally (Maxmen, 2021). In contrast, by 28th May 2021, a total of only 14998 SARS-CoV-2 sequences had been shared by African countries (Figure 3). The Africa CDC PGI, an integrated, cross-continental network of laboratories equipped with the necessary tools, human resources, and data infrastructure aims to fully leverage critical genomic sequencing technologies on the continent. In a collaborative effort with WHO AFRO, it will allow the comprehensive mapping of SARS-CoV-2 mutations and monitoring of virus transmission patterns.
The africa medicine agency (AMA) and the africa regulatory task force: Strengthening regulation and expediting approval
Weak regulatory systems create barriers to the safe and efficient introduction of COVID-19 vaccines on the African continent. To minimize delays, Africa CDC has established the Africa Regulatory Task Force, a joint effort with the African Union Development Agency (AUDA-NEPAD), African Medicines Regulatory Harmonization (AMRH) Initiative, and the WHO African Vaccine Regulatory Forum (AVAREF). This mechanism provides a centralized framework that enables AU Member States to expedite emergency marketing authorizations of new vaccines during the pandemic. Ultimately, the long awaited AMA, a competent continental medicines regulatory body, will be critical to facilitate the review of drugs trial data, including for homegrown vaccine candidates. This harmonized regional approach will ensure timely access to safe and effective new medical products delivered by African health systems for routine care, and approval for emergency use during outbreaks. The pace must however be accelerated to bring AMA to existence; two years after the adoption of the treaty that marked its creation at the 32nd ordinary session of the Assembly AU, only 8 countries out of the of the 15 required have ratified the document (Ncube, Dube and Ward, 2021).
The central regulatory entity will likewise sustain the pharmacovigilance of new vaccines based on locally collected and analyzed data pertaining to potential adverse events following immunization (AEFIs). Local context, evidence, and risk-benefit ratio should underpin the country-level decision to suspend use of a new vaccine due to safety concerns. Repercussions of halting rollout cannot be comparable in HICs with access to a diverse portfolio of vaccines and LMICs with no or only limited alternatives. South Africa is the nation burdened with heaviest caseload and fatalities on the continent (https://africacdc.org/covid-19/), and access to vaccines was vital to provide relief to exposed and overstrained HCW dealing with a resurgence of cases. Yet, a two-week pause in administration of the Johnson & Johnson vaccine consecutive to reports of rare thrombotic events in the USA, which followed the decision to forgo AstraZeneca vaccine rollout due to doubts about its efficacy against the B.1.351 VOC, has disrupted early efforts to immunize HCW and country timelines for achieving herd immunity (Oliver, 2021). This highlights the need for coordinated regional action to track and report AEFI, ensure their prompt review by and issue context relevant and evidence based guidance to member
The AU african vaccine acquisition task team (AVATT): Securing vaccine supplies
Even if it reaches its pre-set delivery target for the year 2021, COVAX will still only allocate enough doses to cover 20% of the population in participating African countries. To overcome limitations in purchasing power, the AU established the AVATT. The mechanism operates through the Africa Medical Supplies Platform (AMSP) to negotiate pooled supply of COVID-19 vaccine doses. A partnership with the African Export-Import Bank (Afreximbank) facilitates payments by providing advance procurement commitment guarantees of up to 2 billion USD to the manufacturers on behalf of the Member States. AVATT aims to supply enough vaccine doses to attain the continental target 60% herd immunity by 2022.
The recent agreement signed between Johnson & Johnson and the AU for delivery of 400 million vaccine doses to member states, including formulation, filling, and packaging at Aspen Pharmacare in South Africa Aspen, is a first step toward domestic capacity scale up to address the needs of the African market. Such initiatives will lay the groundwork toward building autonomous and sustainable vaccine industries on the continent, encompassing the full vaccine development and production chain, and lower over reliance on external suppliers.
On 12th and 13th April, a landmark vaccine summit was convened by the AU and Africa CDC (Irwin, 2021). AU heads of states, acknowledging the central role of vaccine manufacturing for Africa’s public health security, pledged to increase to 60% the share of vaccines produced on the continent by 2040. To achieve this long term ambition, high-level political commitment, international cooperation, and support from international financial organizations will be needed. With support from over 100 countries, India and South Africa have been petitioning the World Trade Organization (WTO) for temporary waivers of intellectual property protections on COVID-19 vaccines and treatments. This decision could facilitate additional vaccine manufacturing, including in LMICS, and help resolve persistent shortages. The support recently expressed by the US administration has created momentum and is an additional significant step toward vaccine equity in the Global South.
Conclusion and way forward
The Lancet COVID-19 Commission has identified new SARS-CoV-2 VOC as the chief menace to pandemic control efforts, including vaccination (Lee et al., 2021). New variants’ potential for increased transmissibility, increased disease severity, and immune escape might overturn the impressive progresses achieved in wealthier parts of the globe, but which the WHO Director General has characterized as a “vaccine euphoria.” In an interconnected world, saving lives and restoring the global economy will not be possible unless vaccines are deployed at scale and at speed to everyone in every part of the world to ensure maximum suppression of the virus. If regions with high disease prevalence and high transmission levels persist, new variants will thrive and the pandemic will never end.
Africa must and can handle this colossal task, but the rules of the game need to change. Current multilateral access models based on solidarity and philanthropy from wealthier nations have proven unsuccessful to ensure equitable vaccine distribution to LMICs in the face of vaccine nationalism. The continent over-reliance on external suppliers and external funders will not allow it to move ahead of the waiting list.
AU member states, multilateral organizations, and partners must rally and advance Africa’s vision to establish sustainable domestic vaccine manufacturing capability at scale, which will, at term, enhance the response to the current COVID-19 pandemic and preparedness for future outbreaks and to achieve continental health security. This is an historic moment and an opportunity not to be missed.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.immuni.2021.06.017.
Supplemental information
References
- Abiodun T., Andersen H., Mamo L.T., Sisay O. 2021. Vaccine Manufacturing in Africa: What It Takes and Why It Matters. Tony Blair Institute for Global Change.https://institute.global/advisory/vaccine-manufacturing-africa-what-it-takes-and-why-it-matters [Google Scholar]
- Algeria Press Services . 2021. Sputnik V vaccine to be produced in Algeria as from September.https://www.aps.dz/en/health-science-technology/38794-sputnik-v-vaccine-to-be-produced-in-algeria-as-from-september [Google Scholar]
- Bridge Consulting . 2021. China COVID-19 Vaccine Tracker.https://bridgebeijing.com/our-publications/our-publications-1/china-covid-19-vaccines-tracker/ [Google Scholar]
- Callaway E. The unequal scramble for coronavirus vaccines - by the numbers. Nature. 2020:506–507. doi: 10.1038/d41586-020-02450-x. [DOI] [PubMed] [Google Scholar]
- Irwin A. How COVID spurred Africa to plot a vaccines revolution. Nature. 2021 doi: 10.1038/d41586-021-01048-1. [DOI] [PubMed] [Google Scholar]
- Kreier F. Unprecedented achievement”: who received the first billion COVID vaccinations? Nature. 2021 doi: 10.1038/d41586-021-01136-2. [DOI] [PubMed] [Google Scholar]
- Kuppalli K., Gala P., Cherabuddi K., Kalantri S.P., Mohanan M., Mukherjee B., Pinto L., Prakash M., Pramesh C.S., Rathi S., et al. India’s COVID-19 crisis: a call for international action. Lancet. 2021;397:2132–2135. doi: 10.1016/s0140-6736(21)01121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Bullen C., Karim S.S.A., Bush S., Lo Y.C., Michie S., Rostila M., Smith L., Amor Y.B., Kim B. SARS-CoV-2 variants: the need for urgent public health action beyond vaccines. The Lancet COVID-19 Commission Task Force on Public Health Measures to Suppress the Pandemic. https://covid19commission.org/commission-publications
- Maxmen A. One million coronavirus sequences: popular genome site hits mega milestone. Nature. 2021;593:21. doi: 10.1038/d41586-021-01069-w. [DOI] [PubMed] [Google Scholar]
- Ncube B.M., Dube A., Ward K. Establishment of the African Medicines Agency: progress, challenges and regulatory readiness. J. Pharm. Policy Pract. 2021;14:29. doi: 10.1186/s40545-020-00281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengasong J.N., Ndembi N., Tshangela A., Raji T. COVID-19 vaccines: how to ensure Africa has access. Nature. 2020;586:197–199. doi: 10.1038/d41586-020-02774-8. [DOI] [PubMed] [Google Scholar]
- Oliver G. South Africa’s daunting COVID-19 vaccine rollout. The New Humanitarian. 2021 https://www.thenewhumanitarian.org/analysis/2021/4/28/south-africas-daunting-COVID-19-vaccine-rollout [Google Scholar]
- Samarasekera U. Feelings towards COVID-19 vaccination in Africa. Lancet Infect. Dis. 2021;21:324. doi: 10.1016/S1473-3099(21)00082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Global Fund . 2021. The impact of COVID-19 on HIV, TB and malaria services and systems for health.https://openknowledge.worldbank.org/bitstream/handle/10986/34496/9781464816024.pd [Google Scholar]
- The World Bank . 2021. Assessing Country Readiness for COVID-19 Vaccines.http://documents1.worldbank.org/curated/en/467291615997445437/pdf/Assessing-Country-Readiness-for-COVID-19-Vaccines-First-Insights-from-the-Assessment-Rollout.pdf [DOI] [Google Scholar]
- Yunus M., Donaldson C., Perron J.-L. COVID-19 Vaccines A Global Common Good. The Lancet Healthy Longevity. 2020 doi: 10.1016/s2666-7568(20)30003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C. Researchers Are Hatching a Low-Cost Covid-19 Vaccine. The New York Times. 2021 https://www.nytimes.com/2021/04/05/health/hexapro-mclellan-vaccine.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.