Abstract
Not available.
Keywords: food allergy, IgE diagnostics, provocation testing, nutritional counseling, therapy
Developmental stage: S2k
AWMF guideline register number: (061-031)
Completion: June 2021
Validity: Until December 31, 2024
ICD-10 codes: T78.0, T78.1, L27.2, L23.6, T78.2
Abbreviations. Abbreviations.
| AAAAI | American Academy of Allergy, Asthma and Immunology |
| AGATE | Working Group on Anaphylaxis – Training and Education |
| ASA | Acetylsalicylic acid |
| BAT | Basophil activation test |
| OD | Occupational disease |
| CCD | Cross-reactive carbohydrate determinants, cross-reactive carbohydrate side chains |
| CSACI | Canadian Society of Allergy and Clinical Immunology |
| DBPCFC | Double-blind placebo-controlled food challenge |
| DGES | Study on the health of adults in Germany |
| DELBI | German instrument for methodological guideline evaluation |
| EAACI | European Academy of Allergy and Clinical Immunology |
| EGID | Eosinophilic gastrointestinal disorders, eosinophil-associated diseases of the gastrointestinal tract |
| FDA | Food and drug administration |
| FPIES | Food protein-induced enterocolitis syndrome, food protein-induced enterocolitis syndrome |
| GIT | Gastrointestinal tract |
| HMO | Human milk oligosaccharides |
| HMW | High molecular weight |
| IgE | Immunoglobulin E |
| IgG | Immunoglobulin G |
| GR | Gastroesophageal reflux |
| CI | Confidence interval |
| CU | Contact urticaria |
| LCPUFA | Long-chain polyunsaturated fatty acids |
| LY | Life year |
| LMIV | Food Information Regulation |
| LoQ | Limit of quantitation |
| LTP | Lipid transfer protein |
| NPV | Negative predictive value |
| NSAID | Non-steroidal anti-inflammatory drug |
| nsLTP | Non-specific lipid transfer protein |
| OAS | Oral allergy syndrome |
| OD | Occupational disease |
| OIT | Oral immunotherapy |
| PCD | Protein contact dermatitis |
| PPI | Proton pump inhibitor |
| PPV | Positive predictive value |
| PR-10 | Pathogenesis-related protein family 10 |
| RWC | Reduction in earning capacity |
| SCIT | Subcutaneous immunotherapy |
| SIT | Specific immunotherapy |
| SLIT | Sublingual immunotherapy |
| WDEIA | Wheat-dependent exercise-induced anaphylaxis |
Preamble
The 2015 guideline was updated by authors of the chapters after literature searches of PubMed, meta-analyses, clinical trials, and other scientific research. Consensus of the revision was accomplished by an interdisciplinary expert panel.
It takes into account the methodological guidelines of the Association of the Scientific Medical Societies (AWMF) for the development of guidelines for diagnostics and therapy and corresponds to an S2k guideline according to the three-stage concept of the AWMF [1]. The DELBI criteria were taken into account [2].
The strengths of the individual recommendations are provided in this guideline by standardized expressions (Table 1) [3].
Table 1. Strengths of recommendation.
| Recommendation strength | Syntax |
|---|---|
| Strong recommendation | shall |
| Recommendation | should |
| Recommendation open | may |
1. Epidemiology and most frequent triggers of food allergy
M. Worm and U. Jappe
How are food allergies differentiated according to their sensitization pathway? How common are food allergies? What are the risk factors of food allergy? What is the prognosis of food allergy? What are the most common food allergies?
Classification
Immunoglobulin E (IgE)-mediated food allergies are divided into primary and secondary food allergies, which can vary in severity.
Primary food allergies arise primarily (most likely) from a gastrointestinal sensitization to predominantly stable food allergens (glyco-/lipo-proteins).
Secondary food allergy results from sensitization to aeroallergens (e.g., pollen allergens) with subsequent reactions (so-called cross-allergies) to structurally related, often unstable allergens in (plant) foods.
Prevalence of food allergies
The prevalence of food allergies varies from region to region and has increased in some countries in recent years. For example, the prevalence of peanut and tree nut allergy has tripled in the United States in recent decades [4]. Recent data from a European prevalence study, involving Switzerland, but not Germany and Austria, confirm previous data on the frequency of food allergy [5]. Food allergy leads to a reduction in the quality of life of those affected and, in rare cases, can be fatal [6].
In order to determine the incidence, prevalence, current developments, potential risks and prognostic factors of food allergy in Europe, studies from 2000 – 2012 regarding this question were reviewed in a meta-analysis [7]. The point prevalence of self-reported food allergy was up to 6 times higher than food allergy verified by provocation testing. The prevalence of primary food allergy was higher in children than in adults. The increase in the incidence of secondary food allergy due to cross-reactivity with inhalant allergens is also due to increased awareness and improved diagnostics.
Studies on the epidemiology of food allergy in Germany are limited. A study from 2004 found a prevalence of food allergy, confirmed by double-blind, placebo-controlled food challenge of 3.7% in adults [8] and 4.2% in children [9]. A study of adult health in Germany (DGES), conducted in 2008 – 2012, found a lifetime prevalence of food allergy of 6.4% in women and 2.9% in men and for the total cohort of adults of 4.7% (95% confidence interval 4.1 – 5.4) [10].
Factors influencing the frequency of food allergy
The frequency of food allergy depends on several factors:
Age and gender,
family history of atopy,
place of residence/geographic location,
dietary habits,
the presence of other allergic diseases.
Geographically, the prevalence is highest in children compared to adults in North-Western Europe. A lower frequency of self-reported and confirmed food allergy was found in Southern Europe. However, data on the frequency of food allergy should be interpreted with caution, because of the heterogeneity of studies regarding methodological or diagnostic differences within and between (different) geographic region(s) of Europe.
The frequency of food allergy is difficult to determine for several reasons:
Presence of augmentation factors (factors that favor the occurrence of food allergy symptoms),
poor reproducibility of described symptoms,
relevance of hidden allergens or novel foods,
consideration of individual sensitization profiles,
natural development of tolerance
Prognosis
Data on the course of food allergy show that early milk protein allergy has a good prognosis in terms of spontaneous tolerance development, whereas peanut and tree nut allergies may persist into adulthood. Further studies are needed to better define the long-term prognosis of food allergy in the future.
Food allergy can be fatal in very rare cases. This mainly affects children and adolescents with peanut and tree nut allergy, but also milk protein allergy [11, 12].
Main triggers of food allergy according to age
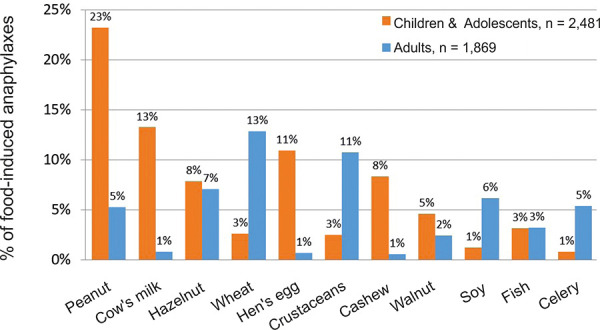
The most common triggers of food allergy in children and adolescents are milk and hen’s egg, soy, wheat, peanut, and tree nuts, and in adults pollen-associated food allergen sources (apple and other pome and stone fruits including hard-shelled fruits, see also Table 7), vegetables (celery, carrot), crustaceans and wheat. The profile of food allergens as triggers of severe allergic reactions is shown in Figure 1.
Figure 1. The most frequent triggers of food-induced anaphylaxis. Anaphylaxis Registry; as of March 2019; total food-induced anaphylaxis n = 4,350 (n = 2,481, children and adolescents 0 – 17 years; n = 1,869, adults 18 years and older.
Consensus statements. Consensus statements.
| The prevalence of food allergy is age-dependent. A study on the prevalence of food allergy in Germany shows a frequency of 4.2% in children and 3.7% in adults. | Strong consensus |
| IgE-mediated food allergy includes primary (predominantly early childhood) and secondary (predominantly pollen-associated) allergies that vary in severity. | Consensus |
| Food allergy can severely limit quality of life and in rare cases can be fatal. | Consensus |
2. Prevention of food allergy
K. Beyer and I. Reese
What measures can be used to influence or reduce the development of food allergy?
Primary prevention aims at reducing the risk for the occurrence of allergic sensitization and allergic diseases. For this purpose, either causative or predisposing factors are changed or the tolerance of the individual is increased. In the prevention of allergic diseases, a few recommendations apply exclusively to at-risk individuals in whom the father, mother, and/or siblings are already affected by an allergic disease. Most recommendations apply equally to non-risk individuals.
The German S3 guideline [13] on allergy prevention is currently being updated. In the systematic literature search for this guideline, all allergic diseases and not explicitly food allergy were considered. Since the prevention of food allergy is now also in the focus of preventive approaches, the results of a systematic review of the EAACI were considered for the current revision of this guideline, which forms the basis for the current European recommendations [14].
A comparison of the German and European recommendations is shown in Table 2. The German recommendations, which were consented for the prevention of food allergy, also consider the prevention of other allergic diseases, whereas, the European recommendations of the EAACI focus exclusively on the prevention of food allergy in infants and young children.
Table 2. Comparison of the German recommendations for the prevention of food allergy and possibly other allergic diseases with the EAACI recommendations for the prevention of food allergy in infants and young children.
| Update Guideline Allergy Prevention DGAKI/ GPA 20/21 | EAACI Recommendation 2020 |
|---|---|
|
Statement: During pregnancy and lactation, a balanced, varied diet that meets nutritional needs is recommended. This includes consumption of vegetables, milk/dairy products (including fermented dairy products such as yogurt), fruits, nuts, eggs, and fish. Recommendation: Dietary restrictions (avoidance of potent food allergen sources) during pregnancy or lactation should not occur for allergy prevention reasons. (A) |
The EAACI Task Force suggests against restricting consumption of potential food allergens during pregnancy or breastfeeding in order to prevent food allergy in infants and young children. |
|
Statement: Any breastfeeding has many benefits for mother and child. Recommendation: If possible, exclusive breastfeeding should be used for the first 4 – 6 months. (A) Breastfeeding should continue with the introduction of complementary foods. (A) |
There is no recommendation for or against using breastfeeding to prevent food allergy in infants and young children, but breastfeeding has many benefits for infants and mothers and should be encouraged wherever possible. |
| Recommendation: Supplemental feeding of cow’s milk-based formula in the first days of life should be avoided if the mother wishes to breastfeed. (B) | The EAACI Task Force suggests avoiding supplementing with cow’s milk formula in breastfed infants in the first week of life to prevent cow’s milk allergy in infants and young children |
| Recommendation: If breastfeeding is not possible or not sufficient, infant formula should be given. For infants at risk, consider whether an infant formula with efficacy demonstrated in allergy prevention studies is available until complementary feeding is introduced. (B) | For infants who need a breastmilk substitute, there is no recommendation for or against the use of regular cow’s milk based infant formula after the first week of life to prevent food allergy. There is no recommendation for or against using partially or extensively hydrolysed formula to prevent food allergy in infants and young children. When exclusive breastfeeding is not possible many substitutes are available for families to choose from, including hydrolysed formulas. |
|
Recommendation: Soy-based infant formulas are not suitable for the purpose of allergy prevention and consequently should not be given for this purpose. (A) Statement: Soy products can be given separately from the purpose of allergy prevention as part of complementary feeding. Recommendation: Since there is no evidence of an allergy-preventive effect of other animal milks, such as goat’s milk (not even as the basis of infant formula), sheep’s milk, or mare’s milk, these should also not be given for the purpose of allergy prevention. (B) |
The EAACI Task Force suggests against introducing soy protein-based formula in the first six months of life to prevent cow’s milk allergy in infants and young children. |
|
Statement: There is evidence that the diversity of the infant’s diet in the first year of life has a protective effect on the development of atopic diseases. A varied diet includes the introduction of fish and a limited amount (up to 200 ml per day) of milk or natural yogurt and hen’s egg as part of complementary feeding.
Recommendation: Depending on the readiness of the infant, complementary feeding should begin no earlier than the beginning of the fifth month of life and no later than the beginning of the seventh month of life. (B) There is no evidence for a preventive effect of dietary restriction by avoiding potent food allergen sources in the first year of life. Therefore, it should not be done. (A) |
|
| Recommendation: For prevention of egg allergy, heated (e.g., baked or hard-boiled) but not „raw“ eggs (including scrambled eggs) should be introduced with complementary feeding and given regularly. (B) | The EAACI Task Force suggests introducing well-cooked hen’s egg, but not pasteurised or raw egg, into the infant diet as part of complementary feeding to prevent egg allergy in infants. |
|
Recommendation: To prevent peanut allergy, consider introducing peanut products in an age-appropriate form (e.g., peanut butter) as part of complementary feeding in infants with atopic dermatitis in families with regular peanut consumption. (C)
Recommendation: Peanut allergy should be ruled out first, especially in infants with moderate to severe AD. (A) |
In populations where there is a high prevalence of peanut allergy, the EAACI Task Force suggests introducing peanuts into the infant diet in an age-appropriate form as part of complementary feeding in order to prevent peanut allergy in infants and young children. |
|
Background: Due to the heterogeneity of studies, no conclusive recommendation can be made on the supplementation of Ω-3 LCPUFAs for pregnant women, breastfeeding women, and infants for allergy prevention.
Statement: Some studies show that a low supply of Ω-3 LCPUFAs in pregnant women, breastfeeding women and infants is associated with a higher risk of allergic diseases in the child, especially asthma and wheezing, and that this risk can be reduced by supplementation of Ω-3 LCPUFAs (1++ to 2++). |
There is no recommendation for or against vitamin supplementation or fish oil supplementation in healthy pregnant and/or breastfeeding women and/or infants to prevent food allergy in infants and young children. |
|
Statement: Data from partly large, randomized, double-blind intervention studies consistently show no preventive effects of pre- and probiotics for the endpoints allergic rhinitis (AR) and bronchial asthma. The vast majority of current intervention studies also show no preventive effect for atopic eczema after administration of prebiotics and/or probiotics.
Recommendation: Prebiotics and/or probiotics should not be given to pregnant women or infants, even as part of infant formula, for allergy prevention purposes. (A) |
There is no recommendation for or against prebiotics, probiotics or synbiotics for pregnant and/or breastfeeding women and/or infants alone or in combination with other approaches to prevent food allergy in infants and young children. |
|
Background: From the point of view of the guideline group, despite heterogeneous interventions in the different studies, it has not been shown that primary prevention in infants with atopic family history can be achieved by daily refatting whole body treatment of healthy skin. Statement: At the present time, based on the available evidence, no recommendation can be made for daily re-lubrication of healthy infant skin with the aim of primary prevention of eczema and allergies – even in families with an increased risk of allergies. Recommendation: Infants and children with visibly dry skin should be creamed regularly – also with the aim of preventing eczema and allergies. (Expert opinion) |
There is no recommendation for or against using emollients as skin barriers to prevent food allergy in infants and young children. |
At this point, only the consented recommendations for the targeted introduction of potent food allergies are presented and explained. Sufficient evidence exists for the foods hen’s egg and peanut.
Hen’s egg
Regarding hen’s egg, favorable effects were shown by early introduction of cooked hen’s egg [15] or high-heated egg powder [16], whereas administration of pasteurized whole egg was associated with the risk of anaphylactic reactions [17, 18], but showed no advantage for the intervention group [17, 18, 19]. Because baked hen’s egg is thought to have a similar effect to hard-boiled chicken egg or high-heat egg powder, the introduction and regular administration of heated-throughegg (baked, hard-boiled) with complementary feeding is recommended. This includes adequately baked egg-containing baked goods (such as hard cookies, bread and roll specialties, and muffins and cakes). In contrast, it is not recommended to introduce “raw” hen’s egg (including scrambled and soft-boiled eggs) with complementary feeding.
Peanut
The EAACI recommendation for targeted introduction of peanut products for countries with high peanut allergy prevalence was not adopted, as Germany is not currently classified as such.
Since infants with atopic dermatitis from families with regular peanut consumption are at increased risk of developing peanut allergy, targeted introduction of peanut products in an age-appropriate form (not whole or in pieces because of the risk of aspiration) followed by regular administration may be considered in this constellation. Due to the fact that to date there are only data on the preventive introduction of peanut in infants with mild or no sensitization in the skin prick test to peanut [20], it is recommended that peanut allergy be ruled out in infants with moderate to severe atopic dermatitis before targeted introduction of peanut.
In addition to the recommendations of the S3 guideline, there is evidence that antacid use may promote sensitization and expression of food allergy [21, 22].
Consensus statements. Consensus statements.
| For prevention of hen’s egg allergy, thoroughly heated (e.g., baked or hard-boiled) but not “raw” hen’s egg (including scrambled egg) should be introduced with complementary feeding and given regularly. | Strong consensus |
| To prevent peanut allergy, infants with atopic dermatitis in families with regular peanut consumption may consider introducing peanut products in an age-appropriate form (e.g., peanut butter) as part of the complementary food introduction and continue to give them regularly. | Consensus |
| Before introduction of peanut allergy should be ruled out first, especially in infants with moderate to severe AD. | Consensus |
3. Clinical symptoms and differential diagnosis of food allergy
L. Lange, B. Koletzko, M. Raithel, and S.C. Bischoff
3.1 Clinical symptoms
What are the (most common) symptoms of food allergy?
Depending on
the ingestion (site of exposure) of the food protein,
the underlying disease,
the frequency and type of exposure, and
the dose
different symptoms of IgE-mediated food allergy can be elicited [23, 24]. Most symptoms are not exclusive to food allergy and may also result from other diseases or non-IgE mediated allergy types.
Contact of food proteins with the immune system happens most commonly via the oral/gastrointestinal mucosa, but can also occur
via the skin (e.g., contact urticaria), e.g., as a sensitization pathway for peanut allergy [20]
the respiratory tract (via the respiratory system, e.g., baker’s asthma, see 7.) or
via the vascular system (e.g., in the case of contamination of injection solution with food proteins).
The route of exposure plays an important role in the outcome of clinical symptoms. Depending on the organ system involved, various symptoms – often in combination – can occur (modified according to [25]) (Table 3 and 4). In seropositive IgE-mediated allergies with positive IgE detection on skin and/or in blood (often atopy), variable symptom patterns consisting of extraintestinal and intestinal symptoms are found. Most frequently, skin and mucous membrane symptoms occur, for example, as urticaria or angioedema. In severe food allergies, respiratory and/or cardiovascular symptoms may occur. In children, respiratory symptoms are more common (e.g., wheezing or dyspnea) in adults, cardiovascular symptoms are more common. Interestingly, gastrointestinal symptoms are not more common in systemic food allergy. In seronegative IgE-mediated food allergy, only localized IgE in the tissues (entopy) can lead to isolated organ reactions (e.g., oral mucosal swelling, etc.) [26, 27, 28, 29, 30]. Although the IgE-mediated response is an immediate reaction, at the gastrointestinal tract (GIT), depending on the site of digestion, resorption, and/or reaction, symptom may be rapid (upper GIT) or delayed for several hours (middle and lower GIT) [26, 27, 29, 31, 32].
Table 3. Symptoms of food allergy.
| Target organ | Symptoms |
|---|---|
| Systemic; Circulation | Anaphylaxis |
| Hypotension, shock | |
| Tachycardia (rarely bradycardia in anaphylaxis) | |
| Drowsiness, dizziness | |
| Syncope | |
| Skin (transient) | Erythema („flush“) |
| Eczema (worsening) | |
| Urticaria | |
| Itching | |
| Angioedema | |
| Exanthema | |
| Eye | Itching |
| Redness (conjunctival injections) | |
| lacrimation | |
| periorbital edema | |
| Upper respiratory tract | Nasal congestion |
| Itching | |
| Rhinorrhea | |
| Laryngeal edema, stridor | |
| Hoarseness | |
| Dry cough | |
| Lower respiratory tract | Cough |
| Thoracic tightness | |
| Heaviness, shortness of breath (dyspnea) | |
| Whistling breath sounds (wheezing) | |
| Cyanosis | |
| Oropharyngeal | Swelling of lips, tongue and/or palate (angioedema) |
| Oral and/or pharyngeal itching (pruritus) | |
| Tongue swelling | |
| Gastrointestinal tract | Nausea |
| Vomiting | |
| Colicky abdominal pain | |
| Gastroesophageal reflux | |
| Diarrhea |
Table 4. Symptoms of delayed reaction or in case of chronic exposure.
| Nausea |
| Vomiting |
| Abdominal pain |
| Gastroesophageal reflux, dysphagia, and bolus events |
| Inappetence and food refusal |
| Diarrhea, malassimilation, enterocolitis |
| Hematochezia (blood in stool) |
| Failure to thrive and weight loss |
Consensus statements. Consensus statements.
| Symptoms of an IgE-mediated food allergy are multifaceted and affect different organ systems (especially skin and oropharyngeal mucosa, gastrointestinal tract, respiratory tract, cardiovascular system). | Strong consensus |
| For the diagnosis of food allergy, a clear and reproducible association of symptoms with the ingestion of defined foods and improvement of symptoms with avoidance, including in association with IgE sensitization on skin, blood or intestine, etc. In blood or skin IgE-negative patients, local seronegative IgE-mediated allergic reactions are possible, among others. |
Strong consensus |
| In cases of intermittent food tolerability a cofactor should be considered, e.g., cofactor-dependent food allergy such as exercise-induced analphylaxis. | Consensus |
3.2 Manifestations and differential diagnoses
What other diseases can cause the symptoms of food allergy? What are the clinical manifestations of food allergy?
Food can cause numerous diseases. These are based on different pathophysiological mechanisms with involvement of different, sometimes several organ systems.
An overview of the manifestations of food allergies and differential diagnoses is given in Table 5.
Table 5. Manifestations and differential diagnoses of food allergy. Modified according to [24].
| Immunopathology | Disease | Clinical characteristics | Typical age group | Prognosis |
|---|---|---|---|---|
| IgE- mediated | Acute urticaria/ angioedema | Triggered by ingestion or direct skin contact | Children > adults | Depending on the triggering food |
| Rhinoconjunctivitis/asthma bronchiale | Accompanied by food protein allergic reactions, rarely isolated respiratory symptoms (exception: inhalation exposure to aerosol of food protein, often occupational) | Infant > adult, except occupational | Dependent on the triggering food substance | |
| Anaphylaxis | Rapidly progressive multisystem reaction | Any age | Depending on triggering food and underlying disease | |
| Delayed food-induced anaphylaxis to mammalian meat [267] | Anaphylaxis three to six hours after ingestion; triggered by antibodies to galactose-α-1,3-galactose | Adults > children | Unclear | |
| Food-dependent, risk factor-dependent anaphylaxis | Food triggers anaphylaxis only if augmentation factors such as exertion, but also alcohol or acetylsalicylic acid (ASA) are present before or after food ingestion | Onset in late childhood/adulthood | Probably permanent | |
| Secondary cross-allergy (mainly pollen-associated food allergies) | Oropharyngeal itching; mild edema confined to oral cavity, less frequently urticaria perioral or generalized, Respiratory symptoms (cough); – rarely systemic reactions (incl. anaphylaxis) in some pollen-associated allergies | Onset after manifestation of pollen allergy (adult > young child) | May persist; may vary with seasons | |
| Gastrointestinal allergic immediate reaction (allergic esophagitis, gastritis, enteritis or colitis) | After ingestion, – depending on resorption and/or reaction site – occurring bolus sensation, vomiting, nausea, or abdominal colic, diarrhea or enterocolitis | Any age | Depending on the triggering food | |
| Mixed IgE- and cell- mediated | Atopic eczema/dermatitis | Associated with food in 30 to 50% [268] of children with moderate/severe eczema | Infant > child > adult | Usually development of tolerance |
| Eosinophil-associated gastrointestinal inflammatory disease (EGID) | Symptoms vary; likely persistent depending on part of gastrointestinal tract affected and degree of eosinophil inflammation | Any age | Unclear | |
| Cell- mediated | Food protein-induced proctitis/proctocolitis | Mucopurulent, bloody stools in infants | Infants | Usually tolerance development |
| Food protein-induced enterocolitis syndrome (FPIES) | Acute exposure: severe manifestation with vomiting, (bloody) diarrhea and exsiccosis to shock; chronic exposure: vomiting, diarrhea, failure to thrive, lethargy, Re-exposure after abstinence: vomiting, diarrhea, hypotension one to three hours after ingestion | Infants – young children, less frequently adults [269] | Usually development of tolerance | |
| Food protein-induced enteropathy | Diarrhea, vomiting, failure to thrive, edema; no colitis | Infants – young children > adults | Usually development of tolerance in children | |
| Celiac disease | Multiple manifestations, mono-, oligo- and polysymptomatic, triggered by gluten in case of genetic predisposition | Persistent at any age (lifelong strict gluten avoidance required) | Permanent | |
| Non-allergic (non-immunological intolerance) | Carbohydrate mal-assimilation/absorption (lactose, fructose, sorbitol, rarely: sucrose, glucose-galactose) | Diarrhea (osmotic), meteorism, abdominal pain one to four hours after ingestion, constipation also possible | Lactase deficiency typically from school age, otherwise any age fructose mal-absorption/sorbitol: any age, very rare: congenital lactase deficiency, glucose-galactose intolerance, sucrose-isomaltase malabsorption | Mostly persistent (lactose, glucose-galactose); fructose, sorbitol |
Non-allergic mechanisms
Food additives and natural flavorings may also activate mast cells and mimic the clinical picture of IgE-mediated food allergy (postulated mechanisms include activation of G protein-coupled receptors, alterations in eicosanoid metabolism, increased mediator formation/secretion). Natural flavoring agents, sulfur compounds, benzoic acid compounds, histamine-containing foods and glutamate have occasionally been described as triggers of non-allergic food intolerance reactions. In addition, augmentation factors may be required, and oral provocations may be negative if these are not considered.
The importance of salicylate-containing foods in acetylsalicylic acid (ASA) intolerance is unlikely due to a low occurrence of salicylic acid in foods [33, 34]. Avoidance of salicylate-containing vegetables and fruits is not recommended in terms of an anti-inflammatory diet [34].
Consensus statements. Consensus statements.
| In the case of suspected food allergy, the differential diagnosis should primarily include infections, chronic inflammatory diseases including eosinophilic gastroenteritis and mastocytosis, carbohydrate malabsorption or functional or somatoform disorders. | Strong consensus |
| For differential diagnosis of suspected food allergy, other diseases should be considered depending on the symptoms and the age of the patient. | Strong consensus |
| If non-IgE-mediated gastrointestinal intolerance reactions are suspected, a gastroenterologist (or pediatric gastroenterologist) should be involved in the diagnostic work-up. | Consensus |
4. Diagnosis of food allergy
J. Kleine-Tebbe
How can food allergy be reliably diagnosed?
Procedure in case of suspected food allergy
If IgE-mediated food allergy is suspected, the diagnostic procedure is based on several components (Figure 2):
Figure 2. Diagnostic procedure for suspected food allergy: in adults, sensitization is often detected by skin tests (left half), in children preferably by specific IgE determination (right half, see text for additional explanation).
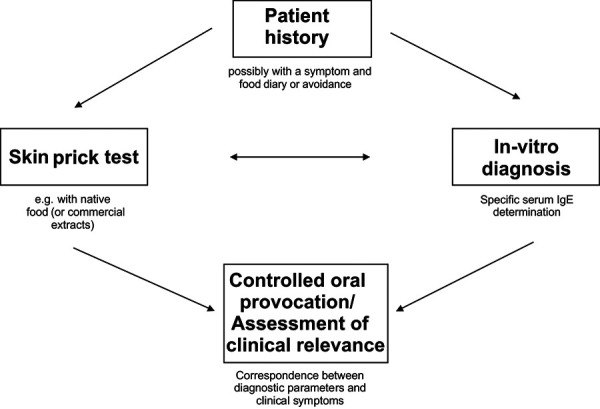
Patient history (if necessary with dietary and symptom protocol) (4.1.),
- Sensitization test (colloquially “allergy test”)
- - IgE determination (4.2.) and/or
- - Skin prick test (4.3.),
Determination of clinical relevance (interpretation)
Plausibility on the basis of the (anamnestic) clinical data,
If necessary, diagnostic elimination diet and
Provocation test (4.4.).
The test sequence and the selection of test reagents are based on
medical history
the age of the patient and
the available testing (presented in the subsections).
The diagnostic tests identify increased allergic susceptibility (i.e., sensitization). This is accomplished by:
direct detection of allergen-specific IgE against food extracts/allergens in serum (4.2.) or through
positive skin tests (prick test) (4.3.) with food (extracts) as an indirect indication of functional, i.e. capable of cross-linking, allergen-specific IgE on mast cells in the skin.
In principle, the qualitative statements (positive vs. negative) of IgE tests and prick tests are equivalent:
A negative result serves to exclude sensitization.
A positive result corresponds to sensitization, which, however, is only clinically relevant in the case of corresponding symptoms.
A single test (IgE test or skin test) may be sufficient to test for sensitization to a food. Multiple tests are often used to detect sensitization (Figure 2). Their results do not always agree qualitatively; in that case, the positive result is more likely to be correct than the (false) negative. In case of concordant results (concordant positive or negative) the diagnostic accuracy is increased, especially since mostly different reagents of a food (native preparations, extracts, single allergens) are used in the skin or IgE test.
Interpretation of the tests
For the interpretation of sensitization tests, the patient history and the clinical symptoms are of central importance: Only if there is a clear agreement between the clinical information of the patient and the test result (prick test/IgE determination), a food allergy can be diagnosed or excluded. If such a match is not or not clearly given (e.g., due to unclear or unproductive patient history), the clinical relevance should be confirmed with oral provocation test (Figure 2) (4.4).
The term “allergy test” (for skin or IgE tests) is misleading in this context and holds the greatest source of misinterpretation of diagnostic results: A positive result, for example, to food (i.e., sensitization) can only be successfully interpreted if the clinical reaction to a given allergy is known.
As a rule of thumb, only half of the atopic sensitizations detectable in the population are really associated with symptoms and thus clinically relevant. Thus, sensitization tests show unsatisfactory diagnostic specificity (~ 50%) and limited positive predictive value (“PPV”), which strongly depends on the particular allergen source and the prevalence of food allergy in the cohorts studied.
In case of gastrointestinal allergy manifestation, specific local diagnostic measures may be considered, such as mucosal or endoscopic provocation and endoscopic lavage.
Consensus statements. Consensus statements.
| Specific testing for IgE sensitization should be guided by the medical history. | Strong consensus |
| Detection of IgE sensitization to foods and aeroallergens should be by specific IgE determination and/or skin prick testing. | Consensus |
| Specific IgE determination and skin prick testing support the diagnosis of food allergy in the context of history and/or food provocation. | Strong consensus |
| Detection of sensitization by specific IgE determination or skin prick test does not prove the clinical relevance of the food tested and should not alone lead to therapeutic elimination. | Strong consensus |
| Lack of evidence of sensitization (negative specific IgE/skin prick test) often, but not certainly, excludes clinically relevant IgE-mediated food allergy. | Consensus |
4.1 Medical history and dietary and symptom protocol
M. Worm, I. Reese, and L. Klimek
What is the importance of the patient history in suspected food allergy?
Which aspects have to be considered in the history of suspected food allergy?
Practical procedure for taking the medical history
The allergy history in cases of suspected food allergy follows basic principles of interviewing. It is helpful to give patients a focused questionnaire before the first appointment, which can be brought to the first interview or filled out during the waiting time.
The medical history (Table 6) includes:
Table 6. Procedure for taking medical history.
| Medical history | |
| Self history | Known allergic diseases Medication Physical exertion Acute infectious diseases Psychological stress |
| Family history | Allergic diseases in first-degree relatives |
| Symptoms or specific triggers | When Where By what How long How often Repeatedly |
| Nutritional history | Record dietary restrictions and extent, tolerance of foods with proven sensitization |
| Dietary and symptom diary | Documentation of diet and symptoms |
the family history regarding atopy,
the patient’s own medical history, and
the specific dietary history.
Reported symptoms should be recorded with their local, temporal and situational occurrence. In order to classify the patient’s data, it is important to know whether periods of complete freedom from symptoms occur, but also which foods are usually consumed and tolerated.
Supporting measures
A diet and symptom diary is useful so that patients can monitor their habits and complaints more specifically themselves. Particularly in the case of chronic complaints, records kept by the patient or their parents over 2 – 3 weeks with the aid of a diet and symptom diary are helpful. Such a diary takes into account the intake of food, drinks, but also sweets, chewing gum, etc., special features (e.g., eating in a restaurant) and complaints occurring in a temporal context. Symptom type and intensity should be listed with date, if necessary time, duration of the complaints. The diary should also record medication consumption. The records should afterwards be evaluated by a dietician with experience in allergy or an allergist. By this procedure, the significance of existing (or missing) sensitizations can be critically reflected and the decision for specific provocation tests or other measures facilitated. Furthermore, it should be considered that certain medications (e.g., proton pump inhibitors (PPI) or alkalizing drugs) may favor the development of sensitization [22, 35]. After the diagnostic work up the further therapeutic procedure is planned including a follow-up history.
Consideration of augmentation factors
Augmentation factors should also be considered in the medical history. These can aggravate an allergic reaction and in some cases are even obligatory for triggering symptoms (e.g., in wheat-dependent exercise-induced anaphylaxis). The best known augmentation factors are:
physical activity and
the use of non-steroidal anti-inflammatory drugs (NSAID).
However, other factors like alcohol, fever, acute infections, allergic symptoms during pollen season and sleep deprivation [36] have been described as augmentation factors as well [37].
Consensus statements. Consensus statements.
| A detailed medical history should be the basis for the diagnosis of food allergy. | Strong consensus |
| The structured history should consider triggers, time course, symptoms, severity, reproducibility, risk and augmentation factors, family history, concomitant diseases and other allergic diseases. | Strong consensus |
| For chronic symptoms, a diet and symptom diary is useful. | Strong consensus |
4.2 Triggering allergens and in vitro diagnostics
J. Kleine-Tebbe, B. Ballmer-Weber, U. Jappe, J. Saloga, and M. Wagenmann
How can the severity of a food-related allergic reaction be determined? What are reasonable indications for sIgE determination? What is the significance of diagnostics with single allergens? What is the significance of sensitization to certain single allergens? Which are the most important allergens in food allergy? What must be considered in the interpretation of serological diagnostics?
4.2.1 Serological IgE determination for the detection of sensitization
Allergen-specific IgE in serum against food corresponds to sensitization. A lack of specific IgE (mostly) excludes it, provided that an extract is used for testing in which all important allergens are contained [38].
Depending on the test setup, reagents and allergens used, specific IgE results from different manufacturers may differ.
For IgE testing individual foods (allergen sources, Table 7), a combination of various foodstuffs (search or panel test) and increasingly single allergens (Table 8, 9, 10, further sources of information in Table 11) used [39].
Table 7. Important allergen sources in food allergies in children and adults.
| Children | Adolescents and adults |
|---|---|
| Cow’s milk | Pollen-associated food allergen sources (e.g., stone and pome fruits, nuts, soy, celery, carrot) |
| Hen’s egg | Nuts and oilseeds (e.g., sesame) |
| Peanut | Peanut |
| Wheat | Fish and shellfish |
| Nuts | Cow’s milk*, Hen’s egg* |
| Soy* | Latex-associated food allergen sources* (e.g., banana, avocado, kiwi, fig) |
| Fish* | Mammalian meat* |
*Rare.
Table 8. List of definitions and abbreviations.
| Allergen | Molecule (protein, e.g., major allergen Gad c 1 from cod, rarely carbohydrate component) that can trigger an allergic immune response. |
| Allergen extract | Mixture of allergenic and non-allergenic components extracted from an allergen source (e.g., fish allergen extract) |
| Allergen source/carrier | Origin/starting material of allergens (e.g., fish). |
| α-Gal | Galactose-α-3-galactose, a disaccharide as a cause of anaphylaxis to mammalian meat, gelatin, and biologics |
| Ara h 2 | 2S albumin, a storage protein of peanut, associated with systemic reactions in peanut allergy |
| Api g 1 | Celery allergen with homology to Bet v 1, responsible for birch pollen-associated cross-reactions |
| Bet v 1 | Immunodominant major allergen in birch pollen (Betula verrucosa) |
| Bet v 2 | Birch pollen profilin, minor allergen, which as a panallergen in numerous pollens and plant foods (e.g., melon) can be responsible for cross-reactions and thus complicates diagnosis |
| CCD | Cross-reactive carbohydrate determinants. They represent epitopes of N-glycans, and less often of O-glycans, as panallergens, are responsible for a pronounced cross-reactivity. |
| Cor a 1.04 | Hazelnut allergen with homology to Bet v 1, responsible for birch pollen-associated cross-reactions |
| Dau c 1 | Carrot allergen with homology to Bet v 1, responsible for birch pollen-associated cross-reactions |
| Gad c 1 | Major cod allergen (Ca2+ transport protein, parvalbumin, major fish allergen) |
| Gly m 4 | Soy allergen with homology to Bet v 1, responsible for birch pollen-associated, sometimes severe cross-reactions |
| Cross-reactive | Similarity-induced immunological reaction with molecular structures that were not responsible for the original sensitization |
| LTP | Lipid transfer proteins; thermo- and digestion-stable allergens of plant origin |
| Mal d 1 | Apple allergen with homology to Bet v 1, responsible for frequent birch pollen-associated, mostly oropharyngeal cross-reactions |
| MUXF3 | Designation of the structure of a carbohydrate side chain of plant glycoproteins and allergens that can potentially be bound by IgE antibodies, corresponds to a specific type of CCD (see above) |
| Oleosins | Lipophilic and thermostable allergens in nuts and oilseeds. |
| Pen a 1 | Tropomyosin (muscle structure protein) of shrimp with homologous proteins in other arthropods and cause of cross-reactions |
| PR-10 | „Pathogenesis-related protein family 10“; Bet-v-1 homologous proteins with defense function in plants (including tree pollen, food) |
| Pru p 3 | Lipid transfer protein in peach responsible for systemic reactions in patients in the Mediterranean region |
| Recombinant | Produced with the aid of genetically modified (micro)organisms |
| Recombinant allergen | Allergenic protein often produced in Escherichia coli without the carbohydrate side chains found in native allergens |
| Sensitization | Allergenicity (only relevant in case of corresponding symptoms) |
| Tri a 19 | ῳ-5-gliadin in wheat, responsible for systemic reactions and effort-dependent anaphylaxis in wheat allergy |
Table 9. Selected food allergens and their sources of plant origina,b.
| Protein families | Storage proteins (protein families, structure) | |||||||
| Prolamins | Cupins | |||||||
| Bet-v-1 homologue | LTP | Profilins | Thaumatins | Oleosins | 2S Albumins | 7/8S-Globulins (Vicilin) | 11S-Globulins (Legumin) | |
| Apple | Mal d 1 | Mal d 3 | Mal d 4 | Mal d 2 | ||||
| Peanut | Ara h 8 |
Ara h 9
Ara h 16 Ara h 17 |
Ara h 5 | Ara h 10 Ara h 11 Ara h 14 Ara h 15 |
Ara h 2
Ara h 6 Ara h 7 |
Ara h 1 | Ara h 3 | |
| Hazelnut | Cor a 1 | Cor a 8 | Cor a 2 | Cor a 12 Cor a 13 |
Cor a 14 | Cor a 11 | Cor a 9 | |
| Carrot | Dau c 1 | Dau c 3 | Dau c 4 | |||||
| Cherry | Pru av 1 | Pru av 3 | Pru av 4 | Pru av 2 | ||||
| Peach | Pru p 1 | Pru p 3 | Pru p 4 | |||||
| Celery | Api g 1 | Api g 4 | ||||||
| Sesame | Ses i 4 Ses i 5 |
Ses i 1
Ses i 2 |
Ses i 3 | Ses i 6 Ses i 7 |
||||
| Soybean | Gly m 4 | Gly m 1 | Gly m 3 | Gly m 8 | Gly m 5 | Gly m 6 | ||
| Wheat | Tri a 14 | Tri a 12 | Tri a 19 (ω-5-Gliadin) | |||||
aAllergen sources (left column) with individual allergens (table columns) and their protein families (header). bBold print: already available for in vitro diagnostics, normal print: not yet available for differentiating diagnostics
Table 10. Selected food allergens of animal origina,c.
| Protein families | ||||
| Parval- bumins | Tropo- myosins | Lysozyms/ α-Lactal- bumins | Other proteins (various families) | |
| Hen’s egg | Gal d 4 (Lysozym C) |
Gal d 1 (ovomucoid, trypsin inhibitor) Gal d 2 (ovalbumin, Serpin) Gal d 3 (ovotransferrin, Conalbumin) |
||
| Fish |
Gad c 1
Cyp c 1 |
Ani s 3b | ||
| Crustaceans/mollusks |
Hom a 6 |
Cha f 1 Hom a 1 Met e 1 Pen a 1 |
||
| Cow’s milk | Bos d 4 (α-lactal- bumin) |
Bos d 5 (β-lactoglobulin, lipocalin) Bos d 6 (bovine thyroglobulin) Bos d 8 (Casein) |
||
| Mammalian meat | Galactose-α-1,3-Galactose (α-GAL) (diasaccharide on proteins (bovine thyro- globulinand glycolipids) | |||
aAllergen sources (left column) with individual allergens (table columns) and their protein families (header row). bDue to infestation with the herring worm (Anisakis), severe allergic reactions have been described after consumption of infested fish. c Bold: already available for in vitro diagnostics, normal/non-bold: not yet available.
Table 11. Open Access internet resources/databases and information on molecular allergology [270].
| Web-Link | Brief description |
| www.allergen.org | Official database of the WHO/IUIS Allergen Nomenclature Sub-committee with simplified search function |
| www.allergenonline.org | Food Allergen Database of the University of Nebraska at Lincoln, Food Allergy Research and Resource Program (FARRP); carefully maintained entries organized by taxonomic affiliation of allergen sources |
| www.allergome.org | Largest database on allergen molecules, initiated by the Italian allergist Adriano Mari and his team; some entries of identified single allergens before their official naming |
| www.meduniwien.ac.at/allergens/allfam/ | Database on allergen families (protein families) of the Medical University of Vienna, Institute of Pathophysiology and Allergy Research in the Center for Pathophysiology, Infectiology and Immunology |
| www.thermofisher.com/diagnostic-education/hcp/de/de/resource- center/allergen-encyclopedia.html | Extensive database for allergen extracts and molecules with additional clinical information from a diagnostic manufacturer |
The diagnostic suitability is evaluated separately according to allergen source, allergen and test method (Table 13).
Table 13. Barriers for the evaluation of specific IgE results.
| Technical and methodological errors (reasons for false-positive and false-negative results) |
| Poor quality of reagents (e.g., allergen extracts or their extraction, coupling and stability) laboratory errors |
| Interpretation errors (reasons for clinically irrelevant results) |
| Strongly increased total IgE with multiple sensitizations high sensitivity of detection cross-reactive IgE antibodies |
IgE = Immunoglobulin E.
4.2.1.1 Indication for IgE determination
Depending on
the age,
the symptoms and
and the suspected allergen sources (Table 7)
different indications for in vitro diagnostics are applicable [40]:
Suspicion/exclusion of food allergy
Specific IgE determination is useful in cases of high suspicion or for a specific exclusion of a food allergy. However, this indication requires that all relevant allergens are represented in the test extract used.
Group tests for specific IgE (e.g., against peanut, fish, hen’s egg white, cow’s milk protein, soy and wheat) allow a rational exclusion or detection of sensitization in the sense of an increased allergic susceptibility. They thus serve as a basis for a further individual allergen source testing. To perform a wide ranged screening without a reasonable suspicion of food allergy is not recommended.
Life threatening reactions to food
In cases of severe anaphylactic reactions, specific IgE determination against the food suspected or to be excluded is preferable and skin testing should be performed according to individual risk-benefit considerations.
Suspicion of sensitization to foods not suitable for skin testing
If the skin test is not suitable as proof of sensitization, a specific IgE determination is recommended (e.g., for skin-irritating foods such as spices).
Conditions that do not allow skin testing or its evaluation
Specific IgE determinations are useful in cases of inadequate skin testing capability. These include urticarial dermographism, skin disease in the test area and medications affecting the skin test. In infants and young children, specific IgE is often determined in serum against allergenic foods instead of skin testing.
Common food allergen sources with a low risk potential
Clinically mild reactions (e.g., oropharyngeal symptoms in pollen-associated food allergy) should be clarified with reasonable effort and in the usual diagnostic sequence (history, skin test, in vitro diagnostics).
Example: If a birch pollen-associated food allergy is suspected, a prick test with a birch pollen extract should be performed and/or a specific IgE test against the main birch pollen allergen Bet v 1. Commercially available fruit or vegetable extracts are often unsuitable for birch pollen-associated food allergy due to unstable allergens. Skin testing with fresh native foods in the prick-to-prick test is more sensitive but less specific. Therefore, an untargeted screening (also serological) of, for example, all fruits and vegetables or the available single allergens in birch pollen-associated cross-sensitization is not recommended [41].
4.2.1.2 Definitions and concepts for allergen selection
Potential advantages and disadvantages of in vitro diagnostics with extracts or single allergens have to be defined separately for each allergen source or single allergen [42] (information in Table 11).
The following arguments speak for the use of single allergens:
Increased test sensitivity [lower limit of quantitation (“LoQ”) [43] by certain single allergens, especially if they are underrepresented or absent in the (food) extract (examples: historically the soy protein Gly m 4 [44], wheat gluten Tri a 19, apple protein Mal d 1, sugar epitope galactose-α-1,3-galactose on proteins and glycolipids in mammalian meat) [45],
Increased test discriminatory power (analytical specificity or selectivity) for single allergens from allergen sources consisting of complex mixtures of numerous allergens associated with increased clinical risk (examples: Ara h 2 of peanut, Pru p 3 of peach, Cor a 9 and 14 of hazelnut, Act d 1 of kiwi),
In case of a lack of the analytical specificity of extracts (cross-reactivity), IgE detection against typical cross-reactive allergen molecules facilitates interpretation (examples: Bet v 1 or homologous representative, Phl p 12 or Pru p 4 as profilin, Pru p 3 as lipid transfer protein (LTP), CCD (“cross-reactive carbohydrate determinant”) component MUXF3).
The current limitation of sIgE determination quantity in the reimbursement of IgE diagnostics may lead to an unacceptable limitation of a necessary more extensive screening in unclear cases of food allergy.
The use of single allergens for IgE determination is mainly justified by their increased test sensitivity (lower LoQ) and (analytical) specificity: If single allergens are thereby able to improve in vitro diagnostics, their use is reasonable and recommendable from an allergological point of view.
4.2.1.3 Foods as allergen sources and their allergens
Foods are complex allergen sources and contain diverse (glyco-/lipo-) proteins, the actual allergens. A relation is thus given by the biological relationship of the foods concerned and by the biochemical similarity of the allergens they contain. The significance of the allergen sources (Table 7) depends on the age of the affected patients and the regional and individual eating habits.
4.2.1.4 Important plant protein families and their allergens
Fruits, vegetables, legumes, tree nuts, oilseeds and cereals can lead to sensitization due to the allergens they contain [46].
Meanwhile, the most important protein families and individual allergens of plant foods have been identified (Table 9) and are increasingly used for IgE diagnostics (Table 9, 12).
Table 12. Examples of clinical patterns and molecular diagnostic recommendations [59].
| Clinical picture | Clinical suspicion | IgE diagnostics |
|---|---|---|
| Anaphylaxis after exertion | Exertion-dependent wheat allergy | Tri a 19 (ω-5-gliadin) |
| „Cat-pork syndrome“ | Allergy to animal serum albumin | Fel d 2 or Bos d 6 |
| Delayed meat allergy (e.g., urticaria) | Sensitization to galactose-α-1,3-galactose (α-GAL) | α-GAL (bovine thyroglobulin) |
| Allergy e.g., to grapes, berries, lettuce | Sensitization to lipid transfer protein (LTP) | Pru p 3 (peach LTP) |
| Oral allergy syndrome (OAS) frequently to nuts, pome and stone fruits, etc., possibly systemic reactions to soy (native) | Sensitization to Bet v 1 homologue (PR 10 proteins) | Bet v 1 and possibly Gly m 4 |
| OAS after atypical plant foods (melon, exotics such as lychee, citrus fruits) | Sensitization to profilins | Pru p 4 (or Bet v 2, Phl p 12, Hev b 8) |
1. Pathogenesis related protein family 10
Birch pollen allergy, which is common in Central Europe, is predominantly caused by sensitization to the main allergen Bet v 1, a natural plant stress protein (“pathogenesis-related protein family 10”, PR-10).
Similar PR-10 proteins are present in tree pollen of hazel, alder, beech and oak, as well as in various fruits and vegetables, nuts and legumes (Table 9). They are the basis of birch pollen-associated cross-reactions, for example against apples, cherries, peaches, hazelnuts and many others [41]. Because of the low proportion of PR-10 proteins in the total mass and their lack of resistance to heat and digestion, the symptoms remain restricted to raw foods and mostly to the mouth and throat. In individual cases, life threatening systemic symptoms can also occur, for example, after the ingestion of larger amounts of the consumed food, the presence of augmentation factors such as physical stress, or matrix effects (protection of the PR-10 protein by other food components) [47] (examples: Gly m 4 in soy, Ara h 8 in peanut [48, 49], more rarely Api g 1 in celery, Dau c 1 in carrots).
2. Lipid transfer proteins
Systemic reactions due to fruits, vegetables, nuts, legumes, and cereals can be caused by sensitization to LTP. Predominantly described in the Mediterranean region, primary sensitization possibly arises from ripe peaches [50]. The structural similarity of peach LTP Pru p 3 with the heat- and acid-stable LTP of other plant foods (pome and stone fruits, but also grapes, blueberries, nuts, lettuce) may be responsible for cross-reactions [51]. Meanwhile, more and more cases are observed also in Northern Europe and elsewhere [52]. For the detection of sensitization, the lead allergen Pru p 3 is often sufficient. The clinical relevance of LTP sensitization must be clarified individually with the patient. The patient’s history (clinical reaction) or, in case of doubt, an oral provocation with the suspected LTP-containing foodstuffs serves this purpose.
3. Seed storage proteins
Storage proteins refer to structurally related, yet variable, stable and clinically significant food allergens, for example in nuts, seeds, legumes (leguminosae), which include peanut, soybean and lupine, and cereals.
Based on their structure, 2S-albumins from the prolamin and globulins from the cupin superfamily are distinguished [53]. Globulins contain vicilins (7/8S globulins) and legumines (11S globulins) (Table 9). Due to their stable structure and high proportion of the total protein, storage proteins rarely cause problems in diagnostics with extracts. Due to their stability to heat and digestion, they are often associated with an increased risk of systemic symptoms and are well suited for the identification of sensitization or exclusion:
Ara h 2, (if negative, Ara h 1, 3, and 6 if appropriate) in peanut allergy,
Cor a 14 (if negative, Cor a 9) in hazelnut allergy,
Jug r 1 in walnut allergy,
Ber e 1 for Brazil nut allergy,
Ana o 3 in cashew and pistachio allergy,
Among the seed storage proteins of wheat, Tri a 19, omega-5 gliadin, is particularly associated with wheat-dependent exercise-induced anaphylaxis (“WDEIA”) [54, 55].
IgE detection against storage proteins of nuts, seeds and legumes, do not allow a reliable prediction of the occurrence of clinical symptoms.
4. Profilins
Profilins are phylogenetically highly conserved proteins and are supposed to be clinically less relevant allergens. Sensitizations are often primarily caused e.g., by high grass pollen exposure, can affect all pollens and numerous plant foods (e.g., apples, carrots) and are caused by cross-reactions. In most cases, one representative (e.g., grass pollen profilin Phl p 12, birch pollen profilin Bet v 2 or peach profilin Pru p 4) is sufficient for IgE diagnostics.
Exotic fruits (e.g., melons, banana, avocado, mango) away from the Bet v 1 food allergen cluster may also have underlying pro lin sensitization as trigger of predominantly oropharyngeal symptoms [41]. Apart from OAS, they may also be responsible for severe allergic reactions in rare cases [56].
Other allergens in plant foods.
Cross-reactive carbohydrate epitopes: many plant food allergens are glycoproteins with cross-reactive carbohydrate side chains (CCD, including those in pollen, plant foods, arthropods, mollusks, and certain pathogenic helminths). Their IgE binding usually remains without clinical relevance [57]. They do not lead to skin test positivity, but can complicate serological IgE diagnostics with extracts or natural CCD-bearing single allergens by clinically mostly irrelevant results. Bromelain, horseradish peroxidase or the N-glycan MUXF (CCD single allergen component of bromelain without peptide content) are suitable for screening CCD-specific IgE (Table 8).
Oleosins: Oleosins occur in lipid-rich plants as allergens. As lipophilic proteins, they are underrepresented in aqueous extracts of legumes (e.g., peanut), seeds (e.g., sesame), and tree nuts (e.g., hazelnut), and extract-based diagnostics may show false-negative results [58]. In this constellation, testing of native foods by skin testing is indicated.
Thaumatins and enzymes: Thaumatin-related proteins are thermo- and digestion-stable plant food allergens [59], for example in cherries (Pru av 2), apples (Mal d 2), kiwi (Act d 2), banana (Mus a 4), peach (Pru p 2), tomato, bell pepper and walnut. So far, they are only sporadically available for diagnostic purposes (Act d 2, ImmunoCAP ISAC). The frequency of sensitization or clinically relevant reactions is unclear. The same applies to a number of enzymes found in plant foods (e.g., exotic fruits).
Examples of component-diagnostic in given allergens
Wheat: Wheat is a relevant food allergen in both childhood and adulthood. Its prevalence has been reported to range from 0.4 to 4% [60, 61]. The sensitizations are more frequently not clinical relevant in children [62]. Baker’s asthma or wheat-dependent exercise-induced anaphylaxis are important clinical pictures of wheat allergy in adults. Since the total extract of wheat gives often false positive results, partly due to strong cross-reactivity to grass pollen, with underrepresentation of other allergens, single allergen component determination is recommended. The most frequently described single allergen is omega-5-gliadin, which, along with other gliadins, may indicate an exercise-dependent wheat allergy. In this case, an allergic reaction is often only triggered after consumption of larger amounts of wheat in combination with physical activity and/or other cofactors [63]. Wheat LTP (Tri a 14) is also an important marker of wheat allergy and presumably not cross-reactive with pollen [64].
Celery: Sensitization to celery is frequently associated with cross-reactivity to birch pollen and less frequently to mugwort pollen. Several allergens, both in celery stalk and bulb, have been described, e.g., a Bet-v-1 homologue (Api g 1) and an nsLTP (Api g 6) in celeriac stalk, but also an nsLTP (Api g 2) in celery stalk[65]. The symptomatology of celery allergy can vary from mild to anaphylactic reactions. Severe clinical reactions to celery have been described in the presence of concomitant mugwort pollen allergy, although the allergen responsible for this is currently not known [64].
4.2.1.5 Common animal food allergens
Animal proteins from diverse allergen sources can also cause sensitization to foods. They are often stable to heat and digestion and usually responsible for systemic allergic reactions.
Their structural similarity causes serological cross-reactions within a protein family, however the clinical relevance cannot be concluded from the test result. Due to the complex sensitization patterns and good representation of the proteins in a given extract, a diagnosis with the extracts only is often sufficient.
Hen’s egg: The major allergens in the egg white have been identified (Gal d 1, 2, 3, 4) [66].
Sensitization to the major allergen Gal d 1 is associated with persistent hen’s egg allergy due to its heat resistance. If IgE is no longer detectable during the course of a hen’s egg allergy, this may indicate incipient tolerance. Despite clinically relevant egg allergy (even in the case of Gal-d-1 sensitization), a large proportion of affected individuals tolerate egg in baked form [67].
Cow’s milk: Complex sensitization patterns against predominantly stable cow’s milk proteins and their good representation in cow’s milk extract are reasons for using the total extract for diagnostics [68]. Certain single allergens such as Bos d 8 (casein) are associated with persistent cow’s milk allergy and reactions to processed milk (products) due to their stability. A decreasing or absent IgE may be an indication of incipient tolerance. Cow’s milk in processed form may also be tolerated by a large proportion of cow’s milk allergic patients.
Meat: Allergies to mammalian meat, especially to raw or insufficiently cooked meat products, may result from sensitization to serum albumin. Due to the high cross-reactivity, IgE determination against a representative serum albumin (e.g., Fel d 2 of cat, Bos d 6 of cow) is sufficient.
Another source of allergic reactions after meat consumption is a carbohydrate epitope (CCD) found in mammals but not in primates: α-Gal. The disaccharide is present in proteins as well as probably in glycolipids and can cause delayed urticarial and severe anaphylactic reactions after red meat [69]; poultry meat, however, is tolerated. If meat allergy is suspected, IgE determinations against albumins, against α-Gal (Ro307, ImmunoCAP, ThermoFisher) and the suspected meat species are useful [70].
Fish: Reactions to fish are often based on a major allergen from the group of parvalbumins (e.g., Gad c 1 from cod, Cyp c 1 from carp), which show a strong homology. Since additional species-specific fish allergens may sensitize, an extract diagnostic with the suspected fish species is recommended [71]. The high stability of most fish allergens to heat and digestion and the large amounts in the total protein explain their hazardous nature: minute amounts can be sufficient to trigger systemic reactions. In the so-called “fish-chicken syndrome”, a clinically relevant cross-allergy between fish and chicken can occur. Parvalbumin, enolase and aldolase have been described as the underlying proteins [72].
Crustaceans, mollusks, and insects: Tropomyosin, a muscle protein with high cross-reactivity, is considered an important major allergen of crustaceans and shellfish. In addition to the determination of this major allergen (e.g., Pen a 1, tropomyosin of shrimp), the use of extracts of the suspected animal is recommended due to additional allergens [73]. Shrimp can also be a trigger of exercise-induced anaphylaxis [74]. House dust mite allergic individuals with sensitizations to tropomyosin, the minor allergen Der p/f 10, may be allergic to crustaceans. However, this is not regularly the case. In dust mite and insect allergic patients, the trend towards “edible insects” can lead to severe food reactions [75].
4.2.1.6 Interpretation of serological IgE diagnostics
Specific IgE against food allergens can only be successfully interpreted if the clinical reaction of the patient is known (Table 13).
The following errors are possible in the interpretation:
Sensitizations without corresponding symptoms are misinterpreted as allergy.
Missing or hardly present allergens in the extract may cause false-negative or too low IgE values.
Laboratory errors can cause both false-negative and false-positive findings.
Total IgE should be considered when interpreting quantitative IgE concentrations: very high total IgE (e.g., > 2000 kU/L in patients with atopic eczema) is often associated with numerous sensitizations of questionable clinical relevance.
If total IgE is low (e.g., < 20 kU/L), even low specific IgE values may be diagnostically significant, and detection or exclusion of sensitization may be difficult.
Conclusion
Positve specific IgE corresponds to an IgE-sensitization, which only becomes clinically relevant in combination with a clear matching history and/or a positive provocation test.
4.2.2 Cellular methods for IgE- dependent sensitization detection
IgE-mediated sensitization can be detected indirectly using a basophil activation test (BAT). These tests are currently laborious, costly, and so far not established with allergens of any food allergen for in vitro diagnosis of suspected food allergies (e.g., when total IgE is unusually low, < 20, < 10, < 5 kU/L).
Recent data suggest that in primary NMA, e.g., to peanut or tree nuts, the results of a BAT (at 10 and 100 ng/mL) are capable to distinguish between clinically relevant and silent sensitizations [76, 77, 78]. Approaches to automate the use of the BAT in a labour- and cost-saving manner are currently under way [283].
Consensus statement. Consensus statements.
| Instead of a quantitative IgE result, the severity of a clinical reaction should be determined by history and/or provocation testing. Strong consensus | Strong consensus |
| Reasonable indications for IgE determination are: - reasonable suspicion of IgE-mediated food allergy, - the specific exclusion of an IgE-mediated food allergy, - a life threatening reaction to food, - suspected sensitization to food,which can not be skin tested - conditions that do not allow skin testing or its evaluation (e.g., urticaria factitia, generalized skin disease, administration of drugs that interfere with skin test results), - very young patients (infants or young children), - an expected diagnostic added value of molecular allergy diagnostics |
Strong consensus |
| Total IgE should be determined as an aid to interpretation. | Consensus |
| For specific questions, IgE diagnostics with single allergens should be used to detect sensitization | Strong consensus |
| In vitro diagnostics with single allergens may increase test sensitivity, especially for unstable or underrepresented food allergens | Majority consensus |
| Sensitization to defined allergen components (see tables in 4.2) may be associated with systemic allergic reactions. Their determination increases analytical specificity compared to food extracts. | Strong consensus |
4.3 Skin testing
T. Zuberbier and Z. Szépfalusi
Which skin test procedure is particularly suitable for the diagnosis of food allergy?
What should be considered in skin testing for the diagnosis of food allergy?
Skin tests are a central component of the diagnosis of a food allergy. The skin prick test is the preferred method. Diagnostic sensitivity and specificity may vary depending on the material used (extract, native food). It is usually safe and results are available within 20 minutes.
Contraindications
Contraindications to skin testing include:
Skin disease in the test area,
Use of medications that affect skin test results (e.g., antihistamines (H1 receptor antagonists)),
Presence of symptomatic dermographism, and
A history of a severe anaphylactic reaction to the suspected food (relative contraindication).
Restrictions on the use of commercial extracts and criteria for their use
Many commercial food extracts are not standardized for their allergen content. In children with atopic eczema and food allergy for example, milk, egg, or peanuts, skin testing has high diagnostic sensitivity and negative predictive value (“NPV”), but limited PPV. Skin tests with extracts of plant foods (fruits, vegetables) often (though not always) have insufficient test sensitivity and diagnostic sensitivity. Endogenous enzymatic processes lead to a degradation of less stable allergenic proteins in the extract (e.g., Bet-v-1 homologous food allergens). In addition, important allergenic components are sometimes present in low concentrations. In these situations, prick-to-prick testing with fresh food offers an alternative to commercial extracts (Table 14).
Table 14. Overview of the suitability of prick test materials [271]c.
| Commercial extract | Suitable for native test a | Limited suitability for native test b | |
|---|---|---|---|
| Food of animal origin | |||
| Fish | + | + | |
| Meat | (+) | + | |
| Chicken egg | + | + | |
| Seafood and snails | + | + | |
| Milk | + | + | |
| Food of vegetable origin | |||
| Pineapple | + | ||
| Apple | + | ||
| Cereals | (+) | + | |
| Strawberries | + | ||
| Peanuts | + | + | |
| Spices | + | ||
| Hazelnuts | + | + | |
| Carrot | + | ||
| Kiwi | + | ||
| Lychee | + | ||
| Mango | + | ||
| Oilseeds (e.g., poppy, sesame) | + | ||
| Peach | + | ||
| Celery | (+) | + | |
| Mustard | + | ||
| Soy | (+) | + | |
| Tomato | + | ||
| Grape | + | ||
| Sugar snap pea | + | ||
aIdeally control subjects should be tested because of possible irritant components (testing of control subjects with not approved test preparations is not legal in Germany according to AMG). bHigher irritant potential. cData on the quality of commercial extracts are only available for individual food prick test solutions from individual manufacturers [271], therefore this table can only provide limited information. It does not allow extrapolation to the performance of test allergens of the same allergen sources from other manufacturers.
In practice, skin testing with pollen extracts is useful when pollen-associated food allergy is suspected. Commercial solutions can be used for those foods that have shown high test sensitivity and diagnostic sensitivity in food allergy diagnostics based on studies, such as fish extract. In contrast, for fruits, vegetables, and meat, prick-to-prick testing with native food is more sensitive, thus diagnostically more sensitive, but also more nonspecific.
Advantages and disadvantages of testing with native material
Skin testing with native material can also be helpful to test original dishes. On the basis of a skin test, for example with a cooked mixed original dish, it can be estimated whether and how then the possible individual components are to be examined. In addition, the skin test offers the possibility to test the foods processed in the meal with possible changes in their allergenicity.
The disadvantage of skin testing with native material is the low diagnostic specificity. For example, false-positive results may occur due to the irritative potential of native food. In rare cases, native food can trigger systemic allergic reactions during skin testing. In addition, this test principle is not standardized or can be standardized.
Other skin tests and their significance
Intracutaneous testing with food has no role in clinical practice, since it represents a considerably higher risk and false-positive reactions can occur. Atopy patch testing with fresh food, for example, based on the suspicion that atopic eczema is aggravated by food allergen sources, rarely provides valuable additional information.
In the future, the use of fresh food in skin testing will become more important, as the number of commercially available extracts is decreasing since they have to be approved as medicinal products due to European legislation. The associated high costs and simultaneously decreasing sales figures lead to the fact that predominantly only the more frequently demanded allergen sources will be offered by manufacturers [41, 79, 80, 81, 82]. An overview of the food test allergens approved in Germany is available at the homepage of the Paul Ehrlich Institute (https://www.pei.de/DE/arzneimittel/allergene/pricktest/pricktest-node.html).
Consensus statements. Consensus statements.
| The preferred skin test method for the diagnosis of IgE-mediated food allergy is the skin prick test. | Strong consensus |
| Scratch testing, friction testing, intradermal testing, and closed epicutaneous testing (atopy patch testing) are not recommended for routine diagnosis of food allergy. | Consensus |
| Depending on the stability and safety of the food allergens, testing should be performed with commercial test solutions or native foods. | Strong consensus |
4.4 Diagnostic elimination diet and provocation testing
L. Lange, S. Lau, I. Reese, and C. Schäfer
What is a diagnostic elimination diet and how long should it be performed? What is the significance of food allergen provocation testing and how should it be performed?
4.4.1 Elimination diet
A diagnostic elimination diet is a controlled avoidance of food for a specific period of time. It should only last longer than one to a maximum of 2 weeks in exceptional cases, even for chronic diseases such as atopic dermatitis. For non-IgE-mediated reactions, longer periods (4 – 6 weeks) may be required. There is evidence that a longer-term elimination in IgE-mediated food allergy, in case of late symptoms only, may increase the risk for the onset of immediate reactions on reintroduction [83, 84, 85, 86, 87]. Elimination can also support the loss of tolerance if sensitization is still present [88] and should be avoided in these cases.
A detailed (complete) documentation by means of a dietary and symptom diary over the period of elimination allows verification with regard to dietary errors. The recurrence of symptoms in the case of dietary errors corroborates the suspected diagnosis, while an absence of symptoms questions intolerance and indicates tolerance.
Following the diagnostic elimination diet in the absence of symptoms or a significant improvement during the diet an oral food provocation test is recommended upon medical supervision.
If symptom improvement does not occur under a diagnostic elimination diet, the extent of the diet should be carefully reviewed. Either are the symptoms food-independent or not all eligible triggers have been identified and subsequently eliminated, or augmentation factors are influencing reactivity.
Use of therapeutic infant formulas during diagnosis
Non-breastfed infants with suspected cow’s milk allergy require cow’s milk substitutes for the period of diagnostic elimination in the form of extensively hydrolyzed infant formula or amino acid formula, which should be selected individually (see also 5.3.). If the symptoms do not change despite a carefully controlled elimination diet, an allergy to the avoided foods is highly unlikely. In this case, these foods should be re-introduced into the diet to ensure nutrient coverage and avoid unnecessary dietary restrictions.
4.4.2 Food provocations
Controlled oral provocations are usually necessary to confirm the diagnosis of food allergy or to prove clinical tolerance (Table 15). In addition, it has been shown that regardless of the outcome of an oral food provocation the quality of life of a given patient can improve [89]. The procedure for food provocations is described in detail in national (GPA manual: https://www.gpau.de/fileadmin/user_upload/GPA/dateien_indiziert/Zeitschriften/GPA-SH_Nahrungsmittelallergie_oA.pdf) and international guidelines (EAACI, PRACTALL consensus paper). The guideline “Food allergy due to immunological cross-reactivity with inhalant allergens” addresses the specifics of provocations in pollen-associated food allergies [41].
Table 15. Procedure for provocation tests.
| Design of the provocation open vs. blinded (single or double blind) titrated vs. one-step | The design should be selected according to indication and purpose of provocation | |
|---|---|---|
| Preparation of the provocation meal | The provocation meal should include, as realistically as possible, the usual edible form of the food that triggered the reaction. A common target amount is 3 – 5 g of the food protein. Processing of the food and incorporation into a matrix can significantly affect allergenicity, e.g., raw or baked egg. In provocations to confirm pollen-associated food allergy, fresh fruits and vegetables should be used if possible, as the triggering proteins are usually heat-labile. | |
| Choice of matrix | Clear care should be taken to ensure that no other allergens to which the patient reacts are included in the meal. As few ingredients as possible should be used. For placebo meals, the sensory characteristics should be as close as possible to those of the test food. | |
| Dosage | Number of doses | In most cases, a titration in seven semi-logarithmic steps should be chosen. If negative provocation is expected and there are no safety concerns, a single dose may be appropriate. |
| Initial Dose | In clinical practice, an initial dose of 3 mg of food protein is appropriate for most foods. Smaller doses should be selected for threshold dose provocations and high-risk patients. | |
| Maximum dose | According to an age-adjusted portion, 3 g of food protein is suitable for most foods. | |
| Cumulative total dose | A cumulative total dose should be administered the next day or another day, as some patients do not respond until repetitive administration. | |
| Time interval between doses | 20 – 30 minutes, but should be adjusted according to history. | |
Decision criteria and influencing factors
The recommendations include various variables that must be considered in order to perform patient-specific individualized provocations:
Patient selection,
safety aspects,
type and amount of food to be administered,
time intervals between individual administrations,
criteria for assessment,
observation periods and
formulations.
When cross-reactive foods to inhalant allergies are provoked further aspects should be considered:
possible accumulation effects during pollen flight,
altered reaction situation due to augmentation factors (physical exertion, infections, medication and alcohol consumption) as well as
concomittant diseases (e.g., unstable bronchial asthma, mastocytosis).
Performance and interpretation of oral provocation tests
Food provocations can be performed open or blind (single- or double-blind). For pollen-associated food allergy, sequential mucosal and systemic provocation may be used. Open oral provocation testing allows a definite conclusion only if the result is negative. Overall, double-blind placebo-controlled food challenge (DBPCFC) is the gold standard for the diagnosis of food allergy.
A negative food challenge should be confirmed by repetitive administration of the cumulative amount on the following day at the earliest. DBPCFC are time and personnel consuming. In this respect, negative open provocation may be a useful first step to rule out food allergy. In patients with moderate or severe atopic eczema, DBPCFC are preferable to open provocations. DBPCFC should also be performed when symptoms are subjective, delayed, or atypical, or when patients or parents are anxious. In addition, their use is useful in scientific investigations, for example, to demonstrate the appropriate clinical relevance and potency of the different allergens and to determine threshold dose values of the different food allergen sources. The food allergen administered in the provocation should not be identifiable regarding
taste,
odor,
texture and
form of presentation (consistency, color and shape) against placebo.
The higher the expectation of a patient for a positive response (overanxious or strongly fixated on a food), the more consideration should be given to changing the standard verum to placebo ratio of 1 : 1 towards a higher number of placebo provocations to better identify and avoid nocebo reactions [90].
To avoid severe reactions during a provocation, patients receive the appropriate food in a titrated manner, usually with semi-logarithmic increments at time intervals of 20 – 30 minutes. For many foods, such as cow’s milk, hen’s egg, peanut, and tree nuts, the amounts between 3 mg and 3 g (based on the protein content of the administered food) have been shown to be sufficient in clinical practice [91].
Food provocations are usually discontinued as soon as clinical objective reactions occur, or are terminated when the last administered amount and a repetitive administration of the total cumulative dose (e.g., on the following day) has been tolerated without clinical symptoms. If subjective symptoms occur, the next dose should be suspended until improvement occurs or the last dose should be repeated. Immediate reactions occur predominantly within 2 hours after the last food intake. Atopic eczema may continue to worsen for several hours or during the next day after food provocation; therefore, skin examination is required the following day. Urticaria and/or angioedema are the most common immediate type reactions, but gastrointestinal, respiratory, or cardiovascular involvement is also common. There are also allergen-specific differences in the frequency of allergic organ-related symptoms; for example, gastrointestinal reactions such as abdominal pain, nausea, and vomiting are commonly seen with raw hen’s egg, but also with peanut [92].
Safety aspects
For safety reasons, oral provocations should only take place where allergic reactions, including anaphylaxis, can be adequately treated in an age-appropriate manner. Staff should be trained and experienced in early recognition of symptoms and performance of emergency management. Age- and weight-adapted emergency medications that may be required should be noted and kept on hand in the chart, for example, before provocation starts. For patients with non-IgE-mediated reactions, provocations should be adapted to suit individual circumstances. ,
Consensus statements. Consensus statements.
| Oral food provocation (especially double-blind placebo-controlled) is the gold standard in the diagnosis of IgE-mediated food allergy. | Strong consensus |
| If there is evidence of augmentation factors, these should be taken into account during provocation. | Strong consensus |
| Food provocations should be performed when indicated to confirm or exclude allergy. Provocation provides the basis for a safe food ingestion, allows counseling regarding appropriate allergen avoidance, and provides an assessment of the risk for severe reactions (anaphylaxis). |
Consensus |
| A negative oral food provocation should be followed by repetitive cumulative administration of the tested food in an age- and daily-adapted amount no earlier than the next day to confirm clinical tolerance. | Strong consensus |
| Oral food provocations should be performed in specialized facilities where emergency measures are immediately available. In addition, for provocation with a high risk of severe allergic reactions, intensive medical support should be available. | Strong consensus |
Rationale of food provocation. Rationale of food provocation .
| Indication | Rationale |
| Common indications for oral food provocation | 1. Basis for informed treatment planning, including a food- and nutrient-based recommendation |
| 2. Basis for informed treatment planning, including a food- and nutrient-based recommendation | |
| 3. Suspected allergic reaction where the trigger remains unclear despite allergy diagnosis (reaction after compounded meal) | |
| 4. Evidence of sensitization, but the corresponding food has never been consumed or has only been consumed in small amounts | |
| 5. Confirmation of clinical relevance after improvement of clinical symptoms, e.g., atopic dermatitis, under elimination diet | |
| 6. Proof of a natural development of tolerance | |
| 7. Evidence of efficacy of a causal therapy, e.g., oral immunotherapy in the context of clinical research | |
| Strong consensus | |
4.5 Mucosal food provocations as an experimental approach
There is a limited experience (especially for children) – also in terms of the number of patients and controls studied – with mucosal food provocations in adults, which are used in cases of isolated gastrointestinal symptoms and suspected local tissue IgE production on mucous membranes of the gastrointestinal tract (esophagus, stomach, duodenum, caecum, sigmoid/rectum) using an appropriate conventional endoscopic or microendoscopic procedure in specialized centers [93, 94, 95, 96, 97, 98, 99, 100]. This experimental approach aims to differentiate between allergy to food and functional gastrointestinal disease. Similar safety issues should be considered as described for oral provocation.
4.6 Alternative diagnostic tests
J. Kleine-Tebbe, L. Klimek, V. Mahler, and K. Nemat
What alternative diagnostic tests are available?
What is the importance of alternative diagnostic tests to confirm food allergy?
A number of alternative diagnostic procedures are used by some physicians and alternative practitioners when food-related symptoms are suspected. These fall into two categories:
1. Tests with questionable theoretical basis, lack of validity, and no reproducibility. These include bioresonance, electroacupuncture, hair analysis, iridology, kinesiology, and cytotoxic food testing (ALCAT test). These methods have not been successfully validated technically or clinically to justify their use [101, 102, 103, 104, 105].
2. Tests with accurate measured data but misleading interpretation: determinations of immunoglobulin G (IgG) or IgG4 antibodies or lymphocyte transformation tests with food do not allow differentiation between healthy and diseased individuals [106], neither in food allergy nor in food intolerance. The lack of diagnostic specificity causes many positive findings in healthy individuals. Food-specific IgG or IgG4 merely indicates that the individual has had repeated contact with the food in question and represents a physiological response of the immune system to a foreign protein. Lymphocyte proliferation after stimulation with food and IgG or IgG4 against food in serum may be elevated in allergic individuals. However, both tests are not suitable for individual diagnosis of food hypersensitivity because of their spread and insufficient specificity [105, 107, 108, 109].
The use of IgG/IgG4 determinations with food in suspected cases of food allergy or intolerance is also discouraged by the EAACI [108], the American (American Academy of Allergy, Asthma & Immunology, AAAAI) and the Canadian Allergy Society of Allergy and Clinical Immunology (CSACI) [39, 110, 111].
Consensus statement. Consensus statement.
| Diagnostic testing procedures such as bioresonance, electroacupuncture, kinesiology, cytotoxic food tests as well as IgG/IgG4 determinations and lymphocyte transformation tests with food should not be performed for the diagnosis of food allergy or intolerance | Strong consensus |
5. Course and treatment of food allergy
5.1 Natural course
L. Lange, U. Lepp, Z. Szépfalusi, and E. Hamelmann
Can a food allergy transmit to spontaneous tolerance?
For which food is a development of tolerance likely, for which unusual?
Most primary IgE-mediated food allergies take the following course:
Onset in infancy and toddlerhood and spontaneous remission sometimes by school age, sometimes in adolescence [25] depending on the food and concomitant disease [112] or cofactors.
A later onset of primary food allergy to staple foods, nuts, legumes, or seeds in school age and adulthood is rare. Only in fish allergy, the onset at any age is possible. From adolescence onwards, cofactor-dependent allergies to various foods are increasing. (Table 16).
Table 16. Typical first onset of food allergy.
| First year of life | Toddler preschool age | Adolescent and adult age |
|---|---|---|
| - Cow’s milk - Hen egg - Wheat - Soy - Peanut - Nuts - Fish |
- Peanut, other legumes (e.g., lentils/chickpeas) - Nuts - Fish |
- Pollen-associated food allergen sources - Cofactor-dependent food allergy (e.g., wheat) - Crustaceans - Fish |
The natural course of food allergy is strongly dependent on the food source: Cow’s milk [113, 114], hen’s egg [115, 116, 117], wheat [118], and soy allergies [119] tend to spontaneously resolve over the first years of life. In contrast, peanut [120, 121, 122, 123, 124, 125], tree nut [126], fish, and crustacean [127] allergies persist more frequently throughout life. However, tolerance development is also possible in these cases (Table 17).
Table 17. Lifetime prevalence of food allergy by self-assessment or food provocation. Importance of spontaneous remission in infancy.
| Lifetime prevalence (self-assessment; 95% CI) [272] | Lifetime prevalence (food provocation; 95% CI) [272] | Spontaneous remission (by LY) | Reference | |
| Cow’s milk | 6.0% (5.7 – 6.4) | 0.6% (0.5 – 0.8) | 50% (5th LY) 57% (2nd LY) 57% (5th LY) |
[112] [113] [273] |
| Chicken egg | 2.5% (2.3 – 2.7) | 0.2% (0.2 – 0.3) 2.01% (1.04 – 3.5) |
50% (6th LY) 47% (2nd LY) 49% (2nd LY) |
[24, 115, 116] [117, 274] |
| Wheat | 3.6% (3.0 – 4.2) | 0.1% (0.01 – 0.2) | 29% (4th LY), 56% (8th LY), 65% (12th LY) 70% (14th LY) |
[118] |
| Soy | − | 0.3% (0.1 – 0.4) | 25% (4th LY), 45% (6th LY), 69% (10th LY) | [275] |
| Peanut | 0.4% (0.3 – 0.6) | 0.2% (0.2 – 0.3) | 0 – 57% 22% (4th LY) |
[120, 121, 122] [125] |
| Fish | 2.2% (1.8 – 2.5) | 0.1% (0.02 – 0.2%) | 0 – 20% (10th LY) | [127, 276] |
| Crustaceans | 1.3% (0.9 – 1.7) | 0.1% (0.06 – 0.3) | 0% | [127] |
Specific IgE antibodies against food can already appear in infancy and early toddlerhood. The values can increase or decrease over time. A drop of sIgE to a given food allergen is often associated with the development of tolerance [125]. Therefore the development of sensitization can be used to decide whether a repeated provocation test is appropriate. Patients with high specific IgE concentrations to a given food allergen are less likely to develop clinical tolerance. Recent data suggest that low specific IgE antibodies, small skin prick test diameters and low atopic dermatitis severity are more likely to be associated with a remission of food allergy [24, 113].
Food allergy in adulthood may represent persistence of a childhood form or a de novo onset. The most frequent triggers are apple, peanut, kiwi, hazelnut, peach, cow’s milk, hen’s egg, wheat, fish, and shrimp [128]. More frequent than primary are secondary food allergies due to cross-reactivities of specific IgE against inhalant allergens. In German-speaking countries the birch pollen-associated food allergy is the most frequent manifestation. These food allergies occurring in adolescence/adulthood may persist throughout life [129].
Consensus statements. Consensus statements.
| Because of the natural history of cow’s milk, hen’s egg, wheat, and soy allergy in children, oral food provocations should be repeated at regular intervals (e.g., every 6, 12, or 24 months) to assess tolerance development. | Strong consensus |
| In children with peanut and primary tree nut allergy and allergy to fish and oilseeds, follow up provocation testing should be performed within longer intervals (e.g., every 3 – 5 years). | Consensus |
| The course of sIgE antibodies over time is a useful parameter to assess whether repetitive provocation is required. | Expert opinion |
5.2 Therapy
Food allergy therapy is based on:
Short-term treatment of acute reactions and
Long-term strategies to reduce the risk of further reactions.
The latter include dietary therapy and education programs. These education programs are designed to support individuals to avoid allergen sources and to learn proper behavior in case of an accidental contact (e.g., use/administration of emergency medications). New perspectives for achieving clinical tolerance seem to be offered by sublingual or oral immunotherapy.
5.2.1 Acute therapy of food allergy
U. Lepp, T. Werfel, M. Raithel, J. Schreiber, S.C. Bischoff, and K. Brockow
What forms of treatment are available for food allergy? When and how are these used?
Key questions
How effective are pharmacologic and non-pharmacologic interventions in treating acute, nonlife-threatening reactions in food allergy? How effective are pharmacologic and non-pharmacologic interventions in the long-term management of food allergic patients?
Therapy of acute reactions
To successfully manage patients with food allergy, a risk assessment for potentially severe reactions is very important. The risk varies in certain subgroups. In particular, patients with
previous anaphylactic reactions,
severe and/or non-controlled bronchial asthma, or
certain underlying diseases (mastocytosis)
show an increased risk.
The guideline for the management of anaphylaxis describes how to recognise and treat anaphylactic reactions [130]. Emergency medications must be applied immediately in addition to other emergency medical measures (e.g., fluid and oxygen administration, circulation monitoring, ABCD measures). They are used as initial medication with immediate effect to avert the pathophysiological effects of anaphylaxis. These include epinephrine, bronchodilators, antihistamines, and glucocorticosteroids [130]. Intramuscular administration of epinephrine for anaphylaxis is considered the first-line agent [131].
There is no solid evidence that antihistamines are effective for respiratory or cardiovascular symptoms. Moveover, a preventive use of antihistamines may mask the onset of early anaphylactic symptoms (e.g., skin flushing), leading to delayed use of necessary epinephrine [130, 132].
Besides epinephrine and antihistamines, glucocorticosteroids are applied in the emergency treatment of food allergy according to the above mentioned guideline [130], although no systematic clinical studies for this indication are available [133, 134, 135]. At moderate doses (1 – 2 mg/kg methylprednisolone), they are effective in the treatment of asthma and may counteract protracted or biphasic reactions [136, 137].
5.2.2 Drug (continuous) therapy of food allergy
Studies on the prophylactic use of mast cell stabilizers have led to different clinical results [138]. Four randomized trials and two non-randomized comparative studies showed that mast cell stabilizers can reduce the onset of symptoms, while three randomized trials found no efficacy. Thus, no uniform recommendation on the use of mast cell stabilizers is currently possible, but a more differentiated approach is required depending on the patient population studied.
The mechanism of action of so-called mast cell stabilizers such as cromoglicic acid or ketotifen is still unclear. While a reduced disease activity of intestinal symptoms has been described by possible positive effects on the intestinal barrier, negative reports on the action of cromoglycic acid have been reported regarding cutaneous and extraintestinal symptoms [139, 140, 141, 142, 143, 144, 145].
There are currently no randomized clinical trials for the use of budesonide in IgE-mediated food allergy. The previous recommendations are based on case and expert reports with an extrapolation of the data derived from patients with eosinophilic disease, which is associated with IgE-mediated allergy in ~ 50% [146].
The aforementioned therapeutic options with mast cell stabilizers and budesonide may be considered individually, if appropriate, for exclusively gastrointestinal symptoms when abstinence measures are not sufficient. They should be critically reviewed primarily by gastroenterologists regarding their efficacy [147].
Consensus statements. Consensus statements.
| Acute therapy | |
| Patients at risk for anaphylaxis should receive emergency medication for self-administration, including an epinephrine autoinjector. | Strong consensus |
| Severe allergic reactions to food should be treated primarily with intramuscular epinephrine. | Strong consensus |
| Antihistamines should be used for acute cutaneous symptoms, especially urticarial reactions and mucosal reactions. | Strong consensus |
| Prophylactic use of antihistamines to prevent food allergic reactions is not recommended. | Consensus |
| Continuous therapy | |
| Cromoglycic acid and ketotifen did not show a consistent therapeutic effect when unselected patient cohorts were treated, therefore currently no consistent treatment recommendation is possible for patients. If gastrointestinal symptoms are present, individual treatment decisions and monitoring are recommended. | Consensus |
5.3 Long-term management of food allergy
5.3.1 Dietary therapy and allergen labeling
I. Reese, K. Brockow, S. Schnadt, and C. Schäfer
How can dietary restriction measures be successfully implemented in everyday life?
Aspects of long-term management
Long-term management of food allergy includes:
the avoidance of the eliciting food,
the replacement with suitable foods
the regular consumption of tolerated foods (especially if sensitization is present), and
the management of therapeutic interventions in daily life [148].
Avoidance is the most important therapeutic intervention to prevent the elicitation of symptoms.
Valid data on efficacy of avoidance are not available due to ethical reasons, because neither randomized controlled trials including individuals without food allergies are possible nor can food allergic individuals be deprived of their dietary therapy.
Therapeutic elimination diets are tailored to the individual allergological requirements and nutritional needs of the affected person. The requirements, goals and expectations of a nutritional therapy vary largely depending on the age of the affected individual and the antigenic structure (primary vs. secondary food allergy).
Ideally, the affected individuals receive the therapeutic counseling from an dietitian specialized in food allergies. It is important to take the individual thresholds into account instead of issuing blanket bans. For example, it has been shown that a large proportion of children with cow’s milk and/or hen’s egg allergy tolerate these foods in baked form [149, 150]. Such partial tolerance allows to expand the diet, and the risk of accidental reactions is lower, a beneficial effect on the development of tolerance is promoted if the food in question is regularly consumed in a baked form [151, 152, 153, 154, 155, 156, 157]. A regular administration of minimal, significantly subthreshold amounts of other allergenic foods is probably also favorable for the promotion of tolerance, but currently not supported by published data [54].
Individual tolerance to a food allergen may vary among affected individuals and may change individually. This applies to primary but also secondary food allergies which may manifest only seasonally in some affected individuals. Augmentation factors for a food allergic reaction are listed in the section “Medical history” (see 4.1), these factors should be taken into account in nutritional therapy.
Cow’s milk (formula) substitution
Cow’s milk allergy, which occurs in the first year of life, requires a therapeutical diet (extensive hydrolysate, amino acid formulas) to ensure age-appropriate growth and thriving. However, an adequate supply of all nutrients can only be guaranteed as long as the infant is mainly bottle-fed [158].
The choice of formula requires an individual approach: Extensive hydrolysates are usually used as the first choice. For patients with severe symptoms (especially also gastrointestinal), amino acid formulas may be beneficial [158, 159, 160, 161].
Soy formulas are not recommended for infants younger than 12 months of age. In addition, their use during the first year of life is viewed critically in Germany due to the content of phytoestrogens, phytate, and aluminum [162]. This is particularly relevant at high intakes per kg body weight, i.e., in the first 6 months of life. With predominant nutrient supply via this milk substitute food and still low consumption of other foods, the risk-benefit ratio of soy formula would be unfavorable.
These limitations are explicitly relevant only regarding soy formula. In contrast, soy products such as soy drink, soy yogurt, tofu, etc. can be used as cow’s milk substitutes. Particularly if these are enriched with calcium, they represent a nutritionally adequate alternative to milk and dairy products. In contrast, other vegetable drinks are not a nutritional substitute due to their low protein and fat content, even when calcium is enriched.
Partially hydrolyzed infant formulas are generally not suitable for the treatment of cow’s milk allergy, nor are sheep’s or goat’s milk [163, 164]. If a partially hydrolyzed infant formula is tolerated [150], it can be maintained.
Food allergen avoidance by the nursing mother
If breastfed infants suffer from allergic symptoms that are clearly attributed to maternal consumption of certain food allergen sources, the breastfeeding mother should eliminate the suspected or triggering food(s) from her diet after dietary counseling. If milk and dairy products are eliminated long term, the mother should be counseled regarding her nutrient coverage. If adequate coverage cannot be achieved by dietary intake, especially for calcium, supplements should be given throughout the day [165].
Monitoring and reevaluation of clinical relevance
Extensive and long-term food avoidance should be carefully monitored, as they may result in an inadequate nutrient intake and and impairment of quality of life.
Consequently, nutritional counseling should cover with a nutriient calculation and, if necessary, an optimization in order to ensure that a given diet is appropriate for everyday life, meets its requirements and is age-appropriate.
A periodic review of clinical relevance is necessary to ensure that avoidance measures are maintained only as long and to the extent that they are appropriate or necessary. In cow’s milk or hen’s egg allergy, a re-evaluation of clinical relevance by oral food challenge is recommended at (6 –) 12 months intervals in young children and at 12 – 18 months intervals in older children.
For allergens with a less favorable prognosis, such as nuts and peanuts, the re-evaluation should be determined on an individual level and should be considered especially if no acute allergic reactions have occurred. In pollen-associated food allergies, a repeated follow-up history is supportive to properly assess the clinically relevant cross-reactions over time.
Patient education and allergen labeling
The most important pillar of nutritional therapy is the education of the patient to implement long-term elimination in daily life.
Patients, their families, relatives and caregivers should:
Know and be able to identify risk situations,
learn to read ingredient lists and
learn to avoid the relevant trigger in an appropriate way both at home and away from home (e.g., in restaurants).
They should be informed about the European Food Information Regulation (FIR [166]):
- The FIR requires the declaration of 14 major triggers of allergies and non-allergic intolerances when they, as well as products thereof, have been added to a food as an ingredient (i.e., intentionally, according to the recipe):
- - Cereals containing gluten (wheat, rye, barley, oats)
- - Crustaceans
- - Egg
- - Fish
- - Peanuts
- - Soy
- - Milk
- - Treenuts (almonds, hazelnuts, walnuts, cashewnuts, pistachios, pecannuts, brazilnuts, macadamia-/queenlandnuts)
- - Celery
- - Mustard
- - Sesame seeds
- - Lupin
- - Mollusks
- - Sulfites (≥ 10 mg/kg)
Mandatory labeling includes packaged as well as non-prepackaged foods.
Not regulated by law is the labeling of the unintentional presence of an allergen in packaged or bulk goods. The so-called “trace” labeling is voluntary and, due to the lack of limit values, does not provide any information about the level (allergen quantity) of contamination or its actual probability, nor does its absence per se indicate a safe food. It must therefore always be interpreted individually with regard to its relevance for the patient.
Patients, their families, close relatives and caregivers should be informed
which substitute products make sense from a nutritional point of view and
what options are available in terms of kitchen technology to enable the patient to continue to enjoy the familiar dishes and preferences despite avoidance.
Therapeutic use of pro- and prebiotics
The use of pro- and prebiotics for the therapy of food allergy is not recommended at present due to limited data.
Consensus statements. Consensus statements.
| An appropriate elimination diet is recommended as a mainstay of food allergy management. | Strong consensus |
| An elimination diet should be based on sound allergy diagnostics. The scope and indication should be re-evaluated regularly. | Strong consensus |
| Individuals with food allergy who are on a long-term elimination diet should be counseled by an allergy-experienced dietitian. | Strong consensus |
| Patients should be educated about allergen labeling (according to the Food Information Regulation) and existing gaps. | Strong consensus |
| Extensive protein hydrolysates or alternatively amino acid formulas are recommended in cases of existing cow’s milk allergy, especially in infants and possibly toddlers. | Strong consensus |
| In the presence of an existing cow’s milk allergy, soy-based infant formulas are second-choice cow’s milk substitutes and are not recommended for infants younger than 12 months of age. Soy-containing foods used as milk substitutes beyond this are not affected by this restriction. | Strong consensus |
Gaps and important areas of research regarding long-term management.
Long-term effect of an elimination diet on nutrition and quality of life,
effect on tolerance development due to altered allergens (baked milk/baked egg),
effect of strict allergen abstinence in patients who have been shown to develop an allergic reaction only after eating larger amounts of the allergenic food (loss of tolerance),
long-term disadvantages of rice and soy formulas in terms of nutrient coverage,
strain-specific (related to specific microorganisms) effects on food allergy management by administration of probiotics or HMOs,
identification of allergen-specific thresholds. Aim: to protect food allergic individuals from severe reactions and to optimize food labeling with respect to ingredient and “trace” (unintended entry) labeling.
5.3.2 Immunotherapy for food allergy
B. Bohle, K. Beyer, L. Lange, R. Treudler, and M. Worm
Is immunotherapy efficacious in patients with food allergy?
Use of specific immunotherapy (SIT) in food allergy
Repeated attempts have been made to treat primary food allergy with the aid of
subcutaneous (SCIT),
sublingual (SLIT) and
oral (OIT) specific immunotherapy with food or food extracts. Epicutaneous immunotherapy (EPIT) is a new treatment for food allergy.
Primary sensitizing pollen extracts have been used sublingually and subcutaneously, but also oral and sublingual application of food directly has been studied for the treatment of pollen-associated food allergy.
The assessment of the efficacy in immunotherapy studies for food allergy is challenging as a standardization of the oral challenge testing, but also the double-blind and placebo-controlled design is required.
Use of SCIT in food allergy
Current data on the efficacy of SCIT for the treatment of food allergy are limited, after this form of treatment for primary food allergy was discontinued decades ago because of severe systemic side effects associated with therapy.
For SCIT with food allergen extracts, two older studies showed evidence for superiority of treatment with verum over placebo in primary food allergy [167, 168]. However, considerable side effects occurred in this case. Attempts have also been made to develop a hypoallergenic recombinant parvalbumin for SCIT of fish allergy [169].
In affected individuals with secondary food allergy, the application of subcutaneously applied pollen allergens has been shown to be effective against pollen-associated food allergy in at least some of the patients in several studies [170, 171, 172, 173, 174]. Here, the effect of SCIT on birch-associated apple, hazelnut, or soy allergy was investigated. Other studies found no efficacy of birch SCIT on birch pollen-associated hazelnut or apple allergy [175].
Currently, a peanut allergen extract is in clinical development. Allergoids contain the protein in a partially denatured form, so better tolerability has been shown [176].
Use of SLIT in food allergy
There are a limited number of studies on the use of SLIT for the treatment of food allergy with single food extracts or recombinant allergens. Daily administration of glycerol-containing liquid allergen extracts under the tongue has been shown to promote desensitization of a food allergen and IgG4 response. For peanut allergy, some studies are available, especially in children/adolescents, showing clinical efficacy with good tolerability [177, 178, 179, 180]. The highest tolerated doses were observed in young children (< 12 years) after a longer duration of therapy (1 year) and are lower than those achievable with OIT. Side effects with this form of therapy are mostly limited to the oral cavity, and systemic reactions are very rare. There are fewer data for other foods. With a hazelnut and Pru p 3-standardized peach extract in 12 and 37 treated patients, respectively, an increase in orally provoked food allergen levels was observed (7.6-fold and 39.8-fold, respectively) [181, 182]. A 16-week SLIT with the main apple allergen Mal d 1 induced an enhanced effect on birch pollen-associated apple allergy than Bet v 1 or placebo [183]. Concomitant immunologic effects on antibody and T-cell response were shown as well [184, 185]. Other studies using a liquid birch pollen extract for SLIT failed to show any or only minor improvements in subjects with apple allergy [186, 187], whereas the SLIT with a standardized allergen tablet containing birch pollen extract was shown to be more efficaceous regarding birch pollen-associated apple allergy in comparison to placebo [188].
Use of EPIT in food allergy
Epicutaneous immunotherapy (EPIT) has been studied in a phase III trial in patients with peanut allergy, and only in a phase II trial for cow’s milk to date. Here, an application system vaporized with food allergen (e.g., “peanut patch”) is applied to the skin. The dosage is increased by increasing the application time. The patch remains on the skin for 24 hours per day. The duration of therapy in the peanut study was up to 3 years. A moderate increase of the threshold in the peanut provocation compared to placebo was observed over the 3-year treatment. However, in some patients the threshold decreased again. The efficacy of this treatment was most pronounced in children aged 4 – 11 years. The side effects were mild with predominantly skin irritation and only very few treatment-related systemic allergic symptoms [189, 190, 191, 192, 193, 194].
Use of OIT in food allergy
OIT with a wide variety of food allergens leads to improved tolerance of the food allergen in childhood and adulthood. This has been shown in various randomized and non-randomized controlled trials – mainly with cow’s milk, hen’s egg and peanut, but also wheat [195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212] – and partly in systematic reviews (“pooled” analyses) based on them [213, 214, 215, 216, 217]. However, side effects occurred in many patients under OIT with the allergen, most of which were not severe.
Especially for peanut allergy, recent studies suggest that OIT is effective in increasing the threshold of reactions to peanut, reducing the acidic reaction, and increasing the quality of life of those affected [218, 219, 220, 221, 222, 223]. Based on a phase III trial, the first oral immunotherapy was approved in Europe for the treatment of peanut allergy in children aged 4 – 17 years. Data from the USA and Canada also show a good efficacy/side-effect profile for younger children, and a phase III trial for the treatment of peanut allergy in 1 – 3 year old children is currently being conducted internationally.
An older randomized trial did not show better efficacy with OIT with cow’s milk or hen’s egg compared with elimination diet in terms of tolerance development, however, this trial was conducted in young children in whom natural tolerance development often occurred [224]. Another study showed for cow’s milk allergy that OIT was more effective than SLIT in a direct comparison, but accompanied by more side effects [199].
Consensus statements. Consensus statements.
| Primary food allergy | |
| Children between 4 and 17 years of age with a confirmed diagnosis of systemic peanut allergy should be offered oral immunotherapy with an approved preparation, taking into account an individual benefit-risk assessment. | Strong consensus |
| Pollen-associated food allergy | |
| Pollen-associated food allergy may improve with subcutaneous or sublingual immunotherapy with pollen allergens. Such treatment may be considered only if there is a concurrent indication for treatment of pollen-related respiratory symptoms. | Strong consensus |
| In pollen-associated food allergy, oral immunotherapy with food allergen sources should only be used in the context of controlled trials. | Strong consensus |
Use of biologics for the treatment of food allergy
The biologic omalizumab (an anti-IgE antibody) was first used to treat peanut allergy in 2003 [225]. Another trial of anti-IgE use in food allergy occurred years later [226]. Anti-IgE can be applied as monotherapy or as adjuvant therapy to oral immunotherapy for the treatment of food allergy. Numerous case series and prospective studies exist in this regard [226, 227, 228]. Potential benefits include a reduction in the duration of therapy, improved efficacy and tolerability of the allergen dose of OIT, acceleration of the increase phase and improved thresholds, and a reduction in the rate of side effects. Recently, a meta-analysis on the use of omalizumab in food allergy was presented. The data show superiority over placebo treatment both as monotherapy, as adjuvant therapy or for multiple food allergen sources [228]. The 2nd generation anti-IgE antibody ligelizumab has stronger IgE receptor affinity than omalizumab. Studies will show its potential regarding efficacy for the treatment of food allergy in the future. In 2018, the FDA in the U.S. granted omalizumab breakthrough treatment designation and recommended accelerated trials for the approval for severe food allergy.
Another antibody of great interest for the treatment of food allergy is dupilumab. This antibody targets the IL-4 receptor α chain and has been approved in Germany for the treatment of atopic dermatitis, TH2-dependent bronchial asthma and polyposis nasi. Clinical trials are currently underway to determine its efficacy in food allergy, thereby this antibody may also represent a new treatment option in the future [229]. Etokimab is another antibody that has been reviewed in a randomized placebo-controlled trial as monotherapy for the treatment of peanut allergy [230]. It is an anti-IL-33 antibody that has been used in patients with peanut allergy. A recently published study demonstrated that the etokimab-treated group achieved higher thresholds to peanut protein after 15 days of treatment.
5.3.3 Day to day management for at risk of anaphylaxis patients
C. Schäfer, S. Schnadt, K. Brockow, and P.J. Fischer
How can a food-allergic patient successfully manage food allergy in everyday life?
Education and risk assessment
Education and training are the essential components of everyday management for patients with food allergies. Risk assessment is necessary for patients at increased risk for severe allergic reactions.
Patients, family members and caregivers receive, in addition to emergency medications:
an individualized nutrition and management plan (see section 5.3.1.),
an anaphylaxis passport and
an anaphylaxis emergency plan (see anaphylaxis guideline [130]).
Emergency plan
The anaphylaxis action plan should consider all variables that may affect the recognition and treatment of allergic reactions to food:
Age of the patient,
Type and extent of food allergy,
Concomitant diseases,
Place of residence, and access to medical help.
Procedure management, especially what to do for which symptoms, should be clear to uninformed third parties.
Training and anaphylaxis training
Training should include the following aspects:
patient-specific avoidance strategies at home and in the social environment,
recognition and interpretation of warning signs and symptoms,
when and how to treat allergic reactions, including use of the epinephrine auto-injector.
Who should be educated on food allergies and anaphylaxis?
All patients in a trainable state of development caregivers, and, if possible, other family members should be included in the training. In addition, training of other occupationally involved individuals is also appropriate. These include:
Family physicians and pediatricians,
other medical specialists,
medical students,
medical assistants,
pharmacists,
nutritionists,
psychologists,
kitchen staff,
teachers and educators, among others, caregivers of children,
first aiders in companies,
paramedics.
In addition to the above-mentioned target groups, there are also other professional groups, such as flight attendants, for whom training may be useful.
A multidisciplinary approach and the provision of written or online information material on food allergies seem to improve the knowledge and correct use of epinephrine auto-injectors and contribute to the reduction of allergic reactions [231]. In addition to face-to-face training courses, “e-learning” has gained importance as a new training route [232].
Patient organizations
It is helpful to refer patients to appropriate patient organizations such as the German Allergy and Asthma Association (DAAB – www.daab.de) for questions regarding everyday management. For severe allergic reactions (anaphylaxis), the standardized AGATE training program (Arbeitsgemeinschaft Anaphylaxie – Training und Edukation – www.anaphylaxieschulung.de) is available in Germany.
Consensus statements. Consensus statements.
| Patients, their relatives and caregivers should be informed about foods to avoid and receive practical advice on avoidance measures, recognition and self-management of allergic reactions. | Strong consensus |
| Patients or those responsible for their medical care (e.g., parents) should receive practical instruction (with AAI trainer) in the use of the emergency kit, including the epinephrine autoinjector. If possible, patients or their relatives, caregivers, and other relevant persons should be offered training in the use of the emergency kit including epinephrine autoinjector. | Strong consensus |
| Patients should be advised to contact an appropriate patient organization. | Consensus |
| Food allergic patients at risk of anaphylaxis should receive an anaphylaxis ID card and or they or their caregivers should attend patient/ or parent education. | Strong consensus |
6. Recommendations for vaccination in patients with hen’s egg allergy
S. Lau
Although there is sufficient evidence for the tolerability of the STIKO-recommended vaccinations of the first 2 years of life for children with hen´s egg allergy, MMR varicella vaccinations are repeatedly postponed unnecessarily or not applied due to their production on chicken fibroblasts or insisted on administration in a clinic.
According to the recommendation of the Robert Koch Institute (as of 2/2020) [233], MMR varicella or MMR vaccination can be administered to hen´s egg allergic patients in a pediatrician’s office. It states on the RKI homepage: “Those who are allergic to chicken egg protein can usually still receive the MMR vaccine, but should seek medical advice before doing so”.
MMR vaccines are grown on so-called chicken fibroblasts, but the vaccine itself contains little or no detectable traces of chicken protein.
International studies have shown [234, 235, 236] that children with proven chicken egg protein allergy can be vaccinated with MMR vaccine without problems. Only children who have very severe chicken egg protein allergy with severe symptoms should be vaccinated under special protective measures and observed afterwards (for example, in hospital).
The indication that only children after severe anaphylaxis (not further specified, but probably grade III and IV) due to chicken egg protein should be vaccinated in hospital or in a specialized practice/day clinic with subsequent observation (2 hours) appreciates the usually great concern of parents and possibly allergologically not so experienced general practitioners. Even in influenza vaccines produced using hen’s eggs, the egg protein content is usually below the dose that normally causes reactions. Therefore, the RKI and the American Center of Disease Control [237] have also published the recommendation to vaccinate patients with rather mild allergic symptoms of a chicken egg allergy normally in practices and to vaccinate only after more severe anaphylaxis under appropriate clinical or specialized outpatient conditions optimized with regard to emergency management, since severe allergic reactions to influenza vaccination are rare or do not occur more frequently than in persons without a hen’s egg protein allergy [238, 239]. However, a chicken protein-free influenza vaccine, i.e., produced in cell culture, is now available that is suitable for allergic individuals.
The situation is different for the yellow fever vaccine, which contains hen’s egg protein concentrations that can lead to symptoms in ~ 5% of hen’s egg allergy sufferers. Here, the indication should be reserved and only given in case of absolute urgency. The yellow fever vaccine is only administered in specialized vaccination centers and by licensed vaccinators. If an indication exists despite hen’s egg allergy, the vaccination should be given under day-case, clinical, or outpatient conditions with the possibility of emergency intervention, with appropriate follow-up.
SARS-CoV2 vaccination can also be given to any food allergic patients [240].
Consensus statements. Consensus statements.
| Hen’s egg allergic children shall be vaccinated as other children according to STIKO. Deferral is not necessary. | Strong consensus |
| Hen’s egg-allergic children shall be vaccinated against MMR varicella like other children according to STIKO; vaccination in a clinic or special outpatient clinic/practice is not necessary. | Strong consensus |
| Influenza vaccination shall be given to hen’s egg allergic outpatients in practices, provided there is sufficient experience in emergency management of allergic/anaphylactic reactions. In patients who have experienced severe anaphylaxis, influenza vaccination may be monitored as an inpatient if necessary. | Strong consensus |
| A yellow fever vaccination contains relevant amounts of hen’s egg protein and should only be performed in hen´s egg allergic patients after careful benefit-risk assessment. If necessary the vaccination should be performed in a day hospital, specialized practice or clinic with appropriate expertise regarding anaphylaxis management. | Strong consensus |
7. Food as an occupational allergen
V. Mahler
How common is occupational food allergy and what are the triggers? How is occupational food allergy diagnosed, and what are the consequences for occupational activity?
Epidemiology and triggers
IgE-mediated sensitization to food allergens can also be acquired and clinically manifest via the skin or respiratory tract, which occurs primarily occupationally but also non-occupationally [41]:
inhalative (work-related) exposure may cause allergic rhinopathy and/or allergic asthma, cutaneous exposure may induce contact urticaria (CU) and/or protein contact dermatitis (PCD) at the site of protein contact (predominantly on the hands) (Table 18) [241, 242] and
rarely after ingestion [243].
Table 18. Forms, clinic, and characteristics of occupational food allergies.
| Immunopathology | Disease/symptoms | Clinical characteristics | Typical age group | Prognosis |
|---|---|---|---|---|
| IgE-mediated | Contact urticaria syndrome (stages I – IV) | Predominantly occupationally triggered by cutaneous contact | Adults, occupationally exposed | Depending on the triggering food and possible avoidance measures |
| Occupational obstructive respiratory diseases caused by allergens (incl. allergic rhinopathy) | Predominantly occupational respiratory symptoms caused by inhalation exposure to allergens | Adults, occupationally exposed persons | Depending on the triggering food and possible avoidance measures | |
| Mixed IgE- and cell-mediated | Protein contact dermatitis | Predominantly occupational lesions on the hands triggered by cutaneous contact | Adults, occupationally exposed | More severe effects and less favorable prognosis than occupational skin eruptions of other etiologies |
| Non-immunological | Non-immunological contact urticaria | Predominantly occupationally triggered lesions on the hands induced by cutaneous contact with benzoic acid, sodium benzoate, sorbic acid, abietic acid, nicotinic acid esters, cinnamic acid, cinnamaldehyde and perubalsam | Adults, occupationally exposed | In contrast to IgE-mediated contact urticaria usually limited to contact area |
Inhalation symptoms to food allergen sources can lead to the development of an occupational disease (OD) of the lung BK 43011, IgE-mediated cutaneous skin symptoms can result in an OD of the skin BK 51012.
1Obstructive respiratory disease 4301, obstructive respiratory disease (including rhinopathy) caused by allergenic substances, which forced the omission of all activities that were or may be causative for the development, exacerbation or recurrence of the disease.*
2Severe or recurrent relapsing skin disease which has compelled omission of all activities which were or may be causative in the development, aggravation or recurrence of the disease.*
*The Seventh Act amending the Fourth Book of the Social Code and other laws, passed by the German Bundestag on 12.06.2020 244. https://www.bmas.de/SharedDocs/Downloads/DE/Gesetze/siebtes-gesetz-zur-aenderung-des-vierten-buches-sozialgesetzbuch-und-anderer-gesetze.pdf?__blob=publicationFile&v=1 (online access: June 28, 2021), which came into force on 01.01.2021, provides in §12 a change in the Annex 1 of the Occupational Diseases Ordinance in the sense that in the occupational disease numbers (BK No.) 1315, 2101, 2104, 2108 to 2110, 4301, 4302 and 5101 the words „... which have forced the omission of all activities that were or may be causal for the development, aggravation or recurrence of the disease“ are deleted. Practical implementation recommendations are currently being developed by representatives of the DGUV and specialist groups.
In the general population, CU and PCD to food allergen sources are very rare; in food processing occupations, the proportion of PCD and CU is significantly higher (1.5 – 20%), depending on the occupation and cohort sample [241, 245, 246, 247]. The prevalence of work-related asthmatic diseases in exposed workers ranges from 1 to 20% and is particularly high among bakers [247, 248, 249, 250]. Flour dust allergy to wheat and rye flour represents the most common cause of occupational allergic obstructive airway disease in Germany [249, 250].
Food allergens from a wide variety of allergen sources have been described as triggers [241, 250, 251, 252, 253]. Asthmatic bakers with sensitization after inhalation exposure to wheat flour show different allergen profiles than individuals with orally acquired wheat-induced food allergy [249, 250]. The extent to which certain food allergens cause specific allergic symptoms depending on the exposure (oral, inhalation, cutaneous) (Table 19) is not yet clear for most allergen sources [249, 254].
Table 19. Allergen profiles and occurrence as occupational allergens (examples).
| Allergen source | Relevant allergens when consumed | Occupational allergens | Occupation | Reference |
| Wheat | ω-5-Gliadin (Tri a 19), and other allergens: „wheat-dependent, exercise-induced anaphylaxis“ (WDEIA); Profilin (Tri a 12), nsLTP (Tri a 14); agglutinin isolectin 1 (Tri a 18), ω-5-gliadin (Tri a 19), γ-gliadin (Tri a 20), thioredoxin (Tri a 25), „high-molecular-weight“(HMW)-glutenin (Tri a 26) and other |
α-Amylase trypsin inhibitors (e.g., Tri a 28, Tri a 29.0101, Tri a 29.0201, Tri a 30, Tri a 15); „thiol reductase“ (Tri a 27); thioredoxin (Tri a 25), triose phosphate isomerase, α-/β-Gliadin, 1-cysperoxiredoxin (Tri a 32), dehydrin (Tri a DH, serpin, glycerinaldehyde-3-phosphate dehydrogenase (GA3PD), ω-5-gliadin (Tri a 19), nsLTP (Tri a 14), Acyl-CoA-oxidase, fructose-bisphosphat aldolase, serine protease inhibitor-like protein (Tri a 39), and other | Bakers | [249, 250, 252, 262] |
| Cattle | Beef (meet): Bos d 6 and α-GAL | Dander: Bos d 2 (Lipocalin) | Farmers, cooks | [277] [243] |
| Soy | Gly m 4 (PR-10-homologue), Gly m 5 (β-conglycinin), Gly m 6 (glycinin) et al. | Soy flour: high molecular weight allergens (Gly m 5 and 6) | Bakers | [278, 279] |
| Dust containing soy: low molecular weight allergens (Gly m 1 and Gly m 2) | Dockworkers | |||
| Fish | Parvalbumin e.g. Gad m 1.0101 Gad m 1.0102 Gad m 1.0201 Gad m 1.0202 Sal s 1.0101 Enolase e.g. Gad m 2.0101 Sal s 2.0101 Aldolase Gad m 3.0101 Sal s 3.0101 |
Skin and inhalation Parvalbumin, glyceraldehyde-3- phosphate dehydrogenase | Fish processing industry, cooks | [280, 281, 282] |
nsLTP = non-specific lipid transfer protein.
Prevention
Protection of workers from allergen exposure and sensitization by minimizing occupational health hazards is necessary for the prevention of IgE-mediated skin and respiratory diseases [250, 255]. Occupational dermatology and occupational medicine guidelines and recommendations are available [242, 246, 248, 255, 256, 257, 258, 259]. In order to optimize preventive measures, the responsible statutory social accident insurance should be informed at an early stage when the disease is suspected:
in the case of skin symptoms by means of a dermatologist’s report,
in the case of respiratory complaints by means of an occupational disease notification.
Clinical picture and differential diagnosis
In food-processing professions, work related skin symptoms of different kinds are common at the hands, with eczematous skin symptoms predominating. Hand eczema can be irritant, allergic and endogenous in origin. Specific occupational and non-occupational triggers should be clarified by history and epicutaneous testing [41, 245, 255].
IgE-mediated contact urticaria to food allergen sources must be distinguished from non-immunologic contact urticaria (e.g., triggered by benzoic acid, sodium benzoate, sorbic acid, abietic acid, nicotinic acid esters, cinnamic acid, cinnamaldehyde, perubalsam) [245]. The latter usually remains confined to the area of contact, whereas systemic forms of manifestation may occur in IgE-mediated contact urticaria [260]. Non-occupational forms of urticaria should be considered for differential diagnosis [261].
Diagnostics
In cases of suspected IgE-mediated work-related allergic diseases, especially work-related rhinopathy/asthma, a diagnosis should be made early, as long as the patient has not yet left the workplace [248].
Stepwise diagnosis includes history, prick test (additionally epicutaneous test in PCD), specific IgE determination and exposure tests [241, 242, 250, 257, 258, 262]. Because extracts for occupationally relevant food allergen sources are often lacking or not sufficiently standardized, in vivo and in vitro diagnostics can be difficult [249, 250]. Diagnostic sensitivity and specificity vary sometimes considerably among currently available occupational allergens depending on the allergen source and test solution [259, 263]. Prick test solutions from different manufacturers should be tested in parallel if available [259]. For the detection of CU and PCD to unstable food allergen sources, a prick-to-prick test with fresh material is recommended [241, 264].
Prick tests for the diagnosis of occupational type I allergies should be performed with a metal lancet, if possible using duplicate determinations. If reproducible, wheals with a small wheal diameter (≥ 1.5 mm) should also be considered positive and serologically confirmed if the control is negative [259]. Allergen avoidance and re-exposure under supervision by a physician as well as workplace-based provocation testing may be required to confirm the diagnosis. The specific inhalation provocation test is the gold standard procedure for many occupational asthma triggers [248]. However, a negative result in this test or after workplace exposure is not sufficient to exclude the diagnosis of occupational asthma when the evidence is otherwise good [248, 250, 257]. Further diagnostic measures are provided in the guideline “Prevention of work-related obstructive airway diseases” [248, 257].
Course and therapy
In occupationally caused IgE-mediated allergies to food components, early allergen avoidance is crucial to prevent increasing symptoms and the development of an OD like BK 5101 (skin symptoms) or an BK 4301 (respiratory symptoms) [248, 256, 265]. Therapeutic measures and the benefit of various management options for work-related allergic rhinopathy and obstructive airway disease are described in the guideline “Prevention of work-related obstructive airway disease” [248].
Allergen avoidance through omittment of exposure or the use of appropriate protective equipment can lead to an improvement or healing of IgE-mediated skin manifestations to food allergen sources, but are not always successful [242]. In the food-processing sector, affected individuals with PCD show more severe courses and a less favorable prognosis than patients with skin manifestations at the hands of other etiologies. Significant differences existed with respect to
the need to consistently wear protective gloves at work,
the duration of incapacity for work, and
the frequency of change of profession [246].
If symptom control by allergen avoidance or reduced exposure by technical or organizational measures or personal protective equipment cannot be achieved, there may be an objective necessity to discontinue work in cases of occupationally acquired IgE-mediated food allergy. In addition to the extent of the clinical manifestations [260], the evaluation of the reduction in earning capacity (MdE) also includes the proportion of jobs on the general labor market that are excluded by the allergy [256, 265].
It can happen that occupational skin and respiratory symptoms are triggered simultaneously by food allergens. Since this is a uniform allergic disease with symptoms in different organs, this constellation is to be treated as one insured event – based on BK 5101 and BK 4301 – and an overall MdE is to be formed, taking into account the consequences of the allergy [265, 266].
Consensus statements. Consensus statements.
| Diagnostic work-up of suspected IgE-mediated work-related allergic diseases should be performed early, as long as the patient has not yet left the workplace, in order to be able to perform, among other measures, workplace-related investigations and exposure tests as needed, in addition to specific step-by-step diagnostics. | Strong consensus |
| Even in cases of occupational food allergy, allergen avoidance should be the primary goal, possibly in the form of exposure avoidance through appropriate protective measures. If this is not possible, the necessity of cessation of the activity should be considered. | Strong consensus |
Methods report
Guideline initiation and stakeholder participation.
The S2k guideline “Management of IgE-mediated food allergy” (AWMF registry number 061-031) was initiated by the German Society of Allergology and Clinical Immunology (DGAKI). The coordination of the guideline project was carried out by Prof. Dr. med. Margitta Worm.
15 professional societies, professional associations and other organizations participated in the preparation of the guideline and delegated mandate holders for the guideline group (Table 20). Patient interests were represented by the German Allergy and Asthma Association.
Table 20. Organizations involved.
| Organization | Representative |
|---|---|
| Medical Association of German Allergists (AeDA) | Dr. med. Katja Nemat |
| Working Group Dietetics in Allergology (ak-dida) | Dr. rer. medic. Imke Reese |
| Professional Association of Pediatricians and Adolescents (BVKJ) | Dr. med. Peter J. Fischer |
| German Allergy and Asthma Association (DAAB) | Sabine Schnadt |
| German Dermatological Society (DDG) | Prof. Dr. med. Regina Treudler Prof. Dr. med. Knut Brockow |
| German Society for Allergology and Clinical Immunology (DGAKI) | Prof. Dr. med. Margitta Worm Prof. Dr. med. Uta Jappe Prof. Barbara Ballmer-Weber Prof. Dr. med. Thomas Werfel Prof. Dr. med. Torsten Zuberbier Prof. Dr. med. Joachim Saloga Prof. Dr. med Jörg Kleine-Tebbe Prof. Dr. med. Eckard Hamelmann |
| German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS) | Prof. Dr. med. Stephan C. Bischoff Prof. Dr. med. Martin Raithel |
| German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNO) | Prof. Dr. med. Ludger Klimek Prof. Dr. med. Martin Wagenmann |
| German Society for Pediatric and Adolescent Medicine (DGKJ) | Prof. Dr. med. Berthold Koletzko |
| Society for Pediatric Allergology and Environmental Medicine (GPA) | Prof. Dr. med. Kirsten Beyer Dr. med. Lars Lange |
| German Society for Pneumology and Respiratory Medicine (DGP) | Dr. med. Ute Lepp Prof. Dr. med. Jens Schreiber |
| German Contact Dermatitis Society (DKG) in the German Dermatological Society (DDG) | Prof. Dr. med. Vera Mahler |
| Austrian Society of Allergology and Immunology (ÖGAI) | Prof. Dr. med. Zsolt Szépfalusi Prof. Dipl. Ing. Dr. Barbara Bohle |
| Professional Association of Oecotrophology e.V. (VDOE) | Dipl. oec. troph. Christiane Schäfer |
| Society for Pediatric Pneumology (GPP) | Prof. Dr. med. Susanne Lau |
| Society of Pediatric Gastroenterology and Nutrition (GPGE) | Dr. med. Martin Claßen |
Formulation of recommendations and structured consensus building
Draft text and recommendations of the guideline chapters were revised by the authors and subsequently submitted to the guideline group by e-mail. In deriving the recommendations, three levels of recommendation, expressing the strength of the recommendations, were distinguished (Table 1).
During two online interdisciplinary consensus conferences on December 14, 2020, and January 11, 2021, the recommendations and key messages were consented using a nominal group process. After presentation of the recommendations for consensus, each group member commented on the draft. Dissenting suggestions were noted. This was followed by the steps of row discussion, pre-voting, debate/discussion, and final voting. Each member of the expert group had one vote. A strong consensus (> 95% agreement) was generally sought. If this could not be achieved even after discussion, adoption was by consensus (> 75% agreement). In the case of a recommendation, only “majority agreement” could be achieved (50 – 74% agreement). The corresponding consensus strengths were documented. For those recommendations or key statements for which consensus could not be reached at the consensus conference due to time constraints, a Delphi process was conducted.
This guideline is intended for all physicians and other health care professionals involved in the acute treatment, diagnosis, and counseling of patients with food allergy.
Adoption by the boards of the participating organizations
On April 22, 2021, the guideline manuscript was sent to the executive boards of all participating professional societies and professional associations as well as the patient organization for their information and request for formal adoption.
As of June 9, 2021, the approval of the different involved organizations took place.
Funding of the guideline
Funded by the DGAKI.
Disclosure and handling of conflicts of interest.
To disclose potential conflicts of interest, all guideline group members completed the Conflict of Interest Declaration form. The declarations were presented and discussed at the consensus conference. No significant conflicts of interest were identified.
A summary of the conflict of in terest declaration is available on the AWMF website at http://www.awmf.org/leitlinien/detail/061-031.html.
Validity period and update procedure
Valid until December 31, 2024, the update should be initiated by the responsible persons of the DGAKI, currently guideline coordinator of this guideline Prof. Margitta Worm, MD.
References
- 1. Muche-Borowski C Selbmann H Nothacker M Müller W Kopp I. Ständige Kommission „Leitlinien“ der Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. 2012.
- 2. Beyer M Geraedts M Gerlach F Gülich M Jäckel W Kopp I Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, Ärztliches Zentrum für Qualität in der Medizin, eds. Deutsches Instrument zur methodischen Leitlinien-Bewertung (DELBI). Arbeitsgemeinschaft der Wissenschaftliche Medizinischen Fachgesellschaften. 2005/2006 + Domäne. 2008.
- 3. Nast A Sporbeck B Jacobs A Erdmann R Roll S Sauerland U Rosumeck S Study of perceptions of the extent to which guideline recommendations are binding: a survey of commonly used terminology. Dtsch Arztebl Int. 2013; 110: 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sicherer SH Sampson HA Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007; 120: 491–503, quiz 504-505.. [DOI] [PubMed] [Google Scholar]
- 5. Grabenhenrich L Trendelenburg V Bellach J Yürek S Reich A Fiandor A Rivero D Sigurdardottir S Clausen M Papadopoulos NG Xepapadaki P Sprikkelman AB Dontje B Roberts G Grimshaw K Kowalski ML Kurowski M Dubakiene R Rudzeviciene O Fernández-Rivas M Frequency of food allergy in school-aged children in eight European countries-The EuroPrevall-iFAAM birth cohort. Allergy. 2020; 75: 2294–2308. [DOI] [PubMed] [Google Scholar]
- 6. Flokstra-de Blok BM Dubois AE Quality of life measures for food allergy. Clin Exp Allergy. 2012; 42: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 7. Nwaru BI Hickstein L Panesar SS Muraro A Werfel T Cardona V Dubois AE Halken S Hoffmann-Sommergruber K Poulsen LK Roberts G Van Ree R Vlieg-Boerstra BJ Sheikh A The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014; 69: 62–75. [DOI] [PubMed] [Google Scholar]
- 8. Zuberbier T Edenharter G Worm M Ehlers I Reimann S Hantke T Roehr CC Bergmann KE Niggemann B Prevalence of adverse reactions to food in Germany – a population study. Allergy. 2004; 59: 338–345. [DOI] [PubMed] [Google Scholar]
- 9. Roehr CC Edenharter G Reimann S Ehlers I Worm M Zuberbier T Niggemann B Food allergy and non-allergic food hypersensitivity in children and adolescents. Clin Exp Allergy. 2004; 34: 1534–1541. [DOI] [PubMed] [Google Scholar]
- 10. Langen U Schmitz R Steppuhn H. Prevalence of allergic diseases in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013; 56: 698–706. [DOI] [PubMed] [Google Scholar]
- 11. Turner PJ Jerschow E Umasunthar T Lin R Campbell DE Boyle RJ Fatal anaphylaxis: Mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017; 5: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pouessel G Turner PJ Worm M Cardona V Deschildre A Beaudouin E Renaudin JM Demoly P Tanno LK Food-induced fatal anaphylaxis: From epidemiological data to general prevention strategies. Clin Exp Allergy. 2018; 48: 1584–1593. [DOI] [PubMed] [Google Scholar]
- 13. Schäfer T Bauer CP Beyer K Bufe A Friedrichs F Gieler U Gronke G Hamelmann E Hellermann M Kleinheinz A Klimek L Koletzko S Kopp M Lau S Müsken H Reese I Schmidt S Schnadt S Sitter H Strömer K Vagts J Vogelberg C Wahn U Werfel T Worm M Muche-Borowski C S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Pediatric and Adolescent Medicine (DGKJ) Allergo J Int. 2014; 23: 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Silva D Halken S Singh C Muraro A Angier E Arasi S Arshad H Beyer K Boyle R du Toit G Eigenmann P Grimshaw K Hoest A Jones C Khaleva E Lack G Szajewska H Venter C Verhasselt V Roberts G Preventing food allergy in infancy and childhood: Systematic review of randomised controlled trials. Pediatr Allergy Immunol. 2020; 31: 813–826. [DOI] [PubMed] [Google Scholar]
- 15. Perkin MR Logan K Tseng A Raji B Ayis S Peacock J Brough H Marrs T Radulovic S Craven J Flohr C Lack G Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016; 374: 1733–1743. [DOI] [PubMed] [Google Scholar]
- 16. Natsume O Kabashima S Nakazato J Yamamoto-Hanada K Narita M Kondo M Saito M Kishino A Takimoto T Inoue E Tang J Kido H Wong GW Matsumoto K Saito H Ohya Y Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389: 276–286. [DOI] [PubMed] [Google Scholar]
- 17. Bellach J Schwarz V Ahrens B Trendelenburg V Aksünger Ö Kalb B Niggemann B Keil T Beyer K Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J Allergy Clin Immunol. 2017; 139: 1591–1599.e2. [DOI] [PubMed] [Google Scholar]
- 18. Palmer DJ Sullivan TR Gold MS Prescott SL Makrides M Randomized controlled trial of early regular egg intake to prevent egg allergy. J Allergy Clin Immunol. 2017; 139: 1600–1607.e2. [DOI] [PubMed] [Google Scholar]
- 19. Palmer DJ Metcalfe J Makrides M Gold MS Quinn P West CE Loh R Prescott SL Early regular egg exposure in infants with eczema: A randomized controlled trial. J Allergy Clin Immunol. 2013; 132: 387–92.e1. [DOI] [PubMed] [Google Scholar]
- 20. Du Toit G Roberts G Sayre PH Bahnson HT Radulovic S Santos AF Brough HA Phippard D Basting M Feeney M Turcanu V Sever ML Gomez Lorenzo M Plaut M Lack G Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015; 372: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pali-Schöll I Herzog R Wallmann J Szalai K Brunner R Lukschal A Karagiannis P Diesner SC Jensen-Jarolim E Antacids and dietary supplements with an influence on the gastric pH increase the risk for food sensitization. Clin Exp Allergy. 2010; 40: 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordakieva G Kundi M Untersmayr E Pali-Schöll I Reichardt B Jensen-Jarolim E Country-wide medical records infer increased allergy risk of gastric acid inhibition. Nat Commun. 2019; 10: 3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burks AW Tang M Sicherer S Muraro A Eigenmann PA Ebisawa M Fiocchi A Chiang W Beyer K Wood R Hourihane J Jones SM Lack G Sampson HA ICON: food allergy. J Allergy Clin Immunol. 2012; 129: 906–920. [DOI] [PubMed] [Google Scholar]
- 24. Sicherer SH Sampson HA Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014; 133: 291–307 quiz 308. [DOI] [PubMed] [Google Scholar]
- 25. Boyce JA Assa’ad A Burks AW Jones SM Sampson HA Wood RA Plaut M Cooper SF Fenton MJ Arshad SH Bahna SL Beck LA Byrd-Bredbenner C Camargo CA Eichenfield L Furuta GT Hanifin JM Jones C Kraft M Levy BD Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010; 126: S1–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin XP Magnusson J Ahlstedt S Dahlman-Höglund A Hanson LÅ Magnusson O Bengtsson U Telemo E Local allergic reaction in food-hypersensitive adults despite a lack of systemic food-specific IgE. J Allergy Clin Immunol. 2002; 109: 879–887. [DOI] [PubMed] [Google Scholar]
- 27. Powe DG Jagger C Kleinjan A Carney AS Jenkins D Jones NS ”Entopy”: localized mucosal allergic disease in the absence of systemic responses for atopy. Clin Exp Allergy. 2003; 33: 1374–1379. [DOI] [PubMed] [Google Scholar]
- 28. Coëffier M Lorentz A Manns MP Bischoff SC Epsilon germ-line and IL-4 transcripts are expressed in human intestinal mucosa and enhanced in patients with food allergy. Allergy. 2005; 60: 822–827. [DOI] [PubMed] [Google Scholar]
- 29. Raithel M Hahn M Donhuijsen K Hagel AF Nägel A Rieker RJ Neurath MF Reinshagen M Eosinophilic gastroenteritis with refractory ulcer disease and gastrointestinal bleeding as a rare manifestation of seronegative gastrointestinal food allergy. Nutr J. 2014; 13: 93–.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoh RA Boyd SD Gut mucosal antibody responses and implications for food allergy. Front Immunol. 2018; 9: 2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sicherer SH Sampson HA Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018; 141: 41–58. [DOI] [PubMed] [Google Scholar]
- 32. Worm M Edenharter G Ruëff F Scherer K Pföhler C Mahler V Treudler R Lang R Nemat K Koehli A Niggemann B Hompes S Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012; 67: 691–698. [DOI] [PubMed] [Google Scholar]
- 33. Wood A Baxter G Thies F Kyle J Duthie G A systematic review of salicylates in foods: estimated daily intake of a Scottish population. Mol Nutr Food Res. 2011; 55: S7–S14. [DOI] [PubMed] [Google Scholar]
- 34. Dölle S Plank-Habibi S Schäfer C Ahrens B Ballmer-Weber B Beyer K Diätische Implikationen bei ASS-Unverträglichkeit. Allergo J. 2020; 29: 93–96. [Google Scholar]
- 35. Diesner SC Pali-Schöll I Jensen-Jarolim E Untersmayr E [Mechanisms and risk factors for type 1 food allergies: the role of gastric digestion]. Wien Med Wochenschr. 2012; 162: 513–518. [DOI] [PubMed] [Google Scholar]
- 36. Dua S Ruiz-Garcia M Bond S Durham SR Kimber I Mills C Roberts G Skypala I Wason J Ewan P Boyle R Clark A Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: A randomized controlled study. J Allergy Clin Immunol. 2019; 144: 1584–1594.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Worm M Babina M Hompes S Causes and risk factors for anaphylaxis. J Dtsch Dermatol Ges. 2013; 11: 44–50. [DOI] [PubMed] [Google Scholar]
- 38. Renz H Biedermann T Bufe A Eberlein B Jappe U Ollert M Petersen A Kleine-Tebbe J Raulf-Heimsoth M Saloga J Werfel T Worm M In-vitro-Allergiediagnostik. Allergo J. 2010; 19: 110–128. [Google Scholar]
- 39. Ansotegui IJ Melioli G Canonica GW Gomez RM Jensen-Jarolim E Ebisawa M A WAO – ARIA – GA2LEN consensus document on molecular-based allergy diagnosis (PAMD@): Update 2020. World Allergy Organ. 2020; 13: 100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleine-Tebbe J Ballmer-Weber B Beyer K Erdmann S Fuchs T Henzgen M In-vitro-Diagnostik und molekulare Grundlagen von IgE-vermittelten Nahrungsmittelallergien. Leitlinie der Deutschen Gesellschaft für Allergologie und klinische Immunologie (DGAKI), des Ärzteverbandes Deutscher Allergologen (ÄDA), der Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA), der Österreichischen Gesellschaft für Allergologie und Immunologie (ÖGAI) und der Schweizerischen Gesellschaft für Allergologie und Immunologie (SGAI). Allergo J. 2009; 18:: 132–146. [Google Scholar]
- 41. Worm M Jappe U Kleine-Tebbe J Schäfer C Reese I Saloga J Treudler R Zuberbier T Waßmann A Fuchs T Dölle S Raithel M Ballmer-Weber B Niggemann B Werfel T Nahrungsmittelallergie infolge immunologischer Kreuzreaktivitäten mit Inhalationsallergenen. Allergo J Int. 2014; 23: 16–31. [Google Scholar]
- 42. Steckelbroeck S Ballmer-Weber BK Vieths S Potential, pitfalls, and prospects of food allergy diagnostics with recombinant allergens or synthetic sequential epitopes. J Allergy Clin Immunol. 2008; 121: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 43. Armbruster DA Pry T Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008; 29 (Suppl 1): S49–52. [PMC free article] [PubMed] [Google Scholar]
- 44. Ballmer-Weber BK Vieths S Soy allergy in perspective. Curr Opin Allergy Clin Immunol. 2008; 8: 270–275. [DOI] [PubMed] [Google Scholar]
- 45. Platts-Mills TA Hilger C Jappe U van Hage M Gadermaier G Spillner E Lidholm J Keshavarz B Aalberse RC Van Ree R Goodman RE Pomés A Carbohydrate epitopes currently recognized as targets for IgE antibodies. Allergy. 2021; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 46. Ballmer-Weber BK Hoffmann-Sommergruber K Molecular diagnosis of fruit and vegetable allergy. Curr Opin Allergy Clin Immunol. 2011; 11: 229–235. [DOI] [PubMed] [Google Scholar]
- 47. Petersen A Rennert S Kull S Becker WM Notbohm H Goldmann T Jappe U Roasting and lipid binding provide allergenic and proteolytic stability to the peanut allergen Ara h 8. Biol Chem. 2014; 395: 239–250. [DOI] [PubMed] [Google Scholar]
- 48. Glaumann S Nopp A Johansson SG Borres MP Lilja G Nilsson C Anaphylaxis to peanuts in a 16-year-old girl with birch pollen allergy and with monosensitization to Ara h 8. J Allergy Clin Immunol Pract. 2013; 1: 698–699. [DOI] [PubMed] [Google Scholar]
- 49. Kleine-Tebbe J Vogel L Crowell DN Haustein UF Vieths S Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1 – related PR-10 protein in soybean, SAM22. J Allergy Clin Immunol. 2002; 110: 797–804. [DOI] [PubMed] [Google Scholar]
- 50. Asero R Antonicelli L Arena A Bommarito L Caruso B Colombo G Crivellaro M De Carli M Della Torre E Della Torre F Heffler E Lodi Rizzini F Longo R Manzotti G Marcotulli M Melchiorre A Minale P Morandi P Moreni B Moschella A Causes of food-induced anaphylaxis in Italian adults: a multi-centre study. Int Arch Allergy Immunol. 2009; 150: 271–277. [DOI] [PubMed] [Google Scholar]
- 51. Sánchez-Monge R Lombardero M García-Sellés FJ Barber D Salcedo G Lipid-transfer proteins are relevant allergens in fruit allergy. J Allergy Clin Immunol. 1999; 103: 514–519. [DOI] [PubMed] [Google Scholar]
- 52. Scheurer S van Ree R Vieths S. The role of lipid transfer proteins as food and pollen allergens outside the Mediterranean area. Curr Allergy Asthma Rep. 2021; 21: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moreno FJ Clemente A 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem J. 2008; 2: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Christensen MJ Eller E Mortz CG Brockow K Bindslev-Jensen C Clinical and serological follow-up of patients with WDEIA. Clin Transl Allergy. 2019; 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kraft M Dölle-Bierke S Renaudin JM Ruëff F Scherer Hofmeier K Treudler R Pföhler C Hawranek T Poziomkowska-Gęsicka I Jappe U Christoff G Müller S Fernandez-Rivas M García BE De Vicente Jiménez TM Cardona V Kleinheinz A Kreft B Bauer A Wagner N Wheat anaphylaxis in adults differs from reactions to other types of food. J Allergy Clin Immunol Pract. 2021. Epub ahead of print. [DOI] [PubMed]
- 56. Alvarado MI Jimeno L De La Torre F Boissy P Rivas B Lázaro MJ Barber D Profilin as a severe food allergen in allergic patients overexposed to grass pollen. Allergy. 2014; 69: 1610–1616. [DOI] [PubMed] [Google Scholar]
- 57. Jappe U Petersen A Raulf-Heimsoth M Allergische Soforttypreaktionen und kreuzreaktive Kohlenhydratepitope (CCD). Allergo J. 2013; 22: 25–32. [Google Scholar]
- 58. Jappe U Schwager C Relevance of lipophilic allergens in food allergy diagnosis. Curr Allergy Asthma Rep. 2017; 17: 61. [DOI] [PubMed] [Google Scholar]
- 59. Breiteneder H Ebner C Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000; 106: 27–36. [DOI] [PubMed] [Google Scholar]
- 60. Longo G Berti I Burks AW Krauss B Barbi E IgE-mediated food allergy in children. Lancet. 2013; 382: 1656–1664. [DOI] [PubMed] [Google Scholar]
- 61. Ostblom E Lilja G Pershagen G van Hage M Wickman M Phenotypes of food hypersensitivity and development of allergic diseases during the first 8 years of life. Clin Exp Allergy. 2008; 38: 1325–1332. [DOI] [PubMed] [Google Scholar]
- 62. Sievers S Rawel HM Ringel KP Niggemann B Beyer K Wheat protein recognition pattern in tolerant and allergic children. Pediatr Allergy Immunol. 2016; 27: 147–155. [DOI] [PubMed] [Google Scholar]
- 63. Scherf KA Brockow K Biedermann T Koehler P Wieser H Wheat-dependent exercise-induced anaphylaxis. Clin Exp Allergy. 2016; 46: 10–20. [DOI] [PubMed] [Google Scholar]
- 64. Matricardi PM Kleine-Tebbe J Hoffmann HJ Valenta R Hilger C Hofmaier S Aalberse RC Agache I Asero R Ballmer-Weber B Barber D Beyer K Biedermann T Bilò MB Blank S Bohle B Bosshard PP Breiteneder H Brough HA Caraballo L EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol. 2016; 27: 1–250. [DOI] [PubMed] [Google Scholar]
- 65. Gadermaier G Hauser M Egger M Ferrara R Briza P Santos KS Zennaro D Girbl T Zuidmeer-Jongejan L Mari A Ferreira F Sensitization prevalence, antibody cross-reactivity and immunogenic peptide profile of Api g 2, the non-specific lipid transfer protein 1 of celery. PLoS One. 2011; 6: e24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hoffman DR Immunochemical identification of the allergens in egg white. J Allergy Clin Immunol. 1983; 71: 481–486. [DOI] [PubMed] [Google Scholar]
- 67. Bartnikas LM Sheehan WJ Tuttle KL Petty CR Schneider LC Phipatanakul W Ovomucoid specific immunoglobulin E as a predictor of tolerance to cooked egg. Allergy Rhinol (Providence). 2015; 6: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reese I Lange L Cow’s milk and hen’s egg allergy: what do molecular-based allergy diagnostics have to offer? Allergo J Int. 2015; 24: 312–319. [Google Scholar]
- 69. Jappe U [Update on meat allergy. α-Gal: a new epitope, a new entity?]. Hautarzt. 2012; 63: 299–306. [DOI] [PubMed] [Google Scholar]
- 70. Wilson JM Platts-Mills TAE Meat allergy and allergens. Mol Immunol. 2018; 100: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sharp MF Lopata AL Fish allergy: in review. Clin Rev Allergy Immunol. 2014; 46: 258–271. [DOI] [PubMed] [Google Scholar]
- 72. Kuehn A Codreanu-Morel F Lehners-Weber C Doyen V Gomez-André SA Bienvenu F Fischer J Ballardini N van Hage M Perotin JM Silcret-Grieu S Chabane H Hentges F Ollert M Hilger C Morisset M Cross-reactivity to fish and chicken meat – a new clinical syndrome. Allergy. 2016; 71: 1772–1781. [DOI] [PubMed] [Google Scholar]
- 73. Pascal M Grishina G Yang AC Sánchez-García S Lin J Towle D Ibañez MD Sastre J Sampson HA Ayuso R Molecular diagnosis of shrimp allergy: Efficiency of several allergens to predict clinical reactivity. J Allergy Clin Immunol Pract. 2015; 3: 521–9.e10. [DOI] [PubMed] [Google Scholar]
- 74. Teo SL Gerez IF Ang EY Shek LP Food-dependent exercise-induced anaphylaxis – a review of 5 cases. Ann Acad Med Singapore. 2009; 38: 905–909. [PubMed] [Google Scholar]
- 75. Pali-Schöll I Blank S Verhoeckx K Mueller RS Janda J Marti E Seida AA Rhyner C DeBoer DJ Jensen-Jarolim E EAACI position paper: Comparing insect hypersensitivity induced by bite, sting, inhalation or ingestion in human beings and animals. Allergy. 2019; 74: 874–887. [DOI] [PubMed] [Google Scholar]
- 76. Schwager C Kull S Behrends J Röckendorf N Schocker F Frey A Homann A Becker WM Jappe U Peanut oleosins associated with severe peanut allergy-importance of lipophilic allergens for comprehensive allergy diagnostics. J Allergy Clin Immunol. 2017; 140: 1331–1338.e8. [DOI] [PubMed] [Google Scholar]
- 77. Mehlich J Fischer J Hilger C Swiontek K Morisset M Codreanu-Morel F Schiener M Blank S Ollert M Darsow U Biedermann T Eberlein B The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization. J Allergy Clin Immunol. 2019; 143: 182–189. [DOI] [PubMed] [Google Scholar]
- 78. Duan L Celik A Hoang JA Schmidthaler K So D Yin X Ditlof CM Ponce M Upton JEM Lee JS Hung L Breiteneder H Palladino C Atkinson AR Kim VHD Berenjy A Asper M Hummel D Wong S Alexanian-Farr M Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study. Allergy. 2021; 76: 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ruëff F Bergmann K-C Brockow K Fuchs T Grübl A Jung K Klimek L Müsken H Pfaar O Przybilla B Sitter H Wehrmann W Hauttests zur Diagnostik von allergischen Soforttypreaktionen. Allergo J. 2010; 19: 402–415. [DOI] [PubMed] [Google Scholar]
- 80. Du Toit G Santos A Roberts G Fox AT Smith P Lack G The diagnosis of IgE-mediated food allergy in childhood. Pediatr Allergy Immunol. 2009; 20: 309–319. [DOI] [PubMed] [Google Scholar]
- 81. Mahler V Testallergene: Aktueller Stand der Verfügbarkeit aus regulatorischer Sicht. Allergologie. 2019; 42: 309–313. [Google Scholar]
- 82. Klimek L Zuberbier T Problematik von Diagnostikallergenen in Deutschland. In: Klimek L, Werfel Th, Vogelberg C (eds). Weißbuch Allergie. Berlin: Springer. 2018. p. 253-261. [Google Scholar]
- 83. Chang A Robison R Cai M Singh AM Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016; 4: 229–36.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Flinterman AE Knulst AC Meijer Y Bruijnzeel-Koomen CA Pasmans SG Acute allergic reactions in children with AEDS after prolonged cow’s milk elimination diets. Allergy. 2006; 61: 370–374. [DOI] [PubMed] [Google Scholar]
- 85. Larramendi CH Martín Esteban M Pascual Marcos C Fiandor A Díaz Pena JM Possible consequences of elimination diets in asymptomatic immediate hypersensitivity to fish. Allergy. 1992; 47: 490–494. [DOI] [PubMed] [Google Scholar]
- 86. Barbi E Gerarduzzi T Longo G Ventura A Fatal allergy as a possible consequence of long-term elimination diet. Allergy. 2004; 59: 668–669. [DOI] [PubMed] [Google Scholar]
- 87. Nachshon L Goldberg MR Elizur A Appel MY Levy MB Katz Y Food allergy to previously tolerated foods: Course and patient characteristics. Ann Allergy Asthma Immunol. 2018; 121: 77–81.e1. [DOI] [PubMed] [Google Scholar]
- 88. Du Toit G Roberts G Sayre PH Plaut M Bahnson HT Mitchell H Radulovic S Chan S Fox A Turcanu V Lack G Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013; 131: 135–43.e1-12. [DOI] [PubMed] [Google Scholar]
- 89. Kansen HM Le TM Meijer Y Flokstra-de Blok BMJ Welsing PMJ van der Ent CK Knulst AC van Erp FC The impact of oral food challenges for food allergy on quality of life: A systematic review. Pediatr Allergy Immunol. 2018; 29: 527–537. [DOI] [PubMed] [Google Scholar]
- 90. Dölle S Grünhagen J Worm M Dem Täter auf der Spur: Indikation und praktische Umsetzung von Nahrungsmittelprovokationen im Erwachsenenalter. Allergologie. 2016; 39: 523–532. [Google Scholar]
- 91. Purington N Chinthrajah RS Long A Sindher S Andorf S O’Laughlin K Woch MA Scheiber A Assa’ad A Pongracic J Spergel JM Tam J Tilles S Wang J Galli SJ Desai M Nadeau KC Eliciting dose and safety outcomes from a large dataset of standardized multiple food challenges. Front Immunol. 2018; 9: 2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ahrens B Niggemann B Wahn U Beyer K Organ-specific symptoms during oral food challenge in children with food allergy. J Allergy Clin Immunol. 2012; 130: 549–551. [DOI] [PubMed] [Google Scholar]
- 93. Reimann HJ Ring J Ultsch B Wendt P Intragastral provocation under endoscopic control (IPEC) in food allergy: mast cell and histamine changes in gastric mucosa. Clin Allergy. 1985; 15: 195–202. [DOI] [PubMed] [Google Scholar]
- 94. Bengtsson U Knutson TW Knutson L Dannaeus A Hällgren R Ahlstedt S Eosinophil cationic protein and histamine after intestinal challenge in patients with cow’s milk intolerance. J Allergy Clin Immunol. 1997; 100: 216–221. [DOI] [PubMed] [Google Scholar]
- 95. Santos J Bayarri C Saperas E Nogueiras C Antolín M Mourelle M Cadahia A Malagelada JR Characterisation of immune mediator release during the immediate response to segmental mucosal challenge in the jejunum of patients with food allergy. Gut. 1999; 45: 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schwab D Raithel M Klein P Winterkamp S Weidenhiller M Radespiel-Troeger M Hochberger J Hahn EG Immunoglobulin E and eosinophilic cationic protein in segmental lavage fluid of the small and large bowel identify patients with food allergy. Am J Gastroenterol. 2001; 96: 508–514. [DOI] [PubMed] [Google Scholar]
- 97. Arslan G Ødegaard S Elsayed S Florvaag E Berstad A Food allergy and intolerance: response to intestinal provocation monitored by endosonography. Eur J Ultrasound. 2002; 15: 29–36. [DOI] [PubMed] [Google Scholar]
- 98. Pickert CN Lorentz A Manns MP Bischoff SC Colonoscopic allergen provocation test with rBet v 1 in patients with pollen-associated food allergy. Allergy. 2012; 67: 1308–1315. [DOI] [PubMed] [Google Scholar]
- 99. Warners MJ Terreehorst I van den Wijngaard RM Akkerdaas J van Esch BCAM van Ree R Versteeg SA Smout AJPM Bredenoord AJ Abnormal responses to local esophageal food allergen injections in adult patients with eosinophilic esophagitis. Gastroenterology. 2018; 154: 57–60.e2. [DOI] [PubMed] [Google Scholar]
- 100. Fritscher-Ravens A Pflaum T Mösinger M Ruchay Z Röcken C Milla PJ Das M Böttner M Wedel T Schuppan D Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology. 2019; 157: 109–118.e5. [DOI] [PubMed] [Google Scholar]
- 101. Benson TE Arkins JA Cytotoxic testing for food allergy: evaluation of reproducibility and correlation. J Allergy Clin Immunol. 1976; 58: 471–476. [DOI] [PubMed] [Google Scholar]
- 102. Committee of Public Health. Statement on cytotoxic testing for food allergy (Bryan’s test). Bull N Y Acad Med. 1988; 64: 117–119. [PMC free article] [PubMed] [Google Scholar]
- 103. Ernst E Iridology: A systematic review. Forsch Komplementarmed. 1999; 6: 7–9. [DOI] [PubMed] [Google Scholar]
- 104. Garrow JS Kinesiology and food allergy. Br Med J (Clin Res Ed). 1988; 296: 1573–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Niggemann B Grüber C Unproven diagnostic procedures in IgE-mediated allergic diseases. Allergy. 2004; 59: 806–808. [DOI] [PubMed] [Google Scholar]
- 106. Zeng Q Dong SY Wu LX Li H Sun ZJ Li JB Jiang HX Chen ZH Wang QB Chen WW Variable food-specific IgG antibody levels in healthy and symptomatic Chinese adults. PLoS One. 2013; 8: e53612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sethi TJ Lessof MH Kemeny DM Lambourn E Tobin S Bradley A How reliable are commercial allergy tests? Lancet. 1987; 1: 92–94. [DOI] [PubMed] [Google Scholar]
- 108. Stapel SO Asero R Ballmer-Weber BK Knol EF Strobel S Vieths S Kleine-Tebbe J Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy. 2008; 63: 793–796. [DOI] [PubMed] [Google Scholar]
- 109. Wüthrich B Unproven techniques in allergy diagnosis. J Investig Allergol Clin Immunol. 2005; 15: 86–90. [PubMed] [Google Scholar]
- 110. Hammond C Lieberman JA Unproven diagnostic tests for food allergy. Immunol Allergy Clin North Am. 2018; 38: 153–163. [DOI] [PubMed] [Google Scholar]
- 111. Ansotegui IJ Melioli G Canonica GW Caraballo L Villa E Ebisawa M Passalacqua G Savi E Ebo D Gómez RM Luengo Sánchez O Oppenheimer JJ Jensen-Jarolim E Fischer DA Haahtela T Antila M Bousquet JJ Cardona V Chiang WC Demoly PM IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020; 13: 100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wood RA Sicherer SH Vickery BP Jones SM Liu AH Fleischer DM Henning AK Mayer L Burks AW Grishin A Stablein D Sampson HA The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013; 131: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schoemaker AA Sprikkelman AB Grimshaw KE Roberts G Grabenhenrich L Rosenfeld L Siegert S Dubakiene R Rudzeviciene O Reche M Fiandor A Papadopoulos NG Malamitsi-Puchner A Fiocchi A Dahdah L Sigurdardottir ST Clausen M Stańczyk-Przyłuska A Zeman K Mills EN Incidence and natural history of challenge-proven cow’s milk allergy in European children – EuroPrevall birth cohort. Allergy. 2015; 70: 963–972. [DOI] [PubMed] [Google Scholar]
- 114. Sampson HA Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999; 103: 717–728. [DOI] [PubMed] [Google Scholar]
- 115. Hattevig G Kjellman B Björkstén B Clinical symptoms and IgE responses to common food proteins and inhalants in the first 7 years of life. Clin Allergy. 1987; 17: 571–578. [DOI] [PubMed] [Google Scholar]
- 116. Boyano-Martínez T García-Ara C Díaz-Pena JM Martín-Esteban M Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol. 2002; 110: 304–309. [DOI] [PubMed] [Google Scholar]
- 117. Xepapadaki P Fiocchi A Grabenhenrich L Roberts G Grimshaw KE Fiandor A Larco JI Sigurdardottir S Clausen M Papadopoulos NG Dahdah L Mackie A Sprikkelman AB Schoemaker AA Dubakiene R Butiene I Kowalski ML Zeman K Gavrili S Keil T Incidence and natural history of hen’s egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy. 2016; 71: 350–357. [DOI] [PubMed] [Google Scholar]
- 118. Keet CA Matsui EC Dhillon G Lenehan P Paterakis M Wood RA The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009; 102: 410–415. [DOI] [PubMed] [Google Scholar]
- 119. Savage JH Kaeding AJ Matsui EC Wood RA The natural history of soy allergy. J Allergy Clin Immunol. 2010; 125: 683–686. [DOI] [PubMed] [Google Scholar]
- 120. Green TD LaBelle VS Steele PH Kim EH Lee LA Mankad VS Williams LW Anstrom KJ Burks AW Clinical characteristics of peanut-allergic children: recent changes. Pediatrics. 2007; 120: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 121. Savage JH Limb SL Brereton NH Wood RA The natural history of peanut allergy: Extending our knowledge beyond childhood. J Allergy Clin Immunol. 2007; 120: 717–719. [DOI] [PubMed] [Google Scholar]
- 122. Neuman-Sunshine DL Eckman JA Keet CA Matsui EC Peng RD Lenehan PJ Wood RA The natural history of persistent peanut allergy. Ann Allergy Asthma Immunol. 2012; 108: 326–331.e3. [DOI] [PubMed] [Google Scholar]
- 123. Spergel JM Beausoleil JL Pawlowski NA Resolution of childhood peanut allergy. Ann Allergy Asthma Immunol. 2000; 85: 473–476. [DOI] [PubMed] [Google Scholar]
- 124. Skolnick HS Conover-Walker MK Koerner CB Sampson HA Burks W Wood RA The natural history of peanut allergy. J Allergy Clin Immunol. 2001; 107: 367–374. [DOI] [PubMed] [Google Scholar]
- 125. Peters RL Allen KJ Dharmage SC Koplin JJ Dang T Tilbrook KP Lowe A Tang ML Gurrin LC Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. J Allergy Clin Immunol. 2015; 135: 1257–66. e1-2. [DOI] [PubMed] [Google Scholar]
- 126. Fleischer DM Conover-Walker MK Matsui EC Wood RA The natural history of tree nut allergy. J Allergy Clin Immunol. 2005; 116: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 127. Lopata AL Lehrer SB New insights into seafood allergy. Curr Opin Allergy Clin Immunol. 2009; 9: 270–277. [DOI] [PubMed] [Google Scholar]
- 128. Röckmann H van Geel MJ Knulst AC Huiskes J Bruijnzeel-Koomen CA de Bruin-Weller MS Food allergen sensitization pattern in adults in relation to severity of atopic dermatitis. Clin Transl Allergy. 2014; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rona RJ Keil T Summers C Gislason D Zuidmeer L Sodergren E Sigurdardottir ST Lindner T Goldhahn K Dahlstrom J McBride D Madsen C The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007; 120: 638–646. [DOI] [PubMed] [Google Scholar]
- 130. Ring J Beyer K Biedermann T Bircher A Fischer M Heller A Huttegger I Jakob T Klimek L Kopp MV Kugler C Lange L Pfaar O Rietschel E Rueff F Schnadt S Seifert R Stöcker B Treudler R Vogelberg C [Guideline (S2k) on acute therapy and management of anaphylaxis: 2021 update: S2k-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Medical Association of German Allergologists (AeDA), the Society of Pediatric Allergology and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Society for Neonatology and Pediatric Intensive Care (GNPI), the German Society of Dermatology (DDG), the Austrian Society for Allergology and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Respiratory Society (DGP), the patient organization German Allergy and Asthma Association (DAAB), the German Working Group of Anaphylaxis Training and Education (AGATE)]. Allergo J. 2021; 30: 20–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Worm M Eckermann O Dölle S Aberer W Beyer K Hawranek T Hompes S Koehli A Mahler V Nemat K Niggemann B Pföhler C Rabe U Reissig A Rietschel E Scherer K Treudler R Ruëff F Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany, Austria and Switzerland. Dtsch Arztebl Int. 2014; 111: 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chong KW Ruiz-Garcia M Patel N Boyle RJ Turner PJ Reaction phenotypes in IgE-mediated food allergy and anaphylaxis. Ann Allergy Asthma Immunol. 2020; 124: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ellis AK Day JH Diagnosis and management of anaphylaxis. CMAJ. 2003; 169: 307–311. [PMC free article] [PubMed] [Google Scholar]
- 134. Greenberger PA Patterson R The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol. 1991; 87: 867–872. [DOI] [PubMed] [Google Scholar]
- 135. Reimers A Müller U. Behandlung des anaphylaktischen Schocks. Therapeutische Umschau. 2001; 58: 325–328. [DOI] [PubMed] [Google Scholar]
- 136. Kaiser H Kley H Cortisontherapie: Corticoide in Klinik und Praxis. Neubearb Aufl. Stuttgart – New York: Thieme. 2002. [Google Scholar]
- 137. Stark BJ Sullivan TJ Biphasic and protracted anaphylaxis. J Allergy Clin Immunol. 1986; 78: 76–83. [DOI] [PubMed] [Google Scholar]
- 138. de Silva D Geromi M Panesar SS Muraro A Werfel T Hoffmann-Sommergruber K Roberts G Cardona V Dubois AE Halken S Host A Poulsen LK Van Ree R Vlieg-Boerstra BJ Agache I Sheikh A Acute and long-term management of food allergy: systematic review. Allergy. 2014; 69: 159–167. [DOI] [PubMed] [Google Scholar]
- 139. Kocoshis S Gryboski JD Use of cromolyn in combined gastrointestinal allergy. JAMA. 1979; 242: 1169–1173. [PubMed] [Google Scholar]
- 140. Ortolani C Pastorello E Zanussi C Prophylaxis of adverse reactions to foods. A double-blind study of oral sodium cromoglycate for the prophylaxis of adverse reactions to foods and additives. Ann Allergy. 1983; 50: 105–109. [PubMed] [Google Scholar]
- 141. Van Elburg RM Heymans HS De Monchy JG Effect of disodiumcromoglycate on intestinal permeability changes and clinical response during cow’s milk challenge. Pediatr Allergy Immunol. 1993; 4: 79–85. [DOI] [PubMed] [Google Scholar]
- 142. Lunardi C Bambara LM Biasi D Cortina P Peroli P Nicolis F Favari F Pacor ML Double-blind cross-over trial of oral sodium cromoglycate in patients with irritable bowel syndrome due to food intolerance. Clin Exp Allergy. 1991; 21: 569–572. [DOI] [PubMed] [Google Scholar]
- 143. Stefanini GF Saggioro A Alvisi V Angelini G Capurso L di Lorenzo G Dobrilla G Dodero M Galimberti M Gasbarrini G Manghisi O Marsigli L Mazzacca G Rigo L Sacerdoti G Scolozzi R Surrenti C Grazioli I Melzi G Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand J Gastroenterol. 1995; 30: 535–541. [DOI] [PubMed] [Google Scholar]
- 144. Klooker TK Braak B Koopman KE Welting O Wouters MM van der Heide S Schemann M Bischoff SC van den Wijngaard RM Boeckxstaens GE The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010; 59: 1213–1221. [DOI] [PubMed] [Google Scholar]
- 145. Burks AW Sampson HA Double-blind placebo-controlled trial of oral cromolyn in children with atopic dermatitis and documented food hypersensitivity. J Allergy Clin Immunol. 1988; 81: 417–423. [DOI] [PubMed] [Google Scholar]
- 146. Raithel M Weidenhiller M Wilken V Hochberger J Mühldorfer S Hahn E 2004; 63–70.
- 147. Kuruvilla ME Mathew S Avadhani V Treatment of refractory mastocytic enterocolitis with budesonide. J Gastrointestin Liver Dis. 2018; 27: 327–329. [DOI] [PubMed] [Google Scholar]
- 148. Lepp U Ballmer-Weber B Beyer K Erdmann S Fuchs T Henzgen M Therapiemöglichkeiten bei der IgE-vermittelten Nahrungsmittelallergie. Allergo J. 2010; 19: 187–195. [Google Scholar]
- 149. Miceli Sopo S Greco M Cuomo B Bianchi A Liotti L Monaco S Dello Iacono I Matrix effect on baked egg tolerance in children with IgE-mediated hen’s egg allergy. Pediatr Allergy Immunol. 2016; 27: 465–470. [DOI] [PubMed] [Google Scholar]
- 150. Miceli Sopo S Greco M Monaco S Bianchi A Cuomo B Liotti L Iacono ID Matrix effect on baked milk tolerance in children with IgE cow milk allergy. Allergol Immunopathol (Madr). 2016; 44: 517–523. [DOI] [PubMed] [Google Scholar]
- 151. Leonard SA Caubet JC Kim JS Groetch M Nowak-Węgrzyn A Baked milk- and egg-containing diet in the management of milk and egg allergy. J Allergy Clin Immunol Pract. 2015; 3: 13–23, quiz 24.. [DOI] [PubMed] [Google Scholar]
- 152. Bird JA Clark A Dougherty I Brown LS Arneson A Crain M Parrish C Baked egg oral immunotherapy desensitizes baked egg allergic children to lightly cooked egg. J Allergy Clin Immunol Pract. 2019; 7: 667–669.e4. [DOI] [PubMed] [Google Scholar]
- 153. Esmaeilzadeh H Alyasin S Haghighat M Nabavizadeh H Esmaeilzadeh E Mosavat F The effect of baked milk on accelerating unheated cow’s milk tolerance: A control randomized clinical trial. Pediatr Allergy Immunol. 2018; 29: 747–753. [DOI] [PubMed] [Google Scholar]
- 154. Goldberg MR Nachshon L Appel MY Elizur A Levy MB Eisenberg E Sampson HA Katz Y Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J Allergy Clin Immunol. 2015; 136: 1601–1606. [DOI] [PubMed] [Google Scholar]
- 155. Kim JS Nowak-Węgrzyn A Sicherer SH Noone S Moshier EL Sampson HA Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J Allergy Clin Immunol. 2011; 128: 125–131.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Leonard SA Sampson HA Sicherer SH Noone S Moshier EL Godbold J Nowak-Węgrzyn A Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012; 130: 473–80.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Upton J Nowak-Wegrzyn A The impact of baked egg and baked milk diets on IgE- and Non-IgE-Mediated Allergy. Clin Rev Allergy Immunol. 2018; 55: 118–138. [DOI] [PubMed] [Google Scholar]
- 158. Reese I Schäfer C Einsatz von therapeutischen Spezialnahrungen im Säuglingsalter – Bedarfsdeckung unter veränderten Voraussetzungen. Allergologie. 2013; 36: 502–509. [Google Scholar]
- 159. Hill DJ Murch SH Rafferty K Wallis P Green CJ The efficacy of amino acid-based formulas in relieving the symptoms of cow’s milk allergy: a systematic review. Clin Exp Allergy. 2007; 37: 808–822. [DOI] [PubMed] [Google Scholar]
- 160. Niggemann B von Berg A Bollrath C Berdel D Schauer U Rieger C Haschke-Becher E Wahn U Safety and efficacy of a new extensively hydrolyzed formula for infants with cow’s milk protein allergy. Pediatr Allergy Immunol. 2008; 19: 348–354. [DOI] [PubMed] [Google Scholar]
- 161. Koletzko S Niggemann B Friedrichs F Koletzko B Vorgehen bei Säuglingen mit Verdacht auf Kuhmilchproteinallergie. Monatsschr Kinderheilkd. 2009; 157: 687–691. [Google Scholar]
- 162. Koletzko B Stellungnahme zur Verwendung von Säuglingsnahrungen auf Sojaeiweißbasis. Monatsschr Kinderheilkd. 2006; 154: 913–916. [Google Scholar]
- 163. Ellis MH Short JA Heiner DC Anaphylaxis after ingestion of a recently introduced hydrolyzed whey protein formula. J Pediatr. 1991; 118: 74–77. [DOI] [PubMed] [Google Scholar]
- 164. Høst A Halken S Hypoallergenic formulas – when, to whom and how long: after more than 15 years we know the right indication! Allergy. 2004; 59: 45–52. [DOI] [PubMed] [Google Scholar]
- 165. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften V. (AWMF); Deutsche Gesellschaft für Osteologie e. V., eds. Prophylaxe, Diagnostik und Therapie der Osteoporose bei Erwachsenen. https://www.awmf.org/uploads/tx_szleitlinien/183-001l_S3_Osteoporose-Prophylaxe-Diagnostik-Therapie_2019-02.pdf. Date of access: June 24, 2020
- 166. Europäische Union, ed. Verordnung (EU) Nr. 1169/2011 des Europäischen Parlaments und des Rates vom 25. Oktober 2011 betreffend die Information der Verbraucher über Lebensmittel und zur Änderung der Verordnungen (EG) Nr. 1924/2006 und (EG) Nr. 1925/2006 des Europäischen Parlaments und des Rates und zur Aufhebung der Richtlinie 87/250/EWG der Kommission, der Richtlinie 90/496/EWG des Rates, der Richtlinie 1999/10/EG der Kommission, der Richtlinie 2000/13/EG des Europäischen Parlaments und des Rates, der Richtlinien 2002/67/EG und 2008/5/EG der Kommission und der Verordnung (EG) Nr. 608/2004 der Kommission. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:de:PDF. Date of access: July 23, 2014.
- 167. Miller JB A double-blind study of food extract injection therapy: a preliminary report. Ann Allergy. 1977; 38: 185–191. [PubMed] [Google Scholar]
- 168. Oppenheimer JJ Nelson HS Bock SA Christensen F Leung DY Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992; 90: 256–262. [DOI] [PubMed] [Google Scholar]
- 169. Zuidmeer-Jongejan L Huber H Swoboda I Rigby N Versteeg SA Jensen BM Quaak S Akkerdaas JH Blom L Asturias J Bindslev-Jensen C Bernardi ML Clausen M Ferrara R Hauer M Heyse J Kopp S Kowalski ML Lewandowska-Polak A Linhart B Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int Arch Allergy Immunol. 2015; 166: 41–51. [DOI] [PubMed] [Google Scholar]
- 170. Asero R Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998; 28: 1368–1373. [DOI] [PubMed] [Google Scholar]
- 171. Bolhaar ST Tiemessen MM Zuidmeer L van Leeuwen A Hoffmann-Sommergruber K Bruijnzeel-Koomen CA Taams LS Knol EF van Hoffen E van Ree R Knulst AC Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004; 34: 761–769. [DOI] [PubMed] [Google Scholar]
- 172. Bucher X Pichler WJ Dahinden CA Helbling A Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004; 59: 1272–1276. [DOI] [PubMed] [Google Scholar]
- 173. Mauro M Russello M Incorvaia C Gazzola G Frati F Moingeon P Passalacqua G Birch-apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011; 156: 416–422. [DOI] [PubMed] [Google Scholar]
- 174. Treudler R Franke A Schmiedeknecht A Ballmer-Weber B Worm M Werfel T Jappe U Biedermann T Schmitt J Brehler R Kleinheinz A Kleine-Tebbe J Brüning H Ruëff F Ring J Saloga J Schäkel K Holzhauser T Vieths S Simon JC BASALIT trial: double-blind placebo-controlled allergen immunotherapy with rBet v 1-FV in birch-related soya allergy. Allergy. 2017; 72: 1243–1253. [DOI] [PubMed] [Google Scholar]
- 175. van Hoffen E Peeters KA van Neerven RJ van der Tas CW Zuidmeer L van Ieperen-van Dijk AG Effect of birch pollen-specific immunotherapy on birch pollen-related hazelnut allergy. J Allergy Clin Immunol. 2011; 127: 100–101. [DOI] [PubMed] [Google Scholar]
- 176. Bindslev-Jensen C de Kam P-J van Twuojver E Boot Boot D El Galta R Pahlow Mose A Tannert LK Opstelten D-J SCIT-treatment with a chemically modified, aluminum hydroxide adsorbed peanut extract (HAL-MPE1) was generally safe and well tolerated and showed immunological changes in peanut allergic patients. J Allergy Clin Immunol. 2017; 139: AB191. [Google Scholar]
- 177. Fleischer DM Burks AW Vickery BP Scurlock AM Wood RA Jones SM Sicherer SH Liu AH Stablein D Henning AK Mayer L Lindblad R Plaut M Sampson HA Sublingual immunotherapy for peanut allergy: A randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013; 131: 119–127.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Burks AW Wood RA Jones SM Sicherer SH Fleischer DM Scurlock AM Vickery AK Liu AK Henning AK Lindblad R Dawson P Plaut M Sampson HA Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol. 2015; 135: 1240–8.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Narisety SD Frischmeyer-Guerrerio PA Keet CA Gorelik M Schroeder J Hamilton RG Wood RA A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015; 135: 1275–82.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kim EH Bird JA Kulis M Laubach S Pons L Shreffler W Steele P Kamilaris J Vickery B Burks AW Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011; 127: 640–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Enrique E Pineda F Malek T Bartra J Basagaña M Tella R Castelló JV Alonso R de Mateo JA Cerdá-Trias T San Miguel-Moncín MM Monzón S García M Palacios R Cisteró-Bahíma A Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005; 116: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 182. Fernández-Rivas M Garrido Fernández S Nadal JA Díaz de Durana MD García BE González-Mancebo E Martín S Barber D Rico P Tabar AI Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009; 64: 876–883. [DOI] [PubMed] [Google Scholar]
- 183. Kinaciyan T Nagl B Faustmann S Frommlet F Kopp S Wolkersdorfer M Wöhrl S Bastl K Huber H Berger U Bohle B Efficacy and safety of 4 months of sublingual immunotherapy with recombinant Mal d 1 and Bet v 1 in patients with birch pollen-related apple allergy. J Allergy Clin Immunol. 2018; 141: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 184. Sánchez Acosta G Kinaciyan T Kitzmüller C Möbs C Pfützner W Bohle B IgE-blocking antibodies following SLIT with recombinant Mal d 1 accord with improved apple allergy. J Allergy Clin Immunol. 2020; 146: 894–900.e2. [DOI] [PubMed] [Google Scholar]
- 185. Kitzmüller C Jahn-Schmid B Kinaciyan T Bohle B Sublingual immunotherapy with recombinant Mal d 1 downregulates the allergen-specific Th2 response. Allergy. 2019; 74: 1579–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hansen KS Khinchi MS Skov PS Bindslev-Jensen C Poulsen LK Malling HJ Food allergy to apple and specific immunotherapy with birch pollen. Mol Nutr Food Res. 2004; 48: 441–448. [DOI] [PubMed] [Google Scholar]
- 187. Kinaciyan T Jahn-Schmid B Radakovics A Zwölfer B Schreiber C Francis JN Ebner C Bohle B Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007; 119: 937–943. [DOI] [PubMed] [Google Scholar]
- 188. Till SJ Stage BS Skypala I Biedermann T Potential treatment effect of the SQ tree SLIT-tablet on pollen food syndrome caused by apple. Allergy. 2020; 75: 2059–2061. [DOI] [PubMed] [Google Scholar]
- 189. Dupont C Bourrier T de Blay F Guénard-Bilbault L Sauvage C Cousin M Peanut epicutaneous immunotherapy (EPIT) in peanut-allergic children: 18 Months treatment in the Arachild study. Allergy Clin Immunol. 2014; 133: AB102. [Google Scholar]
- 190. Sampson HA Shreffler WG Yang WH Sussman GL Brown-Whitehorn TF Nadeau KC Cheema AS Leonard SA Pongracic JA Sauvage-Delebarre C Assa’ad AH de Blay F Bird JA Tilles SA Boralevi F Bourrier T Hébert J Green TD Gerth van Wijk R Knulst AC Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: A randomized clinical trial. JAMA. 2017; 318: 1798–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sampson HA Agbotounou W Thebault C Ruban C Martin L Sussman G Brown-Whitehorn TF Yang WH Nadeau KC Cheema AS Leonard SA Sauvage C Assa’ad AH de Blay F Bird JA Tilles SA Boralevi F Bourrier T Benhamou P-H Dupont C Enhanced efficacy and confirmed safety of a two-year epicutaneous immunotherapy (EPIT) treatment of peanut allergy with Viaskin peanut: The continuation of the vipes phase IIb randomized controlled trial (RCT). J Allergy Clin Immunol. 2016; 137: AB408. [Google Scholar]
- 192. Sampson HA Agbotounou W Thébault C Charles R Martin L Yang WH Sussman GL Brown-Whitehorn TF Nadeau KC Cheema AS Leonard SA Pongracic JA Sauvage C Assa’ad AH de Blay F Bird JA Tilles SA Boralevi F Bourrier T Shreffler WG Epicutaneous immunotherapy (EPIT) is effective and safe to treat peanut allergy: a multi-national double-blind placebo-controlled randomized phase IIb trial. J Allergy Clin Immunol. 2015; 135 (2S): AB390. [Google Scholar]
- 193. Fleischer DM Greenhawt M Sussman G Bégin P Nowak-Wegrzyn A Petroni D Beyer K Brown-Whitehorn T Hebert J Hourihane JO Campbell DE Leonard S Chinthrajah RS Pongracic JA Jones SM Lange L Chong H Green TD Wood R Cheema A Effect of Epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: The PEPITES randomized clinical trial. JAMA. 2019; 321: 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Fleischer DM Shreffler WG Campbell DE Green TD Anvari S Assa’ad A Bégin P Beyer K Bird JA Brown-Whitehorn T Byrne A Chan ES Cheema A Chinthrajah S Chong HJ Davis CM Ford LS Gagnon R Greenhawt M Hourihane JO Long-term, open-label extension study of the efficacy and safety of epicutaneous immunotherapy for peanut allergy in children: PEOPLE 3-year results. J Allergy Clin Immunol. 2020; 146: 863–874. [DOI] [PubMed] [Google Scholar]
- 195. Anagnostou K Islam S King Y Foley L Pasea L Bond S Palmer C Deighton J Ewan P Clark A Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014; 383: 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Burks AW Jones SM Wood RA Fleischer DM Sicherer SH Lindblad RW Stablein D Henning AK Vickery BP Liu AH Scurlock AM Shreffler WG Plaut M Sampson HA Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012; 367: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Caminiti L Passalacqua G Barberi S Vita D Barberio G De Luca R Pajno GB A new protocol for specific oral tolerance induction in children with IgE-mediated cow’s milk allergy. Allergy Asthma Proc. 2009; 30: 443–448. [DOI] [PubMed] [Google Scholar]
- 198. Dello Iacono I Tripodi S Calvani M Panetta V Verga MC Miceli Sopo S Specific oral tolerance induction with raw hen’s egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol. 2013; 24: 66–74. [DOI] [PubMed] [Google Scholar]
- 199. Keet CA Frischmeyer-Guerrerio PA Thyagarajan A Schroeder JT Hamilton RG Boden S Steele P Driggers S Burks AW Wood RA The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012; 129: 448–455 e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Longo G Barbi E Berti I Meneghetti R Pittalis A Ronfani L Ventura A Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol. 2008; 121: 343–347. [DOI] [PubMed] [Google Scholar]
- 201. Mansouri M Movahhedi M Pourpak Z Akramian R Shokohi Shormasti R Mozaffari H Oral desensitization in children with IgE-mediated cow’s milk allergy: a prospective clinical trial. Tehran Univ Med J. 2007; 65: 11–18. [Google Scholar]
- 202. Martorell A De la Hoz B Ibáñez MD Bone J Terrados MS Michavila A Plaza AM Alonso E Garde J Nevot S Echeverria L Santana C Cerdá JC Escudero C Guallar I Piquer M Zapatero L Ferré L Bracamonte T Muriel A Oral desensitization as a useful treatment in 2-year-old children with cow’s milk allergy. Clin Exp Allergy. 2011; 41: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 203. Meglio P Giampietro PG Carello R Gabriele I Avitabile S Galli E Oral food desensitization in children with IgE-mediated hen’s egg allergy: a new protocol with raw hen’s egg. Pediatr Allergy Immunol. 2013; 24: 75–83. [DOI] [PubMed] [Google Scholar]
- 204. Morisset M Moneret-Vautrin DA Guenard L Cuny JM Frentz P Hatahet R Hanss Ch Beaudouin E Petit N Kanny G Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow’s milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol. 2007; 39: 12–19. [PubMed] [Google Scholar]
- 205. Pajno GB Caminiti L Ruggeri P De Luca R Vita D La Rosa M Passalacqua G Oral immunotherapy for cow’s milk allergy with a weekly up-dosing regimen: a randomized single-blind controlled study. Ann Allergy Asthma Immunol. 2010; 105: 376–381. [DOI] [PubMed] [Google Scholar]
- 206. Patriarca G Nucera E Roncallo C Pollastrini E Bartolozzi F De Pasquale T Buonomo A Gasbarrini G Di Campli C Schiavino D Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003; 17: 459–465. [DOI] [PubMed] [Google Scholar]
- 207. Patriarca G Schiavino D Nucera E Schinco G Milani A Gasbarrini GB Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology. 1998; 45: 52–58. [PubMed] [Google Scholar]
- 208. Salmivesi S Korppi M Mäkelä MJ Paassilta M Milk oral immunotherapy is effective in school-aged children. Acta Paediatr. 2013; 102: 172–176. [DOI] [PubMed] [Google Scholar]
- 209. Skripak JM Nash SD Rowley H Brereton NH Oh S Hamilton RG Matsui EC Burks AW Wood RA A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008; 122: 1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Varshney P Jones SM Scurlock AM Perry TT Kemper A Steele P Hiegel A Kamilaris J Carlisle S Yue X Kulis M Pons L Vickery B Burks AW A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011; 127: 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vazquez-Ortiz M Alvaro M Piquer M Dominguez O Machinena A Martín-Mateos MA Plaza AM Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg-allergic children. Clin Exp Allergy. 2014; 44: 130–141. [DOI] [PubMed] [Google Scholar]
- 212. Nowak-Węgrzyn A Wood RA Nadeau KC Pongracic JA Henning AK Lindblad RW Beyer K Sampson HA Multicenter, randomized, double-blind, placebo-controlled clinical trial of vital wheat gluten oral immunotherapy. J Allergy Clin Immunol. 2019; 143: 651–661.e9. [DOI] [PubMed] [Google Scholar]
- 213. Brożek JL Terracciano L Hsu J Kreis J Compalati E Santesso N Fiocchi A Schünemann HJ Oral immunotherapy for IgE-mediated cow’s milk allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2012; 42: 363–374. [DOI] [PubMed] [Google Scholar]
- 214. Calvani M Giorgio V Miceli Sopo S Specific oral tolerance induction for food. A systematic review. Eur Ann Allergy Clin Immunol. 2010; 42: 11–19. [PubMed] [Google Scholar]
- 215. Nurmatov U Devereux G Worth A Healy L Sheikh A Effectiveness and safety of orally administered immunotherapy for food allergies: a systematic review and meta-analysis. Br J Nutr. 2014; 111: 12–22. [DOI] [PubMed] [Google Scholar]
- 216. Nurmatov U Venderbosch I Devereux G Simons FE Sheikh A Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev. 2012. [DOI] [PMC free article] [PubMed]
- 217. Yeung JP Kloda LA McDevitt J Ben-Shoshan M Alizadehfar R Oral immunotherapy for milk allergy. Cochrane Database Syst Rev. 2012; 11: CD009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Soller L Abrams EM Carr S Kapur S Rex GA Leo S First real-world effectiveness analysis of preschool peanut oral immunotherapy. J Allergy Clin Immunol Pract. 2021; 9: 1349–1356.e1. [DOI] [PubMed] [Google Scholar]
- 219. Chinthrajah RS Cao S Dunham T Sampath V Chandra S Chen M Sindher S Nadeau K Oral immunotherapy for peanut allergy: The pro argument. World Allergy Organ J. 2020; 13: 100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Blumchen K Trendelenburg V Ahrens F Gruebl A Hamelmann E Hansen G Heinzmann A Nemat K Holzhauser T Roeder M Rosenfeld L Hartmann O Niggemann B Beyer K Efficacy, safety, and quality of life in a multicenter, randomized, placebo-controlled trial of low-dose peanut oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol Pract. 2019; 7: 479–491.e10. [DOI] [PubMed] [Google Scholar]
- 221. Vickery BP Vereda A Casale TB Beyer K du Toit G Hourihane JO Jones SM Shreffler WG Marcantonio A Zawadzki R Sher L Carr WW Fineman S Greos L Rachid R Ibáñez MD Tilles S Assa’ad AH Nilsson C Rupp N AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018; 379: 1991–2001. [DOI] [PubMed] [Google Scholar]
- 222. Trendelenburg V Blumchen K Bellach J Ahrens F Gruebl A Hamelmann E Hansen G Heinzmann A Nemat K Holzhauser T Röder M Niggemann B Beyer K Peanut oral immunotherapy protects patients from accidental allergic reactions to peanut. J Allergy Clin Immunol Pract. 2020; 8: 2437–2441.e3. [DOI] [PubMed] [Google Scholar]
- 223. O’B Hourihane J Beyer K Abbas A Fernández-Rivas M Turner PJ Blumchen K Nilsson C Ibáñez MD Deschildre A Muraro A Sharma V Erlewyn-Lajeunesse M Zubeldia JM De Blay F Sauvage CD Byrne A Chapman J Boralevi F DunnGalvin A O’Neill C Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health. 2020; 4: 728–739. [DOI] [PubMed] [Google Scholar]
- 224. Staden U Rolinck-Werninghaus C Brewe F Wahn U Niggemann B Beyer K Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007; 62: 1261–1269. [DOI] [PubMed] [Google Scholar]
- 225. Leung DY Sampson HA Yunginger JW Burks AW Schneider LC Wortel CH Davis FM Hyun JD Shanahan WR Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003; 348: 986–993. [DOI] [PubMed] [Google Scholar]
- 226. Sampson HA Leung DY Burks AW Lack G Bahna SL Jones SM Wong DA A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011; 127: 1309–10.e1. [DOI] [PubMed] [Google Scholar]
- 227. Savage JH Courneya JP Sterba PM Macglashan DW Saini SS Wood RA Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012; 130: 1123–1129.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Andorf S Purington N Block WM Long AJ Tupa D Brittain E Rudman Spergel A Desai M Galli SJ Nadeau KC Chinthrajah RS Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018; 3: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Guttman-Yassky E Bissonnette R Ungar B Suárez-Fariñas M Ardeleanu M Esaki H Suprun M Estrada Y Xu H Peng X Silverberg JI Menter A Krueger JG Zhang R Chaudhry U Swanson B Graham NMH Pirozzi G Yancopoulos GD D Hamilton JD Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019; 143: 155–172. [DOI] [PubMed] [Google Scholar]
- 230. Chinthrajah S Cao S Liu C Lyu SC Sindher SB Long A Sampath V Petroni D Londei M Nadeau KC Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight. 2019; 4: e131347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Brockow K Schallmayer S Beyer K Biedermann T Fischer J Gebert N Grosber M Jakob T Klimek L Kugler C Lange L Pfaar O Przybilla B Rietschel E Rueff F Schnadt S Szczepanski R Worm M Kupfer J Gieler U Effects of a structured educational intervention on knowledge and emergency management in patients at risk for anaphylaxis. Allergy. 2015; 70: 227–235. [DOI] [PubMed] [Google Scholar]
- 232. Ring J Brockow K Kugler C Gebert N Grando K Götz D Hutegger I Lüthi H Münch D Spindler T Schmid-Grendelmeier P Gieler U New aspects in allergy education with special emphasis on anaphylaxis. Allergo J Int. 2017; 26: 267–272. [Google Scholar]
- 233. RKI-Homepage: Masernimpfung: Wirksamkeit, Sicherheit und Kontraindikationen. www.rki.de/SharedDocs/FAQ/Impfen/MMR/FAQ_Uebersicht_MSG.html.
- 234. Khakoo G Lack G Recommendations for using MMR vaccine in children allergic to eggs. BMJ. 2000; 320: 929–932. [PubMed] [Google Scholar]
- 235. James JM Burks AW Roberson PK Sampson HA Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med. 1995; 332: 1262–1266. [DOI] [PubMed] [Google Scholar]
- 236. Aickin R Hill D Kemp A Measles immunisation in children with allergy to egg. BMJ. 1994; 309: 223–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Centers for Disease Control and Prevention. Flu vaccine and people with egg allergies. https://www.cdc.gov/flu/prevent/egg-allergies.htm.
- 238. Kelso JM Administering influenza vaccine to egg-allergic persons. Expert Rev Vaccines. 2014; 13: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 239. Turner PJ Southern J Andrews NJ Miller E Erlewyn-Lajeunesse M Safety of live attenuated influenza vaccine in young people with egg allergy: multicentre prospective cohort study. BMJ. 2015; 351: h6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Worm M Ring J Klimek L Jakob T Lange L Treudler R Beyer K Werfel T Biedermann T Bircher A Fischer M Fuchs T Heller AR Hoffmann F Huttegger I Kopp MV Kugler C Lommatzsch M Pfaar O Rietschel E [Covid-19 vaccination and risk of anaphylaxis – Recommendations for practical management]. MMW Fortschr Med. 2021; 163: 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Mahler V Glöckler A Worm M Spornraft-Ragaller P Bauer A Dickel H Proteinkontaktdermatitis. Allergologie. 2013; 36: 219–226. [Google Scholar]
- 242. Nicholson PJ Llewellyn D English JS Evidence-based guidelines for the prevention, identification and management of occupational contact dermatitis and urticaria. Contact Dermat. 2010; 63: 177–186. [DOI] [PubMed] [Google Scholar]
- 243. Anemüller W Mohr M Brans R Homann A Jappe U [Alpha-Gal-associated delayed red meat anaphylaxis as an occupational disease]. Hautarzt. 2018; 69: 848–852. [DOI] [PubMed] [Google Scholar]
- 244. https://www.bmas.de/SharedDocs/Downloads/DE/Gesetze/siebtes-gesetz-zur-aenderung-des-vierten-buches-sozialgesetzbuch-und-anderer-gesetze.pdf?__blob=publicationFile&v=1 (Date of access: June 28, 2021.
- 245. Mahler V Chefs and food handlers. In: Johansen JD, Mahler V, Lepoittevin JP, Frosch PJ (eds.). Contact Dermatitis; 6th edition. Cham, Switzerland: Springer nature Switzerland AG; 2021. p. 471-482. [Google Scholar]
- 246. Vester L Thyssen JP Menné T Johansen JD Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermat. 2012; 67: 328–333. [DOI] [PubMed] [Google Scholar]
- 247. Pesonen M Koskela K Aalto-Korte K Contact urticaria and protein contact dermatitis in the Finnish Register of Occupational Diseases in a period of 12 years. Contact Dermat. 2020; 83: 1–7. [DOI] [PubMed] [Google Scholar]
- 248. Baur X Heutelbeck A Kujath P Stahlkopf H Prävention arbeitsbedingter obstruktiver Atemwegserkrankungen: Interdisziplinäre S1-Leitlinie der Deutschen Gesellschaft für Arbeitsmedizinund Umweltmedizin [Prevention of occupational airway diseases: interdisciplinary guideline of the German Society for Occupational and Environmental Medicine]. Pneumologie. 2011; 65: 263–282. [DOI] [PubMed] [Google Scholar]
- 249. Raulf-Heimsoth M Kespohl S Liebers V Rihs H Rozynek P Sander I van Kampen V Berufbedingte Typ I-Allergien. Allergo J. 2009; 18: 538–550. [Google Scholar]
- 250. Raulf-Heimsoth M van Kampen V Kespohl S Sander I Merget R Brüning T Inhalationsallergien am Arbeitsplatz. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012; 55: 363–372. [DOI] [PubMed] [Google Scholar]
- 251. Barbuzza O Guarneri F Galtieri G Gangemi S Vaccaro M Protein contact dermatitis and allergic asthma caused by Anisakis simplex. Contact Dermat. 2009; 60: 239–240. [DOI] [PubMed] [Google Scholar]
- 252. Matsuo H Uemura M Yorozuya M Adachi A Morita E Identification of IgE-reactive proteins in patients with wheat protein contact dermatitis. Contact Dermat. 2010; 63: 23–30. [DOI] [PubMed] [Google Scholar]
- 253. Raulf M Immediate type hypersensitivity by occupational materials. In: Johansen JD, Mahler V, Lepoittevin JP, Frosch PJ (eds). Contact Dermatitis; 6th edition. Cham, Switzerland: Springer nature Switzerland AG; 2021. p. 499–512. [Google Scholar]
- 254. Jappe U Vieths S Lupine, a source of new as well as hidden food allergens. Mol Nutr Food Res. 2010; 54: 113–126. [DOI] [PubMed] [Google Scholar]
- 255. Adisesh A Robinson E Nicholson PJ Sen D Wilkinson M U.K. standards of care for occupational contact dermatitis and occupational contact urticaria. Br J Dermatol. 2013; 168: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.www.dguv.de/publikationen, Bestellnummer 10196. https://publikationen.dguv.de/versicherungleistungen/berufskrankheiten/2058/bamberger-empfehlung. Deutsche Gesetzliche Unfallversicherung V. (DGUV) (eds) Bamberger Empfehlungen. Empfehlungen zur Begutachtung von arbeitsbedingten Hauterkrankungen und Hautkrebserkrankungen. Ausgabe Juni 2017.
- 257. Moscato G Pala G Barnig C De Blay F Del Giacco SR Folletti I Heffler E Maestrelli P Pauli G Perfetti L Quirce S Sastre J Siracusa A Walusiak-Skorupa J van Wjik RG EAACI consensus statement for investigation of work-related asthma in non-specialized centres. Allergy. 2012; 67: 491–501. [DOI] [PubMed] [Google Scholar]
- 258. Moscato G Vandenplas O Van Wijk RG Malo JL Perfetti L Quirce S Walusiak J Castano R Pala G Gautrin D De Groot H Folletti I Yacoub MR Siracusa A EAACI position paper on occupational rhinitis. Respir Res. 2009; 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. van Kampen V de Blay F Folletti I Kobierski P Moscato G Olivieri M Quirce S Sastre J Walusiak-Skorupa J Raulf-Heimsoth M EAACI position paper: skin prick testing in the diagnosis of occupational type I allergies. Allergy. 2013; 68: 580–584. [DOI] [PubMed] [Google Scholar]
- 260. von Krogh G Maibach HI The contact urticaria syndrome – an updated review. J Am Acad Dermatol. 1981; 5: 328–342. [DOI] [PubMed] [Google Scholar]
- 261. Zuberbier T Aberer W Brockow K Grabbe J Hamelmann E Hartmann K S3-Leitlinie Urtikaria Teil 1: Klassifikation und Diagnostik der Urtikaria – deutschsprachige Version der internationalen S3-Leitlinie. Allergo J. 2011; 20: 249–258. [Google Scholar]
- 262. Mahler V Prick and intracutaneous testing and IgE testing. In: John SM, Johansen JD, Rustemeyer T, Elsner P, Maibach HI (eds) Kanerva’s occupational dermatology. 3rd edition. Cham: Springer International. 2020: p. 1317-1345. [Google Scholar]
- 263. van Kampen V de Blay F Folletti I Kobierski P Moscato G Olivieri M Quirce S Sastre J Walusiak-Skorupa J Kotschy-Lang N Müsken H Mahler V Schliemann S Ochmann U Sültz J Worm M Sander I Zahradnik E Brüning T Merget R Evaluation of commercial skin prick test solutions for selected occupational allergens. Allergy. 2013; 68: 651–658. [DOI] [PubMed] [Google Scholar]
- 264. Mahler V Drexler H [Dermatologic occupationally relevant type I allergies]. Hautarzt. 2004; 55: 34–41. [DOI] [PubMed] [Google Scholar]
- 265. Nowak D Diepgen TL Drexler H [Reduced earning capacity due to IgE-mediated skin and airway allergy. Consensus paper]. Pneumologie. 2004; 58: 365–366. [DOI] [PubMed] [Google Scholar]
- 266. Skudlik C Allmers H John S Becker D Dickel H Geier J Häberle M Lessmann H Mahler V Wagner E Weisshaar E Wehrmann W Werfel T Zagrodnik F Diepgen TL Beurteilung der Auswirkungen einer Allergie gegenüber Naturgummilatex bei der Minderung der Erwerbsfähigkeit im Rahmen der BK 5101. Dermatol Beruf Umw. 2010; 58: 54–60. [Google Scholar]
- 267. Commins SP Platts-Mills TA Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013; 13: 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Martin PE Eckert JK Koplin JJ Lowe AJ Gurrin LC Dharmage SC Vuillermin P Tang ML Ponsonby AL Matheson M Hill DJ Allen KJ Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin Exp Allergy. 2015; 45: 255–264. [DOI] [PubMed] [Google Scholar]
- 269. Nowak-Wegrzyn A Berin MC Mehr S Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol Pract. 2020; 8: 24–35. [DOI] [PubMed] [Google Scholar]
- 270. Kleine-Tebbe J Jappe U Molekulare Allergiediagnostik: Entwicklung und Bedeutung für die klinische Praxis. Allergologie. 2013; 36: 327-349. [Google Scholar]
- 271. Henzgen M Ballmer-Weber B Erdmann S Fuchs T Kleine-Tebbe J Lepp U Niggemann B Raithel M Reese I Saloga J Vieths S Zuberbier T Werfel T Hauttestungen mit Nahrungsmittelallergenen. Allergo J. 2008; 17: 401–406. [Google Scholar]
- 272. Nwaru BI Hickstein L Panesar SS Roberts G Muraro A Sheikh A Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014; 69: 992–1007. [DOI] [PubMed] [Google Scholar]
- 273. Elizur A Rajuan N Goldberg MR Leshno M Cohen A Katz Y Natural course and risk factors for persistence of IgE-mediated cow’s milk allergy. J Pediatr. 2012; 161: 482–487.e1. [DOI] [PubMed] [Google Scholar]
- 274. Peters RL Dharmage SC Gurrin LC Koplin JJ Ponsonby AL Lowe AJ Tang ML Tey D Robinson M Hill D Czech H Thiele L Osborne NJ Allen KJ The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study. J Allergy Clin Immunol. 2014; 133: 485–491. [DOI] [PubMed] [Google Scholar]
- 275. Webb LM Lieberman P Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol. 2006; 97: 39–43. [DOI] [PubMed] [Google Scholar]
- 276. Tsabouri S Triga M Makris M Kalogeromitros D Church MK Priftis KN Fish and shellfish allergy in children: review of a persistent food allergy. Pediatr Allergy Immunol. 2012; 23: 608–615. [DOI] [PubMed] [Google Scholar]
- 277. Santa H Saarela JT Laatikainen R Rautianen J Virtanen T Rytkönen M Mäntyjärvi R A bovine dander allergen, comparative modeling, and similarities and differences in folding with related proteins. J Protein Chem. 1998; 17: 657–662. [DOI] [PubMed] [Google Scholar]
- 278. Holzhauser T Wackermann O Ballmer-Weber BK Bindslev-Jensen C Scibilia J Perono-Garoffo L Utsumi S Poulsen LK Vieths S Soybean (Glycine max) allergy in Europe: Gly m 5 (β-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J Allergy Clin Immunol. 2009; 123: 452–458. [DOI] [PubMed] [Google Scholar]
- 279. Quirce S Polo F Figueredo E González R Sastre J Occupational asthma caused by soybean flour in bakers – differences with soybean-induced epidemic asthma. Clin Exp Allergy. 2000; 30: 839–846. [DOI] [PubMed] [Google Scholar]
- 280. Kuehn A Swoboda I Arumugam K Hilger C Hentges F Fish allergens at a glance: variable allergenicity of parvalbumins, the major fish allergens. Front Immunol. 2014; 5: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Dickel H Bruckner T Altmeyer P Künzlberger B [Seafood allergy in cooks: a case series and review of the literature]. J Dtsch Dermatol Ges. 2014; 12: 891–902. [DOI] [PubMed] [Google Scholar]
- 282. Lopata AL Jeebhay MF Airborne seafood allergens as a cause of occupational allergy and asthma. Curr Allergy Asthma Rep. 2013; 13: 288–297. [DOI] [PubMed] [Google Scholar]
- 283. Behrends J Schwager C Hein M Scholzen T Kull S Jappe U Innovative robust basophil activation test using a novel gating strategy reliably diagnosing allergy with full automation Allergy. 2021. Epub ahead of print. [DOI] [PubMed]


