Abstract
The brain is highly sensitive to auditory regularities and exploits the predictable order of sounds in many situations, from parsing complex auditory scenes, to the acquisition of language. To understand the impact of stimulus predictability on perception, it is important to determine how the detection of predictable structure influences processing and attention. Here, we use pupillometry to gain insight into the effect of sensory regularity on arousal. Pupillometry is a commonly used measure of salience and processing effort, with more perceptually salient or perceptually demanding stimuli consistently associated with larger pupil diameters. In two experiments we tracked human listeners' pupil dynamics while they listened to sequences of 50-ms tone pips of different frequencies. The order of the tone pips was either random, contained deterministic (fully predictable) regularities (experiment 1, n = 18, 11 female) or had a probabilistic regularity structure (experiment 2, n = 20, 17 female). The sequences were rapid, preventing conscious tracking of sequence structure thus allowing us to focus on the automatic extraction of different types of regularities. We hypothesized that if regularity facilitates processing by reducing processing demands, a smaller pupil diameter would be seen in response to regular relative to random patterns. Conversely, if regularity is associated with heightened arousal and attention (i.e., engages processing resources) the opposite pattern would be expected. In both experiments we observed a smaller sustained (tonic) pupil diameter for regular compared with random sequences, consistent with the former hypothesis and confirming that predictability facilitates sequence processing.
SIGNIFICANCE STATEMENT The brain is highly sensitive to auditory regularities. To appreciate the impact that the presence of predictability has on perception, we need to better understand how a predictable structure influences processing and attention. We recorded listeners' pupil responses to sequences of tones that followed either a predictable or unpredictable pattern, as the pupil can be used to implicitly tap into these different cognitive processes. We found that the pupil showed a smaller sustained diameter to predictable sequences, indicating that predictability eased processing rather than boosted attention. The findings suggest that the pupil response can be used to study the automatic extraction of regularities, and that the effects are most consistent with predictability helping the listener to efficiently process upcoming sounds.
Keywords: attention, auditory scene analysis, predictive coding, pupillometry, regularity, statistical learning
Introduction
The sensory environment is laden with regularities. The brain readily exploits this predictable information, using it to drive perceptual experiences (de Lange et al., 2018), guide attention (Zhao et al., 2013), and influence decision-making (Soltani and Izquierdo, 2019). In the domain of hearing, our ability to use these statistics plays many important roles, from auditory scene analysis (Bendixen, 2014; Heilbron and Chait, 2018) to discovering regularities in the speech signal (Erickson and Thiessen, 2015).
Accumulating work demonstrates that listeners automatically detect predictable structure in unfolding sound sequences. In a seminal demonstration, Saffran et al. (1996) showed that infants are able to segment a continuous stream of syllables based only on the statistical relationships (frequency of co-occurrence) between adjacent elements. This paradigm has since been expanded to a variety of statistical structures and behavioral tasks to reveal robust “statistical learning” across the life span (Conway, 2020). Sensitivity to statistical regularities is also exhibited in the brains of naive listeners during passive exposure to sound patterns (Barascud et al., 2016; Southwell et al., 2017) and in other species (Milne et al., 2018).
A key question pertains to understanding how the detection of predictable structure influences processing and attention. The link between regularity and attention has been contentious. On the one hand it is argued that regularity automatically biases attention (Mackintosh, 1975; Feldman and Friston, 2010; Zhao et al., 2013; Alamia and Zénon, 2016). This is consistent with the premise that regular structure in the environment carries important information about behaviorally relevant elements within our surroundings, and should therefore receive perceptual priority and attentional resources. On the other hand, a large body of work demonstrates that the brain exhibits reduced responses to regular, predictable stimuli (Itti and Baldi, 2009; de Lange et al., 2018; Richter et al., 2018), interpreted as reflecting the fact that the detection of regular structure facilitates the conservation of processing and computational resources. Indeed, it has been shown that regular patterns are easier to process (Rohenkohl et al., 2012) and also, critically, easier to ignore (Andreou et al., 2011; Southwell et al., 2017; Makov and Zion Golumbic, 2020), which has been taken as evidence that regularity does not draw on attentional resources.
Here, we use pupillometry to tap into these different cognitive processes. Pupil diameter is a commonly used measure of bottom-up driven salience and processing effort. Non-luminance-mediated pupil dynamics are controlled by a balance between norepinephrine (NE), reflecting the activation of the arousal system (for reviews see Joshi et al., 2016; Larsen and Waters, 2018) and acetylcholine (ACh), hypothesized to correlate with the processing load experienced by the individual (Sarter et al., 2006). By studying pupil responses to structured versus random auditory patterns we sought to determine how sustained pupil diameter, and by proxy the listener's arousal and processing load, change as a function of regularity.
If regularity facilitates processing, a smaller pupil diameter would be predicted in response to regular relative to random patterns. Conversely, if the emergence of regularity is associated with an increased demand on attention, we expect the opposite pattern, a larger pupil diameter associated with more predictable stimuli, reflecting increased salience-evoked arousal and a consequent draw on processing resources.
We studied two types of predictable acoustic structure: in experiment 1, we used deterministic (i.e., fully predictable; Fig. 1) sequences, as described in Barascud et al. (2016), to study the pupil response to regular, relative to randomly-ordered, tone pip sequences. These sequences were generated anew on every trial, tapping into processes that rapidly detect, and exploit, the predictable structure. In experiment 2, we used a more complex probabilistic structure similar to the classic Saffran paradigm (Fig. 2). These sequences did not follow a deterministic order, instead the transitional probabilities (TPs) between tones allowed the stream to be segmented into triplets. Listeners were preexposed to such sequences, and pupil responses were measured subsequently to quantify responses to the preacquired statistical pattern.
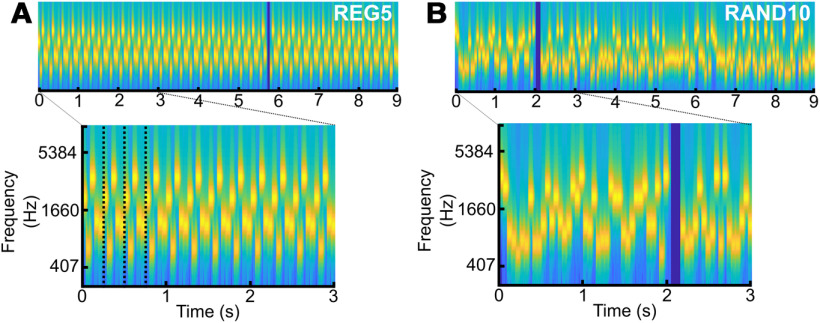
Figure 1.
Stimuli used in experiment 1. Stimuli were sequences of contiguous tone pips (50 ms) with frequencies drawn from a pool of 20 fixed values. The tone pips were arranged according to frequency patterns, generated anew for each subject and on each trial. REG sequences were generated by randomly selecting 5 (REG5), 10 (REG10), or 15 (REG15) frequencies from the pool and iterating that sequence to create a regular repeating pattern. A, Example of a spectrogram for REG5, dotted lines indicate the first three cycles. RAND sequences were generated by randomly sampling 5 (RAND5), 10 (RAND10), or 15 (RAND15) frequencies with replacement. B, Example of a spectrogram for RAND10. A subset of trials were target trials containing a gap generated by the removal of two (REG) or three (RAND) tones, indicated by the dark blue band in the spectrogram.
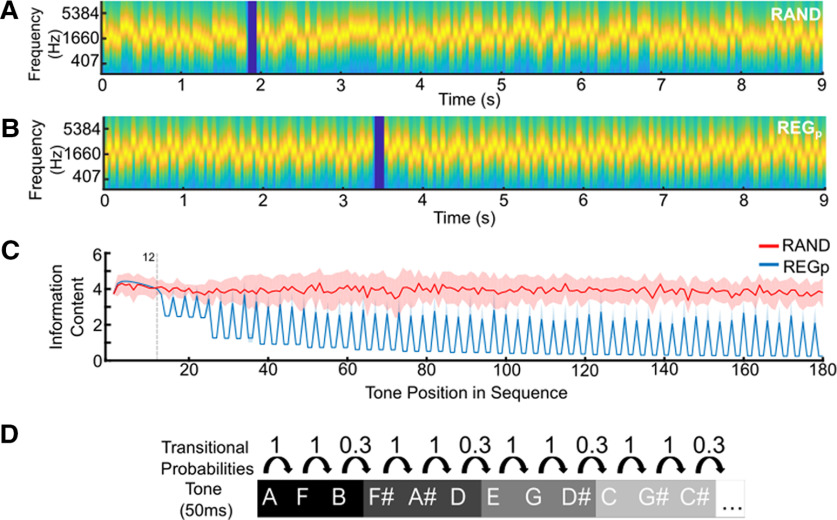
Figure 2.
Stimuli used in experiment 2. Stimuli were sequences of concatenated tone pips (50 ms) with frequencies consisting of 12 different values that correspond to the musical notes shown in D. A, Spectrogram of RAND sequences where the tones do not follow a predictable pattern. A subset of trials was target trials containing a gap generated by the removal of three tones, the gap is indicated by a dark blue band in the spectrogram of A, B. B, Spectrogram of the “regular” (REGp) condition that followed the probabilistic structure shown in D, top row; tones were arranged into four three-item tone “words,” the four words are shown in different shades of gray. The tones within a word always occurred together giving them a TP of 1. Each word could transition to any of the other words, giving tones at word boundaries a TP of ∼0.3. Therefore, these sequences do not have a regular structure in the same way as experiment 1, compare with Figure 1A. C, Ideal observer model response to RAND (red) and REGp (blue) signals shows the IC [negative log probability (-log P); the higher the IC value the more unexpected the tone] of each tone pip (averaged over 24 different sequences). This modeling confirms that while IC remains consistently high for unpredictable sequences (RAND, red), for REGp (blue) it begins to drop on average after 12 tones. Evidence for the predictable structure then continues to accumulate throughout the sequences as indicated by the gradual separation between the REGp and RAND ICs. Shading indicates ±1 SEM (D, bottom row). The random sequences presented the same tones as the regular sequences but in a random order.
Materials and Methods
Results from two experiments are reported. We continuously tracked pupil diameter while participants listened to 9-s-long sequences of contiguous tone pips, that either contained a predictable structure or did not. To control participants' attention, and to make sure it was broadly focused on the auditory stimuli, an incidental, easy gap detection task was used; listeners were required to monitor the stream of tones and indicate when they noticed a silent “gap” within the sequence. The gaps, generated by the removal of several consecutive tones, were placed at a random position in ∼25% (experiment 1) and 20% (experiment 2) of the sequences. Participants were kept naive to the presence of an underlying pattern to enable the study of implicit sequence learning. This study was not preregistered.
Stimuli and procedure
Participants sat with their head fixed on a chinrest in front of a monitor (24-inch BENQ XL2420T with a resolution of 1920 × 1080 pixels and a refresh rate of 60 Hz), in a dimly lit and acoustically shielded room (IAC triple-walled sound-attenuating booth). Sounds were delivered diotically to the participants' ears with Sennheiser HD558 headphones (Sennheiser) via a Roland DUO-CAPTURE EX USB Audio Interface (Roland Ltd), at a comfortable listening level that was adjusted by the participant during the practice phase. Stimulus presentation and response recording were controlled with Psychtoolbox (Psychophysics Toolbox version 3; Brainard, 1997) on MATLAB (The MathWorks).
Experiment 1
Stimuli were 9-s-long tone sequences (Fig. 1A,B) of contiguous 50-ms tone pips (ramped on and off with a 5-ms raised cosine ramp; 180 tone pips per sequence). Tone frequencies were selected from a pool of 20 logarithmically spaced values between 222 and 2000 Hz. Sequences were generated as previously described in Southwell et al. (2017). A unique sequence was presented on each trial. Sequences were defined by two parameters: regularity (whether they consisted of a regularly repeating or random pattern) and alphabet size, the number of frequencies comprising the pattern (5, 10, or 15). In regular (REG) sequences, a subset of frequencies (“alphabet size”) were randomly drawn from the full pool and arranged in repeating cycles. Paired random (RAND) sequences were generated for the same frequency subset by randomly arranging the tones. Therefore, REG and RAND conditions were matched for the occurrence of each frequency. Overall, six conditions were used (RAND/REG × three alphabet sizes; REG5, RAND5; REG10, RAND10; and REG15, RAND15).
Approximately 25% of the stimuli contained a single silent gap anywhere between 1 and 8 s after sequence onset. This was created by removing two tones from REG sequences (100-ms gap) and three tones from RAND sequences (150 ms) to equate task difficulty (Zhao et al., 2019b).
The experiment consisted of seven blocks (∼8 min each) and a practice block. There were 24 trials per block (four trials per condition) for a total of 168 trials (28 trials per condition). Intertrial intervals were jittered between 2500 and 3000 ms. Stimuli were presented in a random order, such that on each trial the specific condition was unpredictable.
Throughout the block a black cross was presented at the center of the screen against a gray background. Participants were instructed to fixate on the cross while monitoring the sequence of tones for gaps, and to respond by button press as quickly as possible when a gap was noticed in the tone stream. At the end of each trial, visual feedback indicated whether gaps were detected correctly. Further feedback was given at the end of each block, indicating the total number of correct responses, false alarms, and average response time. The practice block contained six gap trials (three REG, three RAND) to ensure participants understood the task. In the main blocks only 25% of the trials contained gaps. The experimental session lasted ∼2 h. A break of at least 3 min was imposed between blocks to reduce the effects of fatigue.
Previous work with MEG (Barascud et al., 2016) and EEG (Southwell et al., 2017; Southwell and Chait, 2018) demonstrated that brain responses in naive passive listeners rapidly differentiate RAND from REG signals, with responses to REG diverging from RAND within two regularity cycles. We expected pupil responses to also follow this pattern and show a change in pupil size once the structure has been acquired. Further, we expected the change in pupil size to occur later for larger alphabet sizes, as more information is required to identify a longer pattern.
Experiment 2
Experiment 2 investigated sequences that contained a probabilistic rather than deterministic structure. Sequences were based on the pure tone version of the segmentation paradigm introduced by Saffran and colleagues (Saffran et al., 1999), with the key modification, that instead of the 333-ms-long tones in Saffran et al. (1999), we used 50-ms tones.
To generate the underlying probabilistic structure, 12 different tones were arranged into four tone “words” made from the following musical notes: AFB, F#A#D, EGD#, CG#C# (Fig. 2D), these corresponded to frequencies: A = 440 Hz; A# = 466.16 Hz; B = 493.88 Hz; C = 523.25 Hz; C# = 554.37 Hz; D = 587.33 Hz; D# = 622.25 Hz; E = 659.25 Hz; F = 698.46 Hz; F# = 739.99 Hz; G = 783.99 Hz; G# = 830.31 Hz. As in Saffran et al. (1999), the same tone “words” were used for each subject. Sequences were generated anew for each trial by randomly ordering the tone words, with the constraint that the same word did not occur twice in a row, thus tone words always transitioned to a different tone word. This created a probabilistic structure where the transitional probability (TP; the probability that tone “a” will be followed by tone “b” calculated as the frequency of a to b/frequency of a) between tones within a word was 1, and the TP at word boundaries was 0.33. RAND sequences were generated in the same way as for experiment 1 but using the 12 frequencies listed above.
To formally demonstrate how this probabilistic structure emerged over the course of a sequence we used a PPM (prediction by partial matching) statistical learning model. The model, Information Dynamics of Music (IDYOM; Pearce et al., 2010), uses unsupervised statistical learning to acquire the TPs of tone pips within each sequence. The output of the model shows the information content (IC) for each tone as the negative log probability (-log P) of a tone pip, therefore the higher the IC value the more unexpected the tone. The model output (Fig. 2C) demonstrates that, following presentation of the first 12 tones (each of the four tone “words”) the two types of sequence, regular (REGp, blue) and random (RAND, red), rapidly diverge. While the random sequences remain unpredictable, the tones in REGp gradually become more predictable as the model learns the sequence structure. In contrast to deterministic regularities (see model in Barascud et al., 2016), these probabilistic sequences have a much more gradual change in IC. As a result, we would expect that for this, more complex, regularity listeners will exhibit more variability in learning rate. For this reason, we introduced a familiarization phase to ensure listeners had ample opportunity to become sensitive to the structure. This familiarization phase consisted of only REGp sequences. Participants were then tested on REGp and RAND sequences while recording the pupil response. Following pupillometry measurements, a further behavioral test was administered to more explicitly probe if the subjects had become sensitive to the regularities. Therefore experiment 2 consisted of the following three phases.
Familiarization
The familiarization phase gave listeners ample opportunity to acquire the probabilistic structure. In this phase, trials consisted of 27-s-long REGp sequences (540 individual tones in total) such that each “tone word” was encountered 45 times within each sequence. A gap detection task was used to ensure participants attended to the sequence. Each sequence contained two gaps. The gaps were generated by removing six tones, creating a 300-ms gap. The gap was intentionally longer in the familiarization phase to make the task easy and reduce the effects of fatigue for the next phase. Overall, the familiarization stage lasted ∼7.5 min consisting of 15 trials. Participants were instructed to respond (key press) when they heard a gap. After each trial participants received visual feedback on the number of correct responses and false alarms. No pupil data were collected in this phase.
Pupillometry
Following a minimum 3-min break, participants completed the pupillometry phase. All trials contained a 9-s-long tone sequence (180 tones in total, 60 tone words); 20% of trials (“target trials”; REGp and RAND with equal proportion) contained a single gap that occurred between 1 and 8 s postonset. In all conditions, the gap was 150 ms long (removal of three tones). This phase consisted of two blocks of 30 trials. This provided a total of 24 trials per condition.
Behavioral probe
This phase tested how much knowledge listeners had gained about the structure of the sequence. Pupil responses were not recorded. We conducted two separate probes designed to test familiarity and sensitivity to sequence structure. In the familiarity probe, participants were presented with 60 3-s trials (REGp vs RAND; 50% of each condition). They were instructed to listen carefully to the sounds and decide whether the sequence felt “familiar” based on the initial exposure phase. They were told to use a “gut” feeling if they were unsure. In the structure probe, participants were instructed to listen and identify if the sequence contained any sort of structure, or, appeared to be random. The two probes were completed by the “main” group (those participants who completed the Familiarization and Pupillometry stages), and by a “control” group that was recruited to only complete the behavioral probes. The purpose of this control group was to establish the degree to which the structure could be extracted without prior exposure. As these participants had no prior exposure to the REGp and RAND stimuli in the familiarity probe they were told to use a gut feeling to identify familiar sequences.
Participants
Sample size
We aimed for a sample size of ∼20, based on previous data from a related pupillometry study (Zhao et al., 2019a) where robust pupil response effects were observed using as few as 10 participants.
All participants declared that they had no known otological or neurologic conditions. Experimental procedures were approved by the research ethics committee of University College London and written informed consent was obtained from each participant.
Experiment 1
A total of 22 paid participants were recruited, four were excluded providing a final sample size of 18 participants (11 females, mean age 25.2, range 19–35). In both experiments, exclusion occurred either during data collection, e.g., because of difficulty tracking the eye or excessive blinking or tiredness (eye closure), or because of a high blink rate that was identified in preprocessing, before separating trials by condition.
Experiment 2
For the main group, 24 paid participants were recruited, four were excluded providing a final sample size of 20 participants (17 females, mean age 21.2, range 19–28). The control group consisted of 20 paid participants (10 females, mean age 22.3, range 18–30).
Pupil diameter measurement
An infrared eye-tracking camera (Eyelink 1000 Desktop Mount, SR Research Ltd.) was positioned at a horizontal distance of 65 cm away from the participant. The standard five-point calibration procedure for the Eyelink system was conducted before each experimental block and participants were instructed to avoid head movement after calibration. During the experiment, the eye-tracker continuously tracked gaze position and recorded pupil diameter, focusing binocularly at a sampling rate of 1000 Hz. Participants were instructed to blink naturally during the experiment and encouraged to rest their eyes briefly during intertrial intervals. Where participants blinked excessively during the practice block, additional instructions to reduce blinking were provided. Before each trial, the eye-tracker automatically checked that the participants' eyes were open and fixated appropriately; trials would not start unless this was confirmed.
Statistical analysis
Statistical analysis was conducted in SPSS (IBM SPSS Statistics, version 27) and MATLAB (The MathWorks, 2017a).
Behavioral data
Gap detection task
For experiment 1, sensitivity scores (d') were computed using the hit and false alarm rate [z(hits) – z(false alarms)]. A keypress was classified as a hit if it occurred <1.5 s following a target gap. Where hit rates (HRs) or false alarms were at ceiling (values of 1 and 0, respectively; resulting in an undefined d') a standard correction was applied whereby 1/2t (where t is the number of trials) was added or subtracted. For four out of six of the conditions d' was not normally distributed, therefore Wilcoxon signed-rank tests were used to compare REG versus RAND performance. We first averaged d' across alphabet sizes to test the main effect of regularity (REG vs RAND). As there was a main effect of regularity, we then conducted three pairwise comparisons (Wilcoxon signed-rank) to test whether the effect was present for all alphabet sizes. We were not interested in the effect of alphabet size independent of regularity therefore did not test this as a main effect. p values were adjusted for multiple comparisons using the Holm–Bonferroni method. For experiment 2, no false alarms were made, therefore only HR was computed and analyzed. Because of normality-violating ceiling effects Wilcoxon signed-rank tests were again used to compared REGp versus RAND performance.
Reaction times (RTs) were recorded from each “hit.” For experiment 1, these were analyzed with a repeated measures (RM) ANOVA with factors of regularity (REG vs RAND) and alphabet size (5, 10, 15). For experiment 2, a paired-samples t test was used to contrast RAND and REGp. RTs met the assumptions for parametric tests and α was a priori set to p < 0.05. An additional exploratory RM ANOVA was conducted to compare RTs that occurred early (<4.5 s) or late (>4.5 s) in the trial. Regularity (REG vs RAND) and time (early vs late) were entered as factors. No post hoc tests were run for this analysis.
Behavioral probe (experiment 2 only)
For the two probe tasks, sensitivity scores (d') were computed as described in the previous section. To test whether d' scores were higher in the main group relative to the control group, who were naive to the sequences, an independent samples t test compared group (main vs control) for each probe task. Spearman's correlations were used to test whether performance (d') for the two probes (familiarity vs structure) was correlated across the two tasks. For each probe, exploratory analysis also correlated d' against pupil diameter for each time point in the trial (down-sampled to 20 Hz), using Spearman correlation. We present the correlation coefficient at each time point and indicate time points where p < 0.05, family-wise error (FWE) uncorrected.
Pupillometry data
Trials containing a gap and trials where the participant made a false alarm were excluded from the analysis. Most participants made infrequent false alarms in experiment 1, and only three subjects made more than one false alarm per condition. Between 17 and 21 trials were analyzed per participant per condition [(20–21) for REG5, REG10, REG15; (19–21) for RAND5; (17–21) for RAND10]. There were no false alarms in experiment 2.
Preprocessing
Where possible the left eye was analyzed. To measure the pupil dilation response (PDR) associated with tracking the auditory sequence, the pupil data from each trial were epoched from 1 s before stimulus onset to stimulus offset (9 s postonset).
The data were smoothed with a 150-ms Hanning window and intervals where full or partial eye closure was detected (e.g., during blinks) were treated as missing data and recovered using shape-preserving piecewise cubic interpolation. The blink rate was low overall, with the average blink rate (defined as the proportion of excluded samples because of eye closure) at ∼4% (experiment 1) and 2.6% (experiment 2).
To allow for comparison across trials and subjects, data for each subject in each block were normalized. To do this, the mean and standard deviation across all baseline samples (1 s preonset interval) in that block were calculated and used to z score normalize all data points (all epochs, all conditions) in the block. For each participant, pupil diameter was time-domain averaged across all epochs to produce a single time series per condition.
Time-series statistical analysis of pupil diameter
To identify time intervals where a given pair of conditions, REG5 versus RAND5, REG10 versus RAND10, REG15 versus RAND15 exhibited differences in pupil diameter, a non-parametric bootstrap-based statistical analysis was used (Efron and Tibshirani, 1994). Using the average pupil diameter at each time point, the difference time series between the conditions was computed for each participant and these time series were subjected to bootstrap re-sampling (1000 iterations: with replacement). At each time point, differences were deemed significant if the proportion of bootstrap iterations that fell above or below zero was >95% (i.e., p < 0.05). Any significant differences in the preonset interval would be attributable to noise, therefore the largest number of consecutive significant samples preonset was used as the threshold for the statistical analysis for the entire epoch.
Pupil event rate analysis
In addition to pupil diameter, the incidence of pupil dilation events was also analyzed. Pupil dilation events were defined as instantaneous positive sign-changes of the pupil diameter derivative (i.e., the time points where pupil diameter begins to increase).
This activity was analyzed to focus on phasic pupil activity which has been associated with corresponding phasic activity in the Locus Coeruleus and the release of NE (Joshi et al., 2016; Reimer et al., 2016). Following Joshi et al. (2016) and Zhao et al. (2019b) events were defined as local minima (dilations; PD) with the constraint that continuous dilation is maintained for at least 300 ms. For each condition, each subject, and each trial a causal smoothing kernel ω(τ) = α2 × τ × e-αt was applied with a decay parameter of α = 1/150 ms (Dayan and Abbott, 2001). The mean across trials was computed and baseline corrected. To facilitate the comparison between regular and random sequences, and because pupil dilation events are quite rare (one to two events per second), we collapsed across alphabet size to derive a single mean time series for REG and RAND. To identify periods in which the event rate significantly differed between conditions, a non-parametric bootstrap-based analysis was used. As for the diameter analysis, this involved computation of a difference time series between conditions for each participant, that was then subject to re-sampling with replacement (1000 iterations). At each time point, differences were deemed significant if the proportion of bootstrap iterations that fell above or below zero was >99% (i.e., p < 0.01).
Regressing out behavioral performance
We conducted exploratory analysis to examine whether performance on the incidental gap detection task affected the observed differences in pupil dynamics between REG and RAND patterns. This was achieved by regressing out the variance associated with the gap detection performance from the pupil data. For both experiments each participant's mean RT was used. RT is less limited by ceiling effects and is therefore a good proxy for behavioral difficulty. Sensitivity score (d') was used as a second performance metric for experiment 1. For experiment 2, there were no false alarms and only 5/20 participants were not at ceiling. As a result, it was not appropriate to attempt to model the pupil response to HRs, and only the RT data were analyzed in this way.
Two analysis approaches were taken: the first used average pupil diameter over the latter portion of the trial (4.5–9 s) where robust differences emerged between conditions (see Figs. 3D, 4E). Using mean pupil diameter for this time window as the dependent variable, we conducted a RM analysis of covariance (ANCOVA), with a repeating factor of regularity (REG vs RAND) and the difference (RAND-REG) in RT and d' (experiment 1 only) as covariates. In experiment 1, this analysis was focused on alphabet size 5 (REG5 vs RAND5), as this showed the most robust effect of regularity on the pupil. To increase power, we also combined the datasets from experiments 1 and 2, entering experiment as a between-subjects factor.
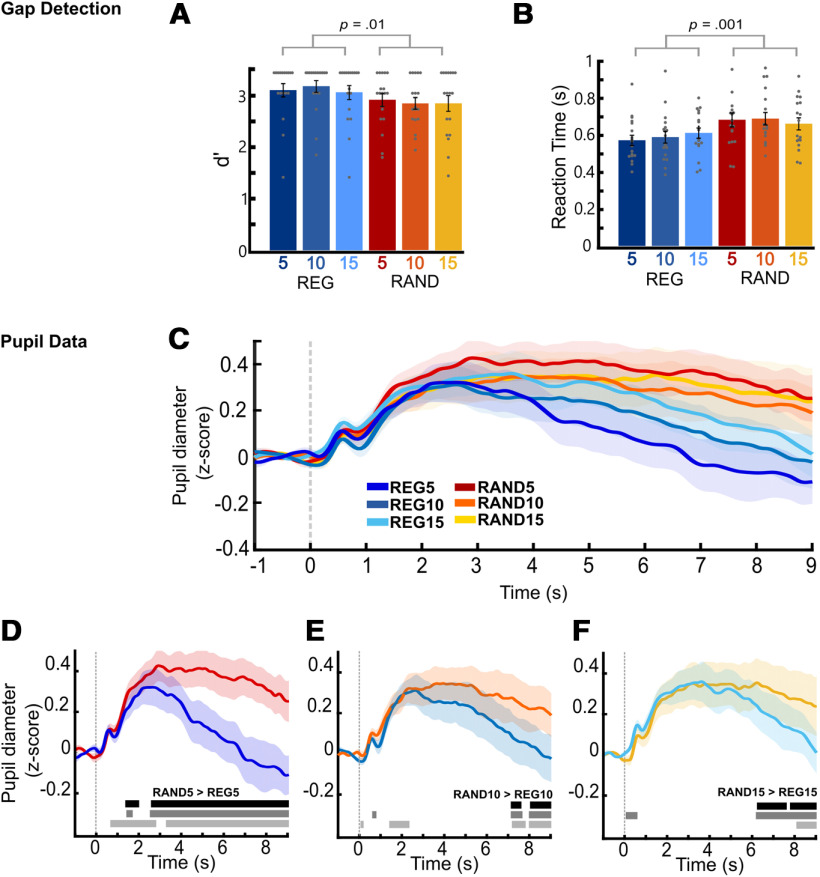
Figure 3.
Experiment 1, regularity modulated pupil size. A, B, The gap detection task showed worse performance for RAND compared with REG sequences. Sensitivity (d') to the gap was significantly higher, and RT shorter for REG relative to RAND sequences. Circles represent individual data points. Error bar shows ±1 SEM. Plots (C–F) show averaged normalized pupil diameter over time, baseline corrected (−1- to 0-s preonset). The shaded area shows ±1 SEM. The horizontal bars show time intervals during which significant differences (bootstrap statistics) were observed. The black bar shows the original results, the gray bars show the significant time intervals after adjusting for the subject-wise difference (RAND-REG) in RT (mid-gray) and d-prime (light-gray). C, Averaged pupil diameter for all conditions. D–F, Average pupil diameters separated by alphabet size 5, 10, and 15 (left to right) showed sustained larger pupil diameters for random conditions (red, orange, and yellow) than regular conditions (shades of blue). D, Alphabet size 5 showed significant differences between REG5 and RAND5 from 2 to 3 s onwards. E, For alphabet size 10, REG10 separates from RAND10 from 3 s onwards with a sustained significant difference from ∼7 to 8 s. F, For alphabet size 15, REG15 separates from RAND15 from 4 s, and is significantly different from 6 s onwards. For E, F, the significant effects at onset are likely artefacts of regressing out the behavioral measures, resulting from low variability between participants at the onset time points.
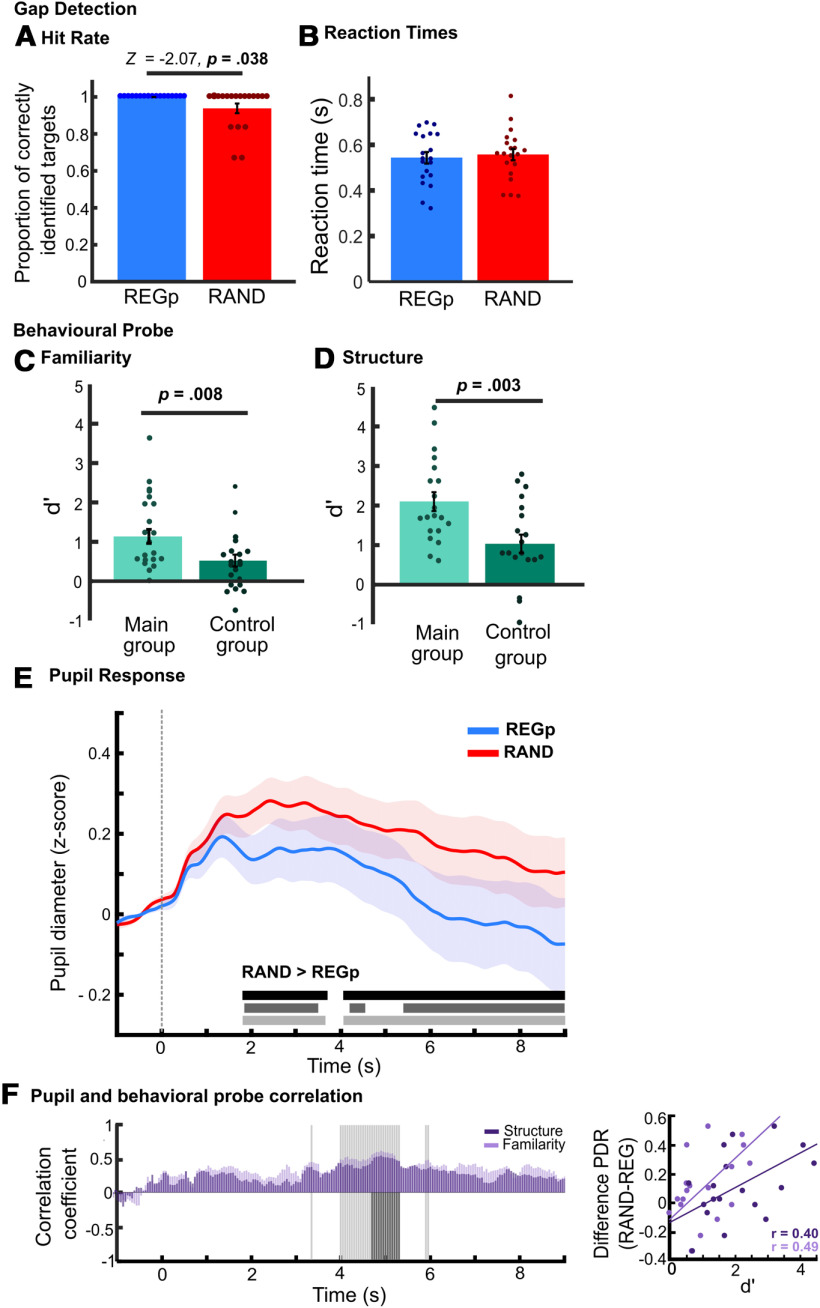
Figure 4.
Experiment 2, probabilistic regularities modulate pupil size. A, HR analysis showed more gaps were detected in REGp (blue) than RAND (red) sequences. There were no false alarms (data not shown). B, RTs for gap detection showed no significant differences. Following the main experiment, two behavioral probes were separately conducted, in one, listeners were asked to judge whether sequences were familiar (C), and in the other whether they contained a structure (D). D prime (d') is plotted for the main group (light green) and a control group who had not conducted the main pupillometry experiment (dark green). Error bars show ±1 SEM, circles show individual subjects. E, Average normalized pupil diameter over time, baseline corrected (−1- to 0-s preonset). The shaded area shows ±1 SEM. The horizontal bars show time intervals during which significant differences (bootstrap statistics) were observed. The black bar shows the original results, the dark gray bar shows significant time intervals when the five participants with below ceiling performance were removed from the analysis (see Materials and Methods), the light gray bar shows the significant time intervals after adjusting for the subject-wise difference (RAND-REGp) in RT. In all cases, the difference between RAND and REG persists suggesting that the main effects are not driven by effort toward the gap detection task (F) Spearman correlation between the difference in pupil diameter (RAND – REGp) and d' from the familiarity probe (light purple) and structure probe (dark purple) conducted sample-by-sample (20 Hz) over the entire trial duration. Each purple bar shows the Spearman correlation coefficients at each time point for the two probe tasks. Gray shaded areas indicate time intervals where a significant correlation (p < 0.05; FWE uncorrected) was observed, light gray corresponds to the correlation with the familiarity probe, significant periods for the structure probe are in dark gray and plotted only on the lower part of the y-axis. For the gray bars, the relationship to the y-axis is for visualization purposes and not meaningful. The plot on the right illustrates the link between pupil size and subsequently assessed sensitivity to regularity by displaying the correlation (Spearman r) between pupil size differences (averaged across 4–6 s) and individual familiarity (light purple) and structure judgments (dark purple).
The second approach involved regressing out the variance related to the behavioral measures from the unfolding pupil diameter data. For each subject, sample-by-sample differences in pupil diameter (RAND-REG) were regressed onto behavioral performance (difference in RT or d' between RAND and REG) to remove variance attributable to this potentially confounding factor. The residual pupil data were then analyzed as described above, Time-series statistical analysis of pupil diameter. This analysis was conducted on all conditions (REG5/RAND5; REG10/RAND10; REG15/RAND15; REGp/RAND in experiment 2). Because extreme values can skew the regression, the behavioral data were checked for outliers and one participant was removed from the regression analysis with d' for REG15/RAND15.
Results
Experiment 1, deterministic regularities
This experiment used sequences of tone pips that were either regularly repeating (REG) or random (RAND; Fig. 1). Previous work showed that brain responses, even from naive listeners, rapidly distinguished regular from random patterns. The differences emerged as early as 400 ms for REG5, 700 ms for REG10, and 1050 ms for REG15, consistent with the prediction of an ideal observer model which indicated that the emergence of regularity should be detectable from roughly one cycle and four tones after the introduction of the regular pattern (for details see Barascud et al., 2016; Southwell et al., 2017). Using the same regular sequence structure, we compared the pupil response to regular (REG), highly predictable deterministic sequences to matched random (RAND) sequences of the same alphabet size.
Two factors were manipulated: (1) whether the sequence contained a repeating pattern (REG vs RAND); (2) the alphabet size (5, 10, or 15), reflecting the number of different tones in the sequence, and thus its complexity in terms of draw on memory and other perceptual resources.
Gap detection results
Sensitivity to the presence of gaps was analyzed using d' (Fig. 3A). However overall performance was high, with HR close to ceiling: (median HR: REG5 = 1; REG10 = 1; REG15 = 1; RAND5 = 0.86; RAND10 = 0.86; RAND15 = 0.86) and false alarm rates close to floor (median all conditions = 0). Parametric tests could not be conducted on d' because of normality violations, therefore, d' was initially averaged across alphabet sizes for REG and RAND and compared using a Wilcoxon signed-rank test. This confirmed that d' was significantly higher for REG (mean = 3.12, SD = 0.50) than RAND (mean = 2.87, SD = 0.48, Z = 2.564, p = 0.010; Fig. 3A). Pairwise Wilcoxon signed-rank tests for each alphabet size (Holm–Bonferroni correction was applied) indicated that the effect may be driven by alphabet size 10, as there was a significant difference between REG10 and RAND10 (Z = 2.836 p = 0.02) but no significant difference between REG5 and RAND5 (Z = 1.536, p = 0.25) or REG15 versus RAND15 (Z = 1.26, p = 0.25).
For RTs (Fig. 3B), a RM ANOVA with two factors, regularity (REG vs RAND) and alphabet size (5, 10, 15) revealed a main effect of regularity, with significantly faster response times in REG (mean = 0.590 s, SEM = 0.027) compared with RAND (mean = 0.677 s, SEM = 0.031), F(1,17) = 41, p < 0.001, ηp2 = 0.71. There was no main effect of alphabet size F(2,34) = 0.263, p = 0.771, ηp2 = 0.015, and no interaction F(2,34) = 1.786, p = 0.183, ηp2 = 0.095.
As an exploratory analysis, we tested whether RTs varied based on the timing of the gap relative to the sequence onset. As will be demonstrated in the next section, the pupil response to regular sequences emerged later in the trial, particularly for larger alphabet sizes. As we show above, RTs were faster for REG sequences, therefore we questioned whether there were faster RTs in the latter portion of the trial in the REG condition that were driving both the behavioral effects and pupil response. As each condition only provided six target trials, and faster RTs and smaller pupil sizes were observed for all regular conditions, we collapsed across alphabet sizes and calculated the average RT for gaps that occurred earlier (<4.5 s postsound onset) versus later in the trial (>4.5 s posttrial onset). An RM-ANOVA was conducted with repeating factor of Time (early vs late) and Regularity (REG vs RAND). RTs showed a clear effect of regularity (F(1,17) = 29.198, p = <0.001, ηp2 = 0.632) but no effect of time (F(1,17) = 1.006, p = 0.316, ηp2 = 0.059) and no interaction (F(1,17) = 0.009, p = 0.925, ηp2 = 0.001).
Sustained pupil dilation is modulated by sequence predictability
Figure 3C plots the average pupil diameter (relative to the preonset baseline) as a function of time. All six conditions share a similar PDR pattern. Immediately after scene onset (t = 0), the pupil diameter rapidly increased, forming an initial peak at ∼0.6 s. Over the next second, pupil diameter slowly increased again to reach a broader peak around ∼3 s after onset. Thereafter, the response entered a sustained phase, which lasted until sequence offset and was associated with a slow continuous decrease in pupil diameter.
Regular sequences elicited a smaller pupil diameter than random sequences, for all alphabet sizes. As can be seen in Figure 3, the REG conditions were associated with a faster decrease in pupil diameter (steeper reduction in the sustained response) than the RAND conditions and this effect was modulated by alphabet size. The comparison across matched REG and RAND pairs (Fig. 3D–F) revealed that the separation between traces occurred substantially earlier for alphabet size 5 (Fig. 3D), where a divergence was observed from ∼1.5 s after onset, than the other two conditions. The average trace for REG diverged from RAND at ∼3 s for REG10 and ∼4.5 s for REG15 (Fig. 3E,F) and became statistically significant later in the trial (>6 s). The staggered divergence is consistent with larger alphabet sizes (i.e., longer REG cycles) requiring more time before a regularity can be established. A similar pattern of divergence latencies has been observed in the brain (Barascud et al., 2016; Southwell et al., 2017), albeit on a faster timescale.
The significant difference between conditions emerged surprisingly late for alphabet size 10, although the conditions separated much earlier. It is likely that a combination of noise and a weaker signal impacted the results for this condition.
Experiment 2, probabilistic regularities
Experiment 2 investigated whether the effects observed in experiment 1 extend to sequences that contain probabilistic rather than deterministic structure. Toward this aim, we focused on a structure that has been extensively used to study statistical learning in the context of language. Saffran et al. (1996) tested whether infants could segment a continuous stream of syllables based only on the statistical regularities between successive items. The streams of syllables had high TPs within “words” consisting of triplets of syllables, and low TPs at word boundaries. Infants were found to spend longer looking at non-words that breached the word boundaries, suggesting they had become sensitive to the distributional cues of the syllable stream. Forms of the paradigm have since been used in behavioral and neuroimaging studies (Batterink and Paller, 2017; Farthouat et al., 2017), in adults (Saffran et al., 1997), infants (Saffran, 2020), and other species (Hauser et al., 2001; Toro and Trobalón, 2005) using a variety of stimuli (Saffran et al., 1999; Kirkham et al., 2002). The current experiment uses the pure tone version of this segmentation paradigm (Saffran et al., 1999), with a key modification. The original study used a tone length of 333 ms to model the length of syllables, in contrast we use 50-ms tones to study this structure at a rate comparable with the sequences in experiment 1.
To generate the underlying probabilistic structure, twelve different tones were arranged into four tone “words” (see Materials and Methods). Following Saffran et al. (1999) the same tone “words” were used for each subject. Probabilistic regular sequences (REGp; 9 s long), generated anew for each trial, were created by randomly ordering the four tone words, with the stipulation that the same tone word could not occur twice in a row (i.e., tone words always transitioned to a different tone word). This created a probabilistic structure where the TP between tones within a word was 1 and the TP at word boundaries was 0.33, see Figure 2 for more details. RAND sequences were generated in the same way as for experiment 1, but using the pool of 12 frequencies from which the tone “words” were created.
The experimental session consisted of three phases. First, participants were familiarized with the REGp sequences. Subsequently, pupil responses were recorded as they listened to REGp or RAND sequences. A gap detection task was used to ensure that participants focused their attention on the sound stream. In a final phase, the same subjects and a control group were asked to make decisions about the familiarity and underlying structure of the different sequence types.
Gap detection results
No false alarms were made but there were significantly more gaps detected in REGp compared with the RAND (Wilcoxon signed-ranks test: Z = 2.07, p = 0.038; Fig. 4A). RTs showed no significant difference between conditions (paired samples t test, t(19) = –0.772, p = 0.450, d = –0.173; Fig. 4B). Therefore, although the effects are weak and most participants performed at ceiling, the gap detection data demonstrate, similar to experiment 1, that performance was facilitated in REGp relative to RAND sequences.
Exposure to REGp sequences improved subsequent sensitivity to structure
Following the main pupillometry task, participants completed two further tasks, in the first identifying whether a 3-s-long sequence was familiar and in the second identifying whether the sequence had a “structure” (see Materials and Methods). These tasks were also completed by a control group who had not participated in the previous phases. The results are shown in Figure 4C,D. In both tasks, the majority of participants in the control group showed d' > 0. This indicates that for some listeners 3 s (60 tones) of exposure to the sequence were sufficient to detect a structure, which the listener then interpreted as feeling familiar. This is in line with previous statistical learning paradigms that show a “familiarity” decision can reflect implicit sequence learning (Forkstam et al., 2008). However, sensitivity in the control group still remained low (d' < 1) suggesting poor sensitivity overall. Importantly, as expected, the main group showed significantly higher sensitivity than the control group in both tasks (independent samples t test, familiarity: t(38) = 2.8, p = 0.008; structure: t(38) = 3.2, p = 0.003), demonstrating that previous exposure improved sensitivity. Unsurprisingly, performance across the familiarity and structure tasks was correlated for the main (Spearman's ρ = 0.797, p < 0.001) and the control group (Spearman's ρ = 0.570, p = 0.009), confirming that both tasks probed sequence learning.
Sustained pupil dilation is modulated by sequence predictability
Figure 4E shows the normalized pupil diameter to REGp (blue) and RAND (red) sequences. As in experiment 1, both conditions showed an increase in diameter after sound onset, followed by a sharp decrease in pupil diameter for REGp but not RAND. Since listeners were preexposed to the regular stimuli, we expected that the pupil response to the REGp condition should rapidly diverge from RAND, as soon as it is statistically possible to differentiate the two sequences (i.e., within two to three “words” after sequence onset). Indeed, a sustained difference between conditions emerged from ∼2 s poststimulus onset, roughly at the same time as that observed for REG5 (repeating cycle of five tones) in experiment 1. We interpret that as indicating that REGp was differentiated from RAND at a similar latency as REG5 (approximately nine tones; see Barascud et al., 2016; Southwell et al., 2017). Although, relative to the neural effects, the pupil response to regularity exhibits a delay linked to slower modulatory pathway effects (i.e., the time it takes for the signal to travel from the cortical network which tracks the regularity, to the LC and from there to the pupil musculature). However, the extent of divergence between REGp and RAND was smaller than that observed for REG5 (compare Figs. 4E and 3D), this was also expected as the probabilistic structure in experiment 2 (Fig. 2D) retains some degree of unpredictability, i.e., at tone word boundaries. In contrast, REG5 can be predicted with 100% certainty once the tone order has been established.
This results pattern was maintained when the five participants who performed below ceiling were excluded from the analysis (Fig. 4E, dark gray shading).
Pupil size correlates with (subsequently obtained) explicit identification of structure
An exploratory analysis was conducted into the relationship between pupil dynamics and sensitivity to sequence structure. We correlated the instantaneous PDR difference between REGp and RAND at every time sample (20 Hz), with the d' for each participant (separately for the familiarity and structure tasks). For this analysis we re-ran the preprocessing to remove blinks without subsequent interpolation to ensure the accuracy of the point-by-point correlations.
As shown above, performance on the two probe tasks was highly correlated, therefore we expected the two measures to have a similar relationship to pupil diameter. In Figure 4F, correlation coefficients (Spearman) are plotted in dark purple (correlation with structure probe) and light purple (correlation with familiarity probe) significant time samples (FWE uncorrected) are marked in gray (light gray = familiarity, dark gray = structure). Significant correlations are observed partway through the epoch, between ∼4 and 6 s after onset, revealing that those participants who later indicated high sensitivity to sequence structure were also those exhibiting a larger PDR regularity effect. That correlations appear to be confined to this interval may be because of the fact that the PDR regularity effect stabilizes around that time. The disappearance of correlations toward the end of the trial is consistent with previous observations (Zhao et al., 2019a) and may be because the expectation of trial offset affects pupil dynamics in a manner that interferes with the correlation with behavior.
Pupil dilation rate is not modulated by predictability
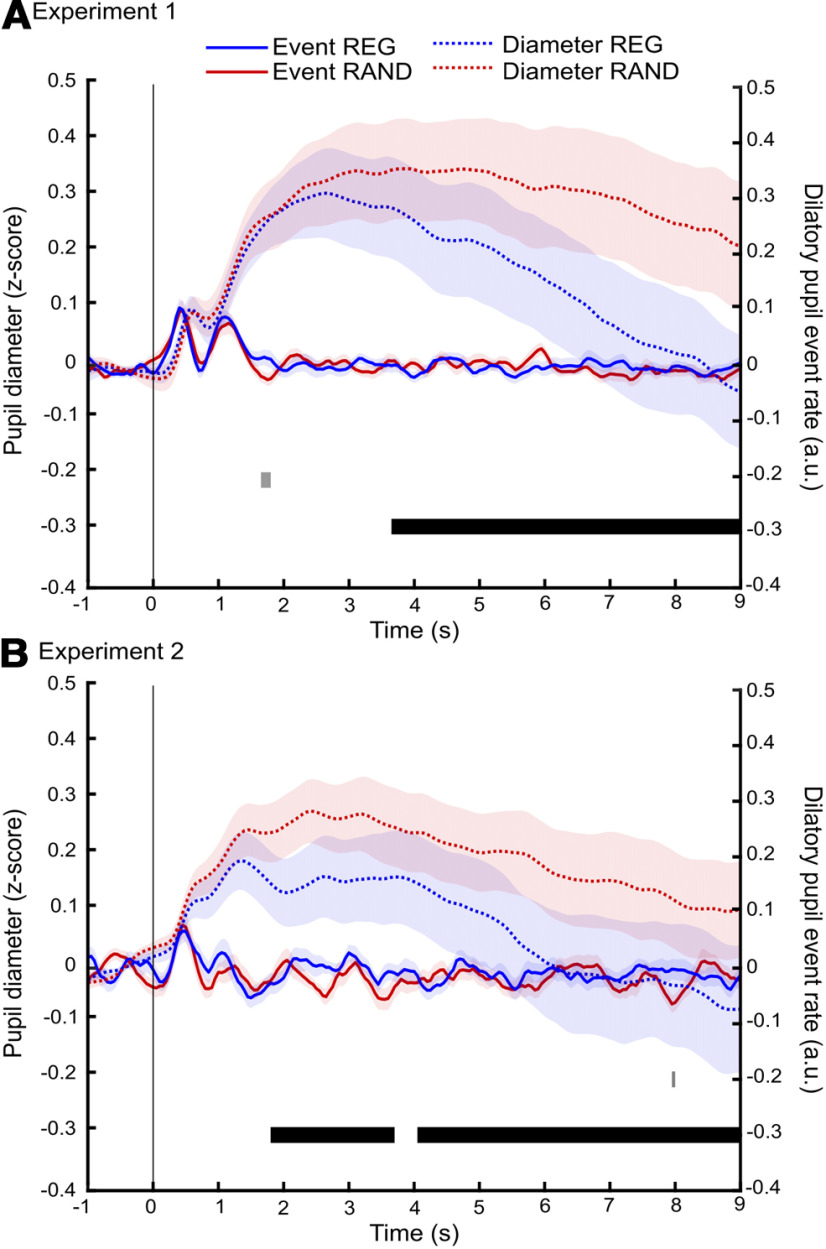
Event rate (instantaneous positive sign-changes of the pupil diameter derivative) was analyzed to focus on phasic pupil activity which has been associated with corresponding phasic activity in the locus coeruleus and the release of NE (Joshi et al., 2016; Reimer et al., 2016). To determine whether the observed pupil response is driven by tonic (sustained) or phasic changes in pupil dynamics, we also analyzed the pupil dilation event rate over the course of the trial (see Materials and Methods). Figure 5 plots both the event rate (solid lines) and dilation response (dotted line) to show how the two measures evolve over time for experiment 1 (top panel) and experiment 2 (bottom panel). To improve power in experiment 1, we collapsed across alphabet size, providing a single time series for REG and RAND.
Figure 5.
Sequence regularity was not associated with differences in incidence of dilatory pupil events. Experiment 1 (A) and experiment 2 (B). Solid lines show pupil dilation event rate. Events were defined as the onset of each pupil dilation with a duration of at least 300 ms. These were collapsed across alphabet sizes for REG (blue) and RAND (red). Gray markers at the bottom of the graph indicate time intervals where bootstrap statistics showed a significant difference between the two conditions. Dotted lines show the pupil diameter REG (blue) and RAND (red) collapsed across alphabet size. Shading indicates ± 1 SEM. The black bar indicates intervals where bootstrap statistics showed a significant difference between the two conditions. Only the pupil diameter data showed a sustained difference between REG and RAND conditions.
For both experiments the dilation event rate data revealed a series of onset peaks, followed by a return to baseline, with no substantial difference between REG and RAND conditions, in contrast to the robust difference observed for pupil diameter. This suggests that the difference in pupil dynamics between REG and RAND signals is driven largely by tonic rather than phasic pupil activity.
Behavioral performance is not driving the pupil effects
Both experiments used a gap detection task to ensure that listeners focused their attention on the tone sequence. The task was deliberately easy so as to reduce possible effects of task difficulty on pupil data. However, at the group level regularity was found to modulate performance, increasing sensitivity to gaps (Figs. 3A, 4A) and reducing RT (experiment 1 only; Fig. 3B). We therefore conducted additional analyses to confirm that the regularity-linked difference in pupil diameter persists after the variance associated with gap detection performance is regressed out.
Regressing out behavioral performance
Two approaches were taken to regress out performance on the gap detection task. First, pupil diameter was averaged over the latter portion of the trial (4.5–9 s) where robust differences emerged between conditions (see Figs. 3D–F, 4E). A RM ANCOVA was conducted on pupil size, with a repeating factor of regularity (REG vs RAND) and the difference (RAND – REG) in RT and dprime (d'; experiment 1 only) as covariates. This analysis on experiment 1 data confirmed that the effect of regularity remained significant, F = 7.307, df = 1,15, p = 0.016, ηp2 = 0.328, with no interaction with either covariate, regularity × RT: F(1,15) = 1.635, p = 0.220, ηp2 = 0.098; regularity versus d': F(1,15) = 0.001, p = 0.977, ηp2 = 0. For experiment 2, the ANCOVA could only be conducted with RT as a covariate (see Materials and Methods). Results confirmed that the effect of regularity persisted: F(1,18) = 0.4.983, p = 0.039, ηp2 = 0.217 and there was no interaction between regularity and RT: F(1,18) = 0.069, p = 0.796, ηp2 = 004. As a further analysis we also collapsed the data across experiment 1 (REG5/RAN5) and experiment 2. As detailed in the previous sections these data yielded similar behavioral effects and pupil dynamics. The ANCOVA confirmed a robust effect of regularity: F(1,35) = 15.347, p < 0.001, ηp2 = 0.968 and no interaction between regularity and RT or experiment (ps > 0.2).
A second approach was based on a point-by-point regression analysis. We focused on the subject-wise point-by-point pupil diameter difference between conditions (RAND-REG) and regressed out the behavioral difference between conditions, this was done separately for RT and performance. For experiment 2, HR could not be regressed out because of ceiling effects (see Materials and Methods), we therefore focus on RT only.
Statistical analysis (see Materials and Methods) was then conducted on the resulting time series. The results are plotted in Figures 3D–F, 4E, gray horizontal bars, and demonstrate that the main effects of regularity remain after the variance associated with the behavioral measures has been removed.
This experiment was designed to involve a task that ensured the tone sequences were behaviorally relevant. Therefore, there is likely to be a degree of shared variability between performance on the gap detection task and the pupil response to regularity. However, the demonstration that the pupil effects remain after accounting for task performance suggests that effort toward the gap detection task is not driving the pupil effects.
Discussion
Over two experiments, we show that pupil diameter is modulated by the statistical structure of rapidly unfolding auditory stimuli, be they deterministic structures that developed anew on each trial, or more complex statistical structures to which the listener had been preexposed. In line with our prediction, we consistently observed a smaller sustained pupil diameter to regular compared with random sequences.
The pupil effects were not correlated with incidental task performance but did reveal a link with subsequently administered familiarity and structure judgements. This demonstrates that pupil dynamics were driven by sequence structure per se, and its draw on processing resources, rather than just effort to perform the incidental task.
Predictability of deterministic sequences modulates sustained pupil size
Previous work has studied pupil responses to deviant stimuli embedded in a predictable structure (Liao et al., 2016; Marois et al., 2018; Quirins et al., 2018; Bianco et al., 2020). Zhao et al. (2019b) showed a transient pupil dilation in response to an unexpected transition from a regular to random pattern. Quirins et al. (2018) used a local-global paradigm, also with rapid tone pips. They found that a deviation from the global but not local structure elicited an increase in pupil diameter, but only when actively attending to the deviants, and only in subjects who subsequently showed an awareness of the global regularity. In contrast, the current study examined the dynamics of the pupil response to ongoing regularity.
Participants performed a task that ensured they were broadly attending to the sound sequences. By manipulating the predictability of the tone pip patterns, we were able to assess the extent to which the processing of each sequence type affects pupil-linked arousal.
Based on previous work that demonstrated increased pupil diameter to salient or behaviorally engaging stimuli (Nieuwenhuis et al., 2011; Wang and Munoz, 2015; Liao et al., 2016), we hypothesized that a larger pupil size in response to regular sequences would indicate that attentional resources were engaged to a greater degree by regular relative to random patterns (Zhao et al., 2013). Conversely, a reduction in pupil diameter would indicate that regularity reduces the draw on processing resources by facilitating sequence processing (Southwell et al., 2017). In both experiments reported here, pupil diameter rapidly decreased once the brain had established the predictable structure of the tone pip sequence, thus supporting the latter hypothesis. In contrast, matched randomly ordered sequences were associated with a largely sustained pupil diameter, suggesting that processing of these stimuli remained more resource demanding.
For highly predictable, deterministic sequences (experiment 1), the pupil response showed a rapid divergence between regular and random sequences, reflecting the quick detection of the regular structure. The emergence of regularity was associated with a sustained decrease in pupil size, relative to that evoked by sequences of the same tones presented in a random order. The effect was modulated by alphabet size, with the simplest regular sequences (REG5) showing the more rapid change in pupil diameter.
The pupil response to regularity was consistent with previous neuroimaging work that revealed a rapid change in neural activity following the emergence of regularity (Barascud et al., 2016; Southwell et al., 2017; Herrmann and Johnsrude, 2018). However, the effects seen here arose substantially later than those observed in the brain responses, consistent with a slower pathway (i.e., delays incurred between the cortical network that detected the regularity and the pupil). The mechanisms driving the neural response to regularity are poorly understood, but emerging work (Barascud et al., 2016; Auksztulewicz et al., 2017) has implicated an interplay between auditory cortical, inferior frontal and hippocampal sources in the discovery of regularity. A similar network has also been implicated in detecting more complex predictable structure (for a summary, see Milne et al., 2018; and also Abla and Okanoya, 2008; Schapiro et al., 2012; Ordin et al., 2020).
Probabilistic sequence structure modulates pupil size
A clear difference between REGp and RAND conditions was also observed for sequences comprised of probabilistic transitions (Saffran et al., 1996, 1999). The relationships between items in the sequence transform it from a stream of individual elements to a series of larger integrated items, in this case triplets of elements, some argue this perceptual shift is a critical component of statistical learning (Batterink and Paller, 2017).
Exploiting this feature of statistical learning, Batterink and Paller (2017) found that as listeners became exposed to the statistical structure they exhibited neural entrainment to not only the rate of individual syllables but also the “words” that were generated using TPs (also, for a similar study, see Farthouat et al., 2017). Furthermore, there was a correlation between entrainment to the words and RT to targets that could be predicted by the structure, supporting a relationship between neural signatures of sequence learning and the influence of sequence learning on subsequent behavior.
To our knowledge, the present study is the first to apply this extensively used probabilistic paradigm to rapid sequences. Our demonstration that the probabilistically structured sequences are associated with reduced pupil size relative to matched random sequences reveals that the statistical structure of these rapidly unfolding sequences was discovered by listeners and facilitated more efficient processing of the regular patterns.
Critically, similar to Batterink and Paller (2017), we also observed a correlation between modulation of pupil size by sequence type and offline sequence classification (familiarity/structural judgment made after pupillometry measurements), suggesting a relationship between the pupil response to the unfolding sequence and the acquired statistical knowledge; those listeners who showed a larger pupil response difference between REGp and RAND patterns were also those who were better at subsequently discriminating statistically structured from random sequences.
Predictability modulates tonic rather than phasic pupil activity
Phasic pupil responses (pupil dilation events) have been linked with phasic firing in the LC-NE system (Joshi et al., 2016) and hypothesized to reflect activation of the arousal system. In contrast, slow (tonic) modulation of pupil diameter has been linked to states of perceptual uncertainty (Nassar et al., 2012; Krishnamurthy et al., 2017) and increased demand on processing resources (Sarter et al., 2006). Here, the analysis of pupil dilation event rate demonstrated no difference between conditions, suggesting that the observed pupil effects arise from tonic rather than phasic pupil dynamics.
Krishnamurthy et al. (2017) created sequences of sounds played from different locations and asked listeners to make decisions about the locations of upcoming sounds. Over the course of the experiment, they manipulated how well the previous sounds could be used to predict the location of an upcoming sound. Where prior information was reliable, the upcoming sound could be accurately predicted. Analysis of baseline pupil dilation, before decision-making, showed smaller tonic pupil sizes when there were more reliable priors. In other words, as with our data, more predictable stimuli were associated with smaller pupil diameters. Unlike these studies (Nassar et al., 2012; Krishnamurthy et al., 2017), the present results demonstrate sustained changes without perceptual judgements related to stimulus likelihood, and with sequences that were too fast for conscious tracking of predictability.
While it may be premature to discuss the underlying brain machinery, the basal forebrain (BF)-ACh system (Joshi and Gold, 2020) could be hypothesized as a possible underpinning for the observed effects. The BF has extensive projections in the brain, including to auditory cortex (Guo et al., 2019). Cholinergic signaling has been implicated in the representation of sensory signal volatility (Marshall et al., 2016), and in supporting the rapid learning of environmental contingencies, for example, by boosting bottom-up sensory processing (Yu and Dayan, 2005; Bentley et al., 2011). In the current paradigm, the rapid decrease in pupil size during predictable sequences is consistent with a reduction in ACh-driven learning once the sequence structure has been established. A related but mechanistically different proposal is that lower levels of ACh for predictable sequences reflect a decrease in processing demands (Witte et al., 1997; Phillips et al., 2000; Sarter et al., 2006). For REG relative to RAND sequences there is a streamlining of processing that is possible when upcoming tones can be accurately predicted. This contrasts with unpredictable sequences (RAND) where learning cannot take place and thus the resources required to process upcoming tones will remain high.
In conclusion, we demonstrate that sustained changes in pupil size can be used to identify the emergence of regularity in rapid auditory tone sequences. The results were robust even with a small number of trials (<25 per condition) and consistent across both deterministic and probabilistic sequences. Furthermore, the effects persisted after regressing out performance on the incidental task, although future studies may wish to further probe the interactions between the pupil, regularity, and task-related effort. Finally, the speed of sequences used in this paradigm prevented conscious sequence structure tracking, and the task did not require decision-making or analysis of the sequence structure. As a result, our findings establish pupillometry as an effective, non-invasive, and fast method to study the automatic extraction of different types of regularities across different populations and even different species.
Footnotes
This work was supported by the Wellcome Trust Grant 213686/Z/18/Z (to A.E.M.), the Biotechnology and Biological Sciences Research Council Grant BB/P003745/1 (to M.C.), and the National Institute for Health Research University College London Hospitals Biomedical Research Centre Deafness and Hearing Problems Theme.
The authors declare no competing financial interests.
References
- Abla D, Okanoya K (2008) Statistical segmentation of tone sequences activates the left inferior frontal cortex: a near-infrared spectroscopy study. Neuropsychologia 46:2787–2795. 10.1016/j.neuropsychologia.2008.05.012 [DOI] [PubMed] [Google Scholar]
- Alamia A, Zénon A (2016) Statistical regularities attract attention when task-relevant. Front Hum Neurosci 10:42. 10.3389/fnhum.2016.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou LV, Kashino M, Chait M (2011) The role of temporal regularity in auditory segregation. Hear Res 280:228–235. 10.1016/j.heares.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Auksztulewicz R, Barascud N, Cooray G, Nobre AC, Chait M, Friston K (2017) The cumulative effects of predictability on synaptic gain in the auditory processing stream. J Neurosci 37:6751–6760. 10.1523/JNEUROSCI.0291-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barascud N, Pearce MT, Griffiths TD, Friston KJ, Chait M (2016) Brain responses in humans reveal ideal observer-like sensitivity to complex acoustic patterns. Proc Natl Acad Sci USA 113:E616–E625. 10.1073/pnas.1508523113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink LJ, Paller KA (2017) Online neural monitoring of statistical learning. Cortex 90:31–45. 10.1016/j.cortex.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendixen A (2014) Predictability effects in auditory scene analysis: a review. Front Neurosci 8:60. 10.3389/fnins.2014.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ (2011) Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog Neurobiol 94:360–388. 10.1016/j.pneurobio.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco R, Ptasczynski LE, Omigie D (2020) Pupil responses to pitch deviants reflect predictability of melodic sequences. Brain Cogn 138:103621. 10.1016/j.bandc.2019.103621 [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997) The psychophysics toolbox. Spat Vis 10:433–436. [PubMed] [Google Scholar]
- Conway CM (2020) How does the brain learn environmental structure? Ten core principles for understanding the neurocognitive mechanisms of statistical learning. Neurosci Biobehav Rev 112:279–299. 10.1016/j.neubiorev.2020.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Abbott L (2001) Theoretical neuroscience: computational and mathematical modeling of neural system. Cambridge: The MIT Press. [Google Scholar]
- de Lange FP, Heilbron M, Kok P (2018) How do expectations shape perception? Trends Cogn Sci 22:764–779. 10.1016/j.tics.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ (1994) An introduction to the bootstrap. New York:CRC press. [Google Scholar]
- Erickson LC, Thiessen ED (2015) Statistical learning of language: theory, validity, and predictions of a statistical learning account of language acquisition. Dev Rev 37:66–108. 10.1016/j.dr.2015.05.002 [DOI] [Google Scholar]
- Farthouat J, Franco A, Mary A, Delpouve J, Wens V, op de Beeck M, de Tiège X, Peigneux P (2017) Auditory magnetoencephalographic frequency-tagged responses mirror the ongoing segmentation processes underlying statistical learning. Brain Topogr 30:220–232. 10.1007/s10548-016-0518-y [DOI] [PubMed] [Google Scholar]
- Feldman H, Friston KJ (2010) Attention, uncertainty, and free-energy. Front Hum Neurosci 4:215. 10.3389/fnhum.2010.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkstam C, Elwér Å, Ingvar M, Petersson KM (2008) Instruction effects in implicit artificial grammar learning: a preference for grammaticality. Brain Res 1221:80–92. 10.1016/j.brainres.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Guo W, Robert B, Polley DB (2019) The cholinergic basal forebrain links auditory stimuli with delayed reinforcement to support learning. Neuron 103:1164–1177.e6. 10.1016/j.neuron.2019.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MD, Newport EL, Aslin RN (2001) Segmentation of the speech stream in a non-human primate: statistical learning in cotton-top tamarins. Cognition 78:B53–B64. 10.1016/S0010-0277(00)00132-3 [DOI] [PubMed] [Google Scholar]
- Heilbron M, Chait M (2018) Great expectations: is there evidence for predictive coding in auditory cortex? Neuroscience 389:54–73. 10.1016/j.neuroscience.2017.07.061 [DOI] [PubMed] [Google Scholar]
- Herrmann B, Johnsrude IS (2018) Neural signatures of the processing of temporal patterns in sound. J Neurosci 38:5466–5477. 10.1523/JNEUROSCI.0346-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Baldi P (2009) Bayesian surprise attracts human attention. Vision Res 49:1295–1306. 10.1016/j.visres.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Gold JI (2020) Pupil size as a window on neural substrates of cognition. Trends Cogn Sci 24:466–480. 10.1016/j.tics.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, Gold JI (2016) Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89:221–234. 10.1016/j.neuron.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham NZ, Slemmer JA, Johnson SP (2002) Visual statistical learning in infancy: evidence for a domain general learning mechanism. Cognition 83:B35–B42. 10.1016/S0010-0277(02)00004-5 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy K, Nassar MR, Sarode S, Gold JI (2017) Arousal-related adjustments of perceptual biases optimize perception in dynamic environments. Nat Hum Behav 1:0107. 10.1038/s41562-017-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Waters J (2018) Neuromodulatory correlates of pupil dilation. Front Neural Circuits 12:21. 10.3389/fncir.2018.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HI, Yoneya M, Kidani S, Kashino M, Furukawa S (2016) Human pupillary dilation response to deviant auditory stimuli: effects of stimulus properties and voluntary attention. Front Neurosci 10:43. 10.3389/fnins.2016.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ (1975) A theory of attention: variations in the associability of stimuli with reinforcement. Psychol Rev 82:276–298. 10.1037/h0076778 [DOI] [Google Scholar]
- Makov S, Zion Golumbic E (2020) Irrelevant predictions: distractor rhythmicity modulates neural encoding in auditory cortex. Cereb Cortex 30:5792–5714. 10.1093/cercor/bhaa153 [DOI] [PubMed] [Google Scholar]
- Marois A, Labonté K, Parent M, Vachon F (2018) Eyes have ears: indexing the orienting response to sound using pupillometry. Int J Psychophysiol 123:152–162. 10.1016/j.ijpsycho.2017.09.016 [DOI] [PubMed] [Google Scholar]
- Marshall L, Mathys C, Ruge D, de Berker AO, Dayan P, Stephan KE, Bestmann S (2016) Pharmacological fingerprints of contextual uncertainty. PLoS Biol 14:e1002575. 10.1371/journal.pbio.1002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne AE, Wilson B, Christiansen MH (2018) Structured sequence learning across sensory modalities in humans and nonhuman primates. Curr Opin Behav Sci 21:39–48. 10.1016/j.cobeha.2017.11.016 [DOI] [Google Scholar]
- Nassar MR, Rumsey KM, Wilson RC, Parikh K, Heasly B, Gold JI (2012) Rational regulation of learning dynamics by pupil-linked arousal systems. Nat Neurosci 15:1040–1046. 10.1038/nn.3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, de Geus EJ, Aston-Jones G (2011) The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology 48:162–175. 10.1111/j.1469-8986.2010.01057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin M, Polyanskaya L, Soto D (2020) Neural bases of learning and recognition of statistical regularities. Ann NY Acad Sci 1467:60–76. 10.1111/nyas.14299 [DOI] [PubMed] [Google Scholar]
- Pearce MT, Müllensiefen D, Wiggins GA (2010) The role of expectation and probabilistic learning in auditory boundary perception: a model comparison. Perception 39:1365–1389. 10.1068/p6507 [DOI] [PubMed] [Google Scholar]
- Phillips JM, McAlonan K, Robb WGK, Brown VJ (2000) Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology (Berl) 150:112–116. 10.1007/s002130000437 [DOI] [PubMed] [Google Scholar]
- Quirins M, Marois C, Valente M, Seassau M, Weiss N, el Karoui I, Hochmann J-R, Naccache L (2018) Conscious processing of auditory regularities induces a pupil dilation. Sci Rep 8:14819. 10.1038/s41598-018-33202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, Tolias AS (2016) Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun 7:13289. 10.1038/ncomms13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Ekman M, FX, de Lange P (2018) Suppressed sensory response to predictable object stimuli throughout the ventral visual stream. J Neurosci 38:7452–7461. 10.1523/JNEUROSCI.3421-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohenkohl G, Cravo AM, Wyart V, Nobre AC (2012) Temporal expectation improves the quality of sensory information. J Neurosci 32:8424–8428. 10.1523/JNEUROSCI.0804-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran JR (2020) Statistical language learning in infancy. Child Dev Perspect 14:49–54. 10.1111/cdep.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, Newport EL (1996) Statistical learning by 8-month-old infants. Science 274:1926–1928. 10.1126/science.274.5294.1926 [DOI] [PubMed] [Google Scholar]
- Saffran JR, Newport EL, Aslin RN, Tunick RA, Barrueco S (1997) Incidental language learning: listening (and learning) out of the corner of your ear. Psychol Sci 8:101–105. 10.1111/j.1467-9280.1997.tb00690.x [DOI] [Google Scholar]
- Saffran JR, Johnson EK, Aslin RN, Newport EL (1999) Statistical learning of tone sequences by human infants and adults. Cognition 70:27–52. 10.1016/s0010-0277(98)00075-4 [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R (2006) More attention must be paid: the neurobiology of attentional effort. Brain Res Rev 51:145–160. 10.1016/j.brainresrev.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Schapiro AC, Kustner LV, Turk-Browne NB (2012) Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr Biol 22:1622–1627. 10.1016/j.cub.2012.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A, Izquierdo A (2019) Adaptive learning under expected and unexpected uncertainty. Nat Rev Neurosci 20:635–644. 10.1038/s41583-019-0180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell R, Chait M (2018) Enhanced deviant responses in patterned relative to random sound sequences. Cortex 109:92–103. 10.1016/j.cortex.2018.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell R, Baumann A, Gal C, Barascud N, Friston K, Chait M (2017) Is predictability salient? A study of attentional capture by auditory patterns. Philos Trans R Soc Lond B Biol Sci 372:20160105. 10.1098/rstb.2016.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro JM, Trobalón JB (2005) Statistical computations over a speech stream in a rodent. Percept Psychophys 67:867–875. 10.3758/bf03193539 [DOI] [PubMed] [Google Scholar]
- Wang CA, Munoz DP (2015) A circuit for pupil orienting responses: implications for cognitive modulation of pupil size. Curr Opin Neurobiol 33:134–140. 10.1016/j.conb.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, Marrocco RT (1997) Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology (Berl) 132:324–334. 10.1007/s002130050352 [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P (2005) Uncertainty, neuromodulation, and attention. Neuron 46:681–692. 10.1016/j.neuron.2005.04.026 [DOI] [PubMed] [Google Scholar]
- Zhao J, Al-Aidroos N, Turk-Browne NB (2013) Attention is spontaneously biased toward regularities. Psychol Sci 24:667–677. 10.1177/0956797612460407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Bury G, Milne A, Chait M (2019a) Pupillometry as an objective measure of sustained attention in young and older listeners. Trends Hear 23:2331216519887815. 10.1177/2331216519887815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Chait M, Dick F, Dayan P, Furukawa S, Liao HI (2019b) Pupil-linked phasic arousal evoked by violation but not emergence of regularity within rapid sound sequences. Nat Commun 10:4030. 10.1038/s41467-019-12048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]