Abstract
Background
Chronic subdural hematoma (cSDH) is a form of intracranial hemorrhage common in older adults. Optimal treatment remains controversial. We conducted a systematic review to identify surgical thresholds, characterize outcomes, and delineate critical considerations in the surgical management of older adults in order to summarize the evidence supporting the best contemporary management of cSDH.
Methods
A systematic review exploring surgical management of cSDH among individuals aged 65 years and older was conducting by searching the PubMed, Embase, and Scopus databases for articles in English. Abstracts from articles were read and selected for full-text review according to a priori criteria. Relevant full-text articles were analyzed for bibliographic data, aim, study design, population, interventions, and outcomes.
Results
Of 1473 resultant articles, 21 were included. Surgery rationale was case-by-case for symptomatic patients with cSDH. Surgery was superior to conservative management and promoted equivalent neurologic outcomes and rates of complications. Recurrence and reoperation rates in older adults were similar to younger individuals. Some studies reported higher mortality rates for older adults, while others reported no difference. Anticoagulation or antiplatelet agent use did not seem to be associated with poorer outcomes in older adults.
Conclusions
Surgery for cSDH in older adults leads to favorable neurologic outcomes without increased risk of overall complications, recurrence, or reoperation compared to younger patients. However, older adults may be at increased risk for mortality after surgery. It is important to determine use of anticoagulant or antiplatelet agents in older adults to optimally manage patients with cSDH.
Keywords: Burr hole, Chronic subdural hematoma, Craniotomy, Neurosurgery, Older adults, Subdural hematoma
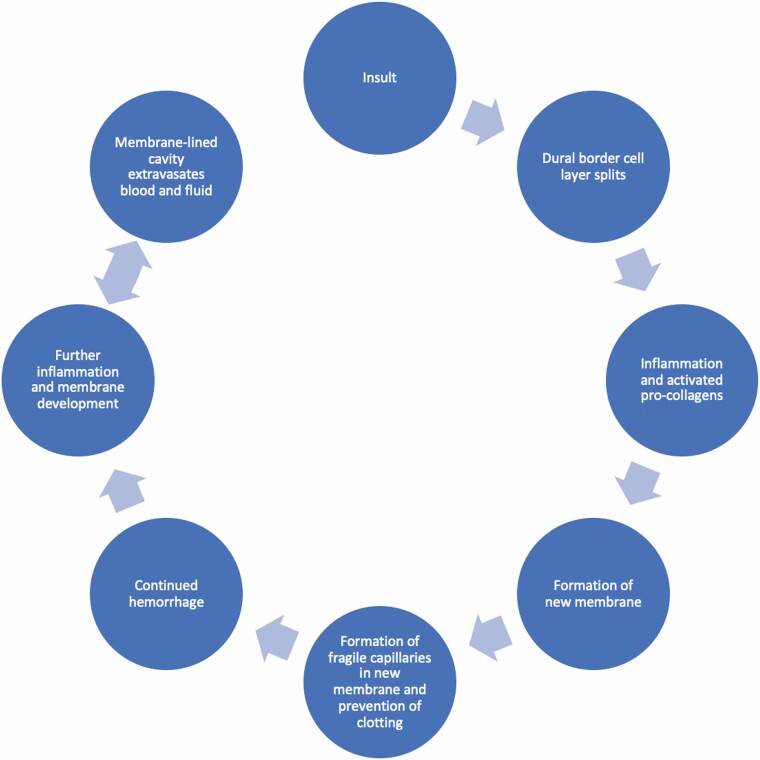
Chronic subdural hematoma (cSDH), defined as an intracranial, extra-axial collection of blood lasting longer than 3 weeks, is a common form of intracranial hemorrhage (1–3). The development of cSDH involves a confluence of pathophysiological processes (Figure 1) (4). Initially, an insult splits the dural border cell layer (4). Inflammatory cells migrate to repair the border cell layer, after which inflammatory processes and activated pro-collagens promote formation of a new membrane (4). Angiogenic factors stimulate formation of fragile capillaries within the new membrane, while fibrinolytic processes prevent clot formation, leading to continued hemorrhage (4). After further inflammation and membrane development occur, the resultant membrane-lined cavity extravasates blood and fluid (4). Although trauma is classically implicated in the development of cSDH, trauma may be absent or minor (4).
Figure 1.
Pathophysiology of chronic subdural hematoma. This diagram indicates the pathophysiological processes involved in the development of chronic subdural hematoma.
The incidence of cSDH increases with age, from 3.4 per 100 000 in people under 65 years of age to a reported range of 8–58.1 per 100 000 in people older than 65 years (1–3). This elevated incidence in older adults has been attributed to increased risk of falls and use of antithrombotic drugs (3). Given the aging global population, the incidence of cSDH is projected to rise, contributing to increased morbidity, mortality, and costs to health care systems (5–7). Increased usage of computed tomography in older adults with a history of a recent fall has led to increased recognition of cSDH in otherwise asymptomatic patients (8–10).
Many small cSDHs may be treated conservatively due to their low propensity to cause neurological morbidity (9). Multiple surgical procedures, such as twist drill or burr hole craniostomy or craniotomy, are commonly used to manage cSDH (9,11). These modalities drain fluid from the cSDH to reduce its size and prevent neurologic morbidity and mortality secondary to compression of brain tissue (9,11). Burr hole craniostomy involves drilling one or more small holes in the skull to allow drainage of fluid, while twist drill craniostomy involves the creation of yet smaller holes in the skull for minimal invasiveness (9,11). Craniotomy involves removing a piece of the skull to enable open evacuation of the cSDH (9,11). Despite acknowledgement of the importance of surgical management in symptomatic cases or those with significant mass effect on imaging, optimal surgical paradigms, thresholds, and timing for older adult with cSDH are unclear (11).
Given increased necessity for clear delineation of surgical thresholds, paradigms, and timing for older adults, we conducted a systematic review to characterize the surgical management of older adults with cSDH. The goals were: (i) identify surgical thresholds, (ii) characterize outcomes of older adults who are managed surgically, and (iii) delineate critical considerations in the surgical management of these patients.
Method
A systematic review using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was conducted to investigate the thresholds for surgery for cSDH in older adults (12). PubMed MEDLINE (National Library of Medicine), Embase (Elsevier), and Scopus (Elsevier) databases were searched in May 2020. No limits on language, date, or study type were applied. Search terms included “chronic subdural hematoma” and variations of the word “elderly” which are shown in Supplementary Table 1. The protocol was not registered, and no funding was received.
All studies that were written in English, had an abstract and full text available, and met prespecified inclusion criteria were included in the review. Inclusion criteria included population of older adults defined as age ≥ 65 years old, diagnosis of cSDH, intervention of surgery, type of surgical intervention, patient-level outcomes, and treatment thresholds. Duplicated articles were removed. Articles were screened by title and abstract by 2 authors independently and conflicts were resolved. The remaining articles were screened via full-text review. Disagreements were reconciled.
After articles were selected for inclusion, a review was conducted of characteristics of each study including aim, design, duration, demographics, surgical interventions used, and outcomes. The primary data of interest were thresholds for surgery in terms of patient-specific factors and clinical picture, readmission, reoperation, and mortality. Secondary factors included neurologic recovery, postoperative complications, and quality of life. Income status of the country from which each paper originated was classified based on the designation of the World Bank (13). The GRADE framework was used to assess the quality of each included study (14). The level of evidence was designated for each included study based on previously delineated guidelines (15). The risk of bias for this systematic review was determined by considering the quality and level of evidence of all included studies.
Results
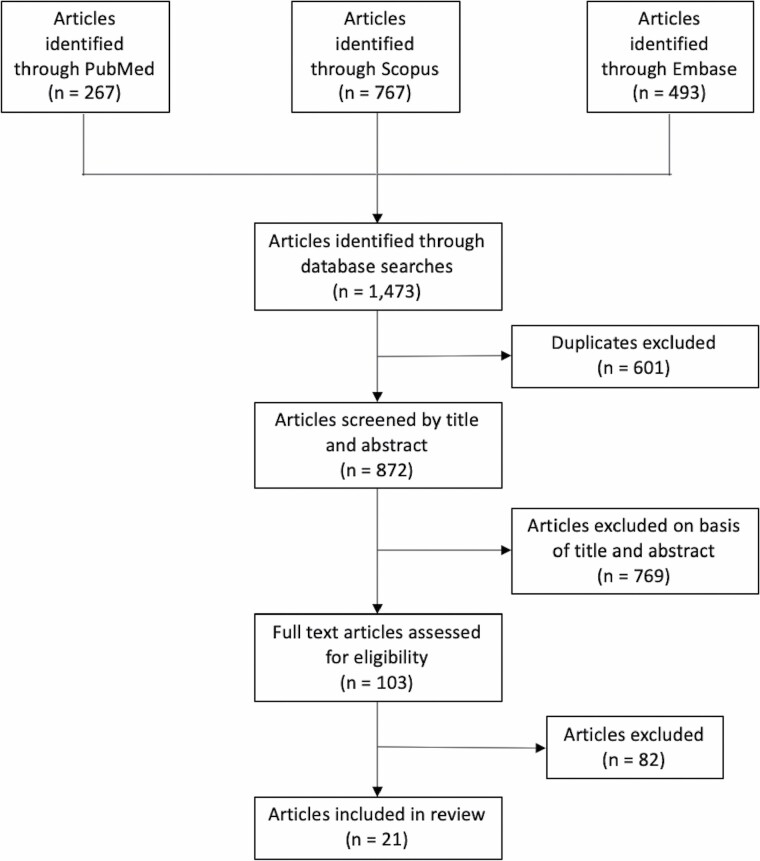
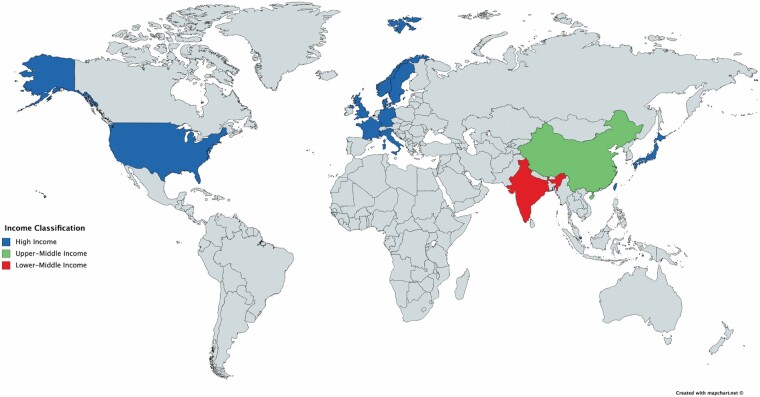
A total of 1473 articles were identified via the search, of which 21 were included in the review (16–36). The PRISMA diagram for this systematic review is shown in Figure 2. No Class I or randomized clinical trials were identified. Study design included 20 (95.2%) retrospective cohorts and one (4.8%) prospective cohort. Evidence level was level II for 14 studies (66.7%) and III for 7 studies (33.3%). There was a moderate risk of bias overall. Data extracted from all included articles are shown in Supplementary Table 2. Studies included originated from 13 countries, of which 11 (84.6%) were high income, one (7.7%) was upper-middle income, and one (7.7%) was lower-middle income (Figure 3). Eight (38.1%) studies examined patients ≥ 65 years, 3 (14.3%) studies examined patients ≥ 70 or 75 years, 4 (19.0%) studies examined patients ≥ 80 or 85 years, and 6 (28.6%) studies examined patients ≥ 90 years.
Figure 2.
PRISMA flowchart. This flowchart delineates the search and review process used to identify and select articles for inclusion in this study.
Figure 3.
Countries represented in this study. This map shows the countries represented in this study by World Bank income status. This map was created using MapChart.
Indications for Surgery
Thirteen studies provided a rationale for conducting surgery in patients with cSDH. Of these studies, 7 offered surgery on a case-by-case basis (18,21,23,25,26,33,34), 5 recommended surgery to symptomatic patients (16,19,20,24,36), and one operated on all patients with cSDH (22). Studies also described the consideration of radiographic factors in recommendations for surgery, namely larger hematoma size in 2 studies (33,34) and midline shift in 2 studies (20,24). Unilateral and bilateral hematomas were indications for surgery in 3 studies (19,24,36). In 2 studies, cSDH with membranes prompted use of mini-craniectomy or craniotomy rather than burr hole cranioplasty (19,25).
Outcomes of Surgery
Of 21 included studies, 2 studies comparing surgery and conservative treatment determined neurologic improvement was greater after surgery (23,34), while another determined there was no difference in disposition (33). Six studies yielded unfavorable neurologic outcomes for older adults compared to younger patients (18,24,27,29,31,36), 4 reported no difference between older adults and younger patients (19,20,25,30), and 3 found generally favorable outcomes in older adults (17,21,32). Five studies found no difference in mortality between older adults and younger patients (16,17,19,25,30), while 2 reported increased mortality for older adults (18,26). Four studies found no difference in complications between older and younger patients (17,19,25,36), while 2 yielded increased complications for older adults (27,32). Three studies found no differences in recurrence between older and younger patients (16,29,36). One study reported no difference in reoperations between older and younger patients (16), while another reported a greater reoperation rate in older adults than younger patients (30).
Outcomes of Surgery by Modality
Fifteen studies examined burr hole craniostomy (16,17,20–23,25,27–29,31,32,34–36). Ten studies reported removal after drains 1–5 days postoperatively (17,20,22,25,27–29,34–36), and 2 studies described positioning patients supine after surgery (22,36). Of the 15 studies, 2 studies reported neurologic improvements after surgery compared to conservative management (23,34), one of which also noted longer survival overall in surgically managed patients (23). Three studies yielded favorable outcomes for older adults (17,21,32), and 2 found no difference between older and younger patients (20,25). Four studies found poorer neurologic outcomes for older adults compared to younger patients (27,29,31,36). Three studies reported no difference in mortality between older adults and younger patients (16,17,25). Three studies reported no difference in complications between older adults and younger patients (17,25,36), while 2 found increased complications for older adults (27,32). Three studies reported no difference in recurrence between older adults and younger patients (16,29,36), and one found no difference in reoperations (16).
Six studies examined 1 burr hole craniostomy (16,22,28,31,34,35). Of the 6 studies, one reported neurologic improvement compared to conservatively treated patients (34). Another study found no difference in mortality, recurrence, or reoperation between older adults and younger patients (16). An additional study found poorer prognosis for cSDH patients with frailty than those without (31). Two studies examined 2 burr hole craniostomy, one of which found no difference in neurologic outcomes between older adults and younger patients (21), while the other found poorer neurologic recovery and greater complication rates in older adults (27).
Five studies examined multiple surgical modalities (19,24,26,30,33). One study determined disposition did not differ between patients managed surgically with any modality than those managed conservatively (33). Two of the 5 studies reported poorer neurologic outcomes for older adults compared to younger patients (18,24), while 2 studies found no difference in neurologic outcome (19,30). Two studies reported greater mortality in older adults (18,26), while 2 studies yielded no difference in comparison to younger patients (19,30). One study found no difference in complications (19), while another found increased reoperation (30). One study did not specify the surgical modality (18).
Outcomes of Surgery by Age
Eight studies examined patients ≥ 65 years of age (17,21,22,25,26,30,31,35). Two studies reported favorable neurologic outcome for older adults (17,21), while another study found patients with frailty, determined using the Clinical Frailty Scale, had poorer neurologic prognosis (31). Three studies reported no difference in mortality between older adults and younger patients (17,25,30), while one found greater mortality for older adults (26). Two studies reported no difference in complications between age groups (17,25). Another study reported increased reoperations for older adults (30).
Three studies examined patients ≥ 70 or 75 years of age (24,28,32). One study found that loss of functional dependence after surgery was associated with older age, initial Glasgow Coma Scale (GCS), and postoperative residual hematoma thickness (24). Another determined patients > 75 years old experienced early postoperative complications more frequently, but that 75% of patients > 75 years had a favorable outcome (32). An additional study noted that temporary acute agitated delirium secondary to intense prolonged hyperperfusion was a unique complication of burr hole craniostomy in patients ≥ 75 years (28).
Four studies examined patients ≥ 80 or 85 years of age (19,27,29,36). Three studies found worse neurologic outcomes after surgery in these patients (27,29,36), while another found no difference in outcomes (19). Two of the studies found no difference in complications between those aged ≥ 80 or 85 years and younger patients (19,36), while one found greater rates of complications in older adults (27). Two studies found no difference in recurrence rates between age groups (29,36).
Six studies examined patients ≥ 90 years of age (16,18,20,23,33,34). Two studies found improved neurologic status after surgery compared to conservative management (23,34), while another found disposition did not differ (33). One study identified worse neurologic outcomes after surgery in patients ≥ 90 years compared to those < 90 (18), while another found no difference (20). One study found increased mortality risk in patients ≥ 90 years after surgery (18), while another found no difference in mortality (16).
Anticoagulation and Antithrombotic Therapy
Fourteen studies discussed the role of anticoagulation or antithrombotic therapy (16,17,19,20,22,24–27,29–31,34,35). Four studies did not find differences in anticoagulant or antiplatelet use between surgically and conservatively managed patients (34) or older adults and younger patients (17,20,29). In studies that found differences, antiplatelet use was more common in older adults and frail, while anticoagulant use was more common in younger patients (16,31). One study found increasing use of anticoagulation with age among older adults (30).
One study reported antiplatelet, but not anticoagulant use, was associated with onset of cSDH in older adults (25). One study found no difference in outcome, mortality, or complications due to antithrombotic drug use, but described a possible increase in length of hospitalization (19), while another study determined greater risk for complications for patients with a history of antithrombotic drug use (29). An additional study found neurologic deterioration was not attributable to anticoagulation use (27), while another study reported no increase in short or long-term mortality due to anticoagulation (26). Three studies determined anticoagulation or antiplatelet use was not associated with cSDH recurrence (22,27,35), while another determined anticoagulation increased risk of recurrence (24). All 14 included studies either did not state the specific anticoagulation agents used or did not differentiate the effects of different anticoagulation agents. One study involved stopping anticoagulation prior to surgery (19), while investigators in another study administered Vitamin K during surgery, to avoid recurrence of cSDH (22).
Other Management Considerations
Five studies discussed presentation of cSDH by age (21,29,30,32,34). Older adults were more likely to present with movement difficulties such as gait disturbance, limb weakness, and cognitive symptoms such as memory deficits. Younger patients were more likely to present with signs and symptoms of increased intracranial pressure such as headache and vomiting. Two studies specifically determined worse neurologic condition at presentation was associated with poorer outcomes (17,24).
Discussion
We present a systematic review of the surgical management of cSDH in older adults defined as age 65 years old or older. While systematic reviews have been conducted to examine different surgical approaches in patients with cSDH (8,10,37–39), existing reviews lack thorough examination of the entire scope of management of these patients and discussion of how management strategies for cSDH may change across age groups. Additionally, this is the first systematic review specifically focused on the surgical management of cSDH in older adults. We highlight surgical indications, outcomes, effects of anticoagulation and antiplatelet agents, and other management considerations for older adults with cSDH. We also emphasize the comprehensive management of older adults with cSDH with appropriate consideration of patient-level, health system, and advocacy factors relevant to this patient population in order to ensure optimal care.
Role of Anticoagulant and Antiplatelet Agents
The use of anticoagulant medications is a risk factor for initial occurrence and recurrence of cSDH (24,40). This is particularly pertinent in planning care for older adults, an increasing percentage of whom take antiplatelet and anticoagulant agents to lower clotting risk associated with underlying hypercoagulability and cardiac arrythmias (16,30,31). Increased use of these agents has been associated with a 5-fold increase in intracerebral hemorrhage (41). Antiplatelet and anticoagulant therapies are associated with increased risk of cSDH in older adults, with patients using a combination of the 2 medications exhibiting the greatest risk of cSDH (42). The use of these agents is often essential in managing underlying comorbidities, and a risk-benefit analysis favors maintaining anticoagulation (41). Clinicians must seek the most balanced treatment plan as safer anticoagulant and antiplatelet agents continue to be developed.
The majority of studies included in this review reported no differences in anticoagulation use between surgically and conservatively managed older adults (34), or between older adults and younger patients (17,20,29). There was no difference in immediate outcomes or recurrence after cSDH in these populations as expected in light of the similarity in anticoagulant use (19,22,27,35). However, some studies included in the review involved measures to avoid recurrence of cSDH, such as stopping anticoagulants preoperatively or administering Vitamin K intraoperatively (19,22). These preemptive measures may allow neurosurgeons to promote better outcomes after cSDH surgery. Further investigation is necessary to determine for which patients stopping anticoagulants or antiplatelet agents preoperatively is indicated, the optimal times to stop these preoperatively and resume these medication regimens postoperatively, and differences in these indications and properties across age groups.
Approach to Management of Older Adults With cSDH
Recognizing differences in presentation between older adults and younger individuals is important to provide optimal care through timely and accurate diagnosis of all afflicted patients. Older adults with cSDH often present with cognitive deficits compared to the presenting symptoms of increased intracranial pressure in younger patient, likely due to brain atrophy, greater potential space, and increased susceptibility of arteries to become friable and rupture (29,30,32,34,43–46). Clinicians should consider that cSDH may mirror conditions common in older adults such as dementia when assessing patients and making a diagnosis (21).
After diagnosis, cSDH often necessitates intervention, and age is generally not a contraindication for such intervention (16,47). Surgery improves neurologic outcomes in older adults without increased complications, recurrence, or reoperation rates compared to younger patients (16,17,19,21,25,29,32,36,43,45,46,48–52). However, the reported possibility of increased mortality for older adults compared to younger patients necessitates careful consideration of surgical candidacy. This is particularly relevant during the current coronavirus disease 2019 pandemic. Accordingly, the rationale for surgery has changed in older adults over time. As the natural history and morphological characteristics of cSDHs and patient-specific factors are better understood, neurosurgeons have moved toward providing surgery on a case-by-case basis or to symptomatic patients. Factors such as comorbidities and general health status, neurologic status at presentation, and history of anticoagulant or antithrombotic agent use, as well as situational factors such as increased baseline risk for nosocomial infection, may change the risk-benefit determination of conducting surgery and should be integrated into a holistic assessment regarding whether the patient is a suitable candidate for surgery (17,19,24,29,47,53).
Similarly, although surgery has been the mainstay of management of cSDH patients, endovascular interventions have grown in popularity (54–57). Middle meningeal artery embolization seeks to devascularize the subdural membranes in cSDH, preventing rupture of the microcapillaries due to direct pressure from the middle meningeal artery and thereby promoting reabsorption of blood rather than continued leakage into the subdural space (54–58). Middle meningeal artery embolization is safe for the treatment of intractable cSDH in the general population and results in low rates of recurrence (54–57). This technique may be performed under light or no sedation and avoid the need for disruption of anticoagulation regimens that occurs in surgical cases and associated risk of ischemic events secondary to reversal of coagulability (55,58). Performing middle meningeal artery embolization with liquid embolic agents will allow casting of the subdural membrane and back reflux of meningeal branches, clear visualization, and durable embolization of the meningeal vasculature (58). No article was identified in this review using endovascular embolization, as the current literature lacks studies focused on the use of middle meningeal artery embolization in older adults. The applicability of endovascular therapies in older adults with cSDH necessitates further investigation.
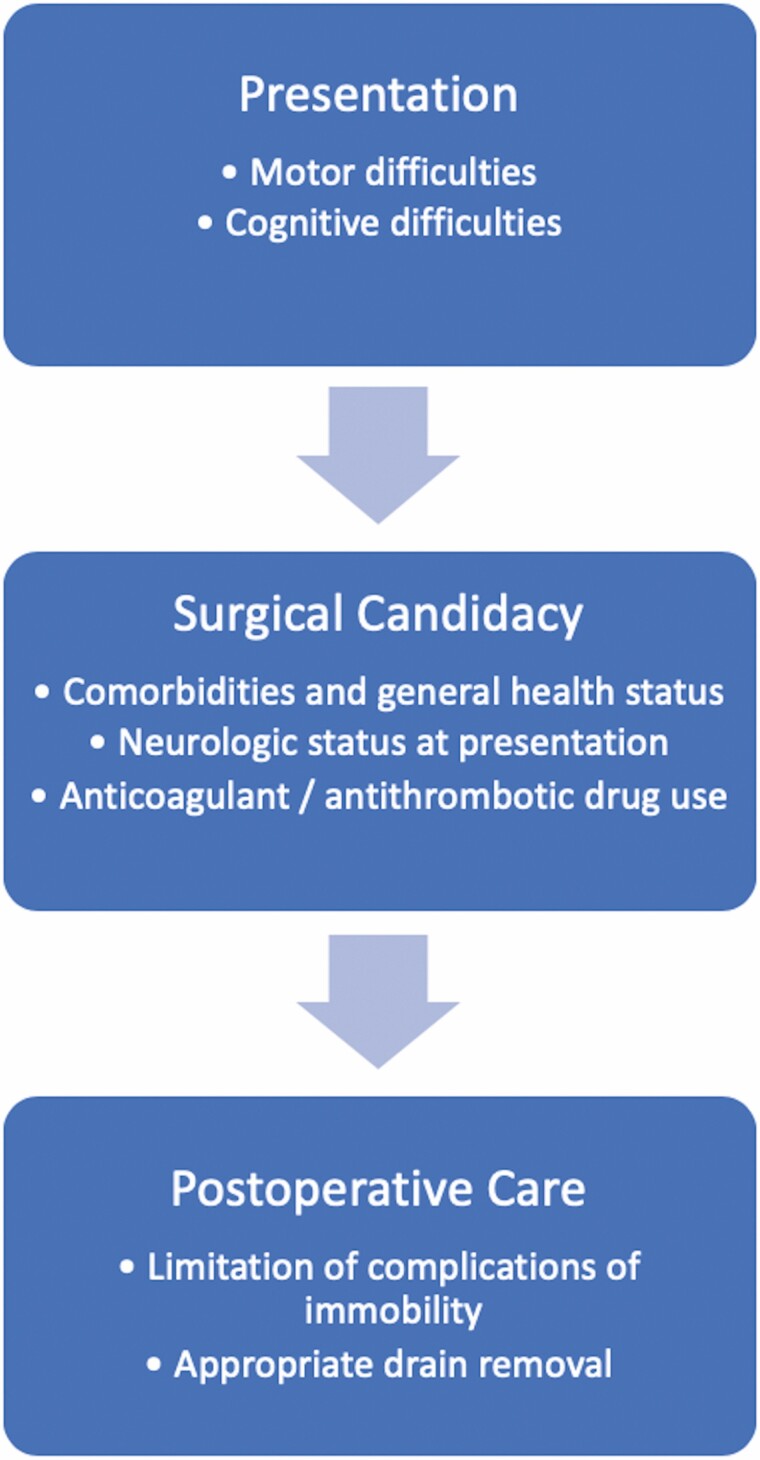
Additionally, mobility-related complications are important to consider in the postoperative management of these patients. It is well known that prolonged bedrest and immobilization leads to loss of muscle strength and joint contractures, limiting movement and promoting skeletal mass loss and fractures in a population already at risk for loss of mobility and fractures (59–61). Immobilization may also spur cognitive deficits such as impaired concentration and psychological deterioration, such as anxiety and depression, which are then coupled with the existing propensity of older adults to experience acute agitated delirium after surgery for cSDH (28,62). Additionally, immobilization may also lead to cardiopulmonary and vascular complications and venous thromboembolic events (28,63,64). It is important to recognize the unique risks this patient population experiences and mitigate the risks for these events with patient-centered care. A comprehensive approach to care of older adults with cSDH is shown in Figure 4.
Figure 4.
Comprehensive approach for surgical management of chronic subdural hematoma in older adults. This diagram represents a comprehensive approach for surgical management of chronic subdural hematoma in older adults consisting of recognition of common presentations, assessment of surgical candidacy, and postoperative care.
Context-Specific Consideration of the Definition of Older Adults
Although conventionally defined as age ≥ 65 years, the definition of older adult varies across contexts (65,66). Studies included in this review classified older adult as ages varying from ≥ 65 to ≥ 90 years old. However, the search also yielded 12 studies with patient populations ≥ 50, 55, or 60 years of age. While the categorization of individuals as older adult is often done in a purely chronological manner, this is inherently limiting (65,66). Life expectancy has increased, and there are regional variations in average life expectancy (5,6,65). Additionally, chronology alone does not consider change in social roles or capabilities, cultural factors, or perspectives toward older adults, all of which vary across cultural and societal contexts (65,66). This effect is reciprocal: the complexity of defining what constitutes an older adult renders examination of which interventions are effective in older adults challenging (65,66). Importantly, consideration of the meaning of older adult based on the local context is important to ensure proper management of these patients from presentation to long-term follow-up (65,66). In turn, this will allow evaluation of the effectiveness of interventions in a given context (65,66).
Neurosurgical Care of Older Adults
Sentinel health events, defined as those that indicate dysfunction of organ systems despite not causing the decline, serve as an important markers of patient prognosis (67). Increasingly, cSDH has begun to be conceptualized as a sentinel heath event by unmasking and exacerbating underlying medical conditions (26,33,68). While high-income countries already have substantial older adult populations, this segment of the population is expected to increase in low- and middle-income countries, due to the large proportion of young people in these countries at present (69). Given that the population of older adults is growing and indications for neurosurgical care in older adults have expanded, increased incidence of cSDH and other neurologic morbidities will be associated with an increased need for neurosurgical care and associated health care expenditures in older adults (70,71).
Current health care infrastructure is unprepared to handle an increasing number of older adults (72). Inadequate access to care is a concern for many older adults (73–76). While this impact of population aging is likely to occur more immediately in high-income countries, low- and middle-income countries will be affected in the future (69). Accordingly, prioritization and development of a global neurosurgical infrastructure, equitable allocation of resources, and training of personnel is increasingly important along with associated neurosurgeon-led advocacy (73,74,77). Increasing the capacity of neurosurgical care in older adults will allow optimal maximization of neurologic recovery and quality of life in this patient population, enhance health equity, and promote associated cost savings by preserving independent living (73,74,78).
Consequently, advocacy and policy efforts aimed at augmenting neurosurgery infrastructure, particularly for older adults, are important (73–75,78,79). As care providers of older adults with neurological disorders, neurosurgeons have a responsibility to advocate for their current and future patients in guiding these efforts through societal and policy change (75,78,79). With a wide breadth of technical knowledge, neurosurgeons may provide expert recommendations focused on policy to provide optimal care to older adults (75,79). Given their responsibility to continually improve care for neurosurgical patients, neurosurgeons can guide surveillance efforts in order to determine the most effective management strategies for older adults (75). Additionally, neurosurgeons exert a disproportionate impact on society via a position of respect, privilege, and prominence, providing them with a platform to champion efforts to expand health care infrastructure and participate in associated advocacy and policymaking efforts aligned with the needs of older adults (75,79). Neurosurgical professional associations represent a collective interest and may be utilized to form collaborative partnerships with other specialties and government agencies aimed at advancing the health of older adults (75,77,80).
Lastly, neurosurgeons should understand a unique ethical challenge relevant to older adults with cSDH. Heroic measures, taken after all other measures have been exhausted, may cause substantial pain, suffering, or injury but have the greatest chance of saving the life of an individual. Given that the clinical course of cSDH in older adults is improved by intervention while conservative intervention is associated with high mortality rates (16,17,19,21,25,29,32,36,43,45,46,48–52), surgical or endovascular management of cSDH may constitute a heroic measure. Living wills that exclude heroic measures often lack consideration of neurosurgical treatments in their categorization of heroic measures. Neurosurgeons should inform patients about the applicability of “no heroic measure” clauses to neurosurgical care in frank discussions about advanced directives.
Limitations
There are several limitations to this study. Only published studies with full-text manuscripts were included, putting results at risk for publication bias due to potential overrepresentation of studies showing favorable outcomes for older adults after cSDH surgery. Studies included were required to specifically discuss surgical management of cSDH in older adults, excluding studies that discussed surgery for cSDH in general. Additionally, the quality of evidence was moderate, as studies were cohort studies with a level of evidence of II or III and no randomized trials were identified. It is unlikely that a randomized trial on this topic could be conducted. The studies included were at high risk of bias due to their retrospective nature and study design. Only studies written in, or translated to, English were included, perhaps excluding other regions in which surgery was received differently in other older adult populations. Studies varied in their definitions of older adults, obfuscating interpretation of conclusions. Many studies did not report rationale for choosing surgery, preventing identification of granular indications for surgery in older adults with cSDH. There is a paucity of literature from low- and middle-income countries, limiting the external validity of this review. Lastly, no meta-analysis was conducted as part of this systematic review due to heterogeneity of the included studies. Thus, some inferences could be made from our findings, but no statistically significant conclusions could be drawn.
Conclusion
Surgery for cSDH in older adults leads more often than not to favorable neurologic outcomes without increased risk of complications, recurrence, or reoperation compared to younger patients. However, older adults may be at increased risk for mortality after surgical treatment of cSDH. After context-appropriate definition of the “older adult” patient, a comprehensive approach is needed to optimally manage these patients. Larger-scale changes to augment neurosurgical care infrastructure for older adults may also help to maximize the recovery and quality of life of this growing patient population.
Supplementary Material
Funding
Dr. Garcia serves as a Fogarty Global Health Trainee and is supported by the Fogarty International Center under grant number D43TW010543 through the Harvard, Boston University, Northwestern University, and University of New Mexico consortium.
Conflict of Interest
None declared.
Acknowledgments
None declared.
References
- 1. Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural haematoma in the elderly–a North Wales experience. J R Soc Med. 2002;95:290–292. doi: 10.1258/jrsm.95.6.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992;32:207–209. doi: 10.2176/nmc.32.207 [DOI] [PubMed] [Google Scholar]
- 3. Kostanian V, Choi JC, Liker MA, Go JL, Zee CS. Computed tomographic characteristics of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:479–489. doi: 10.1016/S1042-3680(18)30111-6 [DOI] [PubMed] [Google Scholar]
- 4. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017;14:108. doi: 10.1186/s12974-017-0881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gavrilov LA, Heuveline P. Aging of population. In: The Encyclopedia of Population. 2003;1:32–37. [Google Scholar]
- 6. DeSA U. World Population Prospects: The 2012 Revision. New York: Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat; 2013:18. [Google Scholar]
- 7. Frontera JA, Egorova N, Moskowitz AJ. National trend in prevalence, cost, and discharge disposition after subdural hematoma from 1998–2007. Crit Care Med. 2011;39(7):1619–1625. doi: 10.1097/CCM.0b013e3182186ed6 [DOI] [PubMed] [Google Scholar]
- 8. Almenawer SA, Farrokhyar F, Hong C, et al. . Chronic subdural hematoma management: a systematic review and meta-analysis of 34829 patients. Ann Surg. 2014;259(3):449–457. doi: 10.1097/SLA.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 9. Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002;78:71–75. doi: 10.1136/pmj.78.916.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu W, Bakker NA, Groen RJ. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg. 2014;121:665–673. doi: 10.3171/2014.5.JNS132715 [DOI] [PubMed] [Google Scholar]
- 11. Cenic A, Bhandari M, Reddy K. Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci. 2005;32:501–506. doi: 10.1017/s0317167100004510 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Bank Country and Lending Groups. The World Bank Group. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed June 14, 2020.
- 14. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–310. doi: 10.1097/PRS.0b013e318219c171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartek J Jr, Sjåvik K, Ståhl F, et al. . Surgery for chronic subdural hematoma in nonagenarians: a Scandinavian population-based multicenter study. Acta Neurol Scand. 2017;136:516–520. doi: 10.1111/ane.12764 [DOI] [PubMed] [Google Scholar]
- 17. Cheng S-Y, Chang C-K, Chen S-J, Lin J-F, Tsai C-C. Chronic subdural hematoma in elderly taiwan patients: a retrospective analysis of 342 surgical cases. Int J Gerontol. 2014;8(1):37–41. doi: 10.1016/j.ijge.2014.01.001 [DOI] [Google Scholar]
- 18. Christopher E, Poon MTC, Glancz LJ, Hutchinson PJ, Kolias AG, Brennan PM; British Neurosurgical Trainee Research Collaborative (BNTRC) . Outcomes following surgery in subgroups of comatose and very elderly patients with chronic subdural hematoma. Neurosurg Rev. 2019;42:427–431. doi: 10.1007/s10143-018-0979-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Bonis P, Olei S, Mongardi L, et al. . Chronic subdural hematoma in patients aged 80 years and older: a two-centre study. Clin Neurol Neurosurg. 2018;170:88–92. doi: 10.1016/j.clineuro.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 20. Dobran M, Marini A, Nasi D, et al. . Clinical outcome of patients over 90 years of age treated for chronic subdural hematoma. J Korean Neurosurg Soc. 2019. doi: 10.3340/jkns.2018.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gill M, Maheshwari V, Narang A, Lingaraju TS. Impact on cognitive improvement following burr hole evacuation of chronic subdural hematoma: a prospective observational study. J Neurosci Rural Pract. 2018;9:457–460. doi: 10.4103/jnrp.jnrp_126_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurabe S, Ozawa T, Watanabe T, Aiba T. Efficacy and safety of postoperative early mobilization for chronic subdural hematoma in elderly patients. Acta Neurochir (Wien). 2010;152:1171–1174. doi: 10.1007/s00701-010-0627-4 [DOI] [PubMed] [Google Scholar]
- 23. Lee L, Ker J, Ng HY, et al. . Outcomes of chronic subdural hematoma drainage in nonagenarians and centenarians: a multicenter study. J Neurosurg. 2016;124:546–551. doi: 10.3171/2014.12.JNS142053 [DOI] [PubMed] [Google Scholar]
- 24. Leroy HA, Aboukaïs R, Reyns N, et al. . Predictors of functional outcomes and recurrence of chronic subdural hematomas. J Clin Neurosci. 2015;22:1895–1900. doi: 10.1016/j.jocn.2015.03.064 [DOI] [PubMed] [Google Scholar]
- 25. Lo W-L, Lee T-C, Fang P-S, Huang Y-H. Chronic subdural hematoma in patients under age 65 years: a comparative study of age cohort. Formos J Surg. 2013;46(1):10–14. doi: 10.1016/j.fjs.2012.10.005 [DOI] [Google Scholar]
- 26. Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011;114:72–76. doi: 10.3171/2010.8.JNS10298 [DOI] [PubMed] [Google Scholar]
- 27. Munoz-Bendix C, Pannewitz R, Remmel D, et al. . Outcome following surgical treatment of chronic subdural hematoma in the oldest-old population. Neurosurg Rev. 2017;40:461–468. doi: 10.1007/s10143-016-0803-y [DOI] [PubMed] [Google Scholar]
- 28. Ogasawara K, Ogawa A, Okuguchi T, Kobayashi M, Suzuki M, Yoshimoto T. Postoperative hyperperfusion syndrome in elderly patients with chronic subdural hematoma. Surg Neurol. 2000;54:155–159. doi: 10.1016/s0090-3019(00)00281-0 [DOI] [PubMed] [Google Scholar]
- 29. Ou Y, Dong J, Wu L, et al. . A comparative study of chronic subdural hematoma in three age ranges: below 40 years, 41-79 years, and 80 years and older. Clin Neurol Neurosurg. 2019;178:63–69. doi: 10.1016/j.clineuro.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 30. Schoedel P, Bruendl E, Hochreiter A, et al. . Restoration of functional integrity after evacuation of chronic subdural hematoma-an age-adjusted analysis of 697 patients. World Neurosurg. 2016;94:465–470. doi: 10.1016/j.wneu.2016.07.027 [DOI] [PubMed] [Google Scholar]
- 31. Shimizu K, Sadatomo T, Hara T, Onishi S, Yuki K, Kurisu K. Importance of frailty evaluation in the prediction of the prognosis of patients with chronic subdural hematoma. Geriatr Gerontol Int. 2018;18:1173–1176. doi: 10.1111/ggi.13436 [DOI] [PubMed] [Google Scholar]
- 32. Spallone A, Giuffrè R, Gagliardi FM, Vagnozzi R. Chronic subdural hematoma in extremely aged patients. Eur Neurol. 1989;29:18–22. doi: 10.1159/000116370 [DOI] [PubMed] [Google Scholar]
- 33. Stippler M, Ramirez P, Berti A, Macindoe C, Villalobos N, Murray-Krezan C. Chronic subdural hematoma patients aged 90 years and older. Neurol Res. 2013;35:243–246. doi: 10.1179/1743132813Y.0000000163 [DOI] [PubMed] [Google Scholar]
- 34. Tabuchi S, Kadowaki M. Chronic subdural hematoma in patients over 90 years old in a super-aged society. J Clin Med Res. 2014;6:379–383. doi: 10.14740/jocmr1907w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wakuta N, Abe H, Fukuda K, et al. . Feasibility and safety of endoscopic procedure in burr-hole surgery for chronic subdural hematoma in patients of very advanced age. World Neurosurg. 2020;134:e1037–e1046. doi: 10.1016/j.wneu.2019.11.080 [DOI] [PubMed] [Google Scholar]
- 36. Watanabe S, Kato N, Sato M, et al. . Treatment outcomes of burr-hole surgery for chronic subdural hematoma in the elderly living beyond life expectancy: a study comparing cure, recurrence, and complications in patients aged ≥80 years versus ≤79 years. World Neurosurg. 2019;132:e812–e819. doi: 10.1016/j.wneu.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 37. Montano N, Ioannoni E, Caricato A, Olivi A. Survey of available literature and meta-analyses on chronic subdural hematoma. Int J Med Rev. 2016;3(4):497–500. doi: 10.15171/ijmr.2016.09 [DOI] [Google Scholar]
- 38. Ivamoto HS, Lemos HP Jr, Atallah AN. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg. 2016;86:399–418. doi: 10.1016/j.wneu.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 39. Teles AR, Falavigna A, Kraemer J. Surgical treatment of chronic subdural hematoma: systematic review and meta-analysis of the literature. Arq Bras Neurocirurg: Braz Neurosurg. 2016;35(2):118–127. doi: 10.1055/s-0035-1571270 [DOI] [Google Scholar]
- 40. Ridwan S, Bohrer AM, Grote A, Simon M. Surgical treatment of chronic subdural hematoma: predicting recurrence and cure. World Neurosurg. 2019;128:e1010–e1023. doi: 10.1016/j.wneu.2019.05.063 [DOI] [PubMed] [Google Scholar]
- 41. Flaherty ML, Kissela B, Woo D, et al. . The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–121. doi: 10.1212/01.wnl.0000250340.05202.8b [DOI] [PubMed] [Google Scholar]
- 42. De Bonis P, Trevisi G, de Waure C, et al. . Antiplatelet/anticoagulant agents and chronic subdural hematoma in the elderly. PLoS One. 2013;8:e68732. doi: 10.1371/journal.pone.0068732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bartek J Jr, Sjåvik K, Dhawan S, et al. . Clinical course in chronic subdural hematoma patients aged 18-49 compared to patients 50 years and above: a multicenter study and meta-analysis. Front Neurol. 2019;10:311. doi: 10.3389/fneur.2019.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang J, Tian Y, Song Y, et al. . Effect of different factors on the short-term outcome of chinese patients with primary chronic subdural hematoma at different age groups: a two-center retrospective study. Front Aging Neurosci. 2019;11:325. doi: 10.3389/fnagi.2019.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Waga S, Otsubo K, Ishikawa M, Handa H. Chronic subdural hematoma in the aged. Neurol Med Chir (Tokyo). 1972;12:84–90. doi: 10.2176/nmc.12.84 [DOI] [PubMed] [Google Scholar]
- 46. Fei X, Wan Y, Wang Z, Chen H, Jiang D. Application of YL-1 needle in chronic subdural hematoma treatment for super-aged patients. J Craniofac Surg. 2018;29:e90–e94. doi: 10.1097/SCS.0000000000004198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Araújo Silva DO, Matis GK, Costa LF, et al. . Chronic subdural hematomas and the elderly: surgical results from a series of 125 cases: old “horses” are not to be shot! Surg Neurol Int. 2012;3:150. doi: 10.4103/2152-7806.104744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48:220–225. doi: 10.1016/s0090-3019(97)80031-6 [DOI] [PubMed] [Google Scholar]
- 49. Raskind R, Metcalf JS, Weiss SR, Doria A. Chronic subdural hematoma in the elderly: a curable lesion. J Am Geriatr Soc. 1968;16:451–457. doi: 10.1111/j.1532-5415.1968.tb02825.x [DOI] [PubMed] [Google Scholar]
- 50. Raskind R, Glover MB, Weiss SR. Chronic subdural hematoma in the elderly: a challenge in diagnosis and treatment. J Am Geriatr Soc. 1972;20:330–334. doi: 10.1111/j.1532-5415.1972.tb00822.x [DOI] [PubMed] [Google Scholar]
- 51. So SC. Chronic subdural haematoma in the elderly. Aust N Z J Surg. 1976;46:166–169. doi: 10.1111/j.1445-2197.1976.tb03225.x [DOI] [PubMed] [Google Scholar]
- 52. Lavy S, Herishianu Y. Chronic subdural haematoma in the aged. J Am Geriatr Soc. 1969;17:380–383. doi: 10.1111/j.1532-5415.1969.tb05354.x [DOI] [PubMed] [Google Scholar]
- 53. Ramachandran R, Hegde T. Chronic subdural hematomas–causes of morbidity and mortality. Surg Neurol. 2007;67:367–372. doi: 10.1016/j.surneu.2006.07.022 [DOI] [PubMed] [Google Scholar]
- 54. Ishihara H, Ishihara S, Kohyama S, et al. . Experience in endovascular treatment of recurrent chronic subdural hematoma. Intervent Neuroradiol. 2007;13(1_suppl):141–144. doi: 10.1177/15910199070130s121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Link TW, Rapoport BI, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: endovascular technique and radiographic findings. Interv Neuroradiol. 2018;24:455–462. doi: 10.1177/1591019918769336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mino M, Nishimura S, Hori E, et al. . Efficacy of middle meningeal artery embolization in the treatment of refractory chronic subdural hematoma. Surg Neurol Int. 2010;1:78. doi: 10.4103/2152-7806.73801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tempaku A, Yamauchi S, Ikeda H, et al. . Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: five cases and a review of the literature. Interv Neuroradiol. 2015;21:366–371. doi: 10.1177/1591019915583224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg. 2019;11:912–915. doi: 10.1136/neurintsurg-2019-014730 [DOI] [PubMed] [Google Scholar]
- 59. Laksmi PW, Harimurti K, Setiati S, Soejono CH, Aries W, Roosheroe AG. Management of immobilization and its complication for elderly. Acta Med Indones. 2008;40:233–240. [PubMed] [Google Scholar]
- 60. Pluijm SM, Graafmans WC, Bouter LM, Lips P. Ultrasound measurements for the prediction of osteoporotic fractures in elderly people. Osteoporos Int. 1999;9:550–556. doi: 10.1007/s001980050275 [DOI] [PubMed] [Google Scholar]
- 61. Akeson WH, Amiel D, Woo SL. Immobility effects on synovial joints the pathomechanics of joint contracture. Biorheology. 1980;17:95–110. doi: 10.3233/bir-1980-171-212 [DOI] [PubMed] [Google Scholar]
- 62. Given CW, Stommel M, Given B, Osuch J, Kurtz ME, Kurtz JC. The influence of cancer patients’ symptoms and functional states on patients’ depression and family caregivers’ reaction and depression. Health Psychol. 1993;12:277–285. doi: 10.1037//0278-6133.12.4.277 [DOI] [PubMed] [Google Scholar]
- 63. Pedersen AB, Ehrenstein V, Szépligeti SK, Sørensen HT. Excess risk of venous thromboembolism in hip fracture patients and the prognostic impact of comorbidity. Osteoporos Int. 2017;28:3421–3430. doi: 10.1007/s00198-017-4213-y [DOI] [PubMed] [Google Scholar]
- 64. Nam JH, Kim DH, Yoo JH, Hwang JH, Chang JD. Does preoperative mechanical prophylaxis have additional effectiveness in preventing postoperative venous thromboembolism in elderly patients with hip fracture?-Retrospective case-control study. PLoS One. 2017;12:e0187337. doi: 10.1371/journal.pone.0187337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly.” Geriatr Gerontol Int. 2006;6(3):149–158. doi: 10.1111/j.1447-0594.2006.00341.x [DOI] [Google Scholar]
- 66. Sabharwal S, Wilson H, Reilly P, Gupte CM. Heterogeneity of the definition of elderly age in current orthopaedic research. Springerplus. 2015;4:516. doi: 10.1186/s40064-015-1307-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Diehr P, Williamson J, Patrick DL, Bild DE, Burke GL. Patterns of self-rated health in older adults before and after sentinel health events. J Am Geriatr Soc. 2001;49:36–44. doi: 10.1046/j.1532-5415.2001.49007.x [DOI] [PubMed] [Google Scholar]
- 68. Dumont TM, Rughani AI, Goeckes T, Tranmer BI. Chronic subdural hematoma: a sentinel health event. World Neurosurg. 2013;80(6):889–892. doi: 10.1016/j.wneu.2012.06.026 [DOI] [PubMed] [Google Scholar]
- 69. Sudharsanan N, Bloom DE, Sudharsanan N. The demography of aging in low-and middle-income countries: chronological versus functional perspectives. In Future directions for the demography of aging: Proceedings of a workshop 2018.National Academies Press; 2018:309–338. [Google Scholar]
- 70. Chibbaro S, Di Rocco F, Makiese O, et al. . Neurosurgery and elderly: analysis through the years. Neurosurg Rev. 2010;34:229–234. doi: 10.1007/s10143-010-0301-6 [DOI] [PubMed] [Google Scholar]
- 71. Maurice-Williams RS, Kitchen N. The scope of neurosurgery for elderly people. Age Ageing. 1993;22:337–342. doi: 10.1093/ageing/22.5.337 [DOI] [PubMed] [Google Scholar]
- 72. Mobley LR, Root E, Anselin L, Lozano-Gracia N, Koschinsky J. Spatial analysis of elderly access to primary care services. Int J Health Geogr. 2006;5:19. doi: 10.1186/1476-072X-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dewan MC, Rattani A, Fieggen G, et al. . Global neurosurgery: the current capacity and deficit in the provision of essential neurosurgical care. Executive Summary of the Global Neurosurgery Initiative at the Program in Global Surgery and Social Change. J Neurosurg. 2018;130(4):1055–1064. doi: 10.3171/2017.11.JNS171500 [DOI] [PubMed] [Google Scholar]
- 74. Park KB, Johnson WD, Dempsey RJ. Global neurosurgery: the unmet need. World Neurosurg. 2016;88:32–35. doi: 10.1016/j.wneu.2015.12.048 [DOI] [PubMed] [Google Scholar]
- 75. Rosseau G, Johnson WD, Park KB, Arráez Sánchez M, Servadei F, Vaughan KA. Global neurosurgery: current and potential impact of neurosurgeons at the World Health Organization and the World Health Assembly. Executive summary of the World Federation of Neurosurgical Societies-World Health Organization Liaison Committee at the 71st World Health Assembly. Neurosurg Focus. 2018;45:E18. doi: 10.3171/2018.7.FOCUS18295 [DOI] [PubMed] [Google Scholar]
- 76. West JL, Fargen KM, Hsu W, Branch CL, Couture DE. A review of big data analytics and potential for implementation in the delivery of global neurosurgery. Neurosurg Focus. 2018;45:E16. doi: 10.3171/2018.7.FOCUS18278 [DOI] [PubMed] [Google Scholar]
- 77. Rosseau G, Johnson WD, Park KB, et al. . Global neurosurgery: continued momentum at the 72nd World Health Assembly. J Neurosurg. 2020;1(aop):1–5. doi: 10.3171/2019.11.JNS191823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Barthélemy EJ, Park KB, Johnson W. Neurosurgery and sustainable development goals. World Neurosurg. 2018;120:143–152. doi: 10.1016/j.wneu.2018.08.070 [DOI] [PubMed] [Google Scholar]
- 79. Estevez-Ordonez D, Davis MC, Hopson B, et al. ; MSHA; MSPM . Reducing inequities in preventable neural tube defects: the critical and underutilized role of neurosurgical advocacy for folate fortification. Neurosurg Focus. 2018;45:E20. doi: 10.3171/2018.7.FOCUS18231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kaufman SR. Decision making, responsibility, and advocacy in geriatric medicine: physician dilemmas with elderly in the community. Gerontologist. 1995;35:481–488. doi: 10.1093/geront/35.4.481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.