ABSTRACT
Neurodevelopmental disorders (NDDs), including intellectual disability (ID), autism and schizophrenia, have high socioeconomic impact, yet poorly understood etiologies. A recent surge of large-scale genome or exome sequencing studies has identified a multitude of mostly de novo mutations in subunits of the protein phosphatase 2A (PP2A) holoenzyme that are strongly associated with NDDs. PP2A is responsible for at least 50% of total Ser/Thr dephosphorylation in most cell types and is predominantly found as trimeric holoenzymes composed of catalytic (C), scaffolding (A) and variable regulatory (B) subunits. PP2A can exist in nearly 100 different subunit combinations in mammalian cells, dictating distinct localizations, substrates and regulatory mechanisms. PP2A is well established as a regulator of cell division, growth, and differentiation, and the roles of PP2A in cancer and various neurodegenerative disorders, such as Alzheimer's disease, have been reviewed in detail. This Review summarizes and discusses recent reports on NDDs associated with mutations of PP2A subunits and PP2A-associated proteins. We also discuss the potential impact of these mutations on the structure and function of the PP2A holoenzymes and the etiology of NDDs.
KEY WORDS: De novo mutations, Intellectual disability, Neurodevelopmental disorders, PP2A, Regulatory subunits
Summary: Mutations in PP2A subunits are associated with neurodevelopmental diseases. Here, we provide an overview of biochemical and functional implications of the de novo mutations reported on PP2A subunits.
Introduction
Protein phosphorylation is arguably the most common post-translational modification (PTM) in cells. It is estimated that there are 230,000 phosphorylation sites in humans (Vlastaridis et al., 2016), the majority of which (>96%) are serine (Ser) and threonine (Thr) residues (Murray, 2011). Interestingly, for Ser/Thr-modifying enzymes, the number of protein kinases are more than 10-fold greater than the number of protein phosphatases (Manning et al., 2002). This difference has contributed to the misconception that protein Ser/Thr phosphatases are promiscuous and unregulated enzymes that merely reset proteins for regulation by kinase. However, the complexity and specificity of the Ser/Thr phosphatases arises from the association of the catalytic subunit with a large array of regulatory subunits. One of the most abundant Ser/Thr phosphatases is protein phosphatase 2A (PP2A) (Clark and Ohlmeyer, 2019; Wlodarchak and Xing, 2016). PP2A is composed of a catalytic subunit (PP2A-C) and a scaffolding subunit (PP2A-A). This core dimer (PP2AD) binds one of several regulatory subunits (PP2A-B) to form the PP2A heterotrimeric holoenzyme (Fig. 1). There are four gene families encoding the canonical regulatory PP2A-B subunit: PP2A-B, PP2A-B′, PP2A-B″ and PP2A-B‴ encoded by PPP2R2, PPP2R5, PPP2R3 and PPP2R6, respectively. The three to five isoforms in each regulatory subunit family display high sequence conservation, and neither sequence nor structural similarities exists between gene families. Regulatory subunits are further diversified by alternative splicing, such that any given cell type likely expresses more than a dozen distinct PP2A holoenzymes (Seshacharyulu et al., 2013).
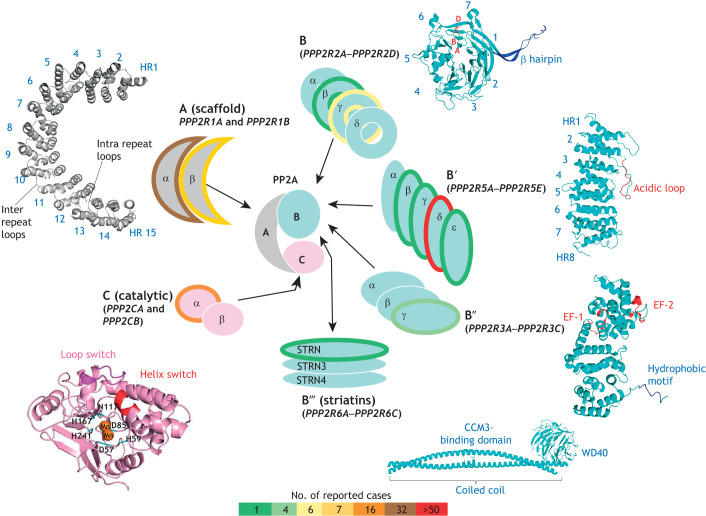
Fig. 1.
PP2A holoenzyme complexity and incidence of NDD mutations. Schematic representation of the multitude of possible PP2A heterotrimers formed by assembly of catalytic (C) in pink, scaffolding (A) in gray and diverse regulatory subunits (B, B′, B″ and B‴) in cyan. The outline of the different PP2A subunits is color-coded from green to red according to numbers of reported NDD cases as per published studies only (see scale on bottom). Ribbon structures of representative members are also shown highlighting important structural features. The PP2A-C subunit folds into an α/β fold, where the conformation of the loop switch determines the active site conformation. Residues colored in cyan chelate the catalytic manganese ions, and helix switch drives the binding to LCMT-1 and PP2A inhibitors (PDB ID: 3DW8; Xu et al., 2008). The PP2A-A subunit comprises 15 tandem heat repeats (HR1–HR15) (PDB ID: 3DW8; Xu et al., 2008). Each HR consist of antiparallel α-helices connected by inter and intra repeat loops. The PP2A-B subunits contain a seven-bladed (1–7) β-propeller; each blade is composed of four anti-parallel β-strands labelled A to D. Blade 2 extends out of the propeller to form a β hairpin arm that binds the A subunit in the holoenzyme (PDB ID: 3DW8; Xu et al., 2008). PP2A-B′ subunits consist of eight tandem HR (1–8) and possesses a highly conserved acidic loop (highlighted in red) (PDB ID: 2IAE; Cho and Xu, 2007). PP2A-B″ subunits are α-helical proteins with an N-terminal hydrophobic region (blue), two Ca2+-binding EF hand domains (red), as well as a C-terminal substrate-binding domain (not shown here) (PDB ID: 4I5L; Wlodarchak et al., 2013). The structure of PP2A-B‴ (STRN3) has an N-terminal coiled-coil domain, a central CCM3- binding domain and a C-terminal WD40 domain (PDB ID: 7K36; Jeong et al., 2021).
PP2A is recognized as a tumor suppressor and negative regulator of cell growth, mostly because of the direct inhibition of PP2A-C by cancer-promoting okadaic acid (Bialojan and Takai, 1988; Janssens et al., 2005; Li et al., 1995; Pallas et al., 1992). Additionally, deletions of PP2A-subunit-encoding genes and inactivating mutations have been identified in various cancers (Calin et al., 2000; Colella et al., 2001; Ruediger et al., 2001; Suzuki and Takahashi, 2003; Takagi et al., 2000; Wang et al., 1998). PP2A dysfunction has also been implicated in Alzheimer's disease (AD). Indeed, PP2A is the main phosphatase targeting the hyperphosphorylated tau, the main component of neurofibrillary tangles (Sontag et al., 2004a,b; Trojanowski and Lee, 1995; Vafai and Stock, 2002). PP2A expression and activity are reported to be much reduced in postmortem AD brains samples, whereas the methylation state of PP2A controls the sensitivity and resistance to β-amyloid-induced cognitive and electrophysiological impairments. Consistent with this, multiple in vivo studies have found that inhibiting PP2A produces AD-like tau pathology and cognitive impairment (Gnanaprakash et al., 2021; Nicholls et al., 2016; Staniszewski et al., 2020).
With whole exome (WES) or genome sequencing (WGS) becoming an affordable diagnostic tool, an increasing number of PP2A subunit mutations have been identified as likely causes of neuropsychiatric and neurodevelopmental disorders (NDDs). Most of these are de novo mutations (DNMs) that are present in the affected individual but not the parents. DNMs typically arise during oogenesis or spermatogenesis, although they can also occur during embryonic development, in which case they result in mosaicism (Acuna-Hidalgo et al., 2015; Biesecker and Spinner, 2013; Campbell et al., 2015; Dal et al., 2014; Lupski, 2010). Here, we review recent discoveries of pathogenic mutations in the PP2A holoenzyme, sourced from peer-reviewed literatures and additionally tabulate novel pathogenic single nucleotide variants (SNVs) identified from publicly available clinical databases (DECIPHER, ClinVar and SFARI; see Table S1). We summarize the genetic and clinical features of the associated NDDs and discuss implications of these mutations on the structure and function of PP2A as a regulator of signaling cascades in neuronal development.
The scaffolding PP2A-A subunit
In mammals, the two isoforms of PP2A-A – PP2A-Aα and PP2A-Aβ (encoded by PPP2R1A and PPP2R1B, respectively) – are ubiquitously expressed and highly conserved (Groves et al., 1999; Hemmings et al., 1990; Khew-Goodall and Hemmings, 1988; Stone et al., 1987; Zhou et al., 2003) (Table 1). PP2A-A is composed of 15 tandem Huntington elongation A-subunit TOR (HEAT) repeats (HRs). Each HR is composed of two α-helices connected by inter- and intra-repeat loops (Fig. 1). HR11 to HR15 anchors PP2A-C, while the variable regulatory B subunit binds to HR1 to HR10. Recent WGS and WES studies (Houge et al., 2015; Lenaerts et al., 2021; Wallace et al., 2019; Zhang et al., 2020) have reported a total of 16 SNVs on the PP2A-Aα isoform (Fig. 2A, red; Table S1). Houge et al. (2015) identified individuals with P179L, R182W and R258H mutations that show severe ID and display neurological deficits such as microcephaly, agenesis of the corpus callosum (ACC), and speech and motor disabilities. These symptoms together in patients are sometimes referred to as autosomal mental retardation 36 (MRD36). Recently, Lenaerts et al. (2021) reported 12 novel mutations in individuals who commonly displayed language delay, hypotonia and hypermobility in joints, but the severity of developmental delay (DD) ranged from mild to severe ID, correlating well with the diverse range of biochemical dysfunctions observed, as is briefly discussed below (Table S1). The one exception was that the individual harboring the S152F mutation did not have ID, possessing a full-scale IQ of 86, but did display autism spectrum disorder (ASD) (Deciphering Developmental Disorders Study, 2015; Houge et al., 2015; Lenaerts et al., 2021; Zhang et al., 2020). Among all these mutations, six recurrent mutations (M180T, M180V, R182W, S219L, V220M and R258H) have been identified in almost 75% of the total PPP2R1A patients (Lenaerts et al., 2021).
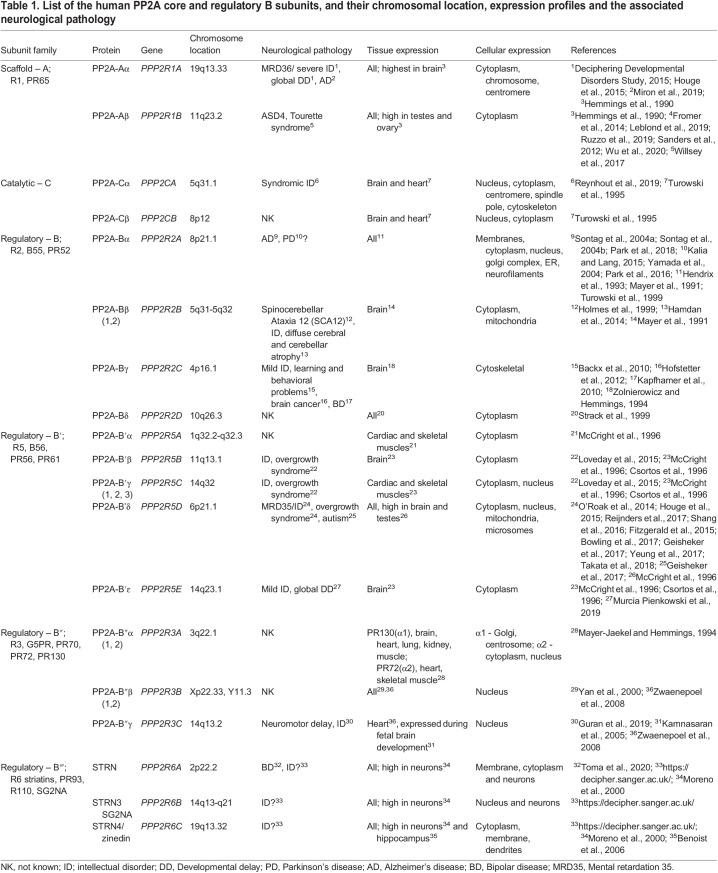
Table 1.
List of the human PP2A core and regulatory B subunits, and their chromosomal location, expression profiles and the associated neurological pathology
Fig. 2.
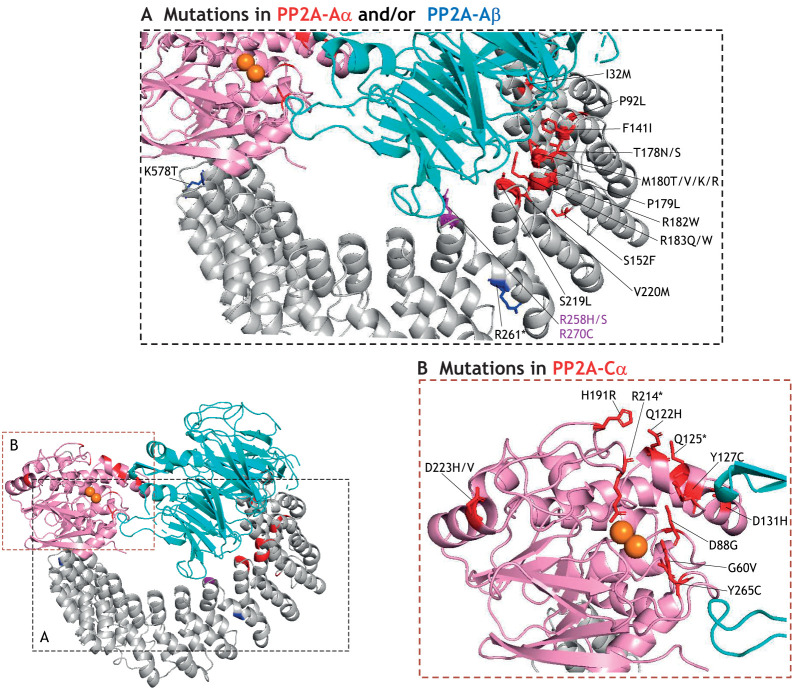
Mutations in the PP2A core dimer associated with NDDs. Ribbon representations of heterotrimeric PP2A complexes containing the scaffold A subunit (gray), catalytic Cα subunit (pink), and regulatory PP2A-Bα subunit (cyan) (PDB ID: 3DW8; Xu et al., 2008). (A) Magnification of the region within the black stippling box highlighting mutations in the PP2A-A subunit, with residues in red and blue representing those mutated in the PP2A-Aα and PP2A-Aβ isoform, respectively. The purple residue is a homologous residue mutated in both PP2A-Aα (R258H/S) and PP2A-Aβ (R270C). (B) Magnification of region within the brown stippling box highlights residues (in red) mutated in the catalytic PP2A-Cα subunit. Catalytic manganese ions are represented by orange spheres. The residue numbers apply to the respective protein sequence with accession numbers NM_014225.6 (Aα), NM_181699 (Aβ) and NM_002715.2 (Cα). Additional mutations of residues that could not be resolved in available crystal structures are listed in Table S1.
Structurally, most PP2A-Aα mutations (Fig. 2A, red) map to the HR1 to HR7 region that engages PP2A-B subunits. Most of these mutations reside adjacent to each other and/or affect the same amino acid within the intra-repeat loops between HR4 and HR7, suggesting this region as a hotspot for the diverse biochemistry and phenotypes associated with PP2A-Aα-mediated NDD. The unique S152F mutation residing within HR4 shows no functional impairments biochemically. However, neurons with this mutation show a significant decrease in dendritic spines indicating its pathogenicity via altered functionality (Lenaerts et al., 2021). Most PP2A-Aα mutations (refer to Table S1 for specific mutation effects) show no or less binding to the PP2A-Bα (encoded by PPP2R2A) but not to PP2A-B′δ (encoded by PPP2R5D) or PP2A-B‴ (encoded by PPP2R6B). Interestingly, these variants bind strongly to STRN3 (encoded by PPP2R6B), but it is unclear how the interaction is associated with the severe ID, seizures and microcephaly in patients. Several PPP2R1A mutations (F141L, P179L, R182W, R183W, S219L, V220M and R258H/S) lead to significantly less binding to PP2A-C despite being present on HR1 to HR8, which are not known to mediate PP2A-C subunit binding, thereby suggesting that some B subunits indirectly mediate binding between PP2A-A and PP2A-C via its interaction with PP2A-A subunit. In a recent cryo-EM structure, STRN3 has been shown to interact via HR1 of the PP2A-A subunit, wherein a mutation within this interface causes diminished PP2A-A and PP2A-C binding suggesting that there would be a similar effect on holoenzyme assembly mediated through the B subunit (Jeong et al., 2021). Notably, functionally disruptive mutations, such as P179L, R182W, R183W, S219L, V220M, R258H/S, are also found in the Catalogue of Somatic Mutations in Cancer (COSMIC), which shows that these exact PPP2R1A mutations cause cancer growth and migration and drug resistance (Haesen et al., 2016; Jeong et al., 2016; O'Connor et al., 2020). Additionally, functionally impaired mutations can cause insufficient dephosphorylation of PP2A substrates (Houge et al., 2015). In HEK293 cells overexpressing the PP2A-Aα R182W mutant, an increased phosphorylation of Ser9 and inactivation of GSK-3β has been observed (Houge et al., 2015). GSK-3β, being an established PP2A-B′δ substrate, plays a pivotal role in controlling the neuronal progenitor proliferation and regulating neuronal polarity (Houge et al., 2015; Jiang et al., 2005; Liu and Eisenman, 2012). With severe biochemical and phenotypic effects of these PPP2R1A mutations, further studies need to be performed in both in vitro and in vivo systems.
In addition to PP2A-Aα, seven de novo or inherited mutations have been reported in the PP2A-Aβ isoform. Six individuals display mild to severe ASD (Fromer et al., 2014; Leblond et al., 2019; Ruzzo et al., 2019; Sanders et al., 2012; Wu et al., 2020) and one individual has Tourette's syndrome (Willsey et al., 2017) (Fig. 2A, blue; Table S1). One missense variant, R270C, is homologous to R258H/S mutation (Fig. 2A, purple) on PP2A-Aα, suggesting a likely similar effect on holoenzyme assembly and pathogenicity. Three frameshift mutations, V115C/G and D211V, and a nonsense mutation, R261*, result in early protein termination of PP2A-Aβ and likely lead to nonsense-mediated decay, hinting towards haploinsufficiency, although this requires further investigation and biochemical characterization (Leblond et al., 2019; Wu et al., 2020).
The catalytic PP2A-C subunit
The two isoforms of PP2A-C, PP2A-Cα and PP2A-Cβ (encoded by PPP2CA and PPP2CB, respectively) are highly conserved among mammals (Table 1) (Orgad et al., 1990; Seshacharyulu et al., 2013). PP2A-C adopts a compact spherical structure composed of α/β folds; the two central β-sheets are connected via protein loops comprised of six highly conserved residues – D57, H59, D85, N117, H167 and H241 – that chelate the catalytic manganese ion (Xing et al., 2006) (Fig. 1). PP2A activity is regulated by two important PTMs – phosphorylation (Chen et al., 1992) and methylation (Lee and Stock, 1993) – on its highly conserved C-terminal tail (Janssens et al., 2008) mediated by an array of proteins (Box 1). PP2A-C has been reported to dephosphorylate and activate various proteins that are critical in neuronal growth, such as actin-depolymerizing factor (ADF), cofilin (Kuhn et al., 2000), collapsin response mediator protein 2 (CRMP2) (Zhu et al., 2010) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Fink et al., 2003; Monroe and Heathcote, 2013). Indeed, overexpression of PP2A-C enhances the formation of neurites in neuroblastoma-2a cells, whereas inhibition of the PP2A-C activity in neuroblastoma-2a cells and hippocampal neurons reduces axonal growth and neurogenesis (Liu et al., 2012; Prinetti et al., 1997). Recently, a WES and microarray-based copy-number analysis have identified 16 patients with 15 different variants caused by DNMs in PP2A-Cα (Reynhout et al., 2019). These include eight missense non-recurrent mutations (G60V, D88G, Q122H, Y127C, D131H, D223V/H, and Y265C), one recurrent missense mutation (H191R), three nonsense mutations (Q125*, R214*, and R295*), a single amino acid duplication (F308dup), a frameshift (F146L fs*29), and a partial gene deletion (del in Chr5) (Reynhout et al., 2019) (Fig. 2B). These individuals have mild to profound ID and DD, severe language impairment, hypotonia, epilepsy, ASD and worsening behavioral problems (Table S1).
Box 1. Regulation of PP2A and the associated NDDs.
The biogenesis and enzymatic activity of PP2A is mediated by a well-coordinated network of regulatory proteins that transiently associate with PP2A-C or the core enzyme PP2AD. These PP2A regulatory proteins include α4, PP2A phosphatase activator (PTPA), leucine carboxy methfyl transferase-1 (LCMT-1), and PP2A methyl esterase-1 (PME-1) (Sents et al., 2013). α4 acts as a chaperone during the early stages of PP2A holoenzyme biogenesis (Kong et al., 2009; Stanevich et al., 2014). α4 may also regulate PP2A turnover, potentially by interacting with the E3 ubiquitin ligase Mid1 (LeNoue-Newton et al., 2011; McConnell et al., 2010). Mutations in α4, c.53delT and c.55T>A at Xq13 have been identified in two brothers with a novel X-linked syndrome presenting with ACC, ID, scoliosis and micrognathia (Graham et al., 2003).
LCMT-1 and PME-1 regulate methylation of the C-terminal tail of PP2A-C, whereby methylation increases the binding affinity of core enzyme towards the regulatory subunit and promotes the biogenesis of active PP2A holoenzymes (Stanevich et al., 2011; Xing et al., 2008). It is also reported that PP2A methylation and its methylating enzyme, LCMT-1, was significantly reduced in substantia nigra and frontal cortex in post-mortem brains of individuals with PD, dementia with lewy bodies (DLB), AD and progressive supranuclear palsy (PSP) (Park et al., 2018), whereas the level of PME-1 and demethylated PP2A was significantly increased, indicating the dysregulation of PP2A in these neurodegenerative disorders.
PTPA is an ATP/Mg2+-dependent PP2A chaperone and proline cis-trans isomerase, which plays a critical role in the biogenesis of an active PP2A (Guo et al., 2014). PTPA was recently implicated in the etiology of Angelman's syndrome (AS), a cognitive disorder characterized by severe DD, ID, epilepsy, seizures and motor dysfunctions (Margolis et al., 2015; Wang et al., 2019). AS is caused by lack of expression of the E3 ubiquitin ligase UBE3A from the maternally inherited chromosome 15 in neurons (Bird, 2014). PTPA was identified as a substrate for UBE3A-mediated ubiquitylation, and proteasomal degradation and loss of maternal UBE3A increased PTPA protein levels, promoted holoenzyme assembly and enhanced PP2A activity, leading to defects in dendritic spine maturation in an AS mouse model (Wang et al., 2019).
Functional studies have shown that the PP2A activity is completely inhibited by the D88G, Y127C, R214*, Y265C, and F308dup mutations, while a significant reduction is seen with the Q122H, H191R, D223H, and R295* mutations (Reynhout et al., 2019). The decrease in catalytic activity is likely due to disruption of methylation by mutations of key residues in the catalytic center (D88, Y127 and Y265), which make direct contact with leucine methyl transferase (LCMT-1), or due to the absence of C-terminal tail in the truncating variants R214* and R295*. These mutations therefore are likely to cause pathologies by a loss-of-function mechanism (Reynhout et al., 2019).
Biochemically, most catalytically impaired mutations retain the ability to interact with endogenous PP2A-A but vary vastly in their ability to form heterotrimers with different PP2A-B regulatory subunits, suggesting different mechanisms of pathogenicity. For example, D88G, R295* and F308dup variants only form heterotrimers with PP2A-B′δ (encoded by PPP2R5D), the Y127C mutation forms heterotrimers only with the PP2A-B and PP2A-B‴ subunits, suggesting a dominant-negative mode of action against PP2A-B′δ substrates and PP2A-B and PP2A-B‴, respectively. Haploinsufficiency is also proposed based on the premature stop mutations, partially deleted PPP2CA and poorly expressing gene product. H191R, a recurrent missense mutation, is located in the conserved ‘loop switch’ (Jiang et al., 2013) (Fig. 2B) region that determines the proper conformation of the active site. A missense mutation therefore would cause PP2A-C inactivation and decrease its binding to PP2A-B′δ, hinting towards haploinsufficiency (Reynhout et al., 2019).
Despite the high expression of PP2A-C in the brain, especially the hippocampus (a region of lifelong neurogenesis) (Liu et al., 2010), and of the role of PP2A-C in neuronal growth, it is still unclear how these specific PP2A-Cα mutations alter signaling cascade to cause pathologies. Brain-specific PPP2CA knockout (KO) mice have phenotypic features similar to individuals with DNMs, including shrinkage of cortical neurons, synaptic plasticity impairments, and learning and memory deficits (Liu et al., 2018), thereby offering a great in vivo model system for further studies.
The PP2A regulatory B subunit family
The mammalian B family of regulatory PP2A-B subunits (also referred to as B55 or PR55) consists of four isoforms: α, β, γ and δ, which are encoded by the genes PPP2R2A–PPP2R2D, respectively. They share high protein sequence similarity (>90% for humans) except at their divergent N-termini, and show specific expression patterns and subcellular localizations (Table 1) (Healy et al., 1991; Mayer et al., 1991; Strack et al., 1999; Zolnierowicz and Hemmings, 1994). The crystal structure of Bα within the PP2A holoenzyme reveals a seven-bladed β-propeller made up of WD40 (tryptophan-aspartate) repeats (Strack et al., 2002). A β-hairpin extending from the second blade contributes to binding of the A subunit, as do residues at the bottom of the centrifuge rotor-shaped structure/β propeller structure of the regulatory subunit (Fig. 1) (Xu et al., 2008). Several kinase signaling cascades are known to be regulated by the different PP2A-B subunits. Specifically, PP2A-Bα inhibits AKT signaling by dephosphorylating phosphorylated T308 (Kuo et al., 2008), PP2A-Bγ promotes neuronal differentiation by activating the MAPK cascade (Strack, 2002), and PP2A-Bα and PP2A-Bδ directly dephosphorylate ERK proteins (Van Kanegan et al., 2005). In Xenopus egg extracts, PP2A-Bα and PP2A-Bβ are involved in multitude of metabolic processes, such as mitochondria function, cell division, apoptosis, cytoskeleton organization and DNA damage repair (Cundell et al., 2016; Wang et al., 2018), whereas PP2A-Bδ controls mitotic entry and exit (Mochida et al., 2009). Among the four B family genes, a possibly pathogenic DNM and a germline breakpoint mutation have been identified in PPP2R2B and PPP2R2C, respectively, as discussed below (Table S1).
The PP2A-Bα and PP2A-Bδ isoforms
PP2A-Bα (encoded by PPP2R2A) and PP2A-Bδ (encoded by PPP2R2D) are expressed in many tissues, including the brain. PPP2R2A is an essential gene, as homozygous KO mice die during embryonic development (Panicker et al., 2020). Specifically, PP2A-Bα is a critical regulator of epidermal stratification and ectoderm development (Panicker et al., 2020). Moreover, dysregulation of PP2A-Bα expression has been implicated in the pathogenesis of neurodegenerative diseases, such as Alzheimer's disease (AD) (Sontag et al., 2004a,b) and Parkinson's disease (PD) (Park et al., 2016). The PP2A-Bα holoenzyme is also the primary tau phosphatase; its downregulation is correlated with increased tau phosphorylation in vivo and in AD patients (Sontag et al., 2004a,b). Similarly, PP2A-Bα plays an important role in dephosphorylating hyperphosphorylated α-synuclein, a hallmark of PD, which leads to misfolding and aggregation of α-synuclein into proto-fibrils (Lee et al., 2011). However, currently, there is no direct link between PP2A-Bα or PP2A-Bδ and NDDs, despite their high expression in brain. However, many commercially available antibodies against the B subunits do not actually differentiate between subunits of this regulatory family, which may lead to difficulty in attributing specific effects of these genes.
The PP2A-Bβ isoform
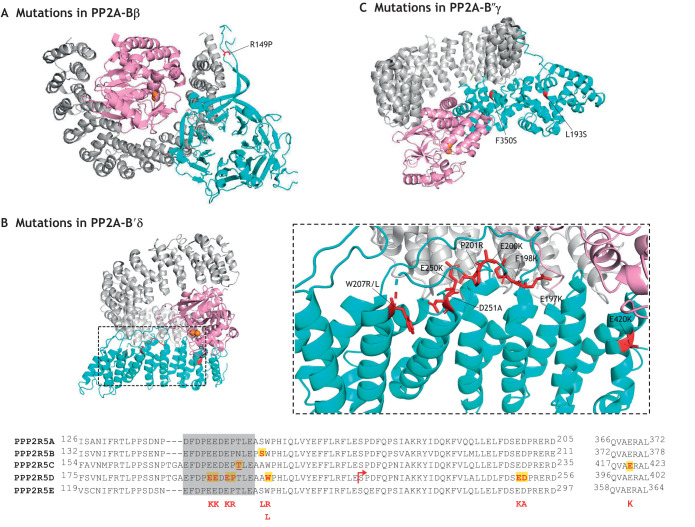
PP2A-Bβ (encoded by PPP2R2B) is expressed specifically in neurons and is associated with the autosomal-dominant neurodegenerative disease spinocerebellar ataxia type 12 (SCA12) (Fujigasaki et al., 2001; Holmes et al., 1999). SCA12 is caused by a CAG repeat expansion (>50 repeats) in a 5′-UTR or promoter region of PPP2R2B, but whether and how the repeat expansion affects PPP2R2B expression is currently unknown. SCA12 patients present cerebral and cerebellar atrophy, bradykinesia, ataxic gait, dysarthria, tremors and dementia (Holmes et al., 2001, 2003; Srivastava et al., 2001). PPP2R2B undergoes alternative splicing and alternative promoter use, which gives rise to several N-terminal sequence variants. The two most abundant isoforms, PP2A-Bβ1 and PP2A-Bβ2, are targeted via their divergent N-terminal tails to the cytosol and the mitochondrial surface, respectively (Dagda et al., 2003; Merrill et al., 2013). During neuronal stress in vitro, PP2A-Bβ2 induces mitochondrial fragmentation by dephosphorylating S637 and activating the mitochondrial fission enzyme Drp1 (also known as DNM1L), which increases susceptibility to neuronal insults and cell death (Dagda, 2006; Dagda et al., 2003, 2008; Merrill et al., 2013). This highly conserved inhibitory phosphorylation site confers neuroprotection by outer-mitochondrial protein kinase A (PKA) in cerebral ischemic stroke (Flippo et al., 2018). Excessive mitochondrial fission is associated with multiple neurodegenerative diseases (Serasinghe and Chipuk, 2017). We recently reported that knocking out PP2A-Bβ2 in mice provides protection against stroke damage by maintaining neuroprotective Drp1 phosphorylation (Flippo et al., 2020). We also showed that this reversible phosphorylation of Drp1 at S637 by PP2A-Bβ2 and AKAP1–PKA (AKAP1 is a kinase anchoring protein for PKA) affects dendrite and synapse development in cultured hippocampal neurons. Phosphorylation of Drp1 by AKAP1–PKA increases mitochondrial length and dendritic occupancy, enhances the dendritic outgrowth, and reduces the synapse number and density, whereas the dephosphorylation of Drp1 by PP2A-Bβ2 has the opposite effect (Dickey and Strack, 2011). A recent WES study has identified a de novo missense mutation at R149P (Bβ1 numbering) in PPP2R2B that is associated with moderate to severe ID and pervasive DD not otherwise specified (PDD-NOS), along with poor eye contact, intractable seizures, autistic features and aggressive behaviors (Hamdan et al., 2014). This mutation is situated within the extended β-hairpin that contacts PP2A-A and thus may block association of PP2A-Bβ with the holoenzyme (Fig. 3A); however, further biochemical and in vivo studies need to be performed.
Fig. 3.
Mutations in PP2A regulatory B, B′ and B″ subunits associated with NDDs. Ribbon representation of the ABαC holoenzyme structure consisting of a scaffold A subunit (gray), a catalytic Cα subunit (pink), and regulatory PP2A-Bα subunit (cyan) (PDB ID: 3DW8; Xu et al., 2008). (A) A single missense mutation in PP2A-Bβ (NM_004576.2) associated with NDD is highlighted in red, which resides within the β hairpin, mapped on the Bα-containing crystal structure of the ABαC holoenzyme. (B) Ribbon representation of the AB′γC holoenzyme (PDB ID: 2IAE; Cho and Xu, 2007). The inset on the right highlights residues mutated in PP2A-B′δ (NM_006245) that are associated with NDDs. The partial alignment of PP2A-B′ subunits emphasizes the sequence conservation of residues affected by recurrent pathogenic DNMs; these are primarily in PP2A-B′δ and concentrated in an acidic loop (shown in a gray box in the sequence alignment of the different B′ isoforms) (reviewed in Mirzaa et al., 2019). The underlined T mutation is an in-frame deletion, while the arrow indicates a frame-shifting mutation that introduces a premature stop codon. The mutations in B′δ are mapped onto to the resolved crystal structure of AB′γC holoenzyme. (C) Highlighted here are NDD-causing mutations associated with B″γ (NM_017917) mapped on the crystal structure of the AB″βC holoenzyme (PDB ID: 4I5L; Wlodarchak et al., 2013). Additional mutations outside of resolved crystal structures are listed in Table S1.
The PP2A-Bγ isoform
PP2A-Bγ (encoded by PPP2R2C) is also predominantly expressed in the brain (Zolnierowicz and Hemmings, 1994). Forced expression of PP2A-Bγ promotes neuronal differentiation via transient activation of MAPK signaling in a neuronal cells line (Strack, 2002). PP2A-Bγ also regulates cancer cell proliferation by suppressing the mTOR signaling pathway, and low levels of PP2A-Bγ activity have been linked to brain cancer (Fan et al., 2013; Li et al., 2015). Additionally, the PPP2R2C gene is located within a region of chromosome 4p16 that is associated with bipolar disorder (BP) and single nucleotide polymorphisms (SNPs), and other BP risk haplotypes have been reported in PPP2R2C (Borsotto et al., 2007). Providing a potential pathogenic mechanism, PP2A-Bγ associates, dephosphorylates and activates the K+ channel KCNQ2 (Borsotto et al., 2007; Hu et al., 2000). In another reported family pedigree, PPP2R2C is disrupted by an autosomal dominant familial reciprocal translocation (4;6) (p.16.1; q22) on chromosome 4 (Backx et al., 2010). Affected individuals suffer from mild ID, learning and behavior problems, including aggressive, obsessive, transgressive behaviors and tonic-clonic epilepsy episodes (Backx et al., 2010).
The PP2A regulatory B′ subunit family
The B′ family members (α, β, γ, δ and ε), encoded by PPP2R5A–PPP2R5E, are highly conserved and contain divergent N- and C-termini that are subject to alternative splicing (see sequence alignment in Fig. S1A). The crystal structure of the PP2A-B′γ holoenzyme reveals that B′-family members are curved superhelical structures containing eight tandem α-helical HEAT-like repeats (Xu et al., 2006) (Fig. 1). The concave surface facing PP2A-C is negatively charged, while the convex side contacting PP2A-A contains conserved hydrophobic residues (Xu et al., 2006). The PP2A-B′-family members have ubiquitous tissue expression with the greatest abundance in the brain and testis (McCright et al., 1996). They also display distinct localization, with PP2A-B′δ and PP2A-B′γ containing a predicted nuclear-localization motif (McCright et al., 1996; Tehrani et al., 1996), suggestive of specific regulatory functions (Nijenhuis et al., 2014; Vallardi et al., 2019). Indeed, the B′ subunits are important for cell cycle regulation, where PP2A-B′γ and PP2A-B′ε have been shown to control anaphase onset and cytokinesis, whereas PP2A-B′α, PP2A-B′β and PP2A-B′δ mediate chromosome alignment and segregation (Bastos et al., 2014). All five B′-family members are phosphorylated proteins (McCright et al., 1996). Both PP2A-B′δ and the splice variant PP2A-B′γ3 contain a consensus motif for PKA-mediated phosphorylation (RRKS) (McCright et al., 1996; Tehrani et al., 1996), which in the case of PP2A-B′δ has been shown to activate the PP2A holoenzyme (Yu and Ahn, 2010).
The mechanisms by which PP2A-B′-containing holoenzymes recognize specific substrates are beginning to be unraveled. We previously reported charge–charge interactions as a general mechanism for substrate recognition by the B′-family members (Jong et al., 2020; Saraf et al., 2010). More recently, the short linear motif (SLiM) LxxIxE has been identified in B′-interacting proteins, such as BuBR1 and KIF4A (Hertz et al., 2016; Kruse et al., 2020; Wang et al., 2016). BuBR1 is a multidomain protein kinase that regulates mitotic spindle checkpoint (Bolanos-Garcia and Blundell, 2011; Takahashi et al., 2016), while KIF4A is a chromokinesin that regulates mitosis, as shown by its function in chromosome condensation and segregation (Takahashi et al., 2016). These SLiMs are recognized by a well-conserved hydrophobic binding pocket on B′ subunit between HEAT repeats 3 and 5 (Hertz et al., 2016). Phosphorylation of residues within the SLiM further strengthens the interaction with B′ subunits (Hertz et al., 2016). A recent study described a highly conserved acidic surface adjacent to the SLiM binding pocket on B′ subunits that electrostatically interacts with a basic patch motif on substrates, suggesting that charge–charge interactions enhance the dephosphorylation of SLiM-containing substrates by PP2A-B′ (Wang et al., 2020). Below, we briefly described each of these five B′ subunit-encoding genes and its associated NDDs (Table 1; Table S1).
The PP2A-B′α isoform
Primarily studied in relation to cancer progression (Janghorban et al., 2017; Liu et al., 2020; Zhuang et al., 2017), the role of PP2A-B′α in neuronal development and physiology is poorly understood. In non-neuronal cells, PP2A-B′α regulates cell cycle progression to promote mitosis (Foley et al., 2011; Porter et al., 2013) and initiates DNA repair response following DNA damage (Freeman et al., 2010). In addition, PP2A-B′α regulates cell growth and proliferation by inhibiting Wnt signaling and mediates degradation of c-Myc and β-catenin (Li et al., 2001). Indeed, several cancer-related studies have identified c-Myc as a direct substrate of PP2A-B′α (Arnold and Sears, 2006; Leonard et al., 2020). At present, no NDDs associated with PP2A-B′α mutations have been reported.
The PP2A-B′β isoform
Primarily expressed in neurons with predominant somatodendritic distribution (Saraf et al., 2007), PP2A-B′β has been implicated in dopamine synthesis (Saraf et al., 2007), hippocampal long-term potentiation (LTP) (Fukunaga et al., 2000) and signal transduction in response to stimulation with various growth factors (Brandt et al., 2008; Van Kanegan and Strack, 2009). PPP2R5B has been recently linked to NDDs as two chromosomal deletions encompassing PPP2R5B have been reported (Boyle et al., 2015; Mohrmann et al., 2011). In addition, one de novo missense mutation in PPP2R5B (S161L) is reported as a pathogenic variant that causes moderate ID and human overgrowth (Loveday et al., 2015) (Table S1). Based on its close proximity to the catalytic subunit, it is postulated that the PP2A-B′β S161L mutation compromises substrate binding and dephosphorylation, thus enhancing phosphoinositide 3-kinase (PI3K)/AKT signaling to cause human overgrowth syndrome. N-terminal phosphorylation of PP2A-B′β has shown to regulate neuronal signaling (Bidinosti et al., 2016). In induced pluripotent stem cell (iPSC)-derived neurons from Phelan–McDermid syndrome (PMDS) patients harboring SHANK3 deletions, PP2A-B′β was found to be hyperphosphorylated at S46 due to elevation of its kinase CLK2, which inhibits AKT–mTORC1 signaling (Bidinosti et al., 2016).
The PP2A-B′γ isoform
The PP2A-B′γ isoform (encoded by PPP2R5C), consists of three splice variants (γ1, γ2, and γ3), is the most widely studied isoform in the B′ family. Various cellular functions of the PP2A-B′γ-containing holoenzyme have been documented, including a role in cell cycle regulation through interaction with shugoshins (Xu et al., 2009), CYK4 (Wu et al., 2017), KIF4A (Bastos et al., 2014), BuBR1 (Varadkar et al., 2017), p53 (also known as TP53) (Li et al., 2007) and the APC/C–CDC20 complex (Fujimitsu and Yamano, 2020). The PP2A-B′γ-containing holoenzyme also regulates Wnt/β-catenin signaling during lung airway morphogenesis (Everett et al., 2002) and vascular remodeling (Jeon et al., 2010), which is likely to be mediated by interaction with Naked cuticle (NKD1), an inhibitor of the Wnt signaling cascade (Creyghton et al., 2005). The PP2A-B′γ subunit has also been suggested to act as an adaptor protein by interacting with liprin-α, a binding partner of the LAR receptor tyrosine phosphatase (Arroyo et al., 2008). When bound to liprin-α, the PP2A-B′γ subunit does not promote cell transformation, but may regulate cell morphology by facilitating the recruitment of substrates to membrane structures containing liprin-α (Arroyo et al., 2008). Pathologically, PP2A-B′γ has been associated with cancer as a tumor suppressor (Chen et al., 2004; Ihara et al., 2002; Ito et al., 2000; Nobumori et al., 2012; Sablina et al., 2010; Shouse et al., 2011; Zheng et al., 2011). While rare, one case of a DNM in PP2A-B′γ is linked to ID and human overgrowth (Loveday et al., 2015) (Table S1). Specifically, an in-frame 3-bp deletion (c.468_470delAAC) causes loss of T157 (based on NP_001133197), which is homologous to T181 (based on NP_001339842), a residue that disrupts the contact of PP2A-B′γ with the PP2A-C subunit and subsequent substrate recognition (Houge et al., 2015; Shang et al., 2016). With regard to the possible pathogenic mechanism, it has been proposed that the PP2A-B′γ T157 deletion disrupts PI3K–AKT–mTOR signaling (Loveday et al., 2015). As there are no published biochemical or functional studies of PP2A-B′γ mutations, further characterization on PPP2R5C mutations is highly warranted.
The PP2A-B′δ isoform
The PP2A-B′δ (encoded by PPP2R5D) isoform is the largest member of the B′ family with unique N- and C-terminal sequence extensions (McCright et al., 1996). PP2A-B′δ contains a PKA phosphorylation motif 570RRKSEL575 (McCright et al., 1996), and phosphorylation at Ser573 has been shown as essential for activation of the PP2A-B′δ-containing holoenzyme (Ahn et al., 2007a; Ranieri et al., 2018; Yu and Ahn, 2010). A number of studies report roles of the PP2A-B′δ-containing holoenzyme. First, the PP2A-B′δ-containing holoenzyme regulates cell cycle progression by dephosphorylating the mitotic activator Cdc25 at Thr138 and releasing 14-3-3 to promote mitosis (Margolis et al., 2006). Second, it facilitates the nuclear translocation of voltage-dependent L-type Ca2+ channel subunit β4 isoform (CACNB4) to regulate neuronal excitability (Ronjat et al., 2013). Third, the PP2A-B′δ subunit interacts with and promotes cytoplasmic localization of PPM1G, a nuclear-localized Ser/Thr type 2C phosphatase family member, to dephosphorylate α-catenin and regulate the proper assembly of adherens junctions (Kumar et al., 2019). Finally, dopaminergic neurotransmission is regulated via cAMP-dependent phosphorylation of the PP2A-B′δ subunit and activation by PKA, leading to dephosphorylation of DARPP-32 at T75 (Ahn et al., 2007a; Yu and Ahn, 2010), or by PKC-mediated phosphorylation of the PP2A-B′δ subunit, which in this case leads to dephosphorylation of tyrosine hydroxylase at S40 (Ahn et al., 2011).
Over the past decade, mutations in PPP2R5D have been reported to cause various pathologies, such as human overgrowth (Loveday et al., 2015), ID (de Ligt et al., 2012; Gilissen et al., 2014; Stessman et al., 2017), Parkinsonism (Hetzelt et al., 2021; Kim et al., 2020; Walker et al., 2021) and ASD (Abrahams et al., 2013; Basu et al., 2009; Firth et al., 2009; Iossifov et al., 2014; Satterstrom et al., 2020). ID caused by de novo PPP2R5D mutations is referred to as MRD35 or Jordan's Syndrome after one of the first patients diagnosed in the United States by WES. Jordan's Guardian Angels (JGA) was established in 2017 to support research on Jordan's Syndrome (https://jordansguardianangels.org/). Currently, JGA has identified more than 130 individuals with Jordan's Syndrome (Biswas et al., 2020; Mirzaa et al., 2019). Ten pathogenic PPP2R5D missense mutations have been reported so far, P53S, E197K, E198K, E200K, E250K, D251A, P201R, W207R, W207L and E420K (Fig. 3B; Table S1) (Houge et al., 2015; Loveday et al., 2015; Mirzaa et al., 2019; Reijnders et al., 2017; Shang et al., 2016; Yeung et al., 2017). E198K mutation is the most common, accounting for approximately one-third of all cases. Except for P53S, E250K, D251A and E420K, most mutations lie in the highly conserved acidic loop of the PP2A-B′δ subunit (Fig. 3B, red). This acidic loop faces and directly contacts PP2A-C (Shang et al., 2016) (Fig. 1). Acidic-to-basic mutations in this region have been reported to destabilize the PP2A-B′δ-containing holoenzyme (Houge et al., 2015), but may also affect substrate specificity (Saraf et al., 2007). Further functional studies have suggested that most PPP2R5D mutations cause Jordan's syndrome by dysregulating PI3K–AKT–mTOR signaling, which regulates cellular proliferation and growth (Loveday et al., 2015; Yeung et al., 2017). Furthermore, a recent study has shown that holoenzyme containing E420K PP2A-B′δ forms a complex with AKT family proteins and suppresses the dephosphorylation of AKT (Papke et al., 2021). Interestingly, mice with global KO of both PPP2R5D alleles are viable and fertile, and display no behavioral or cognitive abnormalities (Louis et al., 2011). In the brain, the KO has no apparent effect on phosphorylation of AKT family proteins although S9 GSK3β phosphorylation is paradoxically decreased (Louis et al., 2011), perhaps because of developmental compensation by other B′ regulatory subunits. PPP2R5D KO mice also exhibit spatially restricted tau hyperphosphorylation that is linked to hyperactivation of CDK5 and GSK3β in the brain (Louis et al., 2011). In addition, hyperactivation of GSK3β is also observed in the liver of the KO mice, which would explain the spontaneous tumor development, validating the role of PP2A as a tumor suppressor (Lambrecht et al., 2018). More recently, the PPP2R5D missense mutations E198K, E200K and E250K have been identified in several patients with early-onset atypical PD (Kim et al., 2020; Walker et al., 2021). These observations suggest that PPP2R5D dysregulation in the brain can cause both neurodevelopmental and neurodegenerative diseases.
The PP2A-B′ε isoform
The functional role of PP2A-B′ε (encoded by PPP2R5E) is not well studied. However, initial studies have described roles for PP2A-B′ε in the regulation of embryonic development via Wnt/β-catenin signaling (Jin et al., 2010; Vinyoles et al., 2017; Yang et al., 2003), microtubule organization through interaction with microtubule crosslinking factor 1 (MTCL1) (Hyodo et al., 2016), vertebrate eye development via the PI3K/AKT pathway (Rorick et al., 2007), and KLHL42-regulated and TGFβ-mediated profibrotic signaling (Lear et al., 2020). While downregulation of PPP2R5E has been associated with cancers (Cristobal et al., 2013, 2014; Liu et al., 2014; Yang et al., 2013), PPP2R5E mutations have recently been linked to mild ID and global DD (Murcia Pienkowski et al., 2019). De novo balanced chromosomal translocations involving chromosomes 5q11.2 and 14q23.2 appears to be caused primarily by the PPP2R5E gene deletion on chromosome 14 (Table S1) (Murcia Pienkowski et al., 2019). Further analyses of the PPP2R5E gene reveal a high probability of loss-of-function intolerant (pLI) score (0.9994) and a high DOMINO score, which measures the likelihood of a dominant inheritance (0.86), both of which are consistent with a dominant effect (Murcia Pienkowski et al., 2019). How PPP2R5E haploinsufficiency alters PP2A-mediated dephosphorylation events in neurodevelopment is not known.
The PP2A regulatory B″ subunit family
The B″ family members are extended α-helical proteins with an N-terminal hydrophobic region, two EF hand Ca2+-binding domains and a substrate-binding domain at their C-terminus (Fig. 1) (Ahn et al., 2007b; Hendrix et al., 1993; Li and Virshup, 2002; Wlodarchak et al., 2013). Both the hydrophobic region and the first EF hand contact PP2A-A, whereas the second EF hand and an adjacent α-helix of B″ contact PP2A-C (Wlodarchak et al., 2013). The B″ family consists of three isoforms encoded by three genes, B″α1/α2 (also known as PR130 and PR72, encoded by PPP2R3A) (Janssens et al., 2003, 2016), B″β1/β2 (PR70, encoded by PPP2R3B) (Dovega et al., 2014; Yan et al., 2000; Zwaenepoel et al., 2008), and B″γ (G5PR, encoded by PPP2R3C) (Xing et al., 2006; Zwaenepoel et al., 2008). B″ subunits are implicated in various cellular events, such as cell cycle progression (Voorhoeve et al., 1999; Wlodarchak et al., 2013; Yan et al., 2000), cell migration (Janssens et al., 2016), B-cell survival (Xing et al., 2005), neuronal signaling (Ahn et al., 2007b) and Wnt signaling (Creyghton et al., 2006). A sequence alignment of B″ family members is shown in Fig. S1B. Among the three isoforms, PPP2R3C has recently been associated with autosomal recessive mutations in four girls with syndromic 46, XY gonadal dysgenesis (Swyer syndrome), who also exhibited extragonadal abnormalities including neuromotor delay and ID (Guran et al., 2019). The parents of these individuals are healthy heterozygous carriers of the PPP2R3C pathogenic mutations L103P, L193S and F350S, of which F350S maps to the first EF hand (Guran et al., 2019) (Fig. 3C; Fig. S1). In the dysgenetic gonads of the affected individuals, SOX9 phosphorylation is greatly diminished (Guran et al., 2019). PKA- and PKC-mediated SOX9 phosphorylation have important roles in testicular development and chondrogenesis (Guran et al., 2019; Huang et al., 2000; Matta and Mobasheri, 2014), and the reduced SOX9 phosphorylation is therefore a plausible mechanism for gonadal dysgenesis and facial deformity in the affected individuals. These findings also further suggest that homozygous PPP2R3C mutations might enhance the catalytic activity of PP2A to impair SOX9 signaling in gonadal and bone development (Guran et al., 2019). Further studies are required to understand the effects of PPP2R3C mutations on PP2A holoenzyme assembly and activity.
The PP2A regulatory B‴ subunit family
PP2AD also forms stable complexes with the large multidomain scaffolding proteins of the striatin family (termed the B‴ family), which consists of three members, striatin (STRN, encoded by PPP2R6A), STRN3 (encoded by PPP2R6B) and STRN4 (encoded by PPP2R6C) (Hwang and Pallas, 2014; Moreno et al., 2000). Striatins contain four protein–protein interaction domains – a caveolin-binding domain, a coiled-coil domain, a calmodulin-binding domain, and a WD-repeat domain, which folds into a β-propeller structure (Bartoli et al., 1999; Castets et al., 2000). The coiled-coil domain mediates striatin dimerization and its association with PP2A-A and PP2A-C (Chen et al., 2014). Striatins are also the eponymous core components of striatin-interacting phosphatase and kinase (STRIPAK) complexes, which include kinases and other signaling and regulatory proteins besides PP2A-A and PP2A-C. A recent cryo-EM structure of human STRIPAK identifies a non-canonical PP2A complex that comprises of four copies of STRN3 coiled-coil, one PP2AD, STRIP1 and MOB4 (Fig. 1) (Jeong et al., 2021).
STRN and STRN4 are major components of the synaptosomal brain fraction (Kuck et al., 2019), and regulate dendritic arborization and spine development in striatal neurons and hippocampal neurons, respectively (Li et al., 2018; Lin et al., 2017). Consistent with them having important roles in synaptic development and function, downregulation of striatin has been reported to impair locomotor activity of rats (Bartoli et al., 1999). The first case of a de novo missense variant of an alanine to valine change in STRN was recently reported to cause a mild bipolar disorder BD (II) (Toma et al., 2020). A recent review has described the function of STRIPAK complexes in numerous developmental signaling pathways (Kuck et al., 2019), and the recent cryo-EM structure of human STRIPAK has revealed that mutations within the interface of the non-canonical PP2A complex are the cause of aberration to the hippo signaling pathway (Jeong et al., 2021). Mutations in STRIPAK complexes have been identified in individuals with autism (Iossifov et al., 2012), specifically a de novo 2-bp deletion in CTTNBP2 causes a frameshift and disruption of protein function (Iossifov et al., 2014). CTTNBP2 is stably localized to neuronal dendrites regulating spinogenesis, and this mutation likely causes an enhanced CTTNBP2 function as evidenced by increased spined density in ASD patients (Chen et al., 2012). Another STRIPAK protein, CCM3, is mutated in familial cerebral carvernous malformations, and causes seizures, recurrent hemorrhages, strokes and focal neurological deficits (Goudreault et al., 2009; Bergametti et al., 2005; Guclu et al., 2005). In addition to neurological disorders, roles of striatins and STRIPAK in heart diseases, diabetes and cancer have been extensively studied and reviewed (Hwang and Pallas, 2014).
Concluding remarks and future perspectives
PP2A has important roles in the development of the nervous system as evidenced by the plethora of recently discovered DNMs associated with NDDs. As discussed in this Review, these mutations occur in the majority of PP2A subunits (A, B and C) and their regulators, most of which are ubiquitously expressed, yet cause largely neurodevelopmental problems. Patients are generally heterozygous for these de novo mutations, indicating either haploinsufficiency of the disease gene, or a dominant-negative or toxic gain-of-function of the mutated gene product. Genetically engineered animals carrying the human mutations will provide useful model systems to tease out the specific roles and substrates of the different PP2A holoenzymes during neurodevelopment. In addition, the use of patient-derived induced pluripotent stem cells, and their differentiation into neurons and organoids, offers complementary opportunities for discovery and validation. Recent advances in phospho-proteomics and other unbiased approaches have finally allowed the protein phosphatase field to catch up with the protein kinase field in terms of insights into regulation and substrate specificity (Peti et al., 2013; Wang et al., 2020; Xing et al., 2006, 2008). In combination with new animal and human model systems, this recently gained understanding can now be leveraged to consider therapeutic interventions into NDDs associated with PP2A.
Supplementary Material
Acknowledgements
The authors acknowledge the support of the National Institute for Health Research, through the Comprehensive Clinical Research Network (https://www.ncbi.nlm.nih.gov/clinvar). This study makes use of data generated by the DECIPHER community. A full list of centres who contributed to the generation of the data is available from https://decipher.sanger.ac.uk/about/stats and via email from decipher@sanger.ac.uk. Funding for the DECIPHER project was provided by Wellcome.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work in this area is supported by National Institutes of Health grants DK116624, MH115673, MH113352 (to S.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support to S.S. was provided by Jordan's Guardian Angels, the Roy J. Carver Charitable Trust, and Iowa Neuroscience Institute. Deposited in PMC for release after 12 months.
References
- Abrahams, B. S., Arking, D. E., Campbell, D. B., Mefford, H. C., Morrow, E. M., Weiss, L. A., Menashe, I., Wadkins, T., Banerjee-Basu, S. and Packer, A. (2013). SFARI Gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism. 4, 36. 10.1186/2040-2392-4-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna-Hidalgo, R., Bo, T., Kwint, M. P., van de Vorst, M., Pinelli, M., Veltman, J. A., Hoischen, A., Vissers, L. E. and Gilissen, C. (2015). Post-zygotic point mutations are an underrecognized source of de novo genomic variation. Am. J. Hum. Genet. 97, 67-74. 10.1016/j.ajhg.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, J. H., Kim, Y., Kim, H. S., Greengard, P. and Nairn, A. C. (2011). Protein kinase C-dependent dephosphorylation of tyrosine hydroxylase requires the B56delta heterotrimeric form of protein phosphatase 2A. PLoS ONE 6, e26292. 10.1371/journal.pone.0026292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, J. H., McAvoy, T., Rakhilin, S. V., Nishi, A., Greengard, P. and Nairn, A. C. (2007a). Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc. Natl. Acad. Sci. USA 104, 2979-2984. 10.1073/pnas.0611532104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, J. H., Sung, J. Y., McAvoy, T., Nishi, A., Janssens, V., Goris, J., Greengard, P. and Nairn, A. C. (2007b). The B″/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc. Natl. Acad. Sci. USA 104, 9876-9881. 10.1073/pnas.0703589104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, H. K. and Sears, R. C. (2006). Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol. Cell. Biol. 26, 2832-2844. 10.1128/MCB.26.7.2832-2844.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo, J. D., Lee, G. M. and Hahn, W. C. (2008). Liprin alpha1 interacts with PP2A B56gamma. Cell Cycle 7, 525-532. 10.4161/cc.7.4.5390 [DOI] [PubMed] [Google Scholar]
- Backx, L., Vermeesch, J., Pijkels, E., de Ravel, T., Seuntjens, E. and Van Esch, H. (2010). PPP2R2C, a gene disrupted in autosomal dominant intellectual disability. Eur. J. Med. Genet. 53, 239-243. 10.1016/j.ejmg.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Bartoli, M., Ternaux, J. P., Forni, C., Portalier, P., Salin, P., Amalric, M. and Monneron, A. (1999). Down-regulation of striatin, a neuronal calmodulin-binding protein, impairs rat locomotor activity. J. Neurobiol. 40, 234-243. [DOI] [PubMed] [Google Scholar]
- Bastos, R. N., Cundell, M. J. and Barr, F. A. (2014). KIF4A and PP2A-B56 form a spatially restricted feedback loop opposing Aurora B at the anaphase central spindle. J. Cell Biol. 207, 683-693. 10.1083/jcb.201409129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, S. N., Kollu, R. and Banerjee-Basu, S. (2009). AutDB: a gene reference resource for autism research. Nucleic Acids Res. 37, D832-D836. 10.1093/nar/gkn835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist, M., Gaillard, S. and Castets, F. (2006). The striatin family: a new signaling platform in dendritic spines. J. Physiol. Paris 99, 146-153. [DOI] [PubMed] [Google Scholar]
- Bergametti, F., Denier, C., Labauge, P., Arnoult, M., Boetto, S., Clanet, M., Coubes, P., Echenne, B., Ibrahim, R., Irthum, B.et al. (2005). Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am. J. Hum. Genet. 76, 42-51. 10.1086/426952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan, C. and Takai, A. (1988). Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 256, 283-290. 10.1042/bj2560283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidinosti, M., Botta, P., Kruttner, S., Proenca, C. C., Stoehr, N., Bernhard, M., Fruh, I., Mueller, M., Bonenfant, D., Voshol, H.et al. (2016). CLK2 inhibition ameliorates autistic features associated with SHANK3 deficiency. Science 351, 1199-1203. 10.1126/science.aad5487 [DOI] [PubMed] [Google Scholar]
- Biesecker, L. G. and Spinner, N. B. (2013). A genomic view of mosaicism and human disease. Nat. Rev. Genet. 14, 307-320. 10.1038/nrg3424 [DOI] [PubMed] [Google Scholar]
- Bird, L. M. (2014). Angelman syndrome: review of clinical and molecular aspects. Appl Clin Genet 7, 93-104. 10.2147/TACG.S57386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, D., Cary, W. and Nolta, J. A. (2020). PPP2R5D-related intellectual disability and neurodevelopmental delay: a review of the current understanding of the genetics and biochemical basis of the disorder. Int. J. Mol. Sci. 21, 1286. 10.3390/ijms21041286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos-Garcia, V. M. and Blundell, T. L. (2011). BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends Biochem. Sci. 36, 141-150. 10.1016/j.tibs.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsotto, M., Cavarec, L., Bouillot, M., Romey, G., Macciardi, F., Delaye, A., Nasroune, M., Bastucci, M., Sambucy, J. L., Luan, J. J.et al. (2007). PP2A-Bgamma subunit and KCNQ2 K+ channels in bipolar disorder. Pharmacogenomics J. 7, 123-132. 10.1038/sj.tpj.6500400 [DOI] [PubMed] [Google Scholar]
- Bowling, K. M., Thompson, M. L., Amaral, M. D., Finnila, C. R., Hiatt, S. M., Engel, K. L., Cochran, J. N., Brothers, K. B., East, K. M., Gray, D. E.et al. (2017). Genomic diagnosis for children with intellectual disability and/or developmental delay. Genome Med. 9, 43. 10.1186/s13073-017-0433-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, M. I., Jespersgaard, C., Nazaryan, L., Ravn, K., Brondum-Nielsen, K., Bisgaard, A. M. and Tumer, Z. (2015). Deletion of 11q12.3-11q13.1 in a patient with intellectual disability and childhood facial features resembling Cornelia de Lange syndrome. Gene 572, 130-134. 10.1016/j.gene.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Brandt, N., Franke, K., Johannes, S., Buck, F., Harder, S., Hassel, B., Nitsch, R. and Schumacher, S. (2008). B56beta, a regulatory subunit of protein phosphatase 2A, interacts with CALEB/NGC and inhibits CALEB/NGC-mediated dendritic branching. FASEB J. 22, 2521-2533. 10.1096/fj.07-096115 [DOI] [PubMed] [Google Scholar]
- Calin, G. A., di Iasio, M. G., Caprini, E., Vorechovsky, I., Natali, P. G., Sozzi, G., Croce, C. M., Barbanti-Brodano, G., Russo, G. and Negrini, M. (2000). Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene 19, 1191-1195. 10.1038/sj.onc.1203389 [DOI] [PubMed] [Google Scholar]
- Campbell, I. M., Shaw, C. A., Stankiewicz, P. and Lupski, J. R. (2015). Somatic mosaicism: implications for disease and transmission genetics. Trends Genet. 31, 382-392. 10.1016/j.tig.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets, F., Rakitina, T., Gaillard, S., Moqrich, A., Mattei, M. G. and Monneron, A. (2000). Zinedin, SG2NA, and striatin are calmodulin-binding, WD repeat proteins principally expressed in the brain. J. Biol. Chem. 275, 19970-19977. 10.1074/jbc.M909782199 [DOI] [PubMed] [Google Scholar]
- Chen, C., Shi, Z., Zhang, W., Chen, M., He, F., Zhang, Z., Wang, Y., Feng, M., Wang, W., Zhao, Y.et al. (2014). Striatins contain a noncanonical coiled coil that binds protein phosphatase 2A A subunit to form a 2:2 heterotetrameric core of striatin-interacting phosphatase and kinase (STRIPAK) complex. J. Biol. Chem. 289, 9651-9661. 10.1074/jbc.M113.529297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Martin, B. L. and Brautigan, D. L. (1992). Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science 257, 1261-1264. 10.1126/science.1325671 [DOI] [PubMed] [Google Scholar]
- Chen, W., Possemato, R., Campbell, K. T., Plattner, C. A., Pallas, D. C. and Hahn, W. C. (2004). Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5, 127-136. 10.1016/S1535-6108(04)00026-1 [DOI] [PubMed] [Google Scholar]
- Chen, Y. K., Chen, C. Y., Hu, H. T. and Hsueh, Y. P. (2012). CTTNBP2, but not CTTNBP2NL, regulates dendritic spinogenesis and synaptic distribution of the striatin-PP2A complex. Mol. Biol. Cell 23, 4383-4392. 10.1091/mbc.e12-05-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, U. S. and Xu, W. (2007). Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 445, 53-57. [DOI] [PubMed] [Google Scholar]
- Clark, A. R. and Ohlmeyer, M. (2019). Protein phosphatase 2A as a therapeutic target in inflammation and neurodegeneration. Pharmacol. Ther. 201, 181-201. 10.1016/j.pharmthera.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella, S., Ohgaki, H., Ruediger, R., Yang, F., Nakamura, M., Fujisawa, H., Kleihues, P. and Walter, G. (2001). Reduced expression of the Aalpha subunit of protein phosphatase 2A in human gliomas in the absence of mutations in the Aalpha and Abeta subunit genes. Int. J. Cancer 93, 798-804. 10.1002/ijc.1423 [DOI] [PubMed] [Google Scholar]
- Creyghton, M. P., Roel, G., Eichhorn, P. J., Hijmans, E. M., Maurer, I., Destree, O. and Bernards, R. (2005). PR72, a novel regulator of Wnt signaling required for Naked cuticle function. Genes Dev. 19, 376-386. 10.1101/gad.328905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton, M. P., Roel, G., Eichhorn, P. J., Vredeveld, L. C., Destree, O. and Bernards, R. (2006). PR130 is a modulator of the Wnt-signaling cascade that counters repression of the antagonist Naked cuticle. Proc. Natl. Acad. Sci. USA 103, 5397-5402. 10.1073/pnas.0507237103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristobal, I., Cirauqui, C., Castello-Cros, R., Garcia-Orti, L., Calasanz, M. J. and Odero, M. D. (2013). Downregulation of PPP2R5E is a common event in acute myeloid leukemia that affects the oncogenic potential of leukemic cells. Haematologica 98, e103-e104. 10.3324/haematol.2013.084731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristobal, I., Manso, R., Rincon, R., Carames, C., Senin, C., Borrero, A., Martinez-Useros, J., Rodriguez, M., Zazo, S., Aguilera, O.et al. (2014). PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential. Mol. Cancer Ther. 13, 938-947. 10.1158/1535-7163.MCT-13-0150 [DOI] [PubMed] [Google Scholar]
- Csortos, C., Zolnierowicz, S., Bako, E., Durbin, S. D. and DePaoli-Roach, A. A. (1996). High complexity in the expression of the B' subunit of protein phosphatase 2A0. Evidence for the existence of at least seven novel isoforms. J. Biol. Chem. 271, 2578-2588. [DOI] [PubMed] [Google Scholar]
- Cundell, M. J., Hutter, L. H., Nunes Bastos, R., Poser, E., Holder, J., Mohammed, S., Novak, B. and Barr, F. A. (2016). A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J. Cell Biol. 214, 539-554. 10.1083/jcb.201606033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda, R. K. (2006). Structure and function of a mitochondrial PP2A holoenzyme that regulates neuronal survival, vol. PhD (Doctor of Philosophy) thesis: University of Iowa. [Google Scholar]
- Dagda, R. K., Zaucha, J. A., Wadzinski, B. E. and Strack, S. (2003). A developmentally regulated, neuron-specific splice variant of the variable subunit Bbeta targets protein phosphatase 2A to mitochondria and modulates apoptosis. J. Biol. Chem. 278, 24976-24985. 10.1074/jbc.M302832200 [DOI] [PubMed] [Google Scholar]
- Dagda, R. K., Merrill, R. A., Cribbs, J. T., Chen, Y., Hell, J. W., Usachev, Y. M. and Strack, S. (2008). The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission. J. Biol. Chem. 283, 36241-36248. 10.1074/jbc.M800989200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal, G. M., Erguner, B., Sagiroglu, M. S., Yuksel, B., Onat, O. E., Alkan, C. and Ozcelik, T. (2014). Early postzygotic mutations contribute to de novo variation in a healthy monozygotic twin pair. J. Med. Genet. 51, 455-459. 10.1136/jmedgenet-2013-102197 [DOI] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study (2015). Large-scale discovery of novel genetic causes of developmental disorders. Nature 519, 223-228. 10.1038/nature14135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ligt, J., Willemsen, M. H., van Bon, B. W., Kleefstra, T., Yntema, H. G., Kroes, T., Vulto-van Silfhout, A. T., Koolen, D. A., de Vries, P., Gilissen, C.et al. (2012). Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 367, 1921-1929. 10.1056/NEJMoa1206524 [DOI] [PubMed] [Google Scholar]
- Dickey, A. S. and Strack, S. (2011). PKA/AKAP1 and PP2A-Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J. Neurosci. 31, 15716-15726. 10.1523/JNEUROSCI.3159-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovega, R., Tsutakawa, S., Quistgaard, E. M., Anandapadamanaban, M., Low, C. and Nordlund, P. (2014). Structural and biochemical characterization of human PR70 in isolation and in complex with the scaffolding subunit of protein phosphatase 2A. PLoS ONE 9, e101846. 10.1371/journal.pone.0101846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett, A. D., Kamibayashi, C. and Brautigan, D. L. (2002). Transgenic expression of protein phosphatase 2A regulatory subunit B56gamma disrupts distal lung differentiation. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L1266-L1271. 10.1152/ajplung.00262.2001 [DOI] [PubMed] [Google Scholar]
- Fan, Y. L., Chen, L., Wang, J., Yao, Q. and Wan, J. Q. (2013). Over expression of PPP2R2C inhibits human glioma cells growth through the suppression of mTOR pathway. FEBS Lett. 587, 3892-3897. 10.1016/j.febslet.2013.09.029 [DOI] [PubMed] [Google Scholar]
- Fidalgo, M., Fraile, M., Pires, A., Force, T., Pombo, C. and Zalvide, J. (2010). CCM3/PDCD10 stabilizes GCKIII proteins to promote Golgi assembly and cell orientation. J. Cell Sci. 123, 1274-1284. 10.1242/jcs.061341 [DOI] [PubMed] [Google Scholar]
- Fink, C. C., Bayer, K. U., Myers, J. W., Ferrell, J. E., Jr., Schulman, H. and Meyer, T. (2003). Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron 39, 283-297. 10.1016/S0896-6273(03)00428-8 [DOI] [PubMed] [Google Scholar]
- Firth, H. V., Richards, S. M., Bevan, A. P., Clayton, S., Corpas, M., Rajan, D., Van Vooren, S., Moreau, Y., Pettett, R. M. and Carter, N. P. (2009). DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 84, 524-533. 10.1016/j.ajhg.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, T. W., Gerety, S. S., Jones, W. D., van Kogelenberg, M., King, D. A., McRae, J., Morley, K. I., Parthiban, V., Al-Turki, S., Ambridge, K.et al. (2015). Large-scale discovery of novel genetic causes of developmental disorders. Nature 519, 223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippo, K. H., Gnanasekaran, A., Perkins, G. A., Ajmal, A., Merrill, R. A., Dickey, A. S., Taylor, S. S., McKnight, G. S., Chauhan, A. K., Usachev, Y. M.et al. (2018). AKAP1 protects from cerebral ischemic stroke by inhibiting Drp1-dependent mitochondrial fission. J. Neurosci. 38, 8233-8242. 10.1523/JNEUROSCI.0649-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippo, K. H., Lin, Z., Dickey, A. S., Zhou, X., Dhanesha, N. A., Walters, G. C., Liu, Y., Merrill, R. A., Meller, R., Simon, R. P.et al. (2020). Deletion of a Neuronal Drp1 Activator Protects against Cerebral Ischemia. J. Neurosci. 40, 3119-3129. 10.1523/JNEUROSCI.1926-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley, E. A., Maldonado, M. and Kapoor, T. M. (2011). Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat. Cell Biol. 13, 1265-1271. 10.1038/ncb2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, A. K., Dapic, V. and Monteiro, A. N. (2010). Negative regulation of CHK2 activity by protein phosphatase 2A is modulated by DNA damage. Cell Cycle 9, 736-747. 10.4161/cc.9.4.10613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer, M., Pocklington, A. J., Kavanagh, D. H., Williams, H. J., Dwyer, S., Gormley, P., Georgieva, L., Rees, E., Palta, P., Ruderfer, D. M.et al. (2014). De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179-184. 10.1038/nature12929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujigasaki, H., Verma, I. C., Camuzat, A., Margolis, R. L., Zander, C., Lebre, A. S., Jamot, L., Saxena, R., Anand, I., Holmes, S. E.et al. (2001). SCA12 is a rare locus for autosomal dominant cerebellar ataxia: a study of an Indian family. Ann. Neurol. 49, 117-121. [DOI] [PubMed] [Google Scholar]
- Fujimitsu, K. and Yamano, H. (2020). PP2A-B56 binds to Apc1 and promotes Cdc20 association with the APC/C ubiquitin ligase in mitosis. EMBO Rep. 21, e48503. 10.15252/embr.201948503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga, K., Muller, D., Ohmitsu, M., Bako, E., DePaoli-Roach, A. A. and Miyamoto, E. (2000). Decreased protein phosphatase 2A activity in hippocampal long-term potentiation. J. Neurochem. 74, 807-817. 10.1046/j.1471-4159.2000.740807.x [DOI] [PubMed] [Google Scholar]
- Geisheker, M. R., Heymann, G., Wang, T., Coe, B. P., Turner, T. N., Stessman, H. A. F., Hoekzema, K., Kvarnung, M., Shaw, M., Friend, K.et al. (2017). Hotspots of missense mutation identify neurodevelopmental disorder genes and functional domains. Nat. Neurosci. 20, 1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen, C., Hehir-Kwa, J. Y., Thung, D. T., van de Vorst, M., van Bon, B. W., Willemsen, M. H., Kwint, M., Janssen, I. M., Hoischen, A., Schenck, A.et al. (2014). Genome sequencing identifies major causes of severe intellectual disability. Nature 511, 344-347. 10.1038/nature13394 [DOI] [PubMed] [Google Scholar]
- Gnanaprakash, M., Staniszewski, A., Zhang, H., Pitstick, R., Kavanaugh, M. P., Arancio, O. and Nicholls, R. E. (2021). Leucine carboxyl methyltransferase 1 overexpression protects against cognitive and electrophysiological impairments in Tg2576 APP transgenic mice. J. Alzheimers Dis. 79, 1813-1829. 10.3233/JAD-200462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudreault, M., D'Ambrosio, L. M., Kean, M. J., Mullin, M. J., Larsen, B. G., Sanchez, A., Chaudhry, S., Chen, G. I., Sicheri, F., Nesvizhskii, A. I.et al. (2009). A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteomics 8, 157-171. 10.1074/mcp.M800266-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, J. M., Jr, Wheeler, P., Tackels-Horne, D., Lin, A. E., Hall, B. D., May, M., Short, K. M., Schwartz, C. E. and Cox, T. C. (2003). A new X-linked syndrome with agenesis of the corpus callosum, mental retardation, coloboma, micrognathia, and a mutation in the Alpha 4 gene at Xq13. Am. J. Med. Genet. A 123A, 37-44. 10.1002/ajmg.a.20504 [DOI] [PubMed] [Google Scholar]
- Groves, M. R., Hanlon, N., Turowski, P., Hemmings, B. A. and Barford, D. (1999). The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell 96, 99-110. 10.1016/S0092-8674(00)80963-0 [DOI] [PubMed] [Google Scholar]
- Guclu, B., Ozturk, A. K., Pricola, K. L., Bilguvar, K., Shin, D., O'Roak, B. J. and Gunel, M. (2005). Mutations in apoptosis-related gene, PDCD10, cause cerebral cavernous malformation 3. Neurosurgery 57, 1008-1013. 10.1227/01.NEU.0000180811.56157.E1 [DOI] [PubMed] [Google Scholar]
- Guo, F., Stanevich, V., Wlodarchak, N., Sengupta, R., Jiang, L., Satyshur, K. A. and Xing, Y. (2014). Structural basis of PP2A activation by PTPA, an ATP-dependent activation chaperone. Cell Res. 24, 190-203. 10.1038/cr.2013.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guran, T., Yesil, G., Turan, S., Atay, Z., Bozkurtlar, E., Aghayev, A., Gul, S., Tinay, I., Aru, B., Arslan, S.et al. (2019). PPP2R3C gene variants cause syndromic 46,XY gonadal dysgenesis and impaired spermatogenesis in humans. Eur. J. Endocrinol. 180, 291-309. 10.1530/EJE-19-0067 [DOI] [PubMed] [Google Scholar]
- Haesen, D., Abbasi Asbagh, L., Derua, R., Hubert, A., Schrauwen, S., Hoorne, Y., Amant, F., Waelkens, E., Sablina, A. and Janssens, V. (2016). Recurrent PPP2R1A mutations in uterine cancer act through a dominant-negative mechanism to promote malignant cell growth. Cancer Res. 76, 5719-5731. 10.1158/0008-5472.CAN-15-3342 [DOI] [PubMed] [Google Scholar]
- Hamdan, F. F., Srour, M., Capo-Chichi, J. M., Daoud, H., Nassif, C., Patry, L., Massicotte, C., Ambalavanan, A., Spiegelman, D., Diallo, O.et al. (2014). De novo mutations in moderate or severe intellectual disability. PLoS Genet. 10, e1004772. 10.1371/journal.pgen.1004772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, A. M., Zolnierowicz, S., Stapleton, A. E., Goebl, M., DePaoli-Roach, A. A. and Pringle, J. R. (1991). CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol. 11, 5767-5780. 10.1128/MCB.11.11.5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings, B. A., Adams-Pearson, C., Maurer, F., Muller, P., Goris, J., Merlevede, W., Hofsteenge, J. and Stone, S. R. (1990). alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry 29, 3166-3173. 10.1021/bi00465a002 [DOI] [PubMed] [Google Scholar]
- Hendrix, P., Mayer-Jackel, R. E., Cron, P., Goris, J., Hofsteenge, J., Merlevede, W. and Hemmings, B. A. (1993). Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J. Biol. Chem. 268, 15267-15276. 10.1016/S0021-9258(18)82465-6 [DOI] [PubMed] [Google Scholar]
- Hertz, E. P. T., Kruse, T., Davey, N. E., Lopez-Mendez, B., Sigurethsson, J. O., Montoya, G., Olsen, J. V. and Nilsson, J. (2016). A conserved motif provides binding specificity to the PP2A-B56 phosphatase. Mol. Cell 63, 686-695. 10.1016/j.molcel.2016.06.024 [DOI] [PubMed] [Google Scholar]
- Hetzelt, K., Kerling, F., Kraus, C., Rauch, C., Thiel, C. T., Winterholler, M., Reis, A. and Zweier, C. (2021). Early-onset parkinsonism in PPP2R5D-related neurodevelopmental disorder. Eur. J. Med. Genet. 64, 104123. 10.1016/j.ejmg.2020.104123 [DOI] [PubMed] [Google Scholar]
- Hofstetter, C. P., Burkhardt, J. K., Shin, B. J., Gursel, D. B., Mubita, L., Gorrepati, R., Brennan, C., Holland, E. C. and Boockvar, J. A. (2012). Protein phosphatase 2A mediates dormancy of glioblastoma multiforme-derived tumor stem-like cells during hypoxia. PLoS ONE 7, e30059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, S. E., Hearn, E. O., Ross, C. A. and Margolis, R. L. (2001). SCA12: an unusual mutation leads to an unusual spinocerebellar ataxia. Brain Res. Bull. 56, 397-403. 10.1016/S0361-9230(01)00596-2 [DOI] [PubMed] [Google Scholar]
- Holmes, S. E., O'Hearn, E. and Margolis, R. L. (2003). Why is SCA12 different from other SCAs? Cytogenet Genome Res. 100, 189-197. 10.1159/000072854 [DOI] [PubMed] [Google Scholar]
- Holmes, S. E., O'Hearn, E. E., McInnis, M. G., Gorelick-Feldman, D. A., Kleiderlein, J. J., Callahan, C., Kwak, N. G., Ingersoll-Ashworth, R. G., Sherr, M., Sumner, A. J.et al. (1999). Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12. Nat. Genet. 23, 391-392. 10.1038/70493 [DOI] [PubMed] [Google Scholar]
- Houge, G., Haesen, D., Vissers, L. E., Mehta, S., Parker, M. J., Wright, M., Vogt, J., McKee, S., Tolmie, J. L., Cordeiro, N.et al. (2015). B56delta-related protein phosphatase 2A dysfunction identified in patients with intellectual disability. J. Clin. Invest. 125, 3051-3062. 10.1172/JCI79860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, P., Yu, L., Zhang, M., Zheng, L., Zhao, Y., Fu, Q. and Zhao, S. (2000). Molecular cloning and mapping of the brain-abundant B1gamma subunit of protein phosphatase 2A, PPP2R2C, to human chromosome 4p16. Genomics 67, 83-86. 10.1006/geno.2000.6219 [DOI] [PubMed] [Google Scholar]
- Huang, W., Zhou, X., Lefebvre, V. and de Crombrugghe, B. (2000). Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol. 20, 4149-4158. 10.1128/MCB.20.11.4149-4158.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, J. and Pallas, D. C. (2014). STRIPAK complexes: structure, biological function, and involvement in human diseases. Int. J. Biochem. Cell Biol. 47, 118-148. 10.1016/j.biocel.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo, T., Ito, S., Asano-Inami, E., Chen, D. and Senga, T. (2016). A regulatory subunit of protein phosphatase 2A, PPP2R5E, regulates the abundance of microtubule crosslinking factor 1. FEBS J. 283, 3662-3671. 10.1111/febs.13835 [DOI] [PubMed] [Google Scholar]
- Ihara, Y., Kato, Y., Bando, T., Yamagishi, F., Minamimura, T., Sakamoto, T., Tsukada, K. and Isobe, M. (2002). Allelic imbalance of 14q32 in esophageal carcinoma. Cancer Genet. Cytogenet. 135, 177-181. 10.1016/S0165-4608(01)00654-9 [DOI] [PubMed] [Google Scholar]
- Iossifov, I., Ronemus, M., Levy, D., Wang, Z., Hakker, I., Rosenbaum, J., Yamrom, B., Lee, Y. H., Narzisi, G., Leotta, A.et al. (2012). De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285-299. 10.1016/j.neuron.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov, I., O'Roak, B. J., Sanders, S. J., Ronemus, M., Krumm, N., Levy, D., Stessman, H. A., Witherspoon, K. T., Vives, L., Patterson, K. E.et al. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216-221. 10.1038/nature13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, A., Kataoka, T. R., Watanabe, M., Nishiyama, K., Mazaki, Y., Sabe, H., Kitamura, Y. and Nojima, H. (2000). A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J. 19, 562-571. 10.1093/emboj/19.4.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janghorban, M., Langer, E. M., Wang, X., Zachman, D., Daniel, C. J., Hooper, J., Fleming, W. H., Agarwal, A. and Sears, R. C. (2017). The tumor suppressor phosphatase PP2A-B56alpha regulates stemness and promotes the initiation of malignancies in a novel murine model. PLoS ONE 12, e0188910. 10.1371/journal.pone.0188910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens, V., Jordens, J., Stevens, I., Van Hoof, C., Martens, E., De Smedt, H., Engelborghs, Y., Waelkens, E. and Goris, J. (2003). Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B″/PR72 subunit of protein phosphatase 2A. J. Biol. Chem. 278, 10697-10706. 10.1074/jbc.M211717200 [DOI] [PubMed] [Google Scholar]
- Janssens, V., Goris, J. and Van Hoof, C. (2005). PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev. 15, 34-41. 10.1016/j.gde.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Janssens, V., Longin, S. and Goris, J. (2008). PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem. Sci. 33, 113-121. 10.1016/j.tibs.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Janssens, V., Zwaenepoel, K., Rosse, C., Petit, M. M., Goris, J. and Parker, P. J. (2016). PP2A binds to the LIM domains of lipoma-preferred partner through its PR130/B'‘ subunit to regulate cell adhesion and migration. J. Cell Sci. 129, 1605-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, K. I., Jono, H., Miller, C. L., Cai, Y., Lim, S., Liu, X., Gao, P., Abe, J., Li, J. D. and Yan, C. (2010). Ca2+/calmodulin-stimulated PDE1 regulates the beta-catenin/TCF signaling through PP2A B56 gamma subunit in proliferating vascular smooth muscle cells. FEBS J. 277, 5026-5039. 10.1111/j.1742-4658.2010.07908.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, A. L., Han, S., Lee, S., Su Park, J., Lu, Y., Yu, S., Li, J., Chun, K. H., Mills, G. B. and Yang, Y. (2016). Patient derived mutation W257G of PPP2R1A enhances cancer cell migration through SRC-JNK-c-Jun pathway. Sci. Rep. 6, 27391. 10.1038/srep27391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, B. C., Bae, S. J., Ni, L., Zhang, X., Bai, X. C. and Luo, X. (2021). Cryo-EM structure of the Hippo signaling integrator human STRIPAK. Nat. Struct. Mol. Biol. 28, 290-299. 10.1038/s41594-021-00564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H., Guo, W., Liang, X. and Rao, Y. (2005). Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell 120, 123-135. [DOI] [PubMed] [Google Scholar]
- Jiang, L., Stanevich, V., Satyshur, K. A., Kong, M., Watkins, G. R., Wadzinski, B. E., Sengupta, R. and Xing, Y. (2013). Structural basis of protein phosphatase 2A stable latency. Nat. Commun. 4, 1699. 10.1038/ncomms2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Z., Wallace, L., Harper, S. Q. and Yang, J. (2010). PP2A:B56{epsilon}, a substrate of caspase-3, regulates p53-dependent and p53-independent apoptosis during development. J. Biol. Chem. 285, 34493-34502. 10.1074/jbc.M110.169581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong, C. J., Merrill, R. A., Wilkerson, E. M., Herring, L. E., Graves, L. M. and Strack, S. (2020). Reduction of protein phosphatase 2A (PP2A) complexity reveals cellular functions and dephosphorylation motifs of the PP2A-B'delta holoenzyme. J. Biol. Chem. 295, 5654-5668. 10.1074/jbc.RA119.011270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia, L. V. and Lang, A. E. (2015). Parkinson's disease. Lancet 386, 896-912. 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- Kamnasaran, D., Chen, C. P., Devriendt, K., Mehta, L. and Cox, D. W. (2005). Defining a holoprosencephaly locus on human chromosome 14q13 and characterization of potential candidate genes. Genomics 85, 608-621. 10.1016/j.ygeno.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Kapfhamer, D., Berger, K. H., Hopf, F. W., Seif, T., Kharazia, V., Bonci, A. and Heberlein, U. (2011). Protein phosphatase 2a and glycogen synthase kinase 3 signaling modulate prepulse inhibition of the acoustic startle response by altering cortical M-type potassium channel activity. J. Neurosci. 30, 8830-8840. 10.1523/JNEUROSCI.1292-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean, M. J., Ceccarelli, D. F., Goudreault, M., Sanches, M., Tate, S., Larsen, B., Gibson, L. C., Derry, W. B., Scott, I. C., Pelletier, L.et al. (2011). Structure-function analysis of core STRIPAK Proteins: a signaling complex implicated in Golgi polarization. J. Biol. Chem. 286, 25065-25075. 10.1074/jbc.M110.214486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khew-Goodall, Y. and Hemmings, B. A. (1988). Tissue-specific expression of mRNAs encoding alpha- and beta-catalytic subunits of protein phosphatase 2A. FEBS Lett. 238, 265-268. 10.1016/0014-5793(88)80493-9 [DOI] [PubMed] [Google Scholar]
- Kim, C. Y., Wirth, T., Hubsch, C., Nemeth, A. H., Okur, V., Anheim, M., Drouot, N., Tranchant, C., Rudolf, G., Chelly, J.et al. (2020). Early-onset parkinsonism is a manifestation of the PPP2R5D p.E200K Mutation. Ann. Neurol. 88, 1028-1033. 10.1002/ana.25863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, M., Ditsworth, D., Lindsten, T. and Thompson, C. B. (2009). Alpha4 is an essential regulator of PP2A phosphatase activity. Mol. Cell 36, 51-60. 10.1016/j.molcel.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, T., Gnosa, S. P., Nasa, I., Garvanska, D. H., Hein, J. B., Nguyen, H., Samsoe-Petersen, J., Lopez-Mendez, B., Hertz, E. P. T., Schwarz, J.et al. (2020). Mechanisms of site-specific dephosphorylation and kinase opposition imposed by PP2A regulatory subunits. EMBO J. 39, e103695. 10.15252/embj.2019103695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck, U., Radchenko, D. and Teichert, I. (2019). STRIPAK, a highly conserved signaling complex, controls multiple eukaryotic cellular and developmental processes and is linked with human diseases. Biol. Chem. 400, 1005-1022. 10.1515/hsz-2019-0173 [DOI] [PubMed] [Google Scholar]
- Kuhn, T. B., Meberg, P. J., Brown, M. D., Bernstein, B. W., Minamide, L. S., Jensen, J. R., Okada, K., Soda, E. A. and Bamburg, J. R. (2000). Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. J. Neurobiol. 44, 126-144. [DOI] [PubMed] [Google Scholar]
- Kumar, P., Tathe, P., Chaudhary, N. and Maddika, S. (2019). PPM1G forms a PPP-type phosphatase holoenzyme with B56delta that maintains adherens junction integrity. EMBO Rep. 20, e46965. 10.15252/embr.201846965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, Y. C., Huang, K. Y., Yang, C. H., Yang, Y. S., Lee, W. Y. and Chiang, C. W. (2008). Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J. Biol. Chem. 283, 1882-1892. 10.1074/jbc.M709585200 [DOI] [PubMed] [Google Scholar]
- Lambrecht, C., Libbrecht, L., Sagaert, X., Pauwels, P., Hoorne, Y., Crowther, J., Louis, J. V., Sents, W., Sablina, A. and Janssens, V. (2018). Loss of protein phosphatase 2A regulatory subunit B56delta promotes spontaneous tumorigenesis in vivo. Oncogene 37, 544-552. 10.1038/onc.2017.350 [DOI] [PubMed] [Google Scholar]
- Lear, T. B., Lockwood, K. C., Larsen, M., Tuncer, F., Kennerdell, J. R., Morse, C., Valenzi, E., Tabib, T., Jurczak, M. J., Kass, D. J.et al. (2020). Kelch-like protein 42 is a profibrotic ubiquitin E3 ligase involved in systemic sclerosis. J. Biol. Chem. 295, 4171-4180. 10.1074/jbc.AC119.012066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond, C. S., Cliquet, F., Carton, C., Huguet, G., Mathieu, A., Kergrohen, T., Buratti, J., Lemiere, N., Cuisset, L., Bienvenu, T.et al. (2019). Both rare and common genetic variants contribute to autism in the Faroe Islands. NPJ Genom. Med. 4, 1. 10.1038/s41525-018-0075-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. and Stock, J. (1993). Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J. Biol. Chem. 268, 19192-19195. 10.1016/S0021-9258(19)36497-X [DOI] [PubMed] [Google Scholar]
- Lee, K. W., Chen, W., Junn, E., Im, J. Y., Grosso, H., Sonsalla, P. K., Feng, X., Ray, N., Fernandez, J. R., Chao, Y.et al. (2011). Enhanced phosphatase activity attenuates alpha-synucleinopathy in a mouse model. J. Neurosci. 31, 6963-6971. 10.1523/JNEUROSCI.6513-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaerts, L., Reynhout, S., Verbinnen, I., Laumonnier, F., Toutain, A., Bonnet-Brilhault, F., Hoorne, Y., Joss, S., Chassevent, A. K., Smith-Hicks, C.et al. (2021). The broad phenotypic spectrum of PPP2R1A-related neurodevelopmental disorders correlates with the degree of biochemical dysfunction. Genet. Med. 23, 352-362. 10.1038/s41436-020-00981-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeNoue-Newton, M., Watkins, G. R., Zou, P., Germane, K. L., McCorvey, L. R., Wadzinski, B. E. and Spiller, B. W. (2011). The E3 ubiquitin ligase- and protein phosphatase 2A (PP2A)-binding domains of the Alpha4 protein are both required for Alpha4 to inhibit PP2A degradation. J. Biol. Chem. 286, 17665-17671. 10.1074/jbc.M111.222414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, D., Huang, W., Izadmehr, S., O'Connor, C. M., Wiredja, D. D., Wang, Z., Zaware, N., Chen, Y., Schlatzer, D. M., Kiselar, J.et al. (2020). Selective PP2A enhancement through biased heterotrimer stabilization. Cell 181, 688-701.e16. 10.1016/j.cell.2020.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. and Virshup, D. M. (2002). Two conserved domains in regulatory B subunits mediate binding to the A subunit of protein phosphatase 2A. Eur. J. Biochem. 269, 546-552. 10.1046/j.0014-2956.2001.02680.x [DOI] [PubMed] [Google Scholar]
- Li, M., Guo, H. and Damuni, Z. (1995). Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry 34, 1988-1996. 10.1021/bi00006a020 [DOI] [PubMed] [Google Scholar]
- Li, X., Yost, H. J., Virshup, D. M. and Seeling, J. M. (2001). Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 20, 4122-4131. 10.1093/emboj/20.15.4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. H., Cai, X., Shouse, G. P., Piluso, L. G. and Liu, X. (2007). A specific PP2A regulatory subunit, B56gamma, mediates DNA damage-induced dephosphorylation of p53 at Thr55. EMBO J. 26, 402-411. 10.1038/sj.emboj.7601519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., Musante, V., Zhou, W., Picciotto, M. R. and Nairn, A. C. (2018). Striatin-1 is a B subunit of protein phosphatase PP2A that regulates dendritic arborization and spine development in striatal neurons. J. Biol. Chem. 293, 11179-11194. 10.1074/jbc.RA117.001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. N., Cao, Y. Q., Wu, X., Han, G. S., Wang, L. X., Zhang, Y. H., Chen, X., Hao, B., Yue, Z. J. and Liu, J. M. (2015). The association between Salt-inducible kinase 2 (SIK2) and gamma isoform of the regulatory subunit B55 of PP2A (B55gamma) contributes to the survival of glioma cells under glucose depletion through inhibiting the phosphorylation of S6K. Cancer Cell Int. 15, 21. 10.1186/s12935-015-0164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. and Eisenman, R. N. (2012). Regulation of c-Myc protein abundance by a protein phosphatase 2A-glycogen synthase kinase 3beta-negative feedback pathway. Genes Cancer 3, 23-36. 10.1177/1947601912448067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L., Lo, L. H., Lyu, Q. and Lai, K. O. (2017). Determination of dendritic spine morphology by the striatin scaffold protein STRN4 through interaction with the phosphatase PP2A. J. Biol. Chem. 292, 9451-9464. 10.1074/jbc.M116.772442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D., Zheng, H. Y., Luo, Z. Z., Wang, Q. and Zhu, L. Q. (2010). Effect of PP-2A on neurite outgrowth in neuronal cells. In Vitro Cell. Dev. Biol. Anim. 46, 702-707. 10.1007/s11626-010-9329-8 [DOI] [PubMed] [Google Scholar]
- Liu, J., Ji, W., Sun, S., Zhang, L., Chen, H. G., Mao, Y., Liu, L., Zhang, X., Gong, L., Deng, M.et al. (2012). The PP2A-Abeta gene is regulated by multiple transcriptional factors including Ets-1, SP1/SP3, and RXRalpha/beta. Curr. Mol. Med. 12, 982-994. 10.2174/156652412802480916 [DOI] [PubMed] [Google Scholar]
- Liu, B., Sun, L. H., Huang, Y. F., Guo, L. J. and Luo, L. S. (2018). Protein phosphatase 2ACalpha gene knock-out results in cortical atrophy through activating hippo cascade in neuronal progenitor cells. Int. J. Biochem. Cell Biol. 95, 53-62. 10.1016/j.biocel.2017.12.015 [DOI] [PubMed] [Google Scholar]
- Liu, X., Liu, Q., Fan, Y., Wang, S., Liu, X., Zhu, L., Liu, M. and Tang, H. (2014). Downregulation of PPP2R5E expression by miR-23a suppresses apoptosis to facilitate the growth of gastric cancer cells. FEBS Lett. 588, 3160-3169. 10.1016/j.febslet.2014.05.068 [DOI] [PubMed] [Google Scholar]
- Liu, Z., Yoshimi, A., Wang, J., Cho, H., Chun-Wei Lee, S., Ki, M., Bitner, L., Chu, T., Shah, H., Liu, B.et al. (2020). Mutations in the RNA splicing factor SF3B1 promote tumorigenesis through MYC stabilization. Cancer Discov 10, 806-821. 10.1158/2159-8290.CD-19-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, J. V., Martens, E., Borghgraef, P., Lambrecht, C., Sents, W., Longin, S., Zwaenepoel, K., Pijnenborg, R., Landrieu, I., Lippens, G.et al. (2011). Mice lacking phosphatase PP2A subunit PR61/B'delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta. Proc. Natl. Acad. Sci. USA 108, 6957-6962. 10.1073/pnas.1018777108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday, C., Tatton-Brown, K., Clarke, M., Westwood, I., Renwick, A., Ramsay, E., Nemeth, A., Campbell, J., Joss, S., Gardner, M.et al. (2015). Mutations in the PP2A regulatory subunit B family genes PPP2R5B, PPP2R5C and PPP2R5D cause human overgrowth. Hum. Mol. Genet. 24, 4775-4779. 10.1093/hmg/ddv182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski, J. R. (2010). New mutations and intellectual function. Nat. Genet. 42, 1036-1038. 10.1038/ng1210-1036 [DOI] [PubMed] [Google Scholar]
- Manning, G., Whyte, D. B., Martinez, R., Hunter, T. and Sudarsanam, S. (2002). The protein kinase complement of the human genome. Science 298, 1912-1934. 10.1126/science.1075762 [DOI] [PubMed] [Google Scholar]
- Margolis, S. S., Perry, J. A., Forester, C. M., Nutt, L. K., Guo, Y., Jardim, M. J., Thomenius, M. J., Freel, C. D., Darbandi, R., Ahn, J. H.et al. (2006). Role for the PP2A-B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell 127, 759-773. 10.1016/j.cell.2006.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis, S. S., Sell, G. L., Zbinden, M. A. and Bird, L. M. (2015). Angelman syndrome. Neurotherapeutics 12, 641-650. 10.1007/s13311-015-0361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta, C. and Mobasheri, A. (2014). Regulation of chondrogenesis by protein kinase C: emerging new roles in calcium signalling. Cell. Signal. 26, 979-1000. 10.1016/j.cellsig.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Mayer, R. E., Hendrix, P., Cron, P., Matthies, R., Stone, S. R., Goris, J., Merlevede, W., Hofsteenge, J. and Hemmings, B. A. (1991). Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry 30, 3589-3597. 10.1021/bi00229a001 [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel, R. E. and Hemmings, B. A. (1994). Protein phosphatase 2A--a 'menage a trois’. Trends Cell Biol. 4, 287-291. [DOI] [PubMed] [Google Scholar]
- McConnell, J. L., Watkins, G. R., Soss, S. E., Franz, H. S., McCorvey, L. R., Spiller, B. W., Chazin, W. J. and Wadzinski, B. E. (2010). Alpha4 is a ubiquitin-binding protein that regulates protein serine/threonine phosphatase 2A ubiquitination. Biochemistry 49, 1713-1718. 10.1021/bi901837h [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCright, B., Rivers, A. M., Audlin, S. and Virshup, D. M. (1996). The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 271, 22081-22089. 10.1074/jbc.271.36.22081 [DOI] [PubMed] [Google Scholar]
- Merrill, R. A., Slupe, A. M. and Strack, S. (2013). N-terminal phosphorylation of protein phosphatase 2A/Bbeta2 regulates translocation to mitochondria, dynamin-related protein 1 dephosphorylation, and neuronal survival. FEBS J. 280, 662-673. 10.1111/j.1742-4658.2012.08631.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, J., Picard, C., Labonte, A., Auld, D., Breitner, J., Poirier, J., United Kingdom Brain Expression Consortium and PREVENT-AD research group (2019). Association of PPP2R1A with Alzheimer's disease and specific cognitive domains. Neurobiol. Aging 81, 234-243. 10.1016/j.neurobiolaging.2019.06.008 [DOI] [PubMed] [Google Scholar]
- Mirzaa, G., Foss, K., Nattakom, M. and Chung, W. K. (2019). PPP2R5D-Related neurodevelopmental disorder. In GeneReviews (Eds. Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Stephens K. and Amemiya A.). University of Washington, Seattle; 1993–2020. [PubMed] [Google Scholar]
- Mochida, S., Ikeo, S., Gannon, J. and Hunt, T. (2009). Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 28, 2777-2785. 10.1038/emboj.2009.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann, I., Gillessen-Kaesbach, G., Siebert, R., Caliebe, A. and Hellenbroich, Y. (2011). A de novo 0.57 Mb microdeletion in chromosome 11q13.1 in a patient with speech problems, autistic traits, dysmorphic features and multiple endocrine neoplasia type 1. Eur. J. Med. Genet. 54, e461-e464. 10.1016/j.ejmg.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Monroe, J. D. and Heathcote, R. D. (2013). Protein phosphatases regulate the growth of developing neurites. Int. J. Dev. Neurosci. 31, 250-257. 10.1016/j.ijdevneu.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Moreno, C. S., Park, S., Nelson, K., Ashby, D., Hubalek, F., Lane, W. S. and Pallas, D. C. (2000). WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem. 275, 5257-5263. 10.1074/jbc.275.8.5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia Pienkowski, V., Kucharczyk, M., Mlynek, M., Szczaluba, K., Rydzanicz, M., Poszewiecka, B., Skorka, A., Sykulski, M., Biernacka, A., Koppolu, A. A.et al. (2019). Mapping of breakpoints in balanced chromosomal translocations by shallow whole-genome sequencing points to EFNA5, BAHD1 and PPP2R5E as novel candidates for genes causing human Mendelian disorders. J. Med. Genet. 56, 104-112. 10.1136/jmedgenet-2018-105527 [DOI] [PubMed] [Google Scholar]
- Murray, S. A. (2011). Protein kinase biochemistry and drug discovery. Bioorg. Chem. 39, 192-210. 10.1016/j.bioorg.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Nicholls, R. E., Sontag, J. M., Zhang, H., Staniszewski, A., Yan, S., Kim, C. Y., Yim, M., Woodruff, C. M., Arning, E., Wasek, B.et al. (2016). PP2A methylation controls sensitivity and resistance to beta-amyloid-induced cognitive and electrophysiological impairments. Proc. Natl. Acad. Sci. USA 113, 3347-3352. 10.1073/pnas.1521018113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis, W., Vallardi, G., Teixeira, A., Kops, G. J. and Saurin, A. T. (2014). Negative feedback at kinetochores underlies a responsive spindle checkpoint signal. Nat. Cell Biol. 16, 1257-1264. 10.1038/ncb3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobumori, Y., Shouse, G. P., Fan, L. and Liu, X. (2012). HEAT repeat 1 motif is required for B56gamma-containing protein phosphatase 2A (B56gamma-PP2A) holoenzyme assembly and tumor-suppressive function. J. Biol. Chem. 287, 11030-11036. 10.1074/jbc.M111.334094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, C. M., Leonard, D., Wiredja, D., Avelar, R. A., Wang, Z., Schlatzer, D., Bryson, B., Tokala, E., Taylor, S. E., Upadhyay, A.et al. (2020). Inactivation of PP2A by a recurrent mutation drives resistance to MEK inhibitors. Oncogene 39, 703-717. 10.1038/s41388-019-1012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgad, S., Brewis, N. D., Alphey, L., Axton, J. M., Dudai, Y. and Cohen, P. T. (1990). The structure of protein phosphatase 2A is as highly conserved as that of protein phosphatase 1. FEBS Lett. 275, 44-48. 10.1016/0014-5793(90)81435-Q [DOI] [PubMed] [Google Scholar]
- O'Roak, B. J., Stessman, H. A., Boyle, E. A., Witherspoon, K. T., Martin, B., Lee, C., Vives, L., Baker, C., Hiatt, J. B., Nickerson, D. A.et al. (2014). Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat. Commun. 5, 5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas, D. C., Weller, W., Jaspers, S., Miller, T. B., Lane, W. S. and Roberts, T. M. (1992). The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J. Virol. 66, 886-893. 10.1128/JVI.66.2.886-893.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker, N., Coutman, M., Lawlor-O'Neill, C., Kahl, R. G. S., Roselli, S. and Verrills, N. M. (2020). Ppp2r2a knockout mice reveal that protein phosphatase 2A regulatory subunit, PP2A-B55alpha, is an essential regulator of neuronal and epidermal embryonic development. Front Cell Dev Biol 8, 358. 10.3389/fcell.2020.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke, C. M., Smolen, K. A., Swingle, M. R., Cressey, L., Heng, R. A., Toporsian, M., Deng, L., Hagen, J., Shen, Y., Chung, W. K.et al. (2021). A disorder-related variant (E420K) of a PP2A-regulatory subunit (PPP2R5D) causes constitutively active AKT-mTOR signaling and uncoordinated cell growth. J. Biol. Chem. 296, 100313. 10.1016/j.jbc.2021.100313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. J., Lee, K. W., Park, E. S., Oh, S., Yan, R., Zhang, J., Beach, T. G., Adler, C. H., Voronkov, M., Braithwaite, S. P.et al. (2016). Dysregulation of protein phosphatase 2A in parkinson disease and dementia with lewy bodies. Ann. Clin. Transl. Neurol. 3, 769-780. 10.1002/acn3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. J., Lee, K. W., Oh, S., Yan, R., Zhang, J., Beach, T. G., Adler, C. H., Voronkov, M., Braithwaite, S. P., Stock, J. B.et al. (2018). Protein phosphatase 2A and its methylation modulating enzymes LCMT-1 and PME-1 are dysregulated in tauopathies of progressive supranuclear palsy and Alzheimer disease. J. Neuropathol. Exp. Neurol. 77, 139-148. 10.1093/jnen/nlx110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti, W., Nairn, A. C. and Page, R. (2013). Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280, 596-611. 10.1111/j.1742-4658.2012.08509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, I. M., Schleicher, K., Porter, M. and Swedlow, J. R. (2013). Bod1 regulates protein phosphatase 2A at mitotic kinetochores. Nat. Commun. 4, 2677. 10.1038/ncomms3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinetti, A., Bassi, R., Riboni, L. and Tettamanti, G. (1997). Involvement of a ceramide activated protein phosphatase in the differentiation of neuroblastoma Neuro2a cells. FEBS Lett. 414, 475-479. 10.1016/S0014-5793(97)00981-2 [DOI] [PubMed] [Google Scholar]
- Ranieri, A., Kemp, E., Burgoyne, J. R. and Avkiran, M. (2018). beta-Adrenergic regulation of cardiac type 2A protein phosphatase through phosphorylation of regulatory subunit B56delta at S573. J. Mol. Cell. Cardiol. 115, 20-31. 10.1016/j.yjmcc.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders, M. R. F., Kousi, M., van Woerden, G. M., Klein, M., Bralten, J., Mancini, G. M. S., van Essen, T., Proietti-Onori, M., Smeets, E. E. J., van Gastel, M.et al. (2017). Variation in a range of mTOR-related genes associates with intracranial volume and intellectual disability. Nat. Commun. 8, 1052. 10.1038/s41467-017-00933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynhout, S., Jansen, S., Haesen, D., van Belle, S., de Munnik, S. A., Bongers, E., Schieving, J. H., Marcelis, C., Amiel, J., Rio, M.et al. (2019). De Novo mutations affecting the catalytic Calpha subunit of PP2A, PPP2CA, cause syndromic intellectual disability resembling other PP2A-related neurodevelopmental disorders. Am. J. Hum. Genet. 104, 139-156. 10.1016/j.ajhg.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronjat, M., Kiyonaka, S., Barbado, M., De Waard, M. and Mori, Y. (2013). Nuclear life of the voltage-gated Cacnb4 subunit and its role in gene transcription regulation. Channels (Austin) 7, 119-125. 10.4161/chan.23895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick, A. M., Mei, W., Liette, N. L., Phiel, C., El-Hodiri, H. M. and Yang, J. (2007). PP2A:B56epsilon is required for eye induction and eye field separation. Dev. Biol. 302, 477-493. 10.1016/j.ydbio.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Ruediger, R., Pham, H. T. and Walter, G. (2001). Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the A alpha subunit gene. Oncogene 20, 10-15. 10.1038/sj.onc.1204059 [DOI] [PubMed] [Google Scholar]
- Ruzzo, E. K., Perez-Cano, L., Jung, J. Y., Wang, L. K., Kashef-Haghighi, D., Hartl, C., Singh, C., Xu, J., Hoekstra, J. N., Leventhal, O.et al. (2019). Inherited and De Novo genetic risk for autism impacts shared networks. Cell 178, 850-866.e26. 10.1016/j.cell.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablina, A. A., Hector, M., Colpaert, N. and Hahn, W. C. (2010). Identification of PP2A complexes and pathways involved in cell transformation. Cancer Res. 70, 10474-10484. 10.1158/0008-5472.CAN-10-2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, S. J., Murtha, M. T., Gupta, A. R., Murdoch, J. D., Raubeson, M. J., Willsey, A. J., Ercan-Sencicek, A. G., DiLullo, N. M., Parikshak, N. N., Stein, J. L.et al. (2012). De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237-241. 10.1038/nature10945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf, A., Virshup, D. M. and Strack, S. (2007). Differential expression of the B'beta regulatory subunit of protein phosphatase 2A modulates tyrosine hydroxylase phosphorylation and catecholamine synthesis. J. Biol. Chem. 282, 573-580. 10.1074/jbc.M607407200 [DOI] [PubMed] [Google Scholar]
- Saraf, A., Oberg, E. A. and Strack, S. (2010). Molecular determinants for PP2A substrate specificity: charged residues mediate dephosphorylation of tyrosine hydroxylase by the PP2A-B′ regulatory subunit. Biochemistry 49, 986-995. 10.1021/bi902160t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterstrom, F. K., Kosmicki, J. A., Wang, J., Breen, M. S., De Rubeis, S., An, J. Y., Peng, M., Collins, R., Grove, J., Klei, L.et al. (2020). Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568-584.e23. 10.1016/j.cell.2019.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sents, W., Ivanova, E., Lambrecht, C., Haesen, D. and Janssens, V. (2013). The biogenesis of active protein phosphatase 2A holoenzymes: a tightly regulated process creating phosphatase specificity. FEBS J. 280, 644-661. 10.1111/j.1742-4658.2012.08579.x [DOI] [PubMed] [Google Scholar]
- Serasinghe, M. N. and Chipuk, J. E. (2017). mitochondrial fission in human diseases. Handb. Exp. Pharmacol. 240, 159-188. 10.1007/164_2016_38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshacharyulu, P., Pandey, P., Datta, K. and Batra, S. K. (2013). Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 335, 9-18. 10.1016/j.canlet.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, L., Henderson, L. B., Cho, M. T., Petrey, D. S., Fong, C. T., Haude, K. M., Shur, N., Lundberg, J., Hauser, N., Carmichael, J.et al. (2016). De novo missense variants in PPP2R5D are associated with intellectual disability, macrocephaly, hypotonia, and autism. Neurogenetics 17, 43-49. 10.1007/s10048-015-0466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse, G. P., Nobumori, Y., Panowicz, M. J. and Liu, X. (2011). ATM-mediated phosphorylation activates the tumor-suppressive function of B56gamma-PP2A. Oncogene 30, 3755-3765. 10.1038/onc.2011.95 [DOI] [PubMed] [Google Scholar]
- Sontag, E., Hladik, C., Montgomery, L., Luangpirom, A., Mudrak, I., Ogris, E. and White, C. L.III. (2004a). Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J. Neuropathol. Exp. Neurol. 63, 1080-1091. 10.1093/jnen/63.10.1080 [DOI] [PubMed] [Google Scholar]
- Sontag, E., Luangpirom, A., Hladik, C., Mudrak, I., Ogris, E., Speciale, S. and White, C. L.III. (2004b). Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J. Neuropathol. Exp. Neurol. 63, 287-301. 10.1093/jnen/63.4.287 [DOI] [PubMed] [Google Scholar]
- Srivastava, A. K., Choudhry, S., Gopinath, M. S., Roy, S., Tripathi, M., Brahmachari, S. K. and Jain, S. (2001). Molecular and clinical correlation in five Indian families with spinocerebellar ataxia 12. Ann. Neurol. 50, 796-800. 10.1002/ana.10048 [DOI] [PubMed] [Google Scholar]
- Stanevich, V., Jiang, L., Satyshur, K. A., Li, Y., Jeffrey, P. D., Li, Z., Menden, P., Semmelhack, M. F. and Xing, Y. (2011). The structural basis for tight control of PP2A methylation and function by LCMT-1. Mol. Cell 41, 331-342. 10.1016/j.molcel.2010.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanevich, V., Zheng, A., Guo, F., Jiang, L., Wlodarchak, N. and Xing, Y. (2014). Mechanisms of the scaffold subunit in facilitating protein phosphatase 2A methylation. PLoS One 9, e86955. 10.1371/journal.pone.0086955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniszewski, A., Zhang, H., Asam, K., Pitstick, R., Kavanaugh, M. P., Arancio, O. and Nicholls, R. E. (2020). Reduced expression of the PP2A methylesterase, PME-1, or the PP2A methyltransferase, LCMT-1, alters sensitivity to beta-amyloid-induced cognitive and electrophysiological impairments in mice. J. Neurosci. 40, 4596-4608. 10.1523/JNEUROSCI.2983-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessman, H. A., Xiong, B., Coe, B. P., Wang, T., Hoekzema, K., Fenckova, M., Kvarnung, M., Gerdts, J., Trinh, S., Cosemans, N.et al. (2017). Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 49, 515-526. 10.1038/ng.3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, S. R., Hofsteenge, J. and Hemmings, B. A. (1987). Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry 26, 7215-7220. 10.1021/bi00397a003 [DOI] [PubMed] [Google Scholar]
- Strack, S. (2002). Overexpression of the protein phosphatase 2A regulatory subunit Bgamma promotes neuronal differentiation by activating the MAP kinase (MAPK) cascade. J. Biol. Chem. 277, 41525-41532. 10.1074/jbc.M203767200 [DOI] [PubMed] [Google Scholar]
- Strack, S., Chang, D., Zaucha, J. A., Colbran, R. J. and Wadzinski, B. E. (1999). Cloning and characterization of B delta, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett. 460, 462-466. 10.1016/S0014-5793(99)01377-0 [DOI] [PubMed] [Google Scholar]
- Strack, S., Ruediger, R., Walter, G., Dagda, R. K., Barwacz, C. A. and Cribbs, J. T. (2002). Protein phosphatase 2A holoenzyme assembly: identification of contacts between B-family regulatory and scaffolding A subunits. J. Biol. Chem. 277, 20750-20755. 10.1074/jbc.M202992200 [DOI] [PubMed] [Google Scholar]
- Suzuki, K. and Takahashi, K. (2003). Reduced expression of the regulatory A subunit of serine/threonine protein phosphatase 2A in human breast cancer MCF-7 cells. Int. J. Oncol. 23, 1263-1268. [PubMed] [Google Scholar]
- Takagi, Y., Futamura, M., Yamaguchi, K., Aoki, S., Takahashi, T. and Saji, S. (2000). Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut 47, 268-271. 10.1136/gut.47.2.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., Wakai, T. and Hirota, T. (2016). Condensin I-mediated mitotic chromosome assembly requires association with chromokinesin KIF4A. Genes Dev. 30, 1931-1936. 10.1101/gad.282855.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata, A., Miyake, N., Tsurusaki, Y., Fukai, R., Miyatake, S., Koshimizu, E., Kushima, I., Okada, T., Morikawa, M., Uno, Y.et al. (2018). Integrative analyses of De Novo mutations provide deeper biological insights into autism spectrum disorder. Cell Rep 22, 734-747. 10.1016/j.celrep.2017.12.074 [DOI] [PubMed] [Google Scholar]
- Tehrani, M. A., Mumby, M. C. and Kamibayashi, C. (1996). Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J. Biol. Chem. 271, 5164-5170. 10.1074/jbc.271.9.5164 [DOI] [PubMed] [Google Scholar]
- Toma, C., Shaw, A. D., Overs, B. J., Mitchell, P. B., Schofield, P. R., Cooper, A. A. and Fullerton, J. M. (2020). De Novo Gene variants and familial bipolar disorder. JAMA Netw Open 3, e203382. 10.1001/jamanetworkopen.2020.3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski, J. Q. and Lee, V. M. (1995). Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. FASEB J. 9, 1570-1576. 10.1096/fasebj.9.15.8529836 [DOI] [PubMed] [Google Scholar]
- Turowski, P., Fernandez, A., Favre, B., Lamb, N. J. and Hemmings, B. A. (1995). Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J. Cell Biol. 129, 397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski, P., Myles, T., Hemmings, B. A., Fernandez, A. and Lamb, N. J. (1999). Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell 10, 1997-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafai, S. B. and Stock, J. B. (2002). Protein phosphatase 2A methylation: a link between elevated plasma homocysteine and Alzheimer's disease. FEBS Lett. 518, 1-4. 10.1016/S0014-5793(02)02702-3 [DOI] [PubMed] [Google Scholar]
- Vallardi, G., Allan, L. A., Crozier, L. and Saurin, A. T. (2019). Division of labour between PP2A-B56 isoforms at the centromere and kinetochore. Elife 8, e42619. 10.7554/eLife.42619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kanegan, M. J. and Strack, S. (2009). The protein phosphatase 2A regulatory subunits B'beta and B'delta mediate sustained TrkA neurotrophin receptor autophosphorylation and neuronal differentiation. Mol. Cell. Biol. 29, 662-674. 10.1128/MCB.01242-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kanegan, M. J., Adams, D. G., Wadzinski, B. E. and Strack, S. (2005). Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J. Biol. Chem. 280, 36029-36036. 10.1074/jbc.M506986200 [DOI] [PubMed] [Google Scholar]
- Varadkar, P., Abbasi, F., Takeda, K., Dyson, J. J. and McCright, B. (2017). PP2A-B56gamma is required for an efficient spindle assembly checkpoint. Cell Cycle 16, 1210-1219. 10.1080/15384101.2017.1325042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinyoles, M., Del Valle-Perez, B., Curto, J., Padilla, M., Villarroel, A., Yang, J., de Herreros, A. G. and Dunach, M. (2017). Activation of CK1varepsilon by PP2A-PR61varepsilon is required for the initiation of Wnt signaling. Oncogene 36, 429-438. 10.1038/onc.2016.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlastaridis, P., Oliver, S. G., Van de Peer, Y. and Amoutzias, G. D. (2016). The challenges of interpreting phosphoproteomics data: a critical view through the bioinformatics lens. computational intelligence Methods for Bioinformatics and Biostatistics. CIBB 2015. Lecture Notes in Computer Science 9874. 10.1007/978-3-319-44332-4_15 [DOI] [Google Scholar]
- Voorhoeve, P. M., Hijmans, E. M. and Bernards, R. (1999). Functional interaction between a novel protein phosphatase 2A regulatory subunit, PR59, and the retinoblastoma-related p107 protein. Oncogene 18, 515-524. 10.1038/sj.onc.1202316 [DOI] [PubMed] [Google Scholar]
- Walker, I. M., Riboldi, G. M., Drummond, P., Saade-Lemus, S., Martin-Saavedra, J. S., Frucht, S., Bardakjian, T. M., Gonzalez-Alegre, P. and Deik, A. (2021). PPP2R5D Genetic Mutations and Early-Onset Parkinsonism. Ann. Neurol. 89, 194-195. 10.1002/ana.25943 [DOI] [PubMed] [Google Scholar]
- Wallace, A., Caruso, P. and Karaa, A. (2019). A newborn with severe ventriculomegaly: expanding the PPP2R1A gene mutation phenotype. J. Pediatr. Genet 8, 240-243. 10.1055/s-0039-1692414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. S., Esplin, E. D., Li, J. L., Huang, L., Gazdar, A., Minna, J. and Evans, G. A. (1998). Alterations of the PPP2R1B gene in human lung and colon cancer. Science 282, 284-287. 10.1126/science.282.5387.284 [DOI] [PubMed] [Google Scholar]
- Wang, X., Bajaj, R., Bollen, M., Peti, W. and Page, R. (2016). Expanding the PP2A interactome by defining a B56-specific SLiM. Structure 24, 2174-2181. 10.1016/j.str.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., Zhu, S., Fisher, L. A., Wang, W., Oakley, G. G., Li, C. and Peng, A. (2018). Protein interactomes of protein phosphatase 2A B55 regulatory subunits reveal B55-mediated regulation of replication protein A under replication stress. Sci. Rep. 8, 2683. 10.1038/s41598-018-21040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Lou, S. S., Wang, T., Wu, R. J., Li, G., Zhao, M., Lu, B., Li, Y. Y., Zhang, J., Cheng, X.et al. (2019). UBE3A-mediated PTPA ubiquitination and degradation regulate PP2A activity and dendritic spine morphology. Proc. Natl. Acad. Sci. USA 116, 12500-12505. 10.1073/pnas.1820131116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Garvanska, D. H., Nasa, I., Ueki, Y., Zhang, G., Kettenbach, A. N., Peti, W., Nilsson, J. and Page, R. (2020). A dynamic charge-charge interaction modulates PP2A:B56 substrate recruitment. Elife 9, e55966. 10.7554/eLife.55966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsey, A. J., Fernandez, T. V., Yu, D., King, R. A., Dietrich, A., Xing, J., Sanders, S. J., Mandell, J. D., Huang, A. Y., Richer, P.et al. (2017). De Novo coding variants are strongly associated with tourette disorder. Neuron 94, 486-499.e9. 10.1016/j.neuron.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarchak, N. and Xing, Y. (2016). PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 51, 162-184. 10.3109/10409238.2016.1143913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarchak, N., Guo, F., Satyshur, K. A., Jiang, L., Jeffrey, P. D., Sun, T., Stanevich, V., Mumby, M. C. and Xing, Y. (2013). Structure of the Ca2+-dependent PP2A heterotrimer and insights into Cdc6 dephosphorylation. Cell Res. 23, 931-946. 10.1038/cr.2013.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. G., Chen, H., Guo, F., Yadav, V. K., McIlwain, S. J., Rowse, M., Choudhary, A., Lin, Z., Li, Y., Gu, T.et al. (2017). PP2A-B′ holoenzyme substrate recognition, regulation and role in cytokinesis. Cell Discov 3, 17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H., Li, H., Bai, T., Han, L., Ou, J., Xun, G., Zhang, Y., Wang, Y., Duan, G., Zhao, N.et al. (2020). Phenotype-to-genotype approach reveals head-circumference-associated genes in an autism spectrum disorder cohort. Clin. Genet. 97, 338-346. 10.1111/cge.13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Y., Igarashi, H., Wang, X. and Sakaguchi, N. (2005). Protein phosphatase subunit G5PR is needed for inhibition of B cell receptor-induced apoptosis. J. Exp. Med. 202, 707-719. 10.1084/jem.20050637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Y., Xu, Y., Chen, Y., Jeffrey, P. D., Chao, Y., Lin, Z., Li, Z., Strack, S., Stock, J. B. and Shi, Y. (2006). Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell 127, 341-353. 10.1016/j.cell.2006.09.025 [DOI] [PubMed] [Google Scholar]
- Xing, Y., Li, Z., Chen, Y., Stock, J. B., Jeffrey, P. D. and Shi, Y. (2008). Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell 133, 154-163. 10.1016/j.cell.2008.02.041 [DOI] [PubMed] [Google Scholar]
- Xu, Y., Xing, Y., Chen, Y., Chao, Y., Lin, Z., Fan, E., Yu, J. W., Strack, S., Jeffrey, P. D. and Shi, Y. (2006). Structure of the protein phosphatase 2A holoenzyme. Cell 127, 1239-1251. 10.1016/j.cell.2006.11.033 [DOI] [PubMed] [Google Scholar]
- Xu, Y., Chen, Y., Zhang, P., Jeffrey, P. D. and Shi, Y. (2008). Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol. Cell 31, 873-885. 10.1016/j.molcel.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., Cetin, B., Anger, M., Cho, U. S., Helmhart, W., Nasmyth, K. and Xu, W. (2009). Structure and function of the PP2A-shugoshin interaction. Mol. Cell 35, 426-441. 10.1016/j.molcel.2009.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, M., Iwatsubo, T., Mizuno, Y. and Mochizuki, H. (2004). Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J. Neurochem. 91, 451-461. 10.1111/j.1471-4159.2004.02728.x [DOI] [PubMed] [Google Scholar]
- Yan, Z., Fedorov, S. A., Mumby, M. C. and Williams, R. S. (2000). PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells. Mol. Cell. Biol. 20, 1021-1029. 10.1128/MCB.20.3.1021-1029.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Wu, J., Tan, C. and Klein, P. S. (2003). PP2A:B56epsilon is required for Wnt/beta-catenin signaling during embryonic development. Development 130, 5569-5578. 10.1242/dev.00762 [DOI] [PubMed] [Google Scholar]
- Yang, R., Yang, L., Qiu, F., Zhang, L., Wang, H., Yang, X., Deng, J., Fang, W., Zhou, Y. and Lu, J. (2013). Functional genetic polymorphisms in PP2A subunit genes confer increased risks of lung cancer in southern and eastern Chinese. PLoS One 8, e77285. 10.1371/journal.pone.0077285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, K. S., Tso, W. W. Y., Ip, J. J. K., Mak, C. C. Y., Leung, G. K. C., Tsang, M. H. Y., Ying, D., Pei, S. L. C., Lee, S. L., Yang, W.et al. (2017). Identification of mutations in the PI3K-AKT-mTOR signalling pathway in patients with macrocephaly and developmental delay and/or autism. Mol Autism 8, 66. 10.1186/s13229-017-0182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, U. Y. and Ahn, J. H. (2010). Phosphorylation on the PPP2R5D B regulatory subunit modulates the biochemical properties of protein phosphatase 2A. BMB Rep 43, 263-267. 10.5483/BMBRep.2010.43.4.263 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Li, H., Wang, H., Jia, Z., Xi, H. and Mao, X. (2020). A De Novo Variant Identified in the PPP2R1A Gene in an Infant Induces Neurodevelopmental Abnormalities. Neurosci. Bull. 36, 179-182. 10.1007/s12264-019-00430-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H., Chen, Y., Chen, S., Niu, Y., Yang, L., Li, B., Lu, Y., Geng, S., Du, X. and Li, Y. (2011). Expression and distribution of PPP2R5C gene in leukemia. J Hematol Oncol 4, 21. 10.1186/1756-8722-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J., Pham, H. T., Ruediger, R. and Walter, G. (2003). Characterization of the Aalpha and Abeta subunit isoforms of protein phosphatase 2A: differences in expression, subunit interaction, and evolution. Biochem. J. 369, 387-398. 10.1042/bj20021244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. Q., Zheng, H. Y., Peng, C. X., Liu, D., Li, H. L., Wang, Q. and Wang, J. Z. (2010). Protein phosphatase 2A facilitates axonogenesis by dephosphorylating CRMP2. J. Neurosci. 30, 3839-3848. 10.1523/JNEUROSCI.5174-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, Z., Hu, F., Hu, J., Wang, C., Hou, J., Yu, Z., Wang, T. T., Liu, X. and Huang, H. (2017). MicroRNA-218 promotes cisplatin resistance in oral cancer via the PPP2R5A/Wnt signaling pathway. Oncol. Rep. 38, 2051-2061. 10.3892/or.2017.5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnierowicz, S. and Hemmings, B. A. (1994). Tethering, targeting and triggering of protein phosphatases. Trends Cell Biol. 4, 61-64. 10.1016/0962-8924(94)90012-4 [DOI] [PubMed] [Google Scholar]
- Zwaenepoel, K., Louis, J. V., Goris, J. and Janssens, V. (2008). Diversity in genomic organisation, developmental regulation and distribution of the murine PR72/B″ subunits of protein phosphatase 2A. BMC Genomics 9, 393. 10.1186/1471-2164-9-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.