Abstract
A growing body of evidence supports the modulation of pain by light exposure. As such, phototherapy is being increasingly utilized for the management of a variety of pain conditions. The modes of delivery, and hence applications of phototherapy, vary by wavelength, intensity, and route of exposure. As such, differing mechanisms of action exist depending upon those parameters. Cutaneous application of red light (660nm) has been shown to reduce pain in neuropathies and complex regional pain syndrome-I, whereas visual application of the same wavelength of red light has been reported to exacerbate migraine headache in patients and lead to the development of functional pain in animal models. Interestingly visual exposure to green light can result in reduction in pain in variety of pain conditions such as migraine and fibromyalgia. Cutaneous application typically requires exposure on the order of minutes, whereas visual application requires exposure on the order of hours. Both routes of exposure elicit changes centrally in the brainstem and spinal cord, and peripherally in the dorsal root ganglia and nociceptors. The mechanisms of photobiomodulation of pain presented in this review provide a foundation in furtherance of exploration of the utility of phototherapy as a tool in the management of pain.
Keywords: Pain management, light therapy, complementary medicine, visual exposure, photoreception
Introduction
The exposure of human biological systems to light is ubiquitous. Importantly, light exposure from the sun determines much of human circadian rhythms and behavior. Sunlight at dawn promotes biological effects including waking, increases in glucocorticoid secretion, and feeding.7 In the modern-day era, however, exposure to light is not limited to sunlight exposure. Artificial indoor lighting and computers present in the workplace and home contribute a significant amount of light exposure.28, 33 Human exposure to light has also increased at night, whether from street lights, night lights, or workplace lights due to night shift work.14 These aberrant light exposures cause circadian rhythm disruptions that have been linked to negative health effects on mood, metabolism, immune defense, and cancer risk.7 Needless to say, the exposure to light has many important implications on human biology and behavior.
The use of light in the form of phototherapy has been increasingly investigated, although the idea has historical precedent.46 In the late 1800s, Nobel laureate Niels Ryberg Finsen reported the use of red light in the treatment of smallpox, as well as the use of ultraviolet light to treat lupus vulgaris.73 Light of various colors and wavelengths has since been used to treat a wide variety of conditions, including neonatal jaundice, seasonal affective disorder, acne, circadian rhythm disruptions, and psoriasis.46 Recently, light has been reported to have an effect on pain, both as a treatment for pain as well as an aggravator of pain.43, 66, 76, 88, 90
In the United States alone, 50 million adults have chronic pain, with an estimated $261–300 billion dollars being spent yearly to manage pain, resulting in an annual economic cost of chronic pain of at least $560–635 billion.18, 44 Current methods of pain management involve pharmacotherapy using agents such as serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, nonsteroidal anti-inflammatory drugs, corticosteroids, benzodiazepines, gabapentinoids, and opioids; however, the use of these drugs does not come without concerning side effects such as sedation, cardiotoxicity, ataxia, addiction, and respiratory depression.27, 98 Therefore, there has been a growing desire for research on complementary, non-pharmacological methods of pain relief therapies and management. Current nonpharmacologic therapies for pain include psychological, behavioral, meditative, and physical therapy approaches, as well as specialized procedural intervention techniques by pain specialists.1, 3, 98 Recently, investigations into the use of light as a treatment for symptomatic pain and pain syndromes have become particularly attractive as they are considered a low-cost, nonpharmacologic alternative with few side effects.
Studies on pain in relation to light administration have reported varying responses depending on the wavelength, intensity, and route of administration of light. For example, exposure to green light via the visual system resulted in lesser pain in an acute migraine episode compared to the exposure of other wavelengths such as white, blue, amber, and red.76 Red light administered through visual pathways caused thermal hyperalgesia and mechanical allodynia in rats.47 However, red light administered cutaneously decreased both thermal and mechanical hyperalgesia in a mouse model of complex regional pain syndrome.90 These reports are just a few examples of the varying effects of light on pain. Many articles report light-induced analgesia as well as hyperalgesia, and novel studies have been conducted to tease out the mechanistic basis of the effects of light on pain.39, 43, 66, 78, 81 Promising results in pre-clinical animal studies have led to the clinical translation of phototherapies for pain management. Although the underlying mechanisms have yet to be fully elucidated, the fact that light can modulate pain is increasingly being acknowledged with more experimental and clinical evidence forthcoming. This review strives to shed light on the effects of light on pain, the biological mechanisms through which light acts on pain, as well as the current applications of phototherapy on pain.
Overview of Photoreception and Phototransduction
It must first be clarified that photoreception, phototransduction, and photobiomodulation all refer to different aspects of light modulating biological mechanisms. Photoreception refers to the process of light detection by photoreceptors.54 Phototransduction involves the conversion of light signals into an intracellular response, such as a membrane depolarization or a phosphorylation, so that the light can exert its effects on biological responses.54 Photobiomodulation, then, is the overall biological effect that is produced in an organism in response to light exposure.39, 49, 88, 90
There are two main routes by which light can act to affect human biological processes, the most apparent being via the visual system.46 Briefly, light travels through the cornea/lens and reaches the retina, where it acts on cone and rod photoreceptors in the outer segment of the retina. Cones and rods are considered as the origin of the visual pathway in the retina, which work together to capture information in the visual field and ultimately respond to light.34, 55 Rods have a low threshold of activation and are activated by low intensity light, whereas cones have a high threshold of activation and are activated by colors.55 Within photoreceptors are photopigments such as opsins, which are G-protein-coupled receptors that respond to photons.34 Upon activation, the intracellular cGMP concentration decreases due to the activation of cGMP-phosphodiesterase (PDE6).34 This ultimately leads to the closure of nucleotide-gated Na+ channels, resulting in hyperpolarization of the photoreceptor cell and phototransduction. Rods and cones function in image formation as well as in the early and transient responses of the pupillary light reflex.8 This is in contrast to non-image-forming cells that are also present in the retina. Intrinsically photosensitive retinal ganglion cells (ipRGCs) are a type of non-image-forming cell that are involved in sensing ambient light. As the name suggests, ipRGCs can depolarize in direct response to light, without any input from rods and cones. The photopigment found in ipRGCs is OPN4 (opsin-4, melanopsin) and has a peak spectral sensitivity of 480nm, which falls in the blue/cyan range of visible light.8 This property of OPN4 explains the function of ipRGCs in circadian rhythm regulation, as it has been widely reported that circadian rhythm is disrupted with excessive blue light exposure.7, 33 From the retina, ipRGCs are able to regulate the circadian rhythm by sending projections via the retino-hypothalamic tract (through the optic nerve) and synapsing on the suprachiasmatic nucleus (SCN) of the hypothalamus. In response to signals from the ipRGCs, the SCN acts on the pineal gland to modulate the secretion of melatonin, a hormone important for circadian rhythm entrainment.7 ipRGCs project to several areas in the brain, including areas in the brainstem and thalamus associated with pain modulation such as the spinal trigeminal nucleus and rostral ventromedial medulla.21, 66, 76, 78, 81, 82 However, it is important to note that ipRGCs are not the only source of retinal projections associated with pain modulation, as it has also been shown that retinal ganglion cells that receive input from rods and cones also contribute to these pathways.78
The second route of photoreception and phototransduction occurs through the integumentary system. The skin in humans is the largest organ in the body, and light that is perceived by the eyes is sensed by the skin as well.46 Interestingly, human skin also possesses photosensory functions involved in multiple biological effects.35, 53 In fact, opsins such as cone opsins (OPN1), rhodopsin (OPN2), encephalopsin (OPN3), melanopsin (OPN4), and neuropsin (OPN5), which are traditionally known for their functions in the eye, have all been found to be expressed in epidermal skin cells.35, 53 The expression of OPN4 in the skin is particularly interesting in that the function of OPN4 in the eye involves modulating the sleep, circadian rhythms, melatonin secretion, pain, and more.7, 8, 77 Blue light irradiation on cultured human skin cells causes Ca2+ influx and ERK1/2 phosphorylation, indicating that skin cells can function in phototransduction by converting a light signal into an intracellular response.53 Skin exposure to ultraviolet radiation (more specifically, ultraviolet B) also increases endogenous vitamin D synthesis in the skin, and vitamin D has been shown to decrease pain. The effects of vitamin D on pain have been elaborated in a literature review19 and meta-analysis.101 Ultraviolet radiation (UVR) exposure has also been implicated in analgesia via increases in β-endorphin (a byproduct of melanocyte stimulating hormone production from the proopiomelanocortin precursor), although there is other literature documenting hyperalgesic effects of UVR.25, 37 Lopes and McMahon have succinctly synthesized the noxious effects of cutaneous UVR exposure in another literature review.62 It is important to note that most cutaneous phototherapies utilize red and infrared light, the latter of which is not sensed by opsins to cause phototransduction (Table 1).
Table 1.
Summary of studies on the effects and mechanisms of light on pain.
| Color | Study | Subjects | Route | Exposure | Intensity/ Irradiance | Pain Condition | Δ Pain | Mechanism of Action |
|---|---|---|---|---|---|---|---|---|
| White 390–740nm | Kowacs et al. 200151 | Human | Visual | 2 sec | 50–20,000 lux | Migraine | ↑ | ↓ trigeminal + cervical pain thresholds |
| Dolgonos et al. 201121 | Rat | Visual | 9 min | 9.1 × 103 μW/cm2 | - | ↑ | Intraretinal trigeminal sensitization | |
| Leichtfried et al. 201459 | Human | Visual | 6 hr | 5000 lux | Chronic nonspecific back pain | ↓ | - | |
| Martenson et al. 201666 | Rat | Visual | 30 sec | 18,000 lux | Fibromyalgia | ↑ | Visual system → OPN → RVM | |
| Noseda et al. 201676 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway | |
| Burgess et al. 201710 | Human | Visual | 1 hr | 3000+ lux | Fibromyalgia | ↓ | Dim light melatonin onset shift | |
| Nir et al. 201875 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway | |
| Burgess et al. 201911 | Human | Visual | 1 hr | 3000+ lux | Chronic LBP | ↓ | Dim light melatonin onset shift | |
| Burns et al. 202012 | Human | Visual | 1 hr | 3000+ lux | Chronic LBP | ↓ | - | |
| Bumgarner et al. 20209 | Mouse | Visual | 10 hr | 5 lux | - | ↑ | ↑ IL-6, NGF; ↑ PAG MOR | |
| Blue 450–500nm | Noseda et al. 201676 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway |
| Nir et al. 201875 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway | |
| Khanna et al. 201947 | Rat | Visual | 8 hr | 4–5 lux | Functional pain syndromes | ↓ | Visual system → RVM | |
| Green 500–565nm | Noseda et al. 201676 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↓ | - |
| Ibrahim et al. 201743 | Rat | Visual | 8 hr | 4 lux | Chronic pain | ↓ | ↑ RVM descending pain
inhibition; ↓ CaV2.2 response; ↑ PENK; Naloxone reversal |
|
| Khanna et al. 201947 | Rat | Visual | 8 hr | 4 lux | Chronic pain | ↓ | Visual system → RVM | |
| Martin et al. 202068 | Human | Visual | 1–2 hr | 4–100 lux | Migraine | ↓ | - | |
| Martin et al. 202067 | Human | Visual | 1–2 hr | 4–100 lux | Fibromyalgia | ↓ | - | |
| Amber 590nm | Noseda et al. 201676 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway |
| Nir et al. 201875 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway | |
| Red 625–740nm | Stelian et al. 199296 | Human | Skin | 15 min | 8 mW/cm2 | Knee osteoarthritis | ↓ | - |
| Hsieh et al. 201241 | Rat | Skin | 60 sec | 150 mW/cm2 | Neuropathic pain | ↓ | ↓ TNF-α, IL1-β, HIF-1α | |
| Noseda et al. 201676 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway | |
| Nir et al. 201875 | Human | Visual | 3 min | 1–100 cd/m2 | Migraine | ↑ | Retino-thalamo-cortical pathway | |
| Langella et al. 201856 | Human | Skin | 300 sec | 16.66 mW/cm2 | Post-total hip arthroplasty | ↓ | ↓ serum IL-8, TNF-α, IL-6 | |
| Khanna et al. 201947 | Rat | Visual | 8 hr | 50 lux | Functional pain syndromes | ↑ | Visual system → RVM | |
| Pigatto et al. 202088 | Mouse | Skin | 60 sec | 84.64 mW/cm2 | - | ↓ | ↓ TRPA1, TRPV1, TRPM8, and ASIC receptor nociception | |
| Herpich et al. 202038 | Human | Skin | 300 sec | 5 mW/cm2 | TMD | ↓ | - | |
| Rodrigues et al. 202090 | Rat | Skin | 20 sec | 316 mW/cm2 | CRPS-I | ↓ | - | |
| Near-Infrared (750–1000nm) | Stelian et al. 199296 | Human | Skin | 15 min | 11 mW/cm2 | Knee osteoarthritis | ↓ | - |
| Cidral-Filho et al. 201315 | Mouse | Skin | 32 sec | 80 mW/cm2 | Neuropathic pain | ↓ | ↓ spinal cord + sciatic nerve TNF-α | |
| Cidral-Filho et al. 201416 | Mouse | Skin | 153 sec | 80 mW/cm2 | Postoperative incisional pain | ↓ | ↑ activation peripheral opioid receptors | |
| Martins et al. 201670 | Mouse | Skin | 50 sec | 80 mW/cm2 | Inflammatory pain | ↓ | ↑ IL-10; ↑ catalase; ↑ superoxide dismutase; ↓ TBARS | |
| Holanda et al. 201739 | Rat | Skin | 120 sec | 300 mW/cm2 | Neuropathic pain | ↓ | ↓ signal via cytoskeletal disruption | |
| Kobiela Ketz et al. 201749 | Rat | Skin | 19 sec | 43.25 mW/cm2 | Neuropathic pain | ↓ | ↑ M2 macrophage activation | |
| Pigatto et al. 201787 | Mouse | Skin | 20 min | 17.3 mW/cm2 | - | ↓ | ↓ PKA, PKC activation | |
| de Sousa et al. 201820 | Mouse | Skin | 120 sec | 50 mW/cm2 | Chronic peripheral pain | ↓ | ↓ DRG mGLuR1; ↑ PAP | |
| Langella et al. 201856 | Human | Skin | 300 sec | 9.66 mW/cm2 | Post-total hip arthroplasty | ↓ | ↓ serum IL-8, TNF-α, IL-6 | |
| Yadav et al. 2018103 | Rat | Skin | 10 min | 0.4 mW/cm2 | Burn wound | ↓ | ↓ TNF-α, NFκB, IL1-β, COX-2, substance-P receptor | |
| Balbinot et al. 20195 | Rat | Skin | 40 sec | 1.43 mW/cm2 | Knee osteoarthritis | ↓ | ↓ spinal cord astrogliosis | |
| Pigatto et al. 201989 | Mouse | Skin | 20 min | 17.4 mW/cm2 | CRPS-I, Neuropathic pain | ↓ | - | |
| Rodrigues et al. 202090 | Rat | Skin | 40 sec | 316 mW/cm2 | CRPS-I | ↓ | - | |
| Herpich et al. 202038 | Human | Skin | 300 sec | 5 mW/cm2 | TMD | ↓ | - |
Abbreviations: ASIC, acid-sensing ion channels; CaV2.2, N-type voltage-gated calcium channel; COX-2, cyclooxygenase-2; CRPS-I, complex regional pain syndrome-1; DRG, dorsal root ganglion; HIF-1α, hypoxia-inducible factor-1α; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; LBP, low back pain; mGLuR1, metabotropic glutamate receptor type 1; MOR, μ-opioid receptor; NFκB, nuclear factor kappa B; NGF, nerve growth factor; OPN, olivary pretectal nucleus; PAG, periaqueductal gray; PAP, prostatic acid phosphatase; PENK, proenkephalin-A; PKA, protein kinase A; PKC, protein kinase C; RVM, rostral ventromedial medulla; TBARS, thiobarbituric acid reactive species; TMD, temporomandibular disorder; TNF-α, tumor necrosis factor α; TRPA1, Transient Receptor Potential Ankyrin 1; TRPM8, Transient Receptor Potential Melastatin 8; TRPV1, Transient Receptor Potential Vanilloid 1.
Instead, red and infrared lights applied cutaneously elicit phototransduction by acting directly on cellular components. For example, red and infrared light can excite chromophores in enzymes which can ultimately result in increases in cellular metabolism and growth.46, 91 Specifically, the excitation of cytochrome c oxidase (a mitochondrial membrane protein) has been shown to result in increases in ATP production, intracellular calcium levels, mitochondrial membrane permeability, reaction oxygen species generation, and vasodilation, ultimately engaging the cell in a regenerative state.36, 45, 46, 84 Although the mechanism of phototransduction through the skin has not yet been as vigorously investigated as in the visual pathway in the context of pain modulation, these findings show that human skin contains functional units through which phototransduction and photobiomodulation can occur, providing the groundwork for a mechanistic understanding of the pain-modulating effects of light when applied to the skin.88, 90
Mechanisms of Visual Phototransduction Leading to Pain Induction or Exacerbation
True photophobia, as defined by Lebensohn, is an abnormal aversion to light in which exposure to light either exacerbates or induces pain.57 Specifically, photophobia has been reported in a wide variety of conditions, including neurological disorders (i.e. multiple sclerosis, posttraumatic brain injury), ophthalmological pathologies (i.e. herpes zoster opthalmicus), and psychiatric disorders (i.e. depression, generalized anxiety).2, 17, 64, 92, 94, 100 However, aversive photophobia is not the only painful phenomenon that can be elicited by light. Exposure to ambient light that is not necessarily aversive has also been reported to cause hyperalgesia and allodynia.9, 47 There are a number of different proposed mechanistic explanations for light inducing or exacerbating pain, and it is likely true that all of these mechanisms mentioned below work in concert to achieve the overall effect of pain (Figure 1).
Figure 1. Neural pathways of visual phototransduction leading to pain induction or exacerbation.
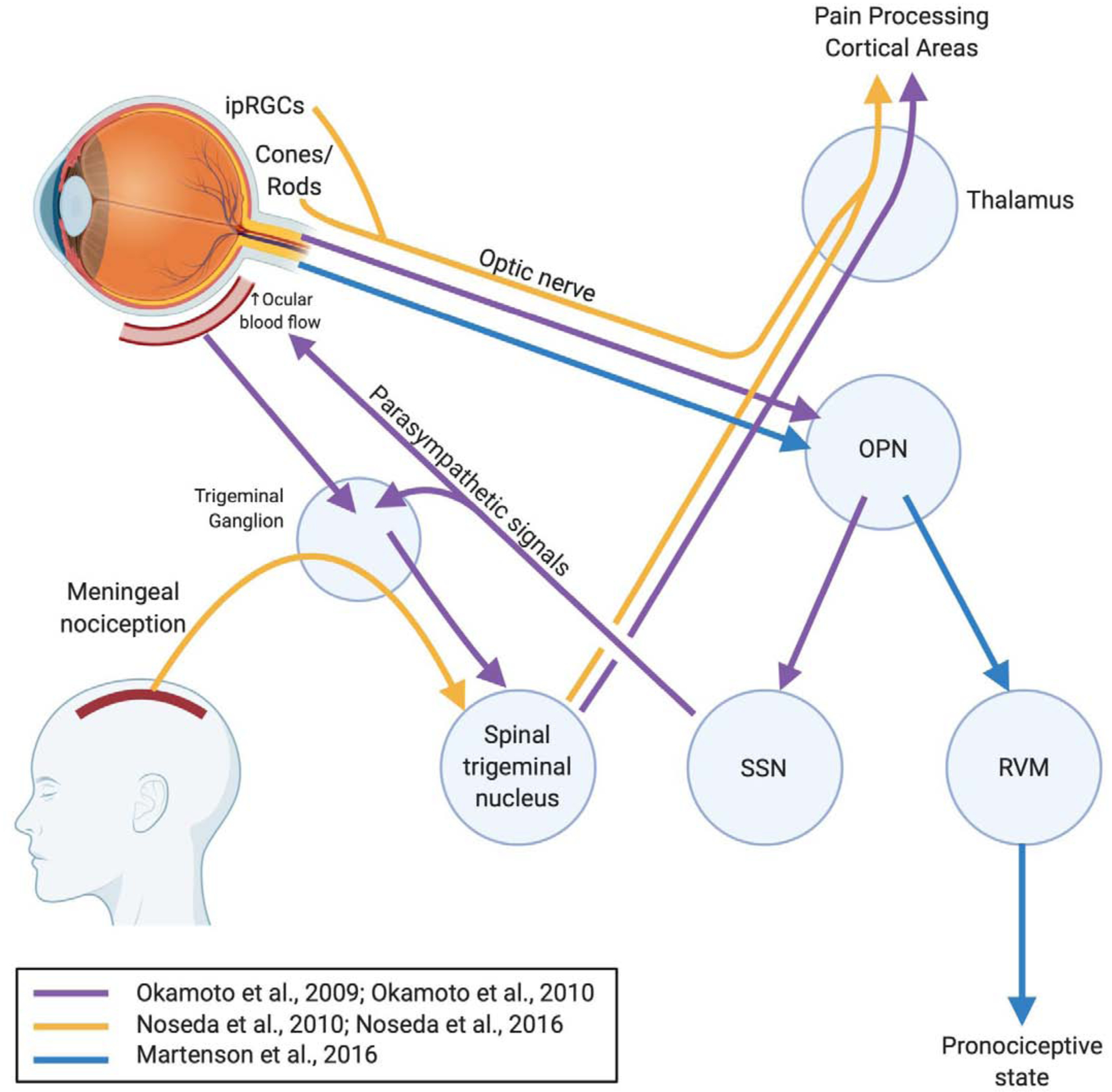
Exposures to light have been reported to induce or exacerbate pain by eliciting changes in pain processing and modulating centers in the brain and brainstem. The importance of the olivary pretectal nucleus in these pathways has been corroborated by multiple studies. The pain-modulating rostral ventromedial medulla can be switched to a pronociceptive state following light exposure. In the setting of migraine, light input and meningeal nociception signals ultimately converge on the thalamus to project to cortical areas of pain processing, resulting in photophobia. Abbreviations: ipRGC, intrinsically photosensitive retinal ganglion cells; OPN, olivary pretectal nucleus; PAG, periaqueductal gray; RVM, rostral ventromedial medulla; SSN, superior salivatory nucleus.
Dolgonos et al. reported a mechanism of photophobia in rodents that doesn’t explicitly involve the central visual pathway.21 They found that even with lesions of the optic nerve, bright light was able to elicit blink modifications characteristic of photophobia. This suggested an intraretinal mechanism that modulates the trigeminal system with bright light exposure. Although the study did not identify a specific intraretinal mechanism, the authors suggested that associational ganglion cells (ie. ipRGCs) are responsible for sensitizing the spinal trigeminal nucleus neurons by projecting axons to the retinal periphery, an area that is richly innervated by trigeminal nociceptors.58, 93, 104
Okamoto et al. reported a series of animal studies documenting the activation of the trigeminal nociceptive pathway by bright light.81, 82 In their first study, they reported light intensity-dependent increases in Fos-like immunoreactivity, a marker of neuronal activation, in the caudal trigeminal brainstem.82 Specifically, the neuronal activation observed in the trigeminal caudalis/cervical cord junction region (Vc/C1) and the nucleus tractus solitarius was associated with regions involving autonomic control, which explained increases in ocular blood flow and activation of nociceptive trigeminal nerves surrounding blood vessels in response to bright light. In their second study, the authors demonstrated that bright light activated nociceptive neurons in Vc/C1 by an intraocular mechanism via the trigeminal root ganglion.81 The proposed intraocular mechanism is as follows: 1) luminance in the eye is relayed to the olivary pretectal nucleus, 2) activation of the olivary pretectal nucleus increases parasympathetic signals to the eye via the superior salivatory nucleus, and 3) nerves of the trigeminal root ganglion are activated either by postganglionic parasympathetic neurotransmitters or by mechanical changes in ocular blood vessels due to changes in ocular blood flow, as suggested in previous studies (Figure 1).82 The authors also noted the importance of the olivary pretectal nucleus in the transmission of light information in this mechanism of the activation of the trigeminal nociceptive pathway, which was also emphasized by Martenson et al. in their report of light acting on the central pain-modulating system in the rostral ventromedial medulla.66
A retino-thalamo-cortical pathway of light exacerbating migraine headache pain has been described by Noseda et al. in both animal and human studies.76, 78 Blind individuals with non-image-forming mechanisms still intact (ie. ipRGCs) who suffer from migraines were still able to experience an increase in headache pain when subjected to light during a migraine.78 This led the authors to hypothesize that non-image-forming signals from the eye could modulate central trigeminovascular neurons. Dura-sensitive neurons in the posterior thalamus were found to be apposed primarily by axons of ipRGCs, and signals from ipRGCs induced by light were able to affect the activity of dura-sensitive thalamocortical neurons. In short, meningeal nociceptors traveling through the trigeminal ganglion convey signals to dura-sensitive neurons in the spinal trigeminal nucleus, relaying the information to the dura-sensitive neurons in the posterior thalamus (Figure 1). Phototransduction via ipRGCs is also relayed to dura/light-sensitive neurons, which can modulate nociceptive information originating from the dura mater before thalamic axons project to pain-processing cortical areas. The significance of this mechanism of photophobia is that it does not involve image-forming components of the eye, such as cones and rods. However, a later study published by the same group reported a cone-driven pathway of migraine photophobia.76 Blue light was found to activate more neurons with a greater magnitude in the “thalamo” portion of the pathway when compared to green light. In fact, green light reduced pain intensity in ~20% of patients having active migraine attacks in the study. The finding that migraine headache exacerbation was color-sensitive suggested that a cone-driven retinal pathway is also important in photophobia. The two mechanistic pathways proposed by these two studies suggest that there are both image-forming and non-image-forming inputs that act together to exacerbate migraine headache pain, further emphasizing that no single mechanistic explanation is sufficient to describe pain exacerbation and induction by light.
Martenson et al. described a more central mechanism of photophobia that contrasted with previous reports of photophobia involving the trigeminal pathway.66 The authors pointed out that although previous reports of photophobia caused by the direct interaction of sensory transmission adequately explain the exacerbation of migraine headache pain, this mechanism does not necessarily explain the pain exacerbation by light in functional pain disorders such as fibromyalgia. They hypothesized that light influences the intrinsic pain-modulating system such as the rostral ventromedial medulla (RVM). The animal study demonstrated that 30 seconds of 18×103 lux light exposure caused the activation of pain-facilitating “ON-cells” and the suppression of pain-inhibiting “OFF-cells” in the RVM, ultimately shifting the pain-modulating system into a pronociceptive state. In the animal behavior studies, this effect manifested as a lowered threshold for noxious heat-evoked paw withdrawal. Furthermore, by using lidocaine to selectively inactivate other potential relays of light information, the authors were able to demonstrate that the pathway of light information transmission acting on the RVM travels through the olivary pretectal nucleus and not the posterior thalamus nor the trigeminal ganglion (Figure 1). These findings suggest a mechanism of light acting on the central pain-modulating system in the RVM, unique from other reports of photophobia mechanisms described above.21, 76, 78, 81, 82
Aberrant light exposure at night causes disruptions in the circadian rhythm by modulation of the melatonin signaling system which ultimately disrupts sleep.7 These disruptions in the diurnal sleep schedule have been shown to increase pain perception, as seen in night-shift workers.52, 71, 80, 86, 97 Although the mechanism has not been fully elucidated, there are some animal studies that might provide a mechanistic explanation. Bumgarner et al. reported that dim light exposure at night in male mice induced cold hyperalgesia and mechanical allodynia.9 Specifically, 28 days of dim light exposure caused increased expression of mu-opioid receptors in the periaqueductal gray and increased IL-6 and NGF expression in the medulla. Although far from a mechanistic explanation, these findings provide important clues to implicate the importance of neuroinflammation caused by light exposure on nociceptive and neuropathic pain states.
A psychological mechanism of pain exacerbation by light has been described by Wiercioch-Kuzianik et al.99 This mechanism involves learned associations of certain colors; for example, red conveys negative information associated with failure, threat, and anger26, 29, whereas green and pink conveys positive information associated with happiness30. In this study, participants were shown a color on a computer screen prior to the administration of a pain stimulus, and then asked to rate the perceived pain.99 Interestingly, pain intensity ratings increased from baseline values for all colors except for green. However, compared to blue and green, red elicited higher pain ratings in response to the pain stimuli. These findings show that the psychological influence of learned associations of colors can change pain perception, with red, a color normally associated to negative emotions, causing a hyperalgesic effect.
The concept of visual phototransduction resulting in the induction and exacerbation of pain has been used to create injury-free animal models of pain. A recent study reported that exposure to red light-emitting diodes (LEDs, 660 nm) caused time- and dose-dependent thermal hyperalgesia and mechanical allodynia in rats.47 Microinjections of bicuculline, a gamma-aminobutyric acid-A receptor antagonist, into the rostroventromedial medulla (RVM) reversed the hyperalgesia and allodynia due to red LED exposure, showing that phototransduction of red light occurred through the visual system and ultimately acted on the RVM to increase descending facilitation of the pain pathway, shifting the pain-modulating system into a pronociceptive state. These findings are in agreement with Martenson et al. and further support the notion that light can modulate pain by acting through the visual system and the RVM.47, 66
Mechanisms of Visual Phototransduction Leading to Analgesia
Although the mechanism of light inducing or exacerbating pain via the visual system has been well-documented, the therapeutic effect of light reducing pain is only recently being studied. There are some clinical studies emphasizing the effect of light exposure on the circadian rhythm in reducing pain. Two proof of concept studies – one in women with fibromyalgia10 and the other in military veterans with chronic low back pain11, showed that sitting before broad-spectrum bright white lights (>3000 lux) for one hour per day upon waking in the morning improved pain sensitivity and behavior. These changes were attributed to approximately an hour earlier shift in circadian timing, indicated by changes in dim light melatonin onset (Figure 2).10, 11. The small number of subjects in these promising pilot studies underscores the need for additional future studies with more subjects to elucidate the effect size and broader application of this therapy to a larger and more diverse patient population. Although these studies do not demonstrate the exact pathway of how shifts in melatonin onset might modulate pain, the role of melatonin in pain modulation has been well-studied and thoroughly reviewed.4, 102 None the less, these studies demonstrate that changes in circadian rhythms by bright light can modulate pain.
Figure 2. Mechanisms of visual phototransduction leading to analgesia.
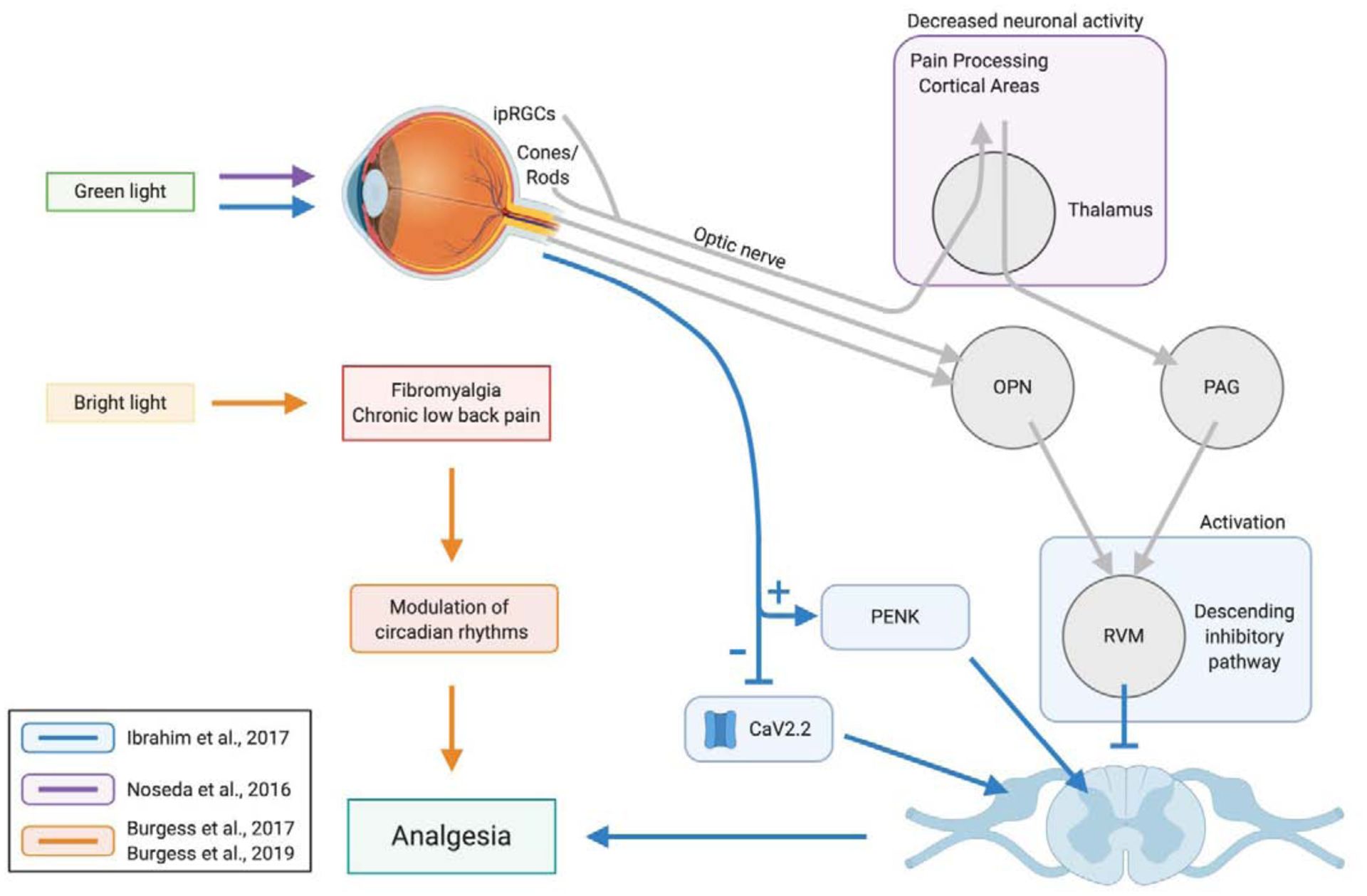
Visual applications of light elicit central changes such as increased enkephalin mRNA expression and decreased calcium influx. Applications of bright light have been shown to modulate circadian rhythms that also contribute to an overall analgesic effect. Abbreviations: CaV2.2, N-type voltage-gated calcium channel; ipRGC, intrinsically photosensitive retinal ganglion cells; OPN, olivary pretectal nucleus; PAG, periaqueductal gray; PENK, proenkephalin-A; RVM, rostral ventromedial medulla.
Another clinical study by Noseda et al. also showed a potential therapeutic effect of light on migraine headache pain.76 They reported that although white, blue, amber, and red lights exacerbated migraine headache pain, green light was found to exacerbate the pain less. The authors proposed that green light did not increase headache pain as much in migraine patients because green light does not activate neurons in the retino-thalamo-cortical pain pathway as much as other colors of light such as blue or white. This explanation defines that green light does not exacerbate migraine headache, but it does not provide a mechanistic explanation as to why ~20% of the migraine patients in the study reported reduced headache pain when exposed to green light during a migraine attack. However, a study from the same group later showed that the effect of light on migraine pain also involves hypothalamic-mediated autonomic responses, further describing the complexity of migraine photophobia.79 A recent clinical study also showed that prophylactic green light exposure for 1–2 hours daily for 10 weeks significantly reduced the number of headache days as well as the intensity and duration of headache attacks in patients with episodic or chronic migraine.68
Studies from our group were also able to elucidate a possible mechanism of light producing antinociceptive effects via the visual system. In a series of animal studies performed in rats, Ibrahim et al. found that 5 days of 8-hour exposure to green (525nm) light, via light emitting diodes (LED) at 4-lux intensity, was able to cause antinociception in rats that persisted up to 4 days after cessation of the green LED exposure.43 The antinociceptive effect of green LED required engagement of the visual system and not the integumentary system by use of colored contact lenses as filters. Microinjections of lidocaine in the RVM and intrathecal administration of naloxone identified that the descending pain inhibitory pathways of the RVM and the mu-opioid receptor pathways were both necessary for the antinociceptive effect of green LED exposure (Figure 2). Quantitative RT-PCR of spinal cords in rats with L5 and L6 spinal nerves ligated that were exposed to green LED found increased expression of proenkephalin-A mRNA, indicating that the analgesia caused by green LED could be partially explained by increased enkephalins in the spinal cord. These results collectively describe a mechanism of antinociception in which light, which travels through the visual system, eventually acts on the central pain modulation system to shift to an antinociceptive state by activating descending pain inhibitory pathways of the RVM, engaging the mu-opioid receptor system, and increasing the expression of enkephalins in the spinal cord. It was also found that green LED treatment in naïve rats caused a decrease in depolarization-induced Ca2+ influx through N-type voltage-gated calcium channels (CaV2.2) in sensory neurons, which are important in antinociception.43, 95 Thus, green LED treatment may have both central and peripheral effects contributing to antinociception. Although it was identified that the visual system was necessary in creating the antinociceptive response due to green light, the exact connection between the visual system and the pain modulation system has not yet been elucidated and requires further investigation. As multiple mechanisms underlie pronociceptive effects of light, it is likely multiple analgesic mechanisms are engaged by green light.66, 76, 78, 81, 82 Further studies in this area may reveal additional antinociceptive mechanisms of light acting via the visual system.
Mechanisms of Cutaneous Phototransduction Leading to Analgesia
Another mechanism by which light can reduce pain is via the skin. Cutaneous applications of light for the treatment of pain conditions have been widely reported, including for neuropathic pain63, chronic low back pain42, 105, fibromyalgia40, and post-surgical pain24. The current mechanistic explanations of the effects of cutaneous application of light for pain alleviation can be divided into peripheral and central effects, both of which likely contribute jointly to decrease pain (Figure 3).
Figure 3. Mechanisms of cutaneous phototransduction leading to analgesia.
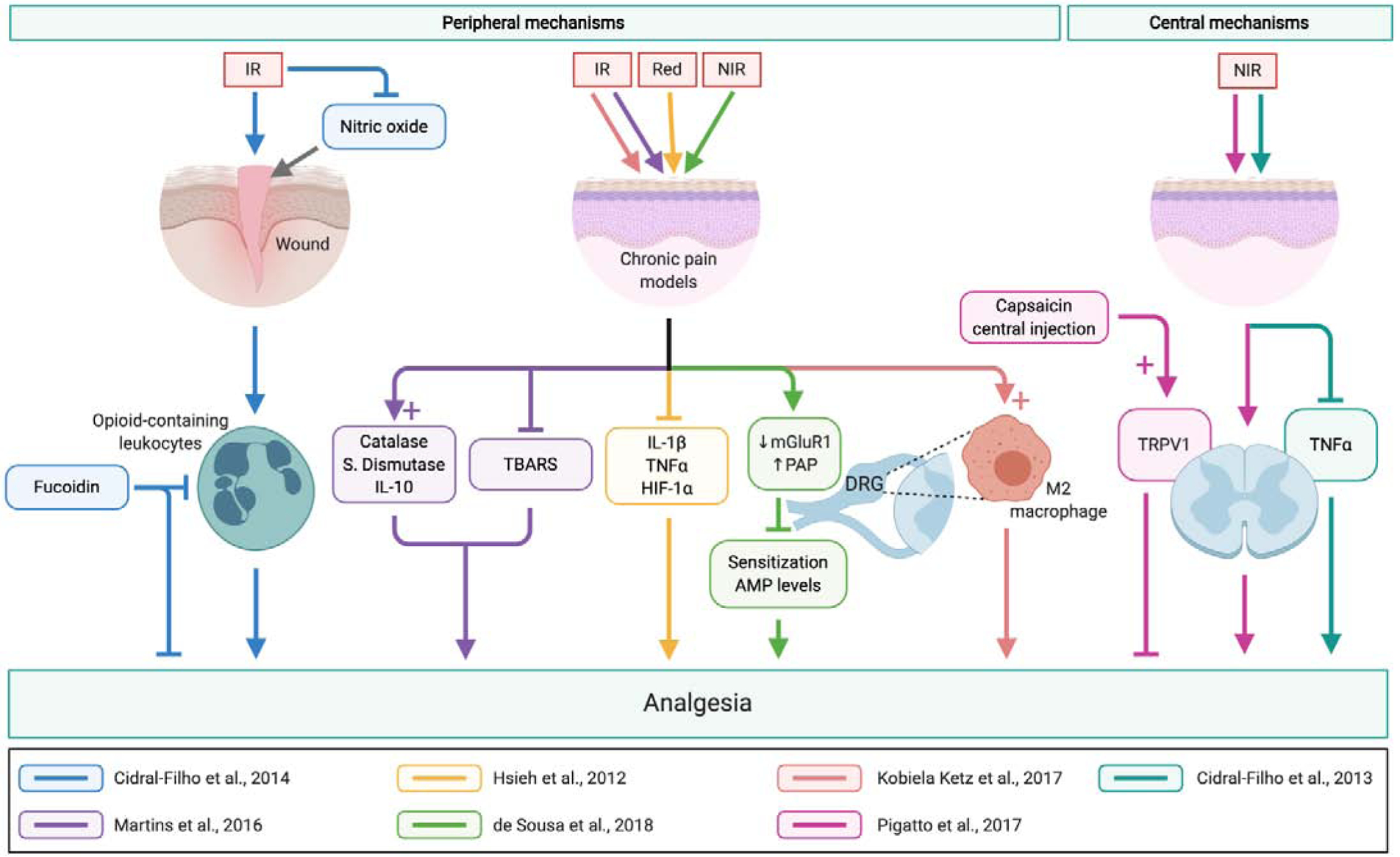
Application of light cutaneously has peripheral and central effects to reduce pain. Peripheral mechanisms include modulation by pro- and anti-inflammatory cytokines, immune cell recruitment and activation, and decreased expression of receptors involved in neuronal transmission. Central mechanisms involve reduction of inflammatory cytokines and receptors responsible for pain transmission. Abbreviations: DRG, dorsal root ganglion; HIF-1α, hypoxia-inducible factor-1α; IL-1β, interleukin 1β; IL-10, interleukin 10; IR, infrared; mGLuR1, metabotropic glutamate receptor type 1; NIR, near-infrared; PAP, prostatic acid phosphatase; TBARS, thiobarbituric acid reactive species; TNF-α, tumor necrosis factor α; TRPV1, Transient Receptor Potential Vanilloid 1.
Peripheral Mechanisms
A mechanism of cutaneously-administered light inducing analgesia involving peripheral opioid receptors in a mouse model of postoperative pain has been described by Cidral-Filho et al.16 In this study, direct contact of 950nm (infrared) LED light to the plantar incision site reduced mechanical hypersensitivity in mice. Injections of naloxone, a nonselective opioid receptor antagonist, were able to prevent the analgesic effects of LED treatment when injected locally in the plantar incision site, but not when injected intrathecally, indicating that the observed analgesic effects involve the activation of peripheral, but not central, opioid receptors. Opioid-containing leukocytes were found to be important in the activation of these peripheral opioid receptors, as administration of fucoidin (an inhibitor of leukocyte rolling) partially prevented the LED-induced analgesia. This study also investigated the involvement of nitric oxide (NO), an inflammatory mediator and messenger of nociceptive transmission, in LED-induced analgesia.32, 74 Experiments using L-arginine (nitric oxide precursor) and Nω-nitro-L-arginine (L-NOARG, nitric oxide inhibitor) found that another mechanism of the analgesia is due to a local reduction of NO in the inflamed site.16 Collectively, these findings demonstrate that the involvement of peripheral opioid receptors and local inflammatory pathways are important in the mechanism of cutaneously-administered LED-induced analgesia (Figure 3).
Another study reported by Martins et al. showed an anti-inflammatory mechanism by cutaneous LED treatment that reduced hyperalgesia in a model of chronic inflammatory pain.70 They found that 950nm (infrared) LED light in direct contact with the plantar aspect of the hindlimb was able to reduce mechanical and thermal hyperalgesia in mice with Complete Freund’s Adjuvant (CFA) injected in the hindlimb. Importantly, cytokine analyses of the subcutaneous paw tissue found no significant changes in pro-inflammatory TNF-α and IL1-β, whereas anti-inflammatory IL-10 was increased (Figure 3). Additionally, thiobarbituric acid reactive species (TBARS, a measure of oxidative stress) was decreased, and catalase and superoxide dismutase (both antioxidants) levels increased in the LED treated group when compared to the controls. These findings demonstrate that activation of anti-inflammatory and antioxidant processes local to the treatment area are important in the analgesic effect of cutaneously-administered LED treatment. The authors also reported the activation of peripheral opioid receptors in response to the LED treatment, further confirming the findings of Cidral-Filho et al.16
Another anti-inflammatory mechanism contributing to cutaneous light treatment analgesia has been proposed by Hsieh et al. in a rat model of neuropathic pain.41 Cutaneous application of 660nm (red) light over the area of loose nerve ligation of the sciatic nerve not only decreased mechanical allodynia, but also decreased the expression of TNF-α, IL1-β, and hypoxia-inducible factor 1α (HIF-1α) in the sciatic nerve when compared to sham controls (Figure 3). Neuroinflammation mediated by pro-inflammatory cytokines and chemokines is known to increase peripheral sensitization to pain.72 The findings in this report show that cutaneous light treatment is able to decrease pro-inflammatory cytokine levels in order to decrease neuroinflammation contributing to pain.
A study by de Sousa et al. reported a peripheral mechanism of analgesia and antinociception involving biomolecular changes in the dorsal root ganglion (DRG).20 In this study, 120 seconds of 810nm (near infrared) irradiation in the lower back was able to increase pain threshold in mice 3 hours after the treatment. More importantly, the changes observed in the lumbar DRGs included a decrease in metabotropic glutamate receptor (mGluR1) expression and an increase in prostatic acid phosphatase (PAP). The authors posited that the decrease in mGluR1 expression contributed to the analgesic effect either by decreasing the sensitization of other neurons or, indirectly, by the decrease of glutamate channel opening. PAP, an enzyme typically used as a marker of prostatic cancer diagnosis and therapy50, can hydrolyze adenosine monophosphate (AMP), which acts as a sensitizer for nociceptive neurons.20 An increase in PAP due to the irradiation therefore reduced nociceptive signaling by decreasing AMP levels. The findings reported in this study describe a mechanism of LED treatment in which intracellular changes within the DRG result in alterations that retard nociceptive transmission.
Cytoskeletal changes in peripheral nerves contributing to light-induced analgesia have also been reported. A number of rodent studies have shown that near-infrared light treatment on the skin over the affected nerve can cause cytoskeletal changes in the nociceptive nerves to decrease the transmission of nociception.20, 39, 61 Specifically, significant increases in axonal β-tubulin varicosities in DRG neurons were found in light-treated groups compared to controls.20, 39 These varicosities were found in axons corresponding to Aδ- and C-fibers responsible for the transmission of chemical, mechanical, and thermal nociception.39 The β-tubulin varicosities formed as a result of light irradiation disrupting microtubules, which are essential in mechanisms involved in neurotransmission.20 The cytoskeletal disruptions reported in these studies only further support a mechanism of light-induced analgesia in which nociceptive transmission is hampered.
Another mechanism described by Kobiela Ketz et al. involves macrophage polarization in the DRG.49 Macrophages can either be activated to a classical M1 phenotype, typically associated with the production of proinflammatory cytokines (TNF-α, IL1-β, IL-6), or an alternative M2 phenotype, typically associated with the production of anti-inflammatory cytokines (TGF-β, IL-10).13, 48, 65, 69 In a spared nerve injury rat model, 980nm (infrared) light treatment reduced mechanical hypersensitivity.49 Moreover, immunohistochemistry staining with M1- and M2-specific antibodies (CD86 and CD206, respectively) found a higher M2 activation in the DRG of the light-treated group, with the activation peaking at 7 days after starting treatment and lasting for 15 days thereafter. No significant differences in M1 activation was observed in either sham or light-treatment groups. Although further cytokine analyses are warranted to elucidate the anti-inflammatory action of the M2-activated macrophages specific to light-treatment analgesia, these results show that macrophage activation to the M2 phenotype in DRG in response to light treatment contribute to the analgesic effect of cutaneous light treatment (Figure 3).
Pigatto et al. proposed a peripheral mechanism of LED treatment analgesia involving hindering the activation of protein kinase A (PKA) and protein kinase C (PKC) by peripheral chemosensors.87 Endogenous noxious signals such as glutamate, prostaglandin E2, and bradykinin bind to G protein-coupled receptors to decrease peripheral nociceptive activation thresholds by phosphorylating different ionic channels (ie. TRPV1) via the PKA or PKC pathways.6, 23, 31, 60, 85 In the animal studies, intraplantar injection of glutamate, prostaglandin E2, and bradykinin all elicited nocifensive behavior in mice, but 890nm (infrared) and 660nm (red) LED applied directly to the skin was able to reduce these nocifensive responses.87, 89 Furthermore, intraplantar injection of forskolin (activator of adenylyl cyclase) and phorbol 12-myristate 13-acetate (PMA, activator of PKC) both caused nocifensive behavior in mice, but LED treatment was also able to reduce these nocifensive responses. These results suggest a mechanism of analgesia by LED treatment by reducing the nociceptive sensitization promoted by endogenous noxious signals ultimately by decreasing the activation of PKA and PKC.
The involvement of peripheral chemosensors in analgesia by LED treatment has also been implicated. Nociceptive responses due to intraplantar injections of noxious chemicals that activate Transient Receptor Potential channels (TRPs) and Acid-Sensing Ion Channels (ASICs) including cinnamaldehyde (activator of TRPA1), capsaicin (activator of TRPV1), menthol (activator of TRPM8), and acidified saline (activator of ASICs) were decreased in mice with cutaneous red LED treatment.87, 89 Though the exact mechanism through which LED treatment affects these channels requires more investigation to be further elucidated, these results show that peripheral ion channels sensitive to noxious stimuli are important in the analgesic effects of LED treatment.
Central Mechanisms
Pigatto et al. have also reported a central mechanism through which cutaneous LED treatment causes analgesia.87 The authors showed that 890nm (infrared) LED treatment in mice not only caused significant decreases in nocifensive responses induced by thermal and chemical noxious stimuli, but also that the analgesic effect was dependent on TRPV1-expressing nociceptors. This was determined due to the fact that only centrally-administered capsaicin (a TRPV1 channel activator) completely reversed the analgesic effects of LED therapy when compared to other routes of administration, indicating that the analgesic effects of LED therapy are dependent on central afferent C fibers that express TRPV1.
Another central mechanism of LED treatment analgesia involving cytokines was shown by Cidral-Filho et al. In that study, 950nm (infrared) LED treatment applied on the skin over the injury site decreased mechanical hyperalgesia, but not cold hyperalgesia, in mice subjected to sciatic nerve crush injury, a model of neuropathic pain.15 Pro-inflammatory cytokine analyses found that LED treatment reduced TNF-α levels in the sciatic nerve as well as in the spinal cord, with no significant changes in IL1-β and IL-10. The significance of this study is that LED treatment was able to decrease levels of TNF-α centrally, whereas other studies report only peripheral changes in cytokine levels due to LED treatment.70
Therapeutic Applications of Different Colors of Light for Pain Treatment
Although light has been shown to modulate pain through a variety of biological mechanisms as described above, the ability of light to elicit photobiomodulation also depends on the physical characteristics of the light itself. For example, light with lower wavelengths of the visible spectrum tend to penetrate tissues less than those of higher wavelengths.91 Since light color is dependent on its wavelength property, light colors with shorter wavelengths on the visible spectrum (ie. violet, 380–450nm) will differ in photobiomodulation utility than lights of longer wavelengths (ie. red, 625–740nm; near-infrared, 750–1000nm). Additionally, the energy delivered by light to tissues depends on the modality through which the light is delivered. For example, lasers emit monochromatic and 100% coherent light, meaning that all light waves emitted are of a single wavelength and that all waves are synchronized in time and space.91 In contrast, LEDs emit light within a narrow range of wavelengths (±4–10nm) that is noncoherent. Due to the noncoherence of its emitted light, LEDs ultimately deliver less energy to tissues than do lasers, explaining why the risk of thermal injury is higher with laser applications than with LED applications.22, 91 Nevertheless, both laser and LED applications of light have been used for the treatment of pain.15, 39, 41, 43, 70, 88
Lights of varying colors and wavelengths across the electromagnetic spectrum have been used in many different therapeutic applications for a wide variety conditions including neonatal jaundice, macular degeneration, acne, cancer, and traumatic brain injuries.46 However, for the treatment of pain, only white, green, red, and near-infrared colors of light have been used in humans and animals (Table 1. Because of the differences in physical characteristics of the different colors of light, each color varies in its route of administration as well as the duration of light exposure per treatment.
Red and near-infrared lights are typically used in cutaneous applications, where the light probe is pressed lightly against the skin of the treatment area.15, 16, 56, 70, 89 Red light has the largest wavelength of all colors within the visible spectrum (625–740nm), and near-infrared light, although outside of the visible spectrum, contains even larger wavelengths (750–1000nm). These large wavelengths allow for very high tissue penetrance of light.91 Because of this property, the duration of light exposure for red and infrared light treatments is short and only requires seconds to minutes to cause photobiomodulation (Table 1). The cutaneous applications of red and near-infrared lights have been used for the treatment of pain associated with knee osteoarthritis5, complex regional pain syndrome-I88, 90, neuropathic pain15, 39, 49, 88, inflammatory pain70, temporomandibular disorder38, and post-surgical pain16, 56.
Unlike red and near-infrared lights, green light (500–565nm) is of a much shorter wavelength and exerts its analgesic effects through the visual pathway.43, 47 Because of the shorter wavelength of green light, the skin penetration it achieves is may be insufficient to elicit robust photobiomodulation in treated tissues. In fact, studies to date have shown that the cutaneous application of green light has been devoid of antinociceptive effects.43 Green light was shown in a clinical study to be as effective in treating actinic keratosis while producing less pain when compared to red light, although the less pain experienced by patients in this study may be related to the visual exposure to green light.83 The effects of green light causing anti-nociception likely act through a cone-driven mechanism in the eye which ultimately elicits downstream analgesic effects.43 It is also interesting to note that the duration of light exposure required for green light to be therapeutic is on the order of hours, which is in contrast to that of red and near-infrared light treatments (seconds to minutes) (Table 1). This further supports the difference in mechanisms engaged by different colors of light and routes of administration, as described above.
The use of bright white light for the treatment of pain conditions has also been reported.10–12, 59 Bright white light is unique from the light colors previously mentioned as it is broad spectrum, meaning that it contains light of all wavelengths in the visible spectrum. This implies that the analgesic effects of bright white light treatment can potentially be the result of light of many different wavelengths. In addition, like green light treatment, bright white light treatment appears to work through the visual route of administration, requiring exposure times of up to an hour.10–12, 59 However, one main difference between these two colors is the intensity at which the light is administered. Bright white light therapy is typically administered at >3000 lux10–12 and even at 5000 lux in one study.59 This is in contrast to green light therapy, which is administered at 4–110 lux.43, 47 Although the route of administration is the same for these two lights, the mechanism engaged by low intensity green light (changes in pain modulation centers of the central nervous system), varies from that engaged by high intensity bright white light (shifts in circadian rhythm).10, 11, 43 Applications of bright white light therapy for pain treatment include as therapy for fibromyalgia10 and chronic low back pain11, 12, 59. Green light therapy has also recently been used in the treatment of migraines, significantly reducing the number of the number of headache days as well as the intensity and duration of headache attacks in episodic or chronic migraineurs.68
Conclusion
Numerous reports in the past decade have now documented the mechanisms of pain modulation by light. The exacerbation of clinical pain by light tends to occur in the setting of a pre-existing pain syndrome, such as fibromyalgia and migraine, and this increase in pain is attributed to visual routes of light exposure which elicit central mechanisms of pain modulation. However, the color of light is also important in these contexts, as white, blue, amber, and red lights increase pain, while green light decreases pain. On the contrary, both cutaneous and visual applications of light treatment induce peripheral and central analgesic effects; however, current evidence shows that cutaneous application acts primarily via peripheral mechanisms, whereas visual application acts via central mechanisms. For the treatment of pain, red and near-infrared light are administered cutaneously with a short duration of exposure. (i.e. seconds to minutes), whereas green and bright white light are administered visually with long duration of exposure (i.e. hours). Current evidence shows the efficacy of light treatment for pain in a variety of pain syndromes, including fibromyalgia, complex regional pain syndrome I and chronic low back pain. Future clinical studies will likely delineate other pain syndromes for which light therapy has applicability.
Current methods of pain management involve pharmacotherapy that, though effective in some clinical settings, contain concerning adverse effect profiles, including sedation, cardiotoxicity, ataxia, addiction, and respiratory depression.27, 98 As such, non-pharmacological methods of pain management are being increasingly explored as complementary therapies to pharmacologic agents.98 In recent years, evidence for the use of light therapy as a nonpharmacologic approach for pain reduction has gained momentum, and both pre-clinical and clinical studies have demonstrated its efficacy and continue to explore its efficacy in a variety of pain syndromes. In addition to its label as a nonpharmacologic therapy, light therapy is attractive to both clinicians and patients due to its noninvasiveness and lack of side effects, ultimately increasing patient compliance.67, 68 When light therapy is administered cutaneously, the main side effect reported is thermal injury, although this issue can be solved by adjusting exposure settings (i.e. decreasing energy density or altering exposure time) so that the same therapeutic dose of energy is delivered to the tissue while reducing the risk of injury.22, 91 No adverse effects were reported by any study participants in clinical studies of light therapy administered visually10, 11, and animal studies support this finding as well.43 Based on these findings, light therapy can increasingly be a useful therapeutic analgesic modality, providing a safe and effective option to reduce the physical, psychological, economic, and societal burden that acute and chronic pain inflicts on so many patients.
Perspective:
This review synopsizes the pathways and mechanisms through which light modulates pain and the therapeutic utility of different colors and exposure modalities of light on pain. Recent advances in photobiomodulation provide a foundation for understanding this novel treatment for pain on which future translational and clinical studies can build upon.
Highlights.
Light provides analgesia through cutaneous application or visual stimulation.
Depending on wavelengths, visual stimuli may induce analgesia or hypersensitivity.
Light-induced analgesia relies on both central and peripheral mechanisms.
ipRGCs, RVM, and inflammation play a crucial role in phototherapy pain management.
Phototherapy for pain management is efficacious with scarce side effects.
Acknowledgements
This work was supported by grants from the University of Arizona MD/PhD Program, NCCIH R01AT009716 (MMI), K08NS104272, R01NS116694 (AMP), the Comprehensive Chronic Pain and Addiction Center-University of Arizona, and the University of Arizona CHiLLi Initiative. Figures created with BioRender.com.
Disclosures:
Dr. Ibrahim has a patent through the University of Arizona for using green light therapy for the management of pain. Dr. Slepian is a partner in a company to commercialize the green light therapy with Dr. Ibrahim. This work was supported by grants from the University of Arizona MD/PhD Program, NCCIH R01AT009716, K08NS104272, R01NS116694 (AMP), the Comprehensive Chronic Pain and Addiction Center-University of Arizona, and the University of Arizona CHiLLi Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agoston AM, Sieberg CB. Nonpharmacologic Treatment of Pain. Semin Pediatr Neurol. 23:220–223, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Albilali A, Dilli E. Photophobia: When Light Hurts, a Review. Curr Neurol Neurosci Rep. 18:62, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Aman MM, Jason Yong R, Kaye AD, Urman RD. Evidence-Based Non-Pharmacological Therapies for Fibromyalgia. Curr Pain Headache Rep. 22:33, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Ambriz-Tututi M, Rocha-Gonzalez HI, Cruz SL, Granados-Soto V. Melatonin: a hormone that modulates pain. Life Sci. 84:489–498, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Balbinot G, Schuch CP, Nascimento PSD, Lanferdini FJ, Casanova M, Baroni BM, Vaz MA. Photobiomodulation Therapy Partially Restores Cartilage Integrity and Reduces Chronic Pain Behavior in a Rat Model of Osteoarthritis: Involvement of Spinal Glial Modulation. Cartilage.1947603519876338, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 139:267–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 7:e1017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benarroch EE. The melanopsin system: Phototransduction, projections, functions, and clinical implications. Neurology. 76:1422–1427, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Bumgarner JR, Walker WH 2nd, Liu JA, Walton JC, Nelson RJ. Dim Light at Night Exposure Induces Cold Hyperalgesia and Mechanical Allodynia in Male Mice. Neuroscience. 434:111–119, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess HJ, Park M, Ong JC, Shakoor N, Williams DA, Burns J. Morning Versus Evening Bright Light Treatment at Home to Improve Function and Pain Sensitivity for Women with Fibromyalgia: A Pilot Study. Pain Med. 18:116–123, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Burgess HJ, Rizvydeen M, Kimura M, Pollack MH, Hobfoll SE, Rajan KB, Burns JW. An Open Trial of Morning Bright Light Treatment Among US Military Veterans with Chronic Low Back Pain: A Pilot Study. Pain Med. 20:770–778, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns JW, Gerhart J, Rizvydeen M, Kimura M, Burgess HJ. Morning Bright Light Treatment for Chronic Low Back Pain: Potential Impact on the Volatility of Pain, Mood, Function, and Sleep. Pain Med. 6, 2020,1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 11:98, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 32:1294–1310, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Cidral-Filho FJ, Martins DF, More AO, Mazzardo-Martins L, Silva MD, Cargnin-Ferreira E, Santos AR. Light-emitting diode therapy induces analgesia and decreases spinal cord and sciatic nerve tumour necrosis factor-alpha levels after sciatic nerve crush in mice. Eur J Pain. 17:1193–1204, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Cidral-Filho FJ, Mazzardo-Martins L, Martins DF, Santos AR. Light-emitting diode therapy induces analgesia in a mouse model of postoperative pain through activation of peripheral opioid receptors and the L-arginine/nitric oxide pathway. Lasers Med Sci. 29:695–702, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Cortese A, Conte A, Ferrazzano G, Sgarlata E, Millefiorini E, Frontoni M, Berardelli A. Photophobia in multiple sclerosis. Mult Scler Relat Disord. 26:55–57, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 67:1001–1006, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira DL, Hirotsu C, Tufik S, Andersen ML. The interfaces between vitamin D, sleep and pain. J Endocrinol. 234:R23–R36, 2017 [DOI] [PubMed] [Google Scholar]
- 20.de Sousa MVP, Kawakubo M, Ferraresi C, Kaippert B, Yoshimura EM, Hamblin MR. Pain management using photobiomodulation: Mechanisms, location, and repeatability quantified by pain threshold and neural biomarkers in mice. J Biophotonics. 11:e201700370, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 52:7852–7858, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douek PC, Correa R, Neville R, Unger EF, Shou M, Banai S, Ferrans VJ, Epstein SE, Leon MB, Bonner RF. Dose-Dependent Smooth-Muscle Cell-Proliferation Induced by Thermal-Injury with Pulsed Infrared-Lasers. Circulation. 86:1249–1256, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 120:3760–3772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezzati K, Fekrazad R, Raoufi Z. The Effects of Photobiomodulation Therapy on Post-Surgical Pain. J Lasers Med Sci. 10:79–85, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta-endorphin mediates addiction to UV light. Cell. 157:1527–1534, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fetterman AK, Robinson MD, Meier BP. Anger as “seeing red”: evidence for a perceptual association. Cogn Emot. 26:1445–1458, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14:162–173, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaston KJ, Bennie J, Davies TW, Hopkins J. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol Rev Camb Philos Soc. 88:912–927, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Gerend MA, Sias T. Message framing and color priming: How subtle threat cues affect persuasion. J Exp Soc Psychol. 45:999–1002, 2009 [Google Scholar]
- 30.Gil S, Le Bigot L. Seeing Life through Positive-Tinted Glasses: Color-Meaning Associations. Plos One. 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 16:1248–1257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes LE, Dalmarco EM, Andre ES. The brain-derived neurotrophic factor, nerve growth factor, neurotrophin-3, and induced nitric oxide synthase expressions after low-level laser therapy in an axonotmesis experimental model. Photomed Laser Surg. 30:642–647, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Green A, Cohen-Zion M, Haim A, Dagan Y. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol Int. 34:855–865, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Grossniklaus HE, Geisert EE, Nickerson JM. Introduction to the Retina. Prog Mol Biol Transl Sci. 134:383–396, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 91:117–123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol. 94:199–212, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison GI, Young AR, McMahon SB. Ultraviolet radiation-induced inflammation as a model for cutaneous hyperalgesia. J Invest Dermatol. 122:183–189, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Herpich CM, Leal-Junior ECP, Politti F, de Paula Gomes CAF, Dos Santos Gloria IP, de Souza Amaral MFR, Herpich G, de Azevedo LMA, de Oliveira Gonzalez T, Biasotto-Gonzalez DA. Intraoral photobiomodulation diminishes pain and improves functioning in women with temporomandibular disorder: a randomized, sham-controlled, double-blind clinical trial : Intraoral photobiomodulation diminishes pain in women with temporomandibular disorder. Lasers Med Sci. 35:439–445, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Holanda VM, Chavantes MC, Wu X, Anders JJ. The mechanistic basis for photobiomodulation therapy of neuropathic pain by near infrared laser light. Lasers Surg Med. 49:516–524, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Honda Y, Sakamoto J, Hamaue Y, Kataoka H, Kondo Y, Sasabe R, Goto K, Fukushima T, Oga S, Sasaki R, Tanaka N, Nakano J, Okita M. Effects of Physical-Agent Pain Relief Modalities for Fibromyalgia Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Res Manag. 2018:2930632, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1alpha (HIF-1alpha). J Comp Neurol. 520:2903–2916, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Huang Z, Ma J, Chen J, Shen B, Pei F, Kraus VB. The effectiveness of low-level laser therapy for nonspecific chronic low back pain: a systematic review and meta-analysis. Arthritis Res Ther. 17:360, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibrahim MM, Patwardhan A, Gilbraith KB, Moutal A, Yang X, Chew LA, Largent-Milnes T, Malan TP, Vanderah TW, Porreca F, Khanna R. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain. 158:347–360, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Institute of Medicine (US) Committee on Advancing Pain Research C, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. 2011 [PubMed] [Google Scholar]
- 45.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 84:1091–1099, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Kemper KJ. “Let there be light.” Research on phototherapy, light therapy, and photobiomodulation for healing - Alternative therapy becomes mainstream. Complement Ther Med. 41:A1–A6, 2018 [DOI] [PubMed] [Google Scholar]
- 47.Khanna R, Patwardhan A, Yang X, Li W, Cai S, Ji Y, Chew LA, Dorame A, Bellampalli SS, Schmoll RW, Gordon J, Moutal A, Vanderah TW, Porreca F, Ibrahim MM. Development and Characterization of An Injury-free Model of Functional Pain in Rats by Exposure to Red Light. J Pain. 20:1293–1306, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 29:13435–13444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobiela Ketz A, Byrnes KR, Grunberg NE, Kasper CE, Osborne L, Pryor B, Tosini NL, Wu X, Anders JJ. Characterization of Macrophage/Microglial Activation and Effect of Photobiomodulation in the Spared Nerve Injury Model of Neuropathic Pain. Pain Med. 18:932–946, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Kong HY, Byun J. Emerging roles of human prostatic Acid phosphatase. Biomol Ther (Seoul). 21:10–20, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowacs PA, Piovesan EJ, Werneck LC, Tatsui CE, Lange MC, Ribas LC, da Silva HP. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 21:184–188, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 66:932–937, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Kusumoto J, Takeo M, Hashikawa K, Komori T, Tsuji T, Terashi H, Sakakibara S. OPN4 belongs to the photosensitive system of the human skin. Genes Cells. 25:215–225, 2020 [DOI] [PubMed] [Google Scholar]
- 54.Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res. 36:52–119, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Lamb TD. Why rods and cones? Eye (Lond). 30:179–185, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langella LG, Casalechi HL, Tomazoni SS, Johnson DS, Albertini R, Pallotta RC, Marcos RL, de Carvalho PTC, Leal-Junior ECP. Photobiomodulation therapy (PBMT) on acute pain and inflammation in patients who underwent total hip arthroplasty-a randomized, triple-blind, placebo-controlled clinical trial. Lasers Med Sci. 33:1933–1940, 2018 [DOI] [PubMed] [Google Scholar]
- 57.Lebensohn JE, Bellows J. The Nature of Photophobia. Archives of Ophthalmology. 12:380–390, 1934 [Google Scholar]
- 58.Lehtosalo JI, Uusitalo H, Palkama A. Sensory supply of the anterior uvea: a light and electron microscope study. Exp Brain Res. 55:562–569, 1984 [DOI] [PubMed] [Google Scholar]
- 59.Leichtfried V, Matteucci Gothe R, Kantner-Rumplmair W, Mair-Raggautz M, Bartenbach C, Guggenbichler H, Gehmacher D, Jonas L, Aigner M, Winkler D, Schobersberger W. Short-term effects of bright light therapy in adults with chronic nonspecific back pain: a randomized controlled trial. Pain Med. 15:2003–2012, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 1772:989–1003, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Liebert AD, Chow RT, Bicknell BT, Varigos E. Neuroprotective Effects Against POCD by Photobiomodulation: Evidence from Assembly/Disassembly of the Cytoskeleton. J Exp Neurosci. 10:1–19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopes DM, McMahon SB. Ultraviolet Radiation on the Skin: A Painful Experience? CNS Neurosci Ther. 22:118–126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.M A, Ummer VS, Maiya AG, Hande M. Low level laser therapy for the patients with painful diabetic peripheral neuropathy - A systematic review. Diabetes Metab Syndr. 13:2667–2670, 2019 [DOI] [PubMed] [Google Scholar]
- 64.Malem A Photophobia and a painful rash. BMJ. 353:i3221, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Martenson ME, Halawa OI, Tonsfeldt KJ, Maxwell CA, Hammack N, Mist SD, Pennesi ME, Bennett RM, Mauer KM, Jones KD, Heinricher MM. A possible neural mechanism for photosensitivity in chronic pain. Pain. 157:868–878, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin L, Porreca F, Mata EI, Salloum M, Goel V, Gunnala P, Killgore WDS, Jain S, Jones-MacFarland FN, Khanna R, Patwardhan A, Ibrahim MM. Green Light Exposure Improves Pain and Quality of Life in Fibromyalgia Patients: A Preliminary One-Way Crossover Clinical Trial. Pain Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin LF, Patwardhan AM, Jain SV, Salloum MM, Freeman J, Khanna R, Gannala P, Goel V, Jones-MacFarland FN, Killgore WD, Porreca F, Ibrahim MM. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial. Cephalalgia.333102420956711, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6:13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martins DF, Turnes BL, Cidral-Filho FJ, Bobinski F, Rosas RF, Danielski LG, Petronilho F, Santos AR. Light-emitting diode therapy reduces persistent inflammatory pain: Role of interleukin 10 and antioxidant enzymes. Neuroscience. 324:485–495, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Matre D, Knardahl S, Nilsen KB. Night-shift work is associated with increased pain perception. Scand J Work Environ Health. 43:260–268, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 33:131–139, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moller KI, Kongshoj B, Philipsen PA, Thomsen VO, Wulf HC. How Finsen’s light cured lupus vulgaris. Photodermatol Photoimmunol Photomed. 21:118–124, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Moriyama Y, Nguyen J, Akens M, Moriyama EH, Lilge L. In vivo effects of low level laser therapy on inducible nitric oxide synthase. Lasers Surg Med. 41:227–231, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Nir RR, Lee AJ, Huntington S, Noseda R, Bernstein CA, Fulton AB, Bertisch SM, Hovaguimian A, Buettner C, Borsook D, Burstein R. Color-selective photophobia in ictal vs interictal migraineurs and in healthy controls. Pain. 159:2030–2034, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM, Hovaguimian A, Cestari DM, Saavedra-Walker R, Borsook D, Doran BL, Buettner C, Burstein R. Migraine photophobia originating in cone-driven retinal pathways. Brain. 139:1971–1986, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noseda R, Copenhagen D, Burstein R. Current understanding of photophobia, visual networks and headaches. Cephalalgia. 39:1623–1634, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 13:239–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noseda R, Lee AJ, Nir RR, Bernstein CA, Kainz VM, Bertisch SM, Buettner C, Borsook D, Burstein R. Neural mechanism for hypothalamic-mediated autonomic responses to light during migraine. Proc Natl Acad Sci U S A. 114:E5683–E5692, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Odegard SS, Omland PM, Nilsen KB, Stjern M, Gravdahl GB, Sand T. The effect of sleep restriction on laser evoked potentials, thermal sensory and pain thresholds and suprathreshold pain in healthy subjects. Clin Neurophysiol. 126:1979–1987, 2015 [DOI] [PubMed] [Google Scholar]
- 81.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 149:235–242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 160:858–864, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Osiecka BJ, Nockowski P, Szepietowski JC. Treatment of Actinic Keratosis with Photodynamic Therapy Using Red or Green Light: A Comparative Study. Acta Derm Venereol. 98:689–693, 2018 [DOI] [PubMed] [Google Scholar]
- 84.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 140:344–358, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 92:1699–1775, 2012 [DOI] [PubMed] [Google Scholar]
- 86.Pieh C, Jank R, Waiss C, Pfeifer C, Probst T, Lahmann C, Oberndorfer S. Night-shift work increases cold pain perception. Sleep Med. 45:74–79, 2018 [DOI] [PubMed] [Google Scholar]
- 87.Pigatto GR, Coelho IS, Aquino RS, Bauermann LF, Santos ARS. Light-Emitting Diode Phototherapy Reduces Nocifensive Behavior Induced by Thermal and Chemical Noxious Stimuli in Mice: Evidence for the Involvement of Capsaicin-Sensitive Central Afferent Fibers. Mol Neurobiol. 54:3205–3218, 2017 [DOI] [PubMed] [Google Scholar]
- 88.Pigatto GR, Quinteiro MHS, Nunes-de-Souza RL, Coimbra NC, Parizotto NA. Low-Intensity Photobiomodulation Decreases Neuropathic Pain in Paw Ischemia-Reperfusion and Spared Nervus Ischiadicus Injury Experimental Models. Pain Pract. 20:371–386, 2020 [DOI] [PubMed] [Google Scholar]
- 89.Pigatto GR, Silva CS, Parizotto NA. Photobiomodulation therapy reduces acute pain and inflammation in mice. J Photochem Photobiol B. 196:111513, 2019 [DOI] [PubMed] [Google Scholar]
- 90.Rodrigues M, Cardoso RB, Kuriki HU, Marcolino AM, de Oliveira Guirro EC, Barbosa RI. Photobiomodulation Decreases Hyperalgesia in Complex Regional Pain Syndrome: An Experimental Mouse Model Subjected to Nicotine. Lasers Surg Med. 2020 [DOI] [PubMed] [Google Scholar]
- 91.Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye Brain. 3:49–67, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rossi HL, Recober A. Photophobia in primary headaches. Headache. 55:600–604, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruskell GL. Trigeminal innervation of the scleral spur in cynomolgus monkeys. J Anat. 184 (Pt 3):511–518, 1994 [PMC free article] [PubMed] [Google Scholar]
- 94.Seidel S, Beisteiner R, Manecke M, Aslan TS, Wober C. Psychiatric comorbidities and photophobia in patients with migraine. J Headache Pain. 18:18, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Snutch TP. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx. 2:662–670, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stelian J, Gil I, Habot B, Rosenthal M, Abramovici I, Kutok N, Khahil A. Improvement of pain and disability in elderly patients with degenerative osteoarthritis of the knee treated with narrow-band light therapy. J Am Geriatr Soc. 40:23–26, 1992 [DOI] [PubMed] [Google Scholar]
- 97.Takahashi M, Matsudaira K, Shimazu A. Disabling low back pain associated with night shift duration: sleep problems as a potentiator. Am J Ind Med. 58:1300–1310, 2015 [DOI] [PubMed] [Google Scholar]
- 98.Tick H, Nielsen A, Pelletier KR, Bonakdar R, Simmons S, Glick R, Ratner E, Lemmon RL, Wayne P, Zador V, Pain Task Force of the Academic Consortium for Integrative M, Health. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. Explore (NY). 14:177–211, 2018 [DOI] [PubMed] [Google Scholar]
- 99.Wiercioch-Kuzianik K, Babel P. Color Hurts. The Effect of Color on Pain Perception. Pain Med. 20:1955–1962, 2019 [DOI] [PubMed] [Google Scholar]
- 100.Wu Y, Hallett M. Photophobia in neurologic disorders. Transl Neurodegener. 6:26, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. Effect of Vitamin D Supplementation on Pain: A Systematic Review and Meta-analysis. Pain Physician. 19:415–427, 2016 [PubMed] [Google Scholar]
- 102.Xie S, Fan W, He H, Huang F. Role of Melatonin in the Regulation of Pain. J Pain Res. 13:331–343, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yadav A, Verma S, Keshri GK, Gupta A. Combination of medicinal honey and 904nm superpulsed laser-mediated photobiomodulation promotes healing and impedes inflammation, pain in full-thickness burn. J Photochem Photobiol B. 186:152–159, 2018 [DOI] [PubMed] [Google Scholar]
- 104.Yazulla S, Studholme KM. Vanilloid receptor like 1 (VRL1) immunoreactivity in mammalian retina: colocalization with somatostatin and purinergic P2X1 receptors. J Comp Neurol. 474:407–418, 2004 [DOI] [PubMed] [Google Scholar]
- 105.Yousefi-Nooraie R, Schonstein E, Heidari K, Rashidian A, Pennick V, Akbari-Kamrani M, Irani S, Shakiba B, Mortaz Hejri SA, Mortaz Hejri SO, Jonaidi A. Low level laser therapy for nonspecific low-back pain. Cochrane Database Syst Rev.CD005107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


