Abstract
Background:
Angiopoietin-1 and 2 (Ang1, Ang2) are important mediators of angiogenesis. Angiopoietin levels are perturbed in cardiovascular disease, but it is unclear whether angiopoietin signaling is causative, an adaptive response, or merely epiphenomenon of disease activity.
Methods and Results:
In a cohort free of cardiovascular disease at baseline (MESA), relationships between angiopoietins, cardiac morphology, and subsequent incidence of heart failure or cardiovascular death were evaluated. In cohorts with pulmonary arterial hypertension (PAH) or left heart disease (LHD), associations between angiopoietins, invasive hemodynamics, and adverse clinical outcomes were evaluated. In MESA, Ang2 was associated with a higher incidence of heart failure or cardiovascular death (HR 1.21 per standard deviation, P<0.001). Ang2 was associated with increased right atrial pressure (PAH cohort) and increased wedge pressure and right atrial pressure (LHD cohort). Elevated Ang2 was associated with mortality in the PAH cohort.
Conclusion:
Ang2 was associated with incident heart failure or death among adults without cardiovascular disease at baseline and with disease severity in individuals with existing heart failure. Our findings that Ang2 is elevated prior to disease onset and that elevations reflect disease severity, suggest Ang2 may contribute to heart failure pathogenesis.
Lay summary:
This study evaluated angiopoietins in three different groups of study participants. The results of the study suggest that high angiopoietin levels in the blood may predict future onset of heart failure. High angiopoietin levels may also correlate with more severe heart failure in people with heart failure. These findings may help physicians to detect heart failure early and may lead to future therapies that could treat heart failure.
Keywords: Angiopoietin, heart failure, clinical outcomes
Introduction
The angiopoietin-Tie system is an important pathway in angiogenesis (1). Angiopoietin-1 (Ang1), a Tie-2 receptor agonist, is thought to have stabilizing and anti-inflammatory effects on the endothelium (1–4). Angiopoietin-2 (Ang2) is primarily a Tie-2 competitive antagonist (5) released in response to endothelial stress, with destabilizing effects that increase vascular permeability and mediate vascular remodeling (1, 3).
Ang1 and Ang2 have been studied in a variety of cardiovascular diseases (CVD). Elevated Ang2 has generally been associated with disease severity and/or cardiovascular outcomes, including mortality, in pulmonary hypertension (2, 4, 6), coronary disease (7–9), peripheral arterial disease (10), stroke (11), and cohorts with high rates of CVD (12–14). With respect to heart failure, studies have demonstrated Ang2 elevation in heart failure exacerbations (15), positive correlation between Ang2 and heart failure severity (16, 17), and prediction of mortality or heart transplant (18). In contrast, elevated Ang1 either has not been associated with CVD or has been a protective factor (2, 4, 9, 15). Despite this body of evidence, it remains unclear whether angiopoietin signaling contributes to heart failure progression, is an adaptive or protective response to heart failure, or is merely an epiphenomenon of heart failure. Studying Ang1 and Ang2 in healthy subjects prior to CVD onset strongly limits the possibility that differences in angiopoietin levels are a response to existing disease, whether as an epiphenomenon or adaptive response.
The aim of this study was to evaluate Ang1 and Ang2 across the spectrum of heart failure, from individuals without clinical heart disease at baseline to individuals with existing left and/or right heart failure. We hypothesized that elevated Ang2 levels would be associated with cardiac morphology and increased heart failure incidence in individuals without heart disease at baseline. We also hypothesized that elevated Ang2 would be associated with worse hemodynamics and mortality in individuals with existing heart failure. We further hypothesized that elevated Ang1 would have opposite relationships.
Methods
This work utilizes three cohorts to evaluate the relationship between angiopoietins and heart failure. Serum angiopoietin levels were measured in each cohort. The Multi-Ethnic Study of Atherosclerosis (MESA) Angiogenesis cohort was used to evaluate relationships between angiopoietins, cardiac morphology, and the risk of incident heart failure or cardiovascular death in individuals without CVD at the time Ang1 and Ang2 were measured. A pulmonary arterial hypertension (PAH) cohort was used to evaluate the relationship between Ang1, Ang2 and severity of isolated right heart failure, and a left heart disease (LHD) cohort was used to evaluate the relationship between Ang2 and severity of biventricular heart failure.
MESA Cohort
MESA is a multicenter prospective cohort study that enrolled 6,814 participants aged 45–84 free of clinically recognized CVD (19). A subset of MESA participants had cardiac magnetic resonance imaging (cMRI) through the MESA-Right Ventricle study (20). MESA-Angiogenesis was an ancillary case-cohort study funded to evaluate markers of angiogenesis and pulmonary vasoconstriction in 1,538 MESA-Right Ventricle participants. All MESA participants with interpretable cMRI who developed incident heart failure or cardiovascular death through 2014 were selected for angiopoietin measurement (cases). Additionally, a random subset of MESA participants with available cMRI was selected as the MESA-Angiogenesis cohort (cohort). Following a typical case-cohort design, the MESA-Angiogenesis cohort included cases at a similar rate to the parent MESA population. This allows baseline cross-sectional cohort characteristics to be described without the distortion of over or under-representing cases, while preserving the power of all cases for weighted longitudinal case-cohort analyses of incident heart failure or cardiovascular death. For the present analysis, all case-cohort participants with measured angiopoietin levels, interpretable cMRI, and all other covariate data were included.
Serum Ang1 and Ang2 (ng/ml) were measured at the baseline exam using a Meso Scale Discovery custom multiplex immunoassay. The inter-assay coefficient of variation was 10.7% for Ang1 and 1.9% for Ang2 on seventy-eight duplicate samples. Cardiac MRIs were performed on all MESA participants included in the present study at the baseline exam. Methods for MRI interpretation in MESA are well described (21). Left and right ventricle measurements included: end-diastolic mass (g), end-diastolic volume (ml), stroke volume (ml), and ejection fraction (%). The clinical event of interest was defined as a composite of either incident heart failure or death due to cardiovascular disease. Time to event was defined by whichever event occurred first. Methodology for adjudicated longitudinal clinical events assessment is described in the MESA Clinical Events Manual of Operations (https://www.mesa-nhlbi.org/manuals.aspx).
Demographic covariates included age, gender (self-reported), height, weight, study site, highest level of education attained, and race/ethnicity, categorized as non-Hispanic white, African American, Hispanic, and Asian (predominantly Chinese American). Cardiovascular risk factors included hypertension, systolic blood pressure (SBP), smoking status, duration of smoking history, diabetes, and total cholesterol. Exploratory models included glomerular filtration rate (GFR), brain natriuretic peptide (BNP) level, and medication use (beta-blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and non-steroidal anti-inflammatory drugs). All covariates were measured as part of the baseline MESA evaluation. Covariate specification is included in the supplemental methods.
Cross-sectional analyses were performed using the MESA-Angiogenesis cohort. Baseline characteristics by angiopoietin tertile were described using mean ± standard deviation (SD) for continuous variables, and percentages for categorical variables. Linear regression was used to estimate associations between Ang1, Ang2, and cardiac morphology. For prospective clinical events, a case-cohort analysis was performed. All 235 participants in the parent cohort with available cMRIs who developed heart failure or cardiovascular death were included; however, only 1,277 of 3,967 participants in the parent cohort without heart failure or cardiovascular death during follow-up were included. To account for this under-sampling of participants without an event, event-free participants were given a weight of 3.11 relative to those with an event (3,967/1,277). Nelson-Aalen cumulative hazards were used to estimate unadjusted non-parametric associations, and Cox proportional hazards were used to estimate adjusted and unadjusted parametric associations between Ang1 and Ang2 and incident heart failure or cardiovascular death.
The same model specification was used for cross-sectional and case-cohort analyses. In limited models, relationships were adjusted for age, gender, race/ethnicity, height, weight, and study site. Full models were also adjusted for education level and cardiovascular risk factors. Exploratory models also account for differences in GFR, BNP, and co-medication use. As Ang1 and Ang2 may have antagonistic effects, additional models included Ang1 and Ang2 simultaneously to evaluate whether their relative levels may be important. Because distributions of Ang1 and Ang2 were positively skewed, hazards models were repeated with log transformations of these variables to ensure that this did not impact results.
Heart Failure Cohorts
Pulmonary arterial hypertension cohort.
Adult participants in the Seattle Right Ventricle Translational Science (SeRVeTuS) study were recruited at the University of Washington from April 2014 through May 2016 (22). All participants were diagnosed with PAH using standard definitions at the time of enrollment (mean pulmonary artery pressure ≥25 mmHg). Servetus participants gave blood, answered questionnaires, and allowed access to their medical record for imaging and hemodynamics from right heart catheterization (RHC). Ang1 and Ang2 were assayed using a multiplex assay as described above. Longitudinal data on lung transplant and mortality were also collected. This comprised a cohort of individuals with predominantly right heart failure.
Left heart disease cohort.
Adult patients undergoing RHC for evaluation of clinical heart failure at Medical University of South Carolina from December 2016 through July 2018 were screened for participation in a longitudinal cohort of patients with advanced heart failure. Clinical and demographic data were obtained from medical records including etiology of heart failure. Ang2 was assayed using a multiplex ELISA (EveTechnologies). Longitudinal data on heart transplant, left ventricular assist device (LVAD) implantation, and mortality were collected. A full description of the LHD cohort methods has been previously published (23). This comprised a cohort of individuals with left heart with or without accompanying right heart failure.
For both heart failure cohorts, linear regression was used to evaluate associations of Ang1 and Ang2 (PAH cohort), or Ang2 only (LHD cohort), with hemodynamics measured or calculated during RHC. Dependent hemodynamic variables included right atrial pressure, mean pulmonary artery pressure, pulmonary artery wedge pressure, pulmonary vascular resistance, cardiac index by thermodilution, and SBP. Cox proportional hazards models were used to estimate associations between angiopoietins and the composite of transplant, LVAD implantation (LHD cohort), and all-cause mortality. In adjusted models, associations were adjusted for age, gender, and race/ethnicity, categorized as white and non-white in the PAH cohort, and black and non-black in the LHD cohort. Etiologies of PAH (idiopathic, connective tissue disease, toxin mediated, congenital, and portopulmonary) and LHD (ischemic vs non-ischemic) were included in adjusted models. Covariate specification is included in the online supplement.
The Institutional Review Boards of participating institutions approved study protocols and all participants provided informed consent. A significance level of 0.05 was used. Analyses were performed using STATA v14.2 (StataCorp LP, College Station, TX).
Results
MESA Cohort
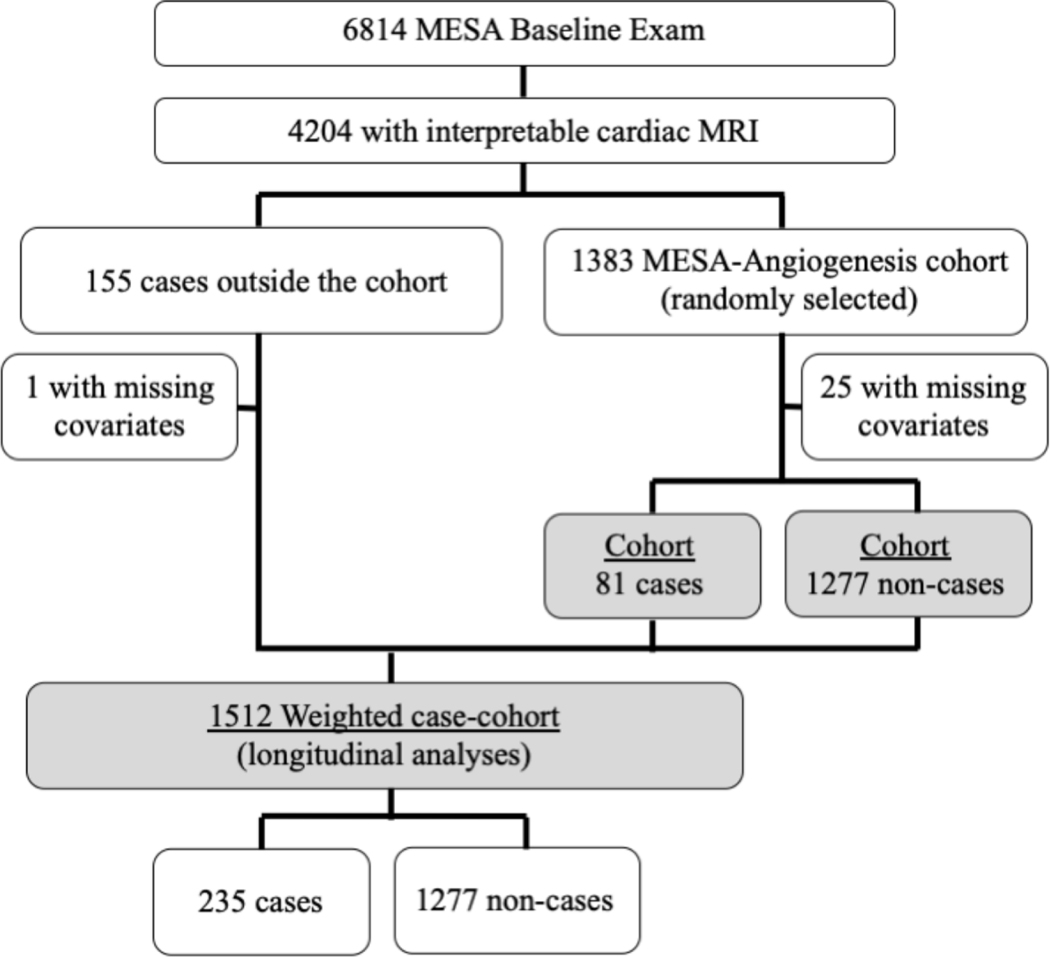
The MESA-Angiogenesis cohort included 1,383 participants selected randomly from the parent MESA cohort. In addition, 155 MESA participants outside the MESA-Angiogenesis cohort experienced incident heart failure or cardiovascular death; these participants were included in longitudinal case-cohort analyses only. In the current study, 1,358 participants in the MESA-Angiogenesis cohort (81 cases, 1,277 non-cases) and 154 additional cases outside the cohort had all available covariates and were included in analyses. The case-cohort selection process is depicted in Figure 1.
Figure 1.
Study sample selection for participants in the MESA analyses without heart failure at the time Ang1 and Ang2 were measured. Sample includes the MESA Cohort (baseline cross-sectional analyses) and the MESA Case-Cohort (longitudinal analyses). Cases were defined as all MESA participants with interpretable cMRI who developed incident heart failure or cardiovascular death through the follow up period. The MESA-Angiogenesis Cohort consisted of a randomly selected subset of MESA participants with available cMRI.
Participants in the highest Ang1 tertile were more likely to be male, Hispanic, and current smokers. Participants in the highest Ang2 tertile were more likely to be older, female, less educated, black or Hispanic, current smokers, and have a higher BNP. Baseline characteristics are presented in Table 1. Mean values of Ang1 and Ang2 in the MESA-Angiogenesis cohort were 1.74 ng/ml and 5.33 ng/ml respectively.
Table 1.
Baseline characteristics of MESA-Angiogenesis cohort (N=1,358)
| Ang1 Tertile | Ang2 Tertile | |||||
|---|---|---|---|---|---|---|
| Low | Middle | High | Low | Middle | High | |
| Protein Range (ng/mL) | (0.3–1.1) | (1.1–1.6) | (1.6–21.2) | (1.4–4.0) | (4.0–5.7) | (5.7–33.8) |
| Age (years) mean ± SD | 62 ± 10 | 60 ± 10 | 61 ± 10 | 59 ± 9 | 61 ± 10 | 63 ± 10 |
| Female % | 55.9 | 50.1 | 51.5 | 44.6 | 53.7 | 60.1 |
| Race/ethnicity % | ||||||
| White | 45.4 | 35.5 | 40.2 | 39.2 | 43.0 | 39.0 |
| Black | 30.4 | 26.9 | 22.9 | 20.8 | 26.6 | 33.4 |
| Hispanic | 13.0 | 22.5 | 27.3 | 19.6 | 19.9 | 23.7 |
| Asian | 11.2 | 15.0 | 9.6 | 20.4 | 10.5 | 3.9 |
| Height (cm) mean ± SD | 167 ± 10 | 166 ± 10 | 166 ± 10 | 167 ± 10 | 166 ± 10 | 166 ± 10 |
| Weight (kg) mean ± SD | 77 ± 17 | 78 ± 16 | 76 ± 16 | 75 ± 15 | 77 ± 16 | 79 ± 17 |
| Education % | ||||||
| No HS diploma | 12.3 | 20.1 | 16.4 | 17.3 | 12.8 | 18.8 |
| HS diploma | 31.8 | 32.9 | 38.2 | 27.5 | 38.0 | 38.1 |
| College degree | 55.9 | 47.0 | 45.4 | 55.2 | 49.2 | 43.2 |
| Smoking status % | ||||||
| Never | 53.0 | 54.3 | 51.3 | 58.1 | 55.0 | 44.8 |
| Former | 39.2 | 34.4 | 35.2 | 36.5 | 36.0 | 36.2 |
| Current | 7.8 | 11.3 | 13.5 | 5.4 | 9.0 | 19.0 |
| Pack years mean ± SD | 11 ± 21 | 9 ± 19 | 11 ± 24 | 7 ± 15 | 9 ± 17 | 16 ± 29 |
| Hypertension % | 43.0 | 44.6 | 43.0 | 38.8 | 40.9 | 51.5 |
| Systolic BP mean ± SD | 126 ± 21 | 125 ± 21 | 125 ± 21 | 122 ± 19 | 125 ± 22 | 128 ± 23 |
| Diabetes % | 11.6 | 11.5 | 11.6 | 7.7 | 10.5 | 16.9 |
| Cholesterol (mg/dl) mean ± SD | 190 ± 33 | 195 ± 33 | 198 ± 33 | 196 ± 31 | 194 ± 33 | 191 ± 36 |
| GFR (ml/min) mean ± SD | 81 ± 17 | 82 ± 18 | 80 ± 16 | 82 ± 16 | 81 ± 16 | 80 ± 19 |
| BNP (pg/ml) mean ± SD | 97 ± 144 | 101 ± 175 | 82 ± 116 | 60 ± 78 | 85 ± 127 | 141 ± 206 |
| Medication use % | ||||||
| Beta blocker | 7.8 | 9.7 | 10.5 | 6.9 | 8.3 | 13.2 |
| ACEI/ARB | 17.2 | 15.0 | 18.1 | 14.8 | 13.7 | 22.3 |
| NSAIDs | 47.4 | 38.6 | 41.3 | 37.5 | 43.4 | 46.9 |
Continuous variables are presented by mean ± SD. Categorical variables are presented as percentages. ACEI=angiotensin converting enzyme inhibitor, Ang1=angiopoietin-1, Ang2=angiopoietin-2, ARB=angiotensin receptor blocker, BNP=brain natriuretic peptide, BP=blood pressure, GFR=glomerular filtration rate, HS=high school, NSAID=non-steroidal anti-inflammatory drug, SD=standard deviation.
In unadjusted cardiac morphology analysis, a SD increase in Ang1 was associated with a 0.4% absolute greater left ventricular ejection fraction (LVEF, 95% confidence interval 0.1% to 0.6%, P=0.002). This association with LVEF remained significant in adjusted models, but Ang1 was not associated with any other metrics of cardiac morphology. A SD increase in Ang2 was associated with a 0.4% absolute greater right ventricular ejection fraction (RVEF, 95% confidence interval 0.1% to 0.8%, P=0.01) in unadjusted analyses; however, there was no association between Ang2 and RVEF in adjusted models. Ang2 was not associated with any other metrics of cardiac morphology in adjusted or unadjusted analyses. Results from cardiac morphology analyses are presented in Table 2. Log transformations of Ang1 and Ang2 did not significantly change model results. Further exploratory models adjusting for GFR, BNP, and co-medication use did not meaningfully impact associations (Supplemental e-Table 1). In addition, sensitivity analyses modelling study site as a random effect, rather than as an adjustment variable, did not significantly impact results.
Table 2.
Multivariable linear regression estimating associations between Ang1, Ang2, and cardiac structure and function in the MESA-Angiogenesis cohort (N=1,358)
| Parameter | Model | Ang1 | Ang2 | ||||
|---|---|---|---|---|---|---|---|
| Coefficient | (95% CI) | P-value | Coefficient | (95% CI) | P-value | ||
| RVEDM (g) | Unadjusted | 0.0 | (−0.2, 0.3) | 0.77 | −0.1 | (−0.3, 0.1) | 0.30 |
| Limited | 0.0 | (−0.2, 0.1) | 0.88 | 0.0 | (−0.2, 0.2) | 0.96 | |
| Full | 0.0 | (−0.2, 0.2) | 0.97 | 0.0 | (−0.2, 0.2) | 0.80 | |
| RVEDV (ml) | Unadjusted | 0.3 | (−1.2, 1.8) | 0.73 | −0.9 | (−2.4, 0.6) | 0.23 |
| Limited | −0.3 | (−1.2, 0.5) | 0.46 | −0.3 | (−1.4, 0.9) | 0.65 | |
| Full | −0.3 | (−1.1, 0.6) | 0.58 | 0.1 | (−1.0, 1.2) | 0.87 | |
| RVSV (ml) | Unadjusted | 0.0 | (−1.0, 1.0) | 0.97 | −0.1 | (−1.1, 1.0) | 0.87 |
| Limited | −0.5 | (−1.2, 0.2) | 0.15 | 0.0 | (−0.9, 0.9) | 1.00 | |
| Full | −0.4 | (−1.1, 0.3) | 0.24 | 0.3 | (−0.5, 1.2) | 0.46 | |
| RVEF (%) | Unadjusted | −0.2 | (−0.5, 0.1) | 0.29 | 0.4 | (0.1, 0.8) | 0.01 |
| Limited | −0.2 | (−0.5, 0.1) | 0.13 | 0.2 | (−0.2, 0.5) | 0.39 | |
| Full | −0.2 | (−0.5, 0.1) | 0.19 | 0.2 | (−0.1, 0.6) | 0.18 | |
| LVEDM (g) | Unadjusted | 0.0 | (−1.5, 1.4) | 0.96 | 1.2 | (−0.5, 2.9) | 0.16 |
| Limited | −0.2 | (−1.2, 0.9) | 0.77 | 0.7 | (−0.6, 2.0) | 0.29 | |
| Full | −0.3 | (−1.2, 0.6) | 0.51 | −0.1 | (−1.4, 1.2) | 0.85 | |
| LVEDV (ml) | Unadjusted | −0.2 | (−1.7, 1.4) | 0.84 | 0.6 | (−0.8, 2.1) | 0.40 |
| Limited | −0.6 | (−1.7, 0.5) | 0.31 | 1.0 | (−0.2, 2.2) | 0.10 | |
| Full | −0.6 | (−1.7, 0.5) | 0.27 | 1.1 | (−0.2, 2.3) | 0.09 | |
All results presented per standard deviation increase in Ang1 (1.67 ng/ml) or Ang2 (2.59 ng/ml). Limited model: adjusted for age, gender, race/ethnicity, height, weight, and study site. Full model: limited model + adjusted for education level, hypertension, systolic blood pressure, smoking status, pack-years smoking history, diabetes, total cholesterol. Ang1=angiopoietin-1, Ang2=angiopoietin-2, CI=confidence interval, EDM=end-diastolic mass, EDV=end-diastolic volume, EF=ejection fraction, LV=left ventricle, RV=right ventricle, SV=stroke volume. Coefficients, confidence intervals, and P values are bold where statistically significant.
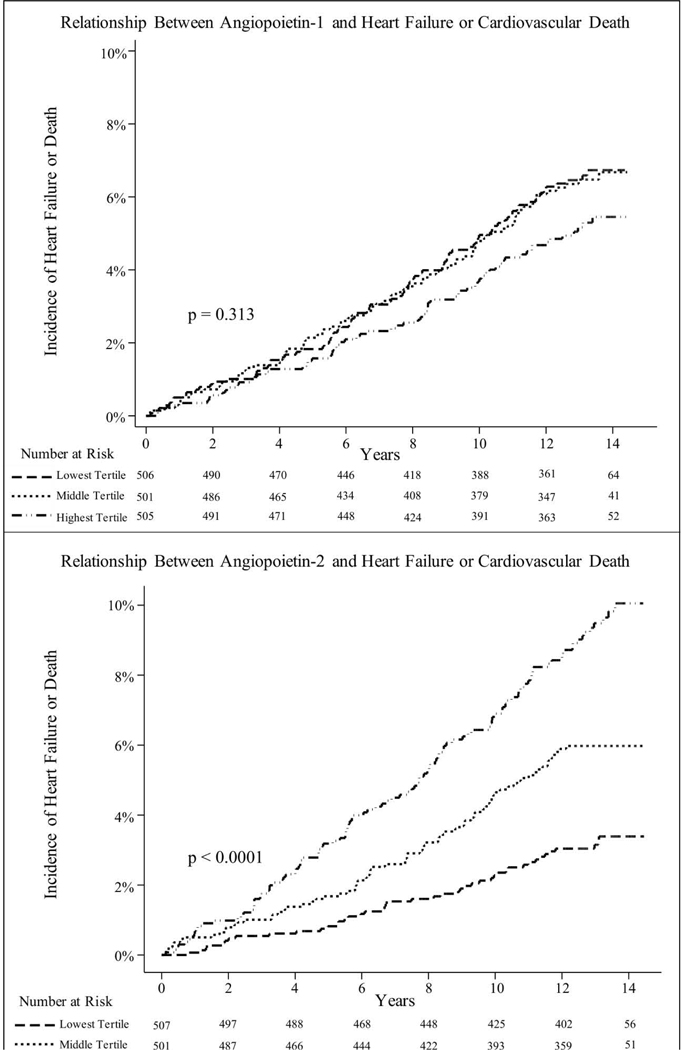
Mean follow-up for MESA participants was 11.4 years, maximum follow-up was 14.5 years, and total follow-up was 17,289 person-years. The incidence of heart failure or cardiovascular death among members of the cohort was 5.0 events per 1,000 person-years, with a median time to event of 7.4 years. There was no association between Ang1 and hazard of heart failure or cardiovascular death (unadjusted hazard ratio 1.02 per SD difference in Ang1, 95% confidence interval 0.78 to 1.34, P=0.88). Conversely, higher Ang2 at baseline was strongly associated with the hazard of heart failure or cardiovascular death (unadjusted hazard ratio 1.37 per SD increase in Ang2, 95% confidence interval 1.27 to 1.47, P<0.001). Incident heart failure accounted for 62% of outcomes. The association between elevated Ang2 and increased incidence of heart failure or cardiovascular death remained strong after adjusting for differences in demographics and cardiovascular risk factors in parametric Cox models (Table 3). Figure 2 shows complementary results from non-parametric analyses (Nelson-Aalen cumulative hazards by tertile). Exploratory models accounting for Ang1 and Ang2 levels simultaneously, GFR, and co-medication use did not strongly influence these results. When accounting for differences in BNP at baseline, the significant association between Ang2 and incident heart failure or cardiovascular death was markedly attenuated. Sensitivity analyses using a log transformation of Ang1 and Ang2 and analyses treating study site as a random effect did not significantly change model results. All results from the clinical events analysis are presented in Table 3.
Table 3.
Hazard of Incident Heart Failure or Cardiovascular Death per Standard Deviation Increase in Angl or Ang2 (MESA Case Cohort) (N= 1512)
| Model | HR | (95% Cl) | P Value |
|---|---|---|---|
| Angl | |||
| Unadjusted | 1.02 | (0.78–1.34) | .88 |
| Limited | 1.03 | (0.84–1.28) | .76 |
| Full | 1.00 | (0.78–1.28) | .996 |
| GFR | 1.00 | (0.79–1.27) | .98 |
| BNP* | 1.02 | (0.78–1.34) | .88 |
| Medic ations | 1.00 | (0.78–1.28) | 1.00 |
| Ang2 | |||
| Unadjusted | 1.37 | (1.27–1.47) | <.001 |
| Limited | 1.27 | (1.19–1J7) | <.001 |
| Full | 1.21 | (1.10—133) | <.001 |
| GFR | 1.23 | (1.11–137) | <.001 |
| BNP* | 1.03 | (0.82–1.28) | .81 |
| Medications | 1.22 | (1.10—135) | <.001 |
All results presented per standard deviation increase in Angl (1.96 ng/ mL> or Ang2 (2.95 ng/mL). The limited model is adjusted for age. sex. race/ethnicity, height, weight, and study site. The full model encompasses the limited model and is adjusted for education level, hypertension, systolic blood pressure, smoking status, pack-years smoking history, diabetes, and total cholesterol. Medication models were adjusted for use of bcta- blockcrs, angiotensin convening enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inilammatoty drugs.
Coefficients, confidence intervals, and P values arc bold where statistically significant.
For models including BNP, N= 1257 owing to missing data.
Figure 2.
(Visual Take Home) Hazard of incident heart failure or cardiovascular death (N=1,512). TOP: Nelson-Aalen cumulative hazards by Ang1 tertile. BOTTOM: Nelson-Aalen cumulative hazard by Ang2 tertile.
PAH cohort
The mean age of PAH cohort participants was 52 (±14) years. Eighty-one percent were female and 92% were white. Thirty-nine percent had idiopathic PAH, 23% had PAH due to connective tissue disease, 17% had toxin-associated PAH, and 21% had PAH from other causes. Mean values of Ang1 and Ang2 in the PAH cohort were 2.83 ng/ml (SD 2.65 ng/ml) and 15.03 ng/ml (SD 12.68 ng/ml), respectively. After excluding individuals with missing data, 73 participants were included in cross-sectional analyses. Increased Ang1 was associated with higher SBP and trends toward lower mean pulmonary artery pressure and higher cardiac index (Table 4). Increased Ang2 was associated with higher right atrial pressure and a trend toward lower SBP (Table 4). All RHC hemodynamic data are presented in Supplemental e-Table 2.
Table 4.
Multivariable linear regression models estimating associations between Ang1, Ang2 and hemodynamic measurements in pulmonary arterial hypertension (N=73) and left heart disease (N=56) cohorts
| Model | PAH Cohort | LHD Cohort | |||||
|---|---|---|---|---|---|---|---|
| Coefficient | (95% CI) | P-value | Coefficient | (95% CI) | P-value | ||
| Ang1 | |||||||
| RAP | Unadjusted | −0.7 | (−2.0, 0.7) | 0.34 | - | - | - |
| (mmHg) | Adjusted | −0.5 | (−2.0, 0.9) | 0.45 | - | - | - |
| mPAP | Unadjusted | −2.8 | (−5.7, 0.1) | 0.06 | - | - | - |
| (mmHg) | Adjusted | −2.6 | (−5.6, 0.4) | 0.09 | - | - | - |
| PAWP | Unadjusted | −0.7 | (−1.8, 0.4) | 0.21 | - | - | - |
| (mmHg) | Adjusted | −0.8 | (−1.9, 0.3) | 0.16 | - | - | - |
| PVR | Unadjusted | −0.8 | (−1.9, 0.3) | 0.17 | - | - | - |
| (Wood units) | Adjusted | −0.8 | (−2.0, 0.3) | 0.15 | - | - | - |
| Cardiac Index | Unadjusted | 0.1 | (0.0, 0.3) | 0.15 | - | - | - |
| (L/min/m2) | Adjusted | 0.2 | (0.0, 0.3) | 0.07 | - | - | - |
| SBP | Unadjusted | 4.8 | (1.1, 8.4) | 0.01 | - | - | - |
| (mmHg) | Adjusted | 5.5 | (1.8, 9.2) | 0.004 | - | - | - |
| Ang2 | |||||||
| RAP | Unadjusted | 1.3 | (0.0, 2.7) | 0.05 | 3.0 | (2.0, 4.0) | <0.001 |
| (mmHg) | Adjusted | 1.8 | (0.5, 3.2) | 0.009 | 2.7 | (1.7, 3.8) | <0.001 |
| mPAP | Unadjusted | 0.8 | (−2.1, 3.8) | 0.57 | 3.6 | (0.5, 6.6) | 0.02 |
| (mmHg) | Adjusted | 1.4 | (−1.7, 4.4) | 0.37 | 2.8 | (−0.2, 5.8) | 0.07 |
| PAWP | Unadjusted | 0.3 | (−0.8, 1.4) | 0.61 | 3.5 | (1.8, 5.2) | <0.001 |
| (mmHg) | Adjusted | 0.8 | (−0.4, 1.9) | 0.18 | 3.4 | (1.6, 5.3) | 0.001 |
| PVR | Unadjusted | 0.6 | (−0.5, 1.7) | 0.27 | 0.2 | (−0.1, 0.6) | 0.23 |
| (Wood units) | Adjusted | 0.6 | (−0.6, 1.8) | 0.30 | 0.1 | (−0.2, 0.5) | 0.41 |
| Cardiac Index | Unadjusted | −0.1 | (−0.3, 0.1) | 0.20 | −0.2 | (−0.4, 0.1) | 0.18 |
| (L/min/m2) | Adjusted | −0.1 | (−0.3, 0.0) | 0.11 | −0.2 | (−0.4, 0.0) | 0.10 |
| SBP | Unadjusted | −4.3 | (−8.0, −0.7) | 0.02 | −2.0 | (−7.9, 3.9) | 0.50 |
| (mmHg) | Adjusted | −3.7 | (−7.5, 0.2) | 0.06 | −1.8 | (−7.9, 4.4) | 0.56 |
All results presented per standard deviation increase in Ang1 (PAH 2.65 ng/ml) or Ang2 (PAH 12.68 ng/ml, LHD 19.58 ng/ml). Adjusted model: adjusted for age, gender, race/ethnicity, and etiology of pulmonary hypertension (PAH cohort) or heart failure (LHD cohort). Ang1=angiopoietin-1, Ang2=angiopoietin-2, CI=confidence interval, LHD=left heart disease, mPAP=mean pulmonary artery pressure, PAH=pulmonary arterial hypertension, PAWP=pulmonary artery wedge pressure, PVR=pulmonary vascular resistance, RAP=right atrial pressure, SBP=systolic blood pressure.
Coefficients, confidence intervals, and P values are bold where statistically significant.
All 116 PAH cohort members were evaluated for the relationship between angiopoietins and the composite of lung transplant or mortality. Mean follow-up was 2.6 years, maximum follow-up was 3 years, and total follow-up was 304 person-years. The incidence of death from any cause was 63 deaths per 1,000 person-years. There were no lung transplants. Higher Ang2 was associated with mortality in unadjusted models (hazard ratio 1.69 per SD difference in Ang2, 95% confidence interval 1.23 to 2.34, P=0.001). This association strengthened after accounting for differences in age, race/ethnicity, gender, and etiology of PAH (hazard ratio 1.91 per SD difference in Ang2, 95% confidence interval 1.34 to 2.70, P<0.001). An exploratory analysis further accounting for resting hemodynamics (right atrial pressure, mean pulmonary artery pressure, and pulmonary vascular resistance) in the limited cohort for whom these covariates were available did not significantly change associations between Ang2 and mortality. There was no association between Ang1 and mortality.
LHD cohort
The mean age of LHD cohort participants was 55 (±14) years. Thirty-eight percent were female and 45% were black. Eighty-two percent had non-ischemic cardiomyopathy. The mean Ang2 value in the LHD cohort was 11.86 ng/ml (SD 19.58 ng/ml). All 56 cohort members were included in the cross-sectional analysis. Increased Ang2 was associated with higher right atrial pressure and pulmonary artery wedge pressure, and a trend toward higher mean pulmonary artery pressure. All results are presented in Table 4, and hemodynamic data is presented in Supplemental e-Table 2.
Fifty-five LHD cohort members were evaluated for the relationship between Ang2 and mortality, heart transplant, or LVAD placement. Mean follow-up was 2.1 years, maximum follow-up was 2.9 years, and total follow-up was 118 person-years. The incidence of heart transplant, LVAD placement, or death from any cause was 93 events per 1,000 person-years. Ang2 was not associated with the composite outcome of mortality, transplant, or LVAD placement.
Discussion
To our knowledge, this is the first study to demonstrate that elevation in Ang2 is associated with a higher risk of heart failure or cardiovascular death in individuals without heart disease at baseline. We also confirm and expand previous observations suggesting that Ang2 is associated with more severe disease in individuals with existing heart failure. Taken together, this suggests Ang2 elevation precedes heart disease, is unlikely to merely represent a reaction to existing disease, and may cautiously suggest that Ang2 contributes to disease pathogenesis. By evaluating both heart failure and non-heart failure cohorts, we were able to characterize the temporality of changes in Ang2 relative to disease onset. Temporality, alongside other aspects such as consistency and plausibility, are important elements which inform the possibility for casual inference in epidemiology as articulated by the Bradford Hill criteria.
We observed a strong association between Ang2 elevation and subsequent incidence of heart failure or cardiovascular death in MESA participants. Conversely, we did not observe associations between Ang2 and cardiac morphology. This joins the observation that the median time until heart failure or cardiovascular death was more than seven years after Ang2 was measured. Although alternative explanations are possible, this reinforces our confidence that Ang2 was measured at a time before overt heart disease was present. In turn, this increases our confidence that the association between Ang2 and heart failure is unlikely to merely represent a response to existing or mild disease. This explanation is potentially challenged by the observation that accounting for differences in BNP markedly attenuated the association between Ang2 and heart failure incidence. This observation, and the very small difference in ejection fraction, might support the alternative hypothesis that Ang2 is not a cause of heart failure but instead a marker of subclinical disease that precedes overt heart failure.
While our results in a population without heart disease at baseline are novel, our results in individuals with existing heart failure confirm and are consistent with other observational studies in LHD (8, 16, 17) and PAH (2, 24). For example, elevated Ang2 was also associated with increased mortality in a previous PAH cohort study (2). Endothelial dysfunction is connected to cardiovascular remodeling and CVD risk factors (25, 26), and may be one mechanism by which Ang2 is related heart disease. Ang2-related endothelial destabilization may promote atherosclerosis and microvascular dysfunction (27, 28) predisposing individuals to ischemic heart disease, cerebrovascular disease, or peripheral arterial disease. In addition, a growing body of research has identified associations between Ang2 and renal disease (25, 29). That having been said, while renal disease may exacerbate existing heart failure it is less likely to mediate the initial development of heart failure independent of a primary cardiac derangement.
Ang2 was predictive of worse hemodynamics in the LHD cohort and with worse hemodynamics and mortality in the PAH cohort. Surprisingly, Ang2 was not associated with incident transplant, LVAD placement, or all-cause mortality in the LHD cohort. This may argue against prognostic significance of Ang2 in LHD relative to PAH and agrees with a prior study suggesting Ang2 levels were not associated with increased risk of death in a left heart failure cohort in adjusted analyses (18). That having been said, the difference in the relationships between Ang2 and vital status in the PAH and LHD cohorts is not fully explained. It is possible that small differences in follow-up time between these cohorts contributed; however, it is perhaps more likely that non-physiologic aspects of LVAD or transplant decisions, including patient goals, insurance, and scheduling, distorted the relationships between angiopoietins, natural history, deranged physiology, and this composite outcome.
Our hemodynamic observations may provide some physiologic insight into the relationship between Ang2 and heart failure. In both heart failure cohorts, higher Ang2 was associated with elevated filling pressures specific to the failing ventricle; however, higher Ang2 was not associated with pulmonary vascular resistance in the PAH cohort nor with pulmonary vascular resistance or SBP in the LHD cohort. This may suggest that the association between Ang2 and heart failure severity may not be mediated by increased afterload.
Importantly, heart failure is not a homogeneous disease, and there are likely a range of different etiologies and pathways that lead to heart failure. Despite this heterogeneity, there are likely cofactors or vulnerabilities that contribute to heart failure regardless of etiology. The fact that Ang2 is elevated prior to the onset of heart failure in community dwelling adults, is elevated in patients with PAH who have more severe right heart failure, and is elevated in patients with left heart disease who have more severe left heart failure is notable. This suggests that differences in Ang2 and angiopoiesis may be one such cofactor or vulnerability that is important across a spectrum of different diseases complicated by heart failure.
Elevated Ang1 was associated with a small, but significant increase in LVEF on cMRI in the MESA cohort. In addition, elevated Ang1 was associated with a trend toward less severe hemodynamic impairment in the PAH cohort. Beyond this, Ang1 was not associated with cardiac morphology or clinical outcomes. This suggests that if Ang1 has a role in the pathogenesis of CVD, it is less prominent relative to Ang2. These findings are consistent with previous research. While some studies have suggested a protective effect (9, 11), others found no association between Ang1 and CVD severity or outcomes (2, 4). Additionally, while some studies showed the ratio of Ang1 to Ang2 may be important (7, 30), our results do not support this notion, as simultaneous adjustment for Ang1 and Ang2 did not change results.
The strengths of this study include the large MESA sample size, the evaluation of angiopoietins in a multi-ethnic cohort, and a presentation of angiopoietin associations across the spectrum of heart failure, including individuals without CVD at baseline. The inclusion of individuals with and without clinical disease is particularly important to minimize the potential for reverse causation to explain observed associations. In addition, the LHD and PAH cohorts were extensively phenotyped and demonstrated relatively strong and consistent associations despite their size.
There were also several limitations. The most prescient are that, while associations with clinical outcomes and heart failure severity were robust after adjustment for a variety of variables, unmeasured or residual confounding certainly may exist and explain the associations independent of a causal mechanism involving angiopoietins. Furthermore, our demonstration of associations across multiple cohorts is important in suggesting causality and supported by our results, but our discussion of mechanism is speculative. The PAH and LHD cohorts were small, particularly for the hemodynamic analyses. In addition, for the MESA and PAH cohorts, serum was not always drawn on the same day as RHC or cMRI measurements and the Ang2 assay used for the MESA and PAH cohorts differed from the LHD cohort. These differences may lead to misclassification and type II error. Stochastic misclassification of the exposure is unlikely to result in false positive associations and does not substantially weaken the observations regarding Ang2 but may impact the inference for null results largely observed for Ang1. Finally, as a result of multiple testing there was an increased likelihood of finding a significant result by chance alone; however, the consistency of results in different cohorts and across different metrics minimizes this possibility.
Conclusions
This study corroborates previous data identifying elevated Ang2 as a biomarker for heart failure severity. Our results suggest elevated Ang2 predicts increased risk of mortality in PAH, and increased risk of incident heart failure or cardiovascular mortality in individuals without cardiovascular disease. Because Ang2 is elevated prior to disease onset in the MESA cohort, and elevations reflect disease severity in right and left heart failure, Ang2 could be a contributor to heart failure pathogenesis and not merely a marker of disease activity.
Supplementary Material
Highlights.
Angiopoietin levels may predict an individual’s likelihood to develop heart failure
Angiopoietin levels may help prognosticate for individuals who have heart failure
This study may highlight a pathway that could be potentially targetable in individuals with heart failure
Acknowledgements
The authors thank the MESA investigators, staff, and participants for their valuable contributions. A full list of participating investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts from the National Heart, Lung, and Blood Institute 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, R01-HL-086719, K24-HL-103844, and R01-HL-071759, and by grants UL1-TR-000040, UL1-TR-001079 and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The American Heart Association Mentored Award in Population Research supported the MESA-Angiogenesis Ancillary Study.
Abbreviations:
- Ang1/2
angiopoietin-1/2
- BNP
brain natriuretic peptide
- cMRI
cardiac magnetic resonance imaging
- CVD
cardiovascular disease
- GFR
glomerular filtration rate
- LHD
left heart disease
- LVAD
left ventricular assist device
- MESA
Multi-Ethnic Study of Atherosclerosis
- PAH
pulmonary arterial hypertension
- RHC
right heart catheterization
- R/LVEF
right/left ventricular ejection fraction
- SBP
systolic blood pressure
- SD
standard deviation
Footnotes
Prior Abstract Presentation:
Oral presentation for online symposium: “What’s new in clinical research in pulmonary hypertension: lessons from the best abstracts.” Presented October 21, 2020 at the 2020 American Thoracic Society Annual Conference.
Disclosures/Conflict of Interest
All authors report no direct conflict of interest related to this manuscript. Dr. Leary receives research support from the NHLBI, PHA, AHA, Chest Foundation, Lung LLC, Bayer, and Actelion, salary support from the Cystic Fibrosis Therapeutic Development network, and honoraria from Bayer. Dr. Tedford has consulting relationships with Medtronic, Aria CV Inc., Acceleron, Arena Pharmaceuticals and United Therapeutics. Dr. Tedford is on a steering committee for Medtronic and Abbott, and a research advisory board for Abiomed. He also does hemodynamic core lab work for Actelion and Merck. Dr. Rayner holds research grants from Actelion and United Therapeutics and is a consultant for Verathon. Dr. Steinberg is a consultant for Medtronic. All other authors report no disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Kumpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, et al. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31(18):2291–300. doi: 10.1093/eurheartj/ehq226. [DOI] [PubMed] [Google Scholar]
- 3.Moss A. The angiopoietin:Tie 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013;24(6):579–92. doi: 10.1016/j.cytogfr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Richter MJ, Tiede SL, Sommer N, Schmidt T, Seeger W, Ghofrani HA, et al. Circulating Angiopoietin-1 Is Not a Biomarker of Disease Severity or Prognosis in Pulmonary Hypertension. PLoS One. 2016;11(11):e0165982. doi: 10.1371/journal.pone.0165982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 6.Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, et al. Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1025–33. doi: 10.1164/rccm.200602-304OC. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Guo L, Cui M, Sun L, Mi L. Dynamic changes in serum angiopoietin-1, angiopoietin-2, and angiopoietin-2/angiopoietin-1 ratio in acute myocardial infarction patients treated with primary percutaneous coronary intervention. Biomarkers. 2012;17(5):441–6. doi: 10.3109/1354750X.2012.684152. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Yong H, Mi L, Bai Y, Guo L, Gao W, et al. Changes and significance of serum angiopoietin-2 levels in patients with coronary heart disease. Biomarkers. 2012;17(8):745–9. doi: 10.3109/1354750X.2012.727028. [DOI] [PubMed] [Google Scholar]
- 9.Liu KL, Lin SM, Chang CH, Chen YC, Chu PH. Plasma angiopoietin-1 level, left ventricular ejection fraction, and multivessel disease predict development of 1-year major adverse cardiovascular events in patients with acute ST elevation myocardial infarction - a pilot study. Int J Cardiol. 2015;182:155–60. doi: 10.1016/j.ijcard.2014.12.172. [DOI] [PubMed] [Google Scholar]
- 10.Findley CM, Mitchell RG, Duscha BD, Annex BH, Kontos CD. Plasma levels of soluble Tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;52(5):387–93. doi: 10.1016/j.jacc.2008.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moxon JV, Trollope AF, Dewdney B, de Hollander C, Nastasi DR, Maguire JM, et al. The effect of angiopoietin-1 upregulation on the outcome of acute ischaemic stroke in rodent models: A meta-analysis. J Cereb Blood Flow Metab. 2019:271678X19876876. doi: 10.1177/0271678X19876876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golledge J, Clancy P, Yeap BB, Hankey GJ, Norman PE. Increased serum angiopoietin-2 is associated with abdominal aortic aneurysm prevalence and cardiovascular mortality in older men. Int J Cardiol. 2013;167(4):1159–63. doi: 10.1016/j.ijcard.2012.03.120. [DOI] [PubMed] [Google Scholar]
- 13.Patel JV, Lim HS, Varughese GI, Hughes EA, Lip GY. Angiopoietin-2 levels as a biomarker of cardiovascular risk in patients with hypertension. Ann Med. 2008;40(3):215–22. doi: 10.1080/07853890701779586. [DOI] [PubMed] [Google Scholar]
- 14.Lorbeer R, Baumeister SE, Dorr M, Nauck M, Grotevendt A, Volzke H, et al. Circulating angiopoietin-2, its soluble receptor Tie-2, and mortality in the general population. Eur J Heart Fail. 2013;15(12):1327–34. doi: 10.1093/eurjhf/hft117. [DOI] [PubMed] [Google Scholar]
- 15.Chong AY, Caine GJ, Freestone B, Blann AD, Lip GY. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol. 2004;43(3):423–8. doi: 10.1016/j.jacc.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Lukasz A, Beutel G, Kumpers P, Denecke A, Westhoff-Bleck M, Schieffer B, et al. Angiopoietin-2 in adults with congenital heart disease and heart failure. PLoS One. 2013;8(6):e66861. doi: 10.1371/journal.pone.0066861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eleuteri E, Di Stefano A, Tarro Genta F, Vicari C, Gnemmi I, Colombo M, et al. Stepwise increase of angiopoietin-2 serum levels is related to haemodynamic and functional impairment in stable chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2011;18(4):607–14. doi: 10.1177/1741826710389410. [DOI] [PubMed] [Google Scholar]
- 18.Eleuteri E, Di Stefano A, Giordano A, Corra U, Tarro Genta F, Gnemmi I, et al. Prognostic value of angiopoietin-2 in patients with chronic heart failure. Int J Cardiol. 2016;212:364–8. doi: 10.1016/j.ijcard.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)--right ventricle study. Circulation. 2012;126(14):1681–8. doi: 10.1161/CIRCULATIONAHA.112.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 Suppl 2):S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 22.Samokhin AO, Hsu S, Yu PB, Waxman AB, Alba GA, Wertheim BM, et al. Circulating NEDD9 is increased in pulmonary arterial hypertension: A multicenter, retrospective analysis. J Heart Lung Transplant. 2019. doi: 10.1016/j.healun.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houston BA, Tedford RJ, Baxley RL, Sykes B, Powers ER, Nielsen CD, et al. Relation of Lymphangiogenic Factor Vascular Endothelial Growth Factor-D to Elevated Pulmonary Artery Wedge Pressure. Am J Cardiol. 2019;124(5):756–62. doi: 10.1016/j.amjcard.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 24.Joshi AA, Davey R, Rao Y, Shen K, Benza RL, Raina A. Association between cytokines and functional, hemodynamic parameters, and clinical outcomes in pulmonary arterial hypertension. Pulm Circ. 2018;8(3):2045894018794051. doi: 10.1177/2045894018794051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells. 2019;8(5). doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shantsila E, Wrigley BJ, Blann AD, Gill PS, Lip GY. A contemporary view on endothelial function in heart failure. Eur J Heart Fail. 2012;14(8):873–81. doi: 10.1093/eurjhf/hfs066. [DOI] [PubMed] [Google Scholar]
- 27.Lorbeer R, Baumeister SE, Dorr M, Felix SB, Nauck M, Grotevendt A, et al. Angiopoietin-2, its soluble receptor Tie-2 and subclinical cardiovascular disease in a population-based sample. Heart. 2015;101(3):178–84. doi: 10.1136/heartjnl-2014-306056. [DOI] [PubMed] [Google Scholar]
- 28.Theelen TL, Lappalainen JP, Sluimer JC, Gurzeler E, Cleutjens JP, Gijbels MJ, et al. Angiopoietin-2 blocking antibodies reduce early atherosclerotic plaque development in mice. Atherosclerosis. 2015;241(2):297–304. doi: 10.1016/j.atherosclerosis.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennings A, Hannemann A, Rettig R, Dorr M, Nauck M, Volzke H, et al. Circulating Angiopoietin-2 and Its Soluble Receptor Tie-2 Concentrations Are Related to Renal Function in Two Population-Based Cohorts. PLoS One. 2016;11(11):e0166492. doi: 10.1371/journal.pone.0166492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukasz A, Hillgruber C, Oberleithner H, Kusche-Vihrog K, Pavenstadt H, Rovas A, et al. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res. 2017;113(6):671–80. doi: 10.1093/cvr/cvx023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.