Abstract
Background.
Depression is strongly associated with chronic disease; yet, the direction of this relationship is poorly understood. Allostatic load (AL) provides a framework for elucidating depression-disease pathways. We aimed to investigate bidirectional, longitudinal associations of baseline depressive symptoms or AL with 5-year AL or depressive symptoms, respectively.
Methods.
Data were from baseline, 2-year, and 5-year visits of 620 adults (45–75 years) enrolled in the Boston Puerto Rican Health Study. The Center for Epidemiology Studies Depression (CES-D) scale (0–60) captured depressive symptoms, which were categorized at baseline as low (<8), subthreshold (8–15), or depression-likely (⩾16) symptoms. AL was calculated from 11 parameters of biological functioning, representing five physiological systems. Baseline AL scores were categorized by the number of dysregulated parameters: low (0–2), moderate (3–5), or high (⩾6) AL. Multivariable, multilevel random intercept and slope linear regression models were used to examine associations between 3-category baseline CES-D score and 5-year continuous AL score, and between baseline 3-category AL and 5-year continuous CES-D score.
Results.
Baseline subthreshold depressive symptoms [(mean (95% CI)): 4.8 (4.5–5.2)], but not depression-likely symptoms [4.5 (4.2–4.9)], was significantly associated with higher 5-year AL scores, compared to low depressive symptoms [4.3 (3.9–4.7)]. Baseline high AL [19.4 (17.6–21.2)], but not low AL [18.5 (16.5–20.6)], was significantly associated with higher 5-year CES-D score, compared to baseline moderate AL [16.9 (15.3–18.5)].
Conclusions.
Depressive symptoms and AL had a bi-directional relationship over time, indicating a nuanced pathway linking depression with chronic diseases among a minority population.
Keywords: Allostatic load, depression, depressive symptoms, Hispanics, Latinos, Puerto Ricans
Introduction
Depression affects 322 million people (4.4%) worldwide, although the prevalence is highest among middle-aged to older adults (55–74 years), particularly women (World Health Organization, 2017). Between 2009 and 2012, 7.6% of US individuals ƒ12 years were categorized with depression, and women, middle-aged to older adults (40–59 years), and persons living below the poverty line experienced higher prevalence (Pratt & Brody, 2014). Consequently, depression is the leading contributor to disability globally (World Health Organization, 2017), and a significant factor affecting chronic disease management (Chapman, Perry, & Strine, 2005). Non-Hispanic black and Hispanic older adults have higher prevalence of subthreshold and major depression compared to non-Hispanic white older adults (Xiang, Leggett, Himle, & Kales, 2018). Furthermore, racial and ethnic minorities have poor access to mental health care, leading to undiagnosed depression and inadequate treatment (Kim, 2014) that can exacerbate chronic disease and its management.
Depression has been viewed both as an independent determinant (Poole & Steptoe, 2018) as well as a comorbidity of chronic diseases (O’Connor et al., 2015), as persons with chronic medical conditions also have a high prevalence of depressive disorders (Benton, Staab, & Evans, 2007). Individuals with chronic diseases are at an increased risk of depression or depressive symptoms (Chang-Quan, Bi-Rong, Zhen-Chan, Ji-Rong, & Qing-Xiu, 2010a; Chang-Quan et al., 2010b), likely triggered by the financial, emotional, and physical strain of managing chronic disease, although physiological mechanisms have also been proposed (Aziz & Steffens, 2013; Katon, 2011). Alternatively, depression and depressive disorders have been considered as contributors to chronic disease etiology, including cardiovascular disease, diabetes, and obesity (Chapman et al., 2005; Mezuk, Eaton, Albrecht, & Golden, 2008). However, limited evidence exists for pathways in which depression, or depressive symptoms, may facilitate disease development. Studies assessing relationships of depression and disease status have shown associations in both directions for some conditions (i.e. cancer, metabolic syndrome, obesity, cardiovascular disease) (Gothe et al., 2012; Luppino et al., 2010; Nemeroff & Goldschmidt-Clermont, 2012; Pan et al., 2012; Spiegel & Giese-Davis, 2003), while others are less clearly defined (e.g. type 2 diabetes) (Renn, Feliciano, & Segal, 2011; Tabák, Akbaraly, Batty, & Kivimäki, 2014).
One understudied pathway in the depression-chronic disease connection is the role of depression in disturbing the body’s allostasis, or process of attaining homeostasis after responding to stressors (Barrett, Quigley, & Hamilton, 2016; McEwen, 2003a). Depression can up-regulate proinflammatory cytokines and cortisol (Ogłodek, Szota, Just, Moś, & Araszkiewicz, 2014) but these, in turn, elicit further depressive symptoms (Dedovic & Ngiam, 2015; Slavich & Irwin, 2014). Inflammatory and cortisol pathways activated independently from depression may also trigger depressive symptoms (Barrett et al., 2016; McEwen, 2003a). Moreover, the sustained presence of neuroendocrine and inflammation biomarkers may disrupt multiple downstream physiological systems (e.g. cardiovascular), leading to allostatic load (AL), a cumulative measure of physiological ‘wear and tear’ that is directly associated with mortality (McEwen, 1998b; McEwen & Stellar, 1993) and chronic disease (Crews, 2007; Mattei, Demissie, Falcon, Ordovas, & Tucker, 2010; Seeman, Singer, Rowe, Horwitz, & McEwen, 1997). AL provides an integrated framework to examine the role of chronic or repeated stress on multiple physiological systems, rather than a single system, and incorporates the physiological responses mediating (e.g. neuroendocrine) eventual disease development (McEwen, 1998a, 2003a). High AL has been associated with depressive symptoms in a cross-sectional community sample of US older adults (Kobrosly, van Wijngaarden, Seplaki, Cory-Slechta, & Moynihan, 2014) and in a cross-sectional, nationally-representative sample of older Taiwanese adults (Seplaki, Goldman, Glei, & Weinstein, 2005), but most study designs prevent establishing relationship directionality. One analysis among older adults found cross-sectional, but not longitudinal, associations between AL and depressive symptoms (Juster et al., 2011); associations between depressive symptoms and eventual AL were not tested. The dearth of studies testing the depressive symptom–AL relationship and its directionality warrants robust, longitudinal investigations, especially in at-risk populations.
US mainland Puerto Ricans are a marginalized population experiencing high prevalence of depression (38%) and depressive symptoms (60%), especially compared to other Hispanic/Latino heritages (Tucker et al., 2010; Wassertheil-Smoller et al., 2014). Simultaneously, mainland Puerto Ricans have high prevalence of cardiometabolic risk factors and disease (Daviglus et al., 2012; Mattei et al., 2016; Schneiderman et al., 2014; Tucker et al., 2010) and high AL (Mattei et al., 2010; Salazar et al., 2016). The Boston Puerto Rican Health Study (BPRHS) longitudinal cohort of Puerto Rican adults (45–75 years) provides a unique opportunity to address these gaps in an at-risk population, as 60% had depressive symptomology (Tucker et al., 2010), and 61.8% had three to five dysregulated AL parameters (15.4% had ⩾6 dysregulated AL parameters) (Mattei et al., 2010) at baseline. The objective of this study was to investigate bidirectional, longitudinal associations of baseline depressive symptoms or AL with 5-year AL or depressive symptoms, respectively.
Methods
Study population
We analyzed data from the BPRHS (n = 1500), a longitudinal cohort study examining psychosocial stress, AL, and health outcomes. Recruitment and data collection methods have been published in detail elsewhere (Tucker et al., 2010). Eligible participants were adults (45–75 years), who self-identified as Puerto Rican, were able to respond to questions in English or Spanish, and were living in the Boston, MA metropolitan area at the time of initial recruitment (2004–2007). Census tracks with at least 25 Puerto Rican adults ages 45–75 years were identified using the 2000 Census and, within these tracks, census blocks with ⩾10 Hispanic/Latino adults ages 45–75 years were randomly selected. Participants were recruited using door-to-door enumeration and community outreach strategies. Among those invited to participate (n = 2093), 86.5% agreed and 72.0% (n = 1500) completed the baseline interview. Compared to those who agreed to participate, those who declined tended to be older or to have lived longer in the USA, but did not differ by sex, birthplace, or language spoken (Tucker et al., 2010). Study visits occurred at baseline, 2-years, and 5-years. Of those participants completing the baseline interview, 83.9% (n = 1258) completed the 2-year and 64.1% (n = 961) completed the 5-year follow-up assessments. Upon obtaining written informed consent from participants, trained bilingual interviewers administered questionnaires and performed anthropometric measures in duplicate and blood pressure measurements in triplicate in the participant’s home; the average readings were used as the final values. Questionnaires included demographics, acculturation, depressive symptoms, perceived stress, self-reported medically-diagnosed conditions, medication use, physical activity, dietary intake, smoking status, and alcohol use. Participants provided a 12-h fasting blood sample (for analysis of high-density lipoprotein-cholesterol (HDL-C), and total cholesterol (TC)), C-reactive protein (CRP), glycated hemoglobin (HbA1c), dehydroepiandrosterone sulfate (DHEA-S)), and a 12-h urine collection for analysis of epinephrine, norepinephrine, and cortisol. The Institutional Review Boards at Tufts Medical Center and Northeastern University approved the study.
Measures
Depressive symptoms
The Center for Epidemiology Studies Depression (CES-D) scale assessed depressive symptoms and has demonstrated consistency and validity in older adults (Radloff, 1986), and reliability among Hispanics/Latinos (Moscicki, Locke, Rae, & Boyd, 1989), including Puerto Ricans (Tucker, Falcon, Bianchi, Cacho, & Bermudez, 2000b). The 20-item CES-D scale captured the frequency over the past week that participants experienced feelings and behaviors associated with depression, including poor appetite, feeling sad and lonely, and restless sleep. Response options were assigned a value of 0 (rarely or none of the time), 1 (some or little of the time), 2 (moderately or much of the time), or 3 (most or almost all the time). Total scores ranged from 0 to 60, with higher scores indicating greater depressive symptoms (Radloff, 1977). Internal consistency of the CES-D in our sample was highly reliable (α = 0.91). Based on previously-defined cutoffs for older black and white adults (Cohen, Magai, Yaffee, & Walcott-Brown, 2005; Vahia et al., 2010), we defined baseline depressive symptoms as low (<8), subthreshold (8–15), or depression-likely (⩾16). When CES-D was the outcome, we used the continuous score. Two additional variables captured the total number of times across the three visits (0–3) that participants had (1) any depressive symptoms (subthreshold or depression-likely) and (2) depression-likely symptoms. In the sensitivity analysis, we also tested a higher cutoff (⩾20) for depression-likely symptoms, as recommended for the general population (Villagut, Forero, Barbaglia, & Alonso, 2016) and tested among a different US mainland Puerto Rican population (Robison, Gruman, Gaztambide, & Blank, 2002). This 3-category depressive symptom variable was: low (<8), subthreshold (8–19), or depression-likely (⩾20). We tested CES-D as a continuous exposure variable, but results were non-significant and did not portray the distinctions of the depressive symptom–AL relationship in our cohort.
Allostatic load
AL was calculated from 11 parameters of biological functioning, representing five physiological systems: hypothalamus-pituitary-adrenal (HPA) axis (DHEA-S, cortisol), sympathetic nervous system (SNS; epinephrine, norepinephrine), inflammation (CRP), metabolic (waist circumference, HbA1c), and cardiovascular (systolic (SBP) and diastolic (DBP) blood pressure, HDL-C, and TC). The following biological parameters had clinically-defined high-risk cutoffs: waist circumference (men: >102 cm; women: >88 cm), HbA1c (>7%), SBP (>140 mmHg), DBP (>90 mmHg), HDL-C (<40 mg/dl), and TC (⩾240 mg/dl). Previously-defined quartile and high-risk cutoffs were used for the remaining parameters: DHEA-S (men: ⩾589.5 ng/ml; women: ⩾368.5 ng/ml), cortisol (men: ⩾41.5 μg/g creatine; women: ⩾49.5 μg/g creatine), epinephrine (men: ⩾2.8 μg/g creatine; women: ⩾3.6 μg/g creatine), norepinephrine (men: ⩾30.5 μg/g creatine; women: ⩾46.9 μg/g creatine), and CRP (>3 mg/l). The cut-offs for these latter parameters have been previously employed in defining AL in this cohort (Mattei et al., 2010, 2013). The sex-specific cut-offs for neuroendocrine parameters represent previously documented sex differentials (Goldman et al., 2004), and the CRP cut-off is based on a cumulation across population studies (Pearson et al., 2003). A point was assigned to each parameter if the value exceeded the cutoff. An additional point was assigned to respective parameters when participants used medications for hypertension, diabetes, hyperlipidemia, or testosterone. Points were summed across all parameters to calculate the composite AL score (0–11). The AL score was further categorized according to previous analysis in this cohort (Mattei et al., 2010): low (0–2), moderate (3–5), or high (⩾6) AL. One additional variable captured the number of times participants were categorized with high AL across the three visits (0–3). Points were also summed across each AL subsystem to produce subsystem scores: HPA-axis (0–2), SNS (0–2), inflammation (0–1), metabolic (0–2), and cardiovascular (0–4). We tested AL as a continuous exposure variable, but results were non-significant and did not capture the nuances of the relationship between AL and CES-D in our cohort.
Potential confounders
Potential confounders included sociodemographic characteristics, health behaviors, perceived stress, and language-based acculturation, based on previous analyses examining AL as an exposure or an outcome (Forrester, Leoutsakos, Gallo, Thorpe, & Seeman, 2019; Kobrosly et al., 2014; Mattei, Bhupathiraju, & Tucker, 2013; Salazar et al., 2016). Sociodemographic data included age, sex, income-to-poverty ratio (IPR), and educational attainment. IPR was calculated as the ratio of total household income to a year-specific federal poverty threshold, a measure of need that considers basic food costs, household size and composition, and age of the household head (U.S. Census Bureau, 2016). We categorized participants as either ⩽120% or >120% of the IPR. Health behaviors included physical activity, diet quality, alcohol use, and smoking status. Physical activity was assessed using a modified Paffenbarger questionnaire. Total hours spent on typical activities in a 24-h period were multiplied by respective weighting, associated with the activity intensity and oxygen consumption, to define a physical activity score (Tucker, Bermudez, & Castaneda, 2000a). Dietary intake was assessed using a valid semiquantitative food frequency questionnaire (FFQ), based off of the National Cancer Institute Block-FFQ and adapted to incorporate appropriate foods and portion sizes for this population (Tucker, Bianchi, Maras, & Bermudez, 1998). The Alternate Healthy Eating Index-2010 (AHEI-2010) captured overall diet quality, consisting of 11 dietary components related to chronic disease risk. AHEI-2010 scores ranged from 0 (lowest diet quality) to 110 (highest diet quality) (Chiuve et al., 2012). We imputed missing baseline AHEI-2010 scores for 60 participants in our analytical sample with multiple imputations for chained equations using predictive mean matching. The Perceived Stress Scale (PSS) measured the extent to which participants regarded their lives as stressful. Higher scores (range: 0–40) indicated greater perceived stress (Cohen, Kamarck, & Mermelstein, 1983). Language-based acculturation was assessed with a questionnaire adapted from the Bi-Dimensional Acculturation Scale for Hispanics (Marin & Gamba, 1996), which captured the use of either Spanish or English language in seven different daily activities (e.g. watching TV, speaking with friends). A summary score (0–100) was calculated. Higher scores indicated greater language-based acculturation (more use of English).
Statistical analyses
Our analytical sample included 620 participants with complete data on CES-D and AL at baseline and 5-year follow-up. Of these, 604 participants had complete CES-D scores at all three visits and 543 participants had complete AL data at all three visits.
Participant characteristics were compared by baseline CES-D symptoms category (low v. subthreshold v. depression-likely) and by baseline AL category (low v. moderate v. high), using ANOVA or Student’s t tests for continuous variables and chi-square tests for categorical variables. Multilevel random intercept and slope (time) linear regression models were used to examine associations between 3-category baseline CES-D score and 5-year continuous AL score, and between baseline 3-category AL and 5-year continuous CES-D score. Model 1 was adjusted for age (time) and sex. Model 2 was adjusted for Model 1 covariates plus baseline IPR category and educational attainment. Model 3 was adjusted for Model 2 covariates plus baseline physical activity level, AHEI-2010, smoking status, and alcohol use. Model 4 was adjusted for Model 3 covariates plus baseline language-based acculturation and PSS. All models accounted for the outcome variable at baseline and 2-year follow-up. We used Tukey’s test to determine the significance of pairwise comparisons. We used multilevel random intercept regression models to test relationships of 3-category baseline CES-D score with each of the five AL subsystem scores (HPA-axis, SNS, inflammation, metabolic, and cardiovascular). Multilevel random intercept and slope (time) linear regression models (n = 604) were also separately examined for the association between the number of times with any depressive symptoms (subthreshold or depression-likely) or with depression-likely symptoms across the three visits and 5-year continuous AL score. Similarly, we tested the association between the number of times categorized with high AL and 5-year depressive symptoms (n = 543). Analyses were conducted in SAS v9.4 (SAS Institute Inc., Cary, NC, USA) with a significance level set at α = 0.05.
Results
Participant characteristics
At baseline, 18.1% and 60.6% of participants were classified with subthreshold and depression-likely symptoms, respectively (Table 1). Compared to low or subthreshold depressive symptoms, participants classified with depression-likely symptoms at baseline were younger, less likely to be currently working, married/living with a partner, physically active, and more likely to be female, living ⩽120% of the IPR, or taking depression medication. These participants also tended to have a higher PSS score and lower language-based acculturation score. Compared with low depressive symptoms, participants with subthreshold depressive symptoms at baseline were more likely to be female, living ⩽120% of the IPR, or physically active, and less likely to be currently working or married/living as married. These participants also tended to have a higher PSS score, lower language-based acculturation score, and higher AL score at 2-year follow-up.
Table 1.
Sample characteristics by baseline depressive symptoms among participants in the Boston Puerto Rican Health Study (n=620)a
| Characteristic | Depressive symptomsb | p value | ||
|---|---|---|---|---|
| Low (<8) n = 132 | Subthreshold (8–15) n = 112 | Depression-likely (≥16) n = 376 | ||
| Age, years | 57.4 (7.3) | 57.4 (7.2) | 55.6 (7.3) | 0.01 |
| Female | 59.9 | 65.2 | 77.9 | <0.0001 |
| Income-to-poverty ratioc | <0.0001 | |||
| ⩾120% | 50.0 | 60.7 | 72.9 | |
| >120% | 46.2 | 36.6 | 20.7 | |
| Missing | 3.8 | 2.7 | 6.4 | |
| Currently working | 36.4 | 24.1 | 13.1 | <0.0001 |
| Educational attainment | 0.09 | |||
| <5thgrade | 16.7 | 24.3 | 20.5 | |
| 5th–8thgrade | 22.7 | 20.7 | 28.5 | |
| 9th–12thgrade/GED | 43.2 | 36.0 | 40.2 | |
| ≥Some college | 17.4 | 18.9 | 10.9 | |
| Marital status | 0.01 | |||
| Married/living together | 47.0 | 33.9 | 29.9 | |
| Divorced/separated | 31.1 | 45.5 | 40.3 | |
| Widowed | 11.4 | 8.9 | 12.3 | |
| Never married | 10.6 | 11.6 | 17.6 | |
| Smoking status | 0.48 | |||
| Never | 50.0 | 45.5 | 44.8 | |
| Past | 29.6 | 32.1 | 27.7 | |
| Current | 20.5 | 22.3 | 27.5 | |
| Alcohol use | 0.38 | |||
| Never | 25.0 | 25.0 | 31.0 | |
| Past | 26.5 | 30.4 | 29.4 | |
| Current | 48.5 | 44.6 | 39.6 | |
| Physical activityd | 0.04 | |||
| Light/sedentary | 40.2 | 36.6 | 48.9 | |
| Moderate | 54.6 | 55.4 | 47.9 | |
| High/vigorous | 5.3 | 8.0 | 3.2 | |
| Alternate Healthy Eating Index-2010e | 55.8 (9.1) | 54.6 (9.8) | 53.7 (9.0) | 0.07 |
| Perceived stress scoref | 13.5 (7.4) | 19.6 (6.3) | 28.3 (7.0) | <0.0001 |
| Language-based acculturation scoreg | 28.3 (24.1) | 23.9 (20.4) | 21.9 (21.3) | 0.02 |
| Depressive symptom scoreb | ||||
| 2 year follow-up | 8.3 (7.6) | 12.4 (8.5) | 22.9 (12.2) | <0.0001 |
| 5 year follow-up | 14.2 (6.1) | 16.5 (7.4) | 22.0 (10.1) | <0.0001 |
| Taking depression medication | <0.0001 | |||
| Yes | 10.6 | 21.4 | 58.2 | |
| No | 74.2 | 60.7 | 35.9 | |
| Missing | 15.2 | 17.9 | 5.9 | |
| Allostatic load scoreh | ||||
| Baseline | 4.3 (1.8) | 4.8 (2.0) | 4.4 (2.0) | 0.09 |
| 2 year follow-up | 4.6 (1.8) | 5.2 (1.8) | 4.6 (1.8) | 0.03 |
| 5 year follow-up | 5.0 (1.7) | 5.3 (2.1) | 5.1 (1.7) | 0.37 |
Values shown as mean (s.d.) for continuous variables or percentages for categorical variables.
Assessed using the Center for Epidemiology Studies Depression (CESD) scale (range: 0–60).
Ratio of total household income to appropriate and year-specific federal poverty threshold. Scores categorized above/below 120%.
Sum of hours spent in typical activities over a 24-h period, multiplied by weight factors associated with oxygen consumption for each activity. Scores categorized as light or sedentary (<30), moderate (30–40), or heavy (>40).
Assessed using 11 dietary components. Higher scores indicate higher diet quality (total score range from 0 to 110).
Measures the degree to which one’s life is viewed as stressful, ranging from 0 to 40, with higher values indicating higher perceived stress.
Higher scores (range 0–100) indicated greater acculturation (more use of English).
Calculated from 11 parameters of biological functioning (range: 0–11).
From baseline to 5-year follow-up, 1.2%, 11.8%, 24.2%, and 62.9% of participants were categorized with any type of depressive symptoms (subthreshold or depression-likely) zero, one, two, and three times, respectively. Across the three visits, 21.0%, 22.9%, 23.5%, and 32.6% of participants were categorized with depression-likely symptoms zero, one, two, and three times, respectively.
At baseline, 53.4% and 30.0% of participants had moderate and high AL, respectively (Table 2). Compared with low AL, participants with moderate or high AL were older and more likely to be a past drinker. Participants with moderate AL had a lower PSS score and CES-D score at baseline, 2-year, and 5-year follow-up.
Table 2.
Sample characteristics by baseline allostatic load category among participants in the Boston Puerto Rican Health Study (n = 620)a
| Characteristic | Allostatic load category | p value | ||
|---|---|---|---|---|
| Low (<3) n = 103 | Moderate (3–5) n = 112 | High (≥16) n = 186 | ||
| Age, years | 53.0 (6.4) | 56.5 (7.1) | 57.8 (7.6) | <0.0001 |
| Female | 78.6 | 70.4 | 70.4 | 0.24 |
| Income-to-poverty ratiob | 0.74 | |||
| ⩾120% | 65.1 | 65.3 | 67.2 | |
| >120% | 31.1 | 28.4 | 29.0 | |
| Missing | 3.9 | 6.3 | 3.8 | |
| Currently working | 21.4 | 23.0 | 14.1 | 0.05 |
| Educational attainment | 0.05 | |||
| <5th grade | 12.6 | 21.5 | 22.6 | |
| 5th–8th grade | 20.4 | 28.8 | 23.7 | |
| 9th–12th grade/GED | 53.4 | 35.8 | 40.3 | |
| ⩾Some college | 13.6 | 13.9 | 13.4 | |
| Marital status | 0.94 | |||
| Married/living together | 37.9 | 32.7 | 35.0 | |
| Divorced/separated | 38.8 | 40.3 | 37.6 | |
| Widowed | 8.7 | 11.8 | 12.4 | |
| Never married | 14.6 | 15.2 | 15.1 | |
| Smoking status | 0.13 | |||
| Never | 48.5 | 48.9 | 39.5 | |
| Past | 22.3 | 28.4 | 33.5 | |
| Current | 29.1 | 22.7 | 27.0 | |
| Alcohol use | 0.04 | |||
| Never | 38.2 | 27.2 | 26.0 | |
| Past | 18.6 | 29.0 | 34.6 | |
| Current | 43.1 | 43.8 | 39.5 | |
| Physical activityc | <0.05 | |||
| Light/sedentary | 38.8 | 46.8 | 44.6 | |
| Moderate | 52.4 | 48.3 | 53.8 | |
| High/vigorous | 8.7 | 4.8 | 1.6 | |
| Alternate Healthy Eating Index-2010d | 53.5 (9.8) | 54.3 (9.3) | 54.8 (8.6) | 0.49 |
| Perceived stress scoree | 25.5 (9.8) | 22.6 (9.2) | 24.2 (9.0) | 0.01 |
| Language-based acculturation scoref | 26.6 (22.7) | 23.0 (21.2) | 23.0 (22.6) | 0.31 |
| Depressive symptom scoreg | ||||
| Baseline | 23.1 (14.6) | 19.2 (12.9) | 21.5 (13.2) | 0.02 |
| 2 year follow-up | 21.1 (13.9) | 16.4 (11.6) | 18.8 (12.8) | 0.002 |
| 5 year follow-up | 20.7 (10.3) | 18.2 (8.8) | 20.7 (10.3) | <0.01 |
| Allostatic load scoreh | ||||
| 2 year follow-up | 3.0 (1.5) | 4.5 (1.5) | 6.1 (1.4) | <0.0001 |
| 5 year follow-up | 3.5 (1.6) | 5.0 (1.6) | 6.2 (1.5) | <0.0001 |
Values shown as mean (s.d.) for continuous variables or percentages for categorical variables.
Ratio of total household income to appropriate and year-specific federal poverty threshold. Scores categorized above/below 120%.
Sum of hours spent in typical activities over a 24-h period, multiplied by weight factors associated with oxygen consumption for each activity. Scores categorized as light or sedentary (<30), moderate (30–40), or heavy (>40).
Assessed using 11 dietary components. Higher scores indicate higher diet quality (total score range from 0 to 110).
Measures the degree to which one’s life is viewed as stressful, ranging from 0 to 40, with higher values indicating higher perceived stress.
Higher scores (range 0–100) indicated greater acculturation (more use of English).
Assessed using the Center for Epidemiology Studies Depression (CESD) scale (range: 0–60).
Calculated from 11 parameters of biological functioning (range: 0–11).
Allostatic load across time
Mean AL score tended to increase from baseline to 5-year follow up. A higher percentage of participants were categorized with moderate or high AL at 5-year follow-up compared to baseline (Table 3). At 5-year follow-up compared to baseline, participants tended to have higher waist circumference, HDL-C, and epinephrine, and lower blood pressure and HbA1c, and were more likely to use antihypertension, antilipidemic, and anti-diabetic medications.
Table 3.
Allostatic load and individual parameters at three time points among participants in the Boston Puerto Rican Health Study (n = 620)a
| Baseline n = 620 | 2-year follow-up n = 543 | p valueb | 5-year follow-up n = 620 | p valuec | p valued | |
|---|---|---|---|---|---|---|
| Allostatic load score | 4.4 ± 1.9 | 4.7 ± 1.8 | 0.0001 | 5.1 ± 1.8 | <0.0001 | <0.0001 |
| Allostatic load categories | <0.0001 | <0.0001 | <0.0001 | |||
| Low (<3) | 16.6 | 12.2 | 7.6 | |||
| Moderate (3-5) | 53.4 | 52.9 | 48.2 | |||
| High (⩾6) | 30.0 | 35.0 | 44.2 | |||
| Parameters | ||||||
| Systolic blood pressure, mmHg | 135 ± 18.7 | 135 ± 18.8 | 0.81 | 133 ± 18.2 | 0.02 | 0.02 |
| Dysregulatede | 35.8 | 35.3 | <0.0001 | 30.7 | <0.0001 | <0.0001 |
| Diastolic blood pressure, mmHg | 81.6 ± 10.2 | 80.6 ± 10.4 | 0.003 | 75.0 ± 9.7 | <0.0001 | <0.0001 |
| Dysregulatede | 20.3 | 17.5 | <0.0001 | 6.3 | <0.0001 | <0.0001 |
| Waist circumference, cm | ||||||
| Male | 102 ± 12.3 | 103 ± 11.9 | 0.01 | 104 ± 15.0 | 0.07 | 0.002 |
| Female | 101 ± 14.7 | 103 ± 14.1 | <0.0001 | 103 ± 17.5 | 0.36 | 0.01 |
| Dysregulatede | 71.8 | 91.1 | <0.0001 | 77.4 | <0.0001 | <0.0001 |
| Total cholesterol, mg/dL | 185 ± 39.6 | 188 ± 42.2 | 0.04 | 182 ± 41.2 | 0.0002 | 0.08 |
| Dysregulatede | 7.4 | 10.0 | <0.0001 | 9.8 | <0.0001 | <0.0001 |
| High density lipoprotein, mg/dL | 44.4 ± 11.5 | 46.3 ± 12.0 | <0.0001 | 46.2 ± 12.0 | 0.96 | <0.0001 |
| Dysregulatede | 34.8 | 29.7 | <0.0001 | 31.1 | <0.0001 | <0.0001 |
| Glycated hemoglobin, % | 6.9 ± 1.7 | 6.8 ± 1.5 | 0.01 | 6.6 ± 1.4 | 0.002 | <0.0001 |
| Dysregulatede | 36.0 | 38.3 | <0.0001 | 41.9 | <0.0001 | <0.0001 |
| Urinary cortisol, μg/g | ||||||
| Male | 35.5 ± 29.6 | 42.2 ± 35.8 | 0.07 | 38.6 ± 24.0 | 0.21 | 0.21 |
| Female | 30.6 ± 27.4 | 34.5 ± 23.6 | 0.02 | 44.1 ± 101 | 0.06 | 0.01 |
| Dysregulatede | 16.6 | 24.7 | <0.0001 | 26.6 | 0.0002 | <0.01 |
| Urinary epinephrine, μg/g | ||||||
| Male | 3.9 ± 3.4 | 4.3 ± 3.7 | 0.29 | 6.0 ± 5.0 | <0.0001 | <0.0001 |
| Female | 3.7 ± 3.4 | 4.2 ± 3.3 | 0.01 | 6.2 ± 5.7 | <0.0001 | <0.0001 |
| Dysregulatede | 42.6 | 52.3 | 0.01 | 66.8 | 0.001 | 0.001 |
| Urinary norepinephrine, μg/g | ||||||
| Male | 36.1 ± 27.7 | 34.8 ± 27.7 | 0.48 | 39.7 ± 28.8 | 0.07 | 0.18 |
| Female | 40.4 ± 28.9 | 44.8 ± 41.6 | 0.09 | 46.4 ± 34.1 | 0.57 | 0.001 |
| Dysregulatede | 33.4 | 36.9 | <0.0001 | 42.3 | <0.0001 | <0.0001 |
| Serum DHEAS, ng/mL | ||||||
| Male | 1,215 ± 801 | 1,237 ± 861 | 0.35 | 1,132 ± 888 | 0.003 | 0.06 |
| Female | 737 ± 493 | 718 ± 504 | 0.11 | 640 ± 480 | <0.0001 | <0.0001 |
| Dysregulatede | 22.7 | 25.1 | <0.0001 | 31.3 | <0.0001 | <0.0001 |
| C-reactive protein, mg/L | 6.0 ± 8.7 | 5.7 ± 10.1 | 0.41 | 6.3 ± 8.6 | 0.04 | 0.40 |
| Dysregulatede | 55.5 | 50.4 | <0.0001 | 54.5 | <0.0001 | <0.0001 |
| Medication use | ||||||
| Anti-hypertension | 54.7 | 60.4 | <0.0001 | 66.9 | <0.0001 | <0.0001 |
| Antilipemic | 42.7 | 50.1 | <0.0001 | 57.3 | <0.0001 | <0.0001 |
| Anti-diabetic | 29.0 | 32.8 | <0.0001 | 37.7 | <0.0001 | <0.0001 |
| Androgen | 0.2 | 0.2 | 1.0 | 0 | – | – |
Values shown as mean (s.d.) for continuous variables or percentages for categorical variables.
Comparison between baseline and 2-year.
Comparison between 2-year and 5-year.
Comparison between baseline and 5-year.
Cut-offs for dysregulated components: waist circumference (men: >102 cm; women: >88 cm), HbA1c (>7%), systolic blood pressure (>140 mmHg), diastolic blood pressure (>90 mmHg), HDL-C (<40 mg/dl), total cholesterol (⩾240 mg/dl), DHEA-S (men: ⩾589.5 ng/ml; women: ⩾368.5 ng/ml), cortisol (men: ⩾41.5 μg/g creatine; women: ⩾49.5 μg/g creatine), epinephrine (men: ⩾2.8 μg/g creatine; women: ⩾3.6 μg/g creatine), norepinephrine (men: ⩾30.5 μg/g creatine; women: ⩾46.9 μg/g creatine), CRP (>3 mg/l).
Multilevel mixed models
Baseline depressive symptoms and 5-year AL
Having baseline subthreshold, compared to low, depressive symptoms were significantly associated with higher 5-year AL score, in minimally-adjusted models (online Supplementary Table S1). In fully-adjusted models, baseline subthreshold depressive symptoms remained associated with higher 5-year AL score [mean (95% CI): 4.8 (4.5–5.2)], compared with low depressive symptoms [4.3 (3.9–4.7)] (Fig. 1). Depression-likely symptoms were not significantly associated with higher 5-year AL score, [4.5 (4.2–4.9)]. Having baseline subthreshold, depressive symptoms were associated with higher SNS [mean (95% CI): 1.0 (0.8–1.1); p < 0.05] and metabolic scores [1.2 (1.0–1.3); p < 0.05] compared to low depressive symptoms [SNS: 0.8 (0.7–0.9)]; metabolic: 1.0 (0.8–1.1) (online Supplementary Table S2). No other baseline depressive symptom categories were associated with AL subsystem scores. In sensitivity analyses using the cutoff of ⩾20, subthreshold depressive symptoms remained associated with 5-year AL score [mean (95% CI): 4.8 (4.5–5.1); p < 0.05] compared to low depressive symptoms [4.3 (3.9–4.7)], but depression-likely symptoms were not associated with 5-year AL score [mean (95% CI): 4.5 (4.1–4.8)] (online Supplementary Table S3).
Fig. 1.
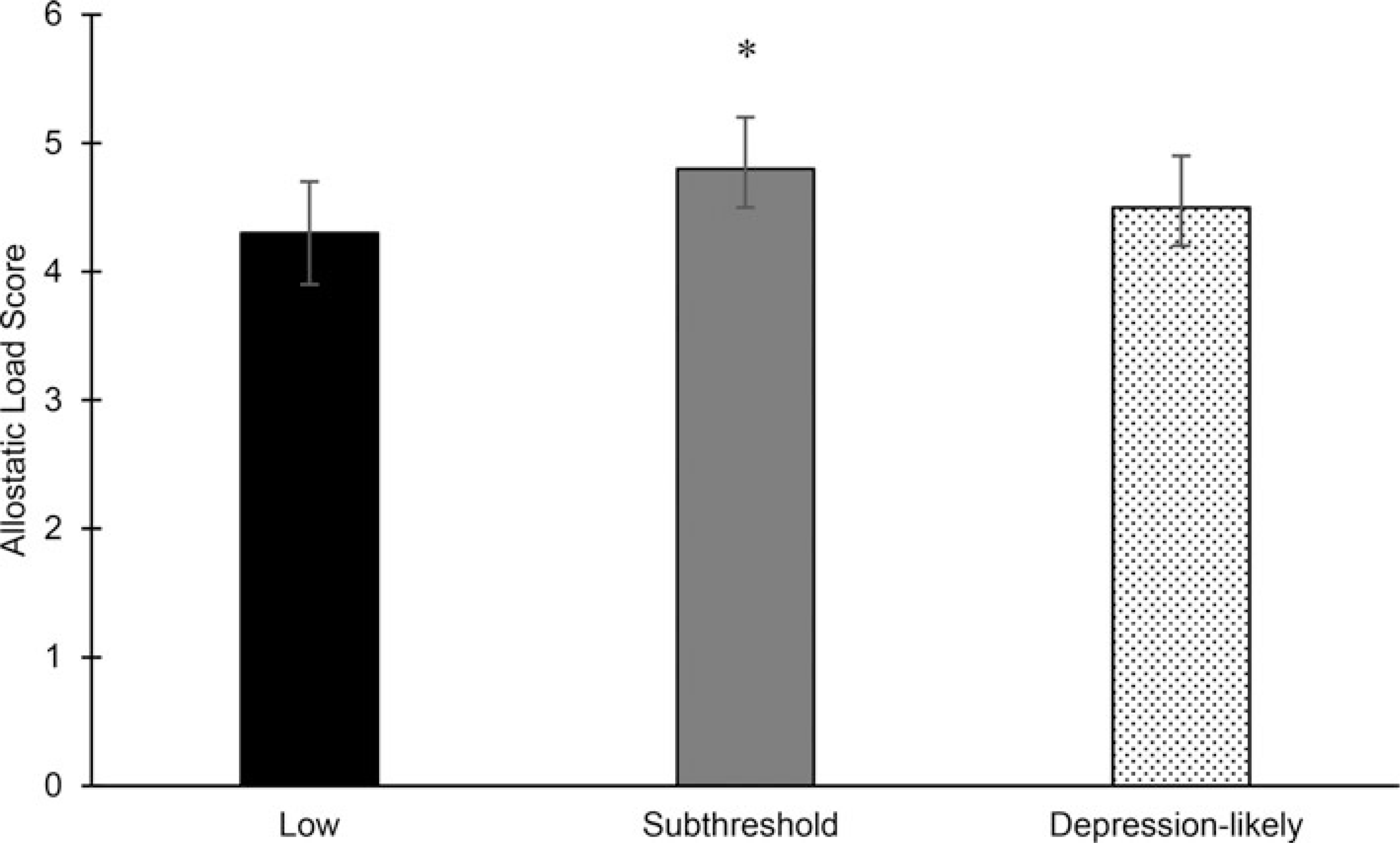
Adjusted mean (95%CI) (Adjusted for baseline and Year 2 allostatic load, age, sex, and baseline 120% of income-to-poverty ratio, education, physical activity, Alternate Healthy Eating Index-2010, smoking, alcohol use, acculturation, and perceived stress. Tukey’s test adjusted for multiple comparisons.) allostatic load score at 5-year follow-up by baseline depressive symptom (Assessed using the Center for Epidemiology Studies Depression (CES-D) scale (range: 0–60). Scores categorized as low (<8), subthreshold (8–15), and depression-likely (⩾16) symptoms.) category in the Boston Puerto Rican Health Study (n = 620). *Significantly different than low category at p < 0.05.
Number of times with depressive symptoms and 5-year AL
Each additional timepoint with any type of depressive symptoms (subthreshold or depression-likely), compared to low depressive symptoms, was associated with a 0.2 (0.1) increase in AL from baseline to 5-year follow-up ( p = 0.047). The number of times classified with depression-likely symptoms, compared to low or subthreshold depressive symptoms, from baseline to year 5-year follow-up was not associated with 5-year AL score [β(S.E.): 0.1 (0.1), p = 0.39].
Baseline AL and 5-year depressive symptoms
Baseline high, compared with moderate, AL was significantly associated with higher 5-year depressive symptom score in minimally-adjusted models (online Supplementary Table S4). In fully-adjusted models, baseline high AL remained associated with higher 5-year depressive symptoms [mean (95% CI): 19.4 (17.6–21.2)], compared to baseline moderate AL [mean (95% CI): 16.9 (15.3–18.5)], but low AL at baselined was not associated with 5-year depressive symptoms [mean (95% CI):18.5 (16.5–20.6)] (Fig. 2).
Fig. 2.
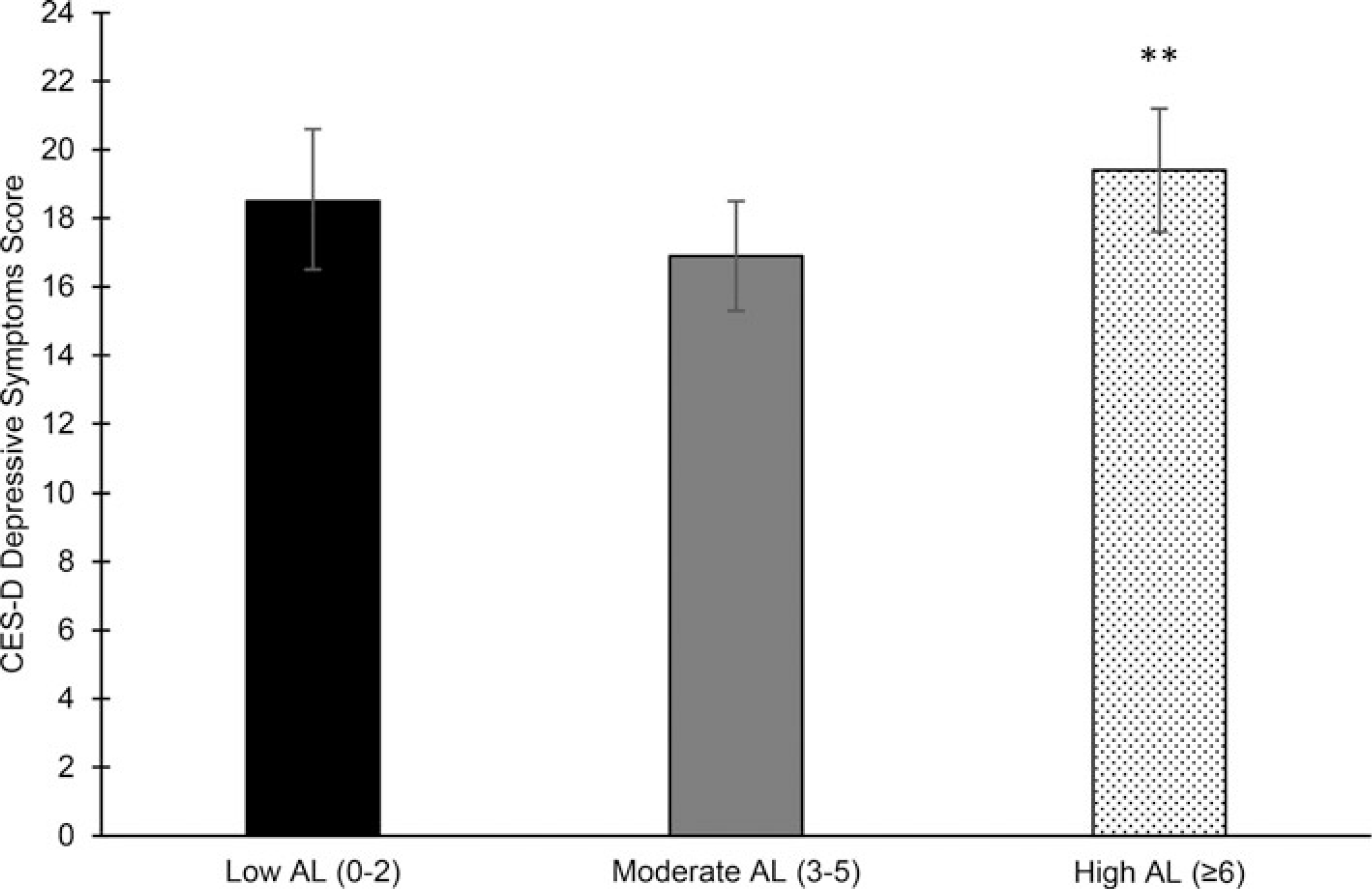
Adjusted mean (95% CI) (Adjusted for baseline and Year 2 depressive symptoms, age, sex, and baseline 120% of income-to-poverty ratio, education, physical activity, Alternate Healthy Eating Index-2010, smoking, alcohol use, acculturation, and perceived stress. Tukey’s test adjusted for multiple comparisons.) Center for Epidemiology Studies Depression (CES-D) depressive symptoms at 5-year follow-up by baseline allostatic load category (Calculated from 11 parameters of biological functioning (range: 0–11). Scores categorized as low (<3), moderate (3–5), or high (⩾6).) in the Boston Puerto Rican Health Study (n = 620). **Significantly different than Moderate category at p < 0.01.
Number of times with high AL and 5-year depressive symptoms
Each additional timepoint with high, compared to low or moderate, AL was associated with a 0.5 point higher depressive symptom score ( p = 0.04).
Discussion
In this sample of older mainland US Puerto Rican adults, we found a bidirectional relationship between AL and depressive symptoms. Subthreshold depressive, compared to low, symptoms at baseline were associated with higher mean AL over 5-year, which may be functioning through the SNS and metabolic system. High, compared to moderate, AL at baseline was associated with higher mean depressive symptoms over 5-year. Furthermore, an increasing number of times with subthreshold or depression-likely symptoms (or high AL) over 5-year was associated with increases in AL (or CES-D) score over 5-year. The bidirectional relationship suggests that low-income and minority populations may be particularly sensitive, as experiences of social inequity can get ‘under the skin’ (APA Working Group on Stress and Health Disparities, 2017).
Existing evidence for the AL–depression relationship is mixed. Juster et al. (2011) found a cross-sectional, but not longitudinal, association between AL and depression, suggesting an acute response. Age was a stronger predictor of depressive symptoms over time than AL in their sample (Juster et al., 2011). However, compared to our analyses, Juster et al. (2011) had fewer AL markers, with only one marker for the SNS (cortisol) and none for inflammation, perhaps limiting their scope for assessing multi-system dysregulation. Their sample was also small (n = 58), older (median: 67.6 years), predominantly non-Hispanic white, with low baseline depression levels (none taking medication), and mean AL (2.2) (Juster et al., 2011), which is in stark comparison to our sample of minority adults (60.6% with depression-likely symptoms and mean AL of 4.5 at baseline). Similar to our findings, Kobrosly et al. (2014) demonstrated in a cross-sectional, predominately non-Hispanic white, well-educated older adult sample that AL was independently associated with overall, affective, and somatic depressive symptom scores (Kobrosly et al., 2014). Their sample had low AL (mean: 1.7) and depressive symptoms (mean: 7.9) but, like our study, their AL score included markers of the HPA-axis, SNS, inflammation, metabolic, and cardiovascular systems and they assessed depressive symptoms using the 20-item CES-D. Other comparable studies have employed scores of multi-system physiological dysregulation, instead of AL, using data from the National Health and Nutrition Examination Survey (NHANES). Multi-system physiological dysregulation was not independently associated with depressive disorder among middle-aged to older adults in any racial/ethnic group (Rodriquez et al., 2018). Although Hispanics/Latinos and non-Hispanic blacks reported higher levels of depression than non-Hispanic whites, physiological dysregulation did not appear to explain these differences, perhaps due to the cross-sectional design or the lack of neuroendocrine biomarkers, important mediators in the stress response which are often dysregulated prior to cardiometabolic markers. However, another NHANES study found that multi-system physiological dysregulation was associated with overall, affective, and somatic depressive symptoms when the sample was restricted to adults aged ⩾60 years (Kobrosly, Seplaki, Cory-Slechta, Moynihan, & van Wijngaarden, 2013). The contrasting findings from Rodriquez et al. (2018) and Kobrosly et al. (2013) may be attributed to the age differences between the two samples, as depressive symptoms are more common among older adults (World Health Organization, 2017), or to the way that depressive symptoms were conceptualized; Rodriquez et al. (2018) used a cutoff for being at risk for depressive disorder, whereas Kobrosly et al. (2013) used a continuous score for depressive symptoms and scores for affective v. somatic symptoms. The culmination of evidence across these studies and ours suggests that older age and minority group membership may concurrently exacerbate the AL-depression link, especially when considering dysregulation of a wider range of physiological systems and various ways to conceptualize depression. Older adults and minority populations have a high prevalence of cardiometabolic conditions (World Health Organization, 2017), and minorities like Hispanics/Latinos are exposed to a greater number of social stressors (APA Working Group on Stress and Health Disparities, 2017).
Our findings for baseline depressive symptoms and 5-year AL are noteworthy. Most hypothesized pathways suggest that AL leads to depression, but potential pathways also exist in reverse. Depression initially leads to amygdala hyperactivity (Juster et al., 2011; McEwen, 2003a). Repeated stress may up-regulate hormone and hormone receptors in the basolateral amygdala (Choi et al., 2015), including glucocorticoid concentration (McEwen, 2003a), which facilitates memory of adverse events, further triggering amygdala reactivity (McEwen, 2012). Eventually, the amygdala, hippocampus, and prefrontal cortex undergo structural changes, resulting in cognitive impairment and increased aggression, anxiety, and fear (McEwen, 2003a). Additionally, chronic perceived or actual threats cause the body to develop resistance to glucocorticoid hormones (Slavich & Irwin, 2014), dysregulating the HPA-axis (Juster et al., 2011; McEwen, 2003a), and subsequently up-regulating the proinflammatory immune response (Leonard & Myint, 2009). Proinflammatory cytokines communicate with the brain to influence mood, cognition, and behavior, aspects foundational to depression (Leonard & Myint, 2009), and increase the risk of inflammation-related disease and viral infection (McEwen, 2003b). These same stress responses are observed during the depression (McEwen, 2003a; Ogłodek et al., 2014), which may help explain why diseases with an inflammatory basis (e.g. metabolic syndrome, coronary heart disease, rheumatoid arthritis) often co-occur with depression (Slavich & Irwin, 2014), and provide evidence to support our findings. Long-term dysregulation of these hormones is associated with heightened blood platelet reactivity and cardiovascular disease risk among those with major depression (McEwen, 2003a). Similarly, recurrent, but not single, episodes of major depression have been associated with greater 5-year risk of cardiovascular disease and diabetes (Windle & Windle, 2013). Subthreshold depression is prevalent among those with cardiac illness and may increase the risk of cardiovascular disease mortality (Iqbal & Iqbal, 2019). Subthreshold depressive symptoms may have exacerbated cardiovascular markers in our older adult population, although we observed more 5-year dysregulation in metabolic markers. Higher baseline depressive symptoms have been associated with longitudinal increases in BMI and waist circumference, and these relationships were not bidirectional (Needham, Epel, Adler, & Kiefe, 2010; van Reedt Dortland, Giltay, van Veen, Zitman, & Penninx, 2013). However, research has shown bidirectional relationships between depressive symptoms and glucose metabolism (Roy & Lloyd, 2012), which are posited to function through the HPA-axis, immune, and inflammatory mediators (Demakakos, Zaninotto, & Nouwen, 2014; Stuart & Baune, 2012). CRP, interleukin (IL)-1, IL-6 (Howren, Lamkin, & Suls, 2009), and nocturnal cortisol, but not DHEA (Hartaigh et al., 2012) may also have bi-directional relationships with depression. We found differences in 5-year SNS, but not inflammation, marker changes by baseline depressive symptoms, which may have led to differences in metabolic marker changes. Co-morbid depression and diabetes are common (Ducat, Philipson, & Anderson, 2014). Longitudinal evidence suggests that depression may be associated with type 2 (Deleskog et al., 2019) and gestational (Hinkle et al., 2016) diabetes development, although the direction of the association is not fully established (Roy & Lloyd, 2012).
Depressive symptomatology in our sample was high and persistent, fitting the model of ‘repeated stressors’ (Slavich & Irwin, 2014). Previous research in this cohort demonstrated that Puerto Ricans may have stressful social ties (Falcon, Todorova, & Tucker, 2009). Social stressors, like interpersonal loss, social rejection (Slavich & Irwin, 2014), and negative family interactions (Priest et al., 2015) are strongly related to depression initiation, to inflammation (Slavich & Irwin, 2014), and to greater subjective and objective biobehavioral reactivity (anxiety/depression and AL) (Priest et al., 2015). Furthermore, stressful relationships are often exacerbated by environmental conditions like low socioeconomic or social status (Slavich & Irwin, 2014), of which affected the majority of our sample. Inflammation may be the link connecting existing cardiometabolic conditions, like metabolic syndrome (Chan, Cathomas, & Russo, 2019), and social stress exposure (Finnell & Wood, 2018) to major depressive disorder. Hypothesized ways this occurs through certain inflammatory factors (e.g. IL-6, TNF) crossing the blood-brain barrier and pro-inflammatory cytokines degrading the integrity of the blood-brain barrier (Finnell & Wood, 2018).
Our findings of increased AL are with subthreshold depressive, but not depression-likely, symptoms may have occurred for several reasons. Persons with subthreshold depression may be less likely to be prescribed medication and/or given appropriate mental health support (Tuithof et al., 2018), compared to persons with major depression (Xiang et al., 2018). At baseline, our participants with depression-likely symptoms were more likely to report taking depression medication. Higher health care utilization by persons with major depression (Luppa et al., 2012) may also indicate that they, compared to those with subthreshold depression, may be more likely to be screened and treated for co-morbid health conditions. Additionally, people with depression may have poorer lifestyle behaviors (Kendzor et al., 2014; Walsh, Senn, & Carey, 2013), poorer quality of life (Sivertsen, Bjørkløf, Engedal, Selbæk, & Helvik, 2015), greater cognitive impairment (Clarke, Skoufalos, Medalia, & Fendrick, 2016), or be less likely to adhere to medications to manage their other chronic conditions (Dirmaier et al., 2010), underscoring the complexity of the depression–AL relationship.
This study has several limitations. First, the CES-D may reflect temporary symptoms rather than clinically-diagnosed depression. However, symptoms can capture perceived feelings which may not otherwise be ascertained in a population, like Puerto Ricans, where depression is often undiagnosed and diagnosis is stigmatized (Lopez, Sanchez, Killian, & Eghaneyan, 2018; Vega, Rodriguez, & Ang, 2010). Our analyses may not capture all ideal AL parameters, as a standardized AL definition does not exist; however, we used biomarkers representing multiple systems, including primary biomarkers (i.e. neuroendocrine) which is strongly recommended (Gallo, Fortmann, & Mattei, 2014). Likewise, primary AL biomarkers have acute responses to external stimuli and changes in circadian rhythms, thus, our single-point assessment may limit their accuracy (Gallo et al., 2014). Additionally, we only had one inflammation marker, CRP, and IL-6 may be more correlated with depression (Bremmer et al., 2008). While our study findings provide a good representation of middle-aged and older Puerto Rican adults in the USA, which may be applicable to similar Hispanic/Latino groups, our bidirectional findings should be confirmed in other populations. Likewise, our small proportion of males in the cohort limited our ability to test sex-specific differences in the AL-depressive symptom relationship, which could provide additional insight for screening and treatment.
Our study has several notable strengths. First, we modeled the bidirectional relationships of depressive symptoms and AL using longitudinal data, which allowed us to investigate the effect of the exposure at baseline with the outcome over 5 years. Most previous studies have used cross-sectional data, limiting causal inference. Longitudinal data also permitted the use of multi-level, random intercept and slope models, accounting for both interindividual and intraindividual variation in outcomes. Depressive symptoms were assessed using the CES-D, a valid and reliable tool among Hispanics/Latinos (Moscicki et al., 1989; Radloff, 1977; Tucker et al., 2000b). Our AL score included neuroendocrine and inflammation biomarkers, capturing upstream, in addition to downstream, dysregulation. We also adjusted for most health behaviors in our models, important confounders (Duivis et al., 2011; Forrester et al., 2019; Mattei et al., 2013).
Through the AL framework, this study provides new insights into bidirectional pathways connecting the commonly comorbid conditions of depression and chronic disease, while underscoring the underestimated consequence of subthreshold depressive symptoms on allostasis. Improved and expanded depressive symptom screenings in clinical and public health settings would cast a wider net to better capture marginalized individuals that need treatment, regardless of existing chronic diseases. Simultaneously, promoting greater access to subthreshold and major depression diagnosis and treatment may provide primordial or primary prevention of chronic diseases, and may connect individuals with existing chronic disease to necessary resources and support to help them reduce their risk of depression. Future research should explore practical ways for clinicians and practitioners to expand depressive symptom screenings and should test the effects of depression treatments on AL and the effects of behavioral interventions for chronic disease on depressive symptoms. Likewise, future investigations into potential moderating factors, such as socioeconomic status, acculturation, and health behaviors, may help identify individuals at highest risk of depression or AL.
Supplementary Material
Acknowledgements.
The Boston Puerto Rican Health Study was supported by the National Institutes of Health (NIH)-National Institute on Aging (P01-AG023394) and by the US Department of Agriculture, Agriculture Research Institute (58–1950–7-707). Dr Amanda C. McClain received support for this study from a NIH-National Heart Lung and Blood (NHLBI) Mentored Research Scientist Development Award (K01-HL150406). Dr Josiemer Mattei received support for this study from a NIH-NHLBI Mentored Career Development Award to Promote Faculty Diversity (K01-HL120951).
Footnotes
Conflicts of interest. None.
Ethical standards. ‘The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.’ and ‘The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.’
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720005139.
References
- APA Working Group on Stress and Health Disparities. (2017). Stress and health disparities report: Contexts, mechanisms, and interventions among racial/ethnic minority and low socioeconomic status populations (pp. 1–66). Washington, D.C: American Psychological Association. [Google Scholar]
- Aziz R, & Steffens DC (2013). What are the causes of late-life depression? The Psychiatric Clinics of North America, 36(4), 497–516. 10.1016/j.psc.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, & Hamilton P (2016). An active inference theory of allostasis and interoception in depression. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160011. 10.1098/rstb.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton T, Staab J, & Evans D (2007). Medical co-morbidity in depressive disorders. Annals of Clinical Psychiatry, 19(4), 289–303. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman ATF, Deeg DJH, Penninx BWJH, Dik MG, Hack CE, & Hoogendijk WJG (2008). Inflammatory markers in late-life depression: Results from a population-based study. Journal of Affective Disorders, 106(3), 249–255. 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Chan KL, Cathomas F, & Russo SJ (2019). Central and peripheral inflammation link metabolic syndrome and major depressive disorder. Physiology, 34(2), 123–133. 10.1152/physiol.00047.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Quan H, Bi-Rong D, Zhen-Chan L, Ji-Rong Y, & Qing-Xiu L (2010a). Chronic diseases and risk for depression in old age: A meta-analysis of published literature. Microbes and Ageing, 9(2), 131–141. 10.1016/j.arr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Chang-Quan H, Xue-Mei Z, Bi-Rong D, Zhen-Chan L, Ji-Rong Y, & Qing-Xiu L (2010b). Health status and risk for depression among the elderly: A meta-analysis of published literature. Age and Ageing, 39(1), 23–30. 10.1093/ageing/afp187. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Perry GS, & Strine TW (2005). The vital link between chronic disease and depressive disorders. Preventing Chronic Disease, i(1), A14. [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, … Willett WC (2012). Alternative dietary indices both strongly predict risk of chronic disease. The Journal of Nutrition, 142(6), 1009–1018. 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kim J, Kim T-K, Park J-Y, Lee J-E, Kim H, … Han P-L (2015). TRH And TRH receptor system in the basolateral amygdala mediate stress-induced depression-like behaviors. Neuropharmacology, 97, 346–356. 10.1016/j.neuropharm.2015.03.030. [DOI] [PubMed] [Google Scholar]
- Clarke JL, Skoufalos A, Medalia A, & Fendrick AM (2016). Improving health outcomes for patients with depression: A population health imperative. Report on an expert panel meeting. Population Health Management, 19 (Suppl 2), S–1. 10.1089/pop.2016.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cohen CI, Magai C, Yaffee R, & Walcott-Brown L (2005). Racial differences in syndromal and subsyndromal depression in an older urban population. Psychiatric Services, 56(12), 1556–1563. 10.1176/appi.ps.56.12.1556. [DOI] [PubMed] [Google Scholar]
- Crews DE (2007). Composite estimates of physiological stress, age, and diabetes in American Samoans. American Journal of Physical Anthropology, 133(3), 1028–1034. 10.1002/ajpa.20612. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, … Stamler J (2012). Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. The Journal of the American Medical Association, 308(17), 1775–1784. 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, & Ngiam J (2015). The cortisol awakening response and major depression: Examining the evidence. Neuropsychiatric Disease and Treatment, 11, 1181–1189. 10.2147/NDT.S62289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleskog A, Ljung R, Forsell Y, Nevriana A, Almas A, & Möller J (2019). Severity of depression, anxious distress and the risk of type 2 diabetes – a population-based cohort study in Sweden. BMC Public Health, 19(1), 1174. 10.1186/s12889-019-7322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakakos P, Zaninotto P, & Nouwen A (2014). Is the association between depressive symptoms and glucose metabolism bidirectional? Evidence from the English Longitudinal Study of Ageing. Psychosomatic Medicine, 76(7), 555–561. 10.1097/PSY.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirmaier J, Watzke B, Koch U, Schulz H, Lehnert H, Pieper L, & Wittchen H-U (2010). Diabetes in primary care: Prospective associations between depression, nonadherence and glycemic control. Psychotherapy and Psychosomatics, 79(3), 172–178. 10.1159/000296135. [DOI] [PubMed] [Google Scholar]
- Ducat L, Philipson LH, & Anderson BJ (2014). The mental health comorbidities of diabetes. The Journal of the American Medical Association, 312(7), 691–692. 10.1001/jama.2014.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, & Whooley MA (2011). Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: Prospective findings from the heart and soul study. American Journal of Psychiatry, 168(9), 913–920. 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- Falcon LM, Todorova I, & Tucker K (2009). Social support, life events, and psychological distress among the Puerto Rican population in the Boston area of the United States. Aging & Mental Health, 13(6), 863–873. 10.1080/13607860903046552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JE, & Wood SK (2018). Putative inflammatory sensitive mechanisms underlying risk or resilience to social stress. Frontiers in Behavioral Neuroscience, 12, 240–240. 10.3389/fnbeh.2018.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SN, Leoutsakos J-M, Gallo JJ, Thorpe RJ, & Seeman TE (2019). Association between allostatic load and health behaviours: A latent class approach. Journal of Epidemiology and Community Health, 73(4), 340–345. 10.1136/jech-2018-211289. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Fortmann AL, & Mattei J (2014). Allostatic load and the assessment of cumulative biological risk in biobehavioral medicine: Challenges and opportunities. Psychosomatic Medicine, 76(7), 478–480. 10.1097/PSY.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Weinstein M, Cornman J, Singer B, Seeman T, Goldman N, & Chang M-C (2004). Sex differentials in biological risk factors for chronic disease: Estimates from population-based surveys. Journal of Women’s Health, 13(4), 393–403. 10.1089/154099904323087088. [DOI] [PubMed] [Google Scholar]
- Gothe F, Enache D, Wahlund LO, Winblad B, Crisby M, Lokk J, & Aarsland D (2012). Cerebrovascular diseases and depression: Epidemiology, mechanisms and treatment. Panminerva Medica, 54(3), 161–170. [PubMed] [Google Scholar]
- Hartaigh BÓ, Loerbroks A, Thomas GN, Engeland CG, Hollands MA, Fischer JE, & Bosch JA (2012). Age-dependent and -independent associations between depression, anxiety, DHEAS, and cortisol: From the MIPH Industrial Cohort Studies (MICS). Psychoneuroendocrinology, 37 (7), 929–936. 10.1016/j.psyneuen.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Hinkle SN, Buck Louis GM, Rawal S, Zhu Y, Albert PS, & Zhang C (2016). A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia, 59(12), 2594–2602. 10.1007/s00125-016-4086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren M, Lamkin D, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine, 71(2), 171–186. 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Iqbal MN, & Iqbal F (2019). Subthreshold depression and its association with cardiovascular risk. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists, 31(2), 130–136. [PubMed] [Google Scholar]
- Juster R-P, Marin M-F, Sindi S, Nair NPV, Ng YK, Pruessner JC, & Lupien SJ (2011). Allostatic load associations to acute, 3-year and 6-year prospective depressive symptoms in healthy older adults. Physiology & Behavior, 104(2), 360–364. 10.1016/j.physbeh.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Katon WJ (2011). Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues in Clinical Neuroscience, 13(1), 7–23. 10.31887/dcns.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzor DE, Chen M, Reininger BM, Businelle MS, Stewart DW, Fisher-Hoch SP, & …McCormick JB (2014). The association of depression and anxiety with glycemic control among Mexican Americans with diabetes living near the U.S.-Mexico border. BMC Public Health, 14, 176–176. 10.1186/1471-2458-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M (2014). Racial/ethnic disparities in depression and Its theoretical perspectives. Psychiatric Quarterly, 85(1), 1–8. 10.1007/s11126-013-9265-3. [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Seplaki CL, Cory-Slechta DA, Moynihan J, & van Wijngaarden E (2013). Multisystem physiological dysfunction is associated with depressive symptoms in a population-based sample of older adults. International Journal of Geriatric Psychiatry, 28(7), 718–727. 10.1002/gps.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, van Wijngaarden E, Seplaki CL, Cory-Slechta DA, & Moynihan J (2014). Depressive symptoms are associated with allostatic load among community-dwelling older adults. Physiology & Behavior, 123, 223–230. 10.1016/j.physbeh.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, & Myint A (2009). The psychoneuroimmunology of depression. Human Psychopharmacology: Clinical and Experimental, 24(3), 165–175. 10.1002/hup.1011. [DOI] [PubMed] [Google Scholar]
- Lopez V, Sanchez K, Killian MO, & Eghaneyan BH (2018). Depression screening and education: An examination of mental health literacy and stigma in a sample of hispanic women. BMC Public Health, 18(1), 646. 10.1186/s12889-018-5516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppa M, Sikorski C, Motzek T, Konnopka A, Konig H-H, & Riedel-Heller SG (2012). Health service utilization and costs of depressive symptoms in late life – a systematic review. Current Pharmaceutical Design, 18(36), 5936–5957. 10.2174/138161212803523572. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, & Zitman FG (2010). Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry, 67(3), 220–229. 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Marin G, & Gamba RJ (1996). A New measurement of acculturation for hispanics: The bidimensional acculturation scale for hispanics (BAS). Hispanic Journal of Behavioral Sciences, 18(3), 297–316. 10.1177/07399863960183002. [DOI] [Google Scholar]
- Mattei J, Bhupathiraju S, & Tucker KL (2013). Higher adherence to a diet score based on American heart association recommendations is associated with lower odds of allostatic load and metabolic syndrome in Puerto Rican adults. The Journal of Nutrition, 143(11), 1753–1759. 10.3945/jn.113.180141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei J, Demissie S, Falcon LM, Ordovas JM, & Tucker K (2010). Allostatic load is associated with chronic conditions in the Boston Puerto Rican health study. Social Science & Medicine, 70(12), 1988–1996. 10.1016/j.socscimed.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei J, Sotres-Alvarez D, Daviglus ML, Gallo LC, Gellman M, Hu FB, & …Kaplan RC (2016). Diet quality and its association with cardiometabolic risk factors vary by hispanic and latino ethnic background in the Hispanic Community Health Study/Study of Latinos. The Journal of Nutrition, 146(10), 2035–2044. 10.3945/jn.116.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998a). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338(3), 171–179. 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998b). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2003a). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. 10.1016/S0006-3223(03)00177-X. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2003b). Interacting mediators of allostasis and allostatic load: Towards an understanding of resilience in aging. Aging: Beneficial Effects on Patients From Recent Advances in Genetics, Neurobiology, and Physiology, 52(2), 10–16. 10.1016/S0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences, 109(2), 17180–17185. 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. 10.1001/archinte.1993.00410180039004. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, & Golden SH (2008). Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care, 31(12), 2383–2390. 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki EK, Locke BZ, Rae DS, & Boyd JH (1989). Depressive symptoms Among Mexican Americans: The Hispanic Health and Nutrition Examination Survey. American Journal of Epidemiology, 130(2), 348–360. 10.1093/oxfordjournals.aje.a115341. [DOI] [PubMed] [Google Scholar]
- Needham BL, Epel ES, Adler NE, & Kiefe C (2010). Trajectories of change in obesity and symptoms of depression: The CARDIA Study. American Journal of Public Health, 100(6), 1040–1046. 10.2105/AJPH.2009.172809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, & Goldschmidt-Clermont PJ (2012). Heartache and heartbreak – The link between depression and cardiovascular disease. Nature Reviews Cardiology, 9, 526–539. 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Vizcaino M, Ibarra JM, Balcazar H, Perez E, Flores L, & Anders RL (2015). Multimorbidity in a Mexican community: Secondary analysis of chronic illness and depression outcomes. International Journal of Nursing (New York, N.Y.), 2(1), 35–47. 10.15640/ijn.v2n1a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogłodek E, Szota A, Just M, Moś D, & Araszkiewicz A (2014). The role of the neuroendocrine and immune systems in the pathogenesis of depression. Pharmacological Reports, 66(5), 776–781. 10.1016/j.pharep.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, & Hu FB (2012). Bidirectional association between depression and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Diabetes Care, 35(5), 1171–1180. 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, … Vinicor F (2003). Markers of inflammation and cardiovascular disease. Circulation, 107(3), 499–511. 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Poole L, & Steptoe A (2018). Depressive symptoms predict incident chronic disease burden 10 years later: Findings from the English Longitudinal Study of Ageing (ELSA). Journal of Psychosomatic Research, 113, 30–36. 10.1016/j.jpsychores.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Pratt LA, & Brody DJ (2014). Depression in the U.S. Household Population, 2009–2012 (NCHS Data Brief No. 172). National Center for Health Statistics. [PubMed] [Google Scholar]
- Priest JB, Woods SB, Maier CA, Parker EO, Benoit JA, & Roush TR (2015). The biobehavioral family model: Close relationships and allostatic load. Social Science & Medicine, 142, 232–240. 10.1016/j.socscimed.2015.08.026. [DOI] [PubMed] [Google Scholar]
- Radloff L (1986). The use of the center for epidemiological studies – depression scale with older adults. Clinical Gerontologist: The Journal of Aging and Mental Health, 5(1–2), 119–136. 10.1300/J018v05n01_06. [DOI] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306. [DOI] [Google Scholar]
- Renn BN, Feliciano L, & Segal DL (2011). The bidirectional relationship of depression and diabetes: A systematic review. Clinical Psychology Review, 31(8), 1239–1246. 10.1016/j.cpr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Robison J, Gruman C, Gaztambide S, & Blank K (2002). Screening for depression in middle-aged and older Puerto Rican primary care patients. The Journals of Gerontology: Series A, 57(5), M308–M314. 10.1093/gerona/57.5.M308. [DOI] [PubMed] [Google Scholar]
- Rodriquez EJ, Livaudais-Toman J, Gregorich SE, Jackson JS, Nápoles AM, & Pérez-Stable EJ (2018). Relationships between allostatic load, unhealthy behaviors, and depressive disorder in U.S. Adults, 2005–2012 NHANES. Preventive Medicine, 110, 9–15. 10.1016/j.ypmed.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy T, & Lloyd CE (2012). Epidemiology of depression and diabetes: A systematic review. Journal of Affective Disorders, 142, S8–S21. 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- Salazar CR, Strizich G, Seeman TE, Isasi CR, Gallo LC, Avilés-Santa LM, … Kaplan RC (2016). Nativity differences in allostatic load by age, sex, and hispanic background from the hispanic community Health Study/Study of Latinos. SSM – Population Health, 2, 416–424. 10.1016/j.ssmph.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, … Aviles-Santa ML (2014). Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care, 37(8), 2233–2239. 10.2337/dc13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, & McEwen BS (1997). Price of adaptation – allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine, 157 (19), 2259–2268. 10.1001/archinte.1997.00440400111013. [DOI] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Glei D, & Weinstein M (2005). A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental Gerontology, 40(5), 438–449. 10.1016/j.exger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Sivertsen H, Bjørkløf GH, Engedal K, Selbæk G, & Helvik A-S (2015). Depression and quality of life in older persons: A review. Dementia and Geriatric Cognitive Disorders, 40(5–6), 311–339. 10.1159/000437299. [DOI] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D, & Giese-Davis J (2003). Depression and cancer: Mechanisms and disease progression. Biological Psychiatry, 54(3), 269–282. 10.1016/S0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, & Baune BT (2012). Depression and type 2 diabetes: Inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neuroscience & Biobehavioral Reviews, 36(1), 658–676. 10.1016/j.neubiorev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Tabák AG, Akbaraly TN, Batty GD, & Kivimäki M (2014). Depression and type 2 diabetes: A causal association? The Lancet Diabetes & Endocrinology, 2(3), 236–245. 10.1016/S2213-8587(13)70139-6. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Bermudez OI, & Castaneda C (2000a). Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. American Journal of Public Health, 90 (8), 1288–1293. 10.2105/AJPH.90.8.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Bianchi LA, Maras J, & Bermudez OI (1998). Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. American Journal of Epidemiology, 148(5), 507–518. 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Falcon LM, Bianchi LA, Cacho E, & Bermudez OI (2000b). Self-reported prevalence and health correlates of functional limitation among Massachusetts elderly Puerto Ricans, Dominicans, and non-Hispanic white neighborhood comparison group. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 55(2), M90–M97. 10.1093/gerona/55.2.M90. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, … Falcon LM (2010). The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: Challenges and opportunities. BMC Public Health, 10(1), 1–12. 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuithof M, ten Have M, van Dorsselaer S, Kleinjan M, Beekman A, & de Graaf R (2018). Course of subthreshold depression into a depressive disorder and its risk factors. Journal of Affective Disorders, 241, 206–215. 10.1016/j.jad.2018.08.010. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2016). How the census bureau measures poverty. U.S. Census Bureau. Washington, D.C. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html. [Google Scholar]
- Vahia IV, Meeks TW, Thompson WK, Depp CA, Zisook S, Allison M, & …Jeste DV (2010). Subthreshold depression and successful aging in older women. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 18(3), 212–220. 10.1097/JGP.0b013e3181b7f10e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reedt Dortland AKB, Giltay EJ, van Veen T, Zitman FG, & Penninx BWJH (2013). Longitudinal relationship of depressive and anxiety symptoms with dyslipidemia and abdominal obesity. Psychosomatic Medicine, 75(1), 83–89. 10.1097/PSY.0b013e318274d30f. [DOI] [PubMed] [Google Scholar]
- Vega WA, Rodriguez MA, & Ang A (2010). Addressing stigma of depression in Latino primary care patients. General Hospital Psychiatry, 32(2), 182–191. 10.1016/j.genhosppsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Villagut G, Forero CG, Barbaglia G, & Alonso J (2016). Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): A systematic review with meta-analysis. PLoS ONE, 11(5), e0155431. 10.1371/journal.pone.0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JL, Senn TE, & Carey MP (2013). Longitudinal associations between health behaviors and mental health in low-income adults. Translational Behavioral Medicine, 3(1), 104–113. 10.1007/s13142-012-0189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Arredondo EM, Cai J, Castaneda SF, Choca JP, Gallo LC, … Zee PC (2014). Depression, anxiety, antidepressant use, and cardiovascular disease among hispanic men and women of different national backgrounds: Results from the Hispanic Community Health Study/Study of Latinos. Annals of Epidemiology, 24(11), 822–830. 10.1016/j.annepidem.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, & Windle RC (2013). Recurrent depression, cardiovascular disease, and diabetes among middle-aged and older adult women. Journal of Affective Disorders, 150(3), 895–902. 10.1016/j.jad.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2017). Depression and other common mental disorders: Global health estimates. World Health Organization. Geneva, Switzerland. [Google Scholar]
- Xiang X, Leggett A, Himle JA, & Kales HC (2018). Major depression and subthreshold depression among older adults receiving home care. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 26(9), 939–949. 10.1016/j.jagp.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


