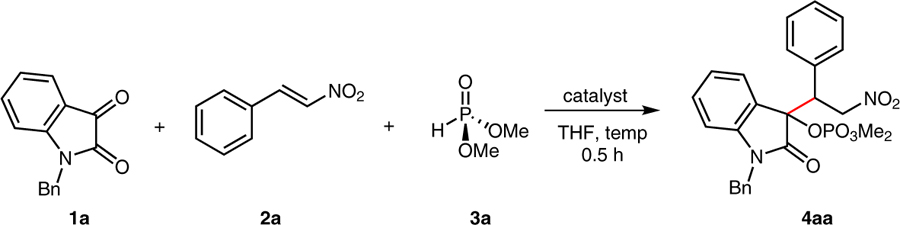
Abstract
A new reductive coupling reaction between N-alkylisatins, dimethyl phosphite, and nitrostyrenes has been developed. The reaction relies on Pudovik addition, subsequent phosphonate–phosphate rearrangement, and Michael-type addition of a transient carbanion on the indolinone with β-nitrostyrenes. This protocol introduces a convenient and versatile method for the construction of polyfunctionalized tertiary phosphates under mild conditions. Chiral general bases catalyze the title reaction with promising levels of enantioselectivity.
Keywords: reduction, phosphates, nucleophilic addition, rearrangement, Michael addition
Reductive C–C coupling reactions of two prochiral π-electrophiles offer attractive and straightforward methods for the synthesis of valuable building blocks in organic chemistry. Transformations of this type can be carried out under many different mechanistic manifolds, many of which rely on low-valent metals;1 however, the emergence of reactions that utilize homogeneous organic reductants offer considerable promise. In this respect, several reactions have been recently developed that rely upon organic phosphites to mediate the reductive coupling of C=O and C=N π-electrophiles. The reactions rely on a multistep three-component coupling mechanism comprised of base-catalyzed Pudovik–Abramov addition,2 [1,2]-phosphonate–phosphate rearrangement,3 and secondary electrophile capture by the nascent carbon nucleophile.4,5 Selectivity for the heterocoupled product is often obtained by using an α-dicarbonyl as the primary electrophile and aldehydes,4 imines,5 or Michael acceptors5a,6 as the secondary electrophile (Scheme 1). Especially germane to the title reaction are the reports of three-component couplings of α-dicarbonyls, diethyl phosphite, and nitrostyrenes or α,β-enones.5a,6 A virtue of these methodologies is the use of inexpensive dialkyl phosphite as the stoichiometric two-electron reductant. Here, we describe methodology for the phosphite-mediated three-component coupling of isatins, nitroolefins, and dimethyl phosphite to provide products of the reductive coupling of C=O and C=C π-electrophiles.
Scheme 1.
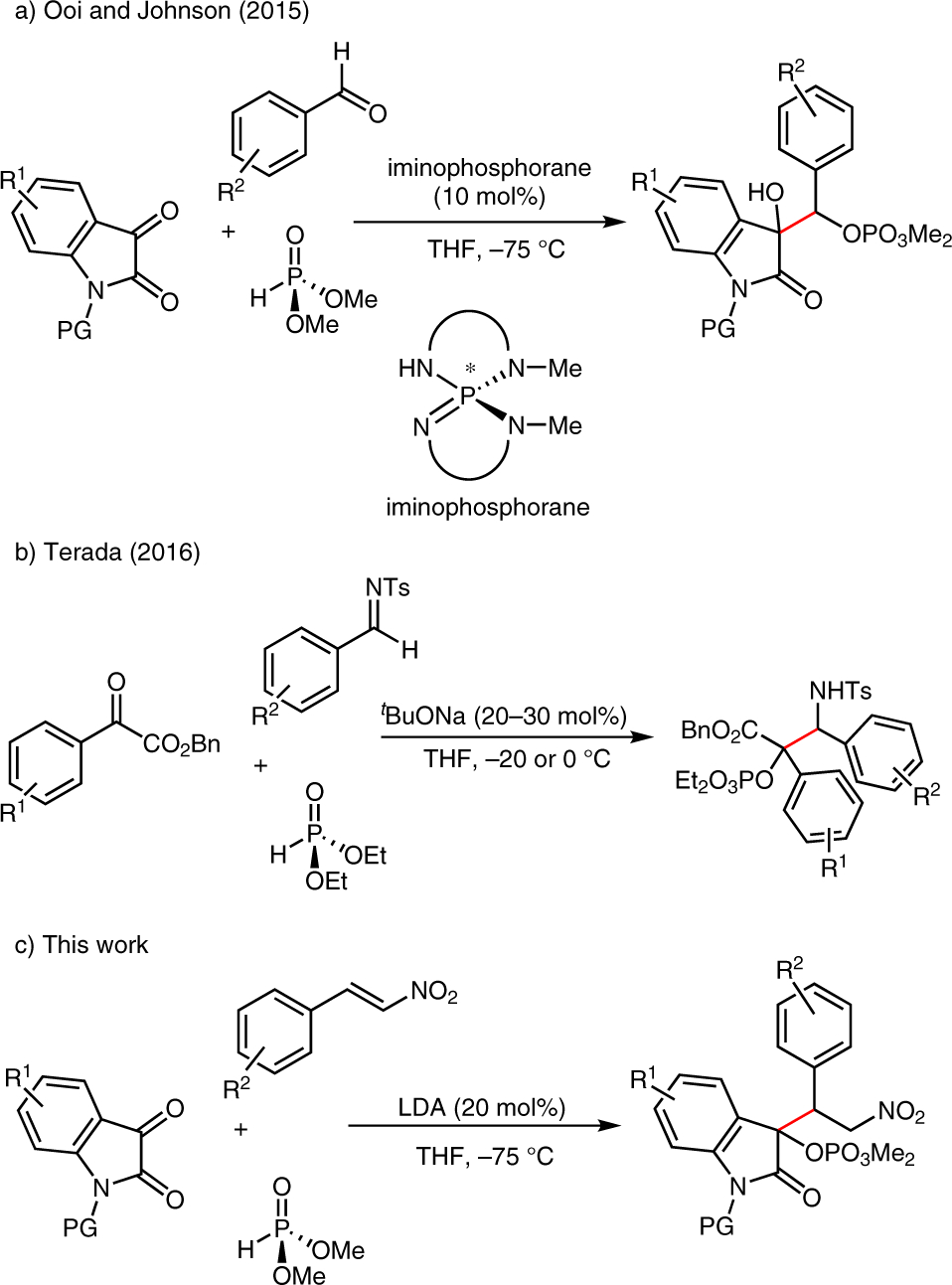
Reductive coupling reactions
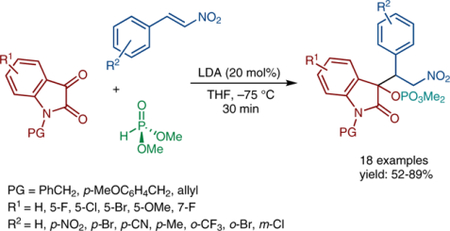
Initial efforts focused on the study of racemic variants of the three-component reductive Michael-type addition, with simple inorganic bases as promotors. N-Benzylisatin (1a), β-nitrostyrene (2a), and dimethyl phosphite (3a) were selected as model substrates for the transformation. A screen of commercial bases, including t-BuOK, sodium hexamethyldisilazide (NaHMDS), and potassium hexamethyldisilazide (KHMDS), in THF at −10 and −60 °C showed that KHMDS provided the best yield and diastereoselectivity (Table 1, entries 1–6). Decreasing the amount of β-nitrostyrene from 5 to 2 equivalents provided a significantly higher yield, albeit with slightly lower diastereoselectivity at −10 °C (entry 7). Varying the order of addition offered no improvement in yield (entry 8). A stoichiometric amount of LDA at −75 °C provided a moderate yield of desired product with lower diastereoselectivity in comparison with KHMDS (Table 1, entry 9); however, catalytic quantities of LDA generated near quantitative yields of 4aa (entries 10, 11). A study on the reaction temperature demonstrated that increasing the reaction temperature to −35 °C did not appreciably alter the diastereomeric ratio (entry 12). The use of chiral lithium amide derived from C17 (Figure 1) engendered similar levels of reaction efficiency but with negligible (<5% ee) enantiocontrol (Table 1, entry 13). Replacing dimethyl phosphite with diethyl phosphite gave the target product in lower yield, while the diastereomeric ratio did not change (entry 14).
Table 1.
Reaction Optimizationa
| Entry | 2a (equiv) | Catalyst (equiv) | Temp (°C) | Yield (%)b | drc |
|---|---|---|---|---|---|
| 1 | 5 | t-BuOK (1) | −10 | 69 | 1.8:1 |
| 2 | 5 | NaHMDS (1) | −10 | 73 | 1.5:1 |
| 3 | 5 | KHMDS (1) | −10 | 85 | 1.9:1 |
| 4 | 5 | t-BuOK (1) | −60 | 69 | 1.5:1 |
| 5 | 5 | NaHMDS (1) | −60 | 78 | 1.5:1 |
| 6 | 5 | KHMDS (1) | −60 | 78 | 1.6:1 |
| 7 | 2 | KHMDS (1) | −10 | 91 | 1.7:1 |
| 8d | 2 | KHMDS (1) | −10 | 76 | nd |
| 9e | 2 | LDA(1) | −75 | 55 | 1.2:1 |
| 10e | 2 | LDA (0.2) | −75 | 97 | 1.2:1 |
| 11e | 2 | LDA(0.1) | −75 | 94 | 1:1 |
| 12e | 2 | LDA (0.2) | −35 | 98 | 1.2:1 |
| 13e | 2 | n-BuLi (0.2)/C1 | −75 | 91 | 1.1:1 |
| 14f | 2 | LDA (0.2) | −75 | 67 | 1.2:1 |
Reaction conditions: N-benzylisatin (1a, 0.1 mmol), β-nitrostyrene (2a), dimethyl phosphite (3a, 0.11 mmol), 1,3,5-trimethoxybenzene as an internal standard (0.1 mmol), THF (1 mL), 30 min.
1H NMR yield versus internal standard.
Diastereomeric ratios were determined through the analysis of crude 1H NMR spectra; nd: not determined.
Order of addition: 1. N-benzylisatin, KHMDS, dimethyl phosphite (15 min); 2. β-nitrostyrene, 1,3,5-trimethoxybenzene.
Order of addition: 1. n-BuLi/DIPA (15 min); 2. N-benzylisatin, dimethyl phosphite (15 min); 3. β-nitrostyrene, 1,3,5-trimethoxybenzene.
Diethyl phosphite was used instead of dimethyl phosphite.
Figure 1.
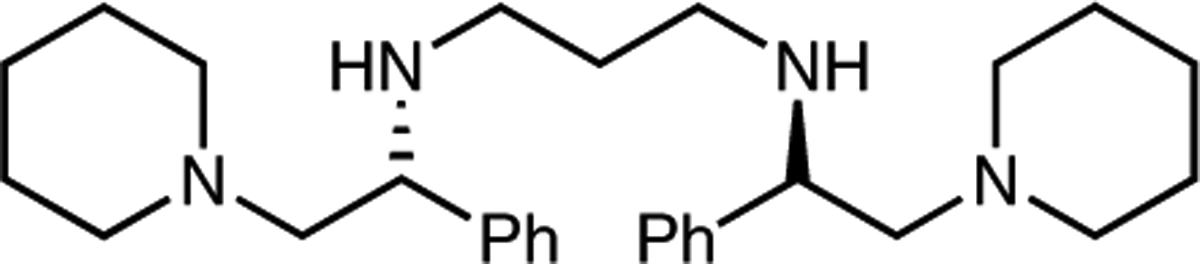
Structure of chiral C1
With optimized reaction conditions in hand (Table 1, entry 10), we initially examined various nitroolefins to gauge the scope of the reaction (Scheme 2). The reaction was tolerant of both electron-withdrawing and electron-donating groups on the arene, delivering coupled products 4ab-4ae in good yields. β-Nitrostyrenes bearing a substituent at the ortho- or meta-position resulted in comparable yields to the para-substituted compounds (4ag, 4ah); the product 4af with an ortho-trifluoromethyl group was formed in excellent yield. Aromatic heterocycles are tolerated in the reaction (4ak, 4am). (E)-5-(2-Nitrovinyl)benzo[d][1,3]dioxole was converted into product 4al in slightly lower yield, most likely due to diminished electrophilicity of the nitroalkene. In all cases, the diastereomeric ratio was largely independent of the arene substitution pattern of the β-nitrostyrene. The relative stereochemistry of the major diastereomer of 4aa was elucidated by X-ray crystallography, revealing the phosphate and the aryl group as being anti to each other (Scheme 2).8
Scheme 2.
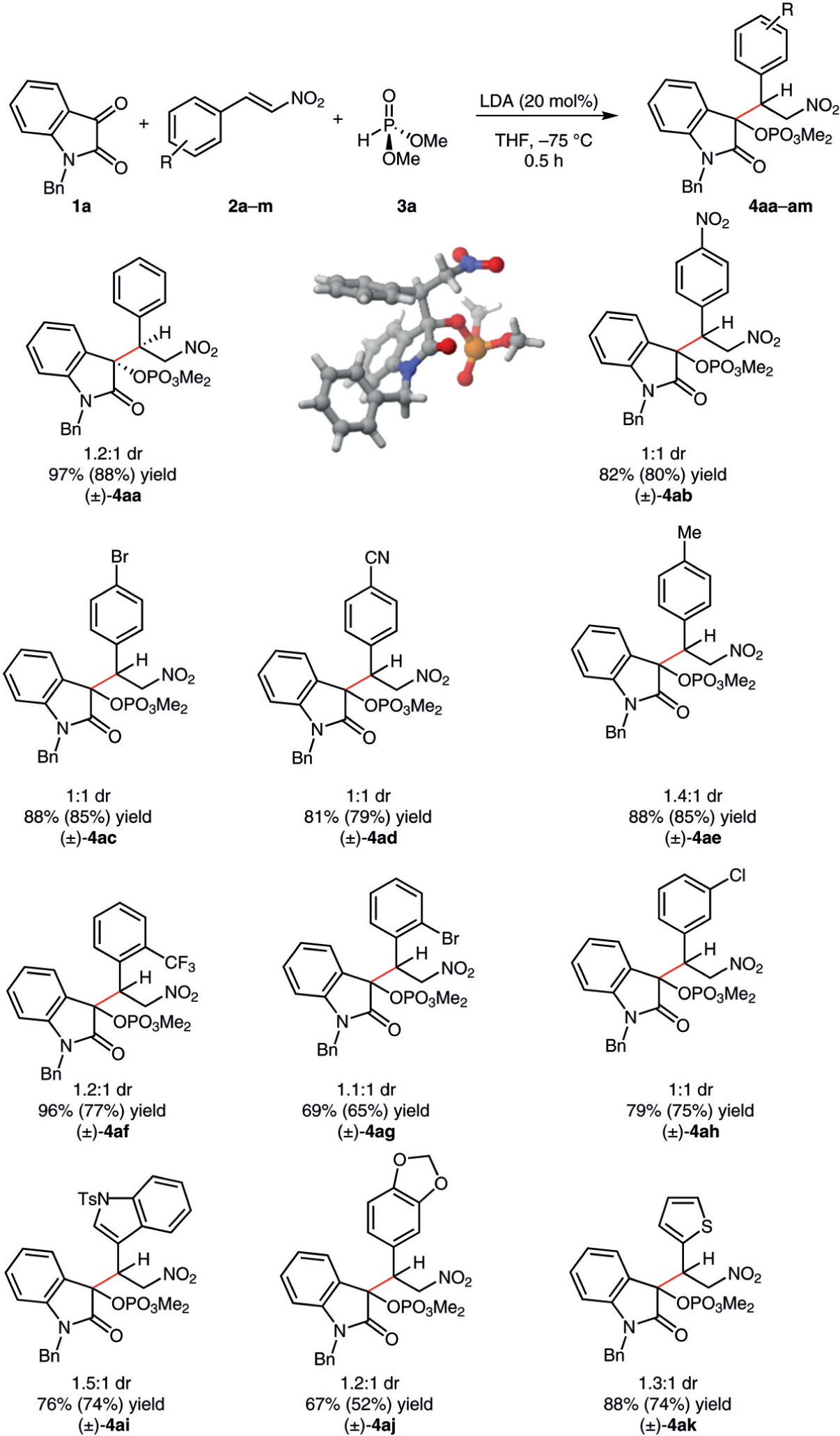
Scope of nitroalkenes in the reductive phospha-Brook rearrangement of N-benzylisatin. Reagents and conditions: N-benzylisatin (1a, 0.2 mmol), β-nitrostyrene 2 (0.4 mmol), dimethyl phosphite (3a, 0.22 mmol), 1,3,5-trimethoxybenzene as an internal standard (0.2 mmol), THF (1 mL), 30 min (1H NMR yields, with isolated yields in parentheses; diastereomeric ratios determined through the analysis of crude 1H NMR spectra).
We next evaluated the effect of a series of N-protected isatins 1 in the reaction with β-nitrostyrene (Scheme 3). The reductive coupling event gave consistently high yields for 5-halogenated N-benzylisatins (4ba, 4ca, 4da), while N-benzyl-5-methoxyisatin delivered a slightly decreased yield of 4ea owing to a relatively slow Pudovik addition. N-Allyl and N-p-methoxybenzyl protecting groups also generated the reductive coupling products 4ga and 4ha in good yields.
Scheme 3.
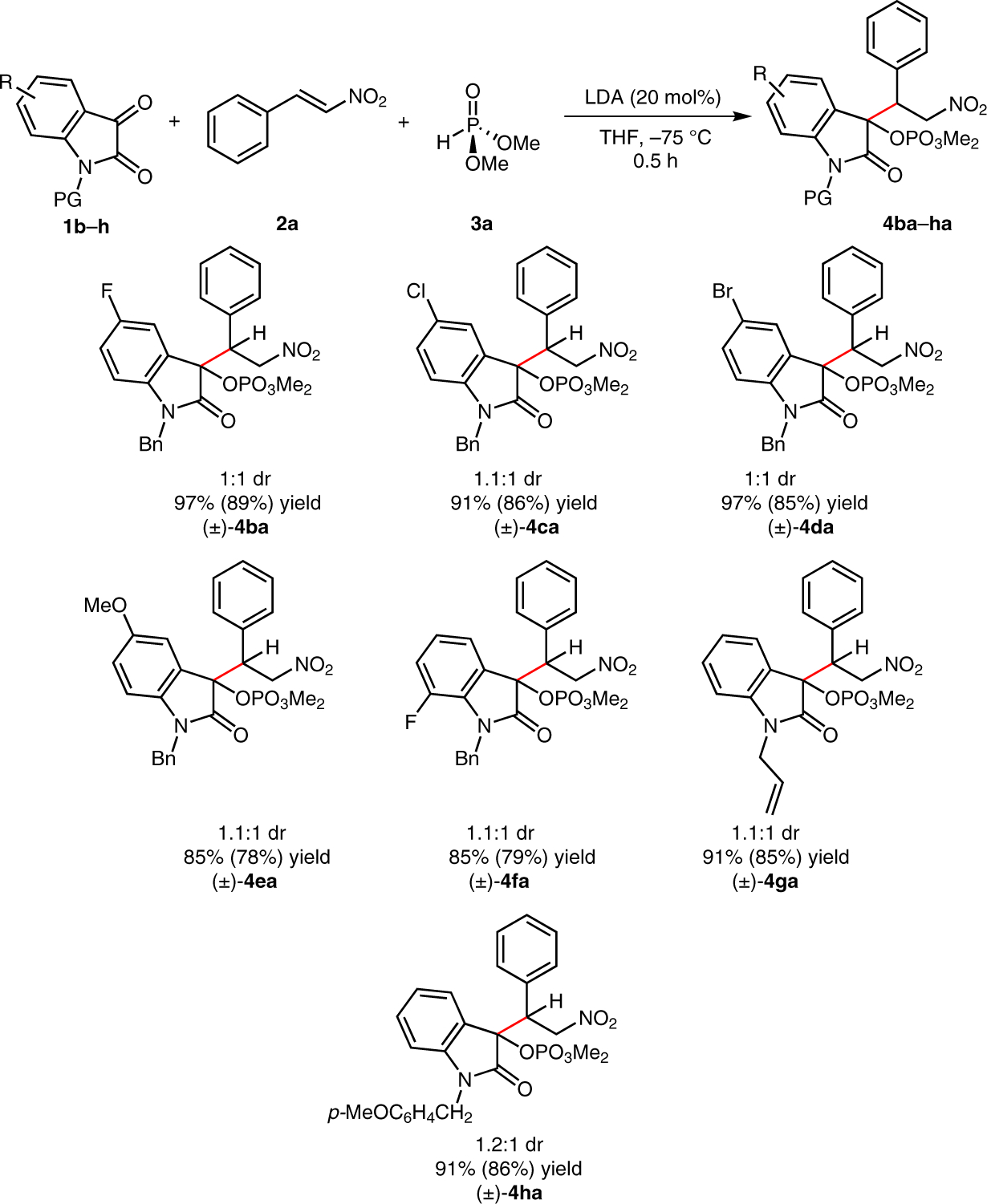
Scope of N-alkylisatins in the reductive phospha-Brook rearrangement. Reagents and conditions: N-alkylisatin 1 (0.2 mmol), β-nitrostyrene (2a, 0.4 mmol), dimethyl phosphite (3a, 0.22 mmol), 1,3,5-trimethoxybenzene as an internal standard (0.2 mmol), THF (1 mL), 30 min (1H NMR yields, with isolated yields in parentheses; diastereomeric ratios determined through the analysis of crude 1H NMR spectra).
A mechanistic scenario similar to that previously proposed by our group and others4,5 for the reductive cross-coupling reaction mediated by phosphonate–phosphate rearrangement is likely operative here. The N-alkylisatin 1 undergoes Pudovik addition with dimethyl phosphite anion formed in the presence of basic catalyst (Scheme 4). Then, [1,2]-phosphonate–phosphate rearrangement (phospha-Brook rearrangement) of intermediate B generates lithium enolate C, which subsequently reacts with β-nitrostyrene 2. Proton exchange of Michael product D with dimethyl phosphite provides the product 4 and regenerates A for reentry into the catalytic cycle. The reaction exhibits a high chemo-selectivity, with respect to possible competing modes of reactivity, including phosphite addition to the nitrostyrene, protonation of the enolate C, or capture of the enolate C by a second equivalent of isatin.
Scheme 4.
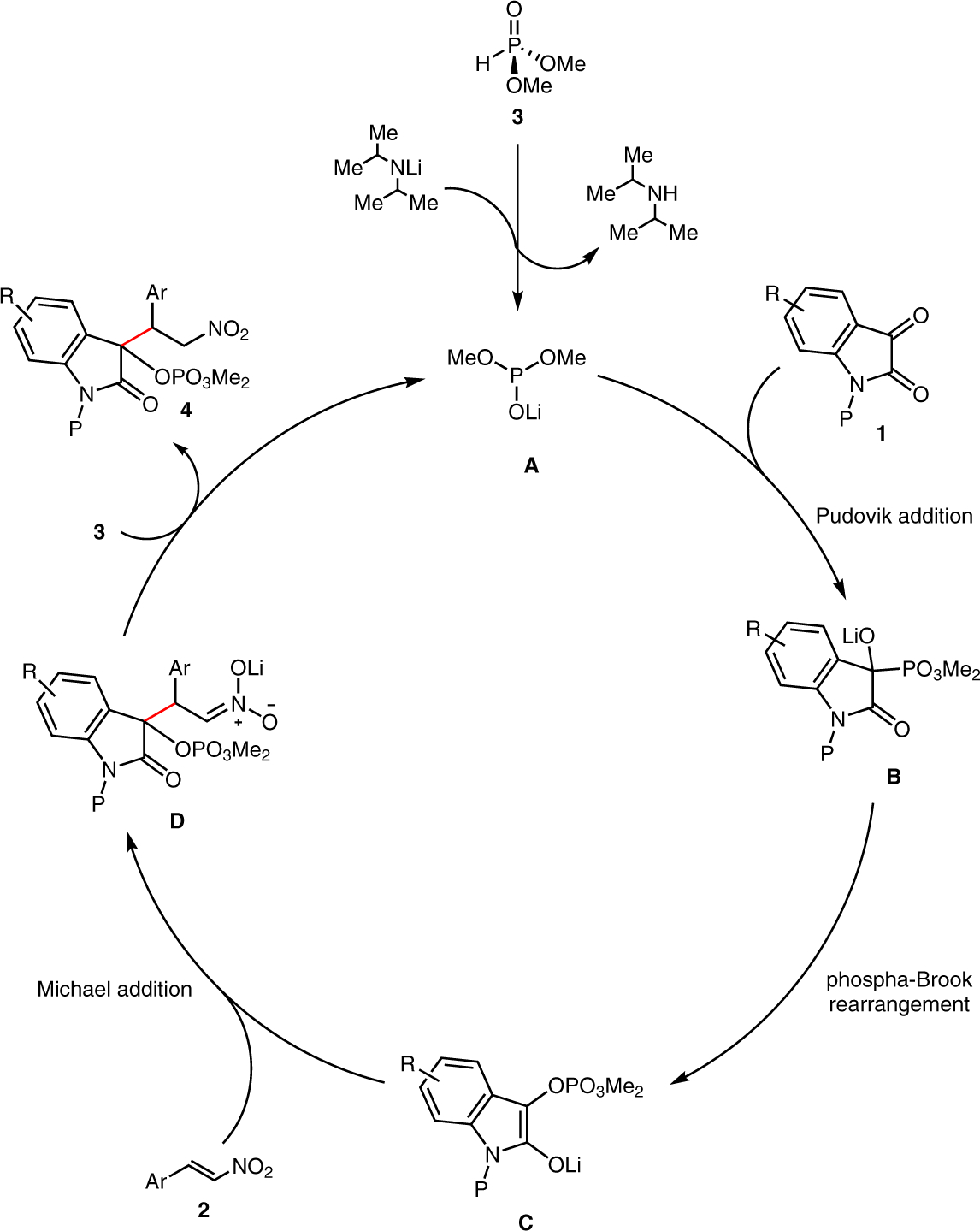
Proposed mechanism
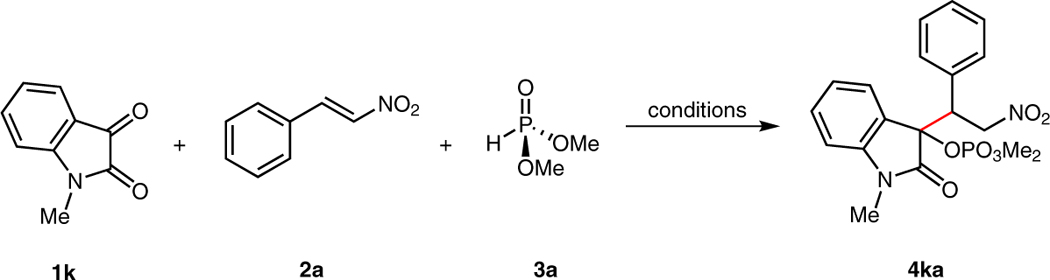
With the racemic reductive coupling reaction in hand, we turned our attention to the possibility of generating two stereogenic centers enantioselectively under the action of a basic chiral catalyst. The envisioned enantioselective process was investigated with a range of well-established chiral alkaloids (C2-C7, Figure 2) and iminophosphorane base C8.9 A screen of solvents revealed that only methanol and toluene allowed for the reaction to occur with C29a under ambient conditions (Table 2, entries 2–9), with the reaction in toluene showing promising levels of stereoselectivity (80.5:19.5 er, 4.1:1 dr). In order to achieve higher selectivities, the reaction was studied with various bases C3-C5, C89b–e (entries 10–13), and the best result was obtained in the presence of base C39b in toluene. Compared with C3, catalyst C2 still displayed better reactivity and potential for the asymmetric induction of reductive coupled product (entries 9, 10). The reaction was further evaluated through the addition of 4 Å molecular sieves with base C2 in toluene at three different temperatures, ranging from −45 °C to room temperature. While the reaction was unchanged by the exclusion of water at room temperature (Table 2, entry 14), the reductive coupling failed at −45 °C (entry 15), and both enantioselectivity and yield were eroded at 0 °C (entry 16).
Figure 2.
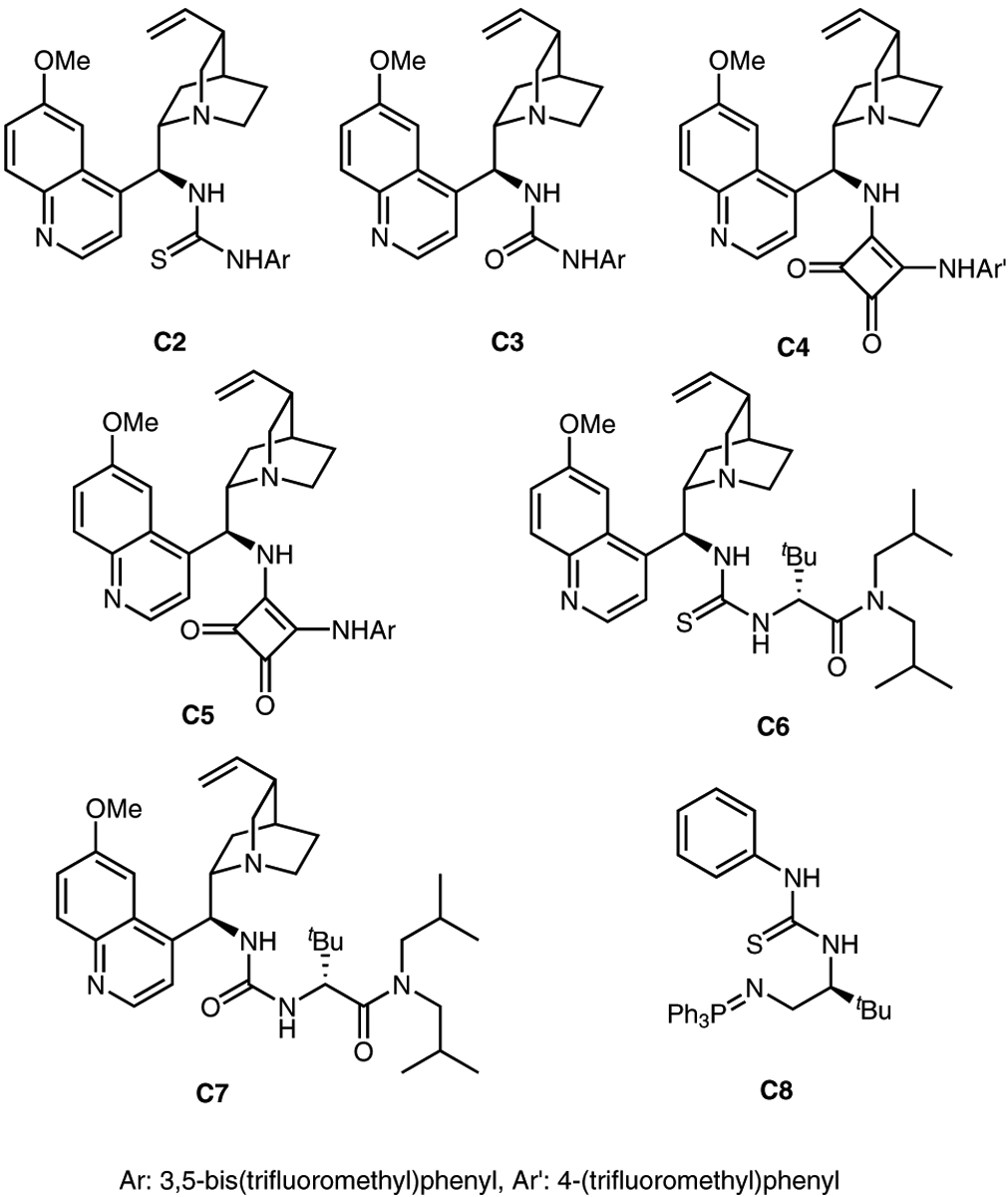
Structures of catalysts C2−C8
Table 2.
Optimization of the Asymmetric Reactiona
| Entry | Catalyst (mol%) | Solvent | Temp (°C) | Yield (%)b | drc | erd |
|---|---|---|---|---|---|---|
| 1 | t-BuOK (50) | THF | r.t. | 63 | 1.7:1 | - |
| 2 | C2 (10) | THF | r.t. | <5 | - | - |
| 3 | C2 (10) | DCM | r.t. | <5 | - | - |
| 4 | C2 (10) | MeCN | r.t. | <5 | - | - |
| 5 | C2 (10) | EtOAc | r.t. | <5 | - | - |
| 6 | C2 (10) | DME | r.t. | <5 | - | - |
| 7 | C2 (10) | o-xylene | r.t. | <5 | - | - |
| 8 | C2 (10) | MeOH | r.t. | 62 | 1.7:1 | 55.5:44.5 |
| 9 | C2 (20) | toluene | r.t. | 89 | 4.1:1 | 80.5:19.5 |
| 10 | C3 (20) | toluene | r.t. | 79 | 5.7:1 | 76.5:23.5 |
| 11 | C8 (20) | toluene | r.t. | - | - | - |
| 12 | C4 (20) | toluene | r.t. | 94 | 2.3:1 | 65:35 |
| 13 | C5 (20) | toluene | r.t. | 94 | 2.9:1 | 68:32 |
| 14 | C2 (20)e | toluene | r.t. | 78 | 3.2:1 | 80.5:19.5 |
| 15 | C2 (20)e | toluene | −45 | <1 | - | - |
| 16 | C2 (20)e | toluene | 0 | 27 | 4.3:1 | 77.8:22.2 |
| 17 | C8 (10)e | THF | −60 | 58 | 1.8:1 | 59.7:40.3 |
| 18 | C8 (10)e | THF | 0 | 89 | 2:1 | 64:36 |
| 19 | C8 (10)e | THF | −20 | 71 | 2.1:1 | 62.5:37.5 |
| 20 | C6 (20)e | toluene | r.t. | 24 | 3.1:1 | 83.5:16.5 |
| 21 | C7 (20)e | toluene | r.t. | 12 | 1.6:1 | nd |
| 22f | C2 (20)e | toluene | r.t. | 72 | 3:1 | 55.5:44.5 |
| 23g | C2 (20)e | toluene | r.t. | 39 | 1.9:1 | nd |
| 24h | C2 (20)e | toluene | r.t. | 41 | 1:1 | nd |
Reaction conditions: N-methylisatin (1k, 0.1 mmol), β-nitrostyrene (2a, 0.2 mmol), dimethyl phosphite (3a, 0.11 mmol), solvent (1 mL), 30 min.
Isolated yield.
Diastereomeric ratios were determined through the analysis of crude 1H NMR spectra.
nd: not determined.
Addition of 4 Å molecular sieves (40 mg).
N-Benzylisatin (0.1 mmol) was used instead of 1k.
N-(p-Methoxybenzyl)isatin (0.1 mmol) was used instead of 1k.
N-Allylisatin (0.1 mmol) was used instead of 1k.
In contrast to a previous report,4a the triaryliminophosphorane failed to deliver meaningful levels of enantioselectivity in this case (Table 2, entries 17–19). Quinine-derived thiourea (C6) and urea (C7) catalysts delivered comparable levels of selectivity to catalyst C2 (entries 9, 20, 21). The influence of the protecting group on isatin was screened; however, replacement of the methyl group with benzyl, p-methoxybenzyl, or an allyl group was deleterious to productive reactivity (entries 22–24).
In summary, we have extended the applicability of N-protected isatins in the catalytic reductive coupling platform with nitroolefins and dialkyl phosphite as an economical reductant. This approach presents high chemoselectivity and promising enantioselectivity through the application of chiral quinine-derived thiourea catalysts. Efforts to improve reaction enantiocontrol and develop new reactions under this general mechanistic paradigm are underway in our laboratories.
IR spectra were obtained using an ASI React IR 1000 FT-IR spectrometer. NMR spectra were recorded on a Bruker DRX 400 or 600 (1H NMR at 600 MHz, 13C NMR at 151 MHz, 19F NMR at 376 MHz, 31P NMR at 162 MHz), or a Bruker AVANCE III-OneBay500 (13C NMR at 151 MHz) spectrometer with solvent resonance as the internal standard (1H NMR: CDCl3 at 7.26 ppm, 13C NMR: CDCl3 at 77.0 ppm). 1H NMR data are reported as follows: chemical shift, multiplicity (standard abbreviations), coupling constant(s) (Hz), and integration. High-resolution mass spectra were obtained with a Thermo Fisher Scientific Exactive or Finnigan LTQ-ICR FT spectrometer (all samples were prepared in MeOH). Melting points were obtained using a Thomas Hoover Un-iMelt capillary melting point apparatus. Analytical TLC was carried out using Whatman 0.25 mm silica gel 60 or Sorbent Technologies 0.20 mm silica gel TLC plates. Visualization was allowed by UV light, phosphomolybdic acid in EtOH, or aqueous CAN solution. Purification of the reaction products was carried out by using SiliaFlash P60 silica gel (40–63 μm) purchased from SiliCycle. NMR yields were calculated using 1,3,5-trimethoxybenzene as an internal standard.
THF was purchased from Sigma Aldrich and purified by passage through an aluminium oxide column under nitrogen. Isatins were purchased from Acros Organics and alkylated according to literature procedures.10 Nitrostyrenes were prepared from aldehydes and nitromethane according to literature procedures.11 Commercially available dimethyl phosphite, diethyl phosphite, and n-BuLi (2.5 M in hexanes) were used as received. DIPA was purchased from Oakwood Products, Inc. and purified by passage through an aluminium oxide column under nitrogen.
Three-Component Reaction Using the Quinine-Derived Thiourea Catalyst C2; General Procedure
An oven-dried test tube under nitrogen atmosphere was charged with β-nitrostyrene (2a; 0.028 g, 0.2 mmol), dimethyl phosphite (3a; 0.01 mL, 0.11 mmol), and N-alkylisatin (0.1 mmol) in toluene (1 mL) at 0 °C and the mixture was stirred for 30 min. The catalyst (0.02 mmol) was added and the reaction mixture was allowed to stir at r.t. for 30 min before concentration in vacuo. The crude material was purified using flash column chromatography (hexanes/EtOAc, 50:50 to 40:60 gradient).
Three-Component Reaction Using LDA; General Procedure
An oven-dried test tube under nitrogen atmosphere was charged sequentially with DIPA (0.71 M in THF, 56 μL, 0.04 mmol) followed by n-BuLi (0.04 mmol) at −75 °C and the mixture was stirred for 15 min. Then, a solution of N-alkylisatin (0.2 mmol) and dialkyl phosphite (0.22 mmol, 1.1 equiv) in THF (1.0 mL) was added to the solution of freshly prepared LDA. After stirring for 15 min, a solution of β-nitrostyrene (0.4 mmol, 2 equiv) and 1,3,5-trimethoxybenzene (1.0 equiv, internal standard) was added. The reaction mixture was stirred at −75 °C for 30 min and was then concentrated in vacuo. The crude material was purified using flash column chromatography (hexanes/EtOAc, 70:30 to 40:60 gradient).
Characterization Data for New Compounds
(±)-(S,S)/(S,R)-1-Benzyl-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4aa)
Prepared according to the general LDA procedure; the dr (1.2:1) was calculated by comparing the resonances at δ 5.63 (major diastereomer) and 5.55 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.63 and 5.55 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 87.3 mg (88%).
Major diastereomer:
White solid; mp 113–116 °C; Rf = 0.29 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2923.5, 1723.0, 1615.0, 1555.3, 1469.4, 1378.8, 1280.5, 1180.2, 1035.5, 992.2, 852.3, 700.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.60–7.58 (m, 1 H), 7.24–7.22 (m, 3 H), 7.17–7.10 (m, 3 H), 7.05 (t, J = 7.8 Hz, 2 H), 6.94–6.91 (m, 4 H), 6.34 (dd, J = 7.2, 1.8 Hz, 1 H), 5.64 (dd, J = 13.8, 6.0 Hz, 1 H), 5.25 (dd, J = 13.8, 8.4 Hz, 1 H), 4.75 (d, J = 16.2 Hz, 1 H), 4.64 (d, J = 16.2 Hz, 1 H), 4.50 (dd, J = 8.4, 6.0 Hz, 1 H), 3.76 (d, J = 11.4 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.7, 143.2, 134.6, 131.8, 131.1, 129.0, 128.6, 128.3, 128.2, 127.4, 127.0, 125.1, 124.3, 122.8, 109.9, 81.5 (d, J = 6.6 Hz), 73.7, 54.6 (d, J = 6.3 Hz), 54.5 (d, J = 5.5 Hz), 50.6 (d, J = 10.5 Hz), 44.2.
31P NMR (162 MHz, CDCl3): δ = −2.68.
HRMS (ESI+): m/z calcd for C25H25N2O7P ([M + Na+]): 519.1297; found: 519.1291.
Minor diastereomer:
Yellowish foam; Rf = 0.18 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2922.5, 1731.7, 1613.1, 1556.2, 1468.5, 1375.9, 1281.4, 1182.1, 1042.3, 1004.7, 852.3 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.31 (t, J = 7.8 Hz, 1 H), 7.24 (td, J = 7.8, 1.2 Hz, 1 H), 7.21–7.14 (m, 6 H), 7.06 (t, J = 7.8 Hz, 1 H), 6.91 (d, J = 7.2 Hz, 2 H), 6.77 (d, J = 6.0 Hz, 2 H), 6.49 (d, J = 7.8 Hz, 1 H), 5.57 (dd, J = 13.2, 4.8 Hz, 1 H), 5.03 (dd, J = 13.2, 10.2 Hz, 1 H), 4.87 (d, J = 16.2 Hz, 1 H), 4.52 (d, J = 16.2 Hz, 1 H), 4.29 (dd, J = 10.2, 4.8 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.60 (d, J = 12.0 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.9, 143.6, 134.7, 132.3, 131.3, 129.4, 128.8, 128.7, 128.6, 127.4, 126.7, 125.2, 123.8, 122.5, 110.2, 81.9 (d, J = 6.4 Hz), 75.0, 54.68 (d, J = 5.8 Hz), 54.62 (d, J = 6.1 Hz), 50.3 (d, J = 10.8 Hz), 44.2.
31P NMR (162 MHz, CDCl3): δ = −2.30.
HRMS (ESI+): m/z calcd for C25H25N2O7P ([M + Na+]): 519.1297; found: 519.1290.
(±)-(S,S)/(S,R)-1-Benzyl-3-(2-nitro-1-(4-nitrophenyl)ethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ab)
Prepared according to the general LDA procedure; the dr (1:1) was calculated by comparing the resonances at δ 6.67 (diastereomer 1) and 6.53 (diastereomer 2). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.63–5.57 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 86.6 mg (80%).
Diastereomer 2:
Yellow foam; Rf = 0.14 (EtOAc/hexanes, 1:2).
IR (thin film): 2959.2, 2856.0, 1723.0, 1614.1, 1556.2, 1524.4, 1469.4, 1378.8, 1349.9, 1283.3, 1180.2, 1035.5, 993.1, 857.2 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.76 (d, J = 8.4 Hz, 2 H), 7.60 (d, J = 7.2 Hz, 1 H), 7.31–7.22 (m, 4 H), 7.15 (t, J = 7.8 Hz, 1 H), 7.05–7.03 (m, 4 H), 6.53 (d, J = 7.8 Hz, 1 H), 5.59 (dd, J = 14.4, 5.4 Hz, 1 H), 5.32 (dd, J = 14.4, 9.6 Hz, 1 H), 4.78 (d, J = 15.6 Hz, 1 H), 4.60 (d, J = 15.6 Hz, 1 H), 4.56 (dd, J = 9.6, 5.4 Hz, 1 H), 3.76 (d, J = 11.4 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.1, 147.5, 143.0, 139.0 (d, J = 1.2 Hz), 134.5, 131.7, 129.9, 128.7, 128.0, 127.5, 124.4, 124.3, 123.3, 123.1, 109.9, 80.7 (d, J = 5.8 Hz), 73.1, 54.7 (d, J = 6.1 Hz), 54.6 (d, J = 5.7 Hz), 50.4 (d, J = 10.8 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.54.
HRMS (ESI+): m/z calcd for C25H24N3O9P ([M + Na+]): 564.1148; found: 564.1142.
(±)-(S,S)/(S,R)-1-Benzyl-3-(1-(4-bromophenyl)-2-nitroethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ac)
Prepared according to the general LDA procedure; the dr (1:1) was calculated by comparing the resonances at δ 5.57 (diastereomer 1) and 5.50 (diastereomer 2). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.55 and 5.48 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 96.8 mg (85%).
Diastereomer 1:
Yellow foam; Rf = 0.16 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2922.5, 1723.0, 1615.0, 1556.2, 1469.4, 1378.8, 1280.5, 1180.2, 1035.5, 993.1, 913.1 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.58 (dd, J = 7.8, 1.2 Hz, 1 H), 7.31–7.28 (m, 3 H), 7.19 (td, J = 7.8, 1.2 Hz, 1 H), 7.15–7.12 (m, 3 H), 6.93–6.91 (m, 2 H), 6.77 (d, J = 8.4 Hz, 2 H), 6.43 (d, J = 7.8 Hz, 1 H), 5.59 (dd, J = 14.4, 6.0 Hz, 1 H), 5.23 (dd, J = 14.4, 8.4 Hz, 1 H), 4.86 (d, J = 16.2 Hz, 1 H), 4.58 (d, J = 16.2 Hz, 1 H), 4.45 (dd, J = 8.4, 6.0 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.57 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.5 (d, J = 0.9 Hz), 143.3, 134.5, 131.6, 131.4, 130.8 (d, J = 1.3 Hz), 130.6, 128.7, 127.6, 127.0, 124.8, 124.2, 122.9, 122.5, 110.0, 81.0 (d, J = 6.3 Hz), 73.4, 54.6 (d, J = 6.3 Hz), 54.5 (d, J = 5.5 Hz), 50.0 (d, J = 10.5 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.63.
HRMS (ESI+): m/z calcd for C25H24N2O7PBr ([M + Na+]): 598.0402; found: 598.0453.
Diastereomer 2:
Yellow foam; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2921.6, 1726.9, 1642.0, 1558.2, 1468.5, 1375.0, 1277.6, 1182.1, 1040.4, 1004.7, 907.3 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.30–7.25 (m, 6 H), 7.20 (d, J = 7.8 Hz, 1 H), 7.10 (t, J = 7.8 Hz, 1 H), 6.78–6.75 (m, 4 H), 6.54 (d, J = 7.8 Hz, 1 H), 5.52 (dd, J = 13.2, 4.8 Hz, 1 H), 4.99–4.95 (m, 2 H), 4.58 (dd, J = 10.2, 4.8 Hz, 1 H), 4.48 (d, J = 15.6 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.59 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.7, 143.7, 134.5, 131.8, 131.5, 131.3, 130.9, 128.7, 127.6, 126.7, 125.1, 123.5, 123.2, 122.7, 110.4, 81.4 (d, J = 6.4 Hz), 74.8, 54.7 (d, J = 5.7 Hz), 54.6 (d, J = 6.1 Hz), 49.8 (d, J = 11.0 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.21.
HRMS (ESI+): m/z calcd for C25H24N2O7PBr ([M + Na+]): 598.0402; found: 598.0471.
(±)-(S,S)/(S,R)-1-Benzyl-3-(1-(4-cyanophenyl)-2-nitroethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ad)
Prepared according to the general LDA procedure; the dr (1:1) was calculated by comparing the resonances at δ 6.62 and 6.50. The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.60–5.54 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 81.6 mg (79%).
Diastereomer 1:
Yellow solid; mp 160–164 °C; Rf = 0.13 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2851.2, 2230.2, 1720.1, 1615.0, 1556.2, 1469.4, 1377.8, 1281.4, 1181.1, 1034.6, 992.1 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.58 (d, J = 7.2 Hz, 1 H), 7.35–7.29 (m, 3 H), 7.23–7.20 (m, 3 H), 7.15 (t, J = 7.8 Hz, 1 H), 7.02–7.00 (m, 2 H), 6.97 (d, J = 8.4 Hz, 2 H), 6.51 (d, J = 7.8 Hz, 1 H), 5.58 (dd, J = 13.8, 5.4 Hz, 1 H), 5.29 (dd, J = 14.4, 9.0 Hz, 1 H), 4.76 (d, J = 15.6 Hz, 1 H), 4.60 (d, J = 15.6 Hz, 1 H), 4.50 (dd, J = 8.4, 5.4 Hz, 1 H), 3.76 (d, J = 12.0 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.2, 143.0, 137.1, 134.5, 131.9, 131.6, 129.7, 128.7, 128.0, 127.4, 124.5, 124.3, 123.1, 117.9, 112.3, 109.9, 80.7 (d, J = 6.0 Hz), 73.0, 54.7 (d, J = 6.1 Hz), 54.6 (d, J = 5.7 Hz), 50.6 (d, J = 10.8 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.56.
HRMS (ESI+): m/z calcd for C26H24N3O7P ([M + Na+]): 544.1250; found: 544.1244.
Diastereomer 2:
Yellow foam; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2959.2, 2856.0, 2231.2, 1731.7, 1613.1, 1558.2, 1469.4, 1375.0, 1281.4, 1182.1, 1045.2, 1003.7, 911.2 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.41 (d, J = 8.4 Hz, 2 H), 7.32–7.24 (m, 4 H), 7.14–7.08 (m, 2 H), 6.99 (d, J = 8.4 Hz, 2 H), 6.87–6.86 (m, 2 H), 6.62 (d, J = 7.8 Hz, 1 H), 5.56 (dd, J = 13.8, 4.8 Hz, 1 H), 5.26 (dd, J = 13.8, 10.8 Hz, 1 H), 4.85 (d, J = 15.6 Hz, 1 H), 4.52 (d, J = 15.6 Hz, 1 H), 4.33–4.29 (m, 1 H), 3.73 (d, J = 11.4 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.4, 143.5, 137.6, 134.5, 132.1, 131.7, 130.1, 128.7, 127.9, 127.0, 125.1, 123.2, 122.8, 118.0, 112.9, 110.3, 81.1 (d, J = 5.8 Hz), 74.3, 54.77, 54.7 (d, J = 5.7 Hz), 50.2 (d, J = 11.0 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.15.
HRMS (ESI+): m/z calcd for C26H24N3O7P ([M + Na+]): 544.1250; found: 544.1244.
(±)-(S,S)/(S,R)-1-Benzyl-3-(2-nitro-1-(p-tolyl)ethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ae)
Prepared according to the general LDA procedure; the dr (1.4:1) was calculated by comparing the resonances at δ 5.61 (major diastereomer) and 5.52 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.52 and 5.61 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 86 mg (85%).
Major diastereomer:
White solid; mp 105–107 °C; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 1723.0, 1644.0, 1615.0, 1555.3, 1469.4, 1377.8, 1279.5, 1180.2, 1034.6, 994.1, 852.3 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.58 (dd, J = 7.2, 1.2 Hz, 1 H), 7.25–7.20 (m, 3 H), 7.16–7.10 (m, 2 H), 6.92 (d, J = 7.8 Hz, 2 H), 6.85 (d, J = 7.8 Hz, 2 H), 6.80 (d, J = 7.8 Hz, 2 H), 6.35 (d, J = 7.8 Hz, 1 H), 5.62 (dd, J = 14.4, 6.0 Hz, 1 H), 5.23 (dd, J = 13.8, 8.4 Hz, 1 H), 4.83 (d, J = 16.2 Hz, 1 H), 4.60 (d, J = 16.2 Hz, 1 H), 4.46 (dd, J = 7.8, 6.0 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.57 (d, J = 11.4 Hz, 3 H), 2.23 (s, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.8 (d, J = 0.9 Hz), 143.3, 137.9, 134.7, 131.0, 129.1, 128.9, 128.6 (d, J = 6.0 Hz), 128.5, 127.4, 127.0, 125.2 (d, J = 0.7 Hz), 124.3, 122.7, 109.9, 81.5 (d, J = 6.7 Hz), 73.9, 54.6 (d, J = 6.1 Hz), 54.4 (d, J = 5.5 Hz), 50.2 (d, J = 10.4 Hz), 44.2, 21.0.
31P NMR (162 MHz, CDCl3): δ = −2.71.
HRMS (ESI+): m/z calcd for C26H27N2O7P ([M + Na+]): 533.1454; found: 533.1448.
Minor diastereomer:
White solid; mp 125–132 °C; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 1731.7, 1613.1, 1557.2, 1515.7, 1468.5, 1375.0, 1281.4, 1182.1, 1041.3, 1003.7, 943.9, 851.4 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.26–7.17 (m, 5 H), 7.07 (t, J = 7.8 Hz, 1 H), 6.97 (d, J = 7.8 Hz, 2 H), 6.78 (d, J = 7.2 Hz, 4 H), 6.49 (d, J = 7.8 Hz, 1 H), 5.53 (dd, J = 13.2, 4.8 Hz, 1 H), 5.00 (dd, J = 13.2, 10.2 Hz, 1 H), 4.93 (d, J = 16.2 Hz, 1 H), 4.49 (d, J = 16.2 Hz, 1 H), 4.26 (dd, J = 10.2, 4.8 Hz, 1 H), 3.74 (d, J = 11.4 Hz, 3 H), 3.59 (d, J = 11.4 Hz, 3 H), 2.32 (s, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.9, 143.7, 138.6, 134.7, 131.2, 129.3, 129.2, 129.1, 128.5, 127.3, 126.8, 125.2, 123.9, 122.5, 110.2, 81.9 (d, J = 6.4 Hz), 75.1, 54.66 (d, J = 5.8 Hz), 54.61 (d, J = 6.0 Hz), 50.0 (d, J = 10.8 Hz), 44.3, 21.2.
31P NMR (162 MHz, CDCl3): δ = −2.31.
HRMS (ESI+): m/z calcd for C26H27N2O7P ([M + Na+]): 533.1454; found: 533.1448.
(±)-(S,S)/(S,R)-1-Benzyl-3-(2-nitro-1-(2-(trifluoromethyl)phenyl)ethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4af)
Prepared according to the general LDA procedure; the dr (1.2:1) was calculated by comparing the resonances at δ 5.72 (major diastereomer) and 6.03 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.97 and 5.65 with the internal standard resonance at δ 6.02.
Combined yield of both diastereomers: 86 mg (77%).
Major diastereomer:
White solid; mp 164–169 °C; Rf = 0.20 (EtOAc/hexanes, 1:2).
IR (thin film): 2959.2, 2923.5, 1723.0, 1614.1, 1559.1, 1469.4, 1376.9, 1310.3, 1161.9, 1037.5, 992.1, 853.3, 701.9 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.50 (d, J = 7.8 Hz, 1 H), 7.47 (d, J = 7.8 Hz, 1 H), 7.37–7.32 (m, 6 H), 7.26 (t, J = 7.8 Hz, 1 H), 7.16 (td, J = 7.8, 0.6 Hz, 1 H), 7.09 (t, J = 7.8 Hz, 1 H), 7.02 (t, J = 7.8 Hz, 1 H), 6.59 (d, J = 7.8 Hz, 1 H), 5.72 (dd, J = 13.2, 6.0 Hz, 1 H), 5.04–5.01 (m, 1 H), 4.95 (d, J = 15.6 Hz, 1 H), 4.85–4.79 (m, 2 H), 3.74 (d, J = 11.4 Hz, 3 h), 3.56 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 173.5, 143.1, 134.8, 131.8, 131.2, 129.6, 129.4, 128.8, 128.5, 128.4, 127.8, 127.1 (d, J = 5.7 Hz), 125.6, 125.5, 124.2, 122.7, 109.5, 81.0 (d, J = 6.0 Hz), 75.0, 54.6 (d, J = 6.6 Hz), 54.5 (d, J = 5.8 Hz), 46.0 (d, J = 2.5 Hz), 45.9 (d, J = 2.4 Hz), 44.7.
31P NMR (162 MHz, CDCl3): δ = −3.04.
19F NMR (376 MHz, CDCl3): δ = −56.3.
HRMS (ESI+): m/z calcd for C26H24N2O7PF3 ([M + Na+]): 587.1171; found: 587.1165.
Minor diastereomer:
White solid; mp 144–147 °C; Rf = 0.15 (EtOAc/hexanes, 1:2).
IR (thin film): 2959.2, 2856.0, 1724.0, 1614.1, 1557.2, 1469.4, 1377.8, 1221.6, 1181.1, 1039.4, 996.0, 853.3, 699.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.98 (d, J = 7.8 Hz, 1 H), 7.72 (t, J = 7.8 Hz, 1 H), 7.60 (d, J = 7.8 Hz, 1 H), 7.54–7.52 (m, 1 H), 7.38–7.28 (m, 5 H), 7.15 (t, J = 7.8 Hz, 1 H), 6.69–6.65 (m, 2 H), 6.05–6.02 (dm, 1 H), 6.03 (d, J = 7.2 Hz, 1 H), 5.13–5.07 (m, 2 H), 4.90 (dd, J = 15.6, 1.8 Hz, 1 H), 4.30–4.27 (m, 1 H), 3.66 (dd, J = 11.4, 2.4 Hz, 3 H), 3.56 (dd, J = 11.4, 2.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 173.5, 142.5, 134.9, 132.1, 131.0, 130.5, 130.3, 129.4, 128.99, 128.91, 127.8, 127.1, 126.6 (d, J = 5.8 Hz), 125.1, 124.5, 122.3, 109.9, 80.9 (d, J = 6.1 Hz), 74.3, 54.7, 54.6 (d, J = 5.4 Hz), 44.5, 44.4, 44.3.
31P NMR (162 MHz, CDCl3): δ = −1.96.
19F NMR (376 MHz, CDCl3): δ = −58.5.
HRMS (ESI+): m/z calcd for C26H24N2O7PF3 ([M + Na+]): 587.1171; found: 587.1199.
(±)-(S,S)/(S,R)-1-Benzyl-3-(1-(2-bromophenyl)-2-nitroethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ag)
Prepared according to the general LDA procedure; the dr (1.1:1) was calculated by comparing the resonances at δ 5.28 (major diastereomer) and 5.54 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.28 and 5.54 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 74 mg (65%).
Major diastereomer:
White solid; mp 178–184 °C; Rf = 0.15 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2923.5, 1721.1, 1643.0, 1615.0, 1554.3, 1469.4, 1375.9, 1293.0, 1180.2, 1040.4, 996.0, 851.4, 754.0, 699.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.57 (d, J = 7.2 Hz, 1 H), 7.28–7.14 (m, 6 H), 7.05 (d, J = 4.8 Hz, 1 H), 6.98–6.97 (m, 2 H), 6.76–6.73 (m, 2 H), 6.44 (d, J = 7.8 Hz, 1 H), 5.61 (dd, J = 13.8, 5.4 Hz, 1 H), 5.25 (dd, J = 14.4, 9.0 Hz, 1 H), 4.83 (d, J = 15.6 Hz, 1 H), 4.76–4.74 (m, 1 H), 4.67 (d, J = 15.6 Hz, 1 H), 3.74 (d, J = 11.4 Hz, 3 H), 3.57 (d, J = 11.4 Hz, 3 H). 13C NMR (151 MHz, CDCl3): δ = 173.4, 142.6, 134.9, 133.5, 133.2, 130.9, 130.1, 128.8, 128.3, 127.8, 127.7, 127.1, 125.4, 124.2, 122.5, 109.9, 80.9 (d, J = 6.3 Hz), 73.6, 54.6, 54.5, 47.1, 47.0, 44.3.
31P NMR (162 MHz, CDCl3): δ = −1.92.
HRMS (ESI+): m/z calcd for C25H24N2O7PBr ([M + Na+]): 597.0402; found: 597.0396.
Minor diastereomer:
White solid; mp 170–176 °C; Rf = 0.21 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2919.7, 1722.1, 1614.1, 1555.3, 1469.4, 1376.9, 1280.5, 1181.1, 1033.6, 992.1, 852.3, 753.0, 700.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.78 (d, J = 7.8 Hz, 1 H), 7.34–7.29 (m, 4 H), 7.24–7.22 (m, 2 H), 7.18–7.13 (m, 2 H), 7.06–7.03 (m, 1 H), 6.98–6.91 (m, 2 H), 6.46 (d, J = 7.8 Hz, 1 H), 5.55 (dd, J = 13.8, 4.8 Hz, 1 H), 5.30 (dd, J = 9.6, 4.8 Hz, 1 H), 5.16 (dd, J = 13.8, 9.0 Hz, 1 H), 4.85 (d, J = 15.6 Hz, 1 H), 4.78 (d, J = 16.2 Hz, 1 H), 3.74 (d, J = 12.0 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 173.0, 142.9, 134.8, 133.5, 132.2 (d, J = 1.6 Hz), 131.3, 129.7, 128.7, 128.0, 127.7, 127.6, 127.5, 126.4, 125.9, 123.8, 122.4, 109.3, 81.4 (d, J = 6.1 Hz), 74.7, 54.6 (d, J = 6.1 Hz), 54.5 (d, J = 11.6 Hz), 47.9 (d, J = 11.3 Hz), 44.6.
31P NMR (162 MHz, CDCl3): δ = −2.73.
HRMS (ESI+): m/z calcd for C25H24N2O7PBr ([M + Na+]): 597.0402; found: 597.0396.
(±)-(S,S)/(S,R)-1-Benzyl-3-(1-(3-chlorophenyl)-2-nitroethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ah)
Prepared according to the general LDA procedure; the dr (1:1) was calculated by comparing the resonances at δ 6.38 and 6.55. The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.62–5.54 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 78.8 mg (75%).
Diastereomer 1:
Yellow foam; Rf = 0.24 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2360.4, 1723.0, 1615.0, 1555.3, 1469.4, 1377.8, 1280.5, 1181.1, 1035.5, 998.9, 853.3, 754.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.57 (dd, J = 7.2, 1.2 Hz, 1 H), 7.27–7.24 (m, 3 H), 7.18–7.12 (m, 3 H), 7.03–7.02 (m, 2 H), 6.97–6.94 (m, 2 H), 6.81 (d, J = 7.8 Hz, 1 H), 6.39 (d, J = 7.2 Hz, 1 H), 5.60 (dd, J = 14.4, 6.0 Hz, 1 H), 5.24 (dd, J = 14.4, 8.4 Hz, 1 H), 4.75 (d, J = 15.6 Hz, 1 H), 4.69 (d, J = 15.6 Hz, 1 H), 4.46 (dd, J = 8.4, 5.4 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.5 (d, J = 1.0 Hz), 143.2, 134.6, 134.2, 134.0 (d, J = 1.3 Hz), 131.3, 129.2, 128.7, 128.6, 127.6, 127.1, 127.0, 126.9, 124.7 (d, J = 0.9 Hz), 124.3, 123.0, 110.1, 81.2 (d, J = 6.3 Hz), 73.4, 54.6 (d, J = 6.3 Hz), 54.5 (d, J = 5.5 Hz), 50.3 (d, J = 10.7 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.66.
HRMS (ESI+): m/z calcd for C25H24N2O7PCl ([M + Na+]): 553.0907; found: 553.0902.
Diastereomer 2:
Yellow foam; Rf = 0.16 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2855.1, 1731.7, 1613.2, 1557.2, 1469.4, 1375.0, 1280.5, 1182.1, 1044.2, 1005.7, 912.1, 853.3, 755.9 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.29–7.23 (m, 5 H), 7.10–7.06 (m, 3 H), 6.89–6.88 (m, 3 H), 6.81 (d, J = 7.2 Hz, 1 H), 6.57 (d, J = 7.8 Hz, 1 h), 5.57 (dd, J = 13.8, 4.8 Hz, 1 H), 4.98 (dd, J = 13.2, 10.2 Hz, 1 H), 4.87 (d, J = 15.6 Hz, 1 H), 4.56 (d, J = 16.2 Hz, 1 H), 4.22 (dd, J = 10.2, 4.2 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.60 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.7, 143.5, 134.6, 134.5, 134.4, 131.5, 129.7, 129.3, 129.1, 128.7, 127.7, 127.6, 126.8, 125.2, 123.5, 122.7, 110.3, 81.5 (d, J = 6.1 Hz), 74.6, 54.7, 54.6 (d, J = 6.0 Hz), 50.0 (d, J = 11.0 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.31.
HRMS (ESI+): m/z calcd for C25H24N2O7PCl ([M + Na+]): 553.0907; found: 553.0902.
(±)-(S,S)/(S,R)-1-Benzyl-3-(2-nitro-1-(1-tosyl-1H-indol-3-yl)ethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ak)
Prepared according to the general LDA procedure; the dr (1.5:1) was calculated by comparing the resonances at δ 5.71 (major diastereomer) and 5.58 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.71 and 5.58 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 101.1 mg (74%).
Minor diastereomer:
Yellow solid; mp 180–185 °C (dec); Rf = 0.35 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 1731.7, 1639.2, 1615.0, 1557.2, 1468.5, 1447.3, 1371.1, 1286.2, 1175.4, 1043.3, 1003.7, 853.3, 738.6 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.90 (d, J = 8.4 Hz, 1 H), 7.52 (d, J = 8.4 Hz, 2 H), 7.31–7.26 (m, 3 H), 7.21–7.18 (m, 2 H), 7.13–7.00 (m, 6 H), 6.93 (br s, 1 H), 6.59 (d, J = 7.2 Hz, 2 H), 6.47 (d, J = 7.8 Hz, 1 H), 5.57 (dd, J = 13.2, 5.4 Hz, 1 H), 4.96 (dd, J = 13.2, 10.2 Hz, 1 H), 4.71–4.66 (m, 2 H), 4.37 (d, J = 16.2 Hz, 1 H), 3.77 (d, J = 11.4 Hz, 3 H), 3.61 (d, J = 11.4 Hz, 3 H), 2.30 (s, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.9, 145.1, 143.9, 134.5, 134.4, 134.1, 131.3, 129.8, 128.5, 127.4, 126.7, 126.3, 125.3, 124.9, 124.0, 123.7, 122.8, 119.6, 113.9, 113.2, 110.3, 81.0, 75.2, 54.7, 54.6 (d, J = 6.3 Hz), 44.4, 21.5 (some signals are overlapped).
31P NMR (162 MHz, CDCl3): δ = −2.21.
HRMS (ESI+): m/z calcd for C34H32N3O9SP ([M + Na+]): 712.1495; found: 712.1489.
(±)-(S,S)/(S,R)-3-(1-(Benzo[d][1,3]dioxol-5-yl)-2-nitroethyl)-1-benzyl-2-oxoindolin-3-yl Dimethyl Phosphate (4al)
Prepared according to the general LDA procedure; the dr (1.2:1) was calculated by comparing the resonances at δ 5.49 (major diastereomer) and 5.55 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.55 and 5.49 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 55.6 mg (52%).
Major diastereomer:
Off-white solid; mp 125–130 °C; Rf = 0.11 (EtOAc/hexanes, 1:2).
IR (thin film): 2959.2, 2921.6, 1730.8, 1644.0, 1557.2, 1489.7, 1375.9, 1278.5, 1249.6, 1182.1, 1039.4, 904.4, 852.3, 700.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.29–7.19 (m, 5 H), 7.08 (t, J = 7.8 Hz, 1 H), 6.86–6.83 (m, 2 H), 6.60 (d, J = 7.8 Hz, 1 H), 6.57 (d, J = 8.4 Hz, 1 H), 6.39 (d, J = 7.8 Hz, 1 H), 6.31 (s, 1 H), 5.90 (d, J = 3.0 Hz, 2 H), 5.50 (dd, J = 13.2, 4.8 Hz, 1 H), 5.00 (d, J = 16.2 Hz, 1 H), 4.94 (dd, J = 13.2, 10.2 Hz, 1 H), 4.50 (d, J = 15.6 Hz, 1 H), 4.20 (dd, J = 10.2, 4.8 Hz, 1 H), 3.74 (d, J = 11.4 Hz, 3 H), 3.59 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.8, 148.0, 147.7, 143.8, 134.8, 131.4, 128.5, 127.5, 126.8, 125.7, 125.2, 123.8, 123.3, 122.6, 110.2, 109.2, 108.3, 101.2, 81.8 (d, J = 6.6 Hz), 75.3, 54.7 (d, J = 5.7 Hz), 54.6 (d, J = 6.1 Hz), 50.0 (d, J = 11.0 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.28.
HRMS (ESI+): m/z calcd for C26H25N2O9P ([M + Na+]): 563.1195; found: 563.1190.
Minor diastereomer:
Off-white solid; mp 121–126 °C; Rf = 0.17 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2919.7, 2341.1, 1722.1, 1615.0, 1555.3, 1505.1, 1469.4, 1378.8, 1278.5, 1248.6, 1181.1, 1035.5, 996.0, 905.4, 853.3, 700.9 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.56 (d, J = 7.2 Hz, 1 H), 7.28–7.24 (m, 3 H), 7.17 (td, J = 7.8, 1.2 Hz, 1 H), 7.12 (t, J = 7.2 Hz, 1 H), 7.02–7.00 (m, 2 H), 6.48 (d, J = 7.8 Hz, 1 H), 6.43–6.39 (m, 3 H), 5.86 (s, 2 H), 5.56 (dd, J = 14.4, 6.0 Hz, 1 H), 5.21 (dd, J = 13.8, 8.4 Hz, 1 H), 4.89 (d, J = 15.6 Hz, 1 H), 4.63 (d, J = 15.6 Hz, 1 H), 4.41 (dd, J = 9.0, 6.0 Hz, 1 H), 3.74 (d, J = 12.0 Hz, 3 H), 3.56 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.7 (d, J = 1.0 Hz), 147.54, 147.50, 143.4, 134.7, 131.2, 128.6, 127.5, 127.0, 125.3 (d, J = 1.3 Hz), 125.1, 124.2, 122.9, 122.7, 110.0, 109.3, 108.1, 101.1, 81.4 (d, J = 6.6 Hz), 74.0, 54.6 (d, J = 6.1 Hz), 54.5 (d, J = 5.5 Hz), 50.2 (d, J = 10.5 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.71.
HRMS (ESI+): m/z calcd for C26H25N2O9P ([M + Na+]): 563.1195; found: 563.1190.
(±)-(S,S)/(S,R)-1-Benzyl-3-(2-nitro-1-(thien-2-yl)ethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4am)
Prepared according to the general LDA procedure; the dr (1.3:1) was calculated by comparing the resonances at δ 6.43 (major diastereomer) and 6.57 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.62–5.55 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 70.7 mg (74%).
Major diastereomer:
Yellow foam; Rf = 0.15 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2922.5, 1721.1, 1644.0, 1616.0, 1556.2, 1469.4, 1377.8, 1280.5, 1181.1, 1030.7, 987.3, 851.4, 700.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.57 (dd, J = 7.2, 0.6 Hz, 1 H), 7.26–7.24 (m, 3 H), 7.21 (td, J = 7.8, 1.2 Hz, 1 H), 7.15 (td, J = 7.8, 0.6 Hz, 1 H), 7.06 (dd, J = 5.4, 1.2 Hz, 1 H), 6.99–6.97 (m, 2 H), 6.77–6.75 (m, 1 h), 6.73 (d, J = 3.6 Hz, 1 H), 6.49 (d, J = 7.8 Hz, 1 H), 5.61 (dd, J = 13.8, 5.4 Hz, 1 H), 5.25 (dd, J = 14.4, 9.0 Hz, 1 H), 4.75 (dd, J = 8.4, 5.4 Hz, 1 H), 4.67 (d, J = 15.6 Hz, 1 H), 4.38 (d, J = 16.2 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.4, 143.7, 134.6, 133.7, 131.4, 128.6, 127.5, 127.2, 127.0, 126.6, 126.0, 125.1, 124.3, 123.0, 110.0, 80.9 (d, J = 9.2 Hz), 74.3, 54.6 (d, J = 6.1 Hz), 54.5 (d, J = 5.7 Hz), 45.9 (d, J = 11.4 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.71.
HRMS (ESI+): m/z calcd for C23H23N2O7SP ([M + Na+]): 525.0861; found: 525.0856.
Minor diastereomer:
Yellow solid; mp 135–141 °C; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2960.0, 2923.1, 1721.6, 1644.5, 1615.0, 1555.9, 1469.4, 1378.7, 1285.6, 1180.1, 1033.7, 989.0, 855.2, 700.1 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.30–7.23 (m, 4 H), 7.19 (d, J = 5.4 Hz, 1 H), 7.13 (d, J = 7.2 Hz, 1 H), 7.07 (t, J = 7.8 Hz, 1 H), 6.93–6.92 (m, 2 H), 6.88 (dd, J = 5.4, 3.6 Hz, 1 H), 6.76 (d, J = 2.4 Hz, 1 H), 6.58 (d, J = 8.4 Hz, 1 H), 5.58 (dd, J = 13.2, 4.8 Hz, 1 H), 4.98 (d, J = 16.2 Hz, 1 H), 4.92 (dd, J = 13.2, 10.2 Hz, 1 H), 4.61–4.59 (m, 2 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.60 (d, J = 12.0 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.7, 144.0, 134.7, 134.1, 131.5, 129.4, 128.7, 127.5, 126.8, 126.6, 126.5, 125.4, 123.6, 122.7, 110.2, 81.3 (d, J = 6.0 Hz), 76.2, 54.7, 54.6 (d, J = 6.0 Hz), 46.3 (d, J = 11.4 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.36.
HRMS (ESI+): m/z calcd for C23H23N2O7SP ([M + Na+]): 525.0861; found: 525.0856.
(±)-(S,S)/(S,R)-1-Benzyl-5-fluoro-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ba)
Prepared according to the general LDA procedure; the dr (1:1) was calculated by comparing the resonances at δ 5.57 and 5.63. The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.57 and 5.63 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 91.5 mg (89%).
Diastereomer 1:
White solid; mp 128–133 °C; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2959.4, 2941.1, 1721.1, 1644.9, 1557.2, 1492.6, 1378.8, 1271.8, 1176.3, 1032.6, 855.5, 741.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.33 (dd, J = 7.2, 2.4 Hz, 1 H), 7.24–7.21 (m, 3 H), 7.19 (t, J = 7.8 Hz, 1 H), 7.09 (t, J = 8.4 Hz, 2 H), 6.95–6.92 (m, 4 H), 6.83 (td, J = 8.4, 2.4 Hz, 1 H), 6.24 (dd, J = 8.4, 4.2 Hz, 1 H), 5.64 (dd, J = 13.8, 6.0 Hz, 1 H), 5.21 (dd, J = 14.4, 8.4 Hz, 1 H), 4.71 (d, J = 16.2 Hz, 1 H), 4.64 (d, J = 16.2 Hz, 1 H), 4.45 (dd, J = 7.8, 6.6 Hz, 1 H), 3.82 (d, J = 11.4 Hz, 3 H), 3.63 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.6, 159.7, 158.1, 139.2 (d, J = 1.5 Hz), 134.3, 131.5 (d, J = 1.5 Hz), 129.0, 128.7, 128.5, 128.4, 127.6, 126.9, 126.8 (d, J = 7.5 Hz), 117.5 (d, J = 24.1 Hz), 112.1 (d, J = 25.6 Hz), 110.8 (d, J = 9.0 Hz), 81.4 (d, J = 6.0 Hz), 73.6, 54.7 (d, J = 6.3 Hz), 54.6 (d, J = 5.7 Hz), 50.7 (d, J = 10.5 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.66.
19F NMR (376 MHz, CDCl3): δ = −118.9.
HRMS (ESI+): m/z calcd for C25H24N2O7PF ([M + Na+]): 537.1203; found: 537.1197.
Diastereomer 2:
White foam; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2959.2, 2921.6, 1731.7, 1627.6, 1557.2, 1491.6, 1377.8, 1277.6, 1181.1, 1046.1, 1014.3, 853.3, 735.7 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.34 (t, J = 7.2 Hz, 1 H), 7.24–7.22 (m, 5 H), 6.97–6.92 (m, 3 H), 6.84–6.83 (m, 2 H), 6.75–6.73 (m, 1 H), 6.42 (dd, J = 9.0, 4.2 Hz, 1 H), 5.57 (dd, J = 13.8, 5.4 Hz, 1 H), 5.01 (dd, J = 13.2, 9.6 Hz, 1 H), 4.86 (d, J = 16.2 Hz, 1 H), 4.56 (d, J = 16.2 Hz, 1 H), 4.22 (dd, J = 9.6, 5.4 Hz, 1 H), 3.82 (d, J = 11.4 Hz, 3 H), 3.63 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.9, 159.3, 157.7, 139.5, 134.4, 132.2, 129.4, 129.0, 128.8, 128.7, 127.5, 126.7, 117.6 (d, J = 23.4 Hz), 113.3 (d, J = 25.5 Hz), 110.9 (d, J = 7.8 Hz), 81.7, 74.6, 54.8 (d, J = 5.7 Hz), 54.6 (d, J = 6.1 Hz), 50.2 (d, J = 10.7 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.20.
19F NMR (376 MHz, CDCl3): δ = −119.2.
HRMS (ESI+): m/z calcd for C25H24N2O7PF ([M + Na+]): 537.1203; found: 537.1197.
(±)-(S,S)/(S,R)-1-Benzyl-5-chloro-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ca)
Prepared according to the general LDA procedure; the dr (1.1:1) was calculated by comparing the resonances at δ 5.62 (major diastereomer) and 5.56 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.62 and 5.56 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 91.3 mg (86%).
Major diastereomer:
White solid; mp 110–116 °C; Rf = 0.39 (EtOAc/hexanes, 1:2).
IR (thin film): 2921.4, 1725.9, 1640.1, 1556.2, 1485.8, 1377.8, 1281.4, 1177.3, 1031.7, 1003.7, 921.8, 700.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.56 (d, J = 1.8 Hz, 1 H), 7.24–7.23 (m, 4 H), 7.11–7.09 (m, 3 H), 6.94–6.90 (m, 4 H), 6.23 (d, J = 8.4 Hz, 1 H), 5.62 (dd, J = 13.8, 6.0 Hz, 1 H), 5.20 (dd, J = 14.4, 8.4 Hz, 1 H), 4.71 (d, J = 16.2 Hz, 1 H), 4.63 (d, J = 16.2 Hz, 1 H), 4.47–4.44 (m, 1 H), 3.83 (d, J = 11.4 Hz, 3 h), 3.62 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.4, 141.8, 134.1, 131.5 (d, J = 1 Hz), 130.9, 129.0, 128.7, 128.6, 128.4, 128.3, 127.6, 127.0, 126.9, 124.4, 111.0, 81.2 (d, J = 6.4 Hz), 73.5, 54.7 (d, J = 6.3 Hz), 54.6 (d, J = 5.7 Hz), 50.6 (d, J = 10.5 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.54.
HRMS (ESI+): m/z calcd for C25H24N2O7PCl ([M + Na+]): 553.0907; found: 553.0934.
Minor diastereomer:
White foam; Rf = 0.28 (EtOAc/hexanes, 1:2).
IR (thin film): 2921.6, 1735.6, 1612.2, 1557.2, 1484.9, 1376.9, 1282.4, 1178.2, 1045.2, 1012.4, 853.3, 700.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.36–7.34 (m, 1 H), 7.285–7.280 (m, 1 H), 7.24–7.20 (m, 5 H), 6.96–6.95 (m, 3 H), 6.80 (br s, 2 H), 6.41–6.40 (m, 1 H), 5.57–5.54 (m, 1 H), 5.04–5.00 (m, 1 H), 4.85 (d, J = 16.2 Hz, 1 H), 4.55 (d, J = 16.2 Hz, 1 H), 4.23–4.22 (m, 1 H), 3.85–3.82 (m, 3 H), 3.62–3.60 (m, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.7, 142.1, 134.2, 132.0, 131.1, 129.3, 129.0, 128.8, 128.7, 127.9, 127.6, 126.6, 125.7, 125.5, 111.2, 81.5 (d, J = 6.4 Hz), 74.6, 54.8 (d, J = 5.4 Hz), 54.6 (d, J = 6.0 Hz), 50.2 (d, J = 10.8 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.0.
HRMS (ESI+): m/z calcd for C25H24N2O7PCl ([M + Na+]): 553.0907; found: 553.0902.
(±)-(S,S)/(S,R)-1-Benzyl-5-bromo-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4da)
Prepared according to the general LDA procedure; the dr (1:1) was calculated by comparing the resonances at δ 5.62 and 5.56. The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.62 and 5.56 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 97.8 mg (85%).
Diastereomer 1:
White foam; Rf = 0.22 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2923.5, 1736.5, 1610.2, 1557.2, 1455.0, 1376.9, 1283.3, 1178.2, 1043.3, 1009.5, 909.2, 853.3, 700.0 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.36–7.34 (m, 2 H), 7.25–7.19 (m, 5 H), 7.08 (br s, 1 H), 6.96 (d, J = 7.2 Hz, 2 H), 6.81–6.79 (m, 2 H), 6.36 (d, J = 8.4 Hz, 1 H), 5.55 (dd, J = 13.2, 4.8 Hz, 1 H), 5.02 (dd, J = 13.8, 10.2 Hz, 1 H), 4.85 (d, J = 15.6 Hz, 1 H), 4.54 (d, J = 16.2 Hz, 1 H), 4.22 (dd, J = 10.2, 5.4 Hz, 1 H), 3.84 (d, J = 11.4 Hz, 3 H), 3.60 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.6, 142.6, 134.2, 134.0, 132.0, 129.4, 129.0, 128.8, 128.7, 128.3, 127.6, 126.6, 126.1, 115.0, 111.7, 81.4 (d, J = 6.1 Hz), 74.6, 54.8 (d, J = 5.7 Hz), 54.6 (d, J = 6.0 Hz), 50.2 (d, J = 10.8 Hz), 44.4.
31P NMR (162 MHz, CDCl3): δ = −2.0.
HRMS (ESI+): m/z calcd for C25H24N2O7PBr ([M + Na+]): 597.0402; found: 597.0396.
(±)-(S,S)/(S,R)-1-Benzyl-5-methoxy-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ea)
Prepared according to the general LDA procedure; the dr (1.1:1) was calculated by comparing the resonances at δ 6.21 (major diastereomer) and 6.39 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 6.39 and 6.21 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 82.1 mg (78%).
Major diastereomer:
Off-white solid; mp 132–137 °C; Rf = 0.17 (EtOAc/hexanes, 1:2).
IR (thin film): 2957.3, 2921.6, 2341.1, 1719.2, 1634.3, 1606.4, 1556.2, 1496.4, 1437.6, 1378.8, 1280.5, 1184.0, 1037.5, 1004.7, 924.7 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.23–7.22 (m, 3 H), 7.19–7.16 (m, 2 H), 7.07 (t, J = 8.4 Hz, 2 H), 6.95–6.92 (m, 4 H), 6.64 (dd, J = 9.0, 3.0 Hz, 1 H), 6.21 (d, J = 8.4 Hz, 1 H), 5.64 (dd, J = 13.8, 6.0 Hz, 1 H), 5.23 (dd, J = 13.8, 8.4 Hz, 1 H), 4.70 (d, J = 16.2 Hz, 1 H), 4.62 (d, J = 16.2 Hz, 1 H), 4.46 (dd, J = 8.4, 6.0 Hz, 1 H), 3.81 (s, 3 H), 3.78 (d, J = 12.0 Hz, 3 H), 3.62 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.4, 155.9, 136.5, 134.7, 131.8, 129.0, 128.6, 128.4, 128.2, 127.4, 126.9, 126.3, 115.4, 111.3, 110.4, 81.8 (d, J = 6.5 Hz), 73.8, 55.9, 54.6 (d, J = 6.3 Hz), 54.5 (d, J = 5.7 Hz), 50.7 (d, J = 10.7 Hz), 44.3.
31P NMR (162 MHz, CDCl3): δ = −2.76.
HRMS (ESI+): m/z calcd for C26H27N2O8P ([M + Na+]): 549.1403; found: 549.1397.
(±)-(S,S)/(S,R)-1-Benzyl-7-fluoro-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4fa)
Prepared according to the general LDA procedure; the dr (1.1:1) was calculated by comparing the 31P NMR resonances at δ −2.86 (major diastereomer) and −2.47 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.50–5.40 with the internal standard resonance at δ 5.98.
Combined yield of both diastereomers: 81.2 mg (79%).
Major diastereomer:
White foam; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2856.0, 1727.9, 1632.4, 1556.2, 1476.2, 1379.8, 1281.4, 1190.8, 1043.3, 988.3 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.39 (dd, J = 7.8, 1.2 Hz, 1 H), 7.28–7.25 (m, 3 H), 7.13–7.06 (m, 4 H), 6.98–6.93 (m, 3 H), 6.82 (d, J = 7.2 Hz, 2 H), 5.60 (dd, J = 13.8, 5.4 Hz, 1 H), 5.17 (dd, J = 13.8, 8.4 Hz, 1 H), 4.84 (d, J = 15.6 Hz, 1 H), 4.79 (d, J = 15.6 Hz, 1 H), 4.43 (dd, J = 8.4, 5.4 Hz, 1 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.61 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.5, 147.8, 146.2, 135.8, 131.3 (d, J = 0.9 Hz), 130.0 (d, J = 8.9 Hz), 128.8, 128.5, 128.4, 128.2 (d, J = 2.8 Hz), 127.4, 123.6 (d, J = 6.3 Hz), 120.3 (d, J = 3.3 Hz), 119.4, 119.2, 81.1 (dd, J = 6.7, 2.4 Hz), 73.6, 54.6 (d, J = 6.1 Hz), 54.5 (d, J = 5.7 Hz), 50.9 (d, J = 10.4 Hz), 45.8 (d, J = 4.5 Hz).
31P NMR (162 MHz, CDCl3): δ = −2.76.
19F NMR (376 MHz, CDCl3): δ = −132.4.
HRMS (ESI+): m/z calcd for C25H24N2O7PF ([M + Na+]): 537.1203; found: 537.1197.
(±)-(S,S)/(S,R)-1-Allyl-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ga)
Prepared according to the general LDA procedure; the dr (1.1:1) was calculated by comparing the resonances at δ 6.50 (major diastereomer) and 6.68 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 6.50 and 6.68 with the internal standard resonance at δ 6.09.
Combined yield of both diastereomers: 75.8 mg (85%).
Major diastereomer:
White foam; Rf = 0.13 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2921.6, 1723.0, 1615.0, 1555.3, 1469.4, 1379.8, 1279.5, 1185.0, 1036.5, 1003.7, 988.3 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.56 (d, J = 7.2 Hz, 1 H), 7.21 (td, J = 7.8, 1.2 Hz, 1 H), 7.12 (t, J = 7.2 Hz, 2 H), 7.07 (t, J = 7.8 Hz, 2 H), 6.50 (d, J = 7.8 Hz, 1 H), 6.19 (d, J = 7.8 Hz, 2 H), 5.60 (dd, J = 14.4, 6.0 Hz, 1 H), 5.52–5.46 (m, 1 H), 5.22 (dd, J = 14.4, 8.4 Hz, 1 H), 5.06 (dd, J = 10.2, 0.6 Hz, 1 H), 4.92 (dd, J = 17.4, 0.6 Hz, 1 H), 4.44 (dd, J = 8.4, 6.0 Hz, 1 H), 4.19–4.15 (m, 1 H), 4.06–4.02 (m, 1 H), 3.77 (d, J = 11.4 Hz, 3 H), 3.57 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.2, 143.0, 131.8 (d, J = 1.2 Hz), 131.0, 130.3, 129.0, 128.3, 128.2, 125.1, 124.2, 122.7, 117.8, 109.6, 81.4 (d, J = 6.4 Hz), 73.7, 54.6 (d, J = 6.1 Hz), 54.5 (d, J = 5.5 Hz), 50.7 (d, J = 10.7 Hz), 42.5.
31P NMR (162 MHz, CDCl3): δ = −2.63.
HRMS (ESI+): m/z calcd for C21H23N2O7P ([M + Na+]): 469.1141; found: 469.1135.
Minor diastereomer:
White foam; Rf = 0.12 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2922.5, 1730.8, 1614.1, 1556.2, 1468.5, 1374.0, 1278.5, 1185.0, 1042.3, 1004.7, 851.4 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.35–7.32 (m, 1 H), 7.26–7.23 (m, 1 H), 7.17–7.15 (m, 2 H), 7.07–7.02 (m, 2 H), 6.88 (d, J = 7.2 Hz, 2 H), 6.69 (d, J = 7.8 Hz, 1 H), 5.55 (dd, J = 13.2, 4.8 Hz, 1 H), 5.37–5.31 (m, 1 H), 5.04–4.99 (m, 2 H), 4.90 (dd, J = 17.4, 0.6 Hz, 1 H), 4.22–4.16 (m, 2 H), 4.01–3.98 (m, 1 h), 3.77 (d, J = 12.0 Hz, 3 H), 3.58 (d, J = 12.0 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.4, 143.4, 132.3, 131.1, 130.5, 129.3, 128.7, 128.4, 125.2, 123.8, 122.4, 117.7, 109.9, 81.8 (d, J = 6.4 Hz), 74.7, 54.6 (d, J = 5.7 Hz), 54.5 (d, J = 6.0 Hz), 50.3 (d, J = 10.7 Hz), 42.5.
31P NMR (162 MHz, CDCl3): δ = −2.2.
HRMS (ESI+): m/z calcd for C21H23N2O7P ([M + Na+]): 469.1141; found: 469.1135.
(±)-(S,S)/(S,R)-1-(4-Methoxybenzyl)-3-(2-nitro-1-phenylethyl)-2-oxoindolin-3-yl Dimethyl Phosphate (4ha)
Prepared according to the general LDA procedure; the dr (1.2:1) was calculated by comparing the resonances at δ 6.20 (major diastereomer) and 6.35 (minor diastereomer). The 1H NMR yield was calculated by comparing the sum of the resonances at δ 5.50–5.40 with the internal standard resonance at δ 5.93.
Combined yield of both diastereomers: 69.7 mg (86%).
Minor diastereomer:
White solid; mp 115–122 °C; Rf = 0.13 (EtOAc/hexanes, 1:2).
IR (thin film): 2958.2, 2359.4, 2341.1, 1722.1, 1644.0, 1615.0, 1556.2, 1513.8, 1469.4, 1378.8, 1277.6, 1248.6, 1180.2, 1034.6, 992.1, 749.2, 701.9 cm−1.
1H NMR (600 MHz, CDCl3): δ = 7.58–7.57 (m, 1 H), 7.16–7.09 (m, 3 H), 7.04 (t, J = 7.8 Hz, 2 H), 6.92–6.90 (m, 4 H), 6.76 (d, J = 8.4 Hz, 2 h), 6.35 (d, J = 7.8 Hz, 1 H), 5.63 (dd, J = 14.4, 6.0 Hz, 1 H), 5.24 (dd, J = 14.4, 8.4 Hz, 1 H), 4.62 (s, 2 h), 4.48 (dd, J = 8.4, 6.0 Hz, 1 H), 3.78 (s, 3 H), 3.75 (d, J = 11.4 Hz, 3 H), 3.58 (d, J = 11.4 Hz, 3 H).
13C NMR (151 MHz, CDCl3): δ = 172.6, 158.9, 143.2, 131.8, 131.0, 129.0, 128.4, 128.3, 128.2, 126.6, 125.1, 124.3, 122.7, 113.9, 109.9, 81.5 (d, J = 6.6 Hz), 73.7, 55.2, 54.6 (d, J = 6.1 Hz), 54.5 (d, J = 5.7 Hz), 50.7 (d, J = 10.5 Hz), 43.7.
31P NMR (162 MHz, CDCl3): δ = −2.71.
HRMS (ESI+): m/z calcd for C26H27N2O8P ([M + Na+]): 549.1403; found: 549.1397.
Supplementary Material
Funding Information
National Institute of General Medical Sciences (R35 GM118055)
Footnotes
Supporting Information
Supporting information for this article is available online at http://dx.doi.org/10.1055/s-0036-1588170.
References
- (1).(a) Boeckman RK Jr.; Hudack RA Jr. J. Org. Chem 1998, 63, 3524. [Google Scholar]; (b) Jung M; Groth U Synlett 2002, 2015. [Google Scholar]; (c) Groth U; Jung M; Vogel T Chem. Eur. J 2005, 11, 3127. [DOI] [PubMed] [Google Scholar]; (d) Fischer S; Groth U; Jung M; Lindenmaier M; Vogel T Tetrahedron Lett. 2005, 46, 6679. [Google Scholar]; (e) Miyoshi N; Fukuma T; Wada M Chem. Lett 1995, 999. [Google Scholar]
- (2).(a) Pudovik AN Dokl. Akad. Nauk SSSR 1950, 73, 499; [Google Scholar]; Chem. Abstr 1951, 45, 2856. [Google Scholar]; (b) Galkin VI; Khabibullina AB; Bakhtiyarova IV; Cherkasov RA; Pudovik AN Zh. Obshch. Khim 1990, 60, 92. [Google Scholar]; (c) Sobanov AA; Zolotukhin AV; Galkina IV; Galkin VI; Cherkasov RA Russ. J. Gen. Chem 2006, 76, 421. [Google Scholar]; (d) Sobanov AA; Zolotukhin AV; Galkin VI; Mostovaya OA; Cherkasov RA; Pudovik AN Russ. J. Gen. Chem 2003, 73, 871. [Google Scholar]; (e) Sobanov AA; Zolotukhin AV; Galkin VI; Cherkasov RA; Pudovik AN Russ. J. Gen. Chem 2002, 72, 1067. [Google Scholar]; (f) Albouy D; Lasperas M; Etemad-Moghadam G; Koenig M Tetrahedron Lett. 1999, 40, 2311. [Google Scholar]; (g) Qian CT; Huang TS Fenzi Cuihua 1997, 11, 455. [Google Scholar]; (h) Yokomatsu T; Yamagishi T; Shibuya SJ Chem. Soc., Perkin Trans 1 1997, 1527. [Google Scholar]; (i) Groeger H; Wilken J; Martens JZ Naturforsch., B: Chem. Sci 1996, 51, 1305. [Google Scholar]; (j) Ovchinnikov VV Zh. Obshch. Khim 1996, 66, 463. [Google Scholar]; (k) Groeger H; Martens J Synth. Commun 1996, 26, 1903. [Google Scholar]; (l) Safina Yu. G.; Malkova G. Sh.; Cherkasov RA Zh. Obshch. Khim 1991, 61, 620. [Google Scholar]; (m) Galkin VI; Galkina IV; Khabibullina AB; Al Kurdi H; Cherkasov RA; Pudovik AN Dokl. Akad. Nauk SSSR 1990, 314, 1408. [Google Scholar]; (n) Nesterov LV; Krepysheva NE; Aleksandrova NA Zh. Obshch. Khim 1984, 54, 54. [Google Scholar]; (o) Malenko DM; Gololobov Yu. G. Zh. Obshch. Khim 1978, 48, 2793. [Google Scholar]; (p) Abramov VS Dokl. Akad. Nauk SSSR 1950, 73, 487; [Google Scholar]; Chem. Abstr 1951, 45, 2855. [Google Scholar]; (q) Chen SJ; Coward JK J. Org. Chem 1998, 63, 502. [DOI] [PubMed] [Google Scholar]; (r) Gawron O; Grelecki C; Reilly W; Sands JJ Am. Chem. Soc 1953, 75, 3591. [Google Scholar]; (s) Tongcharoensirikul P; Suarez AI; Voelker T; Thompson CM J. Org. Chem 2004, 69, 2322. [DOI] [PubMed] [Google Scholar]
- (3).(a) Kondoha A; Terada M Org. Chem. Front 2015, 2, 801. [Google Scholar]; (b) Fitch SJ; Moedritzer KJ Am. Chem. Soc 1962, 84, 1876. [Google Scholar]; (c) Hall LAR; Stephens CW; Drysdale JJ J. Am. Chem. Soc 1957, 79, 1768. [Google Scholar]; (d) Kuroboshi M; Ishihara T; Ando TJ Fluorine Chem. 1988, 39, 293. [Google Scholar]; (e) Demir AS; Reis Ö; İğdir AÇ; Esiringü İ; Eymur SJ Org. Chem 2005, 70, 10584. [DOI] [PubMed] [Google Scholar]; (f) Demir AS; Reis B; Reis Ö; Eymür S; Göllü M; Tural S; Saglam GJ Org. Chem 2007, 72, 7439. [DOI] [PubMed] [Google Scholar]; (g) Demir AS; Esiringü l.; Göllü M; Reis Ö J. Org. Chem 2009, 74, 2197. [DOI] [PubMed] [Google Scholar]; (h) Kondoh A; Terada M Org. Lett 2013, 15, 4568. [DOI] [PubMed] [Google Scholar]; (i) Pallikonda G; Santosh R; Ghosal S; Chakravarty M Tetrahedron Lett. 2015, 56, 3796. [Google Scholar]; (j) Kondoh A; Ishikawa S; Aoki T; Terada M Chem. Commun 2016, 52, 12513. [DOI] [PubMed] [Google Scholar]; (k) Kondoh A; Takei A; Terada M Synlett 2016, 27, 1848. [Google Scholar]; (l) Kondoh A; Aoki T; Terada M Org. Lett 2014, 16, 3528. [DOI] [PubMed] [Google Scholar]
- (4).(a) Horwitz MA; Zavesky BP; Martinez-Alvarado JI; Johnson JS Org. Lett 2016, 18, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Horwitz MA; Tanaka N; Yokosaka T; Uraguchi D; Johnson JS; Ooi T Chem. Sci 2015, 6, 6086. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Corbett M; Uraguchi D; Ooi T; Johnson JS Angew. Chem. Int. Ed 2012, 51, 4685. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bausch CC; Johnson JS Adv. Synth. Catal 2005, 347, 1207. [Google Scholar]
- (5).(a) Kondoh A; Terada M Org. Biomol. Chem 2016, 14, 4704. [DOI] [PubMed] [Google Scholar]; (b) Jiang J; Liu H; Lu C-D; Xu Y-J Org. Lett 2016, 18, 880. [DOI] [PubMed] [Google Scholar]
- (6).Yin D; Liu H; Lu C-D; Xu Y-JJ Org. Chem 2017, 82, 3252. [DOI] [PubMed] [Google Scholar]
- (7).(a) Stivala CE; Zakarian AJ Am. Chem. Soc 2011, 133, 11936. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Frizzle MJ; Caille S; Marshall TL; McRae K; Nadeau K; Guo G; Wu S; Martinelli MJ; Moniz GA Org. Process Res. Dev 2007, 11, 215. [Google Scholar]
- (8). CCDC 1530988 (4aa) contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/getstructures.
- (9).(a) Vakulya B; Varga S; Csampai A; Soos T Org. Lett 2005, 7, 1967. [DOI] [PubMed] [Google Scholar]; (b) Rong Z-Q; Pan H-J; Yan H-L; Zhao Y Org. Lett 2014, 16, 208. [DOI] [PubMed] [Google Scholar]; (c) Yang W; Du D-M Org. Lett 2010, 12, 5450. [DOI] [PubMed] [Google Scholar]; (d) Manna MS; Kumar V; Mukherjee S Chem. Commun 2012, 48, 5193. [DOI] [PubMed] [Google Scholar]; (e) Núñez MG; Farley AJM; Dixon DJ J. Am. Chem. Soc 2013, 135, 16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Overman LE; Peterson EA Tetrahedron 2003, 59, 6905. [Google Scholar]; (b) Vyas DJ; Froehlich R; Oestreich MJ Org. Chem 2010, 75, 6720. [DOI] [PubMed] [Google Scholar]; (c) Bouhfid R; Joly N; Essassi EM; Lequart V; Massoui M; Martin P Synth. Commun 2011, 41, 2096. [Google Scholar]
- (11).(a) Hashmi ASK; Häffner T; Yang W; Pankajakshan S; Schäfer S; Schultes L; Rominger F; Frey W Chem. Eur. J 2012, 18, 10480. [DOI] [PubMed] [Google Scholar]; (b) Ashokkumar V; Siva A Org. Biomol. Chem 2015, 13, 10216. [DOI] [PubMed] [Google Scholar]; (c) Xu C; Du J; Ma L; Li G; Tao M; Zhang W Tetrahedron 2013, 69, 4749. [Google Scholar]; (d) Quan X-J; Ren Z-H; Wang Y-Y; Guan Z-H Org. Lett 2015, 17, 393. [DOI] [PubMed] [Google Scholar]; (e) Kolarovic A; Käslin A; Wennemers H Org. Lett 2014, 16, 4236. [DOI] [PubMed] [Google Scholar]; (f) Park J.-e.; Song C; Choi K; Sim T; Moon B; Roh EJ Bioorg. Med. Chem. Lett 2013, 23, 5515. [DOI] [PubMed] [Google Scholar]; (g) McNulty J; Steere JA; Wolf S Tetrahedron Lett. 1998, 39, 8013. [Google Scholar]; (h) Chen J; Geng Z-C; Li N; Huang X-F; Pan F-F; Wang X-WJ Org. Chem 2013, 78, 2362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.