Abstract
Adipocytes and immune cells are vital for the development of adipose tissue. Adipokines secreted by adipocytes regulate adipogenesis and body metabolism. Chemerin is one of the adipokines. However, the function and mechanism of chemerin in adipose tissue are not fully illuminated. Compared with wild type (WT) mice, Rarres2−/− mice gained weight and significantly increased fat distribution in subcutaneous adipose tissue (SAT), rather than visceral adipose tissue (VAT) on high fat diet (HFD). PPARγ and C/EBPα, the master regulators of adipogenesis, were up-regulated in SAT and down-regulated in VAT in Rarres2−/− mice comparing with WT mice. Inspite of chemerin deficiency or not, the ratio of adipocyte-progenitors to total cells and the differentiation capacity of adipocyte-progenitors were similar in SAT and VAT, but macrophage infiltration in VAT was more severe than in SAT in Rarres2−/− mice. Furthermore, CD45+ immune cells supernatant from Rarres2−/− SAT promoted the differentiation of adipocyte-progenitors and 3T3-L1 cells. Adipokine array assay of CD45+ immune cells supernatant revealed that metalloproteinase inhibitor 1 (TIMP1), an inhibitor of adipogenesis, was reduced in Rarres2−/− SAT, but increased in Rarres2−/− VAT. As we specifically knocked down chemerin in SAT, TIMP1 was down-regulated and adipogenesis was promoted with reducing infiltration of macrophages. The present study demonstrates that the effects of chemerin on adipose tissue is depot different, and specific knock down chemerin in SAT promote adipogenesis and improve glucose tolerance test (GTT) and insulin tolerance test (ITT). This suggests a potential therapeutic target for chemerin in the treatment of obesity related metabolic disorder.
Keywords: Adipogenesis, Adipose tissue depot, Chemerin, Inflammation, TIMP1
Introduction
Obesity is accompanied by expansion of adipose tissue and is highly involved in the development of metabolic diseases, such as cardiovascular disease and type 2 diabetes mellitus,1 which threaten human health.2, 3, 4, 5 Adipose tissue is not only the largest reservoir depot of triglycerides, but also an endocrine organ, which regulates energy and metabolic homeostasis by secreting various adipokines.6,7 The adipokines could affect the function of adipose tissue and other organs by autocrine and paracrine.7
Generally, white adipose tissue is divided into two broad categories according to adipose tissue distribution: subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT).8 Clinical and basic research evidences support many difference between SAT and VAT, such as the release of adipokines and the infiltration of immune cells.9 In obesity, expansion of VAT is tightly associated with metabolic diseases, such as insulin resistance.10,11 However, expansion of SAT potently counteracts the strong trends toward the development of insulin resistance.12,13
Chemerin, the gene name is retinoic acid response factor 2 (Rarres2) or Tazarotene-induced gene 2 (TIG2),14 was identified as a novel adipokine in 2007,15,16 has different effects on different tissues. It has been established that chemerin promotes 3T3-L1 cell differentiation, while down-regulated chemerin impairs 3T3-L1 adipogenesis and adipocyte-progenitors biological functions.16,17 Besides, chemerin altered the fate of myoblast cells from myogenesis to adipogenesis in C2C12 myoblasts.18 Chemerin appears as a potent chemoattractant protein of immune cells in obesity,19,20 especially for macrophages. Chemerin in circulation is positively with BMI,21 exacerbates insulin resistance in human skeletal muscle cells and glucose intolerance in diabetic (db/db) mice model.22, 23, 24 However, the exacerbation of glucose intolerance is a frequent finding in Rarres2−/− mice fed with HFD.25 These controversial data suggest chemerin is not just a byproduct during obesity and is essential for the development of adipose tissue. Meanwhile, it has been reported that SNPs of the chemerin are associated with increased visceral fat mass in lean subjects, which indicated an influence of chemerin on regional fat distribution.26 However, the exact function and mechanism of chemerin in adipose tissue and adipogenesis have not been fully illustrated in vivo.
Here, we found that Rarres2−/− mice had more deposition of fat in SAT rather than VAT, and chemerin deficiency promotes adipogenesis in SAT by reducing TIMP1, but contrary results were observed in VAT. Furthermore, specific down-regulation of chemerin in SAT promotes adipogenesis and ameliorates glucose intolerance and insulin resistance. These data indicate the essential role of chemerin in modulating the development of adipose tissue is depot different.
Materials and methods
Mouse studies
Rarres2−/− mice were kindly provided by Dr. Rui He from the Department of immunology, Fudan University. Targeting strategy for the disruption of the all coding regions of Rarres2 (exon1, 2, 3) with a neomycin-resistant cassette.27 Six to eight-week male mice were maintained under 12 h light and 12 h dark cycles with unlimited access to normal diet (ND) or HFD (60% kcal fat from Research Diet) and water. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All applicable institutional and/or national guidelines for the care and use of animals were followed. All studies were approved by the Animal Care and Use Committee of the Chongqing Medical University and followed the National Institute of Health guidelines on the care and use of animals.
Glucose tolerance tests and insulin tolerance tests
For the glucose tolerance test (GTT), mice were intraperitoneally (i.p.) injected with 2 mg/g glucose after an overnight fast, whereas insulin tolerance test (ITT), 0.75 mU/g insulin was i.p. injected after 4 h fast. The blood glucose concentration in tail blood were detected using a glucometer at baseline and preassigned times after injection.
H&E staining and cell size quantitation
Standard H&E staining were performed on 5-μm paraffin sections of SAT and VAT. Cell diameter was measured in the H&E-stained sections of 6–8 individual samples in each group using Image J.
Isolation of SVF
Stromal vascular fraction (SVF) from the adipose tissue were isolated as described before.28 Briefly, adipose tissue were harvested and cut into small pieces and treated with enzymatic digestion (collagenase VIII; Sigma), filtered in 100 μm mesh filter and collected the SVF after centrifugation, resuspended with an ammonium chloride lysis buffer to remove red blood cells.
Flow cytometry analyses
The stromal vascular fraction cells were stained with mAbs specific for CD45, CD31 and Sca1 (eBioscience) and analyzed on a BD Accuri C6 flow cytometer and the data analysis was performed by using FlowJo.
Cell culture
For adipocyte-progenitors differentiation, adipose tissue was dissected and digested as described above,29 plated in 6-well CellBind plates, adherent cells were grown to confluence. And differentiation as previously described. For CD45+ cells conditional medium, SVF from the adipose tissue were digested, filtered, washed as described above, isolated as per the manufacturer's MACS protocol (Miltenyi) using positive selection for CD45. Briefly, cell suspensions were incubated with Fc block followed by CD45-conjugated magnetic beads, washed and passed over a lineage selection column. Retained cells were eluted, counted, and cultured in medium (DMEM supplemented with 10% heat-inactivated FBS, penicillin, streptomycin) for 24 h. The medium was centrifuged and collected the supernatant. Medium from 60 mm plates with no cells was used as control medium. Supernatant was filtered and used at a 1:1 dilution with fresh medium for 48 h stimulation during the first two days of adipocyte-progenitor differentiation.
Oil red O staining
In vitro differentiated cells were fixed for 20 min in 4% paraformaldehyde and stained with oil red O for 120 min. The stained fat droplets in the cells were visualized by light microscopy and photographed. To quantify the lipid content, the stained cells were treated with 100% isopropyl alcohol to extract the oil red O and measured at 520 nm by spectrophotometer.30
ELISA
TIMP1 concentrations in supernatants of CD45+ immune cells were analyzed by Mouse TIMP1 DuoSet Elisa kits from R&D system according to the manufacturer's protocol.
RNA isolation and quantitative real time-PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). Reverse transcribed involved cDNA synthesis kit (Thermo Fisher). Quantitative PCR (qPCR) involved Power SYBR green PCR master mix (Applied Biosystems, Carlsbad, CA) and a Q3 instrument (Applied Biosystems), with 18s mRNA as an endogenous control. Primer sequences for qPCR were from PrimerBank (http://pga.mgh.harvard.edu/primerbank/).
Western blotting and adipokine array assay
Cells and tissues were homogenized into lysis buffer containing 2% sodium dodecyl sulfate (SDS) and 60 mM Tris·HCl (pH6.8). Lysates were quantitated and equal amounts of protein were loaded to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Antibodies used are C/EBPα and HSP90 (Santa Cruz Biotechnology), PPARγ and Pref-1 (Cell Signaling Technology).
For the adipokine array, cells supernatants were analyzed by Mouse Adipokine Array kit (R&D), and quantified by ImageJ.
Energy expenditure
Energy expenditure was performed using CLAMS chamber (Columbus Instruments). Briefly, mice were housed in a chamber with free access to food and water for 72 h. Parameters of oxygen consumption (mL/kg·h), carbon dioxide production (mL/kg·h) and RER (VCO2/VO2) were calculated for each mouse divided by its body weight.
Generation and administration of recombinant adenovirus
The adenoviral expression vector pAd/BLOCK-iTencoding short hairpin RNA (shRNA) of Rarres2 was constructed, with shLacZ as control. The sequences are as follows: shRarres2: CCGGCATCTATGATGATGA; shLacZ: AATTTAACCGCCAGTCAGGCT. Amplified adenovirus was purified using adenovirus purification kits (Sartorius, Göttingen, Germany) and injected twice a week in SAT in 8-week-old mice for 8 weeks challenged with HFD.
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was measured by Student's unpaired t tests (2-tailed). A P value less than 0.05 was considered significant, for all data, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Results
Chemerin deficiency mice gained weight, and increased fat distribution in SAT rather than VAT
In order to clarify the roles of chemerin in adipogenesis and metabolism in vivo, we employed Rarres2−/− mice to explore the function of chemerin. Rarres2−/− mice displayed increased body weight compared with WT on HFD, but not on ND (Fig. 1A). Notably, its apparent that Rarres2−/− mice displayed increased fat distribution of SAT rather than VAT (Fig. 1B and C). Specifically, the cell size of adipocytes elevated in both SAT and VAT (Fig. 1D).
Figure 1.
Chemerin deficiency regulates adipogenesis differently in SAT and VAT. (A) Body weight of WT, Rarres2 heterozygous (Rarres2+/−) and Rarres2 knockout (Rarres2−/−) mice, fed with ND or HFD. (B) Comparison of SAT and VAT. (C) Fat index (ratio of fat weight to whole body weight) of indicated mice. (D) H&E and quantification of cell size. (E-F) Whole-body oxygen consumption rate (E) and the average values of RER (F). (G) GTT and ITT analysis. (H-I) mRNA expression levels of lipolysis and lipogenic genes. (J-K) Western blot analysis of PPARγ and C/EBPα in SAT (J) and VAT (K). All the mice were male and fed with HFD for 12 weeks if not indicated otherwise, n = 8–12/group. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Next, we assessed glucose homeostasis and oxygen consumption rates in mice. Basal oxygen consumption rates reduced while respiratory exchange ratio (RER) increased in Rarres2−/− mice (Fig. 1E and F). It indicated the increasing utilization of carbohydrate compared with WT mice. Meanwhile, Rarres2−/− mice showed deteriorated glucose tolerance and insulin resistance (Fig. 1G).
As our data show, increased SAT fat accumulation and decreased VAT fat accumulation in Rarres2−/− mice. We proposed that the effect of chemerin deficiency on different adipose depot is different, then we screened for the expression of lipogenic genes by RT-qPCR. Surprisingly, we found that the genes of triglyceride (TG) and fat acid (FA) synthesis were down-regulated while genes of lipolysis were no significant different in SAT of Rarres2−/− mice compared with WT mice (Fig. 1H), data in VAT also show same trend (Fig. 1I). However, the protein levels of PPARγ and C/EBPα (key transcript factor of adipogenesis31) were significantly increased in SAT (Fig. 1J) while decreased in VAT (Fig. 1K) in Rarres2−/− mice compared with WT mice. These data indicate that chemerin deficiency regulate adipogenesis differently in different adipose tissues.
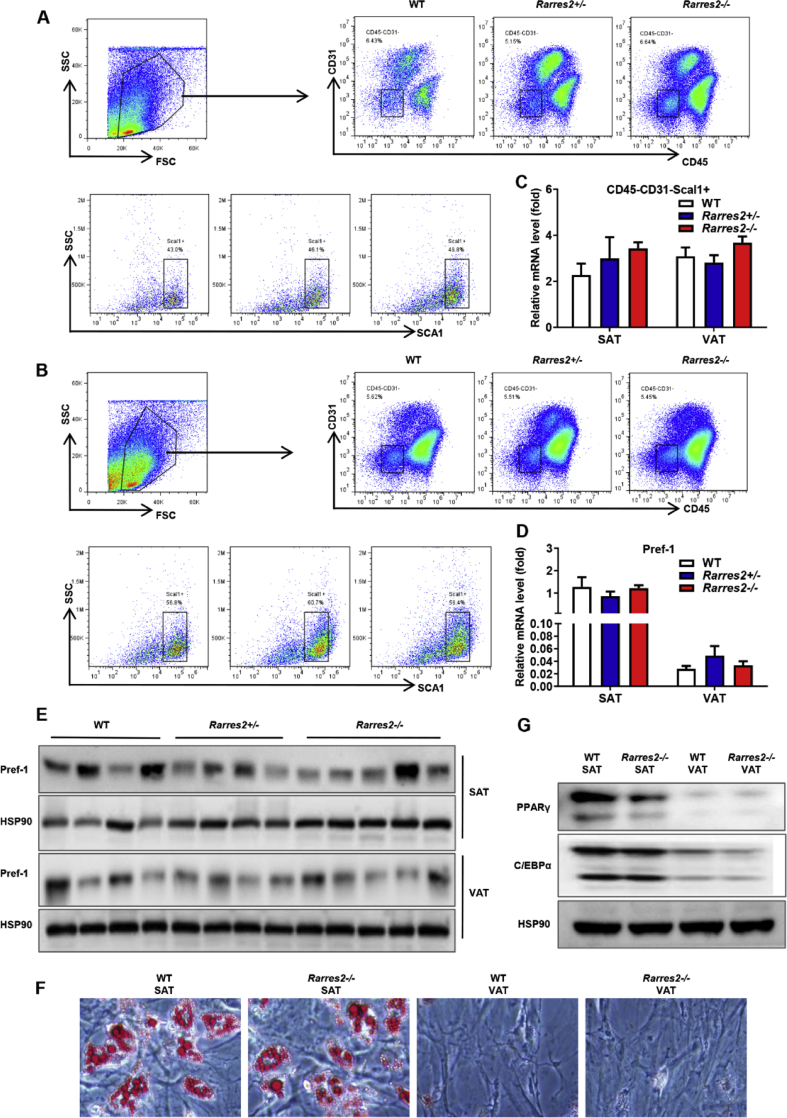
Chemerin deficiency has no effect on the proportion and differentiation capacity of adipocyte-progenitors
The process of adipogenesis is determined by several factors, such as the number, differentiation capability and microenvironment of adipocyte-progenitors. As CD31 is a marker of endothelial cells,32,33 CD45 is a marker of immune cells and SCA1 is stem cells marker,34 previous reports took CD31−CD45−Sca1+ cells as adipocyte-progenitors.35 We tested the proportion of adipocyte-progenitors by flow cytometry, found no significant difference in the proportion of adipocyte-progenitors to total cells in SAT and VAT in WT mice and Rarres2−/− mice (Fig. 2A–C). Meanwhile, the mRNA and protein expression of Pref-1 (marker of adipocyte-progenitors) were also similar in SAT and VAT (Fig. 2D, E). To explore the differentiation capability of adipocyte-progenitors in different adipose depot, we isolated adipocyte-progenitors using MACS magnetic beads and induced into adipocytes. As shown in Fig. 3F, G, there are no different on the fat accumulation and the expression of PPARγ and C/EBPα in SAT and VAT. These data suggested that chemerin deficiency did not affect the adipogenesis of adipocyte-progenitors directly.
Figure 2.
Chemerin had no effect on adipocyte-progenitors of SAT and VAT. (A-B) Representative flow cytometric analysis of adipocyte-progenitors in SAT (A) and VAT (B). (C) Percentage of adipocyte-progenitors measured by flow cytometry. (D-E) mRNA expression levels and Western blot analysis of Pref-1 in SAT and VAT. Oil red O staining (F), Western blot analysis of PPARγ and C/EBPα (G). All the mice were male and fed with HFD for 12 weeks if not indicated otherwise, n = 6–8/group.∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Figure 3.
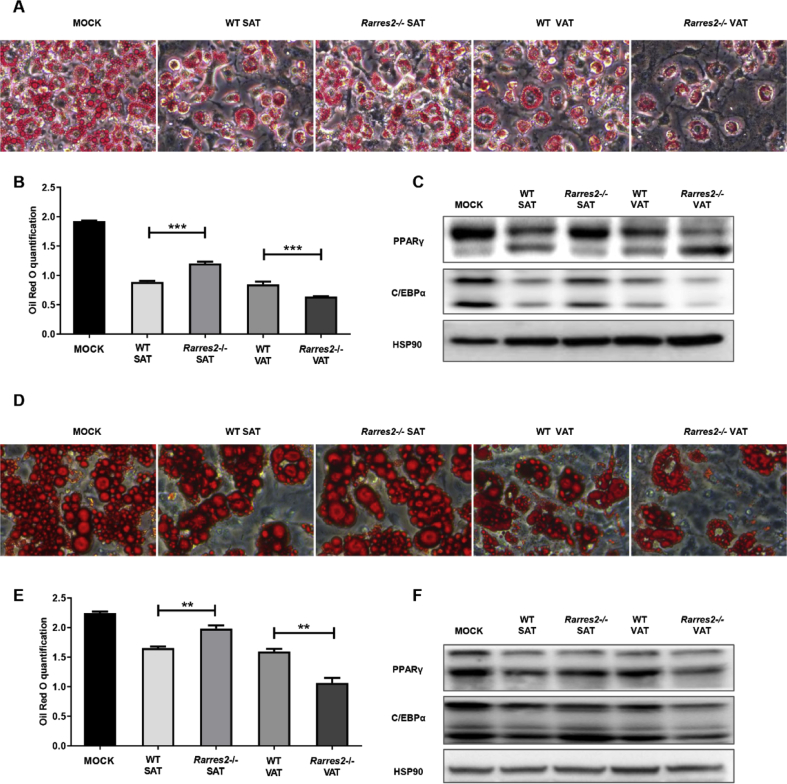
Supernatant of CD45+ cells from different depots have diverse effects on adipogenesis in vitro. 3T3-L1 (A-C) and adipocyte-progenitors sorted from 6-week-old mice (D-F) were induced by conditioned media (details in methods and materials). (A and C) Representative oil red O staining was photographed. (B and E) Oil red O staining in (A) and (C) were quantified. (C and F) Western blot analysis of PPARγ and C/EBPα. All the supernatants of CD45+ cells in adipose tissue of WT or Rarres2−/− male mice fed with HFD for 12 weeks. n = 6–8/group. ∗∗P < 0.01, ∗∗∗P < 0.001.
Supernatant of CD45+ immune cells from different depots has different effects on adipogenesis in vitro
In adipose tissue, there are many other cells excluding adipocytes. Among these cells, CD45+ immune cells contribute much to microenvironment. To confirm our hypothesis that chemerin deficiency may affect adipogenesis by regulating the microenvironment, we induced adipocyte-progenitors of WT with conditioned medium (supernatant of CD45+ immune cells isolated from WT-SAT, WT-VAT, Rarres2−/−-SAT, Rarres2−/−-VAT). In 3T3-L1 cells, lipid content and the expression of key transcription factor (PPARγ and C/EBPα) were increased with the conditioned medium from Rarres2−/−-SAT and decreased from Rarres2−/−-VAT (Fig. 3A–C). Consistent results observed in adipocyte-progenitors, which isolated from stromal vascular fraction of adipose tissue of WT mice (Fig. 3D–F). These data indicated that chemerin deficiency affect the microenvironment through CD45+ cells.
Chemerin deficiency affects the infiltration of macrophage and TIMP1 inhibits adipogenesis
What is the key regulator in the supernatant? We sorted and cultured CD45+ immune cells by magnetic beads in SAT and VAT of Rarres2−/− and WT mice, and detect the supernatant with adipokine array kit. Data revealed that expression of metalloproteinase inhibitor 1 (TIMP1) was down-regulated in CD45+ immune cells conditioned medium from Rarres2−/−-SAT and up-regulated in Rarres2−/−-VAT (Fig. 4A and B). The expression of TIMP1 detected by ELISA was consistant with the data of adipokine array analysis (Fig. 4C).
Figure 4.
TIMP1 secreted by macrophage is involved in the regulation of adipogenesis. (A) Concentration of TIMP1 in supernatant of CD45+ cells in indicated depots of mice. (B) Concentration of TIMP1 were quantified. (C) Concentration of TIMP1 in supernatant of CD45+ cells in indicated depots of mice. (D) Oil red O stain of 3T3-L1 in different concentration of TIMP1. (E) mRNA expression of TIMP1 in adipocyte and macrophage. (F) Representative IHC staining of F4/80 in SAT and VAT from WT and Rarres2−/− mice. All the mice were male and fed with HFD for 12 weeks if not indicated otherwise, n = 6–8/group. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
As chemerin is a chemokine, recent literatures showed chemerin effect macrophage infiltration. Besides, macrophage account for the majority of CD45+ cells in adipose tissue. Furthermore, as Mariana et al reported that TIMP1 is mainly expressed in macrophages but not adipocytes in adipose tissue,36 and our data confirm this (Fig. 4D). Macrophage infiltration is more severe in VAT compared with SAT in Rarres2−/− mice (Fig. 4E). We found that TIMP1 could inhibit the differentiation of 3T3-L1 (Fig. 4F). These data suggested that different levels of TIMP1 may have different effects on adipocyte-progenitors differentiation.
Specific knockdown of chemerin in SAT promotes adipogenesis and improves GTT and ITT
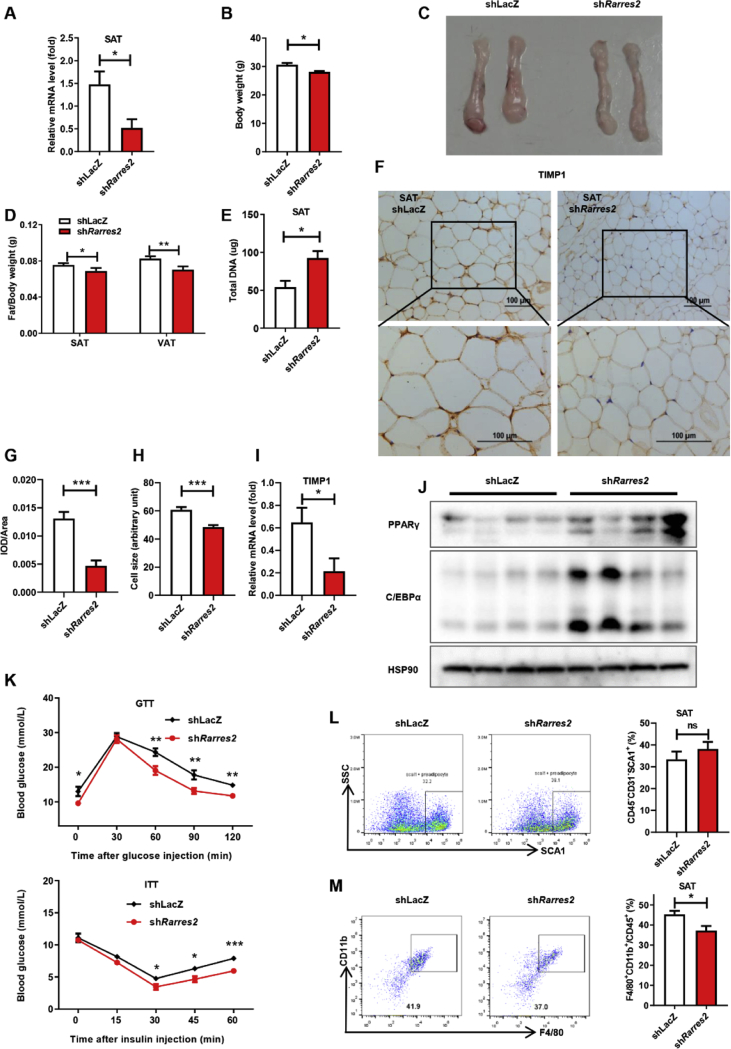
Given that expanse SAT could improve glucose tolerance,13 but our data shows Rarres2−/− mice with expanse SAT exhibiting worse glucose tolerance. Reports showed that chemerin plays an important role in glucose homeostasis by regulating multiple organs including β-cell.25 To exclude the effects of chemerin deficiency on other tissues and verify the roles of chemerin on adipogenesis in SAT, we knocked down chemerin with adenovirus specific in SAT (Fig. 5A). Body weight and the ratio of SAT or VAT to body weight were decreased (Fig. 5B–D). The cell number (based on DNA content) of SAT increased as well as the reduced fat cells volume (Fig. 5D, F–H). Thus, down-regulation of chemerin in SAT increased number of adipocytes in situ, associating with the down-regulated the expression of TIMP1 both in protein and gene level (Fig. 5F, I). Interestingly, the expression of key transcription factors also had slightly but significantly increase (Fig. 5J). Moreover, GTT and ITT analysis showed improved glucose tolerance and insulin resistance (Fig. 5K), consistence with the Rarres2−/− mice, down-regulated chemerin had no effect on the number of adipocyte-progenitors, but reduces the infiltration of macrophages in SAT (Fig. 5L and M).
Figure 5.
Knock down of chemerin specific in SAT promote adipogenesis and improve GTT and ITT. Male mice were fed with HFD and infected with shLacZ or shRarres2 twice weekly via subcutaneous injection adjacent to inguinal SAT for 8 weeks. (A) Gene expression levels of chemerin in SAT. (B) Body weight of mice. (C) Comparison of SAT. (D) Ratio of fat weight to body weight. (E) Total DNA of SAT. (F and H) Adipocyte cell size of SAT. (F and G) IHC of TIMP1 in SAT. (I) mRNA expression levels of TIMP1 in SAT. (J) Western blot analysis of PPARγ and C/EBPα. (K) GTT and ITT analysis. (L-M) Representative flow cytometric analysis of adipocyte-progenitors (L) and macrophage (M) in SAT of indicated mice. n = 6/group. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Discussion
Adipose tissue is composed not only adipocytes, but also of immune cells, endothelial cells and adipocyte-progenitors. Immunity and metabolism are two fundamental systems of adipose tissue. The presence of CD45+ immune cells (macrophages, NK cells, dendritic cells, eosinophils, etc.) recognized as an indispensable component of adipose tissue involved in physiologic and pathologic remodeling.37 In lean conditions, eosinophils, anti-inflammatory macrophages, and innate lymphoid cells, contribute to the maintenance of metabolic homeostasis. In obese conditions, NK cells secret a vast array of active substances, such as interferon gamma (IFN-γ), which promote adipose inflammation,38 pro-inflammatory macrophages are the most abundant immune cell type, which express not only inflammatory cytokines (IL-1β, TNFα)39 which aggravate inflammation and promote insulin resistance via serine phosphorylation of IRS.40 In addition, macrophages secrete osteopontin, which attracts preadipocyte proliferation, promotes the proliferation and differentiation of preadipocytes.41
Chemerin was reported as an enhancer for 3T3-L1 cell differentiation,16 and positively with BMI in circulation.21 Our data shows that development of adipose tissue is not affected in Rarres2−/− mice on ND. Rather, Rarres2−/− mice gained more body weight, and SAT increased, VAT decreased on HFD. As our data showed no significant difference in the proportion of adipocyte-progenitors and the adipogenesis capability in WT and Rarres2−/− mice (Fig. 2). The infiltration of macrophage is significantly different between SAT and VAT, and infiltration in VAT is more severe than SAT (Fig. 4F). The literature showed that the low-grade chronic inflammation perpetuated by HFD promotes the development of obesity.42 This may be the reason for infiltration of macrophages both increased in SAT and VAT, but the adipogenesis was different. Furthermore, we found the expression of TIMP1 were significantly different between SAT and VAT, in adipose tissue, TIMP1 was mainly secreted from macrophages,36 that is consistent with our data that high degree inflammation increasing TIMP1 expression.
Remodeling of extracellular matrix is essential for the differentiation of neonatal adipocytes from fibroblast-like adipocyte-progenitors during body fat formation in vivo.43,44 In this process, as reports revealed that MMP9 promotes angiogenesis45,46 and adipogenesis.47 TIMP1 negatively regulated adipogenesis due to directly inhibit adipocyte differentiation48 and abated adipogenesis by antagonizing the function of MMPs.46
Considering that the increase in SAT improve glucose tolerance.13 We were puzzled by deteriorated glucose tolerance in Rarres2−/− mice which has more SAT. Based on chemerin could affect multiple organs, the chemerin deficiency resulted in the impaired function of hepatic glucose production and islet β-cell.25 In order to clarify the exact roles of chemerin in adipose tissue, we specifically knocked down chemerin in SAT to exclude the effect of chemerin on hepatocyte and islet β-cell, and these mice displayed improved adipogenesis, glucose tolerance and insulin resistance (Fig. 5) accompany with decreased macrophage infiltration and TIMP1.
Conclusion
The study demonstrated that the down-regulated chemerin inhibited the expression of TIMP1 by decreasing the infiltration of macrophage in SAT, we first revealed chemerin deficiency affect adipogenesis is different in SAT and VAT in vivo. The specific down-regulation of chemerin in SAT promotes adipogenesis and improves GTT and ITT. This suggests a potential therapeutic target for chemerin in the treatment of obesity related metabolic disorders.
Conflict of Interests
The authors declare no conflict of interest.
Funding
Fund from Joint International Research Laboratory of Reproduction and Development, Institute of Life Science, Chongqing Medical University. National Key R&D Program of China [grant numbers 2018YFA0800401 to X. Li]; National Natural Science Foundation of China [grant numbers 81770861 and 31571401 to X. Li]; Chongqing Science and Technology Foundation [grant number cstc2018jcyjAX0232]; Science and Technology Research Program of Chongqing Municipal Education Commission [grant number KJZD-K201800402]; The Outstanding Talent Fund of Chongqing Medical University [grant number BJRC201707]; Chongqing education committee [grant number CYB17105].
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Hajer G.R., van Haeften T.W., Visseren F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 2.Haslam D.W., James W.P. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Ng M., Fleming T., Robinson M. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshin A., Forouzanfar M.H., Reitsma M.B. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedrich M.J. Global obesity epidemic worsening. JAMA. 2017;318(7) doi: 10.1001/jama.2017.10693. [DOI] [PubMed] [Google Scholar]
- 6.Galic S., Oakhill J.S., Steinberg G.R. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gesta S., Tseng Y.H., Kahn C.R. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Romacho T., Elsen M., Rohrborn D., Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf) 2014;210(4):733–753. doi: 10.1111/apha.12246. [DOI] [PubMed] [Google Scholar]
- 10.Pouliot M.C., Despres J.P., Nadeau A. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41(7):826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki Y., Glass L., Triplitt C., Wajcberg E., Mandarino L.J., DeFronzo R.A. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metabol. 2002;283(6):E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.Y., van de Wall E., Laplante M. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusminski C.M., Holland W.L., Sun K. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18(10):1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagpal S., Patel S., Jacobe H. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol. 1997;109(1):91–95. doi: 10.1111/1523-1747.ep12276660. [DOI] [PubMed] [Google Scholar]
- 15.Bozaoglu K., Bolton K., McMillan J. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148(10):4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 16.Goralski K.B., McCarthy T.C., Hanniman E.A. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282(38):28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y., Liu P., Jiao W., Meng J., Feng J. Gax suppresses chemerin/CMKLR1-induced preadipocyte biofunctions through the inhibition of Akt/mTOR and ERK signaling pathways. J Cell Physiol. 2018;233(1):572–586. doi: 10.1002/jcp.25918. [DOI] [PubMed] [Google Scholar]
- 18.Li H.X., Chen K.L., Wang H.Y., Tang C.B., Xu X.L., Zhou G.H. Chemerin inhibition of myogenesis and induction of adipogenesis in C2C12 myoblasts. Mol Cell Endocrinol. 2015;414:216–223. doi: 10.1016/j.mce.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Wittamer V., Franssen J.D., Vulcano M. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198(7):977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zabel B.A., Nakae S., Zuniga L. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med. 2008;205(10):2207–2220. doi: 10.1084/jem.20080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst M.C., Sinal C.J. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metabol TEM. 2010;21(11):660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Sell H., Laurencikiene J., Taube A. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 2009;58(12):2731–2740. doi: 10.2337/db09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst M.C., Issa M., Goralski K.B., Sinal C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151(5):1998–2007. doi: 10.1210/en.2009-1098. [DOI] [PubMed] [Google Scholar]
- 24.Chakaroun R., Raschpichler M., Kloting N. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metab Clin Exp. 2012;61(5):706–714. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M., Okimura Y., Iguchi G. Chemerin regulates beta-cell function in mice. Sci Rep. 2011;1 doi: 10.1038/srep00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mussig K., Staiger H., Machicao F. RARRES2, encoding the novel adipokine chemerin, is a genetic determinant of disproportionate regional body fat distribution: a comparative magnetic resonance imaging study. Metab Clin Exp. 2009;58(4):519–524. doi: 10.1016/j.metabol.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y., Yang X., Liu W. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene. 2017;36(25):3599–3608. doi: 10.1038/onc.2016.516. [DOI] [PubMed] [Google Scholar]
- 28.Xiao L., Yang X., Lin Y. Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity. Int J Obes. 2016;40(1):112–120. doi: 10.1038/ijo.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M.W., Odegaard J.I., Mukundan L. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1–2):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S.F., Guo L., Qian S.W. G9a is transactivated by C/EBPbeta to facilitate mitotic clonal expansion during 3T3-L1 preadipocyte differentiation. Am J Physiol Endocrinol Metabol. 2013;304(9):E990–E998. doi: 10.1152/ajpendo.00608.2012. [DOI] [PubMed] [Google Scholar]
- 31.Tang Q.Q., Lane M.D. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 32.Wu S.M., Fujiwara Y., Cibulsky S.M. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Lai L., Alaverdi N., Maltais L., Morse H.C., 3rd Mouse cell surface antigens: nomenclature and immunophenotyping. J Immunol. 1998;160(8):3861–3868. [PubMed] [Google Scholar]
- 34.Staszkiewicz J., Gimble J.M., Manuel J.A., Gawronska-Kozak B. IFATS collection: stem cell antigen-1-positive ear mesenchymal stem cells display enhanced adipogenic potential. Stem cells (Dayton, Ohio) 2008;26(10):2666–2673. doi: 10.1634/stemcells.2008-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S.F., Zhu C.S., Wang Y.M. Downregulation of beta1,4-galactosyltransferase 5 improves insulin resistance by promoting adipocyte commitment and reducing inflammation. Cell Death Dis. 2018;9(2) doi: 10.1038/s41419-017-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toricelli M., Melo F.H., Peres G.B. Timp1 interacts with beta-1 integrin and CD63 along melanoma genesis and confers anoikis resistance by activating PI3-K signaling pathway independently of Akt phosphorylation. Mol Cancer. 2013;12 doi: 10.1186/1476-4598-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee B.C., Kim M.S., Pae M. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metabol. 2016;23(4):685–698. doi: 10.1016/j.cmet.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 40.Hotamisligil G.S., Peraldi P., Budavari A., Ellis R., White M.F., Spiegelman B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271(5249):665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y.H., Petkova A.P., Granneman J.G. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metabol. 2013;18(3):355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilich J.Z., Kelly O.J., Kim Y., Spicer M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh Hig Rada Toksikol. 2014;65(2):139–148. doi: 10.2478/10004-1254-65-2014-2541. [DOI] [PubMed] [Google Scholar]
- 43.Sun K., Tordjman J., Clement K., Scherer P.E. Fibrosis and adipose tissue dysfunction. Cell Metabol. 2013;18(4):470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maquoi E., Munaut C., Colige A., Collen D., Lijnen H.R. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51(4):1093–1101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- 45.Padwal M., Siddique I., Wu L. Matrix metalloproteinase 9 is associated with peritoneal membrane solute transport and induces angiogenesis through beta-catenin signaling. Nephrol Dial Transplant. 2017;32(1):50–61. doi: 10.1093/ndt/gfw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 47.Grippo P.J., Fitchev P.S., Bentrem D.J. Concurrent PEDF deficiency and Kras mutation induce invasive pancreatic cancer and adipose-rich stroma in mice. Gut. 2012;61(10):1454–1464. doi: 10.1136/gutjnl-2011-300821. [DOI] [PubMed] [Google Scholar]
- 48.Meissburger B., Stachorski L., Roder E., Rudofsky G., Wolfrum C. Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) controls adipogenesis in obesity in mice and in humans. Diabetologia. 2011;54(6):1468–1479. doi: 10.1007/s00125-011-2093-9. [DOI] [PubMed] [Google Scholar]