Abstract
Background
Memantine, a noncompetitive N-methyl-D-aspartate receptor antagonist, has been approved for use in Alzheimer’s disease, but an increasing number of studies have investigated its utility for neuropsychiatric disorders. Here, we characterized a novel compound, fluoroethylnormemtantine (FENM), which was derived from memantine with an extra Fluor in an optimized position for in vivo biomarker labeling. We sought to determine if FENM produced similar behavioral effects as memantine and/or if FENM has beneficial effects against fear, avoidance, and behavioral despair.
Methods
We administered saline, FENM, or memantine prior to a number of behavioral assays, including paired-pulse inhibition, open field, light dark test, forced swim test, and cued fear conditioning in male Wistar rats.
Results
Unlike memantine, FENM did not produce nonspecific side effects and did not alter sensorimotor gating or locomotion. FENM decreased immobility in the forced swim test. Moreover, FENM robustly facilitated fear extinction learning when administered prior to either cued fear conditioning training or tone reexposure.
Conclusions
These results suggest that FENM is a promising, novel compound that robustly reduces fear behavior and may be useful for further preclinical testing.
Keywords: FENM, extinction learning, cued fear conditioning, PTSD, NMDAR antagonist
Significance Statement.
N-methyl-D-aspartate (NMDA) receptors have been previously implicated as a potential target for the prevention and treatment of stress-related psychiatric disorders. Recently, a novel NMDA receptor antagonist, fluoroethylnormemantine (FENM), was derived from memantine. Here, using a variety of behavioral assays, we characterized the behavioral properties of FENM in rats. We found that FENM significantly reduced behavioral despair and facilitated extinction learning without altering sensorimotor function. Our findings indicate that FENM may be a suitable compound for further preclinical testing in a variety of stress-based behavioral assays.
Introduction
N-methyl-D-aspartate (NMDA) receptor (NMDAR) function has been increasingly implicated in the pathophysiology of neuropsychiatric diseases, including mood disorders and Alzheimer’s disease (AD) (Paul, 1997; Ravindran and Stein, 2009; Barkus et al., 2010; Steckler and Risbrough, 2012; Ates-Alagoz and Adejare, 2013; Lakhan et al., 2013; Liu et al., 2019). Expressed abundantly throughout the brain, NMDARs play a vital role in excitatory neurotransmission and synaptic plasticity. Accordingly, disruption of NMDAR function, whether due to natural disease causes or pharmacological intervention, can significantly alter cognitive function and emotionality. A number of novel NMDAR antagonists have been demonstrated as efficacious treatments against a variety of neuropsychiatric disorders. For instance, (R,S)-ketamine is widely known for its rapid-acting, long-lasting antidepressant actions as well as its efficacy in treating treatment-resistant depression (Berman et al., 2000; Zarate et al., 2006; aan het Rot et al., 2010; Murrough et al., 2013). Moreover, intranasal (S)-ketamine (Spravato), a stereospecific derivative of (R,S)-ketamine that specifically targets NMDARs, was recently approved by the FDA as an adjunctive treatment for treatment-resistant depression and has shown strong, rapid efficacy for reducing symptoms of depression, including suicidality (Daly et al., 2018, 2019; Fedgchin et al., 2019; Popova et al., 2019; Fu et al., 2020; Ionescu et al., 2020; Nijs et al., 2020; Perez-Ruixo et al., 2020; Saad et al., 2020; Wajs et al., 2020; ClinicalTrials.gov, NCT0260929, NCT02094378, NCT02133001, NCT02343289, NCT02345148, NCT02606084, NCT02611505, NCT02674295, NCT02857777, NCT03298906). Previous studies have also shown that (R,S)-ketamine–induced NMDAR inhibition may also prevent stress-induced behavioral despair as well as attenuate learned fear when administered prior to stress (Amat et al., 2016; Brachman et al., 2016; McGowan et al., 2017; Dolzani et al., 2018; Mastrodonato et al., 2018; Pham et al., 2018; Chen et al., 2020b). Despite these promising studies, (R,S)-ketamine presents a challenge for clinical development because of its nonspecific side effects, including psychotropic actions and high abuse potential. Therefore, efforts are currently underway to develop NMDAR antagonists with similar efficacy but reduced side effects.
Interestingly, although many alternative NMDAR antagonists (e.g., rapastinel, MK-801) have shown promising antidepressant results in both rodents and humans, to date, these compounds have failed to show significant efficacy in reducing depressive symptoms compared with placebo controls in clinical trials (Ates-Alagoz and Adejare, 2013; Moskal et al., 2014; Newport et al., 2015; Gerhard et al., 2016; Yang et al., 2016; Kato et al., 2018; Kadriu et al., 2019; Kato and Duman, 2020; ClinicalTrials.gov, NCT02192099, NCT02267629, NCT02932943, NCT02943564, NCT02943577, NCT02951988, NCT03002077, NCT03352453, NCT03560518, NCT03575776, NCT03614156, NCT03668600, NCT03799900, NCT03814733, NCT03855865). One such drug is memantine, a noncompetitive open-channel NMDAR antagonist (Kishi et al., 2017a). Memantine is clinically used as a treatment for AD and has been shown to improve memory, awareness, and the ability to perform daily tasks (Kishi et al., 2017b). Although some early studies indicated that memantine may exhibit antidepressant efficacy, a longitudinal study showed that memantine is no better than placebo at managing symptoms of depression (Moryl et al., 1993; Kishi et al., 2017a). Therefore, despite its lack of antidepressant actions, memantine does exert pro-cognitive effects. Thus, examining analogues of memantine may reveal novel NMDAR antagonist compounds that can improve cognition as well as exhibit antidepressant and/or anxiolytic efficacy.
Recently, a novel radiolabeled compound, [18F]- fluoroethylnormemantine (FENM), was derived from memantine as a novel positron emission tomography (PET) tracer (Salabert et al., 2015, 2018). With a Ki of 3.510-6 M and high lipophilicity (logD = 1.93), [18F]-FENM stabilized 40 minutes after injection with approximately 0.4% of the original dose present in the brain. Combined ex vivo autoradiography and immunohistochemistry indicated that [18F]-FENM strongly colocalizes with NMDARs in the cortex and cerebellum. Intriguingly, this colocalization is blocked by injection of (R,S)-ketamine, suggesting that FENM exhibits a lower affinity to the NMDAR receptor than (R,S)-ketamine. Moreover, because both compounds bind to phencyclidine sites in the NMDAR channel pore, these data suggest that the compounds may also exert similar behavioral effects. However, while the antidepressant-like effects of FENM remain unknown, recent data indicate that FENM enhances cognitive function and exerts neuroprotective properties in a mouse model of AD (Couly et al., 2020). In this study, Couly and colleagues found that FENM reversed deficits in long-term memory, navigation, and place learning, and object recognition in a pharmacological model of AD. Interestingly, compared with the effects of memantine, the authors showed that FENM improved spatiotemporal orientation in the Hamlet test while memantine did not affect behavior. FENM’s behavioral actions were found to correspond with a reduction in inflammatory cytokines and neuronal cell loss in the hippocampus. Thus, although FENM shows potential for enhancing cognition and protecting age-related brain impairments, it is still unknown whether the drug can reverse stress-related maladaptive behaviors.
Here, we aimed to characterize the effects of FENM on avoidance behavior, behavioral despair, and extinction learning in rats. A single injection of saline, FENM, or memantine was administered acutely prior to a range of behavioral assays, including paired-pulse inhibition (PPI), open field (OF), forced swim test (FST), and cued fear conditioning (FC) and extinction. FENM did not affect sensorimotor behavior or avoidance behavior. FENM decreased behavioral despair in the FST and reduced fear behavior when administered at a variety of timepoints prior to cued FC and extinction training. These data indicate that FENM is a novel compound with robust fear-reducing properties and is a promising candidate for preclinical development as a potential treatment for fear-related disorders.
METHODS
For a full description of Methods, please refer to the Supplemental Methods.
Rats
Three hundred male Wistar rats were purchased from Janvier Labs (Le Genest-Saint-Isle, France). Rats were ordered at 250–274 g and were tested after 1 week of acclimatization. Rats were kept 2 per cage in a 12-hour-light/-dark (6:00 am–6:00 pm) colony room at 22°C. Food and water were provided ad libitum. Rats were used once per behavioral experiment. All procedures were approved by a local ethics committee (French Ministry of Health and Research Authorization N°APAFIS#3571-2015091614517975v7) in accordance with the European Communities Council Directive (2010/63/EU) and the European Union guidelines.
Drugs
All drugs were resuspended in saline and made fresh for each experiment.
FENM
FENM was administered in a single dose at 1, 3, 5, 10, or 20 mg/kg of body weight. FENM was generated by M2i (Saint-Cloud, France).
Memantine
Memantine was administered in a single dose at 1, 3, or 10 mg/kg of body weight. Memantine was provided by M2i (Saint-Cloud, France) and purchased from TCI Chemicals (Tokyo, Japan).
Behavioral Methods
All behavioral tests were performed in the active phase.
PPI
The apparatus consisted of 4 startle chambers (SRLAB, San Diego Instruments, CA) containing a transparent Plexiglas tube (diameter 8.2 cm, length 20 cm) mounted on a Plexiglas frame within a ventilated enclosure. Acoustic noise bursts were presented via a speaker mounted 24 cm above the tube. Throughout the session, a background noise level of 68 dB was maintained. A piezoelectric accelerometer mounted below the frame detected and transduced motion within the tube. Startle amplitudes were defined as the average of 100 1-ms stabilimeter readings collected from stimulus onset. Rats were run in groups of 4. Each rat was put into the PPI chamber for a 5-minute acclimatization period with a 68-dB background noise. Following this period, 10 startle pulses (120 dB, 40-ms duration) were presented with an average inter-trial interval of 15 seconds. Then, no stimulus (background noise, 68 dB), prepulses alone (72, 76, 80, or 84 dB, 20-millisecond duration), startle pulses alone, and prepulses followed (80 millisecond later) by startle pulses, were presented 10 times, randomly distributed over the next 32 minutes. The percentage of PPI induced by each prepulse intensity was calculated as: 100[(SP − SPP)/SP], with SP being the average startle amplitude following the startle pulses and SPP being the average startle response following the combination of a certain prepulse and the startle pulse.
OF
The OF test was conducted in an arena (43.2 cm × 43.2 cm) with transparent acrylic walls and white floor (Med Associates Inc., St. Albans, VT). Rats were individually placed in the OF arena for 120 minutes. Locomotor activity was monitored using a two 16-beam infrared system. Data from the first 30 minutes were included in analysis. Velocity and total distance travelled were analyzed using the Activity Monitor software (Med Associates).
Light Dark Test (LDT)
The apparatus for the light/dark test consisted of a box (40 cm × 40 cm × 32 cm) divided into dark (black walls, floor and roof, 5 lux) and light (white walls and floor, no roof, 600 lux) compartments of equal volume (40 × 40 × 32 cm). The compartments were connected via a small opening (10 × 10 cm) enabling transition between the 2 boxes. Rats were placed in the light compartment, and the time spent in each compartment during the 10 minutes test was assessed online via a video camera located above the box. Behavior was automatically analyzed using video tracking software (View Point, Lyon, France).
FST
For the FST, rats were individually placed into glass cylinders (40 cm high, 18 cm in diameter) containing 28 cm of water at 23°C ± 2°C for 15 minutes (pre-swim) and then gently dried and returned to their home cages. They were placed again in the cylinders 24 hours later, and the 6-minute FST was conducted. All sessions were recorded with an automated video tracking system (View Point). The total duration of immobility as well as bursting were measured throughout the second trial.
Cued FC
Cued FC occurred in a standard conditioning chamber (Context A) (VCF-007, Med Associates) kept inside a sound-attenuating cubicle. This chamber had the internal dimensions of 30 × 24 × 33 cm, with aluminum sidewalls, an opaque polycarbonate rear wall, and a transparent Plexiglas door. The grid floor was connected to a shock scrambler and shock generator, and approximately 0.2 mL of 1% ammonium hydroxide was put in the collection pan. The chamber was dark except for 2 infrared light sources located above the training box (NIR200, Med Associates). Rats received 3 tone-shock pairings at 180 seconds, 381 seconds, and 582 seconds, following placement in the chamber and the launching of the Med Associates program. Each tone was 20 seconds long and each shock, which followed the tone, lasted 1 second. Each foot shock was 1.0 mA based on prior pilot studies.
Extinction
Thirty minutes before either extinction session, rats were injected with saline or FENM and placed into context B. Context B was the same darkened chamber as Context A but with a white plastic floor and curved wall inserts scented with 1% acetic acid in the collection pan. Each extinction session had twenty-four 20-second tone presentations, separated by 35 seconds. Videos were scored by hand by an experimenter blind to treatment group to avoid software-induced false freezing. Freezing was quantified over the entire session.
Statistical Analysis
All data were analyzed using Prism 7.0 and 8.0 (GraphPad Software, La Jolla, CA). Alpha was set to .05 for all analyses. Data were confirmed as normally distributed with equal variance using a Shapiro-Wilk normality test and by calculating the variance. A 1-way, 2-way, or repeated-measures ANOVA (RMANOVA) was conducted where appropriate. Post-hoc analysis was conducted when the overall ANOVA (e.g., drug) was significant or when the interaction between the 2 factors (e.g., drug × tone or drug × time) was significant. Post-hoc Dunnett’s tests were used to determine the statistical significance of multiple experimental groups against a single control group. All statistical tests and P values are listed in supplementary Table 1.
RESULTS
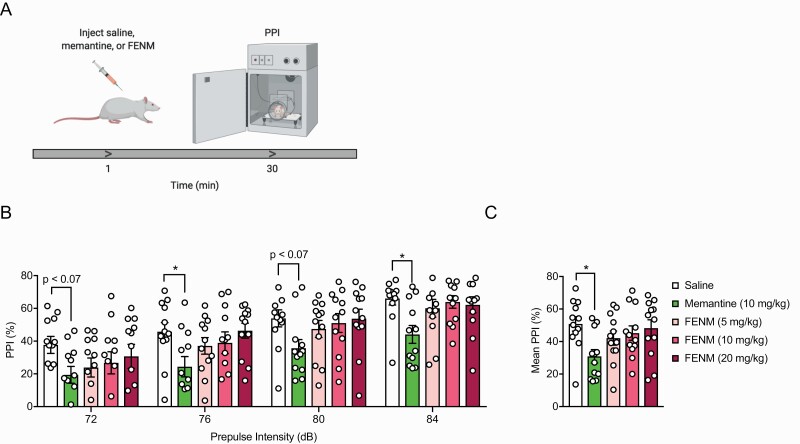
Memantine, But Not FENM, Decreases Prepulse Inhibition in Rats
Reduced PPI is indicative of deficient sensorimotor gating and is present in various neuropsychiatric disorders, including schizophrenia and Tourette’s syndrome. It has previously been shown that memantine can impair normal PPI startle responses (Swerdlow et al., 2009). We therefore sought to determine whether FENM would affect sensorimotor gating similarly to memantine. Rats were administered saline, memantine (10 mg/kg), or FENM (5, 10, or 20 mg/kg). Thirty minutes later, rats were tested in a PPI assay (Figure 1a). Memantine, but not FENM, reduced prepulse inhibition of the startle response compared with saline controls (Figure 1b; RMANOVA drug: F(4,55) = 2.94, P = .0283, pulse: F(3,165) = 68.35, P < .0001, drug × pulse: F(12,165) = 0.5241, P = .8970; Dunnett’s multiple comparisons: Sal vs memantine (10 mg/kg): P = .0103, Sal vs FENM (5 mg/kg): P = .4591, Sal vs FENM (10 mg/kg): P = .7847; Sal vs FENM (20 mg/kg): P = .9824). When mean freezing was averaged across the entire trial, memantine, but not FENM, significantly lowered the mean startle response (Figure 1c; ANOVA drug: F(4,55) = 2.94, P = .0283; Dunnett’s multiple comparisons: Sal vs memantine (10 mg/kg) P = .0103, Sal vs FENM (5 mg/kg) P = .4591, Sal vs FENM (10 mg/kg) P = .7847, Sal vs FENM (20 mg/kg) P = .9824). These results indicate that FENM does not alter sensorimotor gating like its parent compound memantine.
Figure 1.
Fluoroethylnormemantine (FENM) does not alter the startle response during pre-pulse inhibition. (a) Experimental protocol. (b) Memantine consistently reduced the startle response at multiple stimulus intensities compared with saline-administered rats. FENM (5, 10, or 20 mg/kg) did not alter the startle response. (c) Memantine, but not FENM, significantly reduced mean freezing compared with the saline control group (n = 12 male rats/group). Error bars represent ± SEM. **P < .01. Abbreviation: PPI, paired-pulse inhibition.
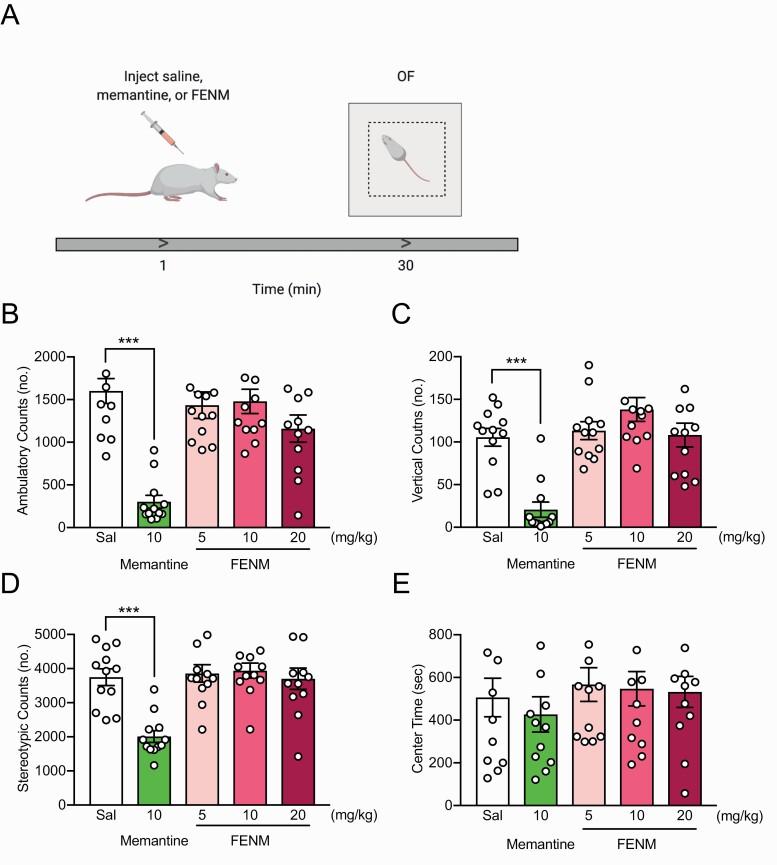
FENM Does Not Alter Locomotion or Avoidance Behavior in Rats
Memantine has previously been shown to reduce ambulatory activity and rearing when administered acutely before the OF (Kos and Popik, 2005). Here, we sought to determine if FENM, similarly to its parent compound, also altered locomotor behavior. Rats were administered saline, memantine (10 mg/kg), or FENM (5, 10, or 20 mg/kg) and 30 minutes later placed into an OF (Figure 2a). Memantine, but not FENM, decreased ambulatory [Figure 2b; ANOVA: drug F(4,55) = 14.19, P < .0001; Dunnett’s multiple comparisons: Sal vs memantine (10 mg/kg) P < .0001, Sal vs FENM (5 mg/kg) P = .8133, Sal vs FENM (10 mg/kg) P = .9259, Sal vs FENM (20 mg/kg) P = .0939], vertical [Figure 2c; ANOVA: drug F(4,55) = 14.430, P < .0001; Dunnett’s multiple comparisons: Sal vs memantine (10 mg/kg) P < .0001, Sal vs FENM (5 mg/kg) P = .9720, Sal vs FENM (10 mg/kg) P = .1678, Sal vs FENM (20 mg/kg) P = .9993], and stereotypic [Figure 2d; ANOVA: F(4,55) = 10.86, P < .0001; Dunnett’s multiple comparisons: Sal vs memantine (10 mg/kg) P < .0001, Sal vs FENM (5 mg/kg) P = .9933, Sal vs FENM (10 mg/kg) P = .95, Sal vs FENM (20 mg/kg) P = .9998] counts in the OF. All groups spent a comparable amount of time in the center (Figure 2e; ANOVA: drug F(4,55) = 0.449, P = .4487). These data show that, unlike memantine, FENM does not alter locomotion.
Figure 2.
Fluoroethylnormemantine (FENM) does not alter locomotion or exploratory behavior in the open field. (a) Experimental protocol. (b–d) Memantine, but not FENM, significantly reduced locomotion, rearing, and stereotypic behavior, respectively, in the open field (OF) compared with saline-administered rats. (e) Time in the center of the OF was comparable across all drug groups. (n = 12 male rats/group). Error bars represent ± SEM. ***P < .0001. Abbreviation: OF, open field.
Next, to further expand on on the locomotor effects, we utilized an LDT. Here, rats were again administered saline, memantine (1, 3, or 10 mg/kg), or FENM (1, 3, or 10 mg/kg) 30 minutes before the LDT (supplementary Figure 1a). The time spent in the light compartment did not differ between the groups (supplementary Figure 1b; ANOVA: drug F(6,76) = 2.13, P = .0595). As observed in the OF assay, memantine (10 mg/kg), but not FENM, significantly reduced the number of transitions between the light and dark compartments [supplementary Figure 1c; ANOVA: drug F(6,76) = 2.314, P = .0416; Dunnett’s multiple comparisons: Sal vs memantine (1 mg/kg) P = .5480, Sal vs memantine (3 mg/kg) P = .0533, Sal vs memantine (10 mg/kg) P = .0174, Sal vs FENM (1 mg/kg) P = .8739, Sal vs FENM (3 mg/kg) P = .9781, Sal vs FENM (10 mg/kg) P = .3863]. These data suggest that FENM does not significantly alter avoidance behavior.
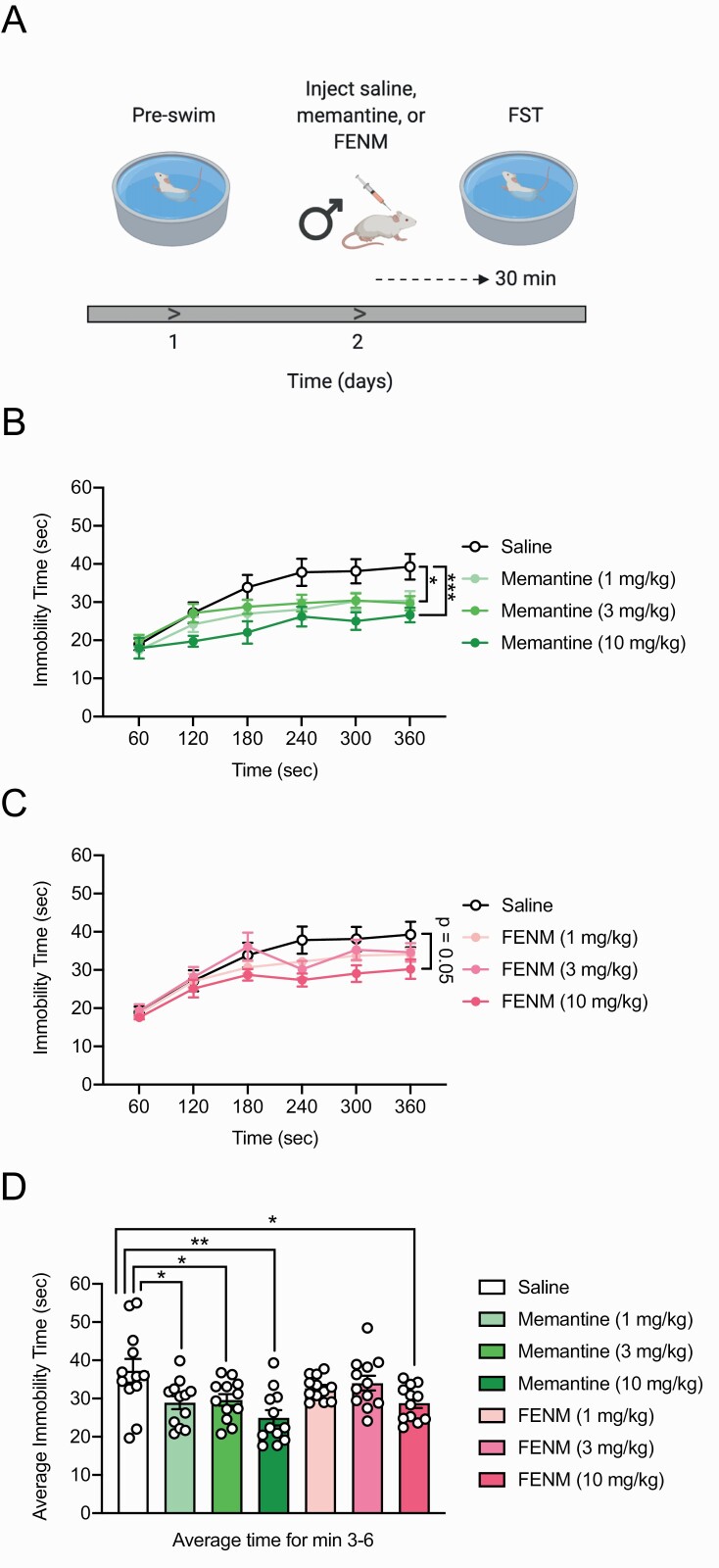
FENM Decreases Behavioral Despair in Rats
Previous data suggest that memantine may reduce behavioral despair in rats (Moryl et al., 1993). Here, we sought to test if FENM altered behavioral despair (Figure 3a). Male Wistar rats were given a 15-minute preswim trial, which functioned as an initial stressor (Porsolt et al., 1977). Twenty-four hours later, saline, memantine (1, 3, or 10 mg/kg), or FENM (1, 3, or 10 mg/kg) was injected, and 30 minutes later, a second FST session was administered for 6 minutes. Here, drug dosages for both memantine and FENM were chosen in accordance with a previous study indicating that lower doses of memantine (e.g., 1, 3, or 10 mg/kg) can reduce behavioral despair in rodents (Almeida et al., 2006). Consistent with this publication, memantine (1, 3, and 10 mg/kg) decreased immobility time [Figure 3b and d; RMANOVA: drug F(6,77) = 3.649, P = .0031, time F(5,385) = 68.02, P < .0001, drug × time F(30,385) = 1.503, P = .0461; Dunnett’s multiple comparisons: Sal vs memantine (1 mg/kg) P = .0435, Sal vs memantine (3 mg/kg) P = .1678, Sal vs memantine (10 mg/kg) P = .0007; ANOVA: drug F(6,77) = 4.558, P = .0005; Dunnett’s multiple comparisons: Sal vs memantine (1 mg/kg) P = .0133, Sal vs memantine (3 mg/kg) P = .0283, Sal vs memantine (10 mg/kg) P = .0001] and increased bursting duration in the FST (supplementary Table 1) (Almeida et al., 2006). FENM (10 mg/kg) exerted a trending reduction in overall immobility time and was effective in reducing mean immobility time (Figure 3c–d; RMANOVA: drug F(6,77) = 3.649, P = .0031, time F(5,385) = 68.02, P < .0001, drug × time F(30,385) = 1.503, P = .0461. Dunnett’s multiple comparisons: Sal vs FENM (1 mg/kg) P = .5903, Sal vs FENM (3 mg/kg) P = .9161, Sal vs FENM (10 mg/kg) P = .0504; ANOVA: drug F(6,77) = 4.558, P = .0005; Dunnett’s multiple comparisons: Sal vs FENM (1 mg/kg) P = .3384, Sal vs FENM (3 mg/kg) P = .6842, Sal vs FENM (10 mg/kg) P = .0125] as well as increasing bursting duration (supplementary Table 1). These data indicate that FENM is effective at reducing behavioral despair.
Figure 3.
Fluoroethylnormemantine (FENM) reduces behavioral despair in the forced swim test. (a) Experimental protocol (b) Memantine at all doses significantly reduced immobility time in the forced swim test (FST) compared with saline-administered rats. (c) FENM (1 and 3 mg/kg) did not alter immobility time compared with saline. FENM (10 mg/kg) reduced overall immobility time, but this decrease was not significant. (d) Memantine (1, 3, and 10 mg/kg) and FENM (10 mg/kg) significantly reduced mean immobility time, averaged over minutes 3–6, in the FST. (n = 12 male rats/group). Error bars represent ± SEM. *P < .05, **P < .01, ***P < .0001. Abbreviation: FST, forced swim test.
FENM Attenuates Learned Fear and Facilitates Extinction Learning in Rats
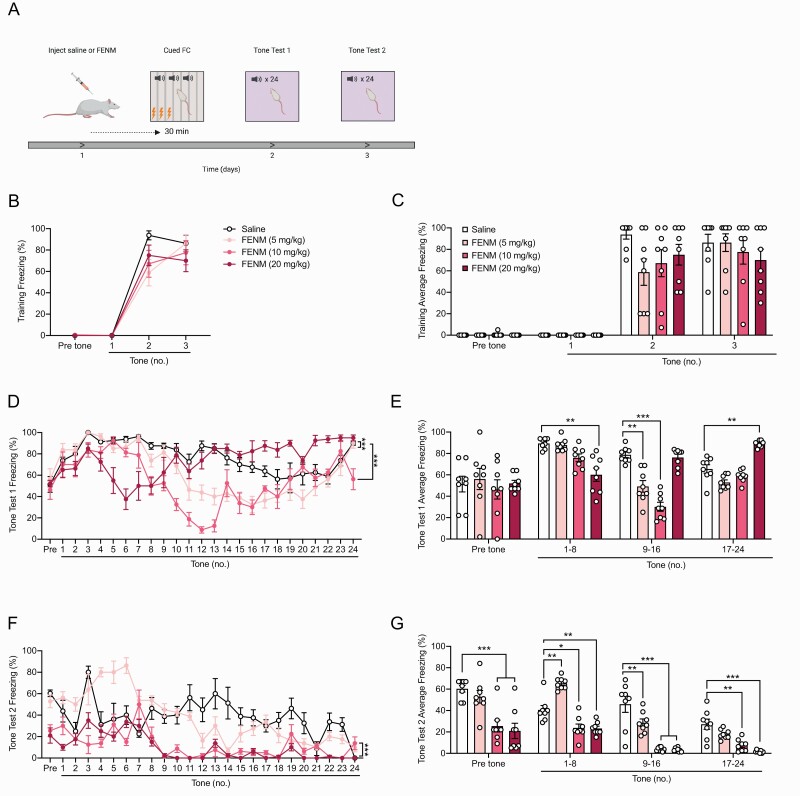
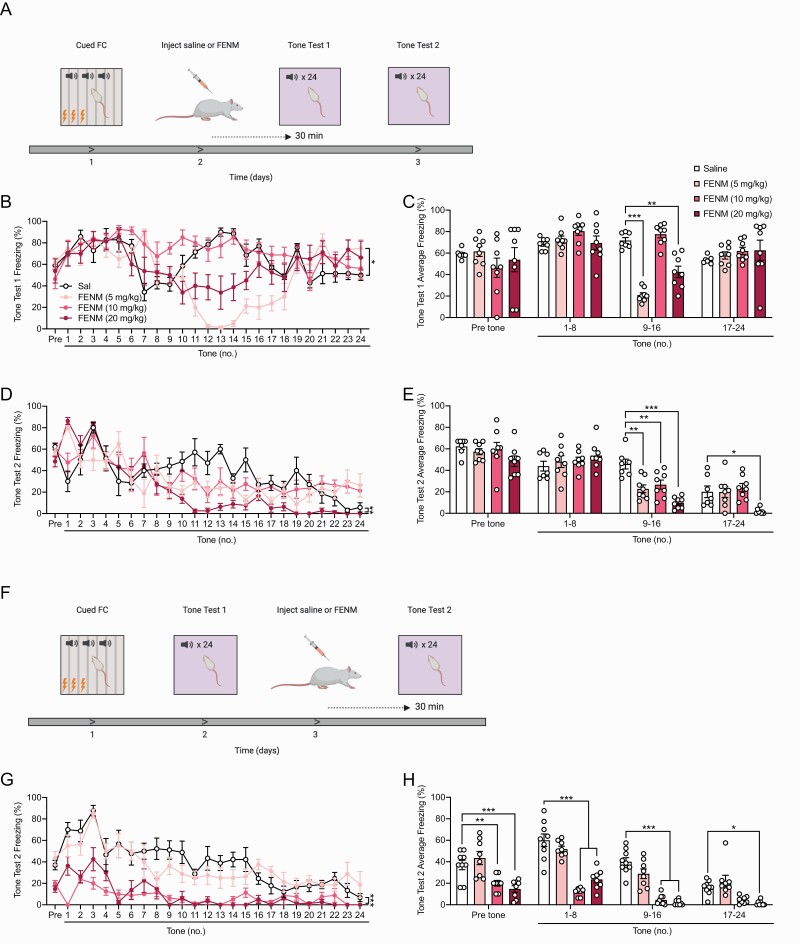
Next, we aimed to determine whether FENM could have effects in attenuating fear behavior when administered at various timepoints before or after stress or extinction learning. In these experiments, rats were not administered memantine due to previous data indicating that memantine does not alter fear learning or memory in wild-type rodents during cued FC (Dong et al., 2008; Ishikawa et al., 2016). Rats were administered a single injection of saline or FENM (5, 10, or 20 mg/kg) 30 minutes prior to 3-shock cued FC. The next 2 days, rats were placed into a novel context and reexposed to the conditioned stimulus. During both tests 1 and 2, freezing was assayed in rats as a measure of learned fear behavior (Figure 4a).
Figure 4.
Fluoroethylnormemantine (FENM) attenuates learned fear when administered directly before cued FC. (a) Rats were administered a single injection of saline or FENM (5, 10, or 20 mg/kg) 30 minutes prior to 3-shock cued FC. The next 2 days, rats were placed into a novel context and reexposed to the conditioned stimulus. During both tests 1 and 2, freezing was assayed in rats as a measure of learned fear behavior. (b–c) In the training session, freezing was comparable across all groups. (d–e) During test 1, FENM (5, 10, and 20 mg/kg) reduced freezing to the conditioned tones at various time points compared with the saline control group. FENM (20 mg/kg) increased freezing compared with saline during the last epoch of the test. (f–g) During test 2, at every time point, FENM (10 and 20 mg/kg) attenuated learned fear compared with the saline control group. FENM (5 mg/kg) increased freezing during tones 1–8, but reduced fear behavior during tones 9–16 (n = 8 male rats/group). Error bars represent ± SEM. *P < .05, **P < .01, ***P < .0001. Abbreviations: FC, fear conditioning; Sal, saline.
During the training session, there was no significant difference in freezing across all groups (Figure 4b–c; RMANOVA: drug F(3,28) = 1.219, P = .3212, time F(3,84) = 178.2, P < .0001, drug × time F(9,84) = 1.574, P = .1364. 2-way ANOVA: drug F(3.112) = 1.562, P = .2027, tone F(3,112) = 161.5, P < .0001, drug × tone F(9,112) = 1.426, P = .1852). During tone test 1, one day after training, rats administered FENM at 5 and 10 mg/kg exhibited significantly reduced freezing compared with controls during tones 9–16 [Figure 4d–e; RMANOVA: drug F(3,28) = 11.62, P < .0001, time F(24,672) = 11.6, P < .0001, drug × time F(72,672) = 6.451, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .0001, Sal vs FENM (10 mg/kg) P < .0001, Sal vs FENM (20 mg/kg) P = .6682; 2-way ANOVA: drug F(3,112) = 11.62, P < .0001, tone F(3,112) = 21.87, P < .0001; drug × tone F(9,112) = 9,659, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .0178, Sal vs FENM (10 mg/kg) P < .0001, Sal vs FENM (20 mg/kg) P = .9040]. Rats given FENM (20 mg/kg) showed reduced freezing during tones 1–8 but increased freezing during tones 17–24 compared with saline controls (Figure 4e). The following day, during tone test 2, FENM at 10 and 20, but not 5 mg/kg, significantly reduced overall freezing compared with saline [Figure 4f; RMANOVA: drug F(3,28) = 46.46, P < .0001, time F(24,672) = 15.35, P < .0001, drug × time F(72,672) = 4.664, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .9857, Sal vs FENM (10 mg/kg) P < .0001, Sal vs FENM (20 mg/kg) P < .0001]. Rats administered FENM at 10 and 20 mg/kg froze significantly less than saline-administered rats during all epochs of the testing session [Figure 4g; 2-way ANOVA: drug F(3,112) = 66.81, P < .0001, tone F(3,112) = 41.62, P < .0001; drug × tone F(9,112) = 4.666, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .7523, Sal vs FENM (10 mg/kg) P < .0001, Sal vs FENM (20 mg/kg) P < .0001]. Rats in the FENM (5 mg/kg) group showed enhanced freezing during tones 1–8 but reduced freezing during tones 9–16 compared with saline controls (Figure 4g). These data show that FENM administered prior to stress can attenuate learned fear in male rats in a dose- and time-specific manner.
We then sought to test whether FENM could alter fear behavior when administered after cued FC, prior to extinction learning. Rats were administered a 3-shock cued FC training session. The next day, rats were administered a single injection of saline or FENM (5, 10, or 20 mg/kg). Thirty minutes later, rats were placed into a novel context and reexposed to the conditioned stimulus. The third day, rats were reexposed to the conditioned stimulus. Freezing behavior was quantified during all training and testing sessions (Figure 5a). During training, there was no difference in freezing between all groups (supplementary Table 1). The following day, during tone test 1, FENM at 5 mg/kg, but no other doses, significantly reduced overall freezing compared with saline [Figure 5b; RMANOVA: drug F(3,27) = 6.298, P=.0022, time F(24,648) = 6.808, P < .0001, drug × time F(72,648) = 4.643, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .0424, Sal vs FENM (10 mg/kg) P = .3284, Sal vs FENM (20 mg/kg) P = .4934]. During tones 9–16, but no other epochs, rats administered FENM (5 and 20 mg/kg) froze significantly less than saline-administered control rats [Figure 5c; 2-way ANOVA: drug F(3,108) = 4.723, P = .0039, tone F(3,108) = 9.777, P < .0001; drug × tone F(9,108) = 6.892, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .0376, Sal vs FENM (10 mg/kg) P = .7276, Sal vs FENM (20 mg/kg) P = .3011]. Subsequently, during tone test 2, FENM (5 or 10 mg/kg) attenuated freezing during tones 9–16 while FENM (20 mg/kg) reduced freezing during the last half of the testing session [Figure 5d–e; RMANOVA: drug F(3,27) = 6.802, P = .0015, time F(24,648) = 18.81, P < .0001, drug × time F(72,648) = 2.906, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .1890, Sal vs FENM (10 mg/kg) P = .654, Sal vs FENM (20 mg/kg) P = .0007; 2-way ANOVA: drug F(3,108) = 8.452, P < .0001, tone F(3,108) = 78.04, P < .0001; drug × tone F(9,108) = 3.822, P = .0003; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .1318, Sal vs FENM (10 mg/kg) P = .5836 Sal vs FENM (20 mg/kg) P < .0001].
Figure 5.
Fluoroethylnormemantine (FENM) facilitates extinction learning. (a) Experimental protocol. (b–c) Freezing prior to the tones and during tones 1–8 was comparable between all the drug groups. FENM at 5 and 20, but not 10 mg/kg, significantly reduced freezing during tones 9–16 compared with saline-administered rats. (d) In tone test 2, FENM (20 mg/kg) significantly reduced overall freezing compared with the control saline group. (e) All doses of FENM significantly reduced freezing during tones 9–16. FENM (20 mg/kg) also reduced freezing during tones 17–24. (f) Experimental protocol. (g–h) During test 2, FENM (5 mg/kg) did not alter fear behavior compared with saline controls. FENM (10 mg/kg) reduced freezing during the pre-tone epoch and during tones 1–16. FENM (20 mg/kg) significantly reduced freezing throughout the entire testing session. (n = 7–9 male rats/group). Error bars represent ± SEM. *P < .05, **P < .01, ***P < .0001. Abbreviations: FC, fear conditioning; sal, saline.
Finally, we sought to test whether FENM could affect fear behavior when administered during extinction learning. Rats were administered a 3-shock cued FC training session. The next day, rats were placed into a novel context and reexposed to the conditioned cues. On the third day, rats were given a single injection of saline or FENM (5, 10, or 20 mg/kg) 30 minutes prior to a second testing session (Figure 5f). There was no significant overall difference freezing during cued FC or during tone test 1 (supplementary Table 1). However, in tone test 2, FENM (10 and 20 mg/kg) significantly reduced freezing throughout the test compared with control saline-administered rats [Figure 5g–h; RMANOVA: drug F(3,29) = 50.01, P < .0001, time F(24,696) = 14.98, P < .0001, drug × time F(72,696) = 2.126, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .5038, Sal vs FENM (10 mg/kg) P < .0001, Sal vs FENM (20 mg/kg) P < .0001; 2-way ANOVA: drug F(3,116) = 76.14, P < .0001, tone F(3,116) = 39.6, P < .0001; drug × tone F(9,116) = 4.368, P < .0001; Dunnett’s multiple comparisons: Sal vs FENM (5 mg/kg) P = .8588, Sal vs FENM (10 mg/kg) P < .0001, Sal vs FENM (20 mg/kg) P < .0001]. Overall, these data suggest that FENM can facilitate extinction learning and reduce fear behavior when administered either before or after stress or extinction learning but may be more effective in attenuating learned fear when administered directly prior to reexposure.
Discussion
In this study, we aimed to test the behavioral effects of FENM administration on sensorimotor gating, avoidance behavior, behavioral despair, and fear behavior in rats. Our experiments show that FENM can effectively reduce behavioral despair as well as facilitate extinction learning without altering locomotion or sensorimotor behavior. These results show that FENM exerts antidepressant-like and fear-attenuating properties. FENM was also effective in attenuating learned fear when administered acutely prior to FC, indicating that it may also be leveraged as a resilience-enhancing prophylactic. Together, our findings indicate that FENM, a novel NMDAR antagonist, may be a suitable candidate for further preclinical testing in a variety of stress-related behavioral assays.
Numerous studies have documented the efficacy of NMDAR antagonists in both treating and preventing stress-related psychiatric disorders. These compounds include, but are not limited to, (R,S)-ketamine, MK-801, and rapastinel (Kadriu et al., 2019). Notably, many of these compounds elicit rapid antidepressant responses in both rodent and human models but are not reported to exhibit anxiolytic properties (Moskal et al., 2014; Newport et al., 2015; Kadriu et al., 2019). A number of these novel compounds are also being tested in clinical trials as either adjunctive or standalone treatments for various depressive disorders, but, to date, many have failed to show significant efficacy in reducing depressive symptoms relative to placebo groups (Kadriu et al., 2019; ClinicalTrials.gov, NCT02192099, NCT02267629, NCT02932943, NCT02943564, NCT02943577, NCT02951988, NCT03002077, NCT03352453, NCT03560518, NCT03575776, NCT03614156, NCT03668600, NCT03799900, NCT03814733, NCT03855865). Additionally, (R,S)-ketamine and group II metabotropic glutamate receptor antagonists have been shown to exhibit prophylactic properties against stress-induced behavioral despair (Highland et al., 2019). Our results, which demonstrate that FENM is antidepressant and may reduce fear, further indicate that targeting NMDARs may be an important strategy to both treating and preventing stress-related psychiatric illness.
Previous research has demonstrated a number of mechanisms by which NDMAR modulation can relieve depressive-like symptoms (Zanos et al., 2018; Kadriu et al., 2019). It is important, however, to note that these mechanisms are not mutually exclusive and may work in a complementary fashion to achieve overall effects on behavior. Notably, NDMAR antagonism can rapidly upregulate the translation of various proteins and neurotrophic factors in the brain, such as brain-derived neurotrophic factor, which supports synaptic plasticity in regions of the brain, including the prefrontal cortex (Björkholm and Monteggia, 2016; Zanos and Gould, 2018; Zanos et al., 2018). This mechanism has been shown to be necessary for the rapid-acting antidepressant effects of (R,S)-ketamine and could potentially contribute to the rapid-acting effects of other NMDAR antagonists, including FENM (Björkholm and Monteggia, 2016). (R,S)-ketamine-mediated NMDAR antagonism has also been shown to reconfigure brain-wide neural networks to restore homeostatic metabolic processes as well as synchronize gamma oscillatory activity by reducing excessive NMDAR-dependent neurotransmission (Wang and Arnsten, 2015; Arnsten et al., 2016; Lv et al., 2016; Kadriu et al., 2019; Nugent et al., 2019). Future studies are therefore necessary to determine whether these and other candidate processes contribute to the neurobiological actions of FENM.
Intriguingly, we also found that FENM, in addition to its antidepressant properties, facilitated extinction learning. In our experiments, when administered at a variety of timepoints prior to both cued FC and extinction training, FENM exerted long-lasting reductions in fear behavior in a dose-specific manner. We speculate that FENM may alter the recall of fear memory traces, ultimately leading to a rapid and long-lasting attenuation of behavioral fear responses. Moreover, FENM was recently shown to improve spatial learning and object recognition as well as reduce spatiotemporal disorientation in a rodent model of AD, suggesting that the results we observed during cued FC were specific to fear expression (Couly et al., 2020). Future studies will be necessary to test whether FENM can alter fear memory traces to attenuate learned fear. Overall, our experiments indicate that, following further preclinical testing, FENM may be effective as a treatment for fear-related disorders such as posttraumatic stress disorder or specific phobias.
Importantly, FENM did not alter sensorimotor gating during PPI or locomotion in the OF acutely after injection. These results are in direct contrast with FENM’s parent compound memantine, which significantly reduced startle response and locomotion 30 minutes after injection. Our data suggest that FENM does not have significant, nonspecific side effects that could confound the data we collected in the FST and cued FC assays. These results show that FENM may be a suitable candidate for further preclinical development. Future studies are, however, necessary to determine potential nonspecific behavioral effects of the compound.
Despite these promising data, it is still unknown if FENM can exhibit antidepressant and fear-attenuating properties in females. We and others have previously shown that both antidepressant and prophylactic drugs may be efficacious at different doses in male and female preclinical models (Franceschelli et al., 2015; Chen et al., 2020b). Additionally, we have also shown that prophylactic compounds that attenuate learned fear in male mice, such as (R,S)-ketamine, (2S,6S)-hydroxynorketamine, an (R,S)-ketamine metabolite, and RS-67,333, a type IV serotonin receptor agonist, fail to alter fear learning or expression in female mice (Chen et al., 2020a, 2020b). Thus, we aim to investigate the prophylactic and fear-reducing efficacy of FENM in females in future studies.
Overall, this series of experiments characterizes a novel NMDAR antagonist, FENM, that exhibits antidepressant properties and facilitates extinction learning in preclinical rat models. The data support the NMDAR as a critical target to better treat and prevent a variety of stress-induced behaviors. Ultimately, further study of this and similar compounds may lead to more efficacious interventions to reduce the burden of stress-related psychiatric disease.
Supplementary Material
Acknowledgments
We thank ReST Therapeutics for the provision of FENM without charge. We also thank Dr Ron Katz and members of the Denny Lab for insightful comments on the manuscript and project. Additional support came from grants from INSERM (Institut National de la Santé et de la Recherche Médicale) and University Paris Descartes. We thank Dr Bill P Godsil for kind advice in the fear conditioning procedures.
This work was supported by the National Institute of Mental Health (F31 MH121023 to B.K.C.), the National Institute on Aging (R56 AG AG058661 and R21 AG064774 to C.A.D.), the National Institute of Neurological Disorders and Stroke (R21 NS114870 to C.A.D.), and the National Institute of Child Health and Human Development (R01 HD101402 to C.A.D.). Figures of behavioral timelines were created with BioRender.com.
Statement of Interest
B.K.C., G.R., and C.A.D. are named on provisional and nonprovisional patent applications for the prophylactic use of (R,S)-ketamine, FENM, and related compounds against stress-related psychiatric disorders. Since the start of 2020, C.A.D. has received honoraria for speaking for Janssen, Janssen Southeast Asia, North American Neuromodulation Society, and Albany Medical College. G.L.P., A.E., and T.M.J. have nothing to disclose. FENM was provided for this study as a gift from ReST Therapeutics.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Almeida RC, Souza DG, Soletti RC, López MG, Rodrigues AL, Gabilan NH (2006) Involvement of PKA, MAPK/ERK and CaMKII, but not PKC in the acute antidepressant-like effect of memantine in mice. Neurosci Lett 395:93–97. [DOI] [PubMed] [Google Scholar]
- Amat J, Dolzani SD, Tilden S, Christianson JP, Kubala KH, Bartholomay K, Sperr K, Ciancio N, Watkins LR, Maier SF (2016) Previous ketamine produces an enduring blockade of neurochemical and behavioral effects of uncontrollable stress. J Neurosci 36:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Murray JD, Seo H, Lee D (2016) Ketamine’s antidepressant actions: potential mechanisms in the primate medial prefrontal circuits that represent aversive experience. Biol Psychiatry 79:713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates-Alagoz Z, Adejare A (2013) NMDA receptor antagonists for treatment of depression. Pharmaceuticals 6:480–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM (2010) Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol 626:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM (2016) BDNF - a key transducer of antidepressant effects. Neuropharmacology 102:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, Gardier AM, Mendez-David I, David DJ, Hen R, Denny CA (2016) Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry 79:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BK, Mendez-David I, Luna VM, Faye C, Gardier AM, David DJ, Denny CA (2020a) Prophylactic efficacy of 5-HT4R agonists against stress. Neuropsychopharmacology 45:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BK, Luna VM, LaGamma CT, Xu X, Deng SX, Suckow RF, Cooper TB, Shah A, Brachman RA, Mendez-David I, David DJ, Gardier AM, Landry DW, Denny CA (2020b) Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology 45:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov (NCT0260929) (2014) A study to evaluate the pharmacokinetics of intranasal esketamine administered with and without a nasal guide on the intranasal device.https://ClinicalTrials.gov/show/NCT02060929. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02094378) (2014) A study to evaluate the effect of intranasal esketamine on cognitive functioning in healthy subjects.https://ClinicalTrials.gov/show/NCT02094378. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02133001) (2020) A double-blind study to assess the efficacy and safety of intranasal esketamine for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in participants who are assessed to be at imminent risk for suicide.https://ClinicalTrials.gov/show/NCT02133001. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02192099) (2019) Open label extension for GLYX13-C-202, NCT01684163.https://ClinicalTrials.gov/show/NCT02192099. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02267629) (2017) Open label trial of rapastinel (formerly GLYX-13) in individuals with obsessive-compulsive disorder.https://ClinicalTrials.gov/show/NCT02267629. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02343289) (2015) A study to evaluate the absolute bioavailability of intranasal and oral esketamine and the effects of clarithromycin on the pharmacokinetics of intranasal esketamine in healthy participants.https://ClinicalTrials.gov/show/NCT02343289. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02345148) (2017) Pharmacokinetic, safety, and tolerability study of intranasally administered esketamine in elderly and healthy younger adult participants.https://ClinicalTrials.gov/show/NCT02345148. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02606084) (2019) A study to assess the effects of renal impairment on the pharmacokinetics, safety, and tolerability of intranasally administered esketamine.https://ClinicalTrials.gov/show/NCT02606084. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02611505) (2017) A study to assess the effects of hepatic impairment on the pharmacokinetics, safety, and tolerability of intranasally administered esketamine.https://ClinicalTrials.gov/show/NCT02611505. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02674295) (2016) A mass balance study with a microtracer dose of 14C-esketamine in healthy male participants.https://ClinicalTrials.gov/show/NCT02674295. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02857777) (2017) Pharmacokinetic, safety, and tolerability study of intranasally administered esketamine in elderly Japanese, and healthy younger adult Japanese subjects.https://ClinicalTrials.gov/show/NCT02857777. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02932943) (2019) A study of rapastinel as adjunctive therapy in major depressive disorder (RAP-MD-01).https://ClinicalTrials.gov/show/NCT02932943. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02943564) (2019) A study of rapastinel as adjunctive therapy in major depressive disorder (RAP-MD-02).https://ClinicalTrials.gov/show/NCT02943564. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02943577) (2019) A study of rapastinel as adjunctive therapy in major depressive disorder (RAP-MD-03).https://ClinicalTrials.gov/show/NCT02943577. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT02951988) (2020) A study of rapastinel as adjunctive therapy in the prevention of relapse in patients with major depressive disorder.https://ClinicalTrials.gov/show/NCT02951988. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03002077) (2020) Long-term safety study of rapastinel as adjunctive therapy in patients with major depressive disorder.https://ClinicalTrials.gov/show/NCT03002077. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03298906) (2017) A study to assess the effect of ticlopidine on the pharmacokinetics, safety, and tolerability of intranasally administered esketamine in healthy participants.https://ClinicalTrials.gov/show/NCT03298906. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03352453) (2020) A study of rapastinel for rapid treatment of depression and suicidality in major depressive disorder.https://ClinicalTrials.gov/show/NCT03352453. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03560518) (2020) Study of rapastinel as monotherapy in patients with MDD.https://ClinicalTrials.gov/show/NCT03560518. Accessed 20 December 2020.
- ClinicalTrials.gov (NCT03575776) (2020) Study of rapastinel as monotherapy in patients with major depressive disorder (MDD).https://ClinicalTrials.gov/show/NCT03675776. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03614156) (2020) Study of monotherapy rapastinel in the prevention of relapse in patients with major depressive disorder (MDD).https://ClinicalTrials.gov/show/NCT03614156. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03668600) (2020) Study of adjunctive or monotherapy rapastinel treatment in patients with major depressive disorder (MDD).https://ClinicalTrials.gov/show/NCT03668600. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03799900) (2019) Assessment of abuse potential of rapastinel in humans.https://ClinicalTrials.gov/show/NCT03799900. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03814733) (2019) Assessment of effect of rapastinel on driving performance.https://ClinicalTrials.gov/show/NCT03814733. Accessed 20 December 2020.
- ClinicalTrials.gov ( NCT03855865) (2019) Study of rapastinel as monotherapy in major depressive disorder (MDD).https://ClinicalTrials.gov/show/NCT03855865. Accessed 20 December 2020.
- Couly S, Denus M, Bouchet M, Rubinstenn G, Maurice T (2020) Anti-amnesic and neuroprotective effects of fluoroethylnormemantine in a pharmacological mouse model of Alzheimer’s disease. Int J Neuropsychopharmacol 24:142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, et al. (2019) Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 76:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Moss JM, Leslie NL, Tilden SG, Sørensen AT, Watkins LR, Lin Y, Maier SF (2018) Inhibition of a descending prefrontal circuit prevents ketamine-induced stress resilience in females. eNeuro 5:ENEURO.0025-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG (2008) Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer’s disease. Neuropsychopharmacology 33:3226–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, Vitagliano D, Blier P, Fava M, Liebowitz M, Ravindran A, Gaillard R, Ameele HVD, Preskorn S, Manji H, Hough D, Drevets WC, Singh JB (2019) Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol 22:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60. [DOI] [PubMed] [Google Scholar]
- Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, Hough D, Manji H, Drevets WC, Canuso CM (2020) Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry 81:19m13191. [DOI] [PubMed] [Google Scholar]
- Gerhard DM, Wohleb ES, Duman RS (2016) Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today 21:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland JN, Zanos P, Georgiou P, Gould TD (2019) Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology 44:1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, Hough D, Drevets WC, Manji H, Canuso CM (2020) Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol 24:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R, Fukushima H, Frankland PW, Kida S (2016) Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. Elife 5:e17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr (2019) Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol 22:119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Duman RS (2020) Rapastinel, a novel glutamatergic agent with ketamine-like antidepressant actions: convergent mechanisms. Pharmacol Biochem Behav 188:172827. [DOI] [PubMed] [Google Scholar]
- Kato T, Fogaça MV, Deyama S, Li XY, Fukumoto K, Duman RS (2018) BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry 23:2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Matsunaga S, Iwata N (2017a) A meta-analysis of memantine for depression. J Alzheimers Dis 57:113–121. [DOI] [PubMed] [Google Scholar]
- Kishi T, Matsunaga S, Oya K, Nomura I, Ikuta T, Iwata N (2017b) Memantine for Alzheimer’s disease: an updated systematic review and meta-analysis. J Alzheimers Dis 60:401–425. [DOI] [PubMed] [Google Scholar]
- Kos T, Popik P (2005) A comparison of the predictive therapeutic and undesired side-effects of the NMDA receptor antagonist, memantine, in mice. Behav Pharmacol 16:155–161. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Caro M, Hadzimichalis N (2013) NMDA receptor activity in neuropsychiatric disorders. Front Psychiatry 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chang L, Song Y, Li H, Wu Y (2019) The role of NMDA receptors in Alzheimer’s disease. Front Neurosci 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q, Yang L, Li G, Wang Z, Shen Z, Yu W, Jiang Q, Hou B, Pu J, Hu H, Wang Z (2016) Large-scale persistent network reconfiguration induced by ketamine in anesthetized monkeys: relevance to mood disorders. Biol Psychiatry 79:765–775. [DOI] [PubMed] [Google Scholar]
- Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, Denny CA (2018) Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol Psychiatry 84:846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JC, LaGamma CT, Lim SC, Tsitsiklis M, Neria Y, Brachman RA, Denny CA (2017) Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology 42:1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moryl E, Danysz W, Quack G (1993) Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol 72:394–397. [DOI] [PubMed] [Google Scholar]
- Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, Leander JD (2014) GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 23:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments (2015) Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Nijs M, Wajs E, Aluisio L, Turkoz I, Daly E, Janik A, Borentain S, Singh JB, DiBernardo A, Wiegand F (2020) Managing esketamine treatment frequency toward successful outcomes: analysis of phase 3 data. Int J Neuropsychopharmacol 23:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA Jr (2019) Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry 24:1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul IA (1997) NMDA Receptors and affective disorders. In: Antidepressants: New Pharmacological Strategies (Skolnick P, ed), pp 145–158. Totowa, NJ: Humana Press. [Google Scholar]
- Perez-Ruixo C, Rossenu S, Zannikos P, Nandy P, Singh J, Drevets WC, Perez-Ruixo JJ (2020) Population pharmacokinetics of esketamine nasal spray and its metabolite noresketamine in healthy subjects and patients with treatment-resistant depression. Clin Pharmacokinet. doi: 10.1007/s40262-020-00953-4 [DOI] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, Landry DW, Brachman RA, Denny CA, Gardier AM (2018) Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry 84:e3–e6. [DOI] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB (2019) Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 176:428–438. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732. [DOI] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB (2009) Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res 1293:24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z, Hibar D, Fedgchin M, Popova V, Furey ML, Singh JB, Kolb H, Drevets WC, Chen G (2020) Effects of Mu-Opiate receptor gene polymorphism rs1799971 (A118G) on the antidepressant and dissociation responses in esketamine nasal spray clinical trials. Int J Neuropsychopharmacol 23:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salabert AS, Fonta C, Fontan C, Adel D, Alonso M, Pestourie C, Belhadj-Tahar H, Tafani M, Payoux P (2015) Radiolabeling of [18F]-fluoroethylnormemantine and initial in vivo evaluation of this innovative PET tracer for imaging the PCP sites of NMDA receptors. Nucl Med Biol 42:643–653. [DOI] [PubMed] [Google Scholar]
- Salabert AS, Mora-Ramirez E, Beaurain M, Alonso M, Fontan C, Tahar HB, Boizeau ML, Tafani M, Bardiès M, Payoux P (2018) Evaluation of [18F]FNM biodistribution and dosimetry based on whole-body PET imaging of rats. Nucl Med Biol 59:1–8. [DOI] [PubMed] [Google Scholar]
- Steckler T, Risbrough V (2012) Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, van Bergeijk DP, Bergsma F, Weber E, Talledo J (2009) The effects of memantine on prepulse inhibition. Neuropsychopharmacology 34:1854–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajs E, et al. (2020) Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry 81:19m12891. [DOI] [PubMed] [Google Scholar]
- Wang M, Arnsten AF (2015) Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neurosci Bull 31:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Ren Q, Ma M, Chen QX, Hashimoto K (2016) Antidepressant effects of (+)-MK-801 and (-)-MK-801 in the social defeat stress model. Int J Neuropsychopharmacol 19:pyw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr, Gould TD (2018) Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32:197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.