Abstract
Background/Objectives:
Gait speed and psychomotor speed slow with age and may predict neuropsychiatric disease such as depression and anxiety. We explored the relative predictive values of gait speed, psychomotor slowing, and a composite index of these two measures on time to new episode depression or anxiety in older adults at risk for these common psychiatric conditions.
Design:
Randomized controlled prevention trial with 15-month follow-up.
Setting:
University-based late-life mental health research clinic.
Participants:
Two hundred thirteen individuals, age 60+ years, with subsyndromal symptoms of depression or anxiety and one of the following risk factors for these common conditions: mild cognitive impairment, knee osteoarthritis, or disabilities requiring home-based care.
Intervention:
Participants in each of the risk factor groups were randomized to a depression-specific preventive intervention or usual care.
Measurements:
Gait speed: 4-meter walk test from the Short Physical Performance Battery. Psychomotor speed: Coding task of the Repeatable Battery for the Assessment of Neuropsychological Status. We created a composite index of slowing by determining whether participants exceeded established cut-offs for slow performance in both gait speed (≤0.8 m/s) and psychomotor speed (< 7 on the coding task). Time to new onset syndromal depression/anxiety was measured using research diagnostic criteria.
Results:
Fifty-four participants developed syndromal depression/anxiety (19.5%) over the course of 15 months. Participants with slowing in both areas were over twice as likely to experience new onset depression/anxiety (HR=2.11, 95% CI=1.02–4.40, p=0.046) compared to participants with no slowing in either area. Slowed gait (HR=1.88, 95% CI=0.992–3.55, p=0.052) or slowed psychomotor speed (HR=0.60, 95% CI=0.14–2.58, p=0.488) alone did not increase risk for depression/anxiety.
Conclusion:
Evaluating both gait and psychomotor speed in older adults with medical comorbidities and sub-syndromal depression may predict incident mental illness and inform prevention planning. Future research is needed to validate our observations and explore shared neurobiological mechanisms that explain this elevated risk.
Keywords: prevention, aging, motor function, cognitive function, mental illness
INTRODUCTION
Depression and anxiety in late-life are common and increase the risk and severity of chronic medical conditions, cognitive impairment and dementia, suicide and all-cause mortality, and health-service utilization.1 Because of limited treatment options and variable treatment response for older adults, it is important to prevent these common psychiatric conditions. For example, it is estimated that between 63-81% of older adults do not reach depression remission with first line pharmacotherapies.2, 3 Switching or augmenting pharmacotherapy results in less than a 50% improvement in more refractory cases.2 Remission from depression in late-life is often fragile, relapse is more common than in younger patients,4 and over half relapse.5 As many as 47% of older adults with depression also meet criteria for generalized anxiety disorder (GAD)6 and when present, is associated with more severe depression, reduced remission rates, and slower pharmacotherapy response time.7 Given the challenges with effective treatment and sustained remission, prevention efforts and establishing easy to measure risk factors should be prioritized.
While there are established medical, psychiatric, and psychosocial risks for becoming depressed and anxious, there are no objective behavioral measures that are routinely used for risk stratification. Both physical and psychomotor slowing are features of aging, frailty, depression,8 and neurodegenerative disease.9 Physical slowing, commonly measured in healthcare settings with gait speed, is associated with elevated anxiety and depression symptoms,10 cognitive impairment,10 falls,11 loss of independence,12 and mortality.13 Slow gait speed is a risk factor for depression.14 Slower gait speed also predicts worse antidepressant treatment response15 and depression chronicity.11 Slower gait speed is also a risk factor for anxiety.16
Psychomotor slowing is a risk factor for falls,17 cognitive impairment,18 worse mobility, and mood,19 and may be a marker of cerebrovascular disease.20 Similar to gait slowing, psychomotor slowing is associated with greater depression severity,21 worse antidepressant treatment response,22 and increased depression chronicity.23 There is also a bidirectional relationship between anxiety and psychomotor slowing.24
To advance objective, easy-to-administer assessments of late-life depression and anxiety risk, we explored the relative predictive value of both gait speed and psychomotor slowing on time to new episode depression or anxiety in older adults at risk for these common psychiatric conditions who were followed for 15 months. We also created a composite index based on these two measures compared to no slowing in either domain.
METHODS
Study Design Overview
This study used data collected from three linked randomized controlled trials (RCTs) for the prevention of depression and anxiety in older adults at elevated risk. Briefly, the primary objective was to determine whether learning-based therapies could prevent incident episodes of major depression, minor depression, and/or generalized anxiety in older adults with: mild cognitive impairment,25 knee osteoarthritis,26, 27 or disabilities requiring home-based care.28, 29 Data were collected at baseline, directly after the intervention period (12 weeks after baseline), and at 3, 6, 9, and 12 months post-intervention between 2012 and 2016. Each RCT had their own study team. However, they administered a similar battery of assessments to participants and followed the same standard operating procedure for gait assessment, neuropsychological testing, and the clinical diagnosis of new onset depression or anxiety. Masters-level clinicians administered assessments and were blinded to treatment assignment and to baseline assessments. They were not blinded to study group (knee pain, MCI, disability).
Participants
Inclusion criteria for the three RCTs required participants to be 60+ years of age, score above 80 on the Modified Mini-Mental Status Exam (3MS)30, and endorse mild (subsyndromal) depressive symptoms (score of 1-9 on the Patient Health Questionnaire-9 with a score of at least 1 for a cardinal symptom of low mood or anhedonia). Exclusion criteria included the regular use of anxiolytics (>4 times/week); major depression or generalized anxiety disorder in the past 12 months as determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition);31 history of central nervous system disease, bipolar disease, or schizophrenia; or drug or alcohol treatment in the past 12 months. Across the three RCTs, 277participants consented and were randomized to either a learning-based and/or physical intervention (e.g., cognitive behavioral therapy, problem-solving therapy, or physical therapy) (n=178); or enhanced usual care (EUC, n=99). The usual care arm meant no additional intervention on part of the study team and was considered “enhanced” because the primary care physicians of participants who reported new medical or psychological symptoms were informed about these symptoms, and participants were encouraged to follow-up with their PCP. Of the 277 who were randomized, 213 completed the gait speed and psychomotor speed tests and are the focus of this report. Of the 213, 80 had knee osteoarthritis (67 randomized to an intervention versus 13 randomized to EUC); 78 had disabilities required home care (37 versus 41), and 55 had mild cognitive impairment (35 versus 20). Participants with missing assessments (n=64) had more mobility limitations (measured with the Late-Life Functional Disability Instrument) and were more socially isolated (measured with the Interpersonal Support Evaluation List) than participants who completed all assessments (n=213).
Measures
New onset syndromal depression/anxiety.
The primary outcome for the present study is time to first onset of major depressive disorder, minor depressive disorder, or generalized anxiety disorder. Major depression, minor depression, and generalized anxiety disorder were identified by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.32
Measures of slowing.
Measures of gait speed and psychomotor speed were evaluated independently and also used to create a composite index of slowing (CIS). Gait speed was measured using the 4-meter walk test from the Short Physical Performance Battery.33 Raw scores were recorded in meters per second. Gait speed scores < 0.8 m/s indicated slow gait speed in late-life; scores ≥ 0.8 m/s indicated average/normal gait speed.13 A score of zero reflects those who did not attempt the test for safety reasons or those who attempted the test but did not complete it and therefore were not included in the CIS. Psychomotor speed was measured using the coding task of the Repeatable Battery for the Assessment of Neuropsychological Status. Scores were standardized (i.e., age corrected) on a scale from 1 to 19 where 7-13 is considered average speed of cognitive function (i.e., within 1 SD of the normative mean). Psychomotor speed scores <7 indicated slow information processing speed in late-life; scores ≥ 7 indicated average/normal processing speed (and scores >13 indicate above-average processing speed). We categorized the sample into 4 groups based on gait speed and psychomotor speed: slow gait speed alone; slow psychomotor speed alone, slowing in both gait and psychomotor speed (the CIS), and no slowing in either area (referent group).
Relevant covariates included in the analysis.
The following baseline covariates were considered likely to affect outcomes and were included in analyses: age, depression symptom severity (PHQ-9), anxiety symptom severity (7-item Generalized Anxiety Disorder Scale),34 and treatment group (learning-based intervention or EUC).
Analytic plan
To compare baseline characteristics of participants who were slow in gait speed alone, slow in psychomotor speed alone, and slow in both gait and psychomotor speed versus those who were not, we used the F-test for continuous variables and the Pearson χ2 test for categorical variables. To examine the relationship between the three slowing groups and 15-month new-onset syndromal depression/anxiety, we performed survival analyses using Cox regression models, censoring follow-up time at 15 months for participants still enrolled at that time. Models were built sequentially, beginning with an unadjusted model, then adjusting for covariates. We included three dummy variables that captured the slowing status of older adults with subsyndromal depression symptoms: slow gait speed (n 599 [28%]); slow psychomotor speed (n=19 [9%]); and the composite index of slowing in both gait and psychomotor speed (n=40 [19%]) (the remaining sample members served as the referent category (n=95 [45%])). These dummy variables were entered as predictors in our cox regression model. We present results for the 3 trials together because 1) there were no differences across the groups on the outcome (see table 3); 2) examining multiple types of comorbidities could increase generalizability; and 2) all participants shared a similar risk for new onset clinical depression/anxiety – subsyndromal depression symptoms including the core symptoms of depressed mood and loss of interest or pleasure in nearly all activities. For all analyses, p values smaller than 0.05 were statistically significant. All analyses were performed using SPSS, version 26.0.
Table 3.
Cox regression analysis examining the association between the composite index of slowing and 15-month incidence of new onset syndromal depression/anxiety
| 95.0% CI for Exp(B) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper |
| Covariates | ||||||||
| Age | 0.004 | 0.016 | 0.064 | 1 | 0.801 | 1.004 | 0.972 | 1.037 |
| Depression symptoms | 0.078 | 0.055 | 2.027 | 1 | 0.154 | 1.081 | 0.971 | 1.203 |
| Anxiety symptoms | 0.115 | 0.052 | 4.877 | 1 | 0.027 | 1.122 | 1.013 | 1.242 |
| Treatment group | 0.103 | 0.288 | 0.127 | 1 | 0.721 | 1.108 | 0.630 | 1.951 |
| Measures of slowing | 6.872 | 3 | 0.076 | |||||
| No slowing in either domain | (ref) | |||||||
| Gait slowing | 0.631 | 0.325 | 3.765 | 1 | 0.052 | 1.880 | 0.994 | 3.555 |
| Psychomotor slowing | −0.519 | 0.749 | 0.480 | 1 | 0.488 | 0.595 | 0.137 | 2.583 |
| Composite index of slowing | 0.748 | 0.374 | 3.998 | 1 | 0.046 | 2.113 | 1.015 | 4.397 |
Notes. There were no differences across the three RCTs on new-onset depression/anxiety:
Mild cognitive impairment*: B=0.10, SE = 0.34, Exp (B) = 1.106, 95% CI – 0.56 – 2.15, p=0.76.
Knee OA*: B=0.73, SE = 0.38, Exp (B) = 2.079, 95% CI – 0.973 – 4.443, p=0.06.
disabilities requiring home care served as the referent category. These p-values were not adjusted for multiple comparisons, given that even with no adjustment, they did not reach traditional levels for statistically reliable effects.
RESULTS
Table 1 shows descriptive statistics for the sample stratified by the four study groups defined by motor and/or psychomotor slowing. The mean age of participants was 74.3 years (SD = 8.9); 69% (n = 191) were women and 22% (n= 62) were Black. Average years of education was 14.2 (SD = 2.6). Several group differences in demographic variables emerged among the four study groups. Compared to participants in the normal range, those who were slow in both gait speed and psychomotor speed were older, more likely to be female, more likely to be Black, and had fewer years of education The groups were similar for depression and anxiety symptoms.
Table 1.
Descriptive statistics for the study sample by slowing status.
| Total sample (N=213) | No slowing (95) | Gait slowing (59) | Psychomotor slowing (19) |
Composite index (40) |
F or x2 | p-value | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, years | 74.31 (8.89) | 72.19 (8.14) | 76.14 (8.57) | 70.45 (8.80) | 77.70 (9.00) | 6.940 | <0.001 |
| Male, % (n) | 31.04 (86) | 36.80 (39) | 15.94 (11) | 50.00 (10) | 25.58 (11) | 13.071 | 0.004 |
| Black/minority status, % (n) | 22.38 (62) | 14.15 (15) | 20.29 (14) | 35.00 (7) | 34.88 (15) | 10.200 | 0.017 |
| Education, years | 14.2 (2.64) | 15.29 (2.48) | 14.18 (2.51) | 13.70 (2.43) | 12.95 (2.61) | 9.904 | <0.001 |
| Psychosocial scales | |||||||
| Depression symptoms | 5.90 (2.44) | 5.90 (2.37) | 5.80 (2.75) | 5.45 (2.14) | 5.74 (1.99) | 0.204 | 0.893 |
| Anxiety symptoms | 3.24 (2.71) | 3.49 (3.26) | 3.29 (2.50) | 2.50 (1.99) | 3.00 (1.99) | 0.881 | 0.451 |
| Pain | 7.95 (4.58) | 7.27 (4.53) | 8.81 (4.34) | 6.35 (4.82) | 8.36 (4.31) | 2.702 | 0.046 |
| Sleep quality | 1.125 (0.74) | 1.12 (0.793) | 1.23 (0.731) | 0.95 (0.686) | 1.17 (0.794) | 0.763 | 0.576 |
| Slowing measures | |||||||
| Gait speed | 0.82 (0.29) | 1.02 (0.16) | 0.60 (0.21) | 1.04 (0.20) | 0.54 (0.18) | 114.475 | <0.001 |
| Psychomotor speed | −0.27 (1.21) | 0.41 (0.79) | 0.14 (0.70) | −1.59 (0.44) | −1.98 (0.73) | 136.83 | <0.001 |
After 15 months of follow-up, 54 (19.5%) participants experienced new onset syndromal depression and/or anxiety. Of the 54 participants, 14 (26%) experienced major depression; 31 (57%) minor depression; and 9 (17%) GAD. Seven participants met criteria for both depression and anxiety diagnosis. The number of events was evenly distributed across the slowing measures (Χ2 [df=3] = 5.62; p = 0.128) (Table 2). After adjusting for covariates, neither slowing in gait speed alone nor slowing in psychomotor speed alone significantly increased the risk of syndromal depression/anxiety, compared to the referent group with no slowing in either area. However, the CIS significantly increased the risk of new-onset depression and/or anxiety relative to the referent group (Hazard Ratio [HR] = 2.11, 95% Confidence Interval (CI) = 1.02 – 4.40, p = 0.046). Participants who were slow in both motor and psychomotor function were twice as likely to experience syndromal depression/anxiety compared to those of average/normal speed (Table 3 and Figure 1). The only other covariate that was associated with increased risk of new onset depression/anxiety in the multivariable model was baseline anxiety symptoms (HR = 1.12, 95% CI = 1.01 – 1.24, p = 0.027).
Table 2.
Number (%) of participants who experienced an incident event within 15 months by slowing status.
| Slowing status | Total N | # (%) of Events |
|---|---|---|
| Average/normal | 95 | 20 (21.1) |
| Gait slowing only | 59 | 19 (32.2) |
| Psychomotor speed slowing only | 19 | 2 (10.5) |
| Composite index of slowing | 40 | 13 (32.5) |
Notes. Fishers exact test, df=3 = 5.598; p = 0.128.
Figure 1.
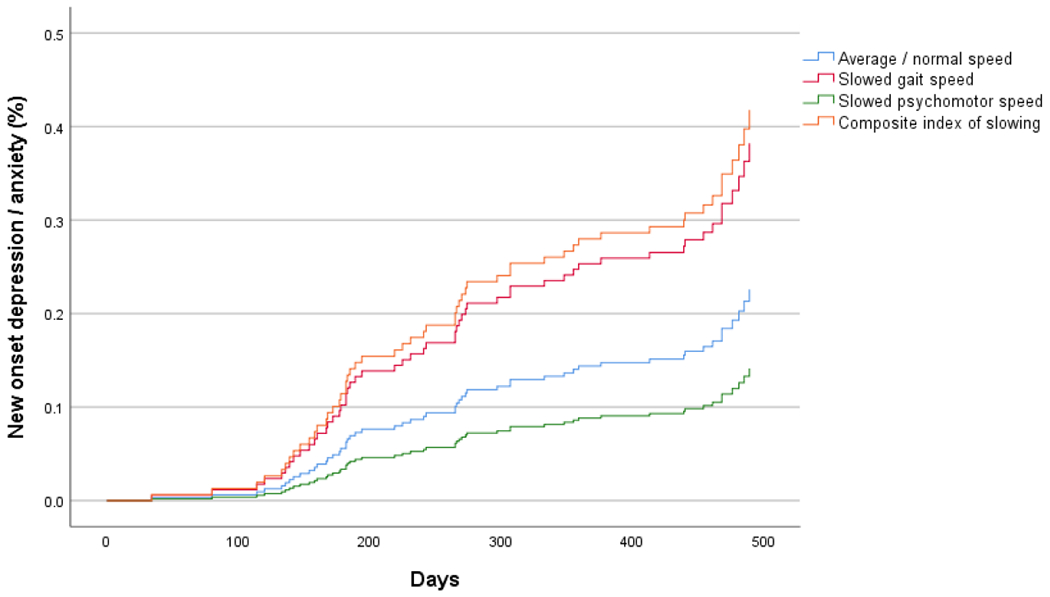
Survival curves for 15-month incidence of new onset syndromal depression/anxiety by slowing group.
Post-hoc Analyses
The survival curves (Figure 1) suggest that the CIS is mostly influenced by slowed gait speed. This raised questions about whether the CIS was superior at predicting incident depression/anxiety versus each measure alone. After adjusting for covariates, the CIS was superior than psychomotor speed alone (HR = 0.473, 95% CI = 0.227 – 0.985, p = 0.046), but not gait slowing alone (HR = 0.282, 95% CI = 0.062 – 1.285, p = 0.102) for prediction of new-onset depression/anxiety.
DISCUSSION
This study shows the predictive value of a composite index of slowing to new onset syndromal depression/anxiety in older adults. Participants who were slow in both motor and psychomotor function were twice as likely to experience syndromal depression/anxiety over 15 months compared to those of average or normal speed in both domains. Follow-up analyses suggest that the CIS is driven by slowness in the gait speed component.
Slowed gait speed represents accelerated biological aging across multiple organ systems; slowing of processing speed signals a reduced ability to cognitively adapt to new situations.35 Mobility and cognitive slowing represents both neural and musculoskeletal changes, possibly representing a vulnerability to illness and reduced neural homeostasis,36 or the tendency of neural circuits to maintain equilibrium. This vulnerability is supported by findings that cognitive slowing is more likely to progress to dementia if accompanied by mobility impairment; and similarly, mobility impairment is more likely to result in falls and fractures if accompanied by cognitive slowing.37, 38 The combination of slow gait with objective cognitive impairments are associated with an increased risk of falls and fractures, dementia, and mortality.39 Our study expands the slowing literature by showing that the co-occurrence of slowed gait with slowed processing speed increases the risk for depression and anxiety (relative to having neither).
Slowed gait and slow processing may represent shared neural circuitry involved in both walking and neurocognitive functions. This neural circuit is related to depression/anxiety risk; for example, evidence suggests that degeneration of neurotransmitter systems—primarily the dopaminergic system—may contribute to age-related gross and fine motor declines, as well as to higher cognitive deficits,40 and depression. Specifically, aging is associated with decreased dopamine receptors and transporters,41 which may be related to cognitive, musculoskeletal, and mood disorder development. For example, loss of the dopamine transporters and dopamine D2 receptors is associated with decreased motor speed and executive functioning.42 Dopaminergic neurons are also involved in cognitive processing and working memory.43 Furthermore, dopamine agonists are effective in some individuals with treatment-resistant depression,44 and individuals diagnosed with depression have lower levels of dopamine metabolites in their cerebral spinal fluid.45 Moreover, the “depression executive dysfunction theory” hypothesizes that dysfunction of the frontal-subcortical networks contributes to depression.46 We have shown, for example, that, in late-life depression, caudate volume is both lower compared with never-depressed individuals, and related to severity of depression.47 The frontal-subcortical networks share parallel pathways and anatomic structures (thalamus), which are involved in motor, executive, motivational (dopamine), and emotional regulation. Thus, it makes sense that cognitive and gait slowing would also parallel “emotional slowing” (depression).
While these findings must be replicated, they have potential clinical implications. Working with rehabilitation or cognitive remediation specialists to minimize further slowing (via physical activity) and/or re-gain lost gait speed and modifying therapeutic and prevention behaviors to accommodate slowing processing speed, may decrease the risk of developing depression/anxiety. While preliminary, these observations support continued research into whether protocolized clinical assessments of both gait speed and psychomotor speed is useful in identifying (and intervening) in older patients at elevated risk for neuropsychiatric disease. While we did identify that participants who scored “positive” on the CIS were twice as likely as participants with neither problem to develop depression/anxiety, we view these findings as hopeful, given the fact that there are interventions to remediate (or at least minimize) both gait speed and psychomotor slowing.48, 49 While hypothetical, a possible biological connection among gait slowing, psychomotor slowing, and depression/anxiety (reduced dopaminergic tone), supports a potential neural or molecular target for future prevention and intervention translational trials in these at-risk older adults. In addition, extracting information about motor and processing speed is something that may be done in the future by smartphones (e.g., GPS tracking of distance traveled for gait speed and time and accuracy between clicks for processing speed).
This study had several limitations. First, our categorical classification of slowing may be a relatively crude assessment of mobility and cognitive impairment. A continuous expression of slowing in both gait and psychomotor speed (with a range of scores) may provide more detailed information of the relation between slowing and depression/anxiety risk. Second, we examined baseline slowing status only. It may be helpful to consider the relative timing of decline in the two domains over a longer follow-up period as there is evidence that slowed gait speed occurs secondarily to slowed processing speed.50 In this sample, gait speed and processing speed did not significantly change/decline over the 15 months. Third, we combined depression and anxiety events into one outcome (due to the low number of events in our sample [54 events or 25% of sample]; depression events = 43 (20%); anxiety events = 9 (4%); comorbid depression and anxiety events = 2 (1%)).). While depression and anxiety commonly co-occur during late life, it may be helpful to examine depression and anxiety outcomes separately, particularly when using interventions that target putative mechanisms of action. Fourth, generalizability may be limited to older adults with subsyndromal depression symptoms. Another limitation is that usual care could have varied for different types of patients (e.g., MCI patients versus knee osteoarthritis patients), but we did not collect this information from participants and therefore were unable to examine those effects in our regression model. However, usual care vs. active study intervention did not matter in terms of our outcome and that lack of effect did not differ by the 3 RCTs. Finally, it was surprising that baseline depression symptoms did not predict new onset depression/anxiety. Prevention interventions for major depressive disorder often follow adults for 2 years. It is possible that our follow-up interval (15 months) was too short to observe new onset of major depressive disorder. It is also possible that baseline symptoms did not predict new onset depression/anxiety because all participants had to have some degree of symptomatology to be eligible for the study. The usual strong association of past and future depression is usually based on having a wider range of symptoms at baseline. Constraining the range at baseline likely reduced the potential to see an association.
Conclusion
To advance objective, easy-to-administer assessments of late-life depression and anxiety risk, we explored the relative predictive value of the combination of both gait speed and psychomotor slowing on time to new episode depression or anxiety in older adults at risk for these common psychiatric conditions. Our composite index of slowing was superior at predicting incident depression or anxiety compared to participants who had neither type of slowing. Future research is needed to explore the neurobiology of slowing to better understand the interplay of age-related changes in motor and cognitive function, and the pathophysiologic changes underlying late life neuropsychiatric disorders.
Why does this paper matter?
While there are established medical, psychiatric, and psychosocial risks for becoming depressed and anxious, there are no objective behavioral measures that are routinely used for risk stratification. Both gait speed and psychomotor speed may predict incident depression and anxiety.
KEY POINTS.
We explored the relative predictive values of gait speed, psychomotor slowing, and a composite index of these two measures on time to new episode depression or anxiety in older adults at risk for these common psychiatric conditions.
The co-occurrence of slowed gait with slowed processing speed increased the risk for new-onset depression or anxiety (relative to having neither type of slowing).
Evaluating both gait and psychomotor speed in at-risk older adults may predict incident mental illness and inform prevention planning.
Funding:
Supported in part by P30 MH090333, CTSI UL1RR024153, UL1TR000005, and MH118270 (Stahl) from the National Institutes of Health.
Sponsor’s Role:
None.
Potential Conflicts of Interest:
JFK has received medication supplies for investigator-initiated trials from Pfizer and Indivior greater than 12 months ago. He receives honoraria from American Journal of Geriatric Psychiatry and Physicians Postgraduate Press. He serves on the scientific advisory board of NightWare and Aifred Health and received honoraria from Otsuka for development and presentation of a webinar (disease-focused, not product-specific). CFR has received research support from the NIH, the Patient Centered Outcomes Research Institute, the Center for Medicare and Medicaid Services, the American Foundation for Suicide Prevention, the Brain and Behavior Research Foundation, and the Commonwealth of Pennsylvania. Bristol Meyer Squib and Pfizer have provided pharmaceutical supplies for his NIH sponsored research. He is a paid consultant to Merck. The other authors have nothing to disclose.
References
- [1].Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5: 363–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163: 1905–1917. [DOI] [PubMed] [Google Scholar]
- [3].Allard P, Gram L, Timdahl K, Behnke K, Hanson M, Søgaard J. Efficacy and tolerability of venlafaxine in geriatric outpatients with major depression: a double-blind, randomised 6-month comparative trial with citalopram. Int J Geriatr Psychiatry. 2004;19: 1123–1130. [DOI] [PubMed] [Google Scholar]
- [4].Mitchell AJ, Subramaniam H. Prognosis of depression in old age compared to middle age: a systematic review of comparative studies. Am J Psychiatry. 2005;162: 1588–1601. [DOI] [PubMed] [Google Scholar]
- [5].Deng Y, McQuoid DR, Potter GG, et al. Predictors of recurrence in remitted late-life depression. Depress Anxiety. 2018;35: 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beekman AT, de Beurs E, van Balkom AJ, Deeg DJ, van Dyck R, van Tilburg W. Anxiety and depression in later life: Co-occurrence and communality of risk factors. Am J Psychiatry. 2000;157: 89–95. [DOI] [PubMed] [Google Scholar]
- [7].Andreescu C, Lenze EJ, Dew MA, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007;190: 344–349. [DOI] [PubMed] [Google Scholar]
- [8].Demakakos P, Cooper R, Hamer M, de Oliveira C, Hardy R, Breeze E. The bidirectional association between depressive symptoms and gait speed: evidence from the English Longitudinal Study of Ageing (ELSA). PLoS One. 2013;8: e68632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sekhon H, Allali G, Beauchet O. The association of anxio-depressive disorders and depression with motoric cognitive risk syndrome: results from the baseline assessment of the Canadian longitudinal study on aging. Geroscience. 2019;41: 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marino FR, Lessard DM, Saczynski JS, et al. Gait Speed and Mood, Cognition, and Quality of Life in Older Adults With Atrial Fibrillation. Journal of the American Heart Association. 2019;8: e013212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sanders JB, Bremmer MA, Comijs HC, van de Ven PM, Deeg DJH, Beekman ATF. Gait Speed and Processing Speed as Clinical Markers for Geriatric Health Outcomes. Am J Geriatr Psychiatry. 2017;25: 374–385. [DOI] [PubMed] [Google Scholar]
- [12].Busch TdA, Duarte YA, Pires Nunes D, et al. Factors associated with lower gait speed among the elderly living in a developing country: a cross-sectional population-based study. BMC Geriatrics. 2015;15: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Studenski S, Perera S, Patel K, et al. Gait Speed and Survival in Older Adults. JAMA. 2011;305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Veronese N, Stubbs B, Trevisan C, et al. Poor Physical Performance Predicts Future Onset of Depression in Elderly People: Progetto Veneto Anziani Longitudinal Study. Physical Therapy. 2017;97: 659–668. [DOI] [PubMed] [Google Scholar]
- [15].Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: A systematic review. Psychiatry Res. 2020;284: 112687. [DOI] [PubMed] [Google Scholar]
- [16].Feldman R, Schreiber S, Pick CG, Been E. Gait, balance, mobility and muscle strength in people with anxiety compared to healthy individuals. Hum Mov Sci. 2019;67: 102513. [DOI] [PubMed] [Google Scholar]
- [17].Chen TY, Peronto CL, Edwards JD. Cognitive function as a prospective predictor of falls. J Gerontol B Psychol Sci Soc Sci. 2012;67: 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gallagher D, Fischer CE, Iaboni A. Neuropsychiatric Symptoms in Mild Cognitive Impairment. Can J Psychiatry. 2017;62: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S. Digit Symbol Substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing. 2016;45: 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kuo HK, Leveille SG, Yu YH, Milberg WP. Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. Gerontology. 2007;53: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amieva H, Meillon C, Proust-Lima C, Dartigues JF. Is Low Psychomotor Speed a Marker of Brain Vulnerability in Late Life? Digit Symbol Substitution Test in the Prediction of Alzheimer, Parkinson, Stroke, Disability, and Depression. Dementia and Geriatric Cognitive Disorders. 2019;47: 297–305. [DOI] [PubMed] [Google Scholar]
- [22].Pimontel MA, Rindskopf D, Rutherford BR, Brown PJ, Roose SP, Sneed JR. A Meta-Analysis of Executive Dysfunction and Antidepressant Treatment Response in Late-Life Depression. Am J Geriatr Psychiatry. 2016;24: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beekman AT, Geerlings SW, Deeg DJ, et al. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59: 605–611. [DOI] [PubMed] [Google Scholar]
- [24].Gulpers B, Ramakers I, Hamel R, Köhler S, Oude Voshaar R, Verhey F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am J Geriatr Psychiatry. 2016;24: 823–842. [DOI] [PubMed] [Google Scholar]
- [25].Gildengers AG, Butters MA, Albert SM, et al. Design and Implementation of an Intervention Development Study: Retaining Cognition While Avoiding Late-Life Depression (ReCALL). The American Journal of Geriatric Psychiatry. 2016;24: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karp JF, Dew MA, Wahed AS, et al. Challenges and Solutions for Depression Prevention Research: Methodology for a Depression Prevention Trial for Older Adults with Knee Arthritis and Emotional Distress. Am J Geriatr Psychiatry. 2016;24: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Karp JF, Zhang J, Wahed AS, et al. Improving Patient Reported Outcomes and Preventing Depression and Anxiety in Older Adults With Knee Osteoarthritis: Results of a Sequenced Multiple Assignment Randomized Trial (SMART) Study. The American Journal of Geriatric Psychiatry. 2019;27: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Albert SM, King J, Anderson S, et al. Depression Agency-Based Collaborative: Effect of Problem-Solving Therapy on Risk of Common Mental Disorders in Older Adults With Home Care Needs. The American Journal of Geriatric Psychiatry. 2019;27: 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Albert SM, King J, Dew MA, et al. Design and Recruitment for a Randomized Controlled Trial of Problem-Solving Therapy to Prevent Depression among Older Adults with Need for Supportive Services. Am J Geriatr Psychiatry. 2016;24: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48: 314–318. [PubMed] [Google Scholar]
- [31].First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). Comprehensive handbook of psychological assessment, Vol 2: Personality assessment. Hoboken, NJ, US: John Wiley & Sons Inc, 2004, pp. 134–143. [Google Scholar]
- [32].Pettersson A, Modin S, Wahlström R, Af Winklerfelt Hammarberg S, Krakau I. The Mini-International Neuropsychiatric Interview is useful and well accepted as part of the clinical assessment for depression and anxiety in primary care: a mixed-methods study. BMC Fam Pract. 2018;19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49: M85–94. [DOI] [PubMed] [Google Scholar]
- [34].Johnson SU, Ulvenes PG, Øktedalen T, Hoffart A. Psychometric Properties of the General Anxiety Disorder 7-Item (GAD-7) Scale in a Heterogeneous Psychiatric Sample. Front Psychol. 2019;10: 1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ebaid D, Crewther SG, MacCalman K, Brown A, Crewther DP. Cognitive Processing Speed across the Lifespan: Beyond the Influence of Motor Speed. Front Aging Neurosci. 2017;9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Andreescu C, Ajilore O, Aizenstein HJ, et al. Disruption of Neural Homeostasis as a Model of Relapse and Recurrence in Late-Life Depression. The American Journal of Geriatric Psychiatry. 2019;27: 1316–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Montero-Odasso M, Speechley M, Muir-Hunter SW, et al. Motor and Cognitive Trajectories Before Dementia: Results from Gait and Brain Study. J Am Geriatr Soc. 2018;66: 1676–1683. [DOI] [PubMed] [Google Scholar]
- [38].Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68: 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Montero-Odasso M, Almeida QJ, Bherer L, et al. Consensus on Shared Measures of Mobility and Cognition: From the Canadian Consortium on Neurodegeneration in Aging (CCNA). J Gerontol A Biol Sci Med Sci. 2019;74: 897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Belujon P, Grace AA. Dopamine System Dysregulation in Major Depressive Disorders. Int J Neuropsychopharmacol. 2017;20: 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 2017;57: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155: 344–349. [DOI] [PubMed] [Google Scholar]
- [43].Matsumoto M, Takada M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron. 2013;79: 1011–1024. [DOI] [PubMed] [Google Scholar]
- [44].Hori H, Kunugi H. Dopamine agonist-responsive depression. Psychogeriatrics. 2013;13: 189–195. [DOI] [PubMed] [Google Scholar]
- [45].Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS. CSF amine metabolites in depression. Biol Psychiatry. 1992;31: 112–118. [DOI] [PubMed] [Google Scholar]
- [46].Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019;9: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Butters MA, Aizenstein HJ, Hayashi KM, et al. Three-dimensional surface mapping of the caudate nucleus in late-life depression. Am J Geriatr Psychiatry. 2009;17: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Van Abbema R, De Greef M, Crajé C, Krijnen W, Hobbelen H, Van Der Schans C. What type, or combination of exercise can improve preferred gait speed in older adults? A meta-analysis. BMC Geriatr. 2015;15: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jiang H, Chen S, Wang L, Liu X. An Investigation of Limbs Exercise as a Treatment in Improving the Psychomotor Speed in Older Adults with Mild Cognitive Impairment. Brain Sci. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]


