Abstract
Methods
We systematically searched articles on electronic databases such as PubMed, Embase, Scopus, and Google Scholar between January 1, 2000 and July 30, 2020. Articles were independently evaluated by two authors. We included observational studies (case-control and cohort) and calculated the risk ratios (RRs) for associated with anemia and PD. Heterogeneity among the studies was assessed using the Q and I2 statistic. We utilized the random-effect model to calculate the overall RR with 95% CI.
Results
A total of 342 articles were identified in the initial searches, and 7 full-text articles were evaluated for eligibility. Three articles were further excluded for prespecified reasons including insufficient data and duplications, and 4 articles were included in our systematic review and meta-analysis. A random effect model meta-analysis of all 4 studies showed no increased risk of PD in patients with anemia (N = 4, RRadjusted = 1.17 (95% CI: 0.94-1.45, p = 0.15). However, heterogeneity among the studies was significant (I2 = 92.60, p = <0.0001). The pooled relative risk of PD in female patients with anemia was higher (N = 3, RRadjusted = 1.14 (95% CI: 0.83-1.57, p = 0.40) as compared to male patients with anemia (N = 3, RRadjusted = 1.09 (95% CI: 0.83-1.42, p = 0.51).
Conclusion
This is the first meta-analysis that shows that anemia is associated with higher risk of PD when compared with patients without anemia. However, more studies are warranted to evaluate the risk of PD among patients with anemia.
1. Introduction
1.1. Rationale
Parkinson disease (PD) is the second most common age-related neurodegenerative disorder, with more than 60,000 newly diagnosed cases yearly in the USA [1]. The incidence of PD has been increasing at an alarming rate and is estimated to be nearly 1.2 million PD cases worldwide by 2030 and 12 million patients worldwide by about 2050 [2]. It is estimated that both direct and indirect costs regarding PD are approximately USD 52 billion only in the USA [2]. The higher amount of financial burden that places on our current health care system highlights the importance of conducting research to curb the incidence and prevalence rate of PD. Therefore, a vivid understanding of PD risk factors can help researchers and policymaker to develop strong strategies for turning down the number of new cases and reducing costs.
The common risk factors of developing PD are exposure to pesticides, genetic variants, environment toxins, idiopathic REM sleep behavior disorder (RBD), and focal cerebrovascular damage [3–6]. However, several epidemiological studies have reported that inflammatory bowel disease (IBD) [7], head injury [8], and autoimmune rheumatic diseases (ARDs) [9] are significantly associated with increased risk of PD. An increased risk of PD was also reported in patients with anemia than those without anemia. Although the exact relationship between anemia and PD risk remains inconclusive, several possible hypotheses have been proposed. A recent study [10] reported that chronic anemia can increase brain hypoxia which is the main risk factor of Alzheimer disease (AD). Previous studies also suggested that patient with AD may experience sequela of epigenetic changes during the development stage [11, 12]. A study showed that neonatal iron deficiency is associated with modification of the AD-related gene expression [13]. Since AD is associated with PD [14], these etiology and link may lead to increased risk of PD. Furthermore, another study demonstrated that rats with iron deficiency diet reduced dopaminergic activity which might induce PD risk [15].
Anemia hampers erythropoiesis and enhances eryptosis, whereas insufficient iron is reported in the substantia nigra of PD patients [16, 17]. Increased serum iron levels are related to decreased risk of PD, and high dietary iron intake reduces PD risk [18, 19]. Moreover, dysregulation of iron metabolism is associated with oxidative stress and cell death [20–22]. Based on biological and epidemiological evidence, it is needed to conduct a study that summarizes the role of anemia on PD risk. However, to our best knowledge, no meta-analysis has been performed to assess the magnitude of association between anemia and PD.
Goal: The aim of this current systematic review and meta-analysis is to evaluate the published epidemiological studies for clarifying the association between anemia and risk of PD.
1.2. Research Questions
Study the magnitude of the risk of PD in patients with anemia and without anemia
Calculate the magnitude difference of the risk of PD in male and female patients with anemia
Reduce the confounding factors by evaluating the risk based on various adjustments
2. Methods
2.1. Search Strategy
We did a systematic search on electronic databases such as PubMed, Embase, Scopus, and Web of Science between January 1, 2000 and July 30, 2020. The following search terms were used to collect relevant articles: “anemia,” OR “low level hemoglobin,” “iron deficiency” And “Parkinson disease.” The initial search was conducted by one author who is an expert in systematic review. Moreover, we searched in the reference lists of retrieved articles to ensure the comprehensiveness.
2.2. Eligibility Criteria
The eligibility criteria were restricted to observational studies (case-control and cohort) and clinical trials (randomized control trial) that evaluated the association between anemia and PD as a primary outcome. Studies were included if they were published in the form of (a) original study, (b) participants at least 200, (c) published in English, (d) provided clear definition of anemia and PD, and (e) provide proper effect size to summarized pool risk.
We excluded studies if they published in the form of review, short report, poster, editorial, case-report, and correspondence.
2.3. Selection Process
Two authors (TNP and YCW) independently reviewed all the titles and abstracts of retrieved articles. They used prespecified selection criteria to select relevant articles to be included in this meta-analysis. Any disagreement in this stage was resolved by the difference by discussing with third author.
2.4. Data Extraction
Same two authors developed data collection form for extracting the required data from the selected studies. They checked the study duplication by comparing authors' names, publication year, and location of study. Two authors collected the information about effect size (odds ratios, hazard ratios with 95% CI), adjusted factors, total number of participants, number of anemia and PD patients, inclusion and exclusion criteria of anemia and PD, age, percentage of male, duration of study period, and location.
2.5. Risk Assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of each study. They calculated the NOS score and divided into two groups: low and high qualities. The heterogeneity was calculated among study-specific RRs using the Q and I2 statistic. Finally, publication bias evaluated by 3 funnel plot–based methods: the Egger's test, the Begg's test, and the trim and fill method.
2.6. Statistical Analysis
The meta-analysis was performed on selected studies that evaluated the magnitude of the association by using adjusted ORs and HRs. The pooled risk ratio (RR) was calculated from selected studies to show the effect size. The random-effect model was used in this meta-analysis. We calculated statistical heterogeneity across the various studies which were tested using the Cochran Q statistic and quantified by the I2 value. The heterogeneity among the studies was categorized into four groups, namely, very low (<25%), low (25~50%), medium (50~75%), and high (>75%) [23–25]. When number of studies is small, the random-effect model is a perfect test to reduce bias and heterogeneity among the studies. We draw forest plot to present effect size and funnel plot to depict the publication bias. However, all the statistical analyses were performed using comprehensive meta-analysis software (V:2). The p value less than 0.5 is considered as a significant.
3. Results
3.1. Study Selection
Initial search in the electronic databases yielded 342 articles. A total of 335 articles were excluded after reviewing the titles and abstracts of retrieved articles. However, 7 articles went to full-text review, and 3 articles were further excluded due to not matching the prespecified selection criteria. Finally, 4 articles were included in the current systematic review and meta-analysis [26–29]. Figure 1 shows our study selection process.
Figure 1.
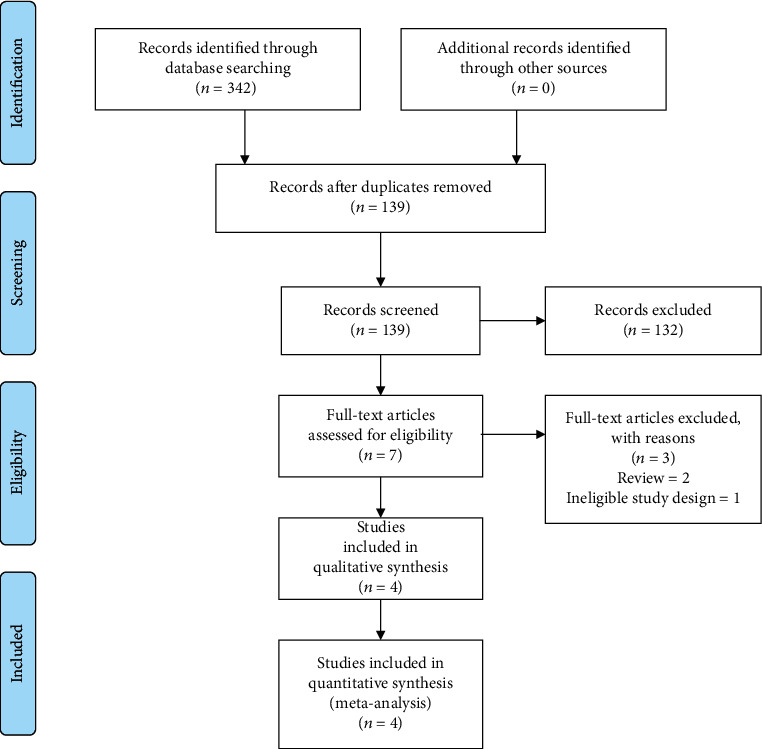
Flow diagram of the study selection process.
3.2. Study Characteristics
This current systematic review and meta-analysis comprised of 4 studies including 3 cohorts and 1 case-control study (Table 1). Included studies published between 2009 and 2019; the average age of patients was from 48.7 to 71. The percentage of male patients was from 24.1 to 63%. Three studies followed World Health Organization (WHO) guidelines to be included in the anemia patients: hemoglobin level < 13 g/dL for men and <12 g/dL for women, and one study used ICD to include anemia patients. On the other hand, three studies used ICD to include PD patients, and one study used PD registry linkage.
Table 1.
Baseline characteristics of included studies.
| Author name | Location | Study duration | Age | Male (%) | Design | PD | Anemia criteria | PD | Mean follow-up (Yrs.) | HR/OR | Major adjustment | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cho 2020 | S. Korea | 2009-2013 | 57.41 ± 7.51 | 63 | Cohort | 3,844 | The World Health Organization (WHO): hemoglobin level < 13 g/dL for men and <12 g/dL for women | ICD-10 | 5 | 0.89 (0.80-0.98) | Smoking, alcohol, physical activity, HTN, DM, dyslipidemia, GFR | 8 |
| Rozani 2019 | Israel | 1999-2012 | 48.7 | 47.4 | Cohort | 2,427 | The World Health Organization (WHO): hemoglobin level < 13 g/dL for men and <12 g/dL for women | ICD-9 | 8.8 ± 3.9 | 1.02 (0.95-1.09) | N/A | 8 |
| Hong 2016 | Taiwan | N/A | 56.4 | 24.1 | Cohort | 86,334 | ICD-9 | ICD-9 | 6.6 | 1.36 (1.22-1.52) | HTN, DM, hyperlipidemia | 8 |
| Savica 2009 | USA | 1976-1995 | 71 | 61.7 | Case-control | 196 | The World Health Organization (WHO): hemoglobin level < 13 g/dL for men and <12 g/dL for women | Records linkage system | n/a | 2.00 (1.31-3.06) | N/A | 8 |
#PD: Parkinson disease; ICD: International Classification of Diseases; HR: hazard ratio; OR: odds ratio; NOS: the Newcastle-Ottawa Scale.
3.3. Anemia and Risk of PD
There were various follow-up durations for each of the 4 studies, ranging from 5 to 8.8 years. Our meta-analysis comprised a total of 92,851 PD patients. A random-effect model meta-analysis of all 4 studies showed no increased risk of PD in patients with anemia (N = 4, RRadjusted = 1.17 (95% CI: 0.94-1.45, p = 0.15). However, heterogeneity among the studies was significant (Q = 40.58, tau2 = 0.04, I2 = 92.60, p = <0.0001) (Figure 2).
Figure 2.
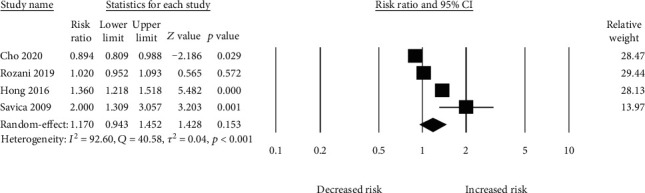
Association between anemia and risk of PD.
3.4. Subgroup Analysis
We pooled 3 studies in order to evaluate the risk of PD based on gender. The pooled relative risk of PD in female patients with anemia was higher (N = 3, RRadjusted = 1.14 (95% CI: 0.83-1.57, p = 0.40) as compared to male patients with anemia (N = 3, RRadjusted = 1.09 (95% CI: 0.83-1.42, p = 0.51) (Figure 3). However, a significant heterogeneity was both in male (I2 = 81.02, p = 0.005, Q = 10.54, tau2 = 0.03) and female (I2 = 82.60, p = 0.003, Q = 11.50, tau2 = 0.05) patients with anemia.
Figure 3.
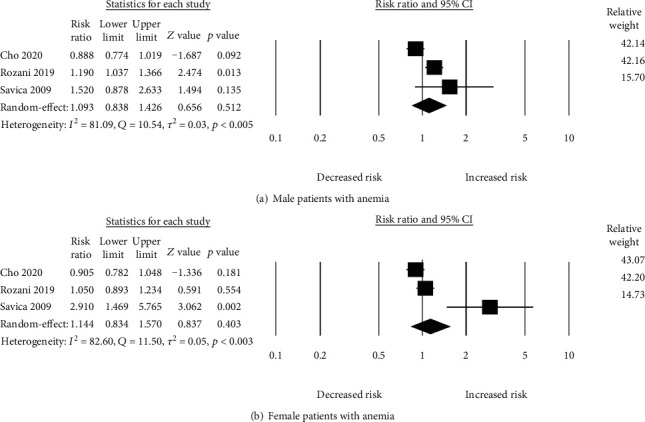
Risk of PD.
We also evaluated the risk of PD in patients among the studies adjusted with most common confounding factors such as diabetes and hypertension. The pooled risk ratio was (N = 3, RRadjusted with diabetes and hypertension = 1.10 (95% CI: 0.73-1.66, p = 0.64), and heterogeneity was significant (I2 = 96.72, p = <0.001, Q = 30.49, tau2 = 0.08).
3.5. Publication Bias
Figure 4 shows the funnel plot indicating possible publication bias. Egger's regression test of the funnel asymmetry presented no possible publication bias.
Figure 4.
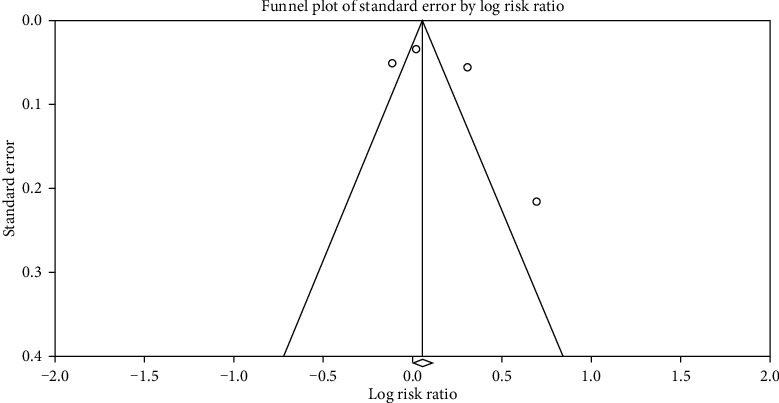
Funnel plot.
4. Discussion
4.1. Main Findings
To our knowledge, this is the first study to assess the risk of PD among the patients with anemia. This current meta-analysis of four observational studies showed that patients with anemia were not significantly associated with 17% increased risk of PD as compared with nonanemia. However, male patients with anemia showed nonsignificant increased risk of PD while comparing with female patients with anemia. The strengths of this study are (a) all included studies quality were high and (b) consider adjusted effect size; therefore, risk of bias is low and (c) clear definition of anemia and PD risk.
4.2. Biological Mechanism
The biological mechanism of PD and anemia is not fully understood yet. However, there are several biological explanations that can define their association. First, the neural degeneration and disease progression among the PD patients can be induced by either apoptosis or necrosis [30]. The brain cell death is caused by DNA fragmentation and typical morphological changes including cell shrinkage and nuclear condensation. However, apoptosis in PD is still debatable. The animals and in vitro studies reported apoptosis as well as promoting the rate of neurodegeneration [31–33]. Second, some assumptions regarding oxidative stress and iron metabolism are likely to dopaminergic neural death in the central nervous system [34, 35]. Several studies analyzed erythrocytes of PD indicating lessened superoxide dismutase, glutathione peroxidase activity, and elevated lipid peroxidation which are responsible for the degeneration of dopaminergic neurons in the substantia nigra. All the process, however, are related to the activity of oxidative stress [36, 37]. Third, α-synuclein and etiology of PD are the main keys that can be used to explain the pathological mechanism of anemia and PD risk. Erythrocytes generate maximum portion of α-synuclein in the human blood [38]; it has shown in the previous study that the ratio of α-synuclein oligomer and total protein is higher in erythrocyte of PD patients when compared to normal patients [39]. Anxiety and depression are most common symptoms in PD that are considered as primary contributors to abnormality, low quality of life, and low survival [40, 41]. Depression in PD patient is associated with several neurotransmitter dysfunctions including serotonin, noradrenaline, and dopamine [42]. Moreover, gut brain axis (GBA) has a positive correlation with increased risk of PD. Accumulation of gut microbiota in α-synuclein in PD has received wide attention over the past years. A previous mice study showed that microbial metabolites may increase the risk of neuroinflammation by expressing proinflammatory cytokines which ultimately lead to the development of motor symptom [43]. Other study also reported a positive link of gut microbiota in neurodegeneration [44].
4.3. Clinical Implications
PD is now one of the leading causes of disability and deaths globally. A significant amount of epidemiological studies have highlighted an increased prevalence of PD which raised sheer concern and urgent need for public health strategies [45–47]. Appropriate planning would help to reduce the number of PD patients as well as healthcare cost over the coming decades [48]. The primary risk factor of PD is age, but PD also appears to be linked to anemia. However, the association between anemia and PD based on duration and severity is less well known. Since anemia and aging population are increasing globally, the prevalence of PD undoubtedly will increase. Conducting a meta-analysis can clarify the effect size in order to prevent and treat the disease both earlier and effectively.
4.4. Strengths and Limitations
Our first and rigorous meta-analysis has several strengths. First, this is the first meta-analysis that investigated the association between anemia and PD risk. Second, this study included four large observation studies from Taiwan, Israel, South Korea, and USA; healthcare data quality of these countries is world standard. They adjusted potential confounding factors to this study to reduce bias of effect size calculation. Third, this present study showed risk difference between male and female patients.
However, this study has some limitations. First, number of included studies is only four, although all are high quality study design with larger number of participants. Second, we were not able to provide risk of PD based on severity of anemia (high, moderate, and low) and BMI (overweight, obese, and underweight). Third, the pooled risk was not calculated based on location, study design, and other factors like smoking status, alcohol consumptions, and duration of anemia; it is because data were limited.
5. Conclusion
Our study shows that the risk of PD was higher among the patients with anemia as compared without anemia. The risk of PD was higher in male patients. Therefore, more protective strategies should be taken in male patients when compared to female patients. The risk of PD among patients with anemia can be explained by some biological mechanisms like oxidative stress and downregulation of iron homeostasis. More observational studies in different regions and biological studies are warranted to clarify the mechanism underlying their association.
Data Availability
The data used to support the findings of this study are from previously reported studies and datasets, which have been cited and included within the article.
Conflicts of Interest
The author(s) declare(s) that they have no conflicts of interest.
References
- 1.Yang W., Hamilton J. L., Kopil C., et al. Current and projected future economic burden of Parkinson's disease in the U.S. npj Parkinson's Disease. 2020;6(1):1–9. doi: 10.1038/s41531-020-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorsey E. R., Elbaz A., Nichols E., et al. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emamzadeh F. N., Surguchov A. Parkinson’s disease: biomarkers, treatment, and risk factors. Frontiers in neuroscience. 2018;12:p. 612. doi: 10.3389/fnins.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller D. B., O’Callaghan J. P. Biomarkers of Parkinson's disease: Present and future. Metabolism. 2015;64(3):S40–S46. doi: 10.1016/j.metabol.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postuma R. B., Lang A. E., Massicotte-Marquez J., Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66(6):845–851. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 6.Postuma R., Gagnon J.-F., Rompre S., Montplaisir J. Y. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74(3):239–244. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S., Kim J., Chun J., et al. Patients with inflammatory bowel disease are at an increased risk of Parkinson’s disease: a South Korean nationwide population-based study. Journal of clinical medicine. 2019;8(8):p. 1191. doi: 10.3390/jcm8081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rugbjerg K., Ritz B., Korbo L., Martinussen N., Olsen J. H. Risk of Parkinson’s disease after hospital contact for head injury: population based case-control study. Bmj. 2008;337, article a2494 doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C.-C., Lin T.-M., Chang Y.-S., et al. Autoimmune rheumatic diseases and the risk of Parkinson disease: a nationwide population-based cohort study in Taiwan. Annals of medicine. 2018;50(1):83–90. doi: 10.1080/07853890.2017.1412088. [DOI] [PubMed] [Google Scholar]
- 10.Atti A., Palmer K., Volpato S., Zuliani G., Winblad B., Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiology of aging. 2006;27(2):278–284. doi: 10.1016/j.neurobiolaging.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Wu J., Basha M. R., Brock B., et al. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. Journal of Neuroscience. 2008;28(1):3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J., Basha M. R., Zawia N. H. The environment, epigenetics and amyloidogenesis. Journal of Molecular Neuroscience. 2008;34(1):1–7. doi: 10.1007/s12031-007-0009-4. [DOI] [PubMed] [Google Scholar]
- 13.Carlson E. S., Magid R., Petryk A., Georgieff M. K. Iron deficiency alters expression of genes implicated in Alzheimer disease pathogenesis. Brain research. 2008;1237:75–83. doi: 10.1016/j.brainres.2008.07.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca W. A., Bower J. H., Ahlskog J. E., et al. Risk of cognitive impairment or dementia in relatives of patients with Parkinson disease. Archives of neurology. 2007;64(10):1458–1464. doi: 10.1001/archneur.64.10.1458. [DOI] [PubMed] [Google Scholar]
- 15.Levenson C. W., Cutler R. G., Ladenheim B., Cadet J. L., Hare J., Mattson M. P. Role of dietary iron restriction in a mouse model of Parkinson's disease. Experimental neurology. 2004;190(2):506–514. doi: 10.1016/j.expneurol.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Martin W. R. W., Wieler M., Gee M. Midbrain iron content in early parkinson disease: A potential biomarker of disease status. Neurology. 2008;70, 16, Part 2:1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- 17.Dexter D., Wells F., Lee A., et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. Journal of Neurochemistry. 1989;52(6):1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 18.Pichler I., Greco F., Gögele M., et al. Serum iron levels and the risk of Parkinson disease: a Mendelian randomization study. PLOS Medicine. 2013;10(6, article e1001462):p. e1001462. doi: 10.1371/journal.pmed.1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyake Y., Tanaka K., Fukushima W., et al. Dietary intake of metals and risk of Parkinson's disease: a case-control study in Japan. Journal of the Neurological Sciences. 2011;306(1-2):98–102. doi: 10.1016/j.jns.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Grune T., Sommerburg O., Siems W. Oxidative stress in anemia. Clinical nephrology. 2000;53(1 Supplement):S18–S22. [PubMed] [Google Scholar]
- 21.Kurtoglu E., Ugur A., Baltaci A. K., Undar L. Effect of iron supplementation on oxidative stress and antioxidant status in iron-deficiency anemia. Biological Trace Element Research. 2003;96(1-3):117–124. doi: 10.1385/BTER:96:1-3:117. [DOI] [PubMed] [Google Scholar]
- 22.Tran P. V., Carlson E. S., Fretham S. J., Georgieff M. K. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. The Journal of nutrition. 2008;138(12):2495–2501. doi: 10.3945/jn.108.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam M. M., Iqbal U., Walther B., et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47(3-4):181–191. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- 24.Poly T. N., Islam M. M. R., Yang H.-C., Li Y.-C. J. Non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease in the elderly population: a meta-analysis. European journal of clinical pharmacology. 2019;75(1, article 2561):99–108. doi: 10.1007/s00228-018-2561-y. [DOI] [PubMed] [Google Scholar]
- 25.Islam M. M., Yang H.-C., Nguyen P.-A., et al. Exploring association between statin use and breast cancer risk: an updated meta-analysis. Archives of gynecology and obstetrics. 2017;296(6):1043–1053. doi: 10.1007/s00404-017-4533-3. [DOI] [PubMed] [Google Scholar]
- 26.Cho I. Y., Shin D. W., Roh Y., et al. Anemia and the risk of Parkinson's disease in Korean older adults: A nationwide population-based study. Scientific reports. 2020;10(1, article 61153):4268–4269. doi: 10.1038/s41598-020-61153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong C. T., Huang Y. H., Liu H. Y., Chiou H.-Y., Chan L., Chien L. N. Newly Diagnosed Anemia Increases Risk of Parkinson's disease: A Population- Based Cohort Study. Scientific reports. 2016;6(1, article 29651) doi: 10.1038/srep29651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozani V., Giladi N., Gurevich T., et al. Anemia in men and increased Parkinson's disease risk: A population-based large scale cohort study. Parkinsonism & related disorders. 2019;64:90–96. doi: 10.1016/j.parkreldis.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Savica R., Grossardt B., Carlin J., et al. Anemia or low hemoglobin levels preceding Parkinson disease: a case-control study. Neurology. 2009;73(17):1381–1387. doi: 10.1212/WNL.0b013e3181bd80c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.KJJont J. Cell death mechanisms in Parkinson's disease. Journal of neural transmission. 2000;107(1):1–29. doi: 10.1007/s007020050001. [DOI] [PubMed] [Google Scholar]
- 31.Ruberg M., France-Lanord V., Brugg B., et al. Neuronal death caused by apoptosis in Parkinson disease. Revue neurologique. 1997;153(8-9):499–508. [PubMed] [Google Scholar]
- 32.Liu W., Zhang Q., Zhang J., Pan W., Zhao J., Xu Y. Long non-coding RNA MALAT1 contributes to cell apoptosis by sponging miR-124 in Parkinson disease. Cell & bioscience. 2017;7(1):1–9. doi: 10.1186/s13578-017-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lev N., Melamed E., Offen D. Apoptosis and Parkinson's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(2):245–250. doi: 10.1016/S0278-5846(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 34.You L.-H., Li F., Wang L., et al. Brain iron accumulation exacerbates the pathogenesis of MPTP-induced Parkinson's disease. Neuroscience. 2015;284:234–246. doi: 10.1016/j.neuroscience.2014.09.071. [DOI] [PubMed] [Google Scholar]
- 35.Ahlskog J. E. Challenging conventional wisdom: the etiologic role of dopamine oxidative stress in Parkinson's disease. Movement disorders. 2005;20(3):271–282. doi: 10.1002/mds.20362. [DOI] [PubMed] [Google Scholar]
- 36.Kilinç A., Yalçin A. S., Yalçin D., Taga Y., Emerk K. Increased erythrocyte susceptibility to lipid peroxidation in human Parkinson's disease. Neuroscience letters. 1988;87(3):307–310. doi: 10.1016/0304-3940(88)90467-3. [DOI] [PubMed] [Google Scholar]
- 37.Urakami K., Sano K., Matsushima E., et al. Decreased superoxide dismutase activity in erythrocyte in Parkinson's disease. Psychiatry and Clinical Neurosciences. 1992;46(4):933–936. doi: 10.1111/j.1440-1819.1992.tb02863.x. [DOI] [PubMed] [Google Scholar]
- 38.Barbour R., Kling K., Anderson J. P., et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegenerative Diseases. 2008;5(2):55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 39.Wang X., Yu S., Li F., Feng T. Detection of α-synuclein oligomers in red blood cells as a potential biomarker of Parkinson's disease. Neuroscience letters. 2015;599:115–119. doi: 10.1016/j.neulet.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 40.Bower J. H., Grossardt B. R., Maraganore D. M., et al. Anxious personality predicts an increased risk of Parkinson's disease. Movement disorders. 2010;25(13):2105–2113. doi: 10.1002/mds.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonso A., Rodriguez L. A. G., Logroscino G., Hernan M. A. Use of antidepressants and the risk of Parkinson’s disease: a prospective study. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(6):671–674. doi: 10.1136/jnnp.2008.152983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postuma R. B., Aarsland D., Barone P., et al. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Movement Disorders. 2012;27(5):617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 43.Sampson T. R., Debelius J. W., Thron T., et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorecki A. M., Preskey L., Bakeberg M. C., et al. Altered gut microbiome in Parkinson’s disease and the influence of lipopolysaccharide in a human α-synuclein over-expressing mouse model. Frontiers in neuroscience. 2019;13:p. 839. doi: 10.3389/fnins.2019.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.on behalf of the Parkinson’s Foundation P4 Group, Marras C., Beck J. C., et al. Prevalence of Parkinson's disease across North America. NPJ Parkinson's disease. 2018;4(1):1–7. doi: 10.1038/s41531-018-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pringsheim T., Jette N., Frolkis A., Steeves T. D. L. The prevalence of Parkinson's disease: A systematic review and meta-analysis. Movement disorders. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 47.Park J.-H., Kim D.-H., Kwon D.-Y., et al. Trends in the incidence and prevalence of Parkinson’s disease in Korea: a nationwide, population-based study. BMC geriatrics. 2019;19(1, article 1332):p. 320. doi: 10.1186/s12877-019-1332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baiano C., Barone P., Trojano L., Santangelo G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson's disease: a meta-analysis. Movement Disorders. 2020;35(1):45–54. doi: 10.1002/mds.27902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are from previously reported studies and datasets, which have been cited and included within the article.


