Abstract
Despite thousands of reported patients with pandemic-associated pernio, low rates of seroconversion and PCR positivity have defied causative linkage to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Pernio in uninfected children is associated with monogenic disorders of excessive IFN-1 immunity, whereas severe COVID-19 pneumonia can result from insufficient IFN-1. Moreover, SARS-CoV-2 spike protein and robust IFN-1 response are seen in the skin of patients with pandemic-associated pernio, suggesting an excessive innate immune skin response to SARS-CoV-2. Understanding the pathophysiology of this phenomenon may elucidate the host mechanisms that drive a resilient immune response to SARS-CoV-2 and could produce relevant therapeutic targets.
Abbreviations: ACE2, angiotensin-converting enzyme 2; HCV, hepatitis C virus; pDC, plasmacytoid dendritic cell; RAS, renin-angiotensin system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Introduction
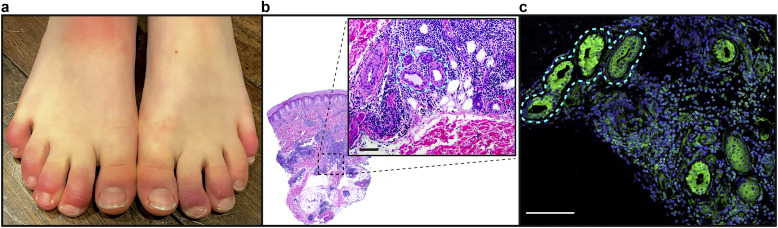
In March 2020, just weeks after the onset of community spread of COVID-19 in Italy, reports of pandemic-associated pernio emerged. Shortly thereafter, dermatologists in the United States were inundated with pernio referrals as the first surge of COVID-19 arrived in the United States (Bouaziz et al., 2020; Cordoro et al., 2020; Duong et al., 2020; Galván Casas et al., 2020; Landa et al., 2020; López-Robles et al., 2020; Piccolo et al., 2020). The phenotype of cool extremities with pain/swelling followed by red‒violaceous discoloration and finally vesiculation of the toes and fingers were strikingly consistent (Figure 1 a). Whereas older age was an important risk factor for severe infection, most patients with pernio were young, with a median age of 25 years in an international dermatology registry (Castelo-Soccio et al., 2021; Freeman et al., 2020). Many had close contact with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected individuals; yet, nearly all were otherwise healthy and denied typical respiratory manifestations of COVID-19 (Castelo-Soccio et al., 2021; Freeman et al., 2020). The spatial and temporal association between pernio and the COVID-19 pandemic has now been independently observed across multiple countries, including Italy, Spain, Germany, the United Kingdom, France, and the United States.
Figure 1.
Pandemic-associated pernio presentation and histopathology. (a) Representative clinical photographs of COVID toes, with red‒violaceous discoloration and swelling on multiple distal digits bilaterally. (b, c) Representative histopathologic section of a patient’s punch biopsy specimen (H&E); (b) demonstrating dense perivascular and periadnexal inflammatory infiltrate; (inset) magnification demonstrating tight inflammation around eccrine structures (outlined). Bar = 100 μM. (c) Staining of eccrine structures (outlined) for ACE2 (green) and nuclei (blue). Bar = 100 μM. ACE2, angiotensin-converting enzyme 2.
The strength of this spatial and temporal association along with its consistency across multiple countries supports a SARS-CoV-2‒triggered phenomenon. Yet, low rates of positive PCR testing of nasopharyngeal samples (0‒20%) and antibody positivity (0‒55%) across 175 publications and thousands of reported patients have led some authors to suggest that this is an epiphenomenon (Baeck and Herman, 2021; Galván Casas et al., 2020). This review will summarize and integrate the growing evidence for a causal relationship with SARS-CoV-2 and construct a mechanistic hypothesis. Pandemic-associated pernio augments the knowledge regarding the spectrum of SARS-CoV-2 infection and reinforces the critical importance of IFN-1 signaling in disease outcomes. A robust IFN-1 response in patients who remain asymptomatic and antibody negative could suggest a population with intrinsic resistance to severe COVID-19. Because the host immune response to SARS-CoV-2 infection is a critical determinant for COVID-19 outcomes, understanding those with natural resiliency to SARS-CoV-2 exposure could produce clinically relevant therapeutic targets.
Inborn errors of IFN-1 and life-threatening infection
IFN-1 responses are tightly regulated to ensure protective immunity while avoiding toxicity from excessive and prolonged IFN signaling. They are largely produced in the blood by plasmacytoid dendritic cells (pDCs) in response to viral infection through the activation of toll-like receptors 3, 7, and 9; RIG-I; and MDA5. IFN-1 is produced in lower amounts by >400 discernable cell types (intrinsic immunity). On binding to IFN-1 receptor, the IFN-1 (13 IFN-α, IFN-β, IFN-ε, IFN- κ, IFN-ω) activates robust antiviral defense programs of INF-stimulated genes, which obstruct various steps of viral replication. Monogenic variants that impair key IFN-1‒related genes are associated with life-threatening infections due to influenza pneumonia, respiratory syncytial virus, rhinovirus, and herpes encephalitis (Asgari et al., 2017; Ciancanelli et al., 2015; Lamborn et al., 2017; Zhang et al., 2007).
IFN-1 signaling is critical in COVID-19 outcomes
Recent investigations of host-specific responses to SARS-CoV-2 have confirmed the central role of IFN-1 signaling in COVID-19 outcomes. An attenuated IFN-1 response was found in critically ill patients with SARS-CoV-1 (Channappanavar et al., 2016). In an international cohort, our team found that 3% of patients with life-threatening COVID-19 harbor loss-of-function variants involved in IFN-1 signaling, with pDCs that did not produce IFN-1 in response to SARS-CoV-2 (Zhang et al., 2020). An accompanying study found that 10% of patients with critical COVID-19 infection had circulating neutralizing autoantibodies against IFN-1. These autoantibodies were pre-existing and were a cause of severe disease rather than a consequence of infection. Remarkably, 94% of these patients were men, half of whom were aged >65 years, and more than a third died from COVID-19 (Bastard et al., 2020).
Exceptional innate immunity may provide resistance to viral infection without engaging the adaptive immune system
In theory, robust innate and intrinsic immune responses may be sufficient to clear a viral exposure without triggering antibody production. This is a difficult phenomenon to study because most patients with viral clearance are identified by their postinfectious seroconversion. However, potential resistance to hepatitis C virus (HCV) infection has been described in high-risk injection drug users who lack HCV-specific T-cell responses and seroconversion despite a long history of HCV exposure, suggesting that individuals may be resistant to viral infection or protected from viral replication by an exceptional innate antiviral response without seroconversion (Shawa et al., 2017). The pandemic provides an opportunity to investigate antiviral resistance through the study of close contacts of patients with critical COVID-19 who remain asymptomatic and seronegative. Patients with pandemic-associated pernio may also serve as a model for a mild or resistant SARS-CoV-2 phenotype and are readily identifiable by their skin findings.
Association of pernio/chilblains with monogenic disorders of constitutively active IFN-1 production
Both clinically and histologically, pandemic-associated pernio mimics the skin lesions of familial chilblain lupus and Aicardi–Goutières syndrome, which are characterized by IFN-1 excess. These monogenic disorders, referred to as type 1 interferonopathies, are caused by mutations associated with impaired nucleic acid sensing that lead to sustained and upregulated IFN-1 signaling (Rice et al., 2007; Uggenti et al., 2019; Zimmermann et al., 2019). In affected patients, pernio develops in early infancy, followed by systemic vasculopathy due to autoinflammation. IFN-1 is profoundly increased in affected skin and blood. Similar to pandemic-associated pernio, cold is a critical precipitant. In familial chilblain lupus, 5-day cold exposure of primary fibroblasts followed by rewarming enhanced ROS, a known trigger of DNA damage, and increased IFN-1 activation, switching cells from a quiescent to a proinflammatory state (Günther et al., 2015).
Investigation of COVID toes identifies spike protein
COVID-19 autopsy studies have shown a SARS-CoV-2 tropism for the skin. Angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 receptor, is expressed on dermal blood vessels, the basal layer of the epidermis, and unexpectedly on eccrine glands (Hamming et al., 2004) (Figure 1b). We hypothesize that this expression may explain the localization of inflammation to hands and feet because these sites harbor the highest concentration of eccrine glands. This is further supported by the recent demonstration of SARS-CoV-2‒associated spike protein in cutaneous vascular endothelium and eccrine glands in biopsies from patients with COVID toes (Colmenero et al., 2020; Ko et al., 2021; Magro et al., 2021; Moon et al., 2021; Santonja et al., 2020). It should be noted that not all biopsy specimens detected spike protein, which could reflect the timing and depth of skin biopsy. Importantly, nucleocapsid antibody staining has been negative.
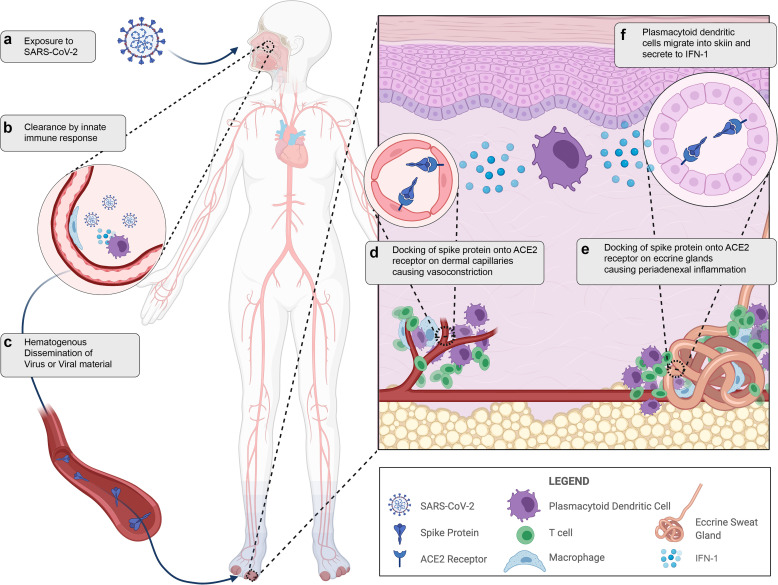
The immunohistochemistry patterns in published studies, coupled with lack of detection of viral RNA by in situ hybridization or PCR from tissue, suggests that pandemic-associated pernio may result from hematogenous spread of viral material and may not require viral replication in the skin (Herman et al., 2020; Ko et al., 2021). Emerging reports of pernio after mRNA vaccination also raise speculation that this could be an immune response to viral proteins or RNA without viral replication (Davido et al., 2021; McMahon et al., 2021). In unaffected skin of patients with critical COVID-19 infection, Magro et al. (2020) found microvascular complement deposition (an end-terminal event driving thrombosis) strongly colocalized with spike protein and the ACE2 receptor but without in situ evidence of viral RNA. The colocalization of the ACE2 receptor and viral capsid proteins suggests that circulating viral debris may dock onto the endothelium/eccrine ducts. This would be consistent with the hypothesis that patients with pandemic-associated pernio clear the SARS-CoV-2 through a robust IFN-1 response but shower viral debris that binds ACE2 receptors in the skin. Finally, the renin-angiotensin system (RAS) is expressed locally in the skin and may be indirectly activated by ACE2 binding from SARS-CoV-2 (Moon et al., 2021; Silva et al., 2020; Steckelings et al., 2004). We hypothesize that persistent vasoconstriction, poor capillary refill, and the chronicity of the response in some patients could also be linked to local cutaneous RAS activation (Figure 2 ).
Figure 2.
Illustrated hypothesis of pandemic-associated pernio pathophysiology. (a) Viral exposure to SARS-CoV-2 in the environment. (b) SARS-CoV-2 is cleared by a robust innate immune response in the nasopharynx. (c) There is hematogenous showering and dissemination of viral material or virus. (d) Docking of spike protein to the ACE2 receptor on dermal capillaries results in vasoconstriction and perivascular inflammation. (e) Docking of spike protein to ACE2 receptors on eccrine glands results in periadnexal inflammation. (f) Plasmacytoid dendritic cells, the major contributor to IFN-1 response, migrate to the skin in response to viral material. ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Evidence of robust IFN-1 response in COVID toes
Pandemic-associated pernio exhibits a lymphocytic infiltrate in a perivascular and perieccrine distribution (Figure 1b), which is composed predominantly of lymphocytes and pDCs. pDCs are responsible for the initiation of IFN-1 signaling in response to recognition of viral RNA. Immunohistochemistry studies have revealed robust MxA staining (a downstream product of IFN-1 activation) in affected specimens in a perivascular and perieccrine distribution (Aschoff et al., 2020). A recent paper suggested a viral-induced interferonopathy in affected patients, demonstrating a significant increase of in vitro IFN-α production after stimulation compared with that in patients with mild or severe acute COVID-19 infection (Hubiche et al., 2021).
Cold feet: Ambient temperature affects virus‒host responses
A cold environment is crucial to the induction of COVID toes. Humans maintain a narrow range of core body temperatures through neural, vascular, and biochemical mechanisms. Increases in body temperature through fever enhance immune function and pathogen killing. Colder ambient temperatures are known to diminish the efficiency of the innate immune response, facilitating viral replication in other infections (Foxman et al., 2015). Indeed, in vitro SARS-CoV-2 replication significantly increases with colder temperatures, demonstrating 10-fold higher infectious titers when incubated at 33 °C versus incubating at 37 °C (V’kovski et al., 2021). Importantly, attenuated IFN-1 expression is responsible for the increased viral replication efficiency at 33 °C. In pandemic-associated pernio, one could hypothesize that after clearance from the warmer respiratory tract, dispersed viral material settles at these colder acral sites owing to skin tropism through ACE2 expression, evading immune clearance. With rewarming of the toes, a local IFN-1 response could be initiated by pDCs after migration into the skin.
Conclusions
The striking spatial and temporal association with the pandemic, the accumulating evidence of both viral material and MxA in the affected skin, and the biologic plausibility of pernio linked to the critical role of IFN-1 signaling in COVID-19 all suggest a causal linkage with SARS-CoV-2. This evidence implicates a robust IFN-1 response in affected patients. The absence of antibody production supports rather than undermines this hypothesis because an exceptional innate and intrinsic immune activity may be enough to clear the viral infection without seroconversion. These findings further intimate IFN-I signaling in host outcomes to COVID-19.
In cooperation with the National Institutes of Health‒funded Human Genome Effort and the International COVID Human Genomic Effort, the COVID toes biobank at the University of Wisconsin-Madison seeks to identify the genetic and immunologic basis to provide clinically relevant insights into SARS-CoV-2‒associated pernio and could provide a framework for considering preventative approaches to SARS-CoV-2 infection utilizing early administration of IFNs.
ORCIDs
Lisa M. Arkin: http://orcid.org/0000-0002-0468-9568
John J. Moon: http://orcid.org/0000-0003-2422-8424
Jennifer M. Tran: http://orcid.org/0000-0003-1505-8099
Samira Asgari: http://orcid.org/0000-0002-2347-8985
Cliona O’Farrelly: http://orcid.org/0000-0002-0616-2874
Jean-Laurent Casanova: http://orcid.org/0000-0002-7782-4169
Edward W. Cowen: http://orcid.org/0000-0003-1918-4324
Jacqueline W. Mays: http://orcid.org/0000-0002-4472-9974
Beth A. Drolet: http://orcid.org/0000-0002-0844-7195
Conflict of Interest
The Authors have no conflict of interest.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Dental and Craniofacial Research (NIDCR) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH). In addition, research at the University of Wisconsin-Madison is supported through a Wisconsin Partnership Program COVID-19 Response Grant. Figure 1c was contributed by Ana Costa da Silva and Clara H. Kim from the JW Mays Lab, NIDCR, NIH with support from the NIDCR Imaging Core: ZIC DE000750. Figure 2 was created with BioRender.com. This review was conducted in Madison, WI. The other authors (COVID Human Genetic Effort) contributed to conceptualization of the review and to the writing (review and editing). The full list of authors (COVID Human Genetic Effort) and their affiliations are given as follows:
Alessandro Aiuti 1,2 , Alexandre Belot 3 , Alexandre Bolze 4 , Anastasiia Bondarenko 5 , Anna Sediva 6 , Anna Shcherbina 7 , Anna M. Planas 8 , Antonio Condino-Neto 9 , Aurora Pujol 10,11,12 , Beth A. Drolet 13 , Biggs Catherine 14 , Carlos Flores 15,16,17,18 , Carlos Rodríguez-Gallego 19,20 , Carolina Prando 21 , Clifton L. Dalgard 22 , Cliona O'Farrelly 23 , Colobran Roger 24 , Davoud Mansouri 25 , Diederik van de Beek 26 , Donald C. Vinh 27,28 , Elena Hsieh 29,30 , Evangelos Andreakos 31 , Filomeen Haerynck 32 , Furkan Uddin 33,34,35 , Giorgio Casari 36 , Giuseppe Novelli 37 , Graziano Pesole 38,39 , Isabelle Meyts 40,41 , Ivan Tancevski 42 , Jacques Fellay 43,44 , Jean-Laurent Casanova 45 , Jordi Tur 46 , Kai Kisand 47 , Keisuke Okamoto 48,49 , Kristina Mironska 50 , Laurent Abel 51 , Laurent Renia 36 , Lisa F.P. Ng 52,53 , Mohammed Shahrooei 54,55 , Pere Soler-Palacín 56 , Petter Brodin 57 , Qiang Pan-Hammarström 58 , Rabih Halwani 59 , Rebeca Perez de Diego 60 , Saleh Al-Muhsen 61 , Sara Espinosa-Padilla 62 , Satoshi Okada 63 , Tayfun Ozcelik 64 , Tayoun Ahmad Abou 65 , Timokratis Karamitros 66 , Trine H. Mogensen 67,68 and Yu-Lung Lau 69
1 San Raffaele Telethon Institute for Gene Therapy, IRCCS Ospedale San Raffaele, Milan, Italy; 2 Vita Salute San Raffaele University, Milan, Italy; 3 Pediatric Nephrology, Rheumatology, Dermatology, HFME, Hospices Civils de Lyon, National Referee Centre RAISE, & INSERM U1111, Université de Lyon, Lyon, France; 4 Helix, San Mateo, California, USA; 5 Department of Pediatric Infectious Diseases and Pediatric Immunology, Shupyk National Medical Academy for Postgraduate Education, Kiev, Ukraine; 6 Department of Immunology, Second Faculty of Medicine, Charles University, University Hospital in Motol, Prague, Czech Republic; 7 Department of Immunology, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia; 8 Department of Brain Ischemia and Neurodegeneration, IIBB-CSIC, IDIBAPS, Barcelona, Spain; 9 Department of Immunology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil; 10 Neurometabolic Diseases Laboratory, Bellvitge Biomedical Research Institute (IDIBELL), L'Hospitalet de Llobregat, Barcelona, Spain; 11 Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain; 12 Center for Biomedical Research on Rare Diseases (CIBERER), ISCIII, Madrid, Spain; 13 School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin, USA; 14 Division of Allergy and Immunology, Faculty of Medicine, The University of British Columbia, Vancouver, British Columbia, Canada; 15 Genomics Division, Instituto Tecnológico y de Energías Renovables (ITER), Santa Cruz de Tenerife, Spain; 16 Research Unit, Hospital Universitario Ntra. Sra. de Candelaria, Santa Cruz de Tenerife, Spain; 17 Instituto de Tecnologías Biomédicas (ITB), Universidad de La Laguna, Santa Cruz de Tenerife, Spain; 18 CIBER de Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain; 19 Department of Immunology, Hospital Universitario de Gran Canaria Dr Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain; 20 Department of Clinical Sciences, University Fernando Pessoa Canarias, Las Palmas de Gran Canaria, Spain; 21 Faculdades Pequeno Príncipe, Instituto de Pesquisa Pelé Pequeno Príncipe, Curitiba, Brazil; 22 Department of Anatomy, Physiology & Genetics, F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA; 23 Comparative Immunology Group, School of Biochemistry and Immunology, Trinity Biomedical Sciences Institute, Trinity College Dublin, The University of Dublin, Dublin, Ireland 24 Immunology Division, Genetics Department, Hospital Universitari Vall d’Hebron, Vall d'Hebron Research Institute, Vall d’Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain; 25 Department of Clinical Immunology and Infectious Diseases, The Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 26 Department of Neurology, Amsterdam Neuroscience, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands; 27 Division of Infectious Diseases, Department of Medicine, Faculty of Medicine and Health Sciences, McGill University Health Centre, Montréal, Québec, Canada; 28 Infectious Disease Susceptibility Program, Research Institute , McGill University Health Centre, Montréal, Québec, Canada; 29 Department of Immunology and Microbiology, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; 30 Division of Allergy and Immunology, Department of Pediatrics, School of Medicine, Children’s Hospital Colorado, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; 31 Laboratory of Immunobiology, Center for Clinical, Experimental Surgery & Translational Research, Biomedical Research Foundation of the Academy of Athens, Athens, Greece; 32 Department of Paediatric Immunology and Pulmonology, Centre for Primary Immunodeficiency Ghent (CPIG), PID Research Laboratory, Jeffrey Modell Diagnosis and Research Centre, Ghent University Hospital, Edegem, Belgium; 33 Holy Family Red Crescent Medical College, Dhaka, Bangladesh; 34 Centre for Precision Therapeutics, NeuroGen Children's Healthcare, Dhaka, Bangladesh; 35 Genetics and Genomic Medicine Centre, NeuroGen Children's Healthcare, Dhaka, Bangladesh; 36 Medical Genetics, IRCCS Ospedale San Raffaele, Milan, Italy; 37 Department of Biomedicine and Prevention, School of Medicine and Surgery, Tor Vergata University of Rome, Rome, Italy; 38 Institute of Biomembranes, Bioenergetics and Molecular Biotechnologies, Consiglio Nazionale delle Ricerche, Bari, Italy; 39 Department of Biosciences, Biotechnology and Biopharmaceutics, University of Bari Aldo Moro, Bari, Italy; 40 Department of Pediatrics, University Hospitals Leuven, Leuven, Belgium; 41 Laboratory for Inborn Errors of Immunity, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium; 42 Department of Internal Medicine II, Medical University of Innsbruck, Innsbruck, Austria; 43 School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland; 44 Precision Medicine Unit, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland; 45 The Rockefeller University, Howard Hughes Medical Institute, Necker Hospital, New York, New York, USA; 46 Institut de Biomedicina de Valencia CSIC, Valencia, Spain; 47 Molecular Pathology, Department of Biomedicine, Institute of Biomedicine and Translational Medicine, University of Tartu, Tartu, Estonia; 48 Laboratory of Clinical Pharmaceutics & Therapeutics, Division of Pharmasciences, Faculty of Pharmaceutical Sciences, Hokkaido University, Sapporo, Japan; 49 Department of Pediatrics and Developmental Biology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan; 50 Department of Pediatric Immunology, Medical Faculty, University Clinic for Children's Diseases, University “ St.Cyril and Methodij” Skopje, Skopje, North Macedonia; 51 INSERM U1163, Imagine Institute, University of Paris, Paris, France; 52 A∗STAR Infectious Diseases Labs (ID labs), Agency for Science, Technology and Research (A∗STAR), Singapore, Singapore; 53 Singapore Immunology Network (SigN), Agency for Science, Technology and Research (A∗STAR), Singapore, Singapore; 54 Saeed Pathobiology and Genetics Lab, Tehran, Iran; 55 Clinical and Diagnostic Immunology, Immunogenetics Research Group, Department of Microbiology and Immunology, KU Leuven, Leuven, Belgium; 56 Pediatric Infectious Diseases and Immunodeficiencies Unit, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain; 57 Science for Life Laboratory (SciLifeLab), Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden; 58 Department of Biosciences and Nutrition, Karolinska Institutet, Stockholm, Sweden; 59 Sharjah Institute of Medical Research, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates; 60 Innate Immunity Group, Laboratory of Immunogenetics of Human Diseases, IdiPAZ Institute for Health Research, La Paz Hospital, Madrid, Spain; 61 Immunology Research Laboratory, Department of Pediatrics, College of Medicine, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia; 62 Instituto Nacional de Pediatria (National Institute of Pediatrics), Mexico City, Mexico; 63 Department of Pediatrics, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan; 64 Department of Molecular Biology and Genetics, Bilkent University, Ankara, Turkey; 65 Al Jalila Children’s Genomics Centre, Al Jalila Children's Specialty Hospital, Dubai, United Arab Emirates; 66 Bioinformatics and Applied Genomics Unit, Department of Microbiology, Hellenic Pasteur Institute, Athens, Greece; 67 Department of Biomedicine, Aarhus University, Aarhus, Denmark; 68 Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark; 69 Department of Paediatrics & Adolescent Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Author Contributions
Conceptualization: BAD, COF, JLC, JJM, JWM, LMA; Project Administration: JJM; Supervision: BAD; Visualization: JJM, JWM; Writing - Original Draft Preparation: BAD, COF, JLC, JJM, LMA; Writing - Review and Editing: AMS, EWC, JWM, JMT, SA
accepted manuscript published online 15 July 2021; corrected proof published online 22 September 2021
Contributor Information
COVID Human Genetic Effort:
Alessandro Aiuti, Alexandre Belot, Alexandre Bolze, Anastasiia Bondarenko, Anna Sediva, Anna Shcherbina, Anna M. Planas, Antonio Condino-Neto, Aurora Pujol, Beth A. Drolet, Biggs Catherine, Carlos Flores, Carlos Rodríguez-Gallego, Carolina Prando, Clifton L. Dalgard, Cliona O'Farrelly, Colobran Roger, Davoud Mansouri, Diederik van de Beek, Donald C. Vinh, Elena Hsieh, Evangelos Andreakos, Filomeen Haerynck, Furkan Uddin, Giorgio Casari, Giuseppe Novelli, Graziano Pesole, Isabelle Meyts, Ivan Tancevski, Jacques Fellay, Jean-Laurent Casanova, Jordi Tur, Kai Kisand, Keisuke Okamoto, Kristina Mironska, Laurent Abel, Laurent Renia, Lisa F.P. Ng, Mohammed Shahrooei, Pere Soler-Palacín, Petter Brodin, Qiang Pan-Hammarström, Rabih Halwani, Rebeca Perez de Diego, Saleh Al-Muhsen, Sara Espinosa-Padilla, Satoshi Okada, Tayfun Ozcelik, Ahmad Abou Tayoun, Timokratis Karamitros, Trine H. Mogensen, and Yu-Lung Lau
References
- Aschoff R., Zimmermann N., Beissert S., Günther C. Type I interferon signature in chilblain-like lesions associated with the COVID-19 pandemic. Dermatopathology (Basel) 2020;7:57–63. doi: 10.3390/dermatopathology7030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S., Schlapbach L.J., Anchisi S., Hammer C., Bartha I., Junier T., et al. Severe viral respiratory infections in children with IFIH1 loss-of-function mutations. Proc Natl Acad Sci USA. 2017;114:8342–8347. doi: 10.1073/pnas.1704259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeck M., Herman A. COVID toes: where do we stand with the current evidence? Int J Infect Dis. 2021;102:53–55. doi: 10.1016/j.ijid.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz J.D., Duong T.A., Jachiet M., Velter C., Lestang P., Cassius C., et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol. 2020;34:e451–e452. doi: 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Soccio L., Lara-Corrales I., Paller A.S., Bean E., Rangu S., Oboite M., et al. Acral changes in pediatric patients during COVID 19 pandemic: registry report from the COVID 19 response task force of the society of pediatric dermatology (SPD) and pediatric dermatology research alliance (PeDRA) Pediatr Dermatol. 2021;38:364–370. doi: 10.1111/pde.14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancanelli M.J., Huang S.X., Luthra P., Garner H., Itan Y., Volpi S., et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero I., Santonja C., Alonso-Riaño M., Noguera-Morel L., Hernández-Martín A., Andina D., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoro K.M., Reynolds S.D., Wattier R., McCalmont T.H. Clustered cases of acral perniosis: clinical features, histopathology, and relationship to COVID-19. Pediatr Dermatol. 2020;37:419–423. doi: 10.1111/pde.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davido B., Mascitti H., Fortier-Beaulieu M., Jaffal K., de Truchis P. ‘Blue toes’ following vaccination with the BNT162b2 mRNA COVID-19 vaccine. J Travel Med. 2021;28:taab024. doi: 10.1093/jtm/taab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong T.A., Velter C., Rybojad M., Comte C., Bagot M., Sulimovic L., et al. Did Whatsapp® reveal a new cutaneous COVID-19 manifestation? J Eur Acad Dermatol Venereol. 2020;34:e348–e350. doi: 10.1111/jdv.16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman E.F., Storer J.A., Fitzgerald M.E., Wasik B.R., Hou L., Zhao H., et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci USA. 2015;112:827–832. doi: 10.1073/pnas.1411030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E.E., McMahon D.E., Lipoff J.B., Rosenbach M., Kovarik C., Takeshita J., et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván Casas C., Català A., Carretero Hernández G., Rodríguez-Jiménez P., Fernández-Nieto D., Rodríguez-Villa Lario A., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther C., Berndt N., Wolf C., Lee-Kirsch M.A. Familial chilblain lupus due to a novel mutation in the exonuclease III domain of 3’ repair exonuclease 1 (TREX1) JAMA Dermatol. 2015;151:426–431. doi: 10.1001/jamadermatol.2014.3438. [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A., Peeters C., Verroken A., Tromme I., Tennstedt D., Marot L., et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998–1003. doi: 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubiche T., Cardot-Leccia N., Le Duff F., Seitz-Polski B., Giordana P., Chiaverini C., et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202–206. doi: 10.1001/jamadermatol.2020.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C.J., Harigopal M., Gehlhausen J.R., Bosenberg M., McNiff J.M., Damsky W. Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2021;48:47–52. doi: 10.1111/cup.13866. [DOI] [PubMed] [Google Scholar]
- Lamborn I.T., Jing H., Zhang Y., Drutman S.B., Abbott J.K., Munir S., et al. Recurrent rhinovirus infections in a child with inherited MDA5 deficiency. J Exp Med. 2017;214:1949–1972. doi: 10.1084/jem.20161759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa N., Mendieta-Eckert M., Fonda-Pascual P., Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739–743. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Robles J., de la Hera I., Pardo-Sánchez J., Ruiz-Martínez J., Cutillas-Marco E. Chilblain-like lesions: a case series of 41 patients during the COVID-19 pandemic. Clin Exp Dermatol. 2020;45:891–892. doi: 10.1111/ced.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C.M., Mulvey J.J., Laurence J., Sanders S., Crowson A.N., Grossman M., et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184:141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C.M., Mulvey J.J., Laurence J., Seshan S., Crowson A.N., Dannenberg A.J., et al. Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019. Hum Pathol. 2020;106:106–116. doi: 10.1016/j.humpath.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.J., Costa da Silva A., Tran J.M., Kim C., Sharma R., Hinshaw M., et al. SARS-CoV-2-associated ‘covid toes:’ multiplex immunofluorescent characterization of its pathophysiology. Society for Investigative Dermatology. 2021. https://www.sidannualmeeting.org/wp-content/uploads/2021/05/SID-2021-Virtual-Meeting-LB-Abstract-Booklet-FINAL.pdf (accessed 24 May 2021)
- Piccolo V., Neri I., Filippeschi C., Oranges T., Argenziano G., Battarra V.C., et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34:e291–e293. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G., Newman W.G., Dean J., Patrick T., Parmar R., Flintoff K., et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;80:811–815. doi: 10.1086/513443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santonja C., Heras F., Núñez L., Requena L. COVID-19 chilblain-like lesion: immunohistochemical demonstration of SARS-CoV-2 spike protein in blood vessel endothelium and sweat gland epithelium in a polymerase chain reaction-negative patient. Br J Dermatol. 2020;183:778–780. doi: 10.1111/bjd.19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawa I.T., Felmlee D.J., Hegazy D., Sheridan D.A., Cramp M.E. Exploration of potential mechanisms of hepatitis C virus resistance in exposed uninfected intravenous drug users. J Viral Hepat. 2017;24:1082–1088. doi: 10.1111/jvh.12720. [DOI] [PubMed] [Google Scholar]
- Silva I.M.S., Assersen K.B., Willadsen N.N., Jepsen J., Artuc M., Steckelings U.M. The role of the renin-angiotensin system in skin physiology and pathophysiology. Exp Dermatol. 2020;29:891–901. doi: 10.1111/exd.14159. [DOI] [PubMed] [Google Scholar]
- Steckelings U.M., Wollschläger T., Peters J., Henz B.M., Hermes B., Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13:148–154. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- Uggenti C., Lepelley A., Crow Y.J. Self-awareness: nucleic acid-driven inflammation and the type I interferonopathies. Annu Rev Immunol. 2019;37:247–267. doi: 10.1146/annurev-immunol-042718-041257. [DOI] [PubMed] [Google Scholar]
- V’kovski P., Gultom M., Kelly J.N., Steiner S., Russeil J., Mangeat B., et al. Disparate temperature-dependent virus-host dynamics for SARS-CoV-2 and SARS-CoV in the human respiratory epithelium. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.Y., Jouanguy E., Sancho-Shimizu V., von Bernuth H., Yang K., Abel L., et al. Human toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N., Wolf C., Schwenke R., Lüth A., Schmidt F., Engel K., et al. Assessment of clinical response to Janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155:342–346. doi: 10.1001/jamadermatol.2018.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]