Abstract
Unrecognized immunodeficiency has been proposed as a possible cause of failure of antibiotics to resolve symptoms of Lyme disease. Here, we examined the efficacy of doxycycline in different immunodeficient mice to identify defects that impair antibiotic treatment outcomes. We found that doxycycline had significantly lower efficacy in the absence of adaptive immunity, specifically B cells. This effect was most pronounced in immunodeficient C3H mice compared with C57BL/6 mice, suggesting a role for genetic background beyond immunodeficiency. Addition of a single dose of ceftriaxone to doxycycline treatment effectively cleared infection in C3H mice with severe combined immunodeficiency.
Keywords: Lyme disease, antibiotics, Borrelia burgdorferi
We show the importance of the immune system in clearance of Borrelia burgdorferi after doxycycline treatment of mice. We found a significant effect of genetic background on bacterial clearance. Differences could be overcome with addition of 1 dose of ceftriaxone.
Lyme disease is caused by Borrelia burgdorferi. The Infectious Diseases Society of America guidelines recommend oral antibiotics as first-line therapy for most manifestations of Lyme disease [1]. Oral doxycycline is the most commonly recommended antibiotic due to its relatively good penetration into the central nervous system and its activity against a commonly co-transmitted pathogen, Anaplasma phagocytophilum. However, doxycycline is less active against B. burgdorferi in vitro than ceftriaxone [2], a β-lactam antibiotic used for severe cases of Lyme disease involving high-grade heart block, central nervous system infection, or Lyme arthritis unresponsive to oral antibiotics [1]. Although doxycycline is less active, the human immune system is likely to eradicate any residual bacteria and the majority of patients treated with doxycycline recover without any long-term sequelae.
Approximately 10%–20% of antibiotic-treated patients continue to have symptoms such as fatigue, myalgias, arthralgias, and memory issues after antibiotic treatment for months to years; this constellation of symptoms is often referred to as posttreatment Lyme disease syndrome (PTLDS) [3]. Of note, given the nonspecific nature of the symptoms, the cause of PTLDS and whether it is linked to Lyme disease remain unknown.
The other group of patients with Lyme disease who do not recover fully after antibiotic therapy comprises those who develop Lyme arthritis and continue to have symptoms after antibiotic therapy. This syndrome has been described as antibiotic refractory Lyme arthritis [4, 5]. The cause of antibiotic refractory Lyme arthritis is also controversial and it typically resolves over time, even without intervention. Possibilities that have been raised include autoimmune responses and the presence of residual antigens such as peptidoglycan in the joint [6, 7]. Another possibility that has been difficult to rule out is that bacteria are not fully eradicated by the antibiotic treatment. DNA can sometimes be detected in the synovial fluid of patients with Lyme arthritis even after prolonged courses of antibiotics [8].
Although there are no good animal models of antibiotic refractory Lyme arthritis or PTLDS, multiple groups have reported the presence of DNA and RNA in animals long after antibiotic treatment [9–13]. Additionally, B. burgdorferi DNA has been detected in a very small number of patients after antibiotic therapy using xenodiagnosis [14]. However, live bacteria have not been detected after antibiotic therapy in animals or humans using xenodiagnosis, and there are no studies linking B. burgdorferi persistence to either PTLDS or antibiotic-resistant Lyme arthritis.
A question that has been raised is whether an unrecognized immunodeficiency may predispose a subset of patients to fail to quickly clear the infection, leading to a build-up of antigens and the development of more prolonged symptoms. The immune system is important for efficacy of bacteriostatic drugs against some other bacteria in vivo [15]. Both innate and adaptive immune systems are activated in response to B. burgdorferi and are important for controlling infection in animal models [16]. Borrelia burgdorferi produces numerous triacylated lipoproteins, which are recognized by Toll-like receptor (TLR) 1/2 heterodimers [17, 18]. Myeloid differentiation factor 88 (MyD88) is a key adaptor for the innate immune response downstream of TLR1/2. Mice deficient in MyD88 or TLR2 show higher pathogen burden and increased ankle swelling compared to control mice [18–21]. Adaptive immunity also plays an important role in clearance of B. burgdorferi [22]. Severe combined immunodeficiency (SCID) mice that lack both B and T cells have higher pathogen burden and increased arthritis severity [13]. Mice deficient in B and T cells had persistent and deteriorating arthritis and carditis, whereas mice deficient in only T cells were able to resolve such swelling, suggesting that B cells are more important for resolution of symptoms of Lyme disease [23].
Recent studies have suggested that decreased B-cell responses may be a risk factor for posttreatment symptoms in Lyme disease [24]. Prior studies have suggested that oral doxycycline is less effective at resolving infection in immunocompromised animals [25]. However, there has not been a systematic examination of effects of different components of the immune system on the efficacy of doxycycline in clearance of B. burgdorferi infection.
Here we report on our studies of the efficacy of doxycycline in animals with immunodeficiencies in different components of their immune function. In addition, we tested the effects of combination of a single dose of ceftriaxone with doxycycline on clearance of infection in severely immunocompromised animals.
MATERIALS AND METHODS
Animal Ethics Statement
This study was approved by the Tufts University Institutional Animal Care and Use Committee (Animal Welfare Assurance number A3775-01).
Mouse and Bacterial Strains
All mice were obtained from Jackson Laboratories. C57BL/6J (catalog number 000664), C57BL/6J TLR2–/– (catalog number 004650), C57BL/6J muMt–/– (catalog number 002288), C57BL/6J MyD88–/– (catalog number 009088), C57BL/6J TCRα –/– (catalog number 002116), C57BL/6J SCID (catalog number 001913), C3H SCID (catalog number 001131), and C3H (catalog number 000474) mice were used for our studies. A mouse-adapted strain of low-passage Borrelia burgdorferi sensu stricto (strain N40, clone D10E9) was used for all experiments [26]. All mice were housed in specific Biosafety Level 1 rooms according to institutional guidelines for the humane care and use of laboratory animals.
Barbour-Stoenner-Kelly H (BSK)–H media was used to culture B. burgdorferi from glycerol stocks [27]. BSK-H media was supplemented with antibiotics, phosphomycin (100 µg/mL), rifampicin (50 µg/mL), and amphotericin B (5 µg/mL) when tissues were used for culture of B. burgdorferi. Cultures were incubated at 37°C.
Antibiotics Used
Phosphomycin, doxycycline, rifampicin, and amphotericin B were purchased from Sigma, and ceftriaxone was purchased from Hospira. All antibiotics used in animal studies were United States Pharmacopeia grade.
Infection and Treatment of Mice
Mice were infected with 1 × 105B. burgdorferi via subcutaneous needle inoculation. Infection was allowed to establish for 3 weeks. Mice were then treated with doxycycline in drinking water (0.2% doxycycline + 5% sucrose) or 5% sucrose water continuously for 5 days as described elsewhere [28]. This dose of doxycycline was reported to produce a serum doxycycline level over the minimum inhibitory concentration (MIC) of doxycycline for B. burgdorferi during the course of treatment [28]. A 2-mm ear punch was collected from each animal every day during treatment and cultured in BSK-II media. Cultures were monitored by dark-field microscopy. Animals were sacrificed 2 days after completion of antibiotic treatment and skin, heart, and ankle were cultured for detection of B. burgdorferi. For experiments involving ceftriaxone, 1 dose of ceftriaxone (156 mg/kg) or saline was administered subcutaneously [25].
Statistical Analysis
Log-rank test was used to determine statistical significance for bacterial clearance over the course of treatment. Fisher exact test was used to calculate significance when comparing data from a single day.
RESULTS
Efficacy of Doxycycline in Mice Deficient in Components of Innate and Adaptive Immune System
MyD88 and TLR2 represent components of the innate immune system important for the recognition of B. burgdorferi by the cells of the innate immune system and for activating cytokine response upon encountering the pathogen. B and T cells are components of the adaptive immune system and are important for pathogen-specific responses. SCID mice lack both B and T cells. muMt knockout mice do not express the membrane-bound immunoglobulin M and lack mature B cells; they are considered a model of B-cell deficiency in humans. TCRα –/– mice are deficient in the αβ T-cell receptor. Their thymus is devoid of CD4+CD8– and CD4–CD8+ cells. Normal numbers of CD4+CD8+ cells are retained without the interleukin 2 receptor. There are normal numbers of CD4–CD8– cells.
Wild-type (WT), TLR2–/–, MyD88–/–, muMt–/–, TCRα –/–, and SCID mice in C57BL/6 background were infected with B. burgdorferi N40 via subcutaneous inoculation. After 3 weeks, mice were treated with doxycycline for 5 days. Ear punch samples were collected daily from the initiation of therapy until completion of antibiotics and cultured in BSK-II media. Mice were killed 2 days after completion of therapy and multiple tissues were cultured for the presence of B. burgdorferi.
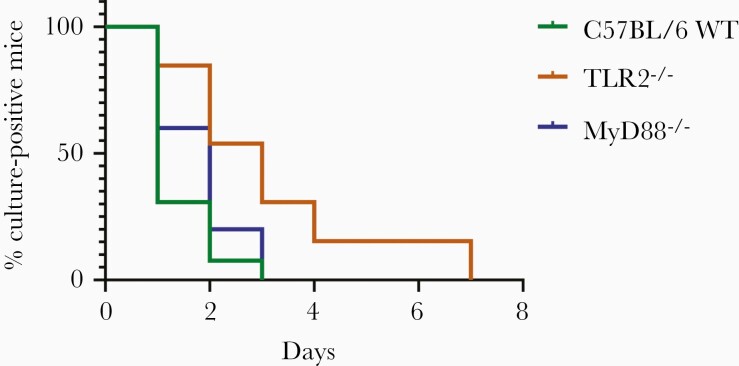
Five days of doxycycline rendered all C57BL/6 WT and immunodeficient mice cultures negative (Figure 1; Supplementary Table 1). Time to negative culture was significantly different between C57BL/6 WT mice and TLR2–/– mice (P = .0017). Time to culture negativity in MyD88–/– mice was not significantly different from that for WT mice (P = .1916).
Figure 1.
Defects in the innate immune system affect the efficacy of doxycycline against Borrelia burgdorferi in vivo. C57BL/6 wild-type (WT) (n = 13), TLR2–/– (n = 13), or MyD88–/– (n = 10) mice were infected with B. burgdorferi N40 for 24 days and treated with 0.2% doxycycline in drinking water for 5 days. Ear punches were taken every day during treatment and cultured in Barbour-Stoenner-Kelly (BSK)–II media. Mice were sacrificed 2 days after the end of treatment and ear, joint, and heart were cultured in BSK-II media. Cultures were examined under dark-field microscope for about 2 weeks. The time to culture negativity was significantly different for C57BL/6 WT vs TLR2–/– (P = .0017) but there was no significant difference between C57BL/6 WT and MyD88–/– (P = .1916). P values were calculated using log-rank test.
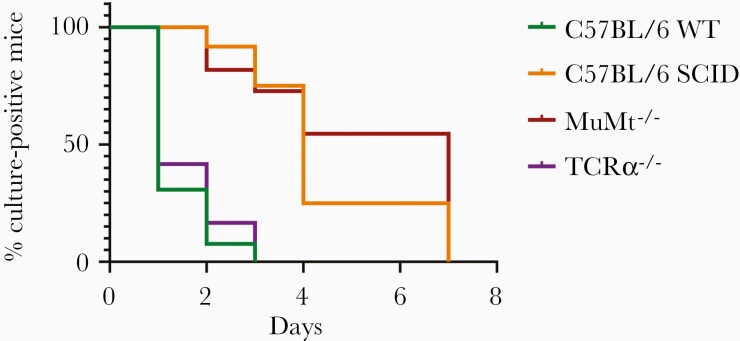
While there was no difference in culture negativity between muMT–/– and C57BL/6 WT mice at the time of sacrifice, there was significantly delayed clearance of B. burgdorferi by cultures in the muMT–/– mice (Figure 2; Supplementary Table 1). About 55% of muMt–/– mice were culture positive after 4 days of treatment compared with 0% of C57BL/6 WT mice (time to clearance P < .0001). The time to culture negativity in C57BL/6 SCID mice was also significantly different than that for WT mice (P < .0001; Figure 2). There was no significant difference between time to culture negativity between muMt–/– and C57BL/6 SCID mice (P = .3238). There was also no difference in time to culture negativity between C57BL/6 WT and TCRα –/– mice (P = .4794).
Figure 2.
B-cell deficiency affects the efficacy of doxycycline against Borrelia burgdorferi in vivo. C57BL/6 wild-type (WT) (n = 13), C57BL/6 severe combined immunodeficiency (SCID) (n = 12), muMt–/– (n = 11), or TCRα –/– (n = 12) mice were infected with B. burgdorferi N40 for 24 days and treated with 0.2% doxycycline in drinking water for 5 days. Ear punches were taken every day during treatment and cultured in Barbour-Stoenner-Kelly (BSK)–II media. Mice were sacrificed 2 days after the end of treatment and ear, joint, and heart were cultured in BSK-II media. Cultures were examined under dark-field microscope for about 2 weeks. Time to culture negativity was significantly different between WT vs SCID (P < .0001) and muMt–/– (P < .0001) but not TCRα –/– (P = .4794). P values were calculated using log-rank test.
Genetic Background Affects Clearance of B. burgdorferi With Doxycycline
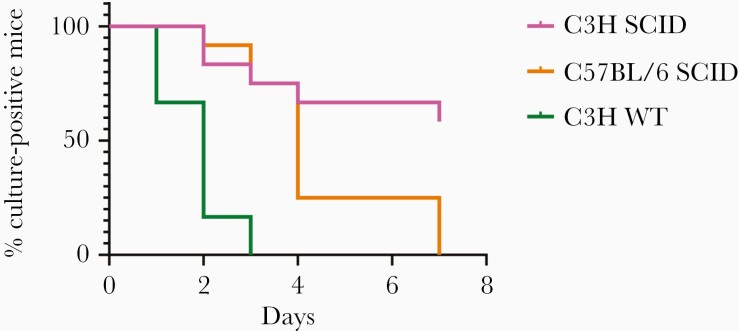
We next sought to determine whether the genetic background of the mice affects clearance of B. burgdorferi with doxycycline treatment (Figures 2 and 3; Supplementary Table 1). C3H mice develop greater inflammatory symptoms than C57BL/6 mice and as a result, C3H are considered B. burgdorferi “susceptible” compared with C57BL/6 mice that are “resistant.” These designations are based only on their inflammatory symptoms and not on their ability to be infected. Although most of the immunodeficient mice we studied are not available on a C3H background, SCID mice are available in both C3H and C57BL/6 backgrounds. Both C3H and C57BL/6 SCID mice contain a point mutation in the Prkdc gene. The C3H SCID contain an additional mutation in Clcc1m1J gene, which causes increased sensitivity to endoplasmic reticulum stress in the cerebellum. However, both the WT and SCID C3H mice contain this mutation. WT C3H and WT C57BL/6 mice were both cleared of B. burgdorferi infection after 3 days of doxycycline. However, C3H SCID and C57BL/6 SCID mice differed significantly in their clearance of B. burgdorferi with doxycycline as 60% of C3H SCID mice remained culture positive after 5 days of antibiotics (P = .0129).
Figure 3.
Immunodeficiencies are enhanced with certain genetic backgrounds. C57BL/6 severe combined immunodeficiency (SCID) (n = 13), C3H SCID (n = 12), and C3H wild-type (WT) (n = 6) mice were infected with Borrelia burgdorferi N40 for 24 days and treated with 0.2% doxycycline in drinking water for 5 days. Ear punches were taken every day during treatment and cultured in Barbour-Stoenner-Kelly (BSK)–II media. Mice were sacrificed 2 days after the end of treatment and ear, joint, and heart were cultured in BSK-II media. Cultures were examined under dark-field microscope for about 2 weeks. Time to culture negativity was significantly different between C3H SCID vs C57BL/6 SCID (P = .0129) and C3H WT (P = .0005). P values were calculated using log-rank test.
Addition of a Single Dose of Ceftriaxone Improves Doxycycline Efficacy in C3H SCID Mice
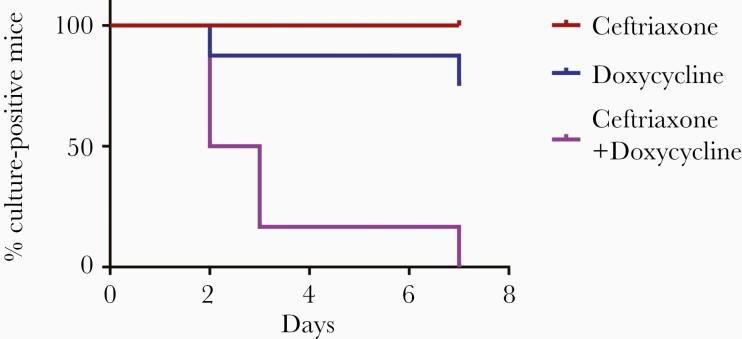
We sought to determine whether the addition of another antibiotic could improve clearance of B. burgdorferi from C3H SCID mice. Ceftriaxone is highly active against B. burgdorferi in vitro, but still requires multiple doses to clear B. burgdorferi from mice when given as a single agent [2, 25]. We tested the effects of a single dose of subcutaneous ceftriaxone combined with oral doxycycline on clearance of B. burgdorferi infection in C3H SCID mice. We chose a dosage of ceftriaxone that maintains the level of ceftriaxone above the MIC of ceftriaxone against B. burgdorferi for at least 12 hours [28]. One hundred percent of mice treated with 1 dose of ceftriaxone and 75% of mice treated with doxycycline alone were culture positive at the end of treatment (Figure 4). However, 100% of mice treated with 1 dose of ceftriaxone followed by 5 days of oral doxycycline were culture negative at the end of treatment (P = .0027; Figure 4).
Figure 4.
One dose of ceftriaxone followed by oral doxycycline clears Borrelia burgdorferi infection in immunocompromised hosts. C3H severe combined immunodeficiency (SCID) mice were infected with B. burgdorferi N40 for 24 days and treated with 1 dose of ceftriaxone (156 mg/kg) or 0.2% doxycycline in drinking water for 5 days, or 1 dose of ceftriaxone followed by doxycycline in drinking water for 5 days. Ear punches were taken every day during treatment and cultured in Barbour-Stoenner-Kelly (BSK)–II media. Mice were sacrificed 2 days after the end of treatment and ear, joint, and heart tissues were cultured in BSK-II media. Cultures were examined under dark-field microscope for about 2 weeks. P value for the doxycycline alone group vs the ceftriaxone and doxycycline combination group was .0027 (n = 6) and was calculated using log-rank test.
DISCUSSION
Previous studies have shown that doxycycline is less efficacious against B. burgdorferi than β-lactam antibiotics in vitro [2, 29]. However, studies in patients and animals have shown equivalence between doxycycline and other antibiotics [30–32]. This is likely due to the ability of the immune system to eliminate any residual infection after antibiotic treatment.
In this study, we have presented a systematic look at the impact of different immunodeficiencies on the efficacy of doxycycline in the clearance of B. burgdorferi from infected animals. The SCID mutation causing both B- and T-cell deficiency produced a pronounced effect, with the majority of animals remaining culture positive for longer time period. Much of the failure of clearance appears to be due to the loss of B cells as muMT–/– mice show delayed clearance of infection that is comparable to SCID mice. Meanwhile, TCRα –/– mice deficient in CD4+CD8– and CD4–CD8+ T cells do not show a significant difference in time to culture negativity compared to WT mice. This is consistent with prior reports suggesting that B cells are most important for clearance of infection [23]. Innate immunity, on the other hand, appears to play a lesser role in clearance by antibiotics as loss of TLR2 resulted in smaller delays in clearance of B. burgdorferi with doxycycline treatment. Interestingly, the loss of the TLR adapter molecule MyD88 resulted in no significant difference with WT mice in time to clearance. Combined, these data suggest that the impact of innate immunity on clearance after doxycycline is small. We suspect that the lack of significance in the MyD88 mice was due to the sample size relative to the size of the difference in time to clearance compared to WT as there was a nonsignificant small trend toward delayed clearance. Also, because in prior reports, both SCID- and MyD88-deficient animals show similarly increased numbers of B. burgdorferi in tissues [20, 33], this suggests that the reduced effect of antibiotics is not due solely to increased numbers of bacteria at initiation of therapy.
Importantly, there appears to be a significant effect of the genetic background of the mice beyond just the immunodeficiency. While all WT mice cleared the infection with antibiotics, there were significant differences in SCID mice depending upon the background, as a majority of the SCID mice on a C3H background were not cleared of infection with 5 days of doxycycline whereas SCID mice on a C57BL/6 background were cleared of infection. This is despite similar numbers of bacteria in the tissues of C3H and C57BL/6 SCID animals. While some genes responsible for the differences between C3H and C57BL/6 mice in arthritis responses have been identified, it is unclear why these would affect antibiotic efficacy. This interesting finding suggests that there may be other genetic reasons for a differential ability to clear infection after doxycycline treatment other than overt B- and T-cell deficiency. A forward genetics approach has previously been used to identify the genes responsible for the phenotypic differences in arthritis response to B. burgdorferi infection between C3H and C57BL/6 mice [34, 35]; a similar approach with testing these mice could be applied to identify the specific genes involved in reduced effectiveness of doxycycline.
Whether genetic background and/or immunodeficiency in humans play a role in failure to clear the bacteria after antibiotic therapy is unknown. In trials of antibiotics for Lyme disease treatment, there is a well-documented incidence of failure of antibiotics in a small minority of patients [5, 30, 31]; however, these were not linked to symptoms of PTLDS. While it is unlikely that these patients had major immunodeficiencies such as SCID, it is unknown whether their genetic background could have affected their susceptibility to B. burgdorferi infection or if they may have had more minor immune deficits that could have affected clearance of the organism. There have been several reports of immunodeficiency in humans that have affected the trajectory of Lyme disease symptoms. Strle et al have reported that a specific inactivating mutation in TLR1, which heterodimerizes with TLR2 to activate immune responses to B. burgdorferi lipoproteins, results in an increased risk for antibiotic refractory Lyme arthritis, particularly in infections with specific strains of B. burgdorferi [36]. However, the mechanism for this is unknown. Another recent study of response to treatment in Lyme disease patients by Blum et al found that an initial robust B-cell response characterized by increased plasmablast levels correlated with reduced risk for persistent symptoms after doxycycline treatment [24].
We also found that the addition of a single dose of ceftriaxone to a course of doxycycline was effective in clearance of infection from C3H SCID animals that did not clear infection with doxycycline alone. We chose to study the addition of a single dose of ceftriaxone because it may be feasible to administer a single dose of this medication in a physician’s office without the need for an indwelling catheter. In fact, the pharmacokinetics of ceftriaxone in humans is more advantageous than in mice, as a single dose will keep levels above the MIC for much longer. The addition of a single dose of ceftriaxone to doxycycline has been studied previously in a randomized, double-blind, placebo-controlled trial of patients with erythema migrans. No statistically significant differences in symptoms were seen between doxycycline (10 days of treatment), doxycycline (20 days of treatment), or doxycycline (10 days) plus 1 dose of ceftriaxone at 3 months, 12 months, or 30 months after treatment [37]. However, the numbers of patients in each group were small (evaluable patients between 45 and 54 per group) given that the majority of patients treated with doxycycline improve without sequelae. As more human genetic information becomes available regarding specific mutations that may impact immune function and the ability to control bacterial loads, it may be possible to identify linkages of mutations to persistent, late symptoms of Lyme disease. At that point, additional studies targeting more effective therapies such as a combination of single-dose ceftriaxone and doxycycline to a narrow group of patients may be warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers R01AI122286 and R21AI126757 to L. T. H.); and the Global Lyme Alliance Deborah and Mark Blackman Fellowship (to B. S.).
Potential conflicts of interest. L. T. H.’s laboratory receives support for contract research services through Tufts University from Sanofi and Moderna for vaccine development unrelated to the content of this manuscript. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Wormser GP, Dattwyler RJ, Shapiro ED, et al. . The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 2. Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother 2015; 59:4616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am 2008; 22:341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22:289–300. [DOI] [PubMed] [Google Scholar]
- 5. Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum 2006; 54:3079–86. [DOI] [PubMed] [Google Scholar]
- 6. Jutras BL, Lochhead RB, Kloos ZA, et al. . Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc Natl Acad Sci U S A 2019; 116:13498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wormser GP, Nadelman RB, Schwartz I. The amber theory of Lyme arthritis: initial description and clinical implications. Clin Rheumatol 2012; 31:989–94. [DOI] [PubMed] [Google Scholar]
- 8. Steere AC. Diagnosis and treatment of Lyme arthritis. Med Clin North Am 1997; 81:179–94. [DOI] [PubMed] [Google Scholar]
- 9. Bockenstedt LK, Mao J, Hodzic E, Barthold SW, Fish D. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi–infected mice after antibiotic treatment. J Infect Dis 2002; 186:1430–7. [DOI] [PubMed] [Google Scholar]
- 10. Embers ME, Barthold SW, Borda JT, et al. . Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 2012; 7:e29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One 2014; 9:e86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Straubinger RK, Summers BA, Chang YF, Appel MJ. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol 1997; 35:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hodzic E, Feng S, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun 2003; 71:5042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marques A, Telford SR 3rd, Turk SP, et al. . Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis 2014; 58:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loree J, Lappin SL.. Bacteriostatic antibiotics. Treasure Island, FL: StatPearls, 2020. [PubMed] [Google Scholar]
- 16. Wang X, Ma Y, Weis JH, Zachary JF, Kirschning CJ, Weis JJ. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect Immun 2005; 73:657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salazar JC, Pope CD, Moore MW, Pope J, Kiely TG, Radolf JD. Lipoprotein-dependent and -independent immune responses to spirochetal infection. Clin Diagn Lab Immunol 2005; 12:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wooten RM, Ma Y, Yoder RA, et al. . Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol 2002; 168:348–55. [DOI] [PubMed] [Google Scholar]
- 19. Behera AK, Hildebrand E, Bronson RT, et al. . MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect Immun 2006; 74:1462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolz DD, Sundsbak RS, Ma Y, et al. . MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J Immunol 2004; 173:2003–10. [DOI] [PubMed] [Google Scholar]
- 21. Liu N, Montgomery RR, Barthold SW, Bockenstedt LK. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi–infected mice. Infect Immun 2004; 72:3195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tufts DM, Hart TM, Chen GF, Kolokotronis SO, Diuk-Wasser MA, Lin YP. Outer surface protein polymorphisms linked to host-spirochete association in Lyme borreliae. Mol Microbiol 2019; 111:868–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKisic MD, Barthold SW. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect Immun 2000; 68:5190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blum LK, Adamska JZ, Martin DS, et al. . Robust B cell responses predict rapid resolution of Lyme disease. Front Immunol 2018; 9:1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu X, Sharma B, Niles S, et al. . Identifying vancomycin as an effective antibiotic for killing Borrelia burgdorferi. Antimicrob Agents Chemother 2018; 62:e01201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petnicki-Ocwieja T, DeFrancesco AS, Chung E, et al. . Nod2 suppresses Borrelia burgdorferi mediated murine Lyme arthritis and carditis through the induction of tolerance. PLoS One 2011; 6:e17414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 1984; 57:521–5. [PMC free article] [PubMed] [Google Scholar]
- 28. Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest 2012; 122:2652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng J, Wang T, Shi W, et al. . Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg Microbes Infect 2014; 3:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barsic B, Maretic T, Majerus L, Strugar J. Comparison of azithromycin and doxycycline in the treatment of erythema migrans. Infection 2000; 28:153–6. [DOI] [PubMed] [Google Scholar]
- 31. Luger SW, Paparone P, Wormser GP, et al. . Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans. Antimicrob Agents Chemother 1995; 39:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dattwyler RJ, Luft BJ, Kunkel MJ, et al. . Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med 1997; 337:289–94. [DOI] [PubMed] [Google Scholar]
- 33. Lederer S, Brenner C, Stehle T, Gern L, Wallich R, Simon MM. Quantitative analysis of Borrelia burgdorferi gene expression in naturally (tick) infected mouse strains. Med Microbiol Immunol 2005; 194:81–90. [DOI] [PubMed] [Google Scholar]
- 34. Bramwell KK, Ma Y, Weis JH, et al. . Lysosomal β-glucuronidase regulates Lyme and rheumatoid arthritis severity. J Clin Invest 2014; 124:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paquette JK, Ma Y, Fisher C, et al. . Genetic control of lyme arthritis by Borrelia burgdorferi arthritis-associated locus 1 is dependent on localized differential production of IFN-beta and requires upregulation of myostatin. J Immunol Res 2017; 199:3525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 2012; 64:1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wormser GP, Ramanathan R, Nowakowski J, et al. . Duration of antibiotic therapy for early Lyme disease. a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2003; 138:697–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.