Abstract
Objective
To review and synthesise evidence on rates of respiratory-associated deaths and associated risk factors in the intellectual disability population.
Design
Systematic review and meta-analysis.
Data sources
Embase, CINAHL, ISI Web of Science (all databases including Medline) and PsychINFO were searched for studies published between 1st January 1985 and 27th April 2020 and examined study and outcome quality. Reference lists and Google Scholar were also hand searched.
Results
We identified 2295 studies, 17 were included in the narrative synthesis and 10 studies (11 cohorts) in the meta-analysis. Data from 90 302 people with intellectual disabilities and 13 808 deaths from all causes in people with intellectual disabilities were extracted. Significantly higher rates of respiratory-associated deaths were found among people with intellectual disabilities (standardised mortality ratio(SMR): 10.86 (95% CI: 5.32 to 22.18, p<0.001) compared with those in the general population, lesser rates for adults with ID (SMR: 6.53 (95% CI: 4.29 to 9.96, p<0.001); and relatively high rates from pneumonia 26.65 (95% CI: 5.63 to 126.24, p<0.001). The overall statistical heterogeneity was I2=99.0%.
Conclusion
Premature deaths due to respiratory disorders are potentially avoidable with improved public health initiatives and equitable access to quality healthcare. Further research should focus on developing prognostic guidance and validated tools for clinical practice to mitigate risks of respiratory-associated deaths.
PROSPERO registration number
CRD42020180479.
Keywords: respiratory medicine (see thoracic medicine), public health, respiratory infections
Strengths and limitations of this study.
To the best of our knowledge, this is the first systematic review and meta-analysis on respiratory-associated deaths among people with intellectual disabilities.
Included studies were limited by sample size.
There was no sufficient data and results provided by studies to investigate predictors or factors associated with respiratory-related deaths; meta-regression or stratification was not possible.
The meta-analysis included mortality ratios from 10 observational studies covering 90 302 people with intellectual disabilities and 13 808 deaths from all causes in people with intellectual disabilities.
A rigorous and systematic analysis process was undertaken which minimised the risk of bias, errors and omissions.
Introduction
People with intellectual disabilities account for approximately 1%–3% of the global population.1 2 The World Health Organisation (WHO)3 defines intellectual disabilities as impairments in adaptive functioning, social functioning and intellectual functioning (IQ<70), requiring a need for daily support, with the onset in the developmental phase (<18 years). While some heterogeneity is to be expected in the definition of intellectual disabilities across studies drawing on administrative data sets, the WHO definition can be applied to all studies included in this review. Life expectancy and mortality rates are important indicators of health inequality.4 People with intellectual disabilities die up to 20 years earlier than the general population.5–8 Respiratory disorders are a leading cause of death among people with intellectual disabilities.6 9 The range of standardised mortality ratios (SMRs) due to respiratory disorders for people with intellectual disabilities are very high in some studies,10–12 and much lower in others.13–15 Despite this, SMRs due to respiratory disorders for people with intellectual disabilities differ widely across studies. Respiratory cause of mortality in people with intellectual disabilities has not been systematically examined. Previous studies have focused on either children and young people (4–19 years)10 or older adults (55+years) on average.12 This systematic review and meta-analysis aims to investigate and quantify the risk of, and factors associated with, respiratory-associated deaths in people with intellectual disabilities.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist was followed.16 This review was prospectively registered with the International Prospective Register of Systematic Reviews.
Eligibility
This systematic review included studies which analysed and presented data on people who were ascertained as having intellectual disabilities and a comparison group of individuals in the general population, with respiratory disorders included as a separate cause of death. For studies that included multiple disabilities, at least 70% of participants had to have intellectual disabilities, if results were not reported separately. Studies also had to be full-text, peer-reviewed and published in English. To be included in the meta-analysis, studies had to report SMRs with 95% CIs for respiratory-associated deaths based on external comparison group or to have presented data allowing such outcomes to be derived. Studies were excluded if they focused on specific etiologies of intellectual disabilities, such as Down syndrome, as these are associated with different health and mortality profiles compared with other people with intellectual disabilities. Studies were excluded if the full paper was not available in English. Studies focussing on postoperative and post-treatment deaths were excluded as these are not representative of the wider population with intellectual disabilities. Studies with small samples (<20 participants) or case series designs were also excluded as these papers are less representative.
Search strategy and selection criteria
We searched Ovid Embase, ISI Web of Science (all databases), CINAHL and PsycINFO from 1 January 1985 to the 27 April 2020, using comprehensive terms related to ‘intellectual disabilities’, ‘mortality’ and ‘respiratory disease’ (full search strategy in online supplemental appendix 1). In addition, a manual bibliography and citation search of included studies was conducted using Google Scholar and key researchers in the field of mortality in individuals with intellectual disabilities were emailed to identify any additional relevant papers. The aforementioned eligibility criteria were used. After duplicates were removed, all records were imported into Covidence software (www.covidence.org) for title and abstract and full-text screening. All titles, abstracts (CM and AMcG) and full-texts (CM, AMcG and ER) were double-screened with inter-rater reliability (Cohen’s kappa) of ĸ = 0.57 and ĸ = 0.58, respectively.
bmjopen-2020-043658supp001.pdf (99.6KB, pdf)
Data extraction
Data extraction was conducted using a structured database created in Excel. Five researchers (GSS, LAH-M, DK, KD and AMcG) each extracted data from 25% of the included studies and, to check reliability, one other researcher (CM) independently extracted data from 20% of included studies. Extracted data were compared in meetings and discrepancies resolved through consensus discussion. Researchers did not extract data on included papers where they were a listed author.
Assessment of study and outcome quality
Study quality was appraised using the Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields.17 Quality ratings were calculated in percentage form using the standard method17 and categorised as weak (<55%), moderate (55%–75%) or strong (>75%) quality. Each paper had quality appraisals completed by two researchers, who then agreed a consensus score for each item (table 1).17 Researchers did not evaluate quality of papers where they were a listed author. Risk of bias score was not used to exclude any studies from either the systematic review or meta-analysis. We evaluated the quality of our own systematic reviews using the Measurement Tool to Assess Systematic Reviews checklist.18
Table 1.
Characteristics of studies reporting mortality rates for respiratory disorders and pneumonia in people with intellectual disabilities (ID)
| Author | Country | Study design, setting and follow-up | Data sources | ID sample (n, % female, age, level of ID) | Deaths in ID sample (n, % female, age at time of death, level of ID) | Comparison sample (n, % female, age) and deaths (n, % female, age) | Respiratory disorder definition (eg, ICD codes or other definitions) | Quality percentage (assessment) |
| Brameld et al (2018)26 | Australia | Retrospective matched cohort study of adults 20 years old and over. Follow-up 2009–2013 | Intellectual Disability Exploring Answers (IDEA) Database. Death certificate data | Total sample characteristics not available | n=591; 43.8% female; mean age* and level of ID not available | Total sample characteristics not available. Number of deaths=62 917; 47.4% female; mean age not available | ICD 10-chapter codes for respiratory disorders | 95.45% (strong) |
| Cooper et al (2020)28 | UK | Population-based cohort study. Follow-up 2001–2018 | Primary care records and health check data; death certificate data. Comparison data from Health Board statistics | n=962; 45.4% female; mean age=44.1 years (range 16–83); ID mild=382 (39.7%), moderate=236 (24.5%), severe=180 (18.7%), profound=163 (17.0%) | n=294/961 (30.6%); 47.5% female; mean age=52.4 (SD 13.6) | Not available | ICD 10-chapter codes for respiratory disorders | 86.36% (strong) |
| Dupont et al (1987)43 | Denmark | Population-based cohort study of adults with mild ID. Follow-up 1976–1984 | Danish National Service for the Mentally Retarded. Death certificate data | n=7134; gender, age and level of ID not available | n=446; 37.9% females; age and level of ID not available | Not available | Not described | 40.90% (weak) |
| Durvasula et al (2002)30 | Australia | Population-based cohort study of children and adults. Follow-up 1989–1999 | ID prevalence study. Death certificate data, medical records and postmortem data. Australian Bureau of Statistics | n=693; 44.6% female; mean age=N/A; ID 40% mild, 35% moderate, 25% severe/profound | n=40 (6%); 45% female; median age=32 (range 10–59); level of ID not available | n=1 25 848; 51% female; mean age not available. Number of deaths=2154; 37.8% female; mean age not available | Not described | 90.91% (strong) |
| Forsgren et al (1996)15 | Sweden | Population-based cohort study of adults with ID. Follow-up 1986–1992 | Board for Provision and Services to the Mentally Retarded. Death certificate data from Swedish National Bureau of Statistics | n=1478; 44.5% female; age and level of ID not available | n=247; 42.1% female; median age=64 years (IQR 52–75 years); ID 39.7% mild, 31.2% moderate, 21.5% severe, 7.7% profound | Not available | ICD 9-chapter codes for respiratory disorders | 81.82% (strong) |
| Glover et al (2017)8 | UK | Population-based case–control study in primary care. Follow-up 2010–2014 | Primary care records (Clinical Practice Research Data, CPRD). Death certificate data | Total sample characteristics not available | n=664 deaths; 44.1% female | Total sample characteristics not available. Number of deaths=97 379; 52.3% female; mean age not available | ICD 10-chapter codes for respiratory disorders | 81.82% (strong) |
| Heslop et al (2014)5 | UK | Population-based audit of deaths of children and adults with ID aged 4 and over. Audit period 2010–2012 | Medical records. Death certificate data from UK Office of National Statistics | Total sample characteristics not available | n=247; 42.1% female; median age=64 years (IQR 52–75 years); ID 39.7% mild, 31.2% moderate, 21.5% severe, 7.7% profound | Total sample characteristics not available. Number of deaths=480 467; 51.6% female; median age not available | 81.82% (strong) | |
| Hollins et al (1998)11 | UK | Cohort study of adults on an ID register. Follow-up 1982–1990 | Learning disability register. Death certificate data. | n=2026; gender, age and ID level not available | n=268 deaths; gender and age not available; 51.5% mild–moderate, 48.5% severe–profound | Not available | Not described | 81.82% (strong) |
| Hosking et al (2016)24 | UK | Population-based case–control study in primary care. Follow-up 2009–2013 | Primary care records (CPRD linkage). Death certificate data | n=16 666; 58.1% female; mean age 39.9 (SD: 16.2). 19.6% of sample had high support needs | n=656 (3.9%); 55.6% female; age and level of ID not available | n=113 562; 58.1% female; mean age not available. Number of deaths=1358 (1.2%); 60.4% female; mean age not available | ICD 10-chapter codes for respiratory disorders | 90.91% (strong) |
| Janicki et al (1999)22 | USA | Cohort of adults with ID 40 years old and over. Follow-up 1984–1993 | Data from state agency with responsibility for reviewing deaths of disabled persons. Health department data | Total sample characteristics not available | n=2752, 48.1% female; mean age- 65.1; ID 18%, 68% Moderate–profound, 4% unspecified, 10% unknown | Total sample characteristics not available. Number of deaths=149, 980; gender not available, mean age=70.0 | ICD 9-chapter codes for respiratory disorders | 77.27% (strong) |
| Ng et al (2017)12 | Sweden | Population-based case–control study of adults with ID 55 years old and over. Follow-up 2002–2015 | National database of hospital admissions and outpatient care. National disability register. Swedish National Cause of Death register | n=15 289; 45.5% females; mean age not available; level of ID not available | n=4728; 44.9% female; age and ID level not available | n=74 445; 45.5% females; mean age not available. Number of deaths=8364 | ICD 10-chapter codes for respiratory disorders | 95.45% (strong) |
| Oppewal et al (2018)27 | Netherlands | Cohort study of adults with ID 50 years old and over living in three care organisations. Follow-up November 2013–March 2018 | Medical case notes of participants with ID who died during study period. Cause specific mortality statistics for 50+ population in the Netherlands | n=1050; 48.7% female; mean age=61.6 (SD: 8.0, range 50–94); ID level=2.9% borderline, 21.2% mild, 48.2% moderate, 16.4% severe, 8.7% profound | n=207 deaths (19.7%) but only 159 with cause of death available. 60.7% female; mean age not available; ID level=5.7% borderline, 18.9% mild, 54.7% moderate, 13.2% severe, 7.5% profound | Not available | ICD 10-chapter codes for respiratory disorders | 50.0% (weak) |
| Patja et al (2001)14 | Finland | Population-based, nationwide cohort study. Follow-up 1963–1997 | Original 1962 population-based study (Amnell et al 1964). Death certificate data | n=2369, gender, age and level of ID not available | 1111 deaths with death certificates available for 1095–51.0% female, mean age=57.7; ID 40.3% mild, 29.4% moderate, 11.5% severe, 18.0% profound, 0.7% unknown | Not available | ICD 9-chapter codes for respiratory disorders | 81.82% (strong) |
| Raitasuo et al (1997)13 | Finland | Cohort study of adults living in an institution. Follow-up 1972–1993 | Medical case notes and death certificate data. General population mortality statistics for population in Finland | N ≈ 2000; gender, age and level of ID not available | 216 deaths, 42.6% female; mean age 26.7 (1–86 years); ID level 2.0% borderline, 15.0% mild, 18.0% moderate, 20.0% severe, 45.0% profound, 20.0% unknown | Not available | ICD 9-chapter codes for respiratory disorders | 54.55% (weak) |
| Smith et al (2020)10 | UK | Nationwide, population-based cohort study of children aged 4–19. Follow-up 2008–2015 | Scottish pupils census: death certificates data | n=18 278; 35% female; mean age not available | n=106; mean age=14.3 (95% CI: 13.4 to 15.1); level of ID not available | n=7 77 912; 50% female; mean age not available. number of deaths=458; mean age=16.1 years (95% CI: 15.8 to 16.5) | ICD 10-chapter codes for respiratory disorders | 100% (strong) |
| Trollor et al (2017)25 | Australia | Population-based cohort study of adults 20 years old and above registered with disability services. Follow-up 2005–2011 | Disability Services Minimal Data set. Australian Bureau of Statistics. Death records | n=19 362; 44% female, mean age=37 (range 27–48); ID not available | n=732 (4%); 41% female; median age=54 (42–64), level of ID not available | Total sample characteristics not available. Number of deaths=305 050; 49% female; median age=81 (70–92). | ICD 10-chapter codes for respiratory disorders | 95.45% (strong) |
| Tyrer and McGrother (2009)23 | UK | Population-based cohort study of individuals with moderate- profound ID on a register. Follow-up 1993–2006 | Leicestershire learning disability register. Death certificate data. National Statistics 1993–2006 | n=2995; 41.9% female; Age and level of ID not available | n=503; gender, age and level of ID not available | Total sample characteristics not available. Number of deaths≈126 000 | ICD 9 and ICD 10-chapter codes for respiratory disorders | 72.73% (moderate) |
*Individuals in the ID cohort died at a significantly younger age than the comparison cohort.
ICD, International Classification of Diseases; ID, intellectual disabilities; N/A, not available.
Summary of outcomes and statistical analysis
Findings of all included studies were combined in a narrative synthesis. The primary goal of the meta-analysis was to investigate if the SMRs of respiratory-associated deaths differ for individuals with and without intellectual disabilities. If SMRs were reported by specific respiratory causes, sex, age group, level of intellectual disability, socioeconomic status or ethnicity, these were collected and presented for potential analysis (see table 2). Random-effects meta-analysis was undertaken using RevMan. Included studies reported either:
Table 2.
All-cause mortality and deaths from respiratory disorders in people with intellectual disabilities
| Author | All-cause mortality | Deaths from respiratory disorders | Between group comparison of deaths from respiratory disorders | Deaths from individual respiratory disorders | Between group comparison of deaths from individual respiratory disorders | Variables associated with risk of death from respiratory disorders |
| Brameld et al (2018)26 | 591 had ID/63 508 out of all deaths (0.93%) | 62/591 (10.5%) deaths | Not available | Emergency Department presentations in the last year of life: influenza and pneumonia RR=2.6 (95% CI: 2.0 to 3.4, p<0.001) Chronic obstructive pulmonary disease (COPD) RR=0.8 (95% CI: 0.5 to 1.6, p=0.596) Asthma RR=4.7 (95% CI: 2.1 to 10.4, p<0.001) Ear, nose and throat infections RR=1.9 (95% CI: 0.8 to 4.0, p=0.122) Pneumonitis due to solids/liquids RR=17.9 (95% CI: 11.3 to 28.3, p<0.001) Hospital admissions in the last year of life: influenza and pneumonia RR=2.3 (95% CI: 1.0 to 5.3, p=0.044) COPD RR=1.4 (95% CI: 0.9 to 2.4, p=0.164) Asthma RR=4.6 (95% CI: 1.4 to 15.0, p=0.011) Ear, nose and throat infections RR=0.0(95% CI: 0.0–, p=0.972) Pneumonitis due to solids/liquids RR=17.6 (95% CI: 11.7 to 26.5, p<0.001) |
Decedents with ID had increased odds of dying of (relative odds of having condition listed as underlying cause of death), adjusted for comorbidity: influenza/pneumonia (OR=5.3, 95% CI: 2.4 to 11.8) Pneumonitis due to solids or liquids (OR=9.9, 95% CI: 5.1 to 19.3) Asthma (OR=2.3, 95% CI: 1.0 to 5.2) (not significant) No difference for COPD as cause of death |
Decedents with ID had increased A&E attendance but received less hospital-based specialist palliative care. For those in hospitals, they were more likely to have hospital stays involving intensive care and ventilator support |
| Cooper et al (2020)28 | 294/961 (30.6%) deaths SMR=2.24 (95% CI: 1.98 to 2.49) |
Underlying cause of death: 57/262 (21.8%) deaths SMR=6.78 (95% CI: 5.02 to 8.54) (adjusted for age and sex) |
Underlying cause of death: Down syndrome (DS): 8/57 (14.0%) deaths Without DS: 49/205 (23.9%) deaths |
All-contributing factors in death: respiratory infection=27.1% deaths Aspiration/ reflux/choking=19.8% deaths |
Underlying cause of death: DS: Aspiration/reflux/choking =<5/57 deaths Respiratory infection=<5/57 deaths Other respiratory conditions=<5/57 deaths Without DS: Aspiration/reflux/choking=22/205 (10.8%) deaths Respiratory infection=21/205 (10.3%) deaths Other respiratory conditions=9/205 (4.4%) deaths All-contributing factors in death: DS: Respiratory infection=22/57 (38.6%) deaths Aspiration/reflux/choking=11/57 (19.3%) deaths Other respiratory conditions =<5/57 deaths Without DS: Respiratory infection=49/205 (23.9%) deaths Aspiration/reflux/choking=41/205 (20.2%) deaths Other respiratory conditions=31/205 (15.1%) deaths |
Not available |
| Dupont et al (1987)43 | n=446 deaths/7134 (5.9%) people with mild ID n=277 males n=169 females |
Respiratory deaths common cause of death in people with ID (all ages) Tests of significance only; respiratory deaths were more common for males with ID (all ages), and females aged 35–64, vs population of Denmark 1977 |
Not available | Not available | Not available | Not available |
| Durvasula (2002)30 | 40/693 (6%) deaths | 14/40 (35%) deaths | For people under 40, respiratory and external deaths were most common, for people over 40, cancer and respiratory deaths were most common Age: 7/14 deaths in under 25-year olds and 6/14 deaths in 40+ year olds Sex: 11/14 deaths in males Conditions: 2/14 had DSanddementia, 1/14 had myelodysplastic syndrome, 1x Battens disease |
Not available | Not available | Age, gender, DS, myelodysplastic syndrome |
| Forsgren et al (1996)15 | n=124/1478 (8.4%) people with ID (all ages), over 9992 person-years SMR=2.0 (95% CI: 1.7 to 2.3) Males 1.6 (95% CI: 1.2, 2.0), females 2.6 (95% CI: 2.0 to 3.3) Additional: SMRs for severity of ID, epilepsy and cerebral palsy are available inonline supplemental appendix |
n=13/124 (10%) deaths were respiratory disease for people with ID vs n=3.9 expected, SMR=3.3 (95% CI: 2.0 to 5.5) (adjusted for age and sex) |
Respiratory disease was common cause of death for people with ID and epilepsy but SMR was not possible due to small sample size | Pneumonia was most common cause of death, but rarely reported as underlying cause Pneumonia was most common cause of death in people with both epilepsy and ID |
Not available | Epilepsy (active seizures) |
| Glover et al (2017)8 | n=664 deaths for people with ID (all ages) over 59 279.7 person-years Crude rate 11.2 (10.4, 12.1) per 1000 person-years SMR=3.18 (2.94, 3.43) Women=3.40 (3.02, 3.81) Men=3.03 (2.73, 3.35) |
n=114 deaths from respiratory causes for people with ID vs 23.3 expected SMR=4.9 (4.0, 5.9) (adjusted for age and sex) |
Not available | n=57/114 (50%) of respiratory deaths (and 8.6% of all deaths) were from influenza and pneumonia, vs expected 7.4 deaths SMR=7.7 (5.8, 9.9) Vast majority of pneumonia were unspecified (organism) n=24/114 (21%) respiratory deaths (3.6% of all deaths) were due to pneumonitis due to solids/liquids vs expected 1.1 deaths SMR=21.8 (13.9, 32.4) n=12 (1.8%) of all deaths were due to respiratory and intrathoracic cancers vs expected 16.6 deaths SMR=0.7 (95% CI: 0.4 to 1.3) |
Not available | Not available |
| Heslop et al (2014)5 | n=247 deaths in people with ID aged 4+ Rate of death 16.2 per 1000 person years Median age of death: 64 (52, 75). Additional; all-cause mortality for sex, ID severity, amenable mortality, patient care, andaccommodation available inonline supplemental appendix |
n=37 (15%) deaths had underlying cause due to respiratory diseases, vs 14.0% EnglandandWales deaths (p=0.66) | Not available | Not available | Not available | Reduced smoking in ID group p=0.02 |
| Hollins et al (1998)11 | 270/2026 (13.3%) deaths 116/1081 (10.7%) deaths on Wandsworth register 154/945 (16.3%) deaths on Kensington register |
Not available | Not available | Bronchopneumonia: n=56 (48%) (Wandsworth) n=69 (45%) (Kensingston) COPD emphysema: n=1 (Wandsworth) n=1 (Kensingston) Asphyxia: n=4 (Wandsworth) n=1 (Kensingston) Respiratory other: n=4 (Wandsworth) n=4 (Kensingston) 52% of all deaths had a diagnosis of pneumonia |
Not available | Not available |
| Hosking et al (2016)24 | 656/16 666 (3.9%) deaths HR=3.62 (95% CI: 3.33 to 3.93) |
123/16 666 (18.8%, rate=24.8) deaths HR=6.68 (95% CI: 5.38 to 8.29) (adjusted for age, sex and general practice) |
Down syndrome=24/1793 (20.3%) deaths. General population: 135/1 13 562 (rate=3.9) deaths |
Pneumonia; n=67/16 666 (rate=13.5) Aspiration pneumonitis; n=21/16 666 (rate=4.2) |
General population: Pneumonia; 39/113 562 (rate=1.1) Aspiration pneumonitis; n=6/113 562 (rate=0.2) |
Not available |
| Janicki et al (1999)22 | 2752 deaths in the group aged 40+/4183 all-age deaths (66%) | 40+ year olds: n=548 (20%), rate: 201 per 100 000 | Increasing by age decade: aged 40s: 343 per 100 000 (16% of those who died) aged 50s: 793 per 100 000 (20%) aged 60s 1660 per 100 000 (25%) aged 70+: 3441 per 100 000 Males with ID rate of death: 257 per 100 000 Females with ID rate of death: 331 per 100 000 respiratory causes did not vary over the 10-year study period. Deaths due to respiratory diseases increased, with increasing age. Gender: breathing obstructions were more prevalent among males. Gender x age: respiratory disease was increased in the oldest groups, for males particularly while respiratory disease remained static as a cause of death for females across ages |
Breathing obstructions: 2.7% average deaths per year across 10 years, n=75, rate=27.5 per 100 000 Respiratory disease types: pneumonia was the most prevalent type of respiratory cause of death, with 43% of respiratory disease deaths in ID group |
Not available | Age, gender |
| Ng et al (2017)12 | 4738/15 289 deaths in people aged 55+ (31%) | 807/4738 (17%) respiratory deaths for those with ID HR=12.5 (10.9, 14.2) (adjusted for sex, year of birth and year of access to services) |
ID rate: 423 per 100 000 DS rate: 3187 per 1000 |
ID group (excludes DS) Pneumonitis due to solids and liquids: 10%, rate 25 per 100 000 Pneumonia: 50%, rate 129 per 100 000 Other COPD: 20%, 49 per 100 000 DS group Pneumonitis due to solids and liquids 31.4%, 181 per 100 000 Pneumonia 20%, 113 per 100 000 Asthma 8%, 45 per 100 000 Bronchitis 8%, 45 per 100 000 Other respiratory disorders 8%, 45 per 100 000 |
Not available | Not available |
| Oppewal et al (2018)27 | 207/1050 ID=19.7%; 54/149DS=26.1% |
69/159 ID=44.3%; 33/45 DS only=73.3%; 36/114 ID with no DS=31.6% Respiratory causes were the top primary causes of ID deaths. Respiratory causes were the top primary cause of DS deaths. General 50+ population, the three largest groups of primary causes of death were neoplasms (31%), circulatory diseases (28%) and respiratory diseases (9%). No SMR available |
5-year age bands: 50–54ID=100%GP=3.3%; 55–59ID=26.5%GP=4.7%; 60–64ID=51.4%GP=6.0%; 65–69ID=30.4%GP=6.7%; 70–74ID=23.8%GP=8.6%; 75–79ID=12.5%GP=9.4%; 80–84ID=26.3%GP=9.4%; 85–90 ID=(0) GP=9.9%; 90–95 ID=40% GP=10.4%; 95+ID=100% GP=10.9% |
Pneumonia ID=80.4%; COPD ID=17.6% |
Not available | Not available |
| Patja et al (2001)14 | 1111/2369 ID=46.9% | Immediate cause 322/1093 ID=29%; Primary cause 241/1095 ID=22% Respiratory diseases second largest cause of ID death SMR=3.76 (CI: 3.31 to 4.27)* (adjusted for age and sex) |
Male: age 2–19 SMR=5.8 (4.4–15.6); age 20–39 SMR=5.4 (2.9–8.0); age 40–59 SMR=5.5 (3.5–7.5); age 60+ SMR=2.7 (2.7–4.8) Female: age 2–19 SMR=4.3 (0.3–4.7); age 20–39 SMR=3.2 (1.1–5.1); age 40–59 SMR=6.2 (4.1–8.2); age 60+SMR=3.3 (1.7–3.0) |
Pneumonia ID=83%; COPDID=11%. |
Pneumonia deaths (%): profound ID=29%; severe ID=13%; moderate ID=33%; mild ID=25%. Risk ratios compared with general population: Mild ID 2.6 times higher; profound ID 5.8 times higher. ID men higher risk than women in younger age groups (<39 years), but at lower risk from 60 years of age onwards |
Age, gender (all respiratory) ID severity (with pneumonia) |
| Raitauso et al (1997)13 | 216 deaths | Immediate cause of death 97/216 ID=45% Primary cause 14/216 ID=6%. Respiratory diseases were the dominant causes of ID death. SMR=2.15 (CI: 1.18 to 3.61) (adjusted for age, and year of death) |
age 0–14 SMR=0.48; age 15–44 SMR=3.46; age 45–74 SMR=2.35; age 75 SMR=0 | Bronchopneumonia (immediate cause) ID=43% Five patients had died of pneumonia caused by aspiration. In one case fatal pneumonia had been caused by a fistula between the bronchus and the pleura. Besides pneumonia, two patients had acute laryngitis and one patient had hyperplasia of the lymph nodes of the lungs as the immediate cause of death. The latter had trisomy of chromosome 13 (Patau’s syndrome) as the basic disorder |
Not available | Age (all respiratory) |
| Smith et al (2020)10 | n=106 (0.6%) deaths SMR=11.6 (95% CI: 9.6 to 14.0) |
Underlying cause of death: n=8/106 (8%) deaths All-contributing factors in death: n=55. CMR=81.7 (95% CI: 62.7 to 106.4) deaths SMR=55.3 (95% CI: 42.5 to 72.1) (adjusted for age and sex) |
Underlying cause of death: General population: 17/458 (4%) deaths All-contributing factors in death: General population: n=51. CMR=1.4 (95% CI: 1.1 to 1.8) deaths |
Underlying cause of death: pneumonia including influenza;<5/106 All-contributing factors in death: pneumonia=27/106 (25.5%) deaths Respiratory failure; 17/106 (16.0%) deaths Respiratory disorders=15/106 (14.2%) deaths Pneumonitis associated with food and vomit=9/106 (8.5%) deaths |
General populations: all-contributing factors in death: pneumonia=21/458 (4.6%) deaths |
Not available |
| Trollor et al (2017)25 | 732/19 362ID=4% SMR=1.3 (1.2 to 1.5) |
632/732 ID=86.3% had cause of death information 78 ID=12% 4th top cause using the ID ABI conversion 130 ID=20% 1st top using the ID revised version 16 ID=3% of respiratory deaths were considered avoidable. 26 242GP=9% 3rd top underling cause |
Not available | Not available | Not available | Not available |
| Tyrer and McGrother (2009)23 | 503/2995 (17%) deaths SMR=2.77 (95% CI: 2.53 to 3.03). |
SMR=5.46 (95% CI: 4.58 to 6.46) (adjusted for age and sex) |
Not available | Bronchopneumonia; SMR=6.47 (95% CI: 5.00 to 8.23), O=66, E=10.2. Other respiratory; SMR=4.64 (CI: 3.58 to 5.91). O=65, E=14.0 |
Male; SMR=2.28 (95% CI: 2.02 to 2.56) O=278, E=121.8. Female; SMR=3.24 (95% CI: 2.83 to 3.69). O=225, E=69.4 |
Gender |
*Only where adjusted specifically for respiratory mortality.
CMF, comparative mortality figure; E, expected death*calculated by authors using data from the study; O, observed deaths; RR, rate ratio; SMR, standardised mortality ratio.
an SMR or HR
bmjopen-2020-043658supp002.pdf (109.8KB, pdf)
bmjopen-2020-043658supp003.pdf (1.1MB, pdf)
OR
The observed number of deaths or expected deaths necessary to calculate a SMR. These were calculated using STATA V.14 by dividing the observed number of deaths in a cohort study group by the expected mortality based on age and gender-specific death rates in the general population comparison group.
Random-effects models were selected for all meta-analyses due to the different populations and measures in the included studies. Inverse of the variance method was used to calculate the weighted mean respiratory mortality log-SMR across studies, as well as for subgroup meta-analyses. As the SMR is a ratio, log transformation was needed to maintain symmetry in the analysis.19 SMRs and HRs from each study were transformed to log values for computations and back transformed for presentation of the results. Weighted mean log-SMRs and their 95% CIs were reported separately for individuals with and without intellectual disabilities. The magnitude of the back transformed ratio and associated CI were also reported. Where data permitted, further subgroup analyses were conducted to examine sources of heterogeneity. Where more than two studies reported subgroup level data, or cause-specific results of causes of respiratory deaths (eg, pneumonia) random-effects models were considered for subgroup meta-analyses.
For the random-effects meta-analysis, heterogeneity was expected in the pooled result. Therefore, the χ2 statistic I2 was chosen to measure level of heterogeneity across the studies, as it allows for interpretation of results regardless of the number of studies included in the meta-analysis, the type of outcome data, or effect measurement.20 Heterogeneity was interpreted as not observed when I2=0%, low when I2=25%, medium when I2=50% and high when I2=75%.20
Sensitivity analysis
Sensitivity analysis was used to assess the impact of risk of bias for each study on the weighted mean SMR. Data were removed one-by-one from the meta-analysis for each study, beginning with the lowest ranked papers, to determine their effect and re-estimate the weighted mean SMR. Cumulative analysis, starting with larger studies and sequentially adding smaller studies, was used to investigate how the weighted mean SMR estimate changes as small studies are added.21
Patient and public involvement
No patient and public involved.
Results
Figure 1 summarises the systematic search, selection and reasons for exclusion. All 17 studies were included in the narrative synthesis and 10 were included in the meta-analysis (studies with relevant SMRs n=8 and HR n=2). A full list of studies excluded from full-text screening is available in online supplemental appendix 2.
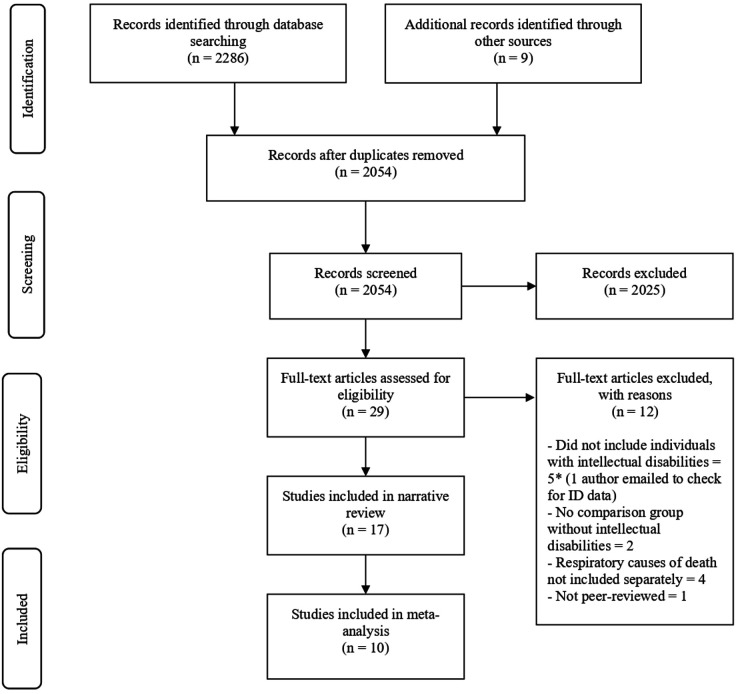
Figure 1.
PRISMA flow diagram of systematic search and selection. A total of 2286 records were retrieved through a search of Embase, ISI Web of Science (all databases), CINAHL and PsycINFO with an additional nine records identified through other sources. After removing 241 duplicates, 2025 records were excluded due to ineligible types, the remaining 29 were retrieved as full-texts. From these, 17 were included in the narrative review and 10 included in the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.
Table 1 illustrates the characteristics of studies reporting mortality rates for respiratory disorders and pneumonia in people with intellectual disabilities and table 2 presents all-cause mortality and deaths from respiratory disorders in people with intellectual disabilities.
Study characteristics
Key features of all studies identified for inclusion in the review were tabulated (table 1). These were cohort studies (n=12), case–control studies (n=4) and one population-based audit of deaths in adults and children. These studies report data on 90 302 people with intellectual disabilities and 27 394 deaths. The average study size was 9250 people. These studies were from the Netherlands (n=1), Finland (n=2), Australia (n=3), the UK (n=7), the USA (n=1), Sweden (n=2) and Denmark (n=1).
Definition of respiratory disorder
Thirteen out of 17 (76%) studies defined the respiratory disorder using International Classification of Diseases (ICD) 9-chapter codes13–15 22 23 and ICD 10-chapter codes for respiratory disorders.8 10 12 23–26 The remaining four studies included in the systematic review did not define respiratory disorders.
Causes of death from respiratory disorders
Thirteen papers reported on cause of deaths from respiratory disorders.8 10–15 22–24 26–28 Pneumonia was reported as a cause of death in 12 studies,8 10–15 22–24 26 27 five studies reported deaths from pneumonitis related to aspiration,8 10 12 14 24 five studies reported on chronic obstructive pulmonary disease (COPD),11 12 14 26 27 one study reported on asthma29 and one reported respiratory cancer deaths.8
Evidence synthesis
Respiratory-associated mortality
Five papers reported that respiratory disorders were the dominant cause of death in people with intellectual disabilities.11 13 27 28 30 A further three studies found that deaths from respiratory disorders were the second most common cause of death.12 14 24 Respiratory-associated deaths were in the top five main causes of deaths for a further four papers.9 10 22 25 Comparative results (intellectual disabilities vs general population) for deaths due to respiratory disorders were reported in 10/17 (59%) of the studies.10 12–15 22 24 27 28 30 In the majority of these studies, rates of death from respiratory disorders were higher for people with intellectual disabilities than for people in the general population. However, Troller et al25 reported that respiratory-associated deaths in the general population were (9%) similar to the population with intellectual disabilities (12%). Hollins et al11 also reported that respiratory disorders were the most commonly cited cause of death for both groups.
Individual respiratory disorders and mortality
Pneumonia was reported as the most common cause of respiratory death in people with intellectual disabilities.8 10–15 22–24 26 27 Contributors to pneumonia deaths included influenza and injury from inhalation and aspiration events.10 14 Pneumonitis featured as an underlying or contributing cause for between 8% and 21% of respiratory-associated deaths in people with intellectual disabilities.8 10 12 Crude comparison data showed people with intellectual disabilities were much more likely (between 10 and 20 times) to die from pneumonitis.24 26 COPD was found to be a common cause of death in two studies focussing on older adults.12 27
Factors associated with respiratory-associated deaths experienced by people with intellectual disabilities
Age, gender and severity of intellectual disability have been found to be associated with risk of respiratory cause of death. Only four out of 17 (23.5%) papers directly reported on factors associated with the risk of respiratory-associated deaths14 22 23 30 (see table 2). Two reported SMRs separately for males and females,14 23 while two reported proportions of respiratory deaths between males and females. None directly compared males versus females or reported tests of significance. While one study reported higher respiratory SMRs among females,23 another study reported separate SMRs for different age-bands which varied widely.14 Group-level analysis was not possible. Level of intellectual disabilities was only reported as associated with respiratory related deaths in one study with 35-year follow-up using relative risk but failed to report confidence or p-values.14 This study found that, when compared with the general population, the relative risk of respiratory related deaths was 2.6 times higher for people with mild intellectual disabilities and 5.8 times higher for people with profound and multiple intellectual disabilities.
Respiratory mortality among children and young people
Respiratory deaths among children and young people with intellectual disabilities were reported in five studies and found to be a common cause of death across all studies.10 13 15 30 31 Four studies included comparison with the general population for respiratory causes of death, while one included the national population without intellectual disabilities.10 All analyses were limited by the small numbers of death. Raitasuo et al reported only one death.13 Patja et al reported higher SMR for males aged 2–19 years but not females.31 Smith et al reported 8% deaths had respiratory disease as the underlying cause but the SMR for underlying cause was not reported.10
Meta-analytical outcomes
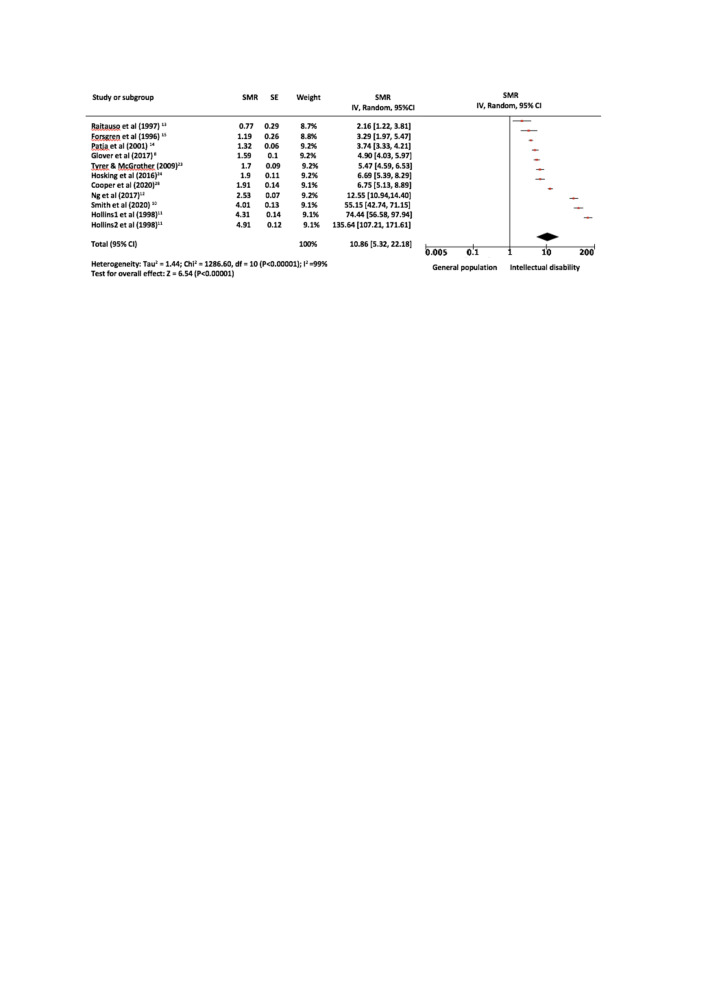
Ten studies8 10–15 23 24 28 reported the necessary data to calculate (SMR, HR or data necessary to calculate these) and were included in the meta-analysis of respiratory mortality of people with intellectual disabilities and the general population. As Hollins et al reported the SMR of two separate cohorts, these are displayed separately in the relevant forest plots.11 The pooled SMRs for respiratory mortality between people with intellectual disabilities and the general population was 10.86 (95% CI: 5.32 to 22.18). The results indicate that respiratory mortality occurs almost 11 times more frequently in the intellectual disabilities group than in the general population group. At the individual study level, this was adjusted for age in all studies and for sex in all studies except for two of these studies,11 13 where this was not clear. There was evidence of considerable statistical heterogeneity between studies in the meta-analyses, with I2=99.0%. Results are displayed in figure 2.
Figure 2.
Forest plot of respiratory-associated mortality. The pooled SMRs for respiratory mortality between people with intellectual disabilities and the general population was 10.86 (95% CI: 5.32 to 22.18). There was considerable statistical heterogeneity between studies in the meta-analyses, with I2=99.0%. SMR, standardised mortality ratio.
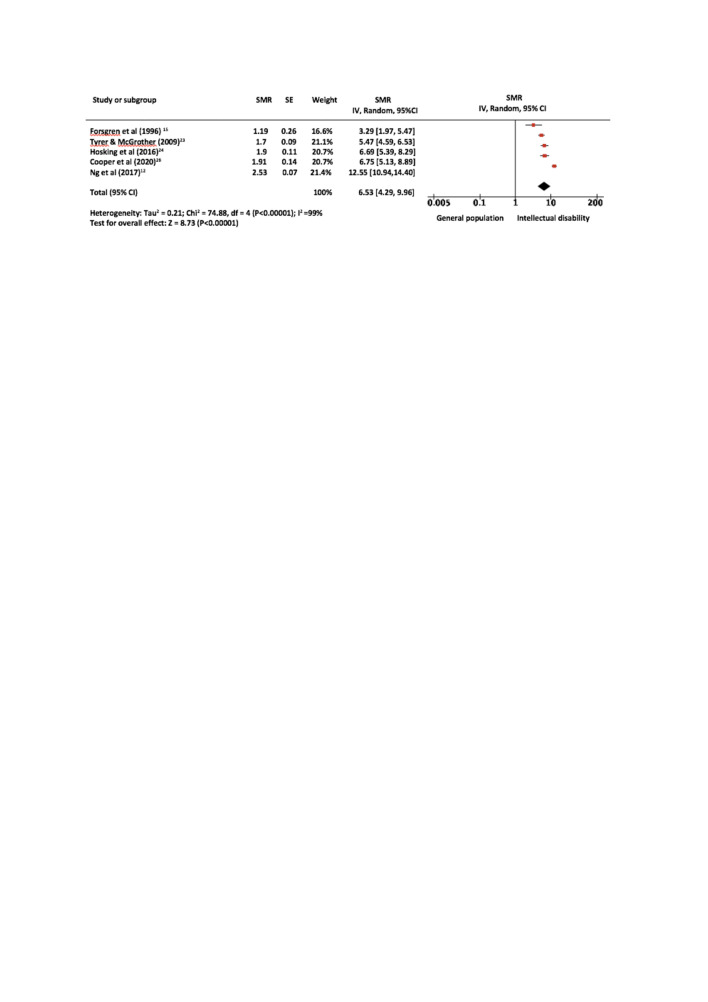
As five studies12 15 23 24 28 focused on adults only, one study10 focused on children only and six8 11–15 included people of all ages, a subanalysis was conducted of studies which reported data on an adult only population. The results of this subanalysis are displayed in figure 3. The pooled SMR reduced slightly from 10.86 (95% CI: 5.32 to 22.18) to 6.53 (95% CI: 4.29 to 9.96), after one study with a sample of primarily children was excluded.10 Studies which included both adults and children in their sample8 11–15 were next removed one at a time. First, both cohorts from Hollins et al were removed and the pooled SMR was reduced by around half, from 915 to 4.80.11 The further removal of studies by Glover et al,8 Patja et al14 and Raitasuo et al13 resulted in a final pooled SMR for adults of 5.85 (95% CI: 4.73 to 7.22, p<0.001). Heterogeneity between studies was also reduced from I2=99% to I2=56% by the exclusion of samples which included children.
Figure 3.
Forest plot for adults only. The pooled SMR for adults only was 5.85 (95% CI: 4.73to 7.22, p<0·001). Heterogeneity between studies was also reduced from I2=99% to I2=56% by the exclusion of samples which included children. SMR, standardised mortality ratio.
A subanalysis was conducted of studies which reported an SMR for pneumonia.8 11 23 The pooled SMR for pneumonia mortality for people with intellectual disabilities compared with the general population was 26.65 (95% CI: 5.63 to 126.24, p<0.001). These results, displayed in figure 4, indicate that pneumonia-related mortality occurs much more frequently in people with intellectual disabilities than in the general population group. Evidence of considerable statistical heterogeneity between studies was also present in this subanalysis with I2=99.0%. SMRs were recalculated excluding the only study to include an adult only sample, Tyrer and McGrother23 resulting in a substantial increase in pooled SMR (95% CI: 26.65 to 42.70).
Figure 4.
Forest plot for pneumonia-related mortality. The pooled SMR for pneumonia mortality for people with intellectual disabilities compared with the general population was 26.65 (95% CI: 5.63 to 126.24, p<0.001). Evidence of considerable statistical heterogeneity between studies was also present in this subanalysis with I2=99.0%. SMR, standardised mortality ratio.
Sensitivity analysis
Sensitivity analysis in relation to quality assessment was run for the 10 studies included in the meta-analysis (online supplemental appendix 3). Studies which were rated as weak13 or moderate23 were removed from the analysis. The pooled SMR for mortality ratios changed slightly as Raitasuo et al13 (from 10.81 to 12.67)27 and then Tyrer and McGrother (from 12.67 to 13.94)23 were removed from the analysis. As the change in SMR was small, this suggests that inclusion of weaker studies did not significantly change the results.
Discussion
This systematic review and meta-analysis highlights that people with intellectual disabilities experience excess respiratory-associated deaths, with a respiratory mortality of almost 11 times greater than for the general population. Respiratory mortality was more prevalent among studies which include children, and pneumonia was a major contributor to the higher respiratory mortality reported in this study. Clinical guidelines have contributed to a reduction in mortality from community-acquired pneumonia.29 We believe the evidence presented here highlights the need for clinical guideline development groups to make recommendations on reducing the risks of premature death due to community-acquired pneumonia among people with intellectual disabilities. Vaccination programmes for influenza can help to reduce respiratory mortality in children32 and adults.33 Although there is a relatively low uptake of influenza vaccine among people with intellectual disabilities, annual health-checks for people with intellectual disabilities have been reported to increase uptake of influenza immunisation.34 People with intellectual disabilities should be identified as a high-risk group and immunisation providers should prioritise the improvement of vaccine uptake, for example through the roll-out of health checks. People with intellectual disabilities are at increased risk of recurrent chest infections which are secondary to dysphagia35 36 with a high proportion of aspiration pneumonia-related deaths occurring among individuals with severe and profound intellectual disabilities.5 22 30 35 37 Increased recognition of the link between dysphagia and respiratory disorders among caregivers and practitioners is critical to ensuring the early identification of individuals with respiratory disorders.
The higher risk of death from respiratory disorders, such as pneumonia, for people with intellectual disabilities is a significant concern in relation to the rapidly developing COVID-19 pandemic.38 39 Urgent action to disaggregate data on deaths from COVID-19 for people with intellectual disabilities and to investigate factors associated with COVID-19-related mortality for people with intellectual disabilities is vital to ensure that clinical guidelines are based on consideration of the specific risks faced by people with intellectual disabilities. Research is urgently required to investigate the risk factors associated with COVID-19 for people with intellectual disabilities to ensure carers and clinicians have access to the best evidence to reduce the risk of infection in those most vulnerable and to inform the clinical management of those who contract COVID-19. Carers and clinical staff must be given training to ensure they understand the human rights and healthcare needs of people with intellectual disabilities to ensure that existing stark disparities in the health of people with intellectual disabilities are not widened during this crisis.
Interventions should focus on the paediatric age group. Among the studies included in this meta-analysis, we found a relationship between inclusion of children and SMRs from respiratory causes, with those studies including children reporting higher SMRs. This is consistent with studies that have reported higher SMRs in children compared with adults in epilepsy15 and cerebral palsy.40 Overall, mortality in childhood is very low relative to adulthood, and in the paediatric age group, chronic disabling conditions such as intellectual disability, epilepsy and cerebral palsy all have a marked impact on SMR. Comorbidity with epilepsy and cerebral palsy are likely to be significant modifiers of the relationship between intellectual disability and respiratory mortality. Children with more severe intellectual disability are more likely to have epilepsy and cerebral palsy, both of which are independent risk factors for respiratory mortality.
Study strengths and limitations
Our study has several strengths. The meta-analysis included mortality ratios from 10 observational studies covering 1844 respiratory deaths in people with intellectual disabilities, which has improved the power and precision to answer this important research question. A rigorous and systematic analysis process was undertaken, and we minimised the risk of bias, errors and omissions by having two or more reviewers conduct comprehensive searches, assess study quality and extract descriptive data. Due to the low prevalence (~1%) of intellectual disabilities among the general population, low sample size was a considerable limitation, relative to other patient groups. However, our meta-analysis included two national,10 12 and five regional intellectual populations in their respective countries.11 15 23 28 While heterogeneity was found, due to methodological and clinical diversity including study design, age and study nationality, this is common in meta-analyses and statistical heterogeneity was inevitable.20 We have not included assessment of non-reporting or publication bias. Most of the research was conducted in Western countries, thus limiting the extent to which the findings may generalise to non-Western countries. Furthermore, ethnicity was not reported widely which prevented further analysis. There was variation among studies on how mortality was examined and how deaths were reported. There is a general lack of evidence on factors associated with the increased risk of respiratory-related deaths in people with intellectual disabilities. As a consequence, we were not able to perform meta-regression on predictors or factors reported in studies which increase SMRs for respiratory deaths (age, sex, place of death or severity of intellectual disabilities). This should be a priority for future research in order to inform the development of targeted interventions to prevent respiratory-related deaths. Although the meta-analysis enables synthesis of data from a large sample, many of the individual studies reported on small samples and are at increased risk of bias. It is encouraging that there have been several larger studies in recent years and future research should focus on reporting respiratory mortality in representative, population-based samples. Furthermore, the majority of the studies included for review relied on death certificate data. One the most reported causes on the death certificate of people with intellectual disabilities is the intellectual disability itself. Given that this problem only exists within this population, true causes of death remain underestimated.41 42 As reporting has improved over the years, and many counties implemented automated coding systems, it is likely that older paper have more bias than more recent studies.
These findings signify the urgent need to develop and implement evidence-informed strategies to reduce premature mortality among people with intellectual disabilities. Respiratory disorders are a major cause of death for people with intellectual disabilities, many of which are avoidable with improved public health initiatives and access to good quality health and social care. However, further research is required to understand both the multifactorial causes of this heightened risk as well as the most effective approaches for the multiprofessional clinical management of these risks.
Supplementary Material
Footnotes
Twitter: @ScotLDO, @lah021_laura, @bhauteshjani
Contributors: MT, CM, AMcG, ER, LAH-M, DK, KD, GSS, AH and FB had full access to all the data, contributed to the systematic review and meta-analysis of studies, interpretation of results and the manuscript. JS and BJ helped to interpret the result of the study and contributed to the manuscript. MT is study guarantor. All authors reviewed the final manuscript and agreed to be accountable for all aspects of the work and approved the final manuscript for submission. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by The Scottish Government grant number (HSCD 169698).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Harris JC. Intellectual disability: understanding its development, causes, classification, evaluation, and treatment. Oxford: Oxford University Press, 2006. [Google Scholar]
- 2.Maulik PK, Mascarenhas MN, Mathers CD, et al. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil 2011;32:419–36. 10.1016/j.ridd.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 3.Organisation W . The ICD-10 classification of mental and behavioural disorders. Clinical descriptions and diagnostic guidelines. Geneva: World Health Organisation, 1992. [Google Scholar]
- 4.World Health Organization . World health statistics 2015. Geneva: World Health Organization, 2015. [Google Scholar]
- 5.Heslop P, Blair P, Fleming P. Confidential inquiry into premature deaths of people with learning disabilities (CIPOLD) final report, 2013. Available: http://www.bristol.ac.uk/cipold/fullfinalreport.pdf [Accessed 25 Jun 2020].
- 6.Heslop P, Blair PS, Fleming P, et al. The Confidential inquiry into premature deaths of people with intellectual disabilities in the UK: a population-based study. Lancet 2014;383:889–95. 10.1016/S0140-6736(13)62026-7 [DOI] [PubMed] [Google Scholar]
- 7.O'Leary L, Cooper S-A, Hughes-McCormack L. Early death and causes of death of people with intellectual disabilities: a systematic review. J Appl Res Intellect Disabil 2018;31:325–42. 10.1111/jar.12417 [DOI] [PubMed] [Google Scholar]
- 8.Glover G, Williams R, Heslop P, et al. Mortality in people with intellectual disabilities in England. J Intellect Disabil Res 2017;61:62–74. 10.1111/jir.12314 [DOI] [PubMed] [Google Scholar]
- 9.Tyrer F, Smith LK, McGrother CW. Mortality in adults with moderate to profound intellectual disability: a population-based study. J Intellect Disabil Res 2007;51:520–7. 10.1111/j.1365-2788.2006.00918.x [DOI] [PubMed] [Google Scholar]
- 10.Smith GS, Fleming M, Kinnear D, et al. Rates and causes of mortality among children and young people with and without intellectual disabilities in Scotland: a record linkage cohort study of 796 190 school children. BMJ Open 2020;10:e034077. 10.1136/bmjopen-2019-034077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollins S, Attard MT, von Fraunhofer N, et al. Mortality in people with learning disability: risks, causes, and death certification findings in London. Dev Med Child Neurol 1998;40:50–6. [PubMed] [Google Scholar]
- 12.Ng N, Flygare Wallén E, Ahlström G. Mortality patterns and risk among older men and women with intellectual disability: a Swedish national retrospective cohort study. BMC Geriatr 2017;17:269. 10.1186/s12877-017-0665-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raitasuo J, Mölsä P, Raitasuo S, et al. Deaths among the intellectually disabled: a retrospective study. J Appl Res Intellect Disabil 1997;10:280–8. 10.1111/j.1468-3148.1997.tb00023.x [DOI] [Google Scholar]
- 14.Patja K, Mölsä P, Iivanainen M. Cause‐specific mortality of people with intellectual disability in a population-based, 35-year follow-up study. J Intellect Disabil Res 2001;45:30–40. 10.1046/j.1365-2788.2001.00290.x [DOI] [PubMed] [Google Scholar]
- 15.Forsgren L, Edvinsson SO, Nyström L, et al. Influence of epilepsy on mortality in mental retardation: an epidemiologic study. Epilepsia 1996;37:956–63. 10.1111/j.1528-1157.1996.tb00533.x [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 17.Kmet LM, Cook LS, Lee RC. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. HTA Initiative #13. Edmonton: Alberta Heritage Foundation for Medical Research, 2004. [Google Scholar]
- 18.Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One 2007;2:e1350. 10.1371/journal.pone.0001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borenstein M, Higgins JPT, Hedges LV, et al. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 2017;8:5–18. 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borenstein M. Common mistakes in meta-analysis and how to avoid them. 388. New Jersey: Biostat Inc, 2019. [Google Scholar]
- 22.Janicki MP, Dalton AJ, Henderson CM, et al. Mortality and morbidity among older adults with intellectual disability: health services considerations. Disabil Rehabil 1999;21:284–94. 10.1080/096382899297710 [DOI] [PubMed] [Google Scholar]
- 23.Tyrer F, McGrother C. Cause‐specific mortality and death certificate reporting in adults with moderate to profound intellectual disability. J Intellect Disabil Res 2009;53:898–904. 10.1111/j.1365-2788.2009.01201.x [DOI] [PubMed] [Google Scholar]
- 24.Hosking FJ, Carey IM, Shah SM, et al. Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health 2016;106:1483–90. 10.2105/AJPH.2016.303240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trollor J, Srasuebkul P, Xu H, et al. Cause of death and potentially avoidable deaths in Australian adults with intellectual disability using retrospective linked data. BMJ Open 2017;7:e013489. 10.1136/bmjopen-2016-013489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brameld K, Spilsbury K, Rosenwax L, et al. Use of health services in the last year of life and cause of death in people with intellectual disability: a retrospective matched cohort study. BMJ Open 2018;8:e020268. 10.1136/bmjopen-2017-020268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppewal A, Schoufour JD, van der Maarl HJK, et al. Causes of mortality in older people with intellectual disability: results from the HA-ID study. Am J Intellect Dev Disabil 2018;123:61–71. 10.1352/1944-7558-123.1.61 [DOI] [PubMed] [Google Scholar]
- 28.Cooper S-A, Allan L, Greenlaw N, et al. Rates, causes, place and predictors of mortality in adults with intellectual disabilities with and without Down syndrome: cohort study with record linkage. BMJ Open 2020;10:e036465. 10.1136/bmjopen-2019-036465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases Society of America/American thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durvasula S, Beange H, Baker W. Mortality of people with intellectual disability in northern Sydney. J Intellect Dev Disabil 2002;27:255–64. 10.1080/1366825021000029311 [DOI] [Google Scholar]
- 31.Patja K, Iivanainen M, Vesala H, et al. Life expectancy of people with intellectual disability: a 35-year follow-up study. J Intellect Disabil Res 2000;44 (Pt 5):591–9. 10.1046/j.1365-2788.2000.00280.x [DOI] [PubMed] [Google Scholar]
- 32.Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics 2017;139:e20164244. 10.1542/peds.2016-4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Backer JA, Wallinga J, Meijer A, et al. The impact of influenza vaccination on infection, hospitalisation and mortality in the Netherlands between 2003 and 2015. Epidemics 2019;26:77–85. 10.1016/j.epidem.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 34.Robertson J, Hatton C, Emerson E, et al. The impact of health checks for people with intellectual disabilities: an updated systematic review of evidence. Res Dev Disabil 2014;35:2450–62. 10.1016/j.ridd.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 35.Chadwick DD, Jolliffe J. A descriptive investigation of dysphagia in adults with intellectual disabilities. J Intellect Disabil Res 2009;53:29–43. 10.1111/j.1365-2788.2008.01115.x [DOI] [PubMed] [Google Scholar]
- 36.Robertson J, Chadwick D, Baines S, et al. Prevalence of dysphagia in people with intellectual disability: a systematic review. Intellect Dev Disabil 2017;55:377–91. 10.1352/1934-9556-55.6.377 [DOI] [PubMed] [Google Scholar]
- 37.Glover G, Ayub M. How people with learning disabilities die. Improving Health and Lives Learning Disabilities Observatory Durham, 2010. [Google Scholar]
- 38.Landes SD, Turk MA, Formica MK, et al. COVID-19 outcomes among people with intellectual and developmental disability living in residential group homes in New York state. Disabil Health J 2020;13:100969. 10.1016/j.dhjo.2020.100969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuypers M, Schalk BWM, Koks-Leensen MCJ, et al. Mortality of people with intellectual disabilities during the 2017/2018 influenza epidemic in the Netherlands: potential implications for the COVID-19 pandemic. J Intellect Disabil Res 2020;64:482–8. 10.1111/jir.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair E, Langdon K, McIntyre S, et al. Survival and mortality in cerebral palsy: observations to the sixth decade from a data linkage study of a total population register and national death index. BMC Neurol 2019;19:111. 10.1186/s12883-019-1343-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landes SD, Peek CW. Death by mental retardation? the influence of ambiguity on death certificate coding error for adults with intellectual disability. J Intellect Disabil Res 2013;57:1183–90. 10.1111/j.1365-2788.2012.01614.x [DOI] [PubMed] [Google Scholar]
- 42.Dunwoodie Stirton F, Heslop P. Medical certificates of cause of death for people with intellectual disabilities: a systematic literature review. J Appl Res Intellect Disabil 2018;31:659–68. 10.1111/jar.12448 [DOI] [PubMed] [Google Scholar]
- 43.Dupont A, Vaeth M, Videbech P. Mortality, life expectancy and causes of death of mildly mentally retarded in Denmark. Ups J Med Sci Suppl 1987;44:76–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-043658supp001.pdf (99.6KB, pdf)
bmjopen-2020-043658supp002.pdf (109.8KB, pdf)
bmjopen-2020-043658supp003.pdf (1.1MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.