Abstract
The hippocampus is a highly plastic brain structure supporting functions central to human cognition. Morphological changes in the hippocampus have been implicated in development, aging, as well as in a broad range of neurological and psychiatric disorders. A growing body of research suggests that hippocampal plasticity is closely linked to the actions of brain-derived neurotrophic factor (BDNF). However, evidence on the relationship between hippocampal volume (HCV) and peripheral BDNF levels is scarce and limited to elderly and patient populations. Further, despite evidence that BDNF expression differs throughout the hippocampus and is implicated in adult neurogenesis specifically in the dentate gyrus, no study has so far related peripheral BDNF levels to the volumes of individual hippocampal subfields. Besides its clinical implications, BDNF-facilitated hippocampal plasticity plays an important role in regulating cognitive and affective processes. In the current registered report, we investigated how serum BDNF (sBDNF) levels relate to volumes of the hippocampal formation and its subfields in a large sample of healthy adults (N = 279, 160 f) with a broad age range (20–55 years, mean 40.5) recruited in the context of the ReSource Project. We related HCV to basal sBDNF and, in a subsample (n = 103, 57 f), to acute stress-reactive change in sBDNF. We further tested the role of age as a moderator of both associations. Contrary to our hypotheses, neither basal sBDNF levels nor stress-reactive sBDNF change were associated with total HCV or volume of the dentate gyrus/cornu ammonis 4 (DG/CA4) subfield. We also found no evidence for a moderating effect of age on any of these associations. Our null results provide a first point of reference on the relationship between sBDNF and HCV in healthy mid-age, in contrast to patient or aging populations. We suggest that sBDNF levels have limited predictive value for morphological differences of the hippocampal structure when notable challenge to its neuronal integrity or to neurotrophic capacity is absent.
Keywords: Hippocampal volume, Brain-Derived Neurotrophic Factor (BDNF), Stress-reactive BDNF, Dentate gyrus, Hippocampal subfields, Neurotrophins
1. Introduction
The human hippocampus displays remarkable potential for structural plasticity throughout lifetime (Gonçalves et al., 2016; Leuner and Gould, 2010; Moreno-Jiménez et al., 2019). While debates on the extent of adult neurogenesis in the dentate gyrus of the hippocampus have recently resparked (see Kempermann et al., 2018 for a review), change in hippocampal structure is well documented as a result of aging (De Flores et al., 2015), chronic stress (Sapolsky, 2000), and in mood disorders (Cole et al., 2011; Malykhin and Coupland, 2015; McEwen et al., 2016). A growing body of research suggests a prominent role of brain-derived neurotrophic factor (BDNF) in mediating such change in hippocampal volume (HCV) (Erickson et al., 2012; Leal et al., 2015; Schmidt and Duman, 2007). BDNF is the most frequently expressed neurotrophin in the human brain, where its release is highly dependent on neuronal activity (Greenberg et al., 2009). Besides promoting neuronal growth and differentiation, the main function of BDNF is the regulation of synaptic plasticity (Huang and Reichardt, 2001; Lu et al., 2014) throughout the (adult) brain. Substantial research has focused specifically on its fundamental action in the hippocampus, including regulation of activity-dependent long-term potentiation (reviewed in Leal et al., 2017). Given the high degree of plasticity in the hippocampus, and its pivotal role in memory, learning, and stress- as well as affect regulation (Bartsch and Wulff, 2015), we aim to critically extend evidence on how serum levels of the plasticity promoter BDNF relate to individual differences in hippocampal structure in a healthy population, aged 20 to 55.
In humans, direct links between BDNF and hippocampal plasticity have mostly been studied in the context of psychopathology and aging: Mondelli et al. (2011) found that reduced levels of BDNF mRNA expression in psychotic patients as compared to healthy controls were associated with smaller left HCV. Relating peripheral levels of BDNF to HCV, Rizos et al. (2011) found a positive association of serum BDNF (sBDNF) levels with right HCV in psychotic patients, whereas both right and left HCV related to sBDNF levels in a small sample of schizophrenia patients (Song et al., 2014). In non-clinical elderly adults, two studies relating plasma levels of BDNF to HCV found no significant association (Driscoll et al., 2012; Kim et al., 2015a). Focusing on BDNF levels in serum rather than plasma, Erickson et al. (2010) showed a positive correlation with left HCV and marginally with right HCV in 142 participants aged 59 to 81 (mean age 66.5). sBDNF further mediated the age-related decline in HCV in this elderly sample (Erickson et al., 2010). Since the hippocampi of older adults undergo accelerated aging-related atrophy (Fraser et al., 2015; Raz et al., 2010), Erickson et al.’s study (2010) describes the relation between sBDNF and HCV in a context of heightened challenge to hippocampal integrity. Overall, available literature lacks a crucial baseline model on the relation between sBDNF and HCV in healthy, middle-aged adults.
The above in vivo imaging studies have investigated the plasticity of the hippocampal formation as a singular structure, averaging across cytoarchitecturally distinct subfields. Following the advancement of high-resolution imaging, studies are now increasingly distinguishing between hippocampal subfields, notably the cornu ammonis (CA1-4), dentate gyrus (DG), and subiculum (SUB) (Kulaga-Yoskovitz et al., 2015; Vos de Wael et al., 2018; Yushkevich et al., 2015). Subfield-specific functions in memory (Yassa and Stark, 2011), as well as differing susceptibility to the effects of aging and pathology (for a review see Small et al., 2011) have been identified. Mixed results have been found regarding subfield-specific atrophy in healthy aging (De Flores et al., 2015; Lowe et al., 2019), but recent evidence suggest strongest associations between age and DG volume loss (Adler et al., 2018). While it has been suggested that BDNF partly mediates aging-related volume loss in the hippocampus (Erickson et al., 2012; von Bohlen und Halbach and von Bohlen und Halbach, 2018), no study has so far investigated if, and how, peripheral BDNF levels are related to hippocampal subfield volumes. Histological post-mortem studies have visualized widespread BDNF gene expression (mRNA) and protein distribution in the adult human and rat hippocampus (Dugich‐Djordjevic et al., 1995; Murer et al., 1999), particularly in the DG (including hilus and fascia dentata) and in the CA (with varying prevalence in CA1-CA4) (Phillips et al., 1990; Webster et al., 2006; Yan et al., 1997; Zhang et al., 2007). There is also evidence that mRNA and BDNF-responsive TrkB receptors reach peak densities in the DG (Webster et al., 2006), which could suggest that BDNF is most active there. Further indirect evidence suggests that the DG could be particularly susceptible to the effects of BDNF: Human post-mortem studies have identified adult neurogenesis in the DG (Eriksson et al., 1998; Spalding et al., 2013), and reported stable numbers of neural progenitors and immature neurons in the DG across age (Boldrini et al., 2018; Moreno-Jiménez et al., 2019). Animal models suggest that BDNF stimulates proliferation of adult neural progenitors (Sairanen et al., 2005), and central BDNF administration has been found to induce synaptic plasticity in the rodent DG (Kuipers et al., 2016). While these studies examined central rather than peripheral levels of BDNF, it has been suggested that peripheral BDNF levels are highly correlated with hippocampal tissue levels in animals (Klein et al., 2011; Sartorius et al., 2009), and with BDNF levels in human cerebrospinal fluid (Pillai et al., 2010). In sum, although animal and post-mortem studies indicate the DG as particularly susceptible to the effects of BDNF, potential in vivo associations with human peripheral BDNF, in the context of aging or otherwise, remain to be investigated.
Complementing research on basal BDNF levels, several studies demonstrated that BDNF levels can be acutely elevated, and linked such increase to morphological and functional plasticity in the hippocampus (Griffin et al., 2011; Hall et al., 2000; Vaynman et al., 2004). Animal models reveal fast increase in hippocampal BDNF mRNA during stressful challenge (Marmigère et al., 2003), and meta-analytic evidence suggests single sessions of physical exercise to robustly stimulate sBDNF levels in humans (Szuhany et al., 2015). Recent research extends this evidence to other acute challenges by showing rapid increase in sBDNF during acute psychosocial stress (Linz et al., 2019; Meng et al., 2011).
While a growing body of research has highlighted the close interaction of BDNF and cortisol in modulating (particularly hippocampal) neuronal plasticity (Gray et al., 2013; Jeanneteau et al., 2018; Suri and Vaidya, 2013), evidence on the interplay of BDNF and cortisol regarding hippocampal plasticity in humans is so far limited to their long-term effects (Schoenfeld and Gould, 2012). The hippocampus is crucial in inhibiting the hypothalamic–pituitary–adrenal (HPA) axis (Ulrich-Lai and Herman, 2009), which is of particular importance in preventing BDNF downregulation and hippocampal atrophy caused by excess cortisol signaling (McEwen et al., 2015; Sapolsky, 2000). Animal studies further suggest that adult hippocampal neurogenesis can buffer stress responses (Snyder et al., 2011), and that hippocampal BDNF mediates resilience to chronic stress (Taliaz et al., 2011).
Indeed, we recently found an inverse relationship between sBDNF and cortisol dynamics during an acute psychosocial stress paradigm (Linz et al., 2019). In particular, higher sBDNF peak levels were associated with a steeper cortisol recovery. Higher reactive sBDNF levels thus appear to be associated with a well-calibrated stress regulatory system, which, in turn, critically implies proper functioning of the hippocampus in terminating the stress response (Herman et al., 2003). We therefore reason that sufficient acute BDNF-related trophic support, which we found to be in accord with a swift cortisol recovery (Linz et al., 2019), may limit the additive adverse effects of repeated stress exposure. Crucially, the hippocampal structure has been shown to be particularly vulnerable to such adverse effects (Bartsch and Wulff, 2015; Kim et al., 2015b). In line with this reasoning, findings from animal models highlight the neuroprotective role of activity-dependent BDNF release in adaptively responding to challenge (reviewed in Rothman and Mattson, 2013), and Leal et al. (2017) suggest that the interplay of BDNF and glucocorticoids may be decisive in determining adaptive or maladaptive plasticity in stress responses.
Examining reactive BDNF levels during acute challenge may signal individuals’ resilience or vulnerability to stressful challenge, which in turn may influence long-term neuronal integrity (Levone et al., 2015; McEwen, Nasca & Gray, 2016). This could provide an intriguing additional window into the link of sBDNF with HCV in humans besides basal sBDNF.
In sum, theoretical accounts and a growing body of evidence suggest that morphological changes in the hippocampus are linked to the actions of BDNF. However, evidence on the association between peripheral BDNF and HCV in vivo is lacking, particularly in healthy populations. No study has to date tested whether sBDNF differentially relates to the volumes of hippocampal subfields, and whether acutely stimulated and non-stimulated (basal) levels of sBDNF relate to HCV differentially. Finally, a potential age-moderation of this association has yet to be tested within a comprehensive age range.
1.1. Current study
In the current study, we aimed to systematically assess if and how basal and reactive sBDNF levels relate to total and subfield volumes of the hippocampus. We examined the association of basal sBDNF and HCV in a large sample of healthy adults (estimated N = 301, age 20–55, mean 40.7) and for the first time assessed associations with subfield volumes. Further, in a subsample of the same population (estimated n = 113), we examined associations with reactive sBDNF levels during an acute psychosocial stress challenge. Acute stress-reactive BDNF has never been related to HCV and may provide a unique insight into the implications of individual differences in acutely stimulated as opposed to basal BDNF levels on structural plasticity.
Data for the current study was collected as part of the ReSource Project (Singer et al., 2016), a longitudinal mental training study. The data assessed here were collected at the pre-intervention baseline measurement and in a control cohort that did not undergo any training. Part of this data is being examined in another project investigating longitudinal effects of mental training on basal sBDNF (Puhlmann et al., in prep.) and part was included in the above discussed study relating acute sBDNF and cortisol release (Linz et al., 2019). The research questions of the current work, however, focus on whether and how individual-differences in sBDNF concentrations are related to unobserved HCV data. None of our previous analyses examined this association (see also 2.2. data status and bias minimization).
1.2. Hypotheses
Based on previous findings from patients and aging populations (Erickson et al., 2010; Mondelli et al., 2011; Rizos et al., 2011; Song et al., 2014), we expected higher basal as well as reactive sBDNF levels to be positively associated with larger hippocampi bilaterally [hypotheses A1 (basal sBDNF) & A2 (reactive sBDNF)]. Further, based on indirect evidence implicating central BDNF particularly in plasticity of the DG (Boldrini et al., 2018; Eriksson et al., 1998), we hypothesized that basal and reactive sBDNF show the strongest associations with DG volumes [hypotheses B1 (basal sBDNF) & B2 (reactive sBDNF)]. Almost all evidence of direct associations between peripheral BDNF and HCV has been found in populations suffering from aging- or disease-related HCV loss. It is possible that individual BDNF concentrations are more relevant for HCV maintenance when neurodegenerative processes occur, such that associations between peripheral sBDNF and HCV also become stronger at older age. Therefore, we predicted that the association of sBDNF and total HCV, as well as DG volume, would be stronger for older participants [hypotheses C1 (basal sBDNF) & C2 (reactive sBDNF) for total HCV; hypotheses D1 (basal sBDNF) & D2 (reactive sBDNF) for DG volume]. Finally, we predicted that basal and reactive sBDNF would independently relate to HCV, such that HCV could best be predicted when using both measures jointly (hypothesis E; see Supplementary Methods A for the statistically formulated hypotheses and corresponding analyses).
2. Methods
2.1. Participants
Three hundred and thirty two healthy participants (age 20–55 years, mean: 40.7, SD: 9.2) were recruited in the context of the ReSource Project (Singer et al., 2016). Out of those, n = 242 participants were assigned to one of three training cohorts (TCs), and n = 90 to a no-training retest control cohort (RCC). All participants were screened for mental and physical health through questionnaires, including the ICD-10 Major Depression Inventory (MDI; Bech et al., 2001) and the Patient Health Questionnaire-D (PHQ-D; Löwe et al., 2002; Spitzer et al., 1999), using predetermined cut-off values as exclusion criteria (for more details see Singer et al., 2016; chapter 7). Additionally, participants underwent two clinical diagnostic interviews with a trained clinical psychologist [Structured Clinical Interview for DSM-IV Axis-I (SCID-I) (Wittchen and Pfister, 1997); SCID-II for Axis-II disorders (Gibbon et al., 1997)], and were excluded if they fulfilled the criteria for an Axis-I disorder within the past two years, or the criteria for an Axis-II disorder at any point in their life. Figure 1 shows the participant flow chart and final sample N for each analysis cluster. For an extensive description of baseline demographic characteristics, see Singer et al. (2016), Appendix C2.
Fig. 1.
Participant flow chart. Adapted from Singer et al., (2016): The Resource Project, chapter 7. The current figure combines numbers from two recruitment periods in 2012/2013 and 2013/2014. SCID, structured clinical interview for DSM-5;TC, training cohort; RCC, retest control cohort; TSST, Trier social stress test; TP, time point; QC, quality control.
The ReSource Project was registered under the title “Plasticity of the Compassionate Brain” with the Protocol Registration System of ClinicalTrials.gov (Identifier: NCT01833104, date of registration: 16/04/2013). The study was approved by the Research Ethics Committees of the University of Leipzig (ethic number: 376/12-ff) and the Humboldt University in Berlin (ethic numbers: 2013–02, 2013–29, 2014–10).
2.2. Data collection procedure
Structural MRI scans and blood for the assessment of basal BDNF used in the current study was collected at study baseline (T0) prior to any intervention for the training cohorts, or in a control cohort that did not undergo any training. Control cohort participants provided basal sBDNF measures at several time points (T0-T3). For the current study, we used the MRI scan and basal sBDNF sample that was collected closest in time to the respective participant's reactive sBDNF sample, which was assessed at only one time-point throughout the study. Specifically, on a separate testing day, blood samples for the measurement of reactive BDNF were collected during an acute laboratory stress challenge. In brief, blood samples were drawn 50 min before stress induction (baseline measure) and immediately after the termination of the stress test (post-stress sample at approximately 15 min after stressor onset). A more detailed study protocol is reported in Engert et al. (2017). In contrast to two previous studies conducted in the context of the ReSource Project, which reported on a range of psychological and physiological stress markers after acute challenge (Engert et al., 2017), and the relation between BDNF and cortisol stress reactivity and recovery (Linz et al., 2019), we here exclusively focused on the reactive sBDNF response. Thus, differences in the respective study samples exist due to different missing data points for different physiological markers.
2.3. Stress induction
Participants underwent the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993). The TSST is the most frequently used psychosocial laboratory stress protocol to reliably elicit both a physiological and psychological stress response (Allen et al., 2014). Following an anticipation phase (15 min in the current study), participants completed a mock job-interview (5 min) and a difficult mental arithmetic task (5 min) in the presence of a committee of alleged behavioral analysts. Key features for the efficacy of the TSST are socio-evaluative threat, unpredictability, and uncontrollability (Dickerson and Kemeny, 2004).
2.4. BDNF assay
To determine serum levels of brain-derived neurotrophic factor (sBDNF), blood (5.5 ml) was collected into serum vacutainers (Sarstedt), allowed to clot for 30 to 45 min, and subsequently centrifuged at 3500 rpm for 15 min. Serum was frozen at −80°C until assay. BDNF concentrations in serum were determined (at the Department of Clinical Biochemistry, “Aghia Sophia” Children's Hospital, Athens, Greece) with a quantitative sandwich enzyme immunoassay technique (R&D Systems, Inc. Minneapolis, MN, USA), using the recommended buffers, diluents and substrates. Optical density of the color reaction was read using a microtiter plate reader set at 450 nm. sBDNF concentrations (in pg/ml) in each sample were calculated according to a standard curve. According to the manufacturer, the minimum detectable dose (MDD) of total sBDNF ranged from 0.372–1.35 pg/mL, with a mean value of 0.997 pg/mL. The intra- and inter-assay coefficients of variation of <7% were determined by duplicate analysis of > 6% of randomly selected samples.
2.5. MRI acquisition
T1-weighted images were acquired on a 3T Siemens Verio scanner (Siemens) with a 32-channel head coil, using a three-dimensional (3D) magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (176 sagittal slices; repetition time (TR), 2300 ms; echo time (TE), 2.98 ms; inversion time (TI), 900 ms; flip angle, 7°; field of view (FOV), 240 × 256 mm2; matrix, 240 × 256; 1 × 1 × 1 mm3 voxels). All data was collected using the same Imaging hardware and console software (Syngo B17).
2.6. Processing of structural data
Based on the available high-resolution T1-weighted images, we segmented CA1-3, CA4/DG, and subiculum (SUB) using a patch-based algorithm in every subject (for details see Caldairou et al., 2016). In brief, the algorithm employs a population-based patch normalization relative to a template library (Kulaga-Yoskovitz et al., 2015), which offers good time and space complexity. In previous validations, this algorithm has shown high segmentation accuracy of hippocampal subfields (Caldairou et al., 2016), and in detecting hippocampal subfield pathology in patients with epilepsy (Bernhardt et al., 2016). It was furthermore demonstrated that these representations can be used to probe subregional functional organization of the hippocampus (Vos de Wael et al., 2018). Hippocampal volumes were estimated based on T1w data that were linearly registered to MNI152, such that intracranial volume was implicitly controlled for.
2.7. Segmentation quality control
To assess successful hippocampus segmentation, an initial quality check was conducted by two independent raters, R.L. and L.P. Both raters were blind to participant characteristics including age, sex, and availability and concentration of sBDNF samples. Each segmentation was rated for quality on a scale of 1–10, with points being subtracted depending on the severity of detected flaws. One point was subtracted for minor flaws, e.g. part of a segmentation extends slightly beyond the hippocampal boundary, or does not cover a small aspect of the hippocampal formation. Two points were subtracted for medium flaws, e.g. gaps between subfield segmentations. Finally, major flaws immediately qualified for resampling, and included e.g. one or more subfield segmentations being clearly misplaced. Given a minimum of 70% inter-rater reliability, segmentation ratings were then averaged and evaluated, with scores of 5 and lower qualifying for reprocessing with the algorithm. Following this second round of processing, segmentations were rated again. Any remaining segmentations with average scores lower than 5 were excluded from the analysis. We had initially planned to add quality ratings of the included volumes as regression weights in follow-up analyses (see 2.9.2. Significance testing).
2.8. Data status and bias minimization
Our two predictor variables basal and reactive sBDNF have been previously observed directly or indirectly as dependent variables. Basal sBDNF was examined directly as part of a project investigating how sBDNF is affected by different types of contemplative mental training (Puhlmann et al., in prep). Reactive sBDNF has been observed indirectly as part of a project investigating how sBDNF levels during the TSST relate to salivary cortisol, and whether this association is affected by different types of contemplative mental training (Linz et al., 2019). HCV data, including total volume and subfield volumes, was unobserved. The current work focuses on the associations between individual differences in basal or reactive sBDNF and HCVs. Previous observation of intervention effects on basal or reactive sBNDF levels did not inform on the relation to the unobserved hippocampal data. To the best of our knowledge, bias in the form of model overfitting could therefore only be derived from including individual-difference variables in our model that have previously been observed together with sBDNF data. To avoid such bias, we adopted the analysis approach by Erickson et al. (2010), limiting the covariates of our main analyses to age and sex.
2.9. Statistical analysis
2.9.1. Preprocessing of variables
The total left and right HCVs were calculated as the sum of the respective subfields. Reactive sBDNF was calculated as the proportion of (pre-TSST) baseline sBDNF. For all measures, data points were excluded if clearly biologically implausible, meaning outside the range of any previously reported values, and thus suggested technical errors. If model residuals suggested the presence of influential cases, we defined any sBDNF and HCV values that diverged more than three standard deviations from the mean as outliers and winsorized them to the respective upper or lower boundary of three standard deviations, avoiding data loss (Dixon, 1960). Additional data processing, for example by means of a log transformation, were considered depending on residual distributions.
2.9.2. Significance testing
Statistical models were calculated in the statistics package R (version 3.5.1, R Core Team 2018). Hypotheses were tested by means of frequentist statistics, using multivariate linear models, which were fit using the function 'lm’ of the r core package ‘stats’. Our model predictor variables of interest were basal sBDNF, reactive sBDNF, and age. We additionally included sex as a covariate in all analyses, since previous studies have found sex to covary with BDNF (Bus et al., 2011), and it was included in Erickson and colleagues’ model. The model testing for a simple relationship of (basal / reactive) sBDNF with HCV (hypotheses A1 & A2) or subfield volumes (hypotheses B1 & B2) thus included the following terms:
HCVi = ß0 + ß1*agei + ß2*sexi + ß3*sBDNF(basal / reactive)i, where i = subject ID, and ß0 = the intercept
To evaluate a possible moderating role of age on the relationships of basal or reactive sBDNF with total HCV (hypotheses C1 & C2), as well as subfield volumes (hypotheses D1 & D2), we added the interaction term sBDNF (basal / reactive) x age to the model. To further address hypotheses B1 / B2 and D1 / D2, we had initially planned to compare the strength of potential associations of basal or reactive sBDNF with DG to the association with CA and SUB (see Stage 1 protocol for details).
Finally, if either total HCV or any of the subfields’ volume had been significantly related to basal as well as reactive sBDNF in the analyses described above, we would have examined whether a combination of both measures would best predict HCV (hypothesis E, see Stage 1 protocol). For a model-by-model list of all prespecified analyses and corresponding hypotheses, see Supplementary Methods A.
Following the detection of any significant associations with sBDNF, we had planned to check the robustness of results by repeating significant analyses with additional control terms. We had planned to apply weighted regressions to account for segmentation quality. Automated segmentations as applied here increase the reproducibility of research, but also remain prone to small inaccuracies. To validate that any potentially observed associations were not driven by systematic segmentation errors, we had planned to rerun all significant analyses with the addition of the quality control ratings as weights. Moreover, in the analysis of basal sBDNF, we had planned to control for the time of day at which blood was sampled, to account for potential effects of diurnal variation on resting levels of sBDNF (Bus et al., 2011; Piccinni et al., 2008). We did not expect sBDNF reactivity to be influenced by diurnal fluctuations. Finally, as outlined above, there is evidence that BDNF and cortisol interact in modulating hippocampal plasticity. We previously identified an antagonistic relationship between sBDNF and cortisol dynamics (Linz et al., 2019) in the context of the TSST. Therefore, if identifying a significant association with reactive sBDNF and HCV in any of the proposed model comparisons, we had planned to investigate whether the explained variance was independent of individual differences in acute cortisol dynamics. To this end, we would have added participants’ cortisol recovery slopes as a covariate to the respective models and repeat the comparisons (see also Supplementary Material). The cortisol recovery slope was selected as an indicator of acute cortisol dynamics because we previously found that it relates to acute peak sBDNF, which is not equal but related to the reactive sBDNF measure we propose to analyse here.
To ensure adequate interpretation of non-significant outcomes (i.e. non-significant p-values in null hypothesis tests), we employed equivalence tests (Wellek, 2010). After defining effect size boundaries deemed equivalent to the absence of a meaningful effect, equivalence tests allow statistical inference on whether an observed effect exceeds a predefined effect size of interest, rather than testing if it equals zero. As recent papers propose that - in the absence of a theoretical limit denoting an effect as meaningful - feasible equivalence boundaries can be based on effect size conventions (Counsell and Cribbie, 2015; Lakens, 2017), we predefined our equivalence boundaries corresponding to the lower end of what is conventionally referred to as small effect sizes (r ≤ .1; Cohen, 1992). Effect size calculation and equivalence tests were carried out using the r package ‘effectsize’ (Ben-Shachar and Lüdecke, 2020).
2.9.3. Outcome-neutral validation tests
Several conditions had to be met to ensure that the proposed statistical analyses could successfully test our research questions. The statistical assumptions of linear models are based on residual distributions. Thus, we only interpreted model summaries after checking residuals for independence, normality of distribution, and equality of variance, and after ruling out potential variance inflation due to (multi)collinearity of predictors.
We planned to analyse total HCV by combining left and right volumes, since we expected to find the same pattern of associations with sBDNF across both hemispheres. This assumption was tested separately for basal and reactive sBDNF, by comparing the respective correlation with lHCV to the correlation with rHCV. To this end, we constructed confidence intervals (CIs) of the difference in correlations using a formula that adjusts for overlapping correlations (Zou, 2007), as implemented in the function ‘cocor()’ of the r package ‘cocor’ (Diedenhofen and Musch, 2015). Left and right HCVs were combined into total HCV unless the above test indicated a difference in correlations (i.e., zero is not in the CI). Based on simulations, this analysis was sufficiently powered (>90%) to detect a difference between correlations that diverge by r ≥ 0.05 for basal sBDNF, or r ≥ 0.08 for reactive sBDNF (see Supplementary Figure S2).
Outcome neutral criteria that have been examined in previous studies included a successful induction of subjective stress and release of the physiological stress marker cortisol (Engert et al., 2017), as well as acute reactivity in sBDNF after stressful challenge (Linz et al., 2019). Below we outline additional outcome-specific criteria, which determined how the subfield analysis was conducted.
2.9.4. Multiple comparisons and outcome-specific analysis criteria
The current study related two measures of sBDNF to HCV and its subfields through a substantial set of analyses. We used a threshold of alpha ≤ 0.05 for the majority of analyses, but applied Bonferroni family-wise error rate corrections to control for false positives due to multiple comparisons within each family of tests that addressed the same hypothesis (Shaffer, 1995; see Supplementary Methods A).
First, if analysing the relation of basal and/or reactive sBDNF to left and right HCVs separately, we had planned to lower the alpha threshold to ≤ 0.05/2 for each analysis performed on both hemispheres, reflecting Bonferroni correction. Further, given the detection of an overall association between basal or reactive BDNF and total HCV, we had planned to subsequently analyse the relationship of the respective sBDNF measure with all three subfields. While we expected the strongest relationship with CA4/DG volume, CA1-3 or SUB could have been associated with basal or reactive sBDNF, particularly in the presence of an association with total HCV. To avoid false positive due to multiple analyses of the likely related subfield volumes, the Bonferroni corrected alpha threshold for these analyses would have been set at ≤ 0.05/3. Alternatively, if finding no significant main association between sBDNF and the left or right HCV, we had planned to subsequently only examine the relation of sBDNF to CA4/DG volume. A significant outcome of this analysis would have been interpreted as evidence for a relation between basal or reactive sBDNF and HCV, even in the absence of an association with total HCV. In this case, the Bonferroni corrected alpha threshold would have been set to ≤ .05/2 for providing an additional opportunity to detect an association between sBDNF and HCV (in relation to the analysis of total HCV). Given a significant relationship of sBDNF with CA4/DG volume, we had planned to use the above proposed interaction model to assess whether this relationship was significantly stronger than sBDNF associations with CA1-3 or SUB. Results were not corrected for multiple comparisons across the two measures of BDNF, which each addressed independent research questions. Basal and reactive BDNF represent distinct temporal dynamics, which we expected to differentially relate to HCV. While basal sBDNF levels were sampled at rest, reactive sBDNF levels indicated acute activity-dependent within-subject changes during challenge. Basal sBDNF levels should thus provide a relatively stable measure of general BDNF concentration, while reactive sBDNF levels can inform BDNF short-term dynamics under challenge. In line with this assumption, basal and reactive BDNF levels were uncorrelated (r = 0.078, p > 0.25).
Finally, age moderation analyses complemented the analyses of simple associations, in that they were designed to assess whether associations with sBDNF were more accurately represented as a function of participant age.
2.9.5. Power analysis
Power was calculated separately for all analyses testing the relationships between basal or reactive sBDNF and total HCV (hypotheses A1, A2, C1 & C2). Power for subfield analyses was not separately calculated, since we could not identify and rely on any previous investigation of sBDNF to subfield volume association.
We opted for a custom simulation approach to adequately represent the potentially interdependent relation of age, sBDNF and HCV, and to be able to combine the full information of the already collected data with simulated hippocampal data. Total HCV was calculated as the sum of separately simulated lHCV and rHCV (see Table 1). Effect sizes were based on Erickson et al. (2010), who detected r = 0.19 and r = 0.14 correlations of basal sBDNF with lHCV and rHCV, respectively. Stronger associations with basal sBDNF have been detected in healthy control groups of smaller studies, i.e., a correlation of r = 0.511 with lHCV in 21 controls (mage = 31; Rizos et al., 2011; correlation with rHCV not reported), and r = 0.231 and r = 0.261 correlations with left and right HCV, respectively, in 30 controls, which were, however, not significant (mage = 24.3; Song et al., 2014). Considering potential publication bias and the comparably small sample sizes of the latter studies, we chose conservative effect size estimates based on Erickson et al. (2010), who reported the smallest effect that was also derived from the largest sample of healthy, albeit older, individuals. Due to lack of studies from which we could derive estimated associations of reactive sBDNF with HCV, we employed the above effect estimates for our simulations of basal as well as reactive sBDNF effects. We considered it possible that compared to Erickson and colleagues’ (2010) findings, we would encounter smaller correlations between HCV and age, given our younger sample. To address this possibility, we modeled expected power based on three different age correlations with HCV (rs for lHCV/rHCV = -0.37/-0.40, -0.32/.0.35, -0.27/.0.30), where the respectively largest associations correspond to Erickson's findings.
Table 1.
Sample descriptives for data simulation (SD in parentheses). Mean (+/-SD) and correlation coefficients of hippocampal volumes are based on work by Erickson and colleagues (2010) and were kindly provided by Prof. Erickson. Data for age, sBDNF and sex were derived from our already collected data. lHCV, left hippocampal volume; rHCV, right hippocampal volume; sBDNF, serum brain-derived neurotrophic factor; SD, standard deviation; BL, baseline (pre TSST).
| Basal BDNF sample | Reactive BDNF sample | |
|---|---|---|
| N | 301 (58% f) | 113 (58% f) |
| HCV (cm3) | ||
| lHCV | 4.82 (0.745) | 4.82 (0.745) |
| rHCV | 4.92 (0.690) (0.915 corr. with lHCV) |
4.92 (0.690) (0.915 corr. with lHCV) |
| Age (yrs) | ||
| Total | 40.48 (9.31) | 39.98 (8.85) |
| f | 41.83 (8.91) | 41.21 (8.91) |
| m | 38.63 (9.56) | 38.38 (9.56) |
| sBDNF | (pg / mL) | (prop. BL) |
| Total | 24755 (5998) | 1.078 (0.186) |
| f | 25022 (6268) | 1.074 (0.171) |
| m | 24388 (6268) | 1.088 (0.171) |
Table 1 shows the parameters used in the simulations. Summary statistics for HCV were based on Erickson et al., 2010. Summary statistics for age, sBDNF and sex describe our data collected before Stage 1 submission (see Supplementary Methods B for a detailed description of the simulation).
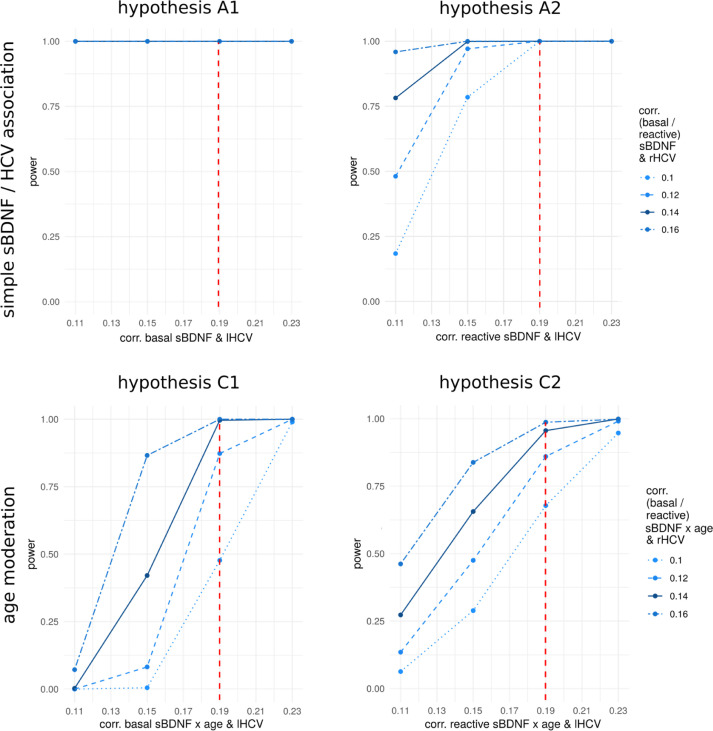
Using an alpha level of 0.05, we calculated an estimated power of 100% to detect a simple association of basal sBDNF with HCV (Fig. 2, hypothesis A1), and of reactive sBDNF with HCV (Fig. 2, hypothesis A2), even when modelling smallest age to HCV correlations. For the age moderation analysis, we assumed fully moderated effects of the same sizes (i.e., r = 0.19 for lHCV and r = 0.14 for rHCV). We calculated an estimated power of 100% to detect an age moderation of basal sBDNF to HCV association for all age to HCV correlations (Fig. 2, hypothesis C1), and power of 95.6% / 88.5% / 80.0% to detect an age moderation of reactive sBDNF to HCV association, depending on the strengths of the correlation between age and HCV (larger correlations corresponding to greater power) (Fig. 2, hypothesis C2). The simulation code and model summary data used to generate Fig. 2 are publicly shared alongside the Stage 1 protocol.
Fig. 2.
Power to reject the null hypothesis in hypotheses A1, A2, C1, and C2, based on different strengths of association with lHCV and rHCV. Displayed power curves were calculated based on age to HCV associations of r = -0.37 (lHCV) and r = -0.40 (rHCV), as detected by Erickson and colleagues (2010). Considering the relative uncertainty regarding which effect sizes may be expected in our hypotheses, particularly regarding age moderation effects, we simulated power for a range of possible effect sizes. The plots illustrate to which magnitude of effects our analyses will be sensitive, given our fixed prospective sample sizes of 301 (basal sBDNF) and 113 (reactive sBDNF). Red vertical dashed lines indicate the assumed effect strength for lHCV (r = 0.19), and solid dark blue lines correspond to the assumed effect strength for rHCV (r = 0.14). While data was modelled separately for lHCV and rHCV, the simulated models evaluated effects on total HCV. The code as well as the model summary data used to generate the above images can be accessed at the Open Science Framework (https://osf.io/3p7da/). lHCV, left hippocampal volume; rHCV, right HCV; sBDNF, serum brain-derived neurotrophic factor.
2.9.6. Stage 1 protocol
The Stage 1 protocol was uploaded after approval and is available on the Open Science Framework at URL https://osf.io/cdqwu.
3. Results
3.1. Quality control
Ratings of segmentation quality following the above-specified procedure were sufficiently consistent between the two independent raters (L.P. & R.L.). Of the initial 301 segmentations, 22 segmentations (7.3 % of the total sample) did not meet the predefined quality standard. Reprocessing and subsequent reevaluation did not result in improved ratings of any of these cases, such that they were excluded from the current analysis.
Although cook's distance values were well below 1 in all models, visual inspection indicated the presence of two potentially influential cases in the analysis of basal sBDNF and one in the analysis of reactive sBDNF. Further data exploration revealed one case with unusual high HCV and one with high basal sBDNF, which were both winsorized, in line with the prespecified procedure. None of the results were affected by this transformation.
3.2. Descriptive statistics
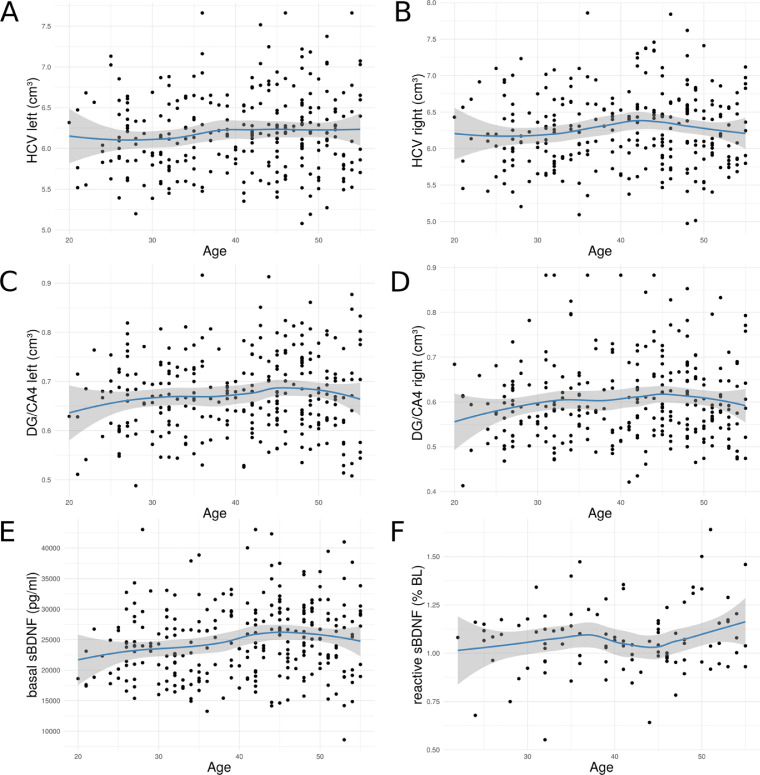
Table 2 displays the descriptives for basal and reactive sBDNF samples. Before hypothesis testing, we explored the association of sBDNF and HCV with our main moderator variable, age. In contrast to findings of Erickson et al. (2010), we observed no association of HCV with age (r(277) = .082, p = .172) (see Figure 3). Basal sBDNF level correlated positively (r(277) = .157, p = .008), while reactive sBDNF levels were not significantly associated with age (r(101) = .152, p = .123). Age associations with HCV and basal sBDNF appeared to become more negative towards older age (median age split, younger: r = .08, p = .32 (HCV), r = .11, p = .18 (basal sBDNF); older: r = -.11, p = .19 (HCV), r = -.09, p = .29 (basal sBDNF)). Independent of sBDNF, females displayed significantly higher HCV in the full sample (β = 357.7, t = 3.07, p = 0.002).
Table 2.
Descriptives of the analysis samples with basal (left) or reactive (right) sBDNF measures. Values shown as mean (+/-SD); HCV, hippocampal volume; DG/CA4, dentate gyrus / cornu ammonis 4; CA, cornu ammonis 1-3; SUB, subiculum; sBDNF, serum brain-derived neurotrophic factor; f, female; m, male.
| Basal sBDNF sample | Reactive sBDNF sample | |
|---|---|---|
| N | 279 (160 f) | 103 (57 f) |
| Age (yrs) | ||
| Total | 40.54 (9.33) | 40.46 (8.84) |
| f | 41.98 (9.00) | 41.82 (8.99) |
| m | 38.61 (9.45) | 38.76 (8.44)] |
| HCV (cm3) | ||
| Total | 12.453 (0.933) | 12.396 (0.973) |
| Left | 6.189 (0.480) | 6.154 (0.508) |
| Right | 6.264 (0.511) | 6.2412 (0.520) |
| DG/CA4 (cm3) | ||
| Total | 1.278 (0.933) | 1.283 (0.157) |
| Left | 0.674 (0.081) | 0.675 (0.087) |
| Right | 0.604 (0.091) | 0.609 (0.096) |
| CA (cm3) | ||
| Total | 7.479 (0.661) | 7.422 (0.634) |
| Left | 3.656 (0.350) | 3.616 (0.348) |
| Right | 3.824 (0.372) | 3.807 (0.353) |
| SUB (cm3) | ||
| Total | 3.699 (0.362) | 3.697 (0.398) |
| Left | 1.860 (0.197) | 1.868 (0.212) |
| Right | 1.838 (0.212) | 1.830 (0.215) |
| sBDNF (pg /mL) | ||
| Total | 24865.42 (6014.17) | 1.08 (0.19) |
| f | 25109.32 (6254.81) | 1.07 (0.20) |
| m | 24537.49 (5684.13) | 11.08 (0.17) |
Fig. 3.
Association of age with total HCV (A, B), DG/CA4 volume (C, D) and with basal (E) and reactive (F) sBDNF levels. Blue lines indicate local polynomial (LOESS) regression, grey area represents 95% CIs. HCV, hippocampal volume; DG, dentate gyrus; CA, cornu ammonis 1-3;sBDNF, serum brain-derived neurotrophic factor; LOESS, Locally Weighted Scatterplot Smoothing, implemented with the function ‘loess’ of the RCore package.
3.3. Confirmatory (pre-registered) analyses
In accordance with the pre-specified analysis plan, we first tested whether associations of sBDNF levels with left and right HCV differed significantly. We found no such indications (95% CIs of differences in correlations, basal sBDNF: -0.113 to 0.045; reactive sBDNF: -0.116 to 0.170) and consequently analysed both hemispheres jointly.
3.3.1. Association of sBDNF with total hippocampal volume (hypotheses A1-A2, C1-C2)
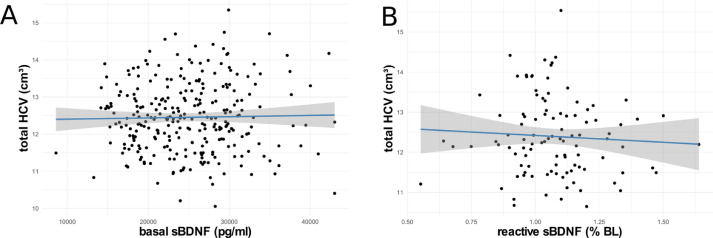
Linear models revealed no significant association of basal sBDNF (hypothesis A1) or reactive sBDNF (hypothesis A2) with total HCV (A1: β = 0.002, F(1,275) = 0.030, p = .865, ω2p = -.003; A2: β = -394.93, F(1, 99) = 0.530, p = .470, ω2p = -.005, see Fig. 4). Similarly, in testing a potential age moderation of the relationships of basal and reactive sBDNF with HCV (hypotheses C1 and C2), linear models revealed no significant interaction of age with basal (C1: β = 0.001, F(1, 274) = 0.268, p = .605, ω2p = -.003) or reactive sBDNF (C2: β = -47.90, F(1, 98) = 0.638, p = .427, ω2p = -.004) in predicting HCV.
Fig. 4.
Association of basal sBDNF (A) and reactive sBDNF (B) with total HCV. Blue lines indicate linear regression, grey areas 95% CIs. HCV, hippocampal volume; sBDNF, serum brain-derived neurotrophic factor.
3.3.2. Association of sBDNF with DG/CA4 (hypotheses B1-B2, D1-D2)
Since the above analyses revealed no significant simple or age-moderated associations of sBDNF with total HCV, subfield analyses were limited to DG/CA4, as specified in the analysis plan. Accordingly, hypotheses B1-2 and D1-2 only examined potential associations with DG/CA4, rather than comparing associations across subfields.
No evidence was found for a significant association of DG/CA4 subfield volume with basal (hypothesis B1) or reactive (hypothesis B2) sBDNF levels (B1: β = 0.002, F(1, 275) = 1.657, p = .199, ω2p = .002; B2: β = -23.40, F(1,99) = 0.078, p = .781, ω2p = -.009).
Similarly, linear models revealed no significant interaction of age with basal (hypothesis D1; β < < 0.001, F(1, 274) = 0.072, p = .789, ω2p = -.003) or reactive (hypothesis D2; β = -13.69, F(1, 98) = 2.218, p = .140, ω2p = .012) sBDNF in predicting DG/CA4 volumes.
3.3.3. Equivalence testing
Equivalence tests were performed to facilitate interpretation of non-significant null hypothesis significance tests. By statistically comparing observed effects to pre-specified effect size boundaries, this approach can provide support for the absence of – or equivalence to – a predetermined ‘meaningful’ effect (Lakens, 2017). We specified a small effect size as meaningful, and consequently tested whether observed effects were statistically equivalent to both the upper and lower boundaries of corresponding partial omega squared values (ω2p >= .01 and ω2p >= .06), which correspond to a very small and a small-to-medium effect (Field et al., 2012; Kirk, 1996). Regarding an association of basal sBDNF with total HCV (hypothesis A1), as well as an age-moderated association of basal sBDNF with DG volume (hypothesis D1), there was statistical support for the absence of even a very small meaningful effect. Tests against the upper boundary supported the absence of a small-to-medium effect for all hypotheses except hypothesis D2. Accordingly, hypothesis D2 was the only tested association that remained undetermined, meaning that the presence of a meaningful effect equivalent to the specified boundaries could not be statistically ruled out (for detailed results of equivalence testing see Supplementary Materials C).
3.4. Exploratory analyses
3.4.1. Joint association of basal and reactive sBDNF with hippocampal volume
Since none of the specified hypotheses revealed significant associations, we could not pursue hypothesis E. Instead, in an exploratory analysis we examined whether jointly modelling basal and reactive sBDNF could explain variance in total HCV or DG/CA4 volume compared to a model lacking both variables; this was not the case (total HCV: F(2, 98) = 0.805, p = .450; DG/CA4: F(2, 98) = 0.998, p = .373).
3.4.2. Non-linear relationship of age with HCV
Confirmatory analyses showed no linear relationship of age with HCV in the present sample. Given the considerable age range, we additionally explored a potential non-linear association of age and HCV. To this end, we compared the fit of the above reported linear model with a model additionally including a quadratic term for age. Adding a quadratic age term did not significantly improve model fit (F (1,276) = 1.0434, p = .31).
3.4.3. Estimation of HCV with Freesurfer 6.0
For completeness and to facilitate comparability of the present results with future and past investigations, we additionally estimated total HCV and subfield volumes by means of a Freesurfer tool for automated segmentation (Iglesias et al., 2015) for all T1 scans included in the main analyses. Results with Freesurfer-based estimates of HCV were consistent with the above confirmatory analyses (section 3.3) and are provided in Supplementary Materials D.
4. Discussion
A growing body of research suggests a prominent role of brain-derived neurotrophic factor (BDNF) in mediating structural change in the hippocampus. Published studies investigating associations between peripheral BDNF levels and hippocampal volume (HCV) in vivo in humans so far focused exclusively on psychopathological or elderly populations. To provide a first baseline for the relationship of serum BDNF (sBDNF) with HCV in the absence of pronounced hippocampal atrophy, the present study investigated this association in a large sample of healthy, young to middle-aged adults. In aiming to further extend the literature, we also investigated associations with hippocampal subfields and, above and beyond basal BDNF levels, examined BDNF dynamics in response to an acute psychosocial laboratory stressor.
Diverging from outcomes with psychopathological and aging populations (Erickson et al., 2010; Rizos et al., 2011; Song et al., 2014), we find no support for an association between basal sBDNF levels and HCV in healthy adults aged 20 to 55. Despite the particular significance of BDNF for dentate gyrus (DG) plasticity (Boldrini et al., 2018; Zagrebelsky et al., 2018), there was also no evidence for a specific association with volume of the DG/CA4 subfield. Furthermore, since previous findings in the present participants showed that sBDNF dynamics inversely related to endocrine responding during acute stress (Linz et al., 2019), we additionally tested the possibility that stress-reactive changes in sBDNF predicted variation in HCV. Like basal levels, stress-reactive changes in sBDNF were unassociated with total HCV and the DG/CA4 subfield. By additionally drawing on equivalence tests, these analyses provide statistical support for the absence of sBDNF to HCV associations that are at least small-to-medium in size in middle-aged healthy adults.
As outlined in the introduction, the present investigation was designed to contribute to a more complete picture of the conditions under which BDNF to HCV associations emerge. In this regard, the above results do not contradict previous findings in patient populations and older adults. Rather, they indicate that sBDNF to HCV associations of meaningful size are only observable under conditions of heightened challenge to neuronal integrity. In other words, a link between sBDNF levels and HCV may more likely be found if hippocampal atrophy – driven by aging- or disease-related neurodegenerative processes – cannot be compensated by an intact neurotrophic system. Accordingly, the here identified lack of an association may signal a ceiling effect in sBDNF availability, and that trophic support may be sufficient to protect hippocampal integrity in the targeted population. Alternatively, the null findings may indicate that the present sample experienced so little age-related atrophy that even individuals with relatively lower BDNF levels did not yet display HCV decline.
Age moderation analyses targeted the role of BDNF on progressive age-related challenge. In the older half of the present sample, age associations with HCV and basal BDNF were negative, but remained non-significant. Perhaps reflecting this limited age-related decline, moderation analyses revealed that age did not amplify BDNF to HCV associations. These observations align with longitudinal and cross-sectional evidence on the structural hippocampus development, which highlights a different trajectory in comparison to many linearly declining cortical areas. The hippocampus shows prolonged development at least until the age of 30 (Ostby et al., 2009), and thereafter reaches a plateau of relative stability in mid-life, characterized by only subtle average volume loss before the age of 50 (Fjell et al., 2013; Raz et al., 2004). The stable phase is followed by a non-linear, progressively accelerating decline for which a recent, exceptionally high-powered study further identified marked sex differences: while men's hippocampi started degeneration earlier, women showed a later onset, but particularly accelerated loss rates after the critical age of 60 years (Nobis et al., 2019) – a range that was not captured by the current study.
An overall positive association of sBDNF and age, as found in our data, has previously been reported (Naegelin et al., 2018), while other studies in comparable age ranges found no association (Lommatzsch et al., 2005; Trajkovska et al., 2007), or described a BDNF decline starting around the age of 40 (Katoh-Semba et al., 2007). In later life, evidence converges in showing a progressively negative relationship (Shimada et al., 2014; Ziegenhorn et al., 2007).
Based on both previous and the present results, middle-aged adults do not seem to experience marked age-related hippocampal atrophy or BDNF decline, which we suggest may be a prerequisite for the detection of BDNF to HCV associations. In our sample, neither sBDNF nor HCV showed a significant negative association with age, even when selectively examining the older half of participants (median split at age 41). One explanation for these results may lie within the specific participant sample, which assembled an unusually healthy set of individuals, despite being recruited from the general population. Because this investigation relied on baseline data from a longitudinal intervention study, recruitment involved a comprehensive screening procedure against health-related and lifestyle risk factors such as smoking and alcohol abuse, as well as drug or medication intake. Additionally, the present sample displayed a socio-economic status well above average, reflected in both substantially higher net income and higher education than local averages (Singer et al., 2016; Statistisches Bundesamt, 2019). Such socio-demographics are an increasingly recognized predictor of brain health (Farah, 2017, 2018; Johnson et al., 2013; McEwen and Gianaros, 2010) and relate to HCV (Janowitz et al., 2014). Thus, the present sample was likely more homogenous and healthier than typical population-based samples, reducing variance in BDNF and HCV. Potentially reflecting this absence of challenge to neuronal integrity, average basal BDNF levels as well as HCV of the present participant sample can be considered at the upper end of population-based scores (Nobis et al., 2019; Polacchini et al., 2015). It should be noted that there are currently no valid BDNF reference values (Naegelin et al., 2018), and between-study comparisons suffer from considerable variability in respective sample collection, treatment and assay (Polacchini et al., 2015). However, lowest sBDNF levels in our study were well above previously found minima (Polacchini et al., 2015). Overall, our sBDNF data show a relatively lower variance and range compared to Erickson et al. (2010) and other studies in comparable age ranges (Lommatzsch et al., 2005; Yoshimura et al., 2016).
Our study was the first to explore reactive sBDNF levels in relation to HCV. Based on our previous finding of an acute inverse relation of sBDNF and cortisol (Linz et al., 2019), we raised the possibility that this acute pattern may reflect the established long-term antagonism of BDNF and cortisol regarding neuronal plasticity (Gray et al., 2013). In more detail, we argued that increased BDNF peaks during acute stress, combined with fast subsequent cortisol recoveries, may reflect a well-attuned HPA axis regulation, which critically implies an intact hippocampus. Acute increases in sBDNF during challenging situations may provide the necessary short-term trophic support counteracting potential neuronal strain induced by excess cortisol signaling. However, the present findings suggest that lower levels of acute TSST-induced sBDNF do not signal reduced hippocampal plasticity. While acute sBDNF dynamics during stress may hint at differences in individuals’ stress regulation, these differences are not captured in HCV of healthy middle-aged adults.
The present study has several limitations. Research suggests that while BDNF levels can be reliably assessed in serum, large samples are needed to allow meaningful investigations (Naegelin et al., 2018). Our simulations indicated that basal sBDNF analyses were sufficiently powered, but the analyses of reactive sBDNF were conducted in a smaller subsample and no a priori effect estimates could be derived from the literature. Likewise, there were no prior reports on the strength of potential associations between sBDNF and subfield volumes. The need for large samples arguably relates to the use of peripheral BDNF levels as an indirect and likely noisier proxy for the actual effects of central BDNF concentrations. Research suggests that peripheral BDNF levels can provide a window into specific aspects of brain functioning, showing that sBDNF levels are linked to general cognitive function and various functional deficits in psychopathology (for a review see Miranda et al., 2019). Regarding direct evidence of central to peripheral associations, BDNF levels in blood and hippocampal tissue are correlated in different non-human species (Klein et al., 2011; Sartorius et al., 2009). In humans, indications for an alignment between central and peripheral measures are provided by studies showing correlations of BDNF protein levels in cerebrospinal fluid and plasma (Pillai et al., 2010), and of serum BDNF with in vivo levels of a neuronal integrity marker (Lang et al., 2007). Nonetheless, the precise extent to which human central and peripheral BDNF concentrations correspond is unknown. Finally and more generally, it remains debated to what extent greater volume of brain structures reflects greater integrity or improved function (e.g., Van Petten, 2004). Measures of functional activity or connectivity may provide more sensitive estimates of hippocampal functioning, particularly for populations that do not display marked neuronal atrophy.
Conclusion
The null findings of the present study provide a baseline for future investigations with vulnerable samples. Although we expected to capture the onset of age-related decline and related compensatory mechanisms, such challenge appeared to be insignificant even at the upper end of the present age range. Based on this outcome, future studies may want to investigate the emergence of BDNF to HCV associations by tracking BDNF levels and neuronal integrity in healthy individuals at risk for developing conditions associated with accelerated neurodegeneration. Our results should not discourage future subfield-specific investigations or further explorations of reactive sBDNF levels in patient or older populations, considering also that hippocampal sub-regions display differential trajectories throughout the lifespan (Daugherty et al., 2016). At present, there is great interest in peripheral BDNF as a predictive marker for various conditions associated with neurodegeneration (Qin et al., 2017), cognitive deficits (Xie et al., 2020), or affective disorders (Fernandes et al., 2014; Molendijk et al., 2014; Polyakova et al., 2015). While there are, to our knowledge, no reports on BDNF to HCV associations in a healthy mid-aged sample, previous null findings may have remained unpublished due to dominant publishing norms and incentive schemes (Nosek et al., 2012). Our findings thus add to a more complete picture of the utility of peripheral BDNF in signaling brain structure differences, by suggesting limited predictive value for HCV in healthy mid-aged adults.
Acknowledgments
Acknowledgements
This study forms part of the ReSource Project, headed by Tania Singer. Data for this project were collected between 2013 and 2016 at the former Department of Social Neuroscience at the Max Planck Institute for Human Cognitive and Brain Sciences Leipzig.
We are thankful to the members of the Social Neuroscience Department involved in the ReSource Project over many years, in particular to Astrid Ackermann, Christina Bochow, Matthias Bolz and Sandra Zurborg for managing the large-scale longitudinal study, to Elisabeth Murzik, Nadine Otto, Sylvia Tydecks, and Kerstin Träger for help with recruiting and data archiving, to Henrik Grunert for technical assistance, and to Hannes Niederhausen and Torsten Kästner for data management.
Funding and Disclosure
Dr. Singer, as the principal investigator, received funding for the ReSource Project from the European Research Council (ERC) under the European Community's Seventh Framework Programme (FP7/2007-2013; ERC grant agreement number 205557), and the Max Planck Society. Dr. Bernhardt acknowledges support from CIHR (FDN-154298), SickKids Foundation (NI17-039), Natural Sciences and Engineering Research Council (NSERC; Discovery-1304413), Azrieli Center for Autism Research of the Montreal Neurological Institute (ACAR), and salary support from FRQS (Chercheur Boursier Junior 1). The authors declare that they have no competing interests.
Data availability & study materials
The present work is based on personal and sensitive physiological data that could be matched to individuals. Participants did not consent to data-sharing with parties outside the MPI CBS, such that in line with the GDPR, data cannot be made publicly available. Data are available upon reasonable request (contact via puhlmann@cbs.mpg.de).
The code used to execute power simulations and analyses can be accessed at the Open Science Framework (https://osf.io/3p7da/).
Author contributions
TS initiated, developed and secured all funding for the ReSource Project with the exception of the BDNF assay. Hippocampal image segmentations were carried out by RvdW and BB, as well as AB, BC, NB. GPC funded and IP overlooked the BDNF assay. VE, SLV and PV provided supervision. LP and RL drafted and all authors contributed critically to writing the manuscript, and approved its final version for submission. Statistical analyses were performed by LP and RL. All authors contributed to the interpretation of the data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.118011.
Appendix. Supplementary materials
References
- Adler D.H., Wisse L.E.M., Ittyerah R., Pluta J.B., Ding S.-L., Xie L., Wang J., Kadivar S., Robinson J.L., Schuck T. Characterizing the human hippocampus in aging and Alzheimer's disease using a computational atlas derived from ex vivo MRI and histology. Proc. Natl. Acad. Sci. 2018;115:4252–4257. doi: 10.1073/pnas.1801093115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.P., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Bartsch T., Wulff P. Elsevier; 2015. The hippocampus in aging and disease: from plasticity to vulnerability. [DOI] [PubMed] [Google Scholar]
- Bech P., Rasmussen N.A., Olsen L.R., Noerholm V., Abildgaard W. The sensitivity and specificity of the major depression inventory, using the present state examination as the index of diagnostic validity. J. Affect. Disord. 2001;66:159–164. doi: 10.1016/s0165-0327(00)00309-8. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar, M.M., D.; Lüdecke, D., 2020. Compute and interpret indices of effect size. R package. CRAN.
- Bernhardt B.C., Bernasconi A., Liu M., Hong S.J., Caldairou B., Goubran M., Guiot M.C., Hall J., Bernasconi N. The spectrum of structural and functional imaging abnormalities in temporal lobe epilepsy. Ann. Neurol. 2016;80:142–153. doi: 10.1002/ana.24691. [DOI] [PubMed] [Google Scholar]
- Boldrini M., Fulmore C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J. Human hippocampal neurogenesis persists throughout aging. Cell stem cell. 2018;22:589–599. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus B.A.A., Molendijk M.L., Penninx B.J.W.H., Buitelaar J.K., Kenis G., Prickaerts J., Elzinga B.M., Voshaar R.C.O. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Caldairou B., Bernhardt B.C., Kulaga-Yoskovitz J., Kim H., Bernasconi N., Bernasconi A. Springer; 2016. A surface patch-based segmentation method for hippocampal subfields; pp. 379–387. [Google Scholar]
- Cohen J. A power primer. Psychol. Bull. 1992;112:155. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cole J., Costafreda S.G., McGuffin P., Fu C.H.Y. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Counsell A., Cribbie R.A. Equivalence tests for comparing correlation and regression coefficients. Br. J. Math. Stat. Psychol. 2015;68:292–309. doi: 10.1111/bmsp.12045. [DOI] [PubMed] [Google Scholar]
- Daugherty A.M., Bender A.R., Raz N., Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26:220–228. doi: 10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flores R., La Joie R., Chételat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer's disease. Neuroscience. 2015;309:29–50. doi: 10.1016/j.neuroscience.2015.08.033. [DOI] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diedenhofen B., Musch J. cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W.J. Simplified estimation from censored normal samples. The Ann. Math. Stat. 1960:385–391. [Google Scholar]
- Driscoll I., Martin B., An Y., Maudsley S., Ferrucci L., Mattson M.P., Resnick S.M. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugich-Djordjevic M.M., Peterson C., Isono F., Ohsawa F., Widmer H.R., Denton T.L., Bennett G.L., Hefti F. Immunohistochemical visualization of brain-derived neurotrophic factor in the rat brain. Eur. J. Neurosci. 1995;7:1831–1839. doi: 10.1111/j.1460-9568.1995.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Engert V., Kok B.E., Papassotiriou I., Chrousos G.P., Singer T. Specific reduction in cortisol stress reactivity after social but not attention-based mental training. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Miller D.L., Roecklein K.A. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Heo S., McLaren M., Pence B.D., Martin S.A., Vieira V.J., Woods J.A. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva E., Björk-Eriksson T., Alborn A.-M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Farah M.J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96:56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Farah M.J. Socioeconomic status and the brain: prospects for neuroscience-informed policy. Nat. Rev. Neurosci. 2018;19:428–438. doi: 10.1038/s41583-018-0023-2. [DOI] [PubMed] [Google Scholar]
- Fernandes B.S., Berk M., Turck C.W., Steiner J., Gonçalves C.A. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol. Psychiatry. 2014;19:750–751. doi: 10.1038/mp.2013.172. [DOI] [PubMed] [Google Scholar]
- Field A., Miles J., Field Z. SAGE Publications; London, England: 2012. Discovering statistics using R. [Google Scholar]
- Fjell A.M., Westlye L.T., Grydeland H., Amlien I., Espeseth T., Reinvang I., Raz N., Holland D., Dale A.M., Walhovd K.B., Alzheimer Disease Neuroimaging I. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol. Aging. 2013;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M.A., Shaw M.E., Cherbuin N. A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. Neuroimage. 2015;112:364–374. doi: 10.1016/j.neuroimage.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S., First M.B. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) Am Psych Pub. 1997 [Google Scholar]
- Gonçalves J.T., Schafer S.T., Gage F.H. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Gray J., Milner T., McEwen B. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.E., Xu B., Lu B., Hempstead B.L. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin É.W., Mullally S., Foley C., Warmington S.A., O'Mara S.M., Kelly Á.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Hall J., Thomas K.L., Everitt B.J. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 2000;3:533. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Figueiredo H., Mueller N.K., Ulrich-Lai Y., Ostrander M.M., Choi D.C., Cullinan W.E. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz D., Schwahn C., Borchardt U., Wittfeld K., Schulz A., Barnow S., Biffar R., Hoffmann W., Habes M., Homuth G., Nauck M., Hegenscheid K., Lotze M., Völzke H., Freyberger H.J., Debette S., Grabe H.J. Genetic, psychosocial and clinical factors associated with hippocampal volume in the general population. Transl. psychiatry. 2014;4:e465. doi: 10.1038/tp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F., Borie A., Chao M.V., Garabedian M.J. Bridging the gap between BDNF and glucocorticoid effects on brain networks. Neuroendocrinology. 2018 doi: 10.1159/000496392. [DOI] [PubMed] [Google Scholar]
- Johnson N.F., Kim C., Gold B.T. Socioeconomic status is positively correlated with frontal white matter integrity in aging. AGE. 2013;35:2045–2056. doi: 10.1007/s11357-012-9493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R., Wakako R., Komori T., Shigemi H., Miyazaki N., Ito H., Kumagai T., Tsuzuki M., Shigemi K., Yoshida F., Nakayama A. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. Int. J. Dev. Neurosci. 2007;25:367–372. doi: 10.1016/j.ijdevneu.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F.H., Aigner L., Song H., Curtis M.A., Thuret S., Kuhn H.G., Jessberger S., Frankland P.W., Cameron H.A. Human adult neurogenesis: evidence and remaining questions. Cell stem cell. 2018 doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A., Fagan A.M., Goate A.M., Benzinger T.L.S., Morris J.C., Head D., Alzheimer's Disease Neuroimaging I. Lack of an association of BDNF Val66Met polymorphism and plasma BDNF with hippocampal volume and memory. Cogn., Affect., & Behav. Neurosci. 2015;15:625–643. doi: 10.3758/s13415-015-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Pellman B., Kim J.J. Stress effects on the hippocampus: a critical review. Learn. Memory. 2015;22:411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk R.E. Practical significance: a concept whose time has come. Educ. Psychol. Meas. 1996;56:746–759. [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The Trier Social Stress Test - a Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klein A.B., Williamson R., Santini M.A., Clemmensen C., Ettrup A., Rios M., Knudsen G.M., Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacolog. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- Kuipers S.D., Trentani A., Tiron A., Mao X., Kuhl D., Bramham C.R. BDNF-induced LTP is associated with rapid Arc/Arg3. 1-dependent enhancement in adult hippocampal neurogenesis. Sci. Rep. 2016;6:21222. doi: 10.1038/srep21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaga-Yoskovitz J., Bernhardt B.C., Hong S.-J., Mansi T., Liang K.E., van der Kouwe A.J.W., Smallwood J., Bernasconi A., Bernasconi N. Multi-contrast submillimetric 3 Tesla hippocampal subfield segmentation protocol and dataset. Sci. Data. 2015;2 doi: 10.1038/sdata.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Equivalence Tests: a practical primer for t tests, correlations, and meta-analyses. Social Psychol. Personal. Sci. 2017;8:355–362. doi: 10.1177/1948550617697177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang U.E., Hellweg R., Seifert F., Schubert F., Gallinat J. Correlation between serum brain-derived neurotrophic factor level and an in vivo marker of cortical integrity. Biol. Psychiatry. 2007;62:530–535. doi: 10.1016/j.biopsych.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Leal G., Afonso P.M., Salazar I.L., Duarte C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101. doi: 10.1016/j.brainres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Leal G., Bramham C.R., Duarte C.B. Elsevier; 2017. BDNF and hippocampal synaptic plasticity. Vitamins and hormones; pp. 153–195. [DOI] [PubMed] [Google Scholar]
- Leuner B., Gould E. Structural plasticity and hippocampal function. Annu. Rev. Psychol. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz R., Puhlmann L.M.C., Apostolakou F., Mantzou E., Papassotiriou I., Chrousos G.P., Engert V., Singer T. Acute psychosocial stress increases serum BDNF levels: an antagonistic relation to cortisol but no group differences after mental training. Neuropsychopharmacology. 2019;44:1797–1804. doi: 10.1038/s41386-019-0391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P., Virchow J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lowe A.J., Paquola C., Vos de Wael R., Girn M., Lariviere S., Tavakol S., Caldairou B., Royer J., Schrader D.V., Bernasconi A., Bernasconi N., Spreng R.N., Bernhardt B.C. Targeting Age-Related Differences in Brain and Cognition with Multimodal Imaging and Connectome Topography Profiling. bioRxiv. 2019 doi: 10.1002/hbm.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe B., Spitzer R.L., Zipfel S., Herzog W. Gesundheitsfragebogen für patienten (PHQ-D). Komplettversion und Kurzform. Testmappe mit Manual. Fragebögen, Schablonen. 2002;2:5–7. [Google Scholar]
- Lu B., Nagappan G., Lu Y. BDNF and Synaptic plasticity, cognitive function, and dysfunction. In: Lewin G.R., Carter B.D., editors. Neurotrophic Factors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. pp. 223–250. [Google Scholar]
- Malykhin N.V., Coupland N.J. Hippocampal neuroplasticity in major depressive disorder. Neuroscience. 2015;309:200–213. doi: 10.1016/j.neuroscience.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Marmigère F., Givalois L., Rage F., Arancibia S., Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18:1353. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B.S., Gianaros, P.J., 2010. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences 1186, 190–222. [DOI] [PMC free article] [PubMed]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D., Wu T., Rao U., North C.S., Xiao H., Javors M.A., Adinoff B. Serum NPY and BNDF response to a behavioral stressor in alcohol-dependent and healthy control participants. Psychopharmacology (Berl.) 2011;218:59. doi: 10.1007/s00213-011-2414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M., Morici J.F., Zanoni M.B., Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M.L., Spinhoven P., Polak M., Bus B.a.A., Penninx B.W.J.H., Elzinga B.M. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484) Mol. Psychiatry. 2014;19:791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- Mondelli V., Cattaneo A., Murri M.B., Di Forti M., Handley R., Hepgul N., Miorelli A., Navari S., Papadopoulos A.S., Aitchison K.J. Stress and inflammation reduce BDNF expression in first-episode psychosis: a pathway to smaller hippocampal volume. J. Clin. Psychiatry. 2011;72:1677. doi: 10.4088/JCP.10m06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jiménez E.P., Flor-García M., Terreros-Roncal J., Rábano A., Cafini F., Pallas-Bazarra N., Ávila J., Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat. Med. 2019:1. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- Murer M.G., Boissiere F., Yan Q., Hunot S., Villares J., Faucheux B., Agid Y., Hirsch E., Raisman-Vozari R. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer's disease. Neuroscience. 1999;88:1015–1032. doi: 10.1016/s0306-4522(98)00219-x. [DOI] [PubMed] [Google Scholar]
- Naegelin Y., Dingsdale H., Säuberli K., Schädelin S., Kappos L., Barde Y.-A. Measuring and validating the levels of brain-derived neurotrophic factor in human serum. eNeuro. 2018;5 doi: 10.1523/ENEURO.0419-17.2018. ENEURO.0419-0417.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis L., Manohar S.G., Smith S.M., Alfaro-Almagro F., Jenkinson M., Mackay C.E., Husain M. Hippocampal volume across age: nomograms derived from over 19,700 people in UK Biobank. NeuroImage. Clin. 2019;23 doi: 10.1016/j.nicl.2019.101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek B.A., Spies J.R., Motyl M. Scientific Utopia: II. Restructuring incentives and practices to promote truth over publishability. Perspect. Psychol. Sci. 2012;7:615–631. doi: 10.1177/1745691612459058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The J. Neurosci. : Off. J. Soc. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips H.S., Hains J.M., Laramee G.R., Rosenthal A., Winslow J.W. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- Piccinni A., Marazziti D., Del Debbio A., Bianchi C., Roncaglia I., Mannari C., Origlia N., Catena Dell'Osso M., Massimetti G., Domenici L. Diurnal variation of Plasma Brain-Derived Neurotrophic Factor (BDNF) in Humans: an analysis of sex differences. Chronobiol. Int. 2008;25:819–826. doi: 10.1080/07420520802387773. [DOI] [PubMed] [Google Scholar]
- Pillai A., Kale A., Joshi S., Naphade N., Raju M., Nasrallah H., Mahadik S.P. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int. J. Neuropsychopharmacolog. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- Polacchini A., Metelli G., Francavilla R., Baj G., Florean M., Mascaretti L.G., Tongiorgi E. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci. Rep. 2015;5:17989. doi: 10.1038/srep17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyakova M., Stuke K., Schuemberg K., Mueller K., Schoenknecht P., Schroeter M.L. BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. J. Affect. Disord. 2015;174:432–440. doi: 10.1016/j.jad.2014.11.044. [DOI] [PubMed] [Google Scholar]
- Qin X.Y., Cao C., Cawley N.X., Liu T.T., Yuan J., Loh Y.P., Cheng Y. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer's disease: a meta-analysis study (N=7277) Mol. Psychiatry. 2017;22:312–320. doi: 10.1038/mp.2016.62. [DOI] [PubMed] [Google Scholar]
- Raz N., Ghisletta P., Rodrigue K.M., Kennedy K.M., Lindenberger U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Gunning-Dixon F., Head D., Rodrigue K.M., Williamson A., Acker J.D. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol. Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Rizos E.N., Papathanasiou M., Michalopoulou P.G., Mazioti A., Douzenis A., Kastania A., Nikolaidou P., Laskos E., Vasilopoulou K., Lykouras L. Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr. Res. 2011;129:201–204. doi: 10.1016/j.schres.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Rothman S.M., Mattson M.P. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience. 2013;239:228–240. doi: 10.1016/j.neuroscience.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M., Lucas G., Ernfors P., Castrén M., Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sartorius A., Hellweg R., Litzke J., Vogt M., Dormann C., Vollmayr B., Danker-Hopfe H., Gass P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270–276. doi: 10.1055/s-0029-1224162. [DOI] [PubMed] [Google Scholar]
- Schmidt H.D., Duman R.S. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schoenfeld T.J., Gould E. Stress, stress hormones, and adult neurogenesis. Exp. Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer J.P. Multiple hypothesis testing. Annu. Rev. Psychol. 1995;46:561–584. [Google Scholar]
- Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Lee S., Park H., Suzuki T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014;6:69. doi: 10.3389/fnagi.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Kok B.E., Bornemann B., Zurborg S., Bolz M., Bochow C. 2016. The ReSource Project: Background, design, samples, and measurements Max Planck Institute for Human Cognitive and Brain Sciences Leipzig. [Google Scholar]
- Small S.A., Schobel S.A., Buxton R.B., Witter M.P., Barnes C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011;12:585. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Fan X., Li X., Kennedy D., Pang L., Quan M., Chen X., Gao J., Zhang W., Zhang J., Lv L. Serum levels of BDNF, folate and homocysteine: In relation to hippocampal volume and psychopathology in drug naïve, first episode schizophrenia. Schizophr. Res. 2014;159:51–55. doi: 10.1016/j.schres.2014.07.033. [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B.W., Patient Health Questionnaire Primary Care Study G. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Statistisches Bundesamt . Einkommen; Verdienst: 2019. Dossier: Gehalt. [Google Scholar]
- Suri D., Vaidya V. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Szuhany K.L., Bugatti M., Otto M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D., Loya A., Gersner R., Haramati S., Chen A., Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovska V., Marcussen A.B., Vinberg M., Hartvig P., Aznar S., Knudsen G.M. Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res. Bull. 2007;73:143–149. doi: 10.1016/j.brainresbull.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vaynman S., Ying Z., Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O., von Bohlen und Halbach V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018;373:729–741. doi: 10.1007/s00441-017-2782-x. [DOI] [PubMed] [Google Scholar]
- Vos de Wael R., Larivière S., Caldairou B., Hong S.-J., Margulies D.S., Jefferies E., Bernasconi A., Smallwood J., Bernasconi N., Bernhardt B.C. Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proc. Natl. Acad. Sci. 2018;115:10154. doi: 10.1073/pnas.1803667115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M.J., Herman M.M., Kleinman J.E., Weickert C.S. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr. Patterns. 2006;6:941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Wellek S. Chapman and Hall/CRC; 2010. Testing statistical hypotheses of equivalence and noninferiority. [Google Scholar]
- Wittchen H.U., Pfister H. Swets & Zeitlinger; Frankfurt: 1997. DIA-X-Interviews: Manual für Screening-Verfahren und Interview. [Google Scholar]
- Xie B., Zhou H., Liu W., Yu W., Liu Z., Jiang L., Zhang R., Cui D., Shi Z., Xu S. Evaluation of the diagnostic value of peripheral BDNF levels for Alzheimer's disease and mild cognitive impairment: results of a meta-analysis. Int. J. Neurosci. 2020;130:218–230. doi: 10.1080/00207454.2019.1667794. [DOI] [PubMed] [Google Scholar]
- Yan Q., Rosenfeld R.D., Matheson C.R., Hawkins N., Lopez O.T., Bennett L., Welcher A.A. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Yassa M.A., Stark C.E.L. Pattern separation in the Hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R., Hori H., Katsuki A., Atake K., Nakamura J. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann. General Psychiatry. 2016;15:1. doi: 10.1186/s12991-015-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich, P.A., Amaral, R.S.C., Augustinack, J.C., Bender, A.R., Bernstein, J.D., Boccardi, M., Bocchetta, M., Burggren, A.C., Carr, V.A., Chakravarty, M.M., Chételat, G., Daugherty, A.M., Davachi, L., Ding, S.-L., Ekstrom, A., Geerlings, M.I., Hassan, A., Huang, Y., Iglesias, J.E., La Joie, R., Kerchner, G.A., LaRocque, K.F., Libby, L.A., Malykhin, N., Mueller, S.G., Olsen, R.K., Palombo, D.J., Parekh, M.B., Pluta, J.B., Preston, A.R., Pruessner, J.C., Ranganath, C., Raz, N., Schlichting, M.L., Schoemaker, D., Singh, S., Stark, C.E.L., Suthana, N., Tompary, A., Turowski, M.M., Van Leemput, K., Wagner, A.D., Wang, L., Winterburn, J.L., Wisse, L.E.M., Yassa, M.A., Zeineh, M.M., Hippocampal Subfields, G., 2015. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. NeuroImage 111, 526–541. [DOI] [PMC free article] [PubMed]
- Zagrebelsky M., Gödecke N., Remus A., Korte M. Cell type-specific effects of BDNF in modulating dendritic architecture of hippocampal neurons. Brain Struct. Funct. 2018;223:3689–3709. doi: 10.1007/s00429-018-1715-0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Shi Y.-G., Woods J.H., Watson S.J., Ko M.-C. Central κ-opioid receptor-mediated antidepressant-like effects of nor-Binaltorphimine: behavioral and BDNF mRNA expression studies. Eur. J. Pharmacol. 2007;570:89–96. doi: 10.1016/j.ejphar.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhorn A.A., Schulte-Herbrüggen O., Danker-Hopfe H., Malbranc M., Hartung H.-D., Anders D., Lang U.E., Steinhagen-Thiessen E., Schaub R.T., Hellweg R. Serum neurotrophins—a study on the time course and influencing factors in a large old age sample. Neurobiol. Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Zou G.Y. American Psychological Association; US: 2007. Toward using confidence intervals to compare correlations; pp. 399–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The present work is based on personal and sensitive physiological data that could be matched to individuals. Participants did not consent to data-sharing with parties outside the MPI CBS, such that in line with the GDPR, data cannot be made publicly available. Data are available upon reasonable request (contact via puhlmann@cbs.mpg.de).
The code used to execute power simulations and analyses can be accessed at the Open Science Framework (https://osf.io/3p7da/).