Abstract
The World Health Organization announced an ambitious call for cervical cancer elimination worldwide. With existing prevention and treatment modalities, cervical cancer elimination is now within reach for high-income countries. Despite limited financing and capacity constraints in low-and-middle-income countries (LMICs), prevention and control efforts can be supported through integrated services and new technologies. We conducted this scoping review to outline a roadmap toward cervical cancer elimination in LMICs and highlight evidence-based interventions and research priorities to accelerate cervical cancer elimination. We reviewed and synthesized literature from 2010 to 2020 on primary and secondary cervical cancer prevention strategies. In addition, we conducted expert interviews with gynecologic and infectious disease providers, researchers, and LMIC health officials. Using these data, we developed a logic model to summarize the current state of science and identified evidence gaps and priority research questions for each prevention strategy. The logic model for cervical cancer elimination maps the needs for improved collaboration between policy makers, production and supply, healthcare systems, providers, health workers, and communities. The model articulates responsibilities for stakeholders and visualizes processes to increase access to and coverage of prevention methods. We discuss the challenges of contextual factors and highlight innovation needs. Effective prevention methods include HPV vaccination, screening using visual inspection and HPV testing, and thermocoagulation. However, vaccine coverage remains low in LMICs. New strategies, including single-dose vaccination could enhance impact. Loss to follow-up and treatment delays could be addressed by improved same-day screen-and-treat technologies. We provide a practical framework to guide cervical cancer elimination in LMICs. The scoping review highlights existing and innovative strategies, unmet needs, and collaborations required to achieve elimination across implementation contexts.
Keywords: cervical cancer, cervical cancer screening, human papillomavirus vaccination, scoping review, cervical cancer prevention, cervical cancer elimination
Introduction
Globally, there are more than half a million new cervical cancer cases and more than a quarter-million cervical cancer-related deaths each year (1). Due to effective screening and treatment of precancerous lesions and cancer, high-income countries have seen marked decreases in cervical cancer incidence and mortality in recent decades (2–4). High coverage of human papillomavirus (HPV) vaccination has also contributed to declines in HPV prevalence and cervical lesions in countries with established national vaccination programs (5). Given the success of effective interventions for prevention and treatment, the World Health Organization (WHO) issued a call in 2018 to eliminate cervical cancer as a public health problem globally, defined as an incidence rate <4 per 100,000 women-years (6). To achieve this goal, the WHO proposes an intermediate 90–70–90 target (also known as the “triple-intervention” strategy), which aims to vaccinate 90% of girls by age 15, screen 70% of women with a high-performance test by age 35 and again by 45, and treat 90% of women with cervical disease (6). Australia is projected to achieve elimination by 2050, and other high-income countries are following close behind (7).
However, the timeline for cervical cancer elimination is significantly longer in low-and-middle-income countries (LMICs) (8). This longer timeline is due in part to the current higher burden of disease. More than 80% of cervical cancer cases occur in LMICs (9), where age-standardized cervical cancer mortality rates are at least 6-fold higher than in high-income countries (10). These disparities reflect low access to prevention and treatment due to limited infrastructure, technical expertise, and resources (10). Of the 118 million women who received the HPV vaccine by 2016, only 1.4 million (1%) lived in LMICs (11). Implementation of successful screening programs in LMICs has been challenged by the lack of equipment and personnel to initiate and maintain the screening program, the financial and logistical burden of multiple visits, high rates of loss-to-follow-up, and lack of resource allocation for specialized training (12).
The science, technology, and implementation of cervical cancer prevention and treatment are changing rapidly, with effective and scalable strategies on the horizon. A recent modeling analysis of 78 LMICs demonstrated the importance of successful implementation and scale-up by predicting that the triple-intervention would reduce cervical cancer mortality of women ages 30–69 years by 33.9% (24.4–37.9 per 100,000 women) by 2030 and almost 99% by 2120 (13). The purpose of this scoping review is to (1) synthesize the evidence on the effectiveness of available and emerging cervical cancer elimination strategies, (2) provide a mechanism for visualizing how primary and secondary prevention methods work together using a logic model framework, and (3) highlight gaps in evidence in primary and secondary prevention and propose research priorities to address these gaps and accelerate progress toward elimination.
Materials and Methods
Our team conducted a scoping review of the literature on primary and secondary cervical cancer prevention strategies using methods adapted from Arksey and O'Malley (14). A scoping review is a technique used to “map” the relevant literature when the field of interest is broad. It differs from a traditional systematic review in that it includes additional study designs as well as randomized trials and other systematic reviews. We chose this method because the topic of cervical cancer elimination in low-resource settings is one that is complex and some aspects of the science (e.g., one-dose HPV vaccine regimen) are still nascent, yet critically important. Our review focuses on primary and secondary prevention to deliver a more in-depth summary on these strategies. The areas of expertise in cervical cancer prevention on our team include: infectious disease specialists (RB, JW, PG, LE), obstetrician gynecologists (LE, LP, NM), LMIC practitioners (PG, NM), epidemiologists (GL, DR), modeling specialists (GL, DR, CB), a gynecologic oncology fellow (LP), and a nurse (MS). The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist is available in Table 1 (15). The scoping review process is described in detail in Table 2.
Table 1.
Preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) checklist (15).
| Section | Item | PRISMA-ScR checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 2 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 2–4 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 2 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 2–4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | N/A |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | Table 2 |
| Information sources* | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | Table 2 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | Supplementary Table 1 |
| Selection of sources of evidence† | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 2, 4, Table 2 |
| Data charting process‡ | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 2, 4, Table 2 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 2, 4, Supplementary Table 2 |
| Critical appraisal of individual sources of evidence§ | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | N/A |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 2, 4, Table 2 |
| RESULTS | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | Numbers of studies retrieved and included reported in Supplementary Table 1 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | Supplementary Table 2 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | N/A |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 4–12 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 4–12 |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 12, 13, Table 6 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 13 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 13, 14 |
| FUNDING | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 14, 20 |
JBI, Joanna Briggs Institute; PRISMA-ScR, preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews.
Where sources of evidence (see second footnote) are compiled from, such as bibliographic databases, social media platforms, and Web sites.
A more inclusive/heterogeneous term used to account for the different types of evidence or data sources (e.g., quantitative and/or qualitative research, expert opinion, and policy documents) that may be eligible in a scoping review as opposed to only studies. This is not to be confused with information sources (see first footnote).
The frameworks by Arksey and O'Malley (6) and Levac et al. (7) and the JBI guidance (4, 5) refer to the process of data extraction in a scoping review as data charting.
The process of systematically examining research evidence to assess its validity, results, and relevance before using it to inform a decision. This term is used for items 12 and 19 instead of “risk of bias” (which is more applicable to systematic reviews of interventions) to include and acknowledge the various sources of evidence that may be used in a scoping review (e.g., quantitative and/or qualitative research, expert opinion, and policy document).
Table 2.
Scoping review framework and description of methods.
| Arksey and O'Malley framework stage | |
|---|---|
| 1. Identifying the research question | • What do we already know? • What are the gaps in evidence? • What are the relevant innovations? • What are the most pressing questions we need to answer to scale-up cervical cancer elimination strategies? |
| 2. Identifying relevant studies | • Search sources: PubMed, Scopus, reference lists, and governmental and non-profit organizational websites • Inclusion criteria: ◦ Programmatic interventions identified by the WHO life course model (16) ◦ English language published between 2010 and 2020 ◦ Peer-reviewed studies and conference abstracts that examined efficacy, effectiveness, sensitivity, and/or specificity of existing and emerging strategies to prevent HPV infection and detect or treat cervical precancers and cervical cancer ◦ Interventional studies that address innovations and implementation gaps |
| 3. Study selection | • Systematic reviews, meta-analysis and randomized controlled trials were prioritized for each intervention. When these were not available, we selected longitudinal and prospective cohort studies with relative risks or odds ratios that address HPV acquisition, progression to precancer and treatment of cancer. Individual cross-sectional studies were reviewed only if sufficient data from the above types of studies were not available. |
| 4. Charting the data | Two authors (MS and GL) screened the search results for relevant articles and independently extracted data relevant to the key questions. The last update of the search was conducted in August 2020, and the following data was extracted using Microsoft Excel sheet (see Supplementary Material): • Primary prevention: author, year, study design, location, population, exposure, unit of exposure, comparison, comparison number of doses, outcomes, sample size, key findings • Secondary prevention: author, year, intervention, study design, location, population, intervention, comparison, outcomes, clinical endpoint, key findings |
| 5. Collating, summarizing, and reporting the results | • As specified by Arksey and O'Malley, a narrative literature review method was used, in which data synthesis and interpretation of the findings were conducted simultaneously, in an iterative manner with the research team. In addition to the narrative synthesis, we followed the Centers for Disease Control and Prevention's Program Evaluation Framework to organize the evidence on the available and emerging strategies for cervical cancer elimination into a logic model (17). |
We chose to review articles published beginning in year 2010, because while the HPV vaccine was introduced to the world in 2006, it was not until 2010 that it became incorporated into the national immunization program in LMIC settings, starting with Bhutan in 2010 and Rwanda in 2011 (18). We used the WHO's “Life-course approach to cervical cancer interventions” as a guide to organize our review (6). We defined primary prevention as the prevention of HPV infection and secondary prevention as the detection and treatment of precancerous cervical lesions.
Two authors (MS and GL) screened the search results for relevant articles and extracted data independently. All authors provided feedback on study selection, data extraction, and synthesis, which informed further search and interpretation of the findings. Our search for primary prevention strategies focused on the effect of HPV vaccination, voluntary medical male circumcision (VMMC), tobacco cessation, condom use as mentioned by the WHO (16), and vaginal dysbiosis. While vaginal dysbiosis is not commonly mentioned as a risk factor for cervical cancer, we decided to include it in our review because there is relatively strong and consistent evidence that the vaginal microbiota play a role in cervical cancer pathology (19–22).
We divided secondary prevention into screening and treatment strategies. For screening, we compared cytology, HPV tests, and visual inspection with acetic acid (VIA). As the WHO recommends screening with a high-performance test equivalent to or better than HPV testing, we reviewed implementation challenges of HPV testing in low-resource settings. We recognize HPV-based screening may be considered a primary prevention strategy as it can detect individuals in whom cervical cancer has not occurred. However, for the purpose of our review and following the WHO guidelines, we reviewed this screening method under secondary prevention. For immediate treatment of precancerous lesions, we focused on cryotherapy, thermal ablation, and loop electrosurgical excision procedure (LEEP). We identified emerging screening and triage options in LMICs as HPV self-sampling, oncogenesis biomarkers, optical techniques, such as portable colposcopes and automated visual evaluation, and therapeutic vaccines. We also dedicated a section to prevention of cervical cancer among women living with HIV to highlight the differences in HPV acquisition and progression in this population.
Results
The Logic Model
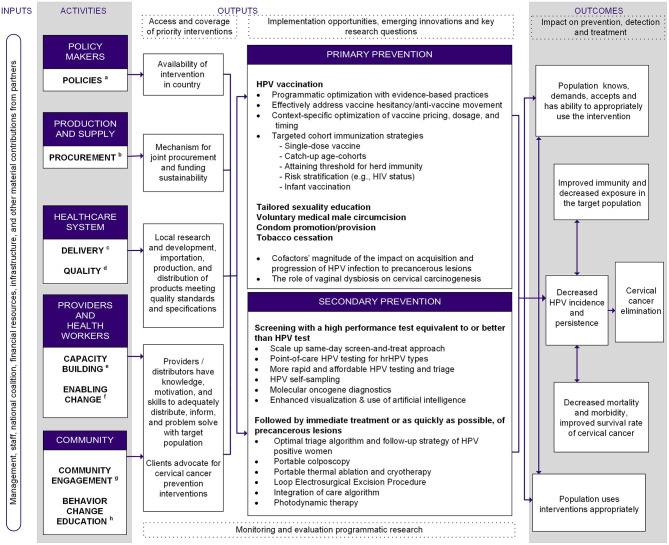
The logic model summarizes and describes the process flow for cervical cancer elimination (Figure 1). Step one is marshaling crucial resources needed for sustainable cervical cancer elimination programs in LMICs: healthcare worker capacity, political commitment, funding, and infrastructure and material support from domestic and global partners (“Inputs”) as explicitly captured in the WHO's “Global strategy toward eliminating cervical cancer as a public health problem” (16). To acquire these resources, key stakeholders must build capacity within their sectors and foster cross-sector collaborations (“Activities”). The intended impact of the program is organized into primary and secondary prevention nodes (“Outputs”). For each prevention strategy, we summarized what is known, the impact of the intervention, and innovations under development. All strategies lead to “Outcomes,” which are the expected intermediate impacts on the path toward cervical cancer elimination. Synthesizing the efficacy of current interventions identified gaps in innovation in primary and secondary prevention, which are summarized in Figure 1.
Figure 1.
Logic model for comprehensive, intersectoral cervical cancer prevention. aPolicies: develop and implement policies, legislation regulations, and registrations. bProcurement: develop and implement provision, production, procurement and training strategies. cDelivery: develop and implement delivery system, strategy for management, training, and maintain motivation among providers and distributors. dQuality: develop and implement an external and internal quality control system. eCapacity building: develop provider and health worker skills training, infrastructure and capacity building. fEnabling change: cultivate dialogue to promote adoption of innovative technologies and approaches (e.g., task-shifting/sharing) to simplify care delivery and break conflicts of interest. gCommunity engagement: demand promotion by empowering local stakeholders and advocacy. hBehavior change education: develop and implement intervention strategy for information, education, and communication for behavior change.
Primary Prevention
Current State of Science
HPV Vaccines
HPV vaccines, which have the potential to prevent 90% of cervical cancer cases (6), are by far the most efficacious primary prevention modality. Among HPV-naïve adolescent girls and young women, the efficacy of available vaccines (bivalent, quadrivalent, and nonavalent) is >95% for preventing HPV infections and cervical lesions caused by vaccine-targeted HPV types (23–28). Since the median age of sexual initiation is 15–17 in many populations (29), the WHO recommends vaccination programs to target 9–13 year-old girls (30). Mathematical modeling analyses from 73 LMICs found that routine vaccination at age 9 and multi-cohort vaccination of girls ages 10–14 reduced cervical cancer deaths by 30–40%, or 1.2–1.8 million, over the lifetime of the vaccinated cohorts in addition to the number of deaths averted with routine vaccination only (31). Unfortunately, as of 2018, only 13 of the 90 countries who had introduced HPV vaccine into their national programs were lower income countries (32). A comprehensive strategy is needed while key interventions, such as HPV vaccination is scaled-up (6).
Further, the cost-effectiveness of vaccination strategies is dependent on the vaccine price, which is likely to drop significantly as new vaccines being developed in India and China increase HPV vaccine supply over the next ten years (33). The economic landscape of HPV vaccines is quickly changing to promote access to the vaccines in LMICs and adoption of national HPV immunization programs. Just prior to the Global Vaccine Summit 2020, five manufacturers of HPV vaccines committed to increasing the supply in Gavi-supported countries (34). The proportion of LMICs with national programs is low (22 of the 78 LMICs vs. 50 of the 57 high-income countries as of 2020) (35, 36), but increasing, since the Gavi Alliance negotiated the price of the vaccine as low as $4.50 USD per dose for the poorest countries in 2013 (37). Economic evaluations from modeling studies suggest that HPV vaccination of adolescent girls is cost-effective for most countries, especially low-income countries (38, 39) and when the vaccine price is affordable for the country's income level (40).
Voluntary Medical Male Circumcision
VMMC for HIV-negative men has been shown to reduce penile HPV viral load of incident infections and the persistence of prevalent HPV infections, which likely reduces male-to-female HPV transmission (41, 42). Even when transmission occurs, female partners of circumcised men had lower HPV viral load (42). The incidence of high-risk HPV (hrHPV) infection was lower among women whose male partners received circumcision than those who did not (incidence rate ratio = 0.77, 95% CI: 0.63–0.93) (43).
Other Cofactors
HPV incidence is inversely associated with the frequency of condom use (44). In a longitudinal study with 8 months of follow-up, female college students who reported using condoms during all vaginal intercourse were 70% less likely to acquire a new infection than those who reported using condoms <5% of the time, after adjusting for the number of new partners and estimated number of previous partners of the male partner (45).
Current smokers were 1.6 times more likely than never smokers to have prevalent hrHPV infection than never smokers (95% CI: 1.2–2.1) and 1.4 times more likely to have newly detected hrHPV infection (95% CI: 1.0 −1.9) (46). This increased risk may be explained by the lack of immune response after a natural infection among people who smoke, which lowers their defense against subsequent infection (46).
Vaginal dysbiosis, including but not limited to bacterial vaginosis, is positively correlated with prevalent HPV infection and cervical intraepithelial neoplasia (CIN) among women with and without HIV (20, 47–49). While vaginal dysbiosis increased risk of persistent HPV infection (50), high grade squamous intraepithelial lesions (HSIL) and cervical cancer (51), a healthy cervicovaginal microbiome, dominated by multiple species of Lactobacillus bacteria, was associated with lower prevalence of hrHPV infection (52). There is a need to further investigate the complex relationship between the microbiome of the female reproductive tract, HPV and cervical carcinogenesis (53).
Table 3 summarizes the efficacies and effect of co-factors in prevention of HPV infection. The clinical endpoints and the intervention effect are not uniform; hence, the potential impact of different preventative strategies cannot be compared to each other. However, this is precisely an evidence gap that can hinder implementation and evaluation prevention efforts. Lastly, the co-factors mentioned in this table do not represent an exhaustive list of risk factors for cervical cancer or HPV infection.
Table 3.
Summary of HPV vaccine efficacy and effect of co-factors on HPV-related clinical endpoints.
| Primary prevention method | Endpoint | Effect (%, 95% CI) | References |
|---|---|---|---|
| HPV VACCINE * | |||
| (HPV)-16/18 AS04-adjuvanted vaccine | CIN2-3 associated with HPV 16/18 [mean follow-up (f/u): 34.9 months] | 92.9 (96.1% CI: 79.9–98.3) | (23) |
| Quadrivalent vaccine (HPV 6, 11, 16, 18) | CIN1-3 or adenocarcinoma in situ associated with HPV 6, 11, 16, 18 (mean f/u: 36 months) | 100.0 (95% CI: 94.0–100.0) | (25) |
| CIN 2 or 3, adenocarcinoma in situ, or cervical cancer related to HPV 16 or 18 (mean f/u: 36 months) | 98.0 (95.89% CI: 86.0–100.0) | (26) | |
| Nonavalent vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58)† | CIN 2 or 3, adenocarcinoma in situ, invasive cervical carcinoma, and vulvar disease related to HPV 31, 33, 45, 52, and 58 (up to 6 years) | 97.4 (95% CI: 85.0–99.9) | (27) |
| High-grade cervical, vulvar, or vaginal disease related to HPV-31, 33, 45, 52, and 58 (up to 54 months) | 96.7 (95% CI: 80.9–99.8) | (28) | |
| VOLUNTARY MEDICAL MALE CIRCUMCISION | |||
| HPV prevalence risk ratios among women partners 24 months after intervention | 0.72 (95% CI: 0.60–0.85) | (43) | |
| Incidence rate ratio of hrHPV‡ | 0.77 (95% CI: 0.63–0.93) | (43) | |
| TOBACCO USE § | |||
| Odds ratio of hrHPV infection at baseline | 1.60 (95% CI: 1.20–2.10) | (46) | |
| Odds ratio of CIN2-3 at baseline | 1.80 (95% CI: 1.30–2.50) | (46) | |
| Risk ratio of incident hrHPV infection | 1.40 (95% CI: 1.00–1.90) | (46) | |
| Risk ratio of incident CIN2-3 | 3.60 (95% CI: 1.50–8.60) | (46) | |
| CONDOM USE ¶ | |||
| Percent reduction of incident genital HPV infection | 70.0% (95% CI: 40.0–90.0) | (45) | |
| VAGINAL DYSBIOSIS | |||
| Risk ratio of incident HPV infection | 1.35 (95% CI: 1.18–1.50) | (19) | |
| Risk ratio of HPV persistence | 1.14 (95% CI: 1.01–1.28) | (19) | |
| Risk ratio of high grade squamous intraepithelial lesion/squamous cell changes | 2.01 (95% CI: 1.40–3.01) | (19) | |
Indicates vaccine efficacy.
The results for HPV 6, 11, 16 and 18 not included in the table because of wide confidence intervals containing 0.
HPV 6, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
Comparing current smokers to never smokers.
Comparing condom use 100% of the time during 8 months to those whose partners used condoms <5% of the time among women who had never had vaginal intercourse or had first had intercourse with one male partner within the previous 3 months of the study.
Priority Research Questions for Primary Prevention Strategies
We prioritized implementation and scale-up of HPV vaccination in primary prevention opportunities, as it directly addresses the known causal agent of most cervical cancer. Several barriers specific to LMICs have been identified, such as costs associated with the vaccine and service delivery, political commitment, challenges specific to the target population of adolescent girls, and global vaccine shortage (36). In addition, the scale-up of HPV vaccination programs has faced challenges of vaccine hesitancy due to concerns about the safety and side effects of the vaccine and the belief that vaccination can lead to sexual promiscuity (54).
Successful implementation and scale-up of HPV vaccine programs depends on achieving and maintaining high rates of vaccine uptake (5), which requires context-informed delivery strategies. Microplanning for HPV vaccination encompasses logistical considerations, such as delivery of multi-dose vaccines, reaching out-of-school girls, and alignment with the school calendar, as well as careful navigation of complex sociopolitical settings where sensitization to reproductive health can be delicate (55). For example, Australia, which has one of the highest coverages in the world (as of 2007, 70% of 12–17-year-old girls nationwide completely vaccinated with the 3-dose regimen), attributes its success to the publicly funded school-based HPV immunization program (56). The high enrollment rate in schools serves as a key facilitator of high coverage, as 98.5% of girls aged 14 are enrolled in schools in Australia (56). In contrast, an estimated 18.6 million girls (23%) aged 6–11 years old are not enrolled in primary schools in sub-Saharan Africa (57). Gavi-eligible countries have proposed many strategies to locate hard-to-reach girls, such as enumeration by community health workers and mapping health facilities (55), but more research is needed to evaluate the currently existing programs and develop reproducible, validated interventions (58).
HPV vaccine introduction efforts have faced more challenges than the pneumococcal conjugate, rotavirus and inactivated polio vaccines, which can be readily integrated into existing infant immunization schedules. While low school attendance in some settings limits school-based routine vaccination, most LMICs have well-established infant immunization programs (59, 60). The competition for resources to scale-up HPV vaccine programs will likely worsen in LMICs due to the COVID-19 pandemic (61), as prevention activities have been disrupted (62), and vaccine coverage is expected to decrease even in high-income countries (63). Development of an HPV vaccine that can be safely administered to infants and maintain immunity into adulthood like the Hepatitis B vaccine would bypass some of the current logistical and financial challenges and expand access and coverage.
Immune correlates of protection against HPV are poorly understood (64, 65), preventing optimization of vaccine dosing schedules, such as through multi-cohort vaccination or reduced-dosing strategies (66, 67). A better understanding of serological correlates of protection can help define the vaccine dosage needed for protection (65). In particular, reduced-dose vaccination, either with a single dose or extended interval dosing (at least 12 months between the first and the second dose) (66), would have significant programmatic implications and allow for increased vaccination coverage in settings with limited healthcare access, infrastructure, personnel, and financial resources (68, 69). Among women who did not complete the required number of HPV vaccine doses, one dose induced robust immune responses with comparable decreases in precancerous lesions compared to women who received multiple doses (70, 71). Clinical trials to evaluate single-dose HPV vaccine efficacy and the duration of protection are underway to inform decisions about adoption of a one-dose regimen (see NCT03675256, NCT03747770, NCT03728881 on ClinicalTrials.gov) (72).
For all scenarios of expanded HPV vaccine coverage, clear communication addressing vaccine hesitancy and increasing parents', caregivers', and adolescents' acceptance of an HPV vaccine are critical. Unfortunately, literature on vaccine hesitancy from LMICs is scarce (73). In their 2014 report, the WHO's Strategic Advisory Group of Experts on Immunization defined vaccine hesitancy as “delay in acceptance or refusal of vaccination despite availability of vaccination services” and posited that it is a “complex and context specific” phenomenon (74). The 2018 Wellcome Global Monitor showed that 95% and 92% of those in South Asia and East Africa perceived the vaccine to be safe, compared to the 59% of the participants in Western Europe (75). As for all vaccines, there needs to be a continued, concerted effort to build trust and send consistent messages about the safety and effectiveness of the HPV vaccines, especially for children (76).
According to the WHO/United Nations International Children's Emergency Fund's joint reporting from 2015 to 2017, factors related to religion, culture, gender, and socioeconomic status were becoming more prominent in vaccine hesitancy in low-income countries, and risk-benefit (scientific evidence) factors in lower-middle-income countries (77). After the launch of a national HPV immunization program in 2012, Colombia reached a first dose coverage level of 94.8% among the target population (girls aged 9–17) by 2014 (78). However, crisis ensued in 2014, during which over 500 girls from a town called Carmen de Bolivar who had received the HPV vaccine months before reported adverse symptoms, such as headache, shortness of breath, and fainting, which was covered widely by the media (79). Although epidemiologic evaluation found no association between the HPV vaccine and the adverse symptoms, public confidence in the vaccine decreased and led to discontinuation of school-based programs (78, 80). The 2016 national coverage rates fell to as low as 14 and 5% for the first and second dose, respectively (79). However, with the help of projects geared toward restoring confidence in HPV vaccination, such as a roundtable of stakeholders and experts including the Ministry of Health and universities, and a communication intervention targeting communities with the highest cervical cancer mortality rates, first dose coverage rose to 34% in 2019 (78). Additionally, media coverage of unconfirmed reports of adverse events in Japan has led their HPV vaccination rate among adolescents born in and after 2002 to drop from about 70% in 2013 to 1% or less in 2019 (81). Experiences in Colombia and Japan demonstrate the importance of community acceptance of the HPV vaccine for achieving and maintaining high coverage. In summary, the priority research questions for primary prevention strategies are:
Do voluntary male circumcision, condoms, smoking cessation, and treatment for bacterial vaginosis reduce persistent HPV infection, and to what extent?
How can monitoring and evaluation provide evidence to support best practices for HPV vaccination programs?
How can we address vaccine hesitancy and the anti-vaccination movement to increase HPV vaccine coverage?
What are the safety, efficacy, durability, and acceptability of an HPV vaccine administered in infancy?
What are the efficacy and durability of one-dose HPV vaccination in routine and catch-up vaccination?
How can we obtain LMIC context-specific estimates of the impact of prevention strategies and make them more reliable and approachable for policy makers?
Secondary Prevention
Current State of Science
Screening of Precancerous Lesions
The WHO's cervical cancer elimination targets are to screen 70% of women with a high-performance test by age 35, and again by age 45 (6). Self-reported lifetime prevalence of cervical cancer screening among women in 55 LMICs was only 43.6%, ranging widely from 0.3 to 97.4% (82). A modeling analysis of LMICs predicted that one lifetime screen could lead to elimination in 96% of LMICs by the end of the century, compared to elimination in 60% of LMICs with HPV vaccination only (8).
Screening for cervical cancer is currently conducted by cytology (Papanicolaou/Pap smear screening), HPV DNA testing, or VIA. VIA is currently the predominant cervical cancer screening method in many LMICs due to its relatively low cost and ease of execution compared to cytology or HPV testing (83). VIA can facilitate same-day screen-and-treat algorithms that minimize loss-to-follow-up when coupled with treatment of detected lesions (84). However, the performance of VIA in detecting HSIL or CIN2-3 varies widely (Table 4), as the interpretation of the results is subjective (92). Quality assurance is another challenge, as the number of micro-invasive cancers that are undetected by the screen-and-treat method of VIA and cryotherapy is unknown (92). About 90% of women who screen positive with VIA and receive treatment are unlikely to have pre-malignant cervical lesions, which raises concern for overtreatment (90, 93–95).
Table 4.
Summary of sensitivity and specificity of cervical cancer screening methods for detecting CIN2-3.
| Sensitivity (%, 95% CI) | Specificity (%, 95% CI) | References |
|---|---|---|
| CYTOLOGY | ||
| 65.9 (54.9–75.3)* | 96.3 (94.7–97.4) | (85) |
| 75.5 (66.6–82.7)† | 91.9 (88.4–94.3) | (86) |
| HPV DNA TESTING | ||
| 88.1(81.4–92.7) | 83.7 (74.9–89.8) | (87) |
| 88.3 (73.1–95.5) | 73.9 (50.7–88.7) | (87) |
| 94.0 (89.0–97.0) | 88.0 (84.0–92.0) | (88) |
| VISUAL INSPECTION WITH ACETIC ACID | ||
| 79.2 | 84.7 | (89) |
| 82.4 (76.3–87.3) | 87.4 (77.1–93.4) | (90) |
| 78.0 (73.0–83.0) | 88.0 (85.0–91.0) | (91) |
| 69.0 (54.0–81.0) | 87.0 (79.0–92.0) | (91) |
| VISUAL INSPECTION WITH ACETIC ACID AND LUGOL'S IODINE | ||
| 89.0 | 85.0 | (90) |
| 90.0 (85.0–94.0) | 83.0 (79.0–86.0) | (91) |
Conventional Pap.
Liquid-based cytology.
Endpoints are all CIN2-3, reference standard = colposcopy with or without biopsy.
While cytology-based screening is used widely in high-income settings, it requires established healthcare infrastructure, repeat client visits to diagnose precancer, training of pathologists and clinicians, and a robust quality assurance program. Cytology-based screening also has lower sensitivity and specificity compared to HPV DNA testing (12). For these reasons, cytology-based screening is not recommended for scale-up of cervical cancer screening in LMICs (96).
The wide range of sensitivity and specificity of subjective tests, such as cytology and VIA leads to variation in the number of women referred to triaged for treatment. For example, estimates of sensitivity to detect CIN2-3 varied between 40.7 and 73.7% using cervical cytology and 21.9–73.6% using VIA in one study conducted in India, Nicaragua and Uganda (97). Variation in the number of women referred to triage for treatment can have significant implications for the risk of overtreatment and for already-overburdened health systems in low-resource settings.
HPV DNA testing has superior sensitivity compared to cytology and VIA in detecting CIN2-3 (Table 4) and has been used to either replaced cytology as a primary screen or been offered as a co-test (98, 99). The test has a high negative predictive value for detecting CIN2-3, which has the advantage of elongating the screening interval to 5 years for screen-negative women compared to 3 years for cytology (6). However, it also has a low specificity and positive predictive value, which can lead to overtreatment especially among younger women (100).
Large-scale randomized controlled trials have demonstrated the efficacy of HPV testing in reducing cervical cancer incidence, and that HPV testing provides 60–70% greater protection against invasive cervical cancer than cytology (101). Despite also demonstrating cost-effectiveness compared to cytology in multiple settings (102–104), HPV testing has been difficult to scale-up in LMICs due to cost (12). However, there have recently been several successful demonstration projects, as well as the launch and the scale-up of national HPV testing projects in Latin America using careHPV (105). Notably, the Ministry of Health of El Salvador has updated its guidelines to recommend HPV testing (106), Guatemala and Honduras are planning to use HPV testing for cervical cancer screening after demonstration projects (105), and Argentina and Mexico have been offering HPV testing in their public health systems for several years (107).
The WHO endorses self-sampled HPV tests as an additional approach to cervical cancer screening for individuals aged 30–60 years (108). In a meta-analysis, sensitivity or specificity for detecting CIN2-3 was not statistically different between self-sampled and clinician-sampled HPV tests based on polymerase chain reaction (PCR) assay (109). However, self-collected hrHPV assays based on signal amplification (careHPV) had lower sensitivity (pooled ratio: 0.85, 95% CI: 0.80–0.89) and lower specificity (pooled ratio: 0.96, 95% CI: 0.93–0.98) to exclude CIN2-3 compared to the clinician-collected samples. Any potential loss in sensitivity is likely outweighed by increased screening uptake (110). Both self-sampled and clinician-sampled HPV tests with a PCR assay have better sensitivity and can allow for longer screening intervals than cytology-based screening programs. For example, a 15-year cohort study in rural China found that while HPV testing with self-sampling was less sensitive than physician-sampling, it performed equally on screening efficiency and predicting cumulative cases, and was as sensitive as high-quality cytology in detecting cumulative CIN2-3 cases (111).
Self-sampling as an alternative method of screening can overcome barriers, such as access to the health facility and fear associated with pelvic examination, opening new possibilities of reaching underscreened women (112). Acceptability of self-sampling has been reported across cultures and resource-settings (113–117). HPV self-sampling has the potential to make HPV testing more affordable and cost-effective by increasing screening coverage (118). One meta-analysis found women who were offered the option of self-sampling were twice as likely to participate in cervical cancer screening services than those who were offered cytology, VIA, or clinician-collected HPV testing (119). The difference was greater when the sampling kits were sent directly to women's homes or offered door-to-door by a health worker (119). Self-sampling has been incorporated into the national screening guidelines in high income countries, such as the Netherlands, Australia, and Finland (112). Among LMICs, self-sampling was successfully scaled-up in Jujuy province in Argentina (120). Additional research is needed on best practices for self-sampling follow up, such as community collection of specimens, delivery of results, and linkage to treatment.
Treatment of Precancerous Lesions
The international consensus is to treat CIN2-3/HSIL by ablation or excision except during pregnancy (121). Cryotherapy and thermal ablation (the latter also known as cold coagulation or thermocoagulation) are the two most commonly used ablative treatments, and LEEP and cold knife conization are commonly used excisional treatments. Meta-analyses that compared the efficacy of cryotherapy, thermal ablation, LEEP, and cold knife conization are summarized in Table 5.
Table 5.
Summary of efficacy of LEEP, cold knife conization, cryotherapy, and thermal ablation.
| Method | Endpoint | Pooled cure proportions (%, 95% CI) | References |
|---|---|---|---|
| LEEP | |||
| CIN2-3 negative after 12 months | 94.7 (96.3–93.1) | (122) | |
| HSIL negative after 12 months follow up | 92.0 (N/A) | (123) | |
| COLD KNIFE CONIZATION | |||
| CIN2-3 negative after 12 months | 98.6 (99.2–98.0) | (124) | |
| CRYOTHERAPY | |||
| CIN2-3 negative after 6 months in LMICs | 82.6 (77.4–87.3) | (125) | |
| CIN2-3 negative at follow up (duration unspecified) | 86.0 (83.0–89.0) | (126) | |
| CIN2-3 negative after 12 months | 94.7 (96.1–93.2) | (124) | |
| HSIL negative after 12 months follow up | 80.9 (N/A) | (123) | |
| THERMAL ABLATION | |||
| CIN2-3 negative after 6+ months | 91.6 (88.2–94.5) | (125) | |
| CIN2-3 negative after 6+ months (LMICs only) | 82.4 (75.4–88.6) | (125) | |
| CIN2-3 negative after 4–6 months | 93.6 (90.8–96.0) | (127) | |
The cervical lesion must be small enough to be covered by the equipment and fully visible with no extension into the endocervix or onto the vaginal wall to be eligible for ablative therapy (121, 128). An estimated 50–80% of cervical lesions found during screening are eligible for ablative therapy (128). In meta-analyses, 81–95% of the women treated with cryotherapy were negative for CIN2-3 12 months after treatment (Table 5) (125, 129). Similarly, 82–94% of the women treated with thermal ablation were negative for CIN2-3 after treatment; however, the duration of post-treatment follow-up was shorter relative to studies on cryotherapy. In LMICs, the efficacy of cryotherapy and thermal ablation was 83 and 82%, respectively (124).
The WHO has recommended thermal ablation for women who have histologically confirmed CIN2-3 or have screened positive in screen-and-treat strategies because of its advantages for implementation in LMICs (128). The traditional gas-based cryotherapy is difficult to implement because refrigerant gas, such as CO2, is expensive and difficult to procure and transport (128). Thermal ablation devices can be battery-operated, are lightweight (2–5 kg), and have a shorter treatment time (128). The use of thermal ablation in low-resource settings has been shown to be effective and acceptable (130). In Zambia, thermal ablation (44%), cryotherapy (48%), and LEEP (47%) achieved similar hrHPV clearance 6 months after treatment (131). It should be noted that up to 54% of the participants in each study arm were seropositive for HIV, and women living with HIV have a higher risk for treatment failure of precancerous lesions (131). The prevalence of treatment failure is unknown in this study, as no histological studies were performed prior to treatment. There are several ongoing and completed clinical trials on development and evaluation of the use of thermal ablation devices for LMIC settings (see NCT02956239, NCT03429582, NCT03510273 on ClinicalTrials.gov) (72).
LEEP is recommended for treating CIN2-3 if there is a medical contraindication for ablative therapy (121) or if the lesion extends into the endocervical canal (132). LEEP is often preferred over ablative techniques in high-resource settings because of its benefit of histopathologic diagnosis (132). Two meta-analyses found that 92–95% of the women treated with LEEP and 99% of the women treated with cold knife conization were free of CIN2-3 or HSIL 12 months post-treatment (Table 5) (123, 124). CIN2-3 persistence (RR: 0.87, 95% CI: 0.76–0.99) and recurrence (RR: 0.91, 95% CI: 0.84–0.99) were lower with LEEP than with cryotherapy (123).
Treatment complication rates differ by the technique. The most common adverse events associated with treatment are bleeding and infection at the cervix, which occur in <5% of treated women (126, 127, 133–135). Relative to LEEP and cold knife conization, cryotherapy and thermal ablation are cheaper, safer, and simpler to use, which makes scale-up and task-shifting more feasible, particularly in LMICs (123, 124, 136). For example, it is easier to train nurses or lay health workers to perform cryotherapy than LEEP or cold knife conization, because of the lower risk of serious complications (129). Women who previously underwent cold knife conization had the highest risk of subsequent perinatal mortality (RR: 2.87, 95% CI: 1.42–5.81) and preterm delivery at <32–34 weeks (RR: 2.78, 95% CI: 1.72–4.51), compared to the women who did not receive this procedure (136). Thermal ablation was not included in the comparisons of complications.
Priority Research Questions for Secondary Prevention Strategies
Screening and Triage of Precancerous Lesions
Point-of-care cervical cancer screening tests that facilitate same-day treatment would minimize loss to follow-up and improve continuity of care. While careHPV meets some of the REASSURED criteria (real-time connectivity, ease of specimen collection, affordable, sensitive, specific, user-friendly, rapid, equipment-free, delivered) (87, 137), and has a point-of-care platform (138), it requires batch testing (106), which enables same-day treatment only under special circumstances, such as health campaigns, and the sensitivity is significantly lower with self-sampling (109). In addition, the final cost after implementation has not been consistently affordable. For example, the per-test cost estimate was reported as $42 USD in a pilot careHPV-based screening program in Myanmar, despite some economic analyses based in low-income countries estimating costs as low as $5 USD (139). Although PCR-based point-of-care tests are currently available [e.g., GeneXpert (Cepheid, Sunnyvale, CA)] and graded pricing exists for LMICs, it is still cost-prohibitive and the required infrastructure is a barrier (140). Real-time PCR-based tests, such as AmpFire (Atila BioSystems, Inc., Mountain View, CA) (141) and Q-POC (QuantuMDx, Newcastle upon Tyne, UK) are being evaluated and developed, which will have important implications for scaling up self-sampling. More innovation is needed to make more point-of-care tests and tools affordable and widely available.
Strategies based on identifying biomarkers of HPV-associated oncogenesis are being developed to improve the precision of current screening methods and prevent the physical, psychological, and financial harms of overtreatment (142). Some examples of biomarkers include, but are not limited to, p16ink4a and E6/E7 oncoproteins (121, 142–144). Tests for E6/E7 oncoproteins present a promising option for triaging screen-positive women given high positive predictive value and limited laboratory equipment (145). However, more than 60% of HPV positive women were also E6/E7 mRNA positive (146), which would present a challenge in identifying women with precancerous lesions. A systematic review of three types of HPV E6/E7 mRNA tests (Aptima, Quantivirus, and PreTect Proofer) found that while the tests have diagnostic relevance to detect CIN2-3, the higher specificity of some tests is due to the limited number of HPV types it detects (147). In a 10-year prospective cohort study in China, HPV methylation and co-testing with E6 oncoprotein showed superior area under curve values compared to cytology, viral load, and VIA (148). However, it has limitations of detecting only two HPV types. Clinician- and self-sampled first-void urine are also being evaluated as sources of viable biomarkers for detecting cervical precancers (149). In a randomized controlled trial among a Pacific Island population, HPV detection in self-collected urine (using Roche Cobas 4800 system, Roche Molecular Systems, Inc.) demonstrated moderate agreement with clinician-collected cervical samples (Kappa = 0.55, 95% CI: 0.43–0.66), with agreement for detection of hrHPV among women ages 40 and older being higher (Kappa = 0.65, 95% CI: 0.46–0.85) than that of women ages 20–39 (Kappa = 0.45, 95% CI: 0.25–0.64) (150). Formal evaluation of biomarker-based triage is needed in LMICs (145).
Several optical techniques are in development, including spectroscopy and other imaging methods (151). Such techniques have the potential to reduce the number of required visits and to save time and cost, which can be helpful especially where infrastructure for laboratories is sparse (151). For example, redesigned portable colposcopes have a high agreement with standard-of-care colposcopy for pathology (see NCT00602368 as an example) (72, 152–154). A smartphone-based colposcope can enhance VIA by taking digital images of the cervix and uploading them to an online repository for remote decision support (152). Additionally, automated visual evaluation of images with machine learning, trained using >60,000 images of the cervix from a Costa Rican tumor registry has shown greater accuracy than traditional VIA or cytology in detecting HSIL (155, 156). This algorithm can increase screening capacity and minimize subjectivity (157). Devices which could be inserted by the women herself for remote visualization of the cervix are also being developed and tested (158). However, a large (n = 9,406) ongoing study in Nigeria noted that the squamocolumnar junction where cancers arise was not fully visible for almost 64.6% of women by age 49 using enhanced visual assessment (MobileODT, Israel) (159). Challenges to adequate visualization of the cervix have pertinent implications for both ablative treatments and visual screening or triage efforts (159).
Treatment of Precancerous Lesions
Identification of women with a precancerous lesion necessitates appropriate linkage to care and treatment to prevent cancer (160). While thermal ablation has been widely recommended and is being adopted in low-resource settings, further data is needed for it to become the new standard for treating patients with precancerous lesions (161).
To optimize screen-and-treat methods, portable treatment tools, such as battery-operated cryotherapy instruments are under development (see NCT03084081) (72, 162). There are also new, portable, solar-powered (128), and battery-powered thermal ablation tools, which can be adapted to low-resource settings without stable electricity (see NCT02956239, NCT03429582) (72, 128, 162).
Photodynamic therapy has shown promising results in the treatment of CIN (163, 164). This novel technology selectively accumulates photosensitizers in pathologic tissue to destroy tumor cells by inducing necrosis (163, 165). Such technology has the potential to be a tissue-preserving treatment alternative and to minimize costs (164).
The development of therapeutic vaccines against hrHPV could help women who are already infected by stopping progression, triggering regression of lesions, and preventing recurrence of disease (166). Currently, there is no therapeutic HPV vaccine approved by the US Food and Drug Administration (167). While the use of therapeutic vaccines to treat invasive cervical cancer or other HPV-related cancers is beyond the scope of this review, many of the completed and currently ongoing trials (see NCT02481414, NCT00054041, NCT01022346, NCT03870113 as examples, not a comprehensive list) use CIN and/or HSIL as the treatment target. Smalley Rumfield et al. recently conducted a review of peptide, protein, viral vector, bacterial vector, cell, DNA, and RNA-based therapeutic vaccines as well as multi-platform and combination therapies, which demonstrate diverse potential therapies that can be useful in LMIC settings, while presenting new and different challenges (167).
In summary, the priority for secondary prevention is the optimization and scale-up of single-visit screen-and-treat modalities. Self-sampling for HPV testing offers several advantages to optimize screening coverage. Subsequent molecular oncogenic evaluation has the potential to detect lesions that are most likely to progress to cancer while reducing overtreatment. Further development is needed to simplify the testing procedure and reduce costs. The feasibility of integrating cervical cancer screening into the existing healthcare system is being explored (168, 169). For example, delivery models that leverage established HIV care infrastructure, such as staff and coordination between the clinics, can screen-and-treat women for cervical cancer to maximize efficiency (170). Other research questions related to secondary prevention based on our gap analysis include:
Is the scale-up of current point-of-care HPV tests and HPV self-sampling effective, feasible, and cost-effective, and how can delivery models adapt sustainably to incorporate them?
What is the most efficient and safe model of task-shifting for providing cervical cancer screening, cervical biopsy and treatment of pre-invasive disease?
What is the optimal triage algorithm and follow-up for hrHPV positive women and those without visible precancerous lesions when biopsy is unavailable?
Are there reliable biomarkers to predict persistent infection with hrHPV?
Would self-visualization of the cervix as a screening tool be feasible, reliable, and acceptable?
What are the strategies for surveillance of HPV positive women with negative oncogenic biomarkers?
Cervical Cancer Elimination Among Women Living With HIV
Current State of Science
Compared to women without HIV, women living with HIV have at least 2-fold higher HPV prevalence (171, 172), experience greater persistence of HPV infection (173, 174) and have more rapid progression of precancerous lesions to HPV-associated cancers (175–178). Women living with HIV are also significantly more likely to have multiple hrHPV types detected in normal cytology, HSIL, and cervical cancer cells or tissue (179, 180). A meta-analysis showed an estimated 33,999 new cases of cervical cancer occurred among women living with HIV in 2018, corresponding to 5.8% of cases (181). In this study, women living with HIV had an overall pooled relative risk of 6.07 (95% CI: 4.40–8.37) of developing cervical cancer compared to their counterparts without HIV. When markedly immune-suppressed (i.e., CD4+ cell count <200 cells/ul), the risk for cervical cancer is 8-fold higher in women living with HIV compared to HIV-negative women (182). The risk of cervical cancer among women living with HIV can be mitigated to some extent with sustained antiretroviral therapy (183, 184). In a population-level analysis in Botswana, women living with HIV on antiretroviral therapy had a lower prevalence of hrHPV than those not on antiretroviral therapy (RR: 0.83, 95% CI: 0.70–0.99) (184). However, in settings with low primary and secondary prevention coverage, cervical cancer incidence and mortality among women living with HIV are high even when antiretroviral therapy is available (185). This is thought to be due to prolonged survival of effectively treated women (184).
HPV vaccines elicit high seroconversion rates and type-specific antibody levels among adolescent girls and young women living with HIV (186–195). CD4+ cell count at vaccination is positively correlated with seroconversion and immune response (188, 191–193). Although seropositivity and antibody levels decline more rapidly among vaccinated women living with HIV compared to vaccinated women without HIV, they are significantly higher compared to unvaccinated women living with HIV and naturally infected women without HIV (187, 190–192). As the correlate of protection against HPV is unknown, the relatively lower antibody level does not necessarily mean lower vaccine efficacy. The bivalent, quadrivalent, and nonavalent vaccines are safe for women living with HIV, as all three are virus-like particle-based vaccines (187, 192, 196).
The endpoints in most vaccine trials in HIV-positive populations are HPV seroconversion rates and immunogenicity. Studies using clinical endpoints (i.e., HPV infections and cervical abnormalities) as the outcome are still needed. The duration of protection in vaccinated adolescent girls and young women living with HIV is unknown. Extending the age of vaccination to include infants and older women could accelerate cervical cancer elimination in HIV-positive populations, although the efficiency and cost-effectiveness of such strategies depend on the prevalence of HPV infection at older ages and the duration of protection afforded by the vaccines. Context-specific modeling work would be valuable to evaluate these outcomes and inform the implementation of effective vaccination programs in settings with high HIV prevalence. In addition, the vaccine efficacy and durability of a reduced dose schedule (two or one doses) among adolescents and young adults living with HIV needs to be determined. The OPTIMO Trial, which aims to see if fewer doses can be used for children/adolescents living with HIV, will begin soon (see NCT04265950) (72).
Prevention strategies, such as VMMC can both help prevent HIV and reduce cases of cervical cancer. A modeling study of HIV prevention and HPV control in Tanzania predicts that VMMC will lower cervical cancer incidence and mortality rates by 28 and 26%, respectively, by 2070 (197).
The American Society for Clinical Oncology and the WHO recommend screening sexually active women living with HIV for HPV or cervical abnormalities as soon as they are diagnosed with HIV and rescreening within 3 years if they are HPV-negative and free of cervical lesions (121). In the United States, women living with HIV below age 30 are recommended to receive Pap screening within 1 year of onset of sexual activity regardless of the mode of HIV transmission and no later than age 21 (198). One study in the United States found that the risk of cervical cancer among regularly-screened women living with HIV was similar to HIV-negative women, highlighting the importance of screening in this population (198).
HIV presents challenges to accurate screening for women. There are more false-positive rates with VIA among women living with HIV than women without HIV (199, 200), likely due to the higher rates of cervical inflammation (201, 202). While HPV testing is an effective screening method among women living with HIV, it could lead to overestimates of cervical lesion prevalence and overtreatment (203).
Treatment failure and recurrence is more common among women living with HIV than in the general population (204). A meta-analysis found that treatment failure was twice as common among women living with HIV as among HIV-negative women (OR: 2.7, 95% CI: 2.0–3.5) (204). Women living with HIV in Sweden were five times more likely to experience recurrence than HIV-negative women (hazard ratio: 5.0, 95% CI: 2.1–11.6) (205). Women living with HIV in Kenya whose high-grade lesions were treated with cryotherapy experienced a significantly higher rate of recurrence than those treated with LEEP over 24 months (206). More research is needed to determine the most appropriate treatment method for precancerous lesions in this population.
Cervical cancer prevention services can be integrated into existing health infrastructure as women already engage in health care throughout their lifetime (e.g., antenatal care, well-child visits, and family planning. Integration of cervical cancer screening with HIV care is acceptable to women living with HIV and feasible on a small scale, however, more data are needed to determine scalability and sustainability (169).
Priority Research Questions for Cervical Cancer Elimination Among Women Living With HIV
What is the duration of protection in vaccinated adolescent girls and young women living with HIV?
What is the clinical efficacy of a reduced HPV vaccine dose (e.g., two-dose or single-dose) schedule in women living with HIV?
What are innovative ways of effectively integrating cervical cancer prevention and treatment into HIV care?
What is the optimal treatment method of precancerous lesions among women living with HIV?
What are the optimal screening modalities and intervals for women living with HIV on antiretroviral therapy?
Discussion
Global cervical cancer elimination is achievable with an increase in HPV vaccine uptake and coverage, implementation of screening and treatment strategies and emerging technologies, and development of innovative delivery approaches. Our scoping review designed a roadmap that prioritizes expanding HPV vaccination and collaborating with global organizations to allocate resources needed to eliminate cervical cancer. The focus of the concerted effort must be (1) scaling up evidence-based interventions, including the application of implementation science and measurement of population-level impact, and (2) filling the gaps through research and harnessing emerging innovations that are simple, effective, and affordable for all settings (Table 6).
Table 6.
Innovative technologies and approaches that may be appropriate for comprehensive prevention packages.
| Priorities | Recommended intervention | Rationale |
|---|---|---|
| PRIMARY PREVENTION | ||
| Increase access to and coverage of HPV vaccination by the sustainable implementation of HPV immunization programs | Reduce vaccination dosage | Evidence that a single-dose is as protective as a multi-dose regimen is emerging (173, 207, 208). A single-dose regimen can be as cost-effective as the two-dose regimen, if high coverage can be achieved in low-resource settings (209, 210). |
| SECONDARY PREVENTION | ||
| Maximize early detection of precancers and micro-invasive disease without the harms of overtreatment by increasing cervical cancer screening coverage with HPV testing and treatment starting at age 30 for at least 35 years for women without HIV | HPV testing, focusing on self-sampling | HPV DNA testing has superior sensitivity compared to cytology and VIA in detecting CIN2-3 (99, 209, 211). Self-sampling can overcome individual and structural level barriers to traditional screening methods (212, 213). It has demonstrated similar accuracy as clinician-collected samples (109, 119), and is accepted across cultures and resource-settings (214). |
| Triage HPV positive women with enhanced visual assessment or a low-cost test for oncogenesis markers | The triage methods used in high-resource settings, such as cytology, colposcopy, and HPV genotyping are not ideal for low-resource settings because of their need for multiple visits, equipment, and personnel (12, 118). Innovations, such as a portable colposcope, enhanced visual assessment that utilizes mHealth and artificial intelligence, and low-cost rapid biomarker tests can accurately stratify women by the risk of progression to invasive cancer and make the process more efficient (145). | |
| Treat eligible precancerous lesions with thermal ablation | Thermal ablation has shown comparable efficacy to cryotherapy in treating ablation-eligible CIN2-3 in a shorter amount of time (125), and is easier to implement in LMICs than cryotherapy because it does not need CO2 (128) and devices are battery-operated and portable. | |
Lessons can be learned from the global response to HIV, which built the infrastructure that allowed for the scale-up of HIV prevention and treatment services. Since the global initiative to eliminate mother-to-child transmission of HIV was announced in 2011 (215), 80% of expecting mothers with HIV received antiretroviral therapy (ART) as part of their antenatal care (compared to 17% in 2010), and transmission dropped below 5% in several high HIV burden countries in sub-Saharan Africa (216). The joint United Nations Programme on HIV/AIDS (UNAIDS) recommended an HIV prevention “package” that combines numerous types of interventions targeting HIV transmission and treatment at multiple levels to address the various interacting risk factors of HIV (215, 217). Similar strategies can be used to combine contextually appropriate cervical cancer prevention and treatment services. Community-based clinical trials may accelerate program scale-up and increase uptake of interventions.
Following the framework of the logic model, key domestic and global stakeholders should work together to prioritize funding to procure vaccines and strengthen healthcare systems. Healthcare practitioners and communities should be engaged at every step of discussion and programmatic planning in order to build capacity and ensure successful implementation (218). Global advocacy and partnerships are needed to continue the ongoing support for HPV vaccine coverage and increased access to low-cost screening and treatment tools. For both primary and secondary prevention strategies, access to and coverage of efficacious interventions over a woman's lifetime must be prioritized. The current disparities in morbidity and mortality are likely to worsen as additional innovations emerge and are more readily adopted in high-resource than low-resource settings.
While reviewing tertiary prevention strategies (e.g., treatment of invasive cervical cancer) is beyond the scope of this article, their importance in low-resource settings cannot be overlooked. Screening implies the capacity and ethical responsibility for health agencies to make the treatment of cervical cancer available. The regions with the highest prevalence of cervical cancer have the lowest availability of skilled personnel and treatment facilities for diagnosis, surgery, chemotherapy and radiation (219, 220). The shortage of radiotherapy equipment and gynecological oncologists is a barrier to care for women with invasive cervical cancer in LMICs (220). Twenty-nine countries in Africa do not have a radiation unit (221). Gynecological oncologists are often limited to tertiary care hospitals, and women with invasive cancer have to travel considerable distances or wait for a long duration to access treatment (222). Twenty-five countries in Africa and two countries in Asia had an extreme shortage of clinical oncologists, defined as more than 1,000 incident cancers per clinical oncologist (222). Decentralization of services, where a local expert at the primary care center is supervised and mentored by a specialist, can increase access to specialty care for women living in rural or remote areas (223). Such a model can also serve as a community-based hub for dissemination of vaccines and screening efforts, such as HPV self-sampling, thereby increasing equitable access to cancer care at all levels of prevention.
The limitations of this review result from its narrative approach. Compared to systematic reviews or meta-analyses, narrative reviews are characterized by subjective study selection. In addition, due to the broad nature of the scoping review, we did not compile an exhaustive list of potentially relevant, innovative strategies and technologies. However, this paper provides an overview of the current landscape of science around cervical cancer elimination and guides the formulation of pertinent questions that deserve further exploration.
Conclusions
The effort to eliminate cervical cancer must focus on sustainable and continuous access to prevention strategies. Large scale demonstration projects have been successfully implemented across resource settings for HPV vaccination and screen-and-treat using HPV testing and thermal ablation. Building on the lessons learned, we propose a demonstration project that combines the above-recommended strategies and provides a comprehensive cervical cancer prevention continuum to show that cervical cancer elimination can be achieved at the local level within LMICs. With a strong evidence base and effective implementation established, strategies can be scaled up more broadly. By strategically and skillfully putting scientific advances to practice, global cervical cancer elimination can be achieved.
Author Contributions
MS and GL: methodology, data curation, writing—original draft, and writing—review and editing. NM, PG, and JW: conceptualization and writing—review and editing. DR, CB, LE, and LP: writing—review and editing. RB: conceptualization, writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Life Sciences Librarian Diana Louden at the University of Washington for assistance with the literature search.
Footnotes
Funding. This publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1 TR002318 and by the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network (CISNET) under Grant number U01CA253912. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Cancer Institute.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.670032/full#supplementary-material
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Cervical Cancer 2018. Available online at: https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/
- 3.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. (2004) 364:249–56. 10.1016/S0140-6736(04)16674-9 [DOI] [PubMed] [Google Scholar]
- 4.Canfell K, Sitas F, Beral V. Cervical cancer in Australia and the United Kingdom: comparison of screening policy and uptake, and cancer incidence and mortality. Med J Aust. (2006) 185:482–6. 10.5694/j.1326-5377.2006.tb00661.x [DOI] [PubMed] [Google Scholar]
- 5.Patel C, Brotherton JM, Pillsbury A, Jayasinghe S, Donovan B, Macartney K, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro Surveill. (2018) 23:1700737. 10.2807/1560-7917.ES.2018.23.41.1700737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Global Strategy Towards the Elimination of Cervical Cancer as a Public Health Problem. World Health Organization; (2020). [Google Scholar]
- 7.Hall MT, Simms KT, Lew JB, Smith MA, Brotherton JM, Saville M, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. (2019) 4:e19–27. 10.1016/S2468-2667(18)30183-X [DOI] [PubMed] [Google Scholar]
- 8.Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. (2020) 395:575–90. 10.1016/S0140-6736(20)30068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol. (2019) 20:394–407. 10.1016/S1470-2045(18)30836-2 [DOI] [PubMed] [Google Scholar]
- 10.Vaccarella S, Laversanne M, Ferlay J, Bray F. Cervical cancer in Africa, Latin America and the Caribbean and Asia: Regional inequalities and changing trends. Int J Cancer. (2017) 141:1997–2001. 10.1002/ijc.30901 [DOI] [PubMed] [Google Scholar]
- 11.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. (2016) 4:e453–63. 10.1016/S2214-109X(16)30099-7 [DOI] [PubMed] [Google Scholar]
- 12.Basu P, Mittal S, Bhadra Vale D, Chami Kharaji Y. Secondary prevention of cervical cancer. Best Pract Res Clin Obstetr Gynaecol. (2018) 47:73–85. 10.1016/j.bpobgyn.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 13.Canfell K, Kim JJ, Brisson M, Keane A, Simms KT, Caruana M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. (2020) 395:591–603. 10.1016/S0140-6736(20)30157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. International J Soc Res Methodol. (2005) 8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 15.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Human Papillomavirus (HPV) and Cervical Cancer 2019. (2019). Available online at: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
- 17.Centers for Disease Control and Prevention . CDC Evaluation Documents, Workbooks and Tools. (2018). Available online at: https://www.cdc.gov/eval/tools/logic_models/index.html
- 18.Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, et al. Human papillomavirus vaccine introduction–the first five years. Vaccine. (2012) 30:F139–48. 10.1016/j.vaccine.2012.05.039 [DOI] [PubMed] [Google Scholar]
- 19.Brusselaers N, Shrestha S, van de Wijgert J, Verstraelen H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstetr Gynecol. (2019) 221:9–18.e8. 10.1016/j.ajog.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. (2016) 4:58. 10.1186/s40168-016-0203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, Martínez-Barnetche J, Cortina-Ceballos B, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS ONE. (2016) 11:e0153274. 10.1371/journal.pone.0153274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilhan ZE, Łaniewski P, Thomas N, Roe DJ, Chase DM, Herbst-Kralovetz MM. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine. (2019) 44:675–90. 10.1016/j.ebiom.2019.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. (2009) 374:301–14. 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- 24.Apter D, Wheeler CM, Paavonen J, Castellsague X, Garland SM, Skinner SR, et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vacc Immunol. (2015) 22:361–73. 10.1128/CVI.00591-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. (2007) 356:1928–43. 10.1056/NEJMoa061760 [DOI] [PubMed] [Google Scholar]
- 26.FUTURE II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. (2007) 356:1915–27. 10.1056/NEJMoa061741 [DOI] [PubMed] [Google Scholar]
- 27.Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. (2017) 390:2143–59. 10.1016/S0140-6736(17)31821-4 [DOI] [PubMed] [Google Scholar]
- 28.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. (2015) 372:711–23. 10.1056/NEJMoa1405044 [DOI] [PubMed] [Google Scholar]
- 29.Castle PE, Maza M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect. (2016) 144:449–68. 10.1017/S0950268815002198 [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . Human papillomavirus vaccines: WHO position paper, October 2014—recommendations. Vaccine. (2015) 33:4383–4. 10.1016/j.vaccine.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 31.Brisson M, Laprise JF. editors. Cost-Effectiveness of Extending HPV Vaccination Above Age 26 Years in the U.S. Advisory Committee on Immunization Practices, Atlanta, GA: (2019). [Google Scholar]
- 32.World Health Organization . 20 Million Children Miss Out on Lifesaving Measles, Diphtheria and Tetanus Vaccines in 2018 New York. Geneva: (2019). Available online at: https://www.who.int/news/item/15–07-2019–20-million-children-miss-out-on-lifesaving-measles-diphtheria-and-tetanus-vaccines-in-2018 [Google Scholar]
- 33.World Health Organization . Global Market Study—HPV. Geneva: World Health Organization; (2018). [Google Scholar]
- 34.Gavi: The Vaccine Alliance . HPV Vaccine Manufacturers Commit to Provide Enough Supply to Immunise At Least 84 Million Girls in Gavi Countries. Geneva: (2020). Available online at: https://www.gavi.org/news/media-room/hpv-vaccine-manufacturers-commit-provide-enough-supply-immunise-least-84-million [Google Scholar]
- 35.World Health Organization . Immunization, Vaccines and Biologicals: Data, Statistics and Graphics. Geneva: (2020). Available online at: https://www.who.int/teams/immunization-vaccines-and-biologicals/data-statistics-and-graphics [Google Scholar]
- 36.Kumar S, Khanduri A, Sidibe A, Morgan C, Torode J, Basu P, et al. Acting on the call: A framework for action for rapid acceleration of access to the HPV vaccination in low- and lower-middle-income countries. Int J Gynaecol Obstet. (2020) 152:32–9. 10.1002/ijgo.13482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GAVI: The Vaccine Alliance . Millions of Girls in Developing Countries to Be Protected Against Cervical Cancer Thanks to New HPV Vaccine Deals. (2013). Available online at: https://www.gavi.org/hpv-price-announcement
- 38.Ginsberg GM, Edejer TTT, Lauer JA, Sepulveda C. Screening, prevention and treatment of cervical cancer—a global and regional generalized cost-effectiveness analysis. Vaccine. (2009) 27:6060–79. 10.1016/j.vaccine.2009.07.026 [DOI] [PubMed] [Google Scholar]
- 39.Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health. (2014) 2:e406–14. 10.1016/S2214-109X(14)70237-2 [DOI] [PubMed] [Google Scholar]
- 40.Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine. (2013) 31:3786–804. 10.1016/j.vaccine.2013.06.060 [DOI] [PubMed] [Google Scholar]
- 41.Grabowski MK, Kong X, Gray RH, Serwadda D, Kigozi G, Gravitt PE, et al. Partner human papillomavirus viral load and incident human papillomavirus detection in heterosexual couples. J Infect Dis. (2016) 213:948–56. 10.1093/infdis/jiv541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson LE, Gravitt P, Tobian AA, Kigozi G, Serwadda D, Nalugoda F, et al. Male circumcision reduces penile high-risk human papillomavirus viral load in a randomised clinical trial in Rakai, Uganda. Sex Transm Infect. (2013) 89:262–6. 10.1136/sextrans-2012-050633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wawer MJ, Tobian AA, Kigozi G, Kong X, Gravitt PE, Serwadda D, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet. (2011) 377:209–18. 10.1016/S0140-6736(10)61967-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis MA, Gray RH, Grabowski MK, Serwadda D, Kigozi G, Gravitt PE, et al. Male circumcision decreases high-risk human papillomavirus viral load in female partners: a randomized trial in Rakai, Uganda. Int J Cancer. (2013) 133:1247–52. 10.1002/ijc.28100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Holmes KK, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. (2006) 354:2645–54. 10.1056/NEJMoa053284 [DOI] [PubMed] [Google Scholar]
- 46.Sarian LO, Hammes LS, Longatto-Filho A, Guarisi R, Derchain SF, Roteli-Martins C, et al. Increased risk of oncogenic human papillomavirus infections and incident high-grade cervical intraepithelial neoplasia among smokers: experience from the Latin American screening study. Sex Transm Dis. (2009) 36:241–8. 10.1097/OLQ.0b013e3181935a7d [DOI] [PubMed] [Google Scholar]
- 47.Eldridge RC, Pawlita M, Wilson L, Castle PE, Waterboer T, Gravitt PE, et al. Smoking and subsequent human papillomavirus infection: a mediation analysis. Ann Epidemiol. (2017) 27:724–30.e1. 10.1016/j.annepidem.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chao XP, Sun TT, Wang S, Fan QB, Shi HH, Zhu L, et al. Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Int J Gynecol Cancer. (2019) 29:28–34. 10.1136/ijgc-2018-000032 [DOI] [PubMed] [Google Scholar]
- 49.Klein C, Gonzalez D, Samwel K, Kahesa C, Mwaiselage J, Aluthge N, et al. Relationship between the cervical microbiome, HIV status, and precancerous lesions. mBio. (2019) 10:e02785-18. 10.1128/mBio.02785-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwasniewski W, Wolun-Cholewa M, Kotarski J, Warchol W, Kuzma D, Kwasniewska A, et al. Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol Lett. (2018) 16:7035–47. 10.3892/ol.2018.9509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kyrgiou M, Mitra A, Moscicki AB. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. (2017) 179:168–82. 10.1016/j.trsl.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Ma Y, Li R, Chen X, Wan L, Zhao W. Associations of cervicovaginal lactobacilli with high-risk human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: a systematic review and meta-analysis. J Infect Dis. (2019) 220:1243–54. 10.1093/infdis/jiz325 [DOI] [PubMed] [Google Scholar]
- 53.Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. (2020) 17:232–50. 10.1038/s41585-020-0286-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attia AC, Wolf J, Núñez AE. On surmounting the barriers to HPV vaccination: we can do better. Ann Med. (2018) 50:209–25. 10.1080/07853890.2018.1426875 [DOI] [PubMed] [Google Scholar]
- 55.Hanson CM, Eckert L, Bloem P, Cernuschi T. Gavi HPV programs: application to implementation. Vaccines. (2015) 3:408–19. 10.3390/vaccines3020408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brotherton JM, Murray SL, Hall MA, Andrewartha LK, Banks CA, Meijer D, et al. Human papillomavirus vaccine coverage among female Australian adolescents: success of the school-based approach. Med J Aust. (2013) 199:614–7. 10.5694/mja13.10272 [DOI] [PubMed] [Google Scholar]
- 57.Unesco Institute for Statistics . Leaving No One Behind: How Far on the Way to Universal Primary and Secondary Education? Contract No.: Fact Sheet 37. Unesco Institute for Statistics; (2016). [Google Scholar]
- 58.Gallagher KE, Howard N, Kabakama S, Mounier-Jack S, Griffiths UK, Feletto M, et al. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low- and middle-income countries. PLoS ONE. (2017) 12:e0177773. 10.1371/journal.pone.0177773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grant LA, Dunne EF, Chesson H, Markowitz LE. Considerations for human papillomavirus (HPV) vaccination of mid-adult women in the United States. Vaccine. (2011) 29:2365–70. 10.1016/j.vaccine.2011.01.032 [DOI] [PubMed] [Google Scholar]
- 60.Tsu VD, Cernuschi T, LaMontagne DS. Lessons learned from HPV vaccine delivery in low-resource settings and opportunities for HIV prevention, treatment, and care among adolescents. J Acquir Immune Defic Syndr (1999). (2014) 66:S209–16. 10.1097/QAI.0000000000000175 [DOI] [PubMed] [Google Scholar]
- 61.Rahman MS, Gultekin M, Lassi ZS. Effective approaches towards eliminating cervical cancer from low-and middle-income countries amid COVID-19 pandemic. Int J Gynecol Cancer. (2020) 30:1848. 10.1136/ijgc-2020-002013 [DOI] [PubMed] [Google Scholar]
- 62.Arbyn M, Bruni L, Kelly D, Basu P, Poljak M, Gultekin M, et al. Tackling cervical cancer in Europe amidst the COVID-19 pandemic. Lancet Public Health. (2020) 5:e425. 10.1016/S2468-2667(20)30122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. (2020) 69:1109–16. 10.15585/mmwr.mm6933a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner TB, Huh WK. HPV vaccines: Translating immunogenicity into efficacy. Hum Vacc Immunother. (2016) 12:1403–5. 10.1080/21645515.2015.1103936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williamson A. S12.1 human papillomavirus vaccines—correlates of protection are not defined. Sex Transm Infect. (2013) 89:A18. 10.1136/sextrans-2013-051184.0057 [DOI] [Google Scholar]
- 66.Secor AM, Driver M, Kharono B, Hergott D, Liu G, Barnabas RV, et al. Immunogenicity of alternative dosing schedules for HPV vaccines among adolescent girls and young women: a systematic review and meta-analysis. Vaccines. (2020) 8:618. 10.3390/vaccines8040618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brotherton JM. Rationalizing the HPV vaccination schedule: a long road to a worthwhile destination. Papillomavirus Res. (2019) 8:100190. 10.1016/j.pvr.2019.100190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonner K, Banura C, Basta NE. HPV vaccination strategies targeting hard-to-reach populations: out-of-school girls in LMICs. Vaccine. (2018) 36:191–3. 10.1016/j.vaccine.2017.11.038 [DOI] [PubMed] [Google Scholar]
- 69.Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. (2018) 36:4761–7. 10.1016/j.vaccine.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 70.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. (2015) 16:775–86. 10.1016/S1470-2045(15)00047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kreimer AR, Herrero R, Sampson JN, Porras C, Lowy DR, Schiller JT, et al. Evidence for single-dose protection by the bivalent HPV vaccine–review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. (2018) 36:4774–82. 10.1016/j.vaccine.2017.12.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.NIH, U,.S. National Library of Medicine. ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/
- 73.Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. (2014) 32:2150–9. 10.1016/j.vaccine.2014.01.081 [DOI] [PubMed] [Google Scholar]
- 74.Strategic Advisory Group of Experts on Immunization . Report on the Sage Working Group on Vaccine Hesitancy. Strategic Advisory Group of Experts on Immunization; (2014). [Google Scholar]
- 75.Gallup . Wellcome Global Monitor—First Wave Findings (2019). Available online at: https://wellcome.org/sites/default/files/wellcome-global-monitor-2018.pdf
- 76.Bhopal S, Nielsen M. Vaccine hesitancy in low- and middle-income countries: potential implications for the COVID-19 response. Archiv Dis Child. (2021) 106:113. 10.1136/archdischild-2020-318988 [DOI] [PubMed] [Google Scholar]
- 77.Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF joint reporting form data-2015–2017. Vaccine. (2018) 36:3861–7. 10.1016/j.vaccine.2018.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vorsters A, Bosch FX, Bonanni P, Franco EL, Baay M, Simas C, et al. Prevention and control of HPV infection and HPV-related cancers in Colombia–a meeting report. BMC Proc. (2020) 14:8. 10.1186/s12919-020-00192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Castro CMM, Muñoz N. HPV Vaccination in Colombia. From a Nightmare to a Bright and Promising Dawn. (2020). Available online at: www.HPVWorld.com
- 80.Simas C, Munoz N, Arregoces L, Larson HJ. HPV vaccine confidence and cases of mass psychogenic illness following immunization in Carmen de Bolivar, Colombia. Hum Vacc Immunother. (2019) 15:163–6. 10.1080/21645515.2018.1511667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanley SJB, Yoshioka E, Ito Y, Kishi R. HPV vaccination crisis in Japan. Lancet. (2015) 385:2571. 10.1016/S0140-6736(15)61152-7 [DOI] [PubMed] [Google Scholar]
- 82.Lemp JM, De Neve J-W, Bussmann H, Chen S, Manne-Goehler J, Theilmann M, et al. Lifetime prevalence of cervical cancer screening in 55 low- and middle-income countries. JAMA. (2020) 324:1532–42. 10.1001/jama.2020.16244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thun M, Thun M, Linet MS, Linet MS, Cerhan JR, Cerhan JR, et al. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; (2017). 10.1093/oso/9780190238667.001.0001 [DOI] [Google Scholar]
- 84.World Health Organization . Comprehensive Cervical Cancer Control: A Guide to Essential Practice. Contract No.: Report. World Health Organization; (2014). [PubMed] [Google Scholar]
- 85.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. (2009) 360:1385–94. 10.1056/NEJMoa0808516 [DOI] [PubMed] [Google Scholar]
- 86.Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. (2017) 8:Cd008587. 10.1002/14651858.CD008587.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelly H, Mayaud P, Segondy M, Pant Pai N, Peeling RW. A systematic review and meta-analysis of studies evaluating the performance of point-of-care tests for human papillomavirus screening. Sex Transm Infect. (2017) 93:S36–45. 10.1136/sextrans-2016-053070 [DOI] [PubMed] [Google Scholar]
- 88.Fokom-Domgue J, Combescure C, Fokom-Defo V, Tebeu PM, Vassilakos P, Kengne AP, et al. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ. (2015) 351:h3084. 10.1136/bmj.h3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mustafa RA, Santesso N, Khatib R, Mustafa AA, Wiercioch W, Kehar R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstetr. (2016) 132:259–65. 10.1016/j.ijgo.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 90.Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. (2008) 123:153–60. 10.1002/ijc.23489 [DOI] [PubMed] [Google Scholar]
- 91.Catarino R, Schafer S, Vassilakos P, Petignat P, Arbyn M. Accuracy of combinations of visual inspection using acetic acid or lugol iodine to detect cervical precancer: a meta-analysis. BJOG. (2018) 125:545–53. 10.1111/1471-0528.14783 [DOI] [PubMed] [Google Scholar]
- 92.Sankaranarayanan R, Nessa A, Esmy PO, Dangou JM. Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstetr Gynaecol. (2012) 26:221–32. 10.1016/j.bpobgyn.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 93.Fokom-Domgue J, Vassilakos P, Petignat P. Is screen-and-treat approach suited for screening and management of precancerous cervical lesions in Sub-Saharan Africa? Prev Med. (2014) 65:138–40. 10.1016/j.ypmed.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 94.Snijders PJ, Verhoef VM, Arbyn M, Ogilvie G, Minozzi S, Banzi R, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J cancer. (2013) 132:2223–36. 10.1002/ijc.27790 [DOI] [PubMed] [Google Scholar]
- 95.Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstetr. (2011) 113:14–24. 10.1016/j.ijgo.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 96.de Sanjose S, Holme F. What is needed now for successful scale-up of screening? Papillomavirus Res. (2019) 7:173–5. 10.1016/j.pvr.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jeronimo J, Bansil P, Lim J, Peck R, Paul P, Amador JJ, et al. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer. (2014) 24:576–85. 10.1097/IGC.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. (2018) 320:43–52. 10.1001/jama.2018.7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Pract Res Clin Obstetr Gynaecol. (2018) 47:107–18. 10.1016/j.bpobgyn.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 100.Goodman A. HPV testing as a screen for cervical cancer. BMJ. (2015) 350:h2372. 10.1136/bmj.h2372 [DOI] [PubMed] [Google Scholar]
- 101.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJF, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. (2014) 383:524–32. 10.1016/S0140-6736(13)62218-7 [DOI] [PubMed] [Google Scholar]
- 102.Campos NG, Maza M, Alfaro K, Gage JC, Castle PE, Felix JC, et al. The cost-effectiveness of implementing HPV testing for cervical cancer screening in El Salvador. Int J Gynaecol Obstetr. (2019) 145:40–6. 10.1002/ijgo.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Campos NG, Mvundura M, Jeronimo J, Holme F, Vodicka E, Kim JJ. Cost-effectiveness of HPV-based cervical cancer screening in the public health system in Nicaragua. BMJ Open. (2017) 7:e015048. 10.1136/bmjopen-2016-015048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Termrungruanglert W, Khemapech N, Tantitamit T, Sangrajrang S, Havanond P, Laowahutanont P. Cost-effectiveness analysis study of HPV testing as a primary cervical cancer screening in Thailand. Gynecol Oncol Rep. (2017) 22:58–63. 10.1016/j.gore.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holme F, Jeronimo J, Maldonado F, Camel C, Sandoval M, Martinez-Granera B, et al. Introduction of HPV testing for cervical cancer screening in Central America: the Scale-Up project. Prev Med. (2020) 135:106076. 10.1016/j.ypmed.2020.106076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alfaro K, Maza M, Felix JC, Gage JC, Castle PE, Alonzo TA, et al. Outcomes for step-wise implementation of a human papillomavirus testing-based cervical screen-and-treat program in El Salvador. JCO Glob Oncol. (2020) 6:1519–30. 10.1200/GO.20.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Almonte M, Murillo R, Sánchez GI, González P, Ferrera A, Picconi MA, et al. Multicentric study of cervical cancer screening with human papillomavirus testing and assessment of triage methods in Latin America: the ESTAMPA screening study protocol. BMJ Open. (2020) 10:e035796. 10.1136/bmjopen-2019-035796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.World Health Organization . WHO Consolidated Guideline on Self-Care Interventions for Health: Sexual and Reproductive Health and Rights. Geneva: World Health Organization; (2019). Available online at: https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf?ua=1 [PubMed] [Google Scholar]
- 109.Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. (2018) 363:k4823. 10.1136/bmj.k4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith MA, Hall MT, Saville M, Brotherton JM, Simms KT, Lew JB, et al. Could HPV testing on self-collected samples be routinely used in an organised cervical screening program? A modelled analysis. Cancer Epidemiol Biomarkers Prev. (2020) 30:268–77. 10.1158/1055-9965.EPI-20-0998 [DOI] [PubMed] [Google Scholar]
- 111.Zhang L, Xu XQ, Hu SY, Chen F, Zhang X, Pan QJ, et al. Durability of clinical performance afforded by self-collected HPV testing: a 15-year cohort study in China. Gynecol Oncol. (2018) 151:221–8. 10.1016/j.ygyno.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 112.Gupta S, Palmer C, Bik EM, Cardenas JP, Nuñez H, Kraal L, et al. Self-sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health. (2018) 6:77. 10.3389/fpubh.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pimple S, Mishra G, Shastri S. Global strategies for cervical cancer prevention. Curr Opin Obstetr Gynecol. (2016) 28:4–10. 10.1097/GCO.0000000000000241 [DOI] [PubMed] [Google Scholar]
- 114.Chatzistamatiou K, Vrekoussis T, Tsertanidou A, Moysiadis T, Mouchtaropoulou E, Pasentsis K, et al. Acceptability of self-sampling for human papillomavirus-based cervical cancer screening. J Womens Health. (2020) 29:1447–56. 10.1089/jwh.2019.8258 [DOI] [PubMed] [Google Scholar]
- 115.Wong EL, Cheung AW, Wong AY, Chan PK. Acceptability and feasibility of HPV self-sampling as an alternative primary cervical cancer screening in under-screened population groups: a cross-sectional study. Int J Environ Res Public Health. (2020) 17:6245. 10.3390/ijerph17176245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Odesanmi TY, Wasti SP, Odesanmi OS, Adegbola O, Oguntuase OO, Mahmood S. Comparative effectiveness and acceptability of home-based and clinic-based sampling methods for sexually transmissible infections screening in females aged 14–50 years: a systematic review and meta-analysis. Sex Health. (2013) 10:559–69. 10.1071/SH13029 [DOI] [PubMed] [Google Scholar]
- 117.Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. (2017) 93:56–61. 10.1136/sextrans-2016-052609 [DOI] [PubMed] [Google Scholar]
- 118.Mezei AK, Armstrong HL, Pedersen HN, Campos NG, Mitchell SM, Sekikubo M, et al. Cost-effectiveness of cervical cancer screening methods in low- and middle-income countries: a systematic review. Int J Cancer. (2017) 141:437–46. 10.1002/ijc.30695 [DOI] [PubMed] [Google Scholar]
- 119.Yeh PT, Kennedy CE, de Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health. (2019) 4:e001351. 10.1136/bmjgh-2018-001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arrossi S, Paolino M, Thouyaret L, Laudi R, Campanera A. Evaluation of scaling-up of HPV self-collection offered by community health workers at home visits to increase screening among socially vulnerable under-screened women in Jujuy Province, Argentina. Implement Sci. (2017) 12:17. 10.1186/s13012-017-0548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jeronimo J, Castle PE, Temin S, Shastri SS. Secondary prevention of cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline summary. J Oncol Pract. (2017) 13:129–33. 10.1200/JOP.2016.017889 [DOI] [PubMed] [Google Scholar]
- 122.Kyrgiou M, Mitra A, Arbyn M, Stasinou SM, Martin-Hirsch P, Bennett P, et al. Fertility and early pregnancy outcomes after treatment for cervical intraepithelial neoplasia: systematic review and meta-analysis. BMJ. (2014) 349:g6192. 10.1136/bmj.g6192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.D'Alessandro P, Arduino B, Borgo M, Saccone G, Venturella R, Di Cello A, et al. Loop electrosurgical excision procedure versus cryotherapy in the treatment of cervical intraepithelial neoplasia: a systematic review and meta-analysis of randomized controlled trials. Gynecol Minim Invasive Ther. (2018) 7:145–51. 10.4103/GMIT.GMIT_56_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Santesso N, Mustafa RA, Wiercioch W, Kehar R, Gandhi S, Chen Y, et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int J Gynaecol Obstetr. (2016) 132:266–71. 10.1016/j.ijgo.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 125.de Fouw M, Oosting RM, Rutgrink A, Dekkers OM, Peters AAW, Beltman JJ. A systematic review and meta-analysis of thermal coagulation compared with cryotherapy to treat precancerous cervical lesions in low- and middle-income countries. Int J Gynaecol Obstetr. (2019) 147:4–18. 10.1002/ijgo.12904 [DOI] [PubMed] [Google Scholar]
- 126.Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the effectiveness of cryotherapy in the treatment of cervical intraepithelial neoplasia. Int J Gynaecol Obstetr. (2013) 120:218–23. 10.1016/j.ijgo.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 127.Randall TC, Sauvaget C, Muwonge R, Trimble EL, Jeronimo J. Worthy of further consideration: an updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions. Prev Med. (2019) 118:81–91. 10.1016/j.ypmed.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 128.World Health Organization . WHO Guidelines for the Use of Thermal Ablation for Cervical Pre-cancer Lesions. (2019). Available online at: https://apps.who.int/iris/bitstream/handle/10665/329299/9789241550598-eng.pdf?sequence=1&isAllowed=y [PubMed]
- 129.Basu P, Taghavi K, Hu S-Y, Mogri S, Joshi S. Management of cervical premalignant lesions. Curr Probl Cancer. (2018) 42:129–36. 10.1016/j.currproblcancer.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 130.Papoutsis D, Underwood M, Parry-Smith W, Panikkar J. Early and late pregnancy outcomes in women treated with cold-coagulation versus LLETZ cervical treatment for cervical intraepithelial neoplasia; a retrospective cohort study. Archiv Gynecol Obstetr. (2018) 297:1015–25. 10.1007/s00404-018-4704-x [DOI] [PubMed] [Google Scholar]
- 131.Pinder LF, Parham GP, Basu P, Muwonge R, Lucas E, Nyambe N, et al. Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual inspection with acetic acid test: pilot phase of a randomised controlled trial. Lancet Oncol. (2020) 21:175–84. 10.1016/S1470-2045(19)30635-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.World Health Organization . WHO Guidelines Approved by the Guidelines Review Committee. WHO Guidelines for Treatment of Cervical Intraepithelial Neoplasia 2–3 and Adenocarcinoma in situ: Cryotherapy, Large Loop Excision of the Transformation Zone, and Cold Knife Conization. Geneva: World Health Organization; (2014). [PubMed] [Google Scholar]
- 133.Naud PS, Muwonge R, Passos EP, Magno V, Matos J, Sankaranarayanan R. Efficacy, safety, and acceptability of thermocoagulation for treatment of cervical intraepithelial neoplasia in a hospital setting in Brazil. Int J Gynaecol Obstetr. (2016) 133:351–4. 10.1016/j.ijgo.2015.09.035 [DOI] [PubMed] [Google Scholar]
- 134.Viviano M, Kenfack B, Catarino R, Tincho E, Temogne L, Benski AC, et al. Feasibility of thermocoagulation in a screen-and-treat approach for the treatment of cervical precancerous lesions in sub-Saharan Africa. BMC Womens Health. (2017) 17:2. 10.1186/s12905-016-0355-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dolman L, Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: a systematic review. BJOG. (2014) 121:929–42. 10.1111/1471-0528.12655 [DOI] [PubMed] [Google Scholar]
- 136.Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. (2008) 337:a1284. 10.1136/bmj.a1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Land KJ, Boeras DI, Chen XS, Ramsay AR, Peeling RW. Reassured diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol. (2019) 4:46–54. 10.1038/s41564-018-0295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sayed S, Chung M, Temmerman M. Point-of-care HPV molecular diagnostics for a test-and-treat model in high-risk HIV populations. Lancet Glob Health. (2020) 8:e171–2. 10.1016/S2214-109X(19)30559-5 [DOI] [PubMed] [Google Scholar]
- 139.Tin-Oo C, Hlaing HN, Nandar CS, Aung T, Fishbein D. Why the cost of purchasing the careHPV test in Myanmar was many times greater than that reported in the international literature. J Glob Oncol. (2018) 4:49s. 10.1200/jgo.18.27900 [DOI] [Google Scholar]
- 140.Cubie HA, Campbell C. Cervical cancer screening—the challenges of complete pathways of care in low-income countries: focus on Malawi. Womens Health. (2020) 16:1745506520914804. 10.1177/1745506520914804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang W, Du H, Huang X, Wang C, Duan X, Liu Y, et al. Evaluation of an isothermal amplification HPV detection assay for primary cervical cancer screening. Infect Agent Cancer. (2020) 15:65. 10.1186/s13027-020-00328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. (2011) 6:1083–98. 10.2217/fmb.11.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wentzensen N, Clarke MA, Bremer R, Poitras N, Tokugawa D, Goldhoff PE, et al. Clinical evaluation of human papillomavirus screening with p16/Ki-67 dual stain triage in a large organized cervical cancer screening program. JAMA Intern Med. (2019) 179:881–8. 10.1001/jamainternmed.2019.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nuovo GJ, de Andrade CV, Wells SI, Brusadelli M, Nicol AF. New biomarkers of human papillomavirus infection in acute cervical intraepithelial neoplasia. Ann Diagn Pathol. (2018) 36:21–7. 10.1016/j.anndiagpath.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 145.Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol. (2016) 76:S49–55. 10.1016/j.jcv.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rossi PG, Carozzi F, Ronco G, Allia E, Bisanzi S, Gillio-Tos A, et al. p16/ki67 and E6/E7 mRNA accuracy and prognostic value in triaging HPV DNA-positive women. J Natl Cancer Inst. (2020) 113:292–300. 10.1093/jnci/djaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Derbie A, Mekonnen D, Woldeamanuel Y, Van Ostade X, Abebe T. HPV E6/E7 mRNA test for the detection of high grade cervical intraepithelial neoplasia (CIN2+): a systematic review. Infect Agents Cancer. (2020) 15:9. 10.1186/s13027-020-0278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong L, Zhang L, Hu S-Y, Feng R-M, Zhao X-L, Zhang Q, et al. Risk stratification of HPV 16 DNA methylation combined with E6 oncoprotein in cervical cancer screening: a 10-year prospective cohort study. Clin Epigenet. (2020) 12:62. 10.1186/s13148-020-00853-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arbyn M, Peeters E, Benoy I, Vanden Broeck D, Bogers J, De Sutter P, et al. VALHUDES: a protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J Clin Virol. (2018) 107:52–6. 10.1016/j.jcv.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 150.Hernandez BY, Tareg AC, Reichhardt M, Agapito A, Zhu X, Sy A, et al. Randomized controlled trial evaluating the utility of urine HPV DNA for cervical cancer screening in a Pacific Island population. J Glob Health Rep. (2018) 2:e2018016. 10.29392/joghr.2.e2018016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Novikova T. Optical techniques for cervical neoplasia detection. Beilstein J Nanotechnol. (2017) 8:1844–62. 10.3762/bjnano.8.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lam CT, Mueller J, Asma B, Asiedu M, Krieger MS, Chitalia R, et al. An integrated strategy for improving contrast, durability, and portability of a pocket colposcope for cervical cancer screening and diagnosis. PLoS ONE. (2018) 13:e0192530. 10.1371/journal.pone.0192530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mueller JL, Lam CT, Dahl D, Asiedu MN, Krieger MS, Bellido-Fuentes Y, et al. Portable Pocket colposcopy performs comparably to standard-of-care clinical colposcopy using acetic acid and Lugol's iodine as contrast mediators: an investigational study in Peru. BJOG. (2018) 125:1321–9. 10.1111/1471-0528.15326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mueller JL, Asma E, Lam CT, Krieger MS, Gallagher JE, Erkanli A, et al. International image concordance study to compare a point-of-Care Tampon colposcope with a standard-of-care colposcope. J Lower Genit Tract Dis. (2017) 21:112–9. 10.1097/LGT.0000000000000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Peterson CW, Rose D, Mink J, Levitz D. Real-time monitoring and evaluation of a visual-based cervical cancer screening program using a decision support job aid. Diagnostics (Basel). (2016) 6:20. 10.3390/diagnostics6020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu L, Bell D, Antani S, Xue Z, Yu K, Horning MP, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. (2019) 111:923–32. 10.1093/jnci/djy225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Asiedu MN, Agudogo J, Krieger MS, Miros R, Proeschold-Bell RJ, Schmitt JW, et al. Design and preliminary analysis of a vaginal inserter for speculum-free cervical cancer screening. PLoS ONE. (2017) 12:e0177782. 10.1371/journal.pone.0177782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Asiedu MN, Agudogo JS, Dotson ME, Krieger MS, Schmitt JW, Huchko M, et al. A novel, versatile speculum-free callascope for clinical examination and self-visualization of the cervix. bioRxiv. (2019) 2019:618348. 10.1101/618348 [DOI] [Google Scholar]
- 159.Desai KT, Ajenifuja KO, Banjo A, Adepiti CA, Novetsky A, Sebag C, et al. Design and feasibility of a novel program of cervical screening in Nigeria: self-sampled HPV testing paired with visual triage. Infect Agent Cancer. (2020) 15:60. 10.1186/s13027-020-00324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.LaVigne AW, Triedman SA, Randall TC, Trimble EL, Viswanathan AN. Cervical cancer in low and middle income countries: addressing barriers to radiotherapy delivery. Gynecol Oncol Rep. (2017) 22:16–20. 10.1016/j.gore.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petignat P, Kenfack B. Is thermal ablation a new standard for cervical pre-cancer treatment in low-income and middle-income countries? Lancet Oncol. (2020) 21:19–20. 10.1016/S1470-2045(19)30732-6 [DOI] [PubMed] [Google Scholar]
- 162.Castle PE, Murokora D, Perez C, Alvarez M, Quek SC, Campbell C. Treatment of cervical intraepithelial lesions. Int J Gynaecol Obstetr. (2017) 138:20–5. 10.1002/ijgo.12191 [DOI] [PubMed] [Google Scholar]
- 163.Hillemanns P, Garcia F, Petry KU, Dvorak V, Sadovsky O, Iversen OE, et al. A randomized study of hexaminolevulinate photodynamic therapy in patients with cervical intraepithelial neoplasia 1/2. Am J Obstetr Gynecol. (2015) 212:465.e1–7. 10.1016/j.ajog.2014.10.1107 [DOI] [PubMed] [Google Scholar]
- 164.Istomin YP, Lapzevich TP, Chalau VN, Shliakhtsin SV, Trukhachova TV. Photodynamic therapy of cervical intraepithelial neoplasia grades II and III with photolon. Photodiagn Photodyn Ther. (2010) 7:144–51. 10.1016/j.pdpdt.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 165.Soergel P, Löning M, Staboulidou I, Schippert C, Hillemanns P. Photodynamic diagnosis and therapy in gynecology. J Environ Pathol Toxicol Oncol. (2008) 27:307–20. 10.1615/JEnvironPatholToxicolOncol.v27.i4.80 [DOI] [PubMed] [Google Scholar]
- 166.Kisling K, Zhang L, Simonds H, Fakie N, Yang J, McCarroll R, et al. Fully automatic treatment planning for external-beam radiation therapy of locally advanced cervical cancer: a tool for low-resource clinics. J Glob Oncol. (2019) 5:1–9. 10.1200/JGO.18.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smalley Rumfield C, Roller N, Pellom ST, Schlom J, Jochems C. Therapeutic vaccines for HPV-associated malignancies. Immunotargets Ther. (2020) 9:167–200. 10.2147/ITT.S273327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rubin R. Artificial intelligence for cervical precancer screening. JAMA. (2019) 321:734. 10.1001/jama.2019.0888 [DOI] [PubMed] [Google Scholar]
- 169.Sigfrid L, Murphy G, Haldane V, Chuah FLH, Ong SE, Cervero-Liceras F, et al. Integrating cervical cancer with HIV healthcare services: a systematic review. PLoS ONE. (2017) 12:e0181156. 10.1371/journal.pone.0181156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Davies N, Chersich M, Mullick S, Naidoo N, Makhoba N, Rees H, et al. Integrating cervical cancer screening into safer conception services to improve women's health outcomes: a pilot study at a primary care clinic in South Africa. Sex Transm Dis. (2019) 46:91–7. 10.1097/OLQ.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morhard R, Nief C, Barrero Castedo C, Hu F, Madonna M, Mueller JL, et al. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Sci Rep. (2017) 7:8750. 10.1038/s41598-017-09371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Poljak M, Sterbenc A, Lunar MM. Prevention of human papillomavirus (HPV)-related tumors in people living with human immunodeficiency virus (HIV). Expert Rev Antiinfect Ther. (2017) 15:987–99. 10.1080/14787210.2017.1392854 [DOI] [PubMed] [Google Scholar]
- 173.Safaeian M, Sampson JN, Pan Y, Porras C, Kemp TJ, Herrero R, et al. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. J Natl Cancer Inst. (2017) 110:205–12. 10.1093/jnci/djx158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Phelan DF, Gange SJ, Ahdieh-Grant L, Mehta SH, Kirk GD, Shah K, et al. Determinants of newly detected human papillomavirus infection in HIV-infected and HIV-uninfected injection drug using women. Sex Transm Dis. (2009) 36:149–56. 10.1097/OLQ.0b013e31818d3df3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. (2005) 97:577–86. 10.1093/jnci/dji073 [DOI] [PubMed] [Google Scholar]
- 176.Kuhn L, Wang C, Tsai WY, Wright TC, Denny L. Efficacy of human papillomavirus-based screen-and-treat for cervical cancer prevention among HIV-infected women. AIDS. (2010) 24:2553–61. 10.1097/QAD.0b013e32833e163e [DOI] [PubMed] [Google Scholar]
- 177.Ezechi OC, Ostergren PO, Nwaokorie FO, Ujah IA, Odberg Pettersson K. The burden, distribution and risk factors for cervical oncogenic human papilloma virus infection in HIV positive Nigerian women. Virol J. (2014) 11:5. 10.1186/1743-422X-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cholli P, Bradford L, Manga S, Nulah K, Kiyang E, Manjuh F, et al. Screening for cervical cancer among HIV-positive and HIV-negative women in Cameroon using simultaneous co-testing with careHPV DNA testing and visual inspection enhanced by digital cervicography: findings of initial screening and one-year follow-up. Gynecol Oncol. (2018) 148:118–25. 10.1016/j.ygyno.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 179.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. (2004) 190:37–45. 10.1086/421467 [DOI] [PubMed] [Google Scholar]
- 180.Clifford GM, de Vuyst H, Tenet V, Plummer M, Tully S, Franceschi S. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. J Acquir Immune Defic Syndr (1999). (2016) 73:332–9. 10.1097/QAI.0000000000001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. (2020) 9:e161–9. 10.1016/S2214-109X(20)30459-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Menon S, Wusiman A, Boily MC, Kariisa M, Mabeya H, Luchters S, et al. Epidemiology of HPV genotypes among HIV positive women in Kenya: a systematic review and meta-analysis. PLoS ONE. (2016) 11:e0163965. 10.1371/journal.pone.0163965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Goedert JJ, Hosgood HD, Biggar RJ, Strickler HD, Rabkin CS. Screening for cancer in persons living with HIV infection. Trends Cancer. (2016) 2:416–28. 10.1016/j.trecan.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kelly H, Weiss HA, Benavente Y, de Sanjose S, Mayaud P. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV. (2018) 5:e45–58. 10.1016/S2352-3018(17)30149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, Chiyapo S, et al. HIV infection and survival among women with cervical cancer. J Clin Oncol. (2016) 34:3749–57. 10.1200/JCO.2016.67.9613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, Seage GR, III, Suneja G, Kayembe MK, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. (2015) 10:e0135602. 10.1371/journal.pone.0135602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kahn JA, Xu J, Kapogiannis BG, Rudy B, Gonin R, Liu N, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis. (2013) 57:735–44. 10.1093/cid/cit319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kojic EM, Kang M, Cespedes MS, Umbleja T, Godfrey C, Allen RT, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis. (2014) 59:127–35. 10.1093/cid/ciu238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Giacomet V, Penagini F, Trabattoni D, Vigano A, Rainone V, Bernazzani G, et al. Safety and immunogenicity of a quadrivalent human papillomavirus vaccine in HIV-infected and HIV-negative adolescents and young adults. Vaccine. (2014) 32:5657–61. 10.1016/j.vaccine.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 190.McClymont E, Lee M, Raboud J, Coutlee F, Walmsley S, Lipsky N, et al. The efficacy of the quadrivalent human papillomavirus vaccine in girls and women living with human immunodeficiency virus. Clin Infect Dis. (2019) 68:788–94. 10.1093/cid/ciy575 [DOI] [PubMed] [Google Scholar]
- 191.Mugo NR, Eckert L, Magaret AS, Cheng A, Mwaniki L, Ngure K, et al. Quadrivalent HPV vaccine in HIV-1-infected early adolescent girls and boys in Kenya: month 7 and 12 post vaccine immunogenicity and correlation with immune status. Vaccine. (2018) 36:7025–32. 10.1016/j.vaccine.2018.09.059 [DOI] [PubMed] [Google Scholar]
- 192.Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA, III, Read JS, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr (1999). (2010) 55:197–204. 10.1097/QAI.0b013e3181de8d26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Weinberg A, Song LY, Saah A, Brown M, Moscicki AB, Meyer WA, III, et al. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis. (2012) 206:1309–18. 10.1093/infdis/jis489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis. (2014) 209:1165–73. 10.1093/infdis/jit657 [DOI] [PubMed] [Google Scholar]
- 195.Faust H, Toft L, Sehr P, Muller M, Bonde J, Forslund O, et al. Human papillomavirus neutralizing and cross-reactive antibodies induced in HIV-positive subjects after vaccination with quadrivalent and bivalent HPV vaccines. Vaccine. (2016) 34:1559–65. 10.1016/j.vaccine.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 196.Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. (2013) 31:5745–53. 10.1016/j.vaccine.2013.09.032 [DOI] [PubMed] [Google Scholar]
- 197.Hall MT, Smith MA, Simms KT, Barnabas RV, Canfell K, Murray JM. The past, present and future impact of HIV prevention and control on HPV and cervical disease in Tanzania: a modelling study. PLoS ONE. (2020) 15:e0231388. 10.1371/journal.pone.0231388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.HIV PoOIiAaAw. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents With HIV: Recommendations From the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. AIDSinfo; (2018). Available online at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf [Google Scholar]
- 199.Sahasrabuddhe VV, Bhosale RA, Kavatkar AN, Nagwanshi CA, Joshi SN, Jenkins CA, et al. Comparison of visual inspection with acetic acid and cervical cytology to detect high-grade cervical neoplasia among HIV-infected women in India. Int J Cancer. (2012) 130:234–40. 10.1002/ijc.25971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mutyaba T, Mirembe F, Sandin S, Weiderpass E. Evaluation of ‘see-see and treat' strategy and role of HIV on cervical cancer prevention in Uganda. Reprod Health. (2010) 7:4. 10.1186/1742-4755-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cervical Cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res. (2012) 5:11–7. 10.1158/1940-6207.CAPR-11-0540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Menon S, Rossi R, Harmon SG, Mabeya H, Callens S. Public health approach to prevent cervical cancer in HIV-infected women in Kenya: issues to consider in the design of prevention programs. Gynecol Oncol Rep. (2017) 22:82–8. 10.1016/j.gore.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mapanga W, Girdler-Brown B, Feresu SA, Chipato T, Singh E. Prevention of cervical cancer in HIV-seropositive women from developing countries through cervical cancer screening: a systematic review. Syst Rev. (2018) 7:198. 10.1186/s13643-018-0874-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Debeaudrap P, Sobngwi J, Tebeu P-M, Clifford GM. Residual or recurrent precancerous lesions after treatment of cervical lesions in human immunodeficiency virus-infected women: a systematic review and meta-analysis of treatment failure. Clin Infect Dis. (2019) 69:1555–65. 10.1093/cid/ciy1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Carlander C, Wagner P, van Beirs A, Yilmaz A, Elfgren K, Dillner J, et al. Suppressive antiretroviral therapy associates with effective treatment of high-grade cervical intraepithelial neoplasia. AIDS. (2018) 32:1475–84. 10.1097/QAD.0000000000001853 [DOI] [PubMed] [Google Scholar]
- 206.Greene SA, De Vuyst H, John-Stewart GC, Richardson BA, McGrath CJ, Marson KG, et al. Effect of cryotherapy vs loop electrosurgical excision procedure on cervical disease recurrence among women with HIV and high-grade cervical lesions in Kenya: a randomized clinical trial. JAMA. (2019) 322:1570–9. 10.1001/jama.2019.14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chido-Amajuoyi OG, Domgue JF, Obi-Jeff C, Schmeler K, Shete S. A call for the introduction of gender-neutral HPV vaccination to national immunisation programmes in Africa. Lancet Glob Health. (2019) 7:e20–1. 10.1016/S2214-109X(18)30405-4 [DOI] [PubMed] [Google Scholar]
- 208.Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. (2016) 17:67–77. 10.1016/S1470-2045(15)00414-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Burger EA, Campos NG, Sy S, Regan C, Kim JJ. Health and economic benefits of single-dose HPV vaccination in a Gavi-eligible country. Vaccine. (2018) 36:4823–9. 10.1016/j.vaccine.2018.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Whitworth HS, Gallagher KE, Howard N, Mounier-Jack S, Mbwanji G, Kreimer AR, et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. (2020) 38:1302–14. 10.1016/j.vaccine.2019.12.017 [DOI] [PubMed] [Google Scholar]
- 211.Umulisa MC, Franceschi S, Baussano I, Tenet V, Uwimbabazi M, Rugwizangoga B, et al. Evaluation of human-papillomavirus testing and visual inspection for cervical cancer screening in Rwanda. BMC Womens Health. (2018) 18:59. 10.1186/s12905-018-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Majid U, Kandasamy S, Farrah K, Vanstone M. Women's preferences and experiences of cervical cancer screening in rural and remote areas: a systematic review and qualitative meta-synthesis. Rural Remote Health. (2019) 19:5190. 10.22605/RRH5190 [DOI] [PubMed] [Google Scholar]
- 213.Ndejjo R, Mukama T, Kiguli J, Musoke D. Knowledge, facilitators and barriers to cervical cancer screening among women in Uganda: a qualitative study. BMJ Open. (2017) 7:e016282. 10.1136/bmjopen-2017-016282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women: focused literature review. Can Fam Physician. (2017) 63:597–601. Available online at: https://www.cfp.ca/content/63/8/597/tab-article-info [PMC free article] [PubMed] [Google Scholar]
- 215.Joint United Nations Programme on HIV/AIDS (UNAIDS) . Global Plan Towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive, 2011–2015. Geneva: UNAIDS; (2011). [Google Scholar]
- 216.United Nations Children's Fund . Women: At the Heart of the HIV Response for Children. New York, NY: UNICEF; (2018). [Google Scholar]
- 217.(UNAIDS) JUNPoHA. Combination HIV Prevention: Tailoring and Coordinating Biomedical, Behavioural and Structural Strategies to Reduce New HIV Infections—A UNAIDS Discussion Paper. Geneva: UNAIDS; (2010). [Google Scholar]
- 218.Tapela NM, Mpunga T, Hedt-Gauthier B, Moore M, Mpanumusingo E, Xu MJ, et al. Pursuing equity in cancer care: implementation, challenges and preliminary findings of a public cancer referral center in rural Rwanda. BMC Cancer. (2016) 16:237. 10.1186/s12885-016-2256-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. (2015) 16:1193–224. 10.1016/S1470-2045(15)00223-5 [DOI] [PubMed] [Google Scholar]
- 220.Wu ES, Jeronimo J, Feldman S. Barriers and challenges to treatment alternatives for early-stage cervical cancer in lower-resource settings. J Glob Oncol. (2017) 3:572–82. 10.1200/JGO.2016.007369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Grover S, Xu MJ, Yeager A, Rosman L, Groen RS, Chackungal S, et al. A systematic review of radiotherapy capacity in low- and middle-income countries. Front Oncol. (2015) 4:380. 10.3389/fonc.2014.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mathew A. Global survey of clinical oncology workforce. J Glob Oncol. (2018) 4:1–12. 10.1200/JGO.17.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gago J, Pendharkar D, Tripathi C, Ginsburg O. Making the best use of resources in global cancer care. Am Soc Clin Oncol Educ Book. (2020) 40:e361–6. 10.1200/EDBK_290311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.