Abstract
Background
Prior studies regarding use of Aromatase inhibitors (AIs) and risk for cardiovascular disease (CVD) have shown conflicting results. This retrospective cohort study aimed to investigate whether AIs use affects risk for CVD events in postmenopausal breast cancer survivors.
Methods
Using a retrospective cohort study design, four CVD outcomes; heart failure or cardiomyopathy, arrhythmia, acute ischemic heart disease and ischemic stroke or Transient Ischemic Attack were compared with uni- and multivariate Cox regression analyses according to exposure to endocrine therapy (use of AI, tamoxifen or AI/tamoxifen sequentially) or no endocrine therapy.
Results
In total 15815 postmenopausal women, surgically treated to early breast cancer during 2006–2012, were included. No significantly increased risk for CVD events was observed in patients with AI use in the whole cohort. However, two subgroup analyses showed increased risk for CVD events in the AI/tamoxifen sequential group; heart failure in patients older than 75 years (Hazard Ratio (HR) 2.44; 95% Confidence Interval (CI): 1.32–4.54) and arrhythmia in patients without prior CVD (HR 1.45; 95% CI: 1.01–2.10). An increased risk for arrhythmia and acute ischemic heart disease in patients with at least four years of AI treatment compared with no or short-time exposure was observed (HR 2.12; 95% CI: 1.40–3.25 for arrhythmia; HR 2.03; 95% CI: 1.15–3.58 for ischemic heart disease).
Conclusion
Our results indicate an increased risk for ischemic heart disease and arrhythmia in patients treated for more than four years with AIs. This should be considered in the risk-benefit assessment concerning endocrine therapy.
Keywords: Breast cancer, Aromatase inhibitors, Cardiovascular disease, Survivorship
Highlights
-
•
A potential negative impact of aromatase inhibitors as adjuvant treatment on risk for cardiovascular events has been proposed.
-
•
We investigated the risk for cardiovascular events in breast cancer patients treated with aromatase inhibitors in a retrospective cohort study.
-
•
Use of aromatase inhibitors for four years or more was associated with increased risk for ischemic heart disease and arrhythmia.
-
•
Our findings support the need to include this information to the risk-benefit assessment concerning endocrine therapy.
1. Introduction
In postmenopausal women with hormone-receptor positive breast cancer, aromatase inhibitors (AIs) as adjuvant treatment reduce both recurrence and breast cancer mortality rates compared to tamoxifen, and are, therefore, the treatment of choice, either as monotherapy or sequentially with tamoxifen [1]. Breast cancer survivors are at increased risk for cardiovascular mortality, an association more evident in older breast cancer patients [2]. Several possible explanations for this association can be considered; some shared risk factors for breast cancer and cardiovascular disease (CVD), the potential cardiotoxic effect of several therapeutic approaches in breast cancer (anthracyclines, anti-HER2 therapy, chest irradiation) and possible cardiometabolic effects of endocrine therapy, such as effects on body composition, fat- and glucose metabolism as well as arterial wall effects [3].
Observational studies comparing AIs with tamoxifen regarding CVD risk have shown conflicting results, with indications of different effects on different CVD events [[4], [5], [6], [7]]. A systematic review including both randomized controlled trials (RCTs) and observational studies found a higher risk for myocardial infarction and angina with AIs compared with tamoxifen [8]. Similar findings were shown in two meta-analyses of RCTs comparing CVD outcomes between AIs and tamoxifen, however, suggesting a potential cardioprotective effect of tamoxifen as explanation rather than a true increased risk for CVD due to AIs [9,10]. To mitigate the potential bias due to the cardioprotective effect of tamoxifen, patients treated with AIs should be compared to patients without active treatment as well. Meta-analyses of RCTs investigating toxicity of extended adjuvant AI compared with no treatment have shown conflicting results with increased risk for CVD with AIs in one [9], but no difference in two other meta-analyses [10,11]. Regarding real-world evidence on CVD risk due to AIs compared to patients without endocrine therapy, few studies are published so far, with conflicting evidence [[12], [13], [14]].
To derive more knowledge regarding the impact of AIs on risk for different CVD outcomes in the real-world setting, we performed a retrospective population-based study, including patients without endocrine therapy to serve as reference for comparison with patients treated with AIs, tamoxifen, or both sequentially. To investigate the potential dose-response relationship between AI treatment and CVD risk, treatment duration was taken into account.
2. Material and methods
2.1. Data sources
The study was based on Breast Cancer Database Sweden (BCBaSe) which is a research database including individuals diagnosed with breast cancer between 1992 and 2012 in three Swedish regions; Stockholm-Gotland, Uppsala-Örebro and the Northern health care regions, comprising approximately 50% of the Swedish population. BCBaSe links breast cancer registers to a number of national population-based registers. Following registers are merged in BCBaSe:
I. The Regional Breast Cancer Clinical Quality Registers (1992–2007), and from 2008 onwards information from The National Quality Register for Breast Cancer (NKBC), with information on tumor characteristics, menopausal status, treatment and follow-up [[15], [16], [17]].
II. The Prescribed Drug Register, which comprises information on all prescribed medications dispensed in Swedish pharmacies since July 1, 2005 classified according to the Anatomic Therapeutical Chemical (ATC) classification system including dates of dispensation and number of defined daily doses (DDD) [18,19].
III. The Longitudinal integrated database for health insurance and labour market studies (LISA), which includes information on socioeconomic status such as educational level as well as personal and family income [20].
IV. The National Patient Register, which comprises data on main and secondary diagnosis on in- and outpatient hospital care, classified according to International Classification of Diseases (ICD). ICD 9 was used before 1997 and ICD 10 was used from 1997 onwards. Diagnoses from the National Patient Register was used to calculate Charlson Comorbidity Index (CCI) in BcBaSe [[21], [22], [23]].
V. The Swedish cause of death register, which comprises information on cause of death according to ICD on Swedish citizens from 1961 and onwards [24].
2.2. Study cohort
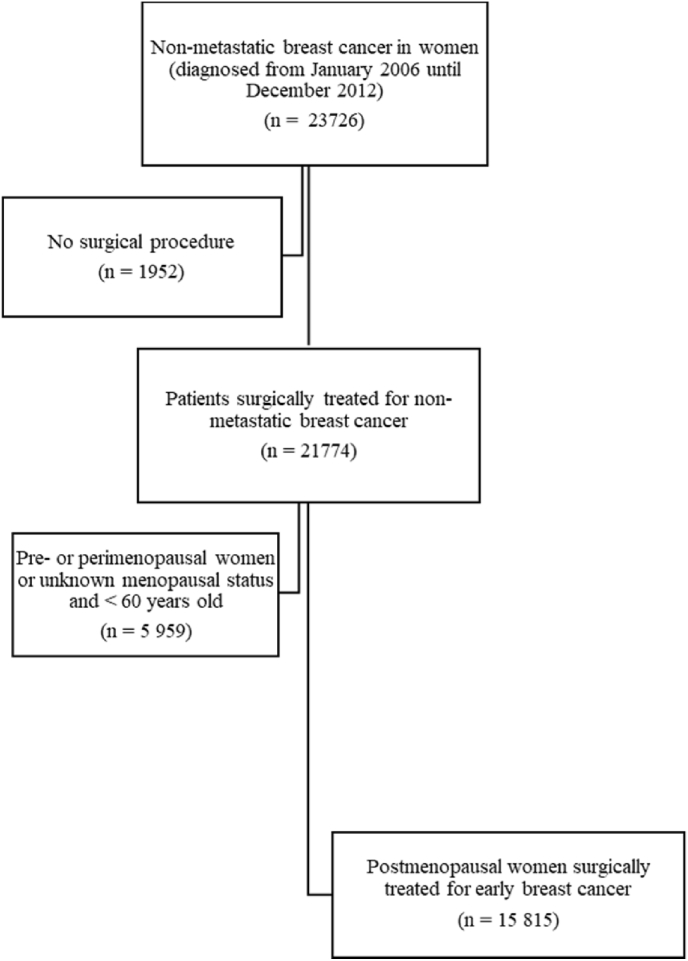
In our study cohort we included patients from BCBaSe, diagnosed with breast cancer from January 1, 2006 until December 31, 2012 irrespective of the breast cancer subtype. We excluded men, patients with evidence of distant metastases, pre- or peri-menopausal status and patients not surgically treated, resulting in a cohort of postmenopausal women surgically treated for early breast cancer. The menopausal status was registered to the database from the treating physician based on patient information. If data regarding menopausal status was missing, patients aged 60 years or older were considered postmenopausal and included.
2.3. Outcomes
Four study outcomes were analyzed separately and defined as hospital care or death due to following conditions from 180 days after breast cancer diagnosis until December 31, 2013: (a) heart failure or cardiomyopathy; (b) arrhythmia; (c) acute ischemic heart disease; (d) Ischemic stroke or Transient Ischemic Attack (TIA). Outcomes were identified by ICD codes in The National Patient Register (main- or secondary diagnosis) and The Cause of death register (only main cause of death). The ICD codes in The National Patient Register are almost exclusively based on diagnostic work-ups of symptomatic patients seeking medical care. For ICD codes used, see Appendix Table 1.
2.4. Follow up
Each woman was followed from day 180 after date of breast cancer diagnosis to last day of follow up or date of outcome, whichever came first. The follow up was split in 180-day periods and exposure was re-evaluated at start of each step. Individuals who reached end of follow up free of outcome, or died of other cause than outcome, were censored.
2.5. Exposure to adjuvant endocrine therapy
Exposure to endocrine treatment was defined and grouped as follows:
-
(1)
“Non-users” - less than six months use of AIs as well as tamoxifen.
-
(2)
“Tamoxifen only” - use of tamoxifen for six months or more and AIs less than six months.
-
(3)
“AI only” - use of AI six months or more and tamoxifen less than six months.
-
(4)
“AI/tamoxifen” - use of both AIs and tamoxifen for six months or more each.
Treatment duration for six months was defined as sum of DDD of at least 180 seen on dispensed drug at start of each time step. Information on DDD regarding tamoxifen (ATC-code: L02A01) and AIs (ATC-code: L02BG) was extracted separately for each outcome. Each patient could serve with exposure time in different exposure groups.
2.6. Covariates
Following data were extracted from described registers: Age at breast cancer diagnosis, two or more dispended prescriptions on CVD medications from 180 days before until 180 days after breast cancer diagnosis, CVD outcomes of interest up to 10 years prior breast cancer diagnosis, CCI, marital status, educational level and income, information on breast cancer regarding tumor characteristics, side, detection mode, surgical procedure and pre-/post-operative oncological treatment (chemotherapy, trastuzumab and radiotherapy).
2.7. Statistical analyses
Covariates were compared based on planned endocrine therapy according to The Regional and The National Quality Register for Breast Cancer, using Chi-square test for categorical variables and Kruskal-Wallis test for continuous, not normally distributed, variables. Univariate and multivariate Cox regression analyses with cluster-robust standard errors were performed separately for each study outcome. Following covariates were included in the multivariate analyses: age, side, stage, chemotherapy, trastuzumab, surgical procedure, radiotherapy, CCI, marital status and family income. Subgroup analyses were performed for patients older than 75 years at breast cancer diagnosis and patients without prior CVD-medication or outcome at breast cancer diagnosis. All statistical analyses were performed in R 3.6.4 [25] and the significance level was considered as α < 0.05.
A sensitivity analysis was performed to investigate a potential dose-response relationship between endocrine therapy and CVD outcomes. Duration of tamoxifen and AI exposure was used to divide into groups for comparison in a multivariate analysis regardless exposure to the other drug (including covariates as described above): (1) Non-users or users less than one year; (2) treatment duration of one to two years; (3) treatment duration of three to four years; (4) treatment duration of four years or more.
3. Results
3.1. Study cohort
In total, 15 815 postmenopausal women were identified and included in the study cohort (Fig. 1). Median age of eligible patients was 66 years (range: 34–101 years old). Median follow up time was 46.8 months (range: 6–97 months). Table 1 summarizes patient-, tumor-, and treatment characteristics of the study cohort divided in comparison groups based on planned endocrine therapy, with additional information supplemented in Appendix Table 2. The “AI only” group contained older patients with lower socioeconomic status, more often comorbid conditions and ongoing CVD medications as well as more advanced breast cancer disease. Numbers of events, person-years studied and incidence rates for cardiovascular outcomes of interest in the whole cohort are presented in Table 2.
Fig. 1.
Flowchart diagram of study cohort.
Table 1.
Cohort characteristics by planned endocrine therapy at baseline.
| Characteristics N (% or as described below) |
All patients 15 815 |
Non-users 3668 |
Tamoxifen only 6235 |
AI only 1481 |
AI/tamoxifen 4431 |
p-value |
|---|---|---|---|---|---|---|
|
Age at diagnosis Median (min-max) ≤60 61–65 66–70 71–75 >75 |
66 (34–101) 3944 (24.9) 3529 (22.3) 2997 (19.0) 2074 (13.1) 3271 (20.7) |
65 (34–97) 1001 (27.3) 849 (23.1) 684 (18.6) 457 (12.5) 677 (18.5) |
66 (34–97) 1584 (25.4) 1416 (22.7) 1163 (18.7) 799 (12.8) 1273 (20.4) |
69 (38–101) 245 (16.5) 274 (18.5) 269 (18.2) 194 (13.1) 499 (33.7) |
66 (38–97) 1114 (25.1) 990 (22.3) 881 (19.9) 624 (14.1) 822 (18.6) |
<0.001 |
| Follow up Median (min-max) |
46.8 (6–97) |
46.8 (6–97) |
55.0 (6–97) |
49.7 (6–97) |
37.7 (6–97) |
<0.001 |
| Marital status | ||||||
| Married | 8057 (50.9) | 1925 (52,4) | 3133 (50.2) | 709 (47.8) | 2290 (51.6) | |
| Not married | 1825 (11.5) | 410 (11.2) | 730 (11.7) | 146 (9.9) | 539 (12.2) | <0.001 |
| Divorced | 3093 (19.6) | 685 (18.7) | 1245 (20.0) | 233 (15.7) | 930 (21.0) | |
| Widowed Missing |
2828 (17.9) 12 (0.1) |
646 (17.6) 2 (0.1) |
1123 (18.0) 4 (0.1) |
392 (26.5) 1 (0.1) |
667 (15.1) 5 (0.1) |
|
| Family income (quartile) | ||||||
| 1 (lowest) | 3924 (24.8) | 948 (25.8) | 1521 (24.4) | 508 (34.3) | 947 (21.4) | |
| 2 | 3947 (25.0) | 903 (24.6) | 1609 (25.8) | 394 (26.6) | 1041 (23.5) | <0.001 |
| 3 | 3952 (25.0) | 895 (24.4) | 1540 (24.7) | 342 (23.1) | 1175 (26.5) | |
| 4 (highest) | 3950 (25.0) | 916 (25.0) | 1550 (24.9) | 231 (15.6) | 1253 (28.3) | |
| Missing |
42 (0.3) |
6 (0.2) |
15 (0.2) |
6 (0.4) |
15 (0.3) |
|
| Charlson Comorbidity Index | ||||||
| 0 | 12953 (81.9) | 2996 (81.7) | 5222 (83.8) | 1092 (73.7) | 3643 (82.2) | |
| 1 | 1494 (9.4) | 363 (9.9) | 529 (8.5) | 193 (13.0) | 409 (9.2) | <0.001 |
| 2 | 901 (5.7) | 201 (5.5) | 332 (5.3) | 111 (7.5) | 257 (5.8) | |
| 3+ |
467 (3.0) |
108 (2.9) |
152 (2.4) |
85 (5.7) |
122 (2.8) |
|
| Breast cancer side | ||||||
| Left | 8220 (52.0) | 1925 (52.5) | 3227 (51.8) | 779 (52.6) | 2289 (51.7) | 0.824 |
| Right |
7595 (48.0) |
1743 (47.5) |
3008 (48.2) |
702 (47.4) |
2142 (48.3) |
|
| Stage of breast cancer | ||||||
| Stage I | 7582 (47.9) | 1896 (51.7) | 4102 (65.8) | 304 (20.5) | 1280 (28.9) | |
| Stage II | 5741 (36.3) | 1038 (28.3) | 1633 (26.2) | 648 (43.8) | 2422 (54.7) | <0.001 |
| Stage III | 1738 (11.0) | 433 (11.8) | 247 (4.0) | 432 (29.2) | 626 (14.1) | |
| Missing |
754 (4.8) |
301 (8.2) |
253 (4.1) |
97 (6.5) |
103 (2.3) |
|
| Estrogen receptor status | ||||||
| Positive | 13477 (85.2) | 1498 (40.8) | 6179 (99.1) | 1444 (97.5) | 4356 (98.3) | |
| Negative | 2083 (13.2) | 1995 (54.4) | 29 (0.5) | 19 (1.3) | 40 (0.9) | <0.001 |
| Missing |
255 (1.6) |
175 (4.8) |
27 (0.4) |
18 (1.2) |
35 (0.8) |
|
|
HER2-status Positive Negative Missing |
1628 (10.3) 12029 (76.1) 2158 (13.6) |
679 (18.5) 2448 (66.7) 541 (14.7) |
230 (3.7) 4683 (75.1) 1322 (21.2) |
222 (15.0) 1170 (79.0) 89 (6.0) |
497 (11.2) 3728 (84.1) 206 (4.6) |
<0.001 |
| Type of surgery | ||||||
| Mastectomy | 6442 (40.7) | 1525 (41.6) | 1937 (31.1) | 909 (61.4) | 2071 (46.7) | <0.001 |
| Breast conserving |
9373 (59.3) |
2143 (58.4) |
4298 (68.9) |
572 (38.6) |
2360 (53.3) |
|
| Postoperative chemotherapy | ||||||
| No | 11031 (69.8) | 2222 (60.6) | 5445 (87.3) | 696 (65.4) | 2395 (54.1) | |
| Anthracycline-taxane based | 2635 (16.7) | 836 (22.8) | 447 (7.2) | 318 (21.5) | 1034 (23.3) | <0.001 |
| Only anthracycline-based | 1186 (7.5) | 357 (9.7) | 195 (3.1) | 107 (7.2) | 527 (11.9) | |
| Only taxane-based | 651 (4.1) | 172 (4.7) | 84 (1.3) | 81 (5.5) | 314 (7.1) | |
| Other | 312 (2.0) | 81 (2.2) | 64 (1.0) | 6 (0.4) | 161 (3.6) | |
|
Trastuzumab |
1182 (7.5) |
500 (13.6) |
124 (2.0) |
141 (9.5) |
417 (9.4) |
<0.001 |
|
Radiotherapy |
11115 (70.3) |
2516 (68.6) |
4348 (69.7) |
879 (59.4) |
3372 (76.1) |
<0.001 |
| Cardiovascular medication at diagnosis | ||||||
| Antidiabetics | 1242 (7.9) | 273 (7.4) | 467 (7.5) | 168 (11.3) | 334 (7.5) | <0.001 |
| Antitrombotics | 3076 (19.4) | 716 (19.5) | 1152 (18.5) | 393 (26.5) | 815 (18.4) | <0.001 |
| Lipid modifiers | 3926 (24.8) | 951 (25.9) | 1457 (23.4) | 418 (28.2) | 1100 (24.8) | <0.001 |
| Cardiac therapy | 1668 (10.5) | 383 (10.4) | 652 (10.5) | 230 (15.5) | 403 (9.1) | <0.001 |
| Antihypertensives | 9520 (60.2) | 2132 (58.1) | 3715 (59.6) | 1020 (68.9) | 2653 (59.9) | <0.001 |
Table 2.
Incidence rates for cardiovascular events in the whole patient cohort.
| Outcome | N of events | Person-Years | Incidence rate per 1000 person-years (95% CI) |
|---|---|---|---|
| Heart failure or cardiomyopathy | 74 | 61 220 | 1.2 (1.0–1.5) |
| Arrythmia | 1140 | 60 420 | 18.9 (17.8–20.0) |
| Acute ischemic heart disease | 311 | 63 597 | 4.9 (4.4–5.5) |
| Ischemic stroke or TIA | 209 | 58 291 | 3.6 (3.1–4.1) |
Abbreviations: N, number; CI, Confidence Interval; TIA, Transient Ischemic Attack.
3.2. CVD risk in the whole study cohort
Table 3 shows the risk for CVD outcomes according to exposure to endocrine therapy. In unadjusted Cox regression analysis regarding the whole cohort, an increased risk for heart failure and arrhythmias was found in patients in the “AI only” group. However, no statistically significant difference was found after adjustment for covariates. Patients in the “tamoxifen only” group were at decreased risk for ischemic heart disease compared to “Non-users”, in both unadjusted and adjusted analyses.
Table 3.
Hazard ratios (95% Confidence interval) for CVD outcomes by exposure to endocrine treatment.
| Outcome | Non-users | Tamoxifen only | AI only | AI/tamoxifen |
|---|---|---|---|---|
|
Heart failure or cardiomyopathy Full cohort - unadjusted Full cohort - adjusted >75 years old - unadjusted >75 years old - adjusted No prior CVD - unadjusted No prior CVD – adjusted |
1 1 1 1 1 1 |
0.87 (0.72–1.07) 0.75 (0.54–1.04) 0.92 (0.71–1.18) 1.06 (0.64–1.77) 0.88 (0.72–1.08) 0.76 (0.55–1.06) |
1.33 (1.10–1.62) 0.82 (0.60–1.13) 1.24 (0.97–1.60) 0.83 (0.50–1.39) 1.32 (1.09–1.61) 0.78 (0.57–1.09) |
1.24 (0.91–1.68) 1.27 (0.81–1.98) 1.40 (0.93–2.10) 2.44 (1.32–4.54) 1.25 (0.92–1.70) 1.26 (0.81–1.98) |
|
Arrythmia Full cohort - unadjusted Full cohort - adjusted >75 years old - unadjusted >75 years old - adjusted No prior CVD - unadjusted No prior CVD - adjusted |
1 1 1 1 1 1 |
0.94 (0.80–1.41) 0.91 (0.73–1.15) 0.81 (0.65–1.02) 0.80 (0.52–1.24) 1.03 (0.85–1.25) 1.12 (0.86–1.46) |
1.35 (1.15–1.59) 1.09 (0.87–1.38) 1.28 (1.03–1.59) 1.20 (0.82–1.75) 1.29 (1.06–1.57) 1.19 (0.90–1.56) |
1.18 (0.92–1.52) 1.19 (0.85–1.66) 0.91 (0.60–1.39) 0.89 (0.46–1.74) 1.23 (0.92–1.63) 1.45 (1.01–2.10) |
|
Acute ischemic heart disease Full cohort unadjusted Full cohort adjusted >75 unadjusted >75 adjusted No prior CVD unadjusted |
1 1 1 1 1 |
0.80 (0.57–1.13) 0.52 (0.28–0.96) 0.96 (0.62–1.47) 0.47 (0.15–1.47) 0.79 (0.55–1.12) |
1.18 (0.86–1.61) 1.25 (0.78–2.00) 1.03 (0.67–1.58) 1.18 (0.51–2.74) 1.16 (0.82–1.62) |
0.94 (0.57–1.56) 0.97 (0.46–2.03) 1.02 (0.50–2.09) 1.13 (0.34–3.83) 0.95 (0.56–1.60) |
|
Ischemic stroke or TIA Full cohort unadjusted Full cohort adjusted >75 unadjusted >75 adjusted No prior CVD unadjusted No prior CVD adjusted |
1 1 1 1 1 1 |
0.84 (0.66–1.08) 0.84 (0.59–1.20) 0.83 (0.59–1.16) 0.67 (0.34–1.34) 0.83 (0.65–1.07) 0.82 (0.57–1.19) |
1.20 (0.95–1.52) 1.14 (0.81–1.61) 1.02 (0.73–1.44) 0.94 (0.51–1.75) 1.17 (0.92–1.49) 1.12 (0.78–1.60) |
1.03 (0.72–1.47) 1.12 (0.70–1.80) 0.86 (0.49–1.51) 0.81 (0.35–1.87) 1.05 (0.73–1.51) 1.12 (0.69–1.82) |
Abbreviations: AI, Aromatase Inhibitor; CVD, Cardiovascular Disease; TIA, Transient Ischemic Attack. Adjustment includes following covariates: Age, side, stage, chemotherapy, Trastuzumab, surgical procedure, radiation therapy, CCI, marital status and family income.
3.3. CVD risk in subgroups
In analyses including patients older than 75 years, exposure to AIs was not associated with increased risk for CVD outcomes compared with “Non-users”, except for heart failure, where an increased risk was seen in the “AI/tamoxifen” group after adjustment for covariates (Table 3).
In analyses regarding patients with no prior CVD event of interest or CVD medication a breast cancer diagnosis, a statistically significant increased risk for arrhythmia was observed in the “AI/tamoxifen” group, whereas a statistically significant decreased risk for ischemic heart disease was seen in the “Tamoxifen only” group (Table 3).
3.4. CVD risk depending on exposure time period
Exposure to AIs for four years or more was associated with significantly increased risk for arrhythmias and ischemic heart disease compared with no AIs or AIs less than one year (Table 4). Usage of tamoxifen for three years or more was associated with statistically significant decreased risk for ischemic heart disease events compared with no tamoxifen or tamoxifen less than one year, whereas an increased risk for stroke or TIA was observed when patients were exposed to tamoxifen for more than four years.
Table 4.
Adjusted Hazard Ratios for cardiovascular events based on duration of endocrine therapy.
| Endocrine therapy | Type of cardiovascular event, HR (95% CI) |
|||
|---|---|---|---|---|
| Heart failure or cardiomyopathy | Arrythmia | Acute ischemic heart disease | Ischemic stroke or TIA | |
|
Tamoxifen duration, years | ||||
| Non-users or < 1 y | 1 | 1 | 1 | 1 |
| 1–2 y | 1.21 (0.13–19.72) | 1.59 (0.88–2.57) | 0.86 (0.57–1.30) | 0.70 (0.27–1.85) |
| 3–4 y | 0.95 (0.06–15.29) | 1.12 (0.74–1.69) | 0.32 (0.19–0.55) | 1.78 (0.86–3.69) |
| >4 y |
0.67 (0.04–10.70) |
1.05 (0.71–1.55) |
0.45 (0.25–0.51) |
2.00 (1.05–3.84) |
|
Aromatase inhibitors, years | ||||
| Non-users or < 1 y | 1 | 1 | 1 | 1 |
| 1–2 y | 1.96 (0.21–19.11) | 1.57 (0.43–2.18) | 1.47 (0.91–2.40) | 1.83 (0.98–3.40) |
| 3–4 y | 2.19 (0.47–17.55) | 1.47 (0.60–1.83) | 1.18 (0.81–1.70) | 1.12 (0.48–2.58) |
| >4 y | 2.72 (0.66–26.09) | 2.12 (1.40–3.25) | 2.03 (1.15–3.58) | 1.16 (0.55–2.46) |
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; TIA, Transient Ischemic Attack; y, years. Adjustment includes following covariates: Age, side, stage, chemotherapy, Trastuzumab, surgical procedure, radiation therapy, CCI, marital status and family income.
4. Discussion
In this population-based study, exposure to AIs was not associated with increased risk for CVD outcomes compared to no endocrine treatment in the main analysis. However, when duration of AI exposure was considered, exposure to AIs for more than four years was associated with increased risk for acute ischemic heart disease and arrhythmias. Furthermore, AI treatment sequentially with tamoxifen increased the risk for heart failure and cardiomyopathy in patients older than 75 years as well as arrhythmias in patients without prior arrhythmia or CVD medication at baseline. A decreased risk for ischemic heart disease was seen in patients treated with tamoxifen only compared to no endocrine treatment whereas tamoxifen was associated with higher risk for stroke or TIA only when tamoxifen exposure exceeded four years.
Considering the evidence supporting a cardioprotective effect of tamoxifen [8,26,27] we argue that exposure to tamoxifen might not be the suitable comparator to investigate a potential impact of AIs on CVD outcomes, but that AI exposure should also be compared to breast cancer patients without endocrine therapy. Three observational studies have investigated the potential role of AIs on CVD outcomes using no endocrine therapy as comparator with conflicting results. Ligibel et al. compared breast cancer patients previously treated with AIs to breast cancer patients without endocrine therapy and found no difference regarding risk for myocardial infarction or ischemic stroke [13]. Matthews et al. analyzed AI exposure in relation to different CVD outcomes in two population-based cohorts from the UK and USA, using two separate analyses, one with tamoxifen exposure as comparator and one with no endocrine therapy as comparator. In the analysis with tamoxifen exposure as comparator, AI exposure was associated with increased risk for coronary artery disease, arrhythmias, and heart failure, whereas this relationship was diminished when no endocrine therapy was used as comparator [14]. On the other hand, Jacobse et al. investigated the risk for heart failure in a population-based cohort of 10 209 breast cancer patients and found that AI exposure was associated with increased risk for heart failure compared to no endocrine therapy [12].
Our results, showing lack of association between AI exposure and CVD outcomes in the analysis including the whole study cohort, are consistent with most of the previously published observational studies comparing AI with no endocrine treatment [[12], [13], [14]]. However, none of the previous studies investigated the duration of AI exposure in relation to CVD outcomes. The observed increased risk for ischemic heart disease and arrythmia in patients treated with at least four years of AIs is plausible from a biological perspective [28] and it is also supported by some indirect evidence where sequential treatment with AI and Tam did not seem to be associated with increased risk for CVD whereas treatment with AI monotherapy did [12] as well as by randomized evidence where extended treatment with AIs has been associated with increased risk for cardiovascular events [9].
Some methodological differences between our study and prior studies using no endocrine therapy as comparator should also be considered as potential sources for different results. First, the definition of exposure to endocrine therapy differed among the studies; Jacobse et al. used planned treatment for exposure, whereas Matthews et al. and Ligibel et al. used prescription data with one prescription to be enough for exposure. In our study, prescription data were also used, but with a treatment period of endocrine therapy for at least six months to define a user, a time period chosen considering the approximated time needed to cause biological changes of clinical significance. Furthermore, the source and definition of outcomes also differed with one study investigating only heart failure (including deaths due to heart failure) using physician-reported outcome whereas the others used ICD-codes for several CVD outcomes without including death due to CVD as event. We used ICD-codes regarding hospital care as well as cause of death, to be able to capture patients with missing ICD-codes for CVD outcomes from The National Patient Register but with suitable ICD-diagnosis as cause of death in The Swedish cause of death register. Finally, the co-variates in the multivariate models varied across the studies, with some variables of interest, such as socioeconomic status, smoking, body mass index (BMI), prior CVD outcome, current medication and lipid status not being included in some models. We were able to adjust for several well-documented risk factors for CVD, such as socioeconomic status [29], whereas we performed subgroup analyses considering prior CVD events of interest and current medication for CVD. However, we were unable to adjust for all potential CVD risk factors, such as smoking status, BMI and lipid status which is a limitation to consider when interpreting our results.
The associations in our study between exposure to sequential treatment with AIs and tamoxifen, but not to AIs only, for certain outcomes in subgroups could probably, at least partially, be explained by the considerably larger cohort of patients exposed to sequential treatment compared to AIs only, which gives more statistical power within this patient group. It should be noted that in subgroup analyses with smaller cohort of patients, there is a higher risk that observations will be due to chance leading to a higher risk for unreliable results. For a more fair interpretation of our results, it should also be noted that all patients with AI exposure, including those with sequential treatment, were included in the analysis based on exposure time period irrespectively the potential exposure to tamoxifen as well.
Our results suggest a protective effect of tamoxifen on heart disease in accordance with previous studies [8,26]. This protective effect of tamoxifen on heart disease is supporting by preclinical data where tamoxifen seems to accelerate endothelial healing through activation of nuclear estrogen receptor (ER)α in smooth muscle cells [30]. On the other hand, an increased risk of ischemic stroke or TIA with longer tamoxifen treatment was observed. A meta-analysis including RCTs has showed an increased relative risk for stroke in patients treated with tamoxifen, although the absolute risk for stroke was small [31]. In a more recent systematic review including both RCTs and observational studies, tamoxifen was not associated with increased risk for stroke [8]. Whether our observation is due to a true association between exposure duration to tamoxifen and risk for stroke or TIA or it depends on potential unmeasured confounders needs to be further investigated.
Interestingly, almost one fourth of study cohort was not planned for endocrine therapy although nearly 40% had stage II-III disease and about 40% were hormone-receptor positive. This observation can be explained by the fact that most of the patients with advanced stage had triple negative breast cancer whereas the majority of patients with hormone-receptor positive disease not planned for endocrine therapy had T1a or T1b breast cancer. Although the relative endocrine treatment benefit for small breast tumors is similar as for larger tumors [32], the absolute benefit is small and the prognosis, especially for luminal A breast cancer, is excellent [33]. As a result, excluding adjuvant endocrine therapy in this patient population is a common practice in Sweden [33].
There are several limitations to our study to discuss. Firstly, the retrospective nature of this study makes the results prone to bias. Secondly, the “AI only” group comprised fewer patients compared with the other exposure groups, which might influence the statistical power as previously described. Thirdly, the source for CVD events does not cover primary care. Therefore, our data lacks information on diagnoses exclusively treated in the primary care. However, only about 17% of heart failure patients in Sweden are treated exclusively in the primary care setting [34], and we have no reason to suspect that a potential misclassification of outcome should affect the different exposure groups differently. Regarding our definitions for CVD outcomes, we chose to merge different CVD events in four distinct categories for analytical purposes which impact our possibility to reveal any association between endocrine therapy exposure and a specific diagnosis. In addition, we had no data regarding the severity of CVD at baseline. Another potential methodological limitation that should be considered is the risk that confounding factors can influence the observed associations between exposure to endocrine therapy and CVD. Although we adjusted our multivariate models for potential patient- and treatment-related confounding factors, the risk for confounding is still present. Another methodological limitation was that planned subgroup analysis on patients with prior CVD events of interest or medication for CVD was not possible due to low number of events. Finally, although the exposure to endocrine therapy was defined using the prescription history through DDD, the actual adherence to endocrine therapy could not be measured.
In conclusion, our study did not find a statistically significant increased risk for CVD in patients treated with AIs compared with no endocrine treatment in general. However, our results indicate an increased risk for ischemic heart disease and arrhythmias in patients treated for more than four years with AIs, an observation which is in line with randomized evidence from extended AI trials [9], as well as for heart failure in patients older than 75 years. This information should be incorporated in the shared decision-making process of risk-benefit assessment concerning endocrine therapy. In particular, sequential treatment approach with two years of AIs followed by three years of tamoxifen should be considered a valid option in patients with higher risk for CVD, considering the randomized evidence of similar efficacy as five years of AI treatment approach [1,35,36]. For extended endocrine therapy, a switching strategy with AIs and tamoxifen might also be beneficial for patients with higher CVD risk to limit the exposure to AIs without jeopardizing the efficacy. Our results urge the need to include strategies to prevent CVD as part of surveillance program in breast cancer survivors [37], especially in women with long AI treatment.
Funding
The project has been supported by Bröstcancerförbundet, The Research Council of Region Örebro County and ALF Funding Region Örebro County.
Availability of data and material
The data are available upon request.
Code availability
Not applicable.
Ethics approval
EPN Stockholm dnr 2013/1272–31/4, with supplements approved dnr 2020–06312.
Declaration of competing interest
The authors have no conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.07.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/s0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 2.Gernaat S.A.M., Ho P.J., Rijnberg N. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Canc Res Treat. 2017;164:537–555. doi: 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung Y.M., Ramchand S.K., Yeo B. Cardiometabolic effects of endocrine treatment of estrogen receptor-positive early breast cancer. J Endocr Soc. 2019;3:1283–1301. doi: 10.1210/js.2019-00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Qadir H., Amir E., Fischer H.D. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Canc. 2016;68:11–21. doi: 10.1016/j.ejca.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Haque R., Shi J., Schottinger J.E. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. 2016;2:1590–1597. doi: 10.1001/jamaoncol.2016.0429. [DOI] [PubMed] [Google Scholar]
- 6.Kamaraju S., Shi Y., Smith E. Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study. Clin Cardiol. 2019;42:93–100. doi: 10.1002/clc.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosrow-Khavar F., Filion K.B., Bouganim N. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: a population-based cohort study. Circulation. 2020;141:549–559. doi: 10.1161/circulationaha.119.044750. [DOI] [PubMed] [Google Scholar]
- 8.Matthews A., Stanway S., Farmer R.E. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review. BMJ. 2018;363:k3845. doi: 10.1136/bmj.k3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldvaser H., Barnes T.A., Seruga B. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110 doi: 10.1093/jnci/djx141. [DOI] [PubMed] [Google Scholar]
- 10.Qian X., Li Z., Ruan G. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials. Breast Canc Res Treat. 2019 doi: 10.1007/s10549-019-05464-w. [DOI] [PubMed] [Google Scholar]
- 11.Xu L., Zhang Z., Xiang Q. Extended adjuvant therapy with aromatase inhibitors for early breast cancer: a meta-analysis of randomized controlled trials. Clin Breast Canc. 2019;19:e578–e588. doi: 10.1016/j.clbc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Jacobse J.N., Schaapveld M., Boekel N.B. Risk of heart failure after systemic treatment for early breast cancer: results of a cohort study. Breast Canc Res Treat. 2021;185:205–214. doi: 10.1007/s10549-020-05930-w. [DOI] [PubMed] [Google Scholar]
- 13.Ligibel J.A., James O'Malley A., Fisher M. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Canc Res Treat. 2012;131:589–597. doi: 10.1007/s10549-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 14.Matthews A.A., Peacock Hinton S., Stanway S. Endocrine therapy use and cardiovascular risk in postmenopausal breast cancer survivors. Heart. 2020 doi: 10.1136/heartjnl-2020-317510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löfgren L., Eloranta S., Krawiec K. Validation of data quality in the Swedish national register for breast cancer. BMC Publ Health. 2019;19:495. doi: 10.1186/s12889-019-6846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Quality Registry for Breast Cancer . 2021. National quality register for breast cancer (NKBC). The Swedish association of local authorities and regions (SALAR)https://statistik.incanet.se/brostcancer/ Accesed April 30 2021. [Google Scholar]
- 17.The Swedish association of local authorities and regions (SALAR). National quality registry for breast cancer. The Swedish association of local authorities and regions (SALAR) 2020. http://kvalitetsregister.se/englishpages/findaregistry/registerarkivenglish/nationalqualityregistryforbreastcancernkbc.2095.html Accesed Jan 8 2021. [Google Scholar]
- 18.Socialstyrelsen . Socialstyrelsen; 2020. The Swedish prescribed drug register.https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-swedish-prescribed-drug-register/ Accesed Jan 8 2021. [Google Scholar]
- 19.Wettermark B., Hammar N., Fored C.M. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 20.Statistics Sweden . Statistics Sweden; 2020. Longitudinal integrated database for health insurance and labour market studies.https://www.scb.se/en/services/guidance-for-researchers-and-universities/vilka-mikrodata-finns/longitudinella-register/longitudinal-integrated-database-for-health-insurance-and-labour-market-studies-lisa Accesed Jan 8 2021. [Google Scholar]
- 21.Charlson M.E., Pompei P., Ales K.L. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson J.F., Andersson E., Ekbom A. External review and validation of the Swedish national inpatient register. BMC Publ Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Socialstyrelsen . Socialstyrelsen; 2020. National patient register.https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/ Accesed Jan 8 2021. [Google Scholar]
- 24.Brooke H.L., Talback M., Hornblad J. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Team RC . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing.https://www.R-project.org/ Accesed April 27 2021. [Google Scholar]
- 26.Onitilo A.A., Kar P., Engel J.M. Long-term cardiac and vascular disease outcomes following adjuvant tamoxifen therapy: current understanding of impact on physiology and overall survival. Minerva Med. 2013;104:141–153. [PubMed] [Google Scholar]
- 27.Silva F.B., Romero W.G., Carvalho A. Effects of treatment with chemotherapy and/or tamoxifen on the biomarkers of cardiac injury and oxidative stress in women with breast cancer. Medicine (Baltim) 2017;96 doi: 10.1097/md.0000000000008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iorga A., Cunningham C.M., Moazeni S. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8:33. doi: 10.1186/s13293-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz W.M., Kelli H.M., Lisko J.C. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/circulationaha.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahreddine R., Davezac M., Smirnova N. Tamoxifen accelerates endothelial healing by targeting ERα in smooth muscle cells. Circ Res. 2020;127:1473–1487. doi: 10.1161/CIRCRESAHA.120.317062. [DOI] [PubMed] [Google Scholar]
- 31.Bushnell C.D., Goldstein L.B. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis. Neurology. 2004;63:1230–1233. doi: 10.1212/01.wnl.0000140491.54664.50. [DOI] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Davies C., Godwin J., Gray R., Clarke M., Cutter D., Darby S., McGale P., Pan H.C., Taylor C., Wang Y.C., Dowsett M., Ingle J., Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaraj D., Höijer J., Widman L. Long-Term prognostication for 20 114 women with small and node-negative breast cancer (T1abN0) JNCI Cancer Spectr. 2020;5 doi: 10.1093/jncics/pkaa084. pkaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarrinkoub R., Wettermark B., Wändell P. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15:995–1002. doi: 10.1093/eurjhf/hft064. [DOI] [PubMed] [Google Scholar]
- 35.De Placido S., Gallo C., De Laurentiis M. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol. 2018;19:474–485. doi: 10.1016/s1470-2045(18)30116-5. [DOI] [PubMed] [Google Scholar]
- 36.Regan M.M., Neven P., Giobbie-Hurder A. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011;12:1101–1108. doi: 10.1016/s1470-2045(11)70270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Runowicz C.D., Leach C.R., Henry N.L. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34:611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request.