Abstract
Hypoxia is a common condition of solid tumors that is mainly caused by enhanced tumor proliferative activity and dysfunctional vasculature. In the treatment of hypoxic human solid tumors, many conventional therapeutic approaches (e.g., oxygen-dependent photodynamic therapy, anticancer drug-based chemotherapy or X-ray induced radiotherapy) become considerably less effective or ineffective. In recent years, various strategies have been explored to deliver or generate oxygen inside solid tumors to overcome tumorous hypoxia and show promising evidence to improve the antitumor efficiency. In this review, the extrinsic regulation of tumor hypoxia via nanomaterial delivery is discussed followed by a summary of the mechanisms through which the modulated tumor hypoxic microenvironment improves therapeutic efficacy. The review concludes with future perspectives, to specifically address the translation of nanomaterial-based therapeutic strategies for clinical applications.
Keywords: chemotherapy, nanomedicine, photodynamic therapy, radiotherapy, tumor hypoxic microenvironment
1. Introduction
Hypoxia, a common attribute of solid tumors, is a combinatory effect from the development of new aberrant blood vessels with poor blood flow and the malignant proliferation of tumor cells.[1] In the interior of solid tumors the insufficient oxygen supply cannot meet the ever-increasing metabolic demands, and consequently leads to a hypoxic tumor microenvironment (typically, pO2 ≤ 2.5 mmHg).[2]
Tumor hypoxia can cause a cascade of changes in intracellular and extracellular metabolic processes.[3] Tumor hypoxia can also trigger the activation of hypoxia inducible factors (HIFs), which in turn give rise to the transcription of some key genes involved in angiogenesis, glucose metabolism, pH regulation, metastasis, and tumor invasion.[3] Indeed, hypoxia can drive tumor cells to adopt anaerobic glycolysis as an alternative metabolic path, which results in a low pH for the tumor. In addition, activation of carbonic anhydrase IX or XII by HIFs can exacerbate the acidity of the tumor microenvironment.[4] Increased tumor acidity can then escalate the degradation of extracellular matrix and subsequently facilitates tumor metastasis.[4] As such, tumor metastasis and invasion are closely regulated by hypoxia. On the other hand, the hypoxic tumor microenvironment can greatly affect treatment outcomes of those therapies involving oxygen as a key element for tumor destruction, such as chemotherapy, radiotherapy (RT), and photodynamic therapy (PDT). Chemotherapy is an essential tumor treatment modality. However, increasing evidence has shown that hypoxic tumors can limit the accumulation of antitumor drugs and frustrate therapeutic efficacy.[5] For example, recent findings indicate that tumor cells grown in hypoxic conditions decrease the bioavailability of doxorubicin (DOX).[6] As a matter of fact, tumor cells adapted to a hypoxic environment overexpress hypoxia-inducible factor-1α (HIF-1α), which participates in the transcriptional activity of a multidrug resistance gene (MDR1).[7] Up-regulation of the MDR1 gene product P-glycoprotein causes efflux of intracellular anticancer drugs and results in chemotherapy resistance.[8] In addition, hypoxia can be a direct cause of chemotherapeutic resistance because some drugs are oxygen-dependent and require oxygen to maximize the cytotoxicity.[9] RT, another widely adopted cancer therapy, induces the breakage of double-stranded DNA and produces massive free radicals to kill tumors.[10] However, minimal killing effects on hypoxic tumors were seen as a result of hypoxia that could attenuate oxidation to protect tumor cells from extensive damage by free radicals.[10] PDT, a promising modality of tumor therapy, utilizes tissue oxygen and photosensitizing molecules upon light irradiation to generate reactive oxygen species such as singlet oxygen (1O2) to kill cancer cells.[11] Thus, during PDT treatment, the oxygen level within the target tissue becomes an important regulator to affect the production of 1O2 for cell destruction. Meanwhile, continuous consumption of O2 mediated by a photosensitizer may further deplete oxygen to exacerbate tumor hypoxia and significantly decreases PDT efficacy. In this regard, relieving the tumor hypoxia microenvironment to improve the therapeutic effect has received tremendous attention.[12]
Nanomedicine, aiming to discover novel solutions for “hard-to-achieve” healthcare needs by taking advantage of rapid advances in nanotechnology, offers great promise in improving the specificity and efficiency of treatment and the resolution and accuracy of diagnosis against tumors.[13] It is well known that nanoparticles exhibit passive tumor targeting as a result of the enhanced permeability and retention (EPR) effect from defective and irregular tumor blood vessels as well as poor lymphatic drainage.[14,15] Built on their intrinsic properties and unique functions, many nanoparticles could be used not only as delivery vehicles to increase retention of therapeutic molecules in tumors, but also as therapeutic agents such as gold nanoparticles in phototherapy, MoS2 nanomaterials in radiotherapy, and polymer micelles in immunotherapy.[16–18] Clearly, nanomedicine opens new opportunities for cancer therapy following different mechanistic routes.
In view of the benefits of oxygen for enhanced tumor therapy, recent efforts have been made to alleviate hypoxic conditions by utilizing various nanomaterials.[19–21] In general, four major strategies are explored, including, 1) the use of catalytic nanoparticles to generate oxygen in situ through decomposing endogenous hydrogen peroxide (H2O2) to effectively conquer tumor hypoxia for enhanced cancer therapies,[22–24] 2) the use of oxygen-loaded nanomaterials that could directly carry oxygen into a tumor site with the help of perfluorocarbon or hemoglobin or the delivery of oxygen precursors by means of engineered nanoparticles to improve tumor oxygenation status,[25,26] 3) relief of the hypoxia by improving blood flow of the tumor sites induced by nanoparticle-enabled local hyperthermia effects or by decompressing blood vessels with nanodrugs,[27,28] and 4) the use of hypoglycemic drugs, such as metformin, to reduce oxygen consumption by tumors via inhibition of respiratory activities to improve the tumor hypoxic microenvironment.[29] In fact, each strategy has its own advantages and disadvantages. Table 1 shows the key characteristics of tumor oxygenation improvement strategies applied in cancer therapy. Due to the elevated level of H2O2 in a tumor microenvironment, the strategies of oxygen generation in situ show high tumor selectivity. However, the amount of endogenous H2O2 is limited, especially in terms of inconsistent supply at tumor sites, which may not meet the need of those treatments requiring a large quantity of O2. Obviously, O2-loaded or H2O2-delivered nanosystems could address this challenge by introducing exogenous oxygen into the tumor.[30,31] In addition, improving blood flow with the help of nanoparticle-enabled local hyperthermia or nanodrugs that cause blood vessel decompression could supply the tumor with endogenous and circulatory O2. Although the strategy of decreasing oxygen consumption could continuously improve O2 status of the tumor by inhibiting respiration activity during drug function period, the oxygen enhancement effect is very limited and often not pronounced. Opposite to the strategies of improving tumor oxygenation, the hypoxic features of tumor could be of advantage to hypoxia-activated drugs delivered to the tumor site for therapy.[32]
Table 1.
Comparison of tumor oxygenation improvement strategies applied in cancer therapy.
| Strategies | Advantages | Disadvantages |
|---|---|---|
| Oxygen generation in situ | High tumor selectivity | Low amount of endogenous H2 O2 in tumor |
| Direct oxygen delivery | Simple and direct | Possible oxygen leakage during circulation |
| Improving blood flow | Endogenous O2 supply | Relative high phototoxicity Poor depth penetration |
| Decreasing oxygen consumption | Sustained | Limited oxygen enhancement effect |
In this review, we summarize the key nanomedicine strategies to regulate the hypoxic microenvironment of tumor for enhanced cancer therapy (Figure 1). The literatures used mainly come from the past 5 years’ reports and those elucidating future directions. To this end, we conclude with future perspectives to specifically address the translational aspects of nanomaterial-based therapeutic strategies for clinical applications in conjunction with hypoxic circumstances.
Figure 1.
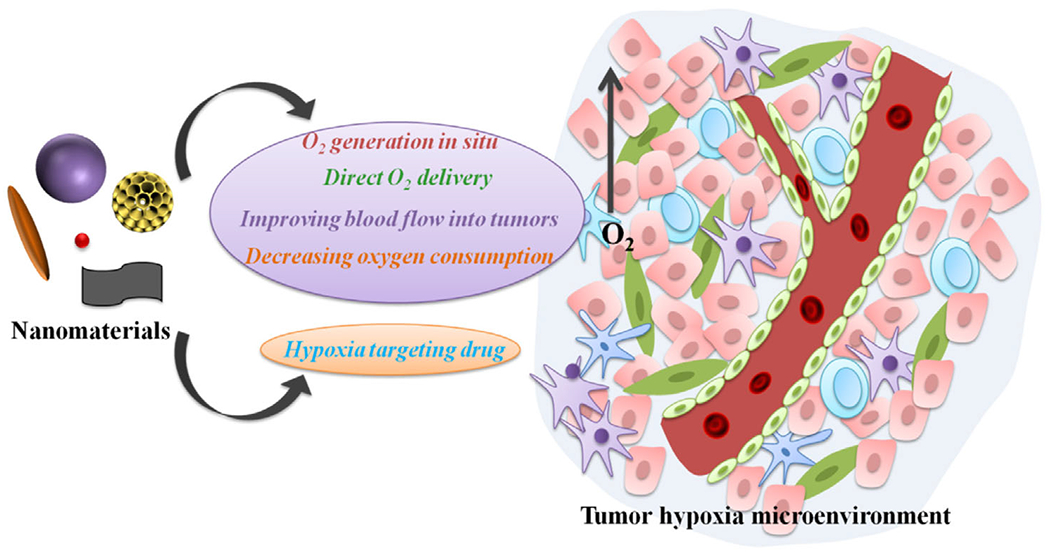
A schematic highlight of the potential utility of nanomaterials for relief of tumor hypoxia and enhancement of tumor therapy.
2. Oxygen Generation In Situ
In living organisms H2O2 is a key signaling mediator, directly involved in regulating cell proliferation, differentiation, migration, or apoptosis.[33] As a result of the unique pathophysiologic processes of tumors in their uncontrollable proliferation, apoptosis resistance and high metastasis, levels of H2O2 are commonly elevated in the tumor microenvironment.[34] Taking advantage of the elevated endogenous H2O2 at tumor sites, hypoxic conditions could be readily improved by decomposing H2O2 into O2 within the tumor with various catalytic nanostructures.[35,36] The detailed decomposition by individual nanostructures is further discussed below.
2.1. Inorganic Catalysis Nanosystems
2 1.1. MnO2 Nanostructures
Besides excessive H2O2 and extreme hypoxia, mild acidity is also often detected in the tumor microenvironment.[37] Built on these features, manganese dioxide (MnO2) nanostructures have been particularly chosen for their high O2-generation efficiency in the acidic and H2O2-rich tumor environment to catalyze and decompose H2O2 to generate O2. In addition, MnO2 nanostructures can be simultaneously reduced to Mn2+ by acidic H2O2, leading to the dissolution of these nanostructures.[38] As such, there is increasing interest in utilizing MnO2-based nanostructures to relieve tumor hypoxia for efficient cancer therapy.[39–44]
The use of MnO2 nanoparticles for modulation of hypoxia and acidosis in tumor microenvironments was first reported by the Wu group in 2014 (Figure 2a).[39] In this study, a one-step fabrication strategy was employed to reduce potassium permanganate (KMnO4) into MnO2 nanoparticles by a cationic polyelectrolyte poly(allylamine hydrochloride) (PAH). To increase the colloidal stability and biocompatibility of the nanoparticles, bovine serum albumin (BSA) was conjugated to the particle surface to form A-MnO2 nanoparticles. In vitro evidence has confirmed their ability to generate oxygen by reacting with endogenous H2O2 produced by cancer cells under hypoxic conditions. In a murine breast tumor model, upon intratumoral (i.t.) injection of a suspension of A-MnO2 nanoparticles the vascular O2 saturation increased promptly by ≈45% as compared to control tumors (Figure 2b). In combination with ionizing radiation, tumor growth was significantly inhibited during the experimental period (Figure 2c). Even though the i.t. injection is not an ideal approach for clinical tumor treatment, the effect of this first Mn-based nanosystem provides a direction for enhanced PDT treatment. In other work, Shi et al. developed intelligent 2D theranostic nanomaterials (UCSMs) based on the MnO2 nanosheets anchored with upconversion nanoparticles (UCSs) for concurrent pH-/H2O2-responsive upconversion luminescence (UCL) imaging and oxygen-elevated synergetic radio/photodynamic therapy (Figure 2d). Upon exposure to the acidic H2O2 in solid tumors, nanosheets of MnO2 could be reduced into soluble Mn2+ along with the generation of a large quantity of oxygen to significantly improve the oxygen-dependent PDT/RT of hypoxic tumors. As shown in Figure 2e, the combination of RT and near infrared-PDT enabled by UCSMs is demonstrated to be the most effective to eradicate tumor. Meanwhile, the released upconversion nanoparticles could turn into bright probe and serve as sensors for diagnosis or monitoring of local environment changes.[40]
Figure 2.
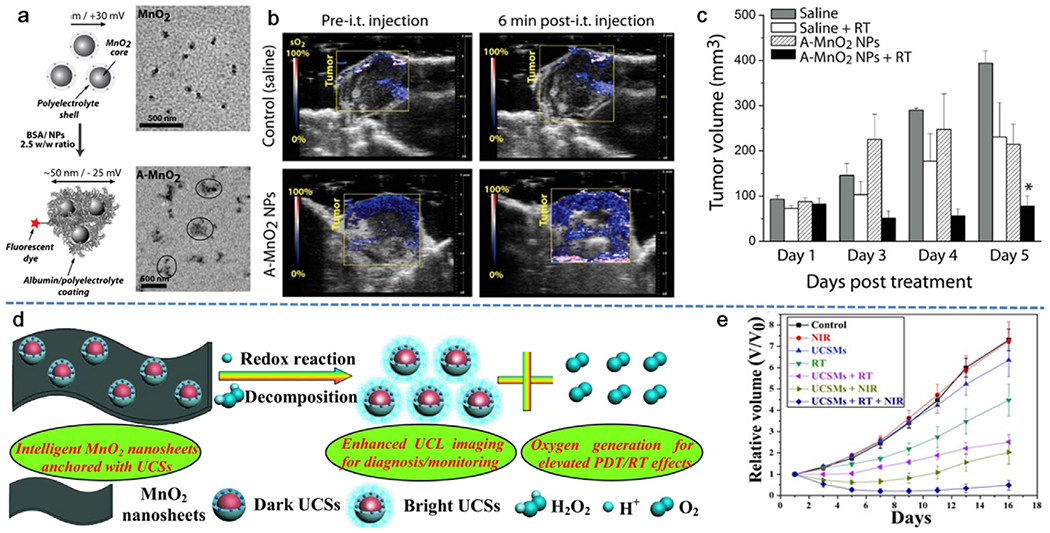
MnO2-catalyzed in situ oxygen generation for enhanced cancer therapy by overcoming tumor hypoxia. a) Diagram and transmission electron microscopy (TEM) images of BSA stabilized MnO2 (A-MnO2) NPs. b) Representative 2D photoacoustic (PA) images of EMT6 solid tumors showing parametric map of estimated oxygen saturation (sO2) after injection with different samples. c) Time-dependent volume changes of tumor after various treatments, demonstrating the efficacy of A-MnO2 in combination with radiation therapy for tumor treatment. Reproduced with permission.[39] Copyright 2014, American Chemical Society. d) A schematic illustration of the decomposition of MnO2 nanosheets anchored with upconversion nanoprobes (UCSM composite) in the presence of acidic H2O2 for enhanced upconversion luminescence (UCL) imaging and elevated PDT and radiotherapy. e) The tumor growth profile in mice after the treatment with UCSM in combination of radiation and near-infrared (NIR) laser irradiation. Reproduced with permission.[40] Copyright 2015, Wiley-VCH.
Wang and his colleagues have also recently fabricated MnO2 nanodots using the redox reaction between MnSO4 and KMnO4 in an aqueous solution and these nanodots were loaded on the surface of g-C3N4- and DOX-encapsulated zeolitic imidazolate framework (ZIF-8) carriers to form MnO2-ZIF@DOX/C3N4 nanomaterial.[41] The surface of the formed nanocomposite was then modified with Pluronic F127 (F127) to increase biocompatibility. In this study, it was anticipated that the loaded MnO2 nanodots could react with endogenous acidic H2O2 within the tumor cells and consequently elevate the concentration of dissolved oxygen, leading to improved cancer chemo-photodynamic therapy efficacy. Experimental evidence from in vivo studies indeed demonstrated that F127-MnO2-ZIF@DOX/C3N4 nanomaterials could effectively relieve tumor hypoxia and enhance the effect of photodynamic and chemotherapeutic treatments. Over an experimental period of 40 days, 100% survival rate of mice was achieved with the combined treatment of nanoparticles and light irradiation. Besides the examples given here, continuous undertakings focus on optimizing the configurations of MnO2-based nanostructures such as hollow nanospheres, as well as the development of nanocomposites together with other nanostructures to yield diverse functionality to further enhance cancer therapy in addition to alleviation of tumor hypoxia.[42–46] Although these MnO2-based nanostructures achieved impressive antitumor effects, their recognized cytotoxicity remained to be addressed.
2.1.2. Pt Nanoparticles
Platinum (Pt) nanoparticles, a well-known metallic catalyst with enzyme mimetic properties, have been noted for their ability to catalyze endogenous H2O2→O2 conversion in tumors.[47–53] For example, Qu et al. decorated Pt nanozymes onto photosensitizer-integrated metal-organic frameworks (MOFs) to form a versatile nanosystem with high catalase-like activity to induce the decomposition of H2O2 into O2 in hypoxic tumors.[48] In more detail, a porous coordination network-224 (PCN-224), consisting of a porphyrinic Zr-cluster MOF, was selected as the nanocarrier to be deposited with Pt nanoparticles (with an average diameter of about 90 nm) by in situ reduction and coated with PEG to form PCN-224-Pt. In recognition of the high expression of HIF-1α and upregulated secretion of vascular endothelial growth factor (VEGF) in hypoxic tumor microenvironments, the ability of PCN-224-Pt to relieve tumor hypoxia was confirmed by staining tumor sections for both HIF-1α and VEGF 24 h post-intratumoral injection with PCN-224-Pt. As shown in Figure 3a, tumors injected with PCN-224-Pt displayed greatly decreased immunofluorescence intensity of HIF-1α and VEGF as a result of the production of O2 in the tumor site through catalytic reaction between intracellular H2O2 and Pt nanoparticles on PCN-224-Pt. Further analysis of the tumor growth upon light radiation revealed great reduction in tumor size with i.t. injection of PCN-224-Pt nanoparticles (Figure 3b). The promoted H2O2→O2 conversion by the presence of Pt was noted, but not dramatic especially at early time points (e.g., 4 days) (Figure 3b). This observation indicates that improvement of tumor therapy via enhancing tumor oxygen status with Pt-loaded nanoparticles may not be as effective as we expect. Zheng et al. designed and fabricated bimetallic nanoplates of Pd@Pt (Pd@Pt nanoplates) by reducing a Pt(acac)2 precursor with hydrazine hydrate onto prepared Pd nanosheets seeds.[49] The Pd@Pt nanoplates were then further modified with bifunctional PEG (SH-PEG-NH2) and covalently conjugated with the photosensitizer chlorin e6 (Ce6) to obtain Pd@Pt-PEG-Ce6 nanocomposite (Figure 3c). Both in vitro and in vivo results clearly indicated that Pd@Pt-PEG-Ce6 effectively delivered Ce6 to tumor sites and decomposed intracellular H2O2 into O2, resulting in a remarkably enhanced PDT efficacy. In another attempt, Pt nanoparticles as a catalyst were allowed to grow on the surface of a polydopamine (Pda) core to form core-shell structures (Pda-Pt).[50] The exterior of Pda-Pt was coated with zirconium-porphyrin (PCN) composed of H2TCPP and Zr4+, and further modified with folic acid (FA) to form Pda-Pt@PCN-FA nanostructures. The obtained hybrid core-shell nanostructures work like a nanofactory to provide sequential products with a temporospatial resolution (Figure 3d). Once endogenous H2O2 diffused through the mesoporous structure of interlayer and reached the Pt nanoparticles, it was decomposed into O2. Under light irradiation, the high concentration of oxygen released from the inner layer was converted into reactive oxygen species (ROS) by the outer PCN shell layer and therefore significantly enhanced the therapeutic effect of PDT. In the absence of light irradiation, the produced O2 could improve hypoxic conditions, thereby inhibiting the invasion and metastasis of cancer. As anticipated, treatment of tumor-bearing mice with Pda-Pt@PCN-FA nanoparticles and light irradiation (660 nm, 220 mW cm−2) did lead to a significant inhibition of tumor progression despite the omission of proper controls such as Pt nanoparticles from this study. In sum, these Pt-based nanoparticles did display improved therapeutic effects, however, they usually suffered from complex fabrication procedures and the need of comprehensive investigation on toxicity as a new material.
Figure 3.
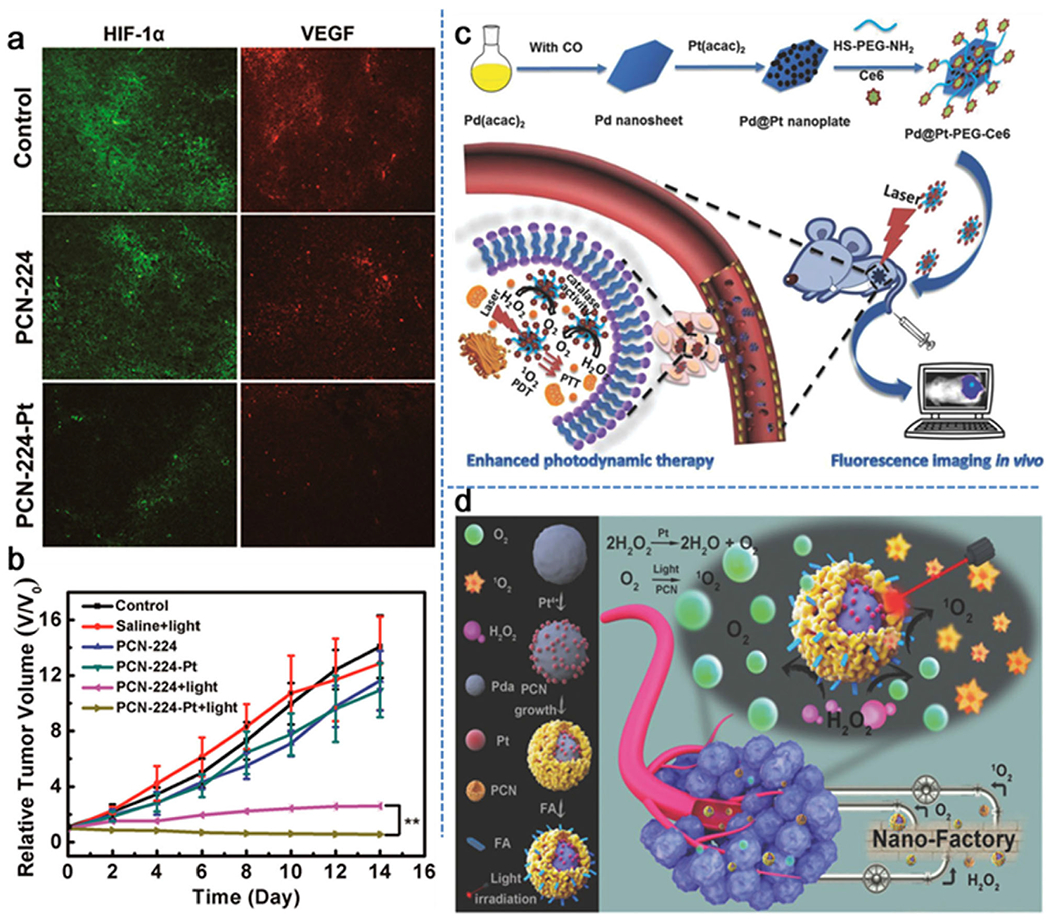
In situ oxygen generation catalyzed by Pt for the relief of tumor hypoxia. a) Immunofluorescence staining for HIF-1α and VEGF of tumor tissues collected from mice after different treatments. b) Time-dependent tumor growth after various treatments, showing the tumor inhibitory effects of both PCN-224 and PCN-224-Pt with light irradiation. Reproduced with permission.[48] Copyright 2018, American Chemical Society. c) Diagrammatic illustration of the preparation and utility of Pd@Pt-PEG-Ce6 for enhanced PDT and fluorescence imaging. Reproduced with permission.[49] Copyright 2018, Wiley-VCH. d) Schematic illustration of the fabrication and use of core-shell nanofactory for enhanced tumor therapy. Reproduced with permission.[50] Copyright 2018, Wiley-VCH.
2.2. Catalase Enzyme Loaded Nanoparticles
Catalase, an enzyme to rapidly decompose H2O2 into H2O and O2, has been explored in recent years to address the tumor hypoxia issue through in situ oxygen generation.[54–57] However, catalase is usually unstable in the presence of proteases during blood circulation after systemic administration, which may raise a significant concern for its further utility.[58] With this regard, various strategies have been taken to protect catalase from protease digestion during its tumor-targeted delivery process, for example, encapsulation of catalase into nanostructures.[59–61] Following this endeavor, Liu et al. designed catalase (Cat)-encapsulated hollow tantalum oxide (TaOx) nanoshells modified with polyethylene glycol (PEG).[62] The obtained TaOx@Cat-PEG served as both a radiosensitizer and a bio-nanoreactor to decompose tumor endogenic H2O2 into O2, rapidly relieving the tumor hypoxia, and simultaneously greatly enhance the efficiency of cancer radiotherapy (Figure 4a). In another work from the Liu group, photosensitizer chlorine e6 (Ce6) together with catalase were loaded into paclitaxel (PTX)-triggered co-assembly of proteins to form HSA-Ce6-Cat-PTX nanoparticles.[63] As shown in Figure 4b, the level of blood oxyhemoglobin in the mouse tumors showed a significant increase after injection with HSA-Ce6-Cat-PTX nanoparticles, indicating the occurrence of H2O2→O2 conversion catalyzed by catalase-containing particles. In the treatment of 4T1 mouse tumor with such nanoparticles, it was found that the efficacy of PDT was enhanced in combination with chemotherapy in the presence of catalase. Clearly, during the treatment with HSA-Ce6-Cat-PTX tumor oxygenation further improved PDT efficiency (Figure 4c). Guo et al. explored the possibility of loading catalase and the photosensitizer methylene blue (MB) into biodegradable and biocompatible poly(d,l-lactic-co-glycolic acid) (PLGA) nanoshells,[64] in which black hole quencher-3 (BHQ-3), as an ultra-efficient energy quencher, was embedded to prevent the excitation of the photosensitizer. Upon light irradiation of such nanosystems, the Förster resonance energy transfer (FRET) effect induced by BHQ-3 “switched off” 1O2 production by MB. Once endogenous H2O2 in the tumor site permeated into the core of the PLGA nanoshells, it was decomposed by the loaded catalase to rapidly generate oxygen gas, leading to the collapse of PLGA nanoshells and subsequent leakage of MB. Here, the cause of nanoshell collapse was solely due to oxygen gas generated from H2O2 without further induction of any intrinsic changes. As such, the 1O2 generation by MB was “switched on” to realize the PDT killing of tumor cells (Figure 4d). It is worth noting that the FRET effect here avoids the side effects of photosensitizer MB. With such a design, the O2-evolving nanoparticles realized self-sufficiency of oxygen during the PDT process to overcome tumor hypoxia, exhibiting a high selectivity for cancer without inducing side effects to normal tissues. However, due to the unstable properties of using proteins like catalase, the feasibility of this kind of nanosystems on large scales needs more efforts in future research. Based on the representative work discussed above, most of these catalase-loaded nanosystems rely on passive EPR effect for tumor targeting, which may not be sufficient to achieve the specificity, thus active targeting nanosystems such as immobilization of ligands specifically interact with the membrane receptors of tumor cells,[65] can significantly increase the tumor selectivity for better therapeutic efficacy with minimal side effects. In another intriguing study, Zhang et al. constructed a tumor homotypic targeted cascade bioreactor (mCGP) for synergistic PDT and starvation therapy by encapsulating catalase and glucose oxidase (GOx) in the porphyrin MOF of PCN-224, which was then coated with tumor cell membrane.[66] The functional mechanism of this system is that the cascade reactions of mCGP could promote catalyzed production of O2 from endogenous H2O2, increase the decomposition of glucose under the action of glucose oxidase, and enhance the production of cytotoxic 1O2 under light irradiation. The catalytic capacity of mCGP in O2 generation from H2O2 is demonstrated by a concentration increase from 5.02 to 24.3 mg L−1 in 500 s. Once internalized by tumor cells, mCGP could improve the microenvironmental oxygen status by initiating the production of O2 from endogenous H2O2, which in turn accelerates the decomposition of intracellular glucose and enhances the generation of cytotoxic singlet oxygen under light irradiation. As a result, tumor inhibition could reach 97.1% when treated with mCGP and light irradiation.
Figure 4.
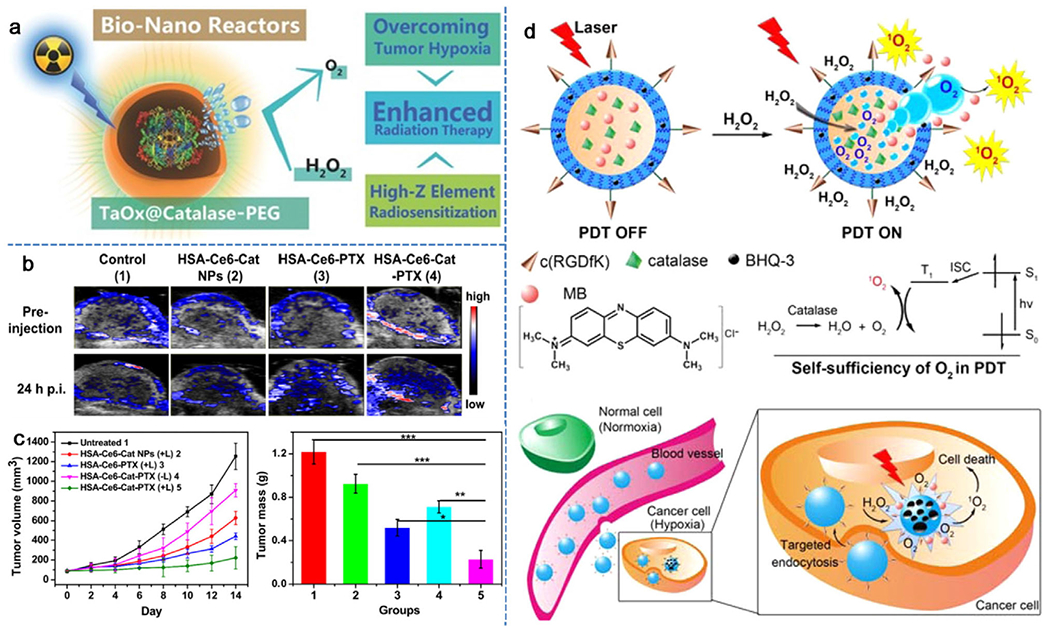
In situ oxygen generation by catalase-loaded nanoparticles for enhanced cancer therapy. a) A schematic illustration of the decomposition of endogenous H2O2 catalyzed by TaOx@Cat-PEG in the tumor region to generate oxygen and synergistically enhance the therapeutic efficacy of radiation therapy. Reproduced with permission.[62] Copyright 2016, Wiley-VCH. b) Representative 2D PA images of 4T1 solid tumors on mice before and 24 h after i.v. injection of different therapeutic drugs. c) Quantification of time-resolved tumor growth and average tumor weight in mice treated with different treatments. Reproduced with permission.[63] Copyright 2017, Elsevier. d) Schematic illustration of the mechanism on H2O2-controlled release of photosensitizer and O2 to implement PDT in the hypoxic tumor microenvironment. Reproduced with permission.[64] Copyright 2015, American Chemical Society.
2.3. Other Nanomaterials
Besides the abovementioned nanostructures, recent efforts have also been made to develop other metallic nanomaterials for catalyzing the decomposition of endogenous H2O2 into O2 to ameliorate the hypoxia of tumors for enhanced cancer therapy.[67–70] Cerium oxide nanoparticles have a similar function to catalase with the ability to reversibly switch from Ce4+ to Ce3+, yet simultaneously converting H2O2 to H2O and O2.[71,72] Based on this mechanism, Zhang and co-workers reported a functional bio-photocatalyst of Yb3+/Tm3+ codoped mesoporous hollow cerium oxide upconversion nanoparticles (Ce-UCNPs), which could simultaneously achieve in situ O2 generation and near-infrared (NIR) laser-induced PDT.[67] Interestingly, the obtained Ce-UCNPs could also realize PDT without the presence of organic photosensitizers. Under 980 nm NIR laser excitation, Yb3+ and Tm3+ dopant could convert NIR light to UV emission and trigger a photodynamic reaction, that is, effectively turning the O2, catalyzed by cerium oxide matrix from H2O2, into oxygen free radicals (O2•−) and hydroxyl free radicals (•OH) and consequently inducing cancer apoptosis. The mesoporous hollow structure of Ce-UCNPs with Arg-Gly-Asp (RGD)-peptide surface modification could be used as a DOX delivery system to realize pH-responsive release to the acidic tumor for targeted chemotherapy. As illustrated in Figure 5a, the signal intensity of blood oxygen saturation in the tumor region was significantly increased from 6.9% to 19.2% in 100 min after i.v. injection with Ce-UCNP. In vivo therapy of glioblastoma in U87MG cell-bearing mice showed that tumor growth was remarkably inhibited upon treatment with DOX-loaded Ce-UCNP and NIR laser irradiation, indicating the combinatory efficiency of PDT and chemotherapy.
Figure 5.
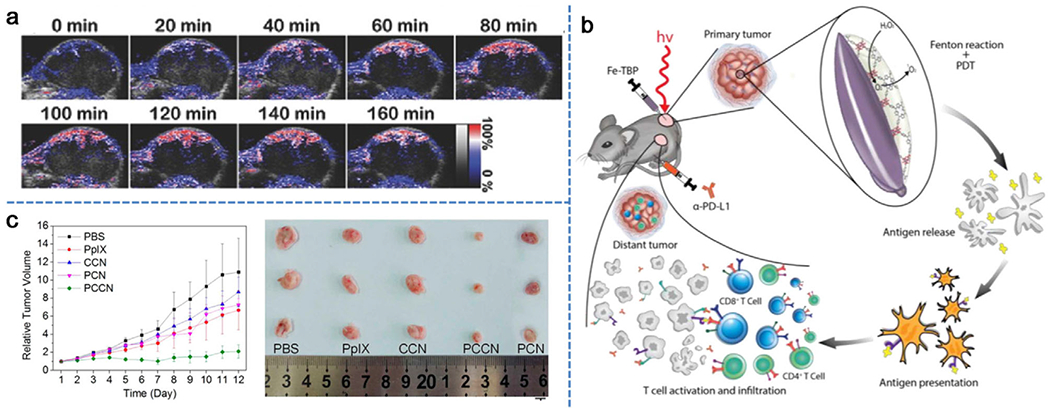
a) Representative PA images of solid tumors by measuring oxygenated hemoglobin after injection of Ce-UCNPs at various time points. Reproduced with permission.[67] Copyright 2018, Wiley-VCH. b) Schematic illustration of overcoming hypoxia using Fe-TBP for PDT primed cancer immunotherapy. Reproduced with permission.[69] Copyright 2018, American Chemical Society. c) Time-dependent tumor growth after different treatments. Reproduced with permission.[73] Copyright 2016, American Chemical Society.
Recent studies have also demonstrated that iron (Fe) associated nanoparticles could initiate a Fenton-like reaction to catalyze O2 generation from intracellular H2O2 under hypoxic conditions.[69,70] The efforts from Lin group in construction of Fe-TBP nanocomposite of Fe3O clusters and 5,10,15,20-tetra(ρ-benzoato)porphyrin (TBP) ligand are considered the pioneering work.[69] In combination with PDT, the competency of Fe-TBP in elevating O2 production by Fe3O clusters via a Fenton-like reaction improved the tumor hypoxia and in turn significantly increased the lethal 1O2 by photoexcited porphyrins (Figure 5b). In this study, Fe-TBP mediated PDT could also activate the host immune system by improving the α-PD-L1 immune checkpoint blockade (ICB) to trigger cancer immunotherapy. In vivo tumor therapy results confirmed the effectiveness of Fe-TBP in regression of locally irradiated tumors after PDT treatment. Further immunotherapy results showed that the combined treatment of Fe-TBP and α-PD-L1 led to significant expansions of cytotoxic T cells (i.e., CD4+ and CD8+), which infiltrated the distant tumors to cause abscopal effects. In a separate undertaking, Hyeon et al. developed a new type of manganese ferrite nanoparticles (MFNs), which could act as a Fenton catalyst.[70] MFNs were then anchored to mesoporous silica nanoparticles (MFMSNs) containing the photosensitizer of chlorin e6. MFMSNs were not consumed during the Fenton-like reaction, which could continuously produce O2 by decomposing H2O2 with a small amount of such nanocomposites. As a result, sO2 in the tumor region increased from 1.5 ± 0.2% to 12.6 ± 1.9% 24 h after i.v. injection of MFMSNs and tumor growth was delayed 10–15-fold under laser irradiation compared to controls. Moreover, MFMSNs also exhibited T2 contrast effect in magnetic resonance imaging (MRI), allowing for in vivo tracking of MFMSNs.
Different from decomposing H2O2 into O2 in other studies, Zheng et al. introduced a very interesting approach that utilized water as an O2 source to relieve tumor hypoxia.[73] In this work, metal-free C3N4 was particularly chosen as the water splitting material. Among various types of water splitting materials, C3N4 has received particular attention for its adjustable band gap and band position. Importantly, due to the absence of metal elements, C3N4 was considered to be a highly biocompatible material for biomedical applications. To enhance the water splitting ability of the C3N4, carbon dots were synthesized and deposited onto C3N4 (CCN) to increase its absorbance in the red light region. Under red light irradiation, the CCN nanocomposite could efficiently produce O2 in water. To achieve the active targeting effect and enhanced PDT, tumor-targeting sequence RGD (Arg-Gly-Asp) and photosensitizer protoporphyrin IX (PpIX) were assembled with CCN using PEG as the linker to obtain polymer-modified, carbon-dot-doped carbon nitride nanoparticles (PCCN). As presented in Figure 5c, CCN and PpIX showed minimal inhibitory effects on 4T1 tumors. The tumor inhibition efficiency of polymer-modified carbon nitride (PCN) without carbon dots was also not very obvious. However, PCCN treatment showed remarkable inhibition of tumor growth with only one injection, which was primarily ascribed to its tumor targeting attributes and O2 generation capability.
3. Direct Delivery of Oxygen into Tumors
To compensate tumor hypoxia and thereby circumvent resistance in O2-dependent cancer therapy, O2-transporting nanomedicine has been investigated as a straightforward strategy to leverage O2 levels in tumors. Exogenous O2 can be delivered in various forms, such as molecular O2 dissolved in perfluorocarbons (PFCs) or bound to hemoglobin, and O2 precursor doped into nanostructures.
3.1. Perfluorocarbon-Based Oxygen Reservoirs
With high electronegative fluorine on the carbon skeleton assuring excellent O2 affinity and good biocompatibility, PFC and its derivatives widely serve as artificial blood substitutes, and some of them have been approved for clinical utilities in the emulsified form.[74–77] Furthermore, PFCs are marked for their superior capability of prolonging 1O2 lifetime compared to water.[78] Therefore, PFCs are extensively explored as O2-suppliers to attenuate tumor hypoxia.
Due to their chemical inertness, PFCs can be incorporated with most synthetic organic or inorganic nanomaterials to deliver oxygen.[79–86] Meanwhile, further modification of such nanomaterials can yield multifunctionality for target delivery, imaging, monitoring, and anticancer efficacy.[87–89] In addition, fluorinated carbon chains could be built into the nanomaterials themselves by incorporating with organic polymers, either to confer the nanoparticles with O2-self-producing function[90] or to expand the versatility of PFCs.[91] Generally speaking, O2 dissolved in PFC-loaded nanoparticles spontaneously diffuses out in a slow gradient-driven manner. Rapid O2 release can be specifically “turned on” by an acidic and reducing environment within tumors.[92,93] In addition, external stimuli including NIR laser and ultrasound are also used to remotely harness the vaporization of PFCs for offloading O2 in a burst-release manner at the focused tumor sites.[94–96]
O2 replenishment allows sensitization of cancer cells toward chemo-cytotoxicity. Considering the relatively longer time required for drug molecules to take effect, sustained hypoxia mitigation is desirable to synergize chemotherapy. Luo and his co-workers ingeniously designed a PFC-loaded hollow Fe3O4 magnetic nanoplatform (PHMNP) featured with aggregation-mediated intracellular retention for prolonged O2 supply. A disulfide-containing copolymer with HepG2 tumor-targeting lactobionic acid as the end group was introduced to the PHMNP surface through hydrophobic interaction. Programmable agglomeration was performed within HepG2 cells, where glutathione (GSH) triggered the cleavage of disulfide followed by exposure of the hydrophobic segments on PHMNP surface. In this way, both drug accumulation and sustained O2 replenishment were achieved, while exocytosis-induced loss of therapeutic activity was efficiently suppressed.[89] As a matter of fact, this concept possesses potential implication to many other types of nanomedicine, while the major drawback lies in the size-impeded renal clearance.
Clearly, the presence of extra O2 is expected to strengthen hypoxia alleviation and ensure O2-dependent therapeutic effectiveness. By screening current major PFCs, Wu et al. found that perfluorotributylamine (PFTBA) could disrupt the barrier functions of tumor blood vessels and enhance tumor permeability. Their tumor-targeting nanosystem constitutes human serum albumin-encapsulated PFTBA (PFTBA@HSA) that could inactivate platelets to induce the rearrangement of endothelial cells and tight junctions (Figure 6a) and eventually led to platelet removal through the reticuloendothelial system. The leaky wall of blood vessels can elevate intratumoral distribution of red blood cells (RBCs) for O2 supply.[97] As shown in Figure 6b, an increased amount of RBCs was indeed observed in the tumor tissue 5 h post-PFTBA@HSA administration. In addition, PFTBA@HSA preferably accumulated at the tumor site due to the high affinity between albumin and highly expressed SPARC (secreted protein acidic and rich in cysteine) on the tumor surface. To validate selective tumor accumulation of IR775-loaded PFTBA@HSA, NIR fluorescence imaging was performed. As shown in Figure 6c, the fluorescence signal collected at tumor locations (black circles) reached the maximum at 48 h post-injection. As a matter of fact, the O2-absorbed PFTBA@HSA provides two-stage O2 delivery, that is, O2 evaporation from PFTBA as the first stage and elevated RBC infiltration as the second stage, which was demonstrated to be effective in strengthening chemo- and radio-therapy.[98]
Figure 6.
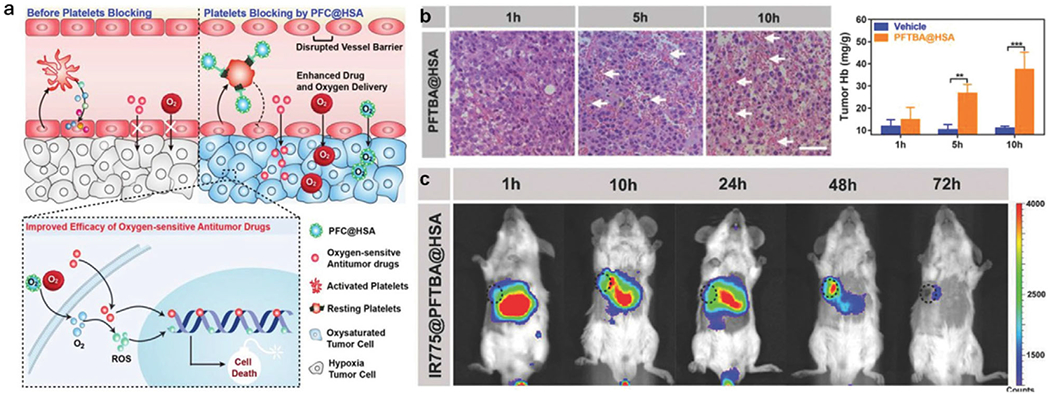
a) Schematic illustration of PFTBA@HSA mediated platelet inhibition to promote oxygen-sensitive chemotherapy. b) Representative images of H&E staining of tumor tissues treated by PFTBA@HSA at different times to highlight the influx of RBCs as indicated by white arrows. c) In vivo NIR fluorescence imaging of IR775-loaded PFTBA@HSA. Black dashed circles indicated tumors. Reproduced with permission.[97] Copyright 2018, Wiley-VCH.
3.2. Hemoglobin-Incorporated Nanomaterials
As the natural O2 carrier of RBCs,[99] hemoglobin (Hb) has been incorporated into nanomedicine for hypoxia reversal and subsequent therapies. The O2 concentration-dependent reversible carrying and offloading behaviors of Hb have been greatly appreciated in recent years. However, proper protection becomes necessary to ensure the intact conformation of binding sites, essential for O2 delivery. Taking advantage of bio-membrane coating technology, nanomaterials could be coated with cell membranes to achieve favorable homologous targeting efficiency and low immunogenic clearance.[100] In that regard, Hb-encapsulated, cell membrane-decorated artifactual RBCs have shown a budding direction for hypoxia reversal.[101,102] In a highlight, man-made RBCs (mmRBCs) with secured Hb activity were designed and fabricated for tumor PDT treatment, which was fueled by sufficient O2 through co-delivery of Hb and the photosensitizer methylene blue (MB).[103] Briefly, abundant Hb, as high as about tenfold above that in natural RBCs, was compacted into RBC membrane (RBCM) vesicles. The vulnerability of Hb toward oxidation was considerably protected by the outer RBCM coating as well as antioxidative enzyme-mimicking polydopamine (PDA) that complexed with the Hb (Figure 7a,b). The oxygen-carrying capacity of mmRBC was comparable to that of natural Hb in an equivalent amount (Figure 7c). Unlike non-targeting natural RBCs, significant accumulation of intravenously injected mmRBCs in tumor was observed (Figure 7d), which assured selective therapy with minimal off-site cell and tissue destruction. The target ability of mmRBC is mostly attributed to the combination of EPR effect and the spindle shape of mmRBC for readily entering the tumor cells. With 660 nm NIR exposure at designed time intervals, distinctively relieved tumor hypoxia was visualized with pimonidazole-based fluorescent hypoxyprobe and evidenced by down-regulation of HIF-α. A profound tumor-suppressing effect was verified by tumor shrinkage or even almost complete elimination (Figure 7e).
Figure 7.
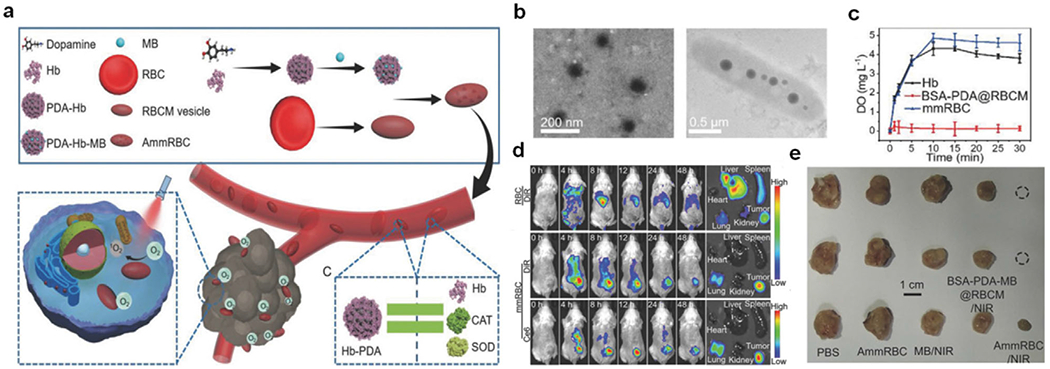
a) Schematic illustration of the preparation of mmRBCs as well as their accumulation and reoxygenation effect at the tumor site. b) TEM images of Hb-PDA complexes (left) and an individual mmRBC (right). c) Concentration of dissolved O2 in PBS-containing O2-saturated Hb, mmRBCs with equivalent Hb content, and BSA-PDA@RBCM as controls. d) Fluorescence images of biodistribution of DiR-labeled mmRBCs and RBCs. e) Digital images of tumor tissues after different treatments. Reproduced with permission.[103] Copyright 2018, Wiley-VCH.
Moreover, an oblate-like polymeric stomatocytes coated with erythrocyte membrane for Hb-Ce6 co-delivery was designed and fabricated by the van Hest group to mimic natural RBCs both structurally and functionally.[104] Similar to this design, a cancer cell membrane-coated nanocarrier was developed with a polymeric core encapsulating Hb and DOX for efficient chemotherapy.[105] As expected, such nanocarriers retained cancer cell adhesion molecules on the surface for homologous targeting and possessed the oxygen-carrying capacity for O2-depedent chemotherapy. Other approaches towards Hb-assisted hypoxia allaying have been explored with the use of liposomes,[106–108] polymers,[109,110] and protein hybridization.[111] Interestingly, during the investigation of liposomal co-delivery of DOX and Hb, You and co-workers identified a potential ion-mediated targeting mechanism of Hb. That is, Hb adsorbed onto the outer surface through hydrophobic interactions that may account for enhanced tumor cell uptake through overexpressed membrane proteins involved in iron uptake.[106]
While enjoying intrinsic biocompatibility and potential targeting merits, Hb-based O2 nanosystems have limited success in clinical translation mainly due to the sophisticated and cautious processing required to retain the conformation of active sites of Hb and more importantly, the serious threat to patient lives from Hb-induced acute nephrotoxicity and acute hypertension.[11]
3.3. Oxygen Precursor-Integrated Nanocomposites
Apart from direct delivery of O2 into a tumor site, efforts have been made to delicately design nanomaterials containing or incorporating oxygen-generating moieties. To elevate the level of H2O2 in tumor, exogenous H2O2, as a precursor of oxygen, was encapsulated in the nanoparticles and then specifically delivered to the tumor sites. For example, Liu et al. developed PEG-modified stealthy liposomes to deliver exogenous catalase and H2O2 (H2O2@liposomes and CAT@liposomes) in order to reverse the immunosuppressive microenvironment caused by hypoxia and, consequently, re-sensitize tumor cells for radio-immunotherapy.[112] The Ge group constructed ROS-responsive polymeric vesicles encapsulating H2O2 and Ce6/cypate-conjugated poly(amidoamine) dendrimers (CC-PAMAM) for enhanced PDT of hypoxic and hypopermeable pancreatic tumors. In this design, cypate induced thermal decomposition of H2O2 through 805-nm-laser irradiation, followed by ROS-mediated disruption of the vesicles upon exposure to a 660-nm laser. Subsequent release of CC-PAMAM could penetrate poorly permeable tumors to achieve deep PDT along with simultaneous supply of oxygen.[113] Other oxygen-generating nanomaterials have also been explored to address hypoxia-induced treatment failure. Inspired by calcium peroxide- and catalase-loaded alginate micropellets established by the Sung group,[114] Zhang et al. encapsulated CaO2 and photosensitizer methylene blue into liposomes to promote PDT following on-demand O2 release.[115] In a similar endeavor, microwave-triggered decomposition of copper peroxide was accordingly adopted to improve the efficacy of microwave thermal therapy enabled by the CuO@ZrO2 nanocomposites developed by the Meng group.[116] Recently, ingeniously designed oxygen and Pt(II) self-generating nanocomposites were evaluated to reverse a hypoxia-induced PDT resistance.[117] To prepare such nanocomposites, amphiphilic oligomers Ce6-PEG-Pt(IV) were self-assembled on UCNPs of NaYbF4:Tm@CaF2 to form UCNP-embedded nanoparticles (UCPPs). UCNPs in the nanocomposites could absorb light in the NIR and convert to UV. Thus, under 980-nm laser irradiation, the UV-sensitive cis-diammine ligands within diazido Pt(IV) (cis, trans, cis-[Pt(N3)2(OH)2(NH3)2]) complexes could be decomposed into O2 and Pt(II). O2 could compensate the consumption of oxygen during the PDT process and Pt(II) could play a toxic role for a synergistic photo-chemotherapy.
4. Improving Blood Flow into Tumors
It is well known that increase of local temperature in a tumor by mild hyperthermia can help improve intratumoral blood flow and therefore overcome hypoxia-associated resistance.[118–120] Studies have shown that photothermal effect of NIR-absorbing agents could produce hyperthermia by converting photonic energy into heat.[121–124] Hence, mild photothermal therapy (PTT) could facilitate blood flow in a tumorous region to alleviate hypoxia. In earlier work, a versatile MoS2 quantum dot@polyaniline (MoS2@PANI) inorganic-organic nanohybrid was developed to accommodate both photoaccoustic (PA)/X-ray computed tomography (CT) guided PTT and RT of cancer.[119] In this nanosystem, MoS2 nanodots acted as the radiosensitizer for RT owing to its high Z number, while the PANI shell acted as a conventional organic photothermal agent for PTT. In this study, hypoxia was relieved through hyperthermia upon treatment of tumor-bearing mice with MoS2@PANI and NIR laser irradiation. Thus, PTT/RT treatment helps decrease hypoxia-associated radiation-resistance. However, evidence that hyperthermia induced by PTT really enhanced tumor oxygen status was insufficient and remained to be demonstrated. Liu et al. fabricated a versatile MnSe@Bi2Se3 core-shell nanostructure via a partial cation exchange method.[125] The MnSe core exhibited magnetic resonance (MR) imaging activity to track the nanoparticles, while the Bi2Se3 shell served as the photothermal agent and radiosensitizer for PTT and RT simultaneously. Both ex vivo immunofluorescence staining and in vivo fluorescence imaging using a hypoxia-specific probe demonstrated that mild PTT by low-power laser irradiation after injection with MnSe@Bi2Se3 nanoparticles mitigated the hypoxia issue through improved blood flow. Clearly, the combined RT and PTT enabled by MnSe@Bi2Se3 nanoparticles provided a strong synergistic contribution to tumor elimination by primarily overcoming hypoxia. In addition to the abovementioned nanoparticles, other nanostructures such as tantalum sulfide nanosheets with mild PTT efficacy were also utilized to improve the hypoxia-restricted radiotherapy or PDT.[126–128]
Besides hyperthermia, delivery of antiangiogenic drugs into the tumorous area to normalize the abnormal structure and function of tumor vasculature approved to be effective in increasing blood flow and subsequently enabling efficient intratumoral perfusion of blood and oxygen.[129–131] Up to now, several molecules such as gemcitabine,[132] cyclophosphamide,[133] taxane[134] have been tested for their ability to decompress blood vessels and improve tumor perfusion. For instance, Liu et al. developed a nanostructure (131I-HSA-PTX) for chemo-radioisotope therapy by simply mixing 131I-labeled human serum albumin (HSA) with paclitaxel (PTX).[135] In this system, PTX enhanced tumor blood flow and oxygenation to overcome hypoxia-associated radio-resistance (Figure 8a). PA imaging of tumor confirmed that sO2 was greatly increased 12 h post-i.v. injection of HSA-PTX and remained at a high level even after 48 h (Figure 8b). In the subsequent work by the Liu group, functional covalent-organic polymers (COPs) were prepared by using a succinic acid-derivatized cisplatin prodrug as a reduction-responsive crosslinker and the photosensitizer of meso-tetra(p-hydroxyphenyl) porphine (THPP) as the connecting moiety.[136] After i.v. injection, the cisplatin released from COPs to the tumor region of tumor-bearing mice was able to normalize the tumor vasculature and improve tumor hypoxia. As anticipated, PDT efficacy induced by THPP was greatly enhanced, exhibiting synergistic suppression to tumor growth.
Figure 8.
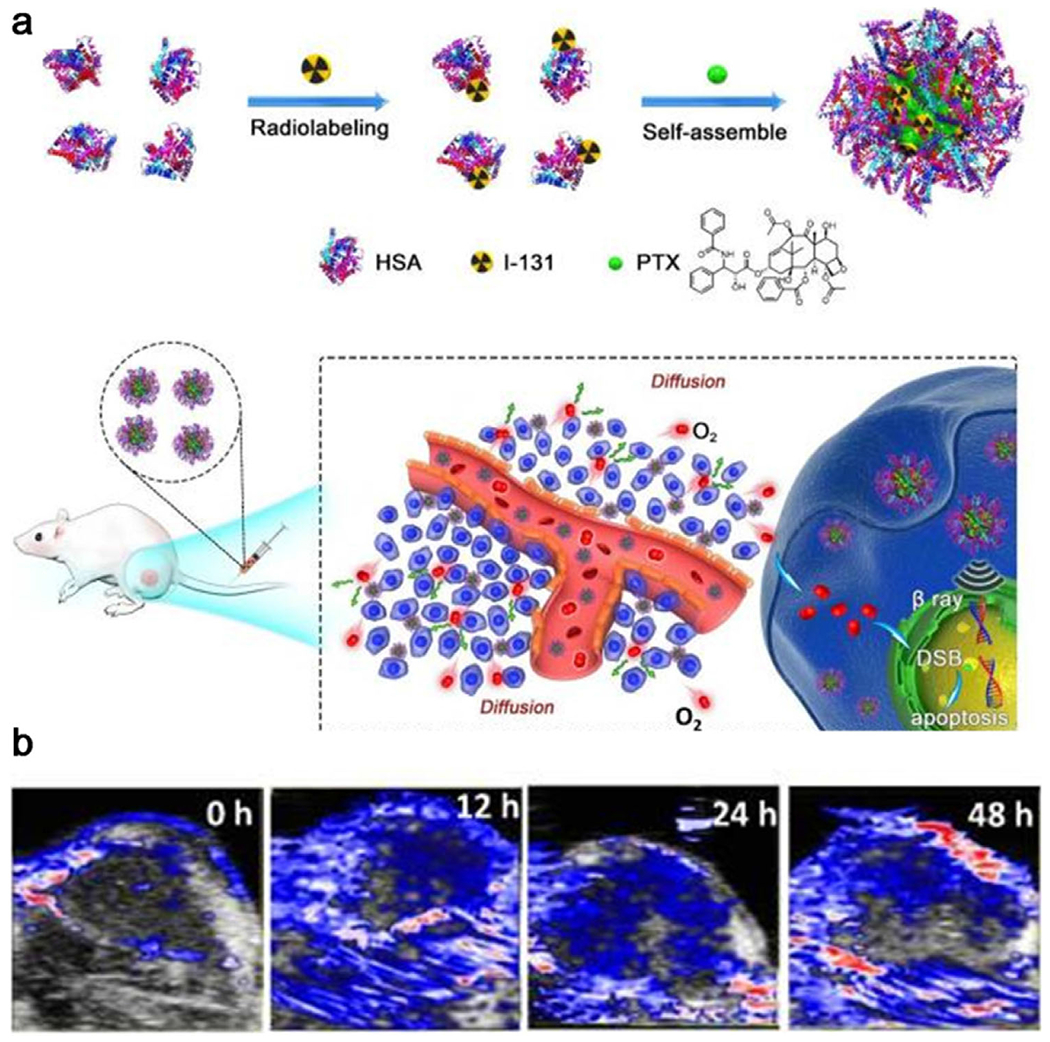
a) A scheme showing the synthesis of 131I-HSA-PTX nanoparticles and in vivo combined chemo-radiotherapy. b) Time-dependent photoacoustic images of tumors after intravenous injection of HSA-PTX. Reproduced with permission.[135] Copyright 2017, Ivyspring International Publisher.
5. Decreasing Oxygen Consumption of Tumors
Converse to supplying oxygen to a tumor site, reduction of oxygen consumption by cancer cells can be considered as a viable solution to tumor hypoxia. Several agents have a high efficacy in decreasing oxygen consumption clinically.[137–139] Papaverine, known as a smooth muscle relaxant, has been identified for its recognized potency in off-target effect limiting mitochondrial oxygen consumption.[137] Metformin (Met), a common oral hypoglycemic agent for type II diabetes, could inhibit respiration by directly suppressing the activity of complex I in the mitochondrial electron transport chain.[139–143]
A recent attempt by the Liu group was to encapsulate Met into the inner cavity of liposomes along with loading the Ce6 photosensitizer to improve tumor oxygenation for circumventing the hypoxia-resisted PDT.[144] As shown in Figure 9a,b, i.v. injection of Met-loaded liposomes into tumor-bearing mice led to the intratumoral oxyhemoglobin saturation and displayed a sustained increase from 14.6% to 30.1%, which exhibited an effective tumor growth inhibition in conjunction with PDT. In a separate study, Met was loaded into platelet membrane-coated W18O49 nanoparticles (PM-W18O49-Met NPs), which reduced oxygen consumption and resulted simultaneously enhanced PDT and PTT (Figure 9c).[145] In this nanosystem, the platelet membranes could protect W18O49 and Met from oxidation and immune evasion and prolong their circulation time to increase the accumulation of Met-loaded W18O49 nanoparticles in tumor site through active adhesion between platelets and cancer cells and a passive EPR effect. The low oxygen consumption rate of cancer cells caused by accumulated Met in the tumor site could alleviate hypoxia. In vivo experimental findings did show that PM-W18O49-Met NPs inhibited tumor growth via the combined treatment of enhanced PDT and PTT (Figure 9d).
Figure 9.
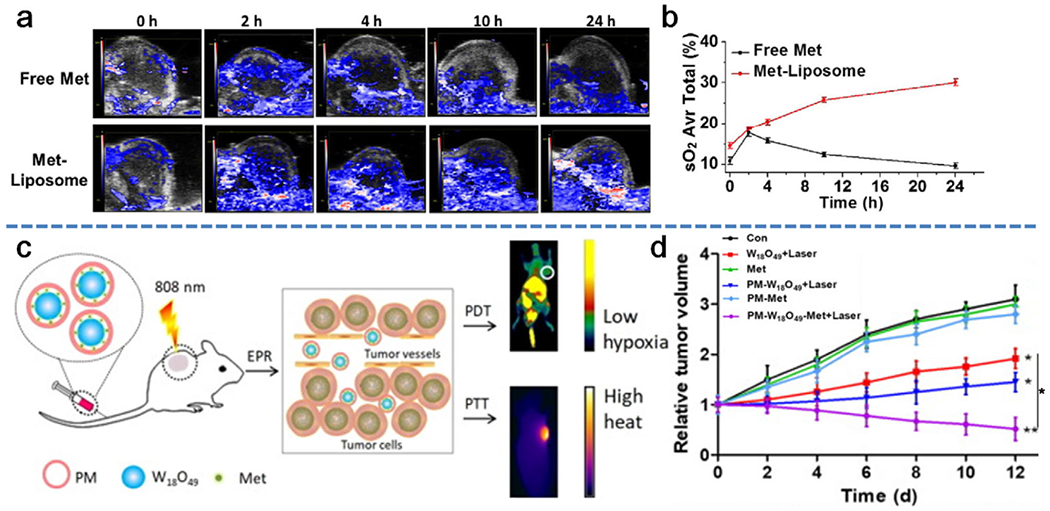
Decrease of oxygen consumption of tumors to relieve hypoxic conditions via metformin-loaded nanoparticles. a) Representative 2D PA images of 4T1 solid tumors at various time points before and after injection with free metformin and metformin-loaded nanoparticles. b) Quantification of the oxyhemoglobin saturation overtime (oxygenated hemoglobin:total hemoglobin), across the total tumor area, based on the PA imaging data shown in (a). Reproduced with permission.[144] Copyright 2017, SpringerLink. c) Schematic illustration of the simultaneously enhanced PDT and PTT induced by PM-W18O49-Met NPs. d) Time-dependent tumor growth after exposure to different treatments. Reproduced with permission.[145] Copyright 2018, Elsevier.
6. Tumor Hypoxia Targeting for Enhanced Cancer Therapy
Aside from the already discussed modalities to improve the hypoxic tumor microenvironment, hypoxia-activated drugs with selective toxicity to cells in the hypoxic environment have also drawn great attention.[146–149] Several hypoxia-activated prodrugs, including banoxantrone, NLCQ-1, tirapazamine, and dinitrobenzamide mustards, are either in preclinical development or early clinical investigation.[150] In the presence of oxygen, the cell-killing effect of these prodrugs does not occur. However, upon activation by hypoxia they become toxic to hypoxic tumor cells. Taking advantages of the unique hypoxic attributes of tumors, the combination of hypoxia-activated drugs with oxygen-dependent treatments is appealing to achieve a high specificity along with the synergistic anticancer efficiency.[151–153]
Liu et al. explored the possibility of encapsulating a hypoxia-activated drug AQ4N and a photosensitizer hexadecylamine conjugated chlorin e6 (hCe6) into liposomes to achieve hypoxia-dependent cell killing specificity.[147] After i.v. injection of tumor-bearing mice with AQ4N-hCe6-liposome and irradiation with a 660 nm light-emitting diode (LED) for PDT treatment, severe hypoxia was observed in the tumor site, which subsequently triggered the activation of AQ4N for chemotherapy. As expected, the treatment with AQ4N-hCe6-liposome plus 660-nm LED light irradiation yielded the most effective inhibition of tumor growth. In a very recent study from Chen et al., the hypoxia-activated prodrug of tirapazamine (TPZ) combined with vascular disrupting combretastatin A4 nanoparticles (CA4-NPs) was used to treat the highly metastatic 4T1 breast carcinoma with an initial volume up to 500 mm3, emulating stage IV triple-negative human breast cancer (Figure 10a).[154] CA4-NPs could selectively disrupt vascular functions and facilitate the development of hypoxia in tumors. As evidenced by fluorescence staining for hypoxia (Figure 10b), the degree of hypoxia was dramatically elevated after systemic administration of CA4-NPs at a dose of 48 mg kg−1, indicating the efficiency of CA4-NPs in cutoff of the vascular supply of oxygen within solid tumors. The cytotoxic benzotriazinyl (BTZ) radical was generated via dehydration of TPZ radical intermediates within a hypoxic microenvironment. In combination, dual-therapy with TPZ and CA4-NPs (Group 8) exhibited the most pronounced suppression of tumor growth (Figure 10c), leading to the tumor shrinkage to ≈140 mm3, as small as 7.7% of its original size.
Figure 10.

a) Schematic illustration of the strategy to combine hypoxia-inducing nanoparticles with hypoxia-activated treatment. b) PA imaging of immunofluorescence staining for hypoxia after intravenous injection of CA4-NPs at 4 and 24 h. c) Time-dependent tumor volume and body weight changes of mice during treatment. Reproduced with permission.[154] Copyright 2019, Wiley-VCH. d) Schematic illustration of SPNpd for hypoxia-activated synergistic photodynamic therapy and chemotherapy. Reproduced with permission.[153] Copyright 2019, Wiley-VCH.
In other recent work, Pu et al. synthesized a semiconducting polymer nanoprodrug (SPNpd), which not only specifically activated its chemotherapeutic effect in a hypoxic tumor microenvironment but also efficiently generated 1O2 under NIR laser irradiation.[153] SPNpd was self-assembled from an amphiphilic photodynamic semiconducting polymer (SPN) grafted with a chemodrug bromoisophosphoramide mustard intermediate (IPM-Br), through the side chains via hypoxia-cleavable linkers. IPM-Br, which could induce DNA crosslinking and cellular apoptosis, has been used for cancer treatment (Figure 10d). Upon irradiation with an 808-nm laser, SPNpd was able to generate 1O2 for PDT of cancer; meanwhile, the oxygen-consuming PDT further exacerbated the hypoxia, therefore facilitating nitroreductase-mediated liberation of IPM-Br.
7. Conclusions and Future Perspectives
Hypoxia is a widely observed phenomenon of solid tumors, which greatly limits the treatment efficiency of therapeutic strategies such as chemotherapy, RT, and PDT. Recent progress in nanotechnology has created a new horizon toward the tumor hypoxia challenge by providing solutions to improve local oxygen level and developing new hypoxia-activated therapeutic nanosystems for enhanced therapy. In this review, we summarized the key nanomedicine-based strategies to overcome the limits of hypoxia in tumor treatment. To address tumor hypoxia, diverse efforts have been made, but mainly focused on the use of nanomedicine to realize: 1) in situ oxygen generation through reactions with endogenous H2O2 with the assistance of catalase or catalase-like nanomaterials; 2) direct O2 delivery to tumors using hemoglobin or perfluorocarbon as a medium; 3) tumor blood flow improvement via mild hyperthermia induced by photothermal nanoagents or antiangiogenic drugs delivered by nanocarriers; and 4) decreased O2 consumption by delivering oxygen expenditure reduction drugs via nanomaterials. Furthermore, hypoxia-activated prodrugs could enhance the efficacy of oxygen-dependent therapeutic approaches.
Based on the exciting results from these studies, we believe that well-designed therapeutic nanosystems specifically targeted to the hypoxic conditions of tumors will continue to grow and may become one of the primary choices together with other therapies for cancer therapy in the future. However, the lack of clinical investigations and convincing evidence indicates that most such therapeutic nanosystems are still at their early development stages. For continued progress, future efforts should clearly address: 1) how to achieve mass production of these functional nanoparticles with quality control for industry; 2) how to precisely quantify the amount of O2 generated via nanomaterials and maintain O2 levels in tumors after treatment; 3) how to fully understand the mechanisms and metabolic processes of nanoparticles in the body, which can provide important guidance to future design in nanomedicine; 4) how to achieve high specificity in translation of nanoagents for clinical applications, that is, destruction of tumors without adverse effects on normal tissues. Nonetheless, as an active option to enhance therapeutic effects, tumor hypoxia modulation can still be rather appealing. From our point of view, future efforts in designing nanosystems for specifically improving tumor hypoxic microenvironments may follow several strategies, including 1) biomimetic O2-replenishing strategies such as the use of hemoglobin as carriers to deliver O2 into tumors; 2) automatic oxygen collection nanosystems during the blood circulation to realize a durable and self-driven oxygen supply for tumor tissue; 3) new inorganic nanosystems which possess high catalytic capacity to generate oxygen from hydrogen peroxide. Based on these trends, development of innovative nanosystems to effectively overcome tumor hypoxia remains an active research field.
Acknowledgements
This work was partially supported by National Science Foundation (NSF-DMR award number 1508511) and NIAMS award number 1R01AR067859. The authors are also grateful for the help from Thomas Cattabiani (Stevens Institute of Technology) for proofreading.
Biographies

Dr. Jinping Wang received her Ph.D. degree from the School of Pharmaceutical Science and Technology, Tianjin University, China. Then she joined Prof. Wang’s group as a postdoctoral fellow at Stevens Institute of Technology, USA. Her current research mainly focuses on the exploration of various functional nanomaterials and nanotechnologies for cancer diagnosis and therapy, with a particular interest in photothermotherapy and photodynamics therapy.

Beilu Zhang is currently a Ph.D. candidate in the Department of Chemistry and Chemical Biology at Stevens Institute of Technology. She received her Master’s degree in biomedical engineering and Bachelor’s degree in bioengineering from Chongqing University, China. Her research includes the design and synthesis of nanomaterials for biomedical applications, in particular as cancer therapeutics.

Dr. Hongjun Wang is professor of biomedical engineering and affiliated professor of chemistry and chemical biology at the Stevens Institute of Technology, USA. The research interests of Wang lab mainly focus on biomimetic materials design, 3Dtissue reconstruction, in vitro tissue-on-a-chip, and nanomedicine. Prior to joining Stevens, he was a research fellow of the Wellman Center for Photomedicine at Massachusetts General Hospital and the postdoctoral fellow of the Department of Dermatology at Harvard Medical School. Dr.Wang received his first doctorate in polymer chemistry and physics from Nankai University and his second doctorate in biomedical engineering from University of Twente.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Jinping Wang, Department of Biomedical Engineering, Stevens Institute of Technology, Hoboken, NJ 07030, USA.
Beilu Zhang, Department of Chemistry and Chemical Biology, Stevens Institute of Technology, Hoboken, NJ 07030, USA.
Jingyu Sun, Department of Chemistry and Chemical Biology, Stevens Institute of Technology, Hoboken, NJ 07030, USA.
Yuhao Wang, Department of Biomedical Engineering, Stevens Institute of Technology, Hoboken, NJ 07030, USA.
Hongjun Wang, Department of Biomedical Engineering, Stevens Institute of Technology, Hoboken, NJ 07030, USA; Department of Chemistry and Chemical Biology, Stevens Institute of Technology, Hoboken, NJ 07030, USA.
References
- [1].Harris AL. Nat. Rev. Cancer 2002, 2, 38. [DOI] [PubMed] [Google Scholar]
- [2].a) Foster TH, Gao L, Radiat. Res 1992, 130, 379; [DOI] [PubMed] [Google Scholar]; b) Song X, Chen Q, Liu Z, Nano Res. 2015, 8, 340. [Google Scholar]
- [3].a) Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Shui QY, Garcia JGN, Semenza GL, Blood 2005, 105, 659; [DOI] [PubMed] [Google Scholar]; b) Semenza GL, Nat. Rev. Cancer 2003, 3, 721. [DOI] [PubMed] [Google Scholar]
- [4].Gilkes DM, Semenza GL, Wirtz D, Nat. Rev. Cancer 2014, 14, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Qiu GZ, Jin MZ, Dai JX, Sun W, Feng JH, Jin WL, Trends Pharmacol. Sci 2017, 38, 669; [DOI] [PubMed] [Google Scholar]; b) Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D, Cancer Treat. Rev 2003, 29, 297. [DOI] [PubMed] [Google Scholar]
- [6].Luo Z, Tian H, Liu L, Chen Z, Liang R, Chen Z, Wu Z, Ma A, Zheng M, Cai L, Theranostics 2018, 8, 3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP, Cancer Res. 2002, 62, 3387. [PubMed] [Google Scholar]
- [8].Su J, Wang C, Kang H, Zhang J, Wang B, Liu M, Zhao J, Liu L, Oncol. Lett 2017, 14, 3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Teicher BA, Cancer Metastasis Rev. 1994, 13, 139. [DOI] [PubMed] [Google Scholar]
- [10].Huo D, Liu S, Zhang C, He J, Zhou Z, Zhang O, Hu Y, ACS Nano 2017, 11, 10159. [DOI] [PubMed] [Google Scholar]
- [11].Yang G, Gong H, Qian X, Tan P, Li Z, Liu T, Liu J, Li Y, Liu Z, Nano Res. 2015, 8, 751. [Google Scholar]
- [12].a) Wilson WR, Hay MP, Nat. Rev. Cancer 2011, 11, 393; [DOI] [PubMed] [Google Scholar]; b) Jansman MMT, Rigau LH, Adv. Colloid Interface Sci 2018, 260, 65; [DOI] [PubMed] [Google Scholar]; c) Rankin EB, Giaccia AJ, Science 2016, 352, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC, Cancer Res. 2013, 73, 2412; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jahanban-Esfahlan R, de la Guardia M, Ahmadi D, Yousefi B, J. Cell. Physiol 2018, 233, 2019. [DOI] [PubMed] [Google Scholar]
- [14].Iyer AK, Khaled G, Fang J, Maeda H, Drug Discovery Today 2006, 11, 812. [DOI] [PubMed] [Google Scholar]
- [15].Ojha T, Pathaka V, Shi Y, Hennink WE, Moonen CTW, Storm G, Kiessling F, Lammers T, Adv. Drug Delivery Rev 2017, 119, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, Tan W, Nanomedicine 2012, 7, 1253. [DOI] [PubMed] [Google Scholar]
- [17].Liang C, Xu L, Song G, Liu Z, Chem. Soc. Rev 2016, 45, 6250. [DOI] [PubMed] [Google Scholar]
- [18].Choi KY, Liu G, Lee S, Chen X, Nanoscale 2012, 4, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li J, Shang W, Li Y, Fu S, Tian J, Lu L, Int. J. Nanomed 2018, 13, 5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saleem J, Wang L, Chen C, Adv. Healthcare Mater 2018, 7, 1800525. [Google Scholar]
- [21].Tang Q, Cheng Z, Yang N, Lia Q, Wang P, Chen D, Wang W, Song X, Dong X, Biomaterials 2019, 205, 1. [DOI] [PubMed] [Google Scholar]
- [22].a) Zhu W, Dong Z, Fu T, Liu J, Chen Q, Li Y, Zhu R, Xu L, Liu Z, Adv. Funct. Mater 2016, 26, 5490; [Google Scholar]; b) Zheng DW, Li, Li CX, Fan JX, Lei Q, Li C, Xu Z, Zhang XZ, ACS Nano 2016, 10, 8715. [DOI] [PubMed] [Google Scholar]
- [23].Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, Peng R, Liu Z, Nat. Commun 2017, 8, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang G, Xu L, Xu J, Zhang R, Song G, Chao Y, Feng L, Han F, Dong Z, Li B, Liu Z, Nano Lett. 2018, 18, 2475. [DOI] [PubMed] [Google Scholar]
- [25].Fu X, Ohta S, Kamihira M, Sakai Y, Ito T, Langmuir 2019, 35, 4094. [DOI] [PubMed] [Google Scholar]
- [26].Liu J, Dua P, Liu T, Wong BJC, Wang W, Jua H, Lei J, Biomaterials 2019, 192, 179. [DOI] [PubMed] [Google Scholar]
- [27].Li X, Kwon N, Guo T, Liu Z, Yoon J, Angew. Chem., Int. Ed 2018, 57, 11522. [DOI] [PubMed] [Google Scholar]
- [28].Cheng L, Yuan C, Shen SD, Yi X, Gong H, Yang K, Liu Z, ACS Nano 2015, 9, 11090. [DOI] [PubMed] [Google Scholar]
- [29].Song X, Feng L, Liang C, Gao M, Song G, Liu Z, Nano Res. 2017, 10, 1200. [Google Scholar]
- [30].Wang P, Lia X, Yao C, Wang W, Zhao M, El-Toni AM, Zhang F, Biomaterials 2017, 125, 90. [DOI] [PubMed] [Google Scholar]
- [31].Wang B, Lin W, Mao Z, Gao C, J. Mater. Chem. B 2018, 6, 3145. [DOI] [PubMed] [Google Scholar]
- [32].Wang Y, Xie Y, Li J, Peng Z-H, Sheinin Y, Zhou J, Oupicky D, ACS Nano 2017, 11,2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Y, Wu C, Zhou X, Wu X, Yang Y, Wu H, Guo S, Zhang J, Nanoscale 2013, 5, 1816. [DOI] [PubMed] [Google Scholar]
- [34].Szatrowski TP, Nathan CF, Cancer Res. 1991, 51, 794. [PubMed] [Google Scholar]
- [35].Wang XS, Zeng JY, Zhang MK, Zeng X, Zhang XZ, Adv. Funct. Mater 2018, 28, 1801783. [Google Scholar]
- [36].Wei J, Li J, Sun D, Li Q, Ma J, Chen X, Zhu X, Zheng N, Adv. Funct. Mater 2018, 28, 1706310. [Google Scholar]
- [37].Quail DF, Joyce JA, Nat. Med 2013, 19, 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen Q, Feng L, Liu J, Zhu W, Dong Z, Wu Y, Liu Z, Adv. Mater 2016, 28, 7129. [DOI] [PubMed] [Google Scholar]
- [39].Prasad P, Gordijo CR, Abbasi AZ, Maeda A, Ip A, Rauth AM, DaCosta RS, Wu XY, ACS Nano 2014, 8, 3202. [DOI] [PubMed] [Google Scholar]
- [40].Fan W, Bu W, Shen B, He Q, Cui Z, Liu Y, Zheng X, Zhao K, Shi J, Adv. Mater 2015, 27.4155. [DOI] [PubMed] [Google Scholar]
- [41].Zhang W, Li S, Liu X, Yang C, Hu N, Dou L, Zhao B, Zhang Q, Suo Y, Wang J, Adv. Funct. Mater 2018, 28, 1706375. [Google Scholar]
- [42].Zhu W, Dong Z, Fu T, Liu J, Chen Q, Li Y, Zhu R, Xu L, Liu Z, Adv. Funct. Mater 2016, 26, 5490. [Google Scholar]
- [43].Yang G, Xu L, Chao Y, Xu J, Sun Xi., Wu Y, Peng R, Liu Z, Nat. Commun 2017, 8, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gordijo CR, Abbasi AZ, Amini MA, Lip HY, Maeda A, Cai P, O’Brien PJ, DaCosta RS, Rauth AM, Wu XY, Adv. Funct. Mater 2015, 25, 1858. [Google Scholar]
- [45].Zhang C, Chen WH, Liu LH, Qiu WX, Yu WY, Zhang XZ, Adv. Funct. Mater 2017, 27, 1700626. [Google Scholar]
- [46].Jia Q, Ge J, Liu W, Zheng X, Chen S, Wen Y, Zhang H, Wang P, Adv. Mater 2018, 30, 1706090. [DOI] [PubMed] [Google Scholar]
- [47].Chen YZ, Wang ZU, Wang H, Lu J, Yu SH, Jiang HL,J. Am. Chem. Soc 2017, 139, 2035. [DOI] [PubMed] [Google Scholar]
- [48].Zhang Y, Wang F, Liu C, Wang Z, Kang L, Huang Y, Dong K, Ren J, Qu X, ACS Nano 2018, 12, 651. [DOI] [PubMed] [Google Scholar]
- [49].Wei J, Li J, Sun D, Li Q, Ma J, Chen X, Zhu X, Zheng N, Adv. Funct. Mater 2018, 28, 1706310. [Google Scholar]
- [50].Wang XS, Zeng JY, Zhang MK, Zeng X, Zhang XZ, Adv. Funct. Mater 2018, 28, 1801783. [Google Scholar]
- [51].Fan J, Yin JJ, Ning B, Xu W, Hu Y, Ferrari M, Anderson GJ, Wei J, Zhao Y, Nie G, Biomaterials 2011, 32, 1611. [DOI] [PubMed] [Google Scholar]
- [52].Moglianetti M, Luca ED, Pedone D, Marotta R, Catelani T, Sartori B, Amenitsch H, Retta SF, Pompa PP, Nanoscale 2016, 8, 3739. [DOI] [PubMed] [Google Scholar]
- [53].Zhang J, Yu Z, Gao Z, Ge H, Zhao S, Chen C, Chen S, Tong X, Wang M, Zheng Z, Qin Y, Angew. Chem., Int. Ed 2017, 56, 816. [DOI] [PubMed] [Google Scholar]
- [54].Zhang R, Song X, Liang C, Yi X, Song G, Chao Y, Yang Y, Yang K, Feng L, Liu Z, Biomaterials 2017, 138, 13. [DOI] [PubMed] [Google Scholar]
- [55].Wang H, Chao Y, Liu J, Zhu W, Wang G, Xu L, Liu Z. Biomaterials 2018, 181, 310. [DOI] [PubMed] [Google Scholar]
- [56].Yang G, Qi W, Apoorva L, Hong V, Chen Z, Thanabalu T, Zhao Y, ACS Nano 2019, 13,4742. [DOI] [PubMed] [Google Scholar]
- [57].Zhao J, Fei J, Du C, Cui W, Ma H, Li J, Chem. Commun 2013, 49, 10733. [DOI] [PubMed] [Google Scholar]
- [58].Chen H, Tian J, He W, Guo Z, J. Am. Chem. Soc 2015, 137, 1539. [DOI] [PubMed] [Google Scholar]
- [59].Chen J, Lei S, Zeng K, Wang M, Asif A, Ge X, Nano Res. 2017, 10, 2351. [Google Scholar]
- [60].Cheng H, Zhu J, Li S, Zeng J, Lei Q, Chen K, Zhang C, Zhang X, Adv. Funct. Mater 2016, 26, 7847. [Google Scholar]
- [61].Anselmo AC, Mitragotri S. J. Controlled Release 2014, 190, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Song G, Chen Y, Liang C, Yi X, Liu J, Sun X, Shen S, Yang K, Liu Z, Adv. Mater 2016, 28, 7143. [DOI] [PubMed] [Google Scholar]
- [63].Chen Q, Chen J, Liang C, Feng L, Dong Z, Song X, Song G, Liu Z, J. Controlled Release 2017, 263, 79. [DOI] [PubMed] [Google Scholar]
- [64].Chen HC, Tian JW, He WJ, Guo ZJ, J. Am. Chem. Soc 2015, 137, 1539. [DOI] [PubMed] [Google Scholar]
- [65].Cui W, Li J, Decher G, Adv. Mater 2016, 28, 1302. [DOI] [PubMed] [Google Scholar]
- [66].Li S, Cheng H, Xie BR, Qiu WX, Zeng JY, Li CX, Wan SS, Zhang L, Liu WL, Zhang XZ, ACS Nano 2017, 11, 7006. [DOI] [PubMed] [Google Scholar]
- [67].Yao C, Wang W, Wang P, Zhao M, Li X, Zhang F, Adv. Mater 2018, 30, 1704833. [DOI] [PubMed] [Google Scholar]
- [68].Xu C, Lin Y, Wang J, Wu L, Wei W, Ren J, Qu X, Adv. Healthcare Mater 2013, 2, 1591. [DOI] [PubMed] [Google Scholar]
- [69].Lan G, Ni K, Xu Z, Veroneau SS, Song Y, Lin W, J. Am. Chem. Soc 2018, 140, 5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kim J, Cho HR, Jeon H, Kim D, Song C, Lee N, Choi SH, Hyeon T,J. Am. Chem. Soc 2017, 139, 10992. [DOI] [PubMed] [Google Scholar]
- [71].Korsvik C, Patil S, Seal S, Self WT, Chem. Commun 2007, 10, 1056. [DOI] [PubMed] [Google Scholar]
- [72].Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JE, Seal S, Self WT, Chem. Commun 2010, 46, 2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zheng DW, Li B, Li CX, Fan JX, Lei Q, Li C, Xu Z, Zhang XZ, ACS Nano 2016, 10,8715. [DOI] [PubMed] [Google Scholar]
- [74].Castro CI, Briceno JC, Artif. Organs 2010, 34, 622. [DOI] [PubMed] [Google Scholar]
- [75].Riess JG, Krafft MP, Biomaterials 1998, 19, 1529. [DOI] [PubMed] [Google Scholar]
- [76].Spiess BD, J. Appl. Physiol 2009, 106, 1444. [DOI] [PubMed] [Google Scholar]
- [77].Spence RK, Artif. Cells, Blood Substitutes, Biotechnol 1995, 23, 367. [DOI] [PubMed] [Google Scholar]
- [78].Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, Yuan A, Wu J, Hu Y, Nat. Commun 2015, 6, 8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hill AV, J. Physiol 1910, 40, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gao M, Liang C, Song X, Chen Q, Jin Q, Wang C, Liu Z, Adv. Mater 2017, 29, 1701429. [DOI] [PubMed] [Google Scholar]
- [81].Yu P, Han X, Yin L, Hui K, Guo Y, Yuan A, Hu Y, Wu J, Adv. Ther 2019, 2, 1900031. [Google Scholar]
- [82].Song G, Liang C, Yi X, Zhao Q, Cheng L, Yang K, Liu Z, Adv. Mater 2016, 28, 2716. [DOI] [PubMed] [Google Scholar]
- [83].Wang J, Liu L, You Q, Song Y, Sun Q, Wang Y, Cheng Y, Tan F, Li N, Theranostics 2018, 8, 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Song G, Ji C, Liang C, Song X, Yi X, Dong Z, Yang K, Liu Z, Biomaterials 2017, 112, 257. [DOI] [PubMed] [Google Scholar]
- [85].Liu C, Dong H, Wu N, Cao Y, Zhang X, ACS Appl. Mater. Interfaces 2018, 10, 6991. [DOI] [PubMed] [Google Scholar]
- [86].Zhao C, Tong Y, Li X, Shao L, Chen L, Lu J, Deng X, Wang X, Wu Y, Small 2018, 14, 1703045. [DOI] [PubMed] [Google Scholar]
- [87].Zhang L, Wang D, Yang K, Sheng D, Tan B, Wang Z, Ran H, Yi H, Zhong Y, Lin H, Adv. Sci 2018, 5, 1800049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tang W, Yang Z, Wang S, Wang Z, Song J, Yu G, Fan W, Dai Y, Wang J, Shan L, Niu G, Fan Q, Chen X, ACS Nano 2018, 12, 2610. [DOI] [PubMed] [Google Scholar]
- [89].Zhou J, Xue C, Hou Y, Li M, Hu Y, Chen Q, Li Y, Li K, Song G, Cai K, Luo Z, Biomaterials 2019, 197, 129. [DOI] [PubMed] [Google Scholar]
- [90].Chen J, Luo H, Liu Y, Zhang W, Li H, Luo T, Zhang K, Zhao Y, Liu J, ACS Nano 2017, 11, 12849. [DOI] [PubMed] [Google Scholar]
- [91].Tao D, Feng L, Chao Y, Liang C, Song X, Wang H, Yang K, Liu Z, Adv. Funct. Mater 2018, 28, 1804901. [Google Scholar]
- [92].Ma S, Zhou J, Zhang Y, Yang B, He Y, Tian C, Xu X, Gu Z, ACS Appl. Mater. Interfaces 2019, 77, 7731. [DOI] [PubMed] [Google Scholar]
- [93].Hu D, Zhong L, Wang M, Li H, Qu Y, Liu Q, Han R, Yuan L, Shi K, Peng J, Adv. Funct. Mater 2019, 29, 1806199. [Google Scholar]
- [94].Tang H, Zheng Y, Chen Y, Adv. Mater 2017, 29, 1604105. [DOI] [PubMed] [Google Scholar]
- [95].Yuan P, Ruan Z, Jiang W, Liu L, Dou J, Li T, Yan L,J. Mater. Chem. B 2018, 6, 2323. [DOI] [PubMed] [Google Scholar]
- [96].Song X, Feng L, Liang C, Yang K, Liu Z, Nano Lett. 2016, 16, 6145. [DOI] [PubMed] [Google Scholar]
- [97].Zhou Z, Zhang B, Wang S, Zai W, Yuan A, Hu Y, Wu J, Small 2018, 14, 1801694. [DOI] [PubMed] [Google Scholar]
- [98].Zhou Z, Zhang B, Wang H, Yuan A, Hu Y,Wu J, Theranostics 2018, 8, 4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jensen FB, J. Exp. Biol 2009, 212, 3387. [DOI] [PubMed] [Google Scholar]
- [100].Fang RH, Kroll AV, Gao W, Zhang L, Adv. Mater 2018, 30, 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chen W, Zeng K, Liu H, Ouyang J, Wang L, Liu Y, Wang H, Deng L, Liu YN, Adv. Funct. Mater 2017, 27, 1605795. [Google Scholar]
- [102].Wang L-Y, Shi X-Y, Yang C-S, Huang D-M, Nanoscale 2013, 5, 416. [DOI] [PubMed] [Google Scholar]
- [103].Liu W-L, Liu T, Zou M-Z, Yu W-Y, Li C-X, He Z-Y, Zhang M-K, Liu M-D, Li Z-H, Feng J, Zhang X-Z, Adv. Mater 2018, 30, 1802006. [DOI] [PubMed] [Google Scholar]
- [104].Shao J, Pijpers IAB, Cao S, Williams DS, Yan X, Li J, Abdelmohsen LKEA, van Hest JCM, Adv. Sci 2019, 6, 1801678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tian H, Luo Z, Liu L, Zheng M, Chen Z, Ma A, Liang R, Han Z, Lu C, Cai L, Adv. Funct. Mater 2017, 27, 1703197. [Google Scholar]
- [106].Yang J, Li W, Luo L, Jiang M, Zhu C, Qin B, Yin H, Yuan X, Yin X, Zhang J, Luo Z, Du Y, You J, Biomaterials 2018, 182, 145. [DOI] [PubMed] [Google Scholar]
- [107].Xu H-L, Shen B-X, Lin M-T, Tong M-Q, Zheng Y-W, Jiang X, Yang W-G, Yuan J-D, Yao Q, Zhao Y-Z, Biomater. Sci 2018, 6, 2410. [DOI] [PubMed] [Google Scholar]
- [108].Murayama C, Kawaguchi AT, Ishikawa K, Kamijo A, Kato N, Ohizumi Y, Sadahiro S, Haida M, Artif. Organs 2012, 36, 170. [DOI] [PubMed] [Google Scholar]
- [109].Li T, Jing X, Huang Y, Macromol. Biosci 2011, 11, 865. [DOI] [PubMed] [Google Scholar]
- [110].Luo Z, Zheng M, Zhao P, Chen Z, Siu F, Gong P, Gao G, Sheng Z, Zheng C, Ma Y, Cai L, Sci. Rep 2016, 6, 23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Luo Z, Tian H, Liu L, Chen Z, Liang R, Chen Z, Wu Z, Ma A, Zheng M, Cai L, Theranostics 2018, 8, 3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Song X, Xu J, Liang C, Chao Y, Jin Q, Wang C, Chen M, Liu Z, Nano Lett. 2018, 18, 6360. [DOI] [PubMed] [Google Scholar]
- [113].Li J, Wei K, Zuo S, Xu Y, Zha Z, Ke W, Chen H, Ge Z, Adv. Funct. Mater 2017, 27, 1702108. [Google Scholar]
- [114].Huang C-C, Chia W-T, Chung M-F, Lin K-J, Hsiao C-W, Jin C, Lim W-H, Chen C-C, Sung H-W, J. Am. Chem. Soc 2016, 138, 5222. [DOI] [PubMed] [Google Scholar]
- [115].Liu L-H, Zhang Y-H, Qiu W-X, Zhang L, Gao F, Li B, Xu L, Fan J-X, Li Z-H, Zhang X-Z, Small 2017, 13, 1701621. [DOI] [PubMed] [Google Scholar]
- [116].Chen Z, Niu M, Chen G, Wu Q, Tan L, Fu C, Ren X, Zhong H, Xu K, Meng X, ACS Nano 2018, 12, 12721. [DOI] [PubMed] [Google Scholar]
- [117].Xu S, Zhu X, Zhang C, Huang W, Zhou Y, Yan D, Nat. Commun 2018, 9, 2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Jin CS, Lovell JF, Chen J, Zheng G, ACS Nano 2013, 7, 2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wang J, Tan X, Pang X, Liu L, Tan F, Li N, ACS Appl. Mater. Interfaces 2016, 8, 24331. [DOI] [PubMed] [Google Scholar]
- [120].Diagaradjane P, Shetty A, Wang JC, Elliott AM, Schwartz J, Shentu S, Park HC, Deorukhkar A, Stafford RJ, Cho SH, Tunnell JW, Hazle JD, Krishnan S, Nano Lett. 2008, 8, 1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhu X, Feng W, Chang J, Tan Y-W, Li J, Chen M, Sun Y, Li F, Nat. Commun 2016, 7, 10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Liu HY, Chen D, Li LL, Liu TL, Tan LF, Wu XL, Tang FQ, Angew. Chem 2011, 123, 921. [Google Scholar]
- [123].Dong WJ, Li YS, Niu DC, Ma Z, Gu JL, Chen Y, Zhao WR, Liu XH, Liu CS, Shi JL, Adv. Mater 2011, 23, 5392. [DOI] [PubMed] [Google Scholar]
- [124].Zhang ZJ, Wang LM, Wang J, Jiang XM, Li XH, Hu ZJ, Ji YL, Wu XC, Chen CY, Adv. Mater 2012, 24, 1418. [DOI] [PubMed] [Google Scholar]
- [125].Song G, Liang C, Gong H, Li M, Zheng X, Cheng L, Yang K, Jiang X, Liu Z, Adv. Mater 2015, 27, 6110. [DOI] [PubMed] [Google Scholar]
- [126].Shen S, Chao Y, Dong Z, Wang G, Yi X, Song G, Yang K, Liu Z, Adv. Funct. Mater 2017, 27, 1700250. [Google Scholar]
- [127].Cheng L, Shen S, Shi S, Yi Y, Wang X, Song G, Yang K, Liu G, Barnhart TE, Cai W, Liu Z, Adv. Funct. Mater 2016, 26, 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Wang S, Li X, Chen Y, Cai X, Yao H, Gao W, Zheng Y, An X, Shi J, Chen H, Adv. Mater 2015, 27, 2775. [DOI] [PubMed] [Google Scholar]
- [129].Jain RK, Nat. Med 2001, 7, 987. [DOI] [PubMed] [Google Scholar]
- [130].Jain RK, Science 2005, 307, 58. [DOI] [PubMed] [Google Scholar]
- [131].Liu J, Chen Q, Feng L, Liu Z, Nano Today 2018, 21, 55. [Google Scholar]
- [132].Cham KKY, Baker JHE, Takhar KS, Flexman JA, Wong MQ, Owen DA, Yung A, Kozlowski P, Reinsberg SA, Chu EM, Chang C-WA, Buczkowski AK, Chung SW, Scudamore CH, Minchinton AI, Yapp DTT, Ng SSW, Br. J. Cancer 2010, 103, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Emmenegger U, Morton GC, Francia G, Shaked Y, Franco M, Weinerman A, Man S, Kerbel RS, Cancer Res. 2006, 66, 1664. [DOI] [PubMed] [Google Scholar]
- [134].Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK, Clin. Implic 1999, 59, 3776. [PubMed] [Google Scholar]
- [135].Tian L, Chen Q, Yi X, Wang G, Chen J, Ning P, Yang K, Liu Z, Theranostics 2017, 7, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang H, Zhu W, Feng L, Chen Q, Chao Y, Dong Z, Liu Z, Nano Res. 2018, 11, 3244. [Google Scholar]
- [137].Benej M, Papandreou I, Hong X, Yu B, Denko N, Cancer Res. 2017, 77, 5841. [Google Scholar]
- [138].Ansiaux R, Dewever J, Gregoire V, Feron O, Jordan BF, Gallez B, Radiat. Res 2009, 172, 584. [DOI] [PubMed] [Google Scholar]
- [139].Zannella VE, Dal Pra A, Muaddi H, McKee TD, Stapleton S, Sykes J, Glicksman R, Chaib S, Zamiara P, Milosevic M, Wouters BG, Bristow RG, Koritzinsky M, Clin. Cancer Res 2013, 19, 6741. [DOI] [PubMed] [Google Scholar]
- [140].Weinberg SE, Chandel NS, Nat. Chem. Biol 2015, 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Loubière C, Goiran T, Laurent K, Djabari Z, Tanti J-F, Bost F, Oncotarget 2015, 6, 15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Morales DR, Morris AD, Annu. Rev. Med 2015, 66, 17. [DOI] [PubMed] [Google Scholar]
- [143].Li HW, Chen XG, Yu Y, Wang ZN, Zuo YF, Li SH, Yang DH, Hu SW, Xiang M, Xu ZM, Yu Z, Oncol. Rep 2014, 32, 2596. [DOI] [PubMed] [Google Scholar]
- [144].Song X, Feng L, Liang C, Gao M, Song G, Liu Z, Nano Res. 2017, 10, 1200. [Google Scholar]
- [145].Zuo H, Tao J, Shi H, He J, Zhou Z, Zhang C, Acta Biomater. 2018, 80, 296. [DOI] [PubMed] [Google Scholar]
- [146].Zhoua Y, Maitib M, Sharmac A, Wonc M, Yua L, Miaoa LX, Shinc J, Podderb A, Bobbab KN, Hand J, Bhuniyab S, Kim JS,J. Controlled Release 2018. 288, 14. [DOI] [PubMed] [Google Scholar]
- [147].Feng L, Cheng L, Dong Z, Tao D, Barnhart TE, Cai W, Chen M, Liu Z, ACS Nano 2017, 11, 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhao P, Ren S, Liu Y, Huang W, Zhang C, He J, ACS Appl. Mater. Interfaces 2018, 10, 3405. [DOI] [PubMed] [Google Scholar]
- [149].He Z, Dai Y,Li X, Guo D,Liu Y,Huang X,Jiang J, Wang S, Zhu G, Zhang F, Lin L, Zhu J, Yu G, Chen X, Small 2019, 15, 1804131. [DOI] [PubMed] [Google Scholar]
- [150].Baran N, Konopleva M, Clin. Cancer Res 2017, 23, 2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wang Y, Liu Y, Wu H, Zhang J, Tian Q, Yang S, Adv. Funct. Mater 2019, 29, 1805764. [Google Scholar]
- [152].Zhang R, Feng L, Dong Z, Wang L, Liang C, Chen J, Ma Q, Zhang R, Chen Q, Wang Y, Liu Z, Biomaterials 2018, 162, 123. [DOI] [PubMed] [Google Scholar]
- [153].Cui D, Huang J, Zhen X, Li J, Jiang Y, Pu K, Angew. Chem., Int. Ed 2019, 58, 5920. [DOI] [PubMed] [Google Scholar]
- [154].Yang S, Tang Z, Hu C, Zhang D, Shen N, Yu H, Chen X, Adv. Mater 2019, 31, 1805955. [DOI] [PubMed] [Google Scholar]


