Abstract
Background
Loss of mitochondrial function contributes to fatigue, exercise intolerance and muscle weakness, and is a key factor in the disability that develops with age and a wide variety of chronic disorders. Here, we describe the impact of a first-in-class cardiolipin-binding compound that is targeted to mitochondria and improves oxidative phosphorylation capacity (Elamipretide, ELAM) in a randomized, double-blind, placebo-controlled clinical trial.
Methods
Non-invasive magnetic resonance and optical spectroscopy provided measures of mitochondrial capacity (ATPmax) with exercise and mitochondrial coupling (ATP supply per O2 uptake; P/O) at rest. The first dorsal interosseous (FDI) muscle was studied in 39 healthy older adult subjects (60 to 85 yrs of age; 46% female) who were enrolled based on the presence of poorly functioning mitochondria. We measured volitional fatigue resistance by force-time integral over repetitive muscle contractions.
Results
A single ELAM dose elevated mitochondrial energetic capacity in vivo relative to placebo (ΔATPmax; P = 0.055, %ΔATPmax; P = 0.045) immediately after a 2-hour infusion. No difference was found on day 7 after treatment, which is consistent with the half-life of ELAM in human blood. No significant changes were found in resting muscle mitochondrial coupling. Despite the increase in ATPmax there was no significant effect of treatment on fatigue resistance in the FDI.
Conclusions
These results highlight that ELAM rapidly and reversibly elevates mitochondrial capacity after a single dose. This response represents the first demonstration of a pharmacological intervention that can reverse mitochondrial dysfunction in vivo immediately after treatment in aging human muscle.
Introduction
Age and disease are associated with a debilitating loss of function that leads to disability [1, 2] underlies morbidity [3] and has a substantial economic impact on society, including the healthcare system [4]. Mitochondrial dysfunction is a key part of the pathophysiology and reduced resilience associated with aging [5] and chronic disease [6] in multiple organ systems. Mitochondrial dysfunction is also associated with sarcopenia and impaired physical endurance across multiple populations [7, 8]. This loss of muscle function is an important contributor to reduced quality of life and increased morbidity with age [7, 9, 10]. Despite observational data indicating the important role of mitochondria in aging and disease there are no approved treatments that directly target dysfunctional mitochondria in skeletal muscle. A new approach has emerged that targets dysfunctional lipids, involves repair rather than replacement of deteriorated components [11, 12], and acts rapidly after a single treatment to reverse dysfunction [13].
Elamipretide (ELAM, also referred to as SS-31 and Bendavia in the literature) is a first-in-class compound that binds reversibly to the phospholipid cardiolipin (CL) located within the inner mitochondrial membrane to stabilize its structure, promote cristae formation and curvature, and positively alter the electrostatic environment. [14–16] Early studies inaccurately described ELAM as having antioxidant properties [17–19], although more recent work has demonstrated that ELAM actually decreases production of superoxide and H2O2 particularly where overproduction resulted from dysfunctional mitochondria [13, 20]. Studies in isolated mitochondria, cells and intact organs reveal improved ETC flux, decreased mitochondrial generation of reactive oxygen species (ROS; H2O2), and elevated ATP generation with ELAM treatment after ischemic insult and heart failure [15, 21, 22]. In skeletal muscle, treatment with ELAM prevented the increase in mitochondrial oxidative stress and muscle wasting associated with disuse atrophy in mouse hindlimb and diaphragm. In aging mice a single IP injection of ELAM elevated the capacity to generate ATP, improved the coupling of oxidative phosphorylation (P/O) in vivo, and increased fatigue resistance in the tibialis anterior in old hindlimb muscle without affecting mitochondrial energetics in young muscle [13]. Running endurance was also elevated in the old mice after 8 days of daily injections of ELAM. More recently, an 8-week treatment with ELAM demonstrated similar increases in in vivo mitochondrial energetics and fatigue resistance in old mice, as well as a reversal of age-related redox stress [23]. A four-month treatment of aged mice with ELAM decreased mtROS emission and improved mitochondrial integrity in muscles similar to the 8-week treatment described above [23, 24]. However, in contrast to the improved in vivo fatigue resistance after 8 weeks, there was no improvement in fatigue resistance in isolated muscles in the 4-month study, suggesting that improvements in performance with ELAM treatment may be potentiated by systemic effects beyond the myofiber fiber level. In a Phase 2 study 5 daily doses of ELAM increased the walking distance over 6 minutes (Six-Minute Walk Test 6MWT]) in individuals with a genetic mitochondrial myopathy [25], although the subsequent expanded Phase 3 trial failed to detect a change in the 6 MWT. Thus, studies from both mouse models and human disease indicate that both a better understanding of the effect of ELAM on in vivo mitochondrial energetics and the link between mitochondrial energetics and skeletal muscle function is necessary. Despite the trials focused on mitochondrial myopathy and other human trials in heart disease, ischemia reperfusion, and ongoing trials in Barth and mitochondrial associated vision disorders, there has been no direct measurement of mitochondrial function in a human clinical trial to determine if the mechanism of action of ELAM is mitochondrial in origin.
Here, we report the results from a randomized, double-blind, placebo controlled clinical trial that evaluated the safety and efficacy of a single dose of ELAM on surrogate endpoints of mitochondrial and muscle function in healthy older adults. Participants were selected on the basis of having evidence of impaired in vivo muscle mitochondrial energetics determined by dynamic, in vivo 31Phosphorus Magnetic Resonance Spectroscopy using a using a standardized protocol [26, 27]. The effects on the first dorsal interosseous (FDI) muscle of the hand were investigated for two reasons. First, handgrip strength is an important clinical measure of frailty and disease burden strongly associated with adverse health related outcomes across populations [28–31]. Second, this particular muscle shows clear changes in mitochondrial function with age [26] providing direct measurement of both mitochondrial energetics and muscle function (S3 Fig in S1 File). We hypothesized that ELAM infusion will lead to immediate improvements in our primary surrogate endpoint, the maximal rate of ATP production (ATPmax) immediately after a 2-hour infusion of ELAM. Two secondary outcomes were evaluated: 1) the resting state coupling of oxidative phosphorylation (ATP/O2) to evaluate mechanisms of improvement and 2) exercise tolerance as evaluated by muscle performance after ELAM treatment. Evidence of efficacy of mitochondrial targeted therapeutics to improve muscle metabolism and function may guide future therapeutic development and inform pharmacologic or lifestyle interventions targeting improvements in quality of life in the older adults.
Materials and methods
Study design and participants
This randomized, double-blind, placebo-controlled trial was conducted at the University of Washington Medical Center. Adults 60 to 85 years of age were recruited through public lectures, mailers, and posted advertisements. Eligible participants were selected based on two criteria found to be linked to low muscle function: 1) muscle mitochondrial ATP synthesis capacity (ATPmax<0.7 mM/sec) and 2) the coupling of oxidative phosphorylation (ATP synthesized per O2 uptake ÷ 2 < 1.9, P/O]) [26, 27]. These fluxes were assessed by in vivo magnetic resonance and optical spectroscopy (S1 and S2 Figs in S1 File) [26, 27]. Inclusion and exclusion criteria are listed in S1 and S2 Tables in S1 File. All procedures and protocols were in accordance with the tenets of the declaration of Helsinki. The study protocol was approved by the University of Washington Human Subjects Division and Western Institutional Review Board (IRB00000533). All subjects provided written informed consent before entering the trial. The study was registered with ClinicalTrials.gov (identifier: NCT02245620).
Power calculations, randomization, and masking
The group size of 20 subjects each for treatment and placebo was based on a mean change in P/O of 0.4 and standard deviation of ± 0.38 to provide a power of 0.9 at the 0.05 level of significance in healthy subjects 65 to 80 years of age [32]. An amended protocol placed ATPmax as the primary outcome and P/O as the secondary outcome.
Subjects were randomized based on a simple randomization technique using the random allocation rule with a desired ratio for participants in each group of 1:1. A random sequence was generated for participants in the entire trial and assigned to participants as they were enrolled. Assignment to treatment groups was determined by a computer-generated random sequence using an Interactive Web-Response System (IWRS). The IWRS was used to assign glass vials containing double-blind investigational product to each subject.
Both participants and study personnel were blinded to treatment assignment during the duration of the study. ELAM was administered intravenously for 2 hours at 0.25 mg/kg body weight per hour, which is the level found to be both safe and effective in two human dose-escalation studies [25, 33]. Placebo consisted of the vehicle used for the active drug, which contained preservative level concentrations of trehalose dihydrate, glutathione, and sodium hydroxide/acetic acid in physiological saline.
Outcomes
The primary endpoint was the change in in vivo skeletal muscle mitochondrial capacity (ATPmax) measured 1–2 hours following 2 hours of infusion compared to baseline. The secondary endpoints were resting mitochondrial coupling (P/O) and exercise tolerance (force-time integral). The coupling of phosphorylation (ATP supply) to oxidation (O2 uptake) by the mitochondria was expressed as P/O (ATP/O2 ÷ 2). The change in muscle performance was measured as the sum of the muscle force (force-time integral, FTI) normalized to the maximum voluntary contraction (MVC) generated during voluntary contractions. This protocol involved step increases of contraction frequency starting at 60 contractions per minute cpm and increasing frequency 10 cpm each minute until exhaustion. Primary and secondary endpoints were reassessed 7 days post-infusion.
Study procedures
The study consisted of 4 visits. A screening visit (Visit 1 or V1) determined eligibility and included a general health exam including ECG, blood tests, and non-invasive measures of muscle mitochondrial energetics and exercise tolerance of the hand muscle (first dorsal interosseous FDI, S3 Fig in S1 File). Those found to qualify for the study returned within 28 days of V1, were infused with either placebo or drug, and all procedures were repeated immediately following infusion (Visit 2 or V2). The exercise tolerance test was repeated a day after the infusion (Visit 3 or V3). All procedures were repeated one week after infusion (Visit 4 or V4).
MRS/OS protocol
The protocol for the phosphorus magnetic resonance spectroscopy (31P MRS) and optical spectroscopy (OS) has been previously published [26, 27]. These two spectroscopic measurements were made on the muscle during a period of ischemia. Probes for each measurement were placed at the same location on the skin surface of the FDI (S3 Fig in S1 File). This allowed us to separate O2 uptake from ATP flux in resting muscle in vivo (S2 Fig in S1 File). P/O is a measure of the efficiency of ATP generation in resting muscle (ATP/O2 ÷ 2). The mitochondrial oxidative phosphorylation capacity (ATPmax) was determined as described [27, 34]. Briefly, a short exercise bout involving the index finger was performed for a period of 20 to 30 seconds so that PCr was reduced by ~50%, while maintaining muscle pH>6.8. The PCr recovery was measured over 6 min to determine a time constant of recovery (tPCr) to yield ATPmax (= PCrrest/ tPCr) where PCrrest = 25.5 mM. Measuring in vivo energetics in the FDI allows a greater focus on the muscle specific effects of the intervention because the increased metabolism in the small muscle will not stress the cardiovascular system. This is particularly important consideration since the treatment with ELAM is systemic and likely to affect the cardiovascular system as well as the skeletal muscle. The ATPmax measured as described above is the standard for characterizing mitochondrial ATP capacity in vivo and is directly related both to mitochondrial markers of oxidative phosphorylation and mitochondrial oxidation in vivo.
Muscle performance testing
Exercise tolerance was tested by measuring the sum of force generated by the FDI muscle during repeated isometric contractions until exhaustion (S3 Fig in S1 File). The FDI is a model system for both energetic and mechanics measures [35] as well as mitochondrial aging [26]. The maximum voluntary contraction (MVC) was measured as the average of 3 maximum contractions separated by 5 sec and sustained for 3 sec each. The exercise level was set at 70% of the MVC and the exercise began at a frequency of 60 contractions per minute (cpm) for the first minute. This frequency was increased at a rate of 10 cpm with each minute until exhaustion. The FTI was measured as the sum of the force generated by these contractions during the test and normalized to the subject’s MVC.
Statistical analysis
The primary analysis set was the intent to treat study population. The primary endpoint test was the difference in the mean change from baseline to immediately after infusion between ELAM and the placebo group in a Student’s t-test in an analysis of covariance (ANCOVA) framework, with baseline as a covariate. The secondary endpoints (P/O and FTI) were tested as a mean change from baseline between treatment groups immediately after and 7 days post-infusion. Statistical tests were 2-sided and evaluated at the 0.05 level of significance. No adjustments for multiple comparisons were made per the pre-specified statistical plan. Because multiple tests were conducted any secondary analyses should be thought of as hypothesis generating, using p-values as a guide rather than a benchmark for significance.
Results
Participant characteristics
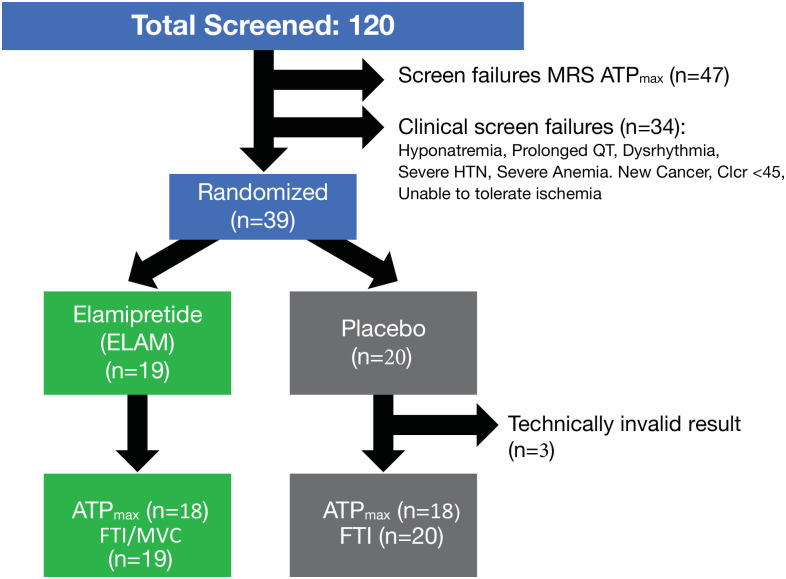
Thirty-nine subjects, from 120 screened potential participants, were randomized to participate in the study based on overall good general health but presenting with low mitochondrial function (ATPmax <0.7 mM sec-1 and P/O <1.9) (Fig 1). Forty-seven subjects (39%) were excluded with high mitochondrial function, while 34 subjects (28%) were excluded due to clinical criteria. ATPmax data was not available for V2 for 1 subject in both the PL and ELAM groups due to problems with data collection. An additional participant in the placebo group had an MRS result that did not meet our quality control criteria (motion artifact) after inclusion in the database; that scan was excluded from the analysis of the primary endpoint. A summary of the demographic and clinical assessments appears in Table 1.
Fig 1. Study schema.
The primary endpoint for the study was change in ATPmax by phosphorus magnetic resonance spectroscopy (31P MRS) after a 2-hour infusion compared with baseline (screening). The secondary endpoints were a change in mitochondrial coupling (P/O) and muscle force time integral (FTI) compared with baseline (screening).
Table 1. Baseline characteristics of study population.
| Placebo (n = 20) | Elamipretide (n = 19) | |
|---|---|---|
| Demographic | ||
| Age (years, mean [±SD]) | 69 (4) | 68 (3.4) |
| Sex, Female (number [%]) | 7 (35.0) | 11 (57.9) |
| White (number [%]) | 20 (100.0) | 18 (94.7) |
| Clinical | ||
| BMI (kg/m2, mean [±SD]) | 26.1 (2.8) | 26.5 (4.3) |
| SBP (mm Hg, mean [±SD]) | 124.3 (13) | 121.1 (12.9) |
| Laboratory | ||
| Sodium (mEq/dL, mean [±SD]) | 137.7 (1.5) | 137.5 (1.6) |
| Hemoglobin (gm/dL, mean [±SD]) | 13.5 (0.9) | 13.1 (0.9) |
| eGFR MDRD, (mL/[min · 1.73m2], mean [±SD]) | 81.5 (14.7) | 82 (10.6) |
| Medications | ||
| Statins (number [%]) | 5 (25.0) | 2 (10.5) |
BMI = body mass index; SBP = systolic blood pressure; eGFR MDRD = estimated glomerular filtration rate.
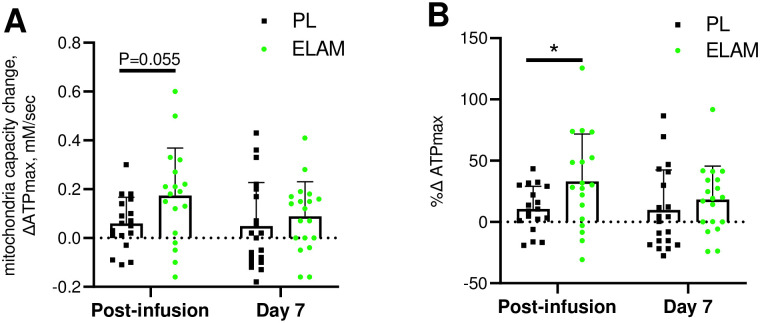
Elevated ATPmax
The primary outcome of this study was an elevated ATPmax in the ELAM group versus the placebo group immediately after a 2-hour ELAM infusion (P = 0.055 for delta ATPmax and P = 0.045 for %change, Fig 2A and 2B). The values for ATPmax at baseline, immediate post-infusion, and 7 days after infusion, as well as the change values used in the ANCOVA analysis are reported in S3 Table in S1 File. The mean increase from baseline to post-infusion was 27% in the ELAM-treated subjects versus 12% in the placebo-treated subjects (Fig 2B). In the ELAM group both post-infusion and 7-day ATPmax values were elevated above baseline, while only the post-infusion ATPmax was higher than baseline in the placebo group (P<0.05, paired t-test). However, the change in ATPmax at 7 days was not different between ELAM and PL groups.
Fig 2. Change in mitochondrial energetic capacity (ΔATPmax) with ELAM treatment.
Change from baseline with ELAM treatment (post-infusion) and from baseline 7 days post-infusion (day 7). A) Absolute change in ATPmax; B) % change relative to baseline value. Error bars show SD. ELAM = elamipretide; PL = placebo.
Resting P/O
A secondary endpoint was the efficiency of oxidative phosphorylation (mitochondrial coupling or P/O), which is one mechanism that could underlie the increased phosphorylation rate with ELAM treatment. No change was seen in this secondary outcome of resting P/O (Fig 3). The baseline, post-infusion and 7-day measurements and ANCOVA analyses are shown in S4 Table in S1 File. This stability of resting P/O indicates that the improvement in ATPmax was the result of higher flux through the ETC rather than more efficient synthesis of ATP per O2 uptake.
Fig 3. Change in mitochondrial coupling (ΔP/O) with ELAM treatment.
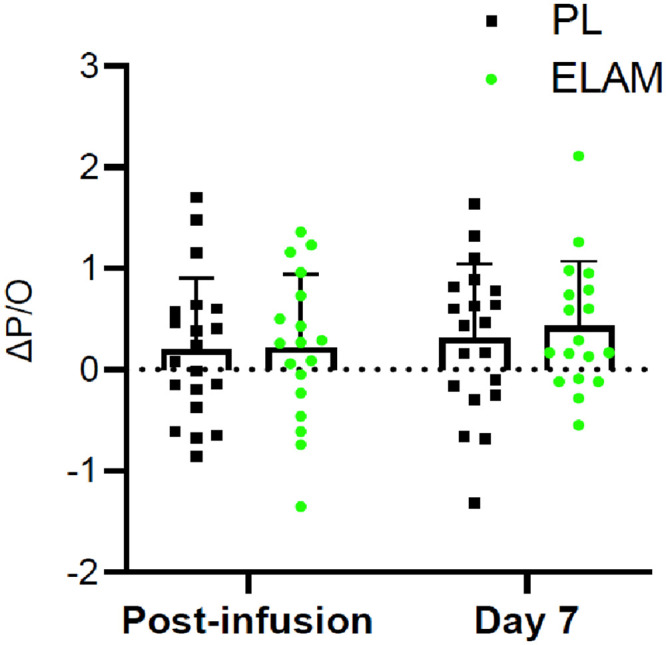
Change from baseline with ELAM treatment (post-infusion) and from baseline 7 days post-infusion (day 7). Error bars show SD. ELAM = elamipretide; PL = placebo.
Muscle function
Exercise tolerance in the hand muscle was a secondary endpoint that assessed the impact of ELAM infusion on muscle function. This endpoint was measured as the sum of force produced during continuous isometric contractions to exhaustion (FTI) normalized to peak strength (FTI per maximum voluntary contraction [MVC]) [35]. No change was apparent in the FTI relative to placebo post-infusion with ELAM in the ANCOVA analysis specified in the protocol (Fig 4; S5 Table in S1 File). However, post-hoc analyses suggest that the study was not sufficiently powered to detect a difference in muscle function in the full ANCOVA model. In addition, a significant rise was found with the two additional days of testing (day 1 and day 7 after infusion, P<0.04) between ELAM versus placebo in ANOVA. In addition, a post-hoc analysis defining muscle fatigue by the number of contractions performed instead of FTI reduced within group variability and indicated a significant treatment effect with a two-way ANOVA (S4 Fig in S1 File).
Fig 4. Change in muscle endurance test (ΔFTI/MVC) with ELAM treatment.
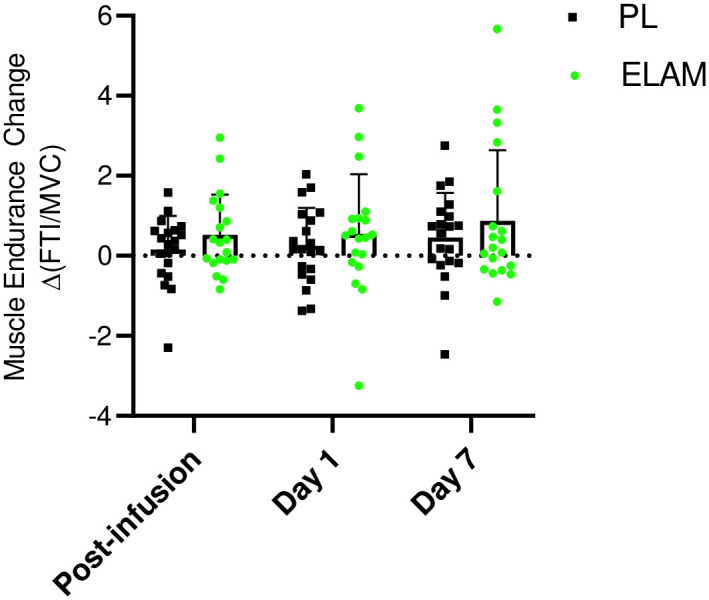
Change from baseline with ELAM treatment post-infusion, from baseline 1 day post-infusion (day 1) and from baseline 7 days post-infusion (day 7). Error bars show SD. ELAM = elamipretide; PL = placebo.
Discussion
In a double-blind placebo-controlled randomized clinical trial a single 2-hour infusion of ELAM improves in vivo ATPmax in the FDI over placebo in skeletal muscle of older adults. Based on published materials that elucidated the mechanism of action of elamipretide, it has been postulated that this significant change in ATPmax can be attributed to the ability of elamipretide to reversibly bind to cardiolipin, resulting in stabilization of the inner mitochondrial membrane and optimization of the electron transport chain in dysfunctional mitochondria [36–38]. This rapid response of in vivo ATPmax to a single ELAM dose in older adult humans reproduces results observed in old (27 month old female) mouse muscle using a similar MRS approach to measure mitochondrial function [13]. A new insight that arose from this study is that the rise in ATPmax on the day of treatment fades by day 7, which is consistent with the 16hour half-time of ELAM in human blood [33]. Thus, ELAM acted to elevate mitochondrial ATP generation capacity in a single dose with a similar magnitude but much faster speed than prolonged interventions, such as exercise, in human muscle from older adults.
In this study we specifically selected community-dwelling older adult participants who demonstrated in vivo deficits in mitochondrial function to focus on the primary endpoint of the study a priori defined as the change in ATPmax with ELAM treatment. Our inclusion criteria included an ATPmax below 0.7 mM ATP/sec, which is above the mean ATPmax of 0.5–0.6 mM ATP/sec from quadriceps muscle we have observed in previous studies of older adults [7, 9, 34]. Previous work in vivo and in vitro suggests that some of this decline in oxidative capacity in the skeletal muscle of older adults is due to poor quality mitochondria as well as the loss of mitochondrial content with age. This is an important point because previous work in both mouse models and human subjects with genetic mitochondrial disease indicate that ELAM (SS-31) provides little or no benefit to well-functioning mitochondria [13, 23]. Despite the data described above, the extent and nature of mitochondrial dysfunction in aging skeletal muscle remains controversial with reports of no differences in oxidative capacity (ATPmax) or maximum respiration in vitro between older and young adult subjects. The disparity in findings between studies underscores the heterogeneity of mitochondrial function with aging, helping to explain the observation that nearly 40% of older adults screened for the current trial were excluded due to normal functioning mitochondria.
Measurement of in vivo ATPmax provides a direct estimate of the capacity for mitochondrial phosphorylation, while other approaches use mitochondrial respiration as a surrogate for the capacity for ATP production [39]. Therefore, the increased ATPmax observed in this study and in previous work in aged mouse skeletal muscles could be due to: 1) increased capacity for flux through the electron transport system (ETS) and 2) improved coupling of oxidation (electron flux, oxygen consumption) to phosphorylation (ATP production) by the mitochondria (P/O) [13]. These two possibilities are not mutually exclusive and are both consistent with the hypothesis that ELAM reversibly binds to cardiolipin to stabilize the cristae structure in the inner mitochondrial membrane [20, 40]. ELAM (or SS-31) has been demonstrated to increase or protect maximum ETS flux in isolated mitochondria and permeabilized muscle fibers in the case of severe dysfunction in multiple models and human subjects associated with heart failure, ischemia-reperfusion, and disuse muscle atrophy. However, in aged mouse skeletal muscle the increased ATPmax after 1 hour or 8 weeks of ELAM (SS-31) treatment was not associated with increased state 3 respiration in permeabilized muscle fibers [13, 23]. It is worth noting that there was also no decrease in state 3 respiration comparing young to old in these studies despite the significant decline in ATPmax with age, suggesting there is a disconnect between maximum rates of respiration measured under saturating substrates and optimal conditions ex vivo and maximum ATP production measured under physiological conditions in vivo. In humans there is a significant correlation between ATPmax and state 3 respiration in permeabilized fibers and in young and old skeletal muscles, although in vivo ATPmax explained only about half of the variation in state 3 respiration in the older adult subjects [7]. These results suggest increased capacity of the ETS may not fully explain the improved ATPmax with ELAM treatment. Recent work also indicates the ELAM directly interacts with mitochondrial proteins involved in substrate supply to the ETS and phosphorylation of ADP to ATP [41] raising the possibility that ELAM could both enhance substrate supply to the ETS and directly affect the phosphorylation system in aged mitochondria.
The lack of an effect of ELAM on the coupling of oxidative phosphorylation (P/O) at rest (Supplemental Methods, S4 Table in S1 File) in this study, despite selection of subjects with low P/O, indicates that changes in the P/O measured under resting conditions is not an important factor in the improved ATPmax observed in this study. The coupling between oxygen consumption and ATP production (P/O) decreases with age in mouse and human skeletal muscle [26]. In aged mice P/O increased with ELAM (SS-31) treatment 1 hour after a single dose and after 8 weeks of continuous treatment [13, 23]. It is important to note that in vivo P/O is measured under resting conditions where membrane potential is high, while ATPmax is measured under high flux conditions and lower membrane potential. Since membrane potential is a key driver of unregulated proton leak across the mitochondrial inner membrane and proton leak is an important mechanism underlying reduced coupling, the P/O is expected to increase as the flux through the ETS increases as has been demonstrated in isolated mitochondria [42]. This is because as the membrane potential decreases with the onset of phosphorylation, the fraction oxygen consumption attributable to proton leak will decline and thereby increase the phosphorylation efficiency and P/O ratio. Therefore, a lower P/O measured under resting conditions may not necessarily have a large impact on ATP production at the high ETS flux rates near ATPmax. This argument and the lack of an effect of ELAM on P/O in this study suggests that mitochondrial coupling is not the primary driver of the increased ATPmax observed in this study. However, this does leave open the possibility that there is a defect in the phosphorylation system (e.g., ADP/ATP exchange, F1Fo ATP synthase function) that impairs the coupling of respiration to ATP production as the mitochondria approach maximum capacity. If ELAM treatment led to reversal of hypothetical dysfunction in the ANT or ATPase this could have the effect of increasing ATPmax without a large effect on state 3 respiration. In support of this possibility is a new study using chemical cross linking with mass spectrometry that demonstrates that ELAM directly interacts with both the ANT and F1Fo ATP synthase in skeletal muscle mitochondria from aged mice [41]. However, the functional significance of these direct protein interactions remains to be tested.
The rapid increase in ATPmax after a 2-hour ELAM infusion agrees with the immediate effect of ELAM treatment on the same measure in the hindlimb of aged mice 1 hour after treatment [13]. Such a fast mitochondrial response is apparent in isolated organ models of disease after ELAM treatment [43]. In comparison, weeks to months are required to achieve a response with treatments that activate mitochondrial biosynthesis pathways. For example, an oral dose of a SIRT1 activator designed to activate mitochondrial biogenesis in older adult subjects involved a 21-day treatment and resulted in a trend toward improved oxidative phosphorylation capacity [44]. In contrast to building new mitochondria, the rapid response in ATPmax in old, but not in young muscle of mice, suggests that ELAM treatment repaired a dysfunction that quickly raised mitochondrial ATP supply [13]. A mechanism of action of ELAM that includes both direct protein interactions and association with cardiolipin to alter membrane structure resulting in improved ETC function and mitochondrial oxidative phosphorylation capacity is consistent with these findings.
Despite the effect on ATPmax, the rise in exercise tolerance did not achieve statistical significance in ELAM versus placebo groups on the infusion day (Fig 4, P = 0.16 S5 Table in S1 File) However, a significant rise was found between ELAM versus placebo with two additional days of testing (one day and one week post-infusion) in an ANOVA test (P<0.04). Both animal and human studies have demonstrated improvement in exercise tolerance with ELAM many days after infusion with daily doses. Greater 6MWT distance was found in patients with genetic mitochondrial disease and impaired mobility after 5 daily infusions at the dose used in this study [25]. Similarly, old mice showed increased endurance (running time to fatigue) on day 8 after daily treatment and after 8 weeks of continuous treatment [13].
This study was originally powered to detect a difference in the effect of treatment on mitochondrial energetics as the primary endpoint. As a result, the study was underpowered in its capacity to detect an effect on muscle fatigue resistance based on the FTI/MVC measure. However, in a post-hoc analysis where the number of contractions, instead of FTI, was used to assess muscle fatigue the ELAM treatment increased muscle fatigue resistance on day 7 (see supplemental material for methods and S4 Fig in S1 File). The absence of significant improvement in muscle endurance despite the increased ATPmax immediately after infusion suggests a disconnect between mitochondrial ATPmax and muscle performance changes with ELAM treatment in older adult human muscle. The direct effects on the mitochondria occurring rapidly, while improvements in muscle performance may take longer to manifest and be due to downstream effects of mitochondrial function not directly linked to ATP supply by the mitochondria.
Despite the intriguing response of skeletal muscle mitochondrial function to a single ELAM treatment demonstrated here, this study has a few important limitations that must be considered. Some caution is warranted in the conclusion that the increase in ATPmax presented is due to a direct effect on skeletal muscle mitochondria. Several studies have demonstrated that treatment with ELAM can protect or preserve blood flow in heart, kidney, and skeletal muscle under multiple disease or acute stress conditions [45–47]. The design of this study did provide for measures of blood flow or independent biochemical measures of mitochondrial function beyond in vivo MRS assays. Therefore, we cannot rule out the possibility that improved substrate delivery to the skeletal muscle contributes to the increase in ATPmax observed after a single ELAM treatment. Another limitation to this clinical trial was the small sample size target of 20 subjects per treatment group that was reduced to 18 per group with a complete ATPmax dataset due to technical problems leading to missing data. This subject number achieved statistical significance for a relative ATPmax change (Δ%ATPmax, P = 0.045) with ELAM treatment, but 25 subjects are needed to reach the threshold for the absolute change in ATPmax based on power calculations derived from this study. Finally, in the absence of ex vivo functional data and redox status from isolated mitochondria or permeabilized fibers. we are left to speculate on the specific nature of the dysfunction with age and reversal with ELAM treatment observed in vivo in this study.
Conclusions
Here, we demonstrate a pharmacological treatment that rapidly reverses mitochondrial deficits of ATP production in skeletal muscle in healthy older adult subjects. Several new insights come from this demonstration. First, the rise in ATPmax with a single infusion identifies the mitochondria as a site of action of ELAM. Second, the speed of the effect suggests that a rapidly reversible process in the mitochondria underlies this improvement. This is in contrast to the biosynthesis and replacement of mitochondrial components that underlie improvements in mitochondrial metabolism observed with exercise training. Third, the decline in ATPmax to placebo levels after one week indicates a reversible process. Thus, ELAM treatment provides new insight into the nature of mitochondrial and muscle dysfunction with age. Most importantly, ELAM treatment holds promise as a new therapy for directly targeting mitochondrial dysfunction associated with age with the potential to improve mobility limitations and disability that come with age and disease.
Supporting information
S1 and S2 Tables list inclusion and exclusion criteria and S3–S5 Tables display muscle energetics and fatigue data. S1–S3 Figs show examples of PCr recovery, resting ATPase and O2 consumption calculations, and muscle fatigue measurements. S4 Fig shows data for muscle fatigue analyzed using the total contractions.
(DOCX)
Approved protocol for this clinical trial, including a description of the primary and secondary endpoints, inclusion and exclusion criteria, and statistical plan can be found in the Supplemental Information.
(PDF)
A checklist specifying the inclusion of key criteria for this randomized controlled trial is included in the Supplemental Information.
(PDF)
Acknowledgments
We thank the volunteers who participated in this study for their time and dedication as well as the contributions by Amy Good, Laura Ferrara, Jeff Finman, Peter Rabinovitch, Doug Weaver, Mark Bamberger, and James Carr.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This work was supported by Stealth Biotherapeutics; National Institutes of Health grants (5T32AG5738, UL1TR000423, 1S10OD016201, K23DK099442, P01AG001751); the Department of Radiology and the Office of the Provost of the University of Washington. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors worked with Stealth BioTherapeutics on the design of this study. Otherwise, the funders played no role in data collection and analysis nor the decision to publish this manuscript.
References
- 1.Guralnik J.M., Ferrucci L., Simonsick E.M., Salive M.E., and Wallace R.B., (1995), Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 332(9): p. 556–61. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roshanravan B., Patel K.V., Fried L.F., Robinson-Cohen C., de Boer I.H., Harris T., et al., (2017), Association of Muscle Endurance, Fatigability, and Strength With Functional Limitation and Mortality in the Health Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 72(2): p. 284–291. doi: 10.1093/gerona/glw210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korpelainen R., Lamsa J., Kaikkonen K.M., Korpelainen J., Laukkanen J., Palatsi I., et al., (2016), Exercise capacity and mortality—a follow-up study of 3033 subjects referred to clinical exercise testing. Ann Med. 48(5): p. 359–66. doi: 10.1080/07853890.2016.1178856 [DOI] [PubMed] [Google Scholar]
- 4.Janssen I., Shepard D.S., Katzmarzyk P.T., and Roubenoff R., (2004), The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 52(1): p. 80–5. doi: 10.1111/j.1532-5415.2004.52014.x [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Freire M., de Cabo R., Bernier M., Sollott S.J., Fabbri E., Navas P., et al., (2015), Reconsidering the Role of Mitochondria in Aging. J Gerontol A Biol Sci Med Sci. 70(11): p. 1334–42. doi: 10.1093/gerona/glv070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorrentino V., Menzies K.J., and Auwerx J., (2017), Repairing Mitochondrial Dysfunction in Disease. Annu Rev Pharmacol Toxicol. [DOI] [PubMed] [Google Scholar]
- 7.Coen P.M., Jubrias S.A., Distefano G., Amati F., Mackey D.C., Glynn N.W., et al., (2013), Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 68(4): p. 447–55. doi: 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kestenbaum B., Gamboa J., Liu S., Ali A.S., Shankland E., Jue T., et al., (2020), Impaired skeletal muscle mitochondrial bioenergetics and physical performance in chronic kidney disease. JCI Insight. 5(5). doi: 10.1172/jci.insight.133289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santanasto A.J., Coen P.M., Glynn N.W., Conley K.E., Jubrias S.A., Amati F., et al., (2016), The relationship between mitochondrial function and walking performance in older adults with a wide range of physical function. Exp Gerontol. 81: p. 1–7. doi: 10.1016/j.exger.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi S., Reiter D.A., Shardell M., Simonsick E.M., Studenski S., Spencer R.G., et al., (2016), 31P Magnetic Resonance Spectroscopy Assessment of Muscle Bioenergetics as a Predictor of Gait Speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 71(12): p. 1638–1645. doi: 10.1093/gerona/glw059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szeto H.H., (2014), First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 171(8): p. 2029–50. doi: 10.1111/bph.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birk A.V., Chao W.M., Bracken C., Warren J.D., and Szeto H.H., (2014), Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol. 171(8): p. 2017–28. doi: 10.1111/bph.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel M.P., Kruse S.E., Percival J.M., Goh J., White C.C., Hopkins H.C., et al., (2013), Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell. 12(5): p. 763–771. doi: 10.1111/acel.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell W., Ng E.A., Tamucci J.D., Boyd K.J., Sathappa M., Coscia A., et al., (2020), The mitochondria-targeted peptide SS-31 binds lipid bilayers and modulates surface electrostatics as a key component of its mechanism of action. J Biol Chem. 295(21): p. 7452–7469. doi: 10.1074/jbc.RA119.012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen M.E., Pennington E.R., Perry J.B., Dadoo S., Makrecka-Kuka M., Dambrova M., et al., (2020), The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats. Commun Biol. 3(389). doi: 10.1038/s42003-020-1101-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szeto H.H., Liu S., Soong Y., Alam N., Prusky G.T., and Seshan S.V., (2016), Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 90(5): p. 997–1011. doi: 10.1016/j.kint.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 17.Nickel A., Kohlhaas M., and Maack C., (2014), Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol. 73: p. 26–33. doi: 10.1016/j.yjmcc.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 18.Szeto H.H. and Schiller P.W., (2011), Novel therapies targeting inner mitochondrial membrane—from discovery to clinical development. Pharm Res. 28(11): p. 2669–79. doi: 10.1007/s11095-011-0476-8 [DOI] [PubMed] [Google Scholar]
- 19.Zhao K., Luo G., Giannelli S., and Szeto H.H., (2005), Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 70(12): p. 1796–806. doi: 10.1016/j.bcp.2005.08.022 [DOI] [PubMed] [Google Scholar]
- 20.Brown D.A., Hale S.L., Baines C.P., del Rio C.L., Hamlin R.L., Yueyama Y., et al., (2014), Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. J Cardiovasc Pharmacol Ther. 19(1): p. 121–32. doi: 10.1177/1074248413508003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai W., Shi J., Gupta R.C., Sabbah H.N., Hale S.L., and Kloner R.A., (2014), Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 64(6): p. 543–53. doi: 10.1097/FJC.0000000000000155 [DOI] [PubMed] [Google Scholar]
- 22.Sabbah H.N., Gupta R.C., Kohli S., Wang M., Hachem S., and Zhang K., (2016), Chronic Therapy With Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ Heart Fail. 9(2): p. e002206. doi: 10.1161/CIRCHEARTFAILURE.115.002206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell M.D., Duan J., Samuelson A.T., Gaffrey M.J., Merrihew G.E., Egertson J.D., et al., (2019), Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol Med. 134: p. 268–281. doi: 10.1016/j.freeradbiomed.2018.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakellariou G.K., Pearson T., Lightfoot A.P., Nye G.A., Wells N., Giakoumaki II, et al., (2016), Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep. 6: p. 33944. doi: 10.1038/srep33944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karaa A., Haas R., Goldstein A., Vockley J., Weaver W.D., and Cohen B.H., (2018), Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology. 90(14): p. e1212–e1221. doi: 10.1212/WNL.0000000000005255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amara C.E., Shankland E.G., Jubrias S.A., Marcinek D.J., Kushmerick M.J., and Conley K.E., (2007), Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci U S A. 104(3): p. 1057–62. doi: 10.1073/pnas.0610131104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amara C.E., Marcinek D.J., Shankland E.G., Schenkman K.A., Arakaki L.S., and Conley K.E., (2008), Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods. 46(4): p. 312–8. doi: 10.1016/j.ymeth.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried L., Tangen C., and Walston J., (2001), Frailty in older adults: evidence for a phenotype. J of Gerontol. 56A(3): p. M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 29.Roshanravan B, C. R.-C., Patel KV., (2013), Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 24(5): p. 822–830. doi: 10.1681/ASN.2012070702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isoyama N., Qureshi A.R., Avesani C.M., Lindholm B., Barany P., Heimburger O., et al., (2014), Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 9(10): p. 1720–8. doi: 10.2215/CJN.10261013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman A.B., Kupelian V., Visser M., Simonsick E.M., Goodpaster B.H., Kritchevsky S.B., et al., (2006), Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 61(1): p. 72–7. doi: 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 32.Conley K., Jubrias S., Cress E., and Esselman P., Elevated energy coupling and aerobic capacity improves exercise performance in endurance trained elderly. Exp Physiol. doi: 10.1113/expphysiol.2012.069633 [DOI] [PubMed] [Google Scholar]
- 33.Daubert M.A., Yow E., Dunn G., Marchev S., Barnhart H., Douglas P.S., et al., (2017), Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ Heart Fail. 10(12). doi: 10.1161/CIRCHEARTFAILURE.117.004389 [DOI] [PubMed] [Google Scholar]
- 34.Conley K.E., Jubrias S.A., and Esselman P.C., (2000), Oxidative capacity and ageing in human muscle. J Physiol. 526 Pt 1: p. 203–10. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega J.O., Lindstedt S.L., Nelson F.E., Jubrias S.A., Kushmerick M.J., and Conley K.E., (2015), Muscle force, work and cost: a novel technique to revisit the Fenn effect. J Exp Biol. 218(Pt 13): p. 2075–82. doi: 10.1242/jeb.114512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claypool S.M. and Koehler C.M., (2012), The complexity of cardiolipin in health and disease. Trends Biochem Sci. 37(1): p. 32–41. doi: 10.1016/j.tibs.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houtkooper R.H. and Vaz F.M., (2008), Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 65(16): p. 2493–506. doi: 10.1007/s00018-008-8030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace D.C., Fan W., and Procaccio V., (2010), Mitochondrial energetics and therapeutics. Annu Rev Pathol. 5: p. 297–348. doi: 10.1146/annurev.pathol.4.110807.092314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell M.D. and Marcinek D.J., (2016), Evaluation of in vivo mitochondrial bioenergetics in skeletal muscle using NMR and optical methods. Biochim Biophys Acta. 1862(4): p. 716–724. doi: 10.1016/j.bbadis.2015.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birk A.V., Liu S., Soong Y., Mills W., Singh P., Warren J.D., et al., (2013), The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 24(8): p. 1250–61. doi: 10.1681/ASN.2012121216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavez J.D., Tang X., Campbell M.D., Reyes G., Kramer P.A., Stuppard R., et al., (2020), Mitochondrial protein interaction landscape of SS-31. Proc Natl Acad Sci U S A. 117(26): p. 15363–15373. doi: 10.1073/pnas.2002250117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gnaiger E., (2009), Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol. 41(10): p. 1837–45. doi: 10.1016/j.biocel.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 43.Szeto H.H., Liu S., Soong Y., Wu D., Darrah S.F., Cheng F.Y., et al., (2011), Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 22(6): p. 1041–52. doi: 10.1681/ASN.2010080808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Libri V., Brown A.P., Gambarota G., Haddad J., Shields G.S., Dawes H., et al., (2012), A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 7(12): p. e51395. doi: 10.1371/journal.pone.0051395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eirin A., Hedayat A.F., Ferguson C.M., Textor S.C., Lerman A., and Lerman L.O., (2018), Mitoprotection preserves the renal vasculature in porcine metabolic syndrome. Exp Physiol. 103(7): p. 1020–1029. doi: 10.1113/EP086988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan F., Hedayat A.F., Ferguson C.M., Lerman A., Lerman L.O., and Eirin A., (2018), Mitoprotection attenuates myocardial vascular impairment in porcine metabolic syndrome. Am J Physiol Heart Circ Physiol. 314(3): p. H669–H680. doi: 10.1152/ajpheart.00431.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan T.E., Schmidt C.A., Alleman R.J., Tsang A.M., Green T.D., Neufer P.D., et al., (2016), Mitochondrial therapy improves limb perfusion and myopathy following hindlimb ischemia. J Mol Cell Cardiol. 97: p. 191–6. doi: 10.1016/j.yjmcc.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 and S2 Tables list inclusion and exclusion criteria and S3–S5 Tables display muscle energetics and fatigue data. S1–S3 Figs show examples of PCr recovery, resting ATPase and O2 consumption calculations, and muscle fatigue measurements. S4 Fig shows data for muscle fatigue analyzed using the total contractions.
(DOCX)
Approved protocol for this clinical trial, including a description of the primary and secondary endpoints, inclusion and exclusion criteria, and statistical plan can be found in the Supplemental Information.
(PDF)
A checklist specifying the inclusion of key criteria for this randomized controlled trial is included in the Supplemental Information.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.