Abstract
Mammalian cardiomyocytes mostly utilize oxidation of fatty acids to generate ATP. The fetal heart, in stark contrast, mostly uses anaerobic glycolysis. During perinatal development, thyroid hormone drives extensive metabolic remodeling in the heart for adaptation to extrauterine life. These changes coincide with critical functional maturation and exit of the cell cycle, making the heart a post-mitotic organ. Here, we review the current understanding on the perinatal shift in metabolism, hormonal status, and proliferative potential in cardiomyocytes. Thyroid hormone and glucocorticoids have roles in adult cardiac metabolism, and both pathways have been implicated as regulators of myocardial regeneration. We discuss the evidence that suggests these processes could be interrelated and how this can help explain variation in cardiac regeneration across ontogeny and phylogeny, and we note what breakthroughs are still to be made.
Keywords: cardiomyocyte, regeneration, thyroid, glucocorticoids, metabolism, development
The complexity of higher mammalian life brings with it a long list of biochemical requirements to fulfill the demands of everyday life. An impressive feat of evolution, the heart can sustain performance to meet this tremendous workload—without interruption—for an entire lifespan. For humans and other mammals, such remarkable functionality is coupled with the loss of regenerative capacity and the mounting of only fibrotic responses to injury. Hence, cardiovascular diseases, such as weakened cardiac ejection, arrhythmias, myocardial infarction (MI), and heart failure, account for about half of deaths caused by noncommunicable diseases worldwide (1). While interventions currently exist to ameliorate heart dysfunction, the need for truly regenerative therapies is clear.
The lack of proliferation in the heart’s functional unit, the cardiomyocyte (CM), has been known of for nearly 100 years (2), when it was first shown that the postnatal heart expands by hypertrophy. Originally thought to be completely unable to proliferate, adult human hearts have been shown to possess around 0.004% to 1.9% proliferative CMs (3-5), depending on donor age and the approach to estimation, which are chiefly derived from pre-existing CMs (6). Although higher vertebrates, including mice, show a similar lack of cardiac proliferative capacity—before which the embryonic heart grows by proliferation and is capable of regeneration (7-11)—several lower vertebrates, for example, zebrafish, have remarkably robust cardiac wound healing even in adulthood (12). During postnatal maturation, mammalian CMs undergo polyploidization and exit the cell cycle (13-15). In mice, this occurs by postnatal day (P) 10 and typically results in more than 90% of CMs being binucleated (13, 16, 17). Human CMs remain majority mononucleated throughout life but are multiploid (18). Mononucleated diploid CMs are capable of proliferation, while multiploidization is associated with its loss (19), and, accordingly, zebrafish CMs remain diploid throughout life (19, 20). Other vertebrates also retain some cardiac regenerative potential throughout life (20, 21), suggesting some conservation of regenerative mechanisms.
Studying the phylogenetic variation in cardiac development has implicated species-dependent endocrine signaling as an underlying reason for the disparity in regenerative capacity (20). While several hormones are suggested to be involved in cardiac regeneration, we noted a particular interest in thyroid hormone (TH) and glucocorticoids as effectors of both metabolic and mitotic remodeling in CMs, which have recently been shown to be interconnected (20, 22-26). We discuss the evidence that suggests myocardial metabolism, proliferation, hypertrophy, and regeneration could be interrelated, and how this may explain variation in cardiac regeneration across ontogeny and phylogeny. We followed recent key reports in the field and reviewed findings from these papers’ citations. Other pertinent information was found through PubMed searches using the key terms cardiomyocyte, metabolism, cell cycle, ploidy, thyroid hormone, glucocorticoids, hypertrophy, and heart regeneration in various combinations, in articles published from 1950 to 2021. Articles were included if they considered key aspects of cardiac metabolism, disease, regeneration, the cardiomyocyte cell cycle, and hormonal regulation thereof.
Perinatal Changes in Metabolism
In the basal state, the heart has the highest oxygen uptake rate in the human body, at about 0.1 mL O2/g/min oxygen (around 60%-70% of which supports contractions) (27, 28). One-third of total CM volume is occupied by mitochondria, from which nearly all of the CMs’ energy is derived via oxidative phosphorylation (OXPHOS) (29). They undergo little dynamics, being highly organized between myofilaments to enable rapid transfer of nascent adenosine triphosphate (ATP), thereby enabling efficient fueling of contraction (29, 30). Although the heart displays considerable metabolic flexibility, its preferred fuel is fatty acids (29, 31), likely due to greater efficiency per gram of substrate.
However, while adult oxygen tension ranges from 100 mmHg in alveolar arterioles to 20 to 40 mmHg in systemic tissues, the arterial pO2 of the fetus is found between 30 and 20 mmHg (28, 30, 32). Due to this relative hypoxia, as well as a low supply (~0.1 mM) of circulating fatty acids, the fetal heart must rely primarily on glycolysis for ATP synthesis (33, 34). At this stage, mitochondria are disorganized, highly dynamic, and possessing low cristae density (29). Nevertheless, the fetal heart can deploy some aerobic ATP synthesis. OXPHOS activity begins to increase around embryonic day (E)10.5 to E14.5 in rodent cardiomyocytes (35, 36). Fetal expression of lactate dehydrogenase A is high, maintaining glycolysis via the regeneration of nicotinamide adenine dinucleotide (NAD)+ from NADH (37). High concentrations of lactate in the intrauterine environment makes it an efficient substrate for OXPHOS, accounting for 45% of oxidative respiration that does occur, while approximately 32% of it uses glucose as a substrate (38). Mitochondrial ATP output is supplied to consumptive sites primarily via diffusion (39).
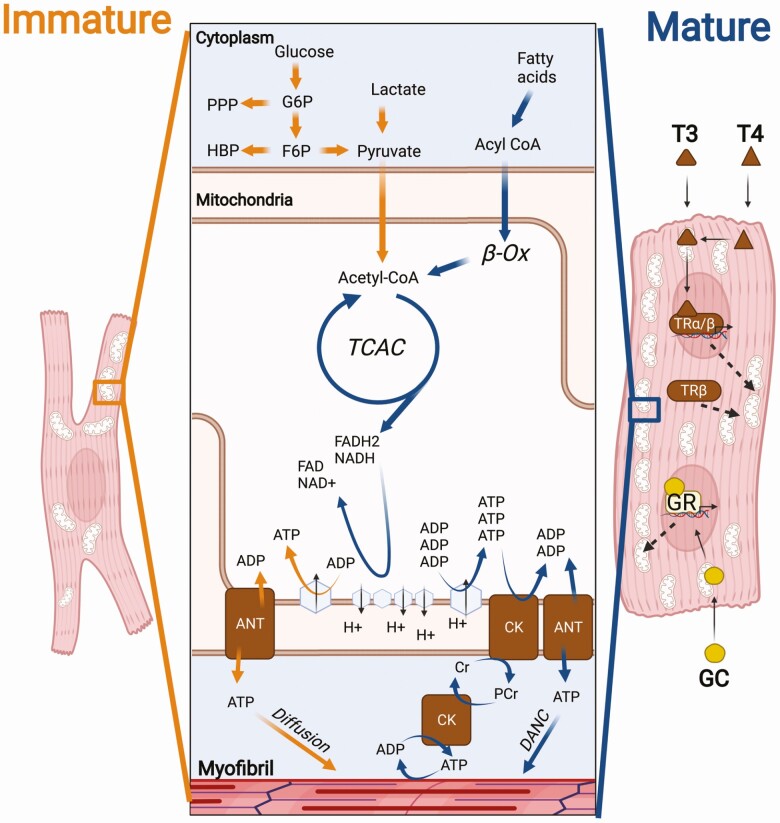
The sizable metabolic difference between fetal and adult hearts (Fig. 1) illustrates the rapid and substantial metabolic remodeling that must occur during early development. In mice, the glycolytic rate is still high immediately after birth. In postnatal day (P)1 rabbits, from 44% to 57% of ATP production is from anaerobic glycolysis (40). Increased O2 and abundance of fatty acids in the extrauterine environment allows the heart to swiftly shift substrate (40). Rapid growth of the whole body outstrips that of the heart, reducing the heart weight to body weight ratio (41). Neonates are largely poikilothermic at birth, but internal thermogenesis initiates to maintain endothermy between days 7 and 15, and muscle shivering begins over days 15 to 17 (42). Around the same time, in order to meet demands, the heart undergoes hypertrophic expansion and the CMs fully mature, myofibril density increases, mature intercalated discs form, ion channels re-localize and Ca2+ handling expands, and T-tubulation occurs (29, 41, 43). As a result of these combined developments, the bioenergetic needs of the postnatal heart surpass the volume of ATP that anaerobic glycolysis can supply. To compensate for this, fetal mitochondria are removed by parkin-mediated mitophagy (44) and extensive mitochondrial biogenesis and maturation occurs, resulting in the more efficient adult mitochondria (44, 45). A concomitant increase in expression of β-oxidation proteins leads to a metabolic switch in neonatal CMs (29, 33, 34). By P7, glycolysis accounts for less than 10% of ATP synthesis in rabbit CMs (27, 40).
Figure 1.
Thyroid hormone and glucocorticoids contribute to the differential metabolism of fetal and adult cardiomyocytes. The predominant ATP generation pathways in the fetal (orange arrows) and adult (blue arrows) cardiomyocytes are shown. The fetal heart primarily relies on glycolysis, with some glucose and lactate oxidation. The adult heart chiefly oxidizes fatty acids but is metabolically flexible. Thyroid hormone in the form of T3 activates TRs α and β directly or downstream of conversion from T4. Upon activation by T3, TRs trigger the expression of several mitochondrial biogenesis, beta-oxidation and electron transport chain related genes. TRβ also promotes oxidative consumption of triglycerides without T3. Glucocorticoid receptor (GR) has similarly been linked to pro-OXPHOS and beta-oxidation pathways in adult CMs and is essential for adult heart function. ATP generated in the mitochondria travels by simple diffusion during development, but creatine kinase (CK) shuttling and direct adenine nucleotide channeling (DANC) ensure rapid transferral in the adult cardiomyocyte to sites of consumption. Abbreviations: ANT, adenine nucleotide translocator; Cr, creatine; PPP, pentose phosphate pathway; F6P, fructose-6-phosphate; β-OX, beta-oxidation; G6P, glucose-6-phosphate; HBP, hexosamine biosynthetic pathway; PCr,
phosphocreatine; TCAC, tricarboxylic acid cycle.
Thyroid Hormone Influence of Neonatal Cardiac Metabolism and Proliferation
Thyroid hormone (TH) is secreted by the thyroid gland in response to the pituitary hormone, thyroid-stimulating hormone (46). Some human TH is secreted as the more active form, triiodothyronine (3,5,3’-triiodo-l-thyronine, T3), but the predominant form, L-thyroxine (T4), is generally later converted into T3 by locally expressed deiodinases (46, 47). The canonical action of TH is by alteration of transcriptional activity via association with nuclear-acting TH receptor (TR) α and β (46). Upon activation by T3, TRα typically acts in a heterodimer with a 9-cis retinoic acid receptor (RXR), and many TRα and β isoforms are functionally associated with various other factors, such as peroxisome proliferator-activated receptors (PPARs) and vitamin D receptor (47, 48). Although it can act on genes directly via TH response elements (TREs), TH also regulates the expression and activity of intermediate activators and coactivators (48), coordinating numerous cellular effects. TRs also have physiological effects in the absence of TH (49). Newborn mice experience a >50-fold increase in circulating TH (50), which triggers important cardiac functional maturation events (51, 52).
It has been known for decades, and demonstrated by quantitative electron micrograph data, that T3 increases mitochondrial content and cristae density in CMs (52). During T3-mediated hypertrophy, sarcolemma growth is proportionate, while the fraction of cellular volume inhabited by mitochondria increases (52). More recently it has been shown that TH and TRs regulate the activity and expression of various genes involved in cardiac metabolism. Primary CM cultures that are induced into hyperthyroidism have an attenuated glucose-uptake response to insulin (53), while prolonged exposure to TH suppresses glucose oxidation directly by increased expression of pyruvate dehydrogenase kinase (PDK) mRNA, thereby inhibiting mitochondrial pyruvate dehydrogenase (PDH) and thus the conversion of pyruvate to acetyl-CoA (54, 55). The cardioselective dominant-negative TRβ1 mutation Δ337T leads to increased PDK 2 protein levels (56). TRβ has been shown to promote oxidative metabolism by noncanonical pathways (49). Likewise, TH signaling promotes mitochondrial biogenesis and aerobic metabolism, via nuclear pathways, such as PPARα, with which TRs share the coactivator, peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α), and binding partner, RXR (57). T3 also activates the orphan receptor, ErbAα, at the mitochondrial genome to promote mitochondrial biogenesis (57).
Previous work has generated mixed results on the role of TH in modulating the CM cell cycle. In vitro stimulation of CMs with TH has resulted in little to no effect, or inhibitory effects, on proliferation (58-60). Meanwhile, in vivo work in fetal sheep has shown that TH stimulates binucleation, but thyroidectomy did not promote CM proliferation (51, 61). In vivo work in mice showed a “second wave” of CM proliferation around P15 in response to T3, while propylthiouracil, which inhibits TH synthesis, between P7 and P14 suppressed the aforementioned wave (62). However, the observation of adolescent CM expansion could not be replicated by 2 other groups (17, 63). An important consideration when understanding the role of TH in hypertrophic and hyperplastic growth is dosage. In vivo work in rats suggests that high doses (5-20 mg/g body weight) of T4 in rats accelerates the shift from mitotic to hypertrophic growth, indicated by increased myocyte volume but decreased number (64).
In addition to dosage, the timing and duration seem to affect the action of TH signaling on CM proliferation. Hirose et al showed that prolonged propylthiouracil inhibition of murine TH extended the perinatal CM proliferative window (20). Mice with cardiac-specific suppression of TRα had higher number of CMs, more of which possessed diploid nuclei and expressed cyclic markers than controls. Likewise, adult TRα mutants showed a 10-fold increase in proliferating, diploid CMs and better functional recovery after ischemic reperfusion injury (20). The heart-specificity of this approach is an important feature of this study, as it suggests that suppression of the cell cycle by thyroid is not dependent on systemic TH suppression. Substantiating this, T3 inhibits zebrafish heart, but not fin, regeneration (20). Conversely, 2 recent papers showed that transient exposure to exogenous T3 increased mononuclear CM number by 10% to 17.5% in mouse hearts when harvested several days after the last exposure of T3 (65, 66). Particularly notable here is the findings that canonical T3 signals promoted mitochondrial H2O2 generation, in turn (via Peroxiredoxin 1) activating c-Jun N-terminal Kinase 2 α2 (65). It then promotes the pro-proliferative insulin-like growth factor-1-dependent extracellular signal-related kinases (ERK)1/2 cascade (64, 67)). They later showed this effect to diminish in all CMs but the left ventricular apex at P8, due to the action of an ERK1/2 phosphatase (66). However, it is well known that the TH pathway is subject to extensive negative feedback regulation to control TH production and signaling (68). Thus, whether the above effects observed by transient perturbations are due to negative feedback, such as decreased circulating TH levels during the period of exogenous TH withdrawal, awaits further investigation.
Glucocorticoids Influence of Neonatal Cardiac Metabolism and Proliferation
As primary stress hormones secreted by the adrenal cortex, the glucocorticoids are steroid hormones that exert their effects by binding to nuclear receptor superfamily transcription factors, the ubiquitous glucocorticoid receptor (GR) and the tissue-dependent mineralocorticoid receptor (MR). In humans, the predominant glucocorticoid is cortisol, the homeostasis of which is mainly controlled by the local interconversion between it and a less active hormone, cortisone, by 11β-hydroxysteroid-dehydrogenase (69). However, in mice, the primary glucocorticoid is corticosterone (69). Due to alternative splicing, translational differences and posttranslational modifications, there are numerous GR isoforms, the most common of which in vertebrates are GRα and GRβ (69). Like most tissues, GRα appears to be the main form in the heart (69).
Although GR and MR display redundancy in the heart, GR activity has specifically been linked to substrate utilization. Via rapid induction of Krüppel-like factor (KLF) 15, a regulator of cardiac lipid metabolism (70), GR signals promote gene expression of branched-chain amino acid transaminase 2, the rate-limiting enzyme in amino acid catabolism, and glucose transporter type 4; this effect could be inhibited by short interfering RNAs against either GR or KLF15 (71). In rat CMs, this GR-KLF15 axis promoted branched-chain amino acid catabolism, indicated by decreased valine, leucine, and isoleucine concentrations (71), which, in other tissues are important in regulating insulin sensitivity, protein synthesis, and cellular bioenergetics (71).
Glucocorticoid signaling is critical for normal heart development. Autonomous adrenal cortex synthesis of glucocorticoids shows a rapid surge around E15.5 that peaks at E16.5 in mice (72). Mice with global deletion or CM and vascular smooth muscle-specific deletion of GR showed impaired heart function and disorganized myofibrils at E17.5 (73). A lack of proper expression of PGC1α, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 (PFKFB2), and hexokinase-1, all important factors in cardiac metabolic maturation in late gestation, was observed in both mutant lines (73). Moreover, fetal CMs stimulated by glucocorticoids in vitro led to induction of beta-oxidation and OXPHOS, as well as myofibrillar maturation (73). This resembles the action of PGC1α (74, 75), itself an important factor for perinatal mitochondrial maturation that promotes expression of genes involved in β-oxidation, OXPHOS, and mitochondrial biogenesis (76, 77). Accordingly, siRNA-mediated knockdown of PGC1α disrupted the myofibrillar organization induced by glucocorticoid stimulation (72). In contrast to mice lacking GR in both CMs and vascular smooth muscles, mice with CM-specific loss of GR are born normally but are rarely viable into adulthood, due to onset of cardiomyopathy (69). The different phenotypes of these 2 mutant mice suggest that fetal metabolic defects in the first mutant line are due to the loss of GR in smooth muscle cells. It is also plausible that only deletion of GR in both CMs and smooth muscle cells would yield severe fetal CM phenotypes.
Glucocorticoids’ role in CM proliferation is not currently defined. In rat CMs, cardiac GR activation is associated with enhanced hypertrophic and suppressed hyperplastic growth (78, 79). Mechanistically, it has been suggested that epigenetic cyclin D2-suppression by GR activation leads to greater populations binucleated of rat CMs (78). In vitro and in vivo pharmacological stimulation of GR in neonatal mouse CMs similarly blocks proliferation, but mice with CM-specific GR deletion have no discernible phenotypes in CM proliferation and binucleation at P14 (80). These results indicate that glucocorticoid signaling is not necessary for CM cell-cycle withdrawal shortly after birth. The GR pathway does not seem to be active during the neonatal window, as supported by the lack of expression of GR downstream target genes in this period (80).
Cardiac Metabolism and Regeneration
Metabolism in Regulating the Cardiomyocyte Cell Cycle
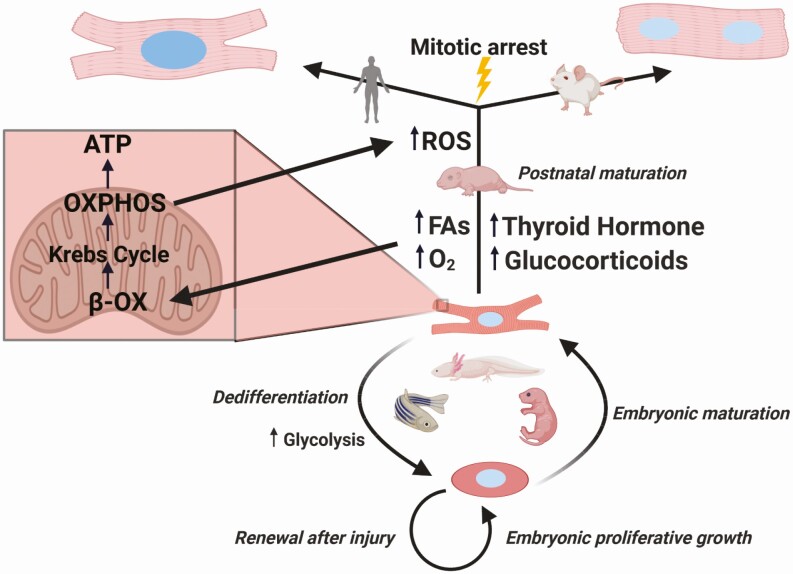
A lingering question remains: to what extent do the metabolic and mitotic effects of TH and glucocorticoids interact in the heart? Comparing phylogeny and ontogeny illustrates how this is conceivable (Fig. 2), showing that certain metabolic states are associated with cardiac regeneration. The vertebrates with the best regenerative capability are aquatic (12, 81, 82) or mostly aquatic (83-85). Life as a wholly terrestrial vertebrate necessitates a higher pressure, closed circulatory system, to supply sufficient blood flow to the whole body, but the primitive circulatory system of fish is more akin to the shunt-based heart of the mammalian fetus (32). Low oxygen tensions are also associated with regenerative potential. Take the zebrafish, which can tolerate as low as 15 mmHg PaO2 (10% air saturation) or 8 mmHg with preconditioning (22, 86), so its environment is analogous to that of the mammalian intrauterine environment; for example, in fetal lambs, the brain and heart supply is only 62% saturated (87). Bringing these trends into sharp focus, Hirose et al’s phylogenetic analysis showed that diploid mononucleated CMs correlated robustly with both standard metabolic rate and body temperature, independent of body weight, heart rate, and blood pressure (20). This suggests that the ecto- to endotherm transition, much like that of perinatal mammalian development, has been a major force in constraining cardiac regeneration in mammals. There was likely very little selection pressure for cardiac regeneration in early endotherms. After all, root causes of cardiovascular disease in humans are linked to very modern concerns, including the aging population (1, 88), poor diet and high rates of obesity (89), poor air quality (1), shift work (90) and job, housing, and financial insecurity–related stress (1, 91). Conversely, there would have been a major selection pressure for a stronger, highly aerobic heart: elevated metabolic activity likely supported niche expansion firstly into colder climate and nocturnal niches (92). A prominent theory is that the appearance of TH was the evolutionary driver of this process (92), and, in line with this, Hirose et al also showed that TH levels inversely correlated with diploid CM populations, a proxy of cardiac regenerative potential (20).
Figure 2.
Interactions of cardiomyocyte metabolism and the cell cycle across ontogeny and phylogeny. Immature cardiomyocytes proliferate rapidly during embryonic development. In organisms such as neonatal mice, zebrafish, and axolotls, cardiomyocytes can respond to injury by glycolysis-dependent reprogramming to a fetal-like state. This allows for repair by myocardium renewal. After birth, mammals rapidly undergo extensive metabolic maturation, stimulated by increased circulating fatty acids (FAs) and oxygen tension. Hormones such as thyroid hormone (TH) and glucocorticoids stimulate increased beta-oxidation (β-OX), which feeds the tricarboxylic acid cycle (Krebs cycle) and oxidative phosphorylation (OXPHOS); subsequent reactive oxygen species (ROS) surging induces the DNA damage response and arrests the cell cycle. Future cycling is primarily endoreduplicative, leading to polyploid cardiomyocytes that are primarily multinucleated in mice and multiploid mononucleated in humans. As a result, cardiomyocytes in the mature mammalian heart cannot mount a regenerative response to injury.
Such lessons from evolution have been explored experimentally. Induced hypoxia (15% O2) or hyperoxia (100% O2) in mice at E18.5 showed that oxygen saturation inversely correlates with perinatal CM proliferation. Lower available O2 results in bigger heart weight to body weight ratios, but smaller CMs that express lower levels of phosphor-Histone3 Serine 10, a marker of G2 to M progression, and cleavage furrow-localization of Aurora B Kinase, a karyokinetic marker (22). In embryonic CMs, hypoxia-inducible factor (HIF)1a signaling stimulates glycolysis and suppresses OXPHOS (93, 94) and promotes proliferation (94). However, glycolytic preference may not be exclusively an adaptation to limited oxygenation, but one that supports proliferation during development—this is highlighted by studies looking at trabeculation. CMs in the ventricular myocardium undergo an epithelial-to-mesenchymal transition in order to form trabeculae (25) and an important mediator of this is avian erythroblastosis oncogene B (ErbB) 2 signals (95, 96). Crucially, Fukuda et al demonstrated that, downstream of Erbb2, glycolysis regulates the cardiomyocyte delamination necessary for trabeculation. Inhibition of PDK3 resulted in fewer trabeculating cells, while the inverse was seen in PDK3 overexpression models. When CMs deficient in pkma2;pkmb, the zebrafish orthologues to the mammalian pyruvate kinase muscle isoenzyme 2 (Pkm2), were transplanted into wildtype embryos, they contributed significantly less to trabeculation than double-het transplants (25). This is notable as, in the zebrafish, the trabeculae are sites of greater proliferation compared to the compact myocardium (97). This differs to the mouse, where proliferation primarily occurs at the base of the trabeculae and compact myocardium (98), with a corresponding inversion of the zebrafish metabolic separation (99).
In recent years, metabolism-derived reactive oxygen species (ROS) have emerged as a predominant factor in CM mitotic arrest. Puente et al (2015) showed that mitotic capacity was also inversely correlated with oxidative DNA damage and the DNA damage response (DDR) (22) suggesting that this is the link between oxygen and CM proliferation. They showed the postnatal surge in mitochondrial biogenesis led to this pattern of oxidative damage (22). The electron transport chain is prone to the premature exit, or leakage, of electrons prior to transferal to complex IV, generating superoxides that rapidly dismutate to H2O2 (29). Though this species is not particularly dangerous to cellular integrity itself, it can react with Fe2+ and Fe3+ in metalloenzymes to generate highly reactive OH∙ radicals, which can cause DNA strand breaks (29). Much like in zebrafish, immediately after birth the mouse heart has relatively little mitochondrial content and complexity, and the low oxidative capacity implies low ROS levels. In mice, ROS generators lead to early cell cycle arrest, while both global and mitochondria-specific scavenging of ROS prolongs the neonatal proliferative window (22). Moreover, pharmacological inhibition of Wee1 kinase, an integral mediator of the DDR, also led to a protracted period of proliferative activity (22). In combination with the fact that several ROS scavenging species and enzymes do not significantly increase postnatally (22, 100), the apparent takeaway from these findings is that the metabolic switch can mediate cell cycle arrest via inducing the DDR. Instead of an apoptotic outcome, as is typical of the DDR, it is possible that polyploidization occurs to preserve the CMs during oxidative stress, which has been shown to induce in vitro CM binucleation through a positive feedback loop with p38 MAPK and mitochondrial connexin 43 (101).
Whether this applies to human CM maturation remains somewhat unclear. A study in human CMs showed that DNA damage does not correlate with age-associated changes in cell cycle activity (102), although it should be noted that the authors only looked at cell cycle markers as proxies for proliferation, so the actual mitotic activity in their CMs is not known. Postnatal ROS surging may be sufficient to irreversibly arrest mitosis in the majority of CMs, regardless of future endoreduplicative cycling. However, other work has shown human CM proliferation to continue during the first 10 years of life (5, 103) despite postnatal oxidative DNA damage apparently peaking around 7 to 12 months (102). An alternate explanation suggests that the number of cycles mammalian CMs undergo is fixed. As many species’ average CM size is similar, larger mammals require more cycles. After all, perinatal cardiac injury ultimately only replaces the lost tissue (7) and blockade of perinatal hormonal and metabolic maturation cannot prolong CM proliferation indefinitely, which might suggest that CM cycles are preprogrammed.
More recently, this model has been expanded in terms of oxidative substrate usage. Cardoso et al (2020) first showed that fatty acid restriction to mouse pups extended the neonatal proliferative window, associated with lower ROS content (23). This suggests that circulating fatty acids are an important trigger for CMs to exit the cell cycle. The effect was not maintained at 10 weeks of life, at which point hepatomegaly, hepatic steatosis, and upregulation of fatty acid-biosynthesis pathways indicated that endogenous fatty acid synthesis had increased to compensate for fatty acid restriction (23). Fatty acid oxidation is maintained by the presence of free fatty acids due to the Randle cycle, whereby fatty acid-derived acetyl-CoA inhibits PDH (104). Thus, endogenous fatty acid synthesis could induce this mechanism in fatty acid–restricted CMs, in turn shutting down the cell cycle. Both glucocorticoids (72) and TH (20) promote beta-oxidation and OXPHOS during neonatal development, meaning this may represent a downstream mechanism for them to induce exit of the cell cycle. Indeed, TH and fatty acids promote PDK4 activity during neonatal development, suggesting that it is a key part of the metabolic switch (54), and Cardoso showed that conditional knockout of PDK4 decreased CM volume, DNA damage, and DDR markers (23).
Metabolism in Regenerating Adult Heart Muscle
Recapitulation of the heart’s fetal metabolic phenotype may benefit the proliferative capacity in adulthood. Nakada et al’s mice exposed to the gradual reduction of oxygen tension—down to the equivalent of the summit of Mt Everest (7% oxygen tension for 2 weeks) – showed cardiac cell cycle reentry, an effect reversed by administration of pharmacological ROS generator, and improved recovery post-MI (24). However, hypoxia was poorly tolerated overall, even less so by the MI than the sham group, resulting in a bias toward smaller infarcts in the former. This suggests their cell cycle measurements may not indicate full mitosis. Indeed, a suggested hallmark of pathological hypertrophy is an increase in cardiomyocyte ploidy (105, 106). As such, it is unclear whether studies reporting proliferation are assessing only full division. Similarly, moderately hypoxic blood saturation (85% to 90% O2 saturation) in the ventricular outflow myocardia of pressure-matched patients was associated with decreased mitochondrial and nuclear oxidative DNA damage, as well as a significant increased cell cycle activity (107), but actual CM division cannot be derived from this study. Furthermore, some findings show that hypoxia can induce mammalian cardiac polyploidy (108).
Contrast this with the adult zebrafish, for which ventricular amputation is more clearly associated with hypoxia during regeneration, which can also be blocked by hyperoxia or HIF1a loss of function (109). Single cell RNA sequencing of proliferating injury-bordering cells and remote, nonproliferating cells shows a distinct recapitulation of fetal metabolism in the former—not unlike the compartmentalization that occurs during trabeculation. After cryoinjury, remote cardiomyocytes displayed a typical OXPHOS program, while border zone cells relied on glycolysis and anaerobic lactate utilization (26). Regeneration could not progress without glycolysis, which could be enhanced by Erbb2 overexpression (26). This illustrates that metabolic remodeling is a component of regeneration and that one of the chief differences in cardiac regeneration between mammals and zebrafish is tolerance of prolonged aerobic metabolism. CM mitosis also involves deconstruction of sarcomeres during cytokinesis (110), required due to their steric hindrance of mitotic architecture. Low-blood pressure organisms like zebrafish can tolerate these impacts but for mammals it is deleterious. Indeed, induction of regenerative pathways in pig hearts can induce serious, even fatal, physiological dysfunction in the heart (111).
This phylogenetic deviation suggests that one of the biggest obstacles is inducing the cell cycle as part of mitosis, and not endoreduplication, in mammalian CMs. The increase in CM nuclear volume during cardiac remodeling (105, 106) is associated with metabolic and hormonal alterations somewhat akin to those of the fetal heart. While variation is seen based on etiology and stage of the pathology, beta and glucose oxidation typically decline, while glycolysis is preferred due to contributions to anaplerosis (112). Concordantly, this is often associated with a hypothyroid heart (113), which has been linked to reduced TR expression (113), and low serum T3 levels (114). Noncanonical activity of TRα1, in the absence of T3 and attenuated by the action of TRα2 and TRβ1, can activate a p38 MAPK pathway to induce hypertrophy (115). However, it is worth noting that much of the literature that infers ploidy from increased nuclear size, particularly in fixed tissue sections. Assessment of polyploidy by 4′,6-diamidino-2-phenylindole (DAPI) staining, the stoichiometric DNA binding of which means fluorescent intensity measurements correlate to DNA content, in CMs isolated from pressure- or volume-overloaded rat hearts showed no significant changes in ploidy (116), while findings from the same group indicate that nuclear hypertrophy accounts for increased nuclear volume in cellular hypertrophy (117). Magnitude and duration of hypertrophy also had no influence (116). As such, the metabolic and hormonal alterations that occur during hypertrophy may not lead to endoreduplication.
Nonetheless, there is evidence that certain metabolic pathways are beneficial for cardiac regeneration. For one, if anaerobic metabolism enhances CM mitotic competency, that there are some mononucleated CMs in adult mammalian hearts suggests that such a persistent population may remain in relative hypoxia. Lineage tracing experiments have corroborated this. Crossing of mice expressing the oxygen degradation domain of HIF1α fused to a tamoxifen-inducible Cre recombinase with a Rosa26 floxed-stop tdTomato line generated pups with fluorescently tagged hypoxic CMs (118). These CMs more closely resembled fetal CMs, being smaller in size, ~50% mononucleated, and having lower DNA damage and cell cycle gene enrichment (118). Hypoxic CMs were able to clonally expand within the myocardium, contributing ~0.3% to 1% to new CMs per year (118). Some papers have shown increased Aurora B Kinase, which associates with the mitotic spindle and thus is a better cytokinetic indicator than cell cycle or DNA replication markers, in glycolytic conditions (22, 24). Gain and loss of function experiments in rat hearts showed Pkm2 to promote regeneration after MI by direct interaction with the pro-regeneration beta-catenin pathway. Ectopic genetic expression of Pkm2 in CMs activated glucose-6-phosphate dehydrogenase, increasing flux through the pentose phosphate pathway, expanding nucleotide pools, and reducing ROS generation (119), illustrating the importance of metabolic alterations alongside mitotic signals during regeneration. Such nodes that integrate both pathways may be crucial targets for clinical settings.
Consistent with findings that suggest that glucose coupling can reduce the severity of cardiac injury (112), Cardoso et al (2020) showed that deletion of PDK4 led to modestly improved recovery from MI (23). This correlated with increased CMs positive for Aurora B Kinase (23). Additionally, the authors used a lineage tracing strategy to specifically mark newly generated daughter CMs; 14 days’ administration of dichloroacetate, a PDK inhibitor, led to significantly higher generation of daughter cells (23). Given that chronic glycolysis activation can promote the progression of heart failure with preserved ejection fraction (112), the finding that coupling it with oxidation may promote cytokinesis is notable. Moreover, PDK4 is one of many metabolic factors regulated by TH, glucocorticoids, and associated PGC-1α pathways (120), suggesting a role for them as upstream signals. As such, much like in development, metabolic remodeling may be a component in hormonal regulation of the CM regenerative status. Indeed, RNA sequencing and chromatin immunoprecipitation sequencing data from Hirose et al’s TRα mutant mice, which possessed a 10-fold increase in proliferating, diploid CMs and better functional recovery after ischemic injury, showed a significant downregulation of Krebs cycle, OXPHOS, and mitochondrial genes (20). Specifically, expression of carnitine palmitoyltransferase 2 (Cpt2), the rate-limiting importer of long-chain fatty acids into the mitochondria, was 60% reduced in TRα mutant hearts. Heterozygous Cpt2 mutants did display modestly enhanced CM proliferative activity, albeit lesser than in the TRα mutants (20). This may also partially explain the difference in findings on the effects of TH on CM proliferation. Overall, investigating the interactions of metabolism and hormonal alterations may uncover targets and mechanisms to induce endogenous regeneration without fatal loss of cardiac function.
Conclusion
Clinical studies on cardiac regeneration thus far have mostly explored use of stem cell therapies, but these are costly. They also pose logistic and biological challenges, such as transport and storage, targeting, low cell survival, immune rejection, and tumorigenicity (121). Although they are still preclinical, cell-free approaches, such as pharmacological or gene therapies, may be used to stimulate endogenous regenerative pathways and thus circumvent the above issues. Manipulation of hormonal pathways may be attractive, in part due to pre-existing drugs, as well as pleiotropic effects, including modulation of CM metabolism.
The endocrine system coordinates and integrates numerous important processes during vertebrate development, establishing the necessary cellular states required for extrauterine life. In contrast to ectothermic vertebrates, most mammals have high energy demand, which seems to be a tradeoff of regenerative capacity (Fig. 2). Strong evidence shows that this is, at least in part, hormonally induced. However, the same knowledge that indicates this also suggests regenerative pathways in the heart are conserved even in those organisms that are incapable of it. Thus, the metabolism-regeneration tradeoff is not a zero-sum game.
Here, we have discussed only interactions between 2 hormonal factors and metabolism. Other hormones, including vitamin D (122), insulin-like growth factor-1 (65, 66, 123), angiotensin II (124), estrogen (125), and oxytocin (126), have also been suggested to regulate regeneration, and understanding combinatory action is important. TH alone, for instance, does not promote every component of the mitochondrial fatty acid oxidation circuitry (127). Cutie et al investigated TH and glucocorticoids in regulating CM proliferation and observed no combinatory effect (80), but it is still possible that they work together in some other way, particularly due to action on metabolic modulators, such as PGC1α (48, 72). Work is also needed to explain the differences in findings in terms of dosage, timing, and experimental variation for these hormones. Specifically, it is necessary to understand their role in mediating ROS status, subsequent signaling events and the DDR in perinatal CMs, as well as key targets in their direct and indirect genomic effects.
Acknowledgments
Financial Support: This work is supported by NIH (R01HL138456), Department of Defense (W81XWH1910206), American Heart Association Transformative Project Award, UCSF Program for Breakthrough Biomedical Research, Edward Mallinckrodt Jr. Foundation and UCSF Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research Seed Grant (G.N.H.).
Glossary
Abbreviations
- ATP
adenosine triphosphate
- CM
cardiomyocyte
- DDR
DNA damage response
- E#
embryonic day #
- Erbb2
erythroblastosis oncogene B
- GR
glucocorticoid receptor
- HIF
hypoxia-inducible factor
- KLF
Krüppel-like factor
- MI
myocardial infarction
- MR
mineralocorticoid receptor
- OXPHOS
oxidative phosphorylation
- P#
postnatal day #
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator 1-α
- Pkm2
pyruvate kinase muscle isoenzyme 2
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- T3
triiodothyronine
- T4
thyroxine
- TH
thyroid hormone
- TR
thyroid hormone receptor
Additional Information
Disclosures: The authors have no conflicts of interest to declare. All figures created with Biorender.com.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Jagannathan R, Patel SA, Ali MK, Narayan KMV. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr Diab Rep. 2019;19(7):44. [DOI] [PubMed] [Google Scholar]
- 2. Karsner HT, Saphir O, Todd TW. The State of the Cardiac Muscle in Hypertrophy and Atrophy. Am J Pathol. 1925;1(4):351-372.1. [PMC free article] [PubMed] [Google Scholar]
- 3. Bergmann O, Zdunek S, Alkass K, Druid H, Bernard S, Frisén J. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp Cell Res. 2011;317(2):188-194. [DOI] [PubMed] [Google Scholar]
- 4. Kajstura J, Rota M, Cappetta D, et al. . Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126(15):1869-1881. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Mollova M, Bersell K, Walsh S, et al. . Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110(4):1446-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Senyo SE, Steinhauser ML, Pizzimenti CL, et al. . Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Porrello ER, Mahmoud AI, Simpson E, et al. . Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haubner BJ, Adamowicz-Brice M, Khadayate S, et al. . Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY). 2012;4(12):966-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zogbi C, Saturi de Carvalho AET, Nakamuta JS, et al. . Early postnatal rat ventricle resection leads to long-term preserved cardiac function despite tissue hypoperfusion. Physiol Rep. 2014;2(8):e12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bryant DM, O’Meara CC, Ho NN, Gannon J, Cai L, Lee RT. A systematic analysis of neonatal mouse heart regeneration after apical resection. J Mol Cell Cardiol. 2015;79:315-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konfino T, Landa N, Ben-Mordechai T, Leor J. The type of injury dictates the mode of repair in neonatal and adult heart. J Am Heart Assoc. 2015;4(1):e001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188-2190. [DOI] [PubMed] [Google Scholar]
- 13. Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28(8):1737-1746. [DOI] [PubMed] [Google Scholar]
- 14. Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li F, Wang X, Bunger PC, Gerdes AM. Formation of binucleated cardiac myocytes in rat heart: I. Role of actin-myosin contractile ring. J Mol Cell Cardiol. 1997;29(6):1541-1551. [DOI] [PubMed] [Google Scholar]
- 16. Patterson M, Barske L, Van Handel B, et al. . Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet. 2017;49(9):1346-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soonpaa MH, Zebrowski DC, Platt C, Rosenzweig A, Engel FB, Field LJ. Cardiomyocyte Cell-Cycle Activity during Preadolescence. Cell. 2015;163(4):781-782. [DOI] [PubMed] [Google Scholar]
- 18. Bergmann O, Zdunek S, Felker A, et al. . Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161(7):1566-1575. [DOI] [PubMed] [Google Scholar]
- 19. González-Rosa JM, Sharpe M, Field D, et al. . Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev Cell. 2018;44(4):433-446.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirose K, Payumo AY, Cutie S, et al. . Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019;364(6436):184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vivien CJ, Hudson JE, Porrello ER. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen Med. 2016;1:16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Puente BN, Kimura W, Muralidhar SA, et al. . The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157(3):565-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cardoso AC, Lam NT, Savla JJ, et al. . Mitochondrial Substrate Utilization Regulates Cardiomyocyte Cell Cycle Progression. Nat Metab. 2020;2(2):167-178. [PMC free article] [PubMed] [Google Scholar]
- 24. Nakada Y, Canseco DC, Thet S, et al. . Hypoxia induces heart regeneration in adult mice. Nature. 2017;541(7636):222-227. [DOI] [PubMed] [Google Scholar]
- 25. Fukuda R, Aharonov A, Ong YT, et al. . Metabolic modulation regulates cardiac wall morphogenesis in zebrafish. Elife. 2019;8. doi: 10.7554/eLife.50161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honkoop H, de Bakker DEM, Aharonov A, et al. . Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. Elife. 2019;8. doi: 10.7554/eLife.50163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lopaschuk GD, Spafford MA. Energy substrate utilization by isolated working hearts from newborn rabbits. Am J Physiol. 1990;258(5 Pt 2):H1274-H1280. [DOI] [PubMed] [Google Scholar]
- 28. Goffart S, von Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: power-plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Res. 2004;64(2):198-207. [DOI] [PubMed] [Google Scholar]
- 29. Pohjoismäki JL, Goffart S. The role of mitochondria in cardiac development and protection. Free Radic Biol Med. 2017;106:345-354. [DOI] [PubMed] [Google Scholar]
- 30. Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med. 2011;50(7):777-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karbassi E, Fenix A, Marchiano S, et al. . Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 2020;17(6):341-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patterson AJ, Zhang L. Hypoxia and Fetal Heart Development. Curr Mol Med. 2010; 10(7):653-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elphick MC, Hudson DG, Hull D. Transfer of fatty acids across the rabbit placenta. J Physiol. 1975;252(1):29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knopp RH, Warth MR, Charles D, et al. . Lipoprotein metabolism in pregnancy, fat transport to the fetus, and the effects of diabetes1. Neonatology. 1986;50(6):297-317. [DOI] [PubMed] [Google Scholar]
- 35. Mackler B, Grace R, Duncan HM. Studies of mitochondrial development during embryogenesis in the rat. Arch Biochem Biophys. 1971;144(2):603-610. [DOI] [PubMed] [Google Scholar]
- 36. Cox SJ, Gunberg DL. Metabolite utilization by isolated embryonic rat hearts in vitro. J Embryol Exp Morphol. 1972;28(2):235-245. [PubMed] [Google Scholar]
- 37. Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56:130-140. [DOI] [PubMed] [Google Scholar]
- 38. Bartelds B, Knoester H, Smid GB, et al. . Perinatal changes in myocardial metabolism in lambs. Circulation. 2000;102(8):926-931. [DOI] [PubMed] [Google Scholar]
- 39. Piquereau J, Novotova M, Fortin D, et al. . Postnatal development of mouse heart: formation of energetic microdomains. J Physiol. 2010;588(Pt 13):2443-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261(6 Pt 2):H1698-H1705. [DOI] [PubMed] [Google Scholar]
- 41. Piquereau J, Ventura-Clapier R. Maturation of Cardiac Energy Metabolism During Perinatal Development. Front Physiol. 2018;9:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lagerspetz KYH. Postnatal development of thermoregulation in laboratory mice. Helgoländer Wissenschaftliche Meeresuntersuchungen. 1966;14(1-4):559-571. [Google Scholar]
- 43. Kannan S, Kwon C. Regulation of cardiomyocyte maturation during critical perinatal window. J Physiol. 2020;598(14):2941-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW II. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015;350(6265):aad2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anmann T, Varikmaa M, Timohhina N, et al. . Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochim Biophys Acta. 2014;1837(8):1350-1361. [DOI] [PubMed] [Google Scholar]
- 46. Grais IM, Sowers JR. Thyroid and the heart. Am J Med. 2014;127(8):691-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Portman MA. Thyroid hormone regulation of heart metabolism. Thyroid. 2008;18(2):217-225. [DOI] [PubMed] [Google Scholar]
- 48. McClure TD, Young ME, Taegtmeyer H, et al. . Thyroid hormone interacts with PPARα and PGC-1 during mitochondrial maturation in sheep heart. Am J Physiol Circ Physiol. 2005;289(5):H2258-H2264. [DOI] [PubMed] [Google Scholar]
- 49. Hönes GS, Rakov H, Logan J, et al. . Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo Published under the PNAS license. Proc Natl Acad Sci U S A. 2017;114(52):E11323-E11332. [DOI] [PMC free article] [PubMed]
- 50. Friedrichsen S, Christ S, Heuer H, et al. . Regulation of iodothyronine deiodinases in the Pax8-/- mouse model of congenital hypothyroidism. Endocrinology. 2003;144(3):777-784. [DOI] [PubMed] [Google Scholar]
- 51. Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. Faseb J. 2012;26(1):397-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Page E, McCallister LP. Quantitative electron microscopic description of heart muscle cells. Application to normal, hypertrophied and thyroxin-stimulated hearts. Am J Cardiol. 1973;31(2):172-181. [DOI] [PubMed] [Google Scholar]
- 53. Orfali KA, Fryer LG, Holness MJ, Sugden MC. Interactive effects of insulin and triiodothyronine on pyruvate dehydrogenase kinase activity in cardiac myocytes. J Mol Cell Cardiol. 1995;27(3):901-908. [DOI] [PubMed] [Google Scholar]
- 54. Sugden MC, Langdown ML, Harris RA, Holness MJ. Expression and regulation of pyruvate dehydrogenase kinase isoforms in the developing rat heart and in adulthood: role of thyroid hormone status and lipid supply. Biochem J. 2000;352(Pt 3):731-738. [PMC free article] [PubMed] [Google Scholar]
- 55. Priestman DA, Donald E, Holness MJ, Sugden MC. Different mechanisms underlie the long-term regulation of pyruvate dehydrogenase kinase (PDHK) by tri-iodothyronine in heart and liver. FEBS Lett. 1997;419(1):55-57. [DOI] [PubMed] [Google Scholar]
- 56. Hyyti OM, Olson AK, Ge M, et al. . Cardioselective dominant-negative thyroid hormone receptor (Δ337T) modulates myocardial metabolism and contractile efficiency. Am J Physiol - Endocrinol Metab. 2008;295(2):E420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Goldenthal MJ, Weiss HR, Marín-García J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol Cell Biochem. 2004;265(1-2):97-106. [DOI] [PubMed] [Google Scholar]
- 58. Chattergoon NN, Giraud GD, Thornburg KL. Thyroid hormone inhibits proliferation of fetal cardiac myocytes in vitro. J Endocrinol. 2007;192(2):R1-R8. [DOI] [PubMed] [Google Scholar]
- 59. Svensson Holm A-CB, Lindgren I, Österman H, Altimiras J. Thyroid hormone does not induce maturation of embryonic chicken cardiomyocytes in vitro. Physiol Rep. 2014;2(12):e12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang X, Rodriguez M, Pabon L, et al. . Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Segar JL, Volk KA, Lipman MH, Scholz TD. Thyroid hormone is required for growth adaptation to pressure load in the ovine fetal heart. Exp Physiol. 2013;98(3):722-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Naqvi N, Li M, Calvert JW, et al. . A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157(4):795-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL, Bergmann O. No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell. 2015;163(4):1026-1036. [DOI] [PubMed] [Google Scholar]
- 64. Gerdes AM, Kriseman J, Bishop SP. Changes in myocardial cell size and number during the development and reversal of hyperthyroidism in neonatal rats. Lab Invest. 1983;48(5):598-602. [PubMed] [Google Scholar]
- 65. Tan L, Bogush N, Naib H, et al. . Redox activation of JNK2α2 mediates thyroid hormone-stimulated proliferation of neonatal murine cardiomyocytes. Sci Rep. 2019;9(1):17731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bogush N, Tan L, Naib H, et al. . DUSP5 expression in left ventricular cardiomyocytes of young hearts regulates thyroid hormone (T3)-induced proliferative ERK1/2 signaling. Sci Rep. 2020;10(1):21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reiss K, Cheng W, Ferber A, et al. . Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci U S A. 1996;93(16):8630-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-Pituitary-Thyroid Axis. Compr Physiol. 2016;6(3):1387-1428. [DOI] [PubMed] [Google Scholar]
- 69. Cruz-Topete D, Oakley RH, Cidlowski JA. Glucocorticoid Signaling and the Aging Heart. Front Endocrinol (Lausanne). 2020;11:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Prosdocimo DA, Anand P, Liao X, et al. . Kruppel-like factor 15 is a critical regulator of cardiac lipid metabolism. J Biol Chem. 2014;289(9):5914-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yoshikawa N, Nagasaki M, Sano M, et al. . Ligand-based gene expression profiling reveals novel roles of glucocorticoid receptor in cardiac metabolism. Am J Physiol Endocrinol Metab. 2009;296(6):E1363-E1373. [DOI] [PubMed] [Google Scholar]
- 72. Rog-Zielinska EA, Craig MA, Manning JR, et al. . Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1α. Cell Death Differ. 2015;22(7):1106-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rog-Zielinska EA, Thomson A, Kenyon CJ, et al. . Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet. 2013;22(16):3269-3282. [DOI] [PubMed] [Google Scholar]
- 74. Birket MJ, Casini S, Kosmidis G, et al. . PGC-1α and reactive oxygen species regulate human embryonic stem cell-derived cardiomyocyte function. Stem Cell Reports. 2013;1(6):560-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lehman JJ, Boudina S, Hausler Banke N, et al. . The transcriptional coactivator PGC-1 is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Hear Circ Physiol. 2008;295(1):185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Duncan JG, Finck BN. The PPARα-PGC-1α Axis Controls Cardiac Energy Metabolism in Healthy and Diseased Myocardium. PPAR Res. Published online November 22, 2007. 2008;2008. doi: 10.1155/2008/253817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106(7):847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gay MS, Dasgupta C, Li Y, Kanna A, Zhang L. Dexamethasone Induces Cardiomyocyte Terminal Differentiation via Epigenetic Repression of Cyclin D2 Gene. J Pharmacol Exp Ther. 2016;358(2):190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gay MS, Li Y, Xiong F, Lin T, Zhang L. Dexamethasone Treatment of Newborn Rats Decreases Cardiomyocyte Endowment in the Developing Heart through Epigenetic Modifications. Plos One. 2015;10(4):e0125033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cutie S, Payumo AY, Lunn D, Huang GN. In vitro and in vivo roles of glucocorticoid and vitamin D receptors in the control of neonatal cardiomyocyte proliferative potential. J Mol Cell Cardiol. 2020;142:126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grivas J, Haag M, Johnson A, et al. . Cardiac repair and regenerative potential in the goldfish (Carassius auratus) heart. Comp Biochem Physiol C Toxicol Pharmacol. 2014;163:14-23. doi: 10.1016/j.cbpc.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lafontant PJ, Burns AR, Grivas JA, et al. . The giant danio (D. aequipinnatus) as a model of cardiac remodeling and regeneration. Anat Rec (Hoboken). 2012;295(2):234-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Flink IL. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat Embryol (Berl). 2002;205(3):235-244. [DOI] [PubMed] [Google Scholar]
- 84. Witman N, Murtuza B, Davis B, Arner A, Morrison JI. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev Biol. 2011;354(1):67-76. [DOI] [PubMed] [Google Scholar]
- 85. Marshall LN, Vivien CJ, Girardot F, et al. . Stage-dependent cardiac regeneration in Xenopus is regulated by thyroid hormone availability. Proc Natl Acad Sci U S A. 2019;116(9):3614-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rees BB, Sudradjat FA, Love JW. Acclimation to hypoxia increases survival time of zebrafish, Danio rerio, during lethal hypoxia. J Exp Zool. 2001;289(4):266-272. [DOI] [PubMed] [Google Scholar]
- 87. Dawes GS, Mott JC, Widdicombe JG. The foetal circulation in the lamb. J Physiol. 1954;126(3):563-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rodgers JL, Jones J, Bolleddu SI, et al. . Cardiovascular Risks Associated with Gender and Aging. J Cardiovasc Dev Dis. 2019;6(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mokdad AH, Ballestros K, Echko M, et al. . The state of US health, 1990–2016: Burden of diseases, injuries, and risk factors among US states. JAMA - J Am Med Assoc. 2018;319(14):1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Crnko S, Du Pré BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. 2019;16(7):437-447. [DOI] [PubMed] [Google Scholar]
- 91. Cuffee Y, Ogedegbe C, Williams NJ, Ogedegbe G, Schoenthaler A. Psychosocial risk factors for hypertension: an update of the literature. Curr Hypertens Rep. 2014;16(10):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Little AG, Seebacher F. The evolution of endothermy is explained by thyroid hormone-mediated responses to cold in early vertebrates. J Exp Biol. 2014;217(Pt 10):1642-1648. [DOI] [PubMed] [Google Scholar]
- 93. Breckenridge RA, Piotrowska I, Ng KE, et al. . Hypoxic regulation of hand1 controls the fetal-neonatal switch in cardiac metabolism. Plos Biol. 2013;11(9):e1001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Guimarães-Camboa N, Stowe J, Aneas I, et al. . HIF1α Represses Cell Stress Pathways to Allow Proliferation of Hypoxic Fetal Cardiomyocytes. Dev Cell. 2015;33(5):507-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu J, Bressan M, Hassel D, et al. . A dual role for ErbB2 signaling in cardiac trabeculation. Development. 2010;137(22):3867-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rasouli SJ, Stainier DYR. Regulation of cardiomyocyte behavior in zebrafish trabeculation by Neuregulin 2a signaling. Nat Commun. 2017;8:15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Uribe V, Ramadass R, Dogra D, et al. . In vivo analysis of cardiomyocyte proliferation during trabeculation. Development. Published online July 30, 2018;145(14):dev164194. doi:10.1242/dev.164194 [DOI] [PubMed] [Google Scholar]
- 98. Park DS, Tompkins RO, Liu F, et al. . Pocket proteins critically regulate cell cycle exit of the trabecular myocardium and the ventricular conduction system. Biol Open. 2013;2(9):968-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Menendez-Montes I, Escobar B, Palacios B, et al. . Myocardial VHL-HIF Signaling Controls an Embryonic Metabolic Switch Essential for Cardiac Maturation. Dev Cell. 2016;39(6):724-739. [DOI] [PubMed] [Google Scholar]
- 100. de Haan JB, Tymms MJ, Cristiano F, Kola I. Expression of copper/zinc superoxide dismutase and glutathione peroxidase in organs of developing mouse embryos, fetuses, and neonates. Pediatr Res. 1994;35(2):188-196. [DOI] [PubMed] [Google Scholar]
- 101. Matsuyama D, Kawahara K. Oxidative stress-induced formation of a positive-feedback loop for the sustained activation of p38 MAPK leading to the loss of cell division in cardiomyocytes soon after birth. Basic Res Cardiol. 2011;106(5):815-828. [DOI] [PubMed] [Google Scholar]
- 102. Huang Y, Hong H, Li M, et al. . Age-Dependent Oxidative DNA Damage Does Not Correlate with Reduced Proliferation of Cardiomyocytes in Humans. Yutzey K, ed. PLoS One. 2017;12(1):e0170351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bergmann O, Bhardwaj RD, Bernard S, et al. . Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785-789. [DOI] [PubMed] [Google Scholar]
- 105. Brodsky VY, Arefyeva AM, Gvasava IG, Sarkisov DS, Panova NW. Polyploidy in cardiac myocytes of normal and hypertrophic human hearts; range of values. Virchows Arch. 1994;424(4):429-435. [DOI] [PubMed] [Google Scholar]
- 106. Herget GW, Neuburger M, Plagwitz R, Adler CP. DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc Res. 1997;36(1):45-51. [DOI] [PubMed] [Google Scholar]
- 107. Ye L, Qiu L, Feng B, et al. . Role of Blood Oxygen Saturation During Post-Natal Human Cardiomyocyte Cell Cycle Activities. JACC Basic Transl Sci. 2020;5(5):447-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jiang YH, Zhu Y, Chen S, et al. . Re-enforcing hypoxia-induced polyploid cardiomyocytes enter cytokinesis through activation of β-catenin. Sci Rep. 2019;9(1):17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jopling C, Suñé G, Faucherre A, Fabregat C, Izpisua Belmonte JC. Hypoxia induces myocardial regeneration in zebrafish. Circulation. 2012;126(25):3017-3027. [DOI] [PubMed] [Google Scholar]
- 110. Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gabisonia K, Prosdocimo G, Aquaro GD, et al. . MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019;569(7756):418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tran DH, Wang ZV. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J Am Heart Assoc. 2019;8(12):e012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kinugawa K, Yonekura K, Ribeiro RC, et al. . Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res. 2001;89(7):591-598. [DOI] [PubMed] [Google Scholar]
- 114. Franklyn JA, Gammage MD, Ramsden DB, Sheppard MC. Thyroid status in patients after acute myocardial infarction. Clin Sci (Lond). 1984;67(6):585-590. [DOI] [PubMed] [Google Scholar]
- 115. Kinugawa K, Jeong MY, Bristow MR, Long CS. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor alpha1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol Endocrinol. 2005;19(6):1618-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kellerman S, Moore JA, Zierhut W, Zimmer HG, Campbell J, Gerdes AM. Nuclear DNA content and nucleation patterns in rat cardiac myocytes from different models of cardiac hypertrophy. J Mol Cell Cardiol. 1992;24(5):497-505. [DOI] [PubMed] [Google Scholar]
- 117. Gerdes AM, Liu Z, Zimmer HG. Changes in nuclear size of cardiac myocytes during the development and progression of hypertrophy in rats. Cardioscience. 1994;5(3):203-208. [PubMed] [Google Scholar]
- 118. Kimura W, Xiao F, Canseco DC, et al. . Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523(7559):226–230. [DOI] [PubMed] [Google Scholar]
- 119. Magadum A, Singh N, Kurian AA, et al. . Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation. 2020;141(15):1249-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab J. 2012;36(5):328-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tang JN, Cores J, Huang K, et al. . Concise Review: Is Cardiac Cell Therapy Dead? Embarrassing Trial Outcomes and New Directions for the Future. Stem Cells Transl Med. 2018;7(4):354-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Han Y, Chen A, Umansky KB, et al. . Vitamin D Stimulates Cardiomyocyte Proliferation and Controls Organ Size and Regeneration in Zebrafish. Dev Cell. 2019;48(6):853-863.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huang Y, Harrison MR, Osorio A, et al. . Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. Hsieh PC, ed. PLoS One. 2013;8(6):e67266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ludwig M, Steinhoff G, Li J. The regenerative potential of angiotensin AT2 receptor in cardiac repair. Can J Physiol Pharmacol. 2012;90(3):287-293. [DOI] [PubMed] [Google Scholar]
- 125. Xu S, Xie F, Tian L, et al. . Estrogen accelerates heart regeneration by promoting the inflammatory response in zebrafish. J Endocrinol. 2020;245(1):39-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jankowski M, Gonzalez-Reyes A, Noiseux N, Gutkowska J. Oxytocin in the heart regeneration. Recent Pat Cardiovasc Drug Discov. 2012;7(2):81-87. [DOI] [PubMed] [Google Scholar]
- 127. Marín-García J. Thyroid hormone and myocardial mitochondrial biogenesis. Vascul Pharmacol. 2010;52(3-4):120-130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.