Keywords: breathing control, desensitization, opioid
Abstract
We have investigated the potential acute desensitizing role of the β arrestin 2 (β-arr2) pathway on the ventilatory depression produced by levels of fentanyl ranging from analgesic to life-threatening (0.1 to 60 mg/kg ip) in control and β-arr2-deficient nonsedated mice. Fentanyl at doses of 0.1, 0.5, and 1 mg/kg ip—corresponding to the doses previously used to study the role of β-arr2 pathway—decreased ventilation, but along the V̇e/V̇co2 relationship established in baseline conditions. This reduction in ventilation was therefore indistinguishable from the decrease in breathing during the periods of spontaneous immobility. Above 1.5 mg/kg, however, ventilation was depressed out of proportion of the changes in metabolic rate, suggesting a specific depression of the drive to breathe. The ventilatory responses were similar between the two groups. At high doses of fentanyl (60 mg/kg ip) 1 out of 20 control mice died by apnea versus 8 out of 20 β-arr2-deficient mice (P = 0.008). In the surviving mice, ventilation was however identical in both groups. The ventilatory effects of fentanyl in β-arr2-deficient mice, reported in the literature, are primarily mediated by the “indirect” effects of sedation/hypometabolism on breathing control. There was an excess mortality at very high doses of fentanyl in the β-arr2-deficient mice, mechanisms of which are still open to question, as the capacity of maintaining a rhythmic, although profoundly depressed, breathing activity remains similar in all of the surviving control and β-arr2-deficient mice.
NEW & NOTEWORTHY When life-threatening doses of fentanyl are used in mice, the β-arrestin 2 pathway appears to play a critical role in the recovery from opioid overdose. This observation calls into question the use of G protein-biased μ-opioid receptor agonists, as a strategy for safer opioid analgesic drugs.
INTRODUCTION
Following a central apnea produced by a rapid intravenous injection of very high doses of fentanyl (up to 300 μg/kg iv) in the rat, breathing activity spontaneously resumes within 1–2 min (1, 2). This emerging regular respiratory activity consists of a severely reduced tidal volume and breathing frequency that can be maintained for very long periods of time, in turn preventing a hypoxic cardiac arrest. An intriguing characteristic of this severely depressed pattern of breathing is that it has become virtually resistant to a new injection of high-dose fentanyl (2). Several conceivable hypotheses could be proposed to account for the mechanisms behind the generation and the maintenance of such an “opioid-insensitive” respiratory rhythm, when μ-opioid receptor occupancy is very high. First, networks of medullary inspiratory neurons already opioid insensitive (3–5) could have taken over the entire respiratory rhythm, whenever populations of respiratory neurons equipped with μ opioid receptors, in and outside the pre-Bötzinger complex, have become incapable of being depolarized. Alternatively, a subset of respiratory neurons, critical for breathing generation could have been rapidly desensitized, resuming their activity despite a persistent μ-opioid receptor high occupancy. The β arrestin 2 (β-arr2)/G-protein-coupled receptor (GPCR) kinase 2 (GRK2) pathway represents a very rapid mechanism of desensitization of G-protein-coupled receptors (6–9) and therefore of opioid receptors. The inhibition of such a pathway has been shown to produce an enhanced analgesic effect following opioid administration (10, 11); such an approach represents a potential new paradigm for treating pain with opioids (12, 13) while reducing the risk for narcotic tolerance and dependence. Our ability to alter the β arrestin 2 (β-arr2)/GPCR kinase 2 (GRK2) pathway would obviously be limited if opioid-induced breathing depression were also mitigated by the β-arr2 pathway, potentiating in turn opioid-induced ventilatory depression. The contribution of the GRK2/β-arr2 pathway induced μ-opioid desensitization and receptor internalization to opioid overdose-induced hypoventilation remains poorly understood (10, 11, 14, 15). Different mouse models of deficit in the GRK2/β-arr2 pathway have been used to determine its potential contribution to the ventilatory response to opioids, showing discrepant results with no consistent differences between control and deficient mice ventilation (10, 11, 14, 15). One of the major limitations of all of these studies is that they used doses of opioids, morphine or fentanyl, that were mostly analgesic/sedative, well below the doses producing life-threatening intoxications. Indeed, as detailed in discussion, doses able to produce a severe, or even potentially lethal, ventilatory depression typically range from 10 mg/kg to 50 mg/kg of fentanyl (16) in mice, two to three orders of magnitude greater than the doses used in all these studies. In addition, breathing was never expressed as a function of the metabolic or pulmonary gas exchange rate. As the level of ventilation in mice is primarily dictated by the changes in their very high metabolic rate, a fundamental mechanism of regulation critical in small mammals (17–19), opioid-induced hypoventilation could certainly, at moderate doses, be accounted for by a reduction in activity/metabolic rate and not a direct effect of opioids on the medullary respiratory neurons. In other words, the ventilatory depression produced by sedative/analgesic doses of opioids could be explained by the consequences of a reduction in vigilance (20) and metabolic rate (17–19). Consequently, if a direct stimulation of μ-opioid receptors on medullary respiratory neurons is not involved, the premises of using models that are able to identify the contribution of desensitizing opioid mechanisms at the respiratory neuronal level (10, 11, 14, 15) can certainly be challenged. In addition, the effects of the desensitization pathway could be much more critical during a life-threatening opioid overdose and be missed when using a sedative dose in a mouse model. Finally, as fentanyl produces rapid bursts of locomotor activity in mice (21, 22), studying ventilation without separating the phases of locomotion from the periods of rest can be quite misleading.
In the present study, we addressed the hypothesis that fentanyl, at moderate and very high doses, alters ventilation in mice in proportion to the changes in metabolic and locomotor activity. Secondly, we investigated whether the absence of GRK2/β-arr2 pathway magnifies the depressed respiratory rhythm produced by an acute administration of fentanyl at doses ranging from moderate to life-threatening intoxications.
METHODS
Animals and Measurements
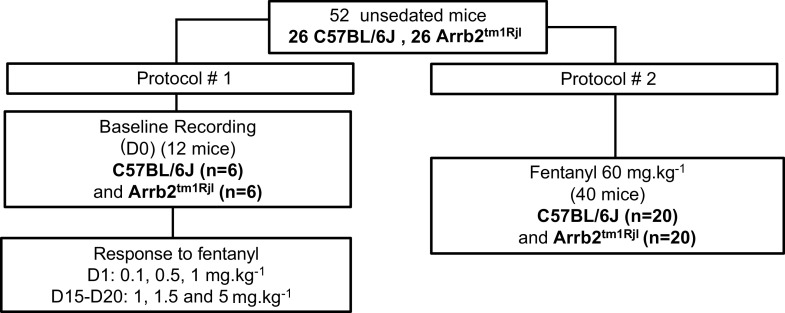
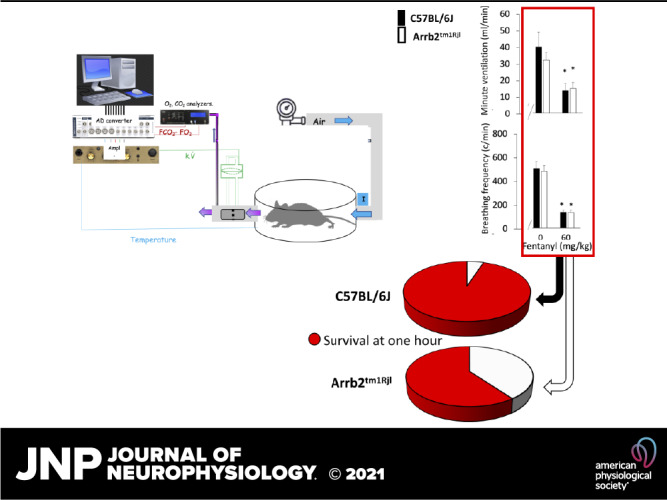
Fifty-two adult male and female control mice (C57BL/6J) and Arrb2 knockout (KO) mice (Arrb2tm1Rjl, Jackson Laboratory) were used in this study. A summary of the protocol along with the number of animals used in each component of the protocol is displayed in Fig. 1. In total, we studied 26 C57BL/6J mice (16 males and 10 females, weight 28.2 ± 5.2 g; age 21 ± 3 wk) and 26 Arrb2tm1Rjl mice (23) (13 males and 13 females; weight 28.4 ± 13.6 g; age 20 ± 6 wk). The description and potential limitations of the Arrb2tm1Rjl mice (Stock No. 011130) obtained through Jackson Laboratory can be found at the following link (https://www.jax.org/strain/011130).
Figure 1.
Number of animals used in each experimental condition. Two different protocols were used: in a first series of tests (protocol 1), a 2-h recording was performed in baseline condition at D0 and data were analyzed for the last hour of recording (see methods for more details). The effects five different dose from 0.1 mg/kg ip to 5 mg/kg ip were investigated on two different days, at D1 and D15–D20. Data were analyzed over the last 15 min after the administration of each dose of fentanyl as well in baseline. Breath-by-breath data were averaged every 5 s, the means and standard deviation data were computed over 15 min, the date presented here consists therefore in 180 data points for each dose. Protocol 2 was performed in two different groups of mice. Ventilation was averaged over the last hour of recording (720 data points).
Each mouse used in the study (both control and Arrb2 tm1Rjl mice) was genotyped (Transnetyx, Cordova, TN). Mouse genotypes from tail biopsies were determined via real-time PCR with specific probes designed for β arrestin 2 gene, using the protocol primer and genotyping database provided by the Jackson Laboratory (https://www.jax.org/Protocol/UrlAsPDF?stockNumber=011130&protocolID=23872).
The absence of β arrestin 2 gene was confirmed in all Arrb2tm1Rjl mice, in contrast to all of our control mice.
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Edition [National Research Council (US) Institute for Laboratory Animal Research]. The Penn State College of Medicine Institutional Animal Care and Use Committee approved the study (PROTO No. 201800353). The mice were housed at the animal resource services at the Penn State College of Medicine, which conforms to the requirements of the US Department of Agriculture and the Department of Health, Education and Welfare. Mice were provided with food and water ad libitum, on a standard 12-h (7 AM to 7 PM) light/dark schedule, under the direct supervision of veterinarians. The personnel involved in the study and the care of the animals received appropriate accreditation by our Institutional Animal Care and Use Committee based on their specific yearly training and experience.
We used a custom-made open flow plethysmographic chamber to determine breath-by-breath respiratory flow and the pulmonary gas exchange rate as previously described (24). The size and shape of the cylindric plethysmographic chamber were designed to minimize the mixing time constant for gas exchange determination, while allowing mice to “circle” in the chamber. A constant flow rate of ambient air (1.2 L/min) was passed through the chamber (24). Respiratory flow (V̇) was determined by a pneumotachograph—connected to a pressure transducer (Sensym, DCLX O1DN, Honeywell)—that was placed in series with the outlet using noncompliant tubing. The fractions of CO2 and O2 were determined from the inlet and outlet ports of the chamber (Model No. 17630 infrared, Vacumed, Ventura, CA and Oxystar-100, CWE). The half time response of the gas exchange measurements, which includes the mixing time of the chamber, the delays, and time constants due to the tubing and the analyzers was ∼12 s. Temperature in the chamber was continuously monitored via a fast-responding thermocouple (Thermalert TH5; Physitemp, Clifton, NJ). The calibration of the flow meter was performed using high-precision rotameters, while linearity of the gas analyzers was established using a 2-points calibration. Calibrations were performed before and after each study; this allowed us to determine that a drift in the CO2 or O2 signals during the studies was always less than 5%. All of the signals were fed into a digital data acquisition system (PowerLab/LabChart; AD Instruments, Colorado Springs, CO). Signals were sampled at 400 Hz, displayed online, and then stored for additional analysis. A high-pass filter (>0.5 Hz) was used to subtract the direct current (DC) component of the flow signal. This “filtered” signal was used for the determination of breathing frequency (f) and estimate minute ventilation (V̇e) as previously described (24, 25). V̇e was obtained from the filtered flow signal by integration of the positive deflections of the flow signal trace that were integrated over 5-s intervals. The result of this integration was then temperature corrected. Due to the complexity of factors involved in quantitative interpretation of the flow signal in an open-flow whole body plethysmograph (26), the determination of V̇e should be seen as a semiquantitative index represented in the same units as are appropriate for direct measurements of ventilation. V̇co2 and V̇o2 were computed in STPD conditions as previously described (24).
Protocol and Data Analysis
Prolonged recordings of baseline conditions.
The 12 mice that were used for protocol 1 (Fig. 1) were placed in the chamber for 2 h without any interference (day 0). The relationship between minute ventilation and V̇co2 was established over the entire last hour of recording.
Effects of fentanyl ip.
Protocol 1.
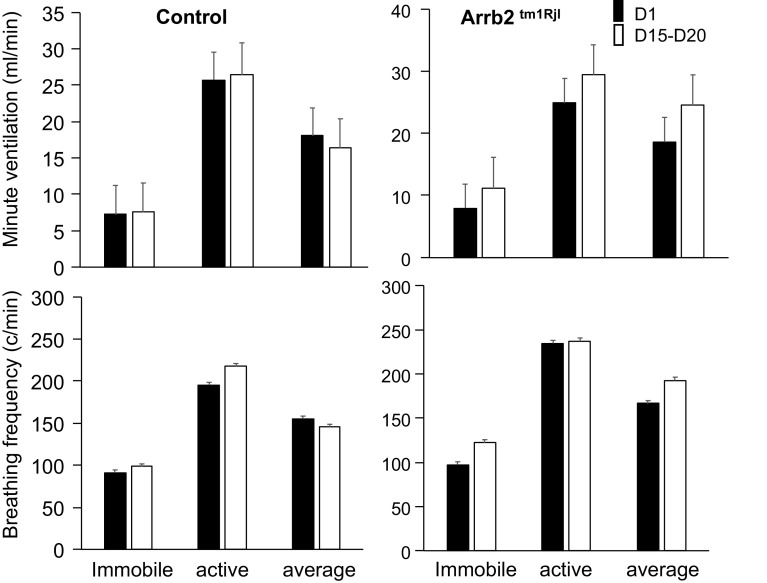
Five different doses of opioid were used: 0.1, 0.5, 1, 1.5, and 5 mg/kg. An equal number of animals (n) was used in each group, that is, control (n = 6) and Arrb2 knockout mice (n = 6) (Fig. 1). The five doses were administered in each of these animals on two different days. The doses 0.1, 0.5, and 1 mg/kg were administered 30 min apart on a given day (D1), whereas the doses of 1, 1.5, and 5 mg/kg were given another day, 15–20 days (D15–D20) apart from the first series of injection. A short period baseline was recorded for 15 min, then the animals were removed from the box, received the first dose of fentanyl intraperitoneally and replaced in the box. The doses 0.1, 0.5, and 1 mg/kg (in this very order) were administered 30 min apart at D1, whereas the doses of 1, 1.5, and 5 mg/kg (in this very order) were given another day at D15–D20 (Fig. 1). The dose of 1 mg/kg was therefore administered twice, once at D1 (as last dose) and then at D15–D20 (as first dose).
All of the animals received naloxone (4 mg ip) at the end of the protocol and were watched every 30 min for the next 6 h, then every 12 h for the next 24 h. None of the animals presented sign of distress, or difficulty eating or ambulating over the next few days.
Protocol 2.
A very large dose, 60 mg/kg, was given in a separate group of Arrb2 knockout mice (n = 20) and control (n = 20). Animals were recorded for 1 h after this unique dose of fentanyl to evaluate early lethality. The rectal temperature of the animals was recorded before and at the end of the protocol. The animals that survived this dose of fentanyl were euthanized 2 h after fentanyl administration by receiving an intraperitoneal injection of a mixture containing barbiturate and phenytoin, that is, Euthasol (at the dose of 150 mg/kg). This method of euthanasia is consistent with the recommendations of the American Veterinary Medical Association guidelines on euthanasia.
Data Analysis
Data were expressed as means ± SD. The relation between V̇e and V̇co2 was established by regression analysis during the last hour of the baseline recording (protocol 1), along with the frequency distribution of minute ventilation. Ventilation was averaged every 10 s, and the frequency distribution was computed for all animals and expressed as percentage of total time spent at any given ventilation.
Protocol 1.
Minute ventilation and breathing frequency were averaged over the last 15 min of recording following each fentanyl administration (12 data/min, corresponding to 180 data point analyzed per dose). In addition, minute ventilation and breathing frequency were also computed during at least 5 periods of immobility and locomotion (when identifiable) for comparison. A cumulative time of 10 min for each condition (immobile vs. circling) was analyzed in each animal. The periods of locomotion and immobility were determined by observing the animals. The distinction between periods of locomotion and rest was only clearly visible above 1 mg/kg and in baseline conditions. Periods during which the animal was not circling but displayed behavioral changes associated with changes in breathing (such as sniffing) were considered as periods of movements or periods of rest.
Nonparametric tests were used to compare the effects of the dose on ventilation and breathing frequency to avoid issues related to the distribution of data. A Friedman test for repeated measurement (paired values) was used to compare the baseline to the different doses up to 5 mg/kg. If significant, a Wilcoxon matched pairs signed-rank test was used to compare each dose to baseline. The effects of 1 mg/kg at D1 were compared with those produced at D15–D20 to determine the presence of desensitization of the response to fentanyl at “low” doses and the effects of prior injection. The comparison between control and Arrb2 knockout mice was done using a Kruskal–Wallis one-way analysis of variance.
Protocol 2.
The effects of 60 mg/kg on the two groups of 20 mice each were compared using a Mann–Whitney U test. Ventilation was averaged over the last hour of recording. A survival curve was established and the occurrence of fatal outcome was computed using a χ2 analysis at 1 h.
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). P < 0.05 was regarded as significant for any of these comparisons.
RESULTS
Prolonged Baseline Studies
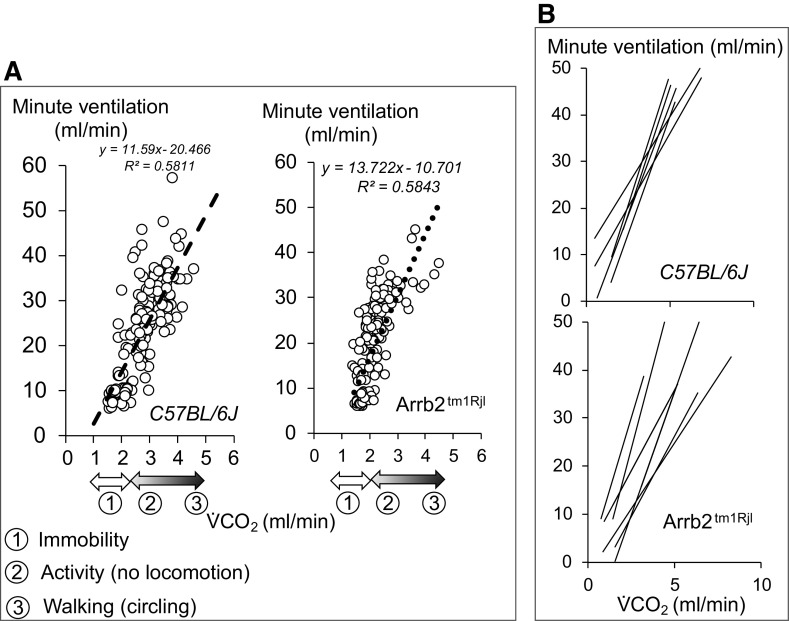
Mice in baseline conditions displayed unpredictable patterns of activity, such as scratching their nose, moving in the chamber, staying motionless for many minutes, or falling asleep. These changes in behavior produced concomitant changes in metabolic rate and ventilation. An example of recording of the instantaneous flow signal is illustrated in Fig. 2 in one mouse. The level of ventilation was significantly correlated to V̇co2 (Fig. 3). The frequency distribution of ventilation and the regression lines of the V̇e/V̇co2 relationships for all mice are given in Figs. 2 and 3, respectively. The range of change in pulmonary gas exchange rate and ventilation, as well as the spontaneous V̇e/V̇co2 relationships were not different between the control and Arrb2tm1Rjl mice as shown in Fig. 3.
Figure 2.
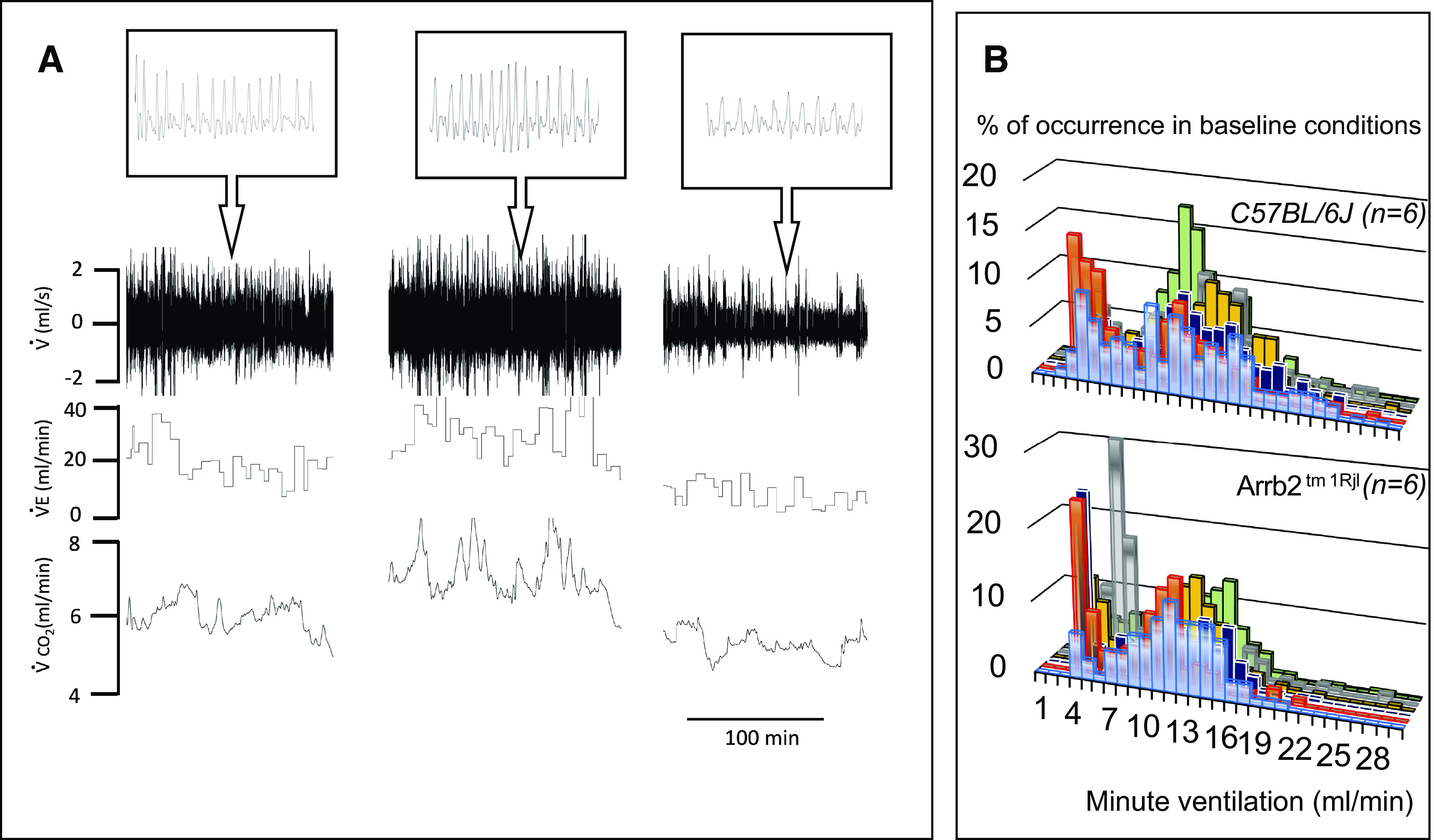
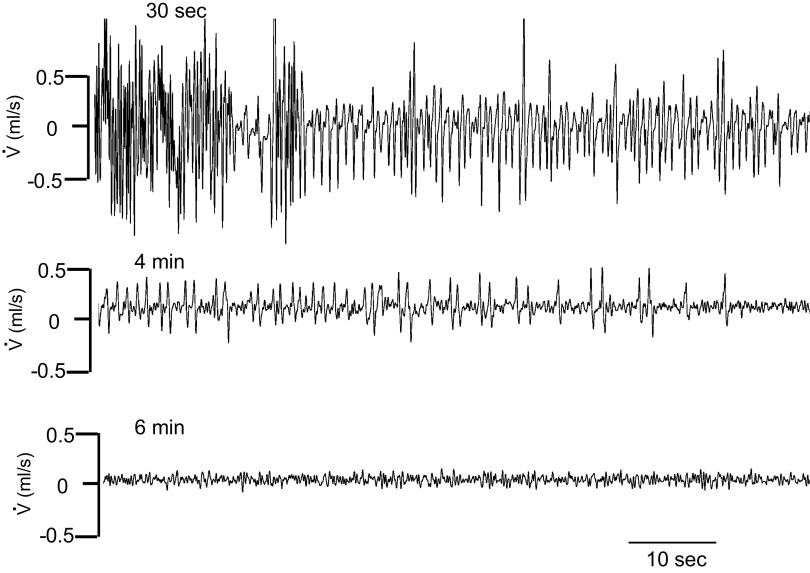
A: example of a recording of baseline spontaneous breathing in one control mouse. The mouse was exposed to dim light and placed in a plethysmographic box for 2 h. Spontaneous instantaneous respiratory flow (V̇), minute ventilation (V̇e), and V̇co2 are shown. Note the spontaneous change in breathing associated with proportional changes in metabolic rate, reflecting fluctuations in the level of vigilance, in behavior and motor activity. B: frequency distribution of ventilation during a 1 h-period of recording in all control (top) and β-arr2-deficient (bottom) mice. Note the first peak corresponds to the lowest levels of ventilation measured when animals were immobile. Arrb2tm1Rjl, Arrb2 knockout mice; β-arr2, β arrestin 2.
Figure 3.
A: relationship between minute ventilation and V̇co2 in the mouse shown in Fig. 2 and in one β-arr2-deficient mouse (data obtained over 1 h). The spontaneous change in breathing was significantly correlated to the metabolic status. B: regression lines obtained in all of the mice (control and β-arr2-deficient mice). Arrb2tm1Rjl, Arrb2 knockout mice; β-arr2, β arrestin 2.
Effects of Fentanyl from 0.1 to 5 mg/kg ip
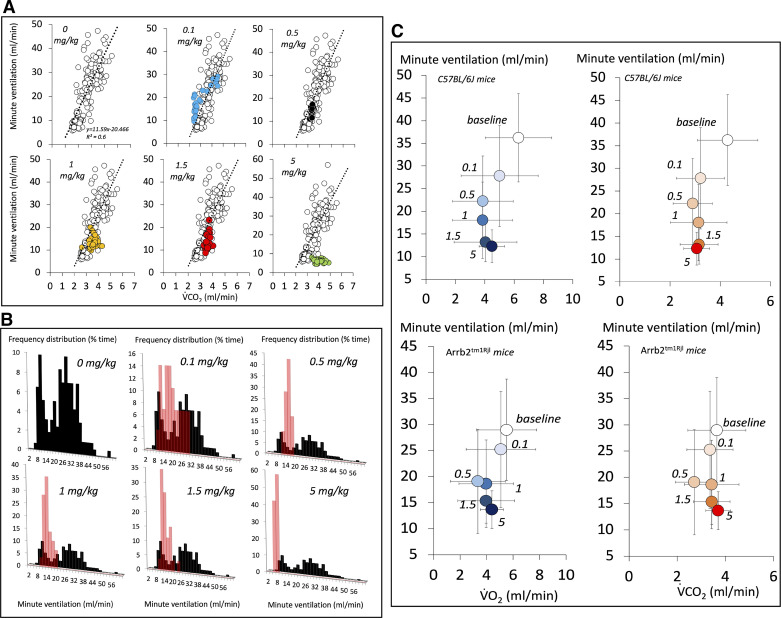
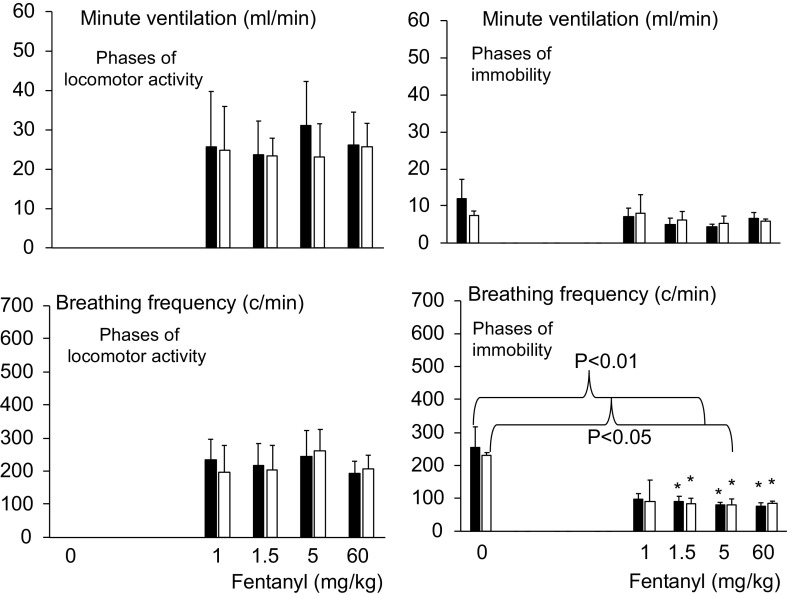
The lowest doses of fentanyl, that is, 0.1, 0.5, and 1 mg/kg, decreased minute ventilation and breathing frequency (Figs. 4, 5, 6, 7, and 8). However, at these doses, minute ventilation was found to fall along the V̇e/V̇co2 relationship established in baseline conditions, as illustrated in Fig. 5. There was no evidence that ventilation was depressed out of proportion of the reduction in metabolic rate produced by a change in behavior/activity. As the doses were increase from 1 mg/kg to 1.5 mg/kg, short and repetitive bouts of locomotor activity (circling) became more and more visible with rhythmic and abrupt transitions between the periods of sedation and activity (Fig. 4). In Figs. 7 and 8, the effects of fentanyl on minute ventilation and breathing frequency were analyzed by either averaging 15 min per recording (Fig. 7) or by separating, when possible, the periods of locomotion and rest (10 min per recording, Fig. 8), as described in methods. Of interest, the effects of opioid on locomotion (circling) led to an average higher level of ventilation after fentanyl than in baseline condition when the animals were immobile. Of note, the identification of the phases of locomotion and immobility was only possible above 1 mg/kg, when a clear rhythmic motor activity could be observed. As shown in Figs. 7 and 8, the periods of immobility were associated with levels of ventilation that were only moderately lower than during the periods of immobility in baseline conditions. Clearly, the ventilatory responses in β-arr2-deficient mice were qualitatively and quantitively similar to those in the control mice. All relevant statistics are given in Figs. 7 and 8. Finally, the administration of 1 mg/kg of fentanyl at D1 following two previous lower doses of fentanyl (0.1 and 0.5 mg/kg) was not different from that at D15–D20, when the dose of 1 mg/kg was administered first, that is, before 1.5 and 5 mg/kg (Fig. 6). Note the lack of difference in the ventilatory response to 1 mg/kg regardless of the timing of fentanyl administration in the control group. In the Arrb2tm1Rjl mice, there was a trend that was not significant for a higher level of minute ventilation and breathing frequency at D15–D20 then at D1 (paired analysis, Wilcoxon test).
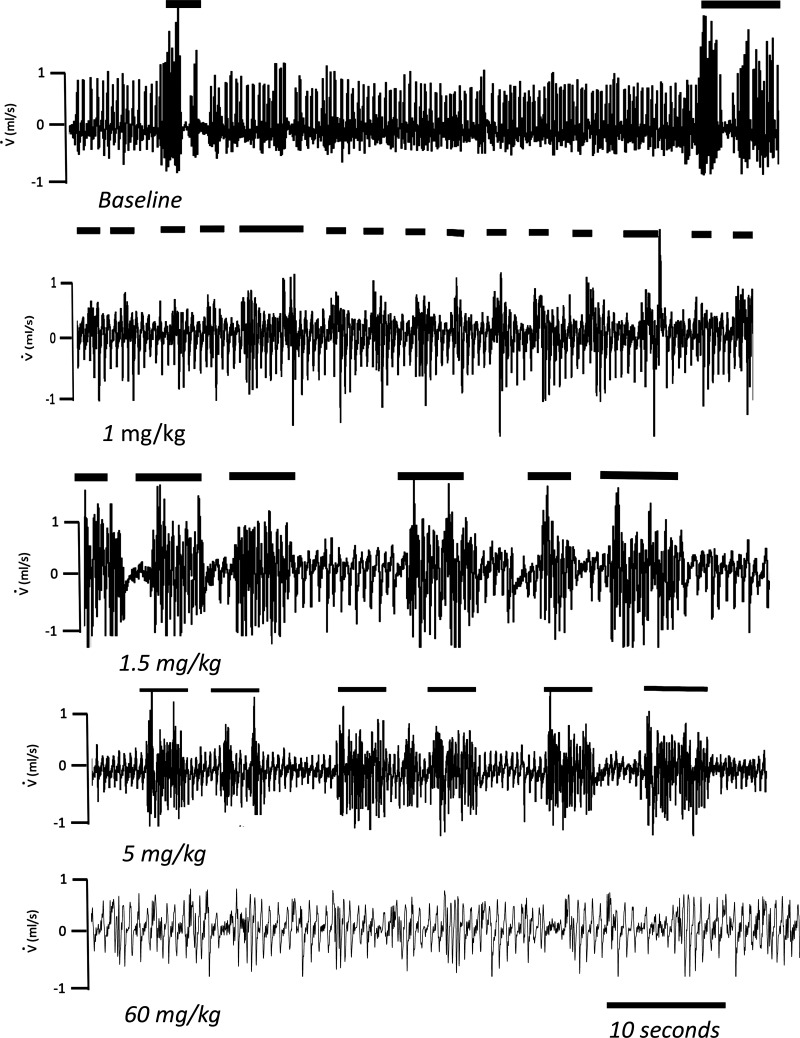
Figure 4.
Examples in two control mice of the effects of an injection of fentanyl (up to 5 mg/kg ip in 1 mouse and at 60 mg/kg ip in another mouse of same weight) on instantaneous respiratory flow (V̇). Note that the depression in breathing produced by fentanyl was “interrupted” by bursts of locomotor activity (horizontal lines) during which ventilation was much higher than in baseline condition when the animal was immobile.
Figure 5.
A: comparison of the V̇e-V̇co2 relationship following fentanyl administration in the control mouse shown in Fig. 2. Note that in up to 1.5 mg/kg dose, this relationship still falls along the regression line established in baseline conditions, whereas at higher fentanyl doses an inhibition of breathing independent of the metabolic rate was observed (see text for more details). Due to the method of average used to compute ventilation, the periods of locomotion and immobility cannot be separated in these figures. B: frequency distribution of ventilation following an injection of fentanyl (from 0.1 mg/kg ip to 5 mg/kg ip) in the control mouse shown in Fig. 2. The frequency distribution of the prolonged baseline data is displayed in black. The effects of fentanyl (up to 1 mg/kg) consisted in a reduction of ventilation (red bars), which overlapped the peak corresponding to the lowest spontaneous levels of ventilation reached when the animals were immobile. Above these concentrations, minute ventilation decreased to levels that were lower than the lowest levels of ventilation reached in baseline breathing. C: average data (means ± SD over 15-min periods) showing the V̇e/V̇o2 and V̇e/V̇co2 relationships before and following fentanyl exposure in keeping with the doses in all of the mice. Arrb2tm1Rjl, Arrb2 knockout mice.
Figure 6.
Minute ventilation and breathing frequency (means ± SD) following the administration of 1 mg/kg of fentanyl at D1 and D15–D20 in the control and β-arr2 deficient animals. At D1, 1 mg/kg fentanyl was administered following two lower doses of fentanyl (0.1 and 0.5 mg/kg), while at D15–D20, 1 mg/kg was administered first (before 1.5 and 5 mg/kg). There was no significant difference between the two injections. Arrb2tm1Rjl, Arrb2 knockout mice; β-arr2, β arrestin 2.
Figure 7.
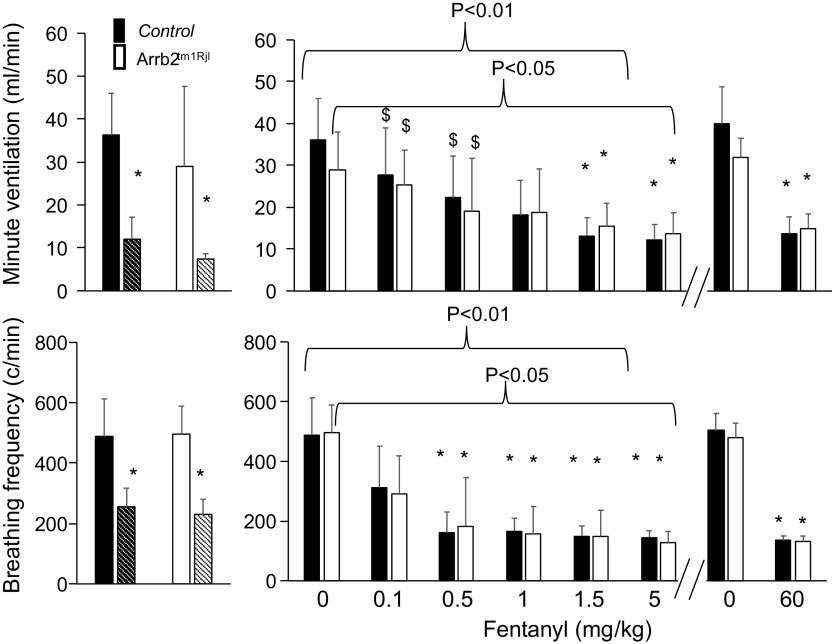
Minute ventilation and breathing frequency (means ± SD) in baseline condition and following fentanyl up to 60 mg/kg in control and in β-arr2-deficient mice. In this figure, data after fentanyl were averaged over a 15-min period without consideration for the animal conditions (locomotion or immobility). The periods of immobility in baseline condition are shown in each group (bars with hatch pattern). Note that 1) the effects of fentanyl on breathing control were similar in both groups, 2) minute ventilation and breathing frequency were more depressed when the animals were immobile in baseline conditions than after the lowest doses of fentanyl. At the highest dose (60 mg/kg), data in the surviving animals are shown. Baseline data used to evaluate the effects of 60 mg/kg were different from those used for the lowest dose (see methods). Despite the same level of minute ventilation in the surviving animals, only 60% of animals of β-arr2-deficient mice survived vs. 95% in the control group (see Fig. 10). *P < 0.05 when compared with baseline, $P < 0.05 when compared with the baseline periods of immobility (bars with hatch pattern). Arrb2tm1Rjl, Arrb2 knockout mice; β-arr2, β arrestin 2.
Figure 8.
Minute ventilation and breathing frequency (means ± SD) in baseline condition and following fentanyl in control and in β-arr2-deficient mice. In contrast to Fig. 7, data obtained during periods of automatic locomotion (circling) and immobility are presented separately. The periods of immobility could be computed in baseline and only above 1 mg/kg fentanyl, there was, however, no period of automatic locomotion in baseline condition. Again, no difference was observed between the two groups. *P < 0.05 when compared with baseline. β-arr2, β arrestin 2.
Effect of 60 mg/kg Fentanyl
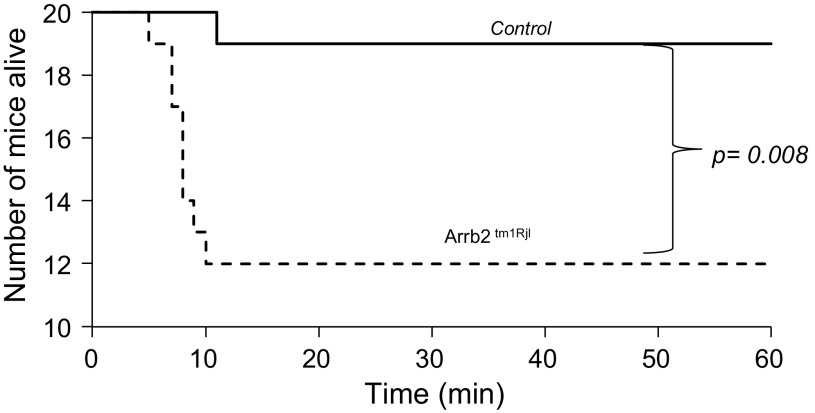
At the highest dose of fentanyl, one control mouse out of 20 presented a fatal central apnea at 7 min (5% mortality), whereas 8 out of 20 Arrb2tm1Rjl mice (40% mortality) presented a fatal apnea (P < 0.008, Figs. 9 and 10) within 12 min. In all of the surviving mice, minute ventilation and breathing frequency reached levels that were no different from those reached after 1.5 or 5 mg/kg, whether the animals were immobile or not and were unchanged by the genetic background of the animals. In all surviving mice, body temperature significantly dropped at 1 h from baseline (Wilcoxon test, P < 0.0001) by 4.1°C ± 1.9°C in the control group (n = 19) versus 3.1°C ± 1.3°C in the Arrb2tm1Rjl mice (n = 12). The difference between the two groups was not significant (Mann–Whitney test, P = 0.215).
Figure 9.
Example of the effects of an injection of the highest dose of fentanyl (60 mg/kg ip) on instantaneous respiratory flow (V̇) in a β-arr2-deficient mouse. This mouse presented a terminal apnea within 5 min. This type of response was observed in 1 out of 20 control mice and 8 out of 20 β-arr2-deficient animals. β-arr2, β arrestin 2.
Figure 10.
Comparison of the survival rate between the groups of animals (n = 20 per group) following the highest doses of fentanyl (60 mg/kg ip). Survival was significantly lower in β-arr2-deficient mice (χ2 test P = 0.008). Note that all animals that died stopped breathing within 10 min. All mice that were alive at 15 min survived the 1-h experiment. Arrb2tm1Rjl, Arrb2 knockout mice.
DISCUSSION
We found that at the doses of opioid previously used in the literature (10, 11, 14, 15) to study the role of β-arr2 pathway on breathing control—i.e., up to 1 mg/kg ip fentanyl or equivalent—opioid-induced breathing depression is indistinguishable from the effects of immobility-induced hypometabolism in baseline conditions. At higher doses, breathing depression becomes independent of the metabolic depression. The responses were identical in control and β-arr2 deficient mice. However, when life-threatening fentanyl concentrations were used, lethality was higher in β-arr2-deficient mice than in control mice.
Opioid-Induced Ventilatory Depression in Mice with a Deficit in the GRK2/β-arr2 Pathway
The study published by Raehal et al. in 2005 (11) was one of the first to look at the ventilatory effects of an acute administration of opioid in β-arr2-deficient mice using analgesic concentrations of morphine (5–20 mg/kg sc), which would correspond to ∼0.02–0.08 mg/kg fentanyl (27). They found that the depression in breathing that was produced in control mice was virtually abolished in β-arr2-deficient animals, whereas the analgesic response was enhanced and prolonged. Neither the change in metabolic rate nor the activity were determined in keeping with the level of breathing that was reported in these animals. This apparently counterintuitive effect was proposed to be accounted for by the fact that, when activated, μ-opioid receptors, like any other G-protein-coupled receptor, bind β-arr2 proteins, in turn activating β-arr2-dependent signaling or ligand bias pathway (12, 13), independently of its desensitizing effect. As a consequence, β-arr2 pathway could autonomously regulate neuronal signaling at the level of the respiratory neurons (13). It was hypothesized (7) that the depression in breathing produced by opioids could result from such a ligand bias pathway. This very intriguing proposition was not confirmed by other recent studies (7, 10, 14). Kliewer et al. (10) studied knock-in mice, wherein a series of serine and threonine-to-alanine mutations prevented opioid receptor phosphorylation by GRK2, making the recruitment of β-arr2 proteins impossible. In contrast to the report from Raehal, and Bohn (7), Kliewer et al. (10) found if anything, a more depressed ventilation in response to fentanyl in mice unable to activate the β-arr2 pathway, while the analgesic effect was magnified. The doses of fentanyl used in that study ranged between 0.1 mg/kg and 0.2 mg/kg, while the doses of morphine were below 22.5 mg/kg. In another study, Kliewer et al. (10) used morphine, at doses ranging from 3.75 mg/kg sc to 100 mg/kg sc or 3 mg/kg ip to 30 mg/kg ip, and fentanyl, at doses ranging from 0.05 mg/kg sc to 0.35 mg/kg sc; they found no difference in the ventilatory depression of β-arr2-deficient or control mice. Similar conclusions were drawn from the study of Hoot et al. (14) who used gallein as a means to produce a selective inhibition of opioid desensitization following the injection of 10 mg/kg morphine.
We found that at these doses of opioid, it is mostly the ventilatory response to opioid-induced sedation/hypometabolism that was investigated in these studies. In mice, Gardocki and Yelnosky (16) have established that the LD50 averaged 62 mg/kg sc and 470 mg/kg sc for fentanyl and morphine, respectively. The doses of fentanyl used in the literature to study the effects of the β-arr2 pathway on respiratory control were 200 times lower than doses potentially lethal. Second, as shown in Fig. 4, mice exposed to fentanyl display repetitive short bouts of locomotor activity, where ventilation and metabolic rate abruptly increased within seconds at a very high rhythm. The well-described opioid-induced automatic locomotion in mice (28) seems to have some similarity with opioid-induced muscle rigidity described in larger mammals (29–32). In mice, opioid-induced automatic locomotion (21, 22) consists of rapid bouts of motor and locomotor excitation that cyclically alternates, every few seconds or so, with phases of complete immobility. We found that these short and abrupt phases of locomotor activity and stillness drove ventilation up and down in a dramatic manner (Figs. 6 and 7). As displayed in these figures, if these phases of activity and rest are averaged, the level of ventilation could be paradoxically higher than the lowest levels of baseline ventilation. As the dose of opioid increases above the analgesic ranges, our mice presented more and more visible periods of locomotion with large swings in breathing. It appears crucial when describing the ventilatory effects of opioid in mice to clearly identify these different phases, as they can explain some of the discrepant results reported in the literature on the effect of opioid, of lack hereof, in β-arr2-deficient mice (10, 11, 14, 15).
As breathing control is under the direct influence of the level of vigilance and arousal (33, 34), a “non-specific” acute inhibition of breathing, in keeping with the level of sedation, produced by the opioids is not unexpected (20). This could occur regardless of, and in addition to, for the highest dose of fentanyl, the direct inhibition by opioids of medullary neurons “equipped” with μ-opioid receptors (5, 35–37).
Methodological Limitations
Several factors can potentially impact the whole body plethysmography method to produce systematic errors in the determination of tidal volume and therefore minute ventilation (26). These errors can result from the frequency response of our measurement system, nonadiabatic conditions, or the fact that the changes in flow or pressure inside the box during respiratory movements are not solely produced by the change in gas temperature during the transfer of gas between the chamber and the lungs (the signal could be affected by compressibility of the gas in the respiratory system). In addition, although tremors and locomotion per se do not produce any measurable artifact, as mice are unable to generate enough pressure (compression and decompression of the gas inside the box) when they move to affect the flow signal, in our experience, artifacts can certainly be produced if the flow of air going into or leaving the box is impeded. Our boxes are designed to prevent mice from blocking the inlet or outlet, so gas can always flow around the animals. To avoid some of these possible errors, periods of movements are usually excluded from analysis, not so much because of the potential artifacts created by movements, but because during these periods, ventilation becomes very rapid and unstable. This makes comparisons between conditions difficult unless these changes in breathing are standardized according to the level of pulmonary gas exchange, for instance. Periods of stable breathing are therefore solely used for analysis. Only focusing on periods of quiet breathing in a small mammal, however, creates a misleading picture, as stillness can be associated with different levels of breathing depending of various physiological factors (temperature, metabolic rate, level of sedation, or sleep) (38). In addition, the striking effect of fentanyl on automatic locomotion in mice leads to repetitive and fast bouts of locomotor activity, which makes it impossible to analyze consecutive periods of rest of more than a few seconds. This fundamental issue was not addressed in previous papers dealing with breathing control in β arrestin 2-deficient mice or in control mice, while the locomotor effect of fentanyl must have been present (Fig. 7).
Finally, we looked at an acute administration of fentanyl in animals that were not addicted. As it is not unusual for patients to overdose after a period of withdrawal, specific protocols mimicking this scenario are warranted. It is possible that periods of withdrawal in previously addicted animals could create conditions of vulnerability during which any desensitizing pathway could have been downregulated. Such an effect was not investigated by the present study.
Limits of the Mouse Models Altering G Protein-Receptor Kinase-β-arr2 Pathway
Different genetically engineered mouse models have been used to investigate the role of the G protein-receptor kinase-β-arr2 pathway in the ventilatory response to opioid (10, 11, 14, 15). They all consist of global deficient KO β-arr2 or KI mutant mice with clear intrinsic limitations: the chronic and constitutional, rather than inducible and acutely modifiable, nature of the deficit allows for redundant and compensatory pathways of “desensitization” to develop in utero and during development of the animals after birth. Although analgesic effects of opioid are usually enhanced in these models, breathing control, which relies on complex mechanisms of redundancy and degeneracy (39, 40), may display a very different behavior. In addition, deficit in the G-protein-receptor kinase-β-arr2 pathway implies a deficit affecting all G-protein-coupled receptors throughout the body, including β receptors in cardiomyocytes (41). All of these models (10, 11, 14, 15), including the one used in our study, can therefore not be translated to mechanisms specifically regulating the respiratory neuron activity following opioid administration without testing the effects of an acute inhibition of the β-arr2 system in the respiratory neurons and perhaps, more importantly, by performing studies in a large mammal (42). In addition, hypothermia, which can be produced by opioid (43), along with hypoxemia, can both lead to a depression in breathing in small mammals (44, 45) through mechanisms which are not opioid mediated (1), and therefore do not involve the β-arr2 pathway.
Potential Clinical Implications of the Present Findings
One important mechanism of acute desensitization of μ-opioid receptors, shared by all G-protein-coupled receptors, is triggered by the phosphorylation of μ-opioid receptors by G-protein-receptor kinase, allowing the very fast binding of β-arr2 proteins (9). This leads to the inhibition of the downstream transduction of μ-opioid receptors signaling and to the internalization of the receptors within seconds or minutes, respectively. KO mice with a deficit in this pathway display measurably enhanced analgesia following morphine or fentanyl (10, 15). This has led to the idea that inhibiting the β-arr2 pathway would represent a promising strategy to decrease the dosage of opioids needed to produce a given analgesic effect (46, 47). For such a strategy to be clinically applicable it would require that, unlike opioid-induced analgesia (23), opioid-induced breathing depression would not be enhanced by inhibiting the β-arr2 pathway. The present results suggest that the β-arr2 pathway could be crucial in allowing “resuscitation” after severe opioid overdose-induced apnea. It is not clear, however, whether such an effect was mediated at the level of medullary neurons. Mechanisms involving blood flow redistributing, heart contractility and arrhythmogenicity, and the sympathetic response to hypoxia are involved during life-threatening acute opioid intoxication (1). Whether some crucial elements of the integrative mechanisms preventing death from opioid overdose are under the influence of the β-arr2 pathway, including the circulatory response to hypoxia, remains an outstanding question.
In conclusion, at the doses of opioids previously used in the literature to investigate the role of β-arr2 pathway on breathing control, the depression in breathing produced by morphine or fentanyl appears to be in large part related to the decrease in vigilance and in metabolic rate. We found that ventilation in β-arr2-deficient mice surviving an opioid intoxication was not different from control animals, even when very high levels of fentanyl were used. However, our present findings suggest that β-arr2 signaling could play a role in the recovery from life-threatening opioid overdose via mechanisms that could be unrelated to a direct effect on respiratory neurons. This conclusion calls into question the suggestion that G-protein-biased μ-opioid receptor agonists are an innocuous strategy for safer opioid analgesic drugs. It also points to different mechanisms of defense against opioid-induced breathing depression, such as the presence of preexisting opioid resistant neuronal networks that would be capable of mitigating the effects of an overdose.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant 1R61HL156248-01 (P. Haouzi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.H. conceived and designed research; P.H. and M.M. performed experiments; P.H., M.M., and N.T. analyzed data; P.H. interpreted results of experiments; P.H., M.M., and N.T. prepared figures; P.H. drafted manuscript; P.H. and N.T. edited and revised manuscript; P.H., M.M., and N.T. approved final version of manuscript.
REFERENCES
- 1.Haouzi P, Guck D, McCann M, Sternick M, Sonobe T, Tubbs N. Severe hypoxemia prevents spontaneous and naloxone-induced breathing recovery after fentanyl overdose in awake and sedated rats. Anesthesiology 132: 1138–1150, 2020. doi: 10.1097/ALN.0000000000003156. [DOI] [PubMed] [Google Scholar]
- 2.Haouzi P, Mellen N, McCann M, Sternick M, Guck D, Tubbs N. Evidence for the emergence of an opioid-resistant respiratory rhythm following fentanyl overdose. Respir Physiol Neurobiol 277: 103428, 2020. doi: 10.1016/j.resp.2020.103428. [DOI] [PubMed] [Google Scholar]
- 3.Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol 545: 1017–1026, 2002. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37: 821–826, 2003. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda S, Eriksson LI, Yamamoto Y, Joensen H, Onimaru H, Lindahl SG. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology 95: 740–749, 2001. doi: 10.1097/00000542-200109000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165: 1704–1716, 2012. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raehal KM, Bohn LM. β-Arrestins: regulatory role and therapeutic potential in opioid and cannabinoid receptor-mediated analgesia. In: Arrestins: Pharmacology and Therapeutic Potential. Handbook of Experimental Pharmacology, edited byGurevich V.Heidelberg, Berlin: Springer, 2014, vol. 219, p. 427–443. doi: 10.1007/978-3-642-41199-1_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trapaidze N, Gomes I, Bansinath M, Devi LA. Recycling and resensitization of delta opioid receptors. DNA Cell Biol 19: 195–204, 2000. doi: 10.1089/104454900314465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA 95: 9914–9919, 1998. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S. Phosphorylation-deficient G-protein-biased mu-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10: 367, 2019. doi: 10.1038/s41467-018-08162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314: 1195–1201, 2005. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 12.Siuda ER, Carr R 3rd, Rominger DH, Violin JD. Biased mu-opioid receptor ligands: a promising new generation of pain therapeutics. Curr Opin Pharmacol 32: 77–84, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28: 416–422, 2007. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Hoot MR, Sypek EI, Reilley KJ, Carey AN, Bidlack JM, McLaughlin JP. Inhibition of Gβγ-subunit signaling potentiates morphine-induced antinociception but not respiratory depression, constipation, locomotion, and reward. Behav Pharmacol 24: 144–152, 2013. doi: 10.1097/FBP.0b013e32835f3d2f. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S. Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol 177: 2923–2931, 2020. doi: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardocki JF, Yelnosky J. A study of some of the pharmacologic actions of fentanyl citrate. Toxicol Appl Pharmacol 6: 48–62, 1964. doi: 10.1016/0041-008x(64)90021-3. [DOI] [PubMed] [Google Scholar]
- 17.Gautier H. Interactions among metabolic rate, hypoxia, and control of breathing. J Appl Physiol (1985) 81: 521–527, 1996. doi: 10.1152/jappl.1996.81.2.521. [DOI] [PubMed] [Google Scholar]
- 18.Haouzi P. Tracking pulmonary gas exchange by breathing control during exercise: role of muscle blood flow. J Physiol 592: 453–461, 2014[Erratum inJ Physiol592: 3161, 2014]. doi: 10.1113/jphysiol.2013.261396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortola JP, Gautier H. Interaction between metabolism and ventilation: Effects of respiratory gases and temperature. In: Regulation of Breathing, edited byDempsey JA, Pack AI.. New York: Marcel Dekker, 1995, p. 1011–1064. [Google Scholar]
- 20.Montandon G, Horner RL. Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci Rep 9: 14122, 2019. doi: 10.1038/s41598-019-50613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mickley GA, Mulvihill MA, Postler MA. Brain mu and delta opioid receptors mediate different locomotor hyperactivity responses of the C57BL/6J mouse. Psychopharmacology (Berl) 101: 332–337, 1990. doi: 10.1007/BF02244050. [DOI] [PubMed] [Google Scholar]
- 22.Murphy NP, Lam HA, Maidment NT. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J Neurochem 79: 626–635, 2001. doi: 10.1046/j.1471-4159.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- 23.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495–2498, 1999. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 24.Haouzi P, Bell HJ, Notet V, Bihain B. Comparison of the metabolic and ventilatory response to hypoxia and H2S in unsedated mice and rats. Respir Physiol Neurobiol 167: 316–322, 2009. doi: 10.1016/j.resp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Bell HJ, Azubike E, Haouzi P. The “other” respiratory effect of opioids: suppression of spontaneous augmented (“sigh”) breaths. J Appl Physiol (1985) 111: 1296–1303, 2011. doi: 10.1152/japplphysiol.00335.2011. [DOI] [PubMed] [Google Scholar]
- 26.Mortola JP, Frappell PB. Measurements of air ventilation in small vertebrates. Respir Physiol Neurobiol 186: 197–205, 2013. doi: 10.1016/j.resp.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Megens AA, Artois K, Vermeire J, Meert T, Awouters FH. Comparison of the analgesic and intestinal effects of fentanyl and morphine in rats. J Pain Symptom Manage 15: 253–257, 1998. doi: 10.1016/s0885-3924(97)00371-0. [DOI] [PubMed] [Google Scholar]
- 28.Brase DA, Loh HH, Way EL. Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther 201: 368–374, 1977. [PubMed] [Google Scholar]
- 29.Burns G, DeRienz RT, Baker DD, Casavant M, Spiller HA. Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clin Toxicol (Phila) 54: 420–423, 2016. doi: 10.3109/15563650.2016.1157722. [DOI] [PubMed] [Google Scholar]
- 30.Kinshella MW, Gauthier T, Lysyshyn M. Rigidity, dyskinesia and other atypical overdose presentations observed at a supervised injection site, Vancouver, Canada. Harm Reduct J 15: 64, 2018. doi: 10.1186/s12954-018-0271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lui PW, Lee TY, Chan SH. Fentanyl-induced muscle rigidity in unanesthetized and ketamine- or thiopental-anesthetized rats. Anesthesiology 70: 984–990, 1989. doi: 10.1097/00000542-198906000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Phua CK, Wee A, Lim A, Abisheganaden J, Verma A. Fentanyl-induced chest wall rigidity syndrome in a routine bronchoscopy. Respir Med Case Rep 20: 205–207, 2017. doi: 10.1016/j.rmcr.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homma I, Masaoka Y. Breathing rhythms and emotions. Exp Physiol 93: 1011–1021, 2008. doi: 10.1113/expphysiol.2008.042424. [DOI] [PubMed] [Google Scholar]
- 34.Orem J, Netick A. Behavioral control of breathing in the cat. Brain Res 366: 238–253, 1986. doi: 10.1016/0006-8993(86)91301-6. [DOI] [PubMed] [Google Scholar]
- 35.Montandon G, Horner R. CrossTalk proposal: the preBotzinger complex is essential for the respiratory depression following systemic administration of opioid analgesics. J Physiol 592: 1159–1162, 2014. doi: 10.1113/jphysiol.2013.261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pattinson KT. Opioids and the control of respiration. Br J Anaesth 100: 747–758, 2008. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 37.Takita K, Herlenius EA, Lindahl SG, Yamamoto Y. Actions of opioids on respiratory activity via activation of brainstem mu-, delta- and kappa-receptors; an in vitro study. Brain Res 778: 233–241, 1997. doi: 10.1016/S0006-8993(97)01105-0. [DOI] [PubMed] [Google Scholar]
- 38.Frappell P, Saiki C, Mortola JP. Metabolism during normoxia, hypoxia and recovery in the newborn kitten. Respir Physiol 86: 115–124, 1991. doi: 10.1016/0034-5687(91)90043-i. [DOI] [PubMed] [Google Scholar]
- 39.Haouzi P. Precedence and autocracy in breathing control. J Appl Physiol (1985) 118: 1553–1556, 2015. doi: 10.1152/japplphysiol.01013.2014. [DOI] [PubMed] [Google Scholar]
- 40.Mellen NM. Degeneracy as a substrate for respiratory regulation. Respir Physiol Neurobiol 172: 1–7, 2010. doi: 10.1016/j.resp.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr R 3rd, Schilling J, Song J, Carter RL, Du Y, Yoo SM, Traynham CJ, Koch WJ, Cheung JY, Tilley DG, Benovic JL. beta-arrestin-biased signaling through the beta2-adrenergic receptor promotes cardiomyocyte contraction. Proc Natl Acad Sci USA 113: E4107–E4116, 2016. doi: 10.1073/pnas.1606267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haouzi P. Murine models in critical care research. Crit Care Med 39: 2290–2293, 2011. doi: 10.1097/CCM.0b013e3182227550. [DOI] [PubMed] [Google Scholar]
- 43.Baker AK, Meert TF. Functional effects of systemically administered agonists and antagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther 302: 1253–1264, 2002. doi: 10.1124/jpet.102.037655. [DOI] [PubMed] [Google Scholar]
- 44.Frappell P. Hypothermia and physiological control: the respiratory system. Clin Exp Pharmacol Physiol 25: 159–164, 1998. doi: 10.1111/j.1440-1681.1998.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 45.Frappell P, Westwood K, Maskrey M. Ventilatory and metabolic responses to hypoxia during moderate hypothermia in anesthetized rats. J Appl Physiol (1985) 79: 256–260, 1995. doi: 10.1152/jappl.1995.79.1.256. [DOI] [PubMed] [Google Scholar]
- 46.DeWire SM, Violin JD. Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ Res 109: 205–216, 2011. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- 47.Rominger DH, Cowan CL, Gowen-MacDonald W, Violin JD. Biased ligands: pathway validation for novel GPCR therapeutics. Curr Opin Pharmacol 16: 108–115, 2014. doi: 10.1016/j.coph.2014.04.002. [DOI] [PubMed] [Google Scholar]