Abstract
One carbon (1C) metabolism is critical for early development as it provides 1C units for both biosynthesis of DNA, proteins, and lipids and epigenetic modification of the genome. Epigenetic marks established early in life can be maintained and exert lasting impacts on gene expression and functions later in life. Animal and human studies have increasingly demonstrated that prenatal 1C nutrient deficiencies impair fetal growth, neurodevelopment and cardiometabolic parameters in childhood, while sufficient maternal 1C nutrient intake is protective against these detrimental outcomes. However, recent studies also highlight the potential risk of maternal 1C nutrient excess or imbalance in disrupting early development. Further studies are needed to delineate the dose-response relationship among prenatal 1C nutrient exposure, epigenetic modifications, and developmental outcomes.
Keywords: one carbon metabolism, methyl donor, epigenetics, fetal programming, early development
1. Importance of one carbon metabolism (OCM) for early development
The prenatal period is characterized with rapid cellular proliferation and differentiation, which makes it susceptible to maternal nutrient supply. OCM, including the folate cycle, methionine cycle and transsulfuration pathway, provides one carbon (1C) units that are used for biosynthesis of nucleic acids, proteins, and lipids required for growth and epigenetic modifications that regulate gene expression (Box 1 and Figure 1) [1]. The influence of OCM on early development and fetal programming of metabolic and cognitive health later in life is studied in various animal models and increasingly observed in human studies (Figure 2. Key Figure).
Box 1. OCM: participating nutrients, pathways, and enzymes.
The Folate Cycle
In the folate cycle, tetrahydrofolate (THF) is reversibly converted to 5,10-methylenetetrahydrofolate (5,10-CH2-THF) by the B6-dependent serine hydroxymethyltransferase (SHMT) and provides 1C units for thymidylate synthesis. 5,10-CH2-THF can be converted back to THF via methylenetetrahydrofolate dehydrogenases (MTHFD). Both 5,10-CH2-THF and THF can be converted to 10-formyl-THF (10fTHF) required for purine synthesis. 5,10-CH2-THF is also reduced to 5-methyl-THF (5mTHF) via the B2-dependent methylenetetrahydrofolate reductase (MTHFR) which commits folate-derived methyl groups to the methionine cycle [1].
The Methionine Cycle
In the methionine cycle, methionine is converted to S-adenosylmethionine (SAM) by methionine adenosyltransferase (MAT). SAM serves as the universal methyl donor for various methyltransferases. SAM turns into SAH after transmethylation. The SAM:SAH ratio is often used as an indicator of cellular methylation potential [27]. SAH is reversibly hydrolyzed to homocysteine (Hcy) by S-adenosyl-L-homocysteine hydrolase (AHCY). Hcy can be remethylated to methionine via two independent pathways using folate or choline. The folate-dependent pathway requires 5mTHF to transfer its methyl group to Hcy by the B12-dependent methionine synthase (MTR) and methionine synthase reductase (MTRR). The choline-dependent pathway requires oxidation of choline to betaine via choline dehydrogenase (CHDH). Betaine transfers its methyl group to Hcy via betaine homocysteine S-methyltransferase 1 (BHMT1). In addition to participating in the methionine cycle, choline is also acetylated to the neurotransmitter acetylcholine via choline acetyltransferase (ChAT) and converted to phosphatidylcholine (PC) via the CDP-choline pathway [44]. PC can also be made from the de novo pathway from sequential methylation of phosphatidylethanolamine (PE) by phosphatidylethanolamine N-methyltransferase (PEMT). Interestingly, PEMT is a major consumer of methyl groups provided by SAM. As such, a methyl group originated from choline for SAM synthesis can also be used for the de novo synthesis of choline [10]. This seemingly futile cycle of choline metabolism may be important for the biosynthesis of specific species of PC, since PC produced by the PEMT pathway is enriched with long-chain polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA). Upregulation of PEMT activity may enhance PC-DHA delivery and enrichment in the fetal brain [10].
The Transsulfuration Pathway
Homocysteine leaves the methionine cycle via the transsulfuration pathway. It is first converted to cystathionine by cystathionine beta synthase (CBS) which is B6-dependent [1]. Cystathionine is converted to cysteine by cystathionine gamma-lyase (CTH) which is also B6-dependent. Cysteine is used to make glutathione and thus links the transsulfuration pathway with redox balance [1].
Figure 1.
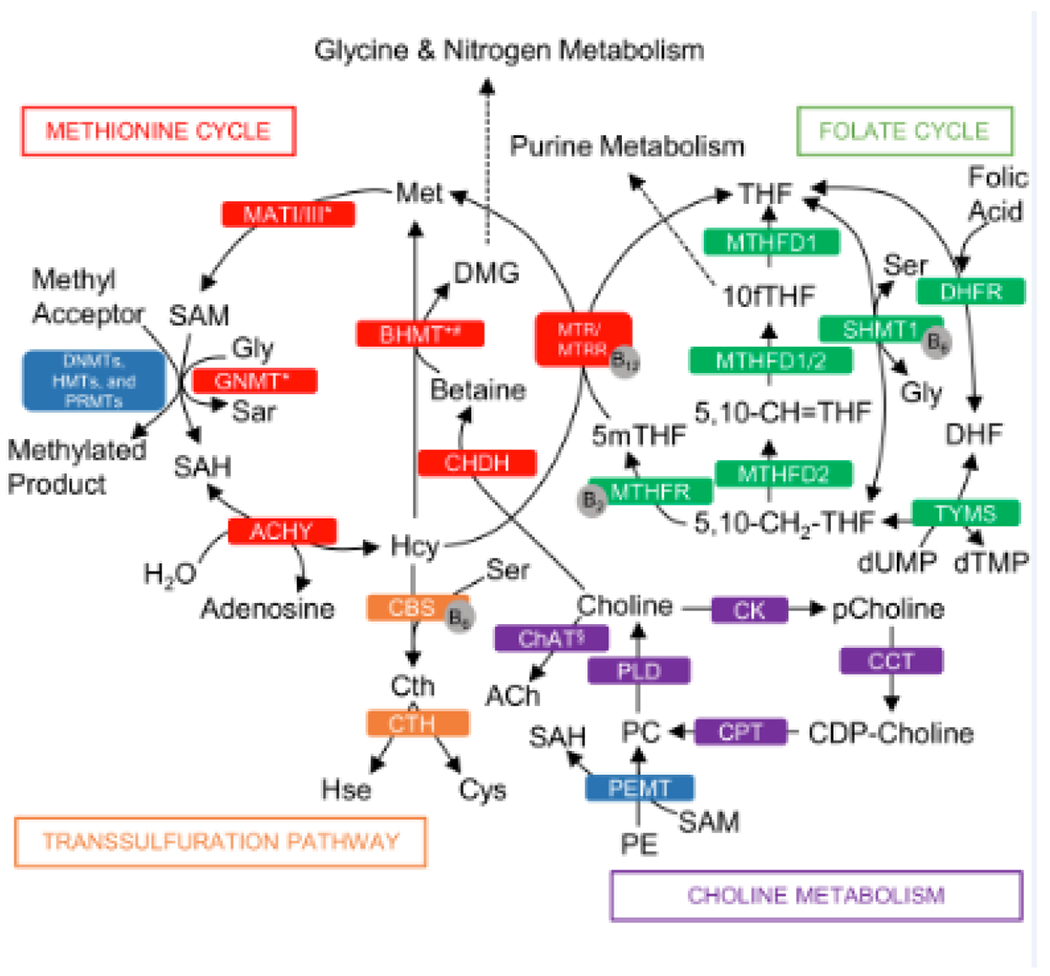
One Carbon Metabolism Metabolic Pathways. Folate cycle metabolites and enzymes (green boxes): 5,10-CH2-THF, 5,10-methylenetetrahydrofolate; 5,10-CH=THF, 5,10-methenyl-tetrahydrofolate; 10fTHF, 10-formyl-tetrahydrofolate; 5mTHF, 5-methyl-tetrahydrofolate; DHF, dihydrofolate; Gly, glycine; Ser, serine; dTMP, thymidine monophosphate; dUMP, deoxyuridine monophosphate; DHFR, dihydrofolate reductase; MTHFD1/2, methylenetetrahydrofolate dehydrogenase; MTHFR, 5,10-methylenetetrahydrofolate reductase; SHMT1, serine hydroxymethyltransferase 1; TYMS, thymidylate synthase. Methionine cycle metabolites and enzymes (red boxes): DMG, dimethylglycine; Hcy, homocysteine; Met, methionine; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine, Sar, sarcosine; AHCY; S-adenosyl-L-homocysteine hydrolase; BHMT, betaine-homocysteine S-methyltransferase; CHDH, choline dehydrogenase; GNMT, glycine N-methyltransferase; MATI/III, methionine adenosyltransferase; MTR, methionine synthase; MTRR, methionine synthase reductase. Transsulfuration pathway metabolites and enzymes (orange boxes): Cth, cystathionine; Cys, cysteine; Hse, homoserine; CBS, cystathionine β-synthase; CTH, cystathionine γ-lyase. Key methyltransferase enzymes (blue box): DNMTs, de novo and maintenance DNA methyltransferases; HMT, histone methyltransferase; PRMT, protein arginine methyltransferase; PEMT, phosphatidylethanolamine N-methyltransferase. Choline metabolites and enzymes (purple boxes): CDP-choline, cytidine diphosphate-choline; PC, phosphatidylcholine; pCholine, phosphocholine; PE, phosphatidylethanolamine; CCT, CTP:phosphocholine cytidylyltransferase; ChAT, choline acetyltransferase; CK, choline kinase; CPT, cholinephosphotransferase; PLD, phospholipase D. Coenzymes (grey circles): vitamin B2, B6 and B12. *enzymes mainly expressed in the liver; # BHMT1 is also expressed in the kidney.
Figure 2.
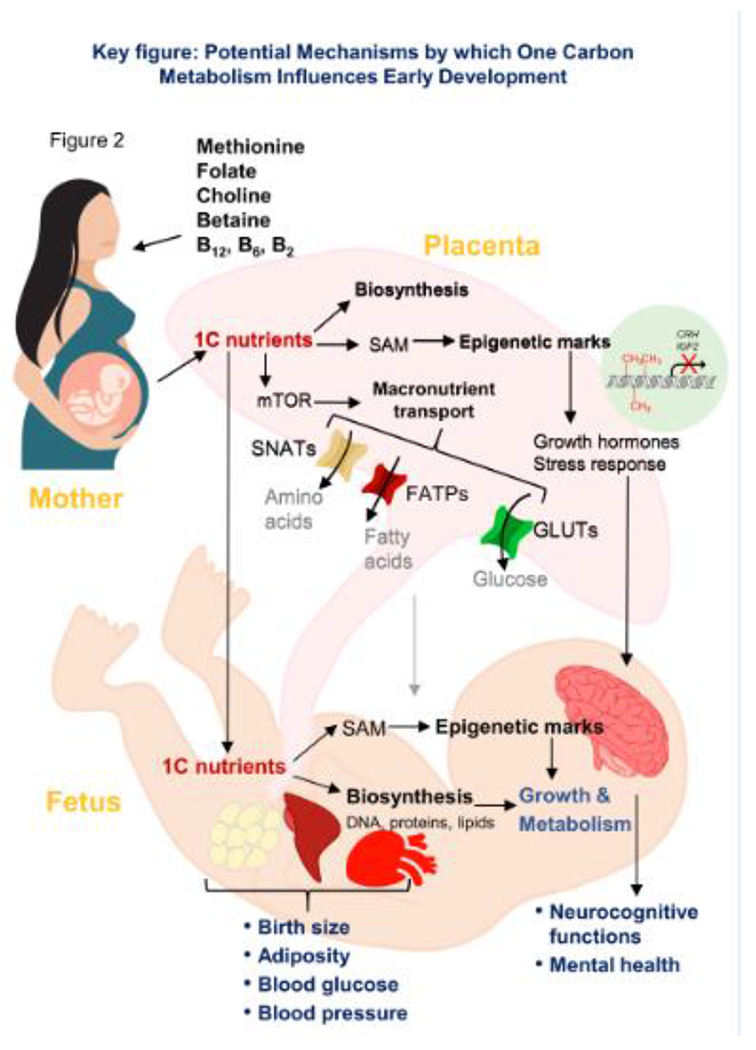
Potential mechanisms by which one carbon metabolism influences early development. Maternal intake of one carbon (1C) nutrients may affect the status of 1C nutrients in the fetus, thereby influencing biosynthesis of nucleic acids, proteins, and lipids and epigenetic regulation, eventually affecting cellular growth and metabolism. Maternal 1C nutrient status may also exert its influence via the placenta by modifying the mTOR pathway activity and subsequently affecting macronutrient transport to the fetus, as well as altering placental epigenetic marks, resulting in secondary epigenetic changes in pathways that regulate growth and stress response in the fetus. These alterations in placental functions by 1C nutrients eventually affect fetal growth and metabolism. Taken together, maternal one carbon metabolism programs developmental and metabolic pathways early in life, thereby exerting lasting impacts on functional outcomes such as neurocognitive development, body size and cardiometabolic differences. CH3: methyl group; CRH, corticotropin-releasing hormone; FATP, fatty acid transport protein; GLUT, glucose transporter; IGF2, insulin-like growth factor 2; mTOR, the mechanistic target of rapamycin; SAM, S-adenosylmethionine; SNAT, sodium-coupled neutral amino acid transporter
Nutrients including methionine, folate (see Glossary), choline and its oxidized metabolite betaine, vitamin B12, B2, and B6 participate in OCM as methyl donors or cofactors. While sufficient supplies of these nutrients are critical for metabolic and cognitive development, recent research has raised the concern of excess 1C metabolites leading to growth and neurodevelopmental abnormalities [2–7]. This review provides an overview on mechanisms by which OCM impacts early development and recent findings in the time-specific and dose-dependent influence of maternal OCM on offspring developmental outcomes.
2. Factors that influence OCM in the prenatal period
OCM in the prenatal period is influenced by the supply of substrates and activity of enzymes. Increasing maternal intakes of folate and vitamin B12 during pregnancy increases maternal and cord blood status of these nutrients [8, 9]. Increasing maternal choline intake leads to higher plasma choline levels and enhanced oxidation of choline to betaine [10]. Since these nutrients are interrelated in OCM, disturbances in one nutrient influence the status of others. Pups from folate-deficient mouse dams had lower betaine levels in the brain [11] , while supplementing choline to these dams partially preserved neurogenesis [12]. Since the enzyme methionine synthase (MTR) that mediates methyl group donation from 5-methyl-tetrahydrofolate (5mTHF) to homocysteine (Hey) requires B12 as a cofactor, a folate-excess and B12- deficient condition leads to the so-called “methyl folate trap” that impedes OCM [1].
Various single nucleotide polymorphisms (SNPs) have been discovered in genes encoding 1C metabolic enzymes such as MTHFR, MTHFD1, MTR, PEMT, and BHMT1, influencing 1C metabolite status in the maternal-fetal dyad and the risk of adverse pregnancy and neonatal outcomes such as preeclampsia, preterm birth, and neural tube defects (NTD) [13, 14]. One carbon metabolic enzymes are also regulated by hormonal changes during pregnancy. For example, PEMT, the enzyme that mediates de novo choline synthesis, contains three estrogen response elements in the promoter, and its expression is increased by 17-ß estradiol [15] and thus has an increase in activity during pregnancy [10].
3. OCM, placental function, and biosynthesis during early development
The folate cycle provides 1C units for nucleotide synthesis in the growing fetus. Moreover, OCM is interrelated with macronutrient biosynthesis and transport. PC is an essential component of very-low-density lipoprotein (VLDL) that mediates endogenous lipid transport [16]. Both choline and folate deficiencies lead to steatosis by impacting PC availability [17]. The 1C nutrients also modify DNA methylation and expression of metabolic genes, which may then exert lasting influence on macronutrient metabolism of offspring [18, 19].
The critical role of 1C nutrients on placental functions, which regulates macronutrient transport to the growing fetus, is recently revealed (Figure 2). Higher maternal choline intake during pregnancy suppressed the anti-angiogenic gene soluble fms-like tyrosine kinase 1 (sFLTI) expression in the placenta in humans and promoted placental angiogenesis in mice [20, 21]. Better placental angiogenesis facilitates macronutrient transport and growth of the fetus [22]. In contrast, maternal B12 deficiency reduced placental expression of fatty acid transporters [23], while maternal folate deficiency inhibited placental mechanistic target of rapamycin (mTOR) signaling and amino acid transporter expression, thereby resulting in fetal growth restriction in rodents [24]. Interestingly, under the condition of energy excess in a mouse model of high-fat feeding, choline supplementation reduced fetal overgrowth potentially by downregulating glucose and lipid transporters and activity of the mTOR pathway in the placenta [25]. Overall, these findings suggest a modulatory role of 1C nutrients in normal fetal growth by maintaining homeostasis of placental metabolism.
4. OCM and epigenetic programming in early life
Maternal exposures during the prenatal period such as famine, nutrient excess or deficiency, seasonality, pollutants and stress, have been associated with alterations in growth and development, as well as long-term health outcomes in the offspring [26]. It is suggested that these maternal exposures program the epigenetic marks such as DNA methylation and histone modifications in the growing fetus [26]. As these epigenetic marks persist after birth, they exert lasting impacts on the health and development of the offspring (Box 2). OCM regulates the supply of methyl groups for epigenetic modifications, and thus plays a critical role in epigenetic programming in early life.
Box 2. Epigenetic modifications in early life.
Epigenetics refers to the heritable changes in gene expression that do not alter DNA sequence. DNA methylation is a common epigenetic modification that requires SAM-derived methyl groups (Figure I). It involves covalent attachment of a methyl group at the C5 position of cytosine by DNA methyltransferases. Histone methylation is another SAM-dependent epigenetic modification which includes methylation of the lysine or arginine residues of histone tails by histone methyltransferases.
The drastic epigenetic remodeling renders the perinatal period a critical window for epigenetic programming by environmental exposures including maternal OCM [119]. The periconceptional period involves rapid demethylation of the genome following fertilization, giving rise to cellular pluripotency. However, the imprinted genes that are expressed in a parent-of-origin-specific manner (e.g., IGF2, H19) resist demethylation in this process. Remethylation occurs after implantation and persists throughout pregnancy in a tissue-specific manner. The primordial germ cells in the developing embryo undergo a second wave of genomic demethylation as they enter the genital ridge. Within this wave the imprint marks are erased, followed with de novo methylation that establishes sex-specific imprints during gametogenesis [119]. Histone methylation, especially tri-methylation at histone 3 lysine 4 (H3K4me3) helps to maintain proliferation and pluripotency of embryonic stem cells [120].
Adequate intake of 1C nutrients influences gametogenesis, gamete stability, and plays an integral role in zygogenesis [1]. Reproductive success is known to be negatively impacted by deficiencies of 1C nutrients and can be defined by elevated Hcy levels (⩾ 20mmol/L) in follicular fluid [1]. Oocyte maturation and zygotic implantation is negatively impacted by hyperhomocysteinaemia. Human embryonic stem cells require sufficient 1C nutrients to maintain H3K4me3 to remain undifferentiated [120].
Studies have investigated whether the maternal SAM:SAH ratio is related to DNA methylation in offspring. A study in Gambia demonstrated that women who conceived in the rainy season (vs. dry season) had higher 1C nutrient status and serum SAM:SAH ratio in early pregnancy [27]. Accordingly, there was higher total methylation of 50 metastable epialleles (MEs) in their children at 3 months [27]. While these results were consistent with the notion that better maternal 1C nutrient status is associated with higher “methylation potential” of the offspring, it should be cautioned that the SAM:SAH ratio may be tissue-specific and subjected to homeostatic regulation [28]. Therefore, the use of serum SAM:SAH to predict methylation changes in other tissues and between the maternal and fetal dyad needs to be further validated. Alterations in DNA methylation in fetal or offspring tissue by maternal 1C nutrient intake may not always be accompanied with tissue SAM:SAH ratio changes. In a randomized controlled trial (RCT) (n=24) among third trimester pregnancy women, the higher choline intake group had higher global DNA methylation in the placenta than the lower intake group, although the placental SAM:SAH ratio did not differ [29].
The influences of maternal 1C nutrient intake on the offspring epigenome and phenotypes are clearly demonstrated in studies of the viable yellow agouti mice. Supplementing 1C nutrients to dams increased DNA methylation of the intracisternal A particle (IAP) retrotransposon at the agouti locus, thereby reducing its expression, which in turn obviates the influence of this gene on offspring fur coat color, food intake, and obesity [30].
Human studies have identified associations between maternal OCM and epigenetic modifications of genes in pathways related to growth (e.g., IGF, H19 and RXRA), brain development (e.g., BDNF), obesity and cardiometabolic function (e.g., POMC, LEP), and stress response (e.g., NR3C1, CRH) [18, 26, 29, 31–34]. The relationship between maternal 1C nutrient intake and offspring DNA methylation seems to be time-specific: for example, cord blood LEP (encoding leptin) methylation was positively associated with periconceptional folic acid (FA) supplementation but inversely related to second trimester folate intake [32]. The influence of maternal 1C nutrients may also be sexually dimorphic. Caffrey et al. demonstrated in an RCT (n = 86) of 400μg/day FA supplementation through the second and third trimesters that IGF2 methylation was lower in the supplemented group only in the female offspring while BDNF methylation was lower only in the male offspring [31]. Although 1C nutrients provide the methyl group for DNA methylation, maternal 1C nutrient intake or status can be either positively or inversely associated with, or unrelated to, global or site-specific DNA methylation (e.g., the imprinted gene IGF2 encoding insulin-like growth factor 2) in the offspring, suggesting regulating mechanisms other than substrate provision [18, 33, 34]. One potential mechanism is related to methylation of the DNA methyltransferase 1 (DNMT1) by 1C nutrients which may lower its expression and activity. Offspring DNMT1 methylation was inversely associated with FA intake up to the second trimester, yet positively associated with FA use that extends into the third trimester [18]. In addition, the influence of maternal OCM is partially mediated through the placenta (Figure 2). An RCT showed that higher maternal choline intake (930 vs. 480 mg/d) in the third trimester increased NR3C1 and CRH DNA methylation (encoding glucocorticoid receptor and corticotropin-releasing hormone, respectively) and thus lowered CRH expression in the placenta. The decrease in placental CRH transport to the fetus may have subsequently caused decreases in methylation of these genes in the fetal cord blood [29]. In summary, although there is a growing body of work interrogating the relationship between maternal OCM and offspring epigenetic programming in humans, results are not consistent. Most of the studies are observational with other lifestyle factors as potential confounders, precluding the possibility to draw a causative conclusion. There are also challenges in the accurate assessment of 1C intake using food frequency questionnaires, while measurements of serum 1C metabolite levels may also be affected by hemodilution during pregnancy and tissue uptake. Current studies mainly examine methylation of a few selected loci in the genome, and thus may not represent changes in the whole methylome. Further, DNA methylation is tissue specific and subjected to dynamic changes during early development. Whether cord blood or buccal cell DNA methylation, as measured in many studies, reflects methylation changes in other tissues remains to be ascertained.
5. Maternal OCM and offspring functional outcomes
5.1. Congenital defects
OCM affects the risk of congenital defects such as NTDs, congenital heart disease and oral clefts [35]. Clinical trials conducted in various countries established the effect of FA supplementation on reducing the risk of NTDs [36, 37]. As a result, mandatory FA fortification in staple foods has been adopted in over 80 countries [38].
Intake and status of other nutrients in OCM, including vitamin B12 and choline, are also associated with NTD risk reduction independent of folate intake or levels in both pre and post FA fortified populations [14, 39, 40]. Polymorphisms in enzymes involved with OCM, such as MTHFR 677C>T, MTRR 66A>G, MTHFD1 1958G>A, and BHMT –742G>A are associated with increased risk of NTD [14], further supporting that disturbances in OCM are detrimental to neural tube development.
5.2. Neurodevelopment
OCM has been demonstrated to affect different aspects of neurodevelopment. Both folate and choline deficiency during late gestation reduces neurogenesis and increases apoptosis of neurons in rodents [12, 41,42]. OCM influences epigenetic modification and DNA repair of genes that are critical for neurodevelopment [43]. OCM nutrients also affect myelination, DHA uptake, neurotransmitter synthesis, and neuroinflammation [44].
Various behavioral studies in rodents have consistently demonstrated that high perinatal choline intake improves offspring cognitive performance and prevents their memory decline during aging [45]. Studies also suggest that low prenatal folate intake or Mthfr deficiency results in more anxiety behavior and worse short-term memory in offspring [11,46]. Supplementation of 1C nutrients reverses neural insults from other maternal exposures: prenatal choline supplementation alleviated behavioral changes, learning, and memory deficits in mice with fetal alcohol spectrum disorders (FASD) [47] or in genetic models of autism, Down syndrome or Alzheimer’s disease [48–50].
In humans, disturbances in OCM which lead to homocysteine accumulation may impair neurodevelopment in children (Figure 3). Maternal hyperhomocysteinemia during the periconceptional period or early pregnancy was associated with lower psychomotor scores in infants, and lower cognitive performance and higher risk of psychological problems in childhood [51–53].
Figure 3.
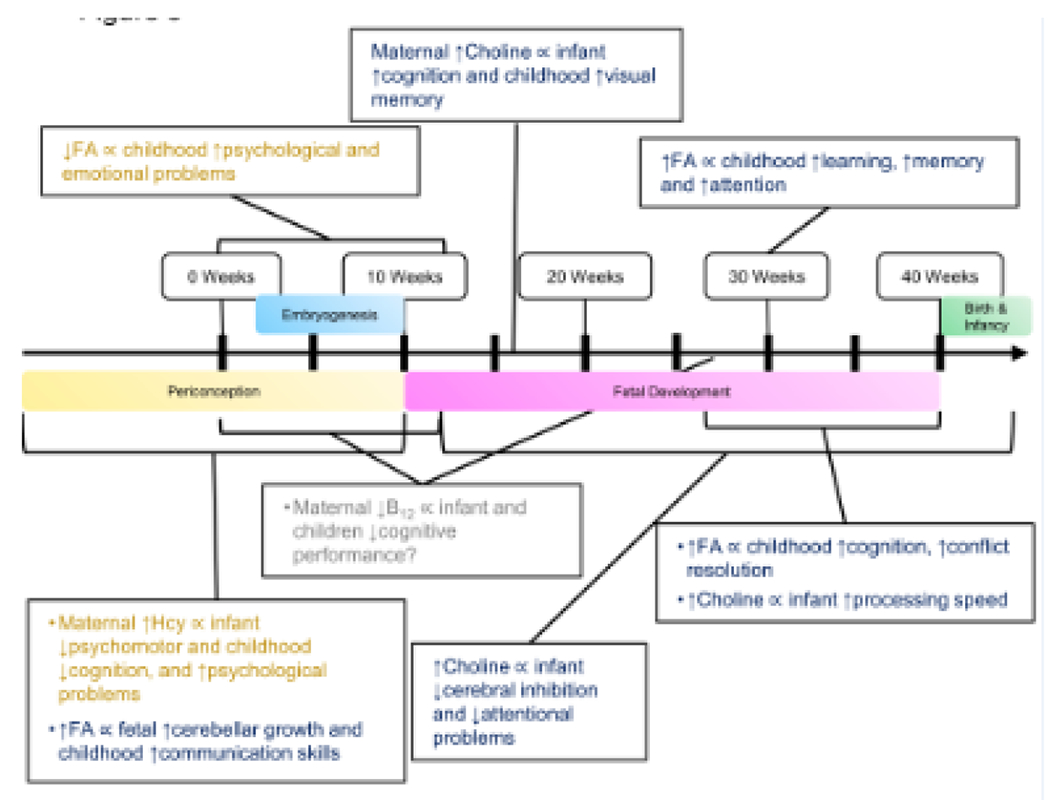
Current human evidence regarding the time-specific associations between maternal one carbon metabolism and offspring neurocognitive outcomes. The brackets represent the timing of exposure and the boxes include the maternal one carbon nutrient intake or status and offspring outcomes. The question mark represents inconsistent results across studies. FA, folic acid; Hcy, homocysteine
Various studies have investigated the relationship between maternal folate intake or status and cognitive outcomes of children (Figure 3). Low maternal folate status in early pregnancy was associated with psychological and emotional problems in two prospective cohorts [54, 55], whereas higher maternal folate status at 30 weeks of gestation was associated with better learning, memory and attention [56]. FA supplementation during periconception and early pregnancy was associated with improved fetal cerebellar growth [57], improved communication ability at 18 months [58], better verbal skills at 3–4 years [59, 60], and reduced omission error at 11 years [61] in observational studies. In two RCTs, supplementing 400 μg of 5-mTHF from gestational week 20 until delivery led to improved ability to solve conflicts at 8.5 years (n = 136), while supplementing 400 μg FA supplementation from gestational week 14 until delivery led to higher cognition scores at 3 years (n = 39) and better reasoning at 7 years (n=70) and higher processing speed at 11 years (n = 68), respectively [62–64]. However, there are also studies that suggest no association between folate status, intake or supplementation and cognitive outcomes [65, 66]. Several studies have investigated the relationship between maternal folate intake or status and autism risk in children, yet results were inconsistent, suggesting either higher or lower risk [67–69].
Results are mixed regarding maternal B12 and cognitive development. Maternal B12 deficiency was associated with lower cognitive performance in infants [70] and 9-year-old children [71] yet another two observational studies did not find an association [56, 72]. An RCT (n = 178) of oral B12 supplementation (50 μg) from early pregnancy to 6 weeks postpartum also did not find an effect on cognitive performance in 9 months old infants [73].
Emerging research suggests a positive relationship between maternal choline intake or status and cognitive outcomes (Figure 3). Maternal plasma choline and betaine at 16 weeks of gestation were associated with better cognitive scores at 18 months [65]. Higher maternal choline intake was also related to better visual memory at 7 years in the Project Viva study [66]. A controlled feeding RCT (n = 24) demonstrated that infants born to mothers consuming 930 versus 480 mg choline/day during their third trimester of pregnancy had faster processing speed [74]. In another RCT (n =100) choline supplementation from the second trimester of pregnancy to infancy improved cerebral inhibition at 5 weeks [75] and reduced attentional problems at 40 weeks of age [76]. Supplementing women who were heavy drinkers with 2g of choline during pregnancy in an RCT (n = 69) improved visual recognition memory of infants at 12 months, providing pilot human evidence that choline prevents the growing fetus from neural insults by alcohol [77]. A recent prospective cohort study showed that higher maternal plasma choline (concentrations ⩾7.07 μM at 16 weeks of gestation) was also associated with less attention problem and withdrawn syndrome in children exposed to maternal cannabis use or infection [78]. However, there are also studies that found no association between maternal choline intake and cognitive scores [60] [79].
Overall, despite promising findings between maternal 1C nutrients and offspring cognitive outcomes in observational cohorts and small trials, large RCTs that validate the causative relationship between the two are still limited. Interpreting the relationship between maternal OCM and offspring cognitive development is complicated by the different timing of exposure, supplementation dosage, SNPs, and the different aspects of cognitive outcomes measured at varied time points of childhood in studies. The null findings in some prospective studies may also be related to the challenge in sensitively estimating 1C nutrient intake in free-living populations. It should also be noted that the influence of 1C nutrient may depend on the dosage levels. The excess of 1C nutrients, especially folate, has also been related to adverse neurodevelopmental outcomes, which is discussed in a later section.
5.3. Early growth
OCM affects cellular biosynthesis, methylation of genes in growth-related pathways (e.g., IGF), and placental macronutrient delivery, thereby influencing growth of the fetus (Figure 2) [20–22, 25]. Maternal supplementation of multiple 1C nutrients or betaine alone increases fetal weight and postnatal growth performance in normal or growth-restricted prenatal environments [80–82]. In contrast, both choline and betaine supplementation prevented fetal overgrowth in high fat-fed obese mouse dams [25, 83].
Low maternal folate status was related to increased risk of fetal growth retardation, small for gestational age (SGA), and lower birth weight [84]. Meta-analyses of cohort studies [85] and RCTs [86] suggest that FA supplementation both before and after conception was associated with lower risk of SGA. Krikke et al. shows that maternal folate levels in early pregnancy were associated with a slight increase in BMI in children at 5-6 years of age, suggesting the positive relationship between maternal folate and growth extends beyond the fetal period [87].
However, high supplemental folate intake along with low vitamin B12 status in the second trimester of pregnancy increases the risk of SGA in a region with high prevalence of B12 deficiency in India [88], suggesting that excess folate or imbalance of 1C nutrients may negatively affect fetal growth. In a more B12 replete population, maternal B12 status was not associated with birth weight [89]. Interestingly, the highest quartile of maternal B12 status was associated with lower weight gain between birth and 3 years [90].
Studies regarding other 1C nutrients and early growth in humans are limited. A recent study demonstrates that a “varied and balanced” 1C nutrient intake pattern at periconception was associated with higher birth length and weight [91]. Two studies associated maternal betaine status with lower birth weight [92, 93]. Maternal choline status was positively related to weight and fat mass at birth but not in early childhood [94]. Overall, while deficiencies in 1C nutrients during pregnancy seem to restrict fetal growth, whether supplementing these nutrients in case of an obesogenic environment may protect against offspring obesity is a new area of exploration.
5.4. Cardiometabolic outcomes
Cardiometabolic diseases, such as atherosclerosis and type 2 diabetes, are present with altered OCM and aberrant epigenetic modifications in multiple tissues or organs, e.g., artery, liver, and pancreas, during pathogenesis [95, 96]. High-fat feeding in dams was demonstrated to disrupt 1C metabolites and enzymes and resulted in genome-wide DNA hypermethylation in offspring mice [97]. Gestational diabetes mellitus (GDM) in human pregnancies was associated with higher folate but lower betaine status in the cord blood [98] and altered DNA methylation of crucial metabolic genes such as LEP in newborns [99]. Therefore, under conditions of OCM derangement secondary to maternal cardiometabolic disturbances, maternal 1C nutrient intake may be critical to overcome the epigenetic programming that predisposes offspring to cardiometabolic diseases later in life.
In rodent studies, maternal 1C supplementation in high-fat fed, obese dams alleviated increases in whole body adiposity and blood glucose levels in offspring [100]. Supplementing 1C nutrients to high-fat dams attenuated higher fat preference and expression of the mu-opioid receptor (MOR) which codes the rewarding properties of food in offspring [101]. Supplementation of the 1C nutrient choline to high-fat dams led to similar relief in excess adiposity and liver fat content by reducing expression of lipogenic genes in fetus, and male offspring also had better glucose tolerance when fed a high-fat diet after weaning [102, 103]. Interestingly, prenatal choline supplementation alleviated weight, adiposity, and insulin resistance in offspring rats faced with postnatal high-fat feeding, yet increased weight gain and leptin levels in offspring receiving a normal-fat control diet [104]. On the contrary, maternal folate depletion led to higher circulating triglyceride levels in mouse offspring when fed a high-fat diet from weaning [105]. The balance between 1C nutrients also seems to matter, as a recent study demonstrates that a low or normal choline/high-folate, but not a high-choline/high-folate gestational diet increased weight gain and plasma insulin and leptin in offspring [106].
Human evidence regarding maternal OCM and cardiometabolic outcomes is relatively limited. Low maternal serum folate levels at early pregnancy were associated with higher BMI whereas low maternal B12 was associated with higher heart rate of children at 5-6 years [87]. In contrast, higher maternal folate intake at 32 weeks of gestation was associated with greater lean body mass in offspring at 9 years [107]. Wang et al. demonstrated 40% lower odds of elevated systolic blood pressure in children whose mothers had folate levels above (vs. below) the median during pregnancy [108]. However, a cohort in India with higher prevalence of B12 deficiency suggests that high maternal erythrocyte folate along with low B12 predicted the highest levels of insulin resistance in children [109]. Consistently, higher maternal folate concentrations were associated with higher insulin resistance in children in another birth cohort in India [110]. An RCT (n=3524) in Nepal suggests that FA supplementation was protective for metabolic syndrome [111] whereas in a subsample of 1132 participants B12 deficiency in mothers was associated with 27% increase in insulin resistance [112]. As for other 1C nutrients, a controlled feeding RCT demonstrated that higher choline intake in the third trimester of pregnancy led to lower cord blood cortisol levels [29]. Since chronic cortisol elevation impairs blood glucose and blood pressure, increasing maternal choline intake may provide a protective mechanism against fetal programming of cardiometabolic diseases.
6. Potential risk of 1C nutrient excess
While ensuring sufficient maternal supply of 1C nutrients is critical for early development, emerging research suggests the potential risk of excess in 1C nutrients, especially excess FA consumption. There is no upper tolerable intake level (UL) for natural folate, but a UL of 1000 μg was established for FA, the synthetic form of folate used in fortified foods and supplements, with concerns that high FA intakes may mask B12 deficiency, increase unmetabolized FA in the body, and inhibit key 1C enzymes such as DHFR and MTHFR [113]. Several studies in North American populations demonstrate that a considerable number (e.g., 26% in one study) of pregnant women exceed the UL from supplement and fortified products [114, 115]. The high folate intake leads to supranutritional folate status (e.g., a serum level over 45.3 nmol/L suggested by the WHO [116]) that suggests metabolic capacity has been exceeded. Several studies supplementing FA in rodent diets at 4-20mg/kg body weight versus control (2mg/kg) suggest that high maternal FA intake increased anxiety behavior and impaired learning and short-term memory of offspring with altered DNA methylation, neurotrophic factor expression and DHA concentration in the brain [117]. The Infancia y Medio Ambiente (INMA) Project which enrolled over 2000 Spanish children suggests that those with maternal FA intake exceeding UL during pregnancy were associated with lower psychomotor and neurocognitive scores [3, 4]. Maternal plasma folate exceeding the 45.3 nmol/L cutoff was associated with greater risk of autism in the Boston Birth Cohort [69].
A recent study by De Crescenzo et al. identified an interesting phenomenon that high maternal FA intake mirrored the detrimental effect of folate deficiency on cortical neurogenesis and behavioral development of offspring, and that both FA excess and deficiency suppressed folate’s participation in the methionine cycle [2]. These results are consistent with a study by Bahous et al. that high dietary folate in pregnant mice led to pseudo-MTHFR deficiency, thereby decreasing the use of folate as a methyl donor [5].
The potential harmful effect of FA excess is also noted for fetal growth and cardiometabolic functions. Excessive gestational FA intake impaired insulin secretion in mouse offspring [6]. FA intake beyond the first trimester of pregnancy was associated with increased risk of large-for-gestational-age (LGA) in a birth cohort in China [7]. High FA and low B12 intake during pregnancy was associated with insulin resistance of children in the PUNE cohort in India [109]. Excess in overall 1C nutrient intake is also related to changes in IGF pathway regulation and leptin secretion [19, 118], suggesting that a bell-shaped curve of benefit versus risk may exist for 1C nutrients in general (Figure 4).
Figure 4.
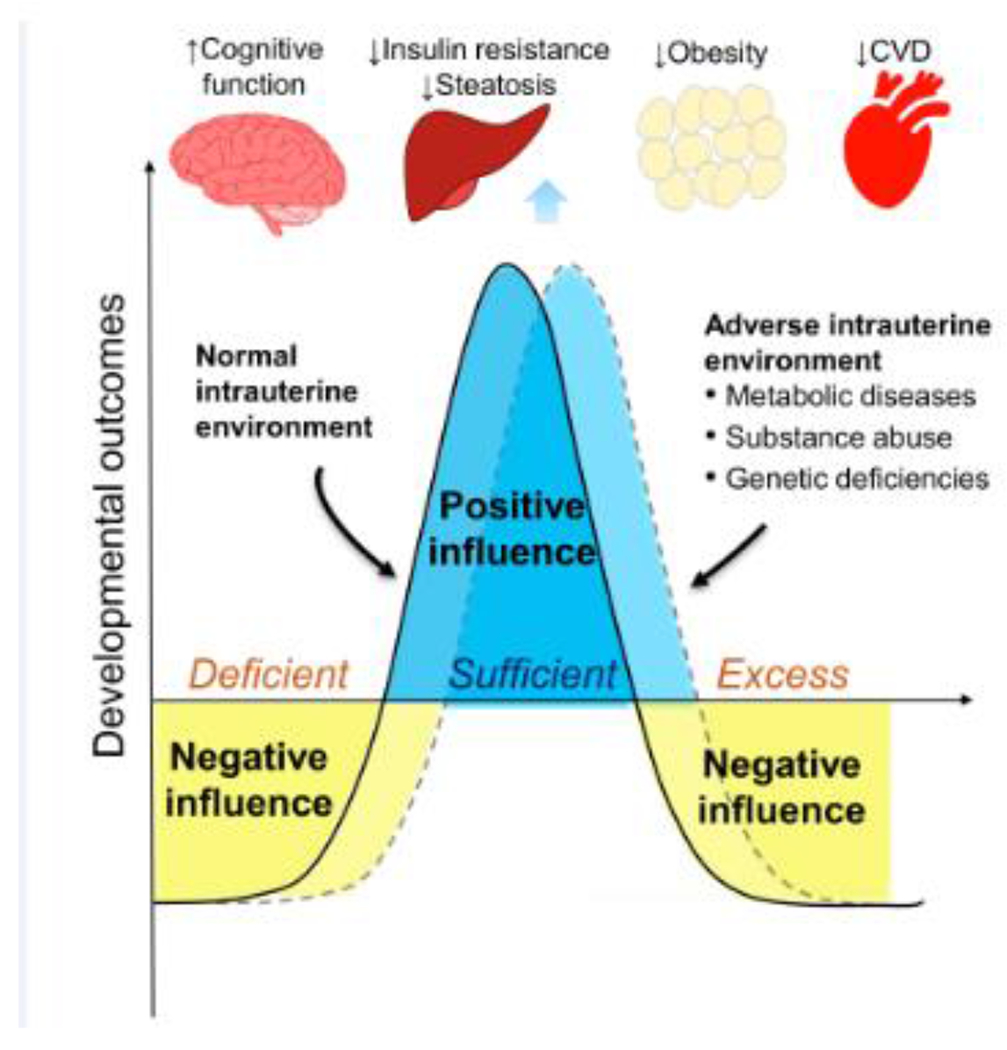
Proposed relationship between prenatal one carbon exposure and developmental outcomes. 1C, one carbon; CVD, cardiovascular disease.
7. Concluding remarks and future perspectives
Altered 1C metabolism due to maternal 1C nutrient intake or genetic deficiencies can result in inappropriate growth at birth and metabolic and cognitive impairments later in life, while increasing maternal 1C nutrient supply may overcome developmental disturbances resulting from other adverse exposures in early life as demonstrated in various animal studies. Emerging observational and interventional studies in humans provide evidence that resonates with findings in animals. Underlying mechanisms of action include direct impacts of OCM on fetal biosynthesis and macronutrient metabolism and epigenetic programming of the fetal genome, as well as modification of the placental epigenome and metabolism which subsequently affects fetal nutrient acquisition and metabolism. However, there are also concerns related to excess maternal 1C nutrient supply and imbalance between 1C nutrients that could also result in OCM derangement, leading to adverse growth and developmental outcomes.
The challenges of research in this area include that: (i) The influence of OCM may be time-specific, demonstrated by the differential DNA methylation changes and growth and cognitive outcomes, with 1C nutrient exposures at different time points of gestation; (ii) The complexity of 1C metabolism regulation by dietary intakes, interactions between 1C pathways and nutrients, and genetic polymorphisms, which may partly explain the inconsistent results in epigenetic and functional outcomes in studies; (iii) The difficulty in conducting human studies that pinpoint the causative relationship among 1C nutrient exposure, epigenomic and cellular changes, and long-term functional outcomes (see Outstanding Questions). Further research is needed to delineate the dose-response relationship of 1C nutrient intakes, in varied forms and combinations, with early development.
Outstanding Questions.
What are the time- and dose-specific effects of prenatal one carbon (1C) nutrient exposures on fetal epigenetic changes in humans? Do these changes have lasting effects on health outcomes?
Is there a bell-shaped curve regarding the relationship between maternal 1C nutrient intakes and benefits on early development?
Can maternal one carbon metabolism (OCM) be modified to avert the negative influence of other maternal exposures (e.gobesity) on offspring health?
What is the right balance of 1C nutrients for a pregnant woman? Should 1C nutrient intakes be personalized with the consideration of genetic polymorphisms to optimize OCM for early development?
How can prenatal vitamin supplements be better designed to complement dietary intake of 1C nutrients in different populations?
How does paternal OCM during the periconceptional period affect early development?
Does 1C nutrient exposure affect early development in a sexually dimorphic manner? How do hormonal treatments such as in cases of in vitro fertilization and transgender pregnancies influence OCM during early development in both sexes?
Figure I.
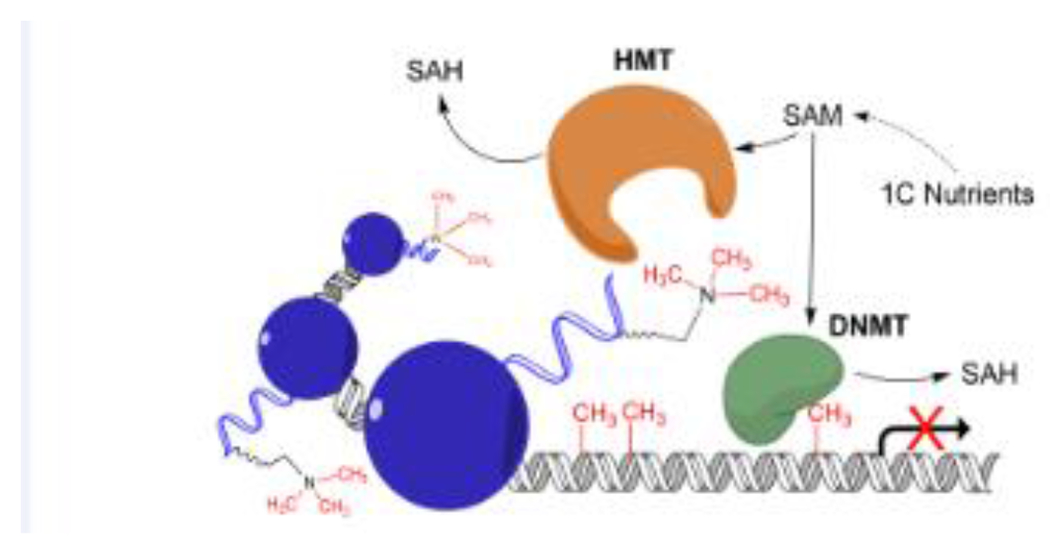
(in Box 2). Influence of one carbon metabolism (OCM) on epigenetic regulation. OCM nutrients provide methyl groups for the universal methyl donor S-adenosylmethionine (SAM) to be used by DNA and histone methyltransferases, thereby influencing DNA and histone methylation.
Trends:
One carbon metabolism (OCM) exerts its impacts on offspring development by participation in biosynthesis, epigenetic modification, and remodeling of placental function.
Dysregulation in maternal OCM impairs early development, leading to abnormalities in growth, body composition, and cardiometabolic and neurocognitive functions in the offspring.
Growing evidence from animal and human studies suggests that sufficient maternal supply of one carbon (1C) nutrients may overcome the adverse influence of other intrauterine exposures, such as maternal obesity and alcohol intake, on early development.
Recent research suggests the potential risk of maternal 1C nutrient excess and imbalance on growth and neurodevelopment. Further studies to delineate the dose-response relationship between maternal 1C nutrient intakes and offspring outcomes are urgently needed.
Acknowledgements
The authors give special thanks to Dr. Marie Caudill (Cornell University) for her insights into conceptualizing and editing this manuscript. This research is funded by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) (grant number: 5SC3GM132010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Steegers-Theunissen RP et al. (2013) The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum. Reprod. Update 19, 640–55 [DOI] [PubMed] [Google Scholar]
- 2.Harlan De Crescenzo A et al. (2021) Deficient or Excess Folic Acid Supply During Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb. Cortex 31,635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valera-Gran D et al. (2017) Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4-5 y of age: the prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am. J. Clin. Nutr 106, 878–887 [DOI] [PubMed] [Google Scholar]
- 4.Compañ Gabucio LM et al. (2021) The Use of Lower or Higher Than Recommended Doses of Folic Acid Supplements during Pregnancy Is Associated with Child Attentional Dysfunction at 4-5 Years of Age in the INMA Project. Nutrients. 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahous RH et al. (2017) High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring. Hum. Mol. Genet 26, 888–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kintaka Y et al. (2020) Excessive folic acid supplementation in pregnant mice impairs insulin secretion and induces the expression of genes associated with fatty liver in their offspring. Heliyon. 6, e03597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S et al. (2016) Maternal Continuing Folic Acid Supplementation after the First Trimester of Pregnancy Increased the Risk of Large-for-Gestational-Age Birth: A Population-Based Birth Cohort Study. Nutrients. 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maulik D et al. (2019) The effect of race and supplementation on maternal and umbilical cord plasma folates. J. Matern. Fetal. Neonatal. Med 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqua TJ et al. (2016) Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur. J. Nutr 55, 281–93 [DOI] [PubMed] [Google Scholar]
- 10.Yan J et al. (2013) Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am. J. Clin. Nutr 98, 1459–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadavji NM et al. (2015) MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring. Neuroscience. 300, 1–9 [DOI] [PubMed] [Google Scholar]
- 12.Craciunescu CN et al. (2010) Dietary choline reverses some, but not all, effects of folate deficiency on neurogenesis and apoptosis in fetal mouse brain. J. Nutr 140, 1162–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankovic-Karasoulos T et al. (2021) Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern. Child. Nutr 17, e13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li K et al. (2016) Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients. 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resseguie M et al. (2007) Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB. J 21, 2622–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vance JE and Vance DE. (1985) The role of phosphatidylcholine biosynthesis in the secretion of lipoproteins from hepatocytes. Can. J. Biochem. Cell. Biol 63, 870–81 [DOI] [PubMed] [Google Scholar]
- 17.da Silva RP et al. (2020) One-Carbon Metabolism in Fatty Liver Disease and Fibrosis: One-Carbon to Rule Them All. J. Nutr 150, 994–1003 [DOI] [PubMed] [Google Scholar]
- 18.Pauwels S et al. (2017) Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin. Epigenetics 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giudicelli F et al. (2013) Excess of methyl donor in the perinatal period reduces postnatal leptin secretion in rat and interacts with the effect of protein content in diet. PLoS. One 8, e68268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X et al. (2013) A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1). Faseb. J 27, 1245–1253 [DOI] [PubMed] [Google Scholar]
- 21.Kwan STC et al. (2017) Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta. 53, 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan STC et al. (2017) Maternal Choline Supplementation Modulates Placental Nutrient Transport and Metabolism in Late Gestation of Mouse Pregnancy. J. Nutr 147, 2083–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadhwani NS et al. (2013) Maternal micronutrients and omega 3 fatty acids affect placental fatty acid desaturases and transport proteins in Wistar rats. Prostaglandins. Leukot. Essent. Fatty. Acids 88, 235–42 [DOI] [PubMed] [Google Scholar]
- 24.Rosario FJ et al. (2017) Maternal folate deficiency causes inhibition of mTOR signaling, down-regulation of placental amino acid transporters and fetal growth restriction in mice. Sci. Rep 7, 3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam J et al. (2017) Choline prevents fetal overgrowth and normalizes placental fatty acid and glucose metabolism in a mouse model of maternal obesity. J. Nutr. Biochem 49, 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James P et al. (2018) Candidate genes linking maternal nutrient exposure to offspring health via DNA methylation: a review of existing evidence in humans with specific focus on one-carbon metabolism. Int. J. Epidemiol 47, 1910–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James P et al. (2019) Maternal One-Carbon Metabolism and Infant DNA Methylation between Contrasting Seasonal Environments: A Case Study from The Gambia. Curr. Dev. Nutr 3, nzy082 [Google Scholar]
- 28.Caudill MA et al. (2001) Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr 131,2811–8 [DOI] [PubMed] [Google Scholar]
- 29.Jiang X et al. (2012) Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB. J 26, 3563–74 [DOI] [PubMed] [Google Scholar]
- 30.Waterland RA et al. (2008) Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes (Lond) 32, 1373–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caffrey A et al. (2018) Gene-specific DNA methylation in newborns in response to folic acid supplementation during the second and third trimesters of pregnancy: epigenetic analysis from a randomized controlled trial. Am. J. Clin. Nutr 107, 566–575. [DOI] [PubMed] [Google Scholar]
- 32.Pauwels S et al. (2017) Dietary and supplemental maternal methyl-group donor intake and cord blood DNA methylation. Epigenetics. 12, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steegers-Theunissen RP et al. (2009) Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS. One 4, e7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haggarty P et al. (2013) Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr 97, 94–9 [DOI] [PubMed] [Google Scholar]
- 35.Bailey LB and Berry RJ (2005) Folic acid supplementation and the occurrence of congenital heart defects, orofacial clefts, multiple births, and miscarriage. Am. J. Clin. Nutr 81, 1213S–1217S [DOI] [PubMed] [Google Scholar]
- 36.Berry RJ et al. (1999) Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med 341, 1485–90 [DOI] [PubMed] [Google Scholar]
- 37.MRC Vitamin Study Research Group. (1991) Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 338, 131–7. [PubMed] [Google Scholar]
- 38.Crider KS et al. (2011) Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 3, 370–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molloy AM et al. (2009) Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic Acid fortification. Pediatrics. 123, 917–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw GM et al. (2009) Choline and risk of neural tube defects in a folate-fortified population. Epidemiology. 20, 714–9. [DOI] [PubMed] [Google Scholar]
- 41.Craciunescu CN et al. (2003) Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr 133, 3614–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Craciunescu CN et al. (2004) Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J. Nutr 134, 162–6. [DOI] [PubMed] [Google Scholar]
- 43.Langie SA et al. (2013) Maternal folate depletion and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring. FASEB. J 27, 3323–34 [DOI] [PubMed] [Google Scholar]
- 44.Korsmo HW et al. (2019) Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blusztajn JK et al. (2017) Neuroprotective Actions of Dietary Choline. Nutrients. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson SA et al. (2005) Behavioral effects of prenatal folate deficiency in mice. Birth. Defects. Res. A. Clin. Mol. Teratol 73, 249–52 [DOI] [PubMed] [Google Scholar]
- 47.Thomas JD et al. (2009) Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol. Teratol 31,303–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langley EA et al. (2015) High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety-like behaviors in the BTBR T+Itpr3tf/J mouse model of autism. Behav. Brain. Res 278, 210–20 [DOI] [PubMed] [Google Scholar]
- 49.Mellott TJ et al. (2017) Perinatal Choline Supplementation Reduces Amyloidosis and Increases Choline Acetyltransferase Expression in the Hippocampus of the APPswePS1dE9 Alzheimer’s Disease Model Mice. PLoS. One 12, e0170450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powers BE et al. (2017) Maternal choline supplementation in a mouse model of Down syndrome: Effects on attention and nucleus basalis/substantia innominata neuron morphology in adult offspring. Neuroscience. 340, 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy MM et al. (2017) Moderately elevated maternal homocysteine at preconception is inversely associated with cognitive performance in children 4 months and 6 years after birth. Matern. Child. Nutr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roigé-Castellví J et al. (2019) Moderately elevated preconception fasting plasma total homocysteine is a risk factor for psychological problems in childhood. Public. Health. Nutr 22, 1615–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ars CL et al. (2019) Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: the Generation R Study. Br. J. Nutr 122, S1–S9 [DOI] [PubMed] [Google Scholar]
- 54.Schlotz W et al. (2010) Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J. Child. Psychol. Psychiatry 51, 594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steenweg-de Graaff J et al. (2012) Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am. J. Clin. Nutr 95, 1413–21 [DOI] [PubMed] [Google Scholar]
- 56.Veena SR, et al. (2010) Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J. Nutr 140, 1014–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koning IV et al. (2015) Periconception Maternal Folate Status and Human Embryonic Cerebellum Growth Trajectories: The Rotterdam Predict Study. PLoS. One 10, e0141089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chatzi L et al. (2012) Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: the mother-child cohort ‘Rhea’ study in Crete, Greece. Public. Health. Nutr 15, 1728–36 [DOI] [PubMed] [Google Scholar]
- 59.J Julvez J et al. (2009) Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr. Perinat. Epidemiol 23, 199–206 [DOI] [PubMed] [Google Scholar]
- 60.Villamor E et al. (2012) Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr. Perinat. Epidemiol 26, 328–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forns J et al. (2012) Longitudinal association between early life socio-environmental factors and attention function at the age 11 years. Environ. Res 117, 54–9 [DOI] [PubMed] [Google Scholar]
- 62.Catena A et al. (2016) Folate and long-chain polyunsaturated fatty acid supplementation during pregnancy has long-term effects on the attention system of 8.5-y-old offspring: a randomized controlled trial. Am. J. Clin. Nutr 103, 115–27 [DOI] [PubMed] [Google Scholar]
- 63.McNulty H et al. (2019) Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: a follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC. Med 17, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caffrey A et al. (2021) Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: an 11-year follow-up from a randomised controlled trial. BMC. Med 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu BT et al. (2012) Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS. One 7, e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boeke CE et al. (2013) Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol 177, 1338–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt RJ et al. (2012) Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr 96, 80–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Surén P et al. (2013) Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 309, 570–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raghavan R et al. (2018) Maternal Multivitamin Intake, Plasma Folate and Vitamin B Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol 32, 100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.del Río Garcia C et al. (2009) Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutr. Neurosci 12, 13–20 [DOI] [PubMed] [Google Scholar]
- 71.Bhate V et al. (2008) Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food. Nutr. Bull 29, 249–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonilla C et al. (2012) Vitamin B-12 status during pregnancy and child’s IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children. PLoS. One 7, e51084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srinivasan K et al. (2017) Effects of maternal vitamin B12 supplementation on early infant neurocognitive outcomes: a randomized controlled clinical trial. Matern. Child. Nutr 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caudill MA et al. (2018) Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB. J 32, 2172–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross RG et al. (2013) Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am. J. Psychiatry 170, 290–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross RG et al. (2016) Perinatal Phosphatidylcholine Supplementation and Early Childhood Behavior Problems: Evidence for CHRNA7 Moderation. Am. J. Psychiatry 173, 509–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobson SW et al. (2018) Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol. Clin. Exp. Res 42, 1327–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hunter SK et al. (2021) Prenatal choline, cannabis, and infection, and their association with offspring development of attention and social problems through 4 years of age. Psychol. Med, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheatham CL et al. (2012) Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr 96, 1465–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y et al. (2019) Effect of maternal or post-weaning methyl donor supplementation on growth performance, carcass traits, and meat quality of pig offspring. J. Sci. Food. Agric 99, 2096–2107 [DOI] [PubMed] [Google Scholar]
- 81.Oster M et al. (2016) Methylating micronutrient supplementation during pregnancy influences foetal hepatic gene expression and IGF signalling and increases foetal weight. Eur. J. Nutr 55, 1717–27 [DOI] [PubMed] [Google Scholar]
- 82.Salahi P et al. (2020) In vivo: maternal betaine supplementation normalized fetal growth in diabetic pregnancy. Arch. Gynecol. Obstet 302, 837–844 [DOI] [PubMed] [Google Scholar]
- 83.Joselit Y et al. (2018) Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr. Diabetes 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bergen NE et al. (2012) Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG. 119, 739–51 [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q et al. (2017) Effect of folic acid supplementation on preterm delivery and small for gestational age births: A systematic review and meta-analysis. Reprod. Toxicol 67, 35–41 [DOI] [PubMed] [Google Scholar]
- 86.Fekete K et al. (2011) Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr. J 11, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krikke GG et al. (2016) Vitamin B12 and folate status in early pregnancy and cardiometabolic risk factors in the offspring at age 5-6 years: findings from the ABCD multi-ethnic birth cohort. BJOG 123, 384–92 [DOI] [PubMed] [Google Scholar]
- 88.Dwarkanath P et al. (2013) High folate and low vitamin B-12 intakes during pregnancy are associated with small-for-gestational age infants in South Indian women: a prospective observational cohort study. Am. J. Clin. Nutr 98, 1450–8 [DOI] [PubMed] [Google Scholar]
- 89.Relton CL et al. (2005) The influence of erythrocyte folate and serum vitamin B12 status on birth weight. Br. J. Nutr 93, 593–9 [DOI] [PubMed] [Google Scholar]
- 90.McCullough LE et al. (2016) Maternal B vitamins: effects on offspring weight and DNA methylation at genomically imprinted domains. Clin. Epigenetics 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lecorguillé M et al. (2020) Association between Dietary Intake of One-Carbon Metabolism Nutrients in the Year before Pregnancy and Birth Anthropometry. Nutrients. 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Lee L et al. (2016) Prospective associations of maternal betaine status with offspring weight and body composition at birth: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am. J. Clin. Nutr 104, 1327–1333 [DOI] [PubMed] [Google Scholar]
- 93.Du YF et al. (2019) Maternal betaine status, but not that of choline or methionine, is inversely associated with infant birth weight. Br. J. Nutr 121, 1279–1286 [DOI] [PubMed] [Google Scholar]
- 94.van Lee L et al. (2019) Prospective associations of maternal choline status with offspring body composition in the first 5 years of life in two large mother-offspring cohorts: the Southampton Women’s Survey cohort and the Growing Up in Singapore Towards healthy Outcomes cohort. Int. J. Epidemiol 48, 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fiorito G et al. (2014) B-vitamins intake, DNA-methylation of One Carbon Metabolism and homocysteine pathway genes and myocardial infarction risk: the EPICOR study. Nutr. Metab. Cardiovasc. Dis 24, 483–8 [DOI] [PubMed] [Google Scholar]
- 96.Spratlen MJ et al. (2018) Arsenic, one carbon metabolism and diabetes-related outcomes in the Strong Heart Family Study. Environ. Int 121,728–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng H et al. (2021) Maternal high-fat diet disrupted one-carbon metabolism in offspring, contributing to nonalcoholic fatty liver disease. Liver. Int [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barzilay E et al. (2018) Fetal one-carbon nutrient concentrations may be affected by gestational diabetes. Nutr. Res 55, 57–64 [DOI] [PubMed] [Google Scholar]
- 99.Allard C et al. (2015) Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics. 10, 342–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiao F et al. (2016) Protective effects of maternal methyl donor supplementation on adult offspring of high fat diet-fed dams. J. Nutr. Biochem 34, 42–51 [DOI] [PubMed] [Google Scholar]
- 101.Carlin J et al. (2013) Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS. One 8, e63549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jack-Roberts C et al. (2017) Choline Supplementation Normalizes Fetal Adiposity and Reduces Lipogenic Gene Expression in a Mouse Model of Maternal Obesity. Nutrients. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Korsmo HW et al. (2020) Prenatal Choline Supplementation during High-Fat Feeding Improves Long-Term Blood Glucose Control in Male Mouse Offspring. Nutrients. 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hammoud R et al. (2021) High Choline Intake during Pregnancy Reduces Characteristics of the Metabolic Syndrome in Male Wistar Rat Offspring Fed a High Fat But Not a Normal Fat Post-Weaning Diet. Nutrients. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McKay JA et al. (2014) Metabolic effects of a high-fat diet post-weaning after low maternal dietary folate during pregnancy and lactation. Mol. Nutr. Food. Res 58, 1087–97 [DOI] [PubMed] [Google Scholar]
- 106.Hammoud R et al. (2021) Choline and Folic Acid in Diets Consumed during Pregnancy Interact to Program Food Intake and Metabolic Regulation of Male Wistar Rat Offspring. J. Nutr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lewis SJ et al. (2009) Body composition at age 9 years, maternal folate intake during pregnancy and methyltetrahydrofolate reductase (MTHFR) C677T genotype. Br. J. Nutr 102, 493–6 [DOI] [PubMed] [Google Scholar]
- 108.Wang H et al. (2017) Association of Maternal Plasma Folate and Cardiometabolic Risk Factors in Pregnancy with Elevated Blood Pressure of Offspring in Childhood. Am. J. Hypertens 30, 532–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yajnik CS et al. (2008) Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 51,29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnaveni GV et al. (2014) Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia. 57, 110–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stewart CP et al. (2009) Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J. Nutr 139, 1575–81 [DOI] [PubMed] [Google Scholar]
- 112.Stewart CP et al. (2011) Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J. Nutr 141, 1912–7 [DOI] [PubMed] [Google Scholar]
- 113.Maruvada P et al. (2020) Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr 112, 1390–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gómez MF et al. (2015) Use of micronutrient supplements among pregnant women in Alberta: results from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Matern. Child. Nutr 11,497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dubois L et al. (2017) Adequacy of nutritional intake from food and supplements in a cohort of pregnant women in Québec, Canada: the 3D Cohort Study (Design, Develop, Discover). Am. J. Clin. Nutr 106, 541–548 [DOI] [PubMed] [Google Scholar]
- 116.WHO. (2012) Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization. (http://www.who.int/nutrition/vmnis/indicators/serum&RBCfolate, accessed May 16, 2021) [Google Scholar]
- 117.Naninck EFG et al. (2019) The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr 10, 502–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amarger V et al. (2017) Perinatal high methyl donor alters gene expression in IGF system in male offspring without altering DNA methylation. Future. Sci. OA 3, FSO164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ishida M and Moore GE (2013) The role of imprinted genes in humans. Mol. Aspects. Med 34, 826–40 [DOI] [PubMed] [Google Scholar]
- 120.Van Winkle LJ and Ryznar R (2019) One-Carbon Metabolism Regulates Embryonic Stem Cell Fate Through Epigenetic DNA and Histone Modifications: Implications for Transgenerational Metabolic Disorders in Adults. Front. Cell. Dev. Biol 7, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]


