Abstract
Nearly half a million people die annually due to mosquito-borne diseases. Despite aggressive mosquito population-control efforts, current strategies are limited in their ability to control these vectors. A better understanding of mosquito metabolism through modern approaches can contribute to the discovery of novel metabolic targets and/or regulators and lead to the development of better mosquito-control strategies. Currently, cutting-edge technologies such as gas or liquid chromatography–mass spectrometry-based metabolomics are considered ‘mature technologies’ in many life-science disciplines but are still an emerging area of research in medical entomology. This review primarily discusses recent developments and progress in the application of mass spectrometry-based metabolomics to answer multiple biological questions related to mosquito metabolism.
Metabolomics: an avenue for studying mosquito metabolic interactions
In spite of extensive worldwide attempts to reduce mosquito populations, current strategies remain only partially effective at controlling these vectors of disease-causing pathogens that infect hundreds of millions of people and cause approximately a half-million deaths annually [1]. Development of complex resistance mechanisms to insecticides through metabolic detoxification or decreased sensitivity of target proteins has contributed to an emergence or resurgence of mosquito-borne diseases [2]. Additionally, it has been reported that environmental changes could also influence the geographical distribution of mosquitoes, their vectorial capacity, and the dynamics of transmission of deadly pathogens through blood feeding [3–6]. Regardless of significant progress in elucidating mosquito metabolism at the molecular level, many research questions have been raised in relation to how changes in metabolite profile and/or abundance can reveal new clues into how mosquitoes regulate different metabolic processes in response to internal and external variations. Clearly, there is a critical need to better understand mosquito metabolism as well as mosquito–host–pathogen interactions at the metabolite level for the identification of novel metabolic targets that can lead to the design and implementation of more effective mosquito-control strategies. To fill this knowledge gap, the application of advanced technologies such as gas chromatography–mass spectrometry (GC/MS) (see Glossary) or liquid chromatography–mass spectrometry (LC/MS)-based metabolomics has emerged as a powerful approach to study mosquito metabolism (Box 1 and Figure 1) and the vector–host–pathogen interplay [7].
Box 1. Metabolomics tinkerer’s toolbox.
Modern metabolomics-based methods encompass a broad set of analytical instrumentation, bioanalytical techniques, and data-processing tools that are routinely used to perform complex biological sample analyses in many areas of the life sciences [61–63]. Even with the diminutive size of a typical mosquito, sample extracts often need to be diluted due to the high sensitivity of modern MS systems.
A diverse palette of MS instrumentation is available to address a multitude of questions in the field of mosquito metabolism (Figure I). MS resolution (Rs) is defined as the centroid mass of the molecular ion divided by the full width at half max of the spectral peak. Low-resolution (Rs ~100–1000) single- or triple-quadrupole mass spectrometers (SQMS and TQMS) are the gold-standard platform for performing quantitative analysis of targeted metabolites.
High-resolution mass spectrometry (HRMS; Rs ~10 000–30 000) systems include hybridized quadrupole time-of-flight (TOF) mass spectrometers (QTOFMS) that can be coupled to both LC- and GC-based separations modules. These instruments are capable of performing a QTOF-based SRM scan mode for targeted metabolomics, or a TOF-only-based full-scan mode of analysis for nontargeted metabolomics.
Ultrahigh-resolution mass spectrometry (UHRMS; Rs >30 000) instruments feature QTOFMS-based options that include a GC-HRT+ system, and a timsTOF system that couples a trapped ion mobility spectrometry (TIMS) device to a QTOFMS. The TIMS device expands the capability of this instrument to resolve coeluting or isobaric substances in the gas phase prior to TOF analysis [64]. The OrbiTrap (OT)-class of MS systems boast maximum mass resolutions from 120 000 up to 1 000 000 in a simple-to-use platform, and these instruments allow for fine isotopolog peak distributions that reveal and improve the fidelity of molecular/product ion formula assignment [65]. The capabilities provided by HRMS and UHRMS systems are important for putative identification in ‘unknown’ discovery work such as in nontargeted metabolomics studies.
For GC/MS- or LC/MS-based methods to be suitably applied to mosquito metabolism studies, the following criteria need to be met: (i) metabolite levels in the mosquito sample extracts must exceed the detection limit of the instrument; (ii)metabolites must be extractable from whole body, tissues/organs, cells, eggs, or excreta; (iii) metabolites must remain soluble and stable throughout the entire extraction procedure; and (iv) metabolites must possess ionizable functional groups.
Figure I. Diversity of state-of-the-art mass spectrometry (MS) instruments on the market at the time of this review.

Red section: low-resolution single-, triple-quadrupole and hybrid triple-quadrupole/linear ion trap (‘QTrap’) MS instruments. Green section: high-resolution quadrupole time-of-flight (TOF) mass spectrometers (QTOFMS). Blue section: ultrahigh-resolution QTOF, timsTOF, and Orbitrap-class MS. Many of the ionization sources for the MS instruments mentioned previously are capable of accommodating special ionization probes and interfaces for high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), super-critical fluid chromatography (SFC), and gas chromatography (GC) separation modules that may be used. *The Leco Pegasus BT GC-TOFMS and the Pegasus GC-HRT+ instruments are GC-only platforms.
Figure 1. Generalized sample-processing methods and instrumentation used for mass spectrometry (MS)-based metabolomics studies in mosquitoes.

(A) General gas chromatography/mass spectrometry (GC/MS)-based methods used for targeted (red section) and untargeted (blue section) metabolomics analysis in mosquito samples. (B) General liquid chromatography–mass spectrometry (LC/MS)-based methods used for targeted (red section), untargeted (blue section), and stable-label isotope tracing (green section) analysis in mosquito samples. Both panels highlight an assortment of GC/MS and LC/MS-based instrumentation used in the listed references. Abbreviations: DI, direct infusion; FTICRMS, Fourier-transform ion cyclotron resonance mass spectrometer; IC, ion chromatography; OTMS, OrbiTrap mass spectrometer; QTOFMS, quadrupole time-of-flight mass spectrometer; SQMS, single-quadrupole mass spectrometer; TQMS, triple-quadrupole mass spectrometer. Figure 1 cites [33–38,40,41,45,47,50–56,58–60].
Metabolomics refers to a multicompound study of chemical processes involving small molecules (<1500 Da) such as substrates, intermediates, and products of cell metabolism [8]. This technique is capable of identifying and quantifying novel metabolic targets and is useful in the exploration of metabolic interactions. Metabolomics is part of the growing and improving ‘systems biology’ effort aimed at integrating genomics, transcriptomics, proteomics, and metabolomics information to provide a better understanding of the cellular biology in an organism as a whole [9]. MS-based metabolomics is capable of performing absolute quantification of individual or collections of metabolites using calibrators and targeted metabolomics-based tandem mass spectrometry (MS/MS) modes that include selected/multiple-reaction monitoring (SRM/MRM) [10,11]. Furthermore, MS-based methods are also capable of performing discovery and relative quantification of large distributions of metabolites using full-scan, nontargeted metabolomics-based approaches [12,13]. In this review, we discuss how recent development and progress in the application of MS-based metabolomics have become highly beneficial to address multiple biological questions related to mosquito metabolism, and to pave the bases towards a better integration of mosquito biological knowledge at the whole-organism level.
Advances in understanding mosquito metabolism
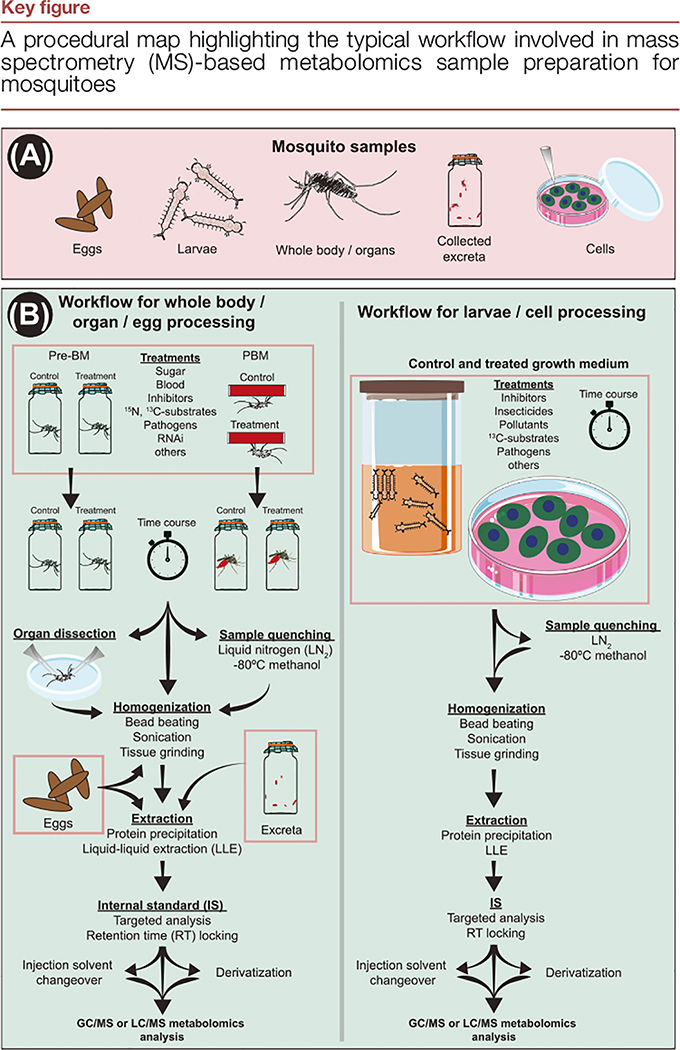
While male mosquitoes feed exclusively on plant nectar or other sugar sources, anautogenous female mosquitoes are both sugar and blood feeders [14,15]. Blood-fed female mosquitoes have evolved efficient mechanisms to digest vertebrate blood meals, absorb nutrients, synthesize new molecules, excrete excess water, ions, and waste, avoid ammonia and free-radical toxicity, and successfully lay eggs within a 72 h period. Although it is known that the concerted action of genes, hormones, proteins, peptides, microRNAs, and nutritional signals modulates each gonotrophic cycle of the female mosquitoes [16–24], the molecular and biochemical bases underlying the mechanistic regulation of the multiple pathways involved have not yet been fully elucidated. Technological innovation in genetic and biochemical methods are expected to be crucial in uncovering the mechanistic framework needed to identify critical regulatory points within mosquito metabolism and the vector–host–pathogen interface. In the following sections we discuss publications in which MS-based metabolomics is driving progress in mosquito metabolism research on several fronts. Additional details related to recent publications discussed in this review are presented in Table 1. A schematic representation of several common steps involved in mosquito sample preparation for MS-based metabolomics is illustrated in Figure 2, Key figure.
Table 1.
MS-based metabolomics studies reported in different mosquito species in recent yearsa
| Mosquito species | Metabolites analyzed | Treatment | Sample type/processing/derivatization | Instrumentation/measurement type | Refs |
|---|---|---|---|---|---|
| Anopheles gambiae G3 strain | AAs + Ders, OAs, sugars + Ders, neurotransmitters, nucleotides, glycerols, glycerates, cholesterol, other metabolites | RNAi; Pre-and PBM | Whole body/ice-cold methanol quench, extraction/MSFTA derivatization | GC-MS for targeted and nontargeted metabolomics | [33] |
| An. coluzzii Ngousso (TEP1*S1) strain | AAs + Ders, FFAs, PLs, SLs, GLs, dipeptides, trehalose, other metabolites | RNAi; Blood meals (+/−) P. falciparum | Whole body/LN2 quench, homogenization, two-step LLE extraction/MSFTA derivatization | GC-HRMS and LC-HRMS for nontargeted metabolomics | [34] |
| Aedes aegypti NIH-Rockefeller strain | AAs + Ders, OAs, sugars + Ders, glycerols, glycerates, nucleic acids, vitamins, other metabolites | Specific larval conditions to produce small and large mosquitoes; Pre- and PBM | Fat body/ice-cold PBS and frozen (−80°C), homogenization, two-stage OSE, IS/MSFTA derivatization | GC-MS for nontargeted metabolomics | [35] |
| Ae. aegypti NIH-Rockefeller strain | Sugars + Ders, OAs | Pre- and PBM | Whole body/PBS wash, LN2 quench, homogenization, two-step OSE/MSFTA derivatization | GC-MS for targeted metabolomics | [36] |
| Ae. aegypti NIH-Rockefeller strain | AAs + Ders, OAs | Blood meal (+/−) Glc or [1,2-13C2]-Glc | Whole body/LN2 quench, homogenization, OSE | SRM LC-MS/MS and LC-HRMS for targeted 13 C-isotope tracing metabolomics | [37] |
| Ae. aegypti NIH-Rockefeller strain | AAs, OAs, sugars + Ders | RNAi; Blood meal + [1,2-13C2]-Glc | Whole body, excreta/LN2 quench, homogenization, OSE | LC-HRMS and IC-HRMS for targeted 13C-isotope tracing metabolomics | [38] |
| Anopheles stephensi | AAs + Ders, sugars + Ders, OAs, FFAs, nucleotides + Ders, other metabolites | Blood meals (+/−) ILP3 or (+/−) ILP4 plus SMIs | Midgut/PBS wash, lysis buffer, homogenization, OSE/MSFTA derivatization | GC-HRMS for nontargeted metabolomics | [40] |
| An. stephensi | AAs + Ders, sugars + Ders, OAs, FFAs, nucleotides + Ders, other metabolites | Blood meals (+/−) specific JNK SMIs. | Midgut/ice-cold buffer + PIC, homogenization, two-step OSE, IS/MSFTA derivatization | GC-HRMS for nontargeted metabolomics | [41] |
| Aedes albopictus colony established from larvae collected in Manassas, VA, USA | AAs + Ders, OAs, carnitine, AcCas, nucleic acids, nucleosides, lipids (GLs, FFAs, SLs, CLs, PLs) other metabolites | Specific conditions to produce eggs in early diapause and nondiapause eggs | Eggs/LN2 quench, homogenization, IS, two distinct OSE | LC-HRMS for nontargeted metabolomics | [45] |
| Anopheles coluzzii colony established from mosquitoes collected in Burkina Faso | AAs + Ders, OAs and inorganic acids, sugars + Ders, glycerols, glycerates, other metabolites | Specific chambers to mimic dry and rainy seasons | Whole body/LN2 quench, lyophilization, homogenization, OSE, IS/MSFTA derivatization | GC-MS for targeted metabolomics | [47] |
| Ae. aegypti Liverpool strain | AAs + Ders, sugars, polyols | Larvae grown in medium (+/−) ibuprofen | Larvae, whole body, eggs/LN2 quench, lyophilization, OSE/BSTFA derivatization | GC-MS for targeted metabolomics | [50] |
| Culex quinquefasciatus (Say) strain from Nuevo Leon, Mexico | AAs + Ders, carnitine, AcCas | Larvae grown in medium with (+/−) chlorpyrifos, (+/−) temephos, or (+/−) permethrin | Larvae/frozen at −80°C, homogenization, sterile-filtration, drying on filter paper, IS, OSE | SRM LC-MS/MS targeted metabolomics using the NeoBase LC-MS/MS kit | [51] |
| Cx. pipiens strain from Berlin, Germany | AAs + Ders, AcCas, lipids (PL, SL), sugars | Larvae grown in medium with (+/−) clothianidin | Larvae/frozen at −80°C, homogenized, IS, OSE | SRM FIA-MS/MS using an Absolute IDQ p180 kit for targeted metabolomics | [52] |
| Ae. aegypti Chetumal strain | AAs + Ders, FFAs + Ders, AcCas, sterols, lipids (PLs, GLs, SLs), other metabolites | Blood meals (+/−) dengue virus | Midgut/ice-cold PBS, homogenization, frozen on dry ice and −80°C storage, IS, OSE/immiscible phases were processed separately | LC-HRMS for nontargeted metabolomics | [53] |
| Ae. aegypti colony established from Singapore Aag2 cells | AAs + Ders, OAs, sugars, nucleotides + Ders, lipids (FFAs, GLs, PLs), other metabolites | Blood meals (+/−) dengue virus Aag2 cells (+/−) dengue virus | Whole body, midgut, Aag2 cells/saline washed (cells), quench with ice-cold organic solvent, homogenization, three-step OSE | LC-HRMS for nontargeted metabolomics | [54] |
| Ae. aegypti colony established from Singapore Aag2 cells | Neurotransmitters, lipids (PLs, SLs), other metabolites | Blood meals, Aag2 cells (+/−) dengue virus (+/−) choline, 13 C2- choline, ethanolamine, or 13C2-ethanolamine | Whole body, midgut, Aag2 cells/saline washed (cells), quench with ice-cold organic solvent, homogenization, OSE x2 | LC-HRMS for nontargeted metabolomics and 13 C-isotope tracing | [55] |
| Ae. albopictus colony established from Suffolk County, NY | AAs + Ders, OAs, sugars + Ders, nucleotides, glycerols, glycerates, nucleosides + Ders, vitamins, sterols, lipids (GLs, FFAs, PLs, prostaglandins) | Blood meal (+/−) Zika virus | Whole body/LN2 quench, homogenization, ice-cold OSE /immiscible phases were processed separately/MSFTA derivatization | GC-HRMS and LC-HRMS for nontargeted metabolomics | [56] |
| Ae. albopictus Aa23 cells | Lipids (SLs, GLs, PLs) | Noninfected Aa23 cells (Aa23-T) or infected with W. pipientis strains (Aa23. wMel, and Aa23.wMelPop) | Cells/quench with −40°C organic solvent, scraped with organic solvent/immiscible phases were processed separately | LC-FT-ICRMS and DI-FT-ICRMS for nontargeted metabolomics | [58] |
| Ae. aegypti colony established from Cairns, Australia | Lipids (GLs, SLs, CLs) | Wolbachia-free (WT), wMel-infected mosquitoes were microinjected with sterile medium (mono infection) or DENV3 (dual infection) | Whole body/quench, extract with ice-cold methanol, homogenization, IS | LC-HRMS for nontargeted metabolomics | [59] |
| Ae. aegypti Aag2 cells | AcCas, carnitine, sterols, Lipids (GLs, SLs, PLs) | Aag2 cells mono-infected with (+/−) Wolbachia (wMet strain), dengue, or Zika virus Aag2 cells dual-infected with wMet and either dengue or Zika virus | Cells/PBS wash, LN2 quench, IS, OSE x 2 | SRM LC-MS/MS for targeted metabolomics | [60] |
Abbreviations: BSTFA, N,O-Bis (trimethylsilyl) trifluoroacetamide; CL, cardiolipin; Ders, derivatives; DENV3, dengue virus serotype 3; DI-FT-ICRMS, direct infusion-Fourier transform ion cyclotron mass spectrometry; FFA, free fatty acid; FIA-MS/MS, flow-injection analysis-tandem mass spectrometry; GL, glycerolipid; ILP3, insulin-like peptide 3; ILP4, insulin-like peptide 4; IC-HRMS, ion chromatography-high-resolution mass spectrometry; IS, internal standard; LC-FT-ICRMS, liquid chromatography-Fourier transform ion cyclotron mass spectrometry; LLE, liquid–liquid extraction; LN2, liquid nitrogen; MSTFA, N-methyl-N-(trimethylsilyl) trifluoroacetamide; OSE, organic solvent extraction; PBS, phosphate-buffered saline; PIC, protease inhibitor cocktail; SIM, single-ion monitoring; SL, sphingolipid.
Figure 2.
(A) The various mosquito-derived samples that are commonly studied. (B) Sample preparation workflows depending on sample type, tissue dissection, quenching, homogenization, and extraction protocols. The orange rectangles indicate locations where mosquito samples of a specific type enter into the workflow. Abbreviations: BM, blood meal; GC/MS, gas chromatography/mass spectrometry; LC/MS, liquid chromatography/mass spectrometry; PBM, post blood meal.
Nitrogen (N) metabolism and related pathways
In mosquitoes and other insects, the absence of the gene encoding ornithine transcarbamylase, also called ornithine carbamoyltransferase [25], prevents them from having a functional urea cycle for ammonia disposal. To elucidate how blood-fed Aedes aegypti mosquitoes detoxify ammonia (NH3, or both) released during the massive amino acid (AA) oxidation that takes place during blood meal digestion, Ae. aegypti females were fed a 15NH4Cl solution during a time-dependent study. The fate of 15N in mosquito whole body was explored by electrospray ionization (ESI)–MS/MS and stable-label isotope tracing. It was found that mosquitoes rapidly incorporate [15N] from 15NH4Cl into [5-15N]-glutamine (Gln) through glutamine synthase (GS). The [5-15N]-Gln and α-ketoglutarate (α-KG) are then converted by glutamate synthase (GltS) into [15N]-glutamate (Glu) and unlabeled Glu. Most of the [15N]-Glu is converted into [15N]-alanine (Ala) and high levels of [15N]-proline (Pro) by specific enzymes. The [15N]-Glu can also be generated by glutamate dehydrogenase (GDH), which can transfer [15N] from 15N-ammonia to unlabeled Glu. Furthermore, [15N] from 15N-ammonia can be transferred to [15N]-Glu and produces [2,5-15N2]-Gln by GS [26] (Figure 3). In addition, ESI–MS/MS experiments using 15NH4Cl and [15N]-AAs coupled with pharmacological inhibitors, led scientists to conclude that Ae. aegypti females differentially metabolize ammonia in fat body and midgut. During ammonia detoxification, [5-15N]-Gln and [15N]-Pro serve as temporary N sinks in fat body, whereas [15N]-Ala serves as a transient N sink in the midgut, where the GS/GltS pathway is absent [27]. The N atom of the amide group of two [5-15N]-Gln molecules is utilized to produce [15N2]-uric acid (UA), an antioxidant but also the main N waste product in mosquitoes. By using stable-label isotope tracing and RNA interference (RNAi) it was also discovered that Ae. aegypti mosquitoes have a functional amphibian-like uricolytic pathway that degrades UA to allantoin, allantoic acid, glyoxylic acid, and two urea molecules [28] (Figure 3). Interestingly, GS/GltS and uricolytic pathways were thought to be absent in mosquitoes. Furthermore, it was demonstrated that crosstalk signaling mechanisms between the pathways involved in ammonia detoxification ensure a tight coordination while preventing ammonia and free-radical toxicity in Ae. aegypti [29–32]. These findings have uncovered how blood-fed female mosquitoes overcome the absence of a functional urea cycle by using multiple metabolic pathways for ammonia disposal (Figure 3).
Figure 3. Metabolic interactions at N and C atomic level during ammonia detoxification in Aedes aegypti females.

Incorporation of 15N from 15N-ammonia into different 15N-metabolites is indicated in green. Incorporation of 13C from [1,2-13C2]-Glucose into different metabolites is indicated in red and blue. [1,2-13C2]-Glucose can be converted to [1,2-13C2]-G6P, which can be metabolized through the glycolysis and produce [13C]-metabolites (indicated in red). [1,2-13C2]-G6P can also be metabolized through the pentose phosphate pathway (PPP). The [1,3-13C2]-F6P and [1-13C1]-F6P produced in the PPP can enter into the glycolysis and contribute to the synthesis of [13C]-metabolites (indicated in blue). Not all the possible reactions between unlabeled, [13C]-metabolites, and [15N]-metabolites are represented in this figure. Abbreviations: α-KG, α-ketoglutarate; Ala, alanine; F6P, fructose-6-phosphate; GABA, γ-aminobutyric acid; GDH, glutamate dehydrogenase; Gln, glutamine; GS, glutamine synthetase; Glu, glutamate; G6P, glucose-6-phosphate; GltS, glutamate synthase; Lac, lactate; PEP, phosphoenolpyruvate; Pro, proline; Pyr, pyruvate; PK, pyruvate kinase; Ser, serine; UA, uric acid. Adapted from Scaraffia et al. (2006) [26], Scaraffia et al. (2008) [28], and Horvath et al. (2018) [37]. Copyright permissions obtained. Copyright (2006) Elsevier. Copyright (2008) National Academy of Sciences, USA. Copyright (2018) John Wiley.
Metabolomics has also contributed to the study of N metabolism and related pathways in Anopheles species. Targeted and nontargeted GC/MS metabolomics approaches have shown an increase in the abundance of certain AAs, organic acids (OAs), nucleotides, and other metabolites in An. gambiae females at 24 h post blood meal (PBM) [33]. RNAi-mediated silencing of phenylalanine hydroxylase (PAH), a gene encoding the enzyme responsible for the conversion of phenylalanine (Phe) into tyrosine (Tyr), led to an increase of α-KG, pyruvate (Pyr), phosphoenolpyruvate (PEP), glucose-6-phosphate (G6P), inosine monophosphate, and cytidine monophosphate, and a decrease of gluconate in non-fed PAH-deficient mosquitoes. On the contrary, a reduction in Tyr, and an increase in Phe, phenyllactate, phenylpyruvate, and phenylacetate were observed in blood-fed PAH-deficient mosquitoes at 24 h PBM. PAH-knockdown also disrupted female fertility and fecundity. A reduction in the number of eggs laid, a retarded vitellogenesis, and a reduction in chorion melanization after oviposition were observed. It was also reported that depletion of PAH resulted in dysfunction of the innate immune system and reduced the ability to mount a melanotic encapsulation response against Plasmodium berghei ookinetes upon ingestion of an infected blood meal [33]. The multiple phenotypes associated with genetic disruption of PAH highlight the key role of Phe metabolism in the reproduction and immunity of An. gambiae females.
A combination of RNAi and nontargeted GC/MS and LC/MS metabolomics has recently shown that high rates of branched-chain AA catabolism are extended in the late phase of the reproductive cycle of microRNA-276 (miR-276)-deficient Anopheles coluzzii females. Silencing of miR-276 also led to an increase in branched-chain amino acid transferase (BCAT) expression and mosquito fertility, a disruption in Plasmodium falciparum development, and a decrease in the number of transmissible sporozoites when females were fed with P. falciparum-infected blood meals [34]. Moreover, increased levels of Glu, histidine, lysine, citrulline, arginine (Arg), argininosuccinate, and ornithine were observed in the whole body of miR-276-deficient mosquitoes at 48 h PBM. The tightly regulated miR-276-BCAT axis shifts the metabolic state of the mosquito from catabolism during oogenesis to anabolism, which is needed to replenish glycogen and lipid reserves in preparation for the next reproductive cycle [34]. These data also provide new insights into the role of AA metabolism in within-vector Plasmodium development and malaria transmission. However, further studies are still necessary to determine how metabolic status and reproductive investment strategies in different mosquito species shape pathogen transmission.
Central carbon (C) metabolism and related pathways
To elucidate differences at the molecular level between large and small mosquitoes generated from larvae raised under standard conditions or nutritionally deficient conditions, transcriptomics and nontargeted GC/MS metabolomics studies were conducted in fat body dissected from large and small Ae. aegypti females before and 24 h PBM [35]. Changes in metabolite profiles and in the expression patterns of genes encoding proteins involved in several physiological processes – including AA metabolism, immunity, reproduction, autophagy, membrane transport, as well as synthesis and catabolism of energy reserves – were observed between unfed and blood-fed large and small mosquitoes. Interestingly, the abundance of certain metabolites – including specific free-AAs, alpha-tocopherol and glycerol – was higher in non-blood-fed small mosquitoes when compared to non-blood-fed large mosquitoes. Transcriptomics and metabolomics data suggest that, prior to blood feeding, small females mainly degrade proteins and reserves to produce energy in lieu of storage. Post-blood-feeding, small females seem to use nutrients released from their first blood meal to replenish their required energy reserves, rather than investing in reproduction. Conversely, large females tend to use the nutrients released from their first blood meal to produce energy to support vitellogenesis and egg production [35].
The energetic demands of Ae. aegypti females were investigated throughout the two phases of the reproductive cycle, post-eclosion (PE) and PBM, by using several techniques, including transcriptomics, targeted GC/MS metabolomics, and RNAi [36]. Profiles of transcripts, proteins, and metabolites related to major carbohydrate metabolism pathways – such as glycogen synthesis and degradation, glycolysis, pentose phosphate pathway (PPP), and Krebs cycle – exhibited variations throughout both phases. In general, the amount of glycogen, trehalose, triacylglycerols (TAGs), glucose (Glc), and fructose increased during the PE phase, but then decreased at different times PBM. In contrast, G6P, fructose-6-phosphate (F6P), Pyr, lactate (Lac), citrate, and malate decreased during the late PE phase, but most of them reached a high level at 6 h PBM. Changes in transcript and metabolite levels related to carbohydrate and intermediate metabolism were well correlated with energetic demands of female mosquitoes throughout the gonotrophic cycle. It was also demonstrated that juvenile hormone and ecdysone receptors play a key role as regulatory switches to synchronize carbohydrate metabolism with energy requirements during PE and PBM phases, respectively [36].
Due to the fact that the majority of the AAs released from blood meal digestion are deaminated, and the C skeleton is oxidized to CO2 for ATP production, it was hypothesized that the C skeleton of Glc (obtained from nectar, blood meal, and/or from degradation of maternal carbohydrates) contributes to the synthesis of OAs necessary to support ammonia clearance in Ae. aegypti blood-fed females. To verify the hypothesis, Ae. aegypti females were fed a blood meal supplemented with [1,2-13C2]-Glc. The [13C]-atom incorporation from [1,2-13C2]-Glc into several [13C]-labeled AAs, AA derivatives, and OAs was monitored in mosquito whole body at different times after feeding by using targeted metabolomics. Several isotopologs and isotopomers were analyzed. It was found that mosquitoes synthesize several [13C]-metabolites that are mainly labeled in two C positions. [13C2]-Ala and [13C2]-Pro were the most abundant and rapidly synthesized. Other AAs (i.e., Glu, Gln, and serine (Ser), AA derivatives (i.e., acetylcholine and γ-aminobutyric acid (GABA), and OAs (i.e., Pyr, Lac, and UA) were [13C]-labeled in one or two C positions. The presence of [13C]-UA suggests that [13C1]-Ser and/or [13C2]-Ser provide the C skeleton to synthesize labeled UA (Figure 3). Altogether, the data provide evidence that the C skeleton of Glc contributes to ammonia detoxification through the concerted action of multiple metabolic pathways. Furthermore, a metabolic link between Glc and ammonia metabolism was uncovered in mosquitoes [37] (Figure 3). More recently, MS-based stable-label isotope tracing has revealed that pyruvate kinase (PK) confers on Ae. aegypti females a metabolic plasticity to control both C and N metabolism. Genetic inhibition of PK by RNAi disrupts Glc and ammonia metabolism in mosquito whole body. PK silencing delays several physiological processes and reduces [13C]-metabolite abundance (i.e., G6P, F6P, Pyr, Lac, α-KG, citrate, isocitrate, Ala, Glu, Gln, Pro, and UA) mainly at 6 h after feeding, by decreasing the flux of Glc to glycolysis, PPP, Krebs cycle, and pathways associated with ammonia detoxification [38] (Figure 3). The application of metabolomics with cutting-edge genome-editing technologies [39] can further facilitate the discovery of metabolic interactions and regulators that control multiple metabolic pathways in mosquitoes.
Intermediate metabolism has been also investigated in Anopheles stephensi females. Genetic and chemical manipulation of signaling pathways as well as nontargeted GC/MS metabolomics in An. stephensi midgut have provided clues on how a midgut-related insulin-like peptide, c-Jun N-terminal kinase (JNK), and intermediate metabolism, mediate anti-P. falciparum resistance [40,41]. Disruption of JNK signaling alters mosquito metabolism, extends mosquito lifespan, and enhances resistance to P. falciparum infection. Shifts in intermediate metabolism in mosquitoes due to JNK inhibition seem to harmfully impact parasite metabolism. Impairment of JNK with specific JNK-small molecule inhibitors suggests that parasites can increase access to essential nutrients from their mosquito host by activating this pathway [41]. Implementation of MS-based stable-label isotope tracing analysis could provide detailed information at the atomic level into vector–parasite metabolism and further assist in the development of metabolism-based strategies to block malaria transmission.
Environmental adaptability
New insights into biological adaptations to cold, dehydration, and desiccation tolerance in mosquitoes have also been gained by MS-based metabolomics approaches.
Diapause is well documented in certain mosquito species [42–44], but the metabolic regulation of embryonic diapause at the metabolite levels is still an emerging area in mosquito research. Distinct profiles of metabolites – including AAs, AA derivatives, OAs, acyl-carnitines (AcCas), nucleic acids, nucleosides, and lipids – were identified by nontargeted LC/MS metabolomics in diapause and non-diapause eggs from Aedes albopictus [45]. Overall, diapause eggs increased lipid abundance, mainly TAGs and diacylglycerols (DAGs), and decreased abundance of several metabolites such as free carnitine, a few AcCas, and phosphocholine (PC), suggesting an activation of lipogenesis and inhibition of lipolysis. Several metabolites involved in different pathways – including AA and AA derivative metabolism, pyrimidine metabolism, Glc-Ala cycle, and neuroregulatory and insulin-like signaling pathways – also significantly decreased in diapause eggs. Moreover, the detection of diapause-specific steroid features suggests that ecdysone signaling could be involved in diapause regulation. These changes in lipid and other metabolite profiles provide new insights to better understand the metabolic regulation of embryonic diapause in Ae. albopictus mosquitoes [45].
Aestivation has also been studied in mosquitoes [44,46]. Metabolic profiles of four An. coluzzii mosquito populations – originally from arid and humid regions of north and southwest of Burkina Faso (West Africa) – were assessed using targeted GC/MS analysis to identify potential metabolite signatures in response to severe desiccating conditions during the dry season [47]. Wild-caught An. coluzzii from Oursi and Déou (permanent and temporary breeding habitats from north of Burkina Faso), and wild-caught An. coluzzii from Bama and Soumousso (permanent and temporary breeding habitats from southwest of Burkina Faso) were first used to establish colonies in the laboratory. Mosquitoes were then maintained in climatic chambers to mimic the onset of the dry season and rainy season. In mosquitoes sampled from Oursi and Bama, an increase in F6P, G6P, glycerol-3-phosphate (G3P), sorbitol, and succinic acid were observed in dry-season-reared females, whereas high levels of adonitol, arabitol, fructose, gluconic acid, and ribose were observed in the rainy-season-reared females. In mosquitoes sampled from Déou and Soumousso, dry-season-reared females showed high abundance of G3P, inositol, Lac, maltose, Pro, and xylitol, whereas rainy-season-reared females exhibited higher amounts of arabitol, GABA, gluconic acid, mannitol, and trehalose. It was suggested that glycolysis, and non-oxidative phase of PPP, could be increased in dry-season-reared females from permanent breeding habitats (Oursi and Bama). Some differences in metabolite abundance among An. coluzzii populations, and under the two specific conditions studied, led authors to suggest that females from temporary northern habitats could have a strong aestivation strategy to overcome desiccation [47]. The molecular mechanisms behind environmental adaptability in mosquitoes, and the impact on disease-transmission, have yet to be further addressed.
Drug pollutant and insecticide impact
It is undeniable that local adaptation of certain mosquito species in urban areas, and the increase in insecticide resistance, represent serious obstacles for current vector-control strategies [48,49].
A combined approach that includes targeted GC/MS metabolomics and transcriptomics has been utilized to monitor the intergenerational impact of the commonly used drug ibuprofen, a surface water pollutant, in Ae. aegypti [50]. It was found that ibuprofen exposure mainly increases generation 0 (F0) of male lifespan and tolerance to starvation in first generation (F1) of first-stage larvae. Ibuprofen also differentially regulates the expression of multiple transcripts in F0 of fourth-stage larvae and females, and F1 of first-stage larvae after direct or parental ibuprofen exposure. Moreover, ibuprofen exposure increases ecdysone signaling and stress-response transcriptional potential in F0 of first-stage larvae, and decreases AAs, carbohydrates, phosphoric acid, and polyol levels in F1 of eggs [50]. Although this study has provided new insights into the intergenerational effects of ibuprofen in mosquitoes, further genetic and biochemical analyses are still needed to fully understand the mechanistic impact of ibuprofen and other pollutants on mosquito metabolism.
Targeted LC/MS metabolomics has also been applied to identify potential key metabolite(s) that can serve as biomarkers for diagnosis of insecticide exposure. Changes in specific AcCas, free carnitine, and AAs were observed in Culex quinquefasciatus Say larvae exposed to 0.002 μg/ml of the pyrethroid permethrin or 0.01 μg/ml of the organophosphate temephos [51]. Overall, specific AcCas, free carnitine, and Arg levels increase significantly in larvae exposed to permethrin, whereas Arg levels increase significantly in larvae exposed to temephos [51].
Variations in AcCas, glycerophospholipids (GPLs) and biogenic amine abundance were observed in Culex pipiens larvae exposed to 1 ng/l, 100 ng/l, or 10 000 ng/l of clothianidin, a neonicotinoid insecticide [52]. After 24 h of clothianidin exposure, the highest dose applied decreases AcCas, GPLs, and biogenic amines, whereas medium and low doses decrease GPLs and biogenic amines but increase AcCas. Notably, 48 h after pesticide exposure, GPLs and AcCas decrease at the low and medium doses, and do not change significantly at the high dose [52]. The mechanism(s) associated with changes in metabolite abundance after insecticide exposure in mosquitoes remain to be revealed.
Metabolite alteration upon viral and/or bacterial infections
Multiple metabolomic changes reported in response to arboviral infections – including dengue, chikungunya, and Zika viruses – in biofluids (i.e., serum, blood, and urine) from humans, mice, and monkeys, as well as in Ae. aegypti midgut and Ae. albopictus cells, were previously reviewed [7].
In correlation with dynamic and temporal lipid alterations observed in mosquito midgut at 3, 7, and 11 days post-dengue infection [53], genetic and nontargeted LC/MS metabolomic analyses in Ae. aegypti cells, and Ae. aegypti midgut and whole body, confirm that the phospholipids (PLs) of mosquitoes are reconfigured throughout dengue infection [54]. Furthermore, the data reveal that the dengue viral infection suppresses mosquito acylglycerolphosphate acyltransferase 1 expression, which yielded an increase in PLs (mainly phosphatidylethanolamines, phosphatidylcholines, and phosphatidylserines) for virus multiplication [54]. These studies suggest that the dengue virus modulates mosquito metabolism for its own benefit [53,54]. In agreement with these findings, recent nontargeted LC/MS metabolomics and stable-label isotope tracing analyses indicate that the dengue virus inhibits the de novo PL pathway and actively reconfigures PLs through the remodeling cycle in mosquito vector to facilitate its replication [55]. It was also found that ethanolamine, a blood metabolite, reduces mosquito infection and serves as a de novo PL precursor and as a determinant of human-to-mosquito virus transmission [55]. Moreover, nontargeted GC/MS and LC/MS metabolomics analyses in Ae. albopictus infected with Zika virus have recently shown changes in the metabolic profile of lipids and biogenic amines that are related to RNA editing, and inflammatory and antiviral responses [56]. Also, an increase in metabolites related to diverse pathways such as glycolysis and uricolysis was observed in Zika-infected mosquitoes [56].
The release of artificially Wolbachia-infected Ae. aegypti or naturally Wolbachia-infected Ae. albopictus is one of the approaches proposed to either suppress mosquito populations or disrupt viral transmissions [57]. In recent years, a metabolomics approach has been applied to study Wolbachia – an intracellular bacterial symbiont – in infected mosquito cells and the impact of antiviral effects in mosquitoes artificially infected with Wolbachia. Interestingly, Wolbachia pipientis-infected Ae. albopictus cells alter the intracellular lipidomic environment by depleting most of the sphingolipids (i.e., ceramides, longer-chain lactosylceramides, phosphoethanolamineceramide), and reducing some DAGs and PLs such as PCs [58], which is the opposite lipid profile shift compared to the one reported in dengue-infected mosquito cells [7,54]. It was proposed that W. pipientis generates a cellular lipid environment that inhibits mosquito-borne viruses [58]. Lipid profiles of Ae. aegypti mosquitoes bearing mono or dual infections of the Wolbachia wMel strain and dengue virus suggest that virus-driven modulation dominates over that of Wolbachia [59]. Data also suggest that Wolbachia-induced alterations are disadvantageous for dengue replication and that the direct competition between dengue and Wolbachia for the same host lipids is limited. It was also discovered that cardiolipins are host factors for dengue and Wolbachia replication [59]. Lipid profiles of Aag2 cells infected with Wolbachia only, either dengue or Zika virus only, and Wolbachia-infected Aag2 cells coinfected with either dengue or Zika virus, were also recently explored [60]. It was reported that AcCas decrease during Wolbachia infection but increase during virus infection. It was also found that the addition of AcCas increases flavivirus infection of Wolbachia-infected cells, suggesting that modulation of AcCas is the mechanism that causes Wolbachia-mediated inhibition of flaviviruses in Ae. aegypti [60]. The elucidation of additional metabolic signatures and/or regulators could lead towards the discovery of target molecule(s) to disrupt or prevent mosquito-borne viral diseases.
Concluding remarks
MS-based metabolomics studies in mosquitoes, with their sensitive, specific, and quantitative capabilities, have already proven to be valuable for understanding key aspects of mosquito metabolism and vector–host–pathogen interactions. In particular, MS-based metabolomics combined with other technologies, such as transcriptomics and/or RNAi, have provided new insights into how certain mosquito species successfully dispose of nitrogen waste, cope with the reduction/depletion of critical proteins relating to different metabolic processes, and endure adverse environmental conditions, exposure to toxic chemicals, and pathogen infections. Identification of specific phenotypes – such as changes in metabolite profile(s) or variations in metabolite abundance – has exposed the mosquito metabolic plasticity in response to internal and external alterations.
The integration of metabolomics with other ‘omics’ and modern genome-editing techniques is anticipated to be expanded to the study of additional mosquito species and be crucial in further advancing mosquito research by uncovering metabolic links between the small-molecule kinetics and pathways present in mosquitoes, and those involved in the mosquito–host–pathogen interface (see Outstanding questions). This approach is also expected to enhance our knowledge of the mosquito as a whole organism rather than as a linear sum of its parts [9]. Collaborations between experts in several fields – including medical entomology, parasitology, biochemistry, bioinformatics, and analytical mass spectrometry – will continue to be essential in this effort.
Outstanding questions.
What are the dynamics of metabolite concentrations in different mosquito tissues/organs? How do metabolic fluxes vary both within and between mosquito tissues/organs? How do diverse factors such as epigenetic regulators, hormones, and signaling cascades impact metabolic fluxes in mosquitoes? What are the biochemical and molecular mechanisms that drive metabolic fluxes in mosquitoes in response to internal or external perturbations?
Can the combination of MS-based metabolomics and stable-label isotope tracers be used to better determine the rates of metabolic reactions and interconnections between metabolites in the metabolic pathways present in mosquitoes? Could these powerful methods be used to define metabolic networks in noninfected and pathogen-infected mosquitoes?
Will advances in modern MS (better resolution, higher sensitivity, improved data processing, expanded reference standard offerings and databases) pave the way to discoveries of new (‘unknown’) metabolites and metabolic routes? Would the development of new computational models, software, and databases specific to mosquitoes accelerate the pace of discovery in this area? Can these tools expand upon existing knowledge about interconnectedness of metabolic pathways in noninfected and pathogen-infected mosquitoes?
How do existing/proposed mosquito-control strategies impact mosquito metabolite concentrations/profiles over time? What are the unique features that exist in the mosquito metabolome compared to close phylogenetic species? Might these differences be leveraged to develop selective mosquito-control strategies? Can MS-based metabolomics approaches be used to discover small-molecule inhibitors that prevent pathogen infection and transmission in the mosquito host?
Many of the targeted metabolomic studies are successfully performed with relatively simple MS instrumentation (such as triple- or even single-quad GC– or LC–MS systems). The more sophisticated high-resolution exact mass systems are beneficial for the pursuit of new (‘unknown’) metabolites carried out in discovery-based studies. The continued improvement of mass spectrometers, databases, and data-processing software for the identification of unknown metabolites will play a major role in unraveling finer mosquito metabolic interactions, novel targets, and key regulatory mechanisms. New discoveries in this field can assist in the development of novel metabolism-based strategies to reduce vector populations and/or to disrupt parasite development or virus replication in both human host and mosquitoes.
Highlights.
Innovations in mass spectrometry (MS)-based hardware, software, and curated databases have improved metabolomics workflows with the capability to advance our understanding of fundamental life processes.
MS-based metabolomics coupled with genetic techniques has shed light on how mosquito metabolism is modulated under different experimental conditions.
Variations in metabolite abundance in eggs, larvae, or adult mosquitoes have provided critical clues to understand how mosquitoes cope with temperature and humidity extremes, and exposure to toxic compounds or pathogens.
The application of MS-based metabolomics combined with 15N or 13C isotope tracing has empowered scientists to perform high-precision dynamic studies, monitor multiple isotopologs simultaneously over time, and reveal metabolic pathways at the atomic level in mosquito samples.
Acknowledgments
P.Y.S. is financially supported by the U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases Grant R01AI146199.
Glossary
- Absolute/relative quantification
absolute quantification requires calibrators, and results are reported in concentration units (i.e., ng/ml or nM), whereas relative quantification is a noncalibrated measurement and comparative results between groups of samples are reported in relative ratios of integrated peak areas (i.e., detector counts)
- Aestivation
a strategy that allows some organisms to endure high temperatures and/or arid conditions
- Calibrator(s)
calibration standard samples prepared with analyte(s) of interest at known concentrations used to calibrate the MS-response for a given analyte(s) concentration
- Diapause
a strategy that allows organisms to enter developmental arrest in anticipation of adverse environmental conditions such as low temperatures and dehydration
- Full-scan
a nontargeted MS mode that uses mass analyzers to scan broad m/z ranges to allow translation of all compounds or all product ions within the selected range for analysis
- Gas chromatography–mass spectrometry (GC/MS)
gas-phase separation that uses stationary-phase coated capillary columns, a carrier gas mobile phase and a temperature gradient to shift adsorption–desorption equilibria of the analyte(s) between the two phases. A mass spectrometer is the detector
- Isotopologs/isotopomers
isotopologs of a specific metabolite contain differing quantities of specific isotope(s) in their molecular formula (i.e., [13C0]-Gln, [13C1]-Gln, [13C2]-Gln). Isotopomers contain a consistent number of specific isotope(s) that are distributed in different atomic locations (i.e., [1,2-13C2]-Gln, [2,3-13C2]-Gln)
- Liquid chromatography–mass spectrometry (LC/MS)
liquid-phase separation that uses stationary-phase coated silica/polymer microparticles, a liquid mobile phase, and a solvent, pH, or salt gradient to shift adsorption–desorption equilibria of analyte(s) between the two phases. A mass spectrometer is the detector
- Nontargeted metabolomics
a full-scan MS approach used to screen the relative levels of metabolites in a sample
- Selected/multiple-reaction monitoring (SRM/MRM)
SRM constitutes a targeted MS/MS mode that monitors a single specific precursor to a single product ion transition. MRM monitors a single or multiple precursor ions into multiple product ion transitions. Parallel-reaction monitoring is analogous to targeted SRM or MRM scan modes on HRMS/UHRMS systems
- Stable-label isotope tracing
a technique that uses metabolic substrates that contain stable atomic isotopes (i.e., 13C or 15N) to map the interconnectedness of metabolic pathways
- Tandem mass spectrometry (MS/MS)
protonated, deprotonated or adducts of molecular ions (precursors) are selected by the first mass analyzer, then are fragmented by collisionally induced processes, and the resultant product ions are selected by the second mass analyzer prior to detection. Most common MS/MS modes are targeted and nontargeted ‘product ion scan’ and targeted MRM/SRM
- Targeted metabolomics
selected-ion monitoring (SIM)-based MS or SRM/MRM-based MS/MS approaches used to quantitatively measure specific metabolite(s) in a sample
Footnotes
Declaration of interests
The authors declare no competing interests.
References
- 1.Chiodini J (2018) Apps from the World Health Organization – The World Malaria Report and more. Travel Med. Infect. Dis 22, 82–84 [DOI] [PubMed] [Google Scholar]
- 2.Dahmana H and Mediannikov O (2020) Mosquito-borne diseases emergence/resurgence and how to effectively control it biologically. Pathogens 9, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina JP et al. (2019) The current and future global distribution and population at risk of dengue. Nat. Microbiol 4, 1508–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellone R and Failloux AB (2020) The role of temperature in shaping mosquito-borne viruses transmission. Front. Microbiol 11, 584846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan SJ et al. (2020) Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malar. J 19, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis C et al. (2021) A regional suitable conditions index to forecast the impact of climate change on dengue vectorial capacity. Environ. Res 195, 110849. [DOI] [PubMed] [Google Scholar]
- 7.Byers NM et al. (2019) Metabolomic insights into human arboviral infections: dengue, chikungunya, and Zika viruses. Viruses 11, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthubharathi BC et al. (2021) Metabolomics: small molecules that matter more. Mol. Omics Published online January 18, 2021. 10.1039/d0mo00176g [DOI] [PubMed] [Google Scholar]
- 9.Klassen A et al. (2017) Metabolomics: definitions and significance in systems biology. In Metabolomics: From Fundamentals to Clinical Applications (Sussulini A, ed.), pp. 3–17, Springer; [DOI] [PubMed] [Google Scholar]
- 10.Zhou J and Yin Y (2016) Strategies for large-scale targeted metabolomics quantification by liquid chromatography-mass spectrometry. Analyst 141, 6362–6373 [DOI] [PubMed] [Google Scholar]
- 11.Yuan M et al. (2012) A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 7, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gika H et al. (2019) Untargeted LC/MS-based metabolic phenotyping (metabonomics/metabolomics): The state of the art. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 1117, 136–147 [DOI] [PubMed] [Google Scholar]
- 13.Gertsman I and Barshop BA (2018) Promises and pitfalls of untargeted metabolomics. J. Inherit. Metab. Dis 41, 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements AN, ed (1992) The Biology of Mosquitoes, Chapman & Hall [Google Scholar]
- 15.Foster WA (1995) Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443–474 [DOI] [PubMed] [Google Scholar]
- 16.Zhu J and Noriega FG (2016) The role of juvenile hormone in mosquito development and reproduction. In Advances in Insect Physiology: Progress in Mosquito Research (Raikhel AS, ed.), pp. 93–113, Elsevier Academic Press [Google Scholar]
- 17.Mitchell SN and Catteruccia F (2017) Anopheline reproductive biology: impacts on vectorial capacity and potential avenues for malaria control. Cold Spring Harb. Perspect. Med 7, a025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng X et al. (2018) microRNA profiles and functions in mosquitoes. PLoS Negl. Trop. Dis 12, e0006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy S et al. (2018) Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol 63, 489–511 [DOI] [PubMed] [Google Scholar]
- 20.Valzania L et al. (2019) Blood feeding activates the vitellogenic stage of oogenesis in the mosquito Aedes aegypti through inhibition of glycogen synthase kinase 3 by the insulin and TOR pathways. Dev. Biol 454, 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isoe J et al. (2019) Identification and characterization of a mosquito-specific eggshell organizing factor in Aedes aegypti mosquitoes. PLoS Biol 17, e3000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling L and Raikhel AS (2021) Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl Acad. Sci. U. S. A 118, e2023470118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oringanje C et al. (2021) Overexpression of activated AMPK in the Anopheles stephensi midgut impacts mosquito metabolism, reproduction and Plasmodium resistance. Genes (Basel) 12, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X et al. (2021) The ecdysone-induced protein 93 is a key factor regulating gonadotrophic cycles in the adult female mosquito Aedes aegypti. Proc. Natl Acad. Sci. U. S. A 118, e2021910118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zdobnov EM et al. (2002) Comparative genome and proteome analysis of Anopheles gambiae and Drosophila melanogaster. Science 298, 149–159 [DOI] [PubMed] [Google Scholar]
- 26.Scaraffia PY et al. (2006) Analysis of whole body ammonia metabolism in Aedes aegypti using [15N]-labeled compounds and mass spectrometry. Insect Biochem. Mol. Biol 36, 614–622 [DOI] [PubMed] [Google Scholar]
- 27.Scaraffia PY et al. (2010) Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J. Insect Physiol 56, 1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaraffia PY et al. (2008) Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U. S. A 105, 518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isoe J and Scaraffia PY (2013) Urea synthesis and excretion in Aedes aegypti mosquitoes are regulated by a unique cross-talk mechanism. PLoS One 8, e65393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzalupo S et al. (2016) Effective disposal of nitrogen waste in blood-fed Aedes aegypti mosquitoes requires alanine aminotransferase. FASEB J 30, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petchampai N and Scaraffia PY (2016) Nitrogen metabolism in mosquitoes: new insights into the nitrogen metabolism in blood-fedmosquitoes. In Advances in Insect Physiology: Progress in Mosquito Research (Raikhel AS, ed.), pp. 363–391, Elsevier Academic Press [Google Scholar]
- 32.Isoe J et al. (2017) Xanthine dehydrogenase-1 silencing in Aedes aegypti mosquitoes promotes a blood feeding-induced adulticidal activity. FASEB J 31, 2276–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs S et al. (2014) Phenylalanine metabolism regulates reproduction and parasite melanization in the malaria mosquito. PLoS One 9, e84865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampe L et al. (2019) Metabolic balancing by miR-276 shapes the mosquito reproductive cycle and Plasmodium falciparum development. Nat. Commun 10, 5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price DP et al. (2015) Small mosquitoes, large implications: crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasit. Vectors 8, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou Y et al. (2015) Temporal coordination of carbohydrate metabolism during mosquito reproduction. PLoS Genet 11, e1005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horvath TD et al. (2018) Positional stable isotope tracer analysis reveals carbon routes during ammonia metabolism of Aedes aegypti mosquitoes. FASEB J 32, 466–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petchampai N et al. (2020) Mass spectrometry-based stable-isotope tracing uncovers metabolic alterations in pyruvate kinase-deficient Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol 121, 103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adolfi A and Lycett GJ (2018) Opening the toolkit for genetic analysis and control of Anopheles mosquito vectors. Curr. Opin. Insect Sci 30, 8–18 [DOI] [PubMed] [Google Scholar]
- 40.Pietri JE et al. (2016) Two insulin-like peptides differentially regulate malaria parasite infection in the mosquito through effects on intermediary metabolism. Biochem. J 473, 3487–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souvannaseng L et al. (2018) Inhibition of JNK signaling in the Asian malaria vector Anopheles stephensi extends mosquito longevity and improves resistance to Plasmodium falciparum infection. PLoS Pathog 14, e1007418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denlinger DL and Armbruster PA (2014) Mosquito diapause. Annu. Rev. Entomol 59, 73–93 [DOI] [PubMed] [Google Scholar]
- 43.Hand SC et al. (2016) Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish. Am. J. Physiol. Regul. Integr. Comp. Physiol 310, R1193–R1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes CJ and Benoit JB (2019) Biological adaptations associated with dehydration in mosquitoes. Insects 10, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batz ZA and Armbruster PA (2018) Diapause-associated changes in the lipid and metabolite profiles of the Asian tiger mosquito, Aedes albopictus. J. Exp. Biol 221, jeb189480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benoit JB (2010) Water management by dormant insects: comparisons between dehydration resistance during summer aestivation and winter diapause. Prog. Mol. Subcell. Biol 49, 209–229 [DOI] [PubMed] [Google Scholar]
- 47.Hidalgo K et al. (2016) Comparative physiological plasticity to desiccation in distinct populations of the malarial mosquito Anopheles coluzzii. Parasit. Vectors 9, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamdem C et al. (2017) Pollutants and insecticides drive local adaptation in African malaria mosquitoes. Mol. Biol. Evol 34, 1261–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adedeji EO et al. (2020) Anopheles metabolic proteins in malaria transmission, prevention and control: a review. Parasit. Vectors 13, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prud’homme SM et al. (2018) Multiscale approach to deciphering the molecular mechanisms involved in the direct and intergenerational effect of ibuprofen on mosquito Aedes aegypti. Environ. Sci. Technol 52, 7937–7950 [DOI] [PubMed] [Google Scholar]
- 51.Martin-Park A et al. (2017) Profiles of amino acids and acylcarnitines related with insecticide exposure in Culex quinquefasciatus (Say). PLoS One 12, e0169514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo R et al. (2018) Identification of pesticide exposure-induced metabolic changes in mosquito larvae. Sci. Total Environ 643, 1533–1541 [DOI] [PubMed] [Google Scholar]
- 53.Chotiwan N et al. (2018) Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathog 14, e1006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vial T et al. (2019) Dengue virus reduces AGPAT1 expression to alter phospholipids and enhance infection in Aedes aegypti. PLoS Pathog 15, e1008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vial T et al. (2020) Mosquito metabolomics reveal that dengue virus replication requires phospholipid reconfiguration via the remodeling cycle. Proc. Natl. Acad. Sci. U. S. A 117, 27627–27636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onyango MG et al. (2020) Zika virus infection results in biochemical changes associated with RNA editing, inflammatory and antiviral responses in Aedes albopictus. Front. Microbiol 11, 559035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogunlade ST et al. (2021) A review: Aedes-borne arboviral infections, controls and Wolbachia-based strategies. Vaccines (Basel) 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molloy JC et al. (2016) Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl. Environ. Microbiol 82, 3109–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koh C et al. (2020) Dengue virus dominates lipid metabolism modulations in Wolbachia-coinfected Aedes aegypti. Commun. Biol. 3, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manokaran G et al. (2020) Modulation of acyl-carnitines, the broad mechanism behind Wolbachia-mediated inhibition of medically important flaviviruses in Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A 117, 24475–24483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Everett JR et al. (2019) A unified conceptual framework for metabolic phenotyping in diagnosis and prognosis. Trends Pharmacol. Sci 40, 763–773 [DOI] [PubMed] [Google Scholar]
- 62.Sindelar M and Patti GJ (2020) Chemical discovery in the era of metabolomics. J. Am. Chem. Soc 142, 9097–9105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacob M et al. (2019) Metabolomics toward personalized medicine. Mass Spectrom. Rev 38, 221–238 [DOI] [PubMed] [Google Scholar]
- 64.Schroeder M et al. (2019) Generation of a collision cross section library for multi-dimensional plant metabolomics using UHPLC-trapped ion mobility-MS/MS. Metabolites 10, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hecht ES et al. (2019) Fundamentals and advances of orbitrap mass spectrometry. In Encyclopedia of Analytical Chemistry (Meyers RA, ed.), pp. 1–40, Wiley [Google Scholar]