Abstract
This review will discuss the interactions of steroids with the gut and vaginal microbiomes within each life phase of adult women and the implications for women’s health. Each phase of a woman’s life is characterized by distinct hormonal states which drive overall physiology of both host and commensal microbes. These host-microbiome interactions underlie disease pathology in disorders affecting women across their lifetime, including bacterial vaginosis, gestational diabetes, polycystic ovary syndrome, anxiety, depression and obesity. While many associations between host health and microbiome composition are well defined, the mechanistic role of the microbiome in women’s health outcomes is largely unknown. This review will address potential mechanisms by which the microbiota influences women’s health and highlight gaps in the current knowledge.
Keywords: gut microbiome, estrogens, progestins, pregnancy, menopause, polycystic ovary syndrome
It is well established that the human microbiome has a dramatic impact on health and disease. In this review, we discuss the interaction of steroids with the gut and vaginal microbiomes and the implications of these interactions on women’s health across three of the major developmental transitions: early adulthood, pregnancy and menopause.
Microbiota refers to the microorganisms that inhabit the body, including bacteria, viruses, archaea, protozoa, and fungi [1]. These microorganisms, their genomes and the metabolites they produce comprise the microbiome. The bacterial composition of the human gut is plastic in infants and becomes adult-like around the age of three [2]. While sex differences in the gut microbiota (GM, see Glossary) exist prior to puberty, these differences are greater after puberty [3] and persist into adulthood [4], suggesting gonadal steroids influence gut microbial composition [5]. While the plasticity and development of the vaginal microbiome in early years remains unclear, the sudden surge in sex steroid levels during puberty is observed to be associated with lower diversity of vaginal microbiota (VM) [6]. Taken together, these findings suggest that the increase in endogenous steroid hormones at puberty creates a new environment and shapes the adult female gut and vaginal microbiomes. This new adult female hormonal milieu establishes a novel equilibrium between host and commensal microbial physiology, which is central to women’s health (Figure 1).
Figure 1. Women’s life phases are characterized by distinct changes in hormonal milieu and vaginal microbiota.
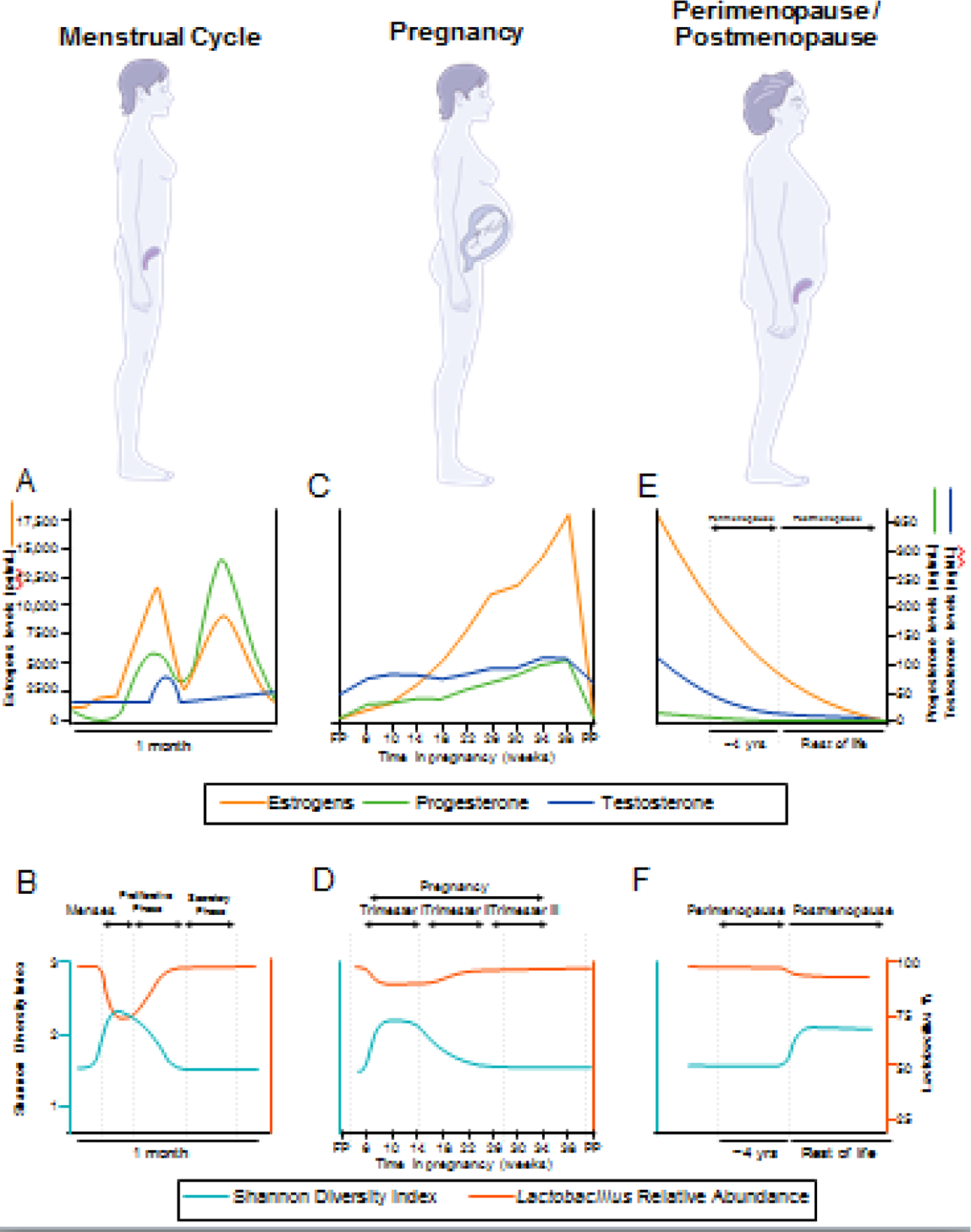
Estrogens and progesterone follow a cyclical pattern of release during the menstrual cycle (A), which influence the composition of the vaginal microbiota (VM), where Shannon index is a measure of diversity (B). Pregnancy (C) is characterized by a lack of hormonal cyclicity and steadily increasing sex steroids to maintain pregnancy. The initial change in hormone levels relative to the follicular phase (FP) alters the composition of the VM during pregnancy (D), which later returns to a state similar to that of the secretory phase of the menstrual cycle. Hormone levels return to pre-pregnancy values postpartum (PP). At menopause (E), ovarian function declines and sex steroid levels drop precipitously. The endocrine changes of menopause coincide with drastic changes to the VM (F), including a decrease in Lactobacillus relative abundance.
Sites of estrogen modification and response by microbiota
Estrobolome and GM
The estrobolome describes the functional collection of all enteric bacterial gene products capable of metabolizing estrogens (Figure 2). In the process of conjugation, estrogens are metabolized in the liver and marked for excretion via urine and feces by the addition of a glucuronic acid moiety [7]. Removal of the glucuronic acid group via deconjugation allows estrogens to remain in the body and exert their effects. Enteric bacteria (e.g. the genera Bifidobacterium, Clostridium, and Lactobacillus [8]) produce β-glucuronidases and β-glucuronides that can determine the fate of estrogens in the gut via deconjugation and conjugation, respectively [7]. The relative ratio of these bacterial enzymes, β-glucuronidases and β-glucuronides, in the gut directly impacts the amount of circulating estrogens. Certain bacteria involved in the estrobolome produce enzymes that differentially deconjugate estradiol (E2) and estrone [9]. The selective deconjugation of certain estrogens by GM could alter the circulating hormonal profile, in turn affecting host physiology. While a direct link between the capacity of the GM to metabolize estrogens and mediate metabolic changes in pregnancy and menopause has yet to be shown, there is the potential that they are functionally linked through the estrobolome.
Figure 2. Systemic effects and modulation of estrogens.
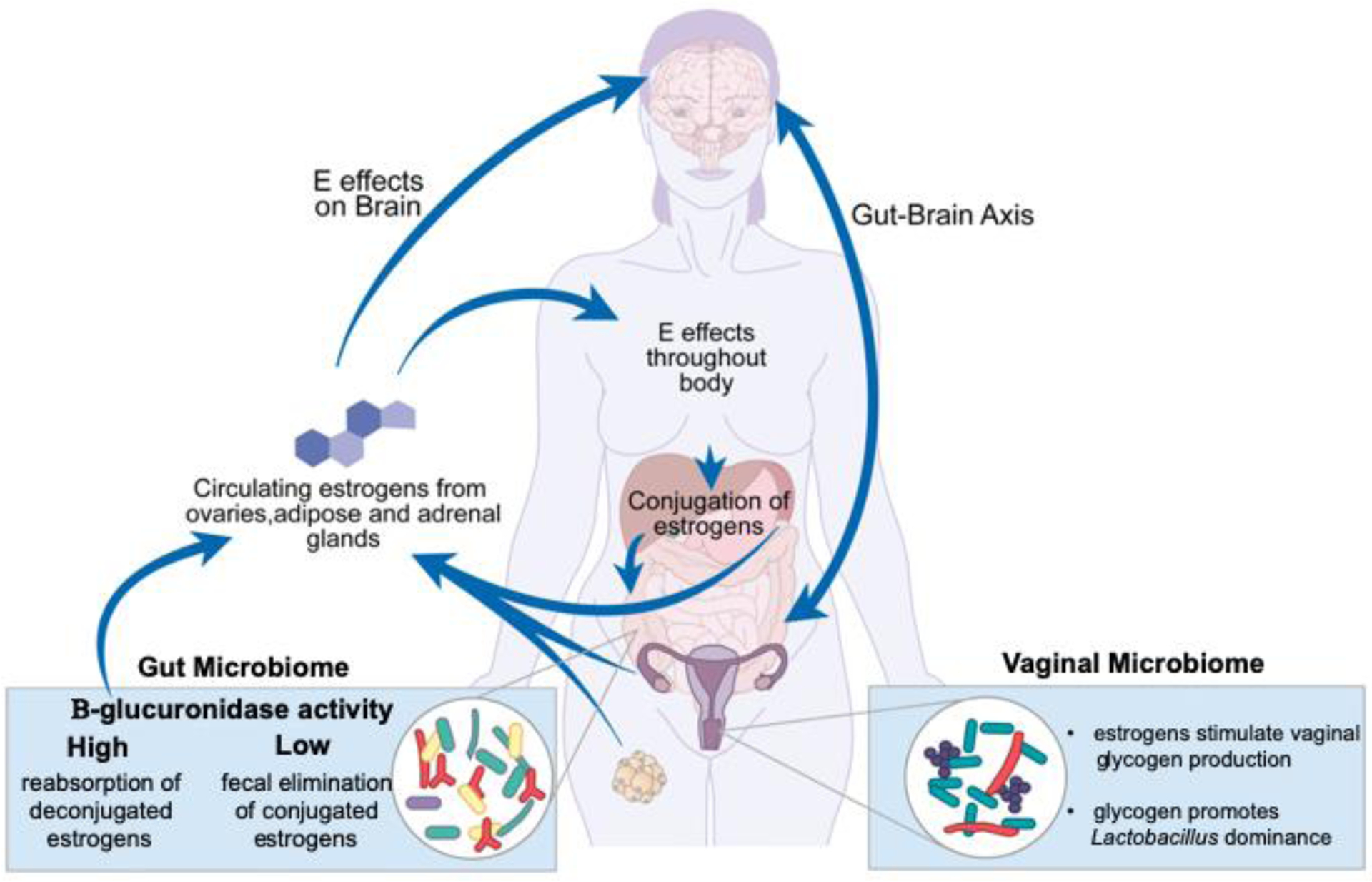
Estrogens have widespread effects throughout the body and are central to many aspects of women’s health. In addition to their central effects, estrogens act on, and are acted on, by the human microbiome. The GM consists of microbes that produce β-glucuronidase. This enzyme can deconjugate estrogens that were bound for excretion, causing them to reenter the body and remain active [7]. The VM composition is influenced by estrogens in the body via its effects on the vaginal epithelium [12]. Vaginal glycogen increases in response to estrogens and drives Lactobacillus spp. dominance in the VM [11].
Glycogen-Estrogen Hypothesis and VM
The hypothesized mechanism by which estrogens establish a high abundance of Lactobacillus spp. in the human VM is through driving an increase in glycogen production by vaginal epithelial cells, which promotes Lactobacillus growth [6, 10, 11] (Figures 1A and 1B). In fact, one evolutionary explanation for how humans developed Lactobacillus dominance suggests that the high starch content of human diets led to high glycogen content in the vaginal tract, creating an ideal environment for lactobacilli [12]. Interestingly, intravaginal estrogen treatment in transgender men appears to increase their typically low vaginal Lactobacillus abundance [13].
Menstrual Cycle
E2 and progesterone levels fluctuate across different phases of the menstrual cycle (Figure 1A). The follicular phase is characterized by rising estrogen levels, in particular E2. During the ovulatory phase, E2 peaks when progesterone levels also begin to rise. During the luteal phase, the follicle transforms into the corpus which secretes progesterone for stimulation of the secretory endometrium. When pregnancy does not occur, the corpus luteum degenerates and estrogen and progesterone levels decline to basal levels, which triggers shedding of the secretory endometrium [14].
Menstrual Cycle and GM
Describing the standard GM in healthy adults is challenging given that each individual’s signature is unique due to a wide variety of factors that influence the GM composition, including sex, age, diet, and BMI [15]. However, across most populations the Firmicutes and Bacteroides phyla are the most abundant. In support, Eubacterium rectale-Clostridium coccoides, Bacteroides-Prevotella and Faecalibacterium prausnitzii are the most prevalent gut microbes across all groups of European adults [16]. It appears that women have lower levels of Bacteroidetes than men [15, 16]. While a variety of rodent studies suggest that ovarian steroids alter GM [5, 17–19], we are only beginning to understand the impact of the menstrual cycle on GM [20]. Given that the menstrual cycle affects a variety of gastrointestinal disorders, including irritable bowel syndrome [21], it will be important for future studies to investigate the effects of the menstrual cycle on GM and the implications for women’s health.
Menstrual Cycle and VM
In the healthy adult vaginal microbiome, Lactobacillus dominance protects the vaginal environment by producing lactic acid [22], creating an exceptionally low pH environment [23]. In addition to creating an unsuitable environment for pathogenic bacterial growth, which can cause bacterial vaginosis (BV), Lactobacillus dominance and low pH contribute to the normal immune response of the vaginal epithelium [24]. A healthy vaginal environment may also be achieved with non-Lactobacillus species, as many women with low or no Lactobacillus have a similar resistance to BV [24]. Moreover, one study reports that transgender men taking testosterone exhibit lower Lactobacillus abundances and higher alpha diversity than cisgender women, yet these men present as asymptomatic for BV [13].
The composition and stability of the vaginal microbiome is dynamic throughout the menstrual cycle, with a notable increase in alpha diversity, decrease in the abundance of Lactobacillus spp., and decrease in community stability during menses [6, 25–27]. Transient and permanent changes in vaginal microbial community state type are also more likely to occur during menses [27]. A variety of changes during menses, including the presence of blood, tampon usage, variations in hygiene and sexual activity, and fluctuations in estrogens and progestins, may contribute to these vaginal microbial alterations [26, 28]. Alpha diversity, relative abundance of Lactobacillus spp., and community stability correlate with changes in E2 levels across the menstrual cycle (Figures 1A and 1B) [25, 27], triggering the proliferation of squamous epithelial cells that produce more glycogen to fuel Lactobacillus spp. growth [11]. Finally, preliminary evidence suggests that progestin-only contraceptives disrupt the usual predictable decline in Lactobacillus during menses [27], and contraceptives containing estrogens promote Lactobacillus proliferation and reduces the risk of BV [29]. However, more work must be done to better understand the effects of contraceptives on VM and the implications for women’s health.
Disorders affecting reproductive age women
Polycystic ovary syndrome (PCOS) is an endocrine disorder that affects approximately 10% of reproductive age women and is characterized by hyperandrogenism, ovarian dysfunction, and metabolic syndrome [30]. While there are several diagnostic criteria, the main symptoms include signs of hyperandrogenism such as hirsutism, irregular menstrual periods, and ovarian cysts [31]. Women with severe symptoms of PCOS have elevated androgen levels, increased triglycerides, low-density lipoprotein cholesterol, fasting insulin, and insulin resistance indices [32]. PCOS is also associated with decreased richness in GM and lower relative abundances of bacteria that produce short-chain fatty acid (SCFAs), including Roseburia and Odoribacter, and increased markers of intestinal dysbiosis and permeability [32, 33]. SCFAs, a product of fiber fermentation by GM, are associated with gut health and have immunomodulating effects [34]. Specifically, the SCFA, butyrate, promotes gut barrier function by providing an energy source for epithelial cells, inducing tight-junction protein expression, and by increasing the expression of antimicrobial peptides, which protect from infection by pathogens [35, 36]. SCFAs reduce inflammation by altering cytokine expression in intestinal epithelial cells and coordinating the recruitment of immune cells, including neutrophils, monocytes, and macrophages [35].
Because of the inherent challenges in translating VM of mouse models to humans, fewer studies have explored the relationship between PCOS and VM. However, one case-control study found that women with PCOS have higher VM alpha diversity, lower abundances of L. crispatus, and elevated Mycoplasma and Prevotella, after adjusting for BMI [37]. In contrast to VM and PCOS, well-established rodent models of PCOS that recapitulate the endocrine, metabolic, and reproductive hallmarks of the disorder have been used to demonstrate a potentially causal role of GM in PCOS. Administration of the aromatase inhibitor, letrozole, in female rodents induces a PCOS-like phenotype [38]. Female mice treated with letrozole have altered GM, including a shift in Firmicutes/Bacteroidetes ratio, similar to that reported in mouse models of metabolic syndrome [39]. These changes in GM in PCOS mice have a causal role in the metabolic and reproductive dysfunction. Letrozole-treated mice cohoused with healthy female mice, and thus exposed to their GM by coprophagy, had marked improvements in metabolic and reproductive phenotype, presumably due to the distinct changes in GM of the PCOS mice [40].
Other reproductive system disorders are associated with VM composition. Lactobacillus-dominated vaginal environments are considered “healthy” is because they confer a lower incidence of BV [28, 41]. BV recurrence is most likely to occur during or just after menses [26], which is a period of decreased Lactobacillus and stability, and increased alpha diversity [6, 25, 27]. Black and Latina women, who are more likely to have low lactobacilli [42], also have higher BV prevalence (>30%) than white (23%) or Asian (11%) women [43]. Studies suggest that estrogen-containing contraceptives are associated with lower risk of BV [29, 44], although a recent randomized controlled trial indicates that combined oral contraceptives did not reduce risk of BV recurrence [45]. Meanwhile, depot medroxyprogesterone acetate, a progestin-only contraceptive injection, may increase BV risk in some individuals [46].
A wide range of brain disorders, including anxiety, depression and Alzheimer’s Disease, involve the gut-brain axis, which is an important communication pathway of the gut microbiome with the brain [5, 47]. Anxiety disorders peak during adolescence, and females have a higher risk and degree of severity than males [48]. This sex difference may be due to the interactions between estrogens and the hypothalamic-pituitary-adrenal (HPA) axis [49]. Although the exact cause is poorly understood, hormonal fluctuations throughout the menstrual cycle do influence mood, particularly anxiety and depression, in premenstrual syndrome and premenstrual dysphoric disorder [50]. Like humans, rodents exhibit sex differences in anxiety [51], likely due to sex hormones such as E2 [52].
Sex differences in GM may also contribute to sex differences in anxiety disorders [53]. Recent studies demonstrate that the gut-brain-axis is sexually dimorphic in regard to the GM composition, resulting in differences in downstream targets (including the immune and neuroendocrine systems) and susceptibility to a variety of disorders [54]. In support of a role for GM in anxiety in females, conventionally raised female mice had greater anxiety-like behavior than germ-free female mice lacking a normal GM [55]. Dietary supplementation with long-chain fatty acid docosahexanoic acid reduces anxiety and depression-like symptoms in mice. Furthermore, male mice challenged with social isolation and treated with docosahexanoic acid had improvements in anxiety-like behavior and different changes in GM compared to females, which did not exhibit improvements in behavior [56]. These findings in mice, taken together with the observations of sex differences in GM and anxiety in humans, support the notion that GM plays a causal role in brain disorders in women.
Pregnancy
Endometrial receptivity is guided by progesterone production during the luteal phase. During implantation, progesterone and estrogens regulate important physiologic mechanisms in preparation for embryonic development [57]. Estrogen and progesterone levels increase throughout pregnancy, peak during the third trimester [58], and return to pre-pregnancy values five days postpartum [59] (Figure 1C).
Pregnancy and GM
The hormonal and physiological changes that occur over the course of pregnancy are accompanied by changes in GM. Between the first and third trimesters of pregnancy, women gain adiposity, have increased insulin levels, and exhibit insulin resistance. The changes in energy metabolism coincide with increases in the relative abundances of the phyla Proteobacteria and Actinobacteria and an overall decrease in GM richness [60]. Germ-free mice inoculated with fecal microbiota collected from pregnant women in their third trimester gained fat, had increased inflammatory markers, and insulin resistance, suggesting that alterations in GM can cause the metabolic changes occurring late in pregnancy [60]. While the effects of specific hormonal changes in pregnancy on GM are poorly understood, an increase in progesterone in late pregnancy mediates an increase in Bifidobacterium relative abundance [61].
Changes in metabolism in late pregnancy can set the stage for the development of glucose intolerance and gestational diabetes (GD). GD is highly prevalent world-wide and is increasing [62]. In addition to the deleterious effects during pregnancy, GD can cause obstetric complications later in pregnancy and increases the risk of type 2 diabetes in mother and baby after delivery [62]. Distinct changes in GM during pregnancy are associated with GD, namely increased abundances of Parabacteroides distasonis and Klebsiella variicola and decreased abundances of Methanobrevibacter smithii and Bidfidobacteria spp. [63]. In another study, women with GD had GM resembling that of non-pregnant individuals with type 2 diabetes, which persisted eight months postpartum [64].
Pregnancy and VM
The normal, healthy VM is distinct during each trimester of pregnancy, and the onset of pregnancy is marked by drastic changes in the VM (Figure 1D)[6]. Microbial diversity decreases and Lactobacillus species abundance increases as pregnancy progresses [65, 66]. However, there are opposing findings on whether VM diversity increases or decreases in pregnant women relative to non-pregnant women [6, 65, 67, 68]. Stratifying the pregnant VM across geographical location and ethnic group reveals differences in species abundance [69], perhaps explaining this controversy. While the prevailing understanding of the role of microbes in the womb posits that the embryonic environment is free of culturable bacteria, recent research indicates that the womb and fallopian tubes are not sterile and microbes may function in fetal health [70, 71]. The postpartum vaginal microbiome is less Lactobacillus dominated and shows increased alpha diversity [69].
Disorders affecting conception, pregnancy, and post-partum health
Unexplained infertility (UI) affects 10% of women undergoing in vitro fertilization (IVF) in the US where assisted reproduction techniques are insufficient for successful pregnancy [72]. This lack of success prompts exploring the vaginal microbiome’s role in IVF [73]. While the vaginal and cervical microbiomes do not differ significantly between fertile and infertile women, the endometrial microbiome differs in community composition [74], and VM may be a predictive tool for IVF outcome [75]. Furthermore, women with UI have distinct cervico-vaginal microbiota from non-idiopathic infertile women [76]. Further work must be done to determine the composition of the endometrial microbiota at the time of implantation and its effect on both the embryo and endometrium morphology specifically in women with UI.
Preterm birth affected 1 in 10 infants in the US in 2018 [77]. The preterm birth vaginal microbiome shifts away from the typical increase in Lactobacillus prevalence [78]. However, increased levels of Lactobacillus iners, in particular, are a risk factor for preterm birth after a gestational age of 16 weeks [79]. Interestingly, translocation of gut microbes to amniotic fluid is associated with preterm rupture of membranes, suggesting that GM composition and its effect on gut integrity influence the intrauterine environment [80]. Furthermore, women receiving vaginal progesterone treatment to mediate risk factors for pre-term birth have unchanged microbial community composition [79]. Preterm rupture of membranes and the accompanying dysbiotic state require temporal examination to deduce why and how these shifts occur and their role in preterm birth.
While the gut microbiome-brain axis has been implicated in depression [81], its role in postpartum depression (PPD) is poorly understood. Antibiotic exposure during pregnancy was a predictive factor for PPD within one month post-birth [82]. Although hormone levels decrease sharply following birth, relative estrogen levels do not correlate with PPD [83]. The role of progesterone is less clear, as progesterone supplementation worsens depression scores, yet decreases recurrent PPD [84]. The modulation of steroid metabolism by GM discussed above [7] could play an important role in PPD and needs to be explored. The VM has not yet been implicated in depression, but given its role in pregnancy and female reproductive health, it could contribute to the interactions between steroid hormones, the microbiome, and the brain.
Across all stages of pregnancy – from before conception to postpartum – the microbiome is a potential mediator for a healthy pregnancy. The distinct challenge of examining the role of both GM and VM is the temporal nature of the experiments required and the sensitivity of embryo-endometrial synchrony to temporal disruptions. Novel in vitro microfluidic culture systems could potentially offer insight into these temporal changes in the reproductive tract and examine interactions between embryo and endometrium while also supporting microbial co-culture [85–87]. While pregnancy is difficult to examine clinically, there are gaps in knowledge that require development of novel techniques and approaches to more fully understand and treat reproductive disorders in the future.
Menopause
Menopause is defined by the permanent cessation of ovarian follicle activity and lack of a menstrual cycle for one year, marking the end of natural reproductive life in women [88]. The period of decline in ovarian function is termed perimenopause and is characterized by increases in follicle stimulating hormone due to the lack of follicles and negative feedback from the ovaries [88]. As ovarian activity continues to decrease, production of E2 and progesterone ceases (Figure 1E). At menopause, estrogens and progestins have declined considerably and women’s E2 levels drop to those similar of age-matched men [89]. Endogenous androgen levels also fluctuate throughout menopause, increasing in some women during the menopause transition, and declining after [89]. Sex hormone-binding globulin (SHBG) begins to increase after the menopause transition and is associated with the levels of the adipokines leptin and adiponectin in postmenopausal women [90]. As discussed below, these hormonal changes have widespread effects on the health of postmenopausal women.
Menopause and GM
In addition to changes in hormonal milieu, menopause also involves alterations in microbiota [4, 91]. While young adult women have more diverse GM than their male counterparts, this sex difference is not observed in older adults, suggesting that the lack of ovarian steroids after menopause impacts the GM [4]. In support of menopause affecting the GM, postmenopausal women had greater abundances of the phylum Firmicutes and the genera Lachnospira and Roseburia than premenopausal women. A higher Firmicutes to Bacteroidetes ratio is associated with obesity in adults [92]. Both Lachnospira and Roseburia produce SCFAs, which exert beneficial effects on host health and metabolism [93]. In addition, postmenopausal women, similar to men, had lower plasma glucagon-like peptide-1 and higher levels of the inflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1 than premenopausal women [91]. Taken together, these findings suggest that menopausal women are more susceptible to metabolic syndrome due to increased inflammation, reduced satiety, and nutrient uptake signaling.
Menopause and VM
The vaginal microbiome changes in tandem with hormonal fluctuations as women enter their menopausal years (Figure 1F). Decreased levels of estrogens can cause vulvovaginal atrophy and decreased vaginal secretions that contain nutrients to support bacterial growth [11, 94, 95]. Consequentially, increased vaginal pH is observed universally across postmenopausal women [94]. However, differences in postmenopausal women’s previous usage of contraceptives and reproductive history can contribute to variation in specific microbial makeup and diversity [11]. Lactobacilli remain prominent in the vaginal microbiomes of healthy Chinese postmenopausal women [94], although other studies suggest that strictly anaerobic bacteria co-dominate vaginal communities [11]. Therefore, inconsistencies in the correlation between levels of serum estrone, vaginal glycogen, lactobacilli [96], and genitourinary symptoms reported in healthy postmenopausal women may imply Lactobacillus dominance or low pH are not necessarily indicative of health in postmenopausal women [94, 96].
Disorders affecting menopausal women
The unopposed estrogen hypothesis posits that exposure to higher circulating levels of estrogens unopposed by the antagonistic effects of progestins is associated with a higher risk for endometrial cancer due to the proliferative effects of estrogens on the endometrium [97]. In support, unopposed exogenous estrogens from oral contraceptives or hormone treatment increase the risk of endometrial cancer, but not when paired with progesterone [98]. Progestins increase dehydrogenase activity in endometrial cells, reduce estrogen receptor concentrations, and induce differentiation of endometrial cells into their secretory state [99]. The specific reproductive tract microbiota in postmenopausal women have significant overlap with microbiota associated with endometrial cancer [100]. Commonly, Lactobacillus spp. no longer dominate the VM postmenopause, leaving the reproductive tract more susceptible to dysbiosis and thus putting women at greater risk for atrophic vaginitis, BV, and endometrial cancer [94, 100]. Hormone treatment is commonly administered to restore Lactobacillus dominance and reduce symptoms of atrophy [11, 94, 95].
Because estrogens are critical in energy homeostasis, the lack thereof results in an increased risk of obesity, metabolic syndrome, diabetes, cardiovascular disease, and cancer [101]. Hormone treatment reduces the risk and severity of type 2 diabetes and metabolic syndrome in menopausal women by improving insulin signaling, but it is not a suitable option for all women because of its risks, including blood clots and steroid-responsive cancers [102]. Interestingly, administration of the probiotic Lactobacillus plantarum from fermented milk improves markers of inflammation and cardiovascular health in postmenopausal women with metabolic syndrome [103]. In addition, obese postmenopausal women treated with flaxseed mucilage, which is rich in prebiotic fibers, altered GM composition and improved insulin sensitivity [104]. Finally, SCFAs from GM metabolism of dietary fibers play a role in protection from lipid metabolism and inflammatory cardiovascular damage [105].
The drastic decline in ovarian steroids during peri-menopause affects mental health, most likely due to the interaction between estrogens and the HPA axis [106]. Across menopausal status, perimenopause is associated with a higher risk of depression symptoms, while post-menopause is associated with greater risk of anxiety-type symptoms [107]. In support of a function of estrogens, a mouse model of menopause suggests that obesity and diabetes during menopause increases the risk of depression-like symptoms, which are alleviated by estrogen treatment [108]. Furthermore, in another mouse model of menopause, the GM was directly linked to depressive symptoms which were alleviated by progesterone supplementation and associated with distinct changes in the GM [109]. The impact of the GM on depression in post-menopausal women is poorly understood, but early results discussed above highlight its importance in future research.
Conclusion
The interaction between sex steroids and the vaginal and gut microbiomes is a bidirectional axis that profoundly impacts women’s health across all life stages. The GM modulate circulating estrogens in the estrobolome, and in turn these circulating estrogens help shape the VM, driving reproductive tract health. The gut and vaginal microbiomes appear to have critical overlapping functions in various disease states in women’s health. However, there are glaring gaps in our understanding of the mechanisms through which women’s health is influenced by the interactions between steroids, the GM, and the VM. In addition, while it is well-established that the GM affects brain health and disease through the gut microbiome-brain axis, it is not known if the VM has similar influences. As highlighted in this review, it will be critical for future research to explore how these interactions, and the mechanisms involved, impact women’s health.
Highlights.
Sex steroids modulate gut and vaginal microbiota, linking their composition and function.
The estrobolome and the glycogen-estrogen hypothesis provide a potential pathway linking the gut and vaginal microbiomes through estrogen signaling.
The gut and vaginal microbiomes are implicated in a wide range of disorders and disease states affecting women across their lifespan, including PCOS, unexplained infertility, obesity, and endometrial cancer. For example, PCOS is characterized by reduced richness and lower relative abundance of SCFA producing microbes in the GM, and increased alpha diversity and lower Lactobacillus spp. abundance in the VM.
Mounting evidence suggests that steroids and gut microbiota acting via the gut brain axis influence mental health changes that can occur throughout women’s life phases, including depression, postpartum depression, and anxiety.
Outstanding Questions.
What effects do hormonal contraceptives have on the hormonal milieu and how might they affect the gut and vaginal microbiomes?
What is the mechanistic role of hormones and the reproductive tract microbiome in embryonic implantation and development?
What is the function of the gut microbiome in mediating the effects of estrogens on mood and energy metabolism in pregnancy and menopause? Could dietary interventions or supplementation of prebiotics ameliorate metabolic dysfunction caused by hormonal shifts?
How do the reproductive tract microbiota and hormonal landscape in menopause drive the pathogenesis of endometrial cancer individually and in concert?
Because of the cyclical nature of female hormones, how can the temporal resolution of gut and vaginal microbial sampling be improved to reflect these changes? How should women’s life phases and/or menstrual phases be taken into account when considering pro- and prebiotic treatments?
If in fact the womb is not sterile, how should treatment of idiopathic infertility, pre-term birth, and fetal surgery evolve? Would it be possible to identify new populations at risk for pre-term birth or birth complications?
Does the vaginal microbiome play a role in mental health? Could pro-and prebiotic supplementation of both GM and VM improve mental health as an alternative to steroid hormone-based therapies in premenstrual dysphoric disorder, PPD, or menopause?
Given that gut microbiota can influence the levels of steroid hormones, what is the causal role of aberrant gut microbiota in disorders such as PCOS, endometrial and other cancers?
Acknowledgments
This work was supported, in part, by CTSA Grant Number KL2 TR002379 from the National Center for Advancing Translational Science and by a career enhancement award from NIH grant P50 CA136393 (M.R.S.W.-A.) and by Wellesley College Jenkins Distinguished Chair in Neuroscience Funds (M.J.T.). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIH (M.R.S.W.-A.).
Glossary
- BV
bacterial vaginosis, a state of perturbed bacterial composition in the vagina, often leading to inflammation
- E2
estradiol, a major ovarian estrogen steroid hormone
- GD
gestational diabetes, the onset of diabetes in pregnancy
- GM
gut microbiota, commensal microbes in the gastrointestinal tract, whereas the gut microbiome constitutes all microbes and their associated gene products
- HPA
hypothalamic-pituitary-adrenal, the primary neuroendocrine pathway comprising the hypothalamus, anterior pituitary gland, and adrenal glands, which regulates homeostasis and the stress response
- IVF
in vitro fertilization, a procedure in which an egg is fertilized by a sperm outside of the body
- PCOS
polycystic ovary syndrome, a common hormonal disorder affecting women of reproductive age, causing elevated androgen levels, irregular menstrual cycles and often small follicular cysts on the ovaries
- PPD
postpartum depression, appearance of depressive symptoms after childbirth
- SCFA
short-chain fatty acid, fatty acids with one to six carbons, produced by bacterial fermentation of non-digestible dietary fibers, with a variety of physiological roles
- SHBG
sex hormone-binding globulin, a glycoprotein that binds estrogens and androgens and modulates their bioavailability
- UI
unexplained infertility, diagnosis of infertility without any underlying conditions
- VM
vaginal microbiota, commensal microbes in the vagina, whereas the vaginal microbiome consists of all microbes and their gene products
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The Mayo Foundation for Medical Education and Research (inventor M.R.S.W.-A.) has been issued a patent “Methods and Materials for Treating Endometrial Cancer”, US10072303B2. The content of the patent relates to the use of the microbiome to address endometrial cancer. M.R.S.W.-A. is a member of the scientific advisory board of LUCA Biologics, Inc. on research related to urinary tract infections, preterm birth, and reproductive medicine.
References
- 1.Sender R, Fuchs S, and Milo R, Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biology, 2016. 14(8): p. e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yatsunenko T, et al. , Human gut microbiome viewed across age and geography. Nature, 2012. 486(7402): p. 222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan X, et al. , Sexual dimorphism of gut microbiota at different pubertal status. Microbial Cell Factories, 2020. 19(1): p. 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Cuesta-Zuluaga J, et al. , Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems, 2019. 4(4): p. e00261–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tetel MJ, et al. , Steroids, stress and the gut microbiome-brain axis. J Neuroendocrinol, 2018. 30(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur H, et al. , Crosstalk Between Female Gonadal Hormones and Vaginal Microbiota Across Various Phases of Women’s Gynecological Lifecycle. Frontiers in Microbiology, 2020. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plottel CS and Blaser MJ, Microbiome and malignancy. Cell host & microbe, 2011. 10(4): p. 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwa M, et al. , The Intestinal Microbiome and Estrogen Receptor–Positive Female Breast Cancer. JNCI: Journal of the National Cancer Institute, 2016. 108(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ervin SM, et al. , Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. Journal of Biological Chemistry, 2019. 294(49): p. 18586–18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunn KL and Forney LJ, Unraveling the Dynamics of the Human Vaginal Microbiome. Yale Journal of Biology and Medicine, 2016. 89: p. 331–337. [PMC free article] [PubMed] [Google Scholar]
- 11.Gliniewicz K, et al. , Comparison of the Vaginal Microbiomes of Premenopausal and Postmenopausal Women. Frontiers in Microbiology, 2019. 10(193). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller EA, et al. , Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front Microbiol, 2016. 7: p. 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winston-McPherson G, et al. , The Vaginal Microbiome of Transgender Men. Clin Chem, 2019. 65(1): p. 199–207. [DOI] [PubMed] [Google Scholar]
- 14.Jennifer Knudtson JM, Female Reproductive Endocrinology. 2019, Gynecology and Obstetrics MSD Manual Professional Edition. [Google Scholar]
- 15.Dominianni C, et al. , Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One, 2015. 10(4): p. e0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller S, et al. , Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol, 2006. 72(2): p. 1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharya KD, et al. , Estradiol and high fat diet associate with changes in gut microbiota in female ob/ob mice. Scientific Reports, 2019. 9(1): p. 20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaliannan K, et al. , Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome, 2018. 6(1): p. 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markle JG, et al. , Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science, 2013. 339(6123): p. 1084–8. [DOI] [PubMed] [Google Scholar]
- 20.Sinha T, et al. , Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes, 2019. 10(3): p. 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulak A, Taché Y, and Larauche M, Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol, 2014. 20(10): p. 2433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boskey ER, et al. , Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Human Reproduction, 2001. 16(9): p. 1809–1813. [DOI] [PubMed] [Google Scholar]
- 23.O’Hanlon DE, Come RA, and Moench TR, Vaginal pH measured in vivo: lactobacilli determine pH and lactic acid concentration. BMC Microbiol, 2019. 19(1): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anahtar MN, et al. , Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity, 2015. 42: p. 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajer P, et al. , Temporal dynamics of the human vaginal microbiota. Sci Transl Med, 2012. 4(132): p. 132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobel JD, et al. , Conventional oral and secondary high dose vaginal metronidazole therapy for recurrent bacterial vaginosis: clinical outcomes, impacts of sex and menses. Infect Drug Resist, 2019. 12: p. 2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song SD, et al. , Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. mSphere, 2020. 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noyes N, et al. , Associations between sexual habits, menstrual hygiene practices, demographics and the vaginal microbiome as revealed by Bayesian network analysis. PLoS One, 2018. 13(1): p. e0191625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradshaw CS, et al. , Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis, 2013. 56(6): p. 777–86. [DOI] [PubMed] [Google Scholar]
- 30.Escobar-Morreale HF, Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol, 2018. 14(5): p. 270–284. [DOI] [PubMed] [Google Scholar]
- 31.Boyle JA and Teede HJ, Refining diagnostic features in PCOS to optimize health outcomes. Nature Reviews Endocrinology, 2016. 12(11): p. 630–631. [DOI] [PubMed] [Google Scholar]
- 32.Qi X, et al. , Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nature Medicine, 2019. 25(8): p. 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres PJ, et al. , Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. The Journal of clinical endocrinology and metabolism, 2018. 103(4): p. 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohoe DR, et al. , The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell metabolism, 2011. 13(5): p. 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrêa-Oliveira R, et al. , Regulation of immune cell function by short-chain fatty acids. Clinical & translational immunology, 2016. 5(4): p. e73–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parada Venegas D, et al. , Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology, 2019. 10(277). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong X, et al. , Association between polycystic ovary syndrome and the vaginal microbiome: A case-control study. Clin Endocrinol (Oxf), 2020. 93(1): p. 52–60. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman AS, et al. , A Novel Letrozole Model Recapitulates Both the Reproductive and Metabolic Phenotypes of Polycystic Ovary Syndrome in Female Mice. Biol Reprod, 2015. 93(3): p. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley ST, et al. , The Gut Microbiome Is Altered in a Letrozole-Induced Mouse Model of Polycystic Ovary Syndrome. PloS one, 2016. 11(1): p. e0146509–e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres PJ, et al. , Exposure to a Healthy Gut Microbiome Protects Against Reproductive and Metabolic Dysregulation in a PCOS Mouse Model. Endocrinology, 2019. 160(5): p. 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onderdonk AB, Delaney ML, and Fichorova RN, The Human Microbiome during Bacterial Vaginosis. Clin Microbiol Rev, 2016. 29(2): p. 223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravel J, et al. , Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci, 2011. 108 Suppl 1: p. 4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peebles K, et al. , High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex Transm Dis, 2019. 46(5): p. 304–311. [DOI] [PubMed] [Google Scholar]
- 44.Crucitti T, et al. , Contraceptive rings promote vaginal lactobacilli in a high bacterial vaginosis prevalence population: A randomised, open-label longitudinal study in Rwandan women. PLoS One, 2018. 13(7): p. e0201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vodstrcil LA, et al. , Combined oral contraceptive pill-exposure alone does not reduce the risk of bacterial vaginosis recurrence in a pilot randomised controlled trial. Sci Rep, 2019. 9(1): p. 3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, et al. , Differential effects of depot medroxyprogesterone acetate administration on vaginal microbiome in Hispanic White and Black women. Emerg Microbes Infect, 2019. 8(1): p. 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaggar M, et al. , You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front Neuroendocrinol, 2020. 56: p. 100815. [DOI] [PubMed] [Google Scholar]
- 48.Altemus M, Sarvaiya N, and Neill Epperson C, Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol, 2014. 35(3): p. 320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeng LY and Milad MR, Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Hormones and behavior, 2015. 76: p. 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lete I and Lapuente O, Contraceptive options for women with premenstrual dysphoric disorder: current insights and a narrative review. Open access journal of contraception, 2016. 7: p. 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scholl JL, et al. , Sex differences in anxiety-like behaviors in rats. Physiol Behav, 2019. 211: p. 112670. [DOI] [PubMed] [Google Scholar]
- 52.Heck AL and Handa RJ, Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 2019. 44(1): p. 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foster JA, Rinaman L, and Cryan JF, Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 2017. 7: p. 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jašarević E, Morrison KE, and Bale TL, Sex differences in the gut microbiome–brain axis across the lifespan. Philosophical Transactions of the Royal Society B: Biological Sciences, 2016. 371(1688): p. 20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neufeld KM, et al. , Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology & Motility, 2011. 23(3): p. 255–e119. [DOI] [PubMed] [Google Scholar]
- 56.Davis DJ, et al. , Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain Behav Immun, 2017. 59: p. 38–48. [DOI] [PubMed] [Google Scholar]
- 57.Tal R, et al. , Endocrinology of Pregnancy. 2015. [Google Scholar]
- 58.Schock H, et al. , Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study. BMC Pregnancy and Childbirth, 2016. 16(1): p. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hendrick V, Altshuler LL, and Suri R, Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics, 1998. 39(2): p. 93–101. [DOI] [PubMed] [Google Scholar]
- 60.Koren O, et al. , Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell, 2012. 150(3): p. 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuriel-Ohayon M, et al. , Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep, 2019. 27(3): p. 730–736.e3. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Y and Zhang C, Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Current diabetes reports, 2016. 16(1): p. 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuang Y-S, et al. , Connections between the human gut microbiome and gestational diabetes mellitus. GigaScience, 2017. 6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crusell MKW, et al. , Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome, 2018. 6(1): p. 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walther-Antonio MR, et al. , Pregnancy’s stronghold on the vaginal microbiome. PLoS One, 2014. 9(6): p. e98514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero R, et al. , The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome, 2014. 2(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freitas AC, et al. , The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Scientific Reports, 2017. 7(1): p. 9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y, et al. , Association between the vaginal microbiome and high-risk human papillomavirus infection in pregnant Chinese women. BMC Infectious Diseases, 2019. 19(1): p. 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacIntyre DA, et al. , The vaginal microbiome during pregnancy and the postpartum period in a European population. Scientific Reports, 2015. 5(1): p. 8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pelzer ES, et al. , The fallopian tube microbiome: implications for reproductive health. Oncotarget, 2018. 9(30): p. 21541–21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perez-Muñoz ME, et al. , A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome, 2017. 5(1): p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.2017 Assisted Reproductive Technology Fertility Clinic Success Rates Report, C.f.D.C.a. Prevention, Editor. 2019, US Dept of Health and Human Services: Atlanta (GA). [Google Scholar]
- 73.Schoenmakers S and Laven J, The vaginal microbiome as a tool to predict IVF success. Curr Opin Obstet Gynecol, 2020. 32(3): p. 169–178. [DOI] [PubMed] [Google Scholar]
- 74.Wee BA, et al. , A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust N Z J Obstet Gynaecol, 2018. 58(3): p. 341–348. [DOI] [PubMed] [Google Scholar]
- 75.Haahr T, et al. , Vaginal Microbiota and In Vitro Fertilization Outcomes: Development of a Simple Diagnostic Tool to Predict Patients at Risk of a Poor Reproductive Outcome. J Infect Dis, 2019. 219(11): p. 1809–1817. [DOI] [PubMed] [Google Scholar]
- 76.Campisciano G, et al. , Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J Cell Physiol, 2017. 232(7): p. 1681–1688. [DOI] [PubMed] [Google Scholar]
- 77.Martin JA, et al. , Births: Final data for 2018. 2019. [PubMed] [Google Scholar]
- 78.Baldwin EA, et al. , Persistent microbial dysbiosis in preterm premature rupture of membranes from onset until delivery. PeerJ, 2015. 3: p. e1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kindinger LM, et al. , The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome, 2017. 5(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DiGiulio DB, et al. , Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. American journal of reproductive immunology (New York, N.Y. : 1989), 2010. 64(1): p. 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foster JA and Neufeld K-AM, Gut–brain axis: how the microbiome influences anxiety and depression. Trends in neurosciences, 2013. 36(5): p. 305–312. [DOI] [PubMed] [Google Scholar]
- 82.Murphy JR, et al. , Maternal peripartum antibiotic exposure and the risk of postpartum depression. Research in Nursing & Health, 2018. 41(4): p. 369–377. [DOI] [PubMed] [Google Scholar]
- 83.Mehta D, et al. , Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychological medicine, 2014. 44(11): p. 2309. [DOI] [PubMed] [Google Scholar]
- 84.Payne JL and Maguire J, Pathophysiological mechanisms implicated in postpartum depression. Frontiers in neuroendocrinology, 2019. 52: p. 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao S, et al. , A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nature Communications, 2017. 8(1): p. 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferraz MAMM, et al. , An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nature Communications, 2018. 9(1): p. 4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang C, et al. , A novel standalone microfluidic device for local control of oxygen tension for intestinal-bacteria interactions. Faseb j, 2021. 35(2): p. e21291. [DOI] [PubMed] [Google Scholar]
- 88.Burger HG, et al. , A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Human Reproduction Update, 2007. 13(6): p. 559–565. [DOI] [PubMed] [Google Scholar]
- 89.Yasui T, et al. , Androgen in postmenopausal women. J Med Invest, 2012. 59(1–2): p. 12–27. [DOI] [PubMed] [Google Scholar]
- 90.Karim R, et al. , Association of Endogenous Sex Hormones with Adipokines and Ghrelin in Postmenopausal Women. The Journal of Clinical Endocrinology & Metabolism, 2015. 100(2): p. 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santos-Marcos JA, et al. , Influence of gender and menopausal status on gut microbiota. Maturitas, 2018. 116: p. 43–53. [DOI] [PubMed] [Google Scholar]
- 92.Koliada A, et al. , Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC microbiology, 2017. 17(1): p. 120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LeBlanc JG, et al. , Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microbial Cell Factories, 2017. 16(1): p. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen J, et al. , Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Scientific Reports, 2016. 6(1): p. 24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muhleisen AL and Herbst-Kralovetz MM, Menopause and the vaginal microbiome. Maturitas, 2016. 91: p. 42–50. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell CM, et al. , Vaginal microbiota and genitourinary menopausal symptoms: a cross-sectional analysis. Menopause (New York, N.Y.), 2017. 24(10): p. 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Key TJ and Pike MC, The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. British journal of cancer, 1988. 57(2): p. 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chlebowski RT, et al. , Continuous Combined Estrogen Plus Progestin and Endometrial Cancer: The Women’s Health Initiative Randomized Trial. JNCI: Journal of the National Cancer Institute, 2015. 108(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lukanova A, et al. , Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. International Journal of Cancer, 2004. 108(3): p. 425–432. [DOI] [PubMed] [Google Scholar]
- 100.Walsh DM, et al. , Postmenopause as a key factor in the composition of the Endometrial Cancer Microbiome (ECbiome). Sci Rep, 2019. 9(1): p. 19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Greendale GA, et al. , Changes in body composition and weight during the menopause transition. JCI insight, 2019. 4(5): p. e124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mauvais-Jarvis F, et al. , Menopausal Hormone Therapy and Type 2 Diabetes Prevention: Evidence, Mechanisms, and Clinical Implications. Endocrine reviews, 2017. 38(3): p. 173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barreto FM, et al. , Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition, 2014. 30(7): p. 939–942. [DOI] [PubMed] [Google Scholar]
- 104.Brahe LK, et al. , Dietary modulation of the gut microbiota--a randomised controlled trial in obese postmenopausal women. Br J Nutr, 2015. 114(3): p. 406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan J, et al. , The role of short-chain fatty acids in health and disease. Adv Immunol, 2014. 121: p. 91–119. [DOI] [PubMed] [Google Scholar]
- 106.Riecher-Rössler A, Menopause and Mental Health, in Mental Health and Illness of Women, Chandra P.S.e.a., Editor. 2020, Springer Nature Singapore Pte Ltd. p. 147–173. [Google Scholar]
- 107.Mulhall S, Andel R, and Anstey KJ, Variation in symptoms of depression and anxiety in midlife women by menopausal status. Maturitas, 2018. 108: p. 7–12. [DOI] [PubMed] [Google Scholar]
- 108.Wada T, et al. , Impact of central and peripheral estrogen treatment on anxiety and depression phenotypes in a mouse model of postmenopausal obesity. PLoS One, 2018. 13(12): p. e0209859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sovijit WN, et al. , Ovarian progesterone suppresses depression and anxiety-like behaviors by increasing the Lactobacillus population of gut microbiota in ovariectomized mice. Neuroscience Research, 2019. [DOI] [PubMed] [Google Scholar]


