Abstract
Alpelisib is a α-selective phosphatidylinositol 3-kinase (PI3K) inhibitor approved for treatment of postmenopausal women, and men, with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2–), PIK3CA-mutated, advanced breast cancer (ABC). Hyperglycemia is a common, on-target adverse effect that impairs treatment efficacy and increases the rate of treatment delays, dose reductions, and discontinuation. Currently, there are no clear guidelines on how to manage hyperglycemia due to alpelisib when metformin is not effective. In this case series, we review 3 subjects with ABC that developed hyperglycemia during alpelisib-fulvestrant therapy and were successfully managed with dietary and pharmacologic interventions. These cases provide anecdotal evidence to support the use of sodium-glucose co-transporter-2 inhibitors (SGLT2i) and very low carbohydrate diets to minimize hyperglycemia during alpelisib therapy.
Keywords: PI3K inhibitor, alpelisib, SGLT2 inhibitor, very-low carbohydrate diet, hyperglycemia
The phosphatidylinositol 3-kinases (PI3Ks) are a family of enzymes that mediate the intracellular effects of insulin. PI3Kα is a heterodimer made of a regulatory subunit (p85α) and a catalytic subunit (p110α), the latter of which is encoded by the gene PIK3CA and is ubiquitously expressed in all cell types. PI3K enhancement is a hallmark of human cancer, and PIK3CA is the most commonly mutated oncogene in breast cancer. 1 Activation of the PI3K pathway contributes to tumor development, progression, and resistance to anticancer therapies.
PI3K inhibitors are a class of small molecules and specifically bind and inhibit PI3K isoforms. Alpelisib (Piqray®) is a PI3Kα-specific inhibitor that is FDA approved in combination with fulvestrant for treatment of postmenopausal women, and men, with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2−), PIK3CA-mutated, advanced breast cancer (ABC). Fulvestrant is an estrogen receptor (ER) antagonist that synergizes with PI3K inhibitors to block the most common pathway of treatment resistance. 2 The approval of this combination was based on the results of the SOLAR-1 trial, a randomized, double-blind, placebo-controlled, international phase 3 trial. 3 In subjects with PIK3CA-mutated ABC, the progression-free survival was nearly doubled in the alpelisib–fulvestrant group, as compared with the placebo–fulvestrant group (11.0 vs 5.7 months). The median overall survival was also increased by 7.9 months in the alpelisib-fulvestrant group; however this analysis did not cross the prespecified boundary for statistical significance. 4
By inhibiting PI3Kα, alpelisib blocks the intracellular action of insulin systemically. This mechanism of action creates a transient state of insulin resistance and hyperglycemia, a common adverse event observed in all large clinical trials of PI3Kα inhibitors.3,5,6 In an attempt to maintain normal glucose homeostasis, the pancreas responds to hyperglycemia by releasing insulin. 7 In preclinical models, the resultant hyperinsulinemia leads to reactivation of the tumor PI3K pathway, which limits the therapeutic efficacy of this approach. 8
Hyperglycemia is identified early during alpelisib treatment. The median time to onset of grade 3 or worse hyperglycemia (greater than 250 mg/dL) is 15 days with some subjects developing it as soon as 5 days after drug initiation. 9 All grades of hyperglycemia occur in as many as 79% of subjects with grade 3 to 4 adverse events observed in 39% of subjects. Rates of grade 3 to 4 hyperglycemia were higher in subjects over 65 years (44% vs 32% in those younger than 65) and those with prediabetes at baseline (48% vs 19% in those with normal baseline glycemic status). Hyperglycemia leads to alpelisib discontinuation and dose-reductions, which can impact its efficacy because higher dose intensities of alpelisib results in longer benefits. 9 These data support the need for optimal management of hyperglycemia during alpelisib therapy.
Currently, there are no clear guidelines on how to manage hyperglycemia during alpelisib therapy if metformin fails. In the SOLAR-1 trial, metformin was the most prescribed drug, administered in 87% of subjects with hyperglycemia. 9 However, more than half of subjects (51%) required more than 1 glucose-lowering medication and a quarter required as many as 3 or more medications for adequate glucose control. It is unclear which agents are most effective in this setting. In the following case series, we present 3 cases of alpelisib-induced hyperglycemia along with the therapeutic strategies designed to normalize hyperglycemia including the use of sodium-glucose-cotransporter-2 (SGLT2) inhibitors and low-carbohydrate diets.
Research Design and Methods
Study Approval
The institutional review board of Weill Cornell Medical Center reviewed the study design and declared it to be non-human subjects research as we requested de-identified data from outside institutions. Permission for publication of each case report and any accompanying images was obtained from the patients’ next of kin. The medical records for each subject were reviewed by clinical providers at Memorial Sloan Kettering Cancer Center, The Ohio State University (OSU) Comprehensive Cancer Center, and the University of California San Francisco’s (UCSF) Helen Diller Family Comprehensive Cancer Center. Subjects were identified with clinically defined ABC who had experienced and were treated for hyperglycemia in the setting of alpelisib. Five subjects were identified. Two subjects were excluded due to lack of glycemic data. Subjects were included if they had adequate glycemic data available. All subjects had routine clinical follow-up.
Results
Subject 1: Carbohydrate Restriction to Limit Alpelisib-Induced Hyperinsulinemia
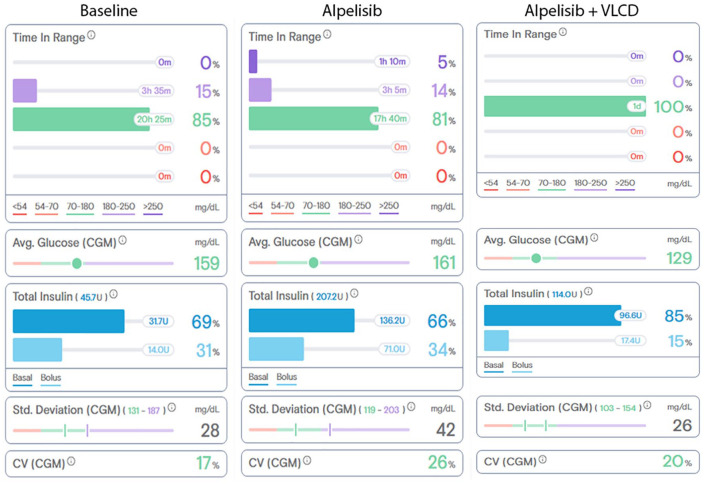
Subject 1 is an obese female (BMI = 36.4 kg/m2) in her 30s with metastatic ER+, PR+, HER2−, PIK3CA-mutated ABC with metastases to the bone and liver, with a medical history of type 1 diabetes mellitus (T1D). As part of her T1D care, the subject used a continuous glucose monitor (CGM) and subcutaneous insulin pump allowing for intensive monitoring and control over her blood glucose. Prior to beginning alpelisib therapy, she maintained a hemoglobin A1c value of 5.8% to 6.3%. She had no history of severe hyperglycemia or microvascular complications. After showing evidence of ABC progression on olaparib and fulvestrant therapy, the decision was made to replace olaparib with alpelisib in December 2019. In the weeks prior to starting alpelisib, the subject had an average glucose of 166 mg/dL with a standard deviation of 56 mg/dL requiring 60 to 70 units of insulin/day, or 0.6 to 0.7 units/kg/day (Figure 1). On the first day of alpelisib, the subject developed hyperglycemia that required additional insulin correction doses, reaching total daily insulin doses of between 160 and 200 units/day (1.6-2.0 units/kg/day). In order to lower the insulin demand, the subject initiated a low-carbohydrate diet (less than 100 g of carbohydrates per day), which progressed to a very low carbohydrate diet (VLCD, <50 g/day). With reduction in dietary carbohydrates, she was able to lower daily insulin requirement down to a minimum of 100 units per day (1.0 unit/kg/day). The additional insulin was not required on days when alpelisib was withheld. Her treatment course was complicated by abnormally elevated liver transaminases, which led to multiple dose interruptions and, ultimately, prompted the medical team to discontinue alpelisib-fulvestrant 3 months later. The rise in transaminases was thought to be related to alpelisib given that this adverse event was found in 8% to 9% of subjects in SOLAR-1. Following discontinuation of alpelisib, the subject’s glycemic control returned to a baseline of 0.6 to 0.7 units/kg/day. Unfortunately, her malignancy progressed, and she died approximately 7 months later.
Figure 1.
Representative single-day glycemic control data from Subject #1 at baseline, 5 days after alpelisib initiation, and 14 days after alpelisib initiation when the subject was consuming a very low carbohydrate diet (VLCD). Time in range, average (Avg.) glucose, standard (Std.) deviation, and coefficient of variation (CV) data were collected from the continuous glucose monitor (CGM) and total insulin data was collected from the subcutaneous insulin pump.
Data was summarized and tabulated by Tidepool™ (Palo Alto, CA), a data visualization software for diabetes devices.
Subject 2: SGLT2 Inhibition to Limit Alpelisib-Induced Hyperglycemia
Subject 2 is female in her 80s (BMI = 24.9 kg/m2) with ER+, PR+, HER2−, PIK3CA-mutated ABC with metastases to the liver and peritoneum, with a history of untreated pre-diabetes. The subject had experienced progression of disease despite treatment with multiple lines of therapy and was ultimately initiated on fulvestrant and alpelisib 300 mg in June 2020. Blood glucose values were normal until day 14 after starting alpelisib when she was noted to have hyperglycemia to 560 mg/dL on routine labs and endorsed 10 days of polydipsia, polyuria, and extreme fatigue. She was referred to her local hospital where she was started on metformin 1000 mg twice daily, pioglitazone 15 mg daily, and instructed to monitor fingerstick blood glucose before meals and at bedtime. Alpelisib was held for 1 week and restarted at a dose of 150 mg daily on day 21. Blood glucose remained well-controlled on the reduced dose of alpelisib and diabetes regimen. On day 52, the alpelisib dose was increased to 200 mg daily and the subject developed hyperglycemia in the range of 300 to 400 mg/dL for which pioglitazone was increased to 30 mg daily. On day 87, capillary blood glucose levels continued to average above 200 mg/dL (Figure 2). On day 91, the team added empagliflozin 10 mg, and subsequently observed marked improvement in the glucose values, which consistently ranged between 90 and 180 mg/dL. On day 101, metformin was reduced to 500 mg twice daily and pioglitazone to 15 mg daily. On day 147, her Hemoglobin A1C was 6.6% reflected an estimated average glucose of about 140 mg/dL. Over the course of her subsequent therapy, the subject developed anorexia, weight loss, anemia, and fatigue. Imaging obtained on day 178 demonstrated progression of disease and it was decided to discontinue alpelisib-fulvestrant as well as the anti-hyperglycemic agents. After alpelisib-fulvestrant was discontinued blood glucose levels normalized and she remained off anti-hyperglycemic agents.
Figure 2.

Average pre-meal capillary blood glucose levels from Subject 2 over the 4 days before (blue circles) and 9 days after (red squares) the addition of empagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor.
Data is mean ± SEM.
Subject 3: Carbohydrate Restriction and SGLT2 Inhibition to Limit Alpelisib-Induced Hyperglycemia
Subject 3 is a female in her 40s (BMI = 41.1 kg/m2) with ER+, PR+, HER2−, and PIK3CA-mutant ABC with metastasis to the bone, with a past medical history of type 2 diabetes mellitus (T2DM) treated with metformin 500 mg daily. The subject had experienced cancer progression despite treatment with multiple lines of therapy including a clinical trial (NCT02684032) studying the use of palbociclib (a CDK4/6 inhibitor) and gedatolisib (a dual PI3K/mTOR inhibitor) with endocrine therapy (letrozole and goserelin). Starting in August 2019, the subject also participated in a clinical trial (NCT03535701) to evaluate the feasibility of implementing a VLCD in women with ABC. The patient received meals prepared by The Ohio State University metabolic kitchen and individualized counseling and support with a registered dietitian. These interventions improved the subject’s average fasting blood glucose from 185 to 120 mg/dL (range 74-174 mg/dL) after 90 days.
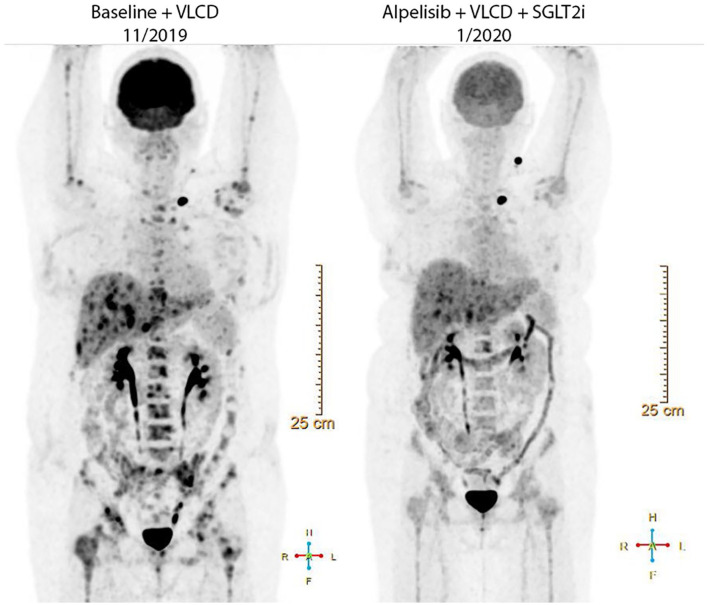
In November 2019, 18F-flurodeoxyglucose positron emission tomography (FDG-PET) revealed marked interval progression of FDG-avid metastatic disease with multiple new liver lesions and progressive/new osseous lesions, and the subject was started on alpelisib with fulvestrant. A clinical decision was made for the subject to continue on the VLCD in an attempt to mitigate the hyperglycemic effects of alpelisib. At the start of therapy, the fasting plasma glucose (FPG) was 100 mg/dL and hemoglobin A1C was 5.2%. By day 2 of treatment, FPG had increased to 264 mg/dL and the average FPG over the first 9 days of therapy was 235 ± 65 mg/dL. Canagliflozin 100 mg daily was added on day 9 of treatment in order to better control the blood sugars. FPG quickly improved to a mean of 132 ± 24 mg/dL and the subject was able to continue therapy.
In January 2020 (day 51), the FDG-PET was repeated (Figure 3). This scan identified an interval response to therapy with improvements in the multifocal FDG avid metastatic disease in the liver and visualized osseous structures. At this time, the hemoglobin A1C was 6.6% consistent with an average glucose around 158 mg/dL. The subject continued therapy for approximately 120 days until grade 3 hyperbilirubinemia was noted and additional imaging demonstrated progression of non-PET avid hepatic metastases. Alpelisib was discontinued and a short course of gemcitabine and carboplatin was attempted prior to transition to hospice care.
Figure 3.
Coronal view of Subject 3ʹs 18F-flurodeoxyglucose positron emission tomography (FDG-PET) imaging before (Baseline + VLCD, 11/2019) and after (1/2020) combination therapy with alpelisib, a very low carbohydrate diet (VLCD), and sodium glucose co-transporter 2 inhibitor (SGTL2i).
Discussion
The survival for HR+, HER2−, ABC remains poor with a 5-year overall survival rate of only 26%.10,11 New targeted therapies like alpelisib improve progression-free survival, however they can place patients at risk for adverse effects that may negatively impact quality of life and physical functioning. Hyperglycemia is common during alpelisib-fulvestrant therapy and there are currently no guidelines for management. Beyond metformin, there is no secondary agent that is widely accepted as the “next step” when hyperglycemia remains uncontrolled. In a preclinical study, preventing hyperglycemia and hyperinsulinemia enhanced the efficacy of PI3K inhibitors in multiple mouse tumor models.7 The authors identified 2 strategies that effectively abrogated these on-target adverse effects in mice: depletion of hepatic glycogen stores using a VLCD and enhancing urinary glucose disposal using SGLT2 inhibitors. Of note, metformin showed no benefit on the host or tumors from this study.
In this case series, we describe 3 subjects with HR+, HER2−, ABC that were treated with alpelisib and developed metabolic complications. Subject 1 had T1DM managed using a CGM and subcutaneous insulin pump. Given the wealth of remote monitoring data provided by these devices, this case provides a detailed look at the effects of alpelisib on glucose homeostasis. Here, we learn that the introduction of alpelisib induces an acute, reversible, and profound insulin resistance with the subject’s daily insulin requirement increasing as much as 4.5-fold in a matter of days. Fortunately, these requirements could be diminished by restricting dietary carbohydrates using a VLCD. Subject 2 had a history of pre-diabetes prior to starting alpelisib and developed uncontrolled hyperglycemia requiring multiple oral agents including metformin and the insulin sensitizer, pioglitazone. However, the blood glucose levels were improved only after initiation of a SGLT2 inhibitor, which allowed this heavily pre-treated subject to continue therapy for an impressive 88 days. Subject 3 had a history of T2DM and developed hyperglycemia on alpelisib despite appropriate implementation of a VLCD. Like subject 2, the addition of a SGLT2 inhibitor rapidly corrected the glucose values. While we did not observe any improvements in tumor outcomes in these subjects, the sample size was very small.
The long-term effects of eating a VLCD have not been evaluated in large, prospective, randomized, controlled clinical trials. However, in an open label study of 349 participants with type 2 diabetes over 2 years, an intervention including telemedicine, health coaching, and guidance improved hemoglobin A1C, body weight, blood pressure, triglycerides, hepatic transaminases, renal function, and markers of systemic inflammation with minimal adverse events.12,13 In patients with advanced cancer, this dietary pattern is safe and produces similar improvements in systemic metabolism. 14
SGLT2 inhibitors reduce renal reabsorption of glucose, which can predispose patients to hypovolemia, hypotension, and genital mycotic infections. Furthermore, the combination of volume depletion and insulin suppression, places patients at risk for euglycemic ketoacidosis, a rare and potentially deadly condition. While ketoacidosis is not common with alpelisib, it has been described in a patient using a SGLT2i and taselisib, another PIKα inhibitor. 13 Prescribing providers should alert patients about the symptoms of ketoacidosis (nausea, vomiting, abdominal pain, and fatigue) and should monitor the anion gap [Na+ − (HCO3− + Cl−)] for signs of an increasing gap metabolic acidosis. Lastly, SGLT2 inhibitors are ineffective in patients with poor renal function (GFR < 30 mL/minutes).
In each of the subjects presented, hyperglycemia was successfully treated with a VLCD, SGLT2 inhibitors, or a combination of both therapies. These interventions were well tolerated and allowed the subjects to maximize the duration of their alpelisib therapy. These anecdotal data support the need for additional prospective clinical trials to determine the optimal strategy to treat hyperglycemia and prevent alpelisib dose reductions and treatment discontinuation.
Acknowledgments
We thank the subjects and their families for sharing their experience with us.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.N. has received research support from Cisco Systems Inc. and Commonwealth Fund; has been a consultant to Steady Health, Medtronic, Eli Lilly, Roche, Intuity Medical, Nokia Growth Partners, WebMD, and Grand Rounds; has received speaking honoraria from Academy Health and Symposia Medicus; and is an uncompensated medical advisor for Tidepool. N.V. is a paid consultant for Novartis, Petra Pharmaceuticals, and Volastra Therapeutics; he is a member of the Scientific Advisory Board for Heligenics, Inc., S.S. was a paid consultant for Novartis in 2019 and 2020. J.S.V. receives royalties for low-carbohydrate nutrition books; he serves on the scientific advisory board for Simply Good Foods and is a founder and shareholder of Virta Health. M.D.G. was paid to attend a Novartis-sponsored advisory board in 2019; his lab received financial support from Pfizer; he is an inventor on a patent (pending) for Combination Therapy for PI3K-associated Disease or Disorder; and he is a co-founder, shareholder, and consultant of Faeth Therapeutics. T.B., P.N.H., J.N.F., R.C., M.A.H., M.B.L., and J.H.F. report no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH P30 CA008748 (J.H.F.), NIH K08 CA230318 (M.D.G.), and the 2020 AACR-The Mark Foundation for Cancer Research “Science of the Patient” (SOP) Grant Number 20-60-51-GONC (M.D.G.).
ORCID iD: Marcus D. Goncalves  https://orcid.org/0000-0002-0784-9248
https://orcid.org/0000-0002-0784-9248
References
- 1. Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol. 2019;30:x3-x11. doi: 10.1093/annonc/mdz281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452-61. doi: 10.1200/JCO.2010.34.4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929-1940. doi: 10.1056/NEJMoa1813904 [DOI] [PubMed] [Google Scholar]
- 4. André F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32:208-217. doi: 10.1016/j.annonc.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 5. Baselga J, Dent SF, Cortés J, et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J Clin Oncol. 2018;36:abstr LBA1006. [Google Scholar]
- 6. Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87-100. doi: 10.1016/S1470-2045(17)30688-5 [DOI] [PubMed] [Google Scholar]
- 7. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018; 379:2052-2062. doi: 10.1056/NEJMra1704560 [DOI] [PubMed] [Google Scholar]
- 8. Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499-503. doi: 10.1038/s41586-018-0343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rugo HS, André F, Yamashita T, et al. Time course and management of key adverse events during the randomized phase III SOLAR-1 study of PI3K inhibitor alpelisib plus fulvestrant in patients with HR-positive advanced breast cancer. Ann Oncol. 2020;31:1001-1010. doi: 10.1016/j.annonc.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 10. Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26:809-815. doi: 10.1158/1055-9965.EPI-16-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 12. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol (Lausanne). 2019;10:348. doi: 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Athinarayanan SJ, Hallberg SJ, McKenzie AL, et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:208. doi: 10.1186/s12933-020-01178-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klement RJ. Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation. Med Oncol. 2017;34:132. doi: 10.1007/s12032-017-0991-5 [DOI] [PubMed] [Google Scholar]