Abstract
Background
Low vitamin D (VitD) status is becoming a global health issue. Previous heterogenous results are urging a meta-analysis to delineate a panorama of VitD conditions in the general population in Mainland of China.
Methods
We performed a systematic review and meta-analysis by searching PubMed, Web of Science, EMBASE, China National Knowledge Infrastructure, WanFang, and VIP databases up to June 4, 2021. The inclusion criteria were as follows: (1) original articles or dissertations focused on VitD status of people in Mainland of China; and (2) studies were population-based, cross-sectional, or longitudinal cohort with baseline data. The outcomes were serum 25(OH)D concentration and the prevalence of low VitD status. Low VitD status included VitD deficiency (< 30 nmol/L) and VitD inadequacy (< 50 nmol/L). Data were estimated by Hierarchical Bayesian methods. All included studies were cross-sectional or longitudinal cohort studies about VitD status of people in Mainland of China. (Registration: PROSPERO CRD42021226130).
Findings
A total of 105 eligible studies including 234,519 subjects were included. In adults, the overall mean 25(OH)D concentration was 44.3 nmol/L (95% Credible Interval [CrI]: 39.8–48.7). The pooled prevalence of VitD deficiency and inadequacy was 20.7% (95% CrI: 11.9–32.9) and 63.2% (95% CrI: 53.5–72.3), respectively. In children and adolescents, the overall mean 25(OH)D concentration was 52.2 nmol/L (95% CrI: 46.7–57.5). The pooled prevalence of VitD deficiency and inadequacy was 23.0% (95% CrI: 8.9–44.3) and 46.8% (95% CrI: 37.2–56.6), respectively. Specially, we identified that the prevalence of VitD inadequacy increased with age in populations with age ≤ 18 years and ≥ 60 years.
Interpretation
Low VitD status is prominent in general population of Mainland of China, especially for adults.
Funding
National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2017ZX09304022).
Keywords: Vitamin D, Deficiency, Mainland of China, Systematic review, Meta-analysis
Research in context.
Evidence before this study
The reported prevalence of low vitamin D (VitD) status in Mainland of China varied from 22.3% to 81.9% in adults, and from 19.6% to 78.1% in children and adolescents. Characteristics of study populations such as geographical disparity, different sampling methods and inconsistent criteria may contribute to the heterogenous results. Although there were some national studies, they often focused on a specific crowd.
Added value of this study
This study presents the largest assessment to delineate the overall VitD conditions of general people in Mainland of China, based on 105 high-quality studies with 234 519 subjects. Results revealed that the prevalence of VitD inadequacy in adults were strikingly up to 63.2%, which was much higher than that in children and adolescents (46.8%). Specially, the estimated prevalence of VitD inadequacy significantly increased with age in populations with age < 18 years or > 60 years.
Implications of all the available evidence
Low VitD status is prominent in Mainland of China, especially for adults. It may help public health experts and officials pay more attention and more encouragement on VitD investigation and control strategies in Chinese adults. In view of significant association between age and VitD inadequacy in children/ adolescents, and in older people, we advocate more detailed guidelines on VitD supplementation in these people.
Alt-text: Unlabelled box
1. Introduction
Low vitamin D (VitD) status including inadequacy and deficiency is becoming a severe public health issue [1]. About 14% of people are estimated as low VitD status worldwide, and the conditions are severer in Middle East, Africa and Asia [2], [3], [4]. VitD is primarily obtained from skin synthesis by sunlight exposure and also absorbed from diet, which is further converted to 25-hydroxyvitamin D (25[OH]D) by 25-hydroxylase in the liver. It plays an essential role in bone metabolism, hormone-related conditions, and even the health of gut microbiome [5], [6], [7], [8]. Low VitD status is associated with various infectious and non-communicable diseases [9].
As a large country including 1.4 billion people, China has a vast territory that stretches from the tropical and subtropical zones in the south to the cold-temperate zones in the north. Although some studies have investigated VitD conditions in Mainland of China, the reported prevalence of low VitD status varied from 22.3 to 81.9% in adults [10,11], and from 19.6 to 78.1% in children and adolescents [12,13]. Differences may result from geographical disparity, dietary habits, and imbalance in urban and rural economic developments. Another considerable issue is no consensus on the definition of VitD sufficiency. Measurement of serum 25(OH)D concentrations is widely considered as the best indicator of VitD nutritional status [14]. The majority of experts recommend serum 25(OH)D above 50 nmol/L as sufficient [15,16], but some experts recommend a concentration of higher than 75 nmol/L [17], [18], [19]. Inconsistent criteria lead to different standards of low VitD status in the previous studies, ranging from 25(OH)D < 25 nmol/L to 25(OH)D < 80 nmol/L [4,20,21]. In addition, several studies were based on hospital and lacked of representativeness of general people, or did not provide design details such as response rate and sampling methods. These factors also contribute to differences of the prevalence of low VitD status in Mainland of China.
Although some national studies have been performed, they often focus on a specific crowd [22,23]. A comprehensive study on VitD conditions of general population is still warranted to guide VitD detection, prevention and control strategies. Therefore, we performed this systematic review and meta-analysis to delineate a panorama of VitD conditions of general population in Mainland of China. The mean serum 25(OH)D concentrations and the prevalence of low VitD status were estimated by a Hierarchical Bayesian (HB) method, and the variations of the outcomes were also assessed based on the potential factors including age, sex and latitude.
2. Methods
2.1. Protocol and guidance
This study was registered with PROSPERO (CRD42021226130). It was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [24].
2.2. Literature search and selection criteria
We searched PubMed, Web of Science, EMBASE databases for relevant articles without language or date restriction. Chinese authoritative databases including China National Knowledge Infrastructure (CNKI), WanFang, and VIP were also searched. The full search strategy was detailed in Supplementary file 1. Papers that occurred in the references of the selected full-text papers were also manually reviewed, but yielded no additional eligible articles. The literature search was updated to June 4, 2021.
Studies were eligible for inclusion if they met the following criteria: (1) original articles or dissertations about measurement of serum 25(OH)D concentrations were published; (2) studies were population-based, cross-sectional, or longitudinal cohort with baseline data; and (3) participants resided in Mainland of China. We excluded studies that were case reports, case series, commentary, review or meeting abstracts; and were measured 25(OH)D concentrations after a clinical intervention. Because we focused on VitD status of general people, studies on special populations were also excluded, including pregnant or lactating women, industry workers, and subjects from hospital, military or nursing home. Besides, studies that not specified the random sampling methods or not provide necessary data were excluded. Two independent investigators (WL and JH) performed these procedures and disagreements in study selection were resolved by discussion and consensus. All data were double checked by another author (YF).
2.3. Data extraction and quality evaluation
Two independent investigators (PW and YL) extracted the following items from each eligible study: first author's name, publication year, sampling method, sample size, age, geographical region, mean 25(OH)D concentrations, prevalence of low VitD status, and so on. If two studies had overlapping data, we kept the one with a larger sample size. The outcomes were mean serum 25(OH)D concentration (nmol/L) and the prevalence of low VitD status. Low VitD status included VitD deficiency (< 30 nmol/L, or < 12 ng/mL) and inadequacy (< 50 nmol/L, or < 20 ng/mL), based on the latest Chinese health industry standard of “Method for vitamin D deficiency screening (WS/T 677–2020)” [16].
The quality of the included studies was evaluated by a tool by Hoy [25]. This tool sets ten items and each with a score of one (yes) or zero (no) for assessment. Quality ratings were reported for each study according to an overall score of high (0–3), moderate (4–6), or low (7–10) risk of bias.
2.4. Statistical analysis
Given the patterns of data sparsity and the hierarchical structure of extracted data, we used HB approaches to perform the meta-analysis [26,27]. HB analysis combines the prior information and sample information to obtain the posterior distribution, and then estimates the pooled effect sizes. However, there is not an “ideal” statistics such as I2 in HB methods to directly quantify the heterogeneity between the included studies. As a compromise, the between-study variances could indicate the heterogeneity to some extent. Therefore, we provided all the between-study variances from each HB model to show the heterogeneity between the studies. In view of obvious differences between children/adolescents and adults, we evaluated their outcomes, respectively. First, overall mean 25(OH)D concentrations and low VitD status were estimated. Second, possible effects of sex, age, sampling frame, latitude, urbanization, season and detection assays on outcomes were evaluated using the HB meta-regression model, separately. For the univariate HB meta-regression analysis, the number of the studies for analysis in each level of a covariate should be 3 or more. The sampling frame of the included studies were classified into three levels as follows: nationwide level, province/city level (≥ 3 districts/counties), and county level (one or two districts/counties). The latitude of Mainland of China is defined as north and south, based on the Qinling Mountains-Huaihe River line boundary. So the latitude of the included studies was divided into north, south, and both. The urbanization of the included studies was divided into Urban, Rural, and both. Seasons were divided into three categories as follows: summer/autumn (June to November), winter/spring (December to May), and both (investigation lasting for 12 months or more). Detection assays were also evaluated, including enzyme-linked immunosorbent assays (ELISA), chemiluminescent assays (CLIA), electrochemiluminescence immunoassays (ECLIA), radioimmunoassays (RIA), and chemical assays. Chemical assays included high-performance liquid chromatography (HPLC) and liquid chromatography coupled with mass spectrometry (LC-MS/MS). It should be noted that the included studies rarely provided sex-age-specific outcomes, so we extracted sex-specific and age-specific outcomes to explore the effects of sex and age on outcomes, respectively. We used the mid-point values of each age group as continuous variables to construct the trend HB model. All HB models were fitted with the Markov chain Monte Carlo (MCMC) algorithm and Gibbs sampling to estimate the posterior distribution of interest outcomes. Difference, the odds ratio (OR) and credible interval (CrI) were used to measure the effects of potential factors on the outcomes of interest. The CrI represents the 2.5–97.5 percentiles of the posterior distribution of the estimation. Inferences were based on 5000 iterations, and the first 2500 of which were used as burn-in. Non-informative prior was specified for all parameters. HB analyses were performed using the R software (version 4.0.3) with “R2jags” package. The HB methods were detailed in Supplementary file 2.
2.5. Role of the funding source
This work was supported by National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2017ZX09304022). The funder had no role in study design, data collection, data analysis, result interpretation, or writing. All authors had full access to the full data in the study and accept responsibility to submit for publication.
3. Results
3.1. Characteristics of the eligible studies
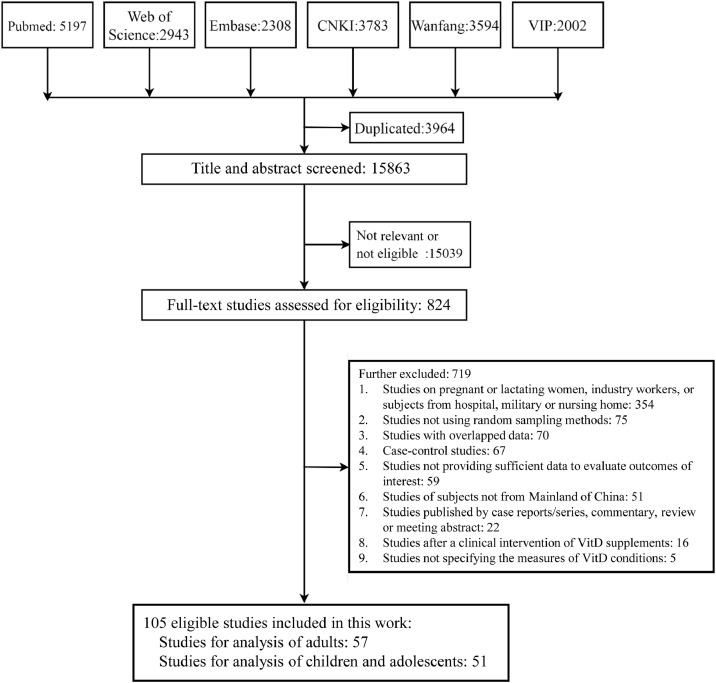
We searched a total of 19,827 records by multiple databases, and then removed 3964 duplicates. After exclusion of irrelevant records by title and abstract screening, we got 824 full-text records for eligibility assessment. Finally, a total of 105 eligible studies including 234,519 subjects were included. A flowchart of study selection is shown in Fig. 1.
Fig. 1.
Study selection. The search date was updated to June 4, 2021. A total of 105 eligible studies were finally included. Of these eligible studies, 54 included only adults, 48 included only children and adolescents, and 3 included both children/adolescents and adults. Abbreviations: VitD = Vitamin D.
These studies were published between 2001 and 2021, with data collection period ranging from 1995 to 2019. 99 studies were cross-sectional design and 6 studies were baseline data of cohorts. All these studies applied the random sampling methods to recruit subjects. 54 studies included only adults, 48 included only children and adolescents, and 3 included both. Other characteristics are shown in Supplementary Table S1. The quality assessment showed that all the included studies had a low risk of bias, based on the overall scores ranging from 8 to 10 (Supplementary Table S2).
3.2. Overall VitD conditions of general population
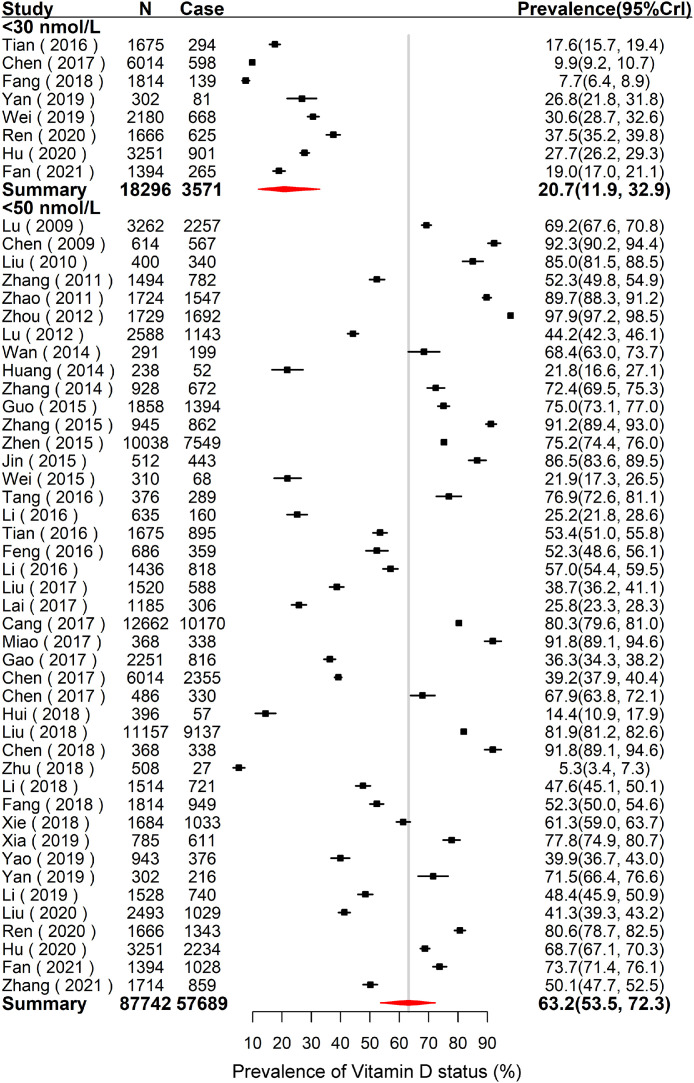
As shown in Supplementary Fig. S1, there were 34 studies including 88,065 adults to estimate the overall mean 25(OH)D concentration, which was 44.3 nmol/L (95% CrI: 39.8–48.7). The results of low VitD status are summarized in Fig. 2. The pooled prevalence of VitD deficiency of adults was 20.7% (95% CrI: 11.9–32.9) based on eight studies including 18,296 subjects. The pooled prevalence of VitD inadequacy of Chinese adults was 63.2% (95% CrI: 53.5–72.3) based on 43 studies including 87,742 subjects.
Fig. 2.
Forest plot of the pooled prevalence of VitD deficiency (< 30 nmol/L) and VitD inadequacy (< 50 nmol/L) of adults in Mainland of China. N is the total number of subjects of the studies, and case is the number of subjects with low VitD status. The forest plot was drawn based on the observational prevalence with 95%CrI of each study, and the pooled prevalence with 95%CrI was estimated using the Hierarchical Bayesian models. For VitD deficiency, the between-study variance () and 95%CrI was 0.8 (0.2–2.9). For VitD inadequacy, the between-study variance () and 95%CrI was 1.8 (1.2–2.8). Abbreviations: VitD = Vitamin D; CrI = Credible Interval.
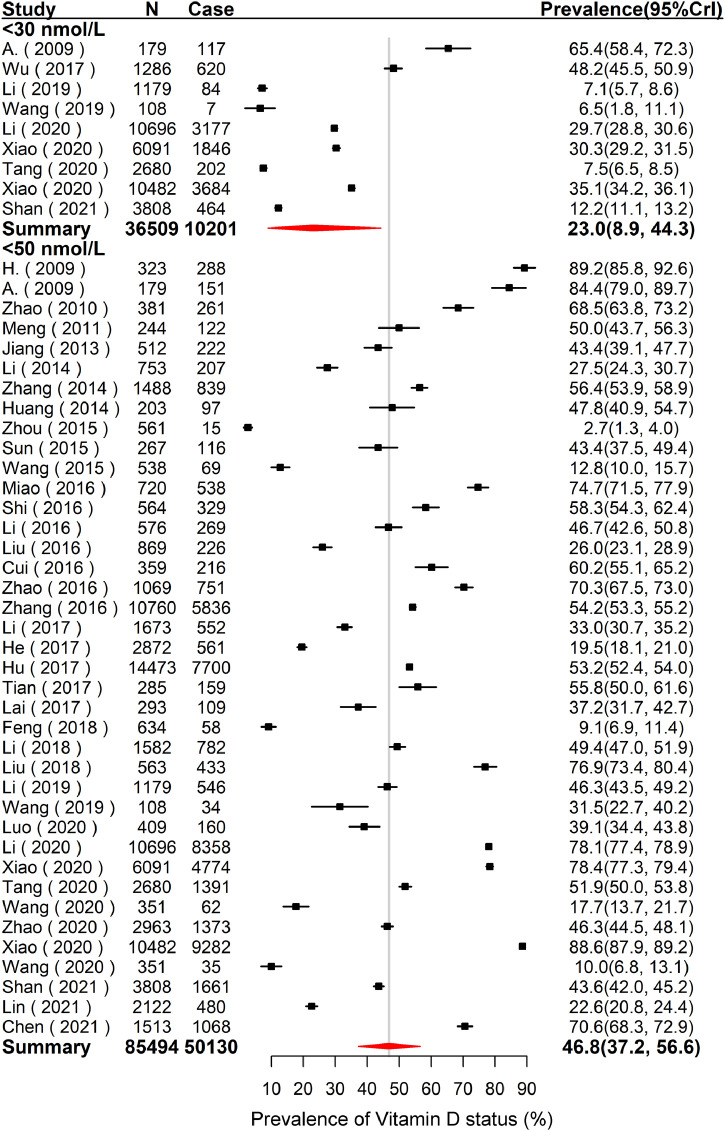
As shown in Supplementary Fig. S2, there were 36 studies including 59,863 children and adolescents to estimate the overall mean 25(OH)D concentration, which was 52.2 nmol/L (95% CrI: 46.7–57.5). The results of low VitD status are summarized in Fig. 3. The pooled prevalence of VitD deficiency of Chinese children and adolescents was 23.0% (95% CrI: 8.9–44.3) based on 9 studies including 36,509 subjects. The pooled prevalence of VitD inadequacy of Chinese children and adolescents was 46.8% (95% CrI: 37.2–56.6) based on 39 studies including 85,494 subjects.
Fig. 3.
Forest plot of the pooled prevalence of VitD deficiency (< 30 nmol/L) and VitD inadequacy (< 50 nmol/L) of children and adolescents in Mainland of China. N is the total number of subjects of the studies, and case is the number of subjects with low VitD status. The forest plot was drawn based on the observational prevalence with 95%CrI of each study, and the pooled prevalence with 95%CrI was estimated using the Hierarchical Bayesian models. For VitD deficiency, the between-study variance () and 95%CrI was 2.4 (0.7–7.5). For VitD inadequacy, the between-study variance () and 95%CrI was 1.6 (1.0–2.5). Abbreviations: VitD = Vitamin D; CrI = Credible Interval.
Between-study heterogeneity of overall VitD conditions was evaluated by and 95% CrI (Supplementary Fig. S1 and S2, Figs. 2 and 3). In view of high levels of heterogeneity, we further explored effects of potential factors contributing to VitD conditions.
3.3. Effects of potential factors on the outcomes of Chinese adults
The effects of some potential factors on the outcomes of Chinese adults were evaluated separately by the HB meta-regression analysis, including sex, age, sampling frame, latitude, urbanization, season and detection assays. For low VitD status, we only evaluated the VitD inadequacy since the number of the studies about VitD deficiency was limited. The results are summarizes in Table 1. Relevant parameters including study number and between-study variance were shown in Supplementary Table S3 and S4. We observed that sex was the most significant factor influencing the outcomes of Chinese adults (the pooled prevalence of VitD inadequacy: OR = 1.7, 95%CrI = 1.5–2.0; the overall mean 25(OH)D concentration: difference = −4.8 nmol/L, 95%CrI = −6.4 ~ −3.2). Compared with men, women had a lower overall mean 25(OH)D concentration and a higher pooled prevalence of VitD inadequacy (Supplementary Fig. S3, female: 42.9 nmol/L [95% CrI: 37.9–48.1], 64.9% [54.8–74.2]; male: 47.7 nmol/L [95% CrI: 42.8–52.8], 52.1% [54.8–74.2]). Relevant forest plots for the VitD outcomes of adults based on sex are shown in Supplementary Fig. S4 and S5. Latitude seemed to be a marginally significant factor of VitD inadequacy, but the effect was not shown on the overall mean 25(OH)D concentration. Other factors such as sampling frame, season and detection assays showed no obvious effect on the outcomes of Chinese adults.
Table 1.
Hierarchical Bayesian meta-regression analyses on the VitD status in Mainland of China.
| Covariates | Children and Adolescents | Adults† |
|---|---|---|
| VitD inadequacy (< 50 nmol/L), OR (95% CrI) | ||
| Sampling frame | ||
| (Province or City) vs. National | 0.4(0.1 to 1.1) | 1.6(0.4 to 4.3) |
| County vs. National | 0.4(0.1 to 1.1) | 2.1(0.4 to 7.0) |
| Latitude | ||
| North vs. South | 4.1(1.8 to 7.9) | 2.7(1.0 to 5.9) |
| Urbanization | ||
| Rural vs. Urban | 1.1(0.4 to 2.2) | 1.1(0.2 to 3.5) |
| Season | ||
| (Summer/Autumn) vs.(Winter/Spring) | 0.9(0.3 to 2.0) | 1.1(0.4 to 2.6) |
| Detection assays | ||
| ELISA vs. chemical assays | 1.5(0.4 to 3.8) | 1.4(0.2 to 4.8) |
| ECLIA vs. chemical assays | 1.2(0.2 to 3.5) | 1.1(0.2 to 4.0) |
| CLIA vs. chemical assays | 6.3(1.3 to 19.7) | 1.8(0.2 to 7.0) |
| RIA vs. chemical assays | 2.6(0.7 to 6.9) | 0.9(0.1 to 3.0) |
| Sex | ||
| Female vs. Male | 1.6(1.4 to 1.9) | 1.7(1.5 to 2.0) |
| Age (years) | ||
| OR per one year | 1.13(1.07 to 1.19) | 1.02(1.01 to 1.04) * |
| Mean 25(OH)D concentration (nmol/L), Diff. (95% CrI) | ||
| Sampling frame | ||
| Province or City vs. National | 10.9(−7.8 to 29.1) | −2.8(−17.3 to 11.0) |
| County vs. National | 11.6(−6.7 to 29.7) | −3.1(−19.5 to 13.3) |
| Latitude | ||
| North vs. South | −2.8(−15.3 to 9.3) | −4.3(−14.4 to 5.3) |
| Urbanization | ||
| Rural vs. Urban | −1.3(−13.6 to 10.8) | NA† |
| Season | ||
| Summer/Autumn vs. Winter/Spring | 5.6(−6.2 to 17.7) | 7.5(−4.1 to 19.0) |
| Detection assays | ||
| ELISA vs. chemical assays | 13.4(−2.6 to 29.4) | 1.0(−16.6 to 19.6) |
| ECLIA vs. chemical assays | −4.4(−19.1 to 10.8) | −4.9(−24.7 to 16.4) |
| CLIA vs. chemical assays | −14.9(−33.4 to 3.2) | −1.8(−21.4 to 17.9) |
| RIA vs. chemical assays | 6.6(−9.9 to 23.5) | 5.4(−13.8 to 25.2) |
| Sex | ||
| Female vs. Male | −4.1(−5.4 to −2.8) | −4.8(−6.4 to −3.2) |
| Age (years) | ||
| Diff. per one year | −1.04(−2.14 to 0.09) | −0.18(−0.36 to −0.01) * |
Abbreviations: VitD = Vitamin D; 25(OH)D = 25-hydroxyvitamin D; OR = Odds Ratio; CrI = Credible Interval; Diff. = Difference; vs. = versus; NA = Not available; ELISA = Enzyme-linked immunosorbent assay; ECLIA = Electrochemiluminescence immunoassay; CLIA = Chemiluminescent assay; RIA = Radioimmunoassay.
Note: We used a Hierarchical Bayesian meta-regression model to estimate the pooled prevalence of VitD inadequacy and mean 25(OH) D concentration based on each covariate (sampling frame, latitude, urbanization, season, assays, sex, and age) separately. Diff., OR, and the corresponding 95%CrI were estimated. If the 95%CrI of the effect sizes included the null effect (OR = 1 or difference = 0), the covariate was not considered as a significant factor contributing to the between-study heterogeneity. Chemical assays included high-performance liquid chromatography (HPLC) and liquid chromatography coupled with mass spectrometry (LC-MS/MS).
† For the univariate Hierarchical Bayesian meta-regression analysis, the number of the studies for analysis in each level of a covariate should be 3 or more. The model was performed without rural category because of only two studies sampling from the rural areas.
* The results showed the data of the elderly people with age ≥ 60 years old. For all the adults, the OR and 95%CrI between age and VitD inadequacy was 1.00 (0.98 to 1.01); the diff. between age and mean 25(OH)D concentration was 0.01 (−0.13 to 0.15).
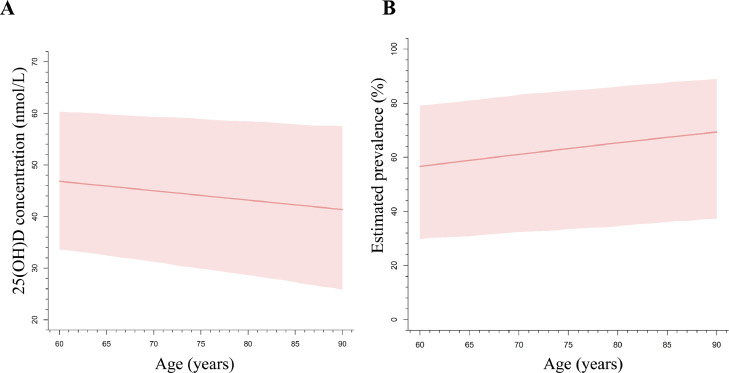
Previous studies have reported that adults with age ≥ 60 years have a higher risk of VitD inadequacy, due to limited sun exposure, inadequate dietary VitD intake, and worse physical function [2,28]. Several studies have reported that elderly people had a poorer VitD condition than that of young and middle-aged people [29,30]. Besides, the expert consensus on clinical application of VitD in Chinese elderly people demonstrated that VitD inadequacy seemed to be severer with age in elderly people [31]. Therefore, when we found no significant effect of age on the outcomes of all adults, we further performed a subgroup analysis on adults with age ≥ 60 years. It is worth noting that a significant negative association between age and the overall mean 25(OH)D concentration was identified in elderly people (≥ 60 years) (Difference = −0.18 nmol/L/year, 95%CrI = −0.36 ~ –0.01), as shown in Fig. 4A. Consistently, our results showed an increased risk of VitD inadequacy with age, with an OR of 1.02 (95% CrI: 1.01–1.04) (Fig. 4B).
Fig. 4.
The relationship of age with the estimated 25(OH)D concentration (A) and with VitD inadequacy (B) of elderly people in Mainland of China. A Hierarchical Bayesian meta-regression model was used to estimate the trend of age with the effect sizes. A random effect of age between studies was specified in the model, and both the between- and within-study variances were taken account in the model. Abbreviations: VitD = Vitamin D; 25(OH)D = 25-hydroxyvitamin D.
3.4. Effects of potential factors on the outcomes of Chinese children and adolescents
Similar analyses were performed in children and adolescents. Results demonstrated a significant sex difference (difference = −4.1 nmol/L, 95%CrI = −5.4 ~ −2.8, as shown in Table 1) that girls had a lower overall mean 25(OH)D concentration (51.3 nmol/L [95% CrI: 44.4–58.4]) than boys (55.4 nmol/L [95% CrI: 48.6–62.2]) (Supplementary Fig. S6).
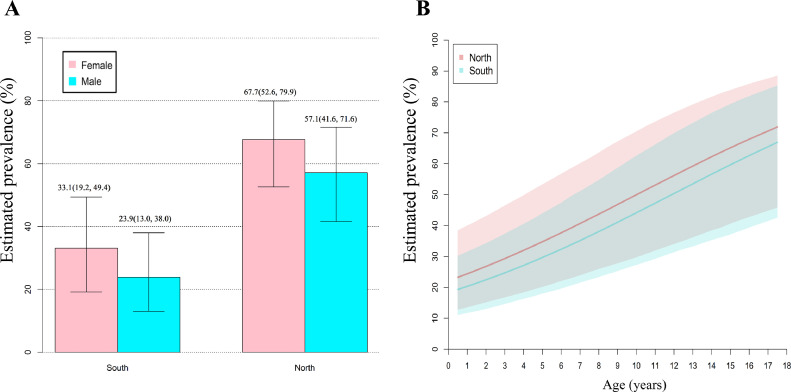
As shown in Table 1, the HB model also suggested that sex, age and latitude were significantly associated with the pooled prevalence of VitD inadequacy. Although CLIA showed a significant effect on VitD inadequacy, the small number of the included studies (n = 4) and the wide range of CrI limited the reliability of this result. Because the included studies rarely provided sex-age-specific prevalence based on latitude, we failed to include all these factors in a fit model. Therefore, we estimated sex-specific prevalence of VitD inadequacy based on latitude (Fig. 5A). The estimated prevalence of VitD inadequacy was highest in northern girls (67.7% [95% CrI: 52.6–79.9]) and lowest in southern boys (23.9% [95% CrI: 13.0–38.0]). Besides, we explored the latitude-specific association between age and the prevalence of VitD inadequacy. As shown Fig. 5B, the estimated prevalence of VitD inadequacy of children and adolescents significantly increased with age, no matter in the north or in the south.
Fig. 5.
Potential effects of sex, latitude and age on VitD inadequacy of children and adolescents in Mainland of China. A shows the estimated prevalence of VitD inadequacy based on sex and latitude. The between-study variance () and 95%CrI was 1.2 (0.7–2.2). B shows the relationship between age and VitD inadequacy based on latitude. The between-study variance () and 95%CrI was 1.0 (0.4–2.2). The detailed model and parameter estimation were described in the Supplementary file 2.2 and 2.3.
4. Discussion
Our work suggests that low VitD status is a public health issue in Mainland of China, especially for adults. We found that 20.7% of the adults were VitD deficiency and 63.2% were VitD inadequacy. Women had a lower overall mean 25(OH)D concentration and a higher prevalence of VitD inadequacy than men. For children and adolescents, the pooled prevalence of VitD deficiency and inadequacy was 23.0% and 46.8%, respectively. The pooled prevalence of VitD inadequacy was highest in northern girls and lowest in southern boys. Particularly, we identified that the prevalence of VitD inadequacy significantly increased with age in populations with age ≤ 18 years or ≥ 60 years.
Prevalence of VitD inadequacy varies throughout the world, with the reported prevalence of 20% in Australia [32], 23.3% in the United States [33], 34.2% in Africa [4], 40.4% in Europe [34], and 53.6% in Japan [35]. Notably, we identified that adults in Mainland of China had a higher prevalence of VitD inadequacy (63.2%). We also found that the overall mean 25(OH)D concentration was 44.3 nmol/L, which was much lower than that of populations in the Asia/Pacific regions (61.39 nmol/L) [36]. Poor VitD conditions in Mainland of China seem to be a public health issue. Plausible explanations are as follows: (1) several factors result in low exposure of sunshine and reduced VitD cutaneous production, such as air pollution, sun-avoiding behavior, and decreased outdoor activities due to busy and heavy work [37,38]; (2) VitD supplements and relative fortification foods are rarely used in Chinese individuals [39]; and (3) Insufficient dietary VitD intake including eggs, fish and sea foods are also prevalent in China [40]. Similarly, poor VitD condition of youth in Mainland of China was also prevalent, with 52.2 nmol/L of overall mean 25(OH)D concentration and 46.8% of VitD inadequacy. That was poorer than condition of children and adolescents in the US or Europe among whom 9%−20% had VitD inadequacy [34,41]. In addition, we observed that the prevalence of VitD inadequacy was higher in adults than that in children and adolescents (63.2% versus 46.8%) in Mainland of China. Our finding suggests that VitD inadequacy in Chinese adults is becoming a public health problem, which requires need of more attentions and interventions. Strategies such as adequate sunshine exposure, more dietary VitD intake, and VitD supplements could be encouraged in Chinese adults.
Another finding in our work is that the pooled prevalence of VitD inadequacy may significantly increase with age in children and adolescents, as well as in older people. In China, regular VitD supplementation is recommended for children from birth, but this health intervention is mainly performed among children under 3 years old. A survey showed that only 16.6% of children with age between 3 and 12 years old had VitD supplementation, and this percentage decreased with increasing age [42]. Additionally, dietary habits and increased schoolwork also reduce outdoor activities, which results in less sunshine exposure for VitD synthesis. That may explain why VitD inadequacy is more prevalent as children grow up. Our work also revealed an increasing trend between VitD inadequacy and age in this population. Chinese older people often advocate a bland diet with heavy on vegetables and seldom take VitD supplements. Besides of less VitD intake and less sunshine, the body function of older people significantly declines with age [43,44]. The evidence above supported our results.
In agreement with the results from other studies, we also found that Chinese women tended to have a lower 25(OH)D concentration and a higher prevalence of low VitD status than men [4,45]. A possible explanation is the effects of different sexual hormones such as testosterone [46]. Another explanation is different lifestyles between males and females, including smoking, alcohol consumption, sun exposure, physical activity and fat intake [47]. In addition, we identified that VitD inadequacy was most prevalent in northern girls and least prevalent in southern boys, suggesting that latitude is another important factor influencing VitD metabolism besides sex. Compared with southern areas, northern areas are in higher latitudes and have short sunshine time, which results in less endogenous production of VitD [22]. However, we did not observe the influence of latitude on VitD inadequacy in adults. That may be because adults have more complex dietary habits and lifestyles.
Detection assays seemed not to influence the pooled outcomes. From older RIA to new chemical assays, they are proven usable to measure serum VitD levels. In general, although there are some variations due to differences of technology and instruments, these assays are comparable; all have similar assay linearity and precision to identify low VitD status [48,49]. That may explain the non-significant effect of detection assays in this study. Curiously, we did not observe the effect of season on the overall mean 25(OH) D concentration and the pooled prevalence of VitD inadequacy. A possible reason is the insufficient number of available studies. About 1/3 of the included studies did not reported data based on seasons, such as investigation across seasons or not specifying seasons. The remaining studies used for evaluating season's effect came from different study populations and geographical regions. The effect of season may be covered by these factors, and this study might have insufficient power for identification.
This study provides the most up-to-date national representative estimates of VitD conditions in the general population in Mainland of China. However, some limitations should be considered when interpreting our findings. First, we found that sex, age and latitude were significantly associated with the prevalence of low VitD status in children and adolescents. However, we failed to establish the fit model including all these factors because of the restricted availability of primary data in eligible studies. Therefore, we explored sex-specific and age-specific prevalence based on latitude respectively. Second, although some studies used complex sampling method, we did not obtain the data adjusted by complex sampling method. We extracted the crude mean 25(OH) D concentration and the crude prevalence of low VitD status, which were handled as if obtained from simple random sampling in meta-analysis. Third, we did not observe the effect of season on the pooled outcomes, which was possibly due to the insufficient study power in this study. Fourth, some factors such as pollution may be potential determinants of serum 25(OH)D. We did not evaluate their effects in this work due to lacking of sufficient data from the included studies. At last, we observed the significant association between VitD conditions and age in children/ adolescents, and in older people, respectively. However, we did not evaluate the association between VitD conditions and all ages. There also may be a quadratic relationship of VitD conditions with age. More studies are needed for this investigation in the future.
In summary, this study suggests that low VitD status is a general public health issue in the population of Mainland of China, particularly in adults. In view of the trend between age and VitD inadequacy in children/ adolescents, and in older people, additional strategies and more detailed guidelines for VitD intake should be provided targeting these populations.
Authors’ contributions
NS conceived the study and designed the protocol. WL, JH and YF performed the literature search and study selection. PW and YL extracted the relevant information and validated the data. WL conducted the data analysis with support from NS. WL wrote the first draft of the paper. All authors critically revised successive drafts of the paper and approved the final version. WL and NS are the study guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This work was supported by National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2017ZX09304022).
Data sharing statement
All the data are available in the paper/supplementary material.
CRediT authorship contribution statement
Wenhua Liu: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Jing Hu: Data curation, Writing – review & editing. Yuanyuan Fang: Data curation, Writing – review & editing. Peng Wang: Investigation, Writing – review & editing. Yanjun Lu: Investigation, Writing – review & editing. Na Shen: Visualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
WL was supported from the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2017ZX09304022) during the conduct of the study. All the other authors declare no competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101017.
Appendix. Supplementary materials
References
- 1.Wilson L.R., Tripkovic L., Hart K.H., Lanham-New S.A. Proceedings of the nutrition society. Vol. 76. 2017. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. pp. 392–399. [DOI] [PubMed] [Google Scholar]
- 2.Holick M.F. Vitamin D deficiency. New Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Van Schoor N., Lips P. Global overview of vitamin D status. Endocrinol Metab Clin North Am. 2017;46(4):845–870. doi: 10.1016/j.ecl.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Mogire R.M., Mutua A., Kimita W., Kamau A., Bejon P., Pettifor J.M. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2020;8(1):e134–ee42. doi: 10.1016/s2214-109x(19)30457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas R.L., Jiang L., Adams J.S., Xu Z.Z., Shen J., Janssen S. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-19793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbáchano A., Fernández-Barral A., Ferrer-Mayorga G., Costales-Carrera A., Larriba M.J., Muñoz A. The endocrine vitamin D system in the gut. Mol Cell Endocrinol. 2017;453:79–87. doi: 10.1016/j.mce.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Norman A.W. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491s–499s. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 8.Raiten D.J., Picciano M.F. Vitamin D and health in the 21st century: bone and beyond. Executive summary. Am J Clin Nutr. 2004;80(6 Suppl):1673s–1677s. doi: 10.1093/ajcn/80.6.1673S. [DOI] [PubMed] [Google Scholar]
- 9.Autier P., Boniol M., Pizot C., Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. doi: 10.1016/s2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 10.Wei Q.S., Chen Z.Q., Tan X., Su H.R., Chen X.X., He W. Relation of age, sex and bone mineral density to serum 25-hydroxyvitamin D levels in Chinese women and men. Orthop Surg. 2015;7(4):343–349. doi: 10.1111/os.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J., Ma W., Wei L., Yang Y., Yang R., Shao F. Adult serum 25(OH)D3 in Gansu province, northwest China: a cross-sectional study. Asia Pac J Clin Nutr. 2018;27(4):832–839. doi: 10.6133/apjcn.092017.06. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Huang T., Xiao P., Zhao X., Liu J., Cheng H. Widespread vitamin D deficiency and its sex-specific association with adiposity in Chinese children and adolescents. Nutrition. 2020;71 doi: 10.1016/j.nut.2019.110646. (Burbank, Los Angeles County, Calif) [DOI] [PubMed] [Google Scholar]
- 13.He Y., Cai M., Huang X., Journal of Central South University Medical sciences [Prevalence of vitamin D insufficiency/deficiency among overweight and obese preschool children in Yuelu District of Changsha] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(5):565–569. doi: 10.11817/j.issn.1672-7347.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Zerwekh J.E. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87(4):1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 15.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Commission of the People's Republic of China. Method for vitamin D deficiency screening. 2020. http://www.nhc.gov.cn/fzs/s7852d/202005/46557f1d399249989b294c5775bdfdc0.shtml
- 17.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 18.Holick M.F., Chen T.C. Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 19.Vieth R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/ml) Best Pract Res Clin Endocrinol Metab. 2011;25(4):681–691. doi: 10.1016/j.beem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Farrokhyar F., Tabasinejad R., Dao D., Peterson D., Ayeni O.R., Hadioonzadeh R. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45(3):365–378. doi: 10.1007/s40279-014-0267-6. (Auckland, NZ) [DOI] [PubMed] [Google Scholar]
- 21.Sommer I., Griebler U., Kien C., Auer S., Klerings I., Hammer R. Vitamin D deficiency as a risk factor for dementia: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):16. doi: 10.1186/s12877-016-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y., Chen J., Wang R., Li M., Yun C., Li W. Vitamin D nutritional status and its related factors for Chinese children and adolescents in 2010-2012. Nutrients. 2017;9(9) doi: 10.3390/nu9091024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang T., Afzal S., Yu C., Guo Y., Bian Z., Yang L. Vitamin D and cause-specific vascular disease and mortality: a Mendelian randomisation study involving 99,012 Chinese and 106,911 European adults. BMC Med. 2019;17(1):160. doi: 10.1186/s12916-019-1401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. (Clinical research Ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Plummer M. JAGS: a program for analysis of bayesian graphical models using gibbs sampling. Proceeding of the 3rd international workshop on distributed statistical computing (DSC 2003); Vienna, Austria; 2003. p. 124. [Google Scholar]
- 27.Gelman A., Carlin J., Stern H., Rubin, D. (2004) Bayesian Data Analysis, Texts in Statistical Science. Chapman and Hall, London.
- 28.MacLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Stoecklin E., Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition. 2013;29(7–8):953–957. doi: 10.1016/j.nut.2013.01.010. (Burbank, Los Angeles County, Calif) [DOI] [PubMed] [Google Scholar]
- 30.Feng X., Guo T., Wang Y., Kang D., Che X., Zhang H. The vitamin D status and its effects on life quality among the elderly in Jinan, China. Arch Gerontol Geriatr. 2016;62:26–29. doi: 10.1016/j.archger.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Group of Bone Metabolism Disease GBoCMA. The expert consensus on clinical application of vitamin D in elderly people. Chin J Geriatr. 2018;37(9):953–961. [Google Scholar]
- 32.Malacova E., Cheang P.R., Dunlop E., Sherriff J.L., Lucas R.M., Daly R.M. Prevalence and predictors of vitamin D deficiency in a nationally representative sample of adults participating in the 2011-2013 Australian Health Survey. Br J Nutr. 2019;121(8):894–904. doi: 10.1017/s0007114519000151. [DOI] [PubMed] [Google Scholar]
- 33.Herrick K.A., Storandt R.J., Afful J., Pfeiffer C.M., Schleicher R.L., Gahche J.J. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110(1):150–157. doi: 10.1093/ajcn/nqz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cashman K.D., Dowling K.G., Skrabakova Z., Gonzalez-Gross M., Valtuena J., De Henauw S. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K., Kitamura K., Takachi R., Saito T., Kobayashi R., Oshiki R. Impact of demographic, environmental, and lifestyle factors on vitamin D sufficiency in 9084 Japanese adults. Bone. 2015;74:10–17. doi: 10.1016/j.bone.2014.12.064. [DOI] [PubMed] [Google Scholar]
- 36.Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D.A. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111(1):23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 37.Mousavi S.E., Amini H., Heydarpour P., Amini Chermahini F., Godderis L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: evidence and potential mechanisms. Environ Int. 2019;122:67–90. doi: 10.1016/j.envint.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 38.He H., Zeng Y., Wang X., Yang L., Zhang M., An Z. Meteorological condition and air pollution exposure associated with vitamin D deficiency: a cross-sectional population-based study in China. Risk Manag Healthc Policy. 2020;13:2317–2324. doi: 10.2147/RMHP.S273145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan L., Zhou B., Wang X., D'Ath S., Laidlaw A., Laskey M.A. Older people in China and the United Kingdom differ in the relationships among parathyroid hormone, vitamin D, and bone mineral status. Bone. 2003;33(4):620–627. doi: 10.1016/s8756-3282(03)00216-3. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter T.O., Herreros F., Zhang J.H., Ellis B.K., Simpson C., Torrealba-Fox E. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr. 2012;95(1):137–146. doi: 10.3945/ajcn.111.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar J., Muntner P., Kaskel F.J., Hailpern S.M., Melamed M.L. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124(3):e362–e370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng S.S., Zhan J.Y., Zhu B.Q., Shao J. Vitamin D status in Chinese children: review of epidemiological studies. [Chinese] Zhonghua Er Ke Za Zhi. 2019;57(3):232–234. doi: 10.3760/cma.j.issn.0578-1310.2019.03.017. Chinese journal of pediatrics. [DOI] [PubMed] [Google Scholar]
- 43.Bischoff-Ferrari H.A., Borchers M., Gudat F., Dürmüller U., Stähelin H.B., Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19(2):265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 44.Ebeling P.R., Sandgren M.E., DiMagno E.P., Lane A.W., DeLuca H.F., Riggs B.L. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75(1):176–182. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- 45.Arabi A., El Rassi R., El-Hajj Fuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nat Rev Endocrinol. 2010;6(10):550–561. doi: 10.1038/nrendo.2010.146. [DOI] [PubMed] [Google Scholar]
- 46.Rafiq R., van Schoor N.M., Sohl E., Zillikens M.C., Oosterwerff M.M., Schaap L. Associations of vitamin D status and vitamin d-related polymorphisms with sex hormones in older men. J Steroid Biochem Mol Biol. 2016;164:11–17. doi: 10.1016/j.jsbmb.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Huang F., Liu Q., Zhang Q., Wan Z., Hu L., Xu R. Sex-specific association between serum vitamin D status and lipid profiles: a cross-sectional study of a middle-aged and elderly chinese population. J Nutr Sci Vitaminol. 2020;66(2):105–113. doi: 10.3177/jnsv.66.105. [DOI] [PubMed] [Google Scholar]
- 48.Arneson W.L., Arneson D.L. Current methods for routine clinical laboratory testing of vitamin D Levels. Lab Med. 2013;44(1):e38–e42. doi: 10.1309/lmonqzq27tin7xfs. [DOI] [Google Scholar]
- 49.Wallace A.M., Gibson S., de la Hunty A., Lamberg-Allardt C., Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75(7):477–488. doi: 10.1016/j.steroids.2010.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.