Key Points
Question
What is the comparative effectiveness and safety of minimally invasive glaucoma surgeries?
Findings
This meta-analysis examined 6 Cochrane systematic reviews of randomized clinical trials that described 6- to 60-month outcomes of minimally invasive glaucoma surgeries. Compared with cataract surgery alone, addition of a trabecular bypass stent (Hydrus microstent or iStent) safely improved glaucoma control without use of medication and the Hydrus also conferred approximately 2.0-mm Hg intraocular pressure lowering; available data were insufficient to compare other minimally invasive glaucoma surgeries techniques.
Meaning
Among currently available minimally invasive glaucoma surgeries for which randomized clinical trial data have been published, Hydrus was associated with greater glaucoma control without medication and lowering of intraocular pressure than iStent, although effect sizes were small.
Abstract
Importance
Glaucoma affects more than 75 million people worldwide. Intraocular pressure (IOP)–lowering surgery is an important treatment for this disease. Interest in reducing surgical morbidity has led to the introduction of minimally invasive glaucoma surgeries (MIGS). Understanding the comparative effectiveness and safety of MIGS is necessary for clinicians and patients.
Objective
To summarize data from randomized clinical trials of MIGS for open-angle glaucoma, which were evaluated in a suite of Cochrane reviews.
Data Sources
The Cochrane Database of Systematic Reviews including studies published before June 1, 2021.
Study Selection
Reviews of randomized clinical trials comparing MIGS with cataract extraction alone, other MIGS, traditional glaucoma surgery, laser trabeculoplasty, or medical therapy.
Data Extraction and Synthesis
Data were extracted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines by one investigator and confirmed by a second. Methodologic rigor was assessed using the AMSTAR 2 appraisal tool and random-effects network meta-analyses were conducted.
Main Outcomes and Measures
The proportion of participants who did not need to use medication to reduce intraocular pressure (IOP) postsurgery (drop-free). Outcomes were analyzed at short-term (<6 months), medium-term (6-18 months), and long-term (>18 months) follow-up.
Results
Six eligible Cochrane reviews were identified discussing trabecular bypass with iStent or Hydrus microstents, ab interno trabeculotomy with Trabectome, subconjunctival and supraciliary drainage devices, and endoscopic cyclophotocoagulation. Moderate certainty evidence indicated that adding a Hydrus safely improved the likelihood of drop-free glaucoma control at medium-term (relative risk [RR], 1.6; 95% CI, 1.4 to 1.8) and long-term (RR, 1.6; 95% CI, 1.4 to 1.9) follow-up and conferred 2.0-mm Hg (95% CI, −2.7 to −1.3 mm Hg) greater IOP reduction at long-term follow-up, compared with cataract surgery alone. Adding an iStent also safely improved drop-free disease control compared with cataract surgery alone (RR, 1.4; 95% CI, 1.2 to 1.6), but the short-term IOP-lowering effect of the iStent was not sustained. Addition of a CyPass microstent improved drop-free glaucoma control compared with cataract surgery alone (RR, 1.3; 95% CI, 1.1 to 1.5) but was associated with an increased risk of vraision loss. Network meta-analyses supported the direction and magnitude of these results.
Conclusions and Relevance
Based on data synthesized in Cochrane reviews, some MIGS may afford patients with glaucoma greater drop-free disease control than cataract surgery alone. Among the products currently available, randomized clinical trial data associate the Hydrus with greater drop-free glaucoma control and IOP lowering than the iStent; however, these effect sizes were small.
This meta-analysis examines systematic reviews conducted by Cochrane Eyes and Vision to evaluate techniques and devices used in minimally invasive glaucoma surgeries alone or with cataract extraction.
Introduction
Glaucoma is a family of diseases characterized by progressive, irreversible optic neuropathy and visual field loss.1 Recent estimates suggest that its most common form, open-angle glaucoma (OAG), affects more than 3% of people aged 40 years or older, and its global prevalence is expected to exceed 111 million cases by 2040.2,3,4 It behooves the international community of eyecare clinicians and vision researchers to seek and rigorously evaluate effective, well-tolerated treatments for this chronic condition.
The only known modifiable risk factor for OAG is intraocular pressure (IOP), so IOP lowering is the mainstay of medical and surgical glaucoma therapy.5 In the past decade, there has been renewed interest in improving the success and safety profile of incisional glaucoma surgery. Traditional filtering surgeries, such as trabeculectomy and insertion of glaucoma drainage devices, place patients at risk for hypotony, diplopia, and infection.6 Minimally invasive glaucoma surgery (MIGS), also known as microincisional or microinvasive glaucoma surgery, refers to a diverse group of relatively new procedures that lower IOP with limited or no disruption to conjunctiva or sclera.7 Some of these procedures involve implantation of devices and all are readily combined with cataract extraction by phacoemulsification. MIGS may lower IOP to a more modest degree than traditional filtering surgeries but pose fewer risks to patients, so although they are not generally considered first-line therapy, MIGS have become widely used in standard glaucoma care.8,9,10,11 However, uncertainty persists about which MIGS are best for which patients. With applications in early glaucoma, MIGS are potentially relevant to an even larger pool of patients than are other glaucoma surgeries and warrant careful assessment.
Cochrane Eyes and Vision formed a consortium in 2015 to conduct a suite of Cochrane systematic reviews of randomized clinical trials (RCTs) (Cochrane reviews) of MIGS procedures.12,13,14,15,16,17 Authors of these reviews harmonized their protocols so that findings from individual reviews could be compared and summarized in an overview. This overview aims to highlight current evidence for MIGS interventions and uncover areas where opportunities for important research remain.
Methods
Eligibility Criteria
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. We included Cochrane reviews of any MIGS published in the Cochrane Database of Systematic Reviews before June 1, 2021. Eligible reviews described patients with OAG, ocular hypertension, or suspected glaucoma and compared MIGS interventions with cataract extraction alone (for those combined with cataract extraction) or with other MIGS techniques, traditional glaucoma surgery, laser trabeculoplasty, or medical therapy. We obtained the list of eligible reviews from the Cochrane Eyes and Vision editorial base.
Outcomes of the Overview
Our primary outcome was the proportion of participants who did not require use of medication to lower IOP postsurgery (drop-free), which has been identified as important to patients with mild to moderate glaucoma for whom most MIGS devices are approved.15,18 Secondary outcomes included mean change in IOP, mean change in number of IOP-lowering drops taken per day, proportion of participants requiring additional glaucoma procedures or experiencing intraoperative or postoperative complications, and health-related quality-of-life (QOL) measures. We evaluated these outcomes at times in each of the following postoperative windows: less than 6 months (short-term), 6 to 18 months (medium-term), 19 to 36 months (long-term), and longer than 36 months.
Data Extraction
For each eligible review, 1 of us (A.K.B.) extracted data on review characteristics, number of included trials and participants, risk of bias assessments, quantitative results, and review authors’ Grading of Recommendations, Assessment, Development and Evaluations (GRADE). GRADE assessments incorporate risk of bias, directness of evidence, consistency and precision of results, and possibility of publication bias to evaluate the certainty of the evidence.19 A second one of us (J.T.L. or T.L.) confirmed the accuracy and completeness of the extracted data. We relied on data presented in the published reviews. We used a classification system developed using the Delphi consensus method to categorize surgical complications: complications graded 7 and above on a scale from 0 (no harm) to 10 (worst possible surgical outcome) were considered severe.20 Two of us (J.T.L. and T.L.) worked independently to assess the methodologic rigor of the included reviews using the AMSTAR 2, a 16-question critical appraisal tool for systematic reviews that include studies of health care interventions.21
Data Synthesis
We summarize quantitative results and provide a narrative description of all relevant comparisons grouped by type of MIGS procedure. If data were meta-analyzed, we report summary estimates, 95% CIs, and measures of statistical heterogeneity; if data were not meta-analyzed, we report trial-level estimates.
We conducted random-effects network meta-analyses (NMAs) for outcomes examined by 2 or more comparisons across the included reviews (proportion of participants drop-free, mean change in unmedicated IOP, and mean change in number of IOP-lowering drops) at the longest follow-up analyzed. Use of NMA enables simultaneous comparisons of multiple interventions. We assumed a common network-specific heterogeneity parameter for each outcome and equal effects of cataract extraction by phacoemulsification when combined with any MIGS. We present summary estimates with their 95% CIs and 95% prediction intervals (PIs) in interval plots. For each outcome, we also estimated the mean rank for each intervention. Statistical significance was set at 2-sided P < .05. We used Stata, version 15 (StataCorp LLC) and the network graphs package of Stata.
Results
Review Assessment
We included 6 Cochrane reviews published between December 1, 2018, and February 28, 2021, that draw together evidence from RCTs on most MIGS interventions currently available for patients with OAG. eTable 1 in the Supplement provides summary characteristics of the reviews. Most RCTs included in these reviews reported outcomes according to US Food and Drug Administration guidelines, recording change in IOP from baseline and describing washout of IOP-lowering medications before baseline and postoperative IOP measurements. In 2 cases—subconjunctival devices and endoscopic cyclophotocoagulation—the Cochrane authors found no RCTs to include in the review.12,15 Randomized clinical trials included in the other 4 Cochrane reviews evaluated our prespecified primary outcome—the proportion of participants who were drop-free—as well as 4 of 5 prespecified secondary outcomes for at least 1 comparison. No trials included in the Cochrane reviews reported any health-related QOL measures. We summarized quantitative results in eTable 2 in the Supplement. All 6 included Cochrane reviews received a rating of high overall confidence in their results on AMSTAR-2 assessment, with 0 weaknesses found (eTable 3 in the Supplement).
Primary Outcome
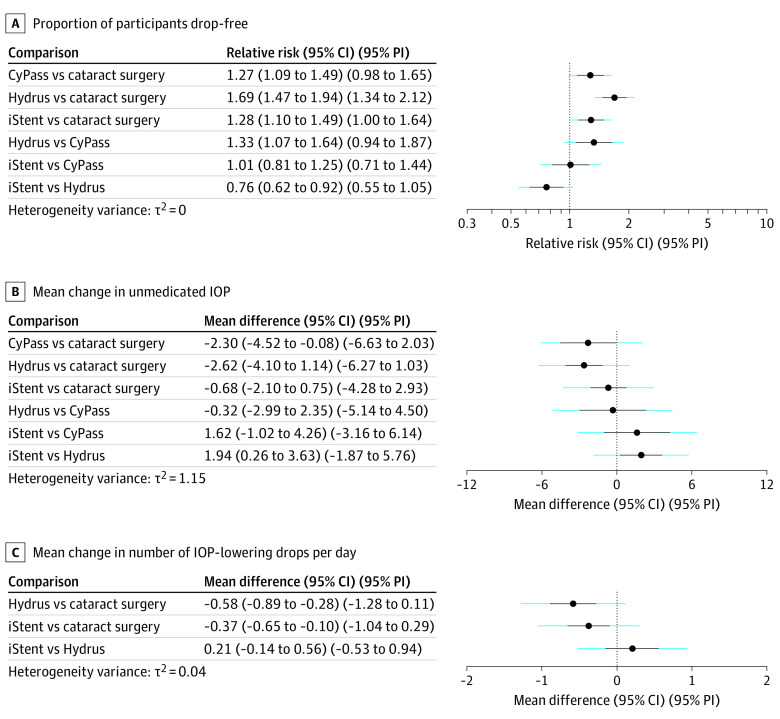
Compared with cataract extraction alone in direct comparisons made in RCTs, addition of trabecular bypass with either Hydrus or iStent increased the likelihood of study participants remaining drop-free at medium-term follow-up (Hydrus, 804 of 1000 vs 502 of 1000: relative risk [RR], 1.6; 95% CI, 1.4-1.8; iStent, 804 of 1000 vs 583 of 1000: RR, 1.4; 95% CI, 1.2-1.6). Each estimate was based on 2 trials, although the certainty of the evidence was moderate for the Hydrus comparison and very low for the iStent comparison. For participants receiving a Hydrus, this effect was sustained at long-term follow-up (2 years) (RR, 1.6; 95% CI, 1.4-1.9); long-term follow-up data were not available for other techniques. There was moderate certainty evidence that addition of a CyPass to cataract surgery also increased the likelihood of remaining drop-free at medium-term follow-up (RR, 1.3; 95% CI, 1.1-1.5); the estimate was based on 1 trial (Figure 1).
Figure 1. Network Meta-analyses of Primary and Select Secondary Outcomes.
Network plots for (A) proportion of participants drop-free, (B) mean change in unmedicated intraocular pressure (IOP) after washout, and (C) mean change in number of IOP-lower drops per day. Hydrus vs iStent comparisons include data from 1 trial (148 participants) that did not involve cataract extraction, assuming an equivalent effect of concurrent cataract extraction on both minimally invasive glaucoma surgery procedures.
In RCTs directly comparing Hydrus and iStent implanted without cataract extraction, study participants who received a Hydrus were more likely to be drop-free at medium-term follow-up than those who received an iStent (Hydrus, 466 of 1000 vs iStent, 240 of 1000: RR, 1.9; 95% CI, 1.2-3.1). In comparisons of 1 vs multiple stand-alone iStents, additional devices did not increase the proportion of participants who remained drop-free. Ab interno trabeculotomy with Trabectome combined with cataract extraction did not result in a greater proportion of drop-free participants than combined trabeculectomy and cataract extraction at medium-term follow-up.
Indirect comparisons were made through common comparators using NMA. Our NMA indicated that drop-free disease control was less likely for patients after iStent than Hydrus (RR, 0.8; 95% CI, 0.6-0.9; 95% PI, 0.6-1.1; mean rank: 2.5 vs 1.0). Indirect comparison with CyPass via NMA suggested that CyPass was as likely as iStent to render patients drop-free (RR, 1.0; 95% CI, 0.8-1.3; 95% PI, 0.7-1.4; mean rank: 2.5 for both) (Table and Figure 2A).
Table. Network Meta-analyses Mean Ranks.
| Outcome | Intervention | |||
|---|---|---|---|---|
| Cataract surgery | CyPass | Hydrus | iStent | |
| Proportion of participants no longer requiring medication | 4.0 | 2.5 | 1.0 | 2.5 |
| Mean change in unmedicated IOP (after washout) | 3.8 | 1.7 | 1.4 | 3.1 |
| Mean change in No. of IOP-lowering drops per day | 3.0 | NA | 1.1 | 1.9 |
Abbreviations: IOP, intraocular pressure; NA, not available.
Figure 2. Network Meta-analyses of Primary and Select Secondary Outcomes.
Interval plots for (A) proportion of participants drop-free; (B) mean change in unmedicated intraocular pressure (IOP) after washout; (C) mean change in number of IOP-lowering drops per day. Hydrus vs iStent comparisons include data from 1 trial (148 participants) that did not involve cataract extraction, assuming equivalent outcome of concurrent cataract extraction with both minimally invasive glaucoma surgery procedures. PI indicates prediction interval.
Secondary Outcomes
Compared with cataract extraction alone, moderate certainty evidence showed adding Hydrus lowered IOP by an additional 2.0 mm Hg at long-term follow-up (95% CI, −2.7 to −1.3 mm Hg; estimate based on 2 trials). Very low-certainty evidence suggested that adding iStent to cataract extraction lowered IOP an additional 5.0 mm Hg (95% CI, −7.5 to −2.5 mm Hg; estimate based on 3 trials) at short-term follow-up, but this outcome was not statistically significant at medium-term follow-up. The addition of a CyPass to cataract surgery conferred an additional 2.3-mm Hg IOP lowering at medium-term follow-up (95% CI, −3.0 to −1.6 mm Hg; estimate based on 1 trial), and the certainty of this evidence was high. Use of NMA suggested similar IOP lowering with CyPass and Hydrus (Figure 2B). In direct comparison of Hydrus and iStent without cataract extraction, moderate-certainty evidence showed Hydrus lowered IOP by 3.1 mm Hg (95% CI, 2.0 to 4.2 mm Hg; estimate based on 1 trial) more than iStent alone. In addition, NMA evidence suggested a statistically significant but more attenuated effect (1.9 mm Hg; 95% CI, 0.3 to 3.6 mm Hg; 95% PI, −1.9 to 5.8) (Figure 2B). Neither the iStents nor the iStent inject lowered IOP more than medical therapy at medium-term follow-up, and although 2 or 3 iStents showed an IOP-lowering benefit over 1 iStent at medium-term follow-up, no statistically significant difference was found at either short-term or long-term follow-up. Compared with ab interno trabeculotomy with Trabectome and cataract extraction, there might be greater IOP lowering after trabeculectomy plus cataract surgery, but this finding was not statistically significant.
Not all reviews contained sufficient data to describe changes in the numbers of IOP-lowering drops required per day, and the certainty of the evidence was low or very low in all cases. Compared with cataract extraction alone, a combination of Hydrus and cataract extraction reduced daily drops required by participants at long-term follow-up by 0.41 (95% CI, −0.6 to −0.3; estimate based on 2 trials) and a combination of iStent and cataract extraction reduced daily drops by 0.42 (range −0.6 to −0.2; estimate based on 3 trials) at medium-term follow-up. Comparing Hydrus and iStent implanted without cataract extraction, Hydrus reduced participants’ daily medication requirement by an additional 0.6 drops (95% CI, −1.0 to −0.2 drops; estimate based on 1 trial) at medium-term follow-up. Evidence from the NMA showed a similar effect (0.2 drops; 95% CI, −0.1 to 0.6 drops; 95% PI, −0.5 to 0.9 drops) (Figure 2). Sandhu et al17 reported a change of −1.2 drops after combined cataract extraction with CyPass vs −0.7 drops after cataract extraction alone, but this effect was not statistically analyzed. No significant difference was seen in mean change in IOP-lowering drops when ab interno trabeculotomy with Trabectome plus cataract extraction was compared with trabeculectomy plus cataract extraction.
Further Procedures and Adverse Outcomes
In most reviews, the number of study participants requiring additional glaucoma procedures was too small in all groups to analyze the relative effect of the MIGS interventions on this outcome. Complications were categorized as mild to moderate or severe for purposes of this overview (eTable 2 in the Supplement). The most prevalent severe complication reported was loss of 2 or more lines of vision. Comparing cataract extraction alone with cataract surgery combined with Hydrus implantation, available low-certainty evidence did not indicate a definite safety difference. High-certainty evidence demonstrated that addition of CyPass to cataract surgery increased the incidence of more than 2 lines of vision loss both at medium-term (11 of 1000 vs 0 of 1000) and long-term (112 of 1000 vs 60 of 1000; estimate based on 1 trial) follow-up, although relative effects were not analyzed. Mild or moderate complications were common in patients undergoing ab interno trabeculotomy with Trabectome (8 of 10) or trabeculectomy (8 of 9) with cataract extraction. The severe complication of hypotony maculopathy was seen in 2 trabeculectomy cases at short-term follow-up, but not at medium-term follow-up. The certainty of this evidence was very low.
Discussion
The suite of Cochrane reviews describing MIGS not only summarizes current evidence of clinically meaningful differences between them, but also reveals opportunities for future research. Among the devices reviewed, adding the iStent, Hydrus, or CyPass to cataract extraction may increase the likelihood of patients remaining drop-free at 6- to 18-month follow-up. For the Hydrus, moderate certainty evidence suggests that drop-free status is sustained at 24 months. Because acceptability to patients is a valuable characteristic of MIGS, selection of a patient-centered primary outcome for these harmonized reviews was appropriate.22 With that said, these results should be understood in the context of the small absolute reduction in the number of IOP-lowering drops reported by each of the reviews: approximately half of 1 drop per day on average for each of the iStent (0.42), Hydrus (0.41), and CyPass (0.5).
For safe IOP control, the Hydrus outperformed the iStent and CyPass. Both Hydrus and CyPass, when combined with cataract surgery, conferred a modest but statistically significant mean reduction in IOP over cataract extraction alone (2.0-2.3 mm Hg); the evidence for these findings was of moderate to high certainty and NMA did not strongly favor one device over the other. The IOP-lowering effect of iStent, on the other hand, was no longer statistically significant at medium-term follow-up. However, neither Hydrus nor iStent increased the risk of severe postoperative complications, but high-certainty evidence from both COMPASS XT23 and the US Food and Drug Administration–mandated safety study24 (NCT03273907) demonstrated that adding CyPass to cataract surgery increased the incidence of 2 or more lines of vision loss both at medium- and long-term follow-up in a setting of endothelial cell density reduction greater than 30% over 5 years in 27.2% of the participants. Other glaucoma surgeries, notably implantation of glaucoma drainage devices, have been associated with loss of endothelial cells at rates exceeding 9% annually.25,26 However, given the emphasis placed on safety as a hallmark of MIGS, this concerning finding led its manufacturer to voluntarily withdraw the CyPass from the global market. With regard to other highly important outcomes (ie, health-related QOL and disease progression measured with visual field testing), there was no comparative evidence available.27
In assembling this overview, we have uncovered several knowledge gaps in the evidence base for MIGS. To our knowledge, the published ophthalmic literature does not contain RCTs comparing either endoscopic cyclophotocoagulation or subconjunctival-draining MIGS devices with other treatments for glaucoma. One ongoing study (NCT01881425) compares PreserFlo to trabeculectomy in persons with OAG; once published, its results are well-positioned to inform glaucoma surgical management.12 A third MIGS approach with limited RCT evidence is ab interno trabeculotomy with Trabectome. The European Glaucoma Society argues that there is insufficient evidence to compare any MIGS with another MIGS or with trabeculectomy, and the American Academy of Ophthalmology’s Preferred Practice Pattern identifies several MIGS techniques not described herein for which evidence is scarce: Kahook Dual Blade (New World Medical), Goniotome (NeoMedix Corp), gonioscopy-assisted transluminal trabeculotomy, and ab interno canaloplasty.28,29 As existing and emerging MIGS techniques become established for appropriate indications in glaucoma surgical practice, clinicians need evidence from well-designed studies that explore all relevant comparisons.
The path for investigators working to meet this need is clear. The US Food and Drug Administration outlines recommended premarket evaluation for MIGS devices, including RCTs reporting effectiveness, health-related QOL, and safety outcomes at 12 to 24 months.30 The IDEAL framework for establishing an evidence base outlines 5 stages of surgical innovation (idea, development, exploration, assessment, and long-term study) that reflect refinement of the equipment and procedure and uptake in the medical community.31 Subconjunctival MIGS devices, for instance, have reached the assessment stage of the IDEAL framework, with stable implantation procedures and widespread use; at this stage, an RCT is the optimal tool for proving their value.30,32 This overview is timely in light of recent updates to the European Glaucoma Society33 and American Academy of Ophthalmology34 clinical practice guidelines for glaucoma management, which discuss MIGS. Moreover, ClinicalTrials.gov reports 2 dozen registered MIGS RCTs planned or recruiting, each representing an opportunity to build the evidence base for MIGS by reporting long-term effectiveness, safety, and health-related QOL outcomes.30,35 Ideally, effectiveness will be assessed using objective measures of glaucoma progression, such as visual field, that are absent from most MIGS trials (reported as a secondary outcome in only 1 of the RCTs presented herein).23 In addition, future studies should consider cost-effectiveness, because use of MIGS will reflect their financial implications for both individuals and health care systems.36 The harmonized outcomes of the Cochrane reviews described might provide a model for future ophthalmic trial design and, ultimately, guide policy based on efficacy.
Strengths and Limitations
A strength of this study is the comprehensiveness of the evidence described. A database maintained by the Cochrane Eyes and Vision group contains 46 non-Cochrane systematic reviews addressing glaucoma surgery, of which 14 pertain to MIGS (eTable 4 in the Supplement).37 Most of these reviews were methodologically flawed and, in 2 reviews, inclusion of observational studies artificially amplified MIGS effects.38 Even so, the main conclusions of these reviews regarding direction and magnitude of MIGS effect were generally in concordance with the Cochrane reviews.
One limitation of this overview flows from the challenge of extracting patient-important outcomes from the published literature on MIGS. Our primary outcome—drop-free disease control—was chosen to reflect a stated patient priority.22 However, detailed discussion with patients with OAG reveals that even those with mild and moderate glaucoma, for whom disease symptoms are minimal, value reduced medication burden but prioritize maintenance of function and health-related QOL more.18,39,40,41,42 None of the reviews in this overview described data gathered using a health-related QOL instrument. For a field such as MIGS, which stands to alter standard care in an effort to improve the lives of patients with glaucoma, consideration of health-related QOL measures is needed.
Conclusions
Among currently available MIGS for which reliable RCT data have been published, Hydrus was associated with greater drop-free glaucoma control and IOP-lowering than iStent, although effect sizes were small. There are important gaps in the evidence base for MIGS, most notably for subconjunctival devices. Vision researchers and device manufacturers might aid in bridging these gaps with well-designed RCTs reporting effectiveness, safety, and health-related QOL outcomes at 24 months and beyond.
eTable 1. Description of Included Cochrane Reviews
eTable 2. Overview of Reviews
eTable 3. Methodological Quality Assessed Using AMSTAR 2
eTable 4. Non-Cochrane Systematic Reviews of MIGS
eReferences
References
- 1.Allingham RR. Introduction. In: Damji KF, Freedman SF, Moroi SE, Rhee DJ, Shields MB, eds. Shields Textbook of Glaucoma. 6th ed. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 2.Friedman DS, Wolfs RCW, O’Colmain BJ, et al. ; Eye Diseases Prevalence Research Group . Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532-538. doi: 10.1001/archopht.122.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 5.Li T, Lindsley K, Rouse B, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123(1):129-140. doi: 10.1016/j.ophtha.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC; Tube Versus Trabeculectomy Study Group . Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804-814.e1. doi: 10.1016/j.ajo.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fellman RL, Mattox C, Singh K, et al. American Glaucoma Society position paper: microinvasive glaucoma surgery. Ophthalmol Glaucoma. 2020;3(1):1-6. doi: 10.1016/j.ogla.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology. 2016;123(1):41-P111. doi: 10.1016/j.ophtha.2015.10.053 [DOI] [PubMed] [Google Scholar]
- 9.Caprioli J, Kim JH, Friedman DS, et al. Supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology. 2015;122(9):1795-1801. doi: 10.1016/j.ophtha.2015.02.029 [DOI] [PubMed] [Google Scholar]
- 10.Francis BA, Singh K, Lin SC, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(7):1466-1480. doi: 10.1016/j.ophtha.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 11.Spaeth GL, Cvintal V, Figueiredo A. Is there a need for new surgical procedures for glaucoma? yes! Open Ophthalmol J. 2015;9:101-103. doi: 10.2174/1874364101509010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King AJ, Shah A, Nikita E, et al. Subconjunctival draining minimally-invasive glaucoma devices for medically uncontrolled glaucoma. Cochrane Database Syst Rev. 2018;12:CD012742. doi: 10.1002/14651858.CD012742.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le JT, Bicket AK, Wang L, Li T. Ab interno trabecular bypass surgery with iStent for open-angle glaucoma. Cochrane Database Syst Rev. 2019;3:CD012743. doi: 10.1002/14651858.CD012743.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otarola F, Virgili G, Shah A, Hu K, Bunce C, Gazzard G. Ab interno trabecular bypass surgery with Schlemm´s canal microstent (Hydrus) for open angle glaucoma. Cochrane Database Syst Rev. 2020;3:CD012740. doi: 10.1002/14651858.CD012740.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tóth M, Shah A, Hu K, Bunce C, Gazzard G. Endoscopic cyclophotocoagulation (ECP) for open angle glaucoma and primary angle closure. Cochrane Database Syst Rev. 2019;2:CD012741. doi: 10.1002/14651858.CD012741.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu K, Shah A, Virgili G, Bunce C, Gazzard G. Ab interno trabecular bypass surgery with Trabectome for open-angle glaucoma. Cochrane Database Syst Rev. 2021;2(2):CD011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandhu A, Jayaram H, Hu K, Bunce C, Gazzard G. Ab interno supraciliary microstent surgery for open-angle glaucoma. Cochrane Database Syst Rev. 2021;5(5):CD012802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le JT, Mohanty K, Bicket AK, Tarver M, Eydelman M, Li T. Identifying outcomes that are important to patients with ocular hypertension or primary open-angle glaucoma: a qualitative interview study. Ophthalmol Glaucoma. 2019;2(6):374-382. doi: 10.1016/j.ogla.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sii S, Barton K, Pasquale LR, Yamamoto T, King AJ, Azuara-Blanco A. Reporting harm in glaucoma surgical trials: systematic review and a consensus-derived new classification system. Am J Ophthalmol. 2018;194:153-162. doi: 10.1016/j.ajo.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 21.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe F, Wormald R, Cable R, et al. The Sight Loss and Vision Priority Setting Partnership (SLV-PSP): overview and results of the research prioritisation survey process. BMJ Open. 2014;4(7):e004905. doi: 10.1136/bmjopen-2014-004905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiss G, Clifford B, Vold S, et al. Safety and effectiveness of CyPass supraciliary micro-stent in primary open-angle glaucoma: 5-year results from the COMPASS XT Study. Am J Ophthalmol. 2019;208:219-225. doi: 10.1016/j.ajo.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 24.Potential Eye Damage from Alcon CyPass Micro-Stent Used to Treat Open-Angle Glaucoma: FDA Safety Communication. US Food and Drug Administration; 2018. [Google Scholar]
- 25.Tan AN, Webers CAB, Berendschot TTJM, et al. Corneal endothelial cell loss after Baerveldt glaucoma drainage device implantation in the anterior chamber. Acta Ophthalmol. 2017;95(1):91-96. doi: 10.1111/aos.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki K, Arimura S, Takihara Y, Takamura Y, Inatani M. Prospective cohort study of corneal endothelial cell loss after Baerveldt glaucoma implantation. PLoS One. 2018;13(7):e0201342. doi: 10.1371/journal.pone.0201342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ismail R, Azuara-Blanco A, Ramsay CR. Consensus on outcome measures for glaucoma effectiveness trials: results from a delphi and nominal group technique approaches. J Glaucoma. 2016;25(6):539-546. doi: 10.1097/IJG.0000000000000301 [DOI] [PubMed] [Google Scholar]
- 28.Azuara-Blanco A, ed. Terminology and Guidelines for Glaucoma. Savona: European Glaucoma Society; 2020. [Google Scholar]
- 29.Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern. Ophthalmology. 2021;128(1):71-150. doi: 10.1016/j.ophtha.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration . Premarket studies of implantable minimally invasive glaucoma surgical (MIGS) devices: guidance for industry and Food and Drug Administration staff. February 11, 2015. https://www.fda.gov/media/90950/download.
- 31.McCulloch P, Altman DG, Campbell WB, et al. ; Balliol Collaboration . No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105-1112. doi: 10.1016/S0140-6736(09)61116-8 [DOI] [PubMed] [Google Scholar]
- 32.Cook JA, McCulloch P, Blazeby JM, Beard DJ, Marinac-Dabic D, Sedrakyan A; IDEAL Group . IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ. 2013;346:f2820. doi: 10.1136/bmj.f2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heijl A, Traverso CE, eds. Terminology and Guidelines for Glaucoma. 5th ed.European Glaucoma Society; 2020. [Google Scholar]
- 34.Gedde SJ, Vinod K, Wright MM, Li T, Mansberger SL; for the American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel . Primary open-angle glaucoma preferred practice pattern. Ophthalmology. 2021;128(1):P71-P150. doi: 10.1016/j.ophtha.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov. http://clinicaltrials.gov/. Accessed November 30, 2020.
- 36.Poitras V, Wells C, Hutnik C, et al. Optimal Use of Minimally Invasive Glaucoma Surgery: A Health Technology Assessment. Canadian Agency for Drugs and Technologies in Health; CADTH Optimal Use Report, No. 8.1b. Canadian Agency for Drugs and Technologies in Health. January 2019. Accessed January 31, 2021. https://www.ncbi.nlm.nih.gov/books/NBK543901 [PubMed]
- 37.Evans J, Li T, Virgili G, Wormald R; Cochrane Eyes and Vision . Cochrane Eyes and Vision: a perspective introducing Cochrane Corner in Eye. Eye (Lond). 2019;33(6):882-886. doi: 10.1038/s41433-019-0357-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qureshi R, Azuara-Blanco A, Michelessi M, et al. What do we really know about the effectiveness of glaucoma interventions? an overview of systematic reviews. Ophthalmol Glaucoma. 2021;S2589-4196(21)00035-1. doi: 10.1016/j.ogla.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le JT, Bicket AK, Janssen E, et al. Prioritizing outcome preferences in patients with open-angle glaucoma using best-worst scaling. Ophthalmol Glaucoma. 2019;2(6):367-373. doi: 10.1016/j.ogla.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bicket AK, Le JT, Yorkgitis C, Li T. Priorities and treatment preferences among surgery-naive patients with moderate to severe open-angle glaucoma. Ophthalmol Glaucoma. 2020;3(5):377-383. doi: 10.1016/j.ogla.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49(5):1907-1915. doi: 10.1167/iovs.07-0559 [DOI] [PubMed] [Google Scholar]
- 42.Kulkarni BB, Leighton P, King AJ. Exploring patients’ expectations and preferences of glaucoma surgery outcomes to facilitate healthcare delivery and inform future glaucoma research. Br J Ophthalmol. 2019;103(12):1850-1855. doi: 10.1136/bjophthalmol-2018-313401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Description of Included Cochrane Reviews
eTable 2. Overview of Reviews
eTable 3. Methodological Quality Assessed Using AMSTAR 2
eTable 4. Non-Cochrane Systematic Reviews of MIGS
eReferences