Abstract
Curing cancer through precision medicine is the paramount aim of the new wave of molecular and genomic therapies. Currently, whether patients with non-reproductive cancers are male or female according to their sex chromosomes is not adequately considered in patient standard of care. This is a matter of consequence because there is growing evidence that these cancer types generally initiate earlier and are associated with higher overall incidence and rates of death in males compared with females. Gender, in contrast to sex, refers to a chosen sexual identity. Hazardous lifestyle choices (notably tobacco smoking) differ in prevalence between genders, aligned with disproportionate cancer risk. These add to underlying genetic predisposition and influences of sex steroid hormones. Together, these factors affect metabolism, immunity and inflammation, and ultimately the fidelity of the genetic code. To accurately understand how human defences against cancer erode, it is crucial to establish the influence of sex. Our Perspective highlights evidence from basic and translational research indicating that including genetic sex considerations in treatments for patients with cancer will improve outcomes. It is now time to adopt the challenge of overhauling cancer medicine based on optimized treatment strategies for females and males.
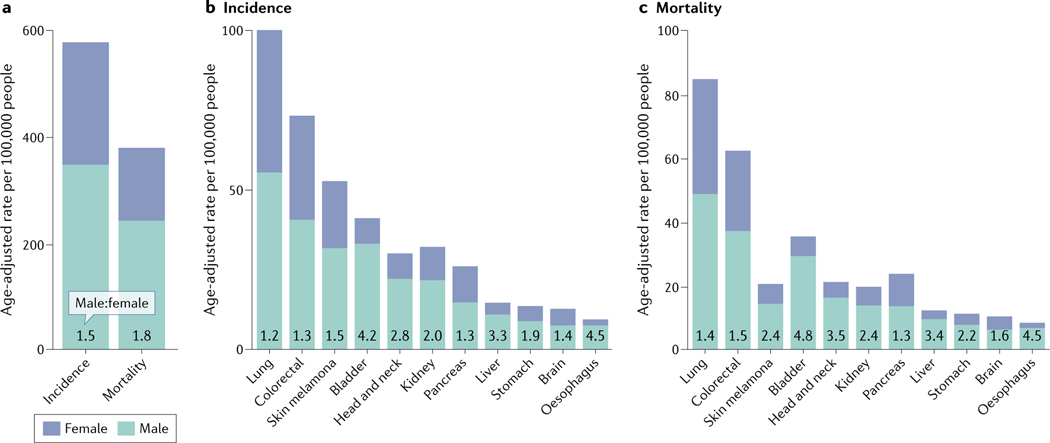
Cancers of non-reproductive tissues occur in males at an overall higher frequency and lead to nearly twice the mortality rate compared with these cancers in females1 (FIG. 1a–c). Surprisingly, comprehensive recognition of cancer sex disparity is relatively recent2. It was only in 2016 that the US National Institutes of Health (NIH) introduced policy changes requiring that investigator-initiated grants make reasonable effort to consider sex as a biological variable for medical research3. The power of this initiative was clear from the subsequent surge in publications reporting patient sex as a fundamental dictator of health, treatment responses and longevity4.
Fig. 1 |. Non-reproductive cancers per 100,000 males and females.
a | Aged-adjusted cancer incidence and mortality per 100,000 people for all non-reproductive cancers for males versus females. b,c | Age-adjusted data for individual cancers that occur at higher rates in males than females for incidence (part b) and mortality (part c). Data taken from the US 2017 Surveillance, Epidemiology, and End Results (SEER) programme1.
Normal male and female development diverges under the influence of both sex and non-sex chromosomes, with formative contribution by sex steroid hormones5,6. The capacity of sex hormones to also influence the inception and progression of non-reproductive cancer has received comprehensive review7. Considering this, we largely limit our mention of sex hormones to a discussion of experimental cancer models (BOX 1). Our focus is on emerging sex-specific genomic differences in non-reproductive cancers.
Box 1 |. Ignorance of the sex of preclinical models jeopardizes robust cancer research.
Fundamental discoveries and translational cancer research commonly spring from a pipeline of cell line testing, validation in preclinical mouse models and patient biopsies. The parameter of sex has been largely ignored across these systems, risking misinterpretation, bias and proper translation to the clinic. We advocate revision of experimental protocols to incorporate considerations of genetic sex across all preclinical steps to provide robust foundations for new cancer therapies.
Cell lines and cell culture
Repositories of human non-reproductive cancer cell lines are stocked with more lines from males than females (Supplementary Table 3). The danger is that studies fail to duly consider male and female responses, owing to: single-sex analyses; averaging of responses across models of both sexes; and mismatching the sex of transplanted cells and their mouse recipients, across cell line and patient-derived xenografts. Sex mismatch is not consistent with faithful modelling.
The sex identity of standard cell culture media components is rarely considered. The sex identity of calf fetuses sourced for sera is not commonly available. Whether sera is from a single sex or a mixture must dictate sex hormone levels, but concentrations are not routinely monitored. Remarkably, the sex of cultured cells and that of sera is seldom matched. Even the hormone content of commercial charcoal-stripped serum varies188. Additional risks lie with unintended oestrogen receptor stimulation arising from the commonly used pH dye indicator phenol red189 and plasticware used for culture and reagents190.
Mouse models of cancer
The widespread adoption of inbred mice to study human cancers raises concerns for modelling sex disparity. The human paradox is that the lifespan is generally shorter for males than females, despite a greater incidence of chronic disease in females. Unexpectedly, across a breadth of facilities, inbred mice display inconsistent longevity between the sexes, indicating the influence of the environment. This highlights the need for strict environmental regulation for comparable sex disparity studies (reviewed elsewhere191).
Species-specific sex differences are also considerations. In females, fewer genes escape X chromosome inactivation in mice than humans (reviewed elsewhere28). Further, gene regulation varies between species (for example, X-linked TLR8 regulation is different in humans and mice148,192). Even between mice strains, immunity132 and metabolism differ193. Adding to this is the general tendency to test drugs in female mice of premenopausal age, whereas human cancers largely manifest at late age (reviewed elsewhere9). Despite the US National Institutes of Health (NIH) call to conduct research across the sexes3, financial considerations often favour single-sex study of young co-caged female mice, rather than males whose aggressive behaviour requires their separation.
Cancers that arise spontaneously reflect a lifetime accumulation of unresolved chromosomal insults. Early-onset brain cancer8 contrasts with late-age diagnosis in the majority of sporadic cancers that exhibit sex disparity9. The time taken to breach a critical tipping point of genomic damage for cancer onset10 generally occurs in males before females11. A few exceptions, such as thyroid cancer12, arise at younger age in females than males, but we leave these for future discussion when they are better understood. Also outside our scope are familial cancers, which tend towards younger emergence, with sex-distinct penetrance — for example, breast cancers predominate in females with inherited mutations in TP53, which encodes the p53 protein in humans13; and gynaecological and prostate cancer risk is associated with Lynch syndrome, which encompasses four clinically different inherited mismatch repair gene defects14.
Cancer is exacerbated by various focus on. First, smoking accounted for ~30% of all US cancer deaths in 2014, of which 62% were males and 38% females15. Second, obesity is credited with fuelling 5.5–20% of cancers16, with males at greater risk. Obesity, as measured by BMI ≥30 kg/m2, poses a 33% higher risk for males and 22% for females for all cancers, relative to counterpart individuals with normal body weight17. Obesity is tightly connected to a third element of inflammation16. Chronic inflammation is a cancer risk18 compounded by genetics, age and environmental causes19 that tend to be more common in males than females.
Understanding differences in molecular circuitry between cancers of males and females is core to rational patient management and treatment. Data mining has uncovered sex differences in vital pathways in human cancers relevant to the ‘hallmarks of cancer’ as defined by Hanahan and Weinberg20. Here, we focus on the key underlying processes of tumour suppression, maintenance of genomic integrity, energy metabolism, immunity and inflammatory responses21. From the angle of risk and treatment, we evaluate how sex impacts the breakdown of these in an age-dependent manner22. Cancers are typically treated by surgery, radiotherapy and/or chemotherapy, as well as with a range of target-directed drugs and immunotherapies23. In this Perspective we provide an evidence-based rationale for customizing cancer treatments according to sex.
Sex chromosomes and cancer
At the centre of sex disparity are the sex chromosomes, the X and Y allosomes (FIG. 2), which encode core genetic differences between females and males6 (FIG. 3; see Supplementary Table 1). In the following sections, we feature allosome genes that confer distinct cancer vulnerabilities for males and females. We describe their involvement in human carcinogenesis and propose how to exploit this knowledge for sex-based customization of cancer therapies.
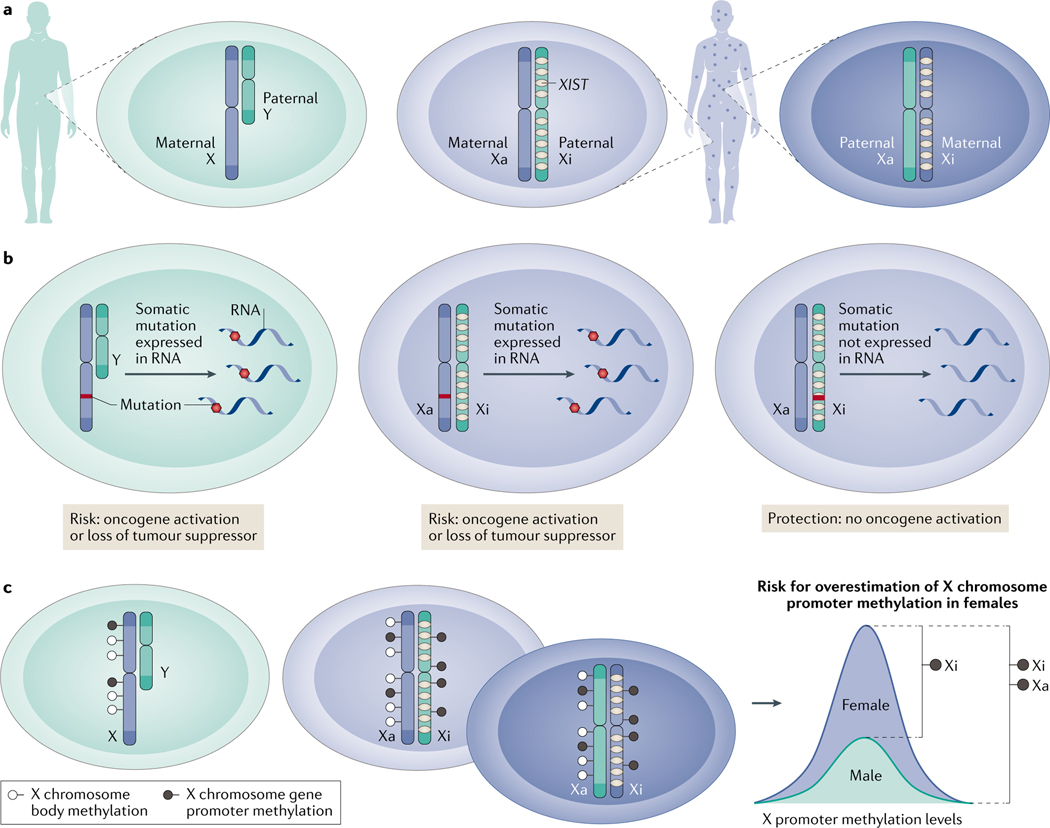
Fig. 2 |. Gene expression from the sex chromosomes differs between males and females.
a | Males inherit their X chromosomes from their mothers and Y chromosomes from their fathers. Females inherit one X chromosome (hereafter X) from each parent but only completely express one chromosome, which is termed the active X chromosome (Xa). Long non-coding RNA X-inactive specific transcript (XIST; marked in yellow) silences one X of each pair at random during early development. The silenced chromosome is the inactive X chromosome (Xi). As the X chromosome inactivation (XCI) process in females is normally random, cells within females have a mosaic expression of either the maternal or paternal X. b | A somatic gene mutation in a male X is a disease risk due to mono-allelic expression. Females will express a mutation on the Xa, but this will remain unexpressed if on the Xi (except for the special case of the escapers; not shown). c | XCI leads to overestimation of female X-methylation levels when compared with males. Promoters of the Xi are methylated to maintain the silencing initiated by XIST. Methylation may either occur on the promoter where it silences genes or in the gene body where it may promote transcription185. The levels of promoter methylation on the Xa are at risk of overestimation in females due to the inability to distinguish between DNA promoter methylation levels of the Xa and the Xi alleles.
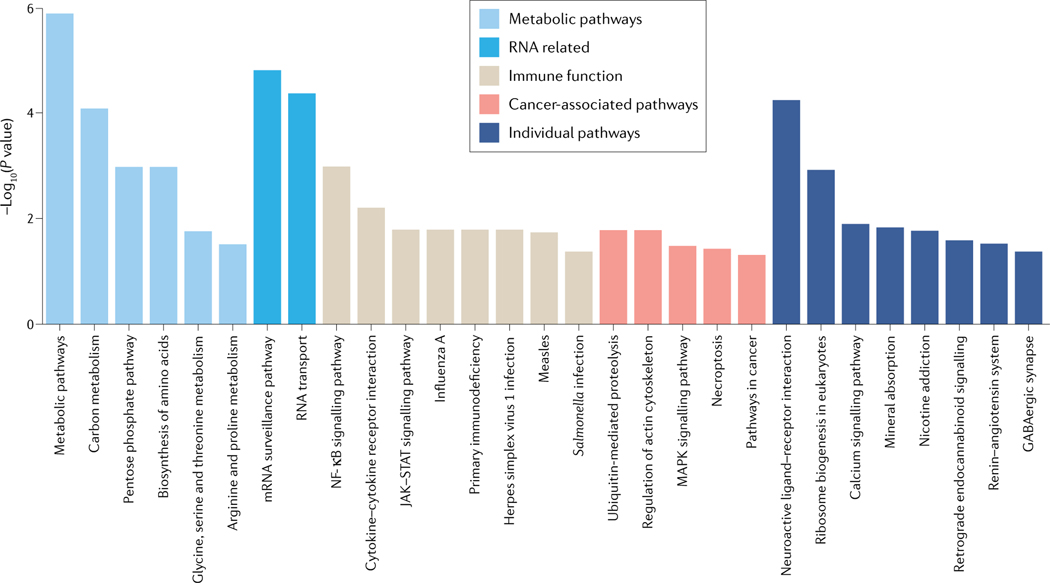
Fig. 3 |. Gene pathways are enriched on the X chromosome.
We conducted over-representation analysis (ORA) of the protein coding X chromosome genes to identify enrichment of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. ORA was applied using the R package gprofiler2 (v0.1.8)186. P values were adjusted for false discovery rate (FDR). Significant gene sets were inferred, using an FDR < 0.05 significance level. The pathway enrichment visualization was created using the R package ggplot2 (v3.3.0). Pathways with their associated genes are listed in Supplementary Table 1. JAK, Janus kinase; NF-κB, nuclear factor-κB; STAT, signal transducer and activator of transcription.
Sex-dependent X chromosome activities in cancer.
Allosome expression differs fundamentally between the typical XY male and XX female in three main ways. In healthy female development, simultaneous expression of two entire X chromosomes is not tolerated. A critical X chromosome inactivation (XCI) event occurs in implanted female embryos at early cell division and is subsequently stably maintained in most somatic cells. XCI is initiated by the long non-coding RNA X-inactive specific transcript (XIST), which coats one of the X chromosome (hereafter X) pair on a random basis, preventing gene transcription24. We identified Xist expression to be crucially regulated during development by the major tumour suppressor p53 (encoded by Trp53 in mice). In female mice embryos lacking Trp53, Xist fails to express, causing fatal bi-allelic gene expression from the X25.
Genomic and expression analyses of the X have been severely understudied because XCI hinders interpretation of whether a gene is expressed from the active X chromosome (Xa) or inactive X chromosome (Xi) (BOX 2; FIG. 2a–c). Measurements of gene expression from the Xi revealed that complete silencing occurs in the majority of the ~800 X-linked protein coding genes26, but up to 1/3 may variably escape inactivation27. Escape genes are expressed from both the Xa and the Xi. The frequency with which genes escape inactivation differs among females, tissues and cells27. These escapers reside largely in the x pseudoautosomal regions, which are able to recombine during meiosis, mimicking bi-allelic autosomal behaviour28. XCI in females introduces phenotypic diversity27 and provides protective benefits from cancer and other diseases, but also adds potential risks29. How XCI impacts the 600 X-linked non-coding RNAs26, including ~10% of the total microRNA repertoire, is yet to be fully determined30,31.
Box 2 |. Sex disparity studies in cancer bioinformatics.
Sex disparity in cancer outcomes remains poorly understood at a genetic level. This is surprising given the wealth of available human sequence data and new genomic analytical capabilities.
One major reason for this lack of understanding is that the sex chromosomes are particularly challenging to analyse.
The exceptional behaviour of the X and Y chromosomes is not catered to by analytical approaches applicable to the autosomal pairs. Tools built for autosomal pairs fail in the analyses of sex chromosomes. Specific problems associated with sex chromosome analyses are as follows:
Traditional measurements of abnormal DNA sequence code, termed DNA variant calling, in the default settings for autosomes do not account for the XX/XY disparities in samples (references elsewhere194). This affects variant detection in the X and Y chromosomes in males where variant allele frequencies are expected to widely differ from autosomal genes.
Tools for detecting DNA copy number variations assume genome-wide chromosomal pairing (diploidy) in humans as the default setting and require properly suited configuration for the X and Y chromosomes in males195.
-
DNA methylation analysis tools do not account for X chromosome inactivation (XCI) in females. XCI is maintained by DNA methylation, which sets a methylation baseline that must be adjusted for, to avoid inaccurate calculations of methylation levels in X chromosome genes196. Furthermore, in addition to the complexities presented by XCI, the female X chromosome contains pseudoautosomal regions that can variably escape XCI197, affecting any chromosome-wide considerations made to account for this process198.
Failure to adjust for sex differences when analysing genomic and transcriptomic data can lead to misinterpretation and false discoveries, for example:
In bulk RNA expression analysis, balancing groups by sex21 and accounting for X and Y sequence homology is necessary to avoid false positive sex chromosome gene identification199.
When quantifying and analysing the DNA mutational burden, females have two X chromosomes and, consequently, are expected to have double the DNA variants in the X chromosome compared with males38. Failure to adjust for this imbalance can lead to inaccurate calculations of the rates of gene mutations pertinent to a cancer context.
XCI in females also affects expression of RNA from the DNA, in contrast to the bi-allelic gene expression of autosomes. XCI renders most DNA variants found in one X chromosome allele silent198, which complicates the identification of cancer driver mutations.
In summary, as a principle of software development for bioinformatics, tools and packages are generally developed as generic and as adaptable as possible to accommodate different taxonomies and organisms. This is posing various challenges in the majority of fundamental analyses undertaken in cancer research, where tools are not set up optimally to account for human sex differences. This adds to the complexities of proper experimental design to account for sex in a data cohort. Statistical and bioinformatics analyses present a wide range of challenges that researchers have avoided by ignoring sex as a confounding variable and the sex chromosomes in genome-wide association studies194. Statistically, accounting for sex requires going a step further from conventional statistical techniques200.
Another phenomenon contributing to heterogeneity of X-linked gene expression between cells in individual females is X mosaicism. This stems from developmental XCI, resulting in cells expressing either the maternal or paternal X (FIG. 2a). Adjacent cells in females may consequently express distinct X gene repertoires, which contrasts the exclusive maternal origin of X-linked gene expression in males. For example, a female with a deleterious recessive X-linked gene mutation, which is not expressed in all cells due to mosaic expression, would be advantaged over a male with obligatory expression of the same mutated maternal X-linked gene32 (FIG. 2b). Furthermore, proliferation of specific mosaic subpopulations expressing one X preferentially can result in skewing. For instance, skewing may contribute advantageous immunomodulation, boosting female cancer defence, which is not an option for males33.
Impaired XCI that results in irregular bi-allelic X expression increases the cancer risk for females34,35. Additional cancer risks are posed in females from the physical loss of the Xi that leaves exclusive Xa expression36 and also from simultaneous disruption of gene copies from both the Xi and the Xa37 (as we discuss). The single X in males defines their particular vulnerability to X disruption, with emerging roles for X homologues on the Y chromosome (hereafter Y)38. These scenarios have ramifications for sex-specific cancer outcomes and treatments.
Developmental XCI requires XIST, and XIST remains actively expressed in adult females. Unexpectedly, after embryonic XCI, the targeted elimination of Xist is well tolerated in many tissues of healthy female mice, despite some evidence of XCI reversal. Cancer develops, however, when Xist is deleted from haematopoietic stem cells39. Dysplastic polyps (likely pre-malignant tumours) arise from mouse gut epithelium lacking Xist, following exposure to chronic carcinogenic stress40. Correspondingly, X gene expression increases in intestinal polyps in these mice. Notably, these two most affected cell types are highly proliferative, in contrast to the brain where no impact was measured40.
Cancer development associated with loss of an Xi is less intuitive. Explanation lies in tumour suppressor genes that normally escape XCI and are expressed from both the Xa and the Xi. Even though escaper gene expression tends to be lower from the Xi than from the Xa28, loss of Xi escapers nonetheless can jeopardize female health. This is evident in a subset of female patients with melanoma with poor survival. Elevated cancer risk in these females aligns with loss of their Xi and, more specifically, loss of the pseudoautosomal X-linked gene PPP2R3B, which encodes the PR70 regulatory subunit of protein phosphatase 2A. The corollary is that poor survival in male patients with melanoma corresponds with loss of Y (discussed in more detail below). PR70 impedes DNA replication and, in turn, cell cycle progression, consistent with a tumour suppressor role that slows cell growth36.
By analogy to PPP2R3B, female cancer risk is expected from loss or dysfunction of other tumour suppressor genes that escape XCI, even those located outside the pseudoautosomal regions. Six such genes, termed escape from X-inactivation tumour suppressors (EXITS), are ATRX, CNKSR2, DDX3X, KDM5C, KDM6A and MAGEC3. The presence of two expressed copies of EXITS in particular female tissues appears to confer unique protection, compared with males38.
EXITS offer sex-specific considerations for cancer therapy and we discuss two in detail. ATRX is a core chromatin remodeller that upholds genomic stability through maintenance of chromatin packing, protection of telomere length and silencing of repetitive genomic sequences. These tumour-suppressive functions of ATRX explain the selection for its mutation or loss across many cancer types in both sexes. Of particular relevance to females, however, is that ATRX binds XIST, contributing to recruitment of Polycomb repressive complex 2 (PRC2) and promoting XCI (see REF.41 and references within). Consistent with tumour-suppressive activities, ATRX is part of an X-linked gene network with functional connections to p53. We found that the frequency at which mutated ATRX mRNA is expressed in female non-reproductive cancers is lower than in male counterparts. This is indicative of exclusive female protection42. Indeed, the disruption of the single X-linked ATRX copy in males is apparent in numerous cancer types that dominate in males, including glioma43 and skin melanoma44. Synthetic lethality using targeted therapies is suggested for neuroblastomas depleted of ATRX, as demonstrated in a preclinical model. Increased DNA damage associated with ATRX loss sensitizes to DNA damaging agents (for example, irinotecan), in combination with DNA repair inhibition, using a poly(ADP-ribose) polymerase inhibitor (PARPi; for example, olaparib); this leads to selective cancer cell death45. Given the male prevalence of ATRX loss or mutation38,43,44, we anticipate the treatment benefit to be of widest application among male patients (FIG. 4).
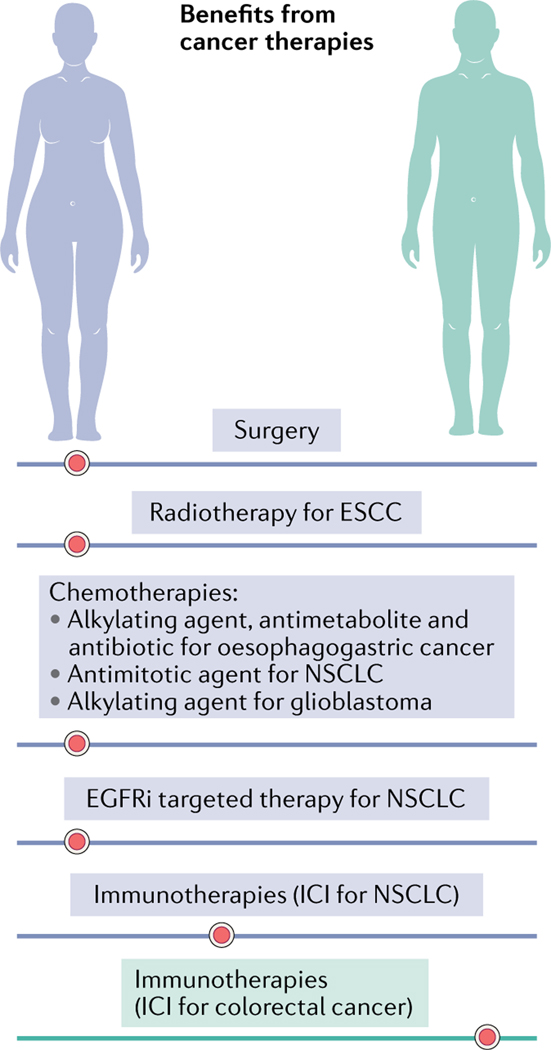
Fig. 4 |. Sex disparity in cancer therapy benefits in humans.
Females have greater overall survival outcomes from surgery for lung cancer142 and less sepsis than males33. Radiotherapy offers survival advantage to females at the expense of toxicity, as evident in patients with oesophageal squamous cell carcinoma (ESCC). Chemotherapies advantage female survival in responses to: combined treatment with an alkylating platinum agent, the antimetabolite pyrimidine and an anthracycline antibiotic in oesophagogastric cancer176; the antimitotic paclitaxel in non-small cell lung cancer (NSCLC)177; and the alkylating agent temozolomide in glioblastoma (with surgery and radiotherapy)178. Targeted therapies show greater efficacy in females for an epidermal growth factor receptor inhibitor (EGFRi) in NSCLC64. Immune checkpoint inhibitors (ICIs; anti-PD1 or anti-PDL1) trend to greater individual female survival benefit in NSCLC, whereas male outcomes are better for colorectal cancer163.
A second EXITS gene, KDM6A (also known as UTX), encodes a histone lysine demethylase. KDM6A is mutated across a breadth of cancers, driving oncogenesis with greater frequency in males than females38. Bi-allelic loss of KDM6A, albeit rare, is a danger for females with metastatic squamous-like pancreatic cancer. This is due to consequent disruption of the COMPASS-like complex of which it is a member, and also deregulated activation of super-enhancers that drive key oncogenes (for example, MYC, RUNX3 and the ΔN isoform of TP63)37. In a mouse model of this cancer, complete Kdm6a loss is prognostic in females for poor outcomes and confers sensitivity to bromodomain (BET) inhibitors37. KDM6A-deficient cells also depend on EZH2, which is the enzymatic component of the gene silencing complex PRC2, and EZH2 is therefore a promising therapeutic target46. Although males are vulnerable to loss of their single copy of KDM6A, their Y homologue KDM6C (also known as UTY) adds a protective dimension38.
In summary, sex disparity in the expression of specific X-linked genes is conspicuous in non-reproductive cancers21,38, with key X-linked genes involved in apoptosis, proliferation47 and crucial genomic regulation38. PPP2R3B exemplifies the female cancer risk associated with losing even a less active escaper from the Xi36. ATRX demonstrates inherent cancer protection afforded to females with a ‘back-up’ second copy38. The third example, KDM6A, illustrates the cancer risk of a complete loss of X-linked genes albeit relatively infrequent in female cancers. Protected EXITS38, together with accumulation of gene mutations in later age11, contribute to lower female cancer incidence compared with age-matched males who encode only a single X-linked gene copy38.
Loss of the Y chromosome in cancers.
The unique male Y is pivotal in determining male sex. Albeit the third smallest chromosome, with ~60 protein coding genes and ~100 non-coding RNAs26, Y encodes functional tumour suppressors48. In 12 solid cancer types, a strong association was uncovered between male cancer risk and extreme downregulation of Y (EDY), relative to autosomal gene expression. EDY is a measure of decreased Y-linked gene expression not only due to somatic complete loss of the Y (LOY) but also reduction due to other mechanisms, such as methylation. EDY is proving to be more strongly associated with cancer risk than LOY alone48. This is relevant as LOY has been a widely used prognostic biomarker, for example correlating with reduced survival in ~25% of male head and neck squamous cell carcinoma49. Beyond LOY, EDY is now predicted to be a valuable biomarker, for both solid50 and haematological51 cancers.
Six Y-linked genes stand out for their reduced transcription levels across 12 major non-reproductive cancers, suggesting a selection against their activity. Their functions are predictive of tumour suppressors: KDM6C and KDM5D (histone demethylases); DDX3Y (RNA helicase); EIF1AY (translation initiation factor); RPS4Y1 (ribosome subunit); and ZFY (transcription factor). KDM6C, KDM5D and DDX3Y are functional homologues of the EXITS introduced above: KDM6A, KDM5C and DDX3X, respectively38.
Male cancer susceptibility associated with loss of these X homologues is also consistent with a tumour-suppressive function. This is suggested by the catalytically weak histone demethylase encoded by KDM6C. Despite its poor enzymatic function52, KDM6C is lost or mutated in multiple male cancers coincidently with its X-linked homologue KDM6A (REF.53). This predicts some additional tumour suppressor competence and, indeed, KDM6C and KDM6A were also found to suppress the oncogenic ETS family of transcription factors, with consequent promotion of a GATA tumour suppression programme (evident in leukaemia)53. In the context of pancreatic cancer, where LOY occurs in ~40% of male cases, loss of both X and Y homologues is necessary for the development of squamous-like tumours, analogous to the female necessity for bi-allelic KDM6A loss (as described earlier). This further predicts sex-specific biomarker relevance and therapeutic opportunities37,46.
Autosomal cancer genes
The complexity of XCI (BOX 2) has led to preferential study of autosomes. Specifically, DNA sequencing does not distinguish between the Xi and the Xa, which prevents straightforward identification of the DNA gene allele being transcribed into the mRNA product. Non-sex chromosomes, on the other hand, offer ready correlation between coding genes and their expressed mRNA. Among the healthy population, differences in gene expression between the sexes are tissue type-dependent, across 44 tissues. The numbers of autosomal genes exhibiting sex-distinct expression exceed those of the X, although variance is greater for the latter. These differences appear to reflect the sum of distinct sex hormone-related transcriptional regulation, plus the influence of other transcription factors and epigenetic regulators6. With this background of healthy tissue gene expression diversity between males and females, it is expected that cancer types will vary depending on the tissue type. An established cancer risk is the epigenetic silencing of tumour suppressor genes mediated by promoter methylation, but there is only limited understanding of how this differs between the sexes54. Instead, we will focus on established molecular findings and their ramifications for tailoring therapies on the basis of sex and cancer type.
Somatic gene mutations are a burden of ageing and begin to accrue in males, on average, 10 years in advance of females11. Males therefore have an earlier risk than females of accumulating the decisive one to five gene mutations necessary to initiate cancer55, referred to as truncal mutations10. Why this disparity occurs is central to this discussion with relevance to therapy.
Mining for molecular explanations of sex disparity in broad pan-cancer studies is exposing divergence in both coding56 and non-coding57 regions of autosomal genes. A greater somatic autosome mutation load in males than females is evident in 28 cancer types57 (although the allosomes were excluded, as relevant to BOX 2). The reason for these mutation variants is unknown57. It is reasonable to consider that age may contribute, given the younger age of mutation acquisition in males11 and the standard practice of age-matching samples.
Mutation of individual tumour suppressor genes and changes in expression between the sexes are largely cancer type-dependent, but a few differ significantly21,58. The most pronounced sex-biased driver gene mutation identified in non-reproductive patient samples of The Cancer Genome Atlas (TCGA) is β-catenin (encoded by CTNNB1) in liver cancers. CTNNB1 mutation incidence in males is 33% versus 12% in females56. Polyclonality and an unexplained single base substitution (SBS) signature (SBS16 (REF.56) involving an adenine mutation59) also dominate male liver cancer (in 16% of males versus 2.2% of females)57. The three times higher liver cancer incidence and mortality rate in males than females (FIG. 1b,c) predicts that males will receive greater therapeutic benefit by targeting, for example, the β-catenin pathway60. By contrast, the less frequent female liver cancers (FIG. 1b,c) associate with defective mismatch DNA repair, gene copy number losses and a distinct mutation signature (SBS1 (REF.57), attributed to deamination of 5-methylcytosine to thymine: C > T59). In liver cancer, 14% of females versus 1.6% of males carry mutations in the gene encoding the tumour suppressor BRCA1-associated protein 1 (BAP1)56. BAP1 is a deubiquitinating enzyme that interacts with proteins that regulate gene expression (such as X-encoded O-linked N-acetylglucosamine transferase and Polycomb group proteins) and impacts cell differentiation, DNA repair, apoptosis and metabolism61. Premised on the high female mutation incidence of BAP1 in liver cancer56, we predict that clinical trials with PARPis that are being tested in BAP1 mutant cancers61 will be of particular female benefit. Additional targeted therapies under consideration in this context include inhibitors of histone deacetylases and EZH2 (reviewed elsewhere61).
Gaining further insight into cancer sex disparity, beyond the comparatively accessible genetic changes, involves the more demanding combination of multiple complementary analyses. In-depth multi-omics study of lung adenocarcinoma segregated four subgroups according to clinical and molecular features, with one showing sex bias. This group was enriched for Chinese females and tumours with epidermal growth factor receptor (EGFR) mutations. EML4–ALK gene fusion (encoding EMAP-like 4 fused to the ALK receptor tyrosine kinase) also clustered in this group62, which offers scope for targeted therapies63. In keeping with this, female patients with non-small cell lung cancer (NSCLC) have better survival in response to EGFR inhibitors (EGFRis)64.
TP53 is the most frequently altered gene in cancer65,66, with a 50% mutation incidence67. We calculated that in the US population more males than females carry TP53 mutations in non-reproductive cancers42. TP53 mutation is strongly selected in cancer not only to abolish its tumour-suppressive functions but also as it can lead to gain of neomorphic oncogenic functions68. Correct p53 function is crucial to maintaining genomic integrity20. In response to DNA damage, p53 transcription activity is enlisted to conduct vital DNA damage response pathways directed to preventing the propagation of cells with damaged genetic code (reviewed elsewhere69). Accordingly, there is close correlation between TP53 mutation and greater mutational burden over the entire genome. This was reflected in more than twice the number of deletions and amplifications, and also more chromosomal instability measured in 32 different cancer types with TP53 mutation, compared with those with wild-type TP53 (REF.68). The contribution of TP53 mutations to cancer progression is strongly supported by the overall poorer survival of patients with cancer with TP53 mutation42. Given the key role that p53 plays in maintaining genomic integrity, it is noteworthy that, in TCGA, DNA repair pathway genes are more highly expressed in cancers of females than of males, in age-adjusted data21. Given the greater numbers of males with TP53 mutated cancers, we predict that they will benefit particularly from TP53 targeted therapies67 compared with females.
Rates of TP53 mRNA expression are remarkably similar among age-matched male and female cancers21,56,58, but these studies do not distinguish whether mutant or wild-type TP53 is expressed. The dynamic cellular levels of human p53 protein required for its normal rapid stress responses are largely controlled post-transcriptionally, not at the level of mRNA expression68. Currently, however, only very few comprehensive protein sequence analyses of biopsy samples from patients with cancer are publicly accessible, in contrast to the extensive banking of DNA sequence and mRNA expression data (for example, in TCGA70). This limitation is relevant to genes that are regulated largely at the protein level, where p53 provides an excellent example. Accumulation of p53 is required for its mutated form to drive the acquisition of oncogenic functions, including metastasis71. Drawing on p53 protein regulation as a prototype68, comprehensive protein mutation analyses of large collections of patient biopsy samples are warranted for a fuller appraisal of post-transcriptional sex disparity in cancer.
Metabolism, obesity and cancer
Many metabolic processes, including glucose and lipid metabolism, differ between healthy females and males. Metabolic X-linked genes contribute to this sexual dimorphism72 (FIG. 3; see Supplementary Table 1). Accordingly, associated disease risks differ between the sexes (reviewed elsewhere73). Within these distinct contexts, cancer cells rewire metabolism to manage the demands of increased proliferation20. Significant sex differences in pathways of glycolysis, fatty acid and bile acid metabolism, and xenobiotics are evident in 13 non-reproductive cancers in TCGA21. Metabolic dependencies acquired during cancer onset define strategic therapeutic targets, and a number of these have proven to be sex-dependent, exemplifying opportunities to customize targeted therapies.
Glucose metabolism differs between males and females.
Upregulation of glycolysis is common in cancer cells, which fuels proliferative growth74 (FIG. 5a). Blood glucose levels are higher in healthy males than females75, which likely contributes to a disproportionate cancer risk between the sexes. Elevated fasting glucose levels in males correlate with increased liver76 and colon77 cancer incidence, but not in females76,77.
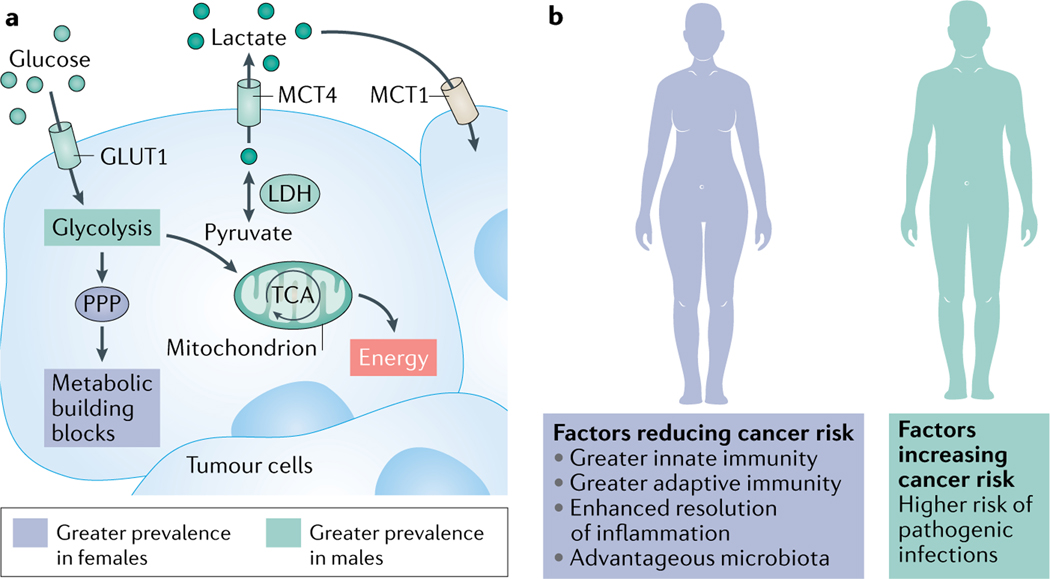
Fig. 5 |. Sex differences in cancer metabolic pathways and immune response contribute to cancer sex disparity.
a | Glucose metabolism may proceed through different pathways in male and female cancers. Glucose metabolism may differ in cancer cells in males and females. In male cancer cells, energy (ATP) is frequently generated from the breakdown of glucose through the glycolysis pathway (Warburg effect187), feeding pyruvate into the TCA cycle and then oxidative phosphorylation. Cancer cells tend to divert pyruvate to lactate that can circulate in the bloodstream and enter other cancer cells. These processes are more prevalent in male cancers than female (for example, in non-small cell lung cancer (NSCLC)) due to higher expression levels of glucose transporter 1 (GLUT1 (REF.80)), the lactate–pyruvate converting enzyme (lactate dehydrogenase (LDH), for which the LDHB subunit is higher in male gliomas91) and the lactate transporter (monocarboxylic acid transporter 4 (MCT4), which is higher in male melanoma93). By contrast, in females, intermediates of glucose metabolism may preferentially be diverted to the less energy-efficient pentose phosphate pathway (PPP; as in right-sided colon cancer (RCC)91) that produces antioxidant NADPH and metabolic building blocks. b | Immunity differs between the sexes. Females have greater adaptive and innate immune responses than males. These confer greater antiviral T cell immunity and anticancer response. A more efficient resolution of inflammation in females protects them from chronic inflammation-associated cancers126–128. Additionally, female microbiota improve responses to anticancer immune therapies116. On the other hand, males have higher risk of infection from pathogenic agents and, consequently, are at greater risk of infection-associated cancers139.
The uniporter glucose transporter 1 (GLUT1; also known as SLC2A1) is crucial and rate-limiting for cellular glucose uptake under basal conditions78. In cultured human cells, wild-type p53 reduces active glucose uptake by inhibiting GLUT1 expression79. GLUT1 levels are higher in tumours in males than in females, as measured in patients with NSCLC, and correspond to the tumour stage and poor survival80. In cancer cells, mutated p53 shunts GLUT1 to the cell membrane81, effectively feeding proliferation. In a feedback loop, glucose maintains mutant p53 stability82. Due to more frequent TP53 mutations in males42, their risk for cancer associated with GLUT1 misregulation is expected to be greater than that for females. This is also anticipated for other p53 partners in the glycolytic pathway whose normal regulation by p53 is disrupted in cancers83, including X-linked glucose-6-phosphate dehydrogenase (G6PD) that is inhibited by wild-type p53 (REF.84). A likely explanation for the greater efficacy of the glucose-reducing drug metformin to lower colorectal cancer mortality rates in females compared with males with type 2 diabetes85 is that the high glucose levels in males cannot be sufficiently reduced by the standard metformin administered dose. The higher male prevalence of TP53 mutations in non-reproductive cancers42 leads us to predict, however, that drugs directed against mutant TP53 (for example, REF.67) will have particular value in males for potentiating the efficacy of metformin85. We anticipate a parallel effect for resveratrol-precursor polydatin, which targets upregulated G6PD (REF.86). Further, as chemotherapy resistance is linked to elevated glucose levels (reviewed elsewhere87), high glucose levels in males75 predict disproportionate risk. An example that begs rigorous testing is resistance to the antimetabolite pyrimidine analogue 5-fluorouracil. High glucose levels in cancers of the intestinal tract demand increased drug dosing for extended time courses for efficacy, which in turn primes resistance87. This is overlaid by higher rates of 5-fluorouracil clearance in males than females with gastrointestinal cancers88 (FIG. 4).
In many types of cancer cells, glucose fed into the glycolytic pathway preferentially generates lactate, in a process termed the Warburg effect89, rather than pyruvate that is normally produced under aerobic conditions. Lactate functions as a fuel that is as energetically beneficial as pyruvate. It can be trafficked around the body, stored in the liver through gluconeogenesis and, then, retrieved on demand and disseminated. Also, lactate can act as a signalling molecule, with ability to promote epithelial– mesenchymal transition and angiogenesis, alter the tumour microenvironment through acidification and regulate immune responses leading to immune evasion90. Disproportionately high levels of lactate production were measured among male compared with female patients with glioma. Specifically, male gliomas with elevated lactate to pyruvate ratios have significantly poorer survival outcomes, compared with those with low lactate to pyruvate. By contrast, females lack this association. Sex disparity is also evident for the intracellular enzyme lactate dehydrogenase (LDH) that reversibly interconverts lactate and pyruvate. Gene expression levels of the LDH subunit LDHB were higher in male gliomas than in female. Such clinically relevant metabolic features argue that genetic sex is a pertinent biological variable to consider in the prognosis and treatment of patients with gliomas91.
Lactate trafficking across cell plasma membranes relies on monocarboxylic acid transporters (MCTs). The efflux activity of MCT4 (also known as SLC16A3) prevents lactate build-up to avoid devastating intracellular acidification in glycolytically active cancer cells. MCT1 (also known as SLC16A1) in turn uptakes lactate into endothelial cells, and also oxidative tumour cells, to fuel energy demands. In liver membranes of healthy rats, protein levels of MCT4 and MCT1 are higher in males compared with premenopausal females, despite hormone-induced fluctuations92. In human melanoma, MCT4 protein levels are highest in males and advanced clinical stage93 (FIG. 5). The non-steroidal anti-inflammatory drug diclofenac is an effective inhibitor of MCT1 and MCT4, reducing lactate efflux and tumour growth94. Specific MCT inhibitors are rational anticancer agents and prototypes are in early-phase clinical trials95. Together, these studies point to particular cancer risks for males posed by elevated glucose and lactate levels, while also emphasizing the value of optimizing drug activity for such contexts.
Obesity and associated inflammation differ between the sexes.
Sex-distinct cancer risks are linked to lipid metabolism. Males with obesity have disproportionately high rates of colon cancer, non-Hodgkin lymphoma and haematological cancers17. Obesity-related, non-reproductive cancers significantly associate with type 2 diabetes in males, but lack association in females96. Regulated calorie restriction (through diets, intermittent fasting or mimetic drugs) has been demonstrated preclinically to improve outcomes in both males and females in conjunction with radiotherapy, chemotherapy and immune therapies97. Future clinical trials warrant the inclusion of patient sex as an outcome determinant.
Chronic low-grade inflammation is frequently associated with excess adipose tissue and poses a cancer risk98. In individuals with obesity, release of the pro-inflammatory adipokine leptin activates pro-inflammatory responses from immune cells. Leptin receptors are present almost universally on immune cells, and their engagement provokes the release of pro-inflammatory cytokines99. Short-term, acute inflammation is remedial but, if chronic, can promote cancer by driving DNA damage100. This is a particular risk for male-dominated liver and colorectal cancers101. Notably, a threefold increased risk of colorectal cancer was measured among males with the highest levels of leptin, versus males with the lowest levels, whereas females showed no association102. Accordingly, we predict that new approaches to therapeutically target leptin with antibodies103 would be particularly beneficial to males. ALK inhibitors63 also beg to be tested in these cancers in males, as mice deleted of ALK resist leptin-linked obesity104.
By contrast, adiponectin, the most abundant adipokine secreted by adipocytes105, protects females from liver cancer106. Adiponectin acts on macrophages and T cells to wield anti-inflammatory impact, while also being anti-proliferative and pro-apoptotic107. In human males, low adiponectin levels in adipose tissue drive liver cancer. Mice studies demonstrate an indirect reduction in adiponectin levels in response to testosterone exposure. Adiponectin protection against liver cancer in females involves activation of the kinase AMPK106. AMPK is a target of metformin108, which is consistent with the greater anticancer properties of metformin in females than males85.
Notably, male mice on a high-fat diet adopt an obesity microbiome more rapidly than female mice109. Associated inflammation is ascribed to epigenetic reprograming in colonic epithelia and silencing of transcription factor hepatocyte nuclear factor 4α (HNF4α). Aligned with this in other contexts, not only is HNF4α linked to anti-inflammatory activity109 but sex disparity of function is also evident from its greater capacity to regulate gene expression in male livers than female110. Regarding the microbiome, crosstalk between microbiota and host lipid metabolism is mediated by bile acids. Compared with males, female microbiota stimulate higher serum levels of these mediators, which in turn feedback to exert greater regulation of host lipid and cholesterol metabolism111.
In humans, the predominant gut microbiota characterized so far include bacteria with some fungi, protozoa, archaea and viruses112. The influence of host age and sex113 prompted the definition ‘microgenderome’114,115. Numbers116 and composition114 also vary according to geographical population, diet117,118 and intestinal location115. Further, microbiota profoundly impact sex-distinct circadian rhythms, as shown in mice119. Deregulation of circadian rhythms is a cancer risk120. As a net sum of these microbiome influences, males and females are prone to different risks of obesity, chronic inflammation and, in turn, cancer. As responses to cancer treatments are affected by gut microbiota (reviewed elsewhere121), evidently influenced by sex, it is timely to consider patient sex when developing treatments that target the microbiota for cancer therapy.
Metabolic niches impact cancer sex disparity.
Whether or not linked to the microbiota, the original concept of sex-specific metabolic tumour niches at discrete intestinal locations was spawned by a seminal metabolomics study of colorectal cancer122. In contrast to the overall higher rates of colorectal cancer in males (FIG. 1b,c), the right-sided colon cancer (RCC) subtype is an exception. RCC is more common in females than males and is more aggressive and lethal than left-sided colon cancer. Comprehensive, untargeted metabolomic analysis suggested that nutrient-poor RCC in females is promoted by increased fatty acid metabolism to provide ATP to fuel glutamine-dependent asparagine synthesis122. Asparagine is able to exchange across the plasma membrane for amino acids (notably serine) and promote nucleotide production; implicating asparagine in protein and nucleotide synthesis123. High levels of asparagine synthetase (ASNS) expression correspond with worse survival among females with colorectal cancer. This adds to the female tendency to metabolize glucose derivatives through the pentose phosphate pathway in RCC (FIG. 5), whereas glycolysis dominates the counterpart male RCC tumours122. Remarkably, despite the more aggressive nature of RCC and the tendency to administer less chemotherapy post surgery to females, their survival is greater than that for males with colorectal cancer124,125. These studies question whether sex-specific metabolic niches with relevance to cancer prognostics and treatments also exist in other locations.
Cancer immunity
How the innate and adaptive immune system and cancer engage under the influence of sex and age is an emerging area of research9,126. Key elements of immune-mediated recognition, elimination of infectious agents linked to cancer causation and, also, purging of cancer cells differ between the sexes, which may influence disease outcomes. Adult female immune responses are generally more robust than those of males and are covered in detail elsewhere126–128. Overall, females are more adept than males at mounting beneficial, acute inflammatory immune responses127,129 (FIG. 5b). In part, this is attributed to the capacity of low physiological levels of oestrogen to stimulate the production of the acute inflammatory cytokines interleukin-6 (IL-6) and tumour necrosis factor (TNF), which are active in infection control. By contrast, higher levels of oestrogen dampen the expression of these cytokines. Testosterone also reduces TNF levels. Overall, females more competently resolve infections than males127, which counters cancer risks associated with sustained exposure to chronic infection-associated inflammation130.
Notably, immune defence against cancer declines with age, in a manner that differs between the sexes. Reduction in T cell numbers occurs in both sexes with age, but a disproportionate decrease in T cell and B cell populations is evident in older males. Corresponding to these alterations, two waves of epigenetic regulation deplete immune cell functions. An initial round in people in their late thirties has similar impact across the sexes, whereas a second cycle is distinct. This occurs in males aged in their early sixties but is delayed by 5–6 years in females. In males aged older than 65 years, this results in the elevation of pro-inflammatory activity and innate immunity. This contrasts with older females, who exhibit greater adaptive immunity131.
Sex chromosomes influence cancer immune defences.
The allosomes critically influence both innate and adaptive immune responses, with significant bearing on carcinogenesis. Of unique relevance to males, our pathway analysis shows that the Y is enriched with wound healing genes that are immune-suppressive, fitting the anti-inflammatory M2-like tumour-associated macrophage signature (Supplementary Table 1). Although macrophages may exhibit various phenotypes beyond M1 and M2 (REF.132), the M2 signature aligns with elevated cancer vascularization, suppressed cancer immunity and poor responses to chemotherapy133. Based on this Y-linked profile, we predict that targeting M2-like macrophages134 in males is a rational approach to potentiate cancer therapy.
From among the ~50 X-linked genes involved in innate and adaptive immunity47 (FIG. 3; see Supplementary Table 1), we discuss key examples that impose sex-distinct influence over cancer. Immune-suppressive tumour microenvironments are driven by CD4+ regulatory T cells (Treg cells135), which characteristically express the X-linked FOXP3 (REF.136). FOXP3 is a transcriptional regulator that is crucial to Treg cell development and function. FOXP3+ Treg cells occur in visceral adipose tissues of male mice at higher incidence and with distinct molecular profiles than in females. Of possible relevance to cancer, these tissues are more prone to inflammation (as introduced above) in males compared with females137.
The X-linked Toll-like receptor 8 (TLR8) also performs a central role in Treg cell biology. Activation of TLR8 in Treg cells impairs immunosuppression. Mechanistically, TLR8 signalling selectively inhibits glucose uptake by the highly glucose-dependent Treg cells, triggering their senescence. This relieves Treg cell inhibition of effector T cells and defines a fundamental X-linked–adaptive immune–metabolic axis135. We expect that the TLR8 agonists that are currently being investigated as new cancer immunotherapies138 will be potent anticancer agents in males137.
X-linked immune genes also dictate sex differences in resilience against infection and relief from chronic inflammation provoked by unresolved infections. This is pertinent to the higher cancer risk associated with infections in males compared with females from both bacteria and viruses139 (also known as oncoviruses140). Among the 18.1 million new non-reproductive cancer cases in 2018 that were credited to infectious pathogens (~13% of the worldwide total, encompassing 36 cancer types), males have twice the incidence of females139 (Supplementary Table 2). An Australian study determined that males are also at higher risk than females of life-threatening responses to infection (sepsis), which stem from cancer intervention procedures. Specifically, within the first year of cancer diagnosis, sepsis occurred at higher incidence and was associated with a significantly higher mortality rate in males141. This is a likely contributor to the overall greater female survival compared with males post surgery for lung cancers142 and melanoma143. Female protection from sepsis has been linked to an advantageous female X-gene repertoire33. Further study of the distinct vulnerabilities to infection and sepsis between the sexes is anticipated to open opportunities for rational therapeutic interventions.
X-linked genes equip the sexes differently against infection and, in turn, cancer. Pattern recognition receptor screening against foreign nucleic acids144 is a central function of X-linked TLR7. Bi-allelic expression of TLR7 is evident in human females145. TLR7 is expressed in key immune defence cells, including monocytes, plasmacytoid dendritic cells (which bridge between innate and adaptive immunity with capability to activate T cells) and B cells145. TLR7 is encoded in tandem with TLR8 (REF.146). From their endosomal location147 in human immune cells148, both of these TLRs have a striking specificity for degraded single-stranded RNA sourced from multiple viruses149 (relevant to the oncoviruses HTLV-1 and HCV150; see Supplementary Table 2), various pathogenic bacteria and archaea (linked to inflammatory disease148).
Activation of these TLRs provokes an acute remedial infection response of pro-inflammatory cytokines and type I interferons. Interferons are key modulators of innate and adaptive immunity. In infected cells, interferons inhibit the spreading of infectious agents151. The TLR–interferon pathway is regulated by two X-linked genes IRAK1 and IKBKG (encoding IL-1 receptor-associated kinase 1; and inhibitor of nuclear factor-κB (NF-κB) kinase regulatory subunit-γ; see Supplementary Table 1), which are part of the NF-κB pathway. Consistent with bi-allelic expression, TLR7 stimulates greater IFNα production in females than in males152. In the context of greater sepsis incidence in males, females are advantaged by skewed IRAK1 expression33. Compounding this, the X-linked helicase DDX3X is an upstream regulator of type I interferon153. Notably, truncating mutations of DDX3X that are exclusive to male melanoma are credited with greater mutational burden and poorer survival outcomes than in females154.
Overall, robust and more heterogeneous immune responses are evident in females than males in response to infection, which may explain greater cancer risks in males139,155 (FIG. 5b). Together, these findings identify a particular need in males to eradicate infections linked to cancer risks. This requires overcoming poor vaccine responses in males66, and their earlier-aged immune degeneration139,156.
X-linked immune regulators are differently regulated between the sexes.
Important details are emerging concerning differences in transcriptional regulation of TLR7 and TLR8 between the sexes, cell types and species. TLR7 bi-allelic expression in human female B cells and myeloid cells is well substantiated145. The situation for T cells requires clarification, with mono-allelic expression evident in mouse CD4+ T cells. Unexpectedly, mono-allelic X-linked TLR7 is more highly expressed in these mice cells of males than females. This is attributed to lower levels of methylation across the maternal X, which is exclusively inherited by males. Females, in contrast, either express their maternal less methylated copy or their paternal more highly methylated X157 (FIG. 2c). How closely mouse and human genetics and immune profiles converge on TLR7 expression remains to be established (BOX 1). Pertinent to this, the numbers of immune cell numbers relative to gene expression is likely to be relevant to infection control. Notably, in humans, there are higher numbers of CD4+ T cells circulating in females than in age-matched males127,129, even as levels decline with ageing131.
Species-distinct gene regulation and sex disparity of expression is evident for TLR8. TLR8 is transcriptionally regulated by p53 in human immune cells in response to radiotherapy or doxorubicin. The level of response is controlled by a single-nucleotide polymorphism in the human TLR8 promoter. Female heterozygosity for this single-nucleotide polymorphism appears to influence TLR8 expression. By contrast, mice lack p53-mediated regulation of TLR8 (REF.158). Mouse TLR8 also fails to be activated by single-stranded RNA148. These phenomena are vital considerations for the selection of appropriate preclinical cancer models (BOX 1). Together, these two examples predict wider lessons for X-linked genes, where we expect single-nucleotide polymorphisms in gene promoters to compound the XCI status of a gene, in a species-specific and cell type-restricted manner. In addition, whether this phenomenon extends beyond immune genes is an important, open question.
Overall, these studies are consistent with sex disparity among immune gene networks identified in the formative in-depth pan-cancer study by Yuan et al.21. Specifically singled out are inflammatory responses, IL-6, Janus kinase (JAK) and signal transducer and activator of transcription 3 (STAT3) signalling, IFNα and IFNγ responses, TNF allograft rejection, IL-2 and STAT5 signalling, and complement. Notably, the exact nature of the sexually dimorphic responses differs between cancer types. A separate pan-cancer analysis reported heterogeneity in immune-related factors between the sexes for tumour purity, CD8+ T cells, immune checkpoint gene expression, cytolytic activity, tumour mutation burden and neoantigens159. In our own study of TCGA NSCLC data, we identified that tumour purity differed between the sexes predominantly due to immune infiltration, rather than stromal contamination. Females with wild-type TP53 had higher immune cell levels than their counterpart males, and these females had extended survival compared with all other males and females with this disease160. Harnessing the immune system to fight cancer is proving powerful but requires crafting to each cancer type and patient sex, as we discuss below.
Sex considerations in therapy
Genetic sex influences immunotherapy outcomes.
Empowering immunity against cancer is core to the new wave of immunotherapies161, and establishing their efficacies between the sexes is of particular interest. Immune checkpoint inhibitors (ICIs) are designed to reinvigorate the immune response in tumours that evade effective T cell-mediated immune surveillance162. Significant sex disparity in reaction to ICIs (for example, inhibitors of programmed cell death protein 1 (PD1), PD1 ligand 1 (PDL1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA4)) is evident from analyses of individual cancer types (FIG. 4). Anti-PD1 or anti-PDL1 treatments resulted in a trend to greater overall survival and better response rates in individual females with NSCLC, compared with males. The opposite was evident for example for colorectal cancer, where individual males outlived females. For skin melanoma, overall survival in response to ICI treatments (anti-PD1 or anti-CTLA4 alone or in combination) was greater for males than females in six out of seven clinical trials. Outcomes for specific therapies on an individual patient with melanoma basis, however, were less consistent between the sexes across trials163, for reasons that are not clear. Sex disparity in melanoma response is likely to reflect the significantly less frequent presentation of peptides derived from cancer drivers, on major histocompatibility complex (MHC) class I and II molecules, in young female patients. This is not attributable to MHC presentation capacity, as this proved comparable between the sexes. The explanation is that the robust immune surveillance in younger females selects for cancer driver mutations that are more adept at evading immune detection162. In addition to the particular benefit for male patients with melanoma receiving monotherapy ICIs, extra advantage is anticipated for patients with highly expressed glycolysis genes and poor prognosis who receive co-treatment with ICIs and MCT inhibitors94 (introduced above). A relevant cohort are males aged older than 65 years with coincident high serum LDH levels, wild-type BRAF status and PD1 expression164.
Robust establishment of sex disparities in responses to ICIs demands the inclusion of immune and molecular features, with adjustment for confounding factors, including race, smoking status, tumour stage, histological type, tumour purity and age at diagnosis163. Pooling across cancer types and therapies in previous studies prevented clear conclusions165,166. Inclusion of sufficient numbers of females is also fundamental163. Under-representation of females is likely to be an issue in many studies for several reasons: there are fewer females with non-reproductive cancers in the general population (FIG. 1); females tend to be less likely to enrol in clinical trials167; older age of females, on average, at cancer diagnosis (exemplified in colorectal cancer124); toxicities associated with exposure to numerous standard chemotherapies168 (discussed below); and greater risk of autoimmune responses triggered by overreaction to immunotherapies156.
A better understanding of the impact of sex on the microbiota is also pertinent to ICI responses. Survival outcomes for patients with melanoma and mouse models treated with ICIs are better in the context of elevated bacterial faecal population diversity. This is evident with greater numbers of the Ruminococcaceae communities including Faecalibacterium (reviewed elsewhere169,170), which are higher in healthy human females116. Of relevance, females are more likely to take antibiotics than males, and at higher dose171. Administration of antibiotics within 1 month prior to, but not simultaneously with, ICI administration diminished survival outcomes by 50% among patients with cancer predominantly diagnosed with melanoma or NSCLC (reviewed elsewhere172). Digging deeper into sex-specific antibiotic usage in the context of ICI treatments begs to be addressed, as does a potential impact on changes in the microbiome and a consequent impact on immunity173.
Consequences of radiotherapy and chemotherapy responses in females and males.
Sex disparity in toxicity responses to radiotherapy and chemotherapy cancer treatments is relevant for treatment optimization (FIG. 4). Remarkably, a 2020 literature survey to investigate sex-dependency of radiotherapy efficacy found that, despite its prevalence as a cancer treatment, only a total of eight papers, and four of these since 2017, were suitable for review on the topic. Among these, radiotherapy treatment of females with oesophageal squamous cell carcinoma significantly extended their survival compared with males. In this cancer type, however, cardiac toxicity in females occurred at significantly lower dose than in males (with median toxic dose 36.6 Gy versus 55.3 Gy, respectively). Similarly, in young children receiving cranial radiotherapy, IQ damage was greater in girls than boys. There is a clear need to expand these studies and ensure that this fundamental treatment is optimized between the sexes174. Chemotherapy toxicity is also evidently greater in females than males for a range of cancer treatments175. Frequently, greater toxicity in females results in fewer doses being administered, compared with males176. Worse female toxicity to chemotherapy is attributed to slow clearance compared with males175. Female patients also tend to be older than their male counterparts (at least for colorectal cancer124), predicting greater fragility.
Evidence that females survive longer than males in response to chemotherapies is growing across the four main classes (FIG. 4): DNA cross-linking alkylating agents, antimetabolites (for example, inhibitory analogues of purine), antimitotics (for example, microtubule stabilizers and microtubule depolymerizers) and anticancer antibiotics (that bind nucleic acids to inhibit either DNA or protein synthesis). Prolonged female survival responses are evident from a number of these cancer treatments. Advantageous female overall survival is evident in patients with oesophagogastric cancer treated with a combination of an alkylating platinum agent, a pyrimidine antimetabolite and an anthracycline antibiotic, despite toxicities176. A modest progression-free survival advantage for females over males is also evident for NSCLC treated with the antimitotic paclitaxel177. The alkylating agent temozolomide extends the survival of female patients with glioblastoma, compared with males, treated with standard of care surgery and focal radiotherapy178.
Factoring genetic sex into new anticancer drug development.
Relevant to the new wave of precision cancer medicine are the sex-based molecular signatures associated with more than 50% of genes identified to be clinically actionable as therapeutic targets or as biomarkers21. It will be propitious to include patient sex in the development of new treatments from the outset. To gain optimal benefit from these treatments, four key sex disparities179 should be factored into future strategies. The first is the slower pharmacokinetics in females caused by retarded elimination of drugs compared with males180. This results from sex differences in the expression of drug-metabolizing enzymes in the liver181 and transporters in the kidney182. The second disparity concerns different efflux rates of chemotherapies and other xenobiotics between the sexes. In female mice, expression of the pump ABCB1 (also known as MDR1) in the ileum mucosa and liver is under greater influence of the circadian clock than in males. This affects both the amplitude and duration of its expression. This expression is further influenced by feeding and fasting in a sex-distinct manner168. Synchronizing dosing in females with respect to these rhythms is anticipated to improve efficacy and reduce toxicity168. The third element of body composition and physiology is pertinent to efficacy. Standard drug dosing regimens based on body surface area do not factor in important sex differences. For females, these include ~10% higher body fat, higher plasma volume and greater organ perfusion as compared with males179. The fourth consideration is the novel concept of targeting appropriate cell populations between the sexes. This is suggested by metabolic niches122 introduced above. It is exemplified further in a mouse model of human glioblastoma, which has sex-distinct biological pathway alterations183. Males have higher levels than females of monocytic myeloid-derived suppressor cells in their tumours, which block antitumour immunity. Males particularly benefit from antimetabolites (for example, 5-fluorouracil). In females, by contrast, granulocyte myeloid-derived suppressor cells circulate at greater levels than in males. Females benefit from IL-1 pathway blockade (for example, rilonacept) to counteract immune suppression and extend survival183. Understanding the basis of different cancer susceptibilities between males and females is fundamental to achieving effective anticancer treatments.
Together, these findings highlight the relevance of customizing therapies to balance efficacy and toxicity as best practice between the sexes. Incorporating consideration of sex-specific chemotherapy pharmacokinetics (for example, bioavailability, metabolism, distribution and clearance) and pharmacodynamics (for example, biological responses), using relevant normalizing parameters (for example, body surface area or BMI) will benefit both male and female patients with cancer175,184.
Conclusions and perspectives
Sex disparity in cancer is undeniable and emerging with complex interplaying elements. Underpinning everything are inherent genetic differences, with overlaying epigenetic alterations and sex hormone influences. The X and Y encode fundamental genetic determinants of regulators of metabolism, immunity and tumour suppression. Exposing sex-specific regulation of genes in cancer is predicting new approaches to customize treatments for males and females individually. The earlier age vulnerabilities of males to genomic damage suggest that they should be prioritized for younger cancer screening, diagnosis and therapeutic intervention than females.
Immune defence in females is stronger than in males, which raises important questions. For example, do females, coming from a position of immune strength, receive a greater boost from immune therapy than males, but at greater adverse risk of autoimmunity? Alternatively, can the more poorly immunologically equipped males experience more dramatic responses to treatment as their lower starting point offers greater capacity to expand? In addition, does the capacity to boost immunity translate into reduced chronic inflammation in a sex-dependent manner? The exciting new analyses of patient responses to ICIs indicate that both sex and cancer type are decisive163. Findings so far indicate that each treatment must be individually profiled.
Epigenetic regulation is emerging as a possible dictator of sex dimorphism in healthy males and females5, but its impact on cancer sex disparity is yet to be fully appreciated54, warranting further investigation. Examining such regulatory mechanisms across the cancer genome promises to be a valuable future research direction for interpreting cancer sex differences. Understanding the basic components of cancer sex disparity is core to developing strategies to achieve comparable rates of optimal outcomes in both male and female patients with cancer. Initiating protocols for strategic inclusion of patient sex in treatment design and standard cancer care is now within reach.
Supplementary Material
Acknowledgements
The authors thank C. Litchfield for undertaking the analyses for Figs 1 and 3 and also for Supplementary Tables 1 and 2. The Haupt laboratory acknowledges funding from the Sister Institution Network Fund (SINF), MD Anderson–Peter MacCallum Cancer Centre and Peter MacCallum Foundation. S.L.K. was supported in part by the National Institutes of Health (NIH) Specialized Center of Research Excellence (U54AG062333) and NIH Center of Excellence in Influenza Research and Surveillance (HHSN272201400007C). Work in the Rubin laboratory is supported by the NIH (R01 CA174737 to J.B.R.), The Children’s Discovery Institute of Washington University, Prayers for Maria Foundation, St Louis Children’s Hospital Foundation, Barnes-Jewish Hospital Foundation, Barnard Research Funds, Joshua’s Great Things Foundation and The American Brain Tumor Association.
Glossary
- Bi-allelic gene expression
Expression from the two chromosomal copies (alleles) of a gene, as opposed to expression from one chromosomal copy (mono-allelic expression)
- COMPASS-like complex
(Complex of proteins associated with Set1). A conserved complex of core proteins that function with specific methyltransferases to catalyse methylation of histone H3 at K4 or demethylation, for example in the context of KDM6A, controlling transcriptional regulation
- Immune checkpoint
A receptor–ligand molecule whose normal function is to regulate the magnitude and duration of immune responses that can be exploited by cancers to prevent anticancer T cell responses. Checkpoint inhibitors block these interactions, thereby restoring the ability of T cells to attack cancer cells
- Mosaic expression
Expression, in females, of different copies of the x chromosome in different cells. This results in a mosaic pattern of expression of different x-linked genes or forms of x-linked genes within a tissue of an individual
- M1 and M2
Two classes of macrophages with different functions. M1 macrophages kill cancer cells and infectious agents, whereas M2 macrophages heal wounds. In many cancer types, M1 gene expression signatures correspond to a favourable outcome, whereas M2 signatures align with poor outcomes
- Pattern recognition receptor
A protein that engages signatures of pathogens or damages and primes the innate immune response. In the instance of Toll-like receptor 7 (TLR7) and TLR8 these proteins function as receptors for single-stranded RNA molecules, as relevant to triggering innate immunity against viral infections
- Skewing
An extreme form of mosaicism in which either the maternal or paternal x chromosome is preferentially active
- Sporadic cancers
Cancers that arise spontaneously from genomic damage that is acquired, in contrast to familial cancers associated with inherited genetic alterations that are predisposing
- Truncal mutations
Mutations that occur in the cell lineage that gave rise to the clonal tumour. These are also called clonal mutations
- X pseudoautosomal regions
The short regions at the end of the x and Y chromosomes that share homology. These regions are important for the pairing and segregation of the x and Y chromosomes during meiosis in males. The behaviour of these regions is similar to that of autosomes
Footnotes
Peer review information
Nature Reviews Cancer thanks G. P. Dotto, J. Feunteun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41568-021-00348-y.
Competing interests
The authors declare no competing interests.
References
- 1.National Cancer Institute. SEER*Explorer. An interactive website for SEER cancer statistics https://seer.cancer.gov/explorer/(2020). [Google Scholar]
- 2.Cook MB, McGlynn KA, Devesa SS, Freedman ND & Anderson WF Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomarkers Prev 20, 1629–1637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clayton JA & Collins FS NIH to balance sex in cell and animal studies. Nature 509, 282–283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F. et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 396, 565–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes-Ramos CM et al. Sex differences in gene expression and regulatory networks across 29 human tissues. Cell Rep. 31, 107795 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliva M. et al. The impact of sex on gene expression across human tissues. Science 369, eaba3066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clocchiatti A, Cora E, Zhang Y. & Dotto GP Sexual dimorphism in cancer. Nat. Rev. Cancer 16, 330–339 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Sun T. et al. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J. Clin. Invest 124, 4123–4133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fane M. & Weeraratna AT How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 20, 89–106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine AJ, Jenkins NA & Copeland NG The roles of initiating truncal mutations in human cancers: the order of mutations and tumor cell type matters. Cancer Cell 35, 10–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podolskiy DI, Lobanov AV, Kryukov GV & Gladyshev VN Analysis of cancer genomes reveals basic features of human aging and its role in cancer development. Nat. Commun 7, 12157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Gosnell JE & Roman SA Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol 16, 17–29 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Levine AJ p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 20, 471–480 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Dominguez-Valentin M. et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet. Med 22, 15–25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lortet-Tieulent J. et al. State-level cancer mortality attributable to cigarette smoking in the United States. JAMA Intern. Med 176, 1792–1798 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Stone TW, McPherson M. & Gail Darlington L. Obesity and cancer: existing and new hypotheses for a causal connection. EBioMedicine 30, 14–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barberio AM et al. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat. Commun 10, 383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med 25, 1822–1832 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian S, Golubnitschaja O. & Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 10, 365–381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D. & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y. et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell 29, 711–722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aunan JR, Cho WC & Soreide K. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. 8, 628–642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falzone L, Salomone S. & Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol 9, 1300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barros de Andrade ESL et al. Kinetics of Xist-induced gene silencing can be predicted from combinations of epigenetic and genomic features. Genome Res. 29, 1087–1099 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delbridge ARD et al. Loss of p53 causes stochastic aberrant X-chromosome inactivation and female-specific neural tube defects. Cell Rep. 27, 442–454. e5 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Aken BL et al. The Ensembl gene annotation system. Database 10.1093/database/baw093 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tukiainen T. et al. Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeters SB, Cotton AM & Brown CJ Variable escape from X-chromosome inactivation: identifying factors that tip the scales towards expression. Bioessays 36, 746–756 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migeon BR X-linked diseases: susceptible females. Genet. Med 22, 1156–1174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Palo A. et al. What microRNAs could tell us about the human X chromosome. Cell Mol Life Sci. 77, 4069–4080 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Care A. et al. Sex disparity in cancer: roles of microRNAs and related functional players. Cell Death Differ. 25, 477–485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamekh M. & Casimir G. Editorial: sexual dimorphism of the immune inflammatory response in infectious and non-infectious diseases. Front. Immunol 10, 107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spolarics Z, Pena G, Qin Y, Donnelly RJ & Livingston DH Inherent X-linked genetic variability and cellular mosaicism unique to females contribute to sex-related differences in the innate immune response. Front. Immunol 8, 1455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaligne R. & Heard E. X-chromosome inactivation in development and cancer. FEBS Lett. 588, 2514–2522 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Wang D. et al. Abnormal X chromosome inactivation and tumor development. Cell Mol. Life Sci 77, 2949–2958 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Kempen LC et al. The protein phosphatase 2A regulatory subunit PR70 is a gonosomal melanoma tumor suppressor gene. Sci. Transl Med 8, 369ra177 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Andricovich J. et al. Loss of KDM6A activates super-enhancers to induce gender-specific squamous-like pancreatic cancer and confers sensitivity to BET inhibitors. Cancer Cell 33, 512–526.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunford A. et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Genet 49, 10–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yildirim E. et al. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 152, 727–742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Yildirim E, Kirby JE, Press W. & Lee JT Widespread organ tolerance to Xist loss and X reactivation except under chronic stress in the gut. Proc. Natl Acad. Sci. USA 117, 4262–4272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren W. et al. Disruption of ATRX–RNA interactions uncovers roles in ATRX localization and PRC2 function. Nat. Commun 11, 2219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haupt S. et al. Identification of cancer sex-disparity in the functional integrity of p53 and its X chromosome network. Nat. Commun 10, 5385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun T, Plutynski A, Ward S. & Rubin JB An integrative view on sex differences in brain tumors. Cell Mol. Life Sci 72, 3323–3342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emran AA et al. Study of the female sex survival advantage in melanoma — a focus on X-linked epigenetic regulators and immune responses in two cohorts. Cancers 12, 2082 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George SL et al. Therapeutic vulnerabilities in the DNA damage response for the treatment of ATRX mutant neuroblastoma. EBioMedicine 59, 102971 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ler LD et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci. Transl Med 9, eaai8312 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Meester I. et al. SeXY chromosomes and the immune system: reflections after a comparative study. Biol. Sex. Differ 11, 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caceres A, Jene A, Esko T, Perez-Jurado LA & Gonzalez JR Extreme down-regulation of chromosome Y and cancer risk in men. J. Natl Cancer Inst 112, 913–920 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollows R. et al. Association between loss of Y chromosome and poor prognosis in male head and neck squamous cell carcinoma. Head Neck 41, 993–1006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loftfield E. et al. Mosaic Y loss is moderately associated with solid tumor risk. Cancer Res. 79, 461–466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown DW, Machiela MJ & Why Y. Down-regulation of chromosome Y genes potentially contributes to elevated cancer risk. J. Natl Cancer Inst 112, 871–872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walport LJ et al. Human UTY(KDM6C) is a male-specific N-methyl lysyl demethylase. J. Biol. Chem 289, 18302–18313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gozdecka M. et al. UTX-mediated enhancer and chromatin remodeling suppresses myeloid leukemogenesis through noncatalytic inverse regulation of ETS and GATA programs. Nat. Genet 50, 883–894 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarne V. et al. Promoter methylation of selected genes in non-small-cell lung cancer patients and cell lines. Int. J. Mol. Sci 21, 4595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iranzo J, Martincorena I. & Koonin EV Cancer-mutation network and the number and specificity of driver mutations. Proc. Natl Acad. Sci. USA 115, E6010–E6019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li CH, Haider S, Shiah YJ, Thai K. & Boutros PC Sex differences in cancer driver genes and biomarkers. Cancer Res. 78, 5527–5537 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Li CH et al. Sex differences in oncogenic mutational processes. Nat. Commun 11, 4330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopes-Ramos CM et al. Gene regulatory network analysis identifies sex-linked differences in colon cancer drug metabolism. Cancer Res. 78, 5538–5547 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alexandrov LB et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung YS & Park JI Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med 52, 183–191 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobrinski DA, Yang H. & Kittaneh M. BAP1: role in carcinogenesis and clinical implications. Transl Lung Cancer Res. 9, S60–S66 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gillette MA et al. Proteogenomic characterization reveals therapeutic vulnerabilities in lung adenocarcinoma. Cell 182, 200–225.e35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuji T. et al. YAP1 mediates survival of ALK-rearranged lung cancer cells treated with alectinib via pro-apoptotic protein regulation. Nat. Commun 11, 74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinto JA et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 3, e000344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soussi T. & Wiman KG TP53: an oncogene in disguise. Cell Death Differ. 22, 1239–1249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia P. & Zhao Z. Characterization of tumor-suppressor gene inactivation events in 33 cancer types. Cell Rep. 26, 496–506.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levine AJ Targeting therapies for the p53 protein in cancer treatments. Annu. Rev. Cancer Biol 3, 21–34 (2019). [Google Scholar]
- 68.Donehower LA et al. Integrated analysis of TP53 gene and pathway alterations in The Cancer Genome Atlas. Cell Rep. 28, 1370–1384.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams AB & Schumacher B. p53 in the DNA-damage–repair process. Cold Spring Harb. Perspect. Med 6, a026070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cancer Genome Atlas Research Network. et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet 45, 1113–1120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olive KP et al. Mutant p53 gain of function in two mouse models of Li–Fraumeni syndrome. Cell 119, 847–860 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Zore T, Palafox M. & Reue K. Sex differences in obesity, lipid metabolism, and inflammation — a role for the sex chromosomes? Mol. Metab 15, 35–44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clegg DJ & Mauvais-Jarvis F. An integrated view of sex differences in metabolic physiology and disease. Mol. Metab 15, 1–2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liberti MV & Locasale JW The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci 41, 211–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keramida G. & Peters AM Fasting hepatic glucose uptake is higher in men than women. Physiol. Rep 5, e13174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han H. et al. Blood glucose concentration and risk of liver cancer: systematic review and meta-analysis of prospective studies. Oncotarget 8, 50164–50173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vulcan A, Manjer J. & Ohlsson B. High blood glucose levels are associated with higher risk of colon cancer in men: a cohort study. BMC Cancer 17, 842 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J. et al. Glucose transporter GLUT1 expression and clinical outcome in solid tumors: a systematic review and meta-analysis. Oncotarget 8, 16875–16886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartzenberg-Bar-Yoseph F, Armoni M. & Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 64, 2627–2633 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Tan Z, Yang C, Zhang X, Zheng P. & Shen W. Expression of glucose transporter 1 and prognosis in non-small cell lung cancer: a pooled analysis of 1665 patients. Oncotarget 8, 60954–60961 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang C. et al. Tumour-associated mutant p53 drives the Warburg effect. Nat. Commun 4, 2935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez OC et al. Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell Cycle 11, 4436–4446 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labuschagne CF, Zani F. & Vousden KH Control of metabolism by p53—cancer and beyond. Biochim. Biophys. Acta Rev. Cancer 1870, 32–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang P. et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 13, 310–316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Xiao J, Zhao Y, Du S. & Du J. Effect of metformin on the mortality of colorectal cancer patients with T2DM: meta-analysis of sex differences. Int. J. Colorectal Dis. 35, 827–835 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Mele L. et al. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 9, 572 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W. et al. Effects of hyperglycemia on the progression of tumor diseases. J. Exp. Clin. Cancer Res 38, 327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mueller F. et al. Gender-specific elimination of continuous-infusional 5-fluorouracil in patients with gastrointestinal malignancies: results from a prospective population pharmacokinetic study. Cancer Chemother. Pharmacol 71, 361–370 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Hu X, Chao M. & Wu H. Central role of lactate and proton in cancer cell resistance to glucose deprivation and its clinical translation. Signal. Transduct. Target. Ther 2, 16047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de la Cruz-Lopez KG, Castro-Munoz LJ, Reyes-Hernandez DO, Garcia-Carranca A. & Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol 9, 1143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ippolito JE, Yim AK, Luo J, Chinnaiyan P. & Rubin JB Sexual dimorphism in glioma glycolysis underlies sex differences in survival. JCI Insight 2, e92142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cao J, Ng M. & Felmlee MA Sex hormones regulate rat hepatic monocarboxylate transporter expression and membrane trafficking. J. Pharm. Pharm Sci 20, 435–444 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Pinheiro C. et al. The metabolic microenvironment of melanomas: prognostic value of MCT1 and MCT4. Cell Cycle 15, 1462–1470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Renner K. et al. Restricting glycolysis preserves T cell effector functions and augments checkpoint therapy. Cell Rep. 29, 135–150.e9 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Beloueche-Babari M. et al. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer 122, 895–903 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hendriks SH et al. Association between body mass index and obesity-related cancer risk in men and women with type 2 diabetes in primary care in the Netherlands: a cohort study (ZODIAC-56). BMJ Open 8, e018859 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Flanagan CH, Smith LA, McDonell SB & Hursting SD When less may be more: calorie restriction and response to cancer therapy. BMC Med. 15, 106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karczewski J. et al. Obesity and the risk of gastrointestinal cancers. Dig. Dis. Sci 64, 2740–2749 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 5, 47–56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wlodarczyk M. & Nowicka G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci 20, 1146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kern L. et al. Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers 11, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chia VM et al. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol. Biomarkers Prev 16, 2697–2703 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Starling S. New therapeutic promise for leptin. Nat. Rev. Endocrinol 15, 625 (2019). [DOI] [PubMed] [Google Scholar]
- 104.Orthofer M. et al. Identification of ALK in thinness. Cell 181, 1246–1262.e22 (2020). [DOI] [PubMed] [Google Scholar]
- 105.Roberts DL, Dive C. & Renehan AG Biological mechanisms linking obesity and cancer risk: new perspectives. Annu. Rev. Med 61, 301–316 (2010). [DOI] [PubMed] [Google Scholar]
- 106.Manieri E. et al. Adiponectin accounts for gender differences in hepatocellular carcinoma incidence. J. Exp. Med 216, 1108–1119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di Zazzo E. et al. Adiponectin as link factor between adipose tissue and cancer. Int. J. Mol. Sci 20, 839 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He L. & Wondisford FE Metformin action: concentrations matter. Cell Metab. 21, 159–162 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Qin Y. et al. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 19, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holloway MG, Miles GD, Dombkowski AA & Waxman DJ Liver-specific hepatocyte nuclear factor-4α deficiency: greater impact on gene expression in male than in female mouse liver. Mol. Endocrinol 22, 1274–1286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baars A. et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep 8, 13426 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Almeida A. et al. A new genomic blueprint of the human gut microbiota. Nature 568, 499–504(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bana B. & Cabreiro F. The microbiome and aging. Annu. Rev. Genet 53, 239–261 (2019). [DOI] [PubMed] [Google Scholar]
- 114.Vemuri R. et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin. Immunopathol 41, 265–275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma ZS & Li W. How and why men and women differ in their microbiomes: medical ecology and network analyses of the microgenderome. Adv. Sci 6, 1902054 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim YS, Unno T, Kim BY & Park MS Sex differences in gut microbiota. World J. Mens Health 38, 48–60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santos-Marcos JA et al. Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol. Nutr. Food Res 63, e1800870 (2019). [DOI] [PubMed] [Google Scholar]
- 118.de la Cuesta-Zuluaga J. et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems 4, e00261–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weger BD et al. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab. 29, 362–382.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Panda S. The arrival of circadian medicine. Nat. Rev. Endocrinol 15, 67–69 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Ma W. et al. Gut microbiota shapes the efficiency of cancer therapy. Front. Microbiol 10, 1050 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cai Y. et al. Sex differences in colon cancer metabolism reveal a novel subphenotype. Sci. Rep 10, 4905 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krall AS, Xu S, Graeber TG, Braas D. & Christofk HR Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun 7, 11457 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmuck R. et al. Gender comparison of clinical, histopathological, therapeutic and outcome factors in 185,967 colon cancer patients. Langenbecks Arch. Surg 405, 71–80 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Y. et al. Gender differences in colorectal cancer survival: a meta-analysis. Int. J. Cancer 141, 1942–1949 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Gubbels Bupp MR, Potluri T, Fink AL & Klein SL The confluence of sex hormones and aging on immunity. Front. Immunol 9, 1269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klein SL & Flanagan KL Sex differences in immune responses. Nat. Rev. Immunol 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Scully EP, Haverfield J, Ursin RL, Tannenbaum C. & Klein SL Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol 20, 442–447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schurz H. et al. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum. Genomics 13, 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taniguchi K. & Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol 18, 309–324 (2018). [DOI] [PubMed] [Google Scholar]
- 131.Marquez EJ et al. Sexual-dimorphism in human immune system aging. Nat. Commun 11, 751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ley K. M1 means kill; M2 means heal. J. Immunol 199, 2191–2193 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Lin Y, Xu J. & Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol 12, 76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown JM, Recht L. & Strober S. The promise of targeting macrophages in cancer therapy. Clin. Cancer Res 23, 3241–3250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li L. et al. TLR8-mediated metabolic control of human Treg function: a mechanistic target for cancer immunotherapy. Cell Metab. 29, 103–123.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ono M. Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology 160, 24–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vasanthakumar A. et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature 579, 581–585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang L, Xu H. & Peng G. TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell Mol. Immunol 15, 428–437 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Martel C, Georges D, Bray F, Ferlay J. & Clifford GM Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health 8, e180–e190 (2020). [DOI] [PubMed] [Google Scholar]
- 140.Guven-Maiorov E, Tsai CJ & Nussinov R. Oncoviruses can drive cancer by rewiring signaling pathways through interface mimicry. Front. Oncol 9, 1236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Te Marvelde L. et al. Epidemiology of sepsis in cancer patients in Victoria, Australia: a population-based study using linked data. Aust. N. Z. J. Public Health 44, 53–58 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Wisnivesky JP & Halm EA Sex differences in lung cancer survival: do tumors behave differently in elderly women? J. Clin. Oncol 25, 1705–1712 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Morgese F. et al. Gender differences and outcomes in melanoma patients. Oncol. Ther 8, 103–114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roers A, Hiller B. & Hornung V. Recognition of endogenous nucleic acids by the innate immune system. Immunity 44, 739–754 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Souyris M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol 3, eaap8855 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Roach JC et al. The evolution of vertebrate Toll-like receptors. Proc. Natl Acad. Sci. USA 102, 9577–9582 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawai T. & Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol 11, 373–384 (2010). [DOI] [PubMed] [Google Scholar]
- 148.Vierbuchen T, Stein K. & Heine H. RNA is taking its toll: impact of RNA-specific Toll-like receptors on health and disease. Allergy 74, 223–235 (2019). [DOI] [PubMed] [Google Scholar]
- 149.Zhang Z. et al. Structural analyses of Toll-like receptor 7 reveal detailed RNA sequence specificity and recognition mechanism of agonistic ligands. Cell Rep. 25, 3371–3381.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Mui UN, Haley CT & Tyring SK Viral oncology: molecular biology and pathogenesis. J. Clin. Med 6, 111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ivashkiv LB & Donlin LT Regulation of type I interferon responses. Nat. Rev. Immunol 14, 36–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Berghofer B. et al. TLR7 ligands induce higher IFN-α production in females. J. Immunol 177, 2088–2096 (2006). [DOI] [PubMed] [Google Scholar]
- 153.Oshiumi H, Sakai K, Matsumoto M. & Seya T. DEAD/H BOX 3 (DDX3) helicase binds the RIG-I adaptor IPS-1 to up-regulate IFN-β-inducing potential. Eur. J. Immunol 40, 940–948 (2010). [DOI] [PubMed] [Google Scholar]
- 154.Phung B. et al. The X-linked DDX3X RNA helicase dictates translation reprogramming and metastasis in melanoma. Cell Rep. 27, 3573–3586.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Libert C, Dejager L. & Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol 10, 594–604 (2010). [DOI] [PubMed] [Google Scholar]
- 156.Klein SL & Morgan R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex. Differ 11, 24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Golden LC et al. Parent-of-origin differences in DNA methylation of X chromosome genes in T lymphocytes. Proc Natl Acad Sci USA 116, 26779–26787 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Menendez D. et al. p53-responsive TLR8 SNP enhances human innate immune response to respiratory syncytial virus. J. Clin. Invest 129, 4875–4884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang PF, Song HF, Zhang Q. & Yan CX Pan-cancer immunogenomic analyses reveal sex disparity in the efficacy of cancer immunotherapy. Eur. J. Cancer 126, 136–138 (2020). [DOI] [PubMed] [Google Scholar]
- 160.Freudenstein D. et al. TP53 status, patient sex, and the immune response as determinants of lung cancer patient survival. Cancers 12, 1535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kruger S. et al. Advances in cancer immunotherapy 2019 — latest trends. J. Exp. Clin. Cancer Res 38, 268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Castro A. et al. Strength of immune selection in tumors varies with sex and age. Nat. Commun 11, 4128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ye Y. et al. Sex-associated molecular differences for cancer immunotherapy. Nat. Commun 11, 1779 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Badami S. et al. Clinical and molecular characteristics associated with survival in advanced melanoma treated with checkpoint inhibitors. J. Oncol 2018, 6279871 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Conforti F. et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J. Natl Cancer Inst 111, 772–781 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wallis CJD et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 5, 529–536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Murthy VH, Krumholz HM & Gross CP Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 291, 2720–2726 (2004). [DOI] [PubMed] [Google Scholar]
- 168.Okyar A. et al. Sex-, feeding-, and circadian time-dependency of P-glycoprotein expression and activity — implications for mechanistic pharmacokinetics modeling. Sci. Rep 9, 10505 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A. & Wargo JA The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 33, 570–580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gong J. et al. The gut microbiome and response to immune checkpoint inhibitors: preclinical and clinical strategies. Clin. Transl Med 8, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schroder W. et al. Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J. Antimicrob. Chemother 71, 1800–1806 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Pinato DJ et al. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J. Immunother. Cancer 7, 287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tibbs TN, Lopez LR & Arthur JC The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microb. Cell 6, 324–334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.De Courcy L, Bezak E. & Marcu LG Gender-dependent radiotherapy: the next step in personalised medicine? Crit. Rev. Oncol. Hematol 147, 102881 (2020). [DOI] [PubMed] [Google Scholar]
- 175.Wagner AD et al. Gender medicine and oncology: report and consensus of an ESMO workshop. Ann. Oncol 30, 1914–1924 (2019). [DOI] [PubMed] [Google Scholar]
- 176.Davidson M. et al. Influence of sex on chemotherapy efficacy and toxicity in oesophagogastric cancer: a pooled analysis of four randomised trials. Eur. J. Cancer 121, 40–47 (2019). [DOI] [PubMed] [Google Scholar]
- 177.Wheatley-Price P. et al. The influence of sex on efficacy, adverse events, quality of life, and delivery of treatment in National Cancer Institute of Canada Clinical Trials Group non-small cell lung cancer chemotherapy trials. J. Thorac. Oncol 5, 640–648 (2010). [DOI] [PubMed] [Google Scholar]
- 178.Yang W. et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl Med 11, eaao5253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ozdemir BC, Csajka C, Dotto GP & Wagner AD Sex differences in efficacy and toxicity of systemic treatments: an undervalued issue in the era of precision oncology. J. Clin. Oncol 36, 2680–2683 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Kim HI, Lim H. & Moon A. Sex differences in cancer: epidemiology, genetics and therapy. Biomol. Ther 26, 335–342 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang L. et al. Sex differences in the expression of drug-metabolizing and transporter genes in human liver. J. Drug. Metab. Toxicol 3, 1000119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Joseph S. et al. Expression of drug transporters in human kidney: impact of sex, age, and ethnicity. Biol. Sex. Differ 6, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bayik D. et al. Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discov. 10, 1210–1225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wagner AD Sex differences in cancer chemotherapy effects, and why we need to reconsider BSA-based dosing of chemotherapy. ESMO Open 5, e000770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Greenberg MVC & Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol 20, 590–607 (2019). [DOI] [PubMed] [Google Scholar]
- 186.Kolberg L, Raudvere U, Kuzmin I, Vilo J. & Peterson H. gprofiler2 — an R package for gene list functional enrichment analysis and namespace conversion toolset g:profiler. F1000Res 10.12688/f1000research.24956.2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Goodwin ML et al. Lactate and cancer: a “lactatic” perspective on spinal tumor metabolism (part 1). Ann. Transl Med 7, 220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sikora MJ, Johnson MD, Lee AV & Oesterreich S. Endocrine response phenotypes are altered by charcoal-stripped serum variability. Endocrinology 157, 3760–3766 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.De Souza Santos R, Frank AP, Palmer BF & Clegg DJ Sex and media: considerations for cell culture studies. ALTEX 35, 435–440 (2018). [DOI] [PubMed] [Google Scholar]
- 190.Bittner GD, Yang CZ & Stoner MA Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environ. Health 13, 41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Austad SN & Fischer KE Sex differences in lifespan. Cell Metab. 23, 1022–1033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Menendez D. et al. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 7, e1001360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Norheim F. et al. Gene-by-sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metab. 29, 932–949.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.[No authors listed] Accounting for sex in the genome. Nat. Med 23, 1243 (2017). [DOI] [PubMed] [Google Scholar]
- 195.Anderson K, Canadas-Garre M, Chambers R, Maxwell AP & McKnight AJ The challenges of chromosome Y analysis and the implications for chronic kidney disease. Front. Genet 10, 781 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Duncan CG et al. Dosage compensation and DNA methylation landscape of the X chromosome in mouse liver. Sci. Rep 8, 10138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garieri M. et al. Extensive cellular heterogeneity of X inactivation revealed by single-cell allele-specific expression in human fibroblasts. Proc. Natl Acad. Sci. USA 115, 13015–13020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cotton AM et al. Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Hum. Mol. Genet 24, 1528–1539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Olney KC, Brotman SM, Andrews JP, Valverde-Vesling VA & Wilson MA Reference genome and transcriptome informed by the sex chromosome complement of the sample increase ability to detect sex differences in gene expression from RNA-seq data. Biol. Sex. Differ 11, 42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rubin JB et al. Sex differences in cancer mechanisms. Biol. Sex. Differ 11, 17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.