Abstract
Declining fitness (VO2peak) is a hallmark of aging and believed to arise from decreased oxygen delivery and reduced muscle oxidative capacity. Physical activity is a modifiable lifestyle factor that is critical when evaluating the effects of age on parameters of fitness and energy metabolism. The objective was to evaluate the effects of age and sex on VO2peak, muscle mitochondrial physiology, and physical activity in young and older adults. An additional objective was to assess the contribution of skeletal muscle oxidative capacity to age-related reductions in VO2peak and determine if age-related variation in VO2peak and muscle oxidative capacity could be explained on the basis of physical activity levels. 23 young and 52 older men and women completed measurements of VO2peak, mitochondrial physiology in permeabilized muscle fibers, and free-living physical activity by accelerometry. Regression analyses were used to evaluate associations between age and VO2peak, mitochondrial function, and physical activity. Significant age-related reductions were observed for VO2peak (P<0.001), but not muscle mitochondrial capacity. Total daily step counts did not decrease with age, but older adults showed lower moderate-to-vigorous physical activity, which was associated with VO2peak (R2=0.323, P<0.001) and muscle oxidative capacity (R2=0.086, P=0.011). After adjusting for sex and physical activity, age was negatively associated with VO2peak but not muscle oxidative capacity. Healthy older adults exhibit lower VO2peak but preserved mitochondrial capacity compared to young. Physical activity, particularly moderate-to-vigorous, is a key factor in observed age-related changes in fitness and muscle oxidative capacity, but cannot entirely explain the age-related reduction in VO2peak.
Keywords: Ageing, Skeletal muscle, Mitochondria, Physical activity
Introduction
The average global life expectancy is rising. Inasmuch, efforts to extend health span require a thorough understanding of the biology of aging and factors that contribute to age-related functional impairments that may limit independence and quality of life. Aging, even healthy aging, is associated with progressive decline in whole-body cardiorespiratory fitness, measured from the maximal rate of oxygen utilization during exercise (VO2peak) (Proctor & Joyner, 1997; Fleg et al., 2005). The negative relationship between VO2peak and all-cause mortality is well-established (Blair et al., 1989; Mandsager et al., 2018). Furthermore, cardiorespiratory fitness is a critical factor in maintaining the ability to perform daily tasks (e.g., walking and stair climbing) and forestalling disability (Fried & Guralnik, 1997; Paterson et al., 2004). Declining VO2peak with aging has been largely attributed to decreased oxygen delivery to peripheral tissues (e.g., decreased cardiac output, decreased muscle blood flow) (Proctor & Joyner, 1997; Betik & Hepple, 2008), compounded by reductions in skeletal muscle oxidative capacity (Lanza et al., 2008), which has been shown to be a determinant of VO2peak in older adults (Coen et al., 2013).
The impact of aging on skeletal muscle mitochondrial biology has been a topic of thoughtful and thorough investigation for several decades using a variety of approaches, including in vivo assessments by 31P magnetic resonance spectroscopy, and ex vivo measurements (enzyme activities, respiration, ATP production) using muscle tissue obtained by biopsy. While some cohorts of older adults exhibit reduced mitochondrial abundance (Conley et al., 2000), enzyme activity (Trounce et al., 1989), respiratory capacity (Lalia et al., 2017; Gonzalez-Freire et al., 2018), and ATP production (Petersen et al., 2003; Short et al., 2005; Lanza et al., 2008; Layec et al., 2013), other painstakingly controlled studies have not revealed any age-related impairments in several different aspects of skeletal muscle mitochondrial physiology using similar methodologies (Rasmussen et al., 2003; Lanza et al., 2005; Hutter et al., 2007; Distefano et al., 2017). These disparate findings have prevented an authoritative consensus on the effects of aging per se on skeletal muscle mitochondrial biology, but have spawned important consideration of a variety of factors that are important to consider such as methodological aspects of muscle biopsy tissue analysis (Picard et al., 2010) and the effects of physical activity and muscle group under investigation (Fitzgerald et al., 2016; Kent & Fitzgerald, 2016).
Lifestyle factors such as habitual physical activity levels are critical when considering the effects of aging on skeletal muscle physiology, cardiorespiratory fitness, and physical function. The effects of exercise on skeletal muscle energy metabolism are solidly established (Holloszy, 1967), and the notion that apparent effects of aging on oxidative capacity are secondary to diminished physical activity in older adults is now becoming mainstream (Fitzgerald et al., 2016; Kent & Fitzgerald, 2016). Indeed, prospective exercise intervention studies demonstrate robust improvements in VO2peak and skeletal muscle metabolism in older adults (Robinson et al., 2017; Berg et al., 2018), yet cross-sectional studies of highly-trained individuals reveal that even chronically endurance trained older adults exhibit declines in VO2peak and modestly altered mitochondrial parameters in skeletal muscle (Proctor & Joyner, 1997; Lanza et al., 2008; Distefano et al., 2018). A recent analysis of data from the Baltimore Longitudinal Study of Aging showed that physical activity was a strong predictor of muscle oxidative capacity measured by 31P-MRS in 384 men and women across a wide age-range (22–92 years) (Adelnia et al., 2019). This report follows on earlier observations that age-related changes in skeletal muscle mitochondrial function can be explained by altered physical activity (Distefano et al., 2017; Distefano et al., 2018). The observations that physical activity patterns explain age-related reductions in muscle oxidative capacity but not whole-body VO2peak lends further support to the notion that central hemodynamics may play a more important role in the age-related reductions of cardiorespiratory fitness than does skeletal muscle mitochondrial capacity. The objective of the current study was to evaluate the effects of age and sex on whole-body cardiorespiratory fitness, skeletal muscle mitochondrial physiology, and habitual physical activity in a group of healthy, untrained young and older adults. An additional objective was to assess the contribution of skeletal muscle oxidative capacity to age-related reductions in VO2peak and determine if age-related variation in VO2peak and muscle oxidative capacity could be explained on the basis of physical activity levels. The main finding of the study is that the age-related decline in VO2peak occurs in the absence of any change in skeletal muscle respiratory capacity, ATP synthesis capacity, or reactive oxygen species production measured in situ in permeabilized muscle fibers. Although total daily step counts did not change with age, older adults had significantly lower levels of moderate-to-vigorous physical activity, which was significantly correlated with VO2peak and muscle oxidative capacity. After adjusting for sex and physical activity levels, age was negatively associated with VO2peak but not muscle oxidative capacity. Together, these results indicate that habitual physical activity, particularly moderate-to-vigorous physical activity, is a key factor in observed age-related changes in fitness and muscle oxidative capacity, but physical inactivity cannot entirely explain the age-related reduction in VO2peak.
Methods
Participants
All study procedures were approved by the Mayo Foundation Institutional Review Board (IRB# 17–004403, ClinicalTrials.gov Identifier: NCT03350906) and conformed to principles outlined in the Declaration of Helsinki. Twenty-three young (20–35 years, 11F/12M) and 52 elderly (65–85 years, 27F/25M) weight-stable adults were recruited from the local community. Participants were independently-living adults who did not participate in structured exercise training (self-reported activity levels <30 min of exercise 3 times per week). Participants with known chronic disease were excluded. Exclusion criteria included anemia (hemoglobin < 11g/dL for females, <12 g/dL for males), diagnosed diabetes (or fasting blood glucose ≥126 mg/dL), cardiovascular disease, impaired coagulation (INR >2.0), liver disease (alanine or aspartate transaminase ≥3 times the upper limit of the normal range), renal disease (serum creatinine >1.4 mg/dL for females, >1.5 mg/dL for males), untreated thyroid disease, or any debilitating musculoskeletal or pulmonary disease that would preclude exercise. Pregnant or breastfeeding females were also excluded from the study. Participants were non-smokers/tobacco-users and did not report any alcohol or substance abuse disorders. Participants taking any medication that may influence the outcomes or increase the risks of the study (e.g., metformin, insulin, tricyclic antidepressants, benzodiazepines, opiates, barbiturates, anticoagulants) were also excluded.
Screening visit
Following an initial screening by phone or email, interested participants reported to the Mayo Clinic Clinical Research and Trials Unit (CRTU) for a screening visit and fasting blood sample used to evaluate eligibility. All study procedures and risks were discussed with the participants, and all participants provided written informed consent. Following consent, height, weight, blood pressure and pulse were recorded, and blood was collected from an antecubital vein. A complete blood count (CBC) with differential and biochemical tests of glucose, insulin, alanine and aspartate transaminase, INR and prothrombin time, creatinine, and thyroid-stimulating hormone were performed by the Mayo Clinic Laboratories to assess participant eligibility. During the screening visit, participants were provided with a 3-axis accelerometer (wGT3X-BT, Actigraph, Pensacola, FL) and instructed to wear the monitor on the hip during all waking hours, except to bathe or during water-based activities, for a period of 2 weeks. Accelerometer data were collected at a 30-Hz sampling frequency and were processed and analyzed in 60-sec epoch lengths using ActiLife software (v6.9.5, Actigraph, Pensacola, FL). Non-wear time was determined using the Choi et al. (Choi et al., 2011) method, and valid days were defined as days with at least 10 hours of validated wear time. Using the cut-points for moderate to vigorous physical activity (MVPA) identified by Freedson et al.(Freedson et al., 1998) MVPA was defined as activity counts ≥1952 counts per minute. For each subject, the average daily step counts and the daily minutes spent in MVPA for 7 valid days, including 2 weekend days, were calculated.
Outpatient Testing Visit
Body composition was assessed by dual-energy X-ray absorptiometry (DEXA; GE Lunar iDXA, GE Healthcare, Chicago, IL). Participants completed a maximal graded treadmill test for the determination of peak whole-body oxygen consumption (VO2peak). Heart rhythm and rate, inspired and expired gases, and oxygen saturation were measured continuously by 12-lead electrocardiogram, breath-by-breath indirect calorimetry, and pulse oximetry, respectively. All tests were monitored by a physician or Ph.D. exercise physiologist trained in clinical exercise testing. The test began after a minute of quiet standing on the treadmill, and treadmill speed and/or incline were increased every 3 minutes in accordance with either the Bruce protocol (Lösse et al., 1979) or the Modified Bruce Protocol (Hossack et al., 1987). Blood pressure was measured during each exercise stage, and the subjects’ rating of perceived exertion (RPE) was assessed in the last 30 seconds of each stage using the Borg 6–20 scale (Borg, 1973). The test was terminated if participants reached volitional exhaustion or at the discretion of the supervising member of the study team based on the following criteria: plateau in VO2 despite increasing intensity, respiratory exchange ratio > 1.10, heart rate > 90% of the age-predicted maximum. The individual VO2peak was identified as the highest average VO2 over an interval lasting at least 15 seconds. Participants met with a dietitian from the Mayo Clinic Metabolic Research Kitchen to discuss food preferences in preparation for 3 days of meals provided to the participants in advance of the inpatient study day. Knee extensor strength was determined from unilateral 1-repetition maximum (1-RM) measurements using a pneumatic resistance leg extension machine (Keiser Air300, Keiser Corporation, Fresno, CA). Participants were habituated to the knee extension exercise and allowed to perform a warm-up set of 10 repetitions at minimal resistance, followed by 3 sets of 5–10 repetitions at progressively increasing resistance prescribed by the investigator based on participant’s perceived exertion of the previous set. Each participant’s 1-RM was determined from a series of single attempts at incremental resistance with 3 minutes of rest between attempts.
Inpatient Study Day
The inpatient study was scheduled at least one week but not more than three months after the VO2 testing visit to avoid potential lingering effects of the exercise testing. For three days prior to the inpatient study day, participants were provided with weight-maintaining meals by the Metabolic Research Kitchen. Participants reported to the metabolic kitchen each morning for weight measurement and breakfast and were given lunch and dinner packages to take with them. On the evening of the third day of the weight-maintaining diet, participants checked into the CRTU at approximately 1700hrs. Following an evening meal at 1800, subjects were fasted until completion of study procedures the following day. At 0830 the following morning, a percutaneous biopsy of the vastus lateralis was performed under local anesthetic (2% lidocaine) using a modified Bergstrom needle. Muscle tissue was rapidly processed at the bedside. A portion of the muscle tissue was prepared fresh for muscle fiber permeabilization and mitochondrial functional measurements and the remainder was frozen in liquid nitrogen and stored at −80°C.
Mitochondrial measurements in permeabilized muscle fibers
Approximately 20mg of muscle tissue was dissected into ~5 mg portions under a dissecting microscope in cold buffer containing 10 mM Ca-EGTA buffer, 0.1 03bcμM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-MES, 0.5 mM DTT, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, pH 7.1 (BIOPS Buffer)(Doerrier et al., 2018). Fiber bundles were mechanically separated using sharp forceps, followed by chemical permeabilization with saponin (50 μg/mL) as previously described (Lanza & Nair, 2009; Lanza et al., 2012). Mitochondrial respiration was assessed in duplicate sets of fibers using an Oxygraph high-resolution respirometer (Oroboros Instruments, Innsbruck, Austria). Fibers were added to the oxygraph chambers containing air-saturated MiR05 buffer, followed by hyperoxygenation of the chamber to ~350 μM. Throughout the subsequent experiment, oxygen concentration was maintained above 200 μM. Oxygen consumption (JO2) was measured throughout a stepwise protocol involving the sequential addition of substrates (10 mM glutamate, 2 mM malate, 10 mM succinate, State 2), 5mM ADP (State 3), 0.5μM rotenone, 2μg/mL oligomycin (state 4), and 2.5 μM antimycin A. Acceptor control (ACR: State 3/State 2) and respiratory control (RCR: State 3 / State 4) ratios were calculated for each sample, although no additional mitochondrial membrane integrity tests were performed. Simultaneous to JO2 measurements, mitochondrial reactive oxygen species (ROS) production (H2O2 flux) was measured by fluorometric monitoring of the oxidation of Amplex Red using the Oxygraph O2K-Fluorescence LED2-Module, as previously described (Abid et al., 2020). The rate of ATP production (JATP) was measured in a separate set of duplicate permeabilized muscle fibers using a spectrofluorometer (Fluorolog 3, Horiba) as described previously (Lark et al., 2016). Importantly, JATP was not measured in hyperoxygenated media. Inasmuch, the measured rates of ATP production are likely to be limited by oxygen diffusion and should be interpreted cautiously alongside JO2 measurements, which were performed at high oxygen saturation. JO2, ATP, and H2O2 production were normalized to tissue wet weight.
Statistical analyses
Data are expressed as mean ± SD. Subject characteristics, body composition, and metabolic parameters were compared between the young and old groups using unpaired 2-tailed student t-tests. Linear regression analyses were used to evaluate associations between age and VO2peak, mitochondrial function, and physical activity. Additional linear regression analysis were performed separately for males and females. Two-way (age, sex) analysis of variance (ANOVA) was used to evaluate the main effects of age, sex, and age-by-sex interactions for all variables. Statistical significance was set a priori at P<0.05. Analyses were performed using the R software environment and GraphPad Prism (v8, GraphPad Software, San Diego, CA).
Results
Participant characteristics
Participant characteristics are provided in Table 1. Anthropometric characteristics, including body mass, and composition, were similar in young and older participants with a non-significant trend toward higher fat mass and lower lean mass in older compared to young. Appendicular skeletal muscle index (ASMI), determined by dividing total appendicular lean mass by the square of height, showed a non-significant trend to be lower in older adults. Leg extension strength, measured from unilateral 1-repetition maximum, was significantly lower in older adults. Systolic blood pressure (SBP) was significantly higher in older adults, as were several blood-based metabolic parameters, including glucose, and total and LDL cholesterol. VO2peak was higher in young compared to older regardless of whether data were presented as absolute oxygen consumption (L/min) or expressed relative to total body mass (mL/kg BW/min) or lean mass (mL/kg lean/min).
Table 1.
Descriptive characteristics.
| Young N=23 | Old N=52 | PAge | |
|---|---|---|---|
| Physical characteristics | |||
| Age (years) | 28 ± 4 | 71 ± 4 | <0.001 |
| Sex (F/M) | 11F/12M | 27F/25M | |
| Height (cm) | 171 ± 9 | 169 ± 10 | 0.36 |
| Weight (kg) | 74.4 ± 11.4 | 74.8 ± 13.6 | 0.92 |
| BMI (kg/m2) | 25.2 ± 2.4 | 26.15 ± 3.6 | 0.25 |
| SBP (mmHg) | 115 ± 9.7 | 129 ± 13 | <0.001 |
| DBP (mmHg) | 71 ± 9 | 74 ± 10 | 0.22 |
| Body composition | |||
| Total body Fat (%) | 32.5 ± 7.4 | 35.4 ± 7.7 | 0.13 |
| Fat, arms (kg) | 2.3 ± 0.7 | 2.6 ± 0.7 | 0.16 |
| Fat, legs (kg) | 8.3 ± 2.5 | 8.0 ± 2.5 | 0.72 |
| Fat, trunk (kg) | 11.7 ± 4.1 | 13.8 ± 5.2 | 0.10 |
| Total lean mass (kg) | 48.1 ± 8.8 | 45.9 ± 9.4 | 0.34 |
| Lean, arms (kg) | 5.5 ± 1.7 | 5.0 ± 1.4 | 0.19 |
| Lean, legs (kg) | 16.7 ± 3.2 | 15.1 ± 3.5 | 0.08 |
| Lean, trunk (kg) | 22.6 ± 3.8 | 22.5 ± 4.4 | 0.92 |
| ASMI (kg/m2) | 7.5 ± 1.2 | 7.0 ± 1.2 | 0.08 |
| Leg extension 1RM (kg) | 65.7 ± 19.3 | 44.0 ± 16.3 | <0.001 |
| Metabolic parameters | |||
| Glucose (mg/dL) | 85.1 ± 7.7 | 93.9 ± 8.0 | <0.001 |
| Insulin (mcIU/mL) | 6.8 ± 2.6 | 7.6 ± 5.4 | 0.51 |
| HOMA-IR | 1.4 ± 0.6 | 1.8 ± 1.5 | 0.24 |
| Cholesterol (mg/dL) | 166 ± 32 | 192 ± 36 | 0.003 |
| HDL (mg/dL) | 56.0 ± 14.8 | 63.3 ± 16.5 | 0.073 |
| LDL (mg/dL) | 94.7 ± 29.4 | 111 ± 30 | 0.038 |
| Triglycerides (mg/dL) | 75.1 ± 30.0 | 92.3 ± 40.2 | 0.070 |
| VO2 peak (L/min) | 2.9 ± 0.8 | 1.9 ± 0.5 | <0.001 |
| VO2 peak (mL/kg BW/min) | 38.6 ± 7.3 | 25.0 ± 5.0 | <0.001 |
| VO2 peak (mL/kg lean/min) | 59.7 ± 8.7 | 40.7 ± 6.4 | <0.001 |
M; Male, F; Female, BMI; body mass index, SBP; systolic blood pressure, DBP; diastolic blood pressure, VO2 peak; peak oxygen uptake, BW; body weight, ASMI; appendicular skeletal muscle index. Data are shown as mean ± SD.
Lower VO2peak in older adults, yet similar skeletal muscle oxidative capacity to young
Increasing age was associated with a decrease in VO2peak normalized to total body weight (Figure 1A) and normalized to lean mass (Figure 1C). When both age and sex were included in the model, there were significant main effects of age (lower in older compared to young) and sex (lower in females compared to males) (Figure 1B, 1D). Although the interaction terms was not statistically significant, there were notable trends whereby males show a non-significant tendency for greater reduction in VO2peak with age compared with females as shown in the boxplots of Figures 1B and 1D. There was no significant association between age and skeletal muscle mitochondrial function, evaluated from 3 distinct parameters measured in permeabilized muscle fibers. Young and older adults demonstrated similar acceptor control ratios (ACR; young = 5.10 ± 0.52, older = 5.08 ± 0.65, P = 0.92) and respiratory control ratios (RCR; young = 4.96 ± 0.71, older = 5.06 ± 1.00, P = 0.66), indicative of no systematic difference in the quality of the permeabilized muscle fiber preparations between young and older adults, although no specific measurements of mitochondrial membrane integrity were performed. The maximal rates of oxygen consumption in permeabilized muscle fibers under ADP-stimulated conditions were not associated with age (Figure 1E), nor were there significant main effects of age or sex or age-by-sex interactions (Figure 1F). Similarly, the maximal rates of ATP production (Figure 1G, 1H) and ROS production (Figure 1I, 1J) showed no significant association with age or sex. There was a significant age-by-sex interaction for maximal ATP production whereby young, but not older females exhibited higher JATP (Figure 1H). Indeed, separate regression analyses for males and females across the age range revealed a significant negative association between age and JATP in females but not males (Figure 1G). In sum, the data in Figure 1 reveal a significant age-related reduction in whole-body cardiorespiratory fitness that was not accompanied by any evidence of altered mitochondrial physiology in skeletal muscle, evaluated from 3 independent assays.
Figure 1. VO2peak declines with aging in the absence of age-related changes in mitochondrial respiratory capacity, ATP production capacity, or hydrogen peroxide emissions in skeletal muscle.
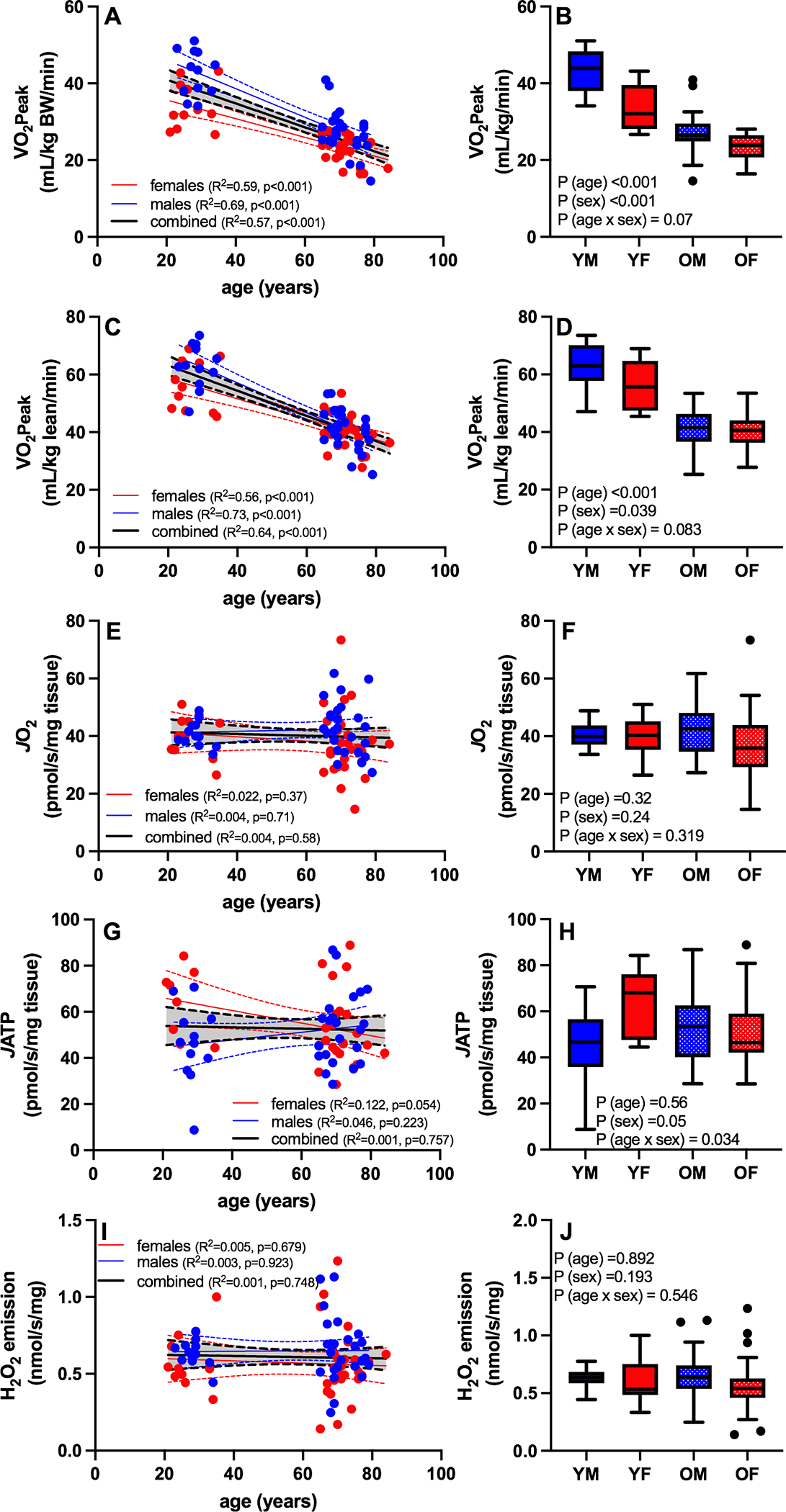
Whole body cardiorespiratory fitness and skeletal muscle mitochondrial function were assessed in young and older males and females, and the relationships between age and age/sex groups were assessed by linear regression. Increasing age was associated with a decrease in VO2peak normalized to total body weight (A,B) or lean mass (C,D). VO2peak was lower in older compared to young and females compared to males regardless of whether normalized to body weight or lean mass (B,D). There was no significant association between age or sex and the maximal rates of oxygen consumption in permeabilized muscle fibers (E,F), ATP production (G, H), or ROS production (I, J). Blue symbols are males and red symbols are females. YM: young male; YF: young female; OM: old male; OF: old female; BW: body weight; JO2: maximal ADP-stimulated oxygen consumption; JATP; maximal ADP-stimulated ATP production. Boxplots show the 25th and 75th percentiles (box), median (line), and whiskers defined by largest and smallest values within 1.5 times the interquartile range with individual points representing values beyond this range.
The role of physical activity, particularly MVPA, in aging
There was no significant association between age and total physical activity, quantified from average daily step counts (Figure 2A), nor did total step counts differ by sex (Figure 2B). Despite similarity in total step counts, the average time spent in moderate-to-vigorous physical activity (MVPA) was negatively associated with age (Figure 2C). When both age and sex were included in the model, there were significant main effects of age (lower in older compared to young) and sex (lower in females compared to males) (Figure 2D). No age-by-sex interaction was observed for MVPA by 2-way ANOVA, but individual regression analyses revealed a significant negative association between age and MVPA in males but not females (Figure 2C).
Figure 2. The role of physical activity, particularly MVPA, in aging.
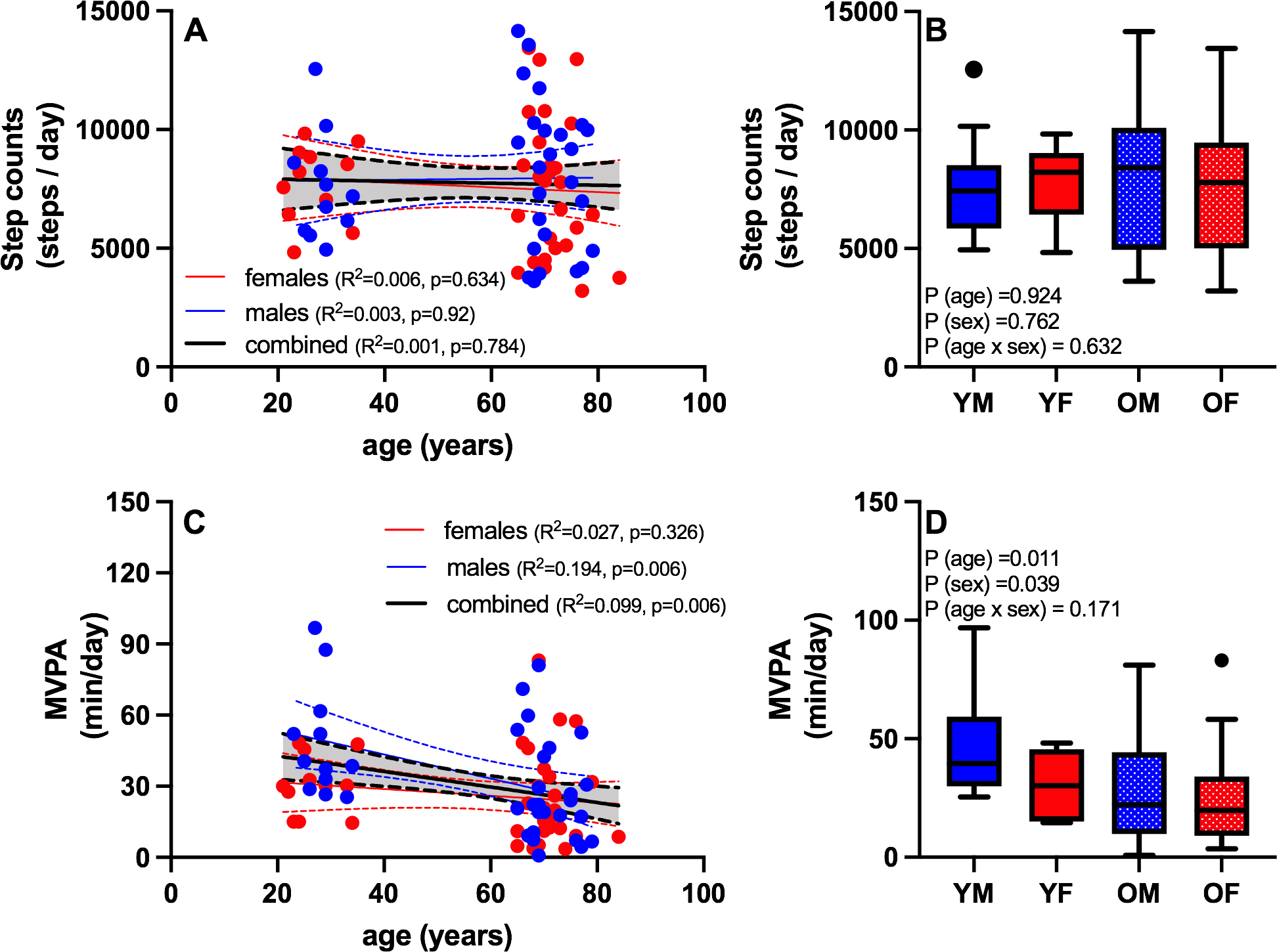
Free-living habitual physical activity levels were assessed by accelerometry. Total physical activity, defined by average daily step counts, was not significantly associated with age (A), nor did total step counts differ by age or sex (B). Despite similarity in total step counts, the average time spent in moderate-to-vigorous physical activity (MVPA) was negatively associated with age (C), and lower in older compared to young and females compared to males (D). Blue symbols are males and red symbols are females. YM: young male; YF: young female; OM: old male; OF: old female. Boxplots show the 25th and 75th percentiles (box), median (line), and whiskers defined by largest and smallest values within 1.5 times the interquartile range with individual points representing values beyond this range.
The relationship between VO2peak, mitochondrial capacity, and physical activity
Whole-body VO2peak was positively associated with total step counts (Figure 3A) and MVPA (Figure 3B), and MVPA was a stronger determinant of VO2peak (R2 = 0.323, P <0.001) than total step counts (R2 = 0.08, P = 0.014). The association between step count and VO2 peak was not statistically significant when males and females were examined with separate regression models (Figure 3A), but interestingly the association between MVPA and VO2 peak was stronger in males compared to females (Figure 3B). Modest but significant positive associations were evident between skeletal muscle oxidative capacity (JO2) and step counts (Figure 3C) and MVPA (Figure 3D). A modest but significant positive association was evident for VO2peak and JO2 (Figure 3E), even after adjusting for age and sex (P=0.0245). The relationship between VO2 peak normalized to lean mass and JO2 was similar but did not reach statistical significance (Figure 3F). Multiple regression was used to determine if age-related changes in cardiorespiratory fitness and muscle oxidative capacity could be explained on the basis of physical activity levels. After adjusting for sex and physical activity levels, age was negatively associated with VO2peak (P < 0.001) but not muscle oxidative capacity (P = 0.546).
Figure 3. The relationship between VO2peak, mitochondrial parameters, and physical activity.
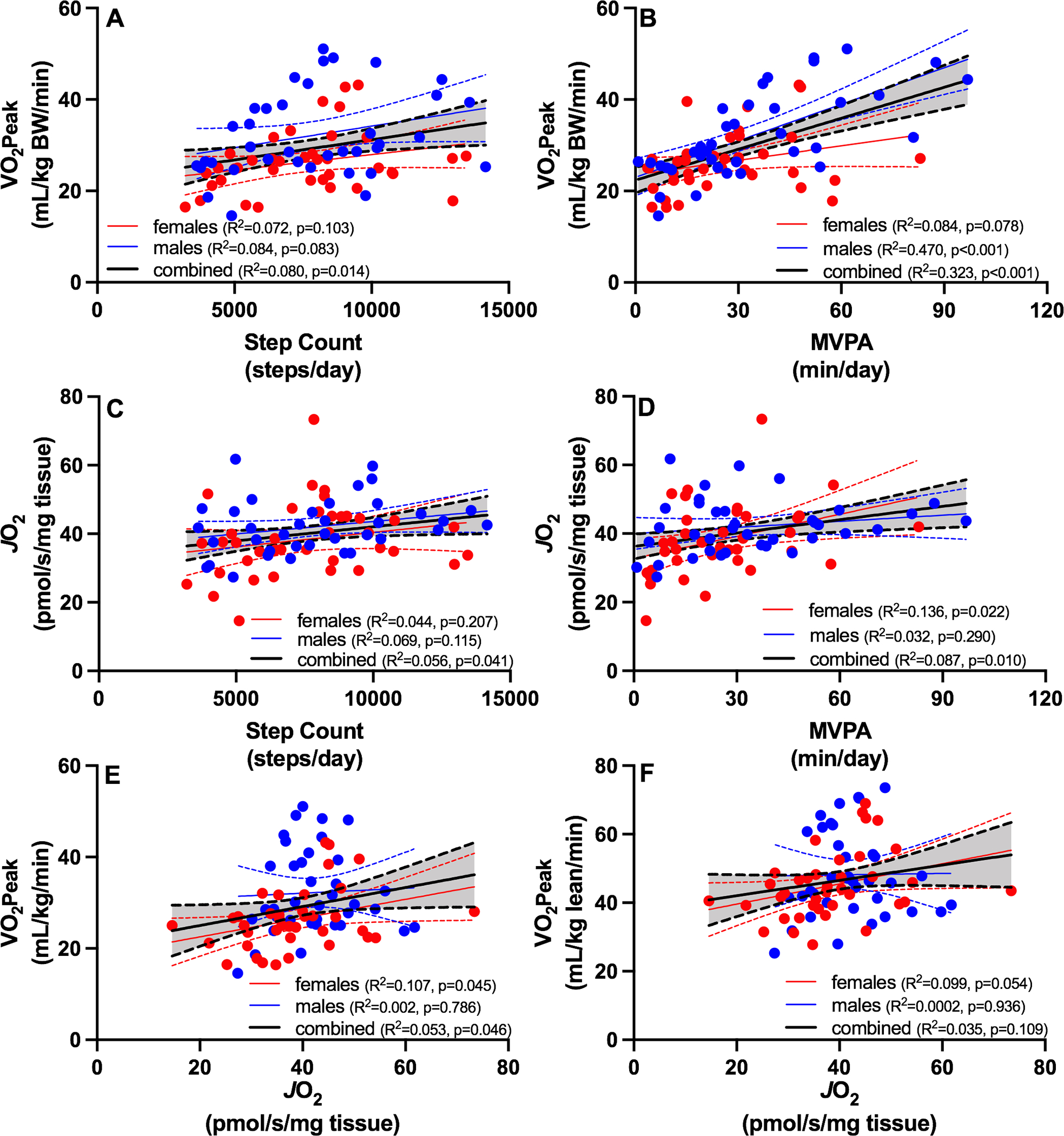
Regression analyses were used to evaluate relationships between physical activity (total and moderate-to-vigorous), cardiorespiratory fitness (VO2peak) and muscle oxidative capacity (JO2). Whole-body VO2peak was positively associated with total step counts (A) and MVPA (B). Modest but significant positive associations were evident between skeletal muscle oxidative capacity (JO2) and step counts (C) and MVPA (D). A modest but significant positive association was evident for VO2peak and JO2 regardless of whether VO2 peak is normalized to total body weight (E) or lean mass (F). Blue symbols are males and red symbols are females. YM: young male; YF: young female; OM: old male; OF: old female. BW: body weight; JO2: maximal ADP-stimulated oxygen consumption; MVPA: moderate to vigorous physical activity.
Discussion
Declining whole-body cardiorespiratory fitness is a hallmark of aging and believed to arise as a result of a combination of decreased oxygen delivery (Proctor & Joyner, 1997; Betik & Hepple, 2008), and reduced muscle oxidative capacity in older adults (Lanza et al., 2008; Coen et al., 2013). Although many studies document skeletal muscle mitochondrial abnormalities with aging (Trounce et al., 1989; Conley et al., 2000; Petersen et al., 2003; Short et al., 2005; Lanza et al., 2008; Layec et al., 2013; Lalia et al., 2017; Gonzalez-Freire et al., 2018), by no means is this universally accepted doctrine as many others have shown that several different muscle mitochondrial parameters are well-maintained into old age (Rasmussen et al., 2003; Lanza et al., 2005; Hutter et al., 2007; Distefano et al., 2017). An emerging concept is that many age-related metabolic and functional derangements are likely secondary to modifiable lifestyle factors such as physical activity rather than predestined by chronological age (Fitzgerald et al., 2016; Kent & Fitzgerald, 2016; Distefano et al., 2017; Adelnia et al., 2019). The current study was designed to further evaluate this concept through rigorous cardiorespiratory fitness testing, comprehensive assessment of salient components of skeletal muscle mitochondrial physiology, and objective quantitation of free-living physical activity levels in a cohort of healthy, community-dwelling young and older men and women. The study demonstrates that VO2peak decreases with aging in the absence of any change in several key skeletal muscle mitochondrial parameters. Furthermore, moderate-to-vigorous physical activity levels decreased with age and were significantly associated with VO2peak and muscle oxidative capacity. Declining physical activity levels with aging could not entirely explain the age-related reduction in VO2peak, but the data indicate that habitual physical activity, particularly moderate-to-vigorous physical activity, is a key determinant of cardiorespiratory fitness and muscle oxidative capacity with aging.
Lower VO2peak in older compared to young adults despite similar mitochondrial capacity in skeletal muscle
It is well-accepted that VO2peak, a benchmark for cardiorespiratory fitness, declines with age even in people who maintain elite levels of physical activity (Proctor & Joyner, 1997; Fleg et al., 2005; Distefano et al., 2018). Less clear, however, are the precise factors that contribute to decreased VO2peak with aging. A primary underlying mechanism is decreased oxygen delivery as a result of decreased cardiac output and blood flow to exercising muscle tissue (Proctor & Joyner, 1997; Betik & Hepple, 2008), which are considered to be the primary factors limiting VO2peak (Saltin & Calbet, 2006; Spurway et al., 2012). Skeletal muscle oxidative capacity (i.e., mitochondrial function) is also an important determinant of VO2peak as suggested from strong associations between muscle succinate dehydrogenase activity and VO2peak in heart failure patients, healthy controls, and trained cyclists (van der Zwaard et al., 2016). Under some circumstances, muscle oxidative capacity may decrease below a threshold where it becomes a limiting factor for VO2peak. It is conceivable that reduced muscle oxidative capacity with aging could contribute to declining VO2peak; a possibility that is supported by precedent literature (Coen et al., 2013). In the current study, we observed a modest yet statistically significant positive association between VO2peak and muscle oxidative capacity (R2=0.053, P=0.046), but the marked decline in VO2peak was observed in the absence of any change in muscle mitochondrial function in older adults. This finding aligns well with a prior study by Distefano and colleagues demonstrating that high-levels of endurance exercise effectively maintains muscle mitochondrial capacity despite lower VO2 peak compared to similarly active young adults (Distefano et al., 2018). Hence, our study does not support the idea that age-related changes in mitochondrial function in skeletal muscle contribute to reductions in whole-body VO2peak and point to central hemodynamics as primary factors, as suggested previously (Proctor & Joyner, 1997; Poole et al., 2003; Betik & Hepple, 2008).
A large number of animal and human studies have examined the influence of aging on skeletal muscle mitochondrial physiology. While some reports provide compelling evidence supporting age-related changes in mitochondrial physiology (Trounce et al., 1989; Conley et al., 2000; Petersen et al., 2003; Short et al., 2005; Lanza et al., 2008; Layec et al., 2013; Lalia et al., 2017; Gonzalez-Freire et al., 2018), others do not (Rasmussen et al., 2003; Lanza et al., 2005; Hutter et al., 2007; Distefano et al., 2017), fueling ongoing debate on this topic. In the current study, we do not find any evidence of altered skeletal muscle mitochondrial physiology from the standpoint of muscle oxidative capacity, ATP production capacity, or reactive oxygen species production. The similarity in mitochondrial capacity in this cohort of young and older adults is at odds with previous studies from our laboratory where we reported significantly decreased ATP production rates (Lanza et al., 2008) and respiratory capacity (Lalia et al., 2017) in mitochondria isolated from muscle biopsy tissue from similar cohorts of older adults (i.e., healthy, community dwelling, non-frail). A key distinction that is likely to explain this disparity is that mitochondrial function was evaluated in permeabilized muscle fibers rather than isolated mitochondrial preparations. This supposition is based on the earlier paper by Picard and colleagues (Picard et al., 2010) where isolated mitochondrial preparations, which strip away important intracellular regulatory systems and selectively harvest damaged organelles, artificially amplify otherwise modest changes in muscle mitochondrial function with age. In contrast, assessment of mitochondrial function in permeabilized fibers preserves organelle morphology, maintains intracellular regulation, and represents the entire population of mitochondria. Indeed, many studies where muscle mitochondrial function was assessed in situ or in vivo have shown modest effects of age (Lanza et al., 2005; Hutter et al., 2007; Picard et al., 2010; Distefano et al., 2017). While we provide evidence for maintained skeletal muscle mitochondrial respiration and ATP production with aging, a key caveat of this study is the absence of any measurements of mitochondrial content, which prevents any conclusions regarding preservation or loss of mitochondrial mass in aging skeletal muscle. Another important consideration is the population of older adults being studied. Here we included healthy, community-dwelling older adults without diagnosis of chronic disease, nor did any participants exhibit evidence of frailty or mobility limitations. A limitation, however, is that we did not formally evaluate the presence of sarcopenia in this cohort. Appendicular skeletal muscle index (ASMI), a purported index for sarcopenia, was not remarkably different between young and older adults, however 10 older adults (5 men, 5 women) had ASMI values that fell below the sex-specific cutoffs associated with sarcopenia (men: 7.26 kg/m2, women: 5.45 kg/m2) (Baumgartner et al., 1998). We interpret this as evidence for some early signs of age-related muscle loss in a fraction of the older adults in this study, but do not observe obvious signs of sarcopenia in this cohort of older adults. It is important to highlight that observations in this segment of the aging population (i.e., successful aging) must not be generalized to the aging population as a whole, particularly since many age-related comorbidities do not develop until the 9th decade of life (Kent & Fitzgerald, 2016). To help achieve consensus, it is critical that future aging studies include older adults with mobility impairments, frailty, and more advanced sarcopenia to maximize external validity. Although invasive procedures are more difficult to rationalize in these populations, non-invasive methods to study skeletal muscle structure, function, and metabolism are well-suited to this purpose.
The role of physical activity, particularly MVPA, in aging.
Muscle mitochondrial function and whole-body cardiorespiratory fitness are exquisitely responsive to physical activity (Holloszy, 1967; Hickson et al., 1977), and exercise has been shown to forestall many cardiovascular and metabolic abnormalities with aging (Proctor & Joyner, 1997; Lanza et al., 2008; Robinson et al., 2017; Berg et al., 2018). Inasmuch, it is important to include objective, sensitive measurements of habitual physical activity levels when evaluating the true effects of aging on VO2peak or muscle mitochondrial function (Russ & Lanza, 2011). Advances in wearable device technology have led to development and validation (Sasaki et al., 2011) of physical activity monitors to allow objective quantitation of free-living physical activity patterns. In the current study we assessed free-living physical activity over a two week period. Although total physical activity (step counts) did not differ in young and older adults, the time spent in moderate-to-vigorous physical activity was significantly lower in older adults and negatively associated with age. Although 7,000–10,000 steps/day is generally recognized as a goal to promote good health in older adults (Tudor-Locke et al., 2011), the time engaged in MVPA was shown to be independently associated with physical function (Wu et al., 2017; Adachi et al., 2018). Extending this concept, we show that MVPA is a stronger predictor of VO2peak and skeletal muscle oxidative capacity than total step counts, underscoring the importance of intensity of physical activity. After adjusting for sex and physical activity levels, age was negatively associated with VO2peak but not muscle oxidative capacity, suggesting that habitual moderate-to-vigorous physical activity is a key factor in age-related changes in fitness and muscle oxidative capacity, but physical inactivity cannot entirely explain age-related reductions in VO2peak.
Supplementary Material
Key Points.
Healthy older adults exhibit lower cardiorespiratory fitness (VO2peak) than young in the absence of any age-related difference in skeletal muscle mitochondrial capacity, suggesting central hemodynamics plays a larger role in age-related declines in VO2peak.
Total physical activity did not differ by age, but moderate-to-vigorous physical activity was lower in older compared to young adults.
Moderate-to-vigorous physical activity is associated with VO2peak and muscle oxidative capacity, but physical inactivity cannot entirely explain the age-related reduction in VO2peak.
Acknowledgments:
The authors thank Bobbie Soderberg and Vicky Wade for biopsy support and assistance and Jocelyn Stepanek for assistance with the accelerometry data analysis. We are grateful to Drs. Adrian Vella, Michael Jensen, and K. Sreekumaran Nair for clinical support of these studies. Work was also supported by the staff at the Mayo Clinic Clinical Research and Trials Unit.
Funding:
Hawley E. Kunz was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases for the Musculoskeletal Research Training Program (T32AR56950). This project was supported by Grant Numbers UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS) and R01 AG054454 from the National Institute on Aging. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Competing interests: No conflicts of interest relevant to this article were reported.
Data availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abid H, Ryan ZC, Delmotte P, Sieck GC & Lanza IR. (2020). Extramyocellular interleukin-6 influences skeletal muscle mitochondrial physiology through canonical JAK/STAT signaling pathways. FASEB J. [DOI] [PubMed] [Google Scholar]
- Adachi T, Kono Y, Iwatsu K, Shimizu Y & Yamada S. (2018). Duration of moderate to vigorous daily activity is negatively associated with slow walking speed independently from step counts in elderly women aged 75 years or over: A cross-sectional study. Archives of gerontology and geriatrics 74, 94–99. [DOI] [PubMed] [Google Scholar]
- Adelnia F, Urbanek J, Osawa Y, Shardell M, Brennan NA, Fishbein KW, Spencer RG, Simonsick EM, Schrack JA & Ferrucci L. (2019). Moderate-to-Vigorous Physical Activity Is Associated With Higher Muscle Oxidative Capacity in Older Adults. J Am Geriatr Soc 67, 1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ & Lindeman RD. (1998). Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147, 755–763. [DOI] [PubMed] [Google Scholar]
- Berg OK, Kwon OS, Hureau TJ, Clifton HL, Thurston T, Le Fur Y, Jeong EK, Amann M, Richardson RS, Trinity JD, Wang E & Layec G. (2018). Maximal strength training increases muscle force generating capacity and the anaerobic ATP synthesis flux without altering the cost of contraction in elderly. Exp Gerontol 111, 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betik AC & Hepple RT. (2008). Determinants of VO2 max decline with aging: an integrated perspective. Appl Physiol Nutr Metab 33, 130–140. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH& Gibbons LW. (1989). Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262, 2395–2401. [DOI] [PubMed] [Google Scholar]
- Borg GA. (1973). Perceived exertion: a note on “history” and methods. Med Sci Sports 5, 90–93. [PubMed] [Google Scholar]
- Choi L, Liu Z, Matthews CE & Buchowski MS. (2011). Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 43, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FG, Shankland E, Conley KE & Goodpaster BH. (2013). Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci 68, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Esselman PC, Jubrias SA, Cress ME, Inglin B, Mogadam C & Schoene RB. (2000). Ageing, muscle properties and maximal O(2) uptake rate in humans. J Physiol 526 Pt 1, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano G, Standley RA, Dube JJ, Carnero EA, Ritov VB, Stefanovic-Racic M, Toledo FG, Piva SR, Goodpaster BH & Coen PM. (2017). Chronological Age Does not Influence Ex-vivo Mitochondrial Respiration and Quality Control in Skeletal Muscle. J Gerontol A Biol Sci Med Sci 72, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano G, Standley RA, Zhang X, Carnero EA, Yi F, Cornnell HH & Coen PM. (2018). Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle 9, 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Meszaros AT & Gnaiger E. (2018). High-Resolution FluoRespirometry and OXPHOS Protocols for Human Cells, Permeabilized Fibers from Small Biopsies of Muscle, and Isolated Mitochondria. Methods Mol Biol 1782, 31–70. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LF, Christie AD & Kent JA. (2016). Heterogeneous effects of old age on human muscle oxidative capacity in vivo: a systematic review and meta-analysis. Appl Physiol Nutr Metab 41, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG & Lakatta EG. (2005). Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112, 674–682. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E & Sirard J. (1998). Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30, 777–781. [DOI] [PubMed] [Google Scholar]
- Fried LP & Guralnik JM. (1997). Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc 45, 92–100. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Freire M, Scalzo P, D’Agostino J, Moore ZA, Diaz-Ruiz A, Fabbri E, Zane A, Chen B, Becker KG, Lehrmann E, Zukley L, Chia CW, Tanaka T, Coen PM, Bernier M, de Cabo R & Ferrucci L. (2018). Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson RC, Bomze HA & Holloszy JO. (1977). Linear increase in aerobic power induced by a strenuous program of endurance exercise. Journal of applied physiology: respiratory, environmental and exercise physiology 42, 372–376. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. (1967). Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242, 2278–2282. [PubMed] [Google Scholar]
- Hossack K, Eldridge J, Wolfel E, Leddy C & Berger N. (1987). Aerobic responses to low level exercise testing following an acute myocardial infarction. Am Heart J 113, 694–699. [DOI] [PubMed] [Google Scholar]
- Hutter E, Skovbro M, Lener B, Prats C, Rabol R, Dela F & Jansen-Durr P. (2007). Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 6, 245–256. [DOI] [PubMed] [Google Scholar]
- Kent JA & Fitzgerald LF. (2016). In vivo mitochondrial function in aging skeletal muscle: capacity, flux, and patterns of use. Journal of applied physiology 121, 996–1003. [DOI] [PubMed] [Google Scholar]
- Lalia AZ, Dasari S, Robinson MM, Abid H, Morse DM, Klaus KA & Lanza IR. (2017). Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging (Albany NY) 9, 1096–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE & Kent-Braun JA. (2005). Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. Journal of applied physiology 99, 1736–1744. [DOI] [PubMed] [Google Scholar]
- Lanza IR & Nair KS. (2009). Functional assessment of isolated mitochondria in vitro. Methods Enzymol 457, 349–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP & Nair KS. (2008). Endurance exercise as a countermeasure for aging. Diabetes 57, 2933–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR 3rd, Dasari S, Walrand S, Short KR, Johnson ML, Robinson MM, Schimke JM, Jakaitis DR, Asmann YW, Sun Z& Nair KS. (2012). Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab 16, 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark DS, Torres MJ, Lin CT, Ryan TE, Anderson EJ & Neufer PD. (2016). Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. Am J Physiol Cell Physiol 311, C239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layec G, Haseler LJ & Richardson RS. (2013). Reduced muscle oxidative capacity is independent of O2 availability in elderly people. Age (Dordr) 35, 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösse B, Krönert H, Rafflenbeul D, Feinendegen LE & Loogen F. (1979). [Sensitivity and accuracy of thallium-201 myocardial scintigraphy in the detection of coronary artery and myocardial disease (author’s transl)]. Z Kardiol 68, 429–435. [PubMed] [Google Scholar]
- Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE & Jaber W. (2018). Association of Cardiorespiratory Fitness With Long-term Mortality Among Adults Undergoing Exercise Treadmill Testing. JAMA Netw Open 1, e183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DH, Govindasamy D, Vidmar M, Cunningham DA & Koval JJ. (2004). Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc 52, 1632–1638. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW & Shulman GI. (2003). Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL, Taivassalo T & Hepple RT. (2010). Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 9, 1032–1046. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C & Richardson RS. (2003). Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284, H1251–1259. [DOI] [PubMed] [Google Scholar]
- Proctor DN & Joyner MJ. (1997). Skeletal muscle mass and the reduction of VO2max in trained older subjects. Journal of applied physiology 82, 1411–1415. [DOI] [PubMed] [Google Scholar]
- Rasmussen UF, Krustrup P, Kjaer M & Rasmussen HN. (2003). Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch 446, 270–278. [DOI] [PubMed] [Google Scholar]
- Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR & Nair KS. (2017). Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab 25, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW & Lanza IR. (2011). The impact of old age on skeletal muscle energetics: supply and demand. Curr Aging Sci 4, 234–247. [DOI] [PubMed] [Google Scholar]
- Saltin B & Calbet JA. (2006). Point: in health and in a normoxic environment, VO2 max is limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol (1985) 100, 744–745. [DOI] [PubMed] [Google Scholar]
- Sasaki JE, John D & Freedson PS. (2011). Validation and comparison of ActiGraph activity monitors. J Sci Med Sport 14, 411–416. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S & Nair KS. (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proceedings of the National Academy of Sciences of the United States of America 102, 5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurway NC, Ekblom B, Noakes TD & Wagner PD. (2012). What limits [V(·)]O(2max)? A symposium held at the BASES Conference, 6 September 2010. J Sports Sci 30, 517–531. [DOI] [PubMed] [Google Scholar]
- Trounce I, Byrne E & Marzuki S. (1989). Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet 1, 637–639. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, Ewald B, Gardner AW, Hatano Y, Lutes LD, Matsudo SM, Ramirez-Marrero FA, Rogers LQ, Rowe DA, Schmidt MD, Tully MA & Blair SN. (2011). How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 8, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaard S, de Ruiter CJ, Noordhof DA, Sterrenburg R, Bloemers FW, de Koning JJ, Jaspers RT & van der Laarse WJ. (2016). Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J Appl Physiol (1985) 121, 636–645. [DOI] [PubMed] [Google Scholar]
- Wu F, Wills K, Laslett LL, Oldenburg B, Jones G & Winzenberg T. (2017). Moderate-to-Vigorous Physical Activity But Not Sedentary Time Is Associated With Musculoskeletal Health Outcomes in a Cohort of Australian Middle-Aged Women. J Bone Miner Res 32, 708–715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


